Abstract
Introduction
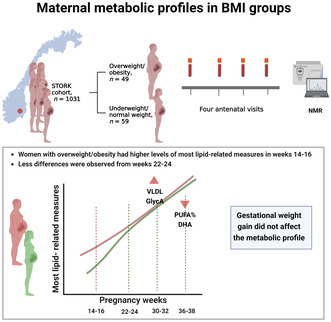
Increased BMI has been identified as a risk factor for most pregnancy complications, but the underlying metabolic factors mediating the detrimental effects of BMI are largely unknown. We aimed to compare metabolic profiles in overweight/obese women (body mass index [BMI] ≥ 25 kg/m2) and normal weight/underweight women (BMI < 25 kg/m2) across gestation. We also explored how gestational weight gain (GWG) affected maternal metabolic profiles.
Material and methods
Exploratory nested case–control study based on a prospective longitudinal cohort of women who were healthy prior to pregnancy and gave birth at Oslo University Hospital from 2002 to 2008. The sample consisted of 48 women who were overweight/obese and 59 normal‐weight/underweight women. Plasma samples from four time points in pregnancy (weeks 14–16, 22–24, 30–32 and 36–38) were analyzed by nuclear magnetic resonance spectroscopy and 91 metabolites were measured. Linear regression models were fitted for each of the metabolites at each time point.
Results
Overweight or obese women had higher levels of lipids in very‐low‐density lipoprotein (VLDL), total triglycerides, triglycerides in VLDL, total fatty acids, monounsaturated fatty acids, saturated fatty acids, leucine, valine, and total branched‐chain amino acids in pregnancy weeks 14–16 compared to underweight and normal‐weight women. Docosahexaenoic acid and degree of unsaturation were significantly lower in overweight/obese women in pregnancy weeks 36–38. In addition, overweight or obese women had higher particle concentration of XXL‐VLDL and glycoprotein acetyls (GlycA) at weeks 14–16 and 30–32. GWG did not seem to affect the metabolic profile, regardless of BMI group when BMI was treated as a dichotomous variable, ≥25 kg/m2 (yes/no).
Conclusions
Overweight or obese women had smaller pregnancy‐related metabolic alterations than normal‐weight/underweight women. There was a trend toward higher triglyceride and VLDL particle concentration in overweight/obese women. As this was a hypothesis‐generating study, the similarities with late‐onset pre‐eclampsia warrant further investigation. The unfavorable development of fatty acid composition in overweight/obese women, with possible implication for the offspring, should also be studied further in the future.
Keywords: body mass index, gestational weight gain, high‐risk pregnancy, metabolomics, molecular biology
Overweight or obese women had elevated levels of most lipid‐related measures in early pregnancy, but the differences were attenuated from weeks 22–24. Particle concentration of very‐low‐density lipoprotein and GlycA were increased throughout pregnancy in overweight or obese women meanwhile the ratio of polyunsaturated fatty acids to total fatty acids (in particular docosahexaenoic acid) were decreased. Regardless of BMI group, gestational weight gain did not affect the metabolic profile.
Abbreviations
- BCAA
branched‐chain amino acids
- BMI
body mass index
- CI
confidence interval
- DHA
docosahexaenoic acid
- GDM
gestational diabetes mellitus
- GlycA
glycoprotein acetyls
- GWG
gestational weight gain
- PUFA
polyunsaturated fatty acids
- SD
standard deviation
- VLDL
very‐low‐density lipoprotein
Key message.
When metabolic profiles in overweight or obese women were compared to normal weight or underweigh women, the largest differences were observed in early pregnancy. Further studies are warranted to evaluate the findings of this explorative study.
1. INTRODUCTION
Obesity in pregnancy is associated with increased risk of many pregnancy complications, including gestational hypertension, pre‐eclampsia, gestational diabetes mellitus (GDM), and giving birth to a large‐for‐gestational‐age (LGA) infant, which in turn may have adverse long‐term effects on the offspring. 1 , 2 The underlying mechanisms are largely unknown, but may be mediated by maternal metabolism.
High body mass index (BMI) is associated with dysregulated metabolism, a common feature of metabolic diseases such as diabetes mellitus and cardiovascular disease. 3 , 4 Pregnancy requires physiological adaptations to secure maternal and fetal nutritional demands, including profound changes in the maternal lipid and glucose metabolism. Maternal insulin sensitivity is decreased to enhance the availability of maternal glucose and fatty acids essential for fetal growth. 5
We have previously shown that women entering pregnancy with a high BMI had dysregulated metabolic status in early pregnancy and differential longitudinal patterns of metabolites during pregnancy compared to women with normal weight. 6 The metabolism of women with overweight and obesity was characterized by less accentuated increase in lipids but exaggerated increase in glucose and insulin compared to normal‐weight women. By detailed metabolic characterization of healthy pregnant women compared to women with late‐onset pre‐eclampsia, we showed differences in metabolites between these two groups already in early pregnancy. 7 Near term, women with pre‐eclampsia developed a metabolic profile that resembled the metabolic alterations observed in atherosclerotic disease, including elevated levels of total lipids, very‐low‐density lipoprotein (VLDL), triglycerides, and total fatty acids. We further showed that the metabolic differences in early pregnancy were largely driven by maternal BMI, whereas there were pre‐eclampsia‐specific differences toward the end of pregnancy.
Gestational weight gain (GWG) comprises the fetus, placenta, amniotic fluid, blood volume, and fat deposits and is a modifiable factor associated with several pregnancy outcomes, such as fetal growth, placental weight, GDM, hypertensive disorders of pregnancy, and long‐term metabolic health in the offspring. 8 , 9 , 10 , 11 However, how GWG affects the maternal metabolism, is largely unknown.
Exploring the metabolic profile throughout pregnancy in overweight and obese women compared to normal‐weight women may identify important metabolic measures with therapeutic potential. In addition, these metabolic profiles may provide valuable knowledge of metabolites mediating the association between maternal metabolism and offspring burden of disease.
We hypothesized that increased maternal BMI and GWG would be associated with atherogenic lipid alterations and that these alterations would be exacerbated close to term.
The main aim of the present study was to make an exploratory comparison of metabolic profiles across gestation between pregnant women within two different BMI groups. In addition, we aimed to explore how GWG affects the maternal plasma metabolome.
2. MATERIAL AND METHODS
2.1. Design and study sample
For this exploratory nested case–control study, we used a subset (n = 107) of the prospective longitudinal STORK cohort (n = 1031), in which all included women were of Scandinavian heritage and healthy prior to pregnancy. The exclusion criteria included multiple pregnancies, pregestational diabetes, and any severe chronic diseases. Each pregnant woman had four study‐related antenatal visits scheduled at weeks 14–16, 22–24, 30–32, and 36–38 and all participants gave birth at Oslo University Hospital between 2002 and 2008. 12 The study sample was previously used in a study investigating metabolic profiles in pre‐eclamptic pregnancies vs healthy pregnancies, where maternal BMI was identified as a strong confounder. 7 The independent metabolic effect of increased BMI was therefore relevant for further investigation.
Only two women (2%) were in the BMI underweight cateogry (BMI < 18.5 kg/m2), and 12 women (11%) were in the BMI obese category (BMI ≥30 kg/m2). Therefore, in the main analysis, we chose to compare overweight/obesity (BMI ≥25 kg/m2) with normal weight/underweight (BMI < 25 kg/m2). A total of 107 women were available for analysis, 59 women (55%) were in the underweight/normal weight group and 48 (45%) were in the overweight/obesity group. A flow chart of inclusion, sample selection and number of samples at each visit can be found in Figure S1. The number of dropouts in the STORK cohort was low (3.3%); however, all women did not attend all four visits. This was especially evident for visit 4 (weeks 36–38) where some women were lost to follow‐up due to delivery. Visit 1 (weeks 14–16) consisted of 106 samples, visit 2 (weeks 22–24) consisted of 107 samples, visit 3 (weeks 30–32) consisted of 102 samples and visit 4 (36–38) consisted of 93 samples.
To confirm that women who developed pre‐eclampsia did not induce an imbalance potentially imposing unfavorable differences between the two BMI groups, we performed a sensitivity analysis including an approximation of what would have been a representative number of women with pre‐eclampsia in a general pregnant population. The proportion of women that developed pre‐eclampsia in the STORK cohort was 3.7%. Hence, in the sensitivity analysis, we only included four randomly chosen women with pre‐eclampsia (107 × 0.037 = 4). The random selection of women with pre‐eclampsia was performed with the sample function in the base package in R, with no additional criterions.
2.2. Data collection
Clinical data was collected at the study related visits and from hospital records. We calculated BMI by height and weight measured at the first visit. Accordingly, GWG was based on the difference between weight measured at visit 1 (weeks 14–16) and visit 4 (weeks 36–38).
Fasting blood samples were drawn in the morning between 07:30 a.m. and 08:30 a.m. into EDTA tubes, centrifuged for 25 min at 3000 G at 4°C, separated, and stored at −80°C until analyzed.
2.3. Definition of variables
Diagnosis of GDM was based on current diagnostic criteria at the time of the STORK study, fasting blood glucose ≥7.0 mmol/L or blood glucose ≥7.8 mmol/L 2 h after an oral glucose tolerance test. 13 The test was performed at visit 1 (gestational weeks 14–16) and visit 3 (gestational weeks 30–32).
Pre‐eclampsia was defined as elevated blood pressure (≥ 140/90 mmHg) and proteinuria, which were clinical diagnostic criteria at the time of the study. The information was obtained from the medical records after delivery and did not differentiate between early‐ and late‐onset pre‐eclampsia.
Definitions of a small‐for‐gestational‐age (SGA) infant, average‐for‐gestational‐age (AGA) infant, and large‐for‐gestational‐age (LGA) infant were based on sex‐ and gestational age‐specific percentiles from a Norwegian population‐based register study. 14
2.4. Quantitive NMR metabolomics
We used a high‐throughput nuclear magnetic resonance (NMR) spectroscopy platform at an accredited laboratory (Nightingale Health, Finland) to measure a large number of standard metabolic measures in addition to quantitative molecular data suitable to form a comprehensive metabolic profile with absolute levels. NMR‐based metabolomics have previously been applied in numerous epidemiological and genetic studies, and the method is described elsewhere. 15 , 16 Although the platform can only provide measures of predefined metabolites and is less sensitive than mass spectrometry, the high degree of automation provides a stable analysis that has been found consistent with clinical chemistry measures. 17
2.5. Outcome measures
We analyzed 91 metabolic measures, which represent each woman's systemic metabolism at the current visit, and include lipid concentration, composition of lipoproteins, fatty acids, amino acids, glycolysis‐related metabolites, and ketone bodies. The four major classes of lipoproteins (VLDL, low‐density lipoprotein [LDL], intermediate‐density lipoprotein [IDL], and high‐density lipoprotein [HDL]) were divided into 14 subclasses based on their size (XXL‐VLDL, XL‐VLDL, L‐VLDL, M‐VLDL, S‐VLDL, and XS‐VLDL; IDL; L‐LDL, M‐LDL, and S‐LDL; XL‐HDL, L‐HDL, M‐HDL, and S‐HDL).
2.6. Statistical analyses
Due to relatively few women in the respective BMI categories for underweight and obesity, BMI was treated as a dichotomous variable for overweight or obesity (yes/no), combining underweight with normal weight and overweight with obesity.
Sex‐ and gestational age‐specific standardized birthweight (i.e., z score) was calculated based on mean and standard deviations (SDs) from a Norwegian population‐based register study. 14
We determined degree of skewness of all metabolites (by BMI group and antenatal visit) by the skewness function in the e1071 package in R, 18 and loge‐transformed all measures with skewness >1.5. To enable visualizations (eg forest plots), all measures were subsequently scaled to number of SDs.
To correct for multiple testing we used a modified Bonferroni method which is sometimes used within metabolomics. 19 , 20 , 21 , 22 The rationale for the method used to correct for multiple testing is described elsewhere. 23 Metabolic data are strongly correlated, hence, we performed principal component analysis (PCA) across all four visits to determine the number of independent tests. More than 90% of the variation in the metabolic data was explained by eight independent principal components (Figure S2). When correcting for multiple testing, the statistical significance level was set at 0.006 (0.05/8). Results with corresponding p‐values below this threshold are thus to be considered statistically significant.
In addition, we performed a sensitivity analysis where we used a Benjamini‐Hochberg procedure to control the false discovery rate (FDR).
We constructed crude linear regression models for each metabolic measure at the four visits, with overweight/obesity (yes/no) as the explanatory variable. Moreover, models were adjusted for potential confounders such as maternal age (continuous), parity (nulliparous, yes/no), pre‐eclampsia (yes/no), GDM (yes/no), and GWG (continuous). The adjustment factors were primarily chosen based on clinical assumptions of factors potentially influencing both exposure and outcome and based on studies of previous literature. 24 , 25 , 26 , 27 As measures were scaled, results were reported as difference in SD units between women with overweight/obesity and underweight/normal‐weight women with associated 95% confidence interval (CI). Quantification in absolute values and number of observations per visit are given in Table S1. Effect estimates with 95% CI, p‐values, and FDR‐adjusted p‐values can also be found in Table S2. Original p‐values are used in the main analysis (statistical significance level < 0.006) and FDR‐adjusted p‐values are used in the sensitivity analysis.
Similarly, crude linear regression models were fitted for each metabolic measure with GWG (continuous) as the explanatory variable, within the respective BMI groups; normal weight/underweight and overweight/obesity. In this model we adjusted for gestational length (possible deviation of ±2 weeks at each study‐related antenatal visit), age (continuous), parity (nulliparous, yes/no), pre‐eclampsia (yes/no) and GDM (yes/no).
Statistical analyses and visualizations were performed in R (version 4.1.1) by using RStudio (version 1.4.1717).
3. RESULTS
Clinical characteristics of the 107 study participants are given in Table 1. Overweight/obese women (n = 48) were more frequently diagnosed with GDM and pre‐eclampsia compared to underweight/normal weight women (n = 59), as expected. GWG was also higher among overweight/obese women. All obese women were in obesity class 1 (BMI: 30.0–34.9 kg/m2), with a mean BMI value of 32.6 kg/m2.
TABLE 1.
Characteristics of the study participants, n = 107.
| Characteristics | All, n = 107 a | Underweight/normal weight, n = 59 (55%) a | Overweight/obesity e n = 48 (45%) a |
|---|---|---|---|
| Age, years | 31.5 (3.9) | 31.8 (4.0) | 31.1 (3.7) |
| Married/cohabiting | 104 (97%) | 58 (98%) | 46 (96%) |
| Higher education | 88 (82%) | 49 (83%) | 39 (81%) |
| Smoking | 1 (0.9%) | 1 (1.7%) | 0 (0%) |
| Nulliparous | 61 (57%) | 36 (61%) | 25 (52%) |
| Gestational diabetes b | 7 (6.7%) | 1 (1.7%) | 6 (13%) |
| Missing, n | 1 | 1 | |
| Pre‐eclampsia c | 37 (35%) | 13 (22%) | 24 (50%) |
| Body mass index (BMI), kg/m2 | 24.5 [5.22] | 22.5 [2.5] | 27.7 [4.2] |
| Body mass index (BMI) category | |||
| Underweight | 2 (1.9%) | 2 (3.4%) | 0 (0%) |
| Normal weight | 57 (53%) | 57 (97%) | 0 (0%) |
| Overweight | 36 (34%) | 0 (0%) | 36 (75%) |
| Obesity | 12 (11%) | 0 (0%) | 12 (25%) |
| Gestational weight gain, visit 1–4, kg | 10.6 [4.7] | 10.1 [3.8] | 12.5 [4.6] |
| Missing, n | 4 | 7 | |
| Male sex | 59 (55%) | 32 (54%) | 27 (56%) |
| Gestational age, week | 40.0 [2.9] | 40.0 [1.5] | 40.0 [3.0] |
| Preterm birth < 34 week | 3 (2.8%) | 2 (3.4%) | 1 (2.1%) |
| Birthweight, g | 3485 (627) | 3399 (616) | 3591 (630) |
| Standardized birthweight d | −0.06 (1.02) | −0.28 (0.94) | 0.21 (1.06) |
| Birthweight category | |||
| Large for gestational age | 13 (12%) | 5 (8.5%) | 8 (17%) |
| Small for gestational age | 11 (10%) | 9 (15%) | 2 (4.2%) |
| Placental weight, g | 696 (152) | 682 (158) | 714 (145) |
| Missing, n | 3 | 2 | |
Note: No missing data unless stated otherwise.
Mean (standard deviation) for continuous variables, median [interquartile range] for skewed distributed variables; n (%) for categorical variables.
Gestational diabetes was diagnosed if fasting blood glucose ≥7.0 mmol/L or blood glucose ≥7.8 mmol/L 2 h after an oral glucose tolerance test at visit 1 or 3.
Pre‐eclampsia was diagnosed if elevated blood pressure (≥140/90 mmHg) and proteinuria.
Standardized birthweight (z‐score) was based on reference values to adjust for gestational age and sex.
BMI ≥ 25 kg/m2.
3.1. Metabolic profiles
Adjusted associations between BMI group and the metabolic measures are shown in Figures 1, 2, 3. Crude estimates are given in Figure S3. As may be seen in Figure S3, adjustment for potential confounders had a significant impact on the results for several of the metabolites under study.
FIGURE 1.
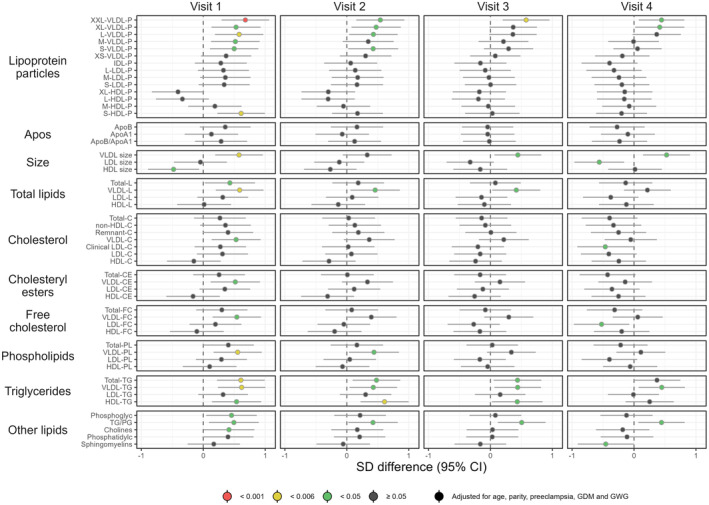
Forest plot illustrating longitudinal associations between the BMI group and the metabolic measures (visits 1–4). Women with overweight/obesity (n = 48) are compared to women with underweight/normal weight (n = 59). Point estimates denote the scaled adjusted differences in SD units with 95% CIs. Robustness of significance is indicated by color: red indicates p‐value <0.001; yellow indicates p‐value <0.006 (significance level after correction for multiple testing); green indicates p‐value <0.05; dark gray indicates p‐value ≥0.05. BMI, body mass index; CI, confidence interval; HDL, high‐density lipoprotein; IDL, intermediate‐density lipoprotein; LDL, low‐density lipoprotein; SD, standard deviation; VLDL, very‐low‐density lipoprotein.
FIGURE 2.
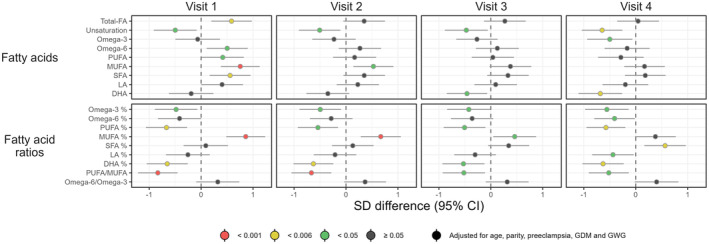
Forest plot illustrating longitudinal associations between the BMI group and the metabolic measures (visits 1–4). Women with overweight/obesity (n = 48) are compared to women with underweight/normal weight (n = 59). Point estimates denote the scaled adjusted differences in SD units with 95% CIs. Robustness of significance is indicated by color: red indicates p‐value <0.001; yellow indicates p‐value <0.006 (significance level after correction for multiple testing); green indicates p‐value <0.05; dark gray indicates p‐value ≥0.05. BMI, body mass index; CI, confidence interval; DHA, docosahexaenoic acid, FA; fatty acid; LA, linoleic acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; SD, standard deviation; SFA, saturated fatty acid.
FIGURE 3.
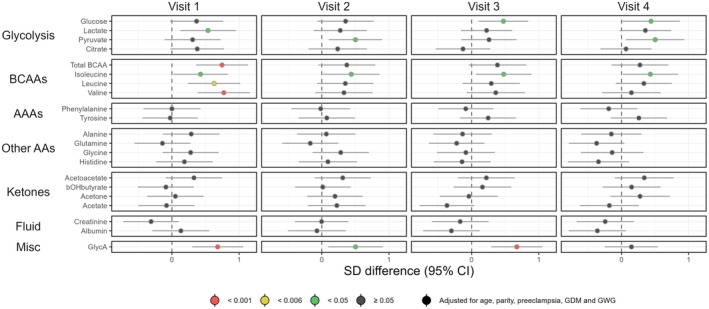
Forest plot illustrating longitudinal associations between the BMI group and the metabolic measures (visits 1–4). Women with overweight/obesity (n = 48) are compared to women with underweight/normal weight (n = 59). Point estimates denote the scaled adjusted differences in SD units with 95% CIs. Robustness of significance is indicated by color: red indicates p‐value <0.001; yellow indicates p‐value <0.006 (significance level after correction for multiple testing); green indicates p‐value <0.05; dark gray indicates p‐value ≥0.05. AA, amino acids; AAA, aromatic amino acids; BCAA, branched‐chain amino acids; BMI, body mass index; CI, confidence interval; GlycA, glycoprotein acetyls; SD, standard deviation.
3.1.1. Lipoprotein particle concentration and lipid‐related measures
We observed statistically significant differences in several metabolic measures between overweight/obese women and underweight/normal weight women at visit 1 (Figure 1), although very few differences were observed from visit 2 onwards.
There was a higher level of the largest VLDL subclass (XXL‐VLDL) at visits 1 and 3 (p < 0.001 and p = 0.003, respectively) combined with a higher level of L‐VLDL (p = 0.004) and the smallest HDL (S‐HDL) (p = 0.002) at visit 1 in overweight and obese women. At visits 2 and 4, there were no differences in particle concentration after adjustment and correction for multiple testing; albeit there appeared to be a trend toward higher concentration of VLDL in overweight/obese women throughout pregnancy. There was also a trend toward increased VLDL size in the overweight/obesity group.
Total triglycerides and triglycerides in VLDL were significantly higher among those with overweight/obesity at visit 1 (p = 0.002 and p = 0.002, respectively). This trend continued throughout pregnancy, but the association was not statistically significant after correction for multiple testing. There was also a trend toward higher levels of triglycerides in HDL in overweight/obese women. However, this was only statistically significant at visit 2 after adjustment and correction for multiple testing (p = 0.002). Overall, the estimated differences were relatively stable throughout pregnancy, although larger differences were observed at visit 1.
3.1.2. Fatty acids and fatty acid ratios
Total fatty acids, monounsaturated fatty acids (MUFA), and saturated fatty acids (SFA) were significantly higher in overweight/obese women than in women with underweight/normal weight at visit 1 (p = 0.004, p < 0.001, and p = 0.006, respectively), but these differences were also attenuated as pregnancy evolved (Figure 2). Differences in degree of unsaturation and docosahexaenoic acid (DHA) were, on the contrary, increased throughout pregnancy, and the associations were statistically significant at visit 4 (p = 0.001 and p = 0.002, respectively). The ratio of polyunsaturated fatty acids (PUFA) to total fatty acids tended to be lower in overweight and obesie women throughout pregnancy, and statistically significant associations were observed at visits 1 (p = 0.001) and 4 (p = 0.003).
3.1.3. Branched‐chain amino acids (BCAA) and the inflammatory marker glycoprotein acetyls (GlycA)
Most BCAAs were observed to be significantly higher at visit 1 among overweight/obese women in comparison to underweight/normal weight women (Figure 3), including leucine (p = 0.002), valine (p < 0.001), and total BCAA (p < 0.001). Again, these differences were attenuated as the pregnancy evolved, and no differences were observed from visit 2 onwards, although estimates were trending toward a higher level among overweight/obese women.
GlycA tended to be higher in overweight/obese women and was highly statistically significant at visits 1 (p < 0.001) and 3 (p < 0.001).
Individual and group mean concentrations of five of the most pronounced metabolites in the two BMI groups are displayed in Figure 4 and show large variations within groups. Mean total triglycerides, total BCAA, and GlycA were higher in women with overweight/obesity compared to women with underweight/normal weight across all four visits. Mean level of DHA was lower in women with overweight/obesity across all four visits.
FIGURE 4.
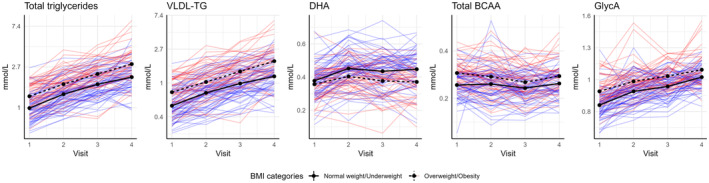
Trend plot illustrating longitudinal plasma concentration in five metabolites from the full analysis shown in Figure 1, 2, 3. Individual concentrations in women with overweight/obesity (n = 48) are given in red and normal weight/underweight (n = 59) in blue. Black lines indicate group means (solid line, normal weight/underweight; dotted line, overweight/obesity).
3.1.4. Sensitivity analyses
Adjusted effect estimates from the main analysis (Figures 1, 2, 3) were strongly correlated with adjusted effect estimates from the sensitivity analysis including only four women with pre‐eclampsia (R 2 ranging between 0.91 and 0.96 at various visits). The correlation plots can be found in Figure S4.
Adjusted effect estimates from the sensitivity analysis where we used FDR‐adjusted p‐values are given in Figures S5 and S6. The alternative approach for dealing with multiple testing led to only minor differences even at a conservative significance level of 0.05.
3.2. Metabolic associations of GWG in women within different BMI groups
Associations between GWG and metabolic measures in normal weight/underweight women and women with overweight/obesity are shown in Figure 5. After adjustment for age, parity, pre‐eclampsia, GDM, and gestational length between visit 1 and visit 4 (possible deviation of ±2 weeks at each study‐related antenatal visit), we observed few metabolic differences. Crude estimates are given in Figure S7. After correction for multiple testing, acetate was the single metabolite with a statistically significant (inverse) association with GWG, and this was only observed in underweight and normal‐weight women (p = 0.004).
FIGURE 5.
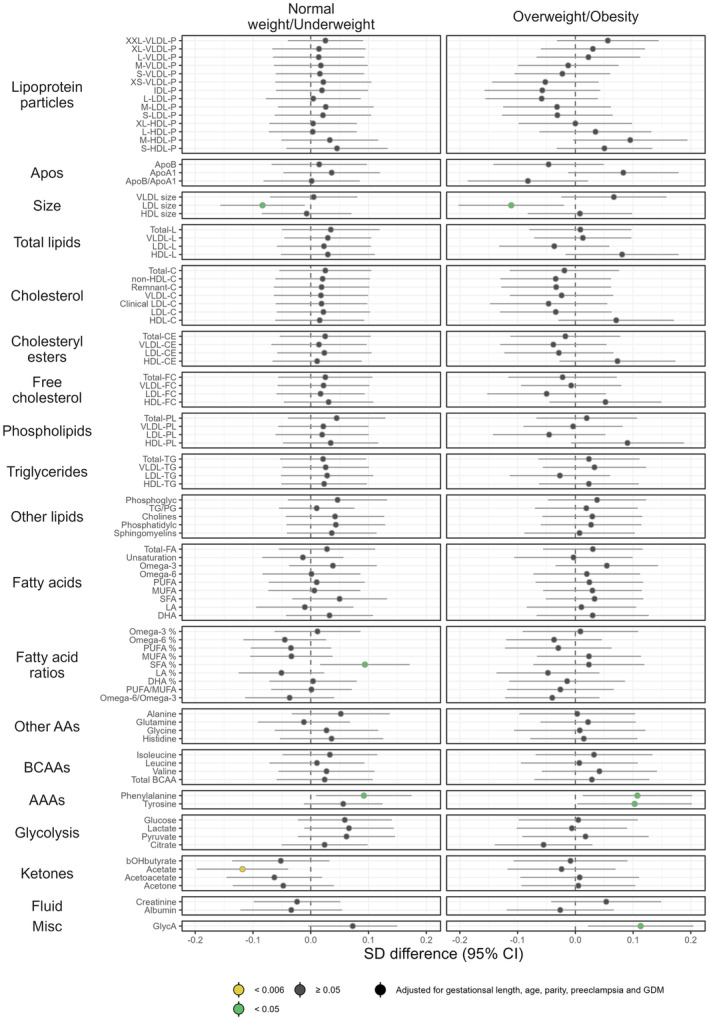
Forest plot illustrating the associations between GWG (estimated as weight difference between visits 1 and 4) and the metabolic measures in two BMI categories: normal weight/underweight (n = 59) and overweight/obesity (n = 48). Point estimates denote the scaled adjusted differences in SD units with 95% CIs. Coefficients are to be interpreted as the differences in mean metabolic measures (in SD units) corresponding to an increase in GWG of 1 kg. Robustness of statistical significance is indicated by color: yellow indicates p‐value <0.006 (significance level after correction for multiple testing); green indicates p‐value <0.05; dark gray indicates p‐value ≥0.05. AA, amino acids; AAA, aromatic amino acids; BCAA, branched‐chain amino acids; BMI, body mass index; CI, confidence interval; GWG, gestational weight gain; HDL, high‐density lipoprotein; IDL, intermediate‐density lipoprotein; LDL, low‐density lipoprotein; SD, standard deviation; VLDL, very‐low‐density lipoprotein.
4. DISCUSSION
In the present study, we compared 91 metabolic measures between overweight/obese women and normal weight/underweight at four time points across gestation. We observed marked differences in metabolic profiles at visit 1, while these differences were attenuated toward the end of pregnancy, indicating that overweight/obese women showed smaller metabolic changes during pregnancy. In addition, we observed a reduction in the ratio of long‐chain PUFA to total fatty acids, and especially DHA, in the overweight/obese group, which was most pronounced toward term. The maternal metabolic profile tended to be almost unaffected by GWG in both BMI groups.
Large variations were observed within each BMI group, and the nonlinear longitudinal trend implies large differences in the metabolic profile of pregnant women. No metabolites were significantly different at all points of time after correction for multiple testing, although a consistent trend was observed.
Our findings are in line with the findings of Kivelä et al., where pregnant women with obesity had higher levels of most VLDL‐related measures, fatty acids, amino acids and had a more adverse metabolic profile. 28 They compared women with obesity (BMI ≥30 kg/m2) and women with normal weight, most likely explaining why differences in the study by Kivelä et al. were more pronounced than in the present study. Importantly, women in the overweight/obese group had a lower ratio of PUFA to total fatty acids in general and DHA to total fatty acids in particular. The difference was most pronounced at visits 3 and 4. These findings are in line with the findings of Vidakovic et al. using data from the Generation R Study. 29 The authors demonstrated high SFA levels and low n‐3 PUFA levels in women with obesity. DHA is an n‐3 PUFA that is considered an essential fatty acid for the fetus. Hence, the growing fetus is dependent on DHA from the maternal circulation. Although there is a preferential transport of DHA across the placenta, lower maternal levels of DHA might correspond to lower fetal levels of DHA. 30 , 31 Given that DHA is necessary for fetal brain development and that fetal uptake of DHA from the placenta is critical in the third trimester of pregnancy, this observation could have important clinical implications. 32 A recent study found that maternal hepatic metabolism and DHA status was improved when participants received a higher dose of choline supplementation administered across second and third trimester when combined with supplemental DHA. 33 Although DHA levels in umbilical cord blood was not significantly increased, this may indicate a therapeutic potential.
Overweight or obese women had higher concentrations of GlycA compared to normal weight or underweight women, with statistically significant differences at visits 1 and 3. GlycA is a novel marker of low‐grade inflammation representing a complex signal originating from multiple acute phase glycoproteins in the circulation. 34 It has been observed in chronic inflammatory diseases with low‐grade inflammation, and suggested as a more sensitive marker than high‐sensitivity C‐reactive protein (hs‐CRP) for identifying metabolic conditions. 35 Altered fatty acid distribution in pregnant women with obesity, as observed in the present study, is associated with a proinflammatory state and increased oxidative stress. 36
In line with the study by Kivelä et al. 28 and Forbes et al., 37 we found that metabolic changes across gestation were smaller in women who were overweight or obese. Pregnancy is a condition with increased energy demands, and a lower metabolic response in women with overweight and obesity may be beneficial, as there is no lack of lipid‐related stores. The smaller response could, however, be interpreted as a relative metabolic maladaptation caused by metabolic overload and chronic inflammation. This relationship is previously described as a vicious cycle in metabolic disorders. 38
Because GWG had a minor impact on the metabolic profile in the present study, the findings imply that preconception measures may be more effective in obtaining a healthy metabolic profile throughout pregnancy. In previous studies on lifestyle interventions aiming to limit GWG, no effect could be determined regarding obstetrical or neonatal outcomes. 39 , 40 At the time of the study, the guidelines on recommendation for GWG from the Institute of Medicine (now the US National Academy of Medicine) had not yet been introduced, 41 and women with overweight/obesity gained more weight than women with normal weight/underweight. Although we studied the effect of GWG on metabolic measures, study populations following current guidelines may demonstrate different effects of GWG on maternal metabolism. We were not able to differentiate women with obesity from women who were overweight, and this may preclude effects that are attributed to obesity. Since the predictive value of GWG in relation to adverse maternal and infant outcomes is limited, 42 the benefit of GWG surveillance in particularly healthy, normal‐weight women should be further investigated.
In relation to our previous study on metabolic differences in pre‐eclamptic pregnancies, 7 we observed a resemblance between the metabolic profiles of women with overweight/obesity in early pregnancy and those found in late pregnancy of late‐onset pre‐eclampsia, especially regarding particle concentration of the largest VLDL and triglycerides. Kivelä et al. found that GDM, pre‐eclampsia, and chronic hypertension were associated with metabolic alterations similar to obesity. 28 This may indicate similar metabolic pathways and may provide valuable insight into the understanding of metabolites mediating the association between maternal health and offspring disease burden.
The strengths of this study include the broad molecular profiling in a prospective longitudinal design across four time points in pregnancy. Although there was a larger proportion of women with pre‐eclampsia than in the underlying STORK cohort, the sensitivity analysis including only four women who developed pre‐eclampsia displayed similar effect estimates as the main analysis. Hence, the higher proportion of women with pre‐eclampsia did not seem to significantly alter the results.
Although the proportions of women within the different BMI categories were representative of the general population of pregnant women in Norway, 43 we acknowledge that the proportion of women with pre‐eclampsia cannot be considered representative. Hence, the results must be interpreted with caution.
There were only a few women in the respective BMI categories for underweight (n = 2) and obesity (n = 12). Thus, we were not able to discriminate between underweight and normal‐weight women or between overweight and obese women in the statistical analyses. Instead, we treated BMI as an indicator variable for overweight/obesity by collapsing underweight with normal weight and obesity with overweight. Furthermore, as all obese women were in obesity class 1 (BMI: 30.0–34.9 kg/m2), the women with overweight/obesity were perceived as a relatively homogenous group. Consequently, the potential associations with the BMI categories might have been obscured, possibly resulting in an underestimation of the metabolic differences between normal‐weight and obese women. The large individual variation within BMI groups may represent biological variation as not all individuals develop metabolic complications. However, combining overweight and obesity into one category is likely to have increased the variation in this group.
The GDM diagnosis was based on the current diagnostic criteria in the study period. We acknowledge that using current diagnostic criteria may affect the number of women with GDM and potentially influence adjusted estimates. Generalizability to other populations may be limited by the fact that the STORK cohort comprised women of Scandinavian heritage who were healthy prior to pregnancy. Furthermore, BMI was based on height and weight measured at visit 1, because self‐reported weight is prone to underestimation, especially among women with obesity. 44 We were not able to discriminate between visceral and subcutaneous fat, which possibly could have been more accurate and were not able to explore differences in physical activity and diet between groups, which is a substantial limitation to this study.
Exploratory observational studies are commonly hindered by challenges related to sample size and multiple testing. A small sample size, obviously increases the risk of type II error as the statistical power of detecting real differences decreases by decreasing number of observations. On the other hand, multiple testing increases the risk of type I error in that the probability of rejecting at least one of the null hypotheses by chance alone may be unduly large. The current study included only 107 women, which might have precluded us from observing real differences between the two BMI groups with respect to the metabolites under study. Furthermore, even though we applied a modified Bonferroni correction for multiple testing through the use of PCA, some of the observed metabolic differences might not represent true findings. With an FDR adjustment, as an alternative approach to the modified Bonferroni, most findings were consistent, but the possibility of false positive results remains.
Matching of underweight/normal‐weight women and overweight/obese women was deemed difficult in the current study due to low numbers of participants with certain characteristics; for example, pre‐eclampsia among women who were underweight or normal weight. Even though we adjusted for potential confounders in the statistical analyses, the possibility for residual confounding remains.
5. CONCLUSION
Our data suggested that overweight/obesity was associated with an altered metabolic profile in early pregnancy and smaller metabolic changes across gestation compared to underweight and normal weight. Women with overweight/obesity appeared to have reduced levels of essential PUFAs, especially DHA, with possible developmental effects on the offspring. In addition, women with overweight and obesity had higher levels of the inflammation marker GlycA. There was a trend toward an atherogenic lipid profile with higher triglyceride and VLDL particle concentrations across gestation in women with overweight/obesity. The results should be considered as tentative and hypothesis generating, and further studies on women with overweight/obesity from a general population are warranted to explore the potential metabolic role in pregnancy complications. GWG had only minor effects on the metabolic profile, regardless of BMI groups in this study. However, the potential role of metabolic alterations related to GWG should be further studied.
AUTHOR CONTRIBUTIONS
Hege Nyhus Skytte: Wrote the original draft. Hege Nyhus Skytte, Marie Cecilie Paasche Roland, Jacob Juel Christensen, and Trond Melbye Michelsen: Conceptualization and methodology. Hege Nyhus Skytte, Jacob Juel Christensen, and Nina Gunnes: Statistical analysis and software. Marie Cecilie Paasche Roland: Investigation. Hege Nyhus Skytte and Jacob Juel Christensen: Visualization. Tove Lekva and Marie Cecilie Paasche Roland: Data curation. Marie Cecilie Paasche Roland, Trond Melbye Michelsen, Nina Gunnes, Jacob Juel Christensen, Tove Lekva, and Kirsten Bjørklund Holven: Critically reviewed and edited the manuscript. Trond Melbye Michelsen: Funding acquisition. Trond Melbye Michelsen, Nina Gunnes and Marie Cecilie Paasche Roland: Supervision. All authors read and approved the final version of the manuscript.
FUNDING INFORMATION
This study was supported by the Norwegian Research Center for Women's Health, Oslo University Hospital, and South‐Eastern Norway Regional Health Authority.
CONFLICT OF INTEREST STATEMENT
The authors report no conflict of interest.
Ethics statement
All study participants gave their written informed consent before taking part in the STORK study. All clinical investigations were conducted in accordance with the principles of the Declaration of Helsinki. This study was approved by the Regional Committee for Medical and Health Research Ethics South East Norway (reference no. S‐01191) on February 18, 2022.
Supporting information
Figure S1.
Figure S2.
Figure S3.
Figure S4.
Figure S5.
Figure S6.
Figure S7.
Table S1.
Table S2.
Skytte HN, Roland MCP, Christensen JJ, et al. Maternal metabolic profiling across body mass index groups: An exploratory longitudinal study. Acta Obstet Gynecol Scand. 2024;103:540‐550. doi: 10.1111/aogs.14750
REFERENCES
- 1. Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. BMJ. 2017;356:j1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Godfrey KM, Reynolds RM, Prescott SL, et al. Influence of maternal obesity on the long‐term health of offspring. Lancet Diabetes Endocrinol. 2017;5:53‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sattar N, Tan CE, Han TS, et al. Associations of indices of adiposity with atherogenic lipoprotein subfractions. Int J Obes. 1998;22:432‐439. [DOI] [PubMed] [Google Scholar]
- 4. Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111‐2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lain KY, Catalano PM. Metabolic changes in pregnancy. Clin Obstet Gynecol. 2007;50:938‐948. [DOI] [PubMed] [Google Scholar]
- 6. Roland MCP, Lekva T, Godang K, Bollerslev J, Henriksen T. Changes in maternal blood glucose and lipid concentrations during pregnancy differ by maternal body mass index and are related to birthweight: a prospective, longitudinal study of healthy pregnancies. PloS One. 2020;15:e0232749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Skytte HN, Christensen JJ, Gunnes N, et al. Metabolic profiling of pregnancies complicated by preeclampsia: a longitudinal study. Acta Obstet Gynecol Scand. 2023;102:334‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Champion ML, Harper LM. Gestational weight gain: update on outcomes and interventions. Curr Diab Rep. 2020;20:11. [DOI] [PubMed] [Google Scholar]
- 9. Roland MCP, Friis CM, Godang K, Bollerslev J, Haugen G, Henriksen T. Maternal factors associated with fetal growth and birthweight are independent determinants of placental weight and exhibit differential effects by fetal sex. PloS One. 2014;9:e87303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Friis CM, Qvigstad E, Paasche Roland MC, et al. Newborn body fat: associations with maternal metabolic state and placental size. PloS One. 2013;8(2):e57467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kristiansen O, Roland MC, Zucknick M, et al. Maternal body mass index and placental weight: a role for fetal insulin, maternal insulin and leptin. J Endocrinol Invest. 2022;45:2105‐2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roland MCP, Friis CM, Voldner N, et al. Fetal growth versus birthweight: the role of placenta versus other determinants. PloS One. 2012;7:e39324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Health Organization Department of Noncommunicable Disease S . Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications: Report of a WHO Consultation. Part 1: Diagnosis and Classification of Diabetes Mellitus. WHO; 1999. [Google Scholar]
- 14. Skjaerven R, Gjessing HK, Bakketeig LS. Birthweight by gestational age in Norway. Acta Obstet Gynecol Scand. 2000;79:440‐449. [PubMed] [Google Scholar]
- 15. Soininen JP, Kangas JA, WГјrtz JP, Suna JT, Ala‐Korpela JM. Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. Circ Cardiovasc Genet. 2015;8:192‐206. [DOI] [PubMed] [Google Scholar]
- 16. Soininen P, Kangas AJ, Würtz P, et al. High‐throughput serum NMR metabonomics for cost‐effective holistic studies on systemic metabolism. Analyst. 2009;134:1781‐1785. [DOI] [PubMed] [Google Scholar]
- 17. Würtz P, Kangas AJ, Soininen P, Lawlor DA, Davey Smith G, Ala‐Korpela M. Quantitative serum nuclear magnetic resonance metabolomics in large‐scale epidemiology: a primer on–Omic technologies. Am J Epidemiol. 2017;186:1084‐1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meyer D, Dimitriadou E, Hornik K, Weingessel A, Leisch F. R Package Version 1.7–8 ed. Vienna, Austria: e1071: Misc Functions of the Department of Statistics, Probability Theory Group. 2021.
- 19. Wang Q, Würtz P, Auro K, et al. Metabolic profiling of pregnancy: cross‐sectional and longitudinal evidence. BMC Med. 2016;14:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang Q, Kangas AJ, Soininen P, et al. Sex hormone‐binding globulin associations with circulating lipids and metabolites and the risk for type 2 diabetes: observational and causal effect estimates. Int J Epidemiol. 2015;44:623‐637. [DOI] [PubMed] [Google Scholar]
- 21. Kujala UM, Mäkinen V‐P, Heinonen I, et al. Long‐term leisure‐time physical activity and serum metabolome. Circulation. 2013;127:340‐348. [DOI] [PubMed] [Google Scholar]
- 22. Christensen JJ, Ulven SM, Retterstøl K, et al. Comprehensive lipid and metabolite profiling of children with and without familial hypercholesterolemia: a cross‐sectional study. Atherosclerosis. 2017;266:48‐57. [DOI] [PubMed] [Google Scholar]
- 23. Sham PC, Purcell SM. Statistical power and significance testing in large‐scale genetic studies. Nat Rev Genet. 2014;15:335‐346. [DOI] [PubMed] [Google Scholar]
- 24. Melzer K, Schutz Y. Pre‐pregnancy and pregnancy predictors of obesity. Int J Obes (Lond). 2010;34(Suppl 2):S44‐S52. [DOI] [PubMed] [Google Scholar]
- 25. Stubert J, Reister F, Hartmann S, Janni W. The risks associated with obesity in pregnancy. Dtsch Arztebl Int. 2018;115:276‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alwash SM, McIntyre HD, Mamun A. The association of general obesity, central obesity and visceral body fat with the risk of gestational diabetes mellitus: evidence from a systematic review and meta‐analysis. Obes Res Clin Pract. 2021;15:425‐430. [DOI] [PubMed] [Google Scholar]
- 27. Hill B, Bergmeier H, McPhie S, et al. Is parity a risk factor for excessive weight gain during pregnancy and postpartum weight retention? A systematic review and meta‐analysis. Obes Rev. 2017;18:755‐764. [DOI] [PubMed] [Google Scholar]
- 28. Kivelä J, Sormunen‐Harju H, Girchenko PV, et al. Longitudinal metabolic profiling of maternal obesity, gestational diabetes, and hypertensive pregnancy disorders. J Clin Endocrinol Metab. 2021;106:e4372‐e4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vidakovic AJ, Jaddoe VWV, Gishti O, et al. Body mass index, gestational weight gain and fatty acid concentrations during pregnancy: the generation R study. Eur J Epidemiol. 2015;30:1175‐1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Basak S, Mallick R, Duttaroy AK. Maternal docosahexaenoic acid status during pregnancy and its impact on infant neurodevelopment. Nutrients. 2020;12:3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Decsi T, Campoy C, Koletzko B. Effect of N‐3 polyunsaturated fatty acid supplementation in pregnancy: the Nuheal trial. Adv Exp Med Biol. 2005;569:109‐113. [DOI] [PubMed] [Google Scholar]
- 32. Brenna JT, Lapillonne A. Background paper on fat and fatty acid requirements during pregnancy and lactation. Ann Nutr Metab. 2009;55:97‐122. [DOI] [PubMed] [Google Scholar]
- 33. Klatt KC, McDougall MQ, Malysheva OV, et al. Prenatal choline supplementation improves biomarkers of maternal docosahexaenoic acid (DHA) status among pregnant participants consuming supplemental DHA: a randomized controlled trial. Am J Clinl Nutr. 2022;116:820‐832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Otvos JD, Shalaurova I, Wolak‐Dinsmore J, et al. GlycA: a composite nuclear magnetic resonance biomarker of systemic inflammation. Clin Chem. 2015;61:714‐723. [DOI] [PubMed] [Google Scholar]
- 35. Mehta NN, Dey AK, Maddineni R, Kraus WE, Huffman KM. GlycA measured by NMR spectroscopy is associated with disease activity and cardiovascular disease risk in chronic inflammatory diseases. Am J Prev Cardiol. 2020;4:100120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Álvarez D, Muñoz Y, Ortiz M, Maliqueo M, Chouinard‐Watkins R, Valenzuela R. Impact of maternal obesity on the metabolism and bioavailability of polyunsaturated fatty acids during pregnancy and breastfeeding. Nutrients. 2020;13:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Forbes S, Barr SM, Reynolds RM, et al. Convergence in insulin resistance between very severely obese and lean women at the end of pregnancy. Diabetologia. 2015;58:2615‐2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860‐867. [DOI] [PubMed] [Google Scholar]
- 39. Sagedal LR, Øverby NC, Bere E, et al. Lifestyle intervention to limit gestational weight gain: the Norwegian fit for delivery randomised controlled trial. BJOG. 2017;124:97‐109. [DOI] [PubMed] [Google Scholar]
- 40. Poston L, Bell R, Croker H, et al. Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol. 2015;3:767‐777. [DOI] [PubMed] [Google Scholar]
- 41. Rasmussen KM, Yaktine AL, eds. Institute of Medicine, National Research Council Committee to Reexamine IOM Pregnancy Weight Guidelines. National Academies Press (US); 2009. PMID: 20669500. [Google Scholar]
- 42. LifeCycle Project‐Maternal Obesity and Childhood Outcomes Study Group . Association of Gestational Weight Gain with Adverse Maternal and Infant Outcomes. Jama. 2019;321:1702‐1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Amberntsson A, Papadopoulou E, Winkvist A, et al. Maternal vitamin D intake and BMI during pregnancy in relation to child's growth and weight status from birth to 8 years: a large national cohort study. BMJ Open. 2021;11:e048980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sharma AJ, Bulkley JE, Stoneburner AB, et al. Bias in self‐reported Prepregnancy weight across maternal and clinical characteristics. Mat Child Health J. 2021;25:1242‐1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.
Figure S2.
Figure S3.
Figure S4.
Figure S5.
Figure S6.
Figure S7.
Table S1.
Table S2.


