Abstract
Background:
Primary aldosteronism (PA) has been associated with atherosclerosis beyond the extent of essential hypertension, but the impact of albuminuria remains unknown.
Objective:
To investigate the effect of concomitant albuminuria on arterial stiffness in PA.
Design:
Prospective cohort study.
Methods:
A prospective cohort study was conducted to evaluate the association of albuminuria (>30 mg/g in morning spot urine) with arterial stiffness, as measured non-invasively by pulse wave velocity (PWV) in patients with PA. Propensity score matching (PSM) with age, sex, diabetes, systolic and diastolic blood pressure, creatinine, potassium, number of antihypertensive medications, and hypertension history was used to balance baseline characteristics. The effects of albuminuria on PWV before and 1 year after treatment were analyzed.
Results:
A total of 840 patients with PA were enrolled, of whom 243 had concomitant albuminuria. After PSM, there were no significant differences in baseline demographic parameters except alpha-blocker and spironolactone use. PWV was greater in the presence of albuminuria (p = 0.012) and positively correlated with urine albumin–creatinine ratio. Multivariable regression analysis identified albuminuria, age, body weight, systolic blood pressure, and calcium channel blocker use as independent predictors of PWV. As for treatment response, only PA patients with albuminuria showed significant improvements in PWV after PSM (p = 0.001). The magnitude of improvement in PWV increased with urine albumin–creatinine ratio and reached plateau when it exceeded 100 mg/g according to restricted cubic spline analysis.
Conclusion:
Concomitant albuminuria in PA was associated with greater arterial stiffness and more substantial improvement after targeted treatment. Both the baseline and the improved extent of PWV increased in correlation with rising urine albumin–creatinine ratio levels, reaching a plateau when the urine albumin–creatinine ratio surpassed 100 mg/g.
Keywords: albuminuria, atherosclerosis, primary aldosteronism, pulse wave velocity
Plain language summary
Albuminuria and primary aldosteronism synergistically induce atherosclerosis
Albuminuria is a common comorbidity in patients with primary aldosteronism (PA), and both has been established to potentiate atherosclerosis. However, the interaction in between remained enigmatic. In this study, we accessed the synergistic vascular impact in a prospectively enrolled cohort. Arterial rigidity was assessed non-invasively by brachial–ankle pulse wave velocity. Concomitant albuminuria in patients with PA was associated with pronouncedly greater arterial stiffness and was further demonstrated as an independent predictor for atherosclerosis. In addition, PA-targeted treatment effectively reversed arterial stiffness, especially in individuals with concomitant albuminuria. The beneficial effect of PA-targeted treatment on PWV increased with rising urine albumin–creatinine ratio levels, eventually plateauing when the UACR surpassed 100 mg/g.
Introduction
Primary aldosteronism (PA) is the leading cause of secondary hypertension, with a prevalence ranging from 5 to 10%, and even up to nearly 30% in resistant hypertension according to a previous epidemiological report. 1 Characterized by the overproduction of aldosterone beyond renin regulation, PA has been associated with more challenging control of elevated blood pressure and also a worse overall prognosis compared with essential hypertension. 2 The overproduction of aldosterone is intertwined with systemic comorbidities including cardiovascular, cerebrovascular, and renal complications. 3 Cardiac remodeling and hypertrophy are associated with suboptimal left ventricular diastolic and even systolic function,4,5 whereas arterial endothelial dysfunction and local inflammation secondary to excessive circulating aldosterone leads to atherosclerosis. 6
Arterial wall stiffening is a major concern in patients with PA, and pulse wave velocity (PWV) is a well-established marker used to assess vascular stiffness non-invasively. 7 Evaluated as the distance between two designated points of the arterial vasculature divided by the travel time of a pulse wave, PWV has been positively correlated with the degree of atherosclerosis and therefore a prognosticator of cardiovascular composite outcomes. 8 Brachial–ankle PWV represents a combination of central elastic artery and peripheral muscular artery stiffness. 9 A recent meta-analysis also suggested that brachial–ankle PWV has better predictive ability for cardiovascular disease compared with Framingham risk score based on traditional risk factors. 10
The results of our previous study suggested that patients with PA exhibit greater PWV in a hemodynamically independent manner compared to those with essential hypertension. 11 PA treatment has also been demonstrated to substantially improve PWV. 12 Albuminuria frequently develops in patients with PA due to hyperaldosteronism through glomerular hypertension, capillary-endothelial damage, and podocyte effacement, which eventually leads to renal damage.13,14 Moreover, the presence of proteinuria conversely aggravated hypertension, forming such vicious cycle. The mutually causative relationship between atherosclerosis and albuminuria highlights the clinical importance of further elucidating the pathophysiology and presentation. However, the impact of concomitant albuminuria on arterial stiffness and how it interacts with PA treatment have never been investigated. Therefore, the aim of this study was to elucidate how albuminuria affects PWV in individuals with PA at baseline and after PA treatment.
Material and methods
Patients
Patients with PA were enrolled prospectively from January 2006 to April 2020 at two medical centers [National Taiwan University Hospital (NTUH), Taipei, Taiwan; Taipei University Hospital, Taipei, Taiwan] and five regional hospitals (Cardinal Tien Hospital, New Taipei City, Taiwan; Taipei Tzu Chi Hospital, New Taipei City, Taiwan; Yun-Lin Branch of NTUH, Douliou City, Taiwan; Hsin-Chu Branch of NTUH, Hsin-Chu City, Taiwan; Zhongxing Branch of Taipei City Hospital, Taipei, Taiwan). All of the patients were registered in the Taiwan Primary Aldosteronism Investigation (TAIPAI) database. 15 Detailed demographic and clinical characteristics along with serum and urine samples were collected at initial encounter and 1 year after PA treatment for analysis. Albuminuria was defined as a urine albumin–creatinine ratio (UACR) > 30 mg/g in morning spot urine. Commercially available radioimmunoassay kits were used to quantify plasma aldosterone concentration (PAC) (ALDO-RIACT RIA kit, Cisbio Bioassays, Codolet, France) and assess plasma renin activity (PRA) (GammaCoat, DiaSorin, Stillwater, MN, USA). Aldosterone-to-renin ratio was determined as PAC divided by PRA. Written informed consent form was obtained from all of the included subjects.
Diagnostic criteria and classification of PA
The diagnosis of PA was confirmed according to the following three criteria 16 : (1) aldosterone-to-renin ratio >35 (ng/dl)/(ng/ml/h); (2) a TAIPAI score >60%; and (3) seated post-saline loading PAC > 16 ng/dl or aldosterone-to-renin ratio (ARR) >35 (ng/dl)/(ng/ml/h) on a post-captopril/losartan test or PAC > 6 ng/dL in a fludrocortisone suppression test. The TAIPAI score, which has been established by logistic regression model to predict PA probability, was calculated as 1/(1 + e − β), where β = [PAC (ng/dL)*0.063] + [PRA (ng/ml/h)*(−0.205)] + [(ARR*0.001) + BMI (kg/m2)*0.067] + [Male*(−0.738) + K + (mmol/l)*(−1.512)] + [eGFR (ml/min/1.73 m2)*0.017] + [propensity score*(−0.539) + 1.851]. The propensity score in the formula was calculated from a logistic regression model for estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73 m2, which contained the following variables: age, sex, Charlson score, hypertension duration, diabetes, history of cardiovascular disease, body mass index (BMI), categories of hypertensive medication, mean blood pressure, post-captopril PRA, PAC, and ARR. 17 Before PAC and PRA quantification, all antihypertensive agents were held for at least 3 weeks. Non-dihydropyridine calcium channel blockers and/or α-blockers were administered to control blood pressure if clinically indicated. The subtypes of PA were classified as either unilateral PA or bilateral PA according to computed tomography, adrenal venous sampling, and NP-59 adrenal scintigraphy results. Laparoscopic adrenalectomy was the treatment of choice for patients with unilateral PA, whereas mineralocorticoid receptor antagonists were given to patients with bilateral PA or those with unilateral PA who were unable or unwilling to receive surgical treatment. 18
PWV measurement
An automatic waveform analyzer (Colin VP-2000, Omron Inc., Kyoto, Japan) was used to assess PWV. After resting in the supine position for at least 15 min, the pressure waveforms of bilateral brachial and tibial arteries were documented. Phonocardiograms and electrocardiograms were recorded by the instrument simultaneously. 19 Occlusive cuffs with oscillometric and plethysmographic sensors were wrapped around the upper extremities and ankles to assess blood pressure and pulse waveform. Body height was used to estimate the distance between the arms and ankles. Wave front theory was used to determine the propagation time interval of arterial pressure travelling between two measurement points. Brachial–ankle PWV, as calculated as the ratio of brachial–ankle distance-to-time interval between the brachial and ankle arterial waveforms, was selected as the outcome parameter in this study. Both right and left sides of the brachial–ankle PWVs were obtained, and the mean value was used for analysis.
Statistical analysis
Continuous variables which were normally distributed were expressed as mean ± standard deviation, and non-normally distributed variables were expressed as median (25th–75th interquartile range). Categorical variables were presented as number with percentage. The independent Student’s t test was used to compare continuous variables between two groups for normally distributed variables, and the Mann–Whitney U-test was used for non-normally distributed variables. The chi-square test or Fisher’s exact test was used to test differences between proportions. Significant heterogeneity of variance in PWV was found at different levels of UACR according to Levene’s test. Therefore, Welch’s analysis of variance (ANOVA) with Games–Howell post hoc analysis was used to test differences between groups.
The sample size was established through a power analysis, factoring in an alpha error of 0.05 and a power of 80%. Based on an effect size of 0.44, as determined from a prior study, 20 a minimum sample size of 144 was necessary. Accounting for potential drop-out rates (approximately 20%) and the potential loss of participants during propensity score matching (PSM) (which could be as high as 70–80% if multiple factors were to be matched), an estimated sample size of 800 was determined.
PSM was conducted to balance the distribution of baseline characteristics between the PA patients with and without albuminuria. Propensity scores were calculated separately by applying non-parsimonious multiple logistic regression for grouping with possible confounding parameters, including age, sex, diabetes, systolic and diastolic blood pressure, serum creatinine and potassium level, number of antihypertensive medication type, and hypertension history. A 1:1 propensity matching ratio with a caliper width equal to 0.05 standard deviations of the logit of the propensity scores was selected, and the balance of variables between the matched groups was checked. The restricted cubic spline (RCS) method was applied to explore linear and nonlinear relationships between UACR with baseline PWV as well as changes in PWV after PA treatment. The paired t-test was used to compare continuous variables before and after PA treatment. Univariable linear regression analysis was performed to test the relationships between PWV and clinical variables. Factors in univariable linear regression analysis with a p value < 0.2 were selected for multivariable linear regression analysis with backward selection to identify the significant independent determinants to predict PWV. 21 A p value < 0.05 was considered as statistically significant.
All statistical analyses were performed using SPSS version 26.0 for Windows (SPSS Inc., Armonk, NY, USA) with the R-3.3 plugin extension for PSM and STATA version 17 (StataCorp LP, College Station, TX, USA). The RCS analysis was performed with R version 4.1.2 (R Development Core Team, Vienna, Austria) with the ‘rms’ package (Version 6.2-0).
Results
Demographic and biochemical characteristics before and after matching
Among the 840 enrolled patients, 243 had concomitant albuminuria. The patients were divided into two groups according to the presence or absence of albuminuria. After 1:1 PSM for age, sex, diabetes, systolic and diastolic blood pressure, serum creatinine and potassium level, number of antihypertensive medication type, and hypertension history, there were 202 individuals in each group. The baseline demographic and clinical parameters before and after matching are summarized (Table 1). Before matching, the patients with albuminuria had higher rate of diabetes, BMI, systolic and diastolic blood pressure, serum creatinine level, percentage of unilateral PA, PAC, log-transformed PAC, number of antihypertensive medication type, hypertension history, and lower serum potassium level. Significantly elevated usage rates of angiotensin-receptor blockers, beta-blockers, calcium channel blockers, vasodilators, and diuretics were found in the PA patients with albuminuria.
Table 1.
Clinical characteristics and PWV of PA with and without albuminuria.
| Demographic and clinical parameters | Original data | Propensity score matching* | ||||
|---|---|---|---|---|---|---|
| With albuminuria (N = 243) | Without albuminuria (N = 597) | p Value | With albuminuria (N = 202) | Without albuminuria (N = 202) | p Value | |
| Sex (Male), n (%) | 118 (49) | 259 (43) | 0.171 | 91 (45) | 91 (45) | 1.000 |
| Age, years | 55 ± 13 | 54 ± 12 | 0.512 | 55 ± 13 | 54 ± 13 | 0.630 |
| Diabetes mellitus | 60 (25) | 69 (12) | <0.001 | 41 (20) | 40 (20) | 1.000 |
| Hypercholesterolemia | 70 (29) | 145 (24) | 0.191 | 54 (27) | 51 (25) | 0.821 |
| Smoking | 28 (12) | 60 (10) | 0.536 | 21 (10) | 25 (12) | 0.639 |
| Body height, cm | 162 ± 9 | 163 ± 8 | 0.167 | 162 ± 9 | 163 ± 8 | 0.155 |
| Body weight, kg | 69 ± 16 | 67 ± 13 | 0.110 | 69 ± 15 | 69 ± 14 | 0.895 |
| Body mass index, kg m−2 | 26 ± 4 | 25 ± 4 | 0.003 | 26 ± 4 | 26 ± 4 | 0.537 |
| SBP, mmHg | 161 ± 23 | 150 ± 19 | <0.001 | 158 ± 22 | 156 ± 20 | 0.181 |
| DBP, mmHg | 95 ± 15 | 90 ± 13 | <0.001 | 94 ± 14 | 92 ± 13 | 0.283 |
| Serum creatinine level, mg dL−1 | 1.1 ± 0.7 | 0.8 ± 0.3 | <0.001 | 0.9 ± 0.4 | 1.0 ± 0.5 | 0.832 |
| Serum potassium level, mmol L−1 | 3.5 ± 0.7 | 3.8 ± 0.5 | <0.001 | 3.6 ± 0.7 | 3.6 ± 0.6 | 0.824 |
| Unilateral PA, n (%) | 176 (72) | 360 (60) | <0.001 | 142 (70) | 138 (68) | 0.746 |
| PAC, ng dL−1 | 46 (39) | 41 (30) | <0.001 | 45 (36) | 43 (31) | 0.262 |
| PRA, ng mL−1 h−1 | 0.3 (0.5) | 0.3 (0.6) | 0.511 | 0.3 (0.6) | 0.3 (0.6) | 0.278 |
| ARR | 178 (473) | 171 (429) | 0.732 | 160 (498) | 204 (698) | 0.362 |
| Log-transformed PAC | 1.7 ± 0.3 | 1.6 ± 0.3 | 0.002 | 1.7 ± 0.3 | 1.6 ± 0.3 | 0.551 |
| Log-transformed PRA | −0.6 ± 0.7 | −0.6 ± 0.7 | 0.515 | −0.6 ± 0.7 | −0.7 ± 0.7 | 0.177 |
| Log-transformed ARR | 2.3 ± 0.7 | 2.3 ± 0.7 | 0.583 | 2.3 ± 0.7 | 2.4 ± 0.8 | 0.286 |
| Number of antihypertensive medication type | 2.3 ± 1.3 | 1.8 ± 1.3 | <0.001 | 2.2 ± 1.3 | 2.3 ± 1.2 | 0.545 |
| Hypertension history, years | 10.4 ± 9.0 | 6.8 ± 7.5 | <0.001 | 9.7 ± 8.8 | 8.4 ± 8.3 | 0.118 |
| Hypertension medication | ||||||
| ACEI, n (%) | 7 (3) | 9 (2) | 0.187 | 5 (3) | 5 (3) | 1.000 |
| ARB, n (%) | 110 (45) | 214 (36) | 0.011 | 89 (44) | 81 (40) | 0.481 |
| Alpha-blocker, n (%) | 53 (22) | 117 (20) | 0.469 | 36 (18) | 59 (29) | 0.010 |
| Beta-blocker, n (%) | 104 (43) | 186 (31) | 0.001 | 80 (40) | 78 (39) | 0.919 |
| CCB, n (%) | 183 (75) | 365 (61) | <0.001 | 152 (75) | 144 (71) | 0.431 |
| Vasodilator, n (%) | 28 (12) | 26 (4) | <0.001 | 20 (10) | 14 (7) | 0.370 |
| Spironolactone, n (%) | 43 (18) | 107 (18) | 0.938 | 32 (16) | 50 (25) | 0.035 |
| Diuretics, n (%) | 35 (14) | 58 (10) | 0.050 | 30 (15) | 28 (14) | 0.887 |
| PWV (cm/s) | 1809 ± 391 | 1656 ± 322 | <0.001 | 1783 ± 374 | 1689 ± 368 | 0.012 |
Matched with age, sex, DM, SBP, DBP, serum creatinine level, serum potassium level, number of antihypertensive medication type, and hypertension history.
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARR, aldosterone-renin ratio; CCB, calcium channel blocker; DBP, diastolic blood pressure; DM, diabetes mellitus; PA, primary aldosteronism; PAC, plasma aldosterone concentration; PRA, plasma renin activity; PWV, pulse wave velocity; SBP, systolic blood pressure.
After PSM, all of the above-mentioned parameters were balanced except for the percentages of alpha-blocker (p = 0.010) and spironolactone (p = 0.035) use.
The association between albuminuria and PWV
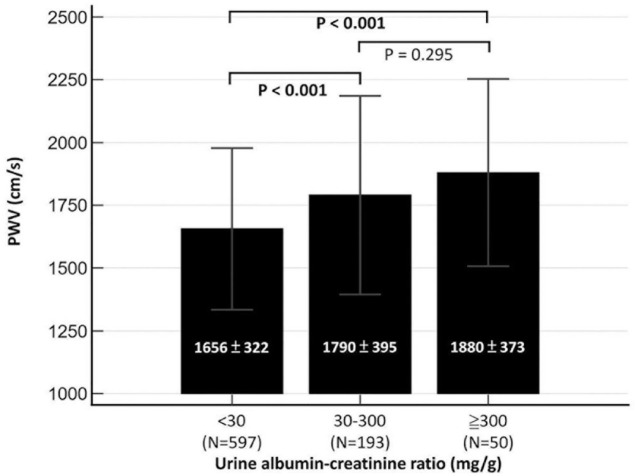
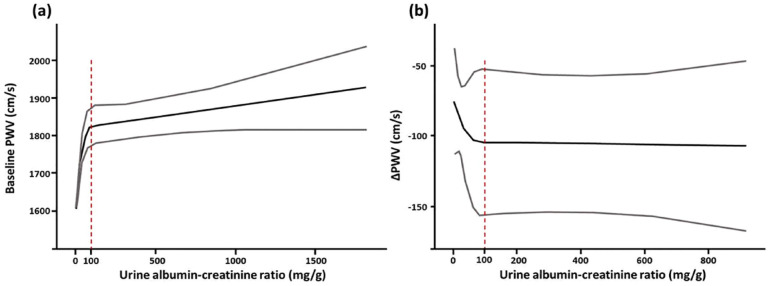
PA patients with concomitant albuminuria presented with greater PWV than those without albuminuria (1809 ± 391 versus 1656 ± 322 cm/s, p < 0.001). The difference in PWV remained statistically significant after PSM (1783 ± 374 versus 1689 ± 368 cm/s, p = 0.012) (Table 1). The overall cohort was further stratified into three groups by the level of UACR according to the traditional definitions of microalbuminuria and macroalbuminuria (UACR < 30 mg/g, 30–300 mg/g and > 300 mg/g). Generally, a greater UACR was associated with a greater elevation in PWV (1656 ± 322 cm/s in the UACR < 30 mg/g group, 1790 ± 395 cm/s in the UACR 30–300 mg/g group, and 1880 ± 373 cm/s in the UACR > 300 mg/g group; ANOVA p < 0.001) (Figure 1). However, the post hoc comparison between the UACR 30–300 mg/g and UACR > 300 mg/g groups did not reach statistical significance (p = 0.295). RCS analysis was further used to illustrate the relationship between UACR and baseline PWV. In general, PWV increased along with the degree of albuminuria; however, the slope was steeper when the UACR was <100 mg/g [Figure 2(a)].
Figure 1.
PWV of primary aldosteronism according to UACR.
PWV, pulse wave velocity; UACR, urine albumin–creatinine ratio.
Figure 2.
The association between (a) baseline (b) the change in PWV (ΔPWV) and UACR, plotted by restricted cubic spline method.
PWV, pulse wave velocity; UACR, urine albumin–creatinine ratio.
To investigate the possible risk factors associated with elevated PWV, univariable regression analysis was performed. The results showed that the presence of albuminuria, diabetes, hypercholesterolemia, age, body height, body weight, systolic and diastolic blood pressure, number of antihypertensive medication type, hypertension duration, as well as angiotensin-receptor blocker, beta-blocker, calcium channel blocker, vasodilator, and diuretic usage, were significantly correlated with PWV level. The presence of albuminuria remained an independent predictor of PWV in multivariable regression analysis (p = 0.002). Other statistically significant predictive factors for PWV included age (p < 0.001), body weight (p < 0.001), systolic blood pressure (p < 0.001), and calcium channel blocker use (p = 0.010) (Table 2).
Table 2.
Univariable and multivariable regression analysis of factors associated with pulse wave velocity.
| PA patients (N = 840) | Univariable analysis* | Multivariable analysis (adjusted R 2 = 0.490) | ||
|---|---|---|---|---|
| Determinants | Standardized β coefficient | p value | β [95% CI] | p value |
| Albuminuria | 0.197 | <0.001 | 63.364 [24.069–102.659] | 0.002 |
| Diabetes mellitus | 0.160 | <0.001 | 41.086 [−7.620–89.792] | 0.098 |
| Hypercholesterolemia | 0.091 | 0.008 | ||
| Smoking | −0.042 | 0.224 | ||
| Sex | 0.019 | 0.587 | ||
| Age | 0.554 | <0.001 | 15.176 [13.705–16.647] | <0.001 |
| Body height | −0.180 | <0.001 | ||
| Body weight | −0.141 | <0.001 | −2.816 [−4.097 to −1.535] | <0.001 |
| Body mass index | −0.066 | 0.054 | ||
| SBP | 0.404 | <0.001 | 6.532 [5.688–7.376] | <0.001 |
| DBP | 0.168 | <0.001 | ||
| Serum potassium level | 0.015 | 0.674 | ||
| Unilateral PA | 0.007 | 0.837 | ||
| Pre-Log-transformed PAC | 0.012 | 0.731 | ||
| Pre-Log-transformed PRA | −0.013 | 0.701 | ||
| Pre-Log-transformed ARR | 0.017 | 0.622 | ||
| Number of antihypertensive medication type | 0.208 | <0.001 | ||
| Hypertension duration | 0.400 | <0.001 | ||
| Hypertension medication | ||||
| ACEI | 0.036 | 0.302 | ||
| ARB | 0.118 | 0.001 | ||
| Alpha-blocker | 0.046 | 0.179 | ||
| Beta-blocker | 0.145 | <0.001 | ||
| CCB | 0.129 | <0.001 | 47.936 [11.468–84.404] | 0.010 |
| Vasodilator | 0.125 | <0.001 | ||
| Spironolactone | −0.022 | 0.527 | ||
| Diuretics | 0.122 | <0.001 | ||
Parameters in univariable analysis with p < 0.2 were included for multivariable regression analysis with backward selection.
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARR, aldosterone-renin ratio; CCB, calcium channel blocker; CI, confidence interval; DBP, diastolic blood pressure; PA, primary aldosteronism; PAC, plasma aldosterone concentration; PRA, plasma renin activity; SBP, systolic blood pressure.
Influence of albuminuria on treatment response for PA
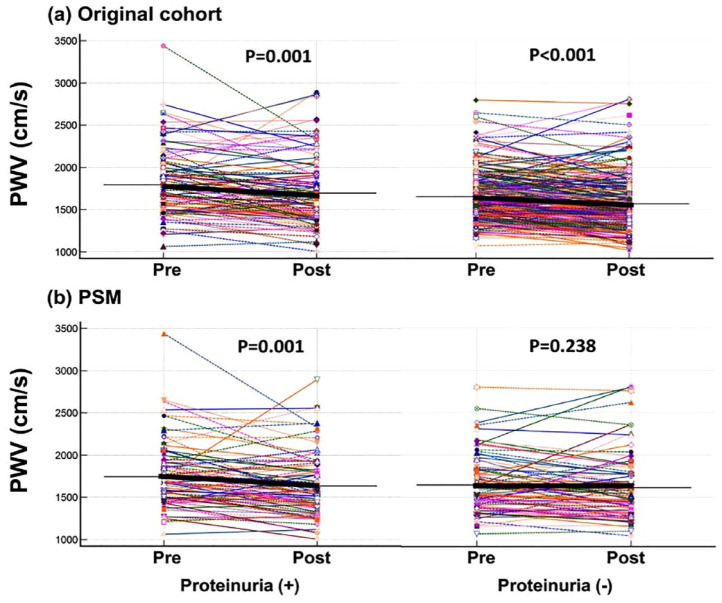
Data on changes in PWV as well as clinical parameters 1 year after PA-targeted treatment were available in 367 PA patients (109 with albuminuria and 258 without albuminuria). By comparisons between two groups before and after PSM (N = 88 in each group), significant improvements in post-treatment blood pressure, serum potassium level, PAC, PRA, and ARR were found in PA patients either with and without albuminuria. However, the change in PWV after PA-targeted treatment remained statistically significant only in patients with albuminuria (p = 0.001) after PSM (Table 3 and Figure 3).
Table 3.
Clinical outcomes of PA with and without albuminuria after treatment.
| Pulse wave analysis | Original data | Propensity score matching* | ||||
|---|---|---|---|---|---|---|
| Baseline | 1 year after treatment | p value | Baseline | 1 year after treatment | p value | |
| Albuminuria (+) | N = 109 | N = 88 | ||||
| SBP, mmHg | 161 ± 25 | 141 ± 19 | <0.001 | 157 ± 24 | 139 ± 19 | <0.001 |
| DBP, mmHg | 95 ± 16 | 85 ± 11 | <0.001 | 94 ± 15 | 85 ± 11 | <0.001 |
| Serum potassium level, mmol L−1 | 3.5 ± 0.7 | 4.4 ± 0.6 | <0.001 | 3.5 ± 0.7 | 4.4 ± 0.5 | <0.001 |
| Number of antihypertensive medication type | 2.4 ± 1.2 | 1.2 ± 1.2 | <0.001 | 2.3 ± 1.3 | 1.0 ± 1.1 | <0.001 |
| PAC, ng dL−1 | 51 (39) | 38 (34) | 0.013 | 50 (39) | 34 (32) | 0.010 |
| PRA, ng mL−1 h−1 | 0.3 (0.6) | 1.8 (4.0) | <0.001 | 0.3 (0.6) | 2.3 (4.4) | <0.001 |
| ARR | 182 (543) | 22 (63) | <0.001 | 177 (437) | 19 (36) | <0.001 |
| PWV, cm/s | 1796 ± 381 | 1696 ± 394 | 0.001 | 1744 ± 375 | 1635 ± 358 | 0.001 |
| Albuminuria (−) | N = 258 | N = 88 | ||||
| SBP, mmHg | 153 ± 18 | 137 ± 19 | <0.001 | 157 ± 19 | 137 ± 19 | <0.001 |
| DBP, mmHg | 92 ± 12 | 84 ± 12 | <0.001 | 92 ± 12 | 83 ± 11 | <0.001 |
| Serum potassium level, mmol L−1 | 3.7 ± 0.6 | 4.3 ± 0.4 | <0.001 | 3.6 ± 0.7 | 4.3 ± 0.4 | <0.001 |
| Number of antihypertensive medication type | 1.9 ± 1.2 | 1.1 ± 1.0 | <0.001 | 2.3 ± 1.1 | 1.2 ± 1.1 | <0.001 |
| PAC, ng dL−1 | 46 (34) | 37 (30) | <0.001 | 45 (43) | 32 (26) | 0.001 |
| PRA, ng mL−1 h−1 | 0.2 (0.5) | 1.6 (4.1) | <0.001 | 0.2 (0.6) | 1.4 (3.5) | <0.001 |
| ARR | 230 (501) | 22 (67) | <0.001 | 229 (705) | 25 (69) | <0.001 |
| PWV, cm/s | 1655 ± 285 | 1572 ± 320 | <0.001 | 1648 ± 327 | 1617 ± 375 | 0.238 |
Matched with age, sex, DM, SBP, DBP, serum creatinine level, serum potassium level, number of antihypertensive medication type, and hypertension history.
ARR, aldosterone-renin ratio; DBP, diastolic blood pressure; DM, diabetes mellitus; PAC, plasma aldosterone concentration; PRA, plasma renin activity; PWV, pulse wave analysis; SBP, systolic blood pressure.
Figure 3.
PWV before and after treatment of primary aldosteronism with and without albuminuria. (a) Original cohort and (b) after PSM.
PSM, propensity score matching; PWV, pulse wave velocity.
In addition, RCS analysis demonstrated that the magnitude of decrease in PWV was nonlinearly associated with UACR. The effectiveness of treatment in reversing arterial stiffness exhibited a more noticeable effect when the UACR was below 100 mg/g and reached a plateau when the UACR surpassed 100 mg/g [Figure 2(b)]. The overall results were summarized (supplementary material).
Discussions
The major findings of this study are that (1) the presence of albuminuria was associated with a greater PWV in the enrolled PA patients, (2) the improvement in PWV was more pronounced in the PA patients with concomitant albuminuria, and (3) baseline and improvements in PWV after PA treatment were positively and nonlinearly correlated with UACR level. To the best of our knowledge, this is the first study to investigate the crosstalk between concomitant albuminuria and atherosclerosis in the setting of PA. Our findings emphasize the significance of the synergistic impact of albuminuria and aldosteronism on arterial stiffening.
PA is a modifiable and independent risk factor for poor cardiovascular outcomes.22,23 The impact of PA on the vasculature exceeds the hemodynamic effect of elevated blood pressure. 24 Circulating aldosterone has been proposed to lead to the pathological remodeling of vascular endothelium, resulting in impaired vascular tone, local inflammation, luminal wall damage, and accelerated atherosclerosis. 6 Moreover, reorganization of the extracellular matrix secondary to aldosterone stimulation has also been shown to contribute to arterial wall stiffening.25,26 PWV is a non-invasive tool which can be used to assess arterial stiffness and evaluate aldosterone-induced vascular damage. PWV has been strongly correlated with cardiovascular outcomes in patients with PA. 27 In previous studies, various clinical parameters have been proposed to alter the improvement in PWV after PA treatment. For example, the presence of diabetes has been correlated with worse atherosclerosis at baseline as well as poorer recovery of arterial stiffness after PA treatment. 28 Autonomous cortisol secretion has also been shown to cause worse arterial stiffness at baseline and recovery after treatment in PA patients. 29 Genetically, the somatic mutation KCNJ5 has been shown to predict greater recovery of arterial stiffness after adrenalectomy. 30
Few studies have investigated the role of concomitant albuminuria on the progression of atherosclerosis. In this study, we investigated the role of albuminuria on arterial stiffness in PA patients at baseline and after PA-targeted treatment. The presence of albuminuria is known to be closely correlated with macroangiopathy. 31 In the Hoorn study, elevated UACR was shown to potentiate arterial stiffening. The diffuse atherosclerosis may not only be caused by traditional cardiovascular risk factors but also by maladaptive vascular remodeling secondary to concomitant albuminuria. 32 A community health study highlighted the relationship between albuminuria and elevated PWV in middle-aged patients, especially those with concomitant diabetes and hypertension. 33 Another recent cohort study also found that albuminuria along with age and blood pressure were independent predictors of atherosclerosis, 34 and implied its role in subclinical target organ damage. In the present study, we found that albuminuria was independently related to worse baseline arterial stiffness, and it could predict better recovery of arterial stiffness after PA treatment. The baseline PWV value and the extent of PWV improvement both rose along with increasing UACR levels and reached a plateau when the UACR exceeded 100 mg/g according to RCS analysis. These findings highlight the importance of early and timely treatment of PA to achieve the largest benefit in arterial stiffness reversal.
Exploring cardiorenal interplay in patients with PA is challenging because atherosclerosis and albuminuria are considered to be mutually causative. A previous study suggested that PA contributes more to excess albuminuria than essential hypertension due to remodeling of the renal vasculature as well as the tubuloglomerular system. 35 A recent study reported that PAC was significantly associated with more substantial proteinuria, and implied that concomitant cardiorenal damage could occur when the PAC is >550 pmol/L (around 20 ng/dL). 36 In our study, the mean PAC was far >20 ng/dL, which may therefore have potentiated the occurrence of albuminuria and cardiorenal damage. A previous study reported that plasminogen activator inhibitor-1 and tumor growth factor β were associated with aldosterone-induced renal vascular damage. 37 The accumulation of reactive oxygen species, endothelial dysfunction with impaired nitric oxide production, and alteration in vascular smooth muscle cells caused by aldosterone 38 have been suggested to have a synergistic effect to anatomically and functionally reshape renal arteries, eventually leading to albuminuria. 39 A recent meta-analysis found that subjects with PA were prone to develop more pronounced macroalbuminuria. 40 Since the presence of albuminuria is known to compromise long-term cardiovascular outcomes in individuals with PA, its effect on arterial viscoelasticity may explain the prognostic implication.
With regard to the treatment response, a reduction in clinical blood pressure and improvements in biochemistry data were seen in both the PA patients with and without albuminuria, whereas the effect of arterial stiffness reversal was more pronounced in the PA patients with albuminuria. In a previous study from our TAIPAI group, Liao et al. 41 demonstrated that baseline PWV was positively correlated with the improvement in arterial stiffness after adrenalectomy. Similarly, the individuals with concomitant albuminuria presented with a greater initial level of PWV and benefited more after PA-targeted treatment. This study provides evidence to consider using albuminuria for PA risk stratification and to predict the effect of PA-targeted treatment. Importantly, the greater vascular benefits should not be misinterpreted as a contraindication for PA treatment in patients without albuminuria.
Although this is the largest cohort study to investigate the association between albuminuria and arterial stiffness reversal, the following limitations exist. First, our study only established the association among albuminuria, PA, and atherosclerosis. The causal relation remained unknown and warranted future investigations to answer. Second, the level of albuminuria was estimated based on the UACR in single spot urine instead of collecting 24-h urine samples. Possible conditions, including physical exercise, prolonged orthostatism, fever, etc., may cause transient increases in albuminuria. Although a previous study validated the predicted value of single spot urine, 42 the results should be interpreted prudently. Third, a proportion of the patients with PA had no PWV measurements after treatment. Also, while RCS analysis was conducted to investigate the correlation between PWV improvement and UACR, a categorical classification based on the severity of albuminuria for comparing PWV improvement was not undertaken due to a substantial reduction in sample size during PSM. Forth, the study did not compare the vascular effect of different therapeutic modalities. An extended follow-up period with stratified analysis of either pharmaceutical or surgical management is needed to address the impact of concomitant albuminuria.
Conclusion
The presence of albuminuria was associated with higher PWV in the enrolled patients with PA. The relationship between PWV level and UACR was nonlinear but positively correlated. PA treatment effectively reversed arterial stiffness, especially in individuals with concurrent albuminuria, with the extent of PWV improvement increasing as UACR levels rose, plateauing when UACR exceeded 100 mg/g.
Supplemental Material
Supplemental material, sj-docx-1-taj-10.1177_20406223231210114 for Synergistic effect of albuminuria on atherosclerosis in patients with primary aldosteronism by Ting-Wei Kao, Che-Wei Liao, Cheng-Hsuan Tsai, Yi-Yao Chang, Chien-Ting Pan, Chin-Chen Chang, Bo-Ching Lee, Wei-Chieh Huang, Kuo-How Huang, Ching-Chu Lu, Tai-Shuan Lai, Chieh-Kai Chan, Jeff S. Chueh, Vin-Cent Wu, Chi-Sheng Hung, Zheng-Wei Chen and Yen-Hung Lin in Therapeutic Advances in Chronic Disease
Acknowledgments
The authors sincerely acknowledged the staff of the Second Core Lab in the Department of Medical Research in National Taiwan University Hospital for technical assistance.
Appendix
Membership of the Taiwan Primary Aldosteronism Investigation (TAIPAI) study group: Che-Hsiung Wu, MD (Chi-Taz Hospital, PI of Committee); Vin-Cent Wu, MD, PhD (NTUH, PI of Committee); Yen-Hung Lin, MD, PhD (NTUH, PI of Committee); Hung-Wei Chang, MD, PhD (Far Eastern Clinics, PI of Committee); Lian-Yu Lin, MD, PhD (NTUH, PI of Committee); Fu-Chang Hu, MS, ScD (Harvard Statistics, Site Investigator); Kao-Lang Liu, MD (NTUH, PI of Committee); Shuo-Meng Wang, MD (NTUH, PI of Committee); Kuo-How Huang, MD (NTUH, PI of Committee); Yung-Ming Chen, MD (NTUH, PI of Committee); Chin-Chen Chang, MD (NTUH, PI of Committee); Shih-Cheng Liao, MD (NTUH, PI of Committee); Ruoh-Fang Yen, MD, PhD (NTUH, PI of Committee); and Kwan-Dun Wu, MD, PhD (NTUH, Director of Coordinating Center).
Footnotes
ORCID iDs: Ting-Wei Kao  https://orcid.org/0000-0002-1069-4558
https://orcid.org/0000-0002-1069-4558
Che-Wei Liao  https://orcid.org/0000-0001-7156-5381
https://orcid.org/0000-0001-7156-5381
Yi-Yao Chang  https://orcid.org/0000-0002-9148-8667
https://orcid.org/0000-0002-9148-8667
Wei-Chieh Huang  https://orcid.org/0000-0003-3759-0131
https://orcid.org/0000-0003-3759-0131
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Ting-Wei Kao, Department of Internal Medicine, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei, Taiwan.
Che-Wei Liao, Department of Internal Medicine, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei, Taiwan; Department of Medicine, National University Cancer Center, Taipei, Taiwan.
Cheng-Hsuan Tsai, Department of Internal Medicine, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei, Taiwan.
Yi-Yao Chang, Department of Cardiovascular Medicine, Far Eastern Memorial Hospital, New Taipei City, Taiwan; Graduate Institute of Medicine, Yuan Ze University, Taoyuan City, Taiwan.
Chien-Ting Pan, Department of Internal Medicine, National University Hospital and National University College of Medicine, Taipei, Taiwan; Department of Internal Medicine, National University Hospital Yun-Lin Branch, Yun-Lin, Taiwan.
Chin-Chen Chang, Department of Medical Imaging, National University Hospital and National University College of Medicine, Taipei, Taiwan.
Bo-Ching Lee, Department of Medical Imaging, National University Hospital and National University College of Medicine, Taipei, Taiwan.
Wei-Chieh Huang, Division of Cardiology, Department of Internal Medicine, Taipei Veterans General Hospital, Taipei, Taiwan.
Kuo-How Huang, Department of Urology, National University Hospital and National University College of Medicine, Taipei, Taiwan.
Ching-Chu Lu, Department of Nuclear Medicine, National University Hospital, Taipei, Taiwan.
Tai-Shuan Lai, Department of Internal Medicine, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei, Taiwan.
Chieh-Kai Chan, Department of Internal Medicine, National University Hospital Hsin-Chu Branch, Hsin-Chu, Taiwan.
Vin-Cent Wu, Department of Internal Medicine, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei, Taiwan.
Chi-Sheng Hung, Department of Internal Medicine, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei, Taiwan.
Zheng-Wei Chen, Division of Cardiology, Department of Internal Medicine, National Taiwan University Hospital Yun-Lin Branch, No. 579, Sec. 2, Yun-Lin Rd., Douliu City, Yun-Lin County 640203.
Yen-Hung Lin, Department of Internal Medicine, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei, Taiwan.
Declarations
Ethics approval and consent to participate: This study was carried out in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of National Taiwan University Hospital (approval number 200611031R).
Consent for publication: Written informed consent form was obtained from all subjects prior to participation.
Author contributions: Ting-Wei Kao: Data curation; Formal analysis; Writing – original draft.
Che-Wei Liao: Data curation; Formal analysis.
Cheng-Hsuan Tsai: Data curation; Formal analysis; Methodology.
Yi-Yao Chang: Data curation; Formal analysis; Validation.
Chien-Ting Pan: Data curation; Formal analysis.
Chin-Chen Chang: Data curation; Formal analysis.
Bo-Ching Lee: Data curation; Formal analysis; Resources.
Wei-Chieh Huang: Data curation; Formal analysis.
Kuo-How Huang: Data curation; Formal analysis; Resources.
Ching-Chu Lu: Data curation; Formal analysis; Resources.
Tai-Shaun Lai: Data curation; Formal analysis; Methodology.
Chieh-Kai Chan: Data curation; Formal analysis; Validation.
Jeff S. Chueh: Data curation; Formal analysis; Supervision.
Vin-Cent Wu: Conceptualization; Data curation; Formal analysis; Funding acquisition; Supervision.
Chi-Sheng Hung: Conceptualization; Data curation; Formal analysis; Funding acquisition; Project administration.
Zheng-Wei Chen: Conceptualization; Data curation; Formal analysis; Funding acquisition; Project administration; Supervision; Validation; Writing – review & editing.
Yen-Hung Lin: Conceptualization; Data curation; Formal analysis; Funding acquisition; Project administration; Supervision; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by grants from the Ministry of Science and Technology (MOST 109-2314-B-002-284-MY3, 109-2314-B-002-247-MY3, 110-2314-B-002-134-MY3, 111-2314-B-002-044), National Taiwan University Hospital (NTUH 110-A141, 110-S5120, 111-N0092, 111UN-0039, VN110-14, VN111-11), National Taiwan University Hospital Yun-Lin branch (NTUHYL 108-N005, 109-N007, 110-S011).
The authors declare that there is no conflict of interest.
Availability of data and materials: The dataset of this study is available under reasonable request to the corresponding author.
References
- 1. Käyser SC, Dekkers T, Groenewoud HJ, et al. Study heterogeneity and estimation of prevalence of primary aldosteronism: a systematic review and meta-regression analysis. J Clin Endocrinol Metab 2016; 101: 2826–2835. [DOI] [PubMed] [Google Scholar]
- 2. Catena C, Colussi G, Nadalini E, et al. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch Intern Med 2008; 168: 80–85. [DOI] [PubMed] [Google Scholar]
- 3. Mulatero P, Monticone S, Bertello C, et al. Long-term cardio- and cerebrovascular events in patients with primary aldosteronism. J Clin Endocrinol Metab 2013; 98: 4826–4833. [DOI] [PubMed] [Google Scholar]
- 4. Chen ZW, Huang KC, Lee JK, et al. Aldosterone induces left ventricular subclinical systolic dysfunction: a strain imaging study. J Hypertens 2018; 36: 353–360. [DOI] [PubMed] [Google Scholar]
- 5. Tsai CH, Pan CT, Chang YY, et al. Left ventricular remodeling and dysfunction in primary aldosteronism. J Hum Hypertens 2021; 35: 131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen ZW, Tsai CH, Pan CT, et al. Endothelial dysfunction in primary aldosteronism. Int J Mol Sci 2019; 20: 5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim HL, Kim SH. Pulse wave velocity in atherosclerosis. Front Cardiovasc Med 2019; 6: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ben-Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol 2014; 63: 636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim ED, Tanaka H, Ballew SH, et al. Associations between kidney disease measures and regional pulse wave velocity in a large community-based cohort: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis 2018; 72: 682–690. [DOI] [PubMed] [Google Scholar]
- 10. Ohkuma T, Ninomiya T, Tomiyama H, et al. Brachial-ankle pulse wave velocity and the risk prediction of cardiovascular disease: an individual participant data meta-analysis. Hypertension 2017; 69: 1045–1052. [DOI] [PubMed] [Google Scholar]
- 11. Hung CS, Sung SH, Liao CW, et al. Aldosterone induces vascular damage. Hypertension 2019; 74: 623–629. [DOI] [PubMed] [Google Scholar]
- 12. Lin YH, Lin LY, Chen A, et al. Adrenalectomy improves increased carotid intima-media thickness and arterial stiffness in patients with aldosterone producing adenoma. Atherosclerosis 2012; 221: 154–159. [DOI] [PubMed] [Google Scholar]
- 13. Wu VC, Kuo CC, Wang SM, et al. Primary aldosteronism: changes in cystatin C-based kidney filtration, proteinuria, and renal duplex indices with treatment. J Hypertens 2011; 29: 1778–1786. [DOI] [PubMed] [Google Scholar]
- 14. Ogata H, Yamazaki Y, Tezuka Y, et al. Renal injuries in primary aldosteronism: quantitative histopathological analysis of 19 patients with primary aldosteronism. Hypertension 2021; 78: 411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu VC, Hu YH, Wu CH, et al. Administrative data on diagnosis and mineralocorticoid receptor antagonist prescription identified patients with primary aldosteronism in Taiwan. J Clin Epidemiol 2014; 67: 1139–1149. [DOI] [PubMed] [Google Scholar]
- 16. Wu VC, Hu YH, Er LK, et al. Case detection and diagnosis of primary aldosteronism – The consensus of Taiwan Society of Aldosteronism. Formos Med Assoc 2017; 116: 993–1005. [DOI] [PubMed] [Google Scholar]
- 17. Wu VC, Yang SY, Lin JW, et al. Kidney impairment in primary aldosteronism. Clin Chim Acta 2011; 412: 1319–1325. [DOI] [PubMed] [Google Scholar]
- 18. Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2016; 101: 1889–1916. [DOI] [PubMed] [Google Scholar]
- 19. Munakata M. Brachial-ankle pulse wave velocity: background, method, and clinical evidence. Pulse 2016; 3: 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smith A, Karalliedde J, De Angelis L, et al. Aortic pulse wave velocity and albuminuria in patients with type 2 diabetes. J Am Soc Nephrol 2005; 16: 1069–1075. [DOI] [PubMed] [Google Scholar]
- 21. Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol 1993; 138: 923–936. [DOI] [PubMed] [Google Scholar]
- 22. Wu VC, Wang SM, Chang CH, et al. Long term outcome of aldosteronism after target treatments. Sci Rep 2016; 6: 32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Monticone S, D’Ascenzo F, Moretti C, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol 2018; 6: 41–50. [DOI] [PubMed] [Google Scholar]
- 24. Strauch B, Petrák O, Wichterle D, et al. Increased arterial wall stiffness in primary adosteronism in comparison with essential hypertension. Am J Hypertens 2006; 19: 909–914. [DOI] [PubMed] [Google Scholar]
- 25. Hung CS, Chou CH, Wu XM, et al. Circulating tissue inhibitor of matrix metalloproteinase-1 is associated with aldosterone-induced diastolic dysfunction. J Hypertens 2015; 33: 1922–1930. [DOI] [PubMed] [Google Scholar]
- 26. Rizzoni D, Paiardi S, Rodella L, et al. Changes in extracellular matrix in subcutaneous small resistance arteries of patients with primary aldosteronism. J Clin Endocrinol Metab 2006; 91: 2638–2642. [DOI] [PubMed] [Google Scholar]
- 27. Hametner B, Wassertheurer S, Mayer CC, et al. Aortic pulse wave velocity predicts cardiovascular events and mortality in patients undergoing coronary angiography. Hypertension 2021; 77: 571–581. [DOI] [PubMed] [Google Scholar]
- 28. Tsai CH, Wu XM, Liao CW, et al. Diabetes mellitus is associated with worse baseline and less post-treatment recovery of arterial stiffness in patients with primary aldosteronism. Ther Adv Chronic Dis 2022; 13: 20406223211066727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tsai CH, Liao CW, Wu XM, et al. Autonomous cortisol secretion is associated with worse arterial stiffness and vascular fibrosis in primary aldosteronism: a cross-sectional study with follow-up data. Eur J Endocrinol 2022; 187: 197–208. [DOI] [PubMed] [Google Scholar]
- 30. Chang YY, Pan CT, Chen ZW, et al. KCNJ5 somatic mutations in aldosterone-producing adenoma are associated with a greater recovery of arterial stiffness. Cancers 2021; 13: 4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, et al. Albuminuria reflects widespread vascular damage. Diabetologia 1989; 32: 219–226. [DOI] [PubMed] [Google Scholar]
- 32. Hermans MM, Henry R, Dekker JM, et al. Estimated glomerular filtration rate and urinary albumin excretion are independently associated with greater arterial stiffness: the Hoorn Study. J Am Soc Nephrol 2007; 18: 1942–1952. [DOI] [PubMed] [Google Scholar]
- 33. Liu CS, Pi-Sunyer FX, Li CI, et al. Albuminuria is strongly associated with arterial stiffness, especially in diabetic or hypertensive subjects–a population-based study (Taichung Community Health Study, TCHS). Atherosclerosis 2010; 211: 315–321. [DOI] [PubMed] [Google Scholar]
- 34. Ye C, Gong J, Wang T, et al. Relationship between high-normal albuminuria and arterial stiffness in Chinese population. J Clin Hypertens 2020; 22: 1674–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Halimi JM, Mimran A. Albuminuria in untreated patients with primary aldosteronism or essential hypertension. J Hypertens 1995; 13: 1801–1802. [PubMed] [Google Scholar]
- 36. Nakamura Y, Kobayashi H, Tanaka S, et al. Association between plasma aldosterone and markers of tubular and glomerular damage in primary aldosteronism. Clin Endocrinol 2021; 94: 920–926. [DOI] [PubMed] [Google Scholar]
- 37. Huang W, Xu C, Kahng KW, et al. Aldosterone and TGF-beta1 synergistically increase PAI-1 and decrease matrix degradation in rat renal mesangial and fibroblast cells. Am J Physiol Renal Physiol 2008; 294: F1287–F1295. [DOI] [PubMed] [Google Scholar]
- 38. Chou CH, Chen YH, Hung CS, et al. Aldosterone impairs vascular smooth muscle function: from clinical to bench research. J Clin Endocrinol Metab 2015; 100: 4339–4347. [DOI] [PubMed] [Google Scholar]
- 39. Hollenberg NK. Aldosterone in the development and progression of renal injury. Kidney Int 2004; 66: 1–9. [DOI] [PubMed] [Google Scholar]
- 40. Monticone S, Sconfienza E, D’Ascenzo F, et al. Renal damage in primary aldosteronism: a systematic review and meta-analysis. J Hypertens 2020; 38: 3–12. [DOI] [PubMed] [Google Scholar]
- 41. Liao CW, Lin LY, Hung CS, et al. Time course and factors predicting arterial stiffness reversal in patients with aldosterone-producing adenoma after adrenalectomy: prospective study of 102 patients. Sci Rep 2016; 6: 20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lambers Heerspink HJ, Brantsma AH, de Zeeuw D, et al. Albuminuria assessed from first-morning-void urine samples versus 24-hour urine collections as a predictor of cardiovascular morbidity and mortality. Am J Epidemiol 2008; 168: 897–905. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-taj-10.1177_20406223231210114 for Synergistic effect of albuminuria on atherosclerosis in patients with primary aldosteronism by Ting-Wei Kao, Che-Wei Liao, Cheng-Hsuan Tsai, Yi-Yao Chang, Chien-Ting Pan, Chin-Chen Chang, Bo-Ching Lee, Wei-Chieh Huang, Kuo-How Huang, Ching-Chu Lu, Tai-Shuan Lai, Chieh-Kai Chan, Jeff S. Chueh, Vin-Cent Wu, Chi-Sheng Hung, Zheng-Wei Chen and Yen-Hung Lin in Therapeutic Advances in Chronic Disease