Abstract
Objectives:
No study has examined the effects of new constipation treatment drugs released in recent years in pregnant women. This prospective cohort study aimed to examine and compare the perinatal prognosis, efficacy rate, and safety of drugs frequently used to treat constipation.
Methods:
The study included 211 perinatally managed individuals who answered a self-administered questionnaire during the second trimester and after delivery. The Japanese version of the constipation evaluation scale (Constipation Assessment Scale [CAS] long-term [LT] version) was used for the subjective evaluation of defecation status.
Results:
Participants aware of constipation had significantly higher CAS scores than those who were unaware. Some participants with a CAS score of 5 points (treatment range) had no subjective symptoms of constipation, whereas some participants with a CAS score of ≤ 5 points were aware of constipation. Regarding the time of onset, 60% of those who had constipation before pregnancy had a high rate of constipation during pregnancy and after delivery. No significant difference was noted in conventional magnesium oxide and polyethylene glycol, a relatively new daily treatment drug, in perinatal prognosis or side effects.
Conclusions:
Polyethylene glycol preparations alleviate constipation without inducing diarrhea, making them an appropriate therapeutic option for pregnant women.
Keywords: Constipation, pregnancy, prospective study, treatment
Introduction
During pregnancy and after childbirth, constipation mainly occurs due to factors such as morning sickness, organic changes in the abdominal cavity, and physical water balance. And increased progesterone during pregnancy suppresses intestinal peristalsis, making constipation more likely. Recently, the relationship between gut microbiota and imminent preterm birth as well as constipation has been reported1).
Many recognized fact-finding surveys and reports on constipation in pregnant women have investigated the dietary content for alleviating constipation2). However, there are very few published articles investigating the onset time of constipation in pregnant women with constipation.
Therefore, this study aimed to investigate the prevalence and treatment of constipation, the prognosis of constipation in the perinatal period, and the persistent rate of constipation in pregnant women. In addition, the Japanese version of the Constipation Assessment Scale (CAS long-term [LT] version), which is an objective evaluation scale, was used to evaluate defecation, and the Bristol scale, which is a subjective evaluation, was used to evaluate shape.
Materials and methods
Study population
We conducted perinatal management from the beginning of pregnancy at our hospital and targeted 211 pregnant women and puerperal women who consented to the survey. Those suffering from gastrointestinal tract diseases such as intestinal obstruction, intestinal perforation, inflammatory bowel disease, irritable bowel syndrome, suspected fetal morphological abnormalities, and eating disorders such as anorexia were excluded.
This study was approved by the Ethics Committee of Iwase General Hospital (approval number: 200704), and conducted in accordance with the tenets of the Declaration of Helsinki. The participants provided written informed consent to participate in the research.
Survey method
Answers were obtained using a self-administered questionnaire (Supplementary Text) during the second trimester (approximately at 28 weeks of gestation) and after delivery (within six days after delivery). During the waiting time, when pregnant or postpartum women visited the hospital for a medical examination, questionnaires were distributed and filled out by those who were informed of the study through a summary and provided informed consent. In the case of postpartum, a questionnaire was distributed from the day after delivery when the condition was stable and collected by the time of discharge. Perinatal medical information was extracted from electronic medical records.
Investigation period
The investigation period was from August 2020 to May 2021.
Investigation
1) Participant background: Age, physical information, and body mass index (BMI) of less than 18.5 were classified as lean, 18.5 to less than 25 as normal, 25 to less than 30 as obese, and 30 or more as severely obese. The history of abdominal surgery, including previous cesarean delivery, was investigated.
2) Medical information: Perinatal prognosis such as treatment history of illness, presence, or absence of obstetric complications of imminent preterm birth, weight gain rate during pregnancy, delivery pattern, birth weight, Apgar score at 1 min and 5 min, pH of umbilical cord arterial blood, and neonatal intensive care unit (NICU) hospitalization rate was obtained.
3) Constipation-related information: We investigated the management of self-styled constipation, the eating status of meals, presence/absence of awareness of constipation, evaluation of objective defecation status, the timing of constipation onset, constipation treatment method, laxatives use, defecation before and after use of laxatives, and the number of times and changes in the shape of the stool. Additionally, the Bristol Scale was used to determine the properties of the stool. The Bristol Scale is a diagnostic medical tool designed to classify human fecal morphology into seven categories. In 1997 he was developed at the Bristol Royal Infirmary as a clinical evaluation tool and is used as a tool to assess the efficacy of treatments for various diseases of the bowel. (Supplementary_Materials Question 9 upper table)
4) Constipation Assessment Scale (CAS): For the objective evaluation of defecation status, we used the Japanese version of the constipation evaluation scale (CAS long-term [LT] version) reported by Fukai et al.3). CAS is scored on a scale of 0-16 points, and the higher the score, the more severe constipation is evaluated. Based on the study results by Fukai et al.3), a CAS score of 5 or higher was defined as constipation requiring medical intervention. In this study, a CAS score of 5 or higher was defined as constipation. We also investigated the postpartum defecation status in consideration of the situation in which it is difficult to apply abdominal pressure due to the effects of perineal laceration during delivery and the cesarean section wound, resulting in constipation.
Statistical analysis
Descriptive statistics were used to analyze the background of the participants, constipation-related matters, and coping strategies for constipation. Additionally, comparisons were made at each period, and defecation status and other related factors were analyzed by the t-test, chi-square (χ2) test, and one-way analysis of variance (ANOVA). We used SPSS version 21 (IBM Corp., Armonk, NY, USA) for analysis. A P value < 0.05 indicated a statistical significance.
Results
Participants background
The participants’ background information (n=211) is shown in Table 1. The participants were classified into two groups according to the presence or absence of constipation: the constipation group and the non-constipation group. There were no differences between the two groups in parameters such as the age of pregnant women, primiparity rate, rate of cesarean delivery, BMI during the first trimester of pregnancy, week of delivery, and infant birth weight.
Table 1.
Participants’ background
| Total (n=211) |
No constipation group (n=116) |
Constipation group (n=95) |
P value | |
| Age (years) | 31.1±5.9 | 31.0±6.3 | 31.3±5.5 | 0.59 |
| Primiparas (%) | 40.8 | 20.4 | 20.4 | 0.55 |
| Cesarean delivery history (%) | 27.3 | 15.6 | 11.7 | 0.64 |
| BMI in first trimester of pregnancy (%) | 21.9±5.1 | 22.1±5.5 | 21.6±4.6 | 0.54 |
| Weeks of delivery (weeks) | 38.9±1.3 | 38.9±1.1 | 38.7±1.1 | 0.10 |
| Infant birth weight (g) | 3,031.6±346 | 3,075.0±332.0 | 3,006.0±352.2 | 0.23 |
BMI, body mass index
Constipation prevention measures
Our results showed that 9.0% of the respondents had the opportunity to exercise, and 46.9%, which accounted for approximately half of the respondents, consumed dietary fiber, such as vegetables and yogurt (containing lactic acid bacteria). In addition, approximately 80% of the respondents actively drank water. When we investigated whether they ate three meals, skipped breakfast, or had an irregular diet, we found that 85.3% ate three meals, and 14.7% did otherwise.
CAS
The participants had a CAS score of 0 to a maximum of 12, with 72 (34.1%) participants having constipation requiring medical intervention (CAS score ≥ 5) (Figure 1). Regarding the presence or absence of constipation in all participants, 108 (51.2%) were aware of constipation with an average CAS score of 6.1 ± 2.8. In contrast, the CAS score of the participants who were unaware of constipation was 2.4 ± 1.9. A significant difference was observed between the participants with or without constipation awareness (p < 0.001, Figure 2).
Fig. 1.
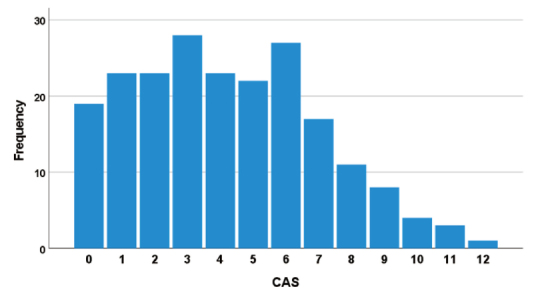
CAS score of mid-pregnancy. The participants had a CAS score of a minimum of 0 to a maximum of 12, with 72 (34.1%) participants having constipation requiring medical intervention (CAS score ≥ 5). CAS, Constipation Assessment Scale.
Fig. 2.
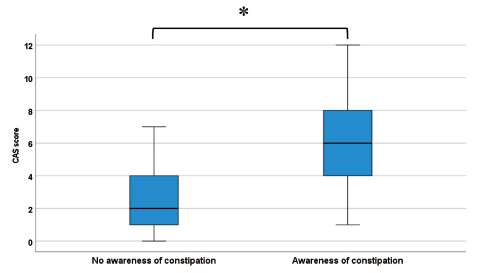
CAS score with or without constipation awareness. The CAS score of participants who were aware of constipation was 6.14 ± 2.80. The CAS score of the participants who were unaware of constipation was 2.44 ± 1.93. A significant difference was observed between the participants with or without constipation awareness (*p < 0.001). CAS, Constipation Assessment Scale.
Among 108 participants with an average CAS score of 6.1 ± 2.8, constipation awareness was in 59 (54.6%) individuals before pregnancy and 38 after pregnancy. Of the constipation symptoms that developed after pregnancy, 16 (14.8%), 21 (19.4%), and 1 (0.9%) were in the first, second (less than 28 gestation weeks), and third trimester s of pregnancy, respectively (Figure 3).
Fig. 3.
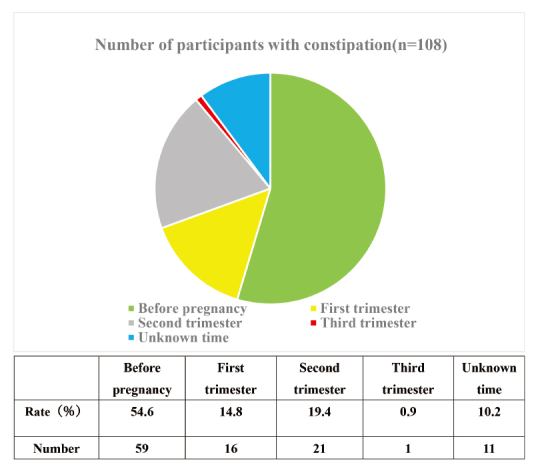
Presence or absence of constipation awareness and incidence rate over time. Among participants with known onset of constipation (n=97) was 59 (60.8%) before pregnancy and 38 after pregnancy. Of the constipation symptoms that developed after pregnancy, 16 (42.1%) were in early pregnancy, 21 (55.3%) were in mid-pregnancy (less than 27 gestation weeks), and 1 (2.6%) was in late pregnancy.
The CAS scores for all those who responded that they developed constipation were 5.9 ±3.3, 5.0 ± 1.6, and 5.7 ± 3.0 after becoming pregnant, in early pregnancy, and in mid -pregnancy, respectively. There was no difference in dominance (p = 0.34).
Furthermore, 16 (7.6% of the total) patients had a CAS score of 5 points, even though there were no subjective symptoms of constipation. In contrast, 29 (13.7%) responded that they were aware of constipation, although the CAS score was less than 5.
Use of laxatives
Magnesium oxide, polyethylene glycol (PEG), and picosulfate sodium were laxatives prescribed in 52 (46.7%), 12 (10.3%), and 2 (1.9%) patients, respectively. Others took butyrate-producing bacteria tablets, over-the-counter laxatives, and supplements claiming the effects of laxatives. Seventeen (26.6%) patients took laxatives daily, and 41 (64.1%) took laxatives on an as-needed basis.
Comparing patients prescribed magnesium oxide and PEG, both the groups had bowel movements once every 2-3 days before taking the drug. However, after dosing, bowel movements were noted 3-7 days per week for patients prescribed magnesium oxide and 3-5 days per week for patients prescribed PEG. A comparison of stool consistency showed that both the magnesium oxide and PEG-prescribed groups had Bristol Scale score s of 1-2 (hard stools) before dosing. However, the majority showed improvement on the Bristol Scale score of 4 (normal stools). On an individual basis, some patients prescribed magnesium oxide increased stool frequency as a result of loose stools or diarrhea.
The overall CAS measurement immediately after delivery was 3.9 ± 2.9, the correlation coefficient before and after delivery was 0.51, and the postpartum CAS score in the group that did not require treatment for clinical constipation with less than 5 points before delivery was 2.8 ± 2.6.
Types of laxatives, especially for perinatal prognosis with magnesium oxide and PEG, showed no difference in superiority. The presence or absence of side effects such as abdominal pain, diarrhea, mood discomfort, abdominal swelling, abdominal discomfort, loss of appetite, and rash was investigated after oral administration. Side effects were observed in 23.4% (11/47) of participants prescribed magnesium oxide and 12.5% (1/8) PEG prescribed participants, respectively. Magnesium oxide prescribed participants experienced abdominal pain and diarrhea most frequently, with few cases of mood disorder and abdominal swelling. However, only one case of diarrhea was seen in the PEG-prescribed participants.
Discussion
More than half of pregnant women try to consume water, yogurt, and dietary fiber such as vegetables to prevent constipation. However, most pregnant women did not exercise (approximately 91%) because they were concerned about its adverse effects on pregnancy.
According to Takai et al.4), fiber-rich diets and intake of lactic acid bacteria containing yogurt are often promoted in the outpatient department; however, they prove ineffective. In addition, the study reported no correlation between dietary fiber and lactic acid bacteria intake and the onset of constipation.
The Japanese Ministry of Health, Labor and Welfare emphasizes the importance of ensuring regular diets (three meals a day) and the number of diets in addition to fiber-rich diets to prevent lifestyle-related diseases5). The ratio of having food three times a day was higher (85% or more) in pregnant women than in non-pregnant women. The food intake in pregnant women was ideal relative to non-pregnant women of the same age6). However, as reported by Takai et al., constipation develops even with regular dietary fiber intake. Therefore, the mechanism of constipation in pregnant women seems to be strongly associated with physiological and functional changes due to pregnancy rather than dietary content and amount.
In our study, approximately 60% of participants developed constipation before pregnancy. Notably, approximately 90% of patients who developed constipation during pregnancy developed it by the middle of pregnancy. According to a survey on the prevalence of constipation during pregnancy7), 35% and 39% of participants developed constipation in the first and second trimesters of pregnancy, respectively. Furthermore, the survey result showed that the number of pregnant women with constipation was higher in the second trimester than in pre-pregnancy and postpartum. In addition, the proportion of women who became aware of constipation during pregnancy in our study was similar to that in a previous study8), which reported that the proportion of women who became aware of constipation during pregnancy was significantly higher during the second trimester of pregnancy than before and one month after childbirth. However, in this survey, the high percentage of pre-pregnancy constipation is thought to be due to the recall bias of the survey itself and some cases that regarded the first trimester of pregnancy as pre-pregnancy.
In 1989, McMillan and Williams9) developed the CAS to quantify the side effects of morphine in patients with cancer. CAS is a self-assessment scale for constipation, with 6 subjective symptoms of constipation ranging from 0 (no symptoms) to 2 (always with symptoms). The higher the score, the worse the symptoms of constipation, up to 16 points. A score of 5 or higher indicates constipation requiring medical intervention (Supplementary Text).
The subjective symptoms commonly seen in our participants were: hunger, reduced number of defecations, and stool excretion status. A total of 34.1% of patients experienced constipation with a CAS score of ≥ 5. Furthermore, there was no difference in CAS for those who developed constipation in the early and middle stages of pregnancy. Therefore, rather than physical factors such as intestinal compression associated with uterine enlargement in the latter half of pregnancy, intestinal motility was suppressed due to the effects of progesterone during pregnancy and sympathetic tone due to stress, and the factor of flaccid constipation was considered to be stronger. Conversely, 16 (7.6%) patients who scored 5 points or more were unaware of constipation and required medical intervention for constipation; most of them had abdominal bloating, decreased stool volume, and defecation. In contrast, those who answered that they had subjective symptoms of constipation often complained of difficulty in defecation but often did not have any other subjective symptoms. This result may support the significance of the abovementioned laxative constipation as the mechanism of constipation during pregnancy.
Recently, several new therapeutic agents for constipation have been launched, and the options for therapeutic agents are expanding. However, many drugs have been excluded from clinical trials prior to application for approval due to ethical considerations for pregnant women; therefore, their efficacy and safety have not been evaluated.
PEG has negligible systemic exposure; therefore, no teratogenic effects have been noted in animal studies10). The most common side effects reported are gastrointestinal disorders such as diarrhea and abdominal pain in 13.5% of clinical trial participants; however, since systemic side effects are rarely observed, few side effects are noted even in pregnant women, and the drug seems to be highly safe. Oral administration of the active ingredient of PEG, Macrogol 4000, increases the water content in the intestinal tract due to osmotic pressure10). Therefore, it can be effective in alleviating rectal constipation in pregnant women. In the investigation of the administered drug, perinatal prognosis, and side effects, there was no difference between the constipation and non-constipation groups; however, a small number of participants reported mood dysregulation and abdominal distension in the magnesium oxide prescribed group.
This is the first study to evaluate constipation in pregnant women using CAS and Bristol scales. However, since these parameters were assessed subjectively by the participants, we did not observe a significant difference between the groups. In future studies, objective evaluation may provide further information. In addition, recall bias is possible because the information from pre-pregnancy to early pregnancy was entered into the questionnaire in the mid-pregnancy, and the information from the mid-pregnancy to the postpartum was entered in the postpartum.
Many pregnant women who suffer from constipation during pregnancy suffer from constipation before pregnancy; however, pregnant women who did not suffer from constipation before pregnancy did not suffer from constipation during the perinatal period despite hormonal problems. Therefore, preventive measures are required to improve pre-pregnancy defecation management in the future.
Conclusions
Treatment before pregnancy is related to the prognosis of constipation during pregnancy and postpartum. In recent years greater importance has been placed on taking care of the body before conception to have a healthy child; therefore, controlling constipation before pregnancy is desirable. Both conventional and new constipation treatments showed similar effects. However, PEG preparations may be an appropriate therapeutic agent to alleviate constipation during pregnancy without inducing diarrhea, a common side-effect observed with conventional constipation treatment. Further research focused on objectively evaluating the differences between conventional and new constipation treatment could prove beneficial to find ing effective treatment with the least side effects.
Disclosed potential conflicts of interest
The authors declare that there is no conflict of interest regarding the publication of this paper. M.I. is currently employed by Mitsuru Munakata, manager of Iwase General Hospital. Neither the employer nor the company was involved in the manuscript writing, editing, approval, or decision to publish.
Supplementary materials
Self-administered questionnaire used during the second trimester and after delivery.
References
- 1.Kirihara N, Kamitomo M, Tabira T, Hashimoto T, Taniguchi H, Maeda T. Effect of probiotics on perinatal outcome in patients at high risk of preterm birth. J Obstet Gynaecol Res, 44: 241-247, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe C. Survey on constipation in pregnant women. Bull Yachiyo Hosp, 26: 49-54, 2006. [Google Scholar]
- 3.Fukai K, Sugita A, Tanaka M. A developmental study of the Japanese version of the constipation assessment scare. The Jpn J Nurs Res, 28: 201-208, 1995. [Google Scholar]
- 4.Takai I, Yoneda M. Actual conditions regarding constipation and coping strategies for pregnant women. Ishikawa J Nurs, 103-110, 2014. [Google Scholar]
- 5.Ministry of Health, Labor and Welfare [Internet]. Health Information Site for Prevention of Lifestyle-Related Diseases, Constipation and Diet | E-Health Net. [Cited 15/5/2021]. Available from: mhlw.go.jp.
- 6.Statistics Bureau. Ministry of internal affairs and communications [Internet]. National Health and Nutrition Survey, 10 Daily meal status by morning, noon, and evening | Statistical table/graph display | General counter for official statistics. [Cited 15/5/2021]. Available from: e-stat.go.jp.
- 7.Kobayashi H. Relationship between constipation symptoms and water intake during pregnancy-examination using the Japanese version of the constipation evaluation scale. J Matern Nurs, 86-88, 2005. [Google Scholar]
- 8.Derbyshire E, Davies J, Costarelli V, Dettmar P. Diet, physical inactivity and the prevalence of constipation throughout and after pregnancy. Matern Child, Nutr, 2: 127-134, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McShane RE, McLane AM. Constipation. Consensual and empirical validation. Nurs Clin North Am, 20: 801-808, 1985. [PubMed] [Google Scholar]
- 10.Pharmaceutical interview form Movicol compounded internal medicine Japanese standard product classification number 872359. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Self-administered questionnaire used during the second trimester and after delivery.


