Abstract
Extracellular matrix (ECM) is a non-cellular constituent found in all tissues and organs. Although ECM was previously recognized as a mere “molecular glue” that supports the tissue structure of organs such as the lungs, it has recently been reported that ECM has important biological activities for tissue morphogenesis, inflammation, wound healing, and tumor progression. Proteoglycans are the main constituent of ECM, with growing evidence that proteoglycans and their associated glycosaminoglycans play important roles in the pathogenesis of several diseases. However, their roles in the lungs are incompletely understood.
Leukocyte migration into the lung is one of the main aspects involved in the pathogenesis of several lung diseases. Glycosaminoglycans bind to chemokines and their interaction fine-tunes leukocyte migration into the affected organs. This review focuses on the role chemokine and glycosaminoglycan interactions in neutrophil migration into the lung. Furthermore, this review presents the role of proteoglycans such as syndecan, versican, and hyaluronan in inflammatory and fibrotic lung diseases.
Keywords: extracellular matrix, lung inflammation, lung fibrosis, syndecan
Introduction
Extracellular matrix (ECM) is a non-cellular constituent found in all tissues and organs. Recently, it has emerged that the ECM not only serves as a physical scaffold for cells, but also has important biological activities for tissue morphogenesis, differentiation, and homeostasis1-5) . ECM includes two main types of macromolecules: fibrous proteins (collagen, elastin, etc.) and proteoglycans.
Proteoglycans are glycoproteins consisting of a core protein with glycosaminoglycan (GAG) side chains. Several types of proteoglycans exist in the lung as components of ECM6) , previously recognized as a mere “molecular glue” providing structural support to tissues. However, growing evidence demonstrates that proteoglycans have a variety of biological activities for fine control of inflammation, wound healing, development, and homeostasis7-12) .
There are four classes of GAGs: heparan sulfate, chondroitin sulfate/dermatan sulfate, keratan sulfate, and hyaluronan. All these classes are found in normal lungs, with heparan sulfate being the predominant GAG (40-60%), followed by chondroitin sulfate/dermatan sulfate (31%), hyaluronan (14%), and heparin (5%)13) . GAGs consist of repeating disaccharide units of a hexosamine (glucosamine of galactosamine) and either a uronic acid (glucuronic acid or iduronic acid) or a galactose14-17) (Figure 1). GAG side chains, which contribute to up to 90% of the molecular weight of proteoglycans, are highly sulfated and bind to a variety of proteins such as chemokines and growth factors6,18-20) .
Fig. 1.
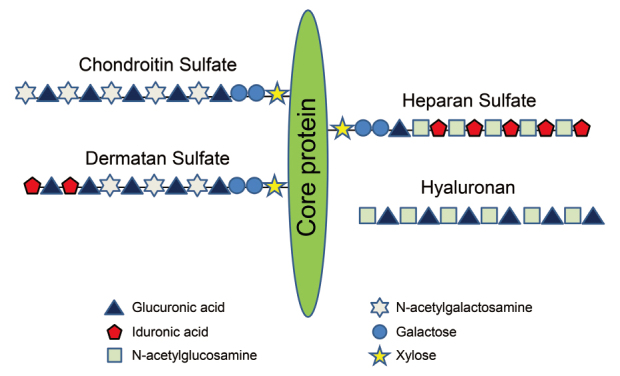
Schematic representations of glycosaminoglycan and proteoglycan. The composition of disaccharide unit repeats is schematically illustrated for heparan sulfate, dermatan sulfate (DS), keratan sulfate (KS), chondroitin sulfate (CS) and hyaluronan (HA). Hyaluronan is the only glycosaminoglycan in a free and unsulfated form. All the other glycosaminoglycans are attached to a protein, forming proteoglycans.
Proteoglycans are named according to the core protein to which constituent GAGs are bound, e.g., heparan sulfate proteoglycan (HSPG), chondroitin sulfate proteoglycan, and dermatan sulfate proteoglycan21,22) . Hyaluronan, a non-sulfated GAG, does not bind to a proteoglycan core protein. Proteoglycans can be classified based on their location as cell surface, pericellular, extracellular, and intracellular proteoglycans23) .
Clinical significance and research implications
Roles of GAGs on neutrophil migration into the lungs
GAGs bind to various cytokines and chemokines, and sulfation of GAGs provides sites to which chemokines bind24-26) . Chemokines are a family of chemotactic cytokines which promote leukocyte migration into the tissues. Several studies have reported that chemokine-GAG interaction is critical for recruitment of leukocytes into the peritoneum and lungs20,27-29) .
Neutrophil migration into the lungs is involved in the pathogenesis of several lung diseases, particularly in lung infection and acute respiratory distress syndrome30,31) . IL-8/CXCL8 is a potent neutrophil chemokine which is produced by alveolar macrophages in the lungs during acute bacterial pneumonia and acute respiratory distress syndrome30-32) . All chemokines have a GAG-binding domain. The binding of chemokines to GAGs plays critical roles in leukocyte recruitment into tissues by facilitating both the formation of tissue-bound chemokine gradients and the presentation of chemokines to leukocytes in tissues25,33,34) .
The GAG-binding domain of CXCL8 includes basic residues located in the proximal loop (K20) and C-terminal α-helix (R60, K64, K67, and R68)35) (Figure 2). In the lungs, CXCL8 binds to heparin sulfate and chondroitin sulfate GAGs, and these interactions promote the dimerization of CXCL8, resulting in an increase in the amount of CXCL8 bound in lung tissues20,24) . Although these results suggest that the interaction of CXCL8 and GAGs plays a critical role in neutrophil migration in the lung and results of in vitro experiments support its possible role36-38) , the precise role of this interaction had not been clarified in vivo. Our research group uncovered the role of CXCL8 in neutrophil migration in the lung by conducting an in vivo experiment using two mutant forms of CXCL8 (R68A-CXCL8 and K64A/K67A/R68A-CXCL8), which do not bind to GAGs39) . When intratracheally instilled into the lungs of mice, the CXCL8 mutants recruited more neutrophils into the lungs and appeared more rapidly in systemic circulation than recombinant CXCL8 (Figure 3a). In addition, the CXCL8 mutants appeared in plasma at significantly higher concentrations (Figure 3b) and diffused more rapidly across the ECM in vitro. Furthermore, when we instilled another mutant CXCL8 (I64K-CXCL8), which has more binding activity to GAGs, into the lungs of mice, it recruited fewer neutrophils than recombinant CXCL8 (unpublished data). These results show that GAGs control the spatiotemporal formation of chemokine gradients and neutrophil migration in the lungs.
Fig. 2.
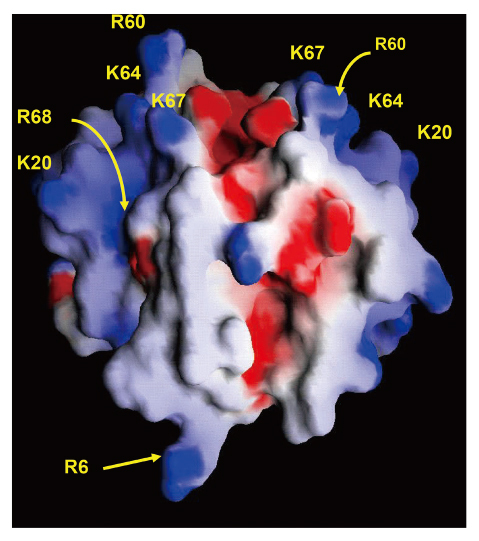
Glycosaminoglycan-binding domain on CXCL8 dimer. CXCL8 has three binding domains: a high-affinity binding domain, which mediates binding to specific receptors on polymorphonuclear neutrophils; the glycosaminoglycan-binding domain (K20, R60, K64, K67, R68); and the dimer interface (R6), where CXCL8 molecules bind to each other to form dimers. Blue and red show positively and negatively charged regions, respectively.
Fig. 3.
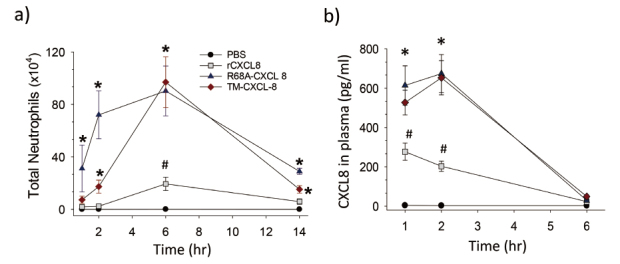
Neutrophil migration in response to rCXCL8 and CXCL8 mutants. a) The CXCL8 mutants (R68A-CXCL8 and K64A/K67A/R68A-CXCL8: TM-CXCL8) recruited more neutrophils into the lungs than recombinant CXCL8 (rCXCL8). b) The CXCL8 mutants appeared more rapidly in plasma after intratracheal instillation than rCXCL8.
*: p < 0.05 vs rCXCL8. #: p < 0.05 vs control.
Syndecan
Syndecan is one of the transmembrane HSPGs and consists of four isoforms. Syndecan-1, -2, and -3 are specifically expressed on the surface of epithelial cells or plasma cells, fibroblasts or endothelium, and nerve cells, respectively. On the other hand, syndecan-4 is expressed on a variety of cells12,40-43) . Heparan sulfate is the most abundant GAG in healthy lungs13) , and several types of proteoglycans exist in the lung as components of ECMs6) . Heparan sulfate GAG side chains of syndecans bind to various proteins such as cytokines, chemokines, and growth factors, and mediate biological activities of these proteins19,20,44,45) . However, the role of HSPGs in the lung had not been clarified in detail.
To clarify the role of HSPGs in acute lung inflammation, we evaluated mRNA expression of HSPGs using an LPS-induced murine lung inflammation model. After LPS instillation, syndecan-4 mRNA was rapidly and selectively upregulated among the HSPGs studied46) (Figure 4a). Therefore, we focused on syndecan-4 for our further studies. In the LPS-induced lung inflammation model, more neutrophils were found in bronchoalveolar lavage fluid (BALF) in syndecan-4 deficient mice compared to wild-type mice46) (Figure 4b, c). Moreover, in a model of lung inflammation induced by S. pneumonia, the survival rate of the syndecan-4 deficient mice was significantly lower than wild-type mice (Figure 5), while their total neutrophil counts in BALF, bacterial counts in blood, and plasma levels of inflammatory cytokines were significantly higher47) . In addition, pretreatment of recombinant syndecan-4 significantly inhibited LPS-induced CXCL8 upregulation in BEAS-2B bronchial epithelial cells46) . These results indicate that syndecan-4 can inhibit acute inflammation in the lungs. Furthermore, we evaluated the role of syndecan-4 in lung fibrosis48) . In a bleomycin-induced lung fibrosis model, the histopathological lung fibrosis score and collagen content in lung tissues were significantly higher in syndecan-4 deficient mice compared to wild-type mice at 21 days after intratracheal bleomycin instillation. In in vitro experiments using lung fibroblasts, TGF-β-induced Smad3 activation as well as collagen and α-smooth muscle actin upregulation were significantly inhibited by co-incubation of recombinant syndecan-4. These results show that syndecan-4 is involved in the pathogenesis of lung fibrosis.
Fig. 4.
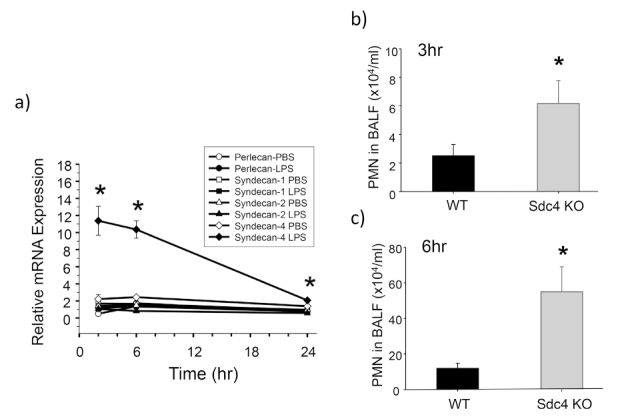
Role of syndecan-4 in lipopolysaccharide-induced lung inflammation. a) Changes in mRNA for the heparan sulfate proteoglycans after intratracheal instillation of lipopolysaccharide (LPS) into wild-type mice. Among heparan sulfate proteoglycans, syndecan-4 mRNA was rapidly and selectively up-regulated. *: p < 0.05 vs syndecan-4-PBS. b, c) Intratracheal instillation of LPS induced more neutrophil recruitment into the lungs in syndecan-4 deficient mice (Sdc4 KO) than wild-type mice (WT). *: p < 0.05 vs WT.
Fig. 5.
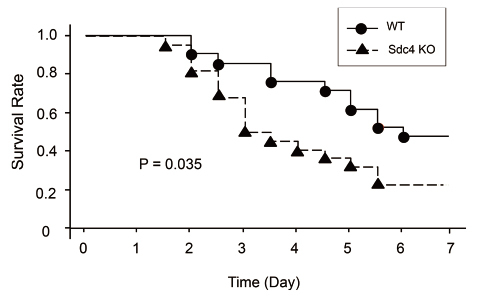
Survival of wild-type and syndecan-4 deficient mice after intranasal instillation of S. pneumoniae. The survival rate of syndecan-4 deficient (Sdc4 KO) mice was significantly worse after intranasal instillation of S. pneumoniae (5.0×106 CFU) than wild-type mice (WT).
To further explore the role of syndecan-4 in lung diseases, we analyzed the serum levels of syndecan-4 in patients with acute pneumonia and idiopathic interstitial pneumonia (IIP)47,49) . Although syndecans exist on cell surfaces, cell surface syndecans can be cleaved by several inflammatory factors such as matrix metalloproteinase (MMP)-7 and -9, or a disintegrin and metalloproteinase 1744,50-54) . In patients with acute pneumonia, serum syndecan-4 levels were significantly higher than those in healthy volunteers and were correlated negatively with the pneumonia severity score. Moreover, among patients who improved with short-term antibiotic therapy, serum syndecan-4 levels on admission were higher than in those who did not improve and gradually increased during the antibiotic therapy47) . IIP, including idiopathic pulmonary fibrosis (IPF), is a chronic, progressive, and intractable fibrosing lung disease55-57) . During its clinical course, acute respiratory failure (also referred to as acute exacerbation) may occur, and this is reported to be the most common cause of death (40%) in Japanese IPF patients58) . We found that serum syndecan-4 levels were significantly lower in patients with acute exacerbation of IIP than in those in the clinically stable phase, and the prognosis after acute exacerbation onset was significantly worse in the patients with higher baseline serum syndecan-4 levels than in those with lower baseline levels49) . Furthermore, the clinical significance of serum syndecan-4 levels in preterm infants with chronic lung disease was recently demonstrated59) . These results show that serum syndecan-4 is a clinically significant biomarker in lung diseases.
Versican and hyaluronan
Versican, a large chondroitin sulfate proteoglycan belonging to the aggrecan family, has a molecular weight >1,000 kDa in its four isoforms, V0-V3, produced by alternative splicing60) . Versican alters the pericellular environment by binding to various mediators via the GAG domain. In addition, versican modifies the bioactivities of ECM proteins, such as hyaluronan, and plays important roles in cell morphology, adhesion, proliferation and migration61,62) . Hyaluronan, a non-sulfated GAG, is a major constituent of the ECM, and growing evidence shows its important roles in inflammation, injury, and repair in the lung63,64) .
In an LPS-induced murine lung inflammation model, we demonstrated that a rapid increase in mRNA expression of versican and hyaluronan synthase was associated with increased immunohistochemical staining for versican and hyaluronan. In addition, in vitro studies showed that LPS caused a rapid increase in versican mRNA, proteins, and hyaluronan synthase in M1 macrophages, but not in M2 macrophages65) . These results show important roles of versican and hyaluronan in the innate immune response to gram-negative lung infection.
In patients with IIP, significantly higher levels of serum hyaluronan were found compared to healthy volunteers, and positive correlations of hyaluronan levels in BALF with the percentage of inflammatory cells and the amount of CXCL8 were shown. In addition, patients with acute exacerbation had significantly higher serum hyaluronan levels compared with those in the stable phase, and patients with the highest serum hyaluronan had the worst 60-day outcomes66) . These results show that hyaluronan is involved in the pathogenesis of IIP, and serum hyaluronan is a possible biomarker in patients with IIP.
Proteases such as MMPs are involved in the pathogenesis of lung fibrosis, and MMP-1 and MMP-7 levels in blood, in particular, are reported to be prognostic biomarkers for IPF67,68) . In addition, it is reported that the serum levels of ECM products degraded by MMPs increased and were related to disease activity in IPF69) . In the PROFILE study, increased serum levels of proteoglycans degraded by MMPs were reported to be associated with disease activity in patients with IPF70) . We analyzed the serum levels of ECM degradation products in IIP patients, and found that type IV and VI collagen degradation products were significantly higher, while elastin and versican degradation products were lower during acute exacerbation than during the stable phase of IIP. Furthermore, lower levels of versican degradation products during acute exacerbation were associated with an increased risk of mortality71) .
Decorin
Decorin is a small, leucine-rich proteoglycan with one chondroitin/dermatan sulfate GAG side chain72) . Decorin binds to collagen and plays important roles in collagen fibril formation and fibrous spacing73-75) . It is reported that decorin-deficient mice have a phenotype of abnormal collagen fibril morphology and skin fragility73,76) . In addition to its role in collagen fibrogenesis, decorin also plays important roles in angiogenesis, innate immunity, inflammation, fibrosis, wound healing, tumor growth and autophagy73,74,77,78) .
In IPF, decorin is reportedly expressed in fibrotic collagen-deposited lesions and fibroblastic foci79) . We analyzed the serum decorin levels in IIP patients in the stable phase and at the time of acute exacerbation, and found that serum decorin levels at the time of acute exacerbation were significantly lower compared with those in the stable phase or in healthy volunteers. In addition, serum decorin levels in clinically stable IIP patients were significantly lower than those in healthy subjects. Moreover, those with serum decorin levels lower than the median, especially the patients with acute exacerbation of IPF, had significantly higher survival rates compared to those with higher-than-median serum decorin levels80).
Conclusion
ECM is involved in the pathogenesis of several lung diseases, and ECM in biological samples, such as serum and BAL fluid, is a potential biomarker in patients with lung diseases.
Acknowledgements
None
Conflict of interest disclosure
The author has no conflicts of interest to declare.
References
- 1.Kyriakopoulou K, Piperigkou Z, Tzaferi K, Karamanos NK. Trends in extracellular matrix biology. Mol Biol Rep, 50: 853-863, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sainio A, Järveläinen H. Extracellular matrix-cell interactions: Focus on therapeutic applications. Cell Signal, 66: 109487, 2020. [DOI] [PubMed] [Google Scholar]
- 3.Theocharis AD, Manou D, Karamanos NK. The extracellular matrix as a multitasking player in disease. FEBS J, 286: 2830-2869, 2019. [DOI] [PubMed] [Google Scholar]
- 4.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Bopl, 15: 786-801, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci, 123: 4195-4200, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gill S, Wight TN, Frevert CW. Proteoglycans: key regulators of pulmonary inflammation and the innate immune response to lung infection. Anat Rec (Hoboken), 293(6): 968-981, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexopoulou AN, Multhaupt HA, Couchman JR. Syndecans in wound healing, inflammation and vascular biology. Int J Biochem Cell Biol, 39: 505-528, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Bartlett AH, Hayashida K, Park PW. Molecular and cellular mechanisms of syndecans in tissue injury and inflammation. Mol and Cells, 24: 153-166, 2007. [PubMed] [Google Scholar]
- 9.Bishop JR, Schuksz M, Esko JD. Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature, 446: 1030-1037, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Celie JW, Beelen RH, van den Born J. Heparan sulfate proteoglycans in extravasation: assisting leukocyte guidance. Front Biosci (Landmark Ed), 14: 4932-4949, 2009. [DOI] [PubMed] [Google Scholar]
- 11.Esko JD, Lindahl U. Molecular diversity of heparan sulfate. Journal Clin Invest, 108: 169-173, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parish CR. The role of heparan sulphate in inflammation. Nat Rev Immunol, 6: 633-643, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Sampson PM, Boyd RB, Pietra GG, Fishman AP. Glycosaminoglycan biosynthesis in the isolated perfused rat lung. J Appl Physiol Respir Environ Exerc Physiol, 57: 1648-1654, 1984. [DOI] [PubMed] [Google Scholar]
- 14.Scott JE. Supramolecular organization of extracellular matrix glycosaminoglycans, in vitro and in the tissues. FASEB J, 6: 2639-2645, 1992. [PubMed] [Google Scholar]
- 15.Alexander CM, Reichsman F, Hinkes MT, Lincecum J, Becker KA, Cumberledge S, et al. Syndecan-1 is required for Wnt-1-induced mammary tumorigenesis in mice. Nat Genet, 25: 329-332, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Colin-Pierre C, El Baraka O, Danoux L, Bardey V, André V, Ramont L, et al. Regulation of stem cell fate by HSPGs: implication in hair follicle cycling. NPJ Regen Med, 7: 77, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merida-de-Barros DA, Chaves SP, Belmiro CLR, Wanderley JLM. Leishmaniasis and glycosaminoglycans: a future therapeutic strategy? Parasit Vectors, 11: 536, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuschert GS, Coulin F, Power CA, Proudfoot AE, Hubbard RE, Hoogewerf AJ, et al. Glycosaminoglycans interact selectively with chemokines and modulate receptor binding and cellular responses. Biochemistry, 38: 12959-12968, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Muramatsu T, Muramatsu H, Kojima T. Identification of proteoglycan-binding proteins. Methods Enzymol, 416: 263-278, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Frevert CW, Kinsella MG, Vathanaprida C, Goodman RB, Baskin DG, Proudfoot A, et al. Binding of interleukin-8 to heparan sulfate and chondroitin sulfate in lung tissue. Am J Respir Cell Mol Biol, 28: 464-472, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Bosman FT, Stamenkovic I. Functional structure and composition of the extracellular matrix. J Pathol, 200: 423-428, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Kinsella MG, Bressler SL, Wight TN. The regulated synthesis of versican, decorin, and biglycan: extracellular matrix proteoglycans that influence cellular phenotype. Crit Rev Eukaryot Gene Expr, 14: 203-234, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Iozzo RV, Schaefer L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol, 42: 11-55, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esko JD, Selleck SB. Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu Rev Biochem, 71: 435-471, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Handel TM, Johnson Z, Crown SE, Lau EK, Proudfoot AE. Regulation of protein function by glycosaminoglycans — as exemplified by chemokines. Annu Rev Biochem, 74: 385-410, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Taylor KR, Gallo RL. Glycosaminoglycans and their proteoglycans: host-associated molecular patterns for initiation and modulation of inflammation. FASEB J, 20: 9-22, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Ishiguro K, Kadomatsu K, Kojima T, Muramatsu H, Iwase M, Yoshikai Y, et al. Syndecan-4 deficiency leads to high mortality of lipopolysaccharide-injected mice. J Biol Chem, 276: 47483-47488, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Minamiya Y, Saito S, Kalina U, Saito H, Terada K, Ogawa J. Antithrombin III diminishes production of oxygen radical in endotoxin-infused rat lung. Shock, 21: 139-143, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Rehberg S, Yamamoto Y, Sousse LE, Jonkam C, Zhu Y, Traber LD, et al. Antithrombin attenuates vascular leakage via inhibiting neutrophil activation in acute lung injury. Crit Care Med, 41: e439-446, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson BT, Chambers RC, Liu KD. Acute Respiratory Distress Syndrome. New Engl J Med, 377: 562-572, 2017. [DOI] [PubMed] [Google Scholar]
- 31.Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, et al. Acute respiratory distress syndrome. Nat Rev Dis Primers, 5: 18, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Standiford TJ, Kunkel SL, Greenberger MJ, Laichalk LL, Strieter RM. Expression and regulation of chemokines in bacterial pneumonia. J Leukoc Biol, 59: 24-28, 1996. [DOI] [PubMed] [Google Scholar]
- 33.Lortat-Jacob H, Grosdidier A, Imberty A. Structural diversity of heparan sulfate binding domains in chemokines. Proc Natl Acad Sci USA, 99: 1229-1234, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bao X, Moseman EA, Saito H, Petryniak B, Thiriot A, Hatakeyama S, et al. Endothelial heparan sulfate controls chemokine presentation in recruitment of lymphocytes and dendritic cells to lymph nodes. Immunity, 33: 817-829, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuschert GS, Hoogewerf AJ, Proudfoot AE, Chung CW, Cooke RM, Hubbard RE, et al. Identification of a glycosaminoglycan binding surface on human interleukin-8. Biochemistry, 37: 11193-11201, 1998. [DOI] [PubMed] [Google Scholar]
- 36.Rot A. Binding of neutrophil attractant/activation protein-1 (interleukin 8) to resident dermal cells. Cytokine, 347: 347-352, 1992. [DOI] [PubMed] [Google Scholar]
- 37.Rot A. Neutrophil attractant/activation protein-1 (interleukin-8) induces in vitro neutrophil migration by haptotactic mechanism. Eur J Immunol, 23: 303-306, 1993. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka Y, Adams DH, Hubscher S, Hirano H, Siebenlist U, Shaw S. Nature, 361: 79-82, 1993. [DOI] [PubMed] [Google Scholar]
- 39.Tanino Y, Coombe DR, Gill SE, Kett WC, Kajikawa O, Proudfoot AE, et al. Kinetics of chemokine-glycosaminoglycan interactions control neutrophil migration into the airspaces of the lungs. J Immunol, 184: 2677-2685, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bernfield M, Kokenyesi R, Kato M, Hinkes MT, Spring J, Gallo RL, et al. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu Rev Cell Biol, 8: 365-393, 1992. [DOI] [PubMed] [Google Scholar]
- 41.Götte M. Syndecans in inflammation. FASEB J, 17: 575-591, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Okuyama E, Suzuki A, Murata M, Ando Y, Kato I, Takagi Y, et al. Molecular mechanisms of syndecan-4 upregulation by TNF-α in the endothelium-like EAhy926 cells. J Biochem, 154: 41-50, 2013. [DOI] [PubMed] [Google Scholar]
- 43.Saphire AC, Bobardt MD, Zhang Z, David G, Gallay PA. Syndecans serve as attachment receptors for human immunodeficiency virus type 1 on macrophages. J Virol, 75: 9187-9200, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernfield M, Götte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, et al. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem, 68: 729-777, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Fears CY, Woods A. The role of syndecans in disease and wound healing. Matrix Biol, 25: 443-456, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Tanino Y, Chang MY, Wang X, Gill SE, Skerrett S, McGuire JK, et al. Syndecan-4 regulates early neutrophil migration and pulmonary inflammation in response to lipopolysaccharide. Am J Respir Cell Mol Biol, 47: 196-202, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nikaido T, Tanino Y, Wang X, Sato S, Misa K, Fukuhara N, et al. Serum Syndecan-4 as a Possible Biomarker in Patients With Acute Pneumonia. J Infect Dis, 212: 1500-1508, 2015. [DOI] [PubMed] [Google Scholar]
- 48.Tanino Y, Wang X, Nikaido T, Misa K, Sato Y, Togawa R, et al. Syndecan-4 Inhibits the Development of Pulmonary Fibrosis by Attenuating TGF-beta Signaling. Int J Mol Sci, 20: 4989, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sato Y, Tanino Y, Wang X, Nikaido T, Sato S, Misa K, et al. Baseline serum syndecan-4 predicts prognosis after the onset of acute exacerbation of idiopathic interstitial pneumonia. PLoS One, 12: e0176789, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brule S, Charnaux N, Sutton A, Ledoux D, Chaigneau T, Saffar L, et al. The shedding of syndecan-4 and syndecan-1 from HeLa cells and human primary macrophages is accelerated by SDF-1/CXCL12 and mediated by the matrix metalloproteinase-9. Glycobiology, 16: 488-501, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell, 111: 635-646, 2002. [DOI] [PubMed] [Google Scholar]
- 52.Pruessmeyer J, Martin C, Hess FM, Schwarz N, Schmidt S, Kogel T, et al. A disintegrin and metalloproteinase 17 (ADAM17) mediates inflammation-induced shedding of syndecan-1 and -4 by lung epithelial cells. J Biol Chem, 285: 555-564, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramnath R, Foster RR, Qiu Y, Cope G, Butler MJ, Salmon AH, et al. Matrix metalloproteinase 9-mediated shedding of syndecan 4 in response to tumor necrosis factor α: a contributor to endothelial cell glycocalyx dysfunction. FASEB J, 28: 4686-4699, 2014. [DOI] [PubMed] [Google Scholar]
- 54.Hayashida K, Parks WC, Park PW. Syndecan-1 shedding facilitates the resolution of neutrophilic inflammation by removing sequestered CXC chemokines. Blood, 114: 3033-3043, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lederer DJ, Martinez FJ. Idiopathic Pulmonary Fibrosis. New Engl J Med, 378: 1811-1823, 2018. [DOI] [PubMed] [Google Scholar]
- 56.Richeldi L, Collard HR, Jones MG. Idiopathic pulmonary fibrosis. Lancet, 389: 1941-1952, 2017. [DOI] [PubMed] [Google Scholar]
- 57.Wijsenbeek M, Cottin V. Spectrum of Fibrotic Lung Diseases. New Engl J Med, 383: 958-968, 2020. [DOI] [PubMed] [Google Scholar]
- 58.Natsuizaka M, Chiba H, Kuronuma K, Otsuka M, Kudo K, Mori M, et al. Epidemiologic survey of Japanese patients with idiopathic pulmonary fibrosis and investigation of ethnic differences. Am J Respir Crit Care Med, 190: 773-779, 2014. [DOI] [PubMed] [Google Scholar]
- 59.Matsuzaki Y, Wang X, Tanino Y, Ikeda K. Insufficient Syndecan-4 is associated with CLD Development in Preterm Infants. Pediatr Int, 65(1): e15413, 2022. [DOI] [PubMed] [Google Scholar]
- 60.Tang F, Brune JE, Chang MY, Reeves SR, Altemeier WA, Frevert CW. Defining the versican interactome in lung health and disease. Am J Physiol Cell physiol, 323: C249-c76, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andersson-Sjöland A, Hallgren O, Rolandsson S, Weitoft M, Tykesson E, Larsson-Callerfelt AK, et al. Versican in inflammation and tissue remodeling: the impact on lung disorders. Glycobiology, 25: 243-251, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sotoodehnejadnematalahi F, Burke B. Structure, function and regulation of versican: the most abundant type of proteoglycan in the extracellular matrix. Acta Med Iran, 51: 740-750, 2013. [PubMed] [Google Scholar]
- 63.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med, 11: 1173-1179, 2005. [DOI] [PubMed] [Google Scholar]
- 64.Hoarau A, Polette M, Coraux C. Lung Hyaluronasome: Involvement of Low Molecular Weight Ha (Lmw-Ha) in Innate Immunity. Biomolecules, 12: 658, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chang MY, Tanino Y, Vidova V, Kinsella MG, Chan CK, Johnson PY, et al. Reprint of: A rapid increase in macrophage-derived versican and hyaluronan in infectious lung disease. Matrix Biol, 35: 162-173, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Inokoshi Y, Tanino Y, Wang X, Sato S, Fukuhara N, Nikaido T, et al. Clinical significance of serum hyaluronan in chronic fibrotic interstitial pneumonia. Respirology, 18: 1236-1243, 2013. [DOI] [PubMed] [Google Scholar]
- 67.Khan FA, Stewart I, Saini G, Robinson KA, Jenkins RG. A systematic review of blood biomarkers with individual participant data meta-analysis of matrix metalloproteinase-7 in idiopathic pulmonary fibrosis. Eur Respir J, 59: 2101612, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guiot J, Moermans C, Henket M, Corhay JL, Louis R. Blood Biomarkers in Idiopathic Pulmonary Fibrosis. Lung, 195: 273-280, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leeming DJ, Sand JM, Nielsen MJ, Genovese F, Martinez FJ, Hogaboam CM, et al. Serological investigation of the collagen degradation profile of patients with chronic obstructive pulmonary disease or idiopathic pulmonary fibrosis. Biomark Insights, 7: 119-126, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jenkins RG, Simpson JK, Saini G, Bentley JH, Russell AM, Braybrooke R, et al. Longitudinal change in collagen degradation biomarkers in idiopathic pulmonary fibrosis: an analysis from the prospective, multicentre PROFILE study. Lancet Respir Med, 3: 462-472, 2015. [DOI] [PubMed] [Google Scholar]
- 71.Sand JMB, Tanino Y, Karsdal MA, Nikaido T, Misa K, Sato Y, et al. A Serological Biomarker of Versican Degradation is Associated with Mortality Following Acute Exacerbations of Idiopathic Interstitial Pneumonia. Respir Res, 19: 82, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weber IT, Harrison RW, Iozzo RV. Model structure of decorin and implications for collagen fibrillogenesis. J Biol Chem, 271: 31767-31770, 1996. [DOI] [PubMed] [Google Scholar]
- 73.Gubbiotti MA, Vallet SD, Ricard-Blum S, Iozzo RV. Decorin interacting network: A comprehensive analysis of decorin-binding partners and their versatile functions. Matrix Biol, 55: 7-21, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Neill T, Schaefer L, Iozzo RV. Decorin: a guardian from the matrix. Am J Pathol, 181: 380-387, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature, 346: 281-284, 1990. [DOI] [PubMed] [Google Scholar]
- 76.Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol, 136: 729-743, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Järveläinen H, Sainio A, Wight TN. Pivotal role for decorin in angiogenesis. Matrix Biol, 43: 15-26, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dong Y, Zhong J, Dong L. The Role of Decorin in Autoimmune and Inflammatory Diseases. J Immunol Res, 2022: 1283383, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bensadoun ES, Burke AK, Hogg JC, Roberts CR. Proteoglycan deposition in pulmonary fibrosis. Am J Respir Crit Care Med, 154: 1819-1828, 1996. [DOI] [PubMed] [Google Scholar]
- 80.Nikaido T, Tanino Y, Wang X, Sato Y, Togawa R, Kikuchi M, et al. Serum decorin is a potential prognostic biomarker in patients with acute exacerbation of idiopathic pulmonary fibrosis. J Thorac Dis, 10: 5346-5358, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]


