Abstract
Study design
Predictive algorithm via decision tree
Objectives
Artificial intelligence (AI) remain an emerging field and have not previously been used to guide therapeutic decision making in thoracolumbar burst fractures. Building such models may reduce the variability in treatment recommendations. The goal of this study was to build a mathematical prediction rule based upon radiographic variables to guide treatment decisions.
Methods
Twenty-two surgeons from the AO Knowledge Forum Trauma reviewed 183 cases from the Spine TL A3/A4 prospective study (classification, degree of certainty of posterior ligamentous complex (PLC) injury, use of M1 modifier, degree of comminution, treatment recommendation). Reviewers’ regions were classified as Europe, North/South America and Asia. Classification and regression trees were used to create models that would predict the treatment recommendation based upon radiographic variables. We applied the decision tree model which accounts for the possibility of non-normal distributions of data. Cross-validation technique as used to validate the multivariable analyses.
Results
The accuracy of the model was excellent at 82.4%. Variables included in the algorithm were certainty of PLC injury (%), degree of comminution (%), the use of M1 modifier and geographical regions. The algorithm showed that if a patient has a certainty of PLC injury over 57.5%, then there is a 97.0% chance of receiving surgery. If certainty of PLC injury was low and comminution was above 37.5%, a patient had 74.2% chance of receiving surgery in Europe and Asia vs 22.7% chance in North/South America. Throughout the algorithm, the use of the M1 modifier increased the probability of receiving surgery by 21.4% on average.
Conclusion
This study presents a predictive analytic algorithm to guide decision-making in the treatment of thoracolumbar burst fractures without neurological deficits. PLC injury assessment over 57.5% was highly predictive of receiving surgery (97.0%). A high degree of comminution resulted in a higher chance of receiving surgery in Europe or Asia vs North/South America. Future studies could include clinical and other variables to enhance predictive ability or use machine learning for outcomes prediction in thoracolumbar burst fractures.
Keywords: thoracolumbar, burst fractures, equipoise, treatment recommendations
Introduction
Artificial intelligence (AI) has gained a lot of interest recently in the medical community. However, the use of predictive modeling remains an emerging field. Predictive analytic algorithms use complex modeling to interpret data to identify correlations, which would not be perceived by researchers using typical statistical models. Linear regression and logistic regression models may fail in situation where the relationship between features and outcome is nonlinear and a complex interaction exists among factors such as the situation that exists surrounding the management decision in treating thoracolumbar (TL) burst fractures without neurological deficits.
A recent literature review summarized the use of predictive analytic modeling in spine surgery with studies mostly analyzing complications, opioid usage and patient-reported outcomes. 1 AI based applications have been described as a diagnostic aid tool in TL fractures. 2 But predictive analytic modeling has not previously been used to guide treatment decision making in TL burst fractures. Predictive models such as decision trees enable the splitting of the data multiple times to identify certain cutoff values for factors used by spinal surgeons in decision-making. Despite strong efforts from researchers, optimal treatment for thoracolumbar burst fractures without neurological deficits remains controversial. Therefore, it is essential to explore other analytic tools such as machine learning to help guide treatment decision making in this commonly encountered spine injury. Building such models is crucial in reducing the variability in decision making and, ultimately, reach consensus on the best management for thoracolumbar burst fractures.
We proposed to use predictive analytic modelling to predict how a group of spine trauma surgeons would treat a patient based on characteristics such as radiographic features and geographic location of the reviewer. The value of such predictive tool would provide valuable support to surgeons to inform and educate patients and their families. Furthermore, this new model could then be used to support a new scoring system to guide treatment.
The goal of this study was to build a mathematical algorithm to predict how spine surgeons would treat TL burst fractures without neurological deficits based on radiographic features including certainty of PLC injury, degree of comminution, use of M1 modifier and geographic location.
Material and Methods
The AO Spine Knowledge Forum Trauma (AOSKFT) completed recruitment for a prospective observational study of TL Fractures; the Spine TL A3/A4 study. Each enrolling center obtained local approval from their local institutional review board. The baseline CT scans and conventional radiographs of 183 patients who were consented and recruited to participate in this study were available. All patients were neurologically intact and had injuries between T11 and L2.
Twenty-two expert spine trauma surgeons from the AOSKFT reviewed the 183 cases. For each case, the expert reviewers were asked to classify the fracture, provide a degree of certainty of posterior ligamentous complex (PLC) injury, evaluate the degree of comminution and provided treatment recommendation. Detailed methodology is available in the article Dandurand et al. “Understanding Decision Making as it Influences Treatment in Thoracolumbar Burst Fractures Without Neurological Deficit: Conceptual Framework and Methodology” in this focus issue. The geographical regions of the reviewers were classified as Europe, North/South America and Asia (Middle East/India).
Classification and regression trees (CART) were used to create a series of predictive models. Classification trees can be used for binary or categorical variables and regression trees can be used for continuous variables. Using this approach, we were able to capture the best cut points in each variable, resulting in a more accurate and efficient algorithm. Decision trees are presented where each fork is split in a predictor variable and each node has prediction for the target variable. The « nodes » identified subgroups that were more homogeneous in terms of the probability of recommending the surgery. Predictor variables were: 1) certainty of PLC injury (0-100%); 2) degree of comminution (0-100%); 3) the use of M1 modifier; and 4) geographic location of the expert reviewers. The target variable was the recommendation for surgery or not (yes/no). We applied the type of decision tree model which is helpful for non-normal distributions of data and specifically for a binary outcome. Several cross-validation techniques were used to validate the multivariable model. P-values of .05 were considered statistically significant. Rstudio and SPSS were used for data analysis.
Results
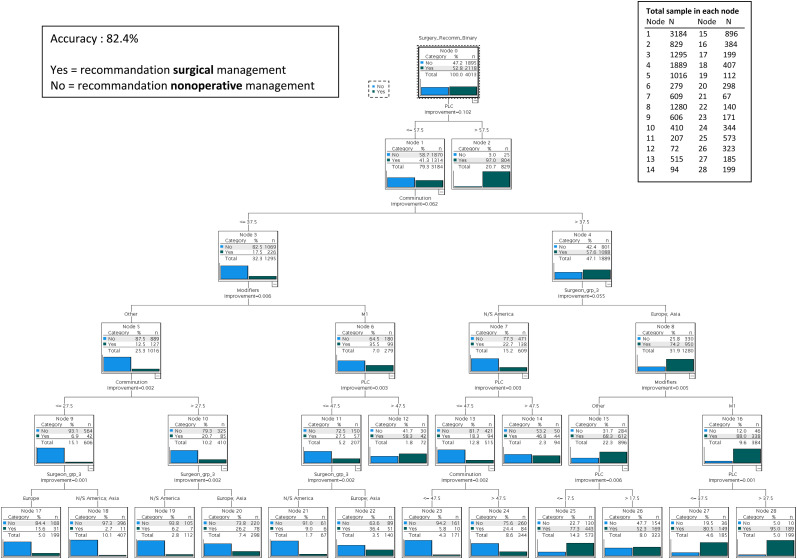
The accuracy of the predictive algorithm was excellent at 82.4% (Figure 1). Twenty-eight nodes were identified. Fifteen terminal nodes (or leaf nodes) were identified.
Figure 1.
Predictive Algorithm.
The first terminal node produced by the algorithm led to the highest probability of receiving surgery with a single predictor variable (Node 2). If a patient had a certainty of PLC injury over 57.5%, then there was a 97.0% chance of receiving surgery.
The terminal node leading to the lowest likelihood of receiving surgery was Node 18. In this node, there was a 97.3% chance of receiving non operative management (only 2.7% chance of receiving surgery) if a patient had a certainty of PLC injury ≤57.5%, a degree of comminution ≤27.5%, no modifier and was treated in North/South America or Asia.
The algorithm identified variability in the influence of comminution on receiving surgery which depended on geographic location if certainty of PLC injury was low (under 57.5%). If degree of comminution was high (> 37.5%), a patient had 74.2% chance of receiving surgery in Europe and Asia (Node 8) while their chance of having surgery recommended was 22.7% if they were treated in North/South America (Node 7). If the degree of comminution was intermediate (between 27.5% and 37.5%), then a patient would have a 26.2% chance of receiving surgery in Europe and Asia (Node 20) compared to only a 6.2% chance of receiving surgery in North/South America (Node 19).
Throughout the algorithm, the use of the M1 modifier increased the probability of receiving surgery by 21.4% on average. Between Node 5 and Node 6, there was a 23% increase of receiving surgery if M1 modifier was used and between Node 15 and Node 16, there was a 19.7% increase.
Discussion
Previous efforts to reach some degree of consensus on what is the best treatment for TL burst fractures relied primarily on the application of statistical modeling such as linear/logistic regressions. This was useful to identify predictors, make associative inferences and test hypothesis. However, the relationship between factors and treatment decision largely consists of nonlinear complex interactions. The necessary next step was to use machine learning models to make accurate predictions based on patterns learned from available data. This study presents an analytic algorithm predicting the treatment decisions of expert spinal trauma surgeons for TL burst fractures without neurological deficits. The model identified cut-off points for the predictor variables certainty of PLC injury, degree of comminution, use of M1 modifier and geographic location of the surgeon. The most important predictive factor for surgical treatment was a certainty of PLC injury above 57.5% with a 97.0% chance of receiving surgery. The second finding was the influence of comminution and geographic location on the prediction of receiving surgical treatment. In the presence of a low suspicion of PLC injury, a higher degree of comminution predicted a higher chance of receiving surgery in Europe or Asia than in North/South America. Lastly, throughout the algorithm, the use of M1 modifier increased the probability of receiving surgery by 21.4%.
The clear biomechanical importance of the PLC among the expert community is further illustrated in our study.3,4 The certainty of PLC injury was the most important predictive variable predicting the highest chance of receiving surgery. With a certainty of PLC injury above 57.5% on review of a patient’s CT scan, there was a 97% chance that a patient would receive surgery regardless of comminution, use of M1 modifier or geographic location. In contrast, the scenario which led to the lowest probably of receiving surgery was certainty of PLC injury less than 57.5%, degree of comminution less than 27.5%, no use of modifier, and being treated in North/South America and Asia. The evaluation of integrity of the PLC relies on many radiographic factors, which create a spectrum of certainty of PLC injury. In this study, PLC injury was treated as a continuous variable on an uncertainty spectrum (0-100%). It may be difficult to quantify the certainty of PLC injury in real-world clinical setting. As per a recent systematic review by Aly et al. the overall accuracy of CT in detecting PLC injury is between 68-90%.5–9 The systematic review suggested that the CT findings of facet joint malalignment, spinous process fractures, interspinous widening, and horizontal laminar fractures are independently associated with PLC injury on MRI. A single positive CT finding yielded a predictive positive value (PPV) of 31% while ≥2 CT findings yielded a PPV of 82-90%. Therefore, it is reasonable to extrapolate that if ≥ 2 CT findings are present, then there is a 97% chance that a patient would receive surgery. Future studies should focus on improving CT accuracy in identifying PLC injury using machine learning as this would significantly improve management of TL burst fractures and have lasting impact of surgeons decision making especially in center’s where MRI access if difficult.
Another finding of this study was the influence of comminution in making a treatment recommendation for surgery and how that is dependent on geographic location. In a patient with a low or intermediate certainty of PLC injury (<57.5%), if more than a third of the vertebral body was comminuted (≥37.5%), a patient would have 74.2% chance of receiving surgery in Europe and Asia vs only 22.7% chance of receiving surgery in North/South America. The rationale behind surgical management for highly comminuted fractures is that the risk of nonoperative management will lead to local kyphosis due to the potential lack of anterior column support. The Load Sharing classification of Spine Fractures included vertebral body comminution in the score to guide the choice between long and short construct but did not account for ligamentous integrity making it incomplete with respect to surgical decision making. 10 Different schools of thought likely exist worldwide and explain this geographic difference in management related to comminution. In Europe, In the mid-1990s, the multicenter study of the Spine Study Group of the German Association of Trauma Surgery showed limitations for isolated posterior instrumentation in cases with a compromised anterior load-bearing column.11-14 Following this, the German Association of Trauma Surgery initiated the second, prospective multicenter study in the 2000s. 11 This new study showed a continuous increase in anterior or combined approaches with an emphasis on correction of posttraumatic deformity. In America, Wood et al. conducted a randomized control trial comparing outcomes between surgical vs nonoperative treatment of stable thoracolumbar burst fractures in the United States. 15 The four-year results showed no difference in clinical or radiographic outcomes, but higher complications in the surgical patients. The results at sixteen to twenty-two years showed significantly better outcomes for nonoperatively treated patients. 16 This and other comparative studies and analyses reduced the enthusiasm for operative management. 17 Additional studies from the United States showed no correlation between initial radiographic severity of injury or residual deformity and symptoms at follow-up.18,19 In one of the earlier papers in this focus issue by Dandurand et al, it was determined that the degree of comminution did not differentiate whether surgeons agree or not on surgical management, but this previous analysis did not take into account geographic regions. Using machine learning, we identified that the degree of comminution influences management decisions differently around the world. These findings provide valuable insight into biases that may be present in surgeon’s current practice. Specifically, those results will help surgeons from Asia and Europe gain awareness towards their operative bias and surgeons from North and South America gain awareness towards their nonoperative bias. These results and the gained intuition could potentially help the spinal surgery community reach a consensus in the treatment of TL burst fractures. Additionally, in the creation of future scoring system aiming at classifying TL spine injury and provide treatment recommendations similarly to Thoracolumbar Injury Classification and Severity Scale (TLICS), geographic location should be considered as potential element. Different schools of thoughts worldwide and specific expertise in nonoperative or operative management could influence outcomes. Otherwise, future studies could further analyze the rates of failure with nonoperative management in highly comminuted fractures and try to identify the factors explaining this.
The elegance of machine learning lies in its ability to create complex mathematical models identifying patterns in perceived heterogenous data for highly accurate predictions for newly acquired data. The analytical dataset was generated with scan review and treatment recommendations. The actual treatment received in the real-world and outcomes were not analyzed. This has the advantage of removing the influence of non-clinical factors on treatment decisions analysis such as access to the operative room or remuneration, which may influence treatment decision in the real-world. Future studies could use predictive modeling to focus on determination of outcomes after surgery or nonoperative management. In this study, review of cases was completed with CT scans. As many centers around the world do not have easy access to MRI for all spine trauma cases, the results are generalizable, but may not hold the same value that it would if MRI is easily obtained. Future work could include further validation using larger sample sizes. As imaging and other clinical factors prove to be important in decision-making, more variables could be included in the machine learning model to enhance predictive ability.
Conclusion
This study presented a predictive analytic algorithm to guide decision-making in the treatment of TL burst fractures without neurological deficits. The model identified important cut-off points. Notably, the certainty of PLC injury above 57.5% was highly predictive of receiving surgery (97.0%). A high degree of comminution was associated with a higher chance of receiving surgery in Europe or Asia compared to North/South America. This new knowledge will be essential in the creation of new scoring system and guidelines as well as the better understanding of surgeon’s xbiases in decision making. Future studies could include more variables to enhance predictive ability or use machine learning for outcomes prediction in thoracolumbar burst fractures.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was organized and funded by AO Spine through the AO Spine Knowledge Forum Trauma, a focused group of international Trauma experts. AO Spine is a clinical division of the AO Foundation, which is an independent medically-guided not-for-profit organization. Study support was provided directly through AO Network Clinical Research.
ORCID iDs
Charlotte Dandurand https://orcid.org/0000-0002-4520-6454
Lorin M. Benneker https://orcid.org/0000-0002-5320-5638
Emiliano Vialle https://orcid.org/0000-0003-1157-4889
Shanmuganathan Rajasekaran https://orcid.org/0000-0001-6043-006X
Mohammad El-Skarkawi https://orcid.org/0000-0001-6177-7145
Rishi M. Kanna https://orcid.org/0000-0001-5817-4909
Mohamed Aly https://orcid.org/0000-0001-5221-8288
Martin Holas https://orcid.org/0000-0002-3763-8767
Jose A. Canseco https://orcid.org/0000-0002-2152-5725
Eugen Cezar Popescu https://orcid.org/0000-0001-5732-5402
Gaston Camino-Willhuber https://orcid.org/0000-0002-5684-7679
Andrei Fernandes Joaquim https://orcid.org/0000-0003-2645-0483
Sebastian Bigdon https://orcid.org/0000-0002-4649-0610
Ulrich Spiegel https://orcid.org/0000-0002-5179-4192
References
- 1.Malik AT, Khan SN. Predictive modeling in spine surgery. Ann Transl Med. 2019;7(Suppl 5):S173. doi: 10.21037/atm.2019.07.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg GS, Cina A, Schiró GR, et al. Artificial intelligence accurately detects traumatic thoracolumbar fractures on sagittal radiographs. Medicina. 2022;58(8):998. doi: 10.3390/medicina58080998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaccaro AR, Lim MR, Hurlbert RJ, et al. Surgical decision making for unstable thoracolumbar spine injuries: Results of a consensus panel review by the Spine Trauma Study Group. J Spinal Disord Tech. 2006;19(1):1-10. doi: 10.1097/01.bsd.0000180080.59559.45. [DOI] [PubMed] [Google Scholar]
- 4.Vaccaro AR, Lehman RA, Hurlbert RJ, et al. A new classification of thoracolumbar injuries: The importance of injury morphology, the integrity of the posterior ligamentous complex, and neurologic status. Spine (Phila Pa 1976). 2005;30(20):2325-2333. doi: 10.1097/01.brs.0000182986.43345.cb [DOI] [PubMed] [Google Scholar]
- 5.Aly MM, Al-Shoaibi AM, Aljuzair AH, Issa TZ, Vaccaro AR. A proposal for a standardized imaging algorithm to improve the accuracy and reliability for the diagnosis of thoracolumbar posterior ligamentous complex injury in computed tomography and magnetic resonance imaging. Global Spine J. 2022. Online ahaed of print. doi: 10.1177/21925682221129220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aly MM, Al-Shoaibi AM, Alzahrani AJ, Al Fattani A. Analysis of the combined computed tomography findings improves the accuracy of computed tomography for detecting posterior ligamentous complex injury of the thoracolumbar spine as defined by magnetic resonance imaging. World Neurosurgery. 2021;151:e760-e770. doi: 10.1016/j.wneu.2021.04.106. [DOI] [PubMed] [Google Scholar]
- 7.Winklhofer S, Thekkumthala-Sommer M, Schmidt D, et al. Magnetic resonance imaging frequently changes classification of acute traumatic thoracolumbar spine injuries. Skeletal Radiol. 2013;42(6):779-786. doi: 10.1007/s00256-012-1551-x. [DOI] [PubMed] [Google Scholar]
- 8.Khurana B, Prevedello LM, Bono CM, et al. CT for thoracic and lumbar spine fractures: can CT findings accurately predict posterior ligament complex injury? Eur Spine J. 2018;27(12):3007-3015. doi: 10.1007/s00586-018-5712-z. [DOI] [PubMed] [Google Scholar]
- 9.Pizones J, Izquierdo E, Álvarez P, et al. Impact of magnetic resonance imaging on decision making for thoracolumbar traumatic fracture diagnosis and treatment. Eur Spine J. 2011;20(3):390. doi: 10.1007/s00586-011-1913-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCormack T, Karaikovic E, Gaines RW. The load sharing classification of spine fractures. Spine (Phila Pa 1976). 1994;19(15):1741-1744. doi: 10.1097/00007632-199408000-00014 [DOI] [PubMed] [Google Scholar]
- 11.Reinhold M, Knop C, Beisse R, et al. Operative treatment of 733 patients with acute thoracolumbar spinal injuries: comprehensive results from the second, prospective, internet-based multicenter study of the Spine Study Group of the German Association of Trauma Surgery. Eur Spine J. 2010;19(10):1657-1676. doi: 10.1007/s00586-010-1451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knop C, Blauth M, Bühren V, et al. [Surgical treatment of injuries of the thoracolumbar transition. 1: epidemiology]. Unfallchirurg. 1999;102(12):924-935. doi: 10.1007/s001130050507. [DOI] [PubMed] [Google Scholar]
- 13.Knop C, Blauth M, Bühren V, et al. [Surgical treatment of injuries of the thoracolumbar transition. 2: operation and roentgenologic findings]. Unfallchirurg. 2000;103(12):1032-1047. doi: 10.1007/s001130050667. [DOI] [PubMed] [Google Scholar]
- 14.Knop C, Blauth M, Bühren V, et al. [Surgical treatment of injuries of the thoracolumbar transition--3: follow-up examination. Results of a prospective multi-center study by the “Spinal” Study Group of the German Society of Trauma Surgery]. Unfallchirurg. 2001;104(7):583-600. doi: 10.1007/s001130170089. [DOI] [PubMed] [Google Scholar]
- 15.Wood K, Buttermann G, Butterman G, et al. Operative compared with nonoperative treatment of a thoracolumbar burst fracture without neurological deficit. A prospective, randomized study. J Bone Joint Surg Am. 2003;85(5):773-781. doi: 10.2106/00004623-200305000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Wood KB, Buttermann GR, Phukan R, et al. Operative compared with nonoperative treatment of a thoracolumbar burst fracture without neurological deficit: a prospective randomized study with follow-up at sixteen to twenty-two years. J Bone Joint Surg Am. 2015;97(1):3-9. doi: 10.2106/JBJS.N.00226. [DOI] [PubMed] [Google Scholar]
- 17.Thomas KC, Bailey CS, Dvorak MF, Kwon B, Fisher C. Comparison of operative and nonoperative treatment for thoracolumbar burst fractures in patients without neurological deficit: a systematic review. J Neurosurg Spine. 2006;4(5):351-358. doi: 10.3171/spi.2006.4.5.351. [DOI] [PubMed] [Google Scholar]
- 18.Mumford J, Weinstein JN, Spratt KF, Goel VK. Thoracolumbar burst fractures. The clinical efficacy and outcome of nonoperative management. Spine. 1993;18(8):955-970. [PubMed] [Google Scholar]
- 19.Weinstein JN, Collalto P, Lehmann TR. Thoracolumbar “burst” fractures treated conservatively: a long-term follow-up. Spine. 1988;13(1):33-38. doi: 10.1097/00007632-198801000-00008. [DOI] [PubMed] [Google Scholar]