Abstract
Introduction
Psidium guajava (guava) is a fruit plant of the Myrtaceae family. Guava roots, leaves, and fruits have traditionally been used to prevent and treat various infections. In the last few decades, there has been exponential growth in herbal medicine. Therefore, the present study was conducted to determine the susceptibility and synergistic properties of the antimicrobial activity of the aqueous leaf extract of guava and other antimicrobial drugs against Escherichia coli (E. coli).
Methodology
A prospective observational study was conducted at the Department of Microbiology, MGM Medical College and Hospital, Navi Mumbai, India, involving 180 urine samples collected from patients who exhibited symptoms of urinary tract infection (UTI). The aim was to evaluate in vitro synergism between leaf extracts of guava and antimicrobial drugs on uropathogenic E. coli, using minimal inhibitory concentration (MIC) and the Kirby-Bauer method. The Kirby-Bauer disc diffusion method was employed to determine the synergistic activity using Muller-Hinton agar (MHA), and the zone of inhibition was measured in millimeters.
Results
The study found that, of the 180 urine samples collected from patients with UTI, significant growth was observed in 93 samples, with the most notable increase seen in E. coli. The antibiotics tobramycin, ofloxacin, and amikacin, each showing a sensitivity of 76% and 70% respectively, were found to be the most sensitive. Conversely, cefuroxime and cephalothin, both at 76%, were the most resistant. Furthermore, the antibiotic sensitivity pattern of E. coli without guava extract demonstrated tobramycin (TOB) at 76.66%, followed by ofloxacin (OF) and amikacin (AK) at 70% each, levofloxacin (LE) at 63.33%, nitrofurantoin (NIT) at 53.33%, trimethoprim (TR) at 43.33%, cefotaxime (CTX) at 36.66%, ceftizoxime (CZX) at 30%, norfloxacin (NR) at 26.66%, cephalothin (CEP) at 23.33%, amoxicillin-clavulanate (AMC) at 20%, and cefuroxime (CXM) at 10%. In contrast, when the antibiotic sensitivity pattern of E. coli with guava extract was examined, the highest sensitivity was noted for OF (100%), followed by LE (96.66%), TOB (93.33%), AK (90%), NIT (76.66%), AMC and TR (66.66% each), CTX (60%), CZX (53.33%), CEP (50%), NX (43.33%), and CXM (26.66%). Therefore, Psidium guajava (guava) extract exhibited a synergistic effect when combined with antibiotics, most notably with ofloxacin.
Conclusion
The study revealed that the highest synergistic activity of guava plant leaf extract was with the antibiotic ofloxacin. This finding indicates that guava extract enhances the effectiveness of commonly used antibiotics for treating UTI, an effect mainly attributed to the flavonoid compounds and their derivatives in the guava leaf extract, which inhibit bacterial growth. This study demonstrated the antibacterial properties of guava, suggesting that combining antibiotics with guava extract can help delay the emergence of bacterial resistance.
Keywords: urinary tract infection, synergistic activity, antimicrobial activity, aqueous extract, escherichia coli, psidium guajava
Introduction
Escherichia coli (E. coli) is one of the most important species encountered clinically and has been associated with various infections such as urinary tract infection (UTI), diarrhea, and meningitis [1]. Plants have long been valuable sources of natural compounds for sustaining human health, with more extensive investigations for natural remedies. The World Health Organization recognizes medicinal plants as the finest source of a wide range of medications [2]. Approximately 80% of people in developed nations utilize plants as traditional medicine [2]. As a result, such plants should be researched to better understand their characteristics, safety features, and efficiency [2]. Multiple drug resistance (MDR) has emerged in human pathogenic bacteria as a result of the widespread application of commercial antimicrobial medications frequently in the treatment of infectious diseases. These circumstances prompted scientists to look for new antimicrobial compounds from other sources as innovative antimicrobial chemotherapeutic agents [3].
Psidium guajava, known as apple guava, has shown a range of biological activities linked to its main constituents, which include flavonoids, phenolic compounds, carotenoids, and terpenoids. It is a plant used in traditional medicine that has phytotherapeutic properties. Psidium guajava phytochemical studies on tannins, flavonoids, essential oils, and proteins have been documented. This plant also has hypoglycemic antibacterial and antimutagenic characteristics [4]. Synergism is defined as the conjugation of discrete agents (such as medications) or instances in such a way that the cumulative effect exceeds the sum of the distinct effects [5]. Hence, this study aimed to evaluate in vitro synergism between leaf extracts of guava (by the maceration method) and antimicrobial drugs against uropathogenic E. coli from UTI by using the minimal inhibitory concentration (MIC) and Kirby-Bauer disc diffusion methods.
Materials and methods
A prospective observational study was conducted in the Department of Microbiology at MGM Medical College and Hospital, Navi Mumbai, India, following approval from the Institutional Ethics Committee (IEC) with reference number N-EC/2022/02/10. A total of 180 urine samples were screened for UTI, among which 93 samples were culture-positive. Of these, 30 samples were E. coli culture-positive, and after obtaining written informed consent, the samples were included in the study. Pathogenic strains of E. coli were obtained from urine samples and isolated on MacConkey agar. The powdered leaf extract of Psidium guajava (50 g) was mixed with 250 ml of distilled water in a conical flask and kept covered for 48 hours in a dark place at room temperature using the Soxhlet apparatus extraction method [6,7].
The MIC is the lowest concentration of an antimicrobial that suppresses bacterial growth. A broth dilution procedure was employed to determine the MIC of the plant extract. The extracts were serially diluted twofold in Mueller-Hinton broth. The guava extract showed an MIC of 25 mg/ml against uropathogenic E. coli from stock concentrations of 200 mg/ml, resulting in 100, 50, 25, and 12.5 mg/ml of the extracts in the broth, respectively. Hence, 0.1 ml of the 25 mg/ml concentration was used to prepare filter paper discs (antibiotic and guava extract) for further testing. The turbidity of the cultures was maintained according to the 0.5 McFarland standard, and the inoculum size was 1 X 10^8 cells [8,9].
The disk diffusion method developed by Kirby and Bauer was utilized for antibiotic susceptibility testing. Following the guidelines of the Clinical Laboratory Standard Institute (CLSI) (2022), Mueller-Hinton agar (MHA) was used for inoculation, and antibiotic discs (Himedia) available for purchase were used. Inoculums were prepared by transferring a single colony with a sterile nichrome loop to 4 ml of peptone water and incubated at 37°C for 2-4 hours to achieve turbidity comparable to a 0.5 McFarland suspension. A sterile cotton swab was then used to seed the MHA plate with the suspension by lawning the entire surface of the media. After lawning, one side of the plates was covered with an antibiotic disc from Himedia using forceps, while the other side was covered with commercially available antimicrobial discs. Subsequently, 20 µl of guava extract was cautiously applied onto the antibiotic disc. The plates were incubated for 24 hours at 37°C, and the zone of inhibition was then measured in mm using a transparent ruler [9-11]. The antibiotics used included ofloxacin (OF) 2 mcg, cephalothin (CEP) 30 mcg, levofloxacin (LE) 5 mcg, ceftizoxime (CZX) 30 mcg, nitrofurantoin (NIT) 30 mcg, cefuroxime (CXM) 30 mcg, trimethoprim (TR) 5 mcg, cefotaxime (CTX) 30 mcg, amoxicillin-clavulanate (AMC) 30 mcg, norfloxacin (NX) 10 mcg, amikacin (AK) 30 mcg, and tobramycin (TOB) 10 mcg.
Results
Overall, 180 urine samples from patients exhibiting symptoms of UTI were screened, among which 93 (51.66%) samples were microbiologically confirmed cases, as they showed significant growth. Of these, 30 samples were E. coli culture-positive UTI samples. The distribution of organisms isolated from the urine samples of the 93 UTI patients is described in Table 1.
Table 1. Organisms isolated from urine samples.
| Organisms | Numbers | Percentage (%) |
| Escherichia coli | 30 | 32.25 |
| Enterococcus | 9 | 9.67 |
| Klebsiella pneumoniae | 6 | 6.52 |
| Citrobacter | 13 | 13.97 |
| Proteus mirabilis | 3 | 3.22 |
| Candida species / albicans | 17 | 18.27 |
| Staphylococcus aureus | 11 | 11.82 |
| Acinetobacter | 4 | 4.30 |
The antibiotic sensitivity pattern of E. coli is demonstrated in Table 2.
Table 2. Antibiotic sensitivity pattern in 30 patients showing growth of E. coli.
E. coli = Escherichia coli, CEP = cephalothin, OF = ofloxacin, CZX = ceftizoxime, LE = levofloxacin, CXM = cefuroxime, NIT = nitrofurantoin, CTX = cefotaxime, TR = trimethoprim, AMC = amoxicillin-clavulanate, and AK = amikacin, NX = norfloxacin, and TOB = tobramycin
| Patients | CEP | OF | CZX | LE | CXM | NIT | CTX | TR | AMC | AK | NX | TOB |
| 1 | _--- | 12 | --- | 22 | 24 | 16 | --- | 16 | --- | 12 | 15 | --- |
| 2 | --- | 17 | --- | 21 | --- | 12 | --- | --- | 14 | 11 | --- | 14 |
| 3 | 5 | 14 | 22 | 14 | --- | 16 | 14 | 10 | 7 | 15 | --- | 15 |
| 4 | --- | 18 | 4 | 17 | --- | 7 | --- | --- | 10 | 16 | 20 | 16 |
| 5 | 6 | 13 | --- | 16 | --- | 19 | 15 | 8 | 19 | 17 | 12 | 15 |
| 6 | --- | 16 | --- | 17 | --- | 19 | 16 | 3 | 13 | 17 | 05 | 15 |
| 7 | --- | 17 | 20 | 22 | --- | 21 | 14 | 7 | 18 | 18 | --- | 16 |
| 8 | --- | 12 | 21 | 14 | 16 | 19 | 18 | 12 | 18 | 18 | 10 | 14 |
| 9 | --- | 16 | --- | --- | 21 | --- | --- | --- | 10 | --- | --- | 17 |
| 10 | --- | 15 | --- | 23 | --- | --- | --- | --- | 7 | --- | --- | 18 |
| 11 | --- | 8 | 12 | 22 | --- | --- | --- | 8 | 13 | 18 | --- | 12 |
| 12 | --- | 13 | --- | 22 | 26 | 19 | --- | --- | 18 | 19 | --- | --- |
| 13 | 7 | 26 | 27 | 33 | --- | 19 | --- | 24 | --- | 24 | 28 | 21 |
| 14 | --- | 16 | --- | 20 | --- | 21 | --- | 18 | 12 | 22 | --- | 20 |
| 15 | 29 | 17 | --- | 21 | --- | 19 | 22 | 06 | --- | 23 | --- | 19 |
| 16 | 22 | 26 | 28 | 28 | --- | --- | 26 | 23 | 16 | 20 | --- | 23 |
| 17 | --- | 19 | 25 | 19 | 22 | --- | 25 | 19 | 17 | 26 | 27 | 22 |
| 18 | 26 | 23 | 26 | 24 | 10 | 18 | 27 | 25 | 07 | 05 | 23 | 12 |
| 19 | --- | 19 | --- | 22 | --- | --- | 22 | 13 | 14 | 11 | --- | 13 |
| 20 | 27 | 30 | 33 | 31 | --- | 21 | 31 | 16 | --- | 25 | 27 | 21 |
| 21 | --- | 20 | --- | 20 | 7 | 23 | --- | 25 | 5 | 23 | --- | 19 |
| 22 | --- | 16 | --- | 20 | --- | 20 | 19 | 27 | --- | 22 | --- | 21 |
| 23 | --- | 20 | 22 | 14 | --- | 23 | 08 | 11 | --- | 23 | --- | 24 |
| 24 | --- | 26 | 29 | 34 | 14 | 18 | --- | 25 | 13 | 24 | 12 | 25 |
| 25 | 27 | 30 | 33 | 31 | 8 | 21 | 31 | 27 | --- | 25 | 28 | 21 |
| 26 | --- | 12 | 27 | 24 | 18 | 23 | 12 | --- | 08 | 23 | --- | 25 |
| 27 | --- | 18 | 23 | 28 | --- | --- | 10 | 19 | --- | 12 | 17 | 18 |
| 28 | 24 | 28 | 23 | 30 | --- | 21 | 31 | 27 | 25 | 28 | 08 | 20 |
| 29 | --- | 14 | 18 | 27 | 24 | 15 | 05 | --- | 19 | 22 | --- | 21 |
| 30 | 22 | 25 | 27 | 29 | --- | 18 | 28 | --- | --- | 22 | 27 | 16 |
| Sensitive | 07 | 21 | 09 | 19 | 03 | 16 | 11 | 13 | 06 | 21 | 08 | 23 |
| % | 23.3 | 70 | 30 | 63.3 | 10 | 53.3 | 36.6 | 43.3 | 20 | 70 | 26.6 | 76.6 |
| Intermediate | 00 | 05 | 04 | 06 | 04 | 05 | 02 | 03 | 04 | 02 | 01 | 03 |
| % | 00 | 16.6 | 13.3 | 20 | 13.3 | 16.6 | 6.66 | 10 | 13.3 | 6.66 | 33.3 | 10 |
| Resistant | 23 | 04 | 17 | 05 | 23 | 09 | 17 | 14 | 20 | 07 | 21 | 04 |
| % | 76.6 | 13.3 | 56.6 | 16.6 | 76.6 | 30 | 56.6 | 46.6 | 66.66 | 23.3 | 70 | 13.3 |
The antibiotic sensitivity pattern of E. coli without guava extract is illustrated in Figure 1.
Figure 1. Sensitivity pattern on E. coli without guava extract.
E. coli = Escherichia coli, CEP = cephalothin, OF = ofloxacin, CZX = ceftizoxime, LE = levofloxacin, CXM = cefuroxime, NIT = nitrofurantoin, CTX = cefotaxime, TR = trimethoprim, AMC = amoxicillin-clavulanate, and AK = amikacin, NX = norfloxacin, and TOB = tobramycin
The synergistic activity of antibiotics with guava extract is depicted in Figure 2.
Figure 2. Synergistic activity of antibiotics with guava extract.
CEP = cephalothin, OF = ofloxacin, CZX = ceftizoxime, LE = levofloxacin, CXM = cefuroxime, NIT = nitrofurantoin, CTX = cefotaxime, TR = trimethoprim, AMC = amoxicillin-clavulanate, and AK = amikacin, NX = norfloxacin, and TOB = tobramycin.
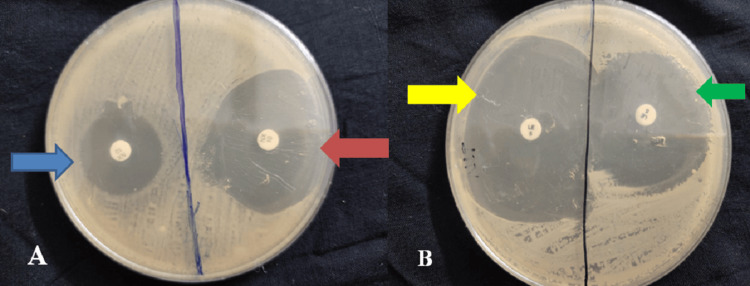
The specimen illustrating sensitivity without guava extract and synergistic activity with guava extract is depicted in Figure 3.
Figure 3. Specimen illustrating sensitivity without guava extract and synergistic activity with guava extract.
A: The blue arrow illustrates sensitivity without guava extract, and the red arrow illustrates sensitivity with guava extract. B: The yellow arrow illustrates sensitivity with guava extract and the green arrow illustrates sensitivity without guava extract.
Discussion
The current study included 180 urine samples from UTI patients, out of which growth of organisms was observed in a total of 93 samples. The most significant growth was of the organism E. coli, found in 30 samples (32.25%), followed by Candida albicans in 17 samples (18.27%), Citrobacter in 13 samples (13.97%), Staphylococcus aureus in 11 samples (11.82%), Enterococcus in nine samples (9.67%), Klebsiella pneumoniae in six samples (6.52%), Acinetobacter in four samples (4.30%), and Proteus mirabilis in three samples (3.22%). In a similar study of 351 samples, 316 showed positive culture growth, with 64% being Escherichia coli, 6.32% Klebsiella pneumoniae, 10.7% Enterococcus species, 3.16% Pseudomonas aeruginosa, 4.43% Candida albicans, 5.06% Staphylococcus aureus, and 5.69% other microorganisms such as Acinetobacter, Staphylococcus epidermidis, and Citrobacter [12].
Another study conducted by Gul and Gurbuz involved 1211 urine samples, in which the organisms isolated were 0.17% Acinetobacter, 0.58% of Acinetobacter baumannii, 0.08% of Alcaligenes faecalis, 0.08% of Burkholderia cepacia, 1.24% of Candida albicans, 0.08% of Candida famata, 0.17% of Candida kefyr, 0.08% of Candida krusei, 0.25% of Candida spherica, 0.25% of Candida tropicalis, 0.17% of Citrobacter freundii, 0.25% of Citrobacter koseri, 0.08% of Enterobacter aerogenes, 0.74% of Enterobacter cloacae, 0.33% of Enterococcus species, 4.5% of Enterococcus faecalis, 0.74% of Enterococcus faecium, 68.4% of Escherichia coli, 2.8% of Klebsiella species, 0.5% of Klebsiella oxytoca, 7.2% of Klebsiella pneumoniae, 0.25% of Morganella morganii, 0.33% of Proteus species, 1.73% of Proteus mirabilis, 0.33% of Providencia rettgeri, 1.2% of Pseudomonas aeruginosa 10.08% of Salmonella species, 0.17% of Serratia fonticola, 0.17% of Serratia liquefaciens, 0.08% of Serratia marcescens, 0.08% of Shigella sonnei, 0.33% of Staphylococcus aureus, 1.9% of Staphylococcus epidermidis, 0.25% of Staphylococcus haemolyticus, 0.08% of Staphylococcus hominis, 0.5% of Staphylococcus saprophyticus, 0.08% of Staphylococcus warneri, 0.17% of Streptococcus species, 15% of Streptococcus agalactiae, 0.08% of Streptococcus constellatus, 0.17% of Streptococcus dysgalactiae, 0.25% of Streptococcus mitis, 0.08% of Streptococcus salivarius, and 0.08% of Streptococcus sanguinis [13]. In the present study, the incidence of E. coli was 51.66%, which was quite similar to the findings reported by Hamza et al., but lower than the incidence reported by Gul and Gurbuz, who found E. coli in 68.4% of 1211 urine samples [12,13].
Additionally, Sabir et al. in their study reported that E. coli were highly resistant to penicillin (100%), amoxicillin (100%), and cefotaxime (89.7%), followed by an intermediate level of resistance to ceftazidime (73.8%), tetracycline (69.4%), cephradine (73.8%), augmentin (62.6%), gentamycin (59.8%), doxycycline (66.6%), ciprofloxacin (54.2%), cefuroxime (58.2%), aztreonam (44.8%), cefaclor (50%), imipenem (43.3%), and ceftriaxone (43.3%), and a low level of resistance to kanamycin (19.9%), streptomycin (30%), amikacin (12.7%), and tazocin (14%), and the lowest to norfloxacin (11.2%). Two hundred sixty-one (81%) of the 321 E. coli isolates were found to be multidrug-resistant, and five (1.5%) were found to be extensively drug-resistant [14]. Furthermore, Ferdosi-Shahandashti's analysis showed that 57 samples of urine contained E. coli growth, and the most sensitive antibiotics were ofloxacin, imipenem, and ciprofloxacin, with 87.7%, 87.7%, and 78.9% sensitivity, respectively. The most resistant antibiotics were cefixime, cotrimoxazole, cefotaxime, and ceftriaxone [15].
Shalini et al. carried out a similar study in a tertiary hospital to examine the shifting patterns of antibiotic sensitivity among uropathogens causing UTIs. A total of 170 urine culture sensitivity reports were examined, where E. coli predominated. Further antibiotic testing showed amoxicillin with a 19.60% sensitivity and 80.40% resistance, amoxiclav with 27.20% sensitivity and 72.80% resistance, cotrimoxazole with 19.60% sensitivity and 80.40% resistance, gentamicin with 66.30% sensitivity and 43.70% resistance, amikacin with 98.91% sensitivity and 1.09% resistance, ciprofloxacin with 69.56% sensitivity and 30.44% resistance, norfloxacin with 73.91% sensitivity and 26.09% resistance, levofloxacin with 75% sensitivity and 25% resistance, nitrofurantoin with 93.48% sensitivity and 6.52% resistance, and ceftazidime with 80.43% sensitivity. Minocycline showed 75% sensitivity and 25% resistance. The results demonstrated that E. coli showed high sensitivity to amikacin, which is 98.91%, nitrofurantoin 93.48%, and ceftazidime 80.43% [16]. In the current study, ofloxacin, tobramycin, and amikacin were reported to be the most sensitive, that is, 70%, 76.6%, and 70%, respectively, and cefuroxime and cephalothin were found to be the most resistant, that is, 76.6% for both.
Additionally, the present study used guava extract to observe the synergistic activity with the combination of 12 antibiotics by the Kirby-Bauer disc diffusion method on MHA and reported that the sensitivity percentage has increased by 7-10% on average, indicating the synergistic activity of Psidium guajava. Furthermore, in a study by Dalee et al., the concentration of tetracycline was fixed (6.25 μg/ml) for E. coli, and it was observed that synergy was achieved as MIC and minimum bactericidal concentration (MBC) values reduced, regardless of the extracting solvent used, as guava MIC decreased twofold and more, and MBC decreased up to eightfold [17].
The guava leaves are two to six inches long and one to two inches broad. They have noticeable veins, a dull-green appearance, and a coriaceous, stiff texture when crushed. The guava leaf contains bioactive ingredients that have antimicrobial, blood-glucose-regulating, and even weight-loss properties. An essential oil rich in cineol, tannins, triterpenes, flavonoids, resin, eugenol, malic acid, fat, cellulose, chlorophyll, and several other fixed compounds can be found in guava leaves [18,19]. In a study by Biswas et al., the antimicrobial potential of guava (Psidium guajava) leaf extracts against two gram-negative bacteria (E. coli and Salmonella enteritidis) and two gram-positive bacteria (Staphylococcus aureus and Bacillus cereus) was evaluated. Only two of the crude solvent extracts made from Psidium guajava leaves, methanol and ethanol, had inhibitory efficacy against bacteria, according to the study's findings. The two extracts only demonstrated inhibition against the two gram-positive bacteria, Bacillus cereus and Staphylococcus aureus, while the gram-negative bacteria did not show any inhibition at all. These results contradicted the outcomes of the present study. Additionally, Hoque et al. found no antibacterial activity of ethanolic extracts of guava against E. coli and S. enteritidis [20]. The outcomes of the above-mentioned studies contradicted the findings of the present study; however, Vieira et al. found guava sprout extracts were effective in inhibiting E. coli [21].
Limitations
The present study was conducted with a very small sample size, and only the antibiotic sensitivity and synergistic activity of E. coli organisms were observed. Therefore, future studies with larger sample sizes that include antibiotic sensitivity and synergistic activity of other organisms can be considered for future scope. Furthermore, future studies highlighting the individual effect of guava leaf extract can be focused on. Additionally, the antibiotic-plant extract synergy mechanism is still unknown. However, advancement in this field can lead to the use of a combination of extracts for the management of diseases, overcoming drug-resistant pathogens, and reducing the use of antibiotics and adverse effects caused by them.
Conclusions
This study demonstrated the antimicrobial potential of Psidium guajava leaf extract by using distilled water as an aqueous solvent. The results reported that the flavonoid compounds and their derivatives in guava plant leaf extract can inhibit the growth of bacteria, and that the cumulative effect of guava leaf extract and antibiotics has a synergistic effect on uropathogenic E. coli. In the present study, the highest sensitivity of antibiotics with guava leaf extract was demonstrated by ofloxacin (100%). Therefore, patients suffering from UTI can be encouraged to consume guava fruit or guava juice as an adjunct to antibiotic treatment. Furthermore, in vivo studies might help to determine whether the consumption of guava juice or extract can increase the effectiveness of antibiotics and whether it can help to avoid the use of expensive, stronger, and toxic antibiotics for UTI treatment.
The authors have declared that no competing interests exist.
Author Contributions
Concept and design: Sohini Mitra
Acquisition, analysis, or interpretation of data: Sohini Mitra, Anahita V. Bhesania Hodiwala, Harapriya Kar
Drafting of the manuscript: Sohini Mitra, Anahita V. Bhesania Hodiwala, Harapriya Kar
Critical review of the manuscript for important intellectual content: Sohini Mitra, Anahita V. Bhesania Hodiwala, Harapriya Kar
Supervision: Anahita V. Bhesania Hodiwala
Human Ethics
Consent was obtained or waived by all participants in this study. MGM Medical College and Hospital, Navi Mumbai, India issued approval N-EC/2022/02/10
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Current trends in antimicrobial resistance of Escherichia coli. Paitan Y. https://pubmed.ncbi.nlm.nih.gov/30088148/ Curr Top Microbiol Immunol. 2018;416:181–211. doi: 10.1007/82_2018_110. [DOI] [PubMed] [Google Scholar]
- 2.Towards advances in medicinal plant antimicrobial activity: a review study on challenges and future perspectives. Vaou N, Stavropoulou E, Voidarou C, Tsigalou C, Bezirtzoglou E. Microorganisms. 2021;9 doi: 10.3390/microorganisms9102041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evaluation of the antibacterial activity of Cocculus hirsutus. Abiramasundari P, Priya V, Jeyanthi GP, Gayathri Devi S. http://www.hygeiajournal.com/downloads/8818372494.gayathri.pdf Hygeia J D Med. 2011;3:26–31. [Google Scholar]
- 4.Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Mostafa AA, Al-Askar AA, Almaary KS, Dawoud TM, Sholkamy EN, Bakri MM. https://pubmed.ncbi.nlm.nih.gov/29472791/ Saudi J Biol Sci. 2018;25:361–366. doi: 10.1016/j.sjbs.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antiviral activity of castor oil plant (Ricinus communis) leaf extracts. Elkousy RH, Said ZN, Abd El-Baseer MA, Abu El Wafa SA. https://pubmed.ncbi.nlm.nih.gov/33515683/ J Ethnopharmacol. 2021;271:113878. doi: 10.1016/j.jep.2021.113878. [DOI] [PubMed] [Google Scholar]
- 6.Antibacterial and antifungal activities and phytochemical profile of leaf extract from different extractants of Ricinus communis against selected pathogens. Suurbaar J, Mosobil R, Donkor AM. BMC Res Notes. 2017;10:660. doi: 10.1186/s13104-017-3001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antibacterial activity of Psidium guajava leaf extract against selected pathogenic bacteria. Ngene AC, Aguiyi JC, Chibuike CJ, et al. Adv Microbiol. 2019;9:1012–1022. [Google Scholar]
- 8.Isolation of antimicrobial compounds from guava (Psidium guajava L.) and their structural elucidation. Arima H, Danno G. Biosci Biotechnol Biochem. 2002;66:1727–1730. doi: 10.1271/bbb.66.1727. [DOI] [PubMed] [Google Scholar]
- 9.The European Committee on Antimicrobial Susceptibility Testing disc diffusion susceptibility testing method for frequently isolated anaerobic bacteria. Matuschek E, Copsey-Mawer S, Petersson S, Åhman J, Morris TE, Kahlmeter G. Clinical Microbiology and Infection. 2023;1:795–791. doi: 10.1016/j.cmi.2023.01.027. [DOI] [PubMed] [Google Scholar]
- 10.Interpretation of antimicrobial susceptibility testing using European Committee on Antimicrobial Susceptibility Testing (EUCAST) and clinical and Laboratory Standards Institute (CLSI) breakpoints: analysis of agreement. Gaur P, Hada V, Rath RS, Mohanty A, Singh P, Rukadikar A. Cureus. 2023;15:0. doi: 10.7759/cureus.36977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The antibacterial effect of some medicinal plant extracts and their synergistic effect with antibiotics. Jouda MM, Elbashiti T, Masad A. https://www.iiste.org/Journals/index.php/ALST/article/view/31568 Adv Life Sci Technol . 2016;46:59–69. [Google Scholar]
- 12.The pattern of organism causing urinary tract infection in diabetic and non diabetic patients in Bangladesh. Hamza M, Barai L, Haq J, Shariful M, Jilani MdSA, Begum M. Bangladesh J Med Microbiol. 19701;4:6–8. [Google Scholar]
- 13.Microorganisms and antibiotic susceptibilities isolated from urine cultures. Gul A, Gurbuz E. https://pagepressjournals.org/index.php/aiua/article/view/aiua.2020.2.146/8855. Arch Ital Urol Androl. 2020;92 doi: 10.4081/aiua.2020.2.146. [DOI] [PubMed] [Google Scholar]
- 14.Isolation and antibiotic susceptibility of E. coli from urinary tract infections in a tertiary care hospital. Sabir S, Ahmad Anjum A, Ijaz T, Asad Ali M, Ur Rehman Khan M, Nawaz M. https://pubmed.ncbi.nlm.nih.gov/24772149/ Pak J Med Sci. 2014;30:389–392. [PMC free article] [PubMed] [Google Scholar]
- 15.Resistance patterns of Escherichia coli causing urinary tract infection. Ferdosi-Shahandashti E, Javanian M, Moradian-Kouchaksaraei M, Yeganeh B, Bijani A, Motevaseli E, Moradian-Kouchaksaraei F. https://pubmed.ncbi.nlm.nih.gov/26644881/ Caspian J Intern Med. 2015;6:148–151. [PMC free article] [PubMed] [Google Scholar]
- 16.Study of antibiotic sensitivity pattern in urinary tract infection at a tertiary hospital. Shalini Shalini, Joshi MC, Rashid MK, Joshi HS. http://njirm.pbworks.com/f/8%20Study%20of%20Antibiotic%20Sensitivity%20Pattern%20In%20Urinary%20Tract%20Infection%2043-46.pdf NJIRM. 2011;2:43–46. [Google Scholar]
- 17.Dalee AD, Ya N, Sali K, Hayeeyusoh N, Hajiwangoh Z, Salaeh P. Yogyakarta State University. Yogyakarta, Indonesia: Yogyakarta State University; 2015. Synergistic Effect of Local Guava, Noni, Carambola and Kariyat Extracts and Tetracycline in Inhibiting the Growth of Escherichia coli and Salmonella sp., Clinically Isolated From Yingo Hospital, Narathiwat Province, South Thailand; pp. 0–28. [Google Scholar]
- 18.Antimicrobial activities of leaf extracts of guava (Psidium guajava L.) on two Gram-negative and Gram-positive bacteria. Biswas B, Rogers K, McLaughlin F, Daniels D, Yadav A. Int J Microbiol. 2013;2013:746165. doi: 10.1155/2013/746165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phytochemical profile and bioactive compounds of beluntas leaves extract (Pluchea indica L.) and its hydrogel preparations. Habibah N, Nugroho HSW, Dhyanaputri IGAS, Dharmawati IGAA, Suyasa IBO. J Med Pharm Allied Sci. 2023;12:6184–6190. [Google Scholar]
- 20.Antibacterial activity of guava (Psidium guajava L.) and neem (Azadirachta indica A. Juss.) extracts against foodborne pathogens and spoilage bacteria. Mahfuzul Hoque MD, Bari ML, Inatsu Y, Juneja VK, Kawamoto S. Foodborne Pathog Dis. 2007;4:481–488. doi: 10.1089/fpd.2007.0040. [DOI] [PubMed] [Google Scholar]
- 21.Microbicidal effect of medicinal plant extracts (Psidium guajava Linn. and Carica papaya Linn.) upon bacteria isolated from fish muscle and known to induce diarrhea in children. Vieira RH, Rodrigues DP, Gonçalves FA, Menezes FG, Aragão JS, Sousa OV. Rev Inst Med Trop Sao Paulo. 2001;43:145–148. doi: 10.1590/s0036-46652001000300005. [DOI] [PubMed] [Google Scholar]