Abstract
Objectives
To examine the 16-year developmental history, research hotspots, and emerging trends of zinc-based biodegradable metallic materials from the perspective of structural and temporal dynamics.
Methods
The literature on zinc-based biodegradable metallic materials in WoSCC was searched. Historical characteristics, the evolution of active topics and development trends in the field of zinc-based biodegradable metallic materials were analyzed using the bibliometric tools CiteSpace and HistCite.
Results
Over the past 16 years, the field of zinc-based biodegradable metal materials has remained in a hotspot stage, with extensive scientific collaboration. In addition, there are 45 subject categories and 51 keywords in different research periods, and 80 papers experience citation bursts. Keyword clustering anchored 3 emerging research subfields, namely, #1 plastic deformation #4 additive manufacturing #5 surface modification. The keyword alluvial map shows that the longest-lasting research concepts in the field are mechanical property, microstructure, corrosion behavior, etc., and emerging keywords are additive manufacturing, surface modification, dynamic recrystallization, etc. The most recent research on reference clustering has six subfields. Namely, #0 microstructure, #2 sem, #3 additive manufacturing, #4 laser powder bed fusion, #5 implant, and #7 Zn–1Mg.
Conclusion
The results of the bibliometric study provide the current status and trends of research on zinc-based biodegradable metallic materials, which can help researchers identify hot spots and explore new research directions in the field.
Keywords: Zinc-based biomaterials, Zn alloys, Biodegradable stent, Bone defect repair, Vascular implant, Bibliometric
Highlights
-
•
Research on zinc-based biodegradable materials has surged remarkably.
-
•
The mechanical properties and corrosion behavior are classic topics.
-
•
The strain softening and creep of zinc alloys should also be considered.
-
•
In vivo performance, dynamic recrystallization, etc., have become hotspots.
-
•
Biomimetic bone repair materials and nerve stents are the latest trends.
1. Introduction
Biomedical metallic materials represented by stainless steel, Co–Cr alloys, and Ti alloys have been widely used as implants, such as bone plates, bone screws, fusion devices, dental implants, vascular stents, and pacemakers, due to their superior mechanical properties and resistance to corrosion, wear, and processability [[1], [2], [3], [4], [5], [6]]. However, the benefits of implants typically diminish as the body recovers, and there is even a possibility of harm to the human body after a certain degree of recovery. For example, bone plates and screws can reduce the gaps between fractured bone tissue, promoting effective fusion, but the presence of bone plates and screws may hinder further fusion of bone tissue and even cause inflammation due to defects in the surface oxide film. Once inflammation occurs, a secondary surgery to remove the implant is needed, resulting in secondary damage and additional costs [7,8]. Additionally, metallic stents lose their beneficial effects after being implanted for six months in the treatment of atherosclerosis, thus necessitating long-term use of anticoagulants to alleviate adverse reactions in the late stage after implantation. However, even with such medication, the presence of stents affects the recovery of vascular dilation and contraction function while also increasing the risk of restenosis [9,10].
Biodegradable metals and alloys are known to degrade gradually in vivo once they have completed their intended function, with the degradation products having no significant adverse effects on the surrounding tissues [[11], [12], [13]]. This is beneficial for restoring bodily functions, reducing complications, and eliminating the need for a secondary surgery to remove the implanted materials, which would cause additional pain and economic burden to the patient. Preferentially selecting metal elements with essential physiological functions as candidates ensure both appropriate degradation rates and adjusts their content through human metabolism, thereby avoiding toxicity caused by accumulation in the body. Among biodegradable metals, Mg-based, Fe-based, and Zn-based alloys have been studied extensively (Fig. 1).
Fig. 1.
Major advantages and disadvantages of Mg-, Zn- and Fe-based biodegradable metals [1,14,15].
Magnesium is the earliest developed and most extensively clinically tested among these three biodegradable metals [15,16]. Compared to traditional alloys (such as Ti or Co-based alloys), magnesium has mechanical properties closer to human bone tissue, which can generate a stress shielding effect and reduce the risk of implant deformation or displacement [17]. Additionally, magnesium ions, as the main cations in organisms and cells, are involved in rhythm regulation, blood pressure, neuro-muscular conduction, as well as ATP hydrolysis, DNA replication, and glucose metabolism within cells [18]. However, magnesium has exhibited a rapid degradation rate and quick release of by-products (hydroxide and hydrogen) in numerous in vitro and in vivo experiments [18]. It leads to inadequate mechanical integrity of implants, hindering the completion of tissue repair at the implant site and potentially causing alkalinization and inflammation. To regulate the degradation rate of magnesium and the release of hydrogen, researchers have employed alloying methods. Bogdan et al. successfully reduced the degradation rate and hydrogen release by decreasing the potential difference between phases by adding calcium and zinc elements to magnesium-based materials [19].
Iron has the highest mechanical strength and excellent formability among these three commonly used metal systems (Fig. 1) [15,16]. Consequently, it offers greater potential for shaping and allows for thinner implant thickness. However, the high elastic modulus of iron-based alloys can lead to stress shielding, and they exhibit poor non-austenitic phase nuclear magnetic compatibility. Most of the iron in the body participates in the formation of hemoglobin to maintain oxygen transport, as well as various redox or hydrolysis reactions in the body. Although iron is essential for life, the Fenton reaction mediated by iron (the reaction between Fe2+ and H2O2 leads to the formation of Fe3+, OH−, and highly destructive HO•) can produce and accumulate reactive oxygen species. Animal experiments have found that the degradation rate of iron-based scaffolds is too slow (more than 2–3 years), and the hydroxide layer formed on the scaffold surface can be penetrated by oxygen, causing HO• to continuously form on the material surface during the entire implantation period, leading to dysfunction of surrounding tissues [20]. Moreover, the final degradation products (mainly iron oxide) may accumulate in surrounding tissues and biological matrices, causing complications [15,21].
Zn, with a standard corrosion potential of 0.76 V vs. SHE, exhibits a degradation rate that falls between that of Mg (2.37 V vs. SHE) and Fe (0.44 V vs. SHE) [22]. Zinc does not produce gas during the degradation process, and its degradation products are non-toxic. It has a low melting point, low molten reactivity, and good processability. Additionally, it shows excellent MRI compatibility. And Zn as an essential nutrient in human physiology, playing a crucial role in protein synthesis, signal transduction, and the normal function of various enzymes. Furthermore, zinc, as a reducing agent, exhibits antioxidant and anti-inflammatory properties both in its elemental form and in the form of zinc compounds. It also has demonstrated characteristics such as promoting wound healing and increasing the blood vessel formation rate, making it a hot topic among biodegradable metals [22]. Despite its excellent fracture elongation (60 %–80 %), pure zinc has a relatively low tensile strength of only 120 MPa and is prone to creep and age hardening. However, with the addition of alloying elements and optimization of processing techniques, these issues are gradually being addressed.
As a biomedical researcher, it can be challenging and time-consuming to comprehend the development history of biodegradable Zn alloys and extract research highlights from over 600 relevant articles published in the last dozen years on the Web of Science. Moreover, due to limitations in one's own experience, memory, and the integrity of literature, researchers are often forced to make subjective judgments about the development in the scientific field. Therefore, different from traditional academic reviews based on viewpoints, literaturebased bibliometric reviews can provide a more objective and comprehensive overview of a field's historical features, research hotspots, and development trends [23]. Bioidentification tools such as CiteSpace, CitNetExplorer, and VOSviewer have been applied to scientometric evaluation to assess the overall picture of an academic field [[24], [25], [26], [27]]. CiteSpace's multiple advantages distinguish it from similar software tools (such as VOSViewer, CitNetExplorer, SCI2, Pajek, and Gephi). As shown in Table 1, while CiteSpace may lag slightly behind Pajek in network analysis and network visualization and VOSViewer in heatmap generation, it excels over other software in various aspects such as automatic cluster naming, dual-map overlay, and timeline functionality. Its high efficiency, user-friendliness, customizability, and powerful visualization capabilities contribute to its wide-ranging applicability and value. This study used CiteSpace software (version 6.1 R4), HistCite Pro 2.1, and burst detection to evaluate research on biodegradable Zn alloys. The purpose of this research includes (1) summarizing the historical characteristics of the biodegradable Zn alloys literature, (2) highlighting articles that have made significant contributions to the field, (3) identifying active topics in the research field, and (4) revealing new trends for future research.
Table 1.
Comparative of bibliometric software.
| Tool | Network Analysis | Network Visualization Analysis | Network Analysis | Turning Points | Automatic cluster naming | Macro theory | Dual-map overlay | Concept tree | Timeline |
|---|---|---|---|---|---|---|---|---|---|
| CiteSpace [28] | ++ | ++ | + | + | + | + | + | + | |
| VOSViewer [29] | + | ||||||||
| CitNetExplorer [30] | + | + | |||||||
| SCI2 [31] | + | ++ | |||||||
| Pajek [32] | +++ | + | |||||||
| Gephi [33] | ++ | +++ |
+: Good; ++: Very good; +++: Excellent.
2. Methods
2.1. Data collection and statistics
The Web of Science Core Collection (WoSCC) from Thomson Reuters includes over 12,000 influential academic journals that are widely recognized as necessary in the international academic community. In this study, we used WoSCC as the target database and employed the search strategy "(((TS=(Zn or Zn-base or zinc or zinc-base)) AND TS=(alloy or metal)) AND TS=(cytotoxicity or biocompatibility or Biocompatible or Bioabsorbability or biodegradable or biodegradation or bioabsorbable or bioresorbable or Degradability)) NOT TS=(pollution or pollute or contamination or contaminate or contaminated or soil or metal organic framework or mofs or mof or wastewater)" to retrieve relevant literature on zinc-based biodegradable metal materials. The first article on zinc-based biodegradable metal materials was published in 2007, and the research period covered complete years from 2007 to 2022. Therefore, the search spanned from January 1, 2007, to December 31, 2022, yielding 5724 documents. After manually selecting relevant articles, 528 documents were retained, and an additional search was conducted on the top 50 most cited documents among the 528 articles. The collected literature records were downloaded and saved in a plain text file in the format of “complete records and cited references” as a sample for data analysis, named DATAZn, totaling 632 entries. In addition, we collected the original data on publication country/region information, such as institutions, journals, authors, and types of articles, and then used Excel (WPS 221214) to conduct data statistics.
2.2. Bibliometric analysis tools
For the present study, CiteSpace was used for co-occurrence analysis, burst analysis, and cluster analysis. Use the citation score in HistCite to evaluate the importance of the article. We utilize CiteSpace to generate a series of separate networks based on co-occurring keywords. These networks are exported from CiteSpace and loaded into the Alluvial Generator, resulting in an alluvial map, to explain how the theme evolves over time. Further details regarding the bibliometric analysis tools can be found in another article on drug-eluting stents in this research group [34].
2.2.1. CiteSpace
The specific steps are as follows: importing data DATAZn into CiteSpace software (6.1. R6), setting ‘Time Slicing’ as ‘2007–2022′ with each slice representing ‘1′ year, selecting term sources such as ‘Title,’ ‘Abstract,’ ‘AuthorKeywords (DE),' and ‘KeywordsPlus,’ choosing node types as needed, while keeping the other settings at default values, generating a knowledge map of national (or regional) institutions or author collaborations automatically, and finally manually adjusting the map for clarity and aesthetics. Similarly, we created a keyword cluster map, with the only difference being the selection of nodes as 'Keywords' and the time slicing set as ‘2007–2022'. Furthermore, when generating citation timeline graphs using ‘Reference,’ we selected the ‘layout’ tab in the ‘control panel’ and continued to choose the ‘timeline view’ to generate the citation timeline graph. In the ‘control panel,’ under the 'Burstness' tab, we clicked ‘view’ to generate a burstness map for keywords, categories, or references.
2.2.2. Hiscite
We imported 632 research articles from DATAZn into HistCite Pro 2.1, set the ‘Limit’ to 30, kept other settings as default, selected ‘Make graph’, and generated the network of the DES research field to locate important literature quickly.
2.2.3. The alluvial generator
To generate the alluvial diagram, we utilize CiteSpace to generate a series of separate networks based on co-occurring keywords. These networks are exported from CiteSpace and loaded into the Alluvial Generator (http://www.mapequation.org/apps/AlluvialGenerator.html).
3. Results
3.1. Historical characteristics of the literature
3.1.1. Literature distribution characteristics
The changes in the quantity of scientific literature at specific time points can reveal the accumulation of knowledge in a particular research field, providing important parameters for us to grasp the development of the field quantitatively. A total of 632 relevant literature on zinc-based biodegradable metallic materials were retrieved, including 553 research articles, 50 reviews, and 29 other papers. In total, 1797 authors and 554 institutions participated in these publications, published in 187 different journals. These publications covered 33 research directions (Table 2).
Table 2.
Basic information on the distribution of the publications.
| Categories | Publications | Research articles | Review articles | Other papers | Authors | institutions | Journals | Subject Categories |
|---|---|---|---|---|---|---|---|---|
| Amount | 52 | 553 | 50 | 29 | 1797 | 554 | 187 | 33 |
The annual research output is shown in Fig. 2A. Sporadic publications on zinc-based biodegradable metallic materials emerged from 2007 to 2013, while the number of publications has shown a sharp increase every year from 2014 to 2022. Fig. 2B lists the top 20 journals with the highest productivity in zinc-based biodegradable metallic materials, serving as a reference for researchers when considering manuscript submissions. Among these, ACTA BIOMATERIALIA published the most papers (46), followed by MATERIALS SCIENCE AND ENGINEERING C MATERIALS FOR BIOLOGICAL APPLICATIONS (32), MATERIALS (29), and JOURNAL OF ALLOYS AND COMPOUNDS (27).
Fig. 2.
(A) The annual distribution of publications (blue columns). (B) The top 20 most fruitful journals (red columns). Numbers on the bar graphs indicate numbers of articles published.
3.1.2. Citation co-occurrence analysis of zinc-based biodegradable metal materials
The citation co-occurrence network graph displays the literature relationship in the field of zinc-based biodegradable metallic materials in the past dozen years (Fig. 3). A total of 769 nodes and 3989 links were present, indicating extensive connections among the literature in this research domain. In the early literature (2007–2016), nodes resembled the roots of a tree. Despite having a lower node density, clear clusters were formed, which provided the foundation for subsequent stages (2017–2022) of research. These later stages formed the main trunk of the tree, with dense node density and abundant connectivity, suggesting a concentration in the research field. Among them, Mostaed E (2018) [35], Mostaed E (2016) [36], and Yang HT (2017) [37] had cocitation frequencies of 148, 143, and 140, respectively. Liu XW (2015) [38] and Li HF (2015) [39] exhibited higher centrality. Furthermore, we utilized hisitePro 2.1 to generate the citation historiography of the research articles (Supplementary Figure). The milestone articles are highlighted in Table 3, with Bowen PK (2013) [40], Vojtech D (2011) [41], and Zheng YF (2014) [42] ranking as the top three local citation score (LCS) articles. Larger nodes indicate greater importance and more connections, resulting in higher intermediary centrality. By employing these two methods, we not only visualized the citation pulse structure of the literature but also focused on high-contributing articles in this field.
Fig. 3.
The citation co-occurrence network.
Table 3.
Information on the top 30 literature sorted by LCS score.
| NO. | Article information | Journal | LCS | GCS |
|---|---|---|---|---|
| 19 | Zinc Exhibits Ideal Physiological Corrosion Behavior for Bioabsorbable Stents [40] | ADV MATER | 324 | 513 |
| 15 | Mechanical and corrosion properties of newly developed biodegradable Zn-based alloys for bone fixation [41] | ACTA BIOMATER | 274 | 413 |
| 26 | Biodegradable metals [42] | MAT SCI ENG R | 224 | 1338 |
| 63 | Novel Zn-based alloys for biodegradable stent applications: Design, development and in vitro degradation [36] | J MECH BEHAV BIOMED | 184 | 243 |
| 126 | Zinc-based alloys for degradable vascular scent applications [35] | ACTA BIOMATER | 149 | 216 |
| 43 | Design and characterizations of novel biodegradable ternary Zn-based alloys with IIA nutrient alloying elements Mg, Ca and Sr [43] | MATER DESIGN | 147 | 182 |
| 51 | Structure, mechanical characteristics and in vitro degradation, cytotoxicity, genotoxicity and mutagenicity of novel biodegradable Zn–Mg alloys [44] | MAT SCI ENG C-MATER | 143 | 182 |
| 100 | Evolution of the degradation mechanism of pure zinc stent in the one-year study of rabbit abdominal aorta model [37] | BIOMATERIALS | 141 | 198 |
| 178 | The influence of alloying and fabrication techniques on the mechanical properties, biodegradability and biocompatibility of zinc: A comprehensive review [1] | ACTA BIOMATER | 134 | 213 |
| 59 | Biodegradable Metals for Cardiovascular Stents: from Clinical Concerns to Recent Zn-Alloys [45] | ADV HEALTHC MATER | 132 | 253 |
| 92 | Potential biodegradable Zn–Cu binary alloys developed for cardiovascular implant applications [46] | J MECH BEHAV BIOMED | 128 | 160 |
| 76 | Research on a Zn–Cu alloy as a biodegradable material for potential vascular stents application [47] | MAT SCI ENG C-MATER | 115 | 152 |
| 89 | Zn–Li alloy after extrusion and drawing: Structural, mechanical characterization, and biodegradation in abdominal aorta of rat [48] | MAT SCI ENG C-MATER | 113 | 130 |
| 53 | Micro-alloying with Mn in Zn–Mg alloy for future biodegradable metals application [49] | MATER DESIGN | 112 | 168 |
| 45 | In vitro biodegradation behavior, mechanical properties, and cytotoxicity of biodegradable Zn–Mg alloy [50] | J BIOMED MATER RES B | 104 | 136 |
| 84 | Design and characterizations of novel biodegradable Zn–Cu–Mg alloys for potential biodegradable implants [51] | MATER DESIGN | 99 | 122 |
| 56 | Effects of alloying elements (Ca and Sr) on microstructure, mechanical property and in vitro corrosion behavior of biodegradable Zn-1.5 Mg alloy [52] | J ALLOY COMPD | 98 | 123 |
| 123 | Novel high-strength, low-alloys Zn–Mg (<0.1 wt% Mg) and their arterial biodegradation [53] | MAT SCI ENG C-MATER | 96 | 113 |
| 46 | Metallic zinc exhibits optimal biocompatibility for bioabsorbable endovascular stents [54] | MAT SCI ENG C-MATER | 95 | 147 |
| 38 | Cytotoxicity evaluation of biodegradable Zn–3Mg alloy toward normal human osteoblast cells [55] | MAT SCI ENG C-MATER | 89 | 124 |
| 50 | Microstructure, mechanical properties, in vitro degradation behavior and hemocompatibility of novel Zn–Mg–Sr alloys as biodegradable metals [56] | MATERIALS LETTERS | 87 | 115 |
| 54 | In Vitro Cytotoxicity, Adhesion, and Proliferation of Human Vascular Cells Exposed to Zinc [57] | ACS BIOMATER SCI ENG | 87 | 109 |
| 44 | Endothelial Cellular Responses to Biodegradable Metal Zinc [58] | ACS BIOMATER SCI ENG | 85 | 122 |
| 127 | In vitro and in vivo studies on zinc-hydroxyapatite composites as novel biodegradable metal matrix composite for orthopedic applications [59] | ACTA BIOMATER | 84 | 135 |
| 83 | Structural Characteristics and In Vitro Biodegradation of a Novel Zn–Li Alloy Prepared by Induction Melting and Hot Rolling [60] | METALL MATER TRANS A | 81 | 95 |
| 144 | Indirectly extruded biodegradable Zn-0.05 wt%Mg alloy with improved strength and ductility: In vitro and in vivo studies [61] | J MATER SCI TECHNOL | 76 | 96 |
| 49 | Mechanical properties, in vitro degradation behavior, hemocompatibility and cytotoxicity evaluation of Zn-1.2 Mg alloy for biodegradable implants [62] | RSC ADV | 74 | 83 |
| 91 | Long-term surveillance of zinc implant in murine artery: Surprisingly, steady biocorrosion rate [63] | ACTA BIOMATER | 74 | 97 |
| 30 | Progress of biodegradable metals [64] | PROG NAT SCI-MATER | 73 | 234 |
| 215 | Challenges in the use of zinc and its alloys as biodegradable metals: Perspective from biomechanical compatibility [65] | ACTA BIOMATER | 72 | 100 |
LCS: local citation score.
GCS: global citation score.
The above literature consists of milestone articles in the field. Among them, the article by Vojtech D (2011) is the first detailed study on the mechanical properties and corrosion behavior of zinc alloys in simulated biological environments [41]. The results showed that the alloy had a maximum strength of 150 MPa and an elongation of 2 % when the Mg content was approximately 1 wt%. The corrosion rate of Zn–Mg alloy was significantly lower than that of Mg and AZ91HP alloys. Based on the corrosion behavior of zinc alloys, the possible zinc dosage and toxicity were estimated, and it was found that these dosages were negligible compared to the tolerable daily intake of zinc [41]. Bowen PK (2013) implanted pure zinc wires, 15 mm long, into the abdominal aorta of rats for a duration of 6 months. Pure zinc remained intact for up to 4 months or longer and then underwent accelerated corrosion, ensuring timely degradation of the implant. The study demonstrated, for the first time in animals, that compared to magnesium alloys with excessively fast degradation rates and the toxic side effects of iron alloy degradation products, zinc alloys have immense potential as bioabsorbable materials for cardiovascular stents [40]. Zheng YF (2014) provided the first definition and classification of biodegradable metals (BM), summarizing their microstructure, mechanical properties, degradation mechanisms, and in vivo studies. The paper also comprehensively discussed the future development directions and challenges from raw materials to semi-finished products to final medical device [42].
Li HF (2015) utilized important elements of bone matrix such as Mg, Ca, and Sr to ensure the biocompatibility of biomedical zinc alloys. This significantly improved the mechanical properties and corrosion behavior of the alloy. Zn–1Mg, Zn–1Ca, and Zn–1Sr alloys were implanted into the femurs of mice, demonstrating the immense potential of different heat-treated Zn-1X alloys as bioabsorbable implants for orthopedic applications [39]. Liu XW (2015) conducted a systematic study on the mechanical properties, in vitro degradation behavior, and biocompatibility of ultra-pure zinc (10mm10 m m2mm) and its microtubes (outer diameter 2 mm, wall thickness 0.15 mm). Compared to high-purity Mg, ultra-pure Zn exhibited similar mechanical properties and cytotoxicity, while its corrosion rate in Hank's solution (0.011 mm/y) was lower than that of pure Mg. Additionally, the hemolysis rate (1.00 %) was lower than 5 %, indicating no significant damage to red blood cells. Compared to flat samples, tubular samples exhibited higher corrosion rates (Zn 0.028–0.037 mm/y, Mg 0.61–0.73 mm/y) and blood compatibility (Zn 1.19 %, Mg 9.50 %). These findings demonstrated that zinc is a promising candidate material for degradable metal scaffolds. However, alloying and advanced processing techniques are necessary to improve the mechanical properties of UP-Zn [38].
Mostaed E (2016) systematically studied the microstructure, mechanical properties, corrosion behavior, and feasibility of microtube processing for Zn–Mg alloys and low-Al content Zn–Al alloys. Laser cutting was used to investigate the microstructural changes that could potentially affect the mechanical properties of the final product [36]. The results showed that extruded alloys exhibited slightly better corrosion resistance compared to cast alloys, with corrosion rates approximately half of those of standard purity Mg alloys. Hot extrusion transformed the corrosion state of Zn–Mg alloy from localized pitting to more uniform corrosion. Zn-0.5 Mg exhibited good strength, ductility, strain hardening index, and appropriate mechanical integrity loss during degradation, making it the most promising material for scaffolds. It was also possible to fabricate tubes with an outer diameter of 4 mm and inner diameter of 1.5 mm as precursors for biodegradable scaffolds. EBSD analysis near the laser-cut Zn-0.5 Mg tubes showed no grain coarsening or texture change, confirming that the grain size and texture orientation of the final scaffold remained unchanged [36]. Yang HT (2017) implanted pure zinc stents (3.0*10 mm, wall thickness 165 mm) into a rabbit abdominal aortic model, demonstrating their mechanical integrity over a period of 6 months. After 12 months, the volume of the stent decreased by 41.75 ± 29.72 %. The degraded pure zinc stents exhibited good biocompatibility, proving that the inherent characteristics of zinc matched the arterial healing process [37]. Mostaed E (2018) further reviewed the effects of different alloy compositions and processing techniques on the microstructure, mechanical properties, corrosion behavior, and in vivo performance, discussing their suitability for biodegradable vascular scaffolds [35].
An ideal biodegradable metallic material in the field of biomedical research should possess a degradation rate that matches tissue healing, sufficient mechanical properties, and good biocompatibility. The evaluation of whether a biodegradable metallic material can meet clinical needs gradually progresses from in vitro exploration to in vivo research. Throughout this process, continuous optimization of alloy composition and processing technology is carried out, enabling the transformation of raw materials into semi-finished products and ultimately into clinically useable finished medical devices. From these milestone articles, it can be seen that the development of zinc alloy as a biodegradable material for cardiovascular stents can be traced back to different stages. Initially, research focused on studying the mechanical and corrosion properties of the alloy in simulated biological environments in vitro. Subsequently, pure zinc wires were implanted into the abdominal aorta of rats to investigate zinc's corrosion behavior in vivo and explore its biocompatibility. Further studies examined the mechanical properties, in vitro degradation behavior, biocompatibility, and the feasibility of laser cutting for ultra-pure zinc and zinc alloy microtubes. Finally, the safety and effectiveness of zinc stents were validated by implanting them into the abdominal aorta of rabbits. These series of studies gradually demonstrated the appropriate degradation behavior, biocompatibility, and processability of zinc alloys. Through the application of alloying and processing strategies, the mechanical properties of zinc alloys have been improved, making them suitable materials for bioabsorbable heart stents. It is worth noting that the ultimate tensile strength of pure zinc varies from 20 MPa to 120 MPa depending on the processing technique, which is far below the required 300 MPa for vascular stents and orthopedic fixation devices [66,67]. Therefore, alloying and processing strategies are essential. Through these strategies, grain refinement, control of second-phase particle size and distribution, and favorable crystallographic orientation can be achieved, enabling zinc alloys to gradually meet the clinical needs of vascular stents and orthopedics [65]. This ensures good biocompatibility while providing sufficient mechanical properties and controllable degradation rate.
In addition to investigating the mechanical properties, biocompatibility, and degradation rate of alloys, it is important to consider the biological activity of the alloys. For example, the incorporation of Mg, Ca, and Sr can not only improve the mechanical properties of the alloy but also potentially induce bone formation [43]. The introduction of Cu can promote the proliferation of endothelial cells and vascular regeneration [46]. Li can be beneficial for nerve protection [68], while Ag can kill bacteria attached to the surface of implants or prevent their adhesion [69]. Considering the multifunctionality of alloys would be advantageous for local tissue reconstruction.
3.1.3. Scientific cooperation
As shown in Fig. 4, many nodes and abundant connections indicate strong scientific collaborations in terms of country, institution, and author dimensions. The national collaboration network consists of 45 nodes and 116 edges, with China, the United States, Germany, Australia, and the Czech Republic being the largest nodes (Fig. 4A). The institution collaboration network exhibits 271 nodes and 572 edges, with Peking University, Beijing University of Science and Technology, Shanghai Jiao Tong University, Northeastern University, and the Chinese Academy of Sciences as the largest nodes, as shown in Fig. 4B. In Fig. 4C, the author's co-authorship graph displays 396 nodes and 1233 edges. Zheng Yufeng, Goldman Jeremy, Vojtech Dalibor, Drelich Jaroslaw W, and Capek Jaroslav are leading in terms of publication quantity in this field, and their research findings have played a crucial role in advancing the field of biodegradable medical zinc alloys, as mentioned in the previous section.
Fig. 4.
The scientific cooperation network. (A) Country cooperation. (B) Institution cooperation. (C) Author cooperation, i: Author clustering as represented by Zheng Yufeng; ii: Author clustering as represented by Vojtech Dalibor; iii: Author clustering as represented by Goldman Jeremy; iv: Author clustering as represented by Wang Luning. Number: frequency of co-occurrence.
It is worth noting that clustering effects can be observed within author nodes. For instance, a cluster is formed by Zheng Yufeng and Zhu Donghui nodes (Fig. 4C–i); another cluster is formed by Vojtech Dalibor, Capek Jaroslav, Kubasek Jiri, and Pinc Jan nodes (Fig. 4C–ii); Goldman Jeremy and Drelich Jaroslaw W nodes form another cluster (Fig. 4C–iii); and Wang Lu-Ning, Zhang Hai-Ju, and Shi Zhang-Zhi nodes form yet another cluster (Fig. 4C–iv), and authors within each cluster have strong academic communication and collaboration.
3.2. Variation of the most active topics
3.2.1. Subject category burst
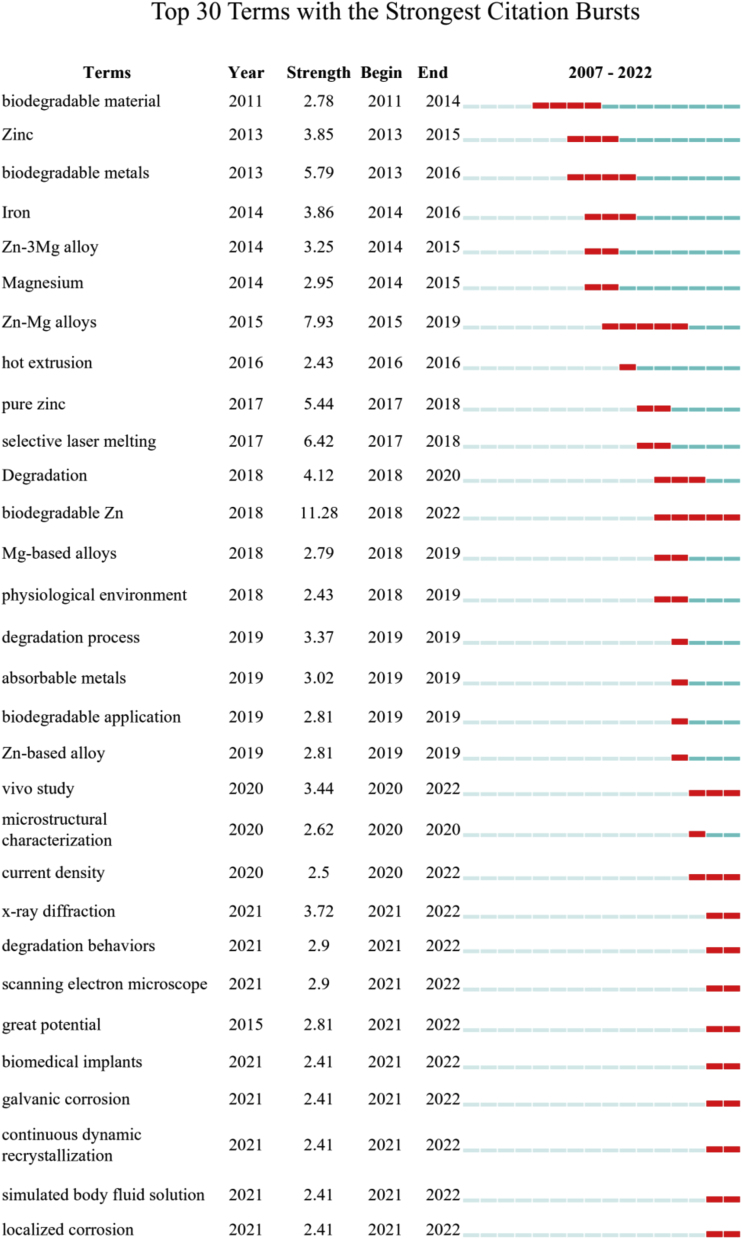
From 2007 to 2022, 45 of the 520 related subject categories experienced citation bursts. The blue line represents the time interval, and the red line segments indicate the time span of citation bursts, including the starting and ending years. Fig. 5 presents the top 30 terms with the highest burst intensity in different periods. The subject category burst progressed from “biodegradable material” (2011) to “biodegradable metals” (2013), “zinc” (2013), “magnesium” (2014), “iron” (2014), and further to “biodegradable Zn” (2018). This progression indicates that zinc-based biodegradable metallic materials have evolved from the foundations of magnesium and iron. The in vitro mechanical properties, degradation rate, biocompatibility, as well as the biological responses and effectiveness in vivo of zinc alloys can be referenced based on the research foundations of magnesium and iron [70]. For instance, when investigating the implantation of zinc wires into the abdominal aorta of rats, prior experiments had already implanted magnesium and iron into the abdominal aorta of rats and validated the feasibility of these materials as absorbable vascular stents. It is worth noting that in vitro research methods cannot replace relevant in vivo studies currently. For example, there is still no satisfactory medium in vitro to simulate the anti-fatigue performance of zinc alloys in vivo. In vitro studies cannot simulate the complex degradation environment in vivo either. Moreover, due to the characteristics of in vivo research, the quantitative and qualitative analysis of degradation products of zinc alloys in vivo is challenging, thus warranting further investigation into the analysis of zinc alloy degradation products and their metabolism and transportation processes in vivo.
Fig. 5.
The top 30 subject categories with the strongest citation bursts. Year: Year of the first occurrence, Strength: Burst's strength, Begin: Burst's beginning year, nd: Burst's ending year.
Fig. 5 also demonstrates that topic emergence transitioned from “hot extrusion” (2016), “selective laser melting” (2017), “degradation process” (2019), to “in vivo study” (2020), “galvanic corrosion” (2021), and “continuous dynamic recrystallization” (2021). This illustrates the evolution of research on zinc-based biodegradable metallic materials from macroscopic properties to a more in-depth exploration of microscopic level and in vivo experiments. Understanding how the alloy microstructure, such as grain and second phase particle size, quantity, shape, distribution, and crystallographic orientation [66], as well as the density and distribution of alloy dislocations, the solubility of alloying elements with zinc metal atoms, and the influence of microstructure (e.g., dynamic recrystallization) [65] on the mechanical properties, degradation rate, and biocompatibility of the product during deformation, shelf life, and implantation degradation processes, is crucial to determine the usability of the developed products. The validation of product safety, effectiveness, and usability through relevant in vivo studies combining large animals with small animals is necessary to accelerate the industrialization of zinc alloys. Furthermore, the 15 topics from 2022 (Table S2) such as “in vivo study,” “current density,” “biomedical implants,” and “continuous dynamic recrystallization” further substantiate the aforementioned observations.
3.2.2. Keywords burst
At a more granular level, keyword bursts were detected to reveal the research progress of zinc-based biodegradable metallic materials across the entire time span (2007–2022). A total of 76 keywords experienced bursts at different time points, with the top 30 keywords exhibiting the highest burst intensities, as shown in Fig. 6. The keyword ‘biodegradable material’ had its peak burst intensity between 2011 and 2015, reaching a value of 3.07. During this time period, researchers have shown a strong interest and emphasis on biodegradable materials. ‘Magnesium alloy’ experienced the highest burst intensity from 2013 to 2017, with a value of 3.91, indicating that magnesium alloys are being prioritized in the development of biodegradable metal materials at this time. ‘Biodegradable stent’ and ‘bioabsorbable stent’ had the highest burst intensities during 2014–2016 and 2015–2018, reaching values of 2.9 and 4, respectively. ‘Elements Mg’ and ‘alloying elements Mg’ exhibited the highest burst intensities during 2016–2019 and 2018–2019, with values of 5.92 and 3.51, respectively. ‘Cardiovascular stent’ had the highest burst intensity in 2017–2018, with a value of 4.01, while the keywords ‘binary alloy’, ‘texture’, and ‘dynamic recrystallization’ had the highest burst intensities during 2019–2020, 2020–2020, and 2021–2022, respectively. The process and intensity of the emergence of these key terms reveal the development trends and evolutionary path of zinc-based biodegradable metal materials research. Researchers have gradually shifted their focus from magnesium alloys to zinc alloys as candidates for biodegradable metals. Simultaneously, they have expanded the scope of their research applications to a wider range of fields, while optimizing alloy elements and controlling the material's microstructure.
Fig. 6.
The top 30 keywords with the strongest citation bursts.
In addition, we specifically focused on 21 key terms that continued to emerge until 2022, as they could represent potential future research hotspots. These key terms include “in vitro corrosion,” “dynamic recrystallization,” “degradation behavior,” “microstructure evolution,” “fracture,” “phase,” “dislocation,” “osteogenic differentiation,” “product,” and “interface” (Table S2). This suggests that alloy processing techniques, compositional elements, changes in the internal microstructure of alloys during use (fracture, phase, dislocation, dynamic recrystallization), studies on alloy degradation behavior, functional activity of alloys, and interface research could now, and perhaps in the forthcoming years, become focal points of research for zinc-based biodegradable metallic materials.
To meet the requirement that absorbable cardiac stents remain mechanically intact within 3–6 months after implantation and are fully absorbed within 12–24 months, research has gradually shifted from early magnesium alloys to zinc alloys with slower degradation rates. The suitable degradation rate of zinc alloys makes them the most promising biodegradable metal materials for absorbable cardiac stents. Meanwhile, to achieve a tensile strength exceeding 300 MPa, alloying becomes an effective approach to enhance the mechanical properties of zinc alloys. How to ensure sufficient elongation at break (at least 20 %) of zinc alloy on the premise of realizing the required strength has become a hot issue of current research. Changes in grain structure, secondary phases, interfaces, and crack filling serve as guidance for the selection of alloying elements and optimization of processing techniques, which are key factors for zinc alloys to meet clinical requirements. Additionally, during the product development process, consideration should be given to strain softening, creep, and room temperature aging caused by dynamic recrystallization.
3.2.3. Reference burst
Through calculation, a total of 80 burst articles were identified. Table 4 presents the top 30 most frequently cited articles from 2007 to 2022. The first article to experience a citation burst was by Vojtech D (2011), which generated significant interest upon its publication with a burst intensity of 28.54. This study continued for 4 years from 2013 to 2016 and provided a comprehensive investigation into the mechanical properties and corrosion behavior of Zn alloys in simulated biological environments [41]. Bowen PK (2013) had a burst intensity of 47.68 from 2014 to 2018, as it examined zinc as a biodegradable material for cardiac stent applications. The authors implanted zinc wires in the abdominal aortas of rats and demonstrated suitable degradation time and good biocompatibility [40]. Zheng YF (2014) experienced its third burst period from 2015 to 2019, with a burst intensity of 37.35. This article introduced the definition and classification of biodegradable metals and summarized the degradation mechanisms and environmental influencing factors, including degradation product metabolism and mechanical integrity degradation. It focused on microstructures, mechanical properties, degradation behavior, in vitro and in vivo performance, as well as preclinical and clinical trials [42]. From 2016 to 2020, Li HF (2015) had its fourth burst period with a burst intensity of 27.59. To enhance their mechanical properties, this study investigated Zn-1X (Mg, Ca, and Sr) alloys under different thermal processing conditions (rolling and extrusion). The microstructure, mechanical properties, corrosion behavior, blood compatibility, in vitro cell compatibility, and in vivo biocompatibility were systematically studied to explore their feasibility as biodegradable implants in orthopedic applications [39]. In 2022, 3 articles emerged, two focused on in vivo studies, and one investigated the influence of alloy immersion media on cellular toxicity. This indicates that biocompatibility research, particularly in vivo studies, has become a hotspot for Zn alloys [[71], [72], [73]]. The endpoint literature obtained from both the reference burst analysis and co-occurrence analysis exhibits a high level of consistency, confirming that these selected articles are key reference sources in the field (see Table 5).
Table 4.
The top 30 references with citation bursts at different period.
| References | Year | Strength | Begin | End | 2007–2022 |
|---|---|---|---|---|---|
| Vojtech D, 2011, ACTA BIOMATER, V7, P3515, DOI 10.1016/j.actbio.2011.05.008, DOI [41] | 2011 | 28.54 | 2013 | 2016 | |
| Pierson D, 2012, J BIOMED MATER RES B, V100B, P58, DOI 10.1002/jbm.b.31922, DOI [74] | 2012 | 10.66 | 2013 | 2017 | |
| Moravej M, 2011, INT J MOL SCI, V12, P4250, DOI 10.3390/ijms12074250, DOI [75] | 2011 | 8.21 | 2013 | 2016 | |
| Bowen PK, 2013, ADV MATER, V25, P2577, DOI 10.1002/adma.201300226, DOI [40] | 2013 | 47.68 | 2014 | 2018 | |
| Cheng J, 2013, J MATER SCI TECHNOL, V29, P619, DOI 10.1016/j.jmst.2013.03.019, DOI [76] | 2013 | 10.07 | 2014 | 2018 | |
| Zheng YF, 2014, MAT SCI ENG R, V77, P1, DOI 10.1016/j.mser.2014.01.001, DOI [42] | 2014 | 37.35 | 2015 | 2019 | |
| Chen YJ, 2014, ACTA BIOMATER, V10, P4561, DOI 10.1016/j.actbio.2014.07.005, DOI [77] | 2014 | 10.12 | 2015 | 2019 | |
| Li HF, 2015, SCI REP-UK, V5, P0, DOI 10.1038/srep10719, DOI [39] | 2015 | 27.59 | 2016 | 2020 | |
| Li HF, 2015, MATER DESIGN, V83, P95, DOI 10.1016/j.matdes.2015.05.089, DOI [66] | 2015 | 22.38 | 2016 | 2020 | |
| Bowen PK, 2015, MAT SCI ENG C-MATER, V56, P467, DOI 10.1016/j.msec.2015.07.022, DOI [49] | 2015 | 15.79 | 2016 | 2020 | |
| Yao CZ, 2014, J ALLOY COMPD, V602, P101, DOI 10.1016/j.jallcom.2014.03.025, DOI [78] | 2014 | 14.44 | 2016 | 2019 | |
| Murni NS, 2015, MAT SCI ENG C-MATER, V49, P560, DOI 10.1016/j.msec.2015.01.056, DOI [50] | 2015 | 13.68 | 2016 | 2020 | |
| Kubasek J, 2016, MAT SCI ENG C-MATER, V58, P24, DOI 10.1016/j.msec.2015.08.015, DOI [67] | 2016 | 13.3 | 2016 | 2019 | |
| Li HF, 2014, PROG NAT SCI-MATER, V24, P414, DOI 10.1016/j.pnsc.2014.08.014, DOI [59] | 2014 | 13.23 | 2016 | 2019 | |
| Ma J, 2015, ACS BIOMATER SCI ENG, V1, P1174, DOI 10.1021/acsbiomaterials.5b00319, DOI [53] | 2015 | 12.28 | 2016 | 2020 | |
| Liu XW, 2015, MATER LETT, V161, P53, DOI 10.1016/j.matlet.2015.06.107, DOI [38] | 2015 | 12.2 | 2016 | 2018 | |
| Liu XW, 2016, MATER LETT, V162, P242, DOI 10.1016/j.matlet.2015.07.151, DOI [51] | 2016 | 10.52 | 2016 | 2019 | |
| Liu XW, 2016, MATER DESIGN, V94, P95, DOI 10.1016/j.matdes.2015.12.128, DOI [69] | 2016 | 9.28 | 2016 | 2019 | |
| Dambatta MS, 2015, MATER DESIGN, V85, P431, DOI 10.1016/j.matdes.2015.06.181, DOI [79] | 2015 | 8.84 | 2016 | 2019 | |
| Seitz JM, 2015, ADV HEALTHC MATER, V4, P1915, DOI 10.1002/adhm.201500189, DOI [80] | 2015 | 6.93 | 2016 | 2020 | |
| Wang JL, 2015, ACTA BIOMATER, V21, P237, DOI 10.1016/j.actbio.2015.04.011, DOI [81] | 2015 | 6.47 | 2016 | 2020 | |
| Mostaed E, 2016, J MECH BEHAV BIOMED, V60, P581, DOI 10.1016/j.jmbbm.2016.03.018, DOI [36] | 2016 | 16.94 | 2017 | 2019 | |
| Bowen PK, 2016, ADV HEALTHC MATER, V5, P1121, DOI 10.1002/adhm.201501019, DOI [65] | 2016 | 15.17 | 2017 | 2019 | |
| Gong HB, 2015, J BIOMED MATER RES B, V103, P1632, DOI 10.1002/jbm.b.33341, DOI [44] | 2015 | 14.74 | 2017 | 2020 | |
| Wang C, 2016, J MATER SCI TECHNOL, V32, P909, DOI 10.1016/j.jmst.2016.06.003, DOI [82] | 2016 | 10.47 | 2017 | 2019 | |
| Chen QZ, 2015, MAT SCI ENG R, V87, P1, DOI 10.1016/j.mser.2014.10.001, DOI [83] | 2015 | 8.43 | 2017 | 2020 | |
| Guillory RJ, 2016, ACS BIOMATER SCI ENG, V2, P2355, DOI 10.1021/acsbiomaterials.6b00591, DOI [84] | 2016 | 7.24 | 2017 | 2019 | |
| Shearier ER, 2016, ACS BIOMATER SCI ENG, V2, P634, DOI 10.1021/acsbiomaterials.6b00035, DOI [52] | 2016 | 7.07 | 2017 | 2020 | |
| Liu XW, 2016, J ALLOY COMPD, V664, P444, DOI 10.1016/j.jallcom.2015.10.116, DOI [47] | 2016 | 6.4 | 2017 | 2018 | |
| Niu JL, 2016, MAT SCI ENG C-MATER, V69, P407, DOI 10.1016/j.msec.2016.06.082, DOI [46] | 2016 | 7.22 | 2018 | 2020 |
Table 5.
References with citation bursts from beginning to 2022.
| References | Year | Strength | Begin | End |
|---|---|---|---|---|
| Li P, 2019, ACTA BIOMATER, V98, P235, DOI 10.1016/j.actbio.2019.03.013, DOI[71] | 2019 | 4.68 | 2020 | 2022 |
| Zhang Y, 2019, MAT SCI ENG C-MATER, V99, P1021, DOI 10.1016/j.msec.2019.01.120, DOI [72] | 2019 | 3.91 | 2020 | 2022 |
| Kafri A, 2018, J MATER SCI-MATER M, V29, P0, DOI 10.1007/s10856-018-6096-7, DOI[73] | 2018 | 2.85 | 2020 | 2022 |
In order to meet the clinical requirements for vascular stents and orthopedic applications, the optimization and characterization of zinc alloys are constantly evolving. From the burst of three articles in 2022, researchers have become aware that the biological environment of zinc alloys varies depending on the implanted part, such as vascular stents coming into contact with serum, hemoglobin, blood cells, etc., which may have an impact on the release of zinc ions and thus lead to differences in cytotoxicity results compared to simple PBS solutions [71]. Although corrosion rate measurements are mainly carried out in Hank's solution at present, referencing ASTM standard G3172 from 2004, it cannot simulate the complex corrosion environment within the human body, and the referenceability of the obtained corrosion rate is limited. It is foreseeable that zinc alloy leachate and degradation media will be continuously optimized for different implant sites. In addition, researchers have also realized that adjusting the types and amounts of alloying elements to form a microcell structure inside the alloy may expand the application of zinc alloys in scenarios where slightly faster degradation rates are required. After more than a decade of research, with the continuous optimization of processing technology and the in-depth study of ternary alloys, more and more zinc alloys that meet clinical requirements have been prepared, such as Zn-0.8%Li-0.2%Mg, Zn-0.8%Li-0.2%Ag, and Zn-0.8Li-0.8Mn, whose ultimate tensile strengths (UTS) are 341.3 MPa, 254.7 MPa, and 515.0 MPa, respectively; yield strengths (YS) are 253.7, 196.2, and 365.0, respectively; and elongations are 30.6 %, 97.9 %, and 103.3 %, respectively [72,85]. The increase in the ultimate tensile strength of these ternary zinc alloys is mainly attributed to nanoprecipitates and grain refinement, while the increase in elongation primarily attributes to grain boundary sliding, dynamic recrystallization, and the grain boundary effect. Among these, the grain boundary effect mainly contributes to enhancing elongation by carrying stress concentration and facilitating crack propagation. In addition, when optimizing the mechanical properties of zinc alloys, the proportion and distribution of brittle phases after the addition of alloying elements should also be considered. As more and more zinc alloy materials that meet clinical requirements are gradually developed, more and more zinc alloy orthopedic and vascular stent products will enter into large animal studies and clinical validations, providing a basis for product launch.
3.3. Emerging trends and new developments
3.3.1. Keyword clustering analysis
There are strong intrinsic correlations among the keywords, and certain keywords can form different clusters based on their affinity. Identifying these clusters provides a more intuitive delineation of the various sub-fields within the research on zinc-based biodegradable metallic materials. The keyword clustering is shown in Fig. 7A. The keyword clustering can be divided into five categories, including traditional alloy composition and microstructure (#2 iron, #8 microstructure), degradation characteristics (#3 biodegradable metal, #6 corrosion resistance), alloy mechanical properties and antibacterial properties (#0 mechanical properties, #1 plastic deformation, #9 antibacterial activity), additive manufacturing processes for alloys (#4 additive manufacturing) and surface modification (#5 surface modification) and activation (#7 activation) related to alloy applications. As shown in Fig. 7B, #0 mechanical performance and #9 antibacterial activity are fundamental requirements for biodegradable materials, and therefore run through the entire development process. Due to its difficulty in degradation, #2 iron has not been overcome, leading to a decrease in research enthusiasm. After experiencing materials such as biodegradable polymers, iron, and magnesium, the selection of #3 biodegradable materials gradually focused on zinc alloys, thus temporarily passing the explosive period. The corrosion resistance of #6 and the microstructure of #8 are in a sustained but not hottest research stage because zinc alloys have been in the selection and development of alloying elements for a long time. Currently, the more ideal alloys selected are being attempted for #1 plastic deformation, #5 surface modification, and #4 additive manufacturing to improve biocompatibility (#7 activation).
Fig. 7.
Keyword cluster snapshots. (A) Clustering form. (B) Timeline form. The Y-axis represents the frequency of occurrence.
Additive manufacturing facilitates the construction of three-dimensional porous structures resembling human bones, which meet the requirements for internal transport of nutrients, tissue fusion, and regeneration in orthopedic implants. The rapid high-temperature heating and cooling process ensures finer grain size and guarantees the mechanical properties of the material. With the optimization of powder, laser, and laser melting space environment, the density of orthopedic implants made from zinc alloys through additive manufacturing has been addressed. Research on the preparation of orthopedic implants using zinc alloys through additive manufacturing will continue to increase. However, further investigation is needed to determine whether their degradation rate and biocompatibility can meet clinical demands. Whether it is for orthopedic materials or vascular stents, achieving tissue fusion and repair while ensuring product biocompatibility is a crucial indicator. Surface modification can functionalize zinc alloy materials, making them “third-generation biodegradable materials.” For example, during the early stages of implantation, surface-modified coatings on zinc alloys control the release rate of zinc ions, increase the biocompatibility of the product, induce bone tissue climbing and endothelialization processes, prevent inflammatory reactions in orthopedic implants, and reduce the probability of clotting in vascular stents. Once they have served their purpose, these coatings can also promote the rapid degradation of the alloy to prevent excessive proliferation of bone tissue or smooth muscle cells and reduce the occurrence of complications.
3.3.2. Keyword alluvial analysis
As shown in Fig. 8, the linked keywords can be combined to form specific research modules. As keywords are recombined, the research modules diverge or converge over time, resulting in new modules. Some influential keywords have a strong lifespan throughout the 12-year period, while others become new research trends, and some fade out from the long history of the research field. Table S4 (Supplementary Material) lists the top five modules with the highest traffic each year. Clearly, in 2022, the keywords contained in Module 4 diverge or converge within our research domain, forming the largest research branch (highlighted in purple). This indicates that Module 4 is the most persistent research module. Additionally, we plotted all the keywords for the top six modules ranked in 2022 (Fig. 9). Fig. 9 shows that Module 4 pertains to the “mechanical property,” signifying a sustained focus on the study of the mechanical properties of zinc-based biodegradable materials. This is related to the fact that the mechanical properties of zinc alloy materials are continuously being optimized to meet clinical demands. It wasn't until recent years that zinc alloys with mechanical properties that truly meet clinical requirements were gradually developed.
Fig. 8.
The keyword alluvial map 2010–2022. X-axis: Time slice. Y-axis: Counting of modules. Number: Order of modules on each time slice sorted by the number of nodes.
Fig. 9.
The keywords of the top 6 modules in 2021. (A) Module 1. (B) Module 2. (C) Module 3. (D) Module 4. (E) Module 5. (F) Module 6.
Module 1 is named ‘laser’ and consists of 11 keywords, including laser, laser powder bed fusion, and steel (Fig. 9A). Module 2 is named ‘strain softening’ and comprises 22 keywords, such as strain softening, plastic deformation, and cytotoxicity (Fig. 9B). Module 3 is named ‘zn implant’ and includes 21 keywords, such as zn implant, composite, and steel (Fig. 9C). Module 4 is named ‘mechanical property’ and consists of 14 keywords, including mechanical property, Mg, and cytotoxicity (Fig. 9D). Module 5 is called ‘in situ reaction’ and comprises nine keywords, such as in situ reaction, selective laser melting, and particle (Fig. 9E). Module 6 is named ‘absorbable metal scaffold’ and includes 12 keywords, such as absorbable metal scaffold, cell viability, and blood compatibility (Fig. 9F). These modules are likely to represent emerging trends in the field of Zn alloys for the next five years or even longer. Based on the above findings, additive manufacturing, strain softening, mechanical property research, implantable materials, and absorbable scaffolds are likely to be emerging trends in the zinc alloy field for the next 5 years or even longer.
As mentioned earlier, the study of mechanical properties of zinc alloys is a long-standing topic, and additive manufacturing, with its flexible processing characteristics, will also become a useful tool for the development of zinc alloy materials, promoting research progress in orthopedic implants and even absorbable stents. It is worth noting that strain softening will limit the application of zinc alloys, and controlling dislocation motion, interface sliding, and dynamic recrystallization in the alloy is a key to control strain softening. The introduction of high-density twinning and coarse grains can suppress strain softening, but considering the impact of strain softening on the product during the product development process is essential.
Simultaneously, we can observe that the term “cell viability/cytotoxicity” appears frequently in different modules, indicating the importance of evaluating cytotoxicity in the medical device field. However, the numerous published cell compatibility test results of zinc alloys currently available demonstrate that the zinc alloy leachate needs to be diluted 2–4 times to exhibit non-toxicity, which is related to the material's degradability [86]. Establishing a universally applicable cytotoxicity evaluation method for degradable metals is also a key area for future research.
3.3.3. Timeline visualization of references
Based on the timeline visualization of citation span, emerging, classical, and relatively outdated topics were predicted. The timeline graph of research on zinc-based biodegradable metal materials consists of 10 clusters within a given timeframe, arranged from top to bottom according to their sizes (Fig. 10A). Among them, the #1 biodegradable stent and #6 Zn–Mg alloys are classical topics, which may not be the latest ones but have intricate connections with other clusters. #8 biodegradable material and #14 equiaxed and dendritic grains are relatively outdated topics, with limited connections to other clusters and no further development on their timelines. The #0 microstructure, #2 sem, #3 additive manufacturing, #4 laser powder bed fusion, #5 implant, and #7 Zn–1Mg are emerging subjects, as their timelines have remained active since their appearance, indicating that these areas will become future research hotspots. Table S5 provides more detailed descriptions of newly emerged clusters. Additionally, some pivotal papers (large nodes with red rings) played a significant role in advancing this subfield.
Fig. 10.
The reference cluster map. (A) Citation timeline visualization. (B) Citation frequency distribution of the burst citations, X-axis: year, Y-axis: citation frequency.
The article published by Mostaed E in 2018 belongs to cluster #0 and has been cited 148 times. The author focused on the development of Zn alloys from 2015 to 2017, based on a review conducted in 2016. Starting from the effects of elemental composition and processing techniques on microstructural features (grain refinement, control of second phase particle size and distribution, and good crystal structure orientation), the suitability of different alloy compositions and treatment techniques for the application of biodegradable vascular stents was discussed in terms of mechanical properties, corrosion behavior, and in vivo performance. The author concluded that extensive research is needed in the development of bioabsorbable Zn alloys to obtain appropriate compositions that meet strict clinical standards for vascular stent applications, such as good biocompatibility, prolonged mechanical integrity, and controlled corrosion rate [35]. The literature published by Yang HT in 2018 belongs to cluster #2 and has a co-occurrence frequency of 84. The study focused on the preparation of metal matrix composites (MMCs) using pure zinc as the matrix and hydroxyapatite (HA) as the reinforcement through spark plasma sintering (SPS). Adding HA to pure zinc was an effective way to regulate its degradation rate and enhance its in vitro and in vivo biocompatibility. In vivo experiments showed better osteogenic effects with the addition of HA over prolonged implantation time. This study demonstrated that zinc-based MMCs are a promising strategy to improve the application performance of pure zinc in orthopedic implants. It is worth mentioning that the author applied SEM techniques in various aspects of the research, such as immersion tests, hemolysis and platelet adhesion, and cross-sectional analysis [59]. In the same year, the literature published by Hermawan H belongs to cluster #5 with a co-occurrence frequency of 38. The author summarized the advances in absorbable metal research progress from 3 aspects: fundamental research, translational research, and standard development. In discussing the use of porous structures as a method to control the corrosion rate, the author suggested the benefits of additive manufacturing for the development of orthopedic materials [87]. The literature published by Han HS in 2019 belongs to cluster #7 with a co-occurrence frequency of 41. The author provided a comprehensive review of the research progress on biodegradable metals in the medical field, focusing on product translation research and clinical trials. Research on the application of biodegradable metals in the orthopedic and cardiovascular fields has been highlighted [88]. We further analyzed the citation distribution of these four articles in recent years (Fig. 10B) and predicted that they may continue to be referenced in the coming years.
The type and content of alloying elements, processing, and heat treatment processes can change the microstructural characteristics of zinc alloys, thereby affecting the degree of obstruction of dislocation motion and stress concentration, and thus changing the ultimate tensile strength and ductility of zinc alloy materials. Similarly, we can also use the microstructural characteristics of zinc alloys to guide us in selecting alloying elements, processing and heat treatment processes to obtain zinc implant materials that meet clinical needs. In this process, guidelines for standardizing zinc alloys are gradually making progress [45], linking the optimization of material alloying elements, processing and heat treatment processes, in vitro performance research, and in vivo research, thereby solving the remaining challenges in the industrialization process of zinc alloys and benefiting patients. In the standardization process, SEM is an essential means of observing the degradation of zinc alloys, platelet adhesion, endothelialization, inflammation response, and restenosis in in vitro and in vivo environments. Although biodegradable zinc alloys have achieved many milestone results, more basic research is still needed as evidence to prove their clinical applicability.
4. Summary and outlook
4.1. Ongoing challenges and emerging themes coexist in the field of Zn alloys biodegradability
Based on bibliometric analysis, this study reviews the structural and temporal characteristics of publications related to biodegradable Zn alloys from 2007 to 2022. Undoubtedly, the field of biodegradable Zn alloys is still in a prominent phase with a rapid increase in the number of papers, extensive scientific collaboration, and an intensive citation network. While the active topics in this field have evolved over time, the analysis of recent subjects, keywords, keyword clustering, and reference clustering indicates that in vivo-related studies (e.g., vivo study in Terms and #5 implant in reference clustering), degradation performance research (e.g., current density, degradation behaviors in Terms and in vitro corrosion, degradation behavior in keywords), microstructural investigations (e.g., X-ray diffraction, scanning electron microscope, fracture, phase, interface in Terms; #8 microstructure in keyword clustering; #0 microstructure, #2 sem in reference clustering), dynamic recrystallization (e.g., continuous dynamic recrystallization in Terms; dynamic recrystallization in keywords clustering), additive manufacturing (e.g., 4# additive manufacturing in keyword clustering; #3 additive manufacturing, #4 laser powder bed fusion in reference clustering), surface modification and activation (e.g., 5# surface modification, 7# activation in keyword clustering) have the potential to become future research hotspots. Recently, cited literature has also revolved around these hot topics.
4.2. Explore emerging fields
Microstructure Research. Alloying elements are crucial factors influencing the mechanical properties, corrosion rate, and biocompatibility of alloys. Previous studies have demonstrated that the ultimate tensile strength of Zn–Mg and Zn–Li alloys is significantly improved, while the addition of Ag, Cu, Fe, Mn, and Zr can enhance the fracture elongation of the alloys [85,[89], [90], [91]]. Second-phase strengthening, grain refinement strengthening, and heterogeneous deformation-induced strengthening are the major strengthening mechanisms in Zn alloys [90,92,93]. The second phase can strengthen the metal either through load transfer mechanisms or by acting as obstacles to dislocation motion. Grain refinement strengthening arises from the hindrance of dislocation motion at grain boundaries, where an increase in grain boundary volume fraction and a decrease in grain size enhance the impediment of dislocation motion. Heterogeneous deformation induces a simple nonhomogeneous structure in Zn alloys, which improves the strength and ductility of the alloys. The improvement of ductility in Zn alloys is usually associated with refining the microstructure and twinning. The addition of alloying elements leads to a reduction in grain size and an increase in grain boundary volume fraction. When the grain size falls below a critical size, grain boundary sliding is activated, promoting ductility and potentially even enabling room-temperature superplasticity [94]. Due to the low recrystallization temperature of Zn alloys, dynamic recrystallization can occur during tensile testing, releasing accumulated stress and improving ductility. Twinning, on the other hand, can initiate crack formation and accelerate crack propagation by inducing localized stress concentration, thus adversely affecting ductility. The addition of alloying elements can activate the critical stress for twinning, which increases with decreasing grain size. Consequently, when the grain size is less than the critical value, twinning can be completely suppressed, thereby enhancing the ductility of the alloy [95]. The addition of alloying elements promotes the refinement of second-phase grains and enhances the compatibility with the Zn matrix, reducing stress concentration and delaying crack nucleation, thus exhibiting higher plastic deformation ability [90]. In addition to alloying elements, processing and heat treatment techniques, such as casting, equal channel angular pressing (ECAP), hot extrusion, rolling, accelerated cooling, and annealing processes, play a crucial role in the mechanical properties and corrosion rate of alloys. Appropriate processing and heat treatment can optimize the mechanical and corrosion properties of alloys to meet clinical requirements [93,[96], [97], [98], [99], [100], [101]]. Regardless of alloy optimization or processing techniques, they both affect the grain refinement and alloy uniformity, thereby influencing the corrosion rate and uniformity. Currently, it is challenging to achieve both high strength and high elongation in binary Zn alloys, even with various processing methods. Consequently, adopting multiple alloying elements on the basis of binary alloy systems, making the microstructure more complex and increasing the diversity of secondary phases, has been explored. Multicomponent alloying has been shown to be effective in adjusting the mechanical properties of Zn-based biomaterials. Zn–Mg and Zn–Li alloys exhibit tensile strengths over 300 MPa, while Zn–Mn and Zn–Cu alloys exhibit fracture elongations exceeding 25 %. Thus, multicomponent alloys based on Zn–Mg, Zn–Li, Zn–Mn, and Zn–Cu have become research focuses. Mn, Ca, Sr, Ag, Fe, and Zr are added to Zn–Mg alloys; Al, Mg, Sr, Cu, and Ca are added to Zn–Li alloys; Ag, Cu, Ca, Mg, and Fe are added to Zn–Mn alloys; and Ag, Mg, Ca, Fe, and Li are added to Zn–Cu alloys. Combined with different processing techniques, the mechanical properties, corrosion rate, and biocompatibility of the alloys can meet clinical requirements [72,90,[102], [103], [104], [105], [106], [107], [108], [109]]. Research on dynamic recrystallization, strain softening, and creep effects has gradually gained attention in the field of biotechnology. Due to the melting point (Tm) of Zn is 692.65 K, dynamic recrystallization (DRX) in Zn alloys is easily induced during processing, with an initial temperature of approximately 288 K (0.42 Tm). Dynamic recrystallization can be categorized into discontinuous dynamic recrystallization (DDRX), continuous dynamic recrystallization (CDRX), twinning-induced dynamic recrystallization (TDRX), and particle-stimulated nucleation (PSN), and the variation in processing parameters can alter the DRX mechanism. The transition from DDRX to CDRX can be achieved by lowering the deformation temperature and increasing the strain rate, while low temperature and high strain rate can promote the activation of TDRX [110]. In addition to DDRX in the initial deformation stage and TDRX at intermediate and high strain rates, CDRX plays a dominant role in the compression of pure Zn at room temperature [111,112]. Dynamic recrystallization helps reduce local stress concentration, lower deformation resistance, and improve alloy ductility. Additionally, DRX can refine alloy grains and enhance mechanical properties by promoting Hall‒Petch strengthening.
The mechanical stability of zinc alloy is of vital importance for its practical applications, and there is a growing body of research focused on investigating strain softening, creep, and ambient temperature aging phenomena associated with it [[113], [114], [115]]. Strain softening refers to the phenomenon where the stress needed for further deformation of the material after one or multiple loading and unloading cycles is smaller than before [114]. Creep is the phenomenon of slow plastic deformation that occurs in materials under constant load over time [116,117]. Ambient temperature aging describes the phenomenon where the mechanical properties of a material change at room temperature. Strain softening, creep, and ambient temperature aging all limit the clinical application of Zn alloy materials. Taking intravascular stents as an example, strain softening can cause a decrease in radial supporting force during balloon expansion, affecting the scaffolding capability of the stent. Vascular stents need to withstand radial pressure from the blood vessels for a long time, and inadequate creep resistance may lead to lumen loss and restenosis. Ambient temperature aging can also impact the shelf life of stents. The low melting point characteristic of zinc alloy is the primary reason for its susceptibility to strain softening, creep, and ambient temperature aging phenomena. The low melting point characteristic of Zn alloys is the main reason for strain softening and creep. When strain or load is applied to the alloy, dislocation motion and grain boundary slip occur due to the low melting point of the Zn alloy, leading to strain softening and creep. Additionally, dynamic recrystallization can cause changes in dislocation density, resulting in the occurrence of these two phenomena [89,90]. Forming high-density twin boundaries to suppress dislocation motion, altering the mode of dislocation motion, improving grain boundary stability through segregation, and introducing low-energy grain boundaries are effective methods to overcome strain softening and creep [65,118]. The low melting point of zinc alloy also leads to extensive atomic migration at room temperature under non-strain and load conditions, resulting in phase transformation during storage and consequently changes in the physical properties. The development direction to overcome ambient temperature aging includes the selection of high melting point alloying elements, regulation of the properties of the second phase (such as softness and hardness), and heat treatment [65].
Surface Modification and Activity Research. The surface characteristics of implants play a crucial role in determining the physiological response upon direct contact with tissues. Surface modification is a simple and effective method for constructing alloys with appropriate corrosion rates, good biocompatibility, and physiological functionality. Surface coating techniques for Zn alloys have been explored for many years, and coatings can be classified into three categories: inorganic, polymer, and composite. Inorganic coatings include oxides, hydroxides, phosphates, ceramics, and metal coatings. Controlling the degradation rate of Zn alloys is crucial to enhance their biocompatibility due to the potential cytotoxicity associated with high zinc ion concentrations. Coatings such as zinc oxide (ZnO), zinc hydroxide (Zn(OH)2), and zinc phosphate (Zn3(PO4)2) can enhance the corrosion resistance of Zn alloy substrates, reduce the release rate of zinc ions, improve the hydrophilicity of the alloy surface, facilitate cell adhesion and enhance the biocompatibility of implants. However, research has shown that zinc phosphate exhibits more significant improvement in alloy biocompatibility compared to ZnO or Zn(OH)2 [119]. Furthermore, zinc phosphate promotes the growth of hydroxyapatite and enhances the cell viability, adhesion, and proliferation of preosteoblasts while inhibiting the adhesion of platelets and Escherichia coli [120]. The role of polymer coatings is similar to that of inorganic coatings, primarily controlling the release rate of zinc ions and enhancing the biocompatibility of the alloy. For example, researchers have used amphiphilic polymers as coatings on zinc-magnesium alloys, significantly improving the corrosion resistance of the alloy [121]. Other researchers have utilized poly(p-xylene) as a coating to enhance the corrosion resistance of stents. Compared to uncoated stents, the poly(p-xylene) coating reduced the corrosion rate by 50 % [122]. To combine the advantages of multiple coatings, composite coatings have become a research hotspot. Composite coatings can include inorganic‒inorganic coatings, inorganic-polymer coatings, and polymer-polymer coatings. The effect of inorganic‒inorganic coatings is similar to that of inorganic coatings, but with multiple layers, it offers more flexibility in adjusting the corrosion resistance of Zn alloys. The corrosion rate can be programmatically set based on the specific application scenario. For example, in the initial stage where mechanical support is needed, the corrosion rate can be reduced to improve effectiveness. In the later stage when tissue regeneration has occurred, the total amount of alloy can be reduced to a certain extent without compromising biocompatibility, thereby accelerating corrosion and minimizing foreign body reactions. Inorganic-polymer and organic-polymer coatings not only control the degradation rate of Zn alloys and enhance their biocompatibility but also impart certain functional properties to the alloy, allowing for better performance within the body. For instance, introducing poly(lactic acid) onto a calcium phosphate coating can improve the corrosion resistance of the alloy while upregulating the expression of osteogenic genes such as RUNX2, OCN, and BMP, thereby promoting cell vitality [123]. Combining biomolecules such as cysteine, phenylalanine, and bovine serum albumin with zinc phosphate allows for a reduction in the release rate of zinc ions, promoting the deposition of hydroxyapatite. This, in turn, enhances the proliferation and adhesion of preosteoblast cells (MC3T3-E1) and rat bone marrow mesenchymal stem cells while upregulating the expression of osteogenic genes [124]. A coating consisting of polycarbonate, tannic acid, and copper ions, applied to Zn alloy scaffolds, exhibited excellent corrosion resistance and long-term stability in both in vitro and in vivo degradation tests. Copper ions act as catalysts for nitric oxide generation, promoting the adhesion and proliferation of endothelial cells on the surface of the Zn alloy. Tannic acid and copper ions synergistically exhibit antibacterial effects and reduce the inflammatory response to the zinc substrate [125]. The zinc ion-crosslinked polycarbonate/heparin composite coating enhances the corrosion resistance of the alloy, decreases the release rate of zinc ions, and enhances endothelial cell adhesion and proliferation on the alloy. Endothelialization occurs at a faster rate compared to bare Zn alloy. Additionally, the surface erosion of the composite coating leads to the uniform and long-term release of heparin, significantly inhibiting platelet adhesion and activation [126]. The PLGA/PDA coating improves the material's corrosion resistance, enhances in vitro biocompatibility, and inhibits hemolysis and smooth muscle cell (SMC) proliferation. The enhanced proliferation of endothelial cells (ECs) is expected to promote re-endothelialization within the scaffold, preventing in-stent restenosis and neointimal hyperplasia [127,128]. Various methods have been employed for coating preparation, including the immersion transformation method [129,130], hydrothermal method [131], plasma electrolytic oxidation (PEO) [[132], [133], [134], [135], [136]], phosphate chemical conversion method (PCC) [120,137], anodic oxidation (AD) [138], magnetron sputtering technique [139], electrophoretic deposition (EPD) [125,126], atomic layer deposition [140], ultrasonic spray coating [141], sol-gel method [142], dip coating method [124], etc.
Research on Additive Manufacturing. Additive manufacturing technologies suitable for metals mainly include direct energy deposition and powder bed fusion techniques. Among them, the powder bed fusion technique, especially laser powder fusion technology (SLM), has been widely used in Zn alloy additive manufacturing research due to its small beam spot diameter, fine powder, thin formed layers, and relatively high dimensional accuracy [143,144]. Laser powder fusion technology allows for the easy construction of three-dimensional (3D) interconnected porous structures similar to human bone, which is beneficial for nutrient transport, tissue integration, and regeneration. Research in this field has focused on porous Zn alloy orthopedic materials. In 2017, Montani et al. first evaluated the process feasibility and applicability of SLM technology for preparing pure zinc. Rapid cooling in SLM resulted in finer grains and stronger mechanical properties than cast and extruded zinc. However, it was found that due to the low melting and boiling points of zinc, it was prone to vaporization during processing, leading to low density (maximum relative density of 88 %) [145]. Addressing the issue of low density in Zn alloy additive manufacturing has become a hot research topic. Factors such as particle shape, particle size, particle composition, laser power, laser scanning speed, laser scanning strategy, layer thickness, hatch spacing, and gas flow rate can all affect the alloy density [143]. Adjustment of these factors is achieved by controlling the input of laser energy per unit area to ensure an appropriate diffusion rate of solute in the melt pool while minimizing alloy evaporation. Additionally, controlling the stability of the fluid in the melt pool and laser stability can enhance alloy density, for example, by reducing the impact of surface-active elements such as oxygen and sulfur on fluid stability and optimizing the gas circulation system to eliminate the influence of evaporated fine particles on laser propagation. The rapid cooling characteristics of laser powder fusion technology result in finer grains and a wider range of dislocations in the alloy, thereby enhancing its strength. Furthermore, the multioriented grain structure improves the alloy's plasticity [146]. The composition of the alloys (such as Mg, Ag, Al, Li, copper, rare earth elements, etc.) [69,[147], [148], [149], [150], [151], [152], [153]], nanoreinforcing materials (such as reduced graphene oxide and nanosilicon carbide) [154,155], 3D configurations [[156], [157], [158]], and reducing defects such as porosity, lack of fusion, inclusions, cracks, and voids through the process are also crucial for ensuring the mechanical properties of additive manufactured Zn alloys. The addition of alloy components also shows a trend toward developing from binary alloys to ternary alloys [152,159]. However, due to the porous nature of additive manufactured zinc-based orthopedic scaffolds, their strength is still insufficient to match cortical bone. It is currently more suitable for application in cancellous bone. The corrosion rate and biocompatibility of porous Zn alloys materials prepared by laser powder fusion technology still require further research. On the one hand, the porous nature increases the specific surface area, accelerating the corrosion rate of the alloy. On the other hand, the formation of surface oxide layers and grain refinement slow the corrosion rate of the alloy. Therefore, different alloy compositions, porosity rates, coatings, etc., can result in significantly different corrosion rates of porous Zn alloys. Similarly, biocompatibility, such as the corrosion rate, needs further investigation. For instance, the porous structure provides climbing space for cells but also increases the release of zinc ions. Current research indicates that the biocompatibility of porous Zn alloys is acceptable [143].
In vivo research. With in-depth research on alloy composition and processing technology, Zn alloys have been manufactured into products for different indications and implanted into animal bodies, providing references for industrialization, such as orthopedic materials, intravascular stents, wound and tissue closure devices, etc. Zheng Yufeng's team screened binary Zn alloys prepared with alloying elements such as Mg, Ca, Sr, Li, Mn, Fe, Cu, and Ag through in vitro and rat femoral implantation studies and developed ternary alloys suitable for load-bearing bones using the best strengthening effect of Zn–Li alloy. The Zn-0.8Li-0.4 Mg alloy had a tensile strength of 646.69 ± 12.79 MPa, and the Zn-0.8Li-0.8Mn alloy had an elongation of 103.27 ± 20 % [85]. Researchers have used 16 rare earth elements to prepare binary alloys, and in vivo experiments on rabbit tibial implants demonstrated that Zn–La, Zn–Ce, and Zn–Nd with stronger performance exhibited good tissue biocompatibility and significant bone integration [160]. The Zn–Mg–Fe bone repair system has been applied to the frontal bone, mandible, and femur of beagle dogs. The Zn–Mg–Fe alloy has good biocompatibility, accumulates only in bone tissue, and does not accumulate in the liver, kidney, and spleen. The alloy exhibits a uniform slow degradation rate in the body, and no degradation difference was observed in the frontal bone, mandible, and femur. It is a bone synthesizing material with significant clinical application value [161]. In addition, alloys such as Zn–Li–Ca, Zn–Li–Ag, Zn–Mg–Sr, Zn–Mn–Mg, and Zn-0.8Li-0.1Sr have been investigated as orthopedic materials, and good experimental results have been achieved in animal validation experiments, demonstrating the great potential of Zn alloys in clinical applications in orthopedics [[162], [163], [164], [165], [166], [167]]. Regarding the application of stents, research has moved beyond the feasibility study of zinc wires or rods implanted in blood vessels. Instead, Zn alloys have been fabricated into stent products and implanted in animal bodies to verify their safety and efficacy, providing evidence for clinical applications. For instance, in our research group, we prepared Φ2.0 × 12 mm stents using Zn–0.02 Mg–0.02Cu and implanted into the left carotid artery of rabbits for 12 months. Rapid endothelialization was observed one week after implantation, and no significant intimal hyperplasia was observed at six months. The stent exhibited slow corrosion during the early stages but accelerated corrosion at 12 months. Throughout the implantation period, all arteries remained unobstructed, and no apparent thrombosis or systemic toxicity was observed, preliminarily demonstrating the feasibility of Zn–0.02 Mg–0.02Cu stents as intravascular implants. Our research group has also developed Zn-0.8Cu coronary artery stents and implanted them in pig coronary arteries for up to 24 months. Endothelialization was completed within one month after implantation, and no signs of inflammation or thrombosis were observed during the implantation period. The stents provided sufficient structural support, exhibited an appropriate degradation rate, and showed no accumulation of degradation products, thrombus formation, or inflammatory responses. Ethical approval and clinical approval from the National Medical Products Administration have been obtained, and clinical trials are about to commence [68,168]. Christoph Hehrlein et al. investigated a highly radiolucent and slowly biodegradable Zn alloys scaffold, which was placed adjacent to a rigid nitinol stent at the bifurcation of the femoral arteries in 21 pigs. Follow-up examinations were conducted at one month and three months. The results showed that the Zn alloys scaffold was accurately positioned at the femoral artery bifurcation and further reduced intimal hyperplasia by 12 % [169]. Amano et al. selected three Zn alloys, namely, Zn-1.0Cu-0.2Mn-0.1Ti (alloy 1), Zn-1.0Mn-0.1Ti (alloy 2), and Zn-1.0Cu-0.1Ti (alloy 3), as candidate materials for gastric resection surgery. These alloys were used for gastric closure in rabbits, and no leakage was detected during implantation. The closure nails made from these three materials demonstrated good in vivo safety and feasibility, with no leakage detected during implantation [170]. Zheng Yufeng's research group also prepared Zn-0.8 wt% Li-0.1 wt% Mn closure nails with a diameter of 0.3 mm and applied them to a porcine model for gastrointestinal anastomosis. Postoperatively, the Zn alloys exhibited closure effects comparable to those of traditional nails. Compared to magnesium-based nails, Zn–Li–Mn nails corrode at a slower rate and retain 89 % of the original implant volume after 12 weeks of implantation, ensuring a longer period of strength maintenance for complete wound healing [171].
Biomimetic bone repair material. After the development of materials, compatibility studies with cells, and verification through experiments on small animals and large animals, porous Zn alloys cancellous bone filling materials have gradually matured. However, biomimetic bone repair materials that can completely replace bone defects, such as cranial bone defects, have not yet been developed. In the future, various alloy processing techniques, such as combining extrusion processing and additive manufacturing processes, are expected to be combined to develop biomimetic bone repair materials with both cancellous and cortical bone structures. The cortical bone structure implanted in the early stage provides sufficient support strength for the bone repair site, while the cancellous bone structure provides space for osteoblast climbing. To promote rapid osteoblast climbing, bone regeneration components such as hydroxyapatite and bioactive glass can be introduced into the matrix during the forming process. After the formation of the matrix, a coating that induces bone growth can be formed on the porous surface, thus achieving bone filling and inducing bone regeneration simultaneously. After osteoblasts climb into the cancellous bone structure, vascularization occurs, and the Zn alloys substrate gradually undergoes calcification to replace the alloy. During this process, the degradation rate of the alloy should match the progress of bone formation, which requires strong coordination of the alloy composition, forming process, and coating process. Ultimately, the biomimetic bone repair material is completely replaced by autogenous bone tissue.
Neurovascular stent. Degradable coronary stents, such as polylactic acid stents and magnesium alloy stents, have been commercialized. Iron alloy stents and Zn alloys stents have been used in large animal experiments, and a clinical trial of Zn alloys stents is about to commence. However, in the field of neurology, only the ReSolv absorbable mesh stent developed by Fluid Biotech has achieved the first clinical implantation. It is noteworthy that the ReSolv stent is composed of both degradable polymers and nondegradable metals. The polymer part gradually becomes absorbed while the singular metal part embeds into the vessel wall, making it a partially absorbable stent. Research on the application of Zn alloys in the nervous system is limited, possibly due to the sensitivity of nerve cells to zinc ions [172]. Elevated zinc levels in the body can cause focal neuronal pathology, while zinc deficiency can lead to mental lethargy. However, studies have shown that zinc may be a potential target for protecting the blood-brain barrier and reducing hemorrhagic transformation, inflammation, and edema in stroke patients [172,173]. Compared to coronary stents, neurology stents require more emphasis on flexibility, anti-thrombotic properties, compliance, and low radial recoil due to the thinner and more curvaceous smooth muscle and outer membrane layers in intracranial blood vessels, as well as their narrower diameter. Based on the current research foundation, Zn alloys are expected to meet the requirements of neurology stents, but further research validation is still needed.
In conclusion, researchers have delved into the study of how alloying elements, processing techniques, and optimization of multi-component alloys can alter the microstructure of zinc alloys. This alteration simultaneously affects their mechanical properties, corrosion rate, and biocompatibility. Moreover, strain softening, creep, and room temperature aging have also been observed and various approaches have been attempted to address these issues. Additionally, to meet clinical demands and expedite industrial application, attention has been given to zinc alloy surface coatings, additive manufacturing, and in vivo studies. Building upon the aforementioned research, clinical trials for zinc alloy bone plates, bone screws, and coronary stents, as well as investigations into biomimetic bone repair materials and neurosurgical scaffolds, hold promise as future directions for zinc alloy product research.
Current research deficiencies. The reliance of CiteSpace on data quality and accuracy is high. While considerable effort has been made to collect relevant literature for this study, the possibility of omissions cannot be entirely ruled out. However, any potential omissions are minimal and have a negligible impact on the conclusions. Additionally, as the year 2023 has not concluded, this study only analyzed data up to 2022, thus the latest literature from 2023 could not be included.
CRediT authorship contribution statement
Kunshan Yuan: Writing – original draft, Visualization, Methodology, Investigation, Formal analysis, Data curation. Chengchen Deng: Writing – review & editing, Writing – original draft, Visualization. Lili Tan: Validation. Xiangxiu Wang: Investigation. Wenhua Yan: Validation. Xiaozhen Dai: Investigation. Ruolin Du: Methodology. Yufeng Zheng: Supervision, Resources. Haijun Zhang: Writing – review & editing, Supervision, Conceptualization. Guixue Wang: Supervision, Funding acquisition, Conceptualization.
Declaration of competing interest
Yufeng Zheng is an editorial board editor-in-chief for Bioactive Materials and was not involved in the editorial review or the decision to publish this article. All authors declare that there are no competing interests.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (12032007, 31971242, 82270535) and the Science and Technology Innovation Project of JinFeng Laboratory, Chongqing, China (jfkyjf202203001). The authors also thank Professor Chaojun Tang of Soochow University for their valuable suggestions on the revision of this article. The authors are also thankful for the First Batch of Key Disciplines on Public Health in Chongqing and the Public Experiment Center of State Bioindustrial Base (Chongqing), China.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2024.01.017.
Contributor Information
Yufeng Zheng, Email: yfzheng@pku.edu.cn.
Haijun Zhang, Email: zhanghaijun@tongji.edu.cn.
Guixue Wang, Email: wanggx@cqu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Venezuela J., Dargusch M.S. The influence of alloying and fabrication techniques on the mechanical properties, biodegradability and biocompatibility of zinc: a comprehensive review. Acta Biomater. 2019;87:1–40. doi: 10.1016/j.actbio.2019.01.035. [DOI] [PubMed] [Google Scholar]
- 2.Jiang P., Zhang Y., Shi B., Zhang L., Huang Q., Yang Y., Tang P., Lin C. Advanced surface engineering of titanium materials for biomedical applications: from static modification to dynamic responsive regulation. Bioact. Mater. 2023;27:15–57. doi: 10.1016/j.bioactmat.2023.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szczesny G., Kopec M., Politis D.J., Kowalewski Z.L., Lazarski A., Szolc T. A review on biomaterials for orthopaedic surgery and traumatology: from past to present. Materials. 2022;15(10):3622. doi: 10.3390/ma15103622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korei N., Solouk A., Nazarpak M.H., Nouri A. A review on design characteristics and fabrication methods of metallic cardiovascular stents. Mater. Today Commun. 2022;31 doi: 10.1016/j.mtcomm.2022.103467. [DOI] [Google Scholar]
- 5.Laubach M., Kobbe P., Hutmacher D.W. Biodegradable interbody cages for lumbar spine fusion: current concepts and future directions. Biomaterials. 2022;288 doi: 10.1016/j.biomaterials.2022.121699. [DOI] [PubMed] [Google Scholar]
- 6.Edlinger C., Paar V., Kheder S.H., Krizanic F., Lalou E., Boxhammer E., Butter C., Dworok V., Bannehr M., Hoppe U.C., Kopp K., Lichtenauer M. Endothelialization and inflammatory reactions after intracardiac device implantation. Adv. Exp. Med. Biol. 2022;1401:1–22. doi: 10.1007/5584_2022_712. [DOI] [PubMed] [Google Scholar]
- 7.Kim K.T., Eo M.Y., Truc Thi Hoang N., Kim S.M. General review of titanium toxicity. Int. J. Implant Dent. 2019;5 doi: 10.1186/s40729-019-0162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pitchai M., Ipe D., Tadakamadla S., Hamlet S. Titanium implant surface effects on adherent macrophage phenotype: a systematic review. Materials. 2022;15(20):7314. doi: 10.3390/ma15207314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Condello F., Spaccarotella C., Sorrentino S., Indolfi C., Stefanini G.G.G., Polimeni A. Stent thrombosis and restenosis with contemporary drug-eluting stents: predictors and current evidence. J. Clin. Med. 2023;12(3):1238. doi: 10.3390/jcm12031238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hou Z., Yan W., Li T., Wu W., Cui Y., Zhang X., Chen Y.-P., Yin T., Qiu J., Wang G. Lactic acid-mediated endothelial to mesenchymal transition through TGF-beta 1 contributes to in-stent stenosis in poly-L-lactic acid stent. Int. J. Biol. Macromol. 2020;155:1589–1598. doi: 10.1016/j.ijbiomac.2019.11.136. [DOI] [PubMed] [Google Scholar]
- 11.Lin S., Ran X., Yan X., Yan W., Wang Q., Yin T., Zhou J.G., Hu T., Wang G. Corrosion behavior and biocompatibility evaluation of a novel zinc-based alloy stent in rabbit carotid artery model. J. Biomed. Mater. Res., Part B. 2019;107(6):1814–1823. doi: 10.1002/jbm.b.34274. [DOI] [PubMed] [Google Scholar]
- 12.Yin T., Du R., Wang Y., Huang J., Ge S., Huang Y., Tan Y., Liu Q., Chen Z., Feng H., Du J., Wang Y., Wang G. Two-stage degradation and novel functional endothelium characteristics of a 3-D printed bioresorbable scaffold. Bioact. Mater. 2022;10:378–396. doi: 10.1016/j.bioactmat.2021.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi W., Fuad A.R.M., Li Y., Wang Y., Huang J., Du R., Wang G., Wang Y., Yin T. Biodegradable polymeric nanoparticles increase risk of cardiovascular diseases by inducing endothelium dysfunction and inflammation. J. Nanobiotechnol. 2023;21(1):65. doi: 10.1186/s12951-023-01808-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rabeeh V.P.M., Hanas T. Progress in manufacturing and processing of degradable Fe-based implants: a review. Prog. Biomater. 2022;11(2):163–191. doi: 10.1007/s40204-022-00189-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kabir H., Munir K., Wen C., Li Y. Recent research and progress of biodegradable zinc alloys and composites for biomedical applications: biomechanical and biocorrosion perspectives. Bioact. Mater. 2021;6(3):836–879. doi: 10.1016/j.bioactmat.2020.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang C.-C., Lam T.-N., Amalia L., Chen K.-H., Yang K.-Y., Muslih M.R., Singh S.S., Tsai P.-I., Lee Y.-T., Jain J., Lee S.Y., Lai H.-J., Huang W.-C., Chen S.-Y., Huang E.W. Tailoring grain sizes of the biodegradable iron-based alloys by pre-additive manufacturing microalloying. Sci. Rep. 2021;11(1):9610. doi: 10.1038/s41598-021-89022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Istrate B., Munteanu C., Baltatu M.-S., Cimpoesu R., Ioanid N. Microstructural and electrochemical influence of Zn in MgCaZn biodegradable alloys. Materials. 2023;16(6):2487. doi: 10.3390/ma16062487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y., Lin T., Meng H., Wang X., Peng H., Liu G., Wei S., Lu Q., Wang Y., Wang A., Xu W., Shao H., Peng J. 3D gel-printed porous magnesium scaffold coated with dibasic calcium phosphate dihydrate for bone repair in vivo. J. Orthop. Transl. 2022;33:13–23. doi: 10.1016/j.jot.2021.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Istrate B., Munteanu C., Antoniac I.-V., Lupescu S.-C. Current research studies of Mg-Ca-Zn biodegradable alloys used as orthopedic implants-review. Crystals. 2022;12(10):1468. doi: 10.3390/cryst12101468. [DOI] [Google Scholar]
- 20.Scarcello E., Lison D. Are Fe-based stenting materials biocompatible? A critical review of in vitro and in vivo studies. J. Funct. Biomater. 2020;11(1) doi: 10.3390/jfb11010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu J., Su Y., Qin Y.-X., Zheng Y., Wang Y., Zhu D. Evolution of metallic cardiovascular stent materials: a comparative study among stainless steel, magnesium and zinc. Biomaterials. 2020;230 doi: 10.1016/j.Biomaterials.2019.119641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su Y., Cockerill I., Wang Y., Qin Y.-X., Chang L., Zheng Y., Zhu D. Zinc-based biomaterials for regeneration and therapy. Trends Biotechnol. 2019;37(4):428–441. doi: 10.1016/j.tibtech.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davarazar M., Kamali M., Lopes I. Engineered nanomaterials for (waste)water treatment-A scientometric assessment and sustainability aspects. Nanoimpact. 2021;22 doi: 10.1016/j.impact.2021.100316. [DOI] [PubMed] [Google Scholar]
- 24.Liu X., Zhao S., Tan L., Tan Y., Wang Y., Ye Z., Hou C., Xu Y., Liu S., Wang G. Frontier and hot topics in electrochemiluminescence sensing technology based on CiteSpace bibliometric analysis. Biosens. Bioelectron. 2022;201 doi: 10.1016/j.bios.2021.113932. [DOI] [PubMed] [Google Scholar]
- 25.Abuin-Porras V., Martinez-Perez C., Romero-Morales C., Cano-de-la-Cuerda R., Martin-Casas P., Palomo-Lopez P., Sanchez-Tena M.A. Citation network study on the use of new technologies in neurorehabilitation. Int. J. Environ. Res. Publ. Health. 2022;19(1):26. doi: 10.3390/ijerph19010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y., Li Y., Guo L., Hong J., Zhao W., Hu X., Chang C., Liu W., Xiong K. Bibliometric analysis of the inflammasome and pyroptosis in brain. Front. Pharmacol. 2021;11 doi: 10.3389/fphar.2020.626502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tagorti G., Kaya B. Publication trends of somatic mutation and recombination tests research: a bibliometric analysis (1984‒2020) Genomics inform. 2022;20(1) doi: 10.5808/gi.21083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abouzid M., Karazniewicz-Lada M., Abdelazeem B., Brasic J.R. Research trends of vitamin D metabolism gene polymorphisms based on a bibliometric investigation. Genes. 2023;14(1):215. doi: 10.3390/genes14010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ageel M. Pandemic critical care research during the COVID-19 (2020-2022): a bibliometric analysis using VOSviewer. BioMed Res. Int. 2022;2022 doi: 10.1155/2022/8564649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Eck N.J., Waltman L. Citation-based clustering of publications using CitNetExplorer and VOSviewer. Scientometrics. 2017;111(2):1053–1070. doi: 10.1007/s11192-017-2300-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yadav S., Chhabra A., Mahesh G. Mapping, clustering, and analysis of research in psychiatric genomics. Psychiatr. Genet. 2022;32(6):221–237. doi: 10.1097/ypg.0000000000000325. [DOI] [PubMed] [Google Scholar]
- 32.Li Q., Yang W., Li J., Shan Z. Emerging trends and hot spots in autoimmune thyroiditis research from 2000 to 2022: a bibliometric analysis. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.953465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goncer-Demiral D., Ince-Yenilmez M. Network analysis of international export pattern. Soc. Netw. Anal. Min. 2022;12(1):156. doi: 10.1007/s13278-022-00984-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan L., Wang X., Yuan K., Yin T., Du R., Shen L., Zhu Z., Yu S., Zhang H., Wang G. Structural and temporal dynamics analysis on drug-eluting stents: history, research hotspots and emerging trends. Bioact. Mater. 2023;23:170–186. doi: 10.1016/j.bioactmat.2022.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mostaed E., Sikora-Jasinska M., Drelich J.W., Vedani M. Zinc-based alloys for degradable vascular scent applications. Acta Biomater. 2018;71:1–23. doi: 10.1016/j.actbio.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mostaed E., Sikora-Jasinska M., Mostaed A., Loffredo S., Demir A.G., Preuitali B., Mantouani D., Beanland R., Vedani M. Novel Zn-based alloys for biodegradable stent applications: design, development and in vitro degradation. J. Mech. Behav. Biomed. Mater. 2016;60:581–602. doi: 10.1016/j.jmbbm.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 37.Yang H., Wang C., Liu C., Chen H., Wu Y., Han J., Jia Z., Lin W., Zhang D., Li W., Yuan W., Guo H., Li H., Yang G., Kong D., Zhu D., Takashima K., Ruan L., Nie J., Li X., Zheng Y. Evolution of the degradation mechanism of pure zinc stent in the one-year study of rabbit abdominal aorta model. Biomaterials. 2017;145:92–105. doi: 10.1016/j.biomaterials.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 38.Liu X., Sun J., Yang Y., Pu Z., Zheng Y. In vitro investigation of ultra-pure Zn and its mini-tube as potential bioabsorbable stent material. Mater. Lett. 2015;161:53–56. doi: 10.1016/j.matlet.2015.06.107. [DOI] [Google Scholar]
- 39.Li H.F., Xie X.H., Zheng Y.F., Cong Y., Zhou F.Y., Qiu K.J., Wang X., Chen S.H., Huang L., Tian L., Qin L. Development of biodegradable Zn-1X binary alloys with nutrient alloying elements Mg, Ca and Sr. Sci. Rep. 2015;5 doi: 10.1038/srep10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bowen P.K., Drelich J., Goldman J. Zinc exhibits ideal physiological corrosion behavior for bioabsorbable stents. Adv. Mater. 2013;25(18):2577–2582. doi: 10.1002/adma.201300226. [DOI] [PubMed] [Google Scholar]
- 41.Vojtech D., Kubasek J., Serak J., Novak P. Mechanical and corrosion properties of newly developed biodegradable Zn-based alloys for bone fixation. Acta Biomater. 2011;7(9):3515–3522. doi: 10.1016/j.actbio.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 42.Zheng Y.F., Gu X.N., Witte F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014;77:1–34. doi: 10.1016/j.mser.2014.01.001. [DOI] [Google Scholar]
- 43.Li H., Yang H., Zheng Y., Zhou F., Qiu K., Wang X. Design and characterizations of novel biodegradable ternary Zn-based alloys with IIA nutrient alloying elements Mg, Ca and Sr. Mater. Des. 2015;83:95–102. [Google Scholar]
- 44.Kubasek J., Vojtech D., Jablonska E., Pospisilova I., Lipov J., Ruml T. Structure, mechanical characteristics and in vitro degradation, cytotoxicity, genotoxicity and mutagenicity of novel biodegradable Zn-Mg alloys. Mater. Sci. Eng., C. 2016;58:24–35. doi: 10.1016/j.msec.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 45.Bowen P.K., Shearier E.R., Zhao S., Guillory R.J., Zhao F., Goldman J., Drelich J.W. Biodegradable metals for cardiovascular stents: from clinical concerns to recent Zn-alloys. Adv. Healthcare Mater. 2016;5(10):1121–1140. doi: 10.1002/adhm.201501019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang Z., Niu J., Huang H., Zhang H., Pei J., Ou J., Yuan G. Potential biodegradable Zn-Cu binary alloys developed for cardiovascular implant applications. J. Mech. Behav. Biomed. Mater. 2017;72:182–191. doi: 10.1016/j.jmbbm.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 47.Niu J., Tang Z., Huang H., Pei J., Zhang H., Yuan G., Ding W. Research on a Zn-Cu alloy as a biodegradable material for potential vascular stents application. Mater. Sci. Eng., C. 2016;69:407–413. doi: 10.1016/j.msec.2016.06.082. [DOI] [PubMed] [Google Scholar]
- 48.Zhao S., Seitz J.-M., Eifler R., Maier H.J., Guillory R.J., Earley E.J., Drelich A., Goldman J., Drelich J.W. Zn-Li alloy after extrusion and drawing: structural, mechanical characterization, and biodegradation in abdominal aorta of rat. Mater. Sci. Eng., C. 2017;76:301–312. doi: 10.1016/j.msec.2017.02.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu X., Sun J., Zhou F., Yang Y., Chang R., Qiu K., Pu Z., Li L., Zheng Y. Micro-alloying with Mn in Zn-Mg alloy for future biodegradable metals application. Mater. Des. 2016;94:95–104. doi: 10.1016/j.matdes.2015.12.128. [DOI] [Google Scholar]
- 50.Gong H., Wang K., Strich R., Zhou J.G. In vitro biodegradation behavior, mechanical properties, and cytotoxicity of biodegradable Zn-Mg alloy. J. Biomed. Mater. Res., Part B. 2015;103(8):1632–1640. doi: 10.1002/jbm.b.33341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang Z., Huang H., Niu J., Zhang L., Zhang H., Pei J., Tan J., Yuan G. Design and characterizations of novel biodegradable Zn-Cu-Mg alloys for potential biodegradable implants. Mater. Des. 2017;117:84–94. doi: 10.1016/j.matdes.2016.12.075. [DOI] [Google Scholar]
- 52.Liu X., Sun J., Qiu K., Yang Y., Pu Z., Li L., Zheng Y. Effects of alloying elements (Ca and Sr) on microstructure, mechanical property and in vitro corrosion behavior of biodegradable Zn-1.5Mg alloy. J. Alloys Compd. 2016;664:444–452. doi: 10.1016/j.jallcom.2015.10.116. [DOI] [Google Scholar]
- 53.Jin H., Zhao S., Guillory R., Bowen P.K., Yin Z., Griebel A., Schaffer J., Earley E.J., Goldman J., Drelich J.W. Novel high-strength, low-alloys Zn-Mg (< 0.1 wt% Mg) and their arterial biodegradation. Mater. Sci. Eng., C. 2018;84:67–79. doi: 10.1016/j.msec.2017.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bowen P.K., Guillory R.J., Shearier E.R., Seitz J.-M., Drelich J., Bocks M., Zhao F., Goldman J. Metallic zinc exhibits optimal biocompatibility for bioabsorbable endovascular stents. Mater. Sci. Eng., C. 2015;56:467–472. doi: 10.1016/j.msec.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murni N.S., Dambatta M.S., Yeap S.K., Froemming G.R.A., Hermawan H. Cytotoxicity evaluation of biodegradable Zn-3Mg alloy toward normal human osteoblast cells. Mater. Sci. Eng., C. 2015;49:560–566. doi: 10.1016/j.msec.2015.01.056. [DOI] [PubMed] [Google Scholar]
- 56.Liu X., Sun J., Yang Y., Zhou F., Pu Z., Li L., Zheng Y. Microstructure, mechanical properties, in vitro degradation behavior and hemocompatibility of novel Zn-Mg-Sr alloys as biodegradable metals. Mater. Lett. 2016;162:242–245. doi: 10.1016/j.matlet.2015.07.151. [DOI] [Google Scholar]
- 57.Shearier E.R., Bowen P.K., He W., Drench A., Drelich J., Goldman J., Zhao F. In vitro cytotoxicity, adhesion, and proliferation of human vascular cells exposed to zinc. ACS Biomater. Sci. Eng. 2016;2(4):634–642. doi: 10.1021/acsbiomaterials.6b00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma J., Zhao N., Zhu D. Endothelial cellular responses to biodegradable metal zinc. ACS Biomater. Sci. Eng. 2015;1(11):1174–1182. doi: 10.1021/acsbiomaterials.5b00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang H., Qu X., Lin W., Wang C., Zhu D., Dai K., Zheng Y. In vitro and in vivo studies on zinc-hydroxyapatite composites as novel biodegradable metal matrix composite for orthopedic applications. Acta Biomater. 2018;71:200–214. doi: 10.1016/j.actbio.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 60.Zhao S., McNamara C.T., Bowen P.K., Verhun N., Braykovich J.P., Goldman J., Drelich J.W. Structural characteristics and in vitro biodegradation of a novel Zn-Li alloy prepared by induction melting and hot rolling. Metall. Mater. Trans. A. 2017;48A(3):1204–1215. doi: 10.1007/s11661-016-3901-0. [DOI] [Google Scholar]
- 61.Xiao C., Wang L., Ren Y., Sun S., Zhang E., Yan C., Liu Q., Sun X., Shou F., Duan J., Wang H., Qin G. Indirectly extruded biodegradable Zn-0.05wt%Mg alloy with improved strength and ductility: in vitro and in vivo studies. J. Mater. Sci. Technol. 2018;34(9):1618–1627. doi: 10.1016/j.jmst.2018.01.006. [DOI] [Google Scholar]
- 62.Shen C., Liu X., Fan B., Lan P., Zhou F., Li X., Wang H., Xiao X., Li L., Zhao S., Guo Z., Pu Z., Zheng Y. Mechanical properties, in vitro degradation behavior, hemocompatibility and cytotoxicity evaluation of Zn-1.2Mg alloy for biodegradable implants. RSC Adv. 2016;6(89):86410–86419. doi: 10.1039/c6ra14300h. [DOI] [Google Scholar]
- 63.Drelich A.J., Zhao S., Guillory R.J., Drelich J.W., Goldman J. Long-term surveillance of zinc implant in murine artery: surprisingly steady biocorrosion rate. Acta Biomater. 2017;58:539–549. doi: 10.1016/j.actbio.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li H., Zheng Y., Qin L. Progress of biodegradable metals. Prog. Nat. Sci. 2014;24(5):414–422. doi: 10.1016/j.pnsc.2014.08.014. [DOI] [Google Scholar]
- 65.Li G., Yang H., Zheng Y., Chen X.-H., Yang J.-A., Zhu D., Ruan L., Takashima K. Challenges in the use of zinc and its alloys as biodegradable metals: perspective from biomechanical compatibility. Acta Biomater. 2019;97:23–45. doi: 10.1016/j.actbio.2019.07.038. [DOI] [PubMed] [Google Scholar]
- 66.Venezuela J., Dargusch M.S. The influence of alloying and fabrication techniques on the mechanical properties, biodegradability and biocompatibility of zinc: a comprehensive review. Acta Biomater. 2019;87:1–40. doi: 10.1016/j.actbio.2019.01.035. [DOI] [PubMed] [Google Scholar]
- 67.Liu Y., Lu B., Cai Z. Recent progress on Mg- and Zn-based alloys for biodegradable vascular stent applications. J. Nanomater. 2019;2019:1–17. doi: 10.1155/2019/1310792. [DOI] [Google Scholar]
- 68.Qiang H., Hou C., Zhang Y., Luo X., Li J., Meng C., Liu K., Lv Z., Chen X., Liu F. CaP-coated Zn-Mn-Li alloys regulate osseointegration via influencing macrophage polarization in the osteogenic environment. Regen. Biomater. 2023;10 doi: 10.1093/rb/rbad051. rbad051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shuai C., Xue L., Gao C., Yang Y., Peng S., Zhang Y. Selective laser melting of Zn-Ag alloys for bone repair: microstructure, mechanical properties and degradation behaviour. Virtual Phys. Prototyp. 2018;13(3):146–154. doi: 10.1080/17452759.2018.1458991. [DOI] [Google Scholar]
- 70.Kabir H., Munir K., Wen C., Li Y. Recent research and progress of biodegradable zinc alloys and composites for biomedical applications: biomechanical and biocorrosion perspectives. Bioact. Mater. 2021;6(3):836–879. doi: 10.1016/j.bioactmat.2020.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li P., Schille C., Schweize E., Kimmerle-Mueller E., Rupp F., Heiss A., Legner C., Klotz U.E., Geis-Gerstorfer J., Scheideler L. Selection of extraction medium influences cytotoxicity of zinc and its alloys. Acta Biomater. 2019;98:235–245. doi: 10.1016/j.actbio.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 72.Zhang Y., Yan Y., Xu X., Lu Y., Chen L., Li D., Dai Y., Kang Y., Yu K. Investigation on the microstructure, mechanical properties, in vitro degradation behavior and biocompatibility of newly developed Zn-0.8%Li-(Mg, Ag) alloys for guided bone regeneration. Mater. Sci. Eng., C. 2019;99:1021–1034. doi: 10.1016/j.msec.2019.01.120. [DOI] [PubMed] [Google Scholar]
- 73.Kafri A., Ovadia S., Yosafovich-Doitch G., Aghion E. In vivo performances of pure Zn and Zn-Fe alloy as biodegradable implants. J. Mater. Sci. Mater. Med. 2018;29(7):94. doi: 10.1007/s10856-018-6096-7. [DOI] [PubMed] [Google Scholar]
- 74.Pierson D., Edick J., Tauscher A., Pokorney E., Bowen P., Gelbaugh J., Stinson J., Getty H., Lee C.H., Drelich J., Goldman J. A simplified in vivo approach for evaluating the bioabsorbable behavior of candidate stent materials. J. Biomed. Mater. Res., Part B. 2012;100B(1):58–67. doi: 10.1002/jbm.b.31922. [DOI] [PubMed] [Google Scholar]
- 75.Moravej M., Mantovani D. Biodegradable metals for cardiovascular stent application: interests and new opportunities. Int. J. Mol. Sci. 2011;12(7):4250–4270. doi: 10.3390/ijms12074250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cheng J., Liu B., Wu Y.H., Zheng Y.F. Comparative in vitro study on pure metals (Fe, Mn, Mg, Zn and W) as biodegradable metals. J. Mater. Sci. Technol. 2013;29(7):619–627. doi: 10.1016/j.jmst.2013.03.019. [DOI] [Google Scholar]
- 77.Chen Y., Xu Z., Smith C., Sankar J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. 2014;10(11):4561–4573. doi: 10.1016/j.actbio.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 78.Yao C., Wang Z., Tay S.L., Zhu T., Gao W. Effects of Mg on microstructure and corrosion properties of Zn-Mg alloy. J. Alloys Compd. 2014;602:101–107. doi: 10.1016/j.jallcom.2014.03.025. [DOI] [Google Scholar]
- 79.Dambatta M.S., Izman S., Kurniawan D., Farahany S., Yahaya B., Hermawan H. Influence of thermal treatment on microstructure, mechanical and degradation properties of Zn-3Mg alloy as potential biodegradable implant material. Mater. Des. 2015;85:431–437. doi: 10.1016/j.matdes.2015.06.181. [DOI] [Google Scholar]
- 80.Seitz J.-M., Durisin M., Goldman J., Drelich J.W. Recent advances in biodegradable metals for medical sutures: a critical review. Adv. Healthcare Mater. 2015;4(13):1915–1936. doi: 10.1002/adhm.201500189. [DOI] [PubMed] [Google Scholar]
- 81.Wang J., Witte F., Xi T., Zheng Y., Yang K., Yang Y., Zhao D., Meng J., Li Y., Li W., Chan K., Qin L. Recommendation for modifying current cytotoxicity testing standards for biodegradable magnesium-based materials. Acta Biomater. 2015;21:237–249. doi: 10.1016/j.actbio.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 82.Wang C., Yang H.T., Li X., Zheng Y.F. In vitro evaluation of the feasibility of commercial Zn alloys as biodegradable metals. J. Mater. Sci. Technol. 2016;32(9):909–918. doi: 10.1016/j.jmst.2016.06.003. [DOI] [Google Scholar]
- 83.Chen Q., Thouas G.A. Metallic implant biomaterials. Mater. Sci. Eng. R Rep. 2015;87:1–57. doi: 10.1016/j.mser.2014.10.001. [DOI] [Google Scholar]
- 84.Guillory R.J., Bowen P.K., Hopkins S.P., Shearier E.R., Earley E.J., Gillette A.A., Aghion E., Bocks M., Drelich J.W., Goldman J. Corrosion characteristics dictate the long-term inflammatory profile of degradable zinc arterial implants. ACS Biomater. Sci. Eng. 2016;2(12):2355–2364. doi: 10.1021/acsbiomaterials.6b00591. [DOI] [PubMed] [Google Scholar]
- 85.Yang H., Jia B., Zhang Z., Qu X., Li G., Lin W., Zhu D., Dai K., Zheng Y. Alloying design of biodegradable zinc as promising bone implants for load-bearing applications. Nat. Commun. 2020;11(1):401. doi: 10.1038/s41467-019-14153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu Y., Xu Y., Zhang W., Li M., Wendel H.P., Geis-Gerstorfer J., Li P., Wan G., Xu S., Hu T. Biodegradable Zn-Cu-Fe alloy as a promising material for craniomaxillofacial implants: an in vitro investigation into degradation behavior, cytotoxicity, and hemocompatibility. Front. Chem. 2022;10 doi: 10.3389/fchem.2022.860040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hermawan H. Updates on the research and development of absorbable metals for biomedical applications. Prog. Biomater. 2018;7(2):93–110. doi: 10.1007/s40204-018-0091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Han H.-S., Loffredo S., Jun I., Edwards J., Kim Y.-C., Seok H.-K., Witte F., Mantovani D., Glyn-Jones S. Current status and outlook on the clinical translation of biodegradable metals. Mater. Today. 2019;23:57–71. doi: 10.1016/j.mattod.2018.05.018. [DOI] [Google Scholar]
- 89.Pola A., Tocci M., Goodwin F.E. Review of microstructures and properties of zinc alloys. Metals. 2020;10(2):253. doi: 10.3390/met10020253. [DOI] [Google Scholar]
- 90.Zhuo X., Wu Y., Ju J., Liu H., Jiang J., Hu Z., Bai J., Xue F. Recent progress of novel biodegradable zinc alloys: from the perspective of strengthening and toughening. J. Mater. Res. Technol. 2022;17:244–269. doi: 10.1016/j.jmrt.2022.01.004. [DOI] [Google Scholar]
- 91.Lin S., Ran X., Yan X., Wang Q., Zhou J.G., Hu T., Wang G. Systematical evolution on a Zn-Mg alloy potentially developed for biodegradable cardiovascular stents. J. Mater. Sci. Mater. Med. 2019;30(11):122. doi: 10.1007/s10856-019-6324-9. [DOI] [PubMed] [Google Scholar]
- 92.Huang H., Liu H., Wang L.-S., Li Y.-H., Agbedor S.-O., Bai J., Xue F., Jiang J.-H. A high-strength and biodegradable Zn-Mg alloy with refined ternary eutectic structure processed by ECAP. Acta Metall. Sin.-Engl. Lett. 2020;33(9):1191–1200. doi: 10.1007/s40195-020-01027-x. [DOI] [Google Scholar]
- 93.Ye L., Huang H., Sun C., Zhuo X., Dong Q., Liu H., Ju J., Xue F., Bai J., Jiang J. Effect of grain size and volume fraction of eutectic structure on mechanical properties and corrosion behavior of as-cast Zn-Mg binary alloys. J. Mater. Res. Technol-JMRT. 2022;16:1673–1685. doi: 10.1016/j.jmrt.2021.12.101. [DOI] [Google Scholar]
- 94.Guo P., Zhu X., Yang L., Deng L., Zhang Q., Li B.Q., Cho K., Sun W., Ren T., Song Z. Ultrafine- and uniform-grained biodegradable Zn-0.5Mn alloy: grain refinement mechanism, corrosion behavior, and biocompatibility in vivo. Mater. Sci. Eng., C. 2021;118 doi: 10.1016/j.msec.2020.111391. [DOI] [PubMed] [Google Scholar]
- 95.Liu S., Kent D., Nghiem D., Dargusch M., Wang G. Effects of deformation twinning on the mechanical properties of biodegradable Zn-Mg alloys. Bioact. Mater. 2019;4:8–16. doi: 10.1016/j.bioactmat.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shi Z., Li C., Li M., Li X., Wang L. Second phase refining induced optimization of Fe alloying in Zn: significantly enhanced strengthening effect and corrosion uniformity. Int. J. Miner. Metall. Mater. 2022;29(4):796–806. doi: 10.1007/s12613-022-2468-6. [DOI] [Google Scholar]
- 97.Ye L., Sun C., Zhuo X., Liu H., Ju J., Xue F., Bai J., Jiang J., Xin Y. Evolution of grain size and texture of Zn-0.5Cu ECAP alloy during annealing at 200 degrees C and its impact on mechanical properties, J. Alloy. Compd. 2022;919 doi: 10.1016/j.jallcom.2022.165871. [DOI] [Google Scholar]
- 98.Li R., Ding Y., Zhang H., Lei J., Shen Y. Effective strengthening and toughening in Zn-1Mg alloy with bimodal grain structure achieved by conventional extrusion. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2022;854 doi: 10.1016/j.msea.2022.143850. [DOI] [Google Scholar]
- 99.Wang X., Ma Y., Meng B., Wan M. Effect of equal-channel angular pressing on microstructural evolution, mechanical property and biodegradability of an ultrafine-grained zinc alloy. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2021;824 doi: 10.1016/j.msea.2021.141857. [DOI] [Google Scholar]
- 100.Li Z., Shi Z.-Z., Hao Y., Li H.-F., Liu X.-F., Volinsky A.A., Zhang H.-J., Wang L.-N. High-performance hot-warm rolled Zn-0.8Li alloy with nano-sized metastable precipitates and sub-micron grains for biodegradable stents. J. Mater. Sci. Technol. 2019;35(11):2618–2624. doi: 10.1016/j.jmst.2019.06.009. [DOI] [Google Scholar]
- 101.Sun S., Liu J., Losu D. Microstructure evolution of as-extruded Zn-0.62Mn alloys during room temperature compression. Mater. Sci. Technol. 2021;37(10):930–934. doi: 10.1080/02670836.2021.1964691. [DOI] [Google Scholar]
- 102.Liu Y., He S., Li Y., Liu Z., Li C., Li J., Miao H., Zhu D., Su L. In vitro degradation behavior and microstructural evolution of a novel biodegradable Zn-Mg-Sr alloy during homogenization. J. Mater. Eng. Perform. 2022:4283–4294. doi: 10.1007/s11665-022-07405-z. [DOI] [Google Scholar]
- 103.Wang L., Lou D., Sun S., Ren Y., Qin G., Bai P., Zhao Z. Microstructure evolution of Zn-0.2Mg-0.8Mn(wt-%) alloys with different initial textures during room-temperature compression. Mater. Sci. Technol. 2022;38(16):1368–1375. doi: 10.1080/02670836.2022.2078936. [DOI] [Google Scholar]
- 104.Vida T.A., Silva C.A.P., Lima T.S., Cheung N., Brito C., Garcia A. Tailoring microstructure and microhardness of Zn-1wt.%Mg-(0.5wt.%Mn, 0.5wt.%Ca) alloys by solidification cooling rate. Trans. Nonferrous Metals Soc. China. 2021;31(4):1031–1048. doi: 10.1016/s1003-6326(21)65559-0. [DOI] [Google Scholar]
- 105.Capek J., Kubasek J., Pinc J., Drahokoupil J., Cavojsky M., Vojtech D. Extrusion of the biodegradable ZnMg0.8Ca0.2 alloy - the influence of extrusion parameters on microstructure and mechanical characteristics. J. Mech. Behav. Biomed. Mater. 2020;108 doi: 10.1016/j.jmbbm.2020.103796. [DOI] [PubMed] [Google Scholar]
- 106.Huang H., Liu H., Wang L., Ren K., Yan K., Li Y., Jiang J., Ma A., Xue F., Bai J. Multi-interactions of dislocations and refined microstructure in a high strength and toughness Zn-Mg-Mn alloy. J. Mater. Res. Technol-JMRT. 2020;9(6):14116–14121. doi: 10.1016/j.jmrt.2020.09.126. [DOI] [Google Scholar]
- 107.Shi Z.-Z., Li H.-Y., Xu J.-Y., Gao X.-X., Liu X.-F. Microstructure evolution of a high-strength low-alloy Zn-Mn-Ca alloy through casting, hot extrusion and warm caliber rolling. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2020;771 doi: 10.1016/j.msea.2019.138626. [DOI] [Google Scholar]
- 108.Shi Z.-Z., Yu J., Liu X.-F., Zhang H.-J., Zhang D.-W., Yin Y.-X., Wang L.-N. Effects of Ag, Cu or Ca addition on microstructure and comprehensive properties of biodegradable Zn-0.8Mn alloy. Mater. Sci. Eng., C. 2019;99:969–978. doi: 10.1016/j.msec.2019.02.044. [DOI] [PubMed] [Google Scholar]
- 109.Xue P., Ma M., Li Y., Li X., Yuan J., Shi G., Wang K., Zhang K. Microstructure, hot deformation behavior, and recrystallization behavior of Zn-1Fe-1Mg alloy under isothermal compression. Materials. 2021;14(7):1735. doi: 10.3390/ma14071735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li M., Shi Z.-Z., Wang Q., Cheng Y., Wang L.-N. Zn-0.8Mn alloy for degradable structural applications: hot compression behaviors, four dynamic recrystallization mechanisms, and better elevated-temperature strength. J. Mater. Sci. Technol. 2023;137:159–175. doi: 10.1016/j.jmst.2022.08.002. [DOI] [Google Scholar]
- 111.Liu S., Kent D., Zhan H., Nghiem D., Dargusch M., Wang G. Dynamic recrystallization of pure zinc during high strain-rate compression at ambient temperature. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2020;784 doi: 10.1016/j.msea.2020.139325. [DOI] [Google Scholar]
- 112.Liu S., Kent D., Zhan H., Doan N., Wang C., Yu S., Dargusch M., Wang G. Influence of strain rate and crystallographic orientation on dynamic recrystallization of pure Zn during room-temperature compression. J. Mater. Sci. Technol. 2021;86:237–250. doi: 10.1016/j.jmst.2020.12.077. [DOI] [Google Scholar]
- 113.Chen C., Yue R., Zhang J., Huang H., Niu J., Yuan G. Biodegradable Zn-1.5Cu-1.5Ag alloy with anti-aging ability and strain hardening behavior for cardiovascular stents. Mater. Sci. Eng., C. 2020;116 doi: 10.1016/j.msec.2020.111172. [DOI] [PubMed] [Google Scholar]
- 114.Xu Z., Liu H., Ren K., Sun C., Zhuo X., Yan K., Ju J., Xue F., Bai J., Jiang J. Revealing the abnormal softening mechanisms of Zn-xCu (x=2, 3) wrought alloys by gradually increasing ECAP numbers. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2022;856 doi: 10.1016/j.msea.2022.143962. [DOI] [Google Scholar]
- 115.Ding F., Zhu X., Guo P., Yang L., Zhang Q., Xu C., Zhang Y., Sun W., Song Z. Softening and structural instability mechanism of biodegradable Zn-0.45Mn alloy at different heat treatment temperatures. Mater. Today Commun. 2022;33 doi: 10.1016/j.mtcomm.2022.104768. [DOI] [Google Scholar]
- 116.Piela K., Blaz L., Bochniak W., Ostachowski P., Lagoda M., Zabinski P., Jaskowski M., Kiper M., Polkowska A. Self-hardening of low-alloyed zinc for biodegradable application. J. Alloys Compd. 2019;810 doi: 10.1016/j.jallcom.2019.151883. [DOI] [Google Scholar]
- 117.Zhu S., Wu C., Li G., Zheng Y., Nie J.-F. Creep properties of biodegradable Zn-0.1Li alloy at human body temperature: implications for its durability as stents. Mater. Res. Lett. 2019;7(9):347–353. doi: 10.1080/21663831.2019.1610106. [DOI] [Google Scholar]
- 118.Huang H., Li G., Jia Q., Bian D., Guan S., Kulyasova O., Valiev R.Z., Rau J.V., Zheng Y. Recent advances on the mechanical behavior of zinc based biodegradable metals focusing on the strain softening phenomenon. Acta Biomater. 2022;152:1–18. doi: 10.1016/j.actbio.2022.08.041. [DOI] [PubMed] [Google Scholar]
- 119.Su Y., Yang H., Gao J., Qin Y.-X., Zheng Y., Zhu D. Interfacial zinc phosphate is the key to controlling biocompatibility of metallic zinc implants. Adv. Sci. 2019;6(14) doi: 10.1002/advs.201900112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Du C.-m., Zuo K.-q., Wang X.-y., Huang S.-y., Liu B., Xiao G.-y., Lu Y.-p. Effect of reaction time on the microstructure and properties of in-situ hopeite chemical conversion coatings formed by self-corrosion on zinc alloy. J. Mater. Res. Technol-JMRT. 2022;18:4445–4455. doi: 10.1016/j.jmrt.2022.04.136. [DOI] [Google Scholar]
- 121.Sheng Y., Yang J., Hou R., Chen L., Xu J., Liu H., Zhao X., Wang X., Zeng R., Li W., Xie Y. Improved biocompatibility and degradation behavior of biodegradable Zn-1Mg by grafting zwitterionic phosphorylcholine chitosan (PCCs) coating on silane pre-modified surface. Appl. Surf. Sci. 2020;527 doi: 10.1016/j.apsusc.2020.146914. [DOI] [Google Scholar]
- 122.Kandala B.S.P.K., Zhang G., Hopkins T.M., An X., Pixley S.K., Shanov V. In vitro and in vivo testing of zinc as a biodegradable material for stents fabricated by photo-chemical etching. Appl. Sci.-Basel. 2019;9(21):4503. doi: 10.3390/app9214503. [DOI] [Google Scholar]
- 123.Su S., Tang Q., Qu D. In vitro study of degradation and cytocompatibility of ceramics/PLA composite coating on pure zinc for orthopedic application. Front. Bioeng. Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.856986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Qian J., Chen Y., Zhang W., Mo X., Zou D., Soliman H., Zhou C., Huang N., Sang H., Zeng H., Zhang H., Wan G. Micro/Nano-structured metal-organic/inorganic hybrid coatings on biodegradable Zn for osteogenic and biocompatible improvement. Adv. Mater. Interfac. 2022;9(6) doi: 10.1002/admi.202101852. [DOI] [Google Scholar]
- 125.Pan K., Zhang W., Shi H., Dai M., Yang Z., Chen M., Wei W., Zheng Y., Liu X., Li X. Facile fabrication of biodegradable endothelium-mimicking coatings on bioabsorbable zinc-alloy stents by one-step electrophoretic deposition. J. Mater. Chem. B. 2022;10(16):3083–3096. doi: 10.1039/d2tb00119e. [DOI] [PubMed] [Google Scholar]
- 126.Pan K., Zhang W., Shi H., Dai M., Wei W., Liu X., Li X. Zinc Ion-crosslinked polycarbonate/heparin composite coatings for biodegradable Zn-alloy stent applications. Colloid Surf. B-Biointerfaces. 2022;218 doi: 10.1016/j.colsurfb.2022.112725. [DOI] [PubMed] [Google Scholar]
- 127.Fang H., Qi X., Zhou S., Yang S., Hang C., Tian Y., Wang C. High-efficient vacuum ultraviolet-ozone assist-deposited polydopamine for poly(lactic-co-glycolic acid)-coated pure Zn toward biodegradable cardiovascular stent applications. ACS Appl. Mater. Interfaces. 2022;14(2):3536–3550. doi: 10.1021/acsami.1c21567. [DOI] [PubMed] [Google Scholar]
- 128.Chen Y., Cai J., Liu D., Liu S., Lei D., Zheng L., Wei Q., Gao M. Zinc-based metal organic framework with antibacterial and anti-inflammatory properties for promoting wound healing. Regen. Biomater. 2022;9 doi: 10.1093/rb/rbac019. rbac019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu L., Meng Y., Volinsky A.A., Zhang H.-J., Wang L.-N. Influences of albumin on in vitro corrosion of pure Zn in artificial plasma. Corrosion Sci. 2019;153:341–356. doi: 10.1016/j.corsci.2019.04.003. [DOI] [Google Scholar]
- 130.Levy G.K., Kafri A., Ventura Y., Leon A., Vago R., Goldman J., Aghion E. Surface stabilization treatment enhances initial cell viability and adhesion for biodegradable zinc alloys. Mater. Lett. 2019;248:130–133. doi: 10.1016/j.matlet.2019.04.006. [DOI] [Google Scholar]
- 131.Jian L., Xie J., Hao X., Li B., Liang C., Peng F., Wang D., Xiao J. Enhanced corrosion resistance and cytocompatibility of zinc by Zn-Al layered double hydroxide films. Mater. Lett. 2022;314 doi: 10.1016/j.matlet.2022.131873. [DOI] [Google Scholar]
- 132.Chen Z., Liu X., Cheng Z., Tan X., Xiang Y., Li J., Zhang Y., Lu Z., Kang E.-T., Xu L., Rao X. Degradation behavior, biocompatibility and antibacterial activity of plasma electrolytic oxidation treated zinc substrates. Surf. Coat. Technol. 2023;455 doi: 10.1016/j.surfcoat.2023.129234. [DOI] [Google Scholar]
- 133.Shishir R., Lokeshkumar E., Manojkumar P., Nasiruddin U., Premchand C., Ponnilavan V., Rama Krishna L., Rameshbabu N. Development of biocompatible and corrosion-resistant plasma electrolytic oxidation coating over zinc for orthopedic implant applications. Surf. Coat. Technol. 2022;450 doi: 10.1016/j.surfcoat.2022.128990. [DOI] [Google Scholar]
- 134.Yuan W., Li B., Chen D., Zhu D., Han Y., Zheng Y. Formation mechanism, corrosion behavior, and cytocompatibility of microarc oxidation coating on absorbable high-purity zinc. ACS Biomater. Sci. Eng. 2019;5(2):487–497. doi: 10.1021/acsbiomaterials.8b01131. [DOI] [PubMed] [Google Scholar]
- 135.Shi Y., Yang L., Wang L., Zhang Q., Zhu X., Sun W., Shen J., Lu T., Song Z., Liu H. Corrosion and biocompatibility of pure Zn with a micro-arc-oxidized layer coated with calcium phosphate. Coatings. 2021;11(11):1425. doi: 10.3390/coatings11111425. [DOI] [Google Scholar]
- 136.Bordbar-Khiabani A., Ebrahimi S., Yarmand B. In-vitro corrosion and bioactivity behavior of tailored calcium phosphate-containing zinc oxide coating prepared by plasma electrolytic oxidation. Corrosion Sci. 2020;173 doi: 10.1016/j.corsci.2020.108781. [DOI] [Google Scholar]
- 137.Du C., Zuo K., Ma Z., Zhao M., Li Y., Tian S., Lu Y., Xiao G. Effect of substrates performance on the microstructure and properties of phosphate chemical conversion coatings on metal surfaces. Molecules. 2022;27(19):6434. doi: 10.3390/molecules27196434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Guillory R.J., Sikora-Jasinska M., Drelich J.W., Goldman J. In vitro corrosion and in vivo response to zinc implants with electropolished and anodized surfaces. ACS Appl. Mater. Interfaces. 2019;11(22):19884–19893. doi: 10.1021/acsami.9b05370. [DOI] [PubMed] [Google Scholar]
- 139.Peng F., Lin Y., Zhang D., Ruan Q., Tang K., Li M., Liu X., Chu P.K., Zhang Y. Corrosion behavior and biocompatibility of diamond-like carbon-coated zinc: an in vitro study. ACS Omega. 2021;6(14):9843–9851. doi: 10.1021/acsomega.1c00531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yuan W., Xia D., Zheng Y., Liu X., Wu S., Li B., Han Y., Jia Z., Zhu D., Ruan L., Takashima K., Liu Y., Zhou Y. Controllable biodegradation and enhanced osseointegration of ZrO2-nanofilm coated Zn-Li alloy: in vitro and in vivo studies. Acta Biomater. 2020;105:290–303. doi: 10.1016/j.actbio.2020.01.022. [DOI] [PubMed] [Google Scholar]
- 141.Wang A., Venezuela J., Dargusch M.S. Enhancing the corrodibility of biodegradable iron and zinc using poly (lactic) acid (PLA) coating for temporary medical implant applications. Prog. Org. Coating. 2023;174 doi: 10.1016/j.porgcoat.2022.107301. [DOI] [Google Scholar]
- 142.Shi Y., Yang L., Zhang Q., Zhu X., Song Z., Liu H. A novel MAO-PLA coating on zinc alloy for potential orthopedic implant material. Mater. Lett. 2022;317 doi: 10.1016/j.matlet.2022.132058. [DOI] [Google Scholar]
- 143.Zhou Y., Wang J., Yang Y., Yang M., Zheng H., Xie D., Wang D., Shen L. Laser additive manufacturing of zinc targeting for biomedical application. Int. J. Bioprinting. 2022;8(1):1–22. doi: 10.18063/ijb.v8i1.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liang W., Zhou C., Zhang H., Bai J., Jiang B., Jiang C., Ming W., Zhang H., Long H., Huang X., Zhao J. Recent advances in 3D printing of biodegradable metals for orthopaedic applications. J. Biol. Eng. 2023;17(1):56. doi: 10.1186/s13036-023-00371-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Montani M., Demir A.G., Mostaed E., Vedani M., Previtali B. Processability of pure Zn and pure Fe by SLM for biodegradable metallic implant manufacturing. Rapid Prototyp. J. 2017;23(3):514–523. doi: 10.1108/rpj-08-2015-0100. [DOI] [Google Scholar]
- 146.Pougis A., Toth L.S., Fundenberger J.J., Borbely A. Extension of the Derby relation to metals severely deformed to their steady-state ultrafine-grain size. Scripta Mater. 2014;72–73:59–62. doi: 10.1016/j.scriptamat.2013.10.020. [DOI] [Google Scholar]
- 147.Ning J., Ma Z.-X., Zhang L.-J., Wang D.-P., Na S.-J. Effects of magnesium on microstructure, properties and degradation behaviors of zinc-based alloys prepared by selective laser melting. Mater. Res. Express. 2022;9(8) doi: 10.1088/2053-1591/ac88b7. [DOI] [Google Scholar]
- 148.Zhao D., Han C., Peng B., Cheng T., Fan J., Yang L., Chen L., Wei Q. Corrosion fatigue behavior and anti-fatigue mechanisms of an additively manufactured biodegradable zinc-magnesium gyroid scaffold. Acta Biomater. 2022;153:614–629. doi: 10.1016/j.actbio.2022.09.047. [DOI] [PubMed] [Google Scholar]
- 149.Voshage M., Megahed S., Schueckler P.G., Wen P., Qin Y., Jauer L., Poprawe R., Schleifenbaum J.H. Additive manufacturing of biodegradable Zn-xMg alloys: effect of Mg content on manufacturability, microstructure and mechanical properties, Mater. Today Commun. 2022;32 doi: 10.1016/j.mtcomm.2022.103805. [DOI] [Google Scholar]
- 150.Shuai C., Cheng Y., Yang Y., Peng S., Yang W., Qi F. Laser additive manufacturing of Zn-2Al part for bone repair: formability, microstructure and properties, J. Alloy. Compd. 2019;798:606–615. doi: 10.1016/j.jallcom.2019.05.278. [DOI] [Google Scholar]
- 151.Qin Y., Yang H., Liu A., Dai J., Wen P., Zheng Y., Tian Y., Li S., Wang X. Processing optimization, mechanical properties, corrosion behavior and cytocompatibility of additively manufactured Zn-0.7Li biodegradable metals. Acta Biomater. 2022;142:388–401. doi: 10.1016/j.actbio.2022.01.049. [DOI] [PubMed] [Google Scholar]
- 152.Zhao D., Yu K., Sun T., Jing X., Wan Y., Chen K., Gao H., Wang Y., Chen L., Guo X., Wei Q. Material-structure-function integrated additive manufacturing of degradable metallic bone implants for load-bearing applications. Adv. Funct. Mater. 2023;33(16) doi: 10.1002/adfm.202213128. [DOI] [Google Scholar]
- 153.Yang Y., Yang M., He C., Qi F., Wang D., Peng S., Shuai C. Rare earth improves strength and creep resistance of additively manufactured Zn implants. Compos. Pt. B-Eng. 2021;216 doi: 10.1016/j.compositesb.2021.108882. [DOI] [Google Scholar]
- 154.Yang Y., Cheng Y., Peng S., Xu L., He C., Qi F., Zhao M., Shuai C. Microstructure evolution and texture tailoring of reduced graphene oxide reinforced Zn scaffold. Bioact. Mater. 2021;6(5):1230–1241. doi: 10.1016/j.bioactmat.2020.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gao C., Yao M., Shuai C., Peng S., Deng Y. Nano-SiC reinforced Zn biocomposites prepared via laser melting: microstructure, mechanical properties and biodegradability. J. Mater. Sci. Technol. 2019;35(11):2608–2617. doi: 10.1016/j.jmst.2019.06.010. [DOI] [Google Scholar]
- 156.Li Y., Li W., Bobbert F.S.L., Lietaert K., Dong J.H., Leeflang M.A., Zhou J., Zadpoor A.A. Corrosion fatigue behavior of additively manufactured biodegradable porous zinc. Acta Biomater. 2020;106:439–449. doi: 10.1016/j.actbio.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 157.Li Y., Pavanram P., Zhou J., Lietaert K., Bobbert F.S.L., Kubo Y., Leeflang M.A., Jahr H., Zadpoor A.A. Additively manufactured functionally graded biodegradable porous zinc. Biomater. Sci. 2020;8(9):2404–2419. doi: 10.1039/c9bm01904a. [DOI] [PubMed] [Google Scholar]
- 158.Lietaert K., Zadpoor A.A., Sonnaert M., Schrooten J., Weber L., Mortensen A., Vleugels J. Mechanical properties and cytocompatibility of dense and porous Zn produced by laser powder bed fusion for biodegradable implant applications. Acta Biomater. 2020;110:289–302. doi: 10.1016/j.actbio.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 159.Shuai C., Xue L., Gao C., Peng S., Zhao Z. Rod-like eutectic structure in biodegradable Zn-Al-Sn alloy exhibiting enhanced mechanical strength. ACS Biomater. Sci. Eng. 2020;6(7):3821–3831. doi: 10.1021/acsbiomaterials.0c00290. [DOI] [PubMed] [Google Scholar]
- 160.Du S., Shen Y., Zheng Y., Cheng Y., Xu X., Chen D., Xia D. Systematic in vitro and in vivo study on biodegradable binary Zn-0.2 at% Rare Earth alloys (Zn-RE: Sc, Y, La-Nd, Sm-Lu) Bioact. Mater. 2023;24:507–523. doi: 10.1016/j.bioactmat.2023.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang X., Shao X., Dai T., Xu F., Zhou J.G., Qu G., Tian L., Liu B., Liu Y. In vivo study of the efficacy, biosafety, and degradation of a zinc alloy osteosynthesis system. Acta Biomater. 2019;92:351–361. doi: 10.1016/j.actbio.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 162.Zhang Z., Jia B., Yan H., Han Y., Wu Q., Dai K., Zheng Y. Biodegradable ZnLiCa ternary alloys for critical-sized bone defect regeneration at load-bearing sites: in vitro and in vivo studies. Bioact. Mater. 2021;6(11):3999–4013. doi: 10.1016/j.bioactmat.2021.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jia B., Zhang Z., Zhuang Y., Yang H., Han Y., Wu Q., Jia X., Yin Y., Qu X., Zheng Y., Dai K. High-strength biodegradable zinc alloy implants with antibacterial and osteogenic properties for the treatment of MRSA-induced rat osteomyelitis. Biomaterials. 2022;287 doi: 10.1016/j.biomaterials.2022.121663. [DOI] [PubMed] [Google Scholar]
- 164.Klima K., Ulmann D., Bartos M., Spanko M., Duskova J., Vrbova R., Pinc J., Kubasek J., Vlk M., Ulmannova T., Foltan R., Brizman E., Drahos M., Beno M., Machon V., Capek J. A complex evaluation of the in-vivo biocompatibility and degradation of an extruded ZnMgSr absorbable alloy implanted into rabbit bones for 360 days. Int. J. Mol. Sci. 2021;22(24) doi: 10.3390/ijms222413444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Klima K., Ulmann D., Bartos M., Spanko M., Duskova J., Vrbova R., Pinc J., Kubasek J., Ulmannova T., Foltan R., Brizman E., Drahos M., Beno M., Capek J. Zn-0.8Mg-0.2Sr (wt.%) absorbable screws-an in-vivo biocompatibility and degradation pilot study on a rabbit model. Materials. 2021;14(12):3271. doi: 10.3390/ma14123271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sun J., Zhang X., Shi Z.-Z., Gao X.-X., Li H.-Y., Zhao F.-Y., Wang J.-Q., Wang L.-N. Development of a high-strength Zn-Mn-Mg alloy for ligament reconstruction fixation. Acta Biomater. 2021;119:485–498. doi: 10.1016/j.actbio.2020.10.032. [DOI] [PubMed] [Google Scholar]
- 167.Zhang Z., Jia B., Yang H., Han Y., Wu Q., Dai K., Zheng Y. Zn0.8Li0.1Sr-a biodegradable metal with high mechanical strength comparable to pure Ti for the treatment of osteoporotic bone fractures: in vitro and in vivo studies. Biomaterials. 2021;275 doi: 10.1016/j.biomaterials.2021.120905. [DOI] [PubMed] [Google Scholar]
- 168.Zhou C., Li H.-F., Yin Y.-X., Shi Z.-Z., Li T., Feng X.-Y., Zhang J.-W., Song C.-X., Cui X.-S., Xu K.-L., Zhao Y.-W., Hou W.-B., Lu S.-T., Liu G., Li M.-Q., Ma J.-Y., Toft E., Volinsky A.A., Wan M., Yao X.-j., Wang C.-b., Yao K., Xu S.-k., Lu H., Chang S.-F., Ge J.-B., Wang L.-N., Zhang H.-J. Long-term in vivo study of biodegradable Zn-Cu stent: a 2-year implantation evaluation in porcine coronary artery. Acta Biomater. 2019;97:657–670. doi: 10.1016/j.actbio.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 169.Hehrlein C., Schorch B., Haberstroh J., Bode C., Mey L., Schwarzbach H., Kinscherf R., Meckel S., Schiestel S., Kovacs A., Fischer H., Nennig E. Bioresorbable zinc stent with ultra-thin center struts attenuates stent jail in porcine femoral artery bifurcations. Minim Invasive Ther. Allied Technol. 2022;31(1):72–79. doi: 10.1080/13645706.2020.1770797. [DOI] [PubMed] [Google Scholar]
- 170.Amano H., Miyake K., Hinoki A., Yokota K., Kinoshita F., Nakazawa A., Tanaka Y., Seto Y., Uchida H. Novel zinc alloys for biodegradable surgical staples. World J. Clin. Cases. 2020;8(3):504–516. doi: 10.12998/wjcc.v8.i3.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Guo H., Hu J., Shen Z., Du D., Zheng Y., Peng J. In vitro and in vivo studies of biodegradable Zn-Li-Mn alloy staples designed for gastrointestinal anastomosis. Acta Biomater. 2021;121:713–723. doi: 10.1016/j.actbio.2020.12.017. [DOI] [PubMed] [Google Scholar]
- 172.Qi Z., Liu K.J. The interaction of zinc and the blood-brain barrier under physiological and ischemic conditions. Toxicol. Appl. Pharmacol. 2019;364:114–119. doi: 10.1016/j.taap.2018.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang J., Xu J., Zang G., Zhang T., Wu Q., Zhang H., Chen Y., Wang Y., Qin W., Zhao S., Qin E., Qiu J., Zhang X., Wen L., Wang Y., Wang G. trans-2-Enoyl-CoA reductase tecr-driven lipid metabolism in endothelial cells protects against transcytosis to maintain blood-brain barrier homeostasis. Research. 2022;2022 doi: 10.34133/2022/9839368. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.