Abstract
Recently, a S168T variant in the acetylcholine receptor subunit ACR-8 was associated with levamisole resistance in the parasitic helminth Haemonchus contortus. Here, we used the Xenopus laevis oocyte expression system and two-electrode voltage-clamp electrophysiology to measure the functional impact of this S168T variant on the H. contortus levamisole-sensitive acetylcholine receptor, L-AChR-1.1. Expression of the ACR-8 S168T variant significantly reduced the current amplitude elicited by levamisole compared to acetylcholine, with levamisole changing from a full to partial agonist on the recombinant L-AChR. Functional validation of the S168T mutation on modulating levamisole activity at the receptor level highlights its critical importance as both a mechanism and a marker of levamisole resistance.
Keywords: Levamisole resistance, S168T, Haemonchus contortus, Resistance mechanism, Functional validation, Acetylcholine receptor
Graphical abstract
1. Introduction
The broad-spectrum anthelmintic levamisole (LEV) is widely used to treat veterinary helminth infections (Kotze and Prichard, 2016) and occasionally used in the treatment of human infections with Ascaris spp., and Taenia spp. (Aribodor et al., 2021). Clinical trials to assess LEV for treating loiasis are ongoing (Campillo et al., 2022). LEV is a cholinergic agonist drug which binds to nematode ligand-gated ion channels, specifically levamisole-sensitive acetylcholine receptors (L-AChRs), in the body wall muscles of the parasite (Aceves et al., 1970; Martin et al., 1997; Kopp et al., 2009). The binding of LEV causes the channel to open, which causes spastic paralysis of the parasite and leads to expulsion from the host (Martin and Robertson, 2007; Martin et al., 2012). The reconstitution of Haemonchus contortus L-AChRs using the Xenopus laevis oocyte expression system has been instrumental in characterising the composition of these pentameric L-AChRs (Fauvin et al., 2010; Neveu et al., 2010; Boulin et al., 2011; Blanchard et al., 2018). The H. contortus L-AChR subunits UNC-29.1, UNC-38, UNC-63, and ACR-8 assemble to form the L-AChR-1.1 subtype (Boulin et al., 2011). In contrast, the substitution of UNC-29.1 by either UNC-29.3 or UNC-29.4 will form the L-AChR-1.3 and L-AChR-1.4 subtypes, respectively (Duguet et al., 2016). Delineating the functional composition of these receptors revealed that ACR-8 is crucial in conferring sensitivity to LEV in vitro and in vivo (Boulin et al., 2011; Blanchard et al., 2018). Indeed, in the absence of ACR-8, the combination of UNC-29.1, UNC-38, and UNC-63 will assemble to form Hco-L-AChR-2, a receptor subtype relatively unresponsive to levamisole but highly sensitive to nicotine and pyrantel (Martin et al., 2012).
Recently, a forward genetic cross between multidrug-resistant and drug-susceptible strains of H. contortus revealed a non-synonymous single nucleotide polymorphism (SNP) in acr-8 that was strongly associated with LEV resistance (Doyle et al., 2022). This SNP conferred a serine-to-threonine substitution (S168T) in exon 4, which was present in all LEV-resistant populations examined to date (including USA, South Africa, and Australia) and absent in all sensitive populations with available sequencing data (Sallé et al., 2019; Antonopoulos et al., 2022; Francis et al., 2024). Furthermore, a serine-to-threonine substitution was present at the analogous position of acr-8 in LEV-resistant T. circumcincta after re-analysis of existing data (Choi et al., 2017; Doyle et al., 2022). This putative convergent evolution would suggest that T168 may play a mechanistic role in LEV resistance in different trichostrongylid species. While S168T represents a robust genetic marker for LEV resistance, the functional relevance of this mutation remained undefined. Here, we have used the X. laevis heterologous expression system to recapitulate the H. contortus L-AChR and measure the functional impact of S168T on the LEV receptor target.
2. Materials and methods
2.1. Cloning the acr-8 S168T variant
Primer sequences to amplify the entire coding sequence of the H. contortus acr-8 gene (HCON_00151270) (Doyle et al., 2022) were designed in Geneious Prime (Biomatters Ltd: 11.1.5) and ordered from Eurofins Genomics: acr8F (ATGCGTGCATTCGGAATTG) and acr8R (TCACAAGCCTTCAGAATTC). Total RNA was extracted from a pool of 20 H. contortus adult males and females of the multi-drug (levamisole, macrocyclic lactone, and benzimidazole) resistant MHco18 (UGA2004) isolate and used for cDNA synthesis following standard methods previously described (Doyle et al., 2022). Phusion Green High-Fidelity PCR was carried out according to manufacturer instructions to amplify the full-length acr-8 gene with the following parameters: 40 cycles: denaturation at 95 °C for 30 s, annealing 58 °C for 30 s, and extension at 72 °C for 120 s, with a final extension at 72 °C for 10 min. The resulting acr-8 amplicon was cloned into the TOPO2.1 vector (Thermo Fisher) and transformed into XL10 gold ultracompetent E. coli (Agilent), as previously described (Antonopoulos et al., 2022). Colonies were screened with an allele-specific PCR for S168T as described previously (Antonopoulos et al., 2022) before plasmid isolation and capillary sequencing at Eurofins Genomics. The full-length acr-8 with S168T in TOPO2.1 was then subcloned into the transcription vector pTB207, which contained the 3′UTR of X. laevis beta-globin (Boulin et al., 2011). The resulting construct was sequence-checked, linearised and used as a template for in vitro cRNA synthesis using the mMessage mMachine T7 transcription kit (Ambion).
2.2. Expression of L-AChR subunits in Xenopus oocytes and electrophysiology
Expression of the L-AChR subunits unc-29.1, unc-38, unc-63 and acr-8 (wild type or S168T variant) in X. laevis oocytes (Ecocyte Bioscience) and two-voltage clamp electrophysiology manipulations were performed as previously described (Boulin et al., 2011; Blanchard et al., 2018). In brief, X. laevis oocytes were injected with 36 nL of cRNA mix containing 50 ng μL−1 of each subunit: unc-63, unc-29.1, unc-38, and acr-8 (S168 or S168T variant), along with 50 ng μL−1 of each H. contortus ancillary proteins ric-3.1, unc-50, and unc-74 (Boulin et al., 2011). Furthermore, a 1:1 mix of S168 and S168T (25 ng μL−1 of each to a total of 50 ng μL−1) was used along with unc-63, unc-29.1, unc-38 and the ancillary protein cRNAs. All transcription vector constructs were generated in these previous studies, other than the construct encoding the S168T variant acr-8 described above. Currents elicited by levamisole (L9756, Sigma-Aldrich) and acetylcholine (A9101, Sigma-Aldrich) were analysed using the pCLAMP 10.4 package (Molecular Devices). All concentration-response relationships were carried out 4–5 days after injection in recording solution consisting of 100 mM NaCl, 2.5 mM KCl, 1 mM CaCl2, and 5 mM HEPES, at pH 7.3 (Boulin et al., 2011). The currents are shown as the ratio of the responses to that for 100 μM ACh. The EC50 value is the concentration giving half of the maximum response and Imax is the relative maximal current obtained at saturating agonist concentration. Results are shown as mean ± SEM. For statistical analysis, a two-sample t-test (https://select-statistics.co.uk/calculators/two-sample-t-test-calculator/) was used to compare the mean recordings of each receptor against the wild-type receptor.
3. Results and discussion
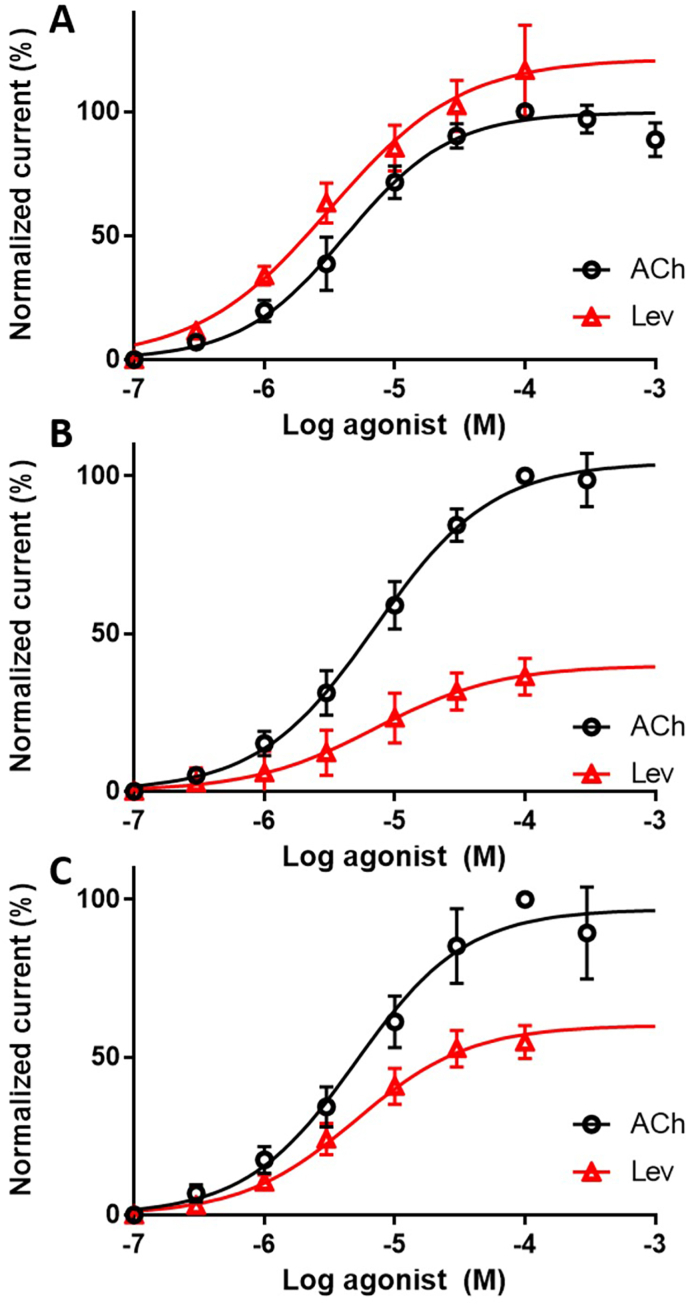
To examine the functional impact of S168T on the AChR, the H. contortus L-AChR-1.1 was reconstituted in Xenopus oocytes by microinjection of the unc-29.1, unc-38, unc-63 and acr-8 cRNAs and assessed with two-electrode voltage-clamp electrophysiology. The expression of H. contortus L-AChR-1.1 with wild-type ACR-8 produced functional channels with acetylcholine (ACh) and LEV EC50 values of 4.2 ± 1.1 (n = 16) and 3.2 ± 1.1 μM (n = 15), respectively. These findings were consistent with previous studies, with LEV acting as a super-agonist (121.4 ± 3.3 % of ACh response) (Fig. 1A, Table 1) (Boulin et al., 2011; Blanchard et al., 2018) (see Table 2).
Fig. 1.
Concentration-response curves of ACh (black) and LEV (red) on the Haemonchus contortus “wild-type” L-AChR-1.1 receptor (A), on the L-AChR-1.1 with the S168T Hco-ACR-8 replacing the wild-type Hco-ACR-8 subunit (“homozygous mutant”) (B), and on the L-AChR-1.1 with S168T Hco-ACR-8 and wild-type Hco-ACR-8 at a 1:1 relative ratio (“heterozygous mutant”) (C). All responses were normalized to 100 μM ACh. The data shown represents mean ± SEM. Sample sizes are shown in Table 1.
Table 1.
Summary of the EC50 and Imax values for acetylcholine (ACh) and levamisole (LEV) on the Hco-L-AChR-1.1 and the different ACR-8 subunits assessed in the context of L-AChR-1.1 expressed in Xenopus oocytes. All currents were normalized to the maximum response obtained by 100 μM ACh. Imax is the relative maximal current obtained at saturating agonist concentration. Results are shown as mean ± SEM.
| L-AChR subunit combination | ACh EC50 (μM) | LEV EC50 (μM) | ACh Imax | LEV Imax | |
|---|---|---|---|---|---|
| UNC-29/UNC-38/UNC-63 | + Hco-ACR-8 (“homozygous wild-type”) | 4.2 ± 1.1 (n = 16) | 3.2 ± 1.1 (n = 15) | 99.7 ± 1.3 (n = 16) | 121.4 ± 3.3 (n = 15) |
| + Hco-ACR-8 S168T (“homozygous mutant”) | 7.1 ± 1.1 (n = 7) | 6.8 ± 1.3 (n = 13) | 104.4 ± 2.4 (n = 7) | 39.5 ± 3.1 (n = 13) | |
| + Hco-ACR-8+ Hco-ACR-8 S168T (“heterozygous”) | 5.3 ± 1.1 (n = 7) | 4.1 ± 1.1 (n = 8) | 97.8 ± 3.1 (n = 7) | 57.0 ± 1.7 (n = 8) | |
Table 2.
Summary of statistical comparisons of EC50 and Imax values for L-AChR subunit combinations. ACh. Imax is the relative maximal current obtained at saturating agonist concentration.
| L-AChR subunit combination (ligand) EC50 | Statistical significance (p-value) |
|---|---|
| S168 vs S168T (ACh) | p=<0.001 |
| S168 vs S168T (LEV) | p=<0.001 |
| S168 vs S168 + S168T (ACh) | p = 0.048 |
| S168 vs S168 + S168T (LEV) | p = 0.082 |
| L-AChR subunit combination (ligand) Imax | Statistical significance (p-value) |
|---|---|
| S168 vs S168T (ACh) | p = 0.001 |
| S168 vs S168T (LEV) | p=<0.001 |
| S168 vs S168 + S168T (ACh) | p = 0.162 |
| S168 vs S168 + S168T (LEV) | p=<0.001 |
To assess if the S168T ACR-8 subunit could assemble into functional L-AChRs, we replaced the wild-type ACR-8 with the S168T variant (Fig. 1B); this resulted in functional channels, evidenced by large ACh-induced currents. Strikingly, the application of 100 μM LEV produced significantly smaller currents than ACh (39.5 ± 3.1 % of ACh response, p=< 0.001), indicating that LEV acts as a partial agonist on channels containing the S168T ACR-8 variant. The ACh and LEV concentration-response curves were characterised by EC50 values of 7.1 ± 1.1 (n = 7) and 6.8 ± 1.3 μM (n = 13), respectively, which are significantly higher than L-AChR containing wild-type ACR-8 (p=< 0.001). With LEV behaving as a partial agonist, it could be hypothesised that these results may be explained by the formation of an L-AChR-2 subtype made of UNC-29.1, UNC-38 and UNC-63 subunits only (Boulin et al., 2011). However, the fact that the ACh and LEV EC50 values were much closer to those of L-AChR-1.1 and were significantly lower than those of L-AChR-2 argues (19.2 ± 0.7 and 48.2 ± 0.9 μM, respectively) against this hypothesis.
To mimic a heterozygous genotype of a worm potentially harbouring mixed populations of L-AChR-1.1, we co-injected cRNAs of S168T acr-8 along with wild-type acr-8 at a 1:1 concentration ratio (Fig. 1C). Surprisingly, the inclusion of both variants led to a significant reduction in the average current to 57.0 ± 1.7 % of the 100 μM ACh response (p=<0.001) after exposure to 100 μM LEV. No significant difference (p = 0.082) in the LEV EC50 values was found between the eggs expressing both variants (4.1 ± 1.1 μM, n = 8) and eggs expressing only the wild-type-L-AChR-1.1 (3.2 ± 1.1 μM, n = 15). However, a significant difference (p = 0.048) was found between the ACh EC50 value of the wild-type L-AChR-1.1 (4.2 ± 1.1 μM, n = 16) and the mixed L-AChRs (5.3 ± 1.1, n = 7). This suggests that by making LEV a partial agonist, the S168T variant could confer a slightly lower susceptibility to LEV in heterozygous worms, although not to a significant degree. The lack of a significant difference observed between the EC50 values for LEV evoked currents in the Xenopus oocytes containing both the S168 and S168T variant, but a significant difference in EC50 values for ACh ACR-8, implies that receptor function could be compromised in heterozygous worms. However, final validation of this observation in vivo would require transgenesis, which is not currently feasible in H. contortus.
4. Conclusion
In summary, we demonstrate that the S168T ACR-8 subunit can substitute for wild-type ACR-8 to form a functional receptor, resulting in a change in the action of LEV from full agonist to partial agonist. This result is consistent with the lack of LEV sensitivity of the C. elegans ACR-8 (Qian et al., 2008; Hernando et al., 2012), which possesses a threonine at the analogous position. Further, the presence of the S168T variant at the equivalent position in LEV-resistant T. circumcincta ACR-8 (Choi et al., 2017) suggests this variant could be the basis for a conserved resistance mechanism in different trichostrongylid species. These data support that the S168T variant in ACR-8 underlies the LEV resistance phenotype in parasites carrying this mutation and represents a significant development toward defining the molecular and genetic basis of LEV resistance.
Declaration of competing interest
The authors report no conflict of interest for this work.
Acknowledgements
This work was funded by a BBSRC International Partnership grant [BB/W510658/1]. CN and CLC are supported by the Institut National de Recherche pour l’Agriculture, l’Alimentation et l’Environnement (INRAE). SRD is supported by a UKRI Future Leaders Fellowship [MR/T020733/1] and the Wellcome Trust (UK) through core funding to the Wellcome Sanger Institute (UK) [206194]. RL is supported by a Wellcome Clinical Research Career Development Fellowship [216614/Z/19/Z] and a University of Glasgow Lord Kelvin Adam Smith Fellowship (LKAS). For the purpose of Open Access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
References
- Aceves J., Erlij D., Martínez-Marañón R. The mechanism of the paralysing action of tetramisole on Ascaris somatic muscle. Br. J. Pharmacol. 1970;38:602–607. doi: 10.1111/j.1476-5381.1970.tb10601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonopoulos A., Doyle S.R., Bartley D.J., Morrison A.A., Kaplan R., Howell S., Neveu C., Busin V., Devaney E., Laing R. Allele specific PCR for a major marker of levamisole resistance in Haemonchus contortus. Int. J. Parasitol.: Drugs Drug Resist. 2022;20:17–26. doi: 10.1016/j.ijpddr.2022.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aribodor O.B., Ekwunife C.A., Sam-Wobo S.O., Aribodor D.N., Ejiofor O.S., Ugwuanyi I.K., Bonney J.H.K. Status of intestinal helminth infection in schools implementing the home-grown school feeding program and the impact of the program on pupils in anambra state, Nigeria. Acta Parasitol. 2021;66:1528–1537. doi: 10.1007/s11686-021-00429-w. [DOI] [PubMed] [Google Scholar]
- Blanchard A., Guégnard F., Charvet C.L., Crisford A., Courtot E., Sauvé C., Harmache A., Duguet T., O'Connor V., Castagnone-Sereno P. Deciphering the molecular determinants of cholinergic anthelmintic sensitivity in nematodes: when novel functional validation approaches highlight major differences between the model Caenorhabditis elegans and parasitic species. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1006996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulin T., Fauvin A., Charvet C.L., Cortet J., Cabaret J., Bessereau J.L., Neveu C. Functional reconstitution of Haemonchus contortus acetylcholine receptors in Xenopus oocytes provides mechanistic insights into levamisole resistance. Br. J. Pharmacol. 2011;164:1421–1432. doi: 10.1111/j.1476-5381.2011.01420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campillo J.T., Bikita P., Hemilembolo M., Louya F., Missamou F., Pion S.D.S., Boussinesq M., Chesnais C. Safety and efficacy of levamisole in loiasis: a randomized, placebo-controlled, double-blind clinical trial. Clin. Infect. Dis. 2022;75:19–27. doi: 10.1093/cid/ciab906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y.J., Bisset S.A., Doyle S.R., Hallsworth-Pepin K., Martin J., Grant W.N., Mitreva M. Genomic introgression mapping of field-derived multiple-anthelmintic resistance in Teladorsagia circumcincta. PLoS Genet. 2017;13 doi: 10.1371/journal.pgen.1006857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle S.R., Laing R., Bartley D., Morrison A., Holroyd N., Maitland K., Antonopoulos A., Chaudhry U., Flis I., Howell S. Genomic landscape of drug response reveals mediators of anthelmintic resistance. Cell Rep. 2022;41 doi: 10.1016/j.celrep.2022.111522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguet T.B., Charvet C.L., Forrester S.G., Wever C.M., Dent J.A., Neveu C., Beech R.N. Recent duplication and functional divergence in parasitic nematode levamisole-sensitive acetylcholine receptors. PLoS Neglected Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauvin A., Charvet C., Issouf M., Cortet J., Cabaret J., Neveu C. cDNA-AFLP analysis in levamisole-resistant Haemonchus contortus reveals alternative splicing in a nicotinic acetylcholine receptor subunit. Mol. Biochem. Parasitol. 2010;170:105–107. doi: 10.1016/j.molbiopara.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Francis E.K., Antonopoulos A., Westman M.E., McKay-Demeler J., Laing R., Slapeta J. A mixed amplicon metabarcoding and sequencing approach for surveillance of drug resistance to levamisole and benzimidazole in Haemonchus spp. Int. J. Parasitol. 2024;54:55–64. doi: 10.1016/j.ijpara.2023.07.002. [DOI] [PubMed] [Google Scholar]
- Kopp S.R., Coleman G.T., Traub R.J., McCarthy J.S., Kotze A.C. Acetylcholine receptor subunit genes from Ancylostoma caninum: altered transcription patterns associated with pyrantel resistance. Int. J. Parasitol. 2009;39:435–441. doi: 10.1016/j.ijpara.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Kotze A., Prichard R. Anthelmintic resistance in Haemonchus contortus: history, mechanisms and diagnosis. Adv. Parasitol. 2016;93:397–428. doi: 10.1016/bs.apar.2016.02.012. [DOI] [PubMed] [Google Scholar]
- Martin R.J., Robertson A.P. Mode of action of levamisole and pyrantel, anthelmintic resistance, E153 and Q57. Parasitology. 2007;134:1093–1104. doi: 10.1017/S0031182007000029. [DOI] [PubMed] [Google Scholar]
- Martin R.J., Robertson A.P., Bjorn H. Target sites of anthelmintics. Parasitology. 1997;114(Suppl. l):S111–S124. [PubMed] [Google Scholar]
- Martin R.J., Robertson A.P., Buxton S.K., Beech R.N., Charvet C.L., Neveu C. Levamisole receptors: a second awakening. Trends Parasitol. 2012;28:289–296. doi: 10.1016/j.pt.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neveu C., Charvet C.L., Fauvin A., Cortet J., Beech R.N., Cabaret J. Genetic diversity of levamisole receptor subunits in parasitic nematode species and abbreviated transcripts associated with resistance. Pharmacogenetics Genom. 2010;20:414–425. doi: 10.1097/FPC.0b013e328338ac8c. [DOI] [PubMed] [Google Scholar]
- Sallé G., Doyle S.R., Cortet J., Cabaret J., Berriman M., Holroyd N., Cotton J.A. The global diversity of Haemonchus contortus is shaped by human intervention and climate. Nat. Commun. 2019;10:4811. doi: 10.1038/s41467-019-12695-4. [DOI] [PMC free article] [PubMed] [Google Scholar]