Abstract
Islet transplantation is the most effective treatment strategy for type 1 diabetes. Long-term storage at ultralow temperatures can be used to prepare sufficient islets of good quality for transplantation. For freezing islets, dimethyl sulfoxide (DMSO) is a commonly used penetrating cryoprotective agent (CPA). However, the toxicity of DMSO is a major obstacle to cell cryopreservation. Hydroxyethyl starch (HES) has been proposed as an alternative CPA. To investigate the effects of two types of nonpermeating CPA, we compared 4 % HES 130 and HES 200 to 10 % DMSO in terms of mouse islet yield, viability, and glucose-stimulated insulin secretion (GSIS). After one day of culture, islets were cryopreserved in each solution. After three days of cryopreservation, islet recovery was significantly higher in the HES 130 and HES 200 groups than in the DMSO group. Islet viability in the HES 200 group was also significantly higher than that in the DMSO group on Day 1 and Day 3. Stimulation indices determined by GSIS were higher in the HES 130 and 200 groups than in the DMSO group on Day 3. After three days of cryopreservation, HES 130 and HES 200 both reduced the expression of apoptosis- and necrosis-associated proteins and promoted the survival of islets. In conclusion, the use of HES as a CPA improved the survival and insulin secretion of cryopreserved islets compared with the use of a conventional CPA.
Keywords: Islet, Hydroxyethyl starch, Islet cryopreservation, Islet cryoprotective agent, Mouse islet cryopreservation
Highlights
-
•
Hydroxyethyl starch maintained islet viability and function after cryopreservation.
-
•
HES reduces apoptosis or necrosis during three-day cryopreservation of islets.
-
•
HES can replace DMSO as a CPA in the cryopreservation of islets.
1. Introduction
Type I diabetes, an autoimmune disease that destroys insulin-producing pancreatic β-cells, is caused by various environmental and genetic factors. Allo-islet transplantation is the most effective strategy for successfully treating type 1 diabetes that is complicated by lability or hypoglycemia unawareness. Initially, transplant of islets from multiple donors resulted in sustained insulin production for normal blood glucose levels; however, exogenous insulin therapy can rarely be withdrawn after islet transplant from a single donor, which suggests that increased islet mass is needed [1]. To solve this problem, islets can be collected and stored in a cell bank using a method such as cryopreservation. Cryopreservation of islets is the best option for transplanting a sufficient number of islets to recipients at remote locations. This method also allows sufficient time for quality control of the isolated islets. In addition, high-quality islets suitable for the patient can be prepared in advance and then selectively transplanted.
However, cryopreserved islets can be nonfunctional or nonviable because of ice crystal formation and cryoprotectant toxicity [[2], [3], [4], [5], [6]]. Several studies have proposed advanced techniques that could increase cell survival and function after cryopreservation and vitrification [7,8]. Many cryoprotective agents (CPAs) have been reported. Among them, permeable CPAs, such as ethylene glycol (EG) and dimethyl sulfoxide (DMSO), have been commonly used for most types of cell cryopreservation, including that of islets [9], but the toxicity of CPAs is a major obstacle to cell survival after the freezing and thawing process [[10], [11], [12]].
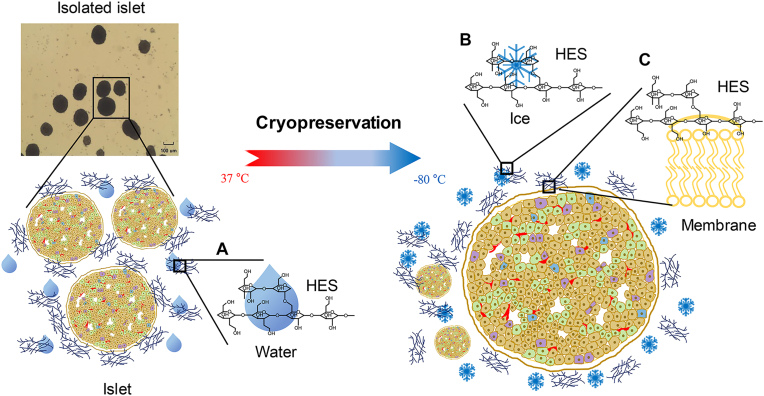
Hydroxyethyl starches (HESs) are polymers that have already been introduced into clinical practice in areas such as the separation of granulocytes from human blood cells, surgical operations, and hemostasis [[13], [14], [15], [16]]. Since their introduction, pharmaceutical improvements have been made, and HESs have been shown to be safe and effective for clinical use [[17], [18], [19]]. They have also been suggested as an alternative CPA [[20], [21], [22]]. These molecules are modified polymers of amylopectin (Fig. 1A) and are synthesized with various properties by hydroxyethyl substitution at carbon positions C2 and C6 (Fig. 1B). In previous studies, a mixture of HES and DMSO was used in the cryopreservation of canine and human islets [23], but no studies have examined the efficacy of HESs alone in islets (only in hamster ovarian cells and human monocytes) [11,24]. In addition, no studies have compared the results of cryopreservation according to the molecular weight (MW) or molar substitution (MS) of HESs in islet experiments.
Fig. 1.
The structure of hydroxyethyl starch (HES). (A) The structural formula for HES. (B) The substitution of the HES hydroxyethyl group (CH2CH2OH) takes place preferentially at the C6 and C2 positions.
In this study, we compared the effects of two different HESs with DMSO alone on mouse islet function after freezing. The HESs used in the experiment were HES 130/0.4, with an average MW of 130 kDa and an average MS of 0.4, and HES 200/0.5, with an average MW of 200 kDa and an average MS of 0.5, at a concentration of 4 % (Table 1). The aim of this study was to determine the potential benefits of new preservation solutions when freezing mouse islets for cryopreservation storage and transplantation.
Table 1.
Features of two different hydroxyethyl starches (HESs).
| MW Range |
Mean MW, kDA | MS Range |
Mean MS | C2/C6 ratio | |
|---|---|---|---|---|---|
| HES 130/0.4 | 110,000–150,000 | 130 | 0.38–0.45 | 0.4 | 9:1 |
| HES 200/0.5 | 180,000–290,000 | 200 | 0.43–0.50 | 0.5 | 5:1 |
MW, molecular weight; MS, molar substitution.
2. Materials and methods
2.1. Preservation solutions
Two types of HESs, HES 130/0.4 (HES 130) and HES 200/0.5 (HES 200) (Ak Scientific, Inc., Union City, CA, USA) were used for islet freezing. Briefly, 10 g of each HES was dissolved separately in 50 mL of Hank's balanced salt solution (HBSS; Sigma-Aldrich, St. Louis, MO, USA, #55021C-1000 ML) to prepare the 20 % stock solutions. The two 20 % stock solutions were diluted with fetal bovine serum (FBS) to make a final 4 % working solution.
2.2. Animals
We obtained 20 male C57BL/6 mice from Orientbio (Sungnam, Korea). The mice were maintained under specific pathogen-free conditions in accordance with the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care in an approved facility (No. 001003) following a protocol approved by the Institutional Animal Care and Use Committee at the Laboratory Animal Research Center of Samsung Biomedical Research Institute. All experimental procedures were conducted in accordance with the guidelines outlined in the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (Publication No. 86–23, revised 2011).
2.3. Mouse islet isolation
Mouse islets were isolated from 10- to 12-week-old male C57BL/6 mice as described previously [25]. Briefly, 0.8 mg/mL collagenase P (Roche, Indianapolis, IN, USA, #45–11213873001) in HBSS was infused into the common bile duct for mouse pancreatic digestion. Islets were purified from the digested pancreas using a discontinuous Ficoll (Biochrom, Berlin, Germany, #L1655) gradient (1.10/1.085/1.069/1.037) and washed several times with 1 × HBSS. Prior to the in vitro studies, isolated islets floating freely in 10 mL of RPMI 1640 medium (Gibco, Grand Island, NY, USA, #11875–093) supplemented with 10 % heat-inactivated FBS (Gibco-Thermo Fisher Scientific, Waltham, MA, USA, #16000–044) were cultured at 37 °C and 5 % CO2.
2.4. Cryopreservation and thawing of islets
After overnight culture, islets were sedimented by centrifugation at 470g for 1 min and resuspended in FBS supplemented with 10 % DMSO, 4 % HES 130, or 4 % HES 200. 200–300 islets were transferred into a 1-mL cryovial and cooled at a rate of −1 °C/min to −80 °C using a Mr. Frosty for 24 h. The samples were then stored in liquid nitrogen at −196 °C. After one or three days of storage in liquid nitrogen, the islets were thawed rapidly in a 37 °C water bath for a few seconds. The islets were washed with RPMI-1640 containing 10 % FBS, sedimented, and placed in fresh culture medium.
2.5. Islet counting and recovery assessment
To assess islet recovery, the quantity of islets was calculated using an islet equivalents number (IEQ) that was obtained by counting the number of islets in an average area of 150 μm after staining them with dithizone (DTZ). The stock solution of DTZ (10 mg/mL) was prepared in DMSO. We diluted the stock solution 10 times in HBSS and then added it to the islet samples for 1–2 min of staining. We examined the samples under a microscope with a 50-μm grid to conduct a morphological evaluation and count the DTZ-stained islets. Islet recovery (%) was calculated as the islet counts after freezing/the islet counts before freezing × 100.
2.6. AO/PI assay
To assess islet viability after culture and thawing, 0.67 μmol/L of acridine orange (AO, Sigma) and 75 μmol/L of propidium iodide (PI, Sigma) were used to identify living and dead islets, respectively. Stained islets were observed on a fluorescence microscope (Eclipse 80i; Nikon, Tokyo, Japan) at emission wavelengths of 488 nm (live) and 594 nm (dead). Both live and dead areas were quantified using Image-Pro Plus 6.0. Islet viability (%) was calculated as living-area cells (488-nm area)/total-area cells (488-nm and 594-nm areas) × 100.
2.7. Glucose-stimulated insulin secretion (GSIS) assay
After the islets were hand-picked and washed with Phosphate buffered saline (PBS), they were seeded on 12-mm diameter insert wells (Merck Millipore, Billerica, MA, USA) with 10 islets per well and preincubated with 3.4 mM (60 mg/dL, basal) glucose in Krebs-Ringer buffer (KRB: 129 mM NaCl, 4.8 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4, 5 mM NaHCO3, 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, and 0.2 % bovine serum albumin) for 90 min at 37 °C. After being washed, the islets were incubated with 17 mM (300 mg/dL, stimulated) glucose-KRB for 1 h followed by an additional incubation with 3.4 mM glucose-KRB for 1 h. Insulin released into the supernatant by the mouse islets was measured using an enzyme-linked immunosorbent assay (ELISA) (ALPCO, Salem, NH, USA, #80-INSMS-E01). The GSIS index was defined as the ratio of the insulin level at 17 mM glucose to that at 3.4 mM glucose.
2.8. Western blot analysis
The islets were washed twice with PBS and then lysed via sonication in lysis buffer (Intron, Seoul, Korea). Equivalent amounts (20 μg) of protein from the mouse islets of each group were separated on 10 % sodium dodecyl sulfate–polyacrylamide gels and then electrically transferred to nitrocellulose membranes (Protran BA83; Whatman, #10600045). Immunoblotting was performed using the following primary antibodies and dilutions: anti-poly ADP-ribose polymerase (anti-PARP) (1:1000; Cell Signaling Technology, Danvers, MA, USA, #9532T), anti-caspase-9 (1:1000; Cell Signaling Technology, Danvers, MA, USA, #9508T), anti-caspase-3 (1:500; Abcam, Cambridge, MA, USA, #ab13585), anti-IκB-α (1:1000; Cell Signaling Technology, Danvers, MA, USA, #9242S), anti–NF–κB (1:1000; Cell Signaling Technology, Danvers, MA, USA, #8242T), and anti-β-actin (1:10000; Sigma, St. Louis, MO, USA, #A5316-2 ML). Horseradish peroxidase–labeled rabbit anti-mouse (1:5000; Abcam, Cambridge, MA, USA, #ab97046) and goat anti-rabbit (1:2000; Santa Cruz, Dallas, TX, USA, #SC-2357) were used as the secondary antibodies. The proteins were visualized using an ECL detection system (Ab Frontier, Seoul, Korea, #LF-QC0101).
2.9. Statistical analysis
The results are expressed as the mean ± standard deviation (SD) or the median and interquartile range, as appropriate. Continuous variables were compared using Student's t-test, one-way analysis of variance (ANOVA), or the Mann-Whitney U test, as appropriate. Longitudinal data were analyzed by two-way ANOVA with Bonferroni post hoc testing using GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA). A p value < 0.05 was accepted as statistically significant for comparisons.
3. Results
3.1. Islet morphology and recovery after cryopreservation
The greatest advantage of preserving islets by cryopreservation is the maintenance of quantity comparable to that of freshly isolated islets. To determine whether we achieved this, we counted the numbers and confirmed the morphology of islets on Day 1 and Day 3 after freezing them in three solutions, DMSO, HES 130, and HES 200, and thawing them. After isolation, the islets were checked and quantified with DTZ staining (Fig. 2A). After cryopreservation for one day, the islets in the HES 130 and 200 groups were more rounded and smoother on the surface than the islets in DMSO. After cryopreservation for three days, the DMSO group had the greatest number of single cells that had detached from the islets. The average ratios of islet numbers after cryopreservation for one day compared with the number of islets before cooling (islet recovery after one day of cryopreservation) did not differ significantly among the groups. However, after three days of cryopreservation, islet recovery was significantly higher in the HES 200 (85.31 ± 1.95 %) group than in the HES 130 (80.28 ± 1.88 %) and DMSO (74.80 ± 3.25 %) groups (Fig. 2B). Thus, the morphology and islet recovery in the HES preservation solutions, especially HES 200, were better than those in DMSO.
Fig. 2.
The survival of mouse islets after cryopreservation in DMSO, HES 130, and HES 200. (A) Islet morphology. Upper: islets immediately after isolation (0 h); middle: islets after freezing (24 h), bottom: islets after freezing (72 h). (B) Recovery after 0, 24 h, and 72 h of islet freezing (N = 4 per group). Data are expressed as the mean ± SD of the islet cell number/initial islet cell number × 100 (%) in each group at 0, 24 h, and 72 h *, p < 0.05; ***, p < 0.001. Bar = 200 μm.
3.2. The effect of preservation solutions on the viability and function of mouse islets
Viability and functionality tests were performed to confirm the state of the mouse islets after freezing and thawing. After cryopreservation for one and three days, PI-stained dead cells were more abundant in the DMSO group than in the HES 130 and 200 groups (Fig. 3A). Compared with those frozen with DMSO, the mouse islets frozen using HES 200 showed greater islet viability after both one and three days, which was demonstrated by the level of green AO-positive cells (Day 1: DMSO 67.30 ± 11.57 % vs. HES 130 82.12 ± 10.22 % and HES 200 86.99 ± 9.40 %; Day 3: DMSO 54.15 ± 20.68 % vs. HES 130 70.29 ± 11.20 % and HES 200 75.94 ± 5.75 %).
Fig. 3.
Islet viability and function. (A) Islets were stained with acridine orange (AO, green) and propidium iodide (PI, red). Representative images show the mean values of the three experimental groups at one and three days. Islet viabilities in the DMSO, HES 130, and HES 200 groups were estimated from photographs (N = 5 to 8 per group). (B) Glucose-stimulated insulin secretion (GSIS) of mouse islets in each group. The GSIS was evaluated using ELISA following ex vivo culture after one day and three days of cryopreservation (N = 3 to 6 per group). The stimulation index was calculated as the ratio of stimulated (300 mg/dL) to basal (60 mg/dL) insulin release. All data are presented as the mean ± SD. *, p < 0.05; **, p < 0.01. #, p < 0.05; ###, p < 0.001; ####, p < 0.0001 vs. Day 0. Bar = 200 μm.
To evaluate the functionality of pancreatic islets after freezing and thawing, the amount of insulin secreted after stimulation with glucose was measured (Basal on Day 1: DMSO 0.23 ± 0.02 ng/mL vs. HES 130 2.12 ± 0.18 ng/mL and HES 200 1.92 ± 0.20 ng/mL; High stimulation on Day 1: DMSO 0.39 ± 0.13 ng/mL vs. HES 130 3.18 ± 0.60 ng/mL and HES 200 3.43 ± 0.19 ng/mL; Basal on Day 3: DMSO 1.89 ± 0.70 ng/mL vs. HES 130 2.53 ± 0.27 ng/mL and HES 200 2.87 ± 0.49 ng/mL; High stimulation on Day 3: DMSO 0.64 ± 0.01 ng/mL vs. HES 130 2.43 ± 0.18 ng/mL and HES 200 3.10 ± 0.50 ng/mL). In terms of the stimulation indices obtained in the GSIS assay, the in vitro functionality of the mouse islets frozen with HES 130 and 200 was significantly higher than that of the DMSO group after cryopreservation for three days (DMSO: 0.38 ± 0.13 vs. HES 130: 0.96 ± 0.06 and HES 200: 1.09 ± 0.06) (Fig. 3B). Together, these results indicate that HES 200 significantly enhanced islet viability and functionality after cryopreservation.
3.3. Levels of apoptosis-associated proteins after cryopreservation
To determine whether the preservation solutions prevented apoptosis, we used western blotting to examine mouse islets after three days of cryopreservation with the different preservation solutions. We first examined the expression levels of apoptosis-related proteins: caspase-9, caspase-3, and PARP. Compared with the cryopreserved islets in the DMSO group, those in the HES 130 and 200 groups showed significantly downregulated expression levels of caspase-9 and caspase-3 (caspase-9: HES 130, p = 0.05; and HES 200, p < 0.001; caspase-3: HES 130, p = 0.001; and HES 200, p < 0.001) (Fig. 4A and B). HES 200 also significantly downregulated the expression of PARP (p < 0.001), which is a substrate of caspase-3 (Fig. 4C and D). To investigate the apoptosis and necrosis of islets, we first measured the expression of NF-κB and then the expression of IκB-α, which is the inhibitory molecule of NF-κB. Compared with the cryopreserved islets in the DMSO group, the HES 200 group showed significantly higher expression of IκB-α (p < 0.0001) and lower expression of NF-κB (p < 0.001). Together, these results indicate that HES 200 reduced apoptosis and necrosis during three days of cryopreservation and promoted the survival of mouse islets, compared with the DMSO group.
Fig. 4.
Expression of proteins related to apoptosis in mouse islet groups after cryopreservation. (A) The protein expression levels of caspase-3 and caspase-9 were assessed by western blotting following cryopreservation. (B) The expression levels of caspase-3 and caspase-9 were quantified relative to β-actin. (C) The protein expression levels of PARP, IκB-α, and NF-κB. (D) The quantified expression levels of PARP, IκB-α, and NF-κB relative to β-actin. DMSO, dimethyl sulfoxide; PARP, poly ADP-ribose polymerase; IκB-α, nuclear factor of kappa light polypeptide gene enhancer in a B-cell inhibitor, alpha; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells. All data are presented as the mean ± SD. *, p < 0.05; **, p < 0.01; ***, p < 0.001; and ****, p < 0.0001 vs. DMSO.
4. Discussion
A sufficient number of highly viable and functioning islets is required for successful islet transplantation. It is difficult to transplant a sufficient number of islets from one donor to one recipient. Therefore, pooling islets isolated from multiple donors and transplanting them together into a recipient could be an alternative. That technique requires the islets to be frozen and thawed while maintaining their viability and functionality through cryopreservation methods.
In this study, we are the first to compare the in vitro use of HES 130 and 200 with results obtained from using the universal CPA (DMSO) in mouse islets. We confirmed islet viability after freezing and thawing by morphological observation and AO/PI staining (Fig. 2, Fig. 3). On the third day of cryopreservation, we found that the recovery of islets after thawing was higher in the HES 200 group than in the DMSO group. The islets in the HES groups were rounder in shape than those in the DMSO group, and dissociated single cells were rare in the HES groups. To evaluate the functional restoration of cryopreserved islets, we investigated the stimulation index (SI) using GSIS. After three days of cryopreservation, the β-cell secretory capacity of the islets decreased significantly in all groups, compared with only one day of cryopreservation. The SI in the HES 200 group was 1.09 ± 0.06 after three days of cryopreservation, which was significantly higher than that in the DMSO group. We performed western blotting on islets that had been frozen and thawed to determine the expression of apoptosis- and necrosis-related proteins in each group. We confirmed that the levels of those proteins were lower in the HES groups than in the DMSO group. These results indicate that HES can replace DMSO as the CPA when freezing islets.
DMSO is a near-universal component of cell cryopreservation solutions. However, the toxicity of DMSO has been previously reported [10,[26], [27], [28]] and can cause significant adverse effects on cell survival, so a new cryopreservation agent is needed. HES is a nonpermeable cryoprotectant, unlike DMSO, which is a penetrating cryoprotectant. Furthermore, HES is currently approved by the FDA for use in clinical trials to increase plasma volume (e.g., Voluven) because it is harmless. It is also used to separate and cryopreserve granulocytes from blood. HES has a relatively high MW and is generally better tolerated and easier to remove than other impermeable protectants [24]. With freezing methods that use a permeable CPA such as DMSO (with or without a low-MW impermeable agent), it is necessary to wash away the CPA inside the cell. This step can be eliminated by using HES, which simplifies the thawing step. Another advantage of using HES as a CPA is its non-toxicity and low antigenicity [29].
Previous studies have shown that HESs have cryoprotective effects similar to those of DMSO in various cells [24,30], and their cryopreservation efficacy was superior to that of DMSO and glycerol in blood cells [11,31]. However, experimental results using HES as a CPA for islets have been insufficient, and the cell death rates of islets after freezing and thawing with HES have rarely been confirmed. Non-invasive CPAs such as EG and HES were previously added to DMSO to reduce its toxicity by lowering its concentration in islet freezing. The viability and function of islets improved after cryopreservation with a mixture of DMSO and a nonpermeable CPA, compared with DMSO alone [23,32]. Furthermore, highly viable cells were obtained after cryopreservation of dissociated human islet cells in 10 % DMSO and 6 % HES [33]. Clinical scalability was achieved by using a mixture of EG and DMSO for the cryopreservation of pancreatic islets by vitrification [34]. In this study, the experimental groups were treated with only HES (mixed with FBS to a final concentration of 4 %) and compared with a DMSO control group, in contrast to a previous cryopreservation study of islets in which HES was mixed with DMSO [23]. HES is generally considered to be less toxic than DMSO, especially at high concentrations [3]. Because cryoprotectants are toxic, their concentration must be minimized to prevent cytotoxic effects and improve recovery. We previously confirmed that the lowest hemolysis ratio after freezing and thawing red blood cells occurred when we used 4 % HES alone (data not shown). This concentration does not deviate significantly from the concentration range generally used in blood cell preservation [35]. The estimated osmotic pressure of 10 % DMSO is 2700–3200 mOsm/L and that of 4 % 130–200 kDa HES is 700–900 mOsm/L. When 10 % DMSO was replaced with 4 % HES, the toxicity of DMSO to cells was effectively reduced, and HES, a relatively high weight molecule, was thought to have created enough osmotic pressure to prevent cell contraction and maintain expansion [36]. HES has lower permeability through cell membranes than DMSO [37], which limits its intracellular accumulation and reduces the risk of potential cytotoxicity or interference with cellular processes. As a result, HES might contribute to osmotic protection primarily by drawing water into the extracellular space and creating a less concentrated environment around the cells. In our experiments freezing islets with HES, the viability and functionality of the islets after thawing were higher with HES than with DMSO. This result is due to the high cell affinity and low cytotoxicity of HES and suggests that HES can be an effective CPA.
The other advantage of using HES is its ability to absorb water molecules [29]. HESs are manufactured to various values of MW, MS, and C2/C6 ratio (indicating whether the hydroxyethyl residue is attached to the C2 or C6 glucose molecule), depending on the clinical purpose [[17], [18], [19]], and they can absorb up to 0.5 g of water per 1 g of HES, depending on those properties [38]. The HESs used in our experiments had an average MW of 130 (HES 130/0.4) and 200 kDa (HES 200/0.5) (Table 1). The cryoprotective effect of HES depends on its ability to absorb water and also affects the viscosity of the solution. When cooling starts, HES absorbs water molecules, which decreases extracellular viscosity (Fig. 5A), dehydrates cells, and prevents osmotic stress and chilling injury (Fig. 5B). The stabilized cell membrane protects against damage from the environment outside the cell (Fig. 5C) [11,[38], [39], [40]], and the increased intracellular viscosity caused by HES suppresses molecular motion and inhibits intracellular ice formation. The underlying mechanism of cryopreservation according to the MW of HES is not yet fully understood. However, the difference in osmotic pressure induction according to the MW produces differences in vitrification [11]; thus, the relatively higher MW HES 200 showed better cryopreservation results than the HES 130. The MS and the C2/C6 ratio determine the HES hydrolysis rate [29], and the concentration and MS of HESs can affect the osmotic pressure in the cells and water solubility around the cells, respectively [41]. The relationship between the hydrolysis of HES and its effectiveness as a CPA needs to be further studied.
Fig. 5.
Schematic illustration of the mechanisms by which hydroxyethyl starch (HES) acts in the process of islet cryopreservation. (A) HES absorbs extracellular water and removes water from the cells. (B) Inhibition of ice formation around the cells. (C) This process stabilizes the islet cell membrane.
We confirmed the expression patterns of apoptosis- and necrosis-related proteins in three-day cryopreserved islets through western blotting. A previous report showed that Caspase-9 and Caspase-3 proteins, which are commonly increased by various stimuli in islets, increase apoptosis in the extrinsic and intrinsic signaling pathways [42]. The expression levels of these proteins were significantly reduced in the HES 200 group compared with the DMSO group. In this process, the decreased expression of PARP, which is a substrate related to DNA breaks, was also confirmed (Fig. 4). In the experiment, we investigated the expression of representative proteins of intrinsic and extrinsic pathways. We also measured the expression of cleaved caspase-9, which is an upstream protein of caspase-3 in an intrinsic (from mitochondria-driven) pathway, and of cleaved PARP, which is substrate of caspase-3. Decreased expression of cleaved caspase-3 in the HES group was expected but was not confirmed. We did confirm that the expression of full-length caspase-3 decreased in the HES group after freezing and thawing. This may be a characteristic of Cas-3, which is involved in both the extrinsic and intrinsic pathways of apoptosis. Further research should investigate the expression patterns of additional proteins related to apoptosis after freezing/thawing of pancreatic islets. The canonical NF-κB signaling pathway has been well studied due to its regulation of islet inflammation. Increased expression of NF-κB induces islet necrosis and apoptosis [43]. Therefore, we also investigated the expression pattern of this protein. In the DMSO group, along with the increase in the expression of this protein, we confirmed that the expression of the inhibitory protein IκB-α was decreased. DMSO infiltration increases the osmotic pressure of cells and the concentration of free radicals and calcium [44]. This change induces mitochondrial damage and cell death during freezing and vitrification [44,45]. The increased survival detected with AO/PI may be due to inhibition of apoptosis, as there was lower expression of cleaved PARP, cleaved caspase-9, and caspase-3 after cooling and thawing with HES. Increased expression of NF-κB and decreased expression of IκB-α also indicates decreased apoptosis/necrosis, which may have increased the survival of islets with HES. Compared with DMSO, HES 200 and HES 130 appear to have effectively protected the islets during the three-day cryopreservation period, enabling us to obtain islets with a relatively high survival rate.
In this experiment, we froze islets for the relatively short periods of one day and three days. This period is sufficient to freeze enough isolated islets and transport them to the country of the patient in need of transplantation. However, long-term cryopreservation is essential not only for clinical islet transplantation but also for the development of further research. Previous studies have examined the cryopreservation of islets for long periods, up to 20 years [46]. Therefore, additional research is needed to determine whether HES produces good results when applied to long-term cryopreservation. Furthermore, optimal cryopreservation methods need to be developed for islets of various species, including humans [47]. In this study, we conducted a basic experiment to confirm the effects of HES in rodent islets; a complementary experiment in large animals (such as pigs) is necessary. It is possible that FBS played an additional role as CPA, which should be further studied. For future clinical applications, FBS should be replaced by human serum or human serum albumin. Various studies on the development of freezing equipment and protocols for cryopreservation are currently in progress. If HES is applied in those studies, further improvements in the cryopreservation of islets can be achieved.
Overall, this study has confirmed that using HES as a CPA improved the survival and insulin secretion function of cryopreserved islets, compared with the use of a conventional CPA. The importance of CPAs in cryopreservation was investigated through a molecular analysis of proteins related to apoptosis. With this improved cryopreservation material, isolated high-quality islets can be stored and collected. Eventually, this method will enable the viable and functional transplantation of islets in quantities sufficient to treat type 1 diabetes.
CRediT authorship contribution statement
Du Yeon Shin: Writing – review & editing, Writing – original draft, Visualization, Funding acquisition, Formal analysis, Data curation, Conceptualization. Jae Suh Park: Investigation, Funding acquisition, Formal analysis, Data curation. Han-Sin Lee: Writing – review & editing, Investigation, Funding acquisition, Formal analysis, Data curation. Wooyoung Shim: Conceptualization. Lauren Jin: Data curation. Kyo Won Lee: Writing – review & editing, Data curation. Jae Berm Park: Supervision, Project administration, Data curation. Dong Hyun Kim: Writing – review & editing, Supervision, Project administration, Conceptualization. Jae Hyeon Kim: Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
DYS designed the study, collected data, and wrote the manuscript. JSP designed the study, collected data, and wrote the manuscript. H-SL designed the study, collected data, and edited the manuscript. WS, LJ, and KWL collected data. JBP, DHK, and JHK designed the study and reviewed the manuscript.
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea, which is funded by the Ministry of Education (NRF-2017R1D1A1B03034272, NRF-2019R1I1A1A01063186, and NRF-2021 R1A2C1093972).
Contributor Information
Dong Hyun Kim, Email: dhx9.kim@skku.edu.
Jae Hyeon Kim, Email: jaehyeon@skku.edu.
References
- 1.McCall M., Shapiro A.M. Update on islet transplantation. Cold Spring Harb. Perspect. Med. 2012;2 doi: 10.1101/cshperspect.a007823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baust J.M., Van B., Baust J.G. Cell viability improves following inhibition of cryopreservation-induced apoptosis, in Vitro Cell. Dev. Biol. Anim. 2000;36:262–270. doi: 10.1290/1071-2690(2000)036<0262:cvifio>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 3.Best B.P. Cryoprotectant toxicity: facts, issues, and questions. Rejuvenation Res. 2015;18:422–436. doi: 10.1089/rej.2014.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fowler A., Toner M. Cryo-injury and biopreservation. Ann. N. Y. Acad. Sci. 2005;1066:119–135. doi: 10.1196/annals.1363.010. [DOI] [PubMed] [Google Scholar]
- 5.Jeong Y.J., Kim M.K., Song H.J., Kang E.J., Ock S.A., Kumar B.M., Balasubramanian S., Rho G.J. Effect of alpha-tocopherol supplementation during boar semen cryopreservation on sperm characteristics and expression of apoptosis related genes. Cryobiology. 2009;58:181–189. doi: 10.1016/j.cryobiol.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Miranda P.M., Mohan V., Ganthimathy S., Anjana R.M., Gunasekaran S., Thiagarajan V., Churchill T.A., Kin T., Shapiro A.M., Lakey J.R. Human islet mass, morphology, and survival after cryopreservation using the Edmonton protocol. Islets. 2013;5:188–195. doi: 10.4161/isl.26304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagaya M., Matsunari H., Kanai T., Maehara M., Nakano K., Umeki I., Katsumata Y., Kasai Y., Sakai R., Kobayashi M., Honda M., Abe N., Watanabe M., Umeyama K., Nagashima H. An effective new cryopreservation procedure for pancreatic islets using hollow fiber vitrification. Horm. Metab. Res. 2016;48:540–549. doi: 10.1055/s-0042-102628. [DOI] [PubMed] [Google Scholar]
- 8.Rawal S., Harrington S., Williams S.J., Ramachandran K., Stehno-Bittel L. Long-term cryopreservation of reaggregated pancreatic islets resulting in successful transplantation in rats. Cryobiology. 2017;76:41–50. doi: 10.1016/j.cryobiol.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Sakonju I., Taura Y., Inayoshi Y., Suzuki T., Takimoto K., Nakaichi M., Nakama S. Cryopreservation of isolated rat islets of Langerhans in the presence of ethylene glycol or dimethyl sulfoxide: evaluation of toxicity and the dynamic pattern of subsequent insulin release in vitro. Cryobiology. 1996;33:354–362. doi: 10.1006/cryo.1996.0036. [DOI] [PubMed] [Google Scholar]
- 10.Szurek E.A., Eroglu A. Comparison and avoidance of toxicity of penetrating cryoprotectants. PLoS One. 2011;6 doi: 10.1371/journal.pone.0027604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi T., Hirsh A., Erbe E., Williams R.J. Mechanism of cryoprotection by extracellular polymeric solutes. Biophys. J. 1988;54:509–518. doi: 10.1016/s0006-3495(88)82983-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weng L., Beauchesne P.R. Dimethyl sulfoxide-free cryopreservation for cell therapy: a review. Cryobiology. 2020;94:9–17. doi: 10.1016/j.cryobiol.2020.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Roy A.J., Franklin A., Simmons W.B., Djerassi I. A method for separation of granulocytes from normal human blood using hydroxyethyl starch. Prep. Biochem. 1971;1:197–203. doi: 10.1080/00327487108081939. [DOI] [PubMed] [Google Scholar]
- 14.Gollub S., Schechter D.C., Hirose T., Bailey C.P. Use of hydroxyethyl starch solution in extensive surgical operations. Surg. Gynecol. Obstet. 1969;128:725–728. [PubMed] [Google Scholar]
- 15.Garzon A.A., Cheng C., Lerner B., Lichtenstein S., Karlson K.E. Hydroxyethyl starch (HES) and bleeding. An experimental investigation of its effect on hemostasis. J. Trauma. 1967;7:757–766. [PubMed] [Google Scholar]
- 16.Knorpp C.T., Merchant W.R., Gikas P.W., Spencer H.H., Thompson N.W. Hydroxyethyl starch: extracellular cryophylactic agent for erythrocytes. Science. 1967;157:1312–1313. doi: 10.1126/science.157.3794.1312. [DOI] [PubMed] [Google Scholar]
- 17.Kozek-Langenecker S.A. Effects of hydroxyethyl starch solutions on hemostasis. Anesthesiology. 2005;103:654–660. doi: 10.1097/00000542-200509000-00031. [DOI] [PubMed] [Google Scholar]
- 18.Boldt J. Modern rapidly degradable hydroxyethyl starches: current concepts. Anesth. Analg. 2009;108:1574–1582. doi: 10.1213/ane.0b013e31819e9e6c. [DOI] [PubMed] [Google Scholar]
- 19.Westphal M., James M.F., Kozek-Langenecker S., Stocker R., Guidet B., Van Aken H. Hydroxyethyl starches: different products--different effects. Anesthesiology. 2009;111:187–202. doi: 10.1097/ALN.0b013e3181a7ec82. [DOI] [PubMed] [Google Scholar]
- 20.Stolzing A., Naaldijk Y., Fedorova V., Sethe S. Hydroxyethylstarch in cryopreservation - mechanisms, benefits and problems. Transfus. Apher. Sci. 2012;46:137–147. doi: 10.1016/j.transci.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Elliott G.D., Wang S., Fuller B.J. Cryoprotectants: a review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures. Cryobiology. 2017;76:74–91. doi: 10.1016/j.cryobiol.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Murray K.A., Gibson M.I. Chemical approaches to cryopreservation. Nat. Rev. Chem. 2022;6:579–593. doi: 10.1038/s41570-022-00407-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kenmochi T., Asano T., Maruyama M., Saigo K., Akutsu N., Iwashita C., Ohtsuki K., Suzuki A., Miyazaki M. Cryopreservation of human pancreatic islets from non-heart-beating donors using hydroxyethyl starch and dimethyl sulfoxide as cryoprotectants. Cell Transplant. 2008;17:61–67. doi: 10.3727/000000008783907026. [DOI] [PubMed] [Google Scholar]
- 24.Ashwood-Smith M.J., Warby C., Connor K.W., Becker G. Low-temperature preservation of mammalian cells in tissue culture with polyvinylpyrrolidone (PVP), dextrans, and hydroxyethyl starch (HES) Cryobiology. 1972;9:441–449. doi: 10.1016/0011-2240(72)90161-7. [DOI] [PubMed] [Google Scholar]
- 25.Kim G., Lee H.S., Oh B.J., Kwon Y., Kim H., Ha S., Jin S.M., Kim J.H. Protective effect of a novel clinical-grade small molecule necrosis inhibitor against oxidative stress and inflammation during islet transplantation. Am. J. Transplant. 2021;21:1440–1452. doi: 10.1111/ajt.16323. [DOI] [PubMed] [Google Scholar]
- 26.Fahy G.M. Cryoprotectant toxicity neutralization. Cryobiology. 2010;60:S45–S53. doi: 10.1016/j.cryobiol.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Fahy G.M. The relevance of cryoprotectant "toxicity" to cryobiology. Cryobiology. 1986;23:1–13. doi: 10.1016/0011-2240(86)90013-1. [DOI] [PubMed] [Google Scholar]
- 28.Rajotte R.V., Lakey J.R.T. In: Adult Islet Cryopreservation. Ricordi C., editor. R. G. Landes Company; Austin, TX: 1995. Methods in cell transplantation; pp. 517–524. [Google Scholar]
- 29.Naaldijk R.N., Yahaira M. 2014. Application of Sugars and Starch in the Cryopreservation of Cells.https://www.db-thueringen.de/servlets/MCRFileNodeServlet/dbt_derivate_00030412/Diss/Dissertation_YN_2014.pdf [Google Scholar]
- 30.Persidsky M.D., Ellett M.H. Hydroxyethyl starch as a cryopreservative for nucleated mammalian cells. Cryobiology. 1971;8:586–588. doi: 10.1016/0011-2240(71)90013-7. [DOI] [PubMed] [Google Scholar]
- 31.Kim H., Tanaka S., Une S., Nakaichi M., Sumida S., Taura Y. A comparative study of the effects of glycerol and hydroxyethyl starch in canine red blood cell cryopreservation. J. Vet. Med. Sci. 2004;66:1543–1547. doi: 10.1292/jvms.66.1543. [DOI] [PubMed] [Google Scholar]
- 32.Kojayan G., Whaley D., Alexander M., Rodriguez S., Lee S., Lakey J.R. Improved cryopreservation yield of pancreatic islets using combination of lower dose permeable cryoprotective agents. Cryobiology. 2019;88:23–28. doi: 10.1016/j.cryobiol.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Marquez-Curtis L.A., Dai X.Q., Hang Y., Lam J.Y., Lyon J., Manning Fox J.E., McGann L.E., MacDonald P.E., Kim S.K., Elliott J.A.W. Cryopreservation and post-thaw characterization of dissociated human islet cells. PLoS One. 2022;17 doi: 10.1371/journal.pone.0263005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhan L., Rao J.S., Sethia N., Slama M.Q., Han Z., Tobolt D., Etheridge M., Peterson Q.P., Dutcher C.S., Bischof J.C., Finger E.B. Pancreatic islet cryopreservation by vitrification achieves high viability, function, recovery and clinical scalability for transplantation. Nat. Med. 2022;28:798–808. doi: 10.1038/s41591-022-01718-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lionetti F.J., Hunt S.M., Gore J.M., Curby W.A. Cryopreservation of human granulocytes. Cryobiology. 1975;12:181–191. doi: 10.1016/0011-2240(75)90016-4. [DOI] [PubMed] [Google Scholar]
- 36.Epstein K.L., Bergren A., Giguère S., Brainard B.M. Cardiovascular, colloid osmotic pressure, and hemostatic effects of 2 formulations of hydroxyethyl starch in healthy horses. J. Vet. Intern. Med. 2014;28:223–233. doi: 10.1111/jvim.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whaley D., Damyar K., Witek R.P., Mendoza A., Alexander M., Lakey J.R. Cryopreservation: an overview of principles and cell-specific considerations. Cell Transplant. 2021;30 doi: 10.1177/0963689721999617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Körber C., Scheiwe M.W. The cryoprotective properties of hydroxyethyl starch investigated by means of differential thermal analysis. Cryobiology. 1980;17:54–65. doi: 10.1016/0011-2240(80)90008-5. [DOI] [PubMed] [Google Scholar]
- 39.Connor W., Ashwood-Smith M.J. Cryoprotection of mammalian cells in tissue culture with polymers; possible mechanisms. Cryobiology. 1973;10:488–496. doi: 10.1016/s0011-2240(73)80002-1. [DOI] [PubMed] [Google Scholar]
- 40.Thomas M.J., Parry E.S., Nash S.G., Bell S.H. A method for the cryopreservation of red blood cells using hydroxyethyl starch as a cryoprotectant. Transfus. Sci. 1996;17:385–396. doi: 10.1016/0955-3886(96)00009-4. [DOI] [PubMed] [Google Scholar]
- 41.Jeon Y.S. Recent findings on the use of hydroxyethyl starch. Anesthesiol. Pain Med. 2014;9:159–164. [Google Scholar]
- 42.Tomita T. Apoptosis of pancreatic β-cells in Type 1 diabetes. Bosn. J. Basic Med. Sci. 2017;17:183–193. doi: 10.17305/bjbms.2017.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhonde R., Shukla R.C., Kanitkar M., Shukla R., Banerjee M., Datar S. Isolated islets in diabetes research. Indian J. Med. Res. 2007;125:425–440. [PubMed] [Google Scholar]
- 44.Kang M.H., Das J., Gurunathan S., Park H.W., Song H., Park C., Kim J.H. The cytotoxic effects of dimethyl sulfoxide in mouse preimplantation embryos: a mechanistic study. Theranostics. 2017;7:4735–4752. doi: 10.7150/thno.21662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chi H.J., Koo J.J., Kim M.Y., Joo J.Y., Chang S.S., Chung K.S. Cryopreservation of human embryos using ethylene glycol in controlled slow freezing. Hum. Reprod. 2002;17:2146–2151. doi: 10.1093/humrep/17.8.2146. [DOI] [PubMed] [Google Scholar]
- 46.Manning Fox J.E., Lyon J., Dai X.Q., Wright R.C., Hayward J., van de Bunt M., Kin T., Shapiro A.M., McCarthy M.I., Gloyn A.L., Ungrin M.D., Lakey J.R., Kneteman N.M., Warnock G.L., Korbutt G.S., Rajotte R.V., MacDonald P.E. Human islet function following 20 years of cryogenic biobanking. Diabetologia. 2015;58:1503–1512. doi: 10.1007/s00125-015-3598-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rajotte R.V. Islet cryopreservation protocols. Ann. N. Y. Acad. Sci. 1999;875:200–207. doi: 10.1111/j.1749-6632.1999.tb08504.x. [DOI] [PubMed] [Google Scholar]