Highlights
-
•
The forming mechanism of volatile compounds from thermal oxidation of (E)-4-decenal was analyzed.
-
•
Density functional theory, thermal desorption cryo-trapping and GC–MS was used.
-
•
24 reactions obtained were grouped into the ROOH, RO• and ROO• mechanisms.
-
•
The priority order of occurrence is RO• mechanism > ROOH mechanism > ROO• mechanism.
Keywords: Unsaturated aldehyde, Theoretical calculation, Flavor, Oxidation mechanism
Abstract
Unsaturated aliphatic aldehyde oxidation plays a significant role in the deep oxidation of fatty acids to produce volatile chemicals. Exposing the oxidation process of unsaturated aliphatic aldehydes is crucial to completely comprehend how food flavor forms. In this study, thermal desorption cryo-trapping in conjunction with gas chromatography-mass spectrometry was used to examine the volatile profile of (E)-4-decenal during heating, and 32 volatile compounds in all were detected and identified. Meanwhile, density functional theory (DFT) calculations were used, and 43 reactions were obtained in the 24 pathways, which were summarized into the peroxide reaction mechanism (ROOH), the peroxyl radical reaction mechanism (ROO·) and the alkoxy radical reaction mechanism (RO·). Moreover, the priority of these three oxidative mechanisms was the RO· mechanism > ROOH mechanism > ROO· mechanism. Furthermore, the DFT results and experimental results agreed well, and the oxidative mechanism of (E)-4-decenal was finally illuminated.
Introduction
Lipid oxidation during heating is one of the most important sources of flavor in food (Calín-Sánchez & Carbonell-Barrachina, 2021, Stier, 2000). Among these volatile compounds, unsaturated ten-carbon aliphatic aldehyde are important substances, such as decanal, (E)-2-decenal and (E)-4-decenal, etc (Angela and Andreas, 2010, Porter et al., 1995, Zaldman, Klsillev, & Sasson, 1988). And (E)-4-decenal is a main unsaturated ten-carbons flavor compound generated in the lipid oxidative process with pineapple aroma, which is also an important part of food flavor formation. It is one of the common volatile flavor substances in the oxidation of cooking oil, and is often used as a high-end flavor in the food industry (Peng, Lan, & Lin, 2017). In our previous experiments, we found that unsaturated ten-carbons aldehydes can be further oxidized to produce shorter carbon-chain volatiles, which are the major components of the deep oxidation of fatty acids (Frankel, 1983, Wang, Zhang, & Lin, 2023). But their oxidative mechanisms are still unclear. Therefore, the in-depth analysis of the thermal oxidation mechanism of (E)-4-decenal is of great significance for the assembly and reduction of the real oleic acid oxidation process and food flavor formation reaction. At the same time, a full understanding of thermal oxidation mechanism of unsaturated aliphatic aldehyde to volatile substances can provide a theoretical basis for the precise regulation of flavor substances in the process of food hot processing, so as to realize the manual intervention and precise regulation of flavor in the food processing, and finally achieve the purpose of improving and enhancing the quality of food flavor.
It is found that the double bonds of unsaturated aldehydes could readily absorb energy during heating, leading to the breaking of the hydrocarbon bond of α-C and the formation of olefin and hydrogen radicals (Al-Otaibi, Mahmoud, & Almuqrin, 2021, Balan and Rajakumar, 2018, Santacesaria et al., 2000, Sheridan, 1946). Olefin radical, as one of the important intermediates of oxidation reaction, could combine with oxygen or peroxyl hydroxyl radicals to form peroxyl oxygen radicals or peroxide. These free radicals may be further oxidized and degraded during heating to generate volatiles with shorter carbon chains.
Theoretical chemistry is an effective approach to study chemical reaction mechanisms, which has been widely used in many fields in recent years (Borges, Colby, & Das, 2021, Hammes-Schiffer, 2017). In theoretical chemistry, one of the most common methods is density functional theory (DFT) (Verma and Truhlar, 2020, Hine Nicholas and Hine, 2016). It can calculate the molecular structure, reaction parameters, transition states and products during chemical reactions. Meanwhile, it can also determine the active sites of functional groups, the activation energy and reaction rate constant of the reaction, which provided the data basis for the kinetic study of the thermal oxidation process by comparing the reaction barrier and the rate constant (Grambow, Pattanaik, & Green, 2020). Therefore, the application of density functional theory to the field of flavor chemistry can deeply elucidate the molecular formation mechanism of food flavor.
In this study the volatile forming mechanism of (E)-4-decenal thermal oxidation was examined by using DFT calculations and experimental data. The volatile compounds generated from the oxidation of (E)-4-decenal during heating were firstly investigated. Then, DFT calculations were used to deeply explore the mechanism of (E)-4-decenal thermal oxidation. By comparing and analyzing the potential energy surface and rate constant of each reaction, the priority of each reaction path was finally determined. Thus, the oxidation mechanism of (E)−4-decenal was finally elucidated.
Materials and methods
Chemicals
Standard chemicals: analytical grade ((E)-4-decenal, 2, 4, 6-trimethyl-pyridine, and C4-C20 alkanes, etc) were purchased from Aladdin (Shanghai, China).
Experimental method
Extraction and collection of volatile compounds from (E)-4-decenal during heating was performed by using a thermal desorption cryo-trapping system, with a qualified microchamber/thermal extractor (M−CTE250 Markes International, UK). (E)-4-decenal was diluted to 100 μL/mL, 20 μL of the sample was taken and 10 μL of diluted cyclohexanone (2 μg/mL) was added as a quantitative internal standard. An Agilent 6890/5975C GC–MS system (Santa Clara, CA, USA) equipped with an HP-5 ms column (30 m × 0.25 mm i.d., 0.25 μm film thickness; J&W Scientific) was used for the analysis of volatile compounds desorbed from the sample (Chai, Li, & Zhang, 2019, Drabova et al., 2013, Dymerski, 2017, Jeleń et al., 2012, Peter, Giorgia, & Mariarosa, 2016). Specific parameters from the experiments are detailed in the Supplementary Material.
Computational methods
In this study, all the reaction mechanism and related structures were calculated on Gussian16 software package (Becke, 1992, Becke, 1993, Elroby, Banaser, & Aziz, 2022, Fukui, 1981, Shabani, Latina, & Li, 2020, Yang and Lee., 1986). The Gibbs free energy was calculated by Shermo 2.0.8 program, and the accurate reaction potential energy surface was obtained (Albaugh, Boateng, & Bradshaw, 2016, Mahmoud, Shiroudi, & Abdel-Rahman, 2020, Ryu, Park, & KiM, 2018, Tian & Chen, 2021). The TST calculator could be used to calculate reaction rate constants (Tian Lu, http://sobereva.com/310). The Condensed Fukui Function (CFF) of molecular structure was calculated using the Multiwfn (Tian & Chen, 2012).
Calculation of molecular orbital
The Chemcraft was used to determine the charge density of each atom in the molecule. The VMD charting application was used to obtain the molecular orbital diagram (Humphrey, Dalke, & Schulten, 1996).
Statistical analysis
There were three duplicates of each experiment. The measured data were expressed as mean ± standard deviation. SPSS 18.0 (Chicago, IL, USA) statistical software was used to analyze the significance among relevant samples at 0.05 (P < 0.05) level by one-way ANOVA.
Results and discussion
Characterization of volatile substances produced by (E)-4-decenal during heating
As shown in Table 1, a total of 32 volatile compounds were identified from (E)-4-decenal oxidation during heating. Meanwhile, Fig. 1 visualises the volatile composition of each temperature point during heating. As shown in Fig. 1, a total of 16 volatiles were detected at 60℃, accounting one half of the total volatiles throughout the whole heating process. Most of them have a good flavor, such as 1-octen-3-ol with its strong mushroom odor and hexanoic acid with the cheese odor (Ama, Fb, & Db, 2019). It can be inferred that the (E)-4-decenal oxidation could take place at lower temperatures, and its oxidative products contribute more to the flavor production.
Table 1.
Volatile substances produced during heating of (E)-4-decenal.
| No. | Compounds | RI | identification method | Content(μg/mg) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| 60 ○C | 90 ○C | 120 ○C | 150 ○C | 180 ○C | 210 ○C | ||||
| Alkanes | |||||||||
| 1 | (E)-3-nonene | 886 | RI, MS | 0.30 ± 0.03b | 0.37 ± 0.11ab | 0.22 ± 0.18b | 0.90 ± 0.63ab | 1.42 ± 0.38a | 0.81 ± 0.37ab |
| 2 | 2-nonene | 902 | RI, MS | nd | nd | nd | 0.04 ± 0.02a | nd | nd |
| 3 | (E)-1,3-nonadiene | 924 | RI, MS | nd | nd | nd | nd | 0.16 ± 0.05a | 0.10 ± 0.04a |
| Aldehydes | |||||||||
| 4 | (E)-4-decenal | 1198 | RI, MS, STD | 330.01 ± 174.51a | 274.19 ± 39.81a | 199.96 ± 71.25a | 199.31 ± 22.48a | 167.14 ± 8.23a | 223.69 ± 112.37a |
| 5 | (Z)-4-decenal | 1193 | RI, MS, STD | 0.33 ± 0.29a | 0.44 ± 0.39a | 0.45 ± 0.38a | 0.48 ± 0.32a | 0.72 ± 0.30a | nd |
| 6 | (E, E)-2,4-decadienal | 1317 | RI, MS, STD | 0.20 ± 0.13a | 0.53 ± 0.41a | 0.68 ± 0.17a | 0.46 ± 0.13a | nd | nd |
| 7 | Hexanal | 800 | RI, MS, STD | nd | 0.21 ± 0.11bc | 0.22 ± 0.14bc | 0.49 ± 0.21a | 0.54 ± 0.14ab | 0.39 ± 0.15ab |
| 8 | (E)-2-heptenal | 958 | RI, MS, STD | nd | 0.07 ± 0.04bc | 0.10 ± 0.07abc | 0.14 ± 0.08abc | 0.41 ± 0.17a | 0.16 ± 0.04ab |
| 9 | (E)-2-octenal | 1060 | RI, MS, STD | nd | nd | 0.21 ± 0.08a | 0.23 ± 0.11a | 0.51 ± 0.14a | 0.34 ± 0.10a |
| Alcohols | |||||||||
| 10 | 1-octen-3-ol | 980 | RI, MS, STD | 0.13 ± 0.03a | 0.18 ± 0.08a | 0.20 ± 0.05a | 0.16 ± 0.12a | 0.46 ± 0.20a | 0.12 ± 0.01a |
| 11 | (E)-3-nonen-1-ol | 1143 | RI, MS, STD | 0.30 ± 0.16a | nd | nd | nd | nd | nd |
| 12 | (E)-2-decenol | 1257 | RI, MS, STD | nd | 0.76 ± 0.41a | 0.36 ± 0.20ab | 0.68 ± 0.55a | nd | nd |
| 13 | (E)-3-decen-1-ol | 1232 | RI, MS | nd | nd | 0.32 ± 0.10a | nd | 0.58 ± 0.10a | nd |
| 14 | Dec-4-en-1-ol | 1257 | RI, MS | nd | nd | nd | nd | nd | 2.44 ± 1.72a |
| 15 | (E)-2-dodecen-1-ol | 1478 | RI, MS | nd | nd | 0.30 ± 0.10a | 0.08 ± 0.06b | nd | nd |
| 16 | 2,4-decadien-1-ol | 1264 | RI, MS | nd | nd | nd | 0.49 ± 0.45a | nd | nd |
| Ketones | |||||||||
| 17 | 3-nonen-2-one | 1142 | RI, MS, STD | nd | nd | nd | 0.25 ± 0.12a | 0.46 ± 0.12a | 0.45 ± 0.26a |
| Acids | |||||||||
| 18 | Hexanoic acid | 990 | RI, MS, STD | 0.12 ± 0.06ab | 0.18 ± 0.14a | nd | nd | nd | nd |
| 19 | (E)-4-decenoic acid | 1366 | RI, MS, STD | 14.98 ± 3.80b | 29.37 ± 9.38a | 71.54 ± 3.90a | 12.63 ± 4.97b | 27.50 ± 9.77b | 8.29 ± 2.18b |
| 20 | Valeric acid | 903 | RI, MS, STD | nd | nd | nd | nd | 0.08 ± 0.02a | nd |
| 21 | (E)-2-decenoic acid | 1392 | RI, MS, STD | nd | nd | 0.53 ± 0.14a | nd | nd | nd |
| Esters | |||||||||
| 22 | Ethyl palmitate | 1974 | RI, MS | 0.02 ± 0.01a | nd | nd | nd | nd | 0.02 ± 0.01a |
| 23 | Isopropyl palmitate | 2023 | RI, MS | 0.05 ± 0.00b | 0.35 ± 0.23a | nd | nd | nd | nd |
| 24 | γ-decalactone | 1470 | RI, MS | nd | nd | 1.27 ± 0.47a | 0.26 ± 0.13bc | 0.84 ± 0.31ab | nd |
| 25 | Methyl tuberate | 1502 | RI, MS, STD | nd | nd | nd | 0.11 ± 0.03a | 0.24 ± 0.10a | nd |
| 26 | Ethyl hexanoate | 1000 | RI, MS, STD | nd | nd | nd | nd | nd | 0.16 ± 0.09a |
| Others | |||||||||
| 27 | 2-pentylfuran | 993 | RI, MS | 0.33 ± 0.06a | 0.49 ± 0.32a | nd | nd | nd | nd |
| 28 | Pentylbenzene | 1157 | RI, MS | 0.02 ± 0.00a | 0.13 ± 0.06a | 0.09 ± 0.04a | 0.05 ± 0.01a | 0.08 ± 0.01a | 0.20 ± 0.15a |
| 29 | Naphthalene | 1182 | RI, MS | 0.24 ± 0.07a | 0.27 ± 0.04a | 0.62 ± 0.31a | 0.18 ± 0.17a | 0.51 ± 0.18a | 0.32 ± 0.11a |
| 30 | 1-(1-ethoxyethoxy)-butane | 872 | RI, MS | 0.03 ± 0.01abc | 0.03 ± 0.02abc | 0.04 ± 0.01bc | nd | 0.08 ± 0.02ab | 0.07 ± 0.04a |
| 31 | 1,1′-[ethylidenebis(oxy)] bis-butane | 1094 | RI, MS | 0.47 ± 0.12b | 0.57 ± 0.15ab | 1.17 ± 0.73ab | 0.90 ± 0.14a | 0.95 ± 0.17ab | nd |
| 32 | 1,1-diethoxyhexane | 1092 | RI, MS | 0.01 ± 0.00b | 0.03 ± 0.01b | nd | nd | nd | 0.08 ± 0.03a |
Nd = Compound not detected in the sample. MS = Identification by MS spectra, RI = Kovat’s retention indexes, STD = Comparison with a standard compound; The content was the average of three replicates ± standard deviation. “a, b, c,” indicated significant difference (P < 0.05).
Fig. 1.
Temperature of volatile chemicals are produced during the thermal oxidation of (E)-4-decenal.
As the temperature increased, 3 and 5 new volatile compounds were produced at 90℃ and 120℃ respectively, including two unsaturated aldehydes and some alcohols (Fig. 1), such as (E)-2-heptenal, (E)-2-octenal and (E)-2-decenol. The presence of longer carbon chains products, such as ethyl palmitate and methyl tuberate, could be attributed to the etherification between oxidative pyrolysis product. At 180℃, the relative contents of some volatile products increased significantly, such as (E)-2-heptenal, (E)-2-octenal, 1-octen-3-ol. They are common volatile substances with good flavor in food. As the temperature continued to rise to 210℃, the relative content of some volatiles showed a decreasing trend, and more volatiles with shorter carbon chains were generated. At the same time, (E)-1,3-nonadiene began to form at this temperature.
Mechanism of (E)-4-decenal theomal oxidation based on DFT calculations
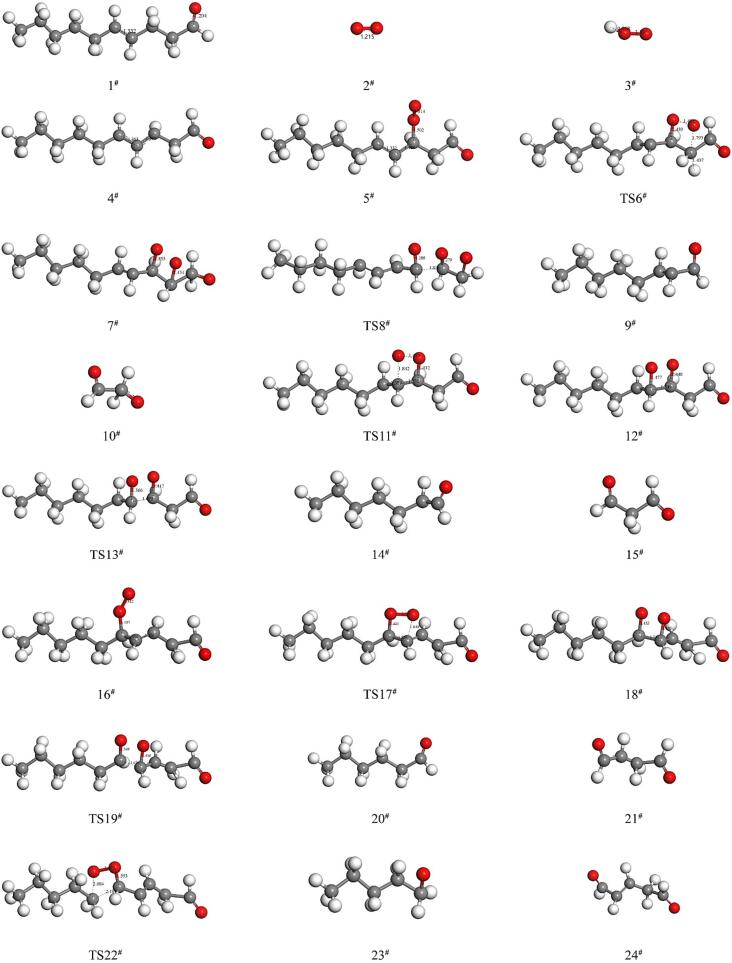
As shown in Fig. 2, 43 reactions were obtained in thermal oxidation process of (E)-4-decenal by using DFT calculations. These 43 reactions were summarized into the peroxyl radical (ROO·) mechanism, the peroxyl (ROOH) mechanism and the alkoxy radical (RO·) mechanism. Fig. 3 displayed the molecular structures of reactants, transition states, intermediates, and end products that were involved in each reaction. Besides, Fig. 4 showed each thermal oxidative decomposition pathways of (E)-4-decenal.
Fig. 2.
Diagram for the whole oxidation of (E)-4-decenal.
Fig. 3.
The composition of the compounds involved in the oxidation of (E)-4-decenal.
Fig. 4.
Decomposition pathways of (E)-4-decenal thermal oxidation.
Analysis of peroxyl radical mechanism (ROO·) in (E)-4-decenal thermal oxidation
As shown in Fig. 4, in the initial stage of oxidation of (E)-4-decenal, the H atom on the α-C (C3, C6) of the carbon–carbon double bond is so reactive that the C—H bond can easily absorb energy and break, forming the corresponding olefinic and hydrogen radicals. Then, electrons could rearrange on the C3, C4, C5 sites or C4, C5, C6 sites to form a π conjugated system. This system could continue to oxidized with oxygen or peroxide (Li and Josef, 1996). Therefore, when oxygen attacks C3 or C5 site of the π conjugated system (C3-C4-C5), the olefinic peroxide radical 3-ROO· (R3, 5#) or 5-ROO· (R4, 16#) are formed, respectively. While oxygen attacks C4 or C6 site of the π conjugated system (C4-C5-C6), the olefinic peroxide radical 4-ROO· (R5, 50#) or 6-ROO· (R6, 58#) are formed, respectively.
As 3-ROO·, 4-ROO·, 5-ROO· and 6-ROO· olefinic peroxide radicals are more active, they could attack the adjacent carbon atom leading to further cleavage of the carbon chain into smaller molecules (Path 1–8). It can be seen from the Fig. 4 (Path 1 and Path 2), the O—O· of 3-ROO· (5#) would attack the C2 or C4 site to form intermediate products (7# or 12#) via transition state TS6# or TS11#. Then, the single bond between C2-C3 or C3-C4 breaks via the TS8# (R19) or TS13# (R20) to form the final products (9#&10#): 2-octenal and CHOCH2O· (Path 1) or the final products (14#&15#): CH3(CH2)5ĊHCHO and malondialdehyde (Path 2), respectively.
The further oxidation of the 4-ROO·, 5-ROO· and 6-ROO· follows a similar pathway as above. With the O—O· of the 4-ROO· attacking C3, 2-heptenal and CȮ(CH2)2CHO (52#&53#, Path 5) are formed via TS51#. If C5 is selected, the intermediate product 55# is firstly formed via TS54#, then final products hexanal and succinaldehyde are formed via R16 and R22 (20#&57#, Path 6). When the O—O· of the 5-ROO· attacks the C4 site to form the intermediate product 18# via TS17# (R13), then hexanal and CHOCH2CHCHO· are formed via TS19# (20#&21#, Path 3). And the O—O· of the 5-ROO· could also attack C6 to form the final products: CH3(CH2)3CH2O· and 2-alkenyl glutaraldehyde (23#&24#, Path 4). For 6-ROO·olefinic peroxide radicals, if the C5 site is selected, the intermediate product 60# was firstly formed via TS59# (R13), then pentanal and CHOĊH(CH2)2CHO are generated via TS61# (62#&63#, Path 7). While, if the C7 site is selected, CH3(CH2)2CH2O·and 2-alkenyl adipaldehyde (65#&66#, Path 8) are generated.
Analysis of peroxide reaction mechanism (ROOH) in (E)-4-decenal thermal oxidation
During the heating process, oxygen can react with hydrogen radical to form peroxy hydroxyl radicals (OOH·, 3#). This radical could continue to attack the π conjugated system (C3-C4-C5 or C4-C5-C6). When OOH· attacks C3-C4-C5 system, 3-ROOH or 5-ROOH are formed via transition states (TS25#or TS32#) respectively. While OOH· attacks C4-C5-C6 conjugated system, 4-ROOH or 5-ROOH are formed via TS67# or TS73#. These peroxides (3-ROOH, 4-ROOH, 5-ROOH and 6-ROOH) are unstable and will further oxidize. As shown in Fig. 4 (Path 9 and Path 10), the hydroxyl group in the peroxyl group of 3-ROOH will attack adjacent carbon atoms C2 or C4. In order to determine which carbon is more active, the molecular orbitals are plotted using Fukui function calculations. As shown in Fig. 5, the density of the electron cloud is expressed in terms of chromaticity, with darker colours indicating a higher density of electron clouds in the region and more active reactions (Charles and Sebens., 2021, Ryu et al., 2018). It can be seen clearly from the Fig. 5 that the closer to the aldehyde group, the darker the color becomes. This is due to the electron absorption effect of the aldehyde group resulting in a relatively high density of electron clouds on its neighbour atoms. As a result, when the hydroxyl group approaches the C2, the hydroxyl group connects to C1 is more active, which leads to the hydrogen on C3 transfers to C2 and the C2-C3 bond breaks simultaneously (R24) to form the final product: 3-nonen-2-one and formic acid via TS27# (28#&29#, path 9). However, if the hydroxyl group is close to C4, the C3-C4 single bond will be broken directly via the TS30# (R25) to form the final products: 2-hepten-1-ol and malondialdehyde (15#&31#, Path 10).
Fig. 5.

Schematic diagram of the molecular orbital for the breaking of the C—H bond at C3.
Similarly, the hydroxyl group of the 4-ROOH attached to C3 is abstracted to form a water molecule, then the C3-C4 bond is broken via TS69# to form the end products: 2-heptenal, 3-butenone and hydrone (Path 13). Whereas if C5 is attacked, the adjacent C4-C5 bond will be broken directly via TS71# to form the final products: (E)-2-hexen-1-ol and butanedione (57#&72#, Path 14). The 5-ROOH is further cleaved via transition state TS34# to form the final products: hexanal and 3-butenal-4-ol (20#&35#, Path 11). Oxidative cleavage via TS36# form glutaric dialdehyde, 1-butene and hydrone (24#& 37#&38#, Path 12). The 6-ROOH is cleaved via TS75# to form the final products: pentanal and 4-pentenal-5-ol (62#&65#, Path 15), and cleavage via TS77# gives the final products: butene, 2-adipaldehyde and hydrone (78#&66#&38#, Path 16).
Analysis of alkoxy radical reaction mechanism (RO·) in (E)-4-decenal thermal oxidation
As shown in the Fig. 2 and Fig. 4, after the formation of peroxides (26#, 33#, 68#, 74#), the structure can continue to absorb energy to break the O-OH bond in the peroxide to form 3-RO· (39#, R32), 4-RO· (79#, R34), 5-RO· (44#, R33), 6-RO·(84#, R35), respectively. These alkoxy radicals would continue to attack the adjacent carbon atom, which causes oxidative breakage of both sites of the alkoxy radicals. As shown in the Fig. 4 (Path 17 and Path 18), in the 3-RO· structure, the O· attacks and breaks C2-C3 bond after going through the TS40# (R36) to form hexanal and CHOCH2CH· group (9#&41#, Path 17). Meanwhile, the O· attacks and breaks the C3-C4 bond directly via the TS42# (R37) to form the final product: 2-pentendialdehyde and CH3(CH2)3CH2· group (15#&43#, Path 18). Similarly, via the TS80# (R40), C3-C4 bond gradually breaks to form the final products: 2-heptenal and CHOCH2CH2· group (52#&81#, Path 21). While the C4-C5 bond of the 4-RO· breaks via the the TS82# (R41), the final products: 2-butanedial and CH3(CH2)3CHCH· group are formed (57#&83#, Path 22). 5-RO· could further oxidize via the TS45#(R38) or TS47#(R39) to form the products hexanal and CHOCH2CHCH· group (20#&46#; Path 19) or 2-pentendialdehyde and CH3(CH2)2CH2· group (24#&48#; Path 20), respectively. 6-RO· would attack C5 or C7 site to form final products: pentanal and CHO(CH2)2CHCH· group (62#&86#; Path 23) or 2-hexendialdehyde and CH3(CH2)2CH· group (66#&88#; Path 24) via the TS85#(R42) or TS87#(R43), respectively.
Analysis of the key reactions and priority of different mechanisms of (E)-4-decenal thermal oxidation
As shown in Fig. 2, compounds 5#, 16#, 26#, 33#, 39#, 44#, 50#, 58#, 68#, 74#, 79#, and 84# are all located at the branching points in the (E)-4-decenal oxidation reaction network, which means that these compounds determine the course of the subsequent oxidation reactions. Therefore, these compounds are considered as the key intermediates in the oxidation of the (E)-4-decenal. Meanwhile, the chemical reactions from the key intermediates are usually considered to be the key reactions in the reaction network. As a result, the reactions R11–R18, R24–R32, and R36–R43 are considered to be the key chemical reactions in their reaction pathways.
Fig. 6(A) shows the energy barrier for the oxidation processes of (E)-4-decanal. Additionally, Fig. 6(B) shows the reaction rate constants for various oxidation reactions. The Tab. S1 contains information on each reaction rate constant at various temperatures. It is well acknowledged that a chemical reaction's energy potential barrier represents the bare minimal amount of energy needed to carry out the reaction. As a result, chemical reactions with high energy barriers are challenging to carry out. Reactions are easier to occur when potential barriers are smaller. It can be seen from Fig. 6(A) that the energy potential barriers for most of key reactions are higher than others in the reaction pathway of (E)-4-decenal. This phenomenon further confirms the conclusion that R11-R18, R24-R32 and R36-R43 are the key chemical reactions in their reaction pathways.
Fig. 6.
Potential energy diagram with (E)-4-decenal oxidation reaction rate constants. (A) Potential energy diagram of (E)-4-decenal oxidation process. The value in brackets are the potential energy of the reaction at 298K. Each color line represents a chemical pathway. (B) The rate constant of various oxidation processes of (E)-4-decenal.
The energy required for reactions of peroxyl radical pathway range from 210 KJ/mol to 420 KJ/mol, as shown in Fig. 6(A), while the energy required for reactions of peroxide pathway range from 20 KJ/mol to 85 KJ/mol. It is obvious that the peroxyl radical reaction pathway has a substantially higher energy barrier than the peroxide reaction pathway. As a result, under the identical circumstances, the peroxide reaction route is easier to react. And the peroxide reaction pathway is preferred over the peroxyl radical reaction pathway. Meanwhile, it can be clearly seen from Fig. 6(A) that all of the reactions in the alkoxyl radical pathway require a lower barrier than that of the peroxide reaction pathway. It means that the alkoxyl radical reaction pathway is preferred over the peroxide reaction pathway. Furthermore, Fig. 6(B) shows that the alkoxy radical pathway's reaction rate constants are significantly larger than those of the other pathways. Additionally, most processes' reaction rate constants show a propensity to rise with temperature. Based on the results above, it can be concluded that in the (E)-4-decenal thermal oxidation process, the alkoxy radical reaction pathway has priority over the peroxide reaction pathway, and the peroxide pathway has priority over the peroxyl radical pathway.
In addition, the calculated reaction priority agrees well with the experimental results based on the fact that the products with higher potential energy barrier have higher formation temperatures than the lower ones in the experiment. For example, the typical products of Path 1 and Path 9 are (E)-2-octenal (9#) and 3-nonene-2-ketone (28#), respectively. The reaction barrier for the creation of 3-nonene-2-ketone (28#) is 129.63 KJ/mol (R7), which is higher in the ROOH reaction pathway. In the experiment, it is found that this substance's formation temperature is likewise higher (150 °C). Similarly, (E)-2-octenal (9#) has an energy barrier of 325.00 KJ/mol (R19). It means that it is more difficult for this reaction to occur than other reactions. The outcomes of the experiment revealed that this compound could be detected at 120 °C. It is further demonstrated that the computed findings and the experimental data agree well.
Conclusion
In the deep oxidation of lipids, (E)-4-decenal is a significant byproduct that can further oxidize to produce volatile flavors with shorter carbon chains. DFT calculations were used to obtain a total of 43 reactions with 24 reaction pathways in the thermal oxidation of (E)-4-decenal. These reactions were categorized into three reaction mechanisms: peroxide (ROOH), alkoxy radical (RO·), and peroxyl radical (ROO·). The outcomes of the DFT calculations and the experiment were generally in agreement. The priority order of these reaction mechanisms is RO· mechanism > ROOH mechanism > ROO· mechanism.
CRediT authorship contribution statement
Binchen Wang: Writing – original draft, Data curation. Shaohua Dou: Software. Shang Wang: Visualization, Software. Yi Wang: Writing – review & editing. Sufang Zhang: Supervision, Methodology. Xinping Lin: Data curation. Yingxi Chen: Formal analysis. Chaofan Ji: Methodology. Yiwei Dai: Formal analysis, Data curation. Liang Dong: Writing – review & editing, Supervision, Resources, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We thank the support of the National Natural Science Foundation of China (No. 31871760).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.101174.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
The authors do not have permission to share data.
References
- Albaugh A., Boateng H.A., Bradshaw R.T., et al. Advanced Potential Energy Surfaces for Molecular Simulation. The journal of physical chemistry. 2016;120(37):9811–9832. doi: 10.1021/acs.jpcb.6b06414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Otaibi J.S., Mahmoud M.A.M., Almuqrin A.H. Ab initio-based kinetics of hydrogen atom abstraction from methyl propionate by h and ch3 radicals: A biodiesel model. Structural Chemistry. 2021;32(5):1857–1872. doi: 10.1007/s11224-021-01746-6. [DOI] [Google Scholar]
- Ama A., Fb A., Db B., et al. RIFM fragrance ingredient safety assessment, 1-octen-3-ol, CAS registry number 3391–86-4-ScienceDirect. Food and Chemical Toxicology. 2019;134 doi: 10.1016/j.fct.2019.110972. [DOI] [PubMed] [Google Scholar]
- Angela K., Andreas M. Oxidation of unsaturated fatty acid derivatives and vegetable oils. European Journal of Lipid Science & Technology. 2010;110(9):812–824. doi: 10.1002/ejlt.200800042. [DOI] [Google Scholar]
- Balan R.C., Rajakumar B. Photo oxidation reaction kinetics of ethyl propionate with cl atom and formation of propionic acid. The Journal of Physical Chemistry A. 2018;122:42. doi: 10.1021/acs.jpca.8b05215. [DOI] [PubMed] [Google Scholar]
- Becke A.D. Density-functional thermochemistry. 1. The effect of the exchange-only gradient correction. Journal of Chemical Physics. 1992;96:2155–2160. doi: 10.1063/1.462066. [DOI] [Google Scholar]
- Becke A.D. Density-functional thermochemistry. 3. The role of exact exchange. Journal of Chemical Physics. 1993;98:5648–5652. doi: 10.1063/1.464913. [DOI] [Google Scholar]
- Borges R.M., Colby S.M., Das S., et al. Quantum chemistry calculations for metabolomics. Chemical Reviews. 2021;10:121. doi: 10.1021/acs.chemrev.0c00901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calín-Sánchez Á., Carbonell-Barrachina Á.A. Flavor and aroma analysis as a tool for quality control of foods. Foods. 2021;10:224. doi: 10.3390/foods10020224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai D., Li C.W., Zhang X.X., et al. Analysis of volatile compounds from wheat flour in the heating process. International Journal of Food Engineering. 2019;15(10):1–13. doi: 10.1515/ijfe-2019-0252. [DOI] [Google Scholar]
- Charles T., Sebens. Electron charge density: A clue from quantum chemistry for quantum foundations. Foundations of Physics. 2021;51:75. doi: 10.1007/s10701-021-00480-7. [DOI] [Google Scholar]
- Drabova L., Tomaniova M., Kalachova K., et al. Application of solid phase extraction and two-dimensional gas chromatography coupled with time-of-flight mass spectrometry for fast analysis of polycyclic aromatic hydrocarbons in vegetable oils. Food Control. 2013;33(2):489–497. doi: 10.1016/j.foodcont.2013.03.018. [DOI] [Google Scholar]
- Dymerski T. Two-dimensional gas chromatography coupled with mass spectrometry in food analysis. Critical Reviews in Analytical Chemistry. 2017 doi: 10.1080/10408347.2017.1411248. [DOI] [PubMed] [Google Scholar]
- Elroby S.A., Banaser B.A., Aziz S.G., et al. Zn2+-schiff’s base complex as an “on-off-on” molecular switch and a fluorescence probe for Cu2+ and Ag+ ions. Journal of Fluorescence. 2022;32(2):691–705. doi: 10.1007/s10895-021-02864-4. [DOI] [PubMed] [Google Scholar]
- Frankel E.N. Volatile lipid oxidation products. Progress in Lipid Research. 1983;22(1):1–33. doi: 10.1016/0163-7827(83)90002-4. [DOI] [PubMed] [Google Scholar]
- Fukui K. The path of chemical reactions-The IRC approach. Accounts of Chemical Research. 1981;14:12. doi: 10.1021/ar00072a001. [DOI] [Google Scholar]
- Grambow C., Pattanaik L., Green W.H. Reactants, products, and transition states of elementary chemical reactions based on quantum. Chemistry. 2020;7:137. doi: 10.1038/s41597-020-0460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes-Schiffer S. A conundrum for density functional theory: DFT studies may sometimes get the right results for the wrong reasons. Science. 2017;355(6320):28–29. doi: 10.1126/science.aal3442. [DOI] [PubMed] [Google Scholar]
- Hine Nicholas D., Hine N.D.M. Applications of large-scale density functional theory in biology. Journal of Physics Condensed Matter. 2016;28 doi: 10.1088/0953-8984/28/39/393001. [DOI] [PubMed] [Google Scholar]
- Humphrey W.F., Dalke A., Schulten K. VMD: Visual molecular dynamics. Journal of Molecular Graphics. 1996;14(1):33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- Jeleń H.H., Majcher M., Dziadas M. Microextraction techniques in the analysis of food flavor compounds: A review. Analytica Chimica Acta. 2012;738:13. doi: 10.1016/j.aca.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Li X., Josef P. Valence bond description of spin properties of π-conjugated systems. Chemical Physics. 1996;204(2–3):447–461. doi: 10.1016/0301-0104(95)00291-X. [DOI] [Google Scholar]
- Mahmoud M., Shiroudi A., Abdel-Rahman M.A. Structures, energetics, and kinetics of h-atom abstraction from methyl propionate by molecular oxygen: Ab initio and dft investigations. Computational and Theoretical Chemistry. 2020;1196:2. doi: 10.1016/j.comptc.2020.113119. [DOI] [Google Scholar]
- Peng C.-Y., Lan C.H., Lin P.C., et al. Effects of cooking method, cooking oil, and food type on aldehyde emissions in cooking oil fumes. Journal of Hazardous Materials. 2017;324:160. doi: 10.1016/j.jhazmat.2016.10.045. [DOI] [PubMed] [Google Scholar]
- Peter Q.T., Giorgia P., Mariarosa M., et al. Impact of comprehensive two-dimensional gas chromatography with mass spectrometry on food analysis. Journal of Separation Science. 2016 doi: 10.1002/jssc.201500379. [DOI] [PubMed] [Google Scholar]
- Porter N.A., Caldwell S., Mills K.A. Mechanisms of free radical oxidation of unsaturated lipids. Lipids. 1995;30:4. doi: 10.1007/BF02536034. [DOI] [PubMed] [Google Scholar]
- Ryu H.o., Park J.Y., KiM H.K. Pitfalls in computational modeling of chemical reactions and how to avoid them. Section B: Structure Science Crystal Engineering Materials. 2018:171–179. doi: 10.1021/acs.organomet.8b00456. [DOI] [Google Scholar]
- Ryu H., Park J., Kim H.K., et al. Pitfalls in computational modeling of chemical reactions and how to avoid them. Organometallics. 2018;37(19):3228–3239. doi: 10.1021/acs.organomet.8b00456. [DOI] [Google Scholar]
- Santacesaria E., Sorrentino A., Rainone F., et al. Oxidative cleavage of the double bond of monoenic fatty chains in two steps: A new promising route to azelaic acid and other industrial products[J] Industrial & Engineering Chemistry Research. 2000;39(8):2766–2771. doi: 10.1021/ie990920u. [DOI] [Google Scholar]
- Shabani M., Latina J., Li Z. Comparison of ultra-high-resolution with conventional ct-derived coronary lumen visibility in patients with coronary artery disease. Journal of Cardiovascular Computed Tomography. 2020;14(3):541. doi: 10.1016/j.jcct.2020.06.063. [DOI] [Google Scholar]
- Sheridan J. Mechanisms of reactions at carbon-carbon double bond. Interscience Publishers. 1946;161:626. doi: 10.1016/j.jhazmat.2016.10.045. [DOI] [Google Scholar]
- Stier R.F. Chemistry of frying and optimization of deep-fat fried food flavour-An introductory review. European Journal of Lipid Science & Technology. 2000;102(8–9):507–514. doi: 10.1002/1438-9312(200009)102:8/9<507::AID-EJLT507>3.0.CO;2-V. [DOI] [Google Scholar]
- Tian L., Chen F. Multiwfn: A multifunctional wavefunction analyzer. Journal of Computational Chemistry. 2012;33(5):580–592. doi: 10.1002/jcc.22885. [DOI] [PubMed] [Google Scholar]
- Tian L., Chen Q.H. Shermo: A general code for calculating molecular thermochemistry properties. Computational and Theoretical. 2021;Chemistry, 1200:113249. doi: 10.1016/j.comptc.2021.113249. [DOI] [Google Scholar]
- Verma P., Truhlar D.G. Status and challenges of density functional theory. Trends Chemistry. 2020;2(4) doi: 10.1016/j.trechm.2020.02.005. [DOI] [Google Scholar]
- Yang P., Lee. Various functionals for the kinetic energy density of an atom or molecule. Physical Review. A, General Physics. 1986;34:6. doi: 10.1103/physreva.34.4586. [DOI] [PubMed] [Google Scholar]
- Wang B., Zhang S., Lin X., et al. Deep exploration of lipid oxidation into flavor compounds: a density functional theory study on (E)-2-decenal thermal oxidative reaction. FoodChemistry. 2023;428 doi: 10.1016/j.foodchem.2023.136725. [DOI] [PubMed] [Google Scholar]
- Zaldman B., Klsillev A., Sasson Y. Double bond oxidation of unsaturated fatty acids. Journal of the American Oil Chemists’ Society. 1988;65(4):611–615. doi: 10.1007/BF02540689. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors do not have permission to share data.