Abstract
T-2 toxin is one of the most widespread and toxic fungal toxins in food and feed. It can cause gastrointestinal toxicity, hepatotoxicity, immunotoxicity, reproductive toxicity, neurotoxicity, and nephrotoxicity in humans and animals. T-2 toxin is physicochemically stable and does not readily degrade during food and feed processing. Therefore, suppressing T-2 toxin-induced organ toxicity through antidotes is an urgent issue. Protective agents against the organ toxicity of T-2 toxin have been recorded widely in the literature, but these protective agents and their molecular mechanisms of detoxification have not been comprehensively summarized. In this review, we provide an overview of the various protective agents to T-2 toxin and the molecular mechanisms underlying the detoxification effects. Targeting appropriate targets to antagonize T-2 toxin toxicity is also an important option. This review will provide essential guidance and strategies for the better application and development of T-2 toxin antidotes specific for organ toxicity in the future.
Keywords: T-2 toxin, Organ toxicity, Protective agent, Detoxification mechanism
1. Introduction
T-2 toxin (T-2) is one of the most toxic fungal toxins and is the most common cause of poisoning in humans and animals resulting from the consumption of contaminated cereal food and feed (Tima et al., 2016; Van Der Fels-Klerx et al., 2012). The food and feedstuff most frequently contaminated with T-2 include wheat, maize, barley, oats and rye, along with their processed products, which results in huge economic losses (Tutelyan et al., 2013; Zakharova et al., 2009). T-2 was detected in 92.59% of maize in Sichuan, China (Halstensen et al., 2006), and caused gastrointestinal absorption disorders, immunosuppression or irritation, reproductive disorders, liver damage, neurotoxicity, cardiac and skeletal muscle toxicity in humans and livestock through contaminated feed and food (Janik et al., 2020). However, owing to the highly stable physicochemical properties of this toxin, 100% degradation is not feasible. Therefore, the use of antagonists to reduce organ toxicity caused by T-2 is an important method of detoxification.
An increasing number of studies have reported various protective agents that mitigate the organ toxicity of T-2. For example, rosmarinic acid (RA) was shown to protect the intestine from T-2-induced damage (Pomothy et al., 2020a). Selenomethionine (SeMet) effectively mitigated T-2-induced immunotoxicity by exerting strong antioxidant and anti-inflammatory effects (Zhang et al., 2022h). Betulinic acid (BA) ameliorated T-2-induced oxidative stress and inflammatory responses, thereby reducing renal damage (Huang et al., 2021). Thus, protective agents could effectively reduce the organ toxicity of T-2.
In recent years, researchers have reviewed findings on T-2 toxicity. Zhang et al. (2022c) systematically described T-2 metabolism, the mechanisms underlying T-2 immunotoxicity, and exposure assessment of the human gut microbiota. Li et al. (2022b) reviewed the mechanism of T-2 toxicity on protein synthesis. Wu et al. (2020) focused on novel findings related to the metabolism, immunotoxicity, and human exposure assessment of T-2 and its modified forms. However, these reviews did not specify antidotes for the organ toxicity of T-2.
Although Li et al. (2022c) outlined the poultry toxicity of T-2 and suggested potential strategies for T-2 detoxification in the poultry diet, to date, no researcher has systematically described the protective agents against the individual organ toxic effects of T-2 and the corresponding detoxification mechanisms and application prospects. Most importantly, previous reviews have barely discussed the protective agents in terms of the toxic targets of T-2.
In this review, we provide a comprehensive overview of protective agents (Table 1) for T-2-induced gastrointestinal toxicity, hepatotoxicity, immunotoxicity, nephrotoxicity, neurotoxicity, reproductive toxicity, cardiotoxicity, dermal toxicity, skeletal toxicity, and muscular toxicity and described in detail the detoxification mechanisms of the various protective agents. Importantly, we also summarize the toxic targets of T-2 (Table 2). This review reveals promising applications of protective agents and provides important strategies and guidance for the better application and development of protective agents against T-2 organ toxicity.
Table 1.
Summary of the protective agents against organ toxicity of T-2.
| Protective agents | Models | Dose and time | Organ toxicity | Effects | Mechanisms | References |
|---|---|---|---|---|---|---|
| Alfalfa meal | Rats | Alfalfa meal (5%, 12.5%, 20%) + T-2 (3 μg/g feed) for 2 weeks. | Gastrointestinal toxicity |
|
|
Carson and Smith (1983a) |
| RA | IPEC-J2 cells | Pre-incubated with RA (50 μmol/L) for 24 h; T-2 (5 nmol/L) for 48 and 72 h. |
|
|
Pomothy et al. (2020a) | |
| BA | Mice | Pretreated with BA (0.25, 0.5, 1 mg/kg BW i.g.) for 2 weeks; T-2 (4 mg/kg BW i.p.) once. |
|
|
Luo et al. (2020) | |
| FWGE | IPEC-J2 cells | FWGE (1% and 2%) + T-2 (5 nmol/L) for 24 h. |
|
|
Pomothy et al. (2020b) | |
| Bentonite | Rats | Pretreated with bentonite (5%, 7.5% or 10%) for 2 weeks; T-2 (3 μg/g feed) once. |
|
|
Carson and Smith (1983b) | |
| Smectite | Mice | T-2 was incubated with smectite for 24 h before oral administration; T-2 (1 mg/kg BW/d, p.o.); Smectite (2 g/kg/d). |
|
|
Fioramonti et al. (1987) | |
| Mineral clays | Caco-2 cells | T-2 (100 μmol/L) + 0.1 mg/mL of each clay (diosmectite, montmorillonite, and illite) for 24 h. |
|
|
Romero et al. (2016) | |
| HSCAS | Broilers | 0.05% HSCAS + T-2 (6.0 mg/kg feed) for 2 weeks. |
|
|
Wei et al. (2019) | |
| MycoRaid | Broilers | MycoRaid (1 or 3 g/kg feed) + T-2 (1 mg/kg feed) for 1–10 d. |
|
|
Riahi et al. (2021) | |
| SeMet | Rabbits | Orally administered with SeMet (0.2, 0.4 and 0.6 mg/kg feed) for 21 d; On 17th d, each group began to take 0.4 mg/kg feed of T-2 orally/d for 5 d. |
|
|
Liu et al. (2020) | |
| FPH | Human colon cancer TC7 cells and Caco-2. | FPH (0.0625 mg/mL) + T-2 (60 nmol/L) for 24 h. |
|
|
Taroncher et al. (2021) | |
| Prednisolone and hydrocortisone | Mice | Pretreated i.p. with 100 mg/kg BW of prednisolone or hydrocortisone for 3 d; T-2 (1.8 mg/kg BW, s.c.) for 4 d. |
|
|
Mutoh et al. (1988) | |
| Dietary nucleotides | Male broiler chickens | Exposed T-2 (10 mg/kg feed) with nucleotides (2 g/kg feed) for 17 d. | Immunotoxicity |
|
|
Frankic et al. (2006) |
| Se | Mice | The sublethal dose of T-2. |
|
|
Ahmadi et al. (2015) | |
| SeMet | Rabbits | Orally administered with SeMet (0.2, 0.4 and 0.6 mg/kg feed) for 21 d. On 17th d, each group began to take 0.4 mg/kg BW of T-2 orally every day for 5 d. |
|
|
Zhang et al. (2022h) | |
| Arginine | Chinese mitten crab | Pretreated with 3.17% arginine for 8 weeks; Injected with T-2 (1.5 mg/kg BW). |
|
|
Zhang et al. (2020a) | |
| BA | Mice | Pretreated with BA (0.25, 0.5, or 1 mg/kg BW i.g.) for 2 weeks, T-2 (4 mg/kg BW single i.p.). |
|
|
Kong et al. (2021) | |
|
|
Zhu et al. (2020) | ||||
| NAC | Neuroblastoma- 2a cells | Pretreated with NAC (5 mmol/L) for 2 h, followed by treatment with T-2 (20 ng/mL) for 24 h. | Neurotoxicity |
|
|
Zhang et al. (2018) |
| Mouse microglia BV2 cell line. | Pretreated with NAC at 2.5 mmol/L for 1 h, followed by co-treatment with T-2 at 2.5 ng/mL for additional 24 h. |
|
|
Sun et al. (2022) | ||
| BA | Mice | Pretreated with BA (0.25, 0.5, and 1.00 mg/kg BW) for 14 d; T-2 (4 mg/kg BW, single i.p.). |
|
|
Huang et al. (2022b) | |
| Minocycline | Mice | Injected with T-2 (4 mg/kg BW) + minocycline (50 mg/kg BW). |
|
|
Li et al. (2023) | |
| L-arginine | Mouse Leydig cells | Treated with T-2 (10 nmol/L) + L-arginine (0.25, 0.5, or 1.0 mmol/L) for 24 h. | Reproductive toxicity |
|
|
Yang et al. (2018) |
|
|
Yang et al. (2019b) | ||||
|
|
Zhang et al. (2020b) | ||||
| Mice | Pretreated with L-arginine (5, 15, 25 g/kg feed) for 7 d; T-2 (10 mg/kg BW/d i.p.) for 7 d. |
|
|
Zhang et al. (2019) | ||
| Melatonin | Bovine ovarian granulosa cells | -Pretreated with melatonin (100 μmol/L) for 12 h; HT-2 toxin 50 nmol/L for 24 h. |
|
|
Yang et al. (2019a) | |
| Quercetin | Porcine ovarian granulosa cells | Quercetin (100 ng/mL) + T-2 (100 ng/mL) for 24 h. |
|
|
Capcarova et al. (2015) | |
| BA | Mice | Pretreated orally with BA (0.25, 0.5, and 1.0 mg/kg feed/d) for 14 d, T-2 (4 mg/kg BW i.p.) once. |
|
|
Wu et al. (2019) | |
| NAC | TM4 cells (The Sertoli cell line) | T-2 (4 nmol/L) + NAC (5 mmol/L) for 24 h. |
|
|
Yang et al. (2021) | |
| VE and Se | Bovine Leydig cells | 100 nmol/L Se+10 nmol/L T-2; 100 μmol/L VE+10 nmol/L T-2; 100 nmol/L Se+100 μmol/L VE+10 nmol/L T-2 for 24 h. |
|
|
Yang et al. (2022) | |
| Se | Rats | Pre-supplementation Se (0.5 and 2.5 mg/kg feed for 6 weeks); T-2 (3.8 mg/kg BW). | Hepatotoxicity |
|
|
Kravchenko et al. (1990) |
| Se, VE, VC | Rats | Received Se (0.15 mg), VE (15 mg) and VC (6 mg) i.g. 16 h before the administration of T-2 (3.6 mg/kg BW orally a single dose). |
|
|
Rizzo et al. (1994) | |
| Modified glucomannans and organic Se | Chicken | Mycosorb (1 g/kg feed) Sel-Plex (Se 0.3 mg/kg feed); T-2 (8.1 mg/kg feed for 21 d). |
|
|
Dvorska et al. (2007) | |
| SeMet | New Zealand rabbits | SeMet (0.2 mg/kg feed) for 21 d. On the 17th day, orally administered with T-2 (0.4 mg/kg BW) for 5 d. |
|
|
Liu et al. (2021c). | |
| CoQ10, VE | Mice | Gavage pretreatment with CoQ10 (6 mg/kg BW) and alpha-tocopherol (6 mg/kg BW) for 4 weeks. T-2 (1.8 or 2.8 mg/kg BW, single oral dose). |
|
|
Atroshi et al. (1997) | |
| Lycopene | Broiler chickens | T-2 (1.5 mg/kg BW/d) + Lycopene (25 mg/kg BW/d) for 7, 14 and 21 d. |
|
|
Leal et al. (1999). | |
| Food indoles | Rats | Fed with 0.1% food indoles for 8 d; T-2 (0.8 mg/kg BW). |
|
|
Kravchenko et al. (2001) | |
| HSCAS | Broilers | 0.05% modified HSCAS adsorbent + T-2 (6.0 mg/kg feed) for 2 weeks. |
|
|
Wei et al. (2019) | |
| Arginine | Chinese mitten crab | Pretreated with 3.17% Arg for 8 weeks; Injected with T-2 (1.5 mg/kg BW). |
|
|
Zhang et al. (2020a) | |
| YCWE and PYCW | Broilers | Contaminated diet containing T-2 (104 g/kg feed) + 0.2% YCWE or 0.2% PYCW. |
|
Kudupoje et al. (2022) | ||
| CC2 | Mice | 20% CC-2 formulation was applied on the exposed dorsal surface at 5, 15, 30 and 60 min after T-2 (11.8 mg/kg BW percutaneous exposure). |
|
Agrawal et al. (2012a) | ||
| L-Carnitine | Rats, primary rat hepatocytes | Received L-carnitine (50 or 500 mg/kg i.p.) for 5 d, rat hepatocytes were isolated and treated with T-2 (640 ng/mL) for 2 h. |
|
|
Moosavi et al. (2016) | |
| CA | Broiler chickens | 1% CA-supplemented diet for 4 d; T-2 (2.0 mg/kg BW) oral administration for 4 d. |
|
|
Dai et al. (2020) | |
| Curcumin and taurine | Rats | Administrated T-2 sublethal oral dose (0.1 mg/kg BW i.p.) for 2 months, followed by curcumin (80 mg/kg BW) and taurine (50 mg/kg BW) for 3 weeks. |
|
|
Al-Zahrani et al. (2023) | |
| SeMet | Rabbits | Fed diets containing SeMet (0.2 mg/kg) for 21 d; On the 17th day, perfused with T-2 (0.4 mg/kg BW) for 5 d. | Nephrotoxicity |
|
|
Liu et al. (2021b) |
| Se | Mice | Pretreated with Se (0.2 mg/kg BW/d) for 2 h, then exposed to T-2 (1.0 mg/kg BW/d) for 28 d. |
|
|
Zhang et al. (2022f) | |
| BA | Porcine kidney cells | Pretreated with BA (0.25, 0.5, and 1 μmol/L) for 24 h, continued with subsequent T-2 (1 μmol/L) for 24 h. |
|
|
Li et al. (2021) | |
| Mice | Pretreated with BA (0.25, 0.5, and 1 mg/kg BW i.g.) for 14 d; T-2 (4 mg/kg BW) was injected intraperitoneally at the 9th h after the last oral administration of BA. |
|
|
Huang et al. (2021). | ||
| Catalase and VC | Rat cardiomyocytes | T-2 (6.0 × 10−3 and 6.0 × 10−4 μmol/L) + VC (10 μg/mL) or catalase (10 U/mL) for 24 h. | Cardiomyopathy |
|
|
Ngampongsa et al. (2013) |
| Se | Primary cardiomyocytes | T-2 (0.25−1 μmol/L) |
|
|
Chen et al. (2019) | |
| Methylprednisolone | Rats | Methylprednisolone (a total single dose of 40 mg/kg i.m.) was given immediately after T-2 (0.23 mg/kg s.c.). |
|
|
Jacevic et al. (2019) | |
| Se | Chondrocytes | T-2 (0.001–2 mg/L). | Skeletal toxicity |
|
Chen et al. (2006; Li et al. (2008) | |
|
||||||
| An artificial cartilage model | Se (0.1 μg/mL) for 14 d; T-2 (1, 10 and 20 ng/mL). |
|
|
Chen et al. (2011) | ||
| Cultured chondrocytes | T-2 (5, 10, 20, 40 and 80 ng/mL) supplemented with Na2SeO3 (50, 100 and 150 ng/mL), and incubated for 6, 12, 24, 36 and 48 h. |
|
|
Yu et al. (2017) | ||
| Rats, primary epiphyseal chondrocytes | Administered with low Se (0.09 ng/g) and/or T-2 (100 ng/g BW per day) for 4 weeks to establish a KBD animal model. |
|
|
Shi et al. (2021) | ||
| Rats | Administered with T-2 (200 ng/g BW per day) for 4 weeks under the Se-deficient diet. |
|
|
Zhang et al. (2021a) | ||
| NAC | Chicken tibial growth plate chondrocytes | NAC (0.5 mmol/L) was co-administered with T-2 (5, 50, and 500 nmol/L) for 48 h. |
|
|
He et al. (2012) | |
| Mycotoxin adsorbents: EGM, HSCAS, CMA | Broilers | The mycotoxins-contaminated feed containing T-2 (320.5 μg/kg) + 0.05% EGM or +0.2% HSCAS or + 0.1% CMA for 10, 21, 35 and 42 d. | Muscle toxicity |
|
|
Liu et al. (2011) |
| Quercetin | Shrimps | Quercetin (2.00 to 32.00 g/kg feed) or tea polyphenols or rutin + T-2 (4.80–24.30 mg/kg feed) for 20 d. |
|
|
Huang et al. (2022a) | |
| Tea polyphenols and rutin |
|
|
||||
| Menthol | Mice | T-2 (2.97 mg/kg BW) for 72 h and 120 h; 0.25% and 0.5% menthol. |
Skin toxicity |
|
- Anti-inflammatory effect. | Rachitha et al. (2023) |
| CC-2 | Mice | The subcutaneous application of 20% CC-2 within 5 and 15 min of treatment with T-2 (23.76 mg/kg BW topical percutaneous smearing). |
|
Agrawal et al. (2012a) | ||
| Quince seed | Rabbits | 100 μg T-2 was dissolved in 12 μL methanol and applied on the shaved skin of rabbit for 2 d. Quince seed mucilage (15%). |
|
Hemmati et al. (2012) |
p.o. = oral administration; s.c. = subcutaneous injection; i.g. = intragastrical administration; i.p. = intraperitoneal injection; i.m. = intramuscular injection; ALT = alanine aminotransferase; AST = aspartate aminotransferase; BA = betulinic acid; BW = body weight; CA = cholic acid; CC-2 = N,N′-dichloro-bis (2,4,6-trichlorophenyl) urea; CMA = compound mycotoxin adsorbent; CoQ10 = coenzyme Q10; EGM = esterified glucomannan; FPH = fish protein hydrolysates; FWGE = fermented wheat germ extract; FXR = farnesoid X receptor; GSH = glutathione; GSH-Px = glutathione peroxidase; HSCAS = hydrated sodium calcium alumino silicate; IPEC-J2 = intestinal porcine epithelial cell line-J2 cells; JAK2 = Janus kinase 2; KBD = Kashin-Beck disease; LPO = lipid peroxidation; MAPK = mitogen-activated protein kinase; MDA = malondialdehyde; MycoRaid = A novel multicomponent MYC; NAC = N-acetylcysteine; Nrf2 = nuclear factor erythroid 2-related factor 2; RA = rosmarinic acid; ROS = reactive oxygen species; Se = selenium; SeMet = selenomethionine; SOD = superoxide dismutase; StAR = steroidogenic acute regulatory protein; STAT3 = signal transducer and activator of transcription 3; T-2 = T-2 toxin; VC = vitamin C; VE = vitamin E; YCWE = yeast cell wall extract.
Table 2.
Primary targets and antagonistic effect for T-2 organ toxicity.
| Toxic targets | Model | Toxicity | The role of targets | Antagonistic effect | References |
|---|---|---|---|---|---|
| GLP-1 and CCK | Mice | Gastrointestinal toxicity |
|
|
Wu et al. (2018) |
| GIP and NPY2 | Nocturnal mouse food refusal model |
|
|
Sheng et al. (2018) | |
| IRE1a | Human intestinal Caco-2 cells and HT-29 cells; mice |
|
|
Lin et al. (2019) | |
| 5-HT and NK-1R | Nocturnal mouse food refusal model |
|
|
Sheng et al. (2019) | |
| HIF-1α | RAW264.7 cells | Immunotoxicity |
|
|
You et al. (2022) |
| IGF-1R | Human C28/I2 chondrocytes and mouse hypertrophic ATDC5 chondrocytes | Skeletal toxicity |
|
|
Zhang et al. (2021b) |
| MMP-10 | Hypertrophic chondrocytes (ATDC5 cells) |
|
|
Zhang et al. (2022a) | |
| Wnt/beta-catenin signaling | Mice |
|
__ | Zhang et al. (2022d) | |
| MGMT | KBD patients and chondrocytes |
|
|
Zhang et al. (2022b) | |
| TGF-βRs | Sprague Dawley rats; C28/I2 chondrocytes |
|
|
Zhang et al. (2022g) | |
| HSP47 | C28/I2 and ATDC5 cells; rats. |
|
|
Zhang et al. (2021a) | |
| YAP | Rat chondrocyte |
|
|
Li et al. (2022a) | |
| HMGB1 | PC12 cell | Neurotoxicity |
|
|
Pei et al. (2021) |
| Nrf2 | Human neuroblastoma SH-SY5Y cells |
|
|
Pang et al. (2022) | |
| HO-1 | Mouse neuroblastoma-2a cells | __ |
|
Zhang et al. (2018) | |
| NF-κB and MAPK | BV-2 cells | __ |
|
Li et al. (2023) | |
| CYP1A5 | Chicken primary hepatocytes | Hepatotoxicity |
|
|
Liu et al. (2019) |
| CYP1A1 | HepG2 cells |
|
__ | Ye et al. (2019) | |
| SIRT1 | HepG2 cells |
|
|
Ma et al. (2017) | |
| FXR | Broiler chicken livers |
|
|
Dai et al. (2020) | |
| TET3 | HepG2 cells |
|
|
Zhu et al. (2023) | |
| Nrf2 | Mice | Nephrotoxicity |
|
__ | Zhang et al. (2021c) |
| PINK1/Parkin | C57BL/6N mice |
|
|
Zhang et al. (2022e) | |
| PERK | PK-15 cells |
|
|
Liu et al. (2021a) | |
| mTORC2 | TM3 cells | Reproductive toxicity |
|
|
Wang et al. (2018) |
| PPAR-γ | Rats | Cardiomyopathy |
|
|
Lu et al. (2021b) |
5-HT = 5-hydroxytryptamine; AKT = protein kinase B; CCK = cholecystokinin; CYP1A5 = cytochrome P450 1A5; CYP1A1 = cytochrome P450 1A1; FXR = farnesoid X receptor; GLP-1 = glucagon-like peptide-17-36 amide; GIP = glucose-dependent insulinotropic polypeptide; HIF-1α = hypoxia-inducible factors-1 alpha; HMGB1 = high mobility group B1; HO-1 = heme oxygenase 1; HSP47 = heat shock protein 47; IRE1a = inositol-requiring enzyme 1 alpha; IGF-1R = insulin like growth factor 1 receptor; KBD = Kashin-Beck disease; MAPK = mitogen-activated protein kinase; MGMT = O6-methylguanine-DNA methyltransferase; MMP-10 = matrix metalloproteinase-10; MMP-13 = matrix metalloproteinase-13; mTORC2 = mammalian target of rapamycin complex 2; NF-κB = nuclear factor -kappa B; NK-1R = neurokinin-1 receptor; NPY2 = neuropeptide Y2; Nrf2 = nuclear factor erythroid 2 related factor 2; PINK1 = phosphatase and tensin homolog (PTEN)-induced putative kinase1; Parkin = E3 ubiquitin ligase PARK2; PD-1 = programmed cell death protein-1; PD-L1 = programmed cell death-ligand 1; PERK = protein kinase RNA-like ER kinase; PPAR-γ = peroxisome proliferator-activated receptor-γ; SIRT1 = silent information regulator sirtuin 1; TET3 = eleven translocation family protein 3; TGF-βRs = transforming growth factor-beta receptors; YAP = Yes-associated protein.
2. Effective protective agents against organ toxicity of T-2 and their mechanisms of action
2.1. Protective agents against gastrointestinal toxicity
2.1.1. Protective agents
2.1.1.1. Protective agents from plants
Alfalfa meal (5%, 10%, 15%, 20% or 25%) with lignin could overcome T-2 (3 μg/g feed)-induced feed refusal and growth depression in rats by binding T-2 in the intestine and thus promoting fecal excretion (Carson and Smith, 1983a). Pomothy et al. (2020a) found that the pretreatment of intestinal porcine epithelial cell line-J2 cells (IPEC-J2) with RA (50 μmol/L) prevented the reduction of trans-epithelial electrical resistance (TEER) under T-2 exposure (5 nmol/L), prevented T-2-induced oxidative stress and interleukin (IL)-6 and IL-8 production (Fig. 1), and alleviated intestinal cell damage. Luo et al. (2020) reported that BA (0.25, 0.5, or 1 mg/kg BW) supplementation inhibited the loss of intestinal antioxidant capacity in T-2 (4 mg/kg BW)-exposed mice by increasing the levels of catalase (CAT), glutathione peroxidase (GSH-Px) and glutathione (GSH), and reducing malondialdehyde (MDA) formation (Fig. 1). In addition, pretreatment with BA (0.5 and 1 mg/kg BW) alleviated T-2-triggered intestinal immune barrier dysregulation, inhibited the expression of pro-inflammatory cytokines interleukin-1 beta (IL-1β), IL-6, and tumor necrosis factor-alpha (TNF-α) and increased the expression of the anti-inflammatory cytokine interleukin-10 (IL-10), thus improving the T-2-induced intestinal inflammatory response (Luo et al., 2020). Thus, BA exerts a protective effect against T-2-stimulated intestinal mucosal destruction in mice by enhancing intestinal antioxidant capacity, inhibiting the secretion of inflammatory cytokines, and repairing intestinal mucosal barrier function. In IPEC-J2 cells, 1% and 2% fermented wheat germ extract (FWGE) significantly reduced T-2 (5 nmol/L)-induced reactive oxygen species (ROS) levels, inhibited oxidative stress (Fig. 1), and increased cell viability and cell monolayer integrity (Pomothy et al., 2020b).
Fig. 1.
The mechanisms of rosmarinic acid (RA), betulinic acid (BA), fermented wheat germ extract (FWGE), selenomethionine (SeMet) and illite to mitigate T-2-induced enterotoxicity. CAT = catalase; Cla-3/4 = Claudin-3/4; GSH = glutathione; GSH-Px = glutathione peroxidase; H2O2 = hydrogen peroxide; IL-6 = interleukin-6; IL-8 = interleukin-8; IL-10 = interleukin-10; IL-1β = interleukin-1 beta; MDA = malondialdehyde; ROS = reactive oxygen species; SOD = superoxide dismutase; TNF-α = tumour necrosis factor alpha; T-2 = T-2 toxin; ZO-1 = Zonula occludens protein 1.
2.1.1.2. Adsorbents and other protective agents
Bentonite (10%) shortened the transit time of T-2 in the intestine to overcome the toxic symptoms of growth inhibition and food refusal in rats provoked by T-2 (3 μg/g feed) (Carson and Smith, 1983b). When T-2 was incubated with smectite for 24 h before oral administration, smectite slowed mice gastric emptying and small intestinal transmission induced by T-2 (1 mg/kg BW per day) by enhancing the natural defenses of the gastric mucosa (Fioramonti et al., 1987). Mineral clays, like diosmectite, montmorillonite, and illite protected Caco-2 cells from T-2 (1 to 100 μmol/L)-induced toxic effects, such as reduced viability and altered epithelial barrier function. Illite treatment reversed the T-2-induced reduction in TEER and in the expression of tight junction constituents like claudin-3, claudin-4, and occludin (Fig. 1), thereby making the intestinal barrier less susceptible to increased permeability and disruption (Romero et al., 2016). The addition of 0.05% modified hydrated sodium calcium aluminosilicate (HSCAS) sorbent prevented the reduction of body weight and feed intake in broilers exposed to T-2 (6.0 mg/kg) and also prevented T-2-induced intestinal damage in broilers (Wei et al., 2019).
A novel multicomponent mycotoxin detoxifying agent (MycoRaid) (3 g/kg feed) (containing modified zeolite, Bacillus subtilis, B. licheniformis, Saccharomyces cerevisiae cell walls, silymarin) accelerated the slow growth of broiler chickens in response to T-2 treatment (1 mg/kg feed) and effectively counteracted the harmful effects of T-2 (Riahi et al., 2021). SeMet (0.2 mg/kg feed) pre-supplementation reduced the ROS and MDA levels and enhanced superoxide dismutase (SOD) and GSH-Px activities, thereby alleviating oxidative stress caused by T-2 (0.4 mg/kg feed) in rabbits (Fig. 1). SeMet also attenuated T-2-induced jejunal inflammation and preserved the integrity of the intestinal barrier by upregulating the expression of zonula occludens protein 1 and occludin proteins (Liu et al., 2020). Fish protein hydrolysates (containing bioactive peptide) inhibited lipid peroxidation (LPO) and protected against T-2-induced cytotoxicity in human colon cancer TC7 cells (Taroncher et al., 2021). Pretreatment of prednisolone or hydrocortisone (100 mg/kg BW) reduced the lethal toxicity of T-2 (1.8 mg/kg BW) in mice; pre-administration of prednisolone or hydrocortisone (50 mg/kg BW) also inhibited T-2 (1.5 mg/kg BW)-induced increment of intestinal fluid volume in rats (Mutoh et al., 1988).
In summary, alfalfa meal, RA, BA, FWGE, adsorbents (bentonite, smectite, mineral clays, and HSCAS), MycoRaid, SeMet, fish protein hydrolysates, prednisolone or hydrocortisone administered at a range of doses could improve T-2-induced gastrointestinal toxicity (Fig. 1). Among these components, RA, BA, FWGE, and SeMet alleviated intestinal damage under T-2 exposure primarily through antioxidant and anti-inflammatory effects, whereas adsorbents and feed additives improved intestinal damage primarily by weakening the intestinal uptake of T-2. However, the potential of protective agents to counter the intestinal toxicity of T-2 needs to be validated in livestock, and the dose of protective agents needs to be controlled.
2.1.2. Toxic targets
Glucagon-like peptide-17-36 amide (GLP-1) and cholecystokinin (CCK) mediated the T-2-induced anorexia response, whereas the GLP-1 receptor antagonist (Exendin9-39) or the CCK receptor antagonists (SR 27897 and L-365 260) attenuated the T-2-induced anorexia response (Wu et al., 2018). Glucose-dependent insulinotropic polypeptide (GIP) mediated T-2-exposed anorexia; GIP receptor antagonist (Pro3GIP) dose-dependently alleviated T-2-triggered anorexia (Sheng et al., 2018). The neurotransmitters 5-hydroxytryptamine (5-HT) and substance P were shown to be involved in T-2-driven anorexia, whereas the 5-HT3 receptor antagonist granisetron or neurokinin-1 receptor (NK-1R) antagonist Emend was shown to suppress the T-2-induced anorexia response (Sheng et al., 2019). Therefore, GLP-1, CCK, GIP, 5-HT, NK-1R, NPY2 and inositol-requiring enzyme 1 alpha (IRE1α) (Table 2) may be used as potential targets of T-2 gastrointestinal toxicity, and inhibition or activation of these targets may alleviate gastrointestinal damage.
2.2. Protective agents for immunotoxicity
2.2.1. Protective agents
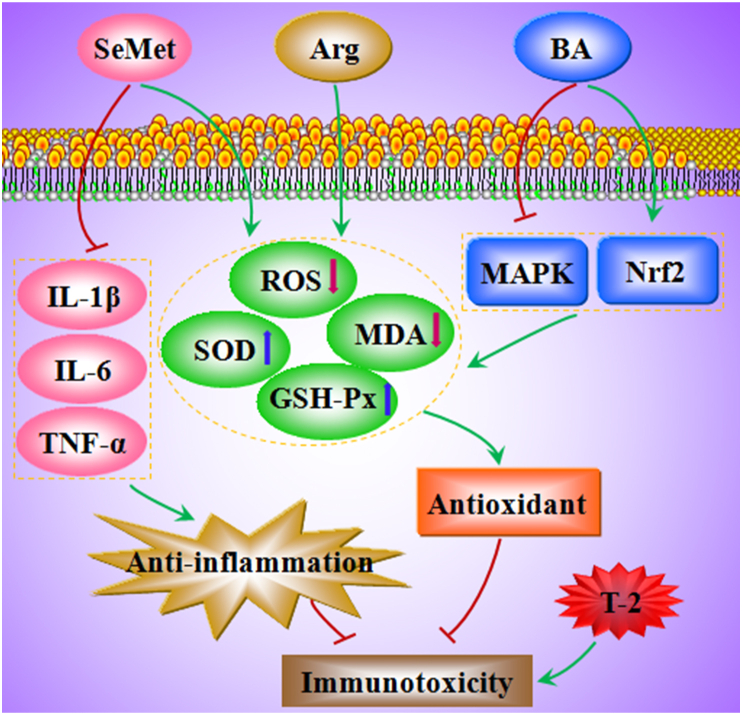
Dietary nucleotides reduced the extent of DNA damage induced by T-2 (10 mg/kg feed) in chicken leukocytes, suggesting that nucleotides benefit the immune system under T-2 exposure (Frankic et al., 2006). Selenium (sodium selenite) may exert a protective effect against the immunotoxic effects of T-2 on T lymphocyte sub-population (CD3+, CD4+ and CD8+ cells) in mice (Salimian et al., 2014). Ahmadi et al. (2015) found that selenium (sodium selenite) pre-supplementation suppressed the T-2-induced reduction in peripheral blood B-cell (CD19+) abundance in mice. Zhang et al. (2022h) observed that the treatment of SeMet (0.2 mg/kg feed) increased the levels of GSH-Px and SOD and decreased the levels of ROS and MDA in the spleen and the serum levels of IL-1β, IL-6, and TNF-α, thereby increasing the antioxidant and anti-inflammatory capacity and attenuating T-2 (0.4 mg/kg BW)-induced immunotoxicity in rabbits (Fig. 2). Notably, SeMet (0.4 and 0.6 mg/kg feed) did not effectively alleviate the immunotoxic effects caused by T-2 and caused some damage (Zhang et al., 2022h), which may be toxic effects of SeMet.
Fig. 2.
The mechanisms of selenomethionine (SeMet), arginine (Arg) and betulinic acid (BA) to attenuate T-2-induced immunotoxicity. GSH-Px = glutathione peroxidase; IL-1β = interleukin-1 beta; IL-6 = interleukin-6; MAPK = mitogen-activated protein kinase; MDA = malondialdehyde; Nrf2 = nuclear factor E2 related factor 2; ROS = reactive oxygen species; SOD = superoxide dismutase; T-2 = T-2 toxin; TNF-α = tumour necrosis factor alpha.
The addition of 3.17% arginine (Arg) to the diet increased the SOD and GSH-Px activities and decreased the concentration of MDA in the hepatopancreas of Chinese mitten crab, thereby resisting oxidative and immune damage caused by T-2 (1.5 mg/kg BW injected) (Fig. 2) (Zhang et al., 2020a). Feed supplementation with 0.5% Green Tea Powder (GTP) increased body weight gain, reduced plasma blood urea nitrogen and glutamate oxaloacetate transaminase concentrations, and mitigated T-2 (0.5 or 5 mg/kg feed, 3 weeks) toxicity in ducks (Tso et al., 2021). Moreover, GTP could improve humoral immune response in broiler chickens (Aziz-Aliabadi et al., 2023). Therefore, GTP may enhance immunity in broilers under T-2 exposure, however, the exact regulatory mechanism has yet to be explored.
In mouse mononuclear macrophages (RAW264.7) cells, silibinin dietary supplementation provided significant protection against T-2-induced cytotoxicity (Tran et al., 2020). BA exerted a protective effect against T-2-induced oxidative damage in the spleen. Specifically, BA restored the number of lymphocytes, reduced the accumulation of ROS and MDA and inflammatory cell infiltration, and enhanced SOD activity in the spleen of mice subjected to T-2. BA also significantly attenuated T-2-induced splenocyte apoptosis (Kong et al., 2021). In addition, BA protected the spleen and thymus from oxidative damage caused by T-2 through activation of the nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway and inhibition of mitogen-activated protein kinase (MAPK) signaling (Fig. 2) (Kong et al., 2021; Zhu et al., 2020).
In summary, selenium/SeMet, Arg, and BA ameliorated T-2-induced immunotoxicity primarily through antioxidant and anti-inflammatory effects (Fig. 2). Dietary nucleotides, GTP and silibinin could also reduce the immunotoxicity of T-2. However, the level of additives has to be controlled strictly when using these protective agents.
2.2.2. Toxic targets
T-2 can evade host immune surveillance and disrupt immune repair, exhibiting an “immune evasion” effect. There is evidence that hypoxia inducible factor-1α (HIF-1α) inhibited T-2-mediated “immune evasion” by negatively regulating programmed cell death protein-1 (PD-1)/programmed cell death-ligand 1 (PD-L1) signaling in RAW264.7 cells, indicating HIF-1α is an important toxicity target of T-2 immunotoxicity (You et al., 2022).
2.3. Protective agents against neurotoxicity
2.3.1. Protective agents
In mouse neuroblastoma 2a cells, n-acetylcysteine (NAC) pretreatment significantly inhibited T-2 (10, 20, 40, and 80 ng/mL)-induced caspase activation and apoptosis, thereby reducing neurotoxicity (Zhang et al., 2018). In a mouse microglia BV2 cell line, NAC supplementation prevented T-2 (1.25–5 ng/mL)-induced ROS production and mitochondrial dysfunction, reduced mitochondrial transmembrane potential, upregulated Bcl-2-associated X protein (Bax) protein expression, and suppressed B-cell lymphoma 2 (Bcl-2) protein expression. Overall, NAC reduced oxidative stress and apoptosis (Sun et al., 2022).
BA improved cognitive function by increasing the levels of the brain neurotransmitters dopamine, 5-HT, and acetylcholine, thereby protecting against brain damage induced by T-2 (4 mg/kg BW). BA also enhanced antioxidant and anti-inflammatory capacity by increasing SOD, CAT, GSH-Px, and GSH levels, inhibiting ROS and MDA production, suppressing the secretion of pro-inflammatory cytokines (IL-1β, IL-6, TNF-α) in the brain, and eventually suppressing brain damage caused by T-2 (Huang et al., 2022b). T-2 (4 mg/kg BW) induced microglia activation and led to learning and memory deficits and motor inhibition in mice, whereas minocycline (50 mg/kg BW) inhibited microglia activation and suppressed the toxic effects of T-2 (Li et al., 2023).
Altogether, NAC, BA and minocycline can narrow the neurotoxicity of T-2 to some extent by antioxidant, anti-apoptotic and anti-inflammatory effects.
2.3.2. Toxic targets
T-2 (10 ng/mL) induced apoptosis in human neuroblastoma cells, whereas pretreatment with caspase inhibitor (Z-VAD-FMK) and MAPK inhibitors (PD 98059, SB 203580 and ZM 336372) decreased the level of apoptosis (Agrawal et al., 2015). Pretreatment of Nrf2 inhibitor (brusatol) or heme oxygenase 1 (HO-1) inhibitor (zinc protoporphyrin IX) enhanced T-2 (20 ng/mL)-induced neurotoxicity in mouse neuroblastoma-2a (N2a) cells (Zhang et al., 2018). Mitochondria-related p53 signaling exacerbated T-2-induced neuronal cell death (Dai et al., 2019). Silencing of high mobility group B1 (HMGB1) reduced the toxic effects of oxidative stress, apoptosis and inflammation in PC12 nerve cells caused by T-2 (Pei et al., 2021). Knockdown of Nrf2 exacerbated T-2-induced cytotoxicity, oxidative stress and mitochondrial dysfunction in human neuroblastoma SH-SY5Y, thereby inducing neurotoxicity (Pang et al., 2022). T-2 (5 ng/mL) induced activation of MAPKs and downstream NF-kappaB in BV-2 cells, and inhibitors of NF-kappaB and MAPKs could reverse T-2-induced microglia activation, thereby alleviating neurocytotoxicity (Li et al., 2023). Therefore, MAPK, NF-kappaB, Nrf2, HO-1, p53, and HMGB1 may be significant targets of T-2 neurotoxicity.
2.4. Protective agent for reproductive toxicity
2.4.1. Protective agents
L-Arg (0.25, 0.5, and 1.0 mmol/L) pretreatment upregulated activities of GSH-Px, SOD and CAT in T-2 (10 nmol/L)-treated mouse Leydig cells, thereby alleviating T-2-induced oxidative damage (Yang et al., 2018). In vivo studies have demonstrated that dietary supplementation with L-Arg protected mice from T-2-induced reproductive damage by improving semen quality and serum testosterone levels (Zhang et al., 2019). In isolated Leydig cells, L-Arg (0.25, 0.5, and 1.0 mmol/L) increased the testosterone levels and upregulated the activity of cholesterol side-chain cleavage enzyme, 3 beta-hydroxysteroid dehydrogenase/isomerase-1 and steroidogenic acute regulatory protein (StAR), thereby ameliorating T-2 (10 nmol/L)-induced reproductive toxicity (Yang et al., 2019b). It was further observed that L-Arg (0.25, 0.5, and 1.0 mmol/L) upregulated Bcl-2 expression, downregulated Bax and caspase-3 expression, and promoted Leydig cell proliferation and mitochondrial activity, thereby preventing T-2 (10 nmol/L)-induced apoptosis (Zhang et al., 2020b).
Quercetin (Que) (100 ng/mL) effectively increased total antioxidant status and the activities of SOD and glutathione peroxidase family (GPX) in T-2 (100 ng/mL)-exposed porcine granulosa cells in vitro (Capcarova et al., 2015). BA (0.25, 0.5, and 1.0 mg/kg feed) pretreatment significantly increased the total antioxidant capacity, the activities of SOD and CAT, and decreased GSH depletion, MDA content and caspase-3 and Bax protein expression, thereby reducing the rate of apoptosis and alleviating T-2 (4 mg/kg BW)-induced oxidative damage in mouse testes (Wu et al., 2019). BA also increased testosterone levels and sperm motility, thereby reversing the adverse effects of T-2 on reproduction (Wu et al., 2019). NAC (5 mmol/L) attenuated T-2 (8.10 nmol/L)-induced oxidative stress, apoptosis, and functional impairment in TM4 cells (testes-supporting cells) (Yang et al., 2021). In isolated bovine testicular mesenchymal cells, vitamin E and selenium significantly upregulated GSH-Px, SOD, and CAT activities, thereby reducing oxidative damage to ameliorate T-2-induced germ cell toxicity (Yang et al., 2022).
Additionally, melatonin protected against HT-2 toxin (a metabolite of T-2)-induced apoptosis and oxidative stress in bovine ovarian granulosa cells by reducing the expression of Bax/Bcl-2 and caspase-3, as well as the levels of ROS and MDA, and promoting the expression of SOD and CAT (Yang et al., 2019a).
In summary, L-Arg, BA, NAC, vitamin E, selenium, Que and melatonin ameliorated T-2-induced reproductive toxicity primarily through antioxidant, anti-inflammatory, and anti-apoptotic effects.
2.4.2. Toxic targets
T-2 promoted TM3 cell apoptosis by increasing Ca2+ accumulation through the inhibition of the mammalian target of rapamycin complex 2 (mTORC2)/protein kinase B pathway. Meanwhile, BAPTA-AM (a Ca2+ chelator) and MHY1485 (an mTOR activator) prevented T-2-induced apoptosis (Wang et al., 2018). Pretreatment of endoplasmic reticulum (ER) stress inhibitor 4-phenylbutyric acid (4-PBA, 1 μmol/L for 2 h) alleviated ER stress and decreased apoptosis rate, thereby alleviating T-2 exposure (10 nmol/L for 24 h)-induced toxicity in goat endometrium epithelial cells (Yi et al., 2018). T-2 (0.5, 1 or 2 mg/kg BW, orally, 28 d) affected fertility in mice by interrupting the hypothalamic-pituitary-testicular (HPT) axis and impairing testicular function (Yang et al., 2019c). In porcine granulosa cells, T-2 (1, 10 and 100 nmol/L for 24 h) decreased the expression of cAMP-stimulated StAR which is a rate limiting protein in progesterone synthesis, suggesting StAR is a sensitive target for T-2 (Wu et al., 2015). Therefore, abnormal changes in Ca2+ concentration, mTORC2, ER stress, HPT axis and StAR may be important links in mitigating T-2 reproductive toxicity.
2.5. Protective agent for hepatotoxicity
2.5.1. Protective agents
2.5.1.1. Protective agents from plants
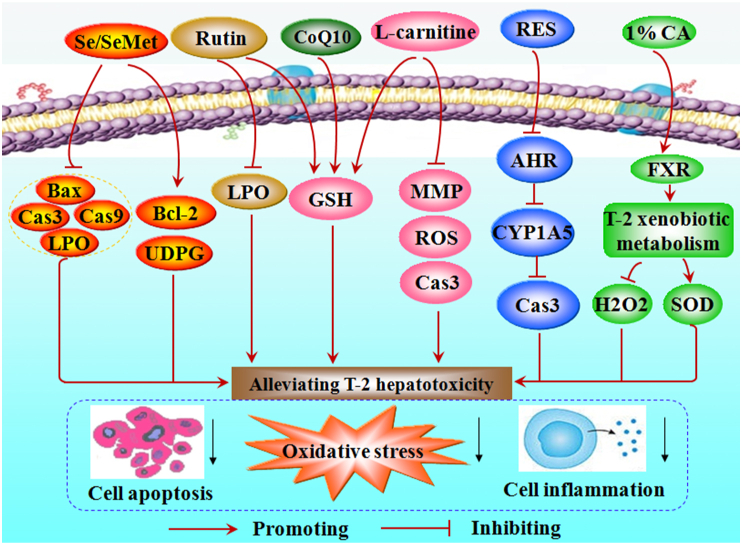
Lycopene (25 mg/kg BW per day) from fresh tomatoes reduced GSH depletion and alleviated oxidative damage in the liver of male broiler chickens fed T-2 (1.5 mg T-2/kg BW per day) (Leal et al., 1999). Diets containing 0.1% concentrate of food indoles (indole-3-carbinole and ascorbigen) fed to rats for 3 weeks increased the activity of hepatic microsomal carboxylesterase and UDP-glucuronosyltransferase (UDPG), thereby attenuating the toxic effects of T-2 (Kravchenko et al., 2001). Rutin reversed the T-2-mediated increase of LPO and SOD, as well as the decrease in GSH levels, in rat liver homogenate (Sawi and Seeni, 2009). Resveratrol (RES, 20 μmol/L) inhibited the aryl hydrocarbon receptor (AHR) – cytochrome P450 family 1 subfamily A polypeptide 5 (CYP1A5) pathway to enhance cell viability and reduce oxidative stress, DNA damage and apoptosis, thereby alleviating T-2 (100 nmol/L) cytotoxicity in leghorn male hepatocellular carcinoma epithelial cells (Fig. 3) (Liu et al., 2019). Treatment with curcumin (80 mg/kg BW per day) and taurine (50 mg/kg BW per day) ameliorated T-2 (0.1 mg/kg BW, intraperitoneal injections)-induced hepatotoxicity in Wistar rats through anti-inflammatory, antioxidant and anti-apoptotic effects (Al-Zahrani et al., 2023).
Fig. 3.
The mechanisms of selenium (Se), L-carnitine, resveratrol (RES) and 1% cholic acid (1%CA) to reduce T-2-triggered hepatotoxicity. AHR = aryl hydrocarbon receptor; Bcl-22 = B-cell lymphoma 2; Bax = Bcl-2-associated X protein; Cas3 = Caspase 3; Cas9 = Caspase 9; CoQ10 = coenzyme Q10; CYP1A5 = cytochrome P450 family 1 subfamily A polypeptide 5; FXR = farnesoid X receptor; GSH = glutathione; H2O2 = hydrogen peroxide; LPO = lipid peroxidation; MMP = membrane potential; ROS = reactive oxygen species; SeMet = selenomethionine; SOD = superoxide dismutase; T-2 = T-2 toxin; UDPG = UDP-glucuronosyltransferase.
2.5.1.2. Selenium/SeMet
Selenium (sodium selenate) supplementation (0.5 and 2.5 mg/kg feed) increased hepatic epoxide hydrolase and UDPG activity, thereby reducing T-2 (3.8 mg/kg BW)-induced toxicity and mortality in male rats (Kravchenko et al., 1990). The dietary use of selenium (sodium selenate), vitamin E and vitamin C eased T-2-induced hepatic lipid peroxide and GSH depletion, thereby alleviating liver injury in rats (Fig. 3) (Rizzo et al., 1994). The combination of selenium and modified glucomannan prevented T-2 (8.1 mg/kg feed)-induced oxidative stress damage and LPO in chicken liver (Dvorska et al., 2007). Low doses of SeMet (0.2 mg/kg feed) downregulated Bax, caspase-3, and caspase-9 and upregulated Bcl-2, thereby reducing the levels of oxidative stress and apoptosis in T-2 (0.4 mg/kg BW)-treated rabbit hepatocytes (Fig. 3) (Liu et al., 2021c).
2.5.1.3. Adsorbents
The dietary addition of HSCAS adsorbent blocked T-2-induced aspartate aminotransferase (AST) elevation and mitigates hepatotoxicity in chicks (Wei et al., 2019). Yeast cell wall extract (YCWE) supplemented with a post-biotic yeast cell wall-based blend improved the negative effects of T-2 (3.0 mg/kg feed) on growth performance and liver function in broilers (Kudupoje et al., 2022).
2.5.1.4. Amino acids and other protective agents
High-dose L-carnitine (500 mg/kg diet) pretreatment reduced GSH depletion, ROS overproduction, and mitochondrial membrane potential collapse and lowered caspase 3 activity and apoptosis in primary rat hepatocytes caused by T-2 exposure, thereby alleviating liver injury (Fig. 3) (Moosavi et al., 2016). Dietary 3.17% Arg administration significantly decreased alanine aminotransferase (ALT) and AST activity in the haemolymph, thereby alleviating T-2 (1.5 mg/kg BW)-induced hepatotoxicity in Chinese mitten crab (Zhang et al., 2020a).
Pretreatment with the antioxidant coenzyme Q10 (CoQ10) (6 mg/kg) and vitamin E (6 mg/kg) reduced DNA damage, cell death and GSH depletion in the liver of T-2 (1.8 or 2.8 mg/kg BW)-exposed mice (Atroshi et al., 1997). Dietary supplementation with 1% cholic acid (CA) promoted the xenobiotic metabolism of T-2 by inducing the expression of farnesoid X receptor (FXR), thereby enhancing SOD activity and reducing hepatic hydrogen peroxide (H2O2) level and oxidative stress, ultimately alleviating T-2-induced liver damage in broiler chickens (Fig. 3) (Dai et al., 2020). N,N′-dichloro-bis (2,4,6-trichlorophenyl) urea (CC-2) (20%) protected mice from T-2 (11.8 mg/kg BW)-induced hepatic LPO and reduced mortality (Agrawal et al., 2012a). Silibinin dietary supplementation protected HepG2 cells from T-2 toxicity (Tran et al., 2020).
In summary, selenium/SeMet, CoQ10, vitamin E, vitamin C, lycopene, food indoles, Rutin, RES, curcumin, taurine, L-carnitine, 1% CA, and 20% CC-2 ameliorated T-2-induced hepatotoxicity primarily through antioxidant, anti-apoptotic, or anti-inflammatory effects (Fig. 3). In addition, 3.17% Arg, HSCAS, YCWE, and silymarin dietary supplements may also alleviate hepatocyte injury caused by T-2 exposure. T-2 can induce hepatic LPO which is also one of the main features of ferroptosis (Ursini and Maiorino, 2020). The liver is an important organ for iron accumulation (Shah and Belonje, 1976); however, it remains unclear whether ferroptosis mediates T-2-induced hepatotoxicity and whether ferroptosis inhibitors alleviate hepatotoxicity.
2.5.2. Toxic targets
T-2-induced CYP1A5 in chicken embryonic hepatocyte cells catalyzed T-2 into a more toxic product, 3′–OH–T-2 (Shang et al., 2013). Therefore, inhibition of CYP1A5 activity may be a key step in alleviating T-2 hepatotoxicity. Upregulation of silent information regulator sirtuin 1 (SIRT1) is involved in T-2-induced mitochondrial dysfunction and ROS production in HepG2 (Ma et al., 2017). Thus, CYP1A5, cytochrome P450 1A1 (CYP1A1), FXR, SIRT1 and eleven translocation family protein 3 (TET3) (Table 2) may be targets of T-2 hepatotoxicity.
2.6. Protective agent for nephrotoxicity
2.6.1. Protective agent
SeMet (0.2 mg/kg feed) reduced T-2 (0.4 mg/kg feed)-induced ROS and inflammatory factor levels, thereby restoring renal function in rabbits (Liu et al., 2021b). Selenium (0.2 mg/kg diet) protected mouse kidney from T-2 (1.0 mg/kg diet)-induced damage by inhibiting ROS-mediated apoptosis in kidney cells, as evidenced by reduced cytochrome-c protein expression and caspase-3/9 activity (Zhang et al., 2022f). Short-term selenium-deficiency synergistic with T-2 (10 ng/g BW) could lead to rat kidney damage and even kidney fibrosis, which in turn proves that Se supplementation could mitigate nephrotoxicity under T-2 exposure (Guo et al., 2023).
In porcine kidney cells (PK-15), BA (0.25, 0.5, and 1 μmol/L) pretreatment ameliorated T-2 (1 μmol/L)-induced oxidative stress damage and apoptosis by increasing SOD, GSH-Px, and CAT activity and reducing intracellular ROS and MDA production, thereby alleviating renal cytotoxicity (Li et al., 2021). Huang et al. (2021) further showed that BA (0.25, 0.5, and 1 mg/kg BW) pretreatment attenuated T-2-induced oxidative stress and inflammatory responses (low expression of IL-1β, TNF-α) by activating the Nrf2 signaling pathway. This reduced T-2 (4 mg/kg BW)-triggered mice glomerular hemorrhage and inflammatory cell infiltration. Therefore, selenium/SeMet and BA in a certain concentration range could reduce the nephrotoxicity of T-2.
2.6.2. Toxic targets
Protein kinase RNA-like ER kinase (PERK)-mediated ER stress was involved in PK-15 apoptosis under T-2 treatment, whereas the ER stress inhibitor 4-PBA and the PERK-selective inhibitor (GSK2606414) prevented the T-2-induced decrease in cell activity and apoptosis, providing a new perspective to inhibit the nephrotoxicity of T-2 (Liu et al., 2021a). T-2 (0.5, 1.0, 2.0 mg/kg BW) increased nuclear Nrf2 protein expression and induced oxidative stress as well as apoptosis in the kidney of male mice (Zhang et al., 2021c). The phosphatase and tensin homolog (PTEN)-induced putative kinase1 (PINK1)/E3 ubiquitin ligase PARK2 (Parkin)-mediated mitophagy alleviated T-2-induced nephrotoxicity in mice (Zhang et al., 2022e). Thus, PERK, ER stress, Nrf2 and PINK1/Parkin may be targets to alleviate T-2 nephrotoxicity.
2.7. Protective agent for cardiotoxicity
2.7.1. Protective agent
CAT (10 U/mL) and vitamin C (10 μg/mL) improved the decrease in oxygen consumption rate in cardiomyocytes exposed to T-2 (6.0 × 10−3 and 6.0 × 10−4 μmol/L) (Ngampongsa et al., 2013). Selenium supplementation improved the effects of Keshan disease, which include congestive cardiomyopathy caused by T-2 (0.25–1 μmol/L) exposure in primary cardiomyocytes (Chen et al., 2019). Methylprednisolone (Meth) reduced the intensity of T-2 (0.23 mg/kg BW, subcutaneous injection)-induced myocardial degeneration and hemorrhage, thus protecting the myocardium from T-2-induced damage (Jacevic et al., 2019). Therefore, a range of concentrations of catalase, vitamin C, selenium and Meth can antagonize the cardiotoxicity of T-2.
2.7.2. Toxic targets
Lu et al. (2021) found that peroxisome proliferator-activated receptor (PPAR)-γ agonist (pioglitazone) alleviated T-2-induced H9C2 (rat cardiomyocyte) injury, indicating PPAR-γ may be a potential target for T-2 (2 mg/kg BW)-induced cardiac fibrosis in rats.
2.8. Protective agent for skeletal toxicity
2.8.1. Protective agent
T-2 exposure has been strongly associated with skeletal deformities such as incomplete ossification and bone loss and fusion, Kashin-Beck disease (Zhou et al., 2016). Thus, it is important to counter its toxic effects on the skeleton. Selenium partially inhibited the degradation of aggregated proteoglycans in chondrocytes and the increase in pro-inflammatory cytokines (IL-1β and TNF-α) caused by T-2 (0.001 to 2 mg/L) (Chen et al., 2006; Li et al., 2008). The addition of selenium (100 ng/mL) enhanced the metabolic transformation of T-2 and reduced its cytotoxicity to human chondrosarcoma cell lines (Yu et al., 2017). Selenium treatment rescued T-2-induced apoptosis and inflammation and reversed T-2-induced damage to primary epiphyseal chondrocytes (Shi et al., 2021). In addition, Selenium supplementation could ease the inhibitory effect of T-2 on chondrocytes (C28/I2 and ATDC5) and cartilage (Zhang et al., 2021a). NAC protected tibial growth plate chondrocytes in chickens against the cytotoxicity of T-2 (5, 50, and 500 nmol/L) in vitro by reducing oxidative stress (He et al., 2012). Therefore, certain concentrations of selenium and NAC could reduce the skeletal toxicity of T-2 through anti-inflammatory, anti-apoptotic or antioxidant mechanisms.
2.8.2. Toxic targets
Inhibition of insulin like growth factor 1 receptor (IGF-1R) may mediate chondrocyte death and extracellular matrix degeneration under T-2 exposure (Zhang et al., 2021b). Heat shock protein 47 (HSP47) is involved in T-2-driven reduced type II collagen expression, further promoting matrix degradation in chondrocytes (Zhang et al., 2021a). The matrix metalloproteinase-10 (MMP-10) deficiency rendered chondrocytes more sensitive to T-2-induced apoptosis, terminal differentiation and death (Zhang et al., 2022a). Yes-associated protein (YAP) was one of the targets of T-2-induced chondrocyte injury in rats, and the YAP-specific inhibitor (vetiporfin) increased MMP-13 expression and reduced human collagen Type II and proliferating cell nuclear antigen levels, thus ameliorating T-2-caused cartilage injury (Li et al., 2022a). T-2 (0.5, 1 or 2 mg/kg BW) induced femoral head lesions in mice accompanied by autophagy and apoptosis, which are associated with Wnt/beta-catenin signaling (Zhang et al., 2022d). Thus, IGF-1R, HSP47, MMP-10, YAP, Wnt/beta-catenin signaling, O6-methylguanine-DNA methyltransferase (MGMT) and transforming growth factor-beta receptorss (TGF-βRs) (Table 2) may be targets for antagonizing the skeletal toxicity of T-2.
2.9. Protective agent for dermal toxicity and muscle toxicity
T-2 (0.5, 1.5, 4.5 and 13.5 mg/kg feed) affected shrimp muscle fatty acid and water distribution, muscle histopathology, resulting in a decrease in quality and nutritional value of shrimp (Bi et al., 2019). Que (2.00 to 32.00 g/kg feed), tea polyphenols and rutin could prevent abnormal changes in the target protein (enolase, malate dehydrogenase, elongation factor 1-alpha and eukaryotic translation initiation factor 2 subunit alpha, actin 2, fast-type skeletal muscle actin 1), and muscle composition (the sarcoplasmic, myofibrillar and the alkali-soluble protein) of shrimp caused by T-2 (4.80 to 24.30 mg/kg feed) and ameliorated the degree of muscle protein deterioration (Huang et al., 2022a). Mycotoxins in feed (containing T-2 at 320.5 μg/kg) could retard broiler growth, nutrient retention and meat quality; while the addition of 0.05% esterified glucomannan, 0.2% HSCAS or 0.1% compound mycotoxin adsorbent (CMA) prevented the adverse effects of mycotoxins on chicken meat (Liu et al., 2011). Therefore, Que, tea polyphenols, rutin, esterified glucomannan, HSCAS, and CMA in a range of doses alleviated the muscle toxicity of T-2.
T-2 is lipophilic, easily penetrates the skin, and causes skin irritation and blistering in the body (Hayes and Schiefer, 1990). Research has shown that aqueous soap solution was largely effective in removing low doses of T-2 from the skin of rabbits, whereas polyethylene glycol 300 (PG300) washing is effective in removing large doses of T-2 from the skin of rabbits (Fairhurst et al., 1987). A topical skin protectant (ICD 2289) effectively relieved skin erythema and edema in animals exposed to T-2 (Liu et al., 1999). The quince seed mucilage (15%) effectively alleviated T-2 (8.34 μg/mL)-induced skin toxicity in rabbits (Hemmati et al., 2012). Mice handled by T-2 (23.76 mg/kg BW topical percutaneous smearing on the back of rat) died within 36 h of exposure. The subcutaneous application of CC-2 within 5 and 15 min of treatment with T-2 resulted in survival rates of 100% and 50%. Therefore, CC-2 may be an effective skin decontaminant against lethal topical exposure to T-2 (Agrawal et al., 2012a). T-2 (2.97 mg/kg BW) caused skin inflammation, erythema and skin tissue necrosis in mice; whereas topical application of 0.25% and 0.5% menthol prevented the development of erythema or inflammation through anti-inflammatory effects (Rachitha et al., 2023). Therefore, topical application of aqueous soap solution, PG300, skin protectant, quince seed mucilage, CC-2 and menthol may reduce the dermal toxicity of T-2 to some extent.
2.9.1. Toxic targets
High transforming growth factor-beta 1 (TGF-β1) levels may be closely related to T-2-induced apoptosis of epidermal basal cells and intradermal infiltration of mast cells and fibroblasts (Albarenque et al., 2000). Increased TNF-α mRNA expression may play an important part in T-2-caused apoptosis in epidermal cells (Albarenque et al., 2001a). Enhanced expression of c-fos and c-jun may be associated with epidermal apoptosis induced by topical application of T-2 (0.5 μg/μL) (Albarenque et al., 2001b). The activation of MMP9/2 and MAPK pathways mediated T-2 (2.97, 5.94 and 11.88 mg/kg BW)-induced skin inflammation and cutaneous damage in Swiss albino mice (Agrawal et al., 2012b). T-2 induced human skin-fibroblast Hs68 cell necrosis accompanied by increased concentration of released cytokeratin 18 (Janik-Karpinska et al., 2022). In summary, TGF-β1, TNF-α, c-fos and c-jun, MMP and p38 MAPK and cytokeratin 18 may be the potential targets of T-2 dermal toxicity.
Where T-2 (1.2 mg/kg BW) exposure affects the protein composition and quality of shrimp muscle, arginine kinase (spot 27) was particularly responsive to T-2 and could potentially be used as a biomarker protein for T-2 intoxication by shrimp (Huang et al., 2020).
3. Conclusions and perspectives
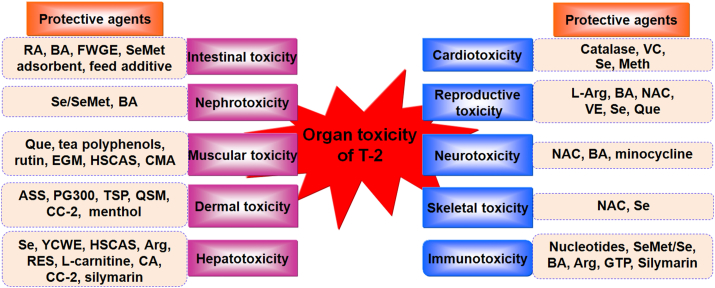
The long-term consumption of T-2-contaminated food and feed can cause gastrointestinal toxicity, immunotoxicity, hepatotoxicity, reproductive toxicity, neurotoxicity, nephrotoxicity, cardiotoxicity, skeletal toxicity, muscular toxicity, and dermal toxicity in humans and animals. Thus, the application of protective agents to reduce the organ toxicity of T-2 is of particular importance. Evidence from available studies has demonstrated that BA, selenium/SeMet and T-2 adsorbents effectively alleviated the majority of organ toxicity caused by T-2 (Fig. 4). Protective agents alleviated toxicity primarily through antioxidant, anti-inflammatory, or anti-apoptotic pathways or by reducing toxin uptake in organs. Although different strategies of protective agents have been developed for T-2, further validation of their utility and safety in animal production is needed.
Fig. 4.
Protective agents of organ toxicity under T-2 exposure. Arg = arginine; ASS = aqueous soap solution; BA = betulinic acid; CA = cholic acid; CC-2 = N,N′-dichloro-bis (2,4,6-trichlorophenyl) urea; CMA = compound mycotoxin adsorbent; EGM = esterified glucomannan; FWGE = fermented wheat germ extract; GTP = green tea powder; HSCAS = hydrated sodium calcium aluminosilicate; Meth = methylprednisolone; NAC = N-acetylcysteine; PG300 = polyethylene glycol 300; Que = quercetin; QSM = quince seed mucilage; RA = rosmarinic acid; RES = resveratrol; Se = selenium; SeMet = selenomethionine; TSP = topical skin protectant; VC = vitamin C; VE = vitamin E; YCWE = yeast cell wall extract.
In addition to the protective agents, inhibition or activation of T-2 toxicity targets can also help mitigate toxicity to an extent. However, the detoxifying effects of toxicity targets need to be validated in vivo and in vitro. The multi-component protectants may protect against T-2 toxicity more comprehensively. The development of antagonists of T-2 toxicity in the future, based on one target or multiple targets, is also an alternative strategy.
Biotransformation and degradation by bacteria and yeast leaves neither toxic residues nor any undesirable by-products. Therefore, microbial biotransformation of T-2 to non-toxic or less toxic metabolites has been recognized as a promising approach. Researchers have now found that bacterial enzyme ZenA hydrolyzes zearalenone, and sodium metabisulfite inactivates deoxynivalenol by sulfonation (Danicke et al., 2023). Curtobacterium sp. (Ueno et al., 1983), Blastobacter sp. (Beeton and Bull, 1989), and Lactobacillus plantarum (Cserháti et al., 2013) could 100% degrade T-2. Deeper mining of bacterial enzymes or compounds that degrade T-2 from these microorganisms is also necessary. In addition, proper processing of T-2 contaminated animal feed has some positive effects on reducing the toxicity of T-2.
Notably, ferroptosis could mediate T-2-induced cell toxicity by increasing ROS levels, whereas NAC significantly blocked ferroptosis under T-2 exposure (Wang et al., 2021). T-2 exposure resulted in decreased expression of GPX4, (a key negative regulatory protein of ferroptosis), accompanied by degeneration of articular cartilage in rats (Sun et al., 2021). Therefore, exploring the role of ferroptosis in T-2 organ toxicity would provide another new detoxification strategy.
Author contributions
Pengju Wang: Topic selection, Outline, Figures, and Writing – original draft. Qinghua Wu: Outline of figures and tables, and Writing – review & editing. Lv-hui Sun: Outline of figures and tables, and Writing – review & editing. Xu Wang: Outline of figures and tables, and Writing – review & editing. Aimei Liu: Writing – review & editing, Supervision, and Funding acquisition.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This work was supported by the Doctoral Initiation Fund Project Grant (BK202315) and Medical Research Special Fund (2022YKY17) of Hubei University of Science and Technology, and Hubei Provincial Natural Science Foundation Programme (2023AFB537).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Agrawal M., Bhaskar A.S., Lakshmana Rao P.V. Involvement of mitogen-activated protein kinase pathway in T-2 toxin-induced cell cycle alteration and apoptosis in human neuroblastoma cells. Mol Neurobiol. 2015;51:1379–1394. doi: 10.1007/s12035-014-8816-4. [DOI] [PubMed] [Google Scholar]
- Agrawal M., Pardasani D., Lakshmana Rao P.V. Evaluation of protective efficacy of CC-2 formulation against topical lethal dose of T-2 toxin in mice. Food Chem Toxicol. 2012;50:1098–1108. doi: 10.1016/j.fct.2011.12.027. [DOI] [PubMed] [Google Scholar]
- Agrawal M., Yadav P., Lomash V., Bhaskar A.S., Lakshmana Rao P.V. T-2 toxin induced skin inflammation and cutaneous injury in mice. Toxicology. 2012;302:255–265. doi: 10.1016/j.tox.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Ahmadi A., Poursasan N., Amani J., Salimian J. Adverse effect of T-2 toxin and the protective role of selenium and vitamin E on peripheral blood B lymphocytes. Iran J Immunol. 2015;12:64–69. [PubMed] [Google Scholar]
- Al-Zahrani M.H., Balgoon M.J., El-Sawi N.M., Alshubaily F.A., Jambi E.J., Khojah S.M., et al. A biochemical, theoretical and immunohistochemical study comparing the therapeutic efficacy of curcumin and taurine on T-2 toxin induced hepatotoxicity in rats. Front Mol Biosci. 2023;10 doi: 10.3389/fmolb.2023.1172403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albarenque S.M., Shinozuka J., Suzuki K., Nakayama H., Doi K. Kinetics and distribution of transforming growth factor (TGF)-beta 1 mRNA in the dorsal skin of hypotrichotic WBN/ILA-Ht rats following topical application of T-2 toxin. Exp Toxicol Pathol. 2000;52:297–301. doi: 10.1016/s0940-2993(00)80050-0. [DOI] [PubMed] [Google Scholar]
- Albarenque S.M., Suzuki K., Nakayama H., Doi K. Kinetics of cytokines mRnas expression in the dorsal skin of WBN/ILA-Ht rats following topical application of T-2 toxin. Exp Toxicol Pathol. 2001;53:271–274. doi: 10.1078/0940-2993-00189. [DOI] [PubMed] [Google Scholar]
- Albarenque S.M., Suzuki K., Shinozuka J., Nakayama H., Doi K. Kinetics of apoptosis-related genes mRNA expression in the dorsal skin of hypotrichotic WBN/ILA-ht rats after topical application of T-2 toxin. Exp Toxicol Pathol. 2001;52:553–556. doi: 10.1016/s0940-2993(01)80016-6. [DOI] [PubMed] [Google Scholar]
- Atroshi F., Rizzo A., Biese I., Veijalainen P., Antila E., Westermarck T. T-2 toxin-induced DNA damage in mouse livers: the effect of pretreatment with coenzyme Q10 and alpha-tocopherol. Mol Aspect Med. 1997;18(Suppl):S255–S258. doi: 10.1016/s0098-2997(97)00032-0. [DOI] [PubMed] [Google Scholar]
- Aziz-Aliabadi F., Noruzi H., Hassanabadi A. Effect of different levels of green tea (Camellia sinensis) and mulberry (Morus alba) leaves powder on performance, carcass characteristics, immune response and intestinal morphology of broiler chickens. Vet Med Sci. 2023;9:1281–1291. doi: 10.1002/vms3.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeton S., Bull A.T. Biotransformation and detoxification of T-2 toxin by soil and freshwater bacteria. Appl Environ Microbiol. 1989;55:190–197. doi: 10.1128/aem.55.1.190-197.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi S., Huang Z., Wang Y., Nie F., Wang X., Sun L., et al. Effects of T-2 toxin on histopathology, fatty acid and water distribution of shrimp (Litopenaeus vannamei) muscle. J Environ Sci Health B. 2019;54:416–423. doi: 10.1080/03601234.2019.1574172. [DOI] [PubMed] [Google Scholar]
- Capcarova M., Petruska P., Zbynovska K., Kolesarova A., Sirotkin A.V. Changes in antioxidant status of porcine ovarian granulosa cells after quercetin and T-2 toxin treatment. J Environ Sci Health B. 2015;50:201–206. doi: 10.1080/03601234.2015.982425. [DOI] [PubMed] [Google Scholar]
- Carson M.S., Smith T.K. Effect of feeding alfalfa and refined plant fibers on the toxicity and metabolism of T-2 toxin in rats. J Nutr. 1983;113:304–313. doi: 10.1093/jn/113.2.304. [DOI] [PubMed] [Google Scholar]
- Carson M.S., Smith T.K. Role of bentonite in prevention of T-2 toxicosis in rats. J Anim Sci. 1983;57:1498–1506. doi: 10.2527/jas1983.5761498x. [DOI] [PubMed] [Google Scholar]
- Chen J., Chu Y., Cao J., Wang W., Liu J., Wang J. Effects of T-2 toxin and selenium on chondrocyte expression of matrix metalloproteinases (MMP-1, MMP-13), alpha2-macroglobulin (alpha2M) and TIMPs. Toxicol Vitro. 2011;25:492–499. doi: 10.1016/j.tiv.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Chen J.H., Cao J.L., Chu Y.L., Yang Z.T., Shi Z.L., Wang H.L., et al. [Protective effect of selenium against T-2 toxin-induced inhibition of chondrocyte aggrecan and collagen II synthesis] Nan Fang Yi Ke Da Xue Xue Bao. 2006;26:381–385. [PubMed] [Google Scholar]
- Chen X., Xu J., Liu D., Sun Y., Qian G., Xu S., et al. The aggravating effect of selenium deficiency on T-2 toxin-induced damage on primary cardiomyocyte results from a reduction of protective autophagy. Chem Biol Interact. 2019;300:27–34. doi: 10.1016/j.cbi.2019.01.009. [DOI] [PubMed] [Google Scholar]
- Cserháti M., Kriszt B., Krifaton C., Szoboszlay S., Kukolya J. Mycotoxin-degradation profile of Rhodococcus strains. Int J Food Microbiol. 2013;166:176–185. doi: 10.1016/j.ijfoodmicro.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Dai C., Xiao X., Sun F., Zhang Y., Hoyer D., Shen J., et al. T-2 toxin neurotoxicity: role of oxidative stress and mitochondrial dysfunction. Arch Toxicol. 2019;93:3041–3056. doi: 10.1007/s00204-019-02577-5. [DOI] [PubMed] [Google Scholar]
- Dai D., Pan Y., Zeng C., Liu S., Yan Y., Wu X., et al. Activated FXR promotes xenobiotic metabolism of T-2 toxin and attenuates oxidative stress in broiler chicken liver. Chem Biol Interact. 2020;316 doi: 10.1016/j.cbi.2019.108912. [DOI] [PubMed] [Google Scholar]
- Danicke S., Carlson L., Heymann A.K., Grumpel-Schluter A., Doupovec B., Schatzmayr D., et al. Inactivation of zearalenone (ZEN) and deoxynivalenol (DON) in complete feed for weaned piglets: efficacy of ZEN hydrolase ZenA and of sodium metabisulfite (SBS) as feed additives. Mycotoxin Res. 2023;39:201–218. doi: 10.1007/s12550-023-00486-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorska J.E., Pappas A.C., Karadas F., Speake B.K., Surai P.F. Protective effect of modified glucomannans and organic selenium against antioxidant depletion in the chicken liver due to T-2 toxin-contaminated feed consumption. Comp Biochem Physiol C Toxicol Pharmacol. 2007;145:582–587. doi: 10.1016/j.cbpc.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Fairhurst S., Maxwell S.A., Scawin J.W., Swanston D.W. Skin effects of trichothecenes and their amelioration by decontamination. Toxicology. 1987;46:307–319. doi: 10.1016/0300-483x(87)90211-3. [DOI] [PubMed] [Google Scholar]
- Fioramonti J., Fargeas M.J., Bueno L. Action of T-2 toxin on gastrointestinal transit in mice: protective effect of an argillaceous compound. Toxicol Lett. 1987;36:227–232. doi: 10.1016/0378-4274(87)90190-1. [DOI] [PubMed] [Google Scholar]
- Frankic T., Pajk T., Rezar V., Levart A., Salobir J. The role of dietary nucleotides in reduction of DNA damage induced by T-2 toxin and deoxynivalenol in chicken leukocytes. Food Chem Toxicol. 2006;44:1838–1844. doi: 10.1016/j.fct.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Guo Z., Chilufya M.M., Deng H., Qiao L., Liu J., Xiao X., et al. Single and combined effects of short-term selenium deficiency and T-2 toxin-induced kidney pathological injury through the MMPs/TIMPs system. Biol Trace Elem Res. 2023;201:4850–4860. doi: 10.1007/s12011-023-03566-7. [DOI] [PubMed] [Google Scholar]
- Halstensen A.S., Nordby K.C., Klemsdal S.S., Elen O., Clasen P.E., Eduard W. Toxigenic Fusarium spp. as determinants of trichothecene mycotoxins in settled grain dust. J Occup Environ Hyg. 2006;3:651–659. doi: 10.1080/15459620600987431. [DOI] [PubMed] [Google Scholar]
- Hayes M.A., Schiefer H.B. A method for accurate measurement of cutaneous irritancy of trichothecenes. J Environ Pathol Toxicol Oncol. 1990;10:103–105. [PubMed] [Google Scholar]
- He S.J., Hou J.F., Dai Y.Y., Zhou Z.L., Deng Y.F. N-acetyl-cysteine protects chicken growth plate chondrocytes from T-2 toxin-induced oxidative stress. J Appl Toxicol. 2012;32:980–985. doi: 10.1002/jat.1697. [DOI] [PubMed] [Google Scholar]
- Hemmati A.A., Kalantari H., Jalali A., Rezai S., Zadeh H.H. Healing effect of quince seed mucilage on T-2 toxin-induced dermal toxicity in rabbit. Exp Toxicol Pathol. 2012;64:181–186. doi: 10.1016/j.etp.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Huang L., Zhu L., Ou Z., Ma C., Kong L., Huang Y., et al. Betulinic acid protects against renal damage by attenuation of oxidative stress and inflammation via Nrf2 signaling pathway in T-2 toxin-induced mice. Int Immunopharm. 2021;101 doi: 10.1016/j.intimp.2021.108210. [DOI] [PubMed] [Google Scholar]
- Huang X., Huang Z., Sun L., Qiu M., Deng Q., Fang Z., et al. Protective mechanisms of three antioxidants against T-2 toxin-induced muscle protein deterioration in shrimp. J Sci Food Agric. 2022;102:4883–4891. doi: 10.1002/jsfa.11851. [DOI] [PubMed] [Google Scholar]
- Huang Y., Zhu Z., Luo C., Ma C., Zhu L., Kong L., et al. Betulinic acid attenuates cognitive dysfunction, oxidative stress, and inflammation in a model of T-2 toxin-induced brain damage. Environ Sci Pollut Res Int. 2022;29:52098–52110. doi: 10.1007/s11356-022-19498-z. [DOI] [PubMed] [Google Scholar]
- Huang Z., Wang Y., Sun L., Wang X., Lu P., Liang G., et al. Effects of T-2 toxin on the muscle proteins of shrimp (Litopenaeus vannamei) - a proteomics study. J Sci Food Agric. 2020;100:119–128. doi: 10.1002/jsfa.10001. [DOI] [PubMed] [Google Scholar]
- Jacevic V., Wu Q., Nepovimova E., Kuca K. Efficacy of methylprednisolone on T-2 toxin-induced cardiotoxicity in vivo: a pathohistological study. Environ Toxicol Pharmacol. 2019;71 doi: 10.1016/j.etap.2019.103221. [DOI] [PubMed] [Google Scholar]
- Janik-Karpinska E., Ceremuga M., Wieckowska M., Szyposzynska M., Niemcewicz M., Synowiec E., et al. Direct T-2 toxicity on human skin-fibroblast Hs68 cell line-in vitro study. Int J Mol Sci. 2022;23:4929. doi: 10.3390/ijms23094929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janik E., Niemcewicz M., Ceremuga M., Stela M., Saluk-Bijak J., Siadkowski A., et al. Molecular aspects of mycotoxins-A serious problem for human health. Int J Mol Sci. 2020;21:8187. doi: 10.3390/ijms21218187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L., Zhu L., Yi X., Huang Y., Zhao H., Chen Y., et al. Betulinic acid alleviates spleen oxidative damage induced by acute intraperitoneal exposure to T-2 toxin by activating Nrf2 and inhibiting MAPK signaling pathways. Antioxidants. 2021;10:158. doi: 10.3390/antiox10020158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravchenko L.V., Avren'eva L.I., Guseva G.V., Posdnyakov A.L., Tutel'yan V.A. Effect of nutritional indoles on activity of xenobiotic metabolism enzymes and T-2 toxicity in rats. Bull Exp Biol Med. 2001;131:544–547. doi: 10.1023/a:1012346401315. [DOI] [PubMed] [Google Scholar]
- Kravchenko L.V., Kuz'mina E.E., Avren'eva L.I., Tutel'ian V.A. [Protective effect of selenium in acute T-2 mycotoxicosis] Vopr Med Khim. 1990;36:36–38. [PubMed] [Google Scholar]
- Kudupoje M.B., Malathi V., Yiannikouris A. Impact of a natural fusarial multi-mycotoxin challenge on broiler chickens and mitigation properties provided by a yeast cell wall extract and a postbiotic yeast cell wall-based blend. Toxins. 2022;14:315. doi: 10.3390/toxins14050315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal M., Shimada A., Ruiz F., Gonzalez de Mejia E. Effect of lycopene on lipid peroxidation and glutathione-dependent enzymes induced by T-2 toxin in vivo. Toxicol Lett. 1999;109:1–10. doi: 10.1016/s0378-4274(99)00062-4. [DOI] [PubMed] [Google Scholar]
- Li H.N., Jin B.M., Hua Z., Le-Le L., Meng-Yuan L., Xiu-Juan Z., et al. YAP plays a protective role in T-2 toxin-induced inhibition of chondrocyte proliferation and matrix degradation. Toxicon. 2022;215:49–56. doi: 10.1016/j.toxicon.2022.06.005. [DOI] [PubMed] [Google Scholar]
- Li J., Wang Y., Deng Y., Wang X., Wu W., Nepovimova E., et al. Toxic mechanisms of the trichothecenes T-2 toxin and deoxynivalenol on protein synthesis. Food Chem Toxicol. 2022;164 doi: 10.1016/j.fct.2022.113044. [DOI] [PubMed] [Google Scholar]
- Li N., Yao C.Y., Diao J., Liu X.L., Tang E.J., Huang Q.S., et al. The role of MAPK/NF-kappaB-associated microglial activation in T-2 toxin-induced mouse learning and memory impairment. Food Chem Toxicol. 2023;174 doi: 10.1016/j.fct.2023.113663. [DOI] [PubMed] [Google Scholar]
- Li S.J., Zhang G., Xue B., Ding Q., Han L., Huang J.C., et al. Toxicity and detoxification of T-2 toxin in poultry. Food Chem Toxicol. 2022;169 doi: 10.1016/j.fct.2022.113392. [DOI] [PubMed] [Google Scholar]
- Li S.Y., Cao J.L., Shi Z.L., Chen J.H., Zhang Z.T., Hughes C.E., et al. Promotion of the articular cartilage proteoglycan degradation by T-2 toxin and selenium protective effect. J Zhejiang Univ - Sci B. 2008;9:22–33. doi: 10.1631/jzus.B071322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Wang X., Liu S., Wang J., Liu X., Zhu Y., et al. Betulinic acid attenuates T-2 toxin-induced cytotoxicity in porcine kidney cells by blocking oxidative stress and endoplasmic reticulum stress. Comp Biochem Physiol C Toxicol Pharmacol. 2021;249 doi: 10.1016/j.cbpc.2021.109124. [DOI] [PubMed] [Google Scholar]
- Lin R., Sun Y., Ye W., Zheng T., Wen J., Deng Y. T-2 toxin inhibits the production of mucin via activating the IRE1/XBP1 pathway. Toxicology. 2019;424 doi: 10.1016/j.tox.2019.06.001. [DOI] [PubMed] [Google Scholar]
- Liu D.K., Wannemacher R.W., Snider T.H., Hayes T.L. Efficacy of the topical skin protectant in advanced development. J Appl Toxicol. 1999;19:S40–S45. doi: 10.1002/(sici)1099-1263(199912)19:1+<s40::aid-jat614>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Liu Q., Wen J., Zhu J., Zhang T., Deng Y., Jiang J. Aromatic hydrocarbon receptor regulates chicken cytochrome P450 1A5 transcription: a novel insight into T-2 toxin-induced gene expression and cytotoxicity in LMH cells. Biochem Pharmacol. 2019;168:319–329. doi: 10.1016/j.bcp.2019.07.023. [DOI] [PubMed] [Google Scholar]
- Liu X., Wang Z., Wang X., Yan X., He Q., Liu S., et al. Involvement of endoplasmic reticulum stress-activated PERK-eIF2alpha-ATF4 signaling pathway in T-2 toxin-induced apoptosis of porcine renal epithelial cells. Toxicol Appl Pharmacol. 2021;432 doi: 10.1016/j.taap.2021.115753. [DOI] [PubMed] [Google Scholar]
- Liu Y., Dong R., Yang Y., Xie H., Huang Y., Chen X., et al. Protective effect of organic selenium on oxidative damage and inflammatory reaction of rabbit kidney induced by T-2 toxin. Biol Trace Elem Res. 2021;199:1833–1842. doi: 10.1007/s12011-020-02279-5. [DOI] [PubMed] [Google Scholar]
- Liu Y., Wang H., Zhang M., Wang J., Zhang Z., Wang Y., et al. Protective effect of selenomethionine on T-2 toxin-induced liver injury in New Zealand rabbits. BMC Vet Res. 2021;17:153. doi: 10.1186/s12917-021-02866-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Yang Y., Dong R., Zhang Z., Jia F., Yu H., et al. Protective effect of selenomethionine on intestinal injury induced by T-2 toxin. Res Vet Sci. 2020;132:439–447. doi: 10.1016/j.rvsc.2020.07.018. [DOI] [PubMed] [Google Scholar]
- Liu Y.L., Meng G.Q., Wang H.R., Zhu H.L., Hou Y.Q., Wang W.J., et al. Effect of three mycotoxin adsorbents on growth performance, nutrient retention and meat quality in broilers fed on mould-contaminated feed. Br Poultry Sci. 2011;52:255–263. doi: 10.1080/00071668.2011.559453. [DOI] [PubMed] [Google Scholar]
- Lu Q., Hu S., Guo P., Zhu X., Ren Z., Wu Q., et al. PPAR-gamma with its anti-fibrotic action could serve as an effective therapeutic target in T-2 toxin-induced cardiac fibrosis of rats. Food Chem Toxicol. 2021;152 doi: 10.1016/j.fct.2021.112183. [DOI] [PubMed] [Google Scholar]
- Luo C., Huang C., Zhu L., Kong L., Yuan Z., Wen L., et al. Betulinic acid ameliorates the T-2 toxin-triggered intestinal impairment in mice by inhibiting inflammation and mucosal barrier dysfunction through the NF-kappaB signaling pathway. Toxins. 2020;12:794. doi: 10.3390/toxins12120794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S., Zhao Y., Sun J., Mu P., Deng Y. miR449a/SIRT1/PGC-1alpha is necessary for mitochondrial biogenesis induced by T-2 toxin. Front Pharmacol. 2017;8:954. doi: 10.3389/fphar.2017.00954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosavi M., Rezaei M., Kalantari H., Behfar A., Varnaseri G. l-carnitine protects rat hepatocytes from oxidative stress induced by T-2 toxin. Drug Chem Toxicol. 2016;39:445–450. doi: 10.3109/01480545.2016.1141423. [DOI] [PubMed] [Google Scholar]
- Mutoh A., Ishii K., Ueno Y. Effects of radioprotective compounds and anti-inflammatory agents on the acute toxicity of trichothecenes. Toxicol Lett. 1988;40:165–174. doi: 10.1016/0378-4274(88)90158-0. [DOI] [PubMed] [Google Scholar]
- Ngampongsa S., Hanafusa M., Ando K., Ito K., Kuwahara M., Yamamoto Y., et al. Toxic effects of T-2 toxin and deoxynivalenol on the mitochondrial electron transport system of cardiomyocytes in rats. J Toxicol Sci. 2013;38:495–502. doi: 10.2131/jts.38.495. [DOI] [PubMed] [Google Scholar]
- Pang Y., Zhang L., Liu Q., Peng H., He J., Jin H., et al. NRF2/PGC-1alpha-mediated mitochondrial biogenesis contributes to T-2 toxin-induced toxicity in human neuroblastoma SH-SY5Y cells. Toxicol Appl Pharmacol. 2022;451 doi: 10.1016/j.taap.2022.116167. [DOI] [PubMed] [Google Scholar]
- Pei X., Jiang H., Liu X., Li L., Li C., Xiao X., et al. Targeting HMGB1 inhibits T-2 toxin-induced neurotoxicity via regulation of oxidative stress, neuroinflammation and neuronal apoptosis. Food Chem Toxicol. 2021;151 doi: 10.1016/j.fct.2021.112134. [DOI] [PubMed] [Google Scholar]
- Pomothy J.M., Barna R.F., Paszti E.A., Babiczky A., Szoladi A., Jerzsele A., et al. Beneficial effects of rosmarinic acid on IPEC-J2 cells exposed to the combination of deoxynivalenol and T-2 toxin. Mediat Inflamm. 2020;2020 doi: 10.1155/2020/8880651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomothy J.M., Paszti-Gere E., Barna R.F., Prokoly D., Jerzsele A. The impact of fermented wheat germ extract on porcine epithelial cell line exposed to deoxynivalenol and T-2 mycotoxins. Oxid Med Cell Longev. 2020;2020 doi: 10.1155/2020/3854247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachitha P., Krupashree K., Jayashree G.V., Raghavendra V.B., Pal A., Chinnathambi A., et al. T-2 toxin induces dermal inflammation and toxicity in mice: the healing potential of menthol. Environ Res. 2023;228 doi: 10.1016/j.envres.2023.115838. [DOI] [PubMed] [Google Scholar]
- Riahi I., Ramos A.J., Raj J., Jakovcevic Z., Farkas H., Vasiljevic M., et al. Effect of a mycotoxin binder (mmda) on the growth performance, blood and carcass characteristics of broilers fed ochratoxin A and T-2 mycotoxin contaminated diets. Animals. 2021;11:3205. doi: 10.3390/ani11113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo A.F., Atroshi F., Ahotupa M., Sankari S., Elovaara E. Protective effect of antioxidants against free radical-mediated lipid peroxidation induced by DON or T-2 toxin. Zentralblatt für Veterinarmed A. 1994;41:81–90. doi: 10.1111/j.1439-0442.1994.tb00070.x. [DOI] [PubMed] [Google Scholar]
- Romero A., Ares I., Ramos E., Castellano V., Martinez M., Martinez-Larranaga M.R., et al. Mycotoxins modify the barrier function of Caco-2 cells through differential gene expression of specific claudin isoforms: protective effect of illite mineral clay. Toxicology. 2016;353–354:21–33. doi: 10.1016/j.tox.2016.05.003. [DOI] [PubMed] [Google Scholar]
- Salimian J., Arefpour M.A., Riazipour M., Poursasan N. Immunomodulatory effects of selenium and vitamin E on alterations in T lymphocyte subsets induced by T-2 toxin. Immunopharmacol Immunotoxicol. 2014;36:275–281. doi: 10.3109/08923973.2014.931420. [DOI] [PubMed] [Google Scholar]
- Sawi N.E., Seeni M.N.A. Assessment of flavonoids as rutin for detoxification of T-2 toxin. J Appl Anim Res. 2009;35:57–60. [Google Scholar]
- Shah B., Belonje B. Liver storage iron in Canadians. Am J Clin Nutr. 1976;29:66–69. doi: 10.1093/ajcn/29.1.66. [DOI] [PubMed] [Google Scholar]
- Shang S., Jiang J., Deng Y. Chicken cytochrome P450 1A5 is the key enzyme for metabolizing T-2 toxin to 3'OH-T-2. Int J Mol Sci. 2013;14:10809–10818. doi: 10.3390/ijms140610809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng K., Lu X., Yue J., Gu W., Gu C., Zhang H., et al. Role of neurotransmitters 5-hydroxytryptamine and substance P in anorexia induction following oral exposure to the trichothecene T-2 toxin. Food Chem Toxicol. 2019;123:1–8. doi: 10.1016/j.fct.2018.10.041. [DOI] [PubMed] [Google Scholar]
- Sheng K., Zhang H., Yue J., Gu W., Gu C., Zhang H., et al. Anorectic response to the trichothecene T-2 toxin correspond to plasma elevations of the satiety hormone glucose-dependent insulinotropic polypeptide and peptide YY3-36. Toxicology. 2018;402–403:28–36. doi: 10.1016/j.tox.2018.04.007. [DOI] [PubMed] [Google Scholar]
- Shi M., He Y., Zhang Y., Guo X., Lin J., Wang W., et al. LncRNA MIAT regulated by selenium and T-2 toxin increases NF-kappaB-p65 activation, promoting the progress of Kashin-Beck Disease. Hum Exp Toxicol. 2021;40:869–881. doi: 10.1177/0960327120975122. [DOI] [PubMed] [Google Scholar]
- Sun J., Min Z., Zhao W., Hussain S., Zhao Y., Guo D., et al. T-2 toxin induces epiphyseal plate lesions via decreased SECISBP2-mediated selenoprotein expression in DA rats, exacerbated by selenium deficiency. Cartilage. 2021;12:121–131. doi: 10.1177/1947603518809406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T., Zhang Q., Li M., Tang S., Dai C. T-2 toxin induces apoptotic cell death and protective autophagy in mouse microglia BV2 cells. J Fungi (Basel) 2022;8:761. doi: 10.3390/jof8080761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taroncher M., Rodriguez-Carrasco Y., Aspevik T., Kousoulaki K., Barba F.J., Ruiz M.J. Cytoprotective effects of fish protein hydrolysates against H(2)O(2)-induced oxidative stress and mycotoxins in caco-2/TC7 cells. Antioxidants. 2021;10:975. doi: 10.3390/antiox10060975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tima H., Racz A., Guld Z., Mohacsi-Farkas C., Kisko G. Deoxynivalenol, zearalenone and T-2 in grain based swine feed in Hungary. Food Addit Contam Part B Surveill. 2016;9:275–280. doi: 10.1080/19393210.2016.1213318. [DOI] [PubMed] [Google Scholar]
- Tran V.N., Viktorova J., Augustynkova K., Jelenova N., Dobiasova S., Rehorova K., et al. Silico and in vitro studies of mycotoxins and their cocktails; their toxicity and its mitigation by silibinin pre-treatment. Toxins. 2020;12:148. doi: 10.3390/toxins12030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso K.H., Lumsangkul C., Cheng M.C., Ju J.C., Fan Y.K., Chiang H.I. Differential effects of green tea powders on the protection of Brown tsaiya and kaiya ducklings against trichothecene T-2 toxin toxicity. Animals. 2021;11:2541. doi: 10.3390/ani11092541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutelyan V.A., Zakharova L.P., Sedova I.B., Perederyaev O.I., Aristarkhova T.V., Eller K.I. Fusariotoxins in Russian Federation 2005-2010 grain harvests. Food Addit Contam Part B Surveill. 2013;6:139–145. doi: 10.1080/19393210.2013.767862. [DOI] [PubMed] [Google Scholar]
- Ueno Y., Nakayama K., Ishii K., Tashiro F., Minoda Y., Omori T., et al. Metabolism of T-2 toxin in Curtobacterium sp. strain 114-2. Appl Environ Microbiol. 1983;46:120–127. doi: 10.1128/aem.46.1.120-127.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursini F., Maiorino M. Lipid peroxidation and ferroptosis: the role of GSH and GPx4. Free Radic Biol Med. 2020;152:175–185. doi: 10.1016/j.freeradbiomed.2020.02.027. [DOI] [PubMed] [Google Scholar]
- Van Der Fels-Klerx H.J., Klemsdal S., Hietaniemi V., Lindblad M., Ioannou-Kakouri E., Van Asselt E.D. Mycotoxin contamination of cereal grain commodities in relation to climate in North West Europe. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2012;29:1581–1592. doi: 10.1080/19440049.2012.689996. [DOI] [PubMed] [Google Scholar]
- Wang G., Qin S., Zheng Y., Xia C., Zhang P., Zhang L., et al. T-2 toxin induces ferroptosis by increasing lipid reactive oxygen species (ROS) and downregulating solute carrier family 7 member 11 (SLC7A11) J Agric Food Chem. 2021;69:15716–15727. doi: 10.1021/acs.jafc.1c05393. [DOI] [PubMed] [Google Scholar]
- Wang J., Yang C., Yuan Z., Yi J., Wu J. T-2 toxin exposure induces apoptosis in TM3 cells by inhibiting mammalian target of rapamycin/serine/threonine protein kinase(mTORC2/AKT) to promote Ca(2+)Production. Int J Mol Sci. 2018;19:3360. doi: 10.3390/ijms19113360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J.T., Wu K.T., Sun H., Khalil M.M., Dai J.F., Liu Y., et al. A novel modified hydrated sodium calcium aluminosilicate (HSCAS) adsorbent can effectively reduce T-2 toxin-induced toxicity in growth performance, nutrient digestibility, serum biochemistry, and small intestinal morphology in chicks. Toxins. 2019;11:199. doi: 10.3390/toxins11040199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Tu D., Yuan L.Y., Yi J.E., Tian Y. T-2 toxin regulates steroid hormone secretion of rat ovarian granulosa cells through cAMP-PKA pathway. Toxicol Lett. 2015;232:573–579. doi: 10.1016/j.toxlet.2014.12.016. [DOI] [PubMed] [Google Scholar]
- Wu J., Yang C., Liu J., Chen J., Huang C., Wang J., et al. Betulinic acid attenuates T-2-toxin-induced testis oxidative damage through regulation of the JAK2/STAT3 signaling pathway in mice. Biomolecules. 2019;9:787. doi: 10.3390/biom9120787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q., Qin Z., Kuca K., You L., Zhao Y., Liu A., et al. An update on T-2 toxin and its modified forms: metabolism, immunotoxicity mechanism, and human exposure assessment. Arch Toxicol. 2020;94:3645–3669. doi: 10.1007/s00204-020-02899-9. [DOI] [PubMed] [Google Scholar]
- Wu W., Sheng K., Xu X., Zhang H., Zhou G. Potential roles for glucagon-like peptide-17-36 amide and cholecystokinin in anorectic response to the trichothecene mycotoxin T-2 toxin. Ecotoxicol Environ Saf. 2018;153:181–187. doi: 10.1016/j.ecoenv.2018.02.003. [DOI] [PubMed] [Google Scholar]
- Yang F., Li L., Chen K., Li C., Wang Y., Wang G. Melatonin alleviates beta-zearalenol and HT-2 toxin-induced apoptosis and oxidative stress in bovine ovarian granulosa cells. Environ Toxicol Pharmacol. 2019;68:52–60. doi: 10.1016/j.etap.2019.03.005. [DOI] [PubMed] [Google Scholar]
- Yang J.Y., Du J.J., Zhang Y.F., Li Y.X. Vitamin E and selenium partially prevent cytotoxicity, oxidative stress and DNA damage induced by T-2 toxin in bovine Leydig cells. Theriogenology. 2022;189:255–261. doi: 10.1016/j.theriogenology.2022.06.028. [DOI] [PubMed] [Google Scholar]
- Yang J.Y., Zhang Y.F., Li Y.X., Meng X.P., Bao J.F. l-arginine protects against oxidative damage induced by T-2 toxin in mouse Leydig cells. J Biochem Mol Toxicol. 2018;32 doi: 10.1002/jbt.22209. [DOI] [PubMed] [Google Scholar]
- Yang J.Y., Zhang Y.F., Nie N., Feng W.P., Bao J.F., Meng X.P., et al. Protective effects of l-arginine against testosterone synthesis decreased by T-2 toxin in mouse Leydig cells. Theriogenology. 2019;134:98–103. doi: 10.1016/j.theriogenology.2019.05.023. [DOI] [PubMed] [Google Scholar]
- Yang X., Liu P., Zhang X., Zhang J., Cui Y., Song M., et al. T-2 toxin causes dysfunction of Sertoli cells by inducing oxidative stress. Ecotoxicol Environ Saf. 2021;225 doi: 10.1016/j.ecoenv.2021.112702. [DOI] [PubMed] [Google Scholar]
- Yang X., Zhang X., Yao Q., Song M., Han Y., Shao B., et al. T-2 toxin impairs male fertility by disrupting hypothalamic-pituitary-testis axis and declining testicular function in mice. Chemosphere. 2019;234:909–916. doi: 10.1016/j.chemosphere.2019.06.145. [DOI] [PubMed] [Google Scholar]
- Ye W., Lin R., Chen X., Chen J., Chen R., Xie X., et al. T-2 toxin upregulates the expression of human cytochrome P450 1A1 (CYP1A1) by enhancing NRF1 and Sp1 interaction. Toxicol Lett. 2019;315:77–86. doi: 10.1016/j.toxlet.2019.08.021. [DOI] [PubMed] [Google Scholar]
- Yi Y., Zhao F., Wang N., Liu H., Yu L., Wang A., et al. Endoplasmic reticulum stress is involved in the T-2 toxin-induced apoptosis in goat endometrium epithelial cells. J Appl Toxicol. 2018;38:1492–1501. doi: 10.1002/jat.3655. [DOI] [PubMed] [Google Scholar]
- You L., Wang X., Wu W., Nepovimova E., Wu Q., Kuca K. HIF-1alpha inhibits T-2 toxin-mediated "immune evasion" process by negatively regulating PD-1/PD-L1. Toxicology. 2022;480 doi: 10.1016/j.tox.2022.153324. [DOI] [PubMed] [Google Scholar]
- Yu F.F., Lin X.L., Wang X., Liu H., Yang L., Goldring M.B., et al. Selenium promotes metabolic conversion of T-2 toxin to HT-2 toxin in cultured human chondrocytes. J Trace Elem Med Biol. 2017;44:218–224. doi: 10.1016/j.jtemb.2017.08.009. [DOI] [PubMed] [Google Scholar]
- Zakharova L.P., Sedova I.B., Aristarkhova T.V., Perederiaev O.I., Selifanov A.V., Eller K.I., et al. [Fusariotoxins in the food production in the Russian Federation: situation 2006-2008 years] Vopr Pitan. 2009;78:26–31. [PubMed] [Google Scholar]
- Zhang C., Chi C., Liu J., Ye M., Zheng X., Zhang D., et al. Protective effects of dietary arginine against oxidative damage and hepatopancreas immune responses induced by T-2 toxin in Chinese mitten crab (Eriocheir sinensis) Fish Shellfish Immunol. 2020;104:447–456. doi: 10.1016/j.fsi.2020.06.034. [DOI] [PubMed] [Google Scholar]
- Zhang D., Deng X., Liu Y., Zhang Y., Wang H., Zhang M., et al. MMP-10 deficiency effects differentiation and death of chondrocytes associated with endochondral osteogenesis in an endemic osteoarthritis. Cartilage. 2022;13 doi: 10.1177/19476035221109226. 19476035221109226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Zhang D., Wang C., Zhang R., Li Q., Xiong Y. Mechanism of DNA methylation-mediated downregulation of O6-methylguanine-DNA methyltransferase in cartilage injury of Kashin-Beck disease. Rheumatology. 2022;61:3471–3480. doi: 10.1093/rheumatology/keab913. [DOI] [PubMed] [Google Scholar]
- Zhang J., Liu X., Su Y., Li T. An update on T2-toxins: metabolism, immunotoxicity mechanism and human assessment exposure of intestinal microbiota. Heliyon. 2022;8 doi: 10.1016/j.heliyon.2022.e10012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Song M., Cui Y., Shao B., Zhang X., Cao Z., et al. T-2 toxin-induced femur lesion is accompanied by autophagy and apoptosis associated with Wnt/beta-catenin signaling in mice. Environ Toxicol. 2022;37:1653–1661. doi: 10.1002/tox.23514. [DOI] [PubMed] [Google Scholar]
- Zhang M., Wang M., Wang H., Zhang Y., Li Z., Feng Y., et al. Decreased expression of Heat shock protein 47 is associated with T-2 toxin and low selenium-induced matrix degradation in cartilages of kashin-beck disease. Biol Trace Elem Res. 2021;199:944–954. doi: 10.1007/s12011-020-02237-1. [DOI] [PubMed] [Google Scholar]
- Zhang M., Wang W., Wang H., Liu Y., Li Z., Yi C., et al. Downregulation of insulin-like growth factor-1 receptor mediates chondrocyte death and matrix degradation in kashin-beck disease. Cartilage. 2021;13:809S–817S. doi: 10.1177/19476035211021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Du J., Li B., Huo S., Zhang J., Cui Y., et al. PINK1/Parkin-mediated mitophagy mitigates T-2 toxin-induced nephrotoxicity. Food Chem Toxicol. 2022;164 doi: 10.1016/j.fct.2022.113078. [DOI] [PubMed] [Google Scholar]
- Zhang X., Wang Q., Zhang J., Song M., Shao B., Han Y., et al. The protective effect of selenium on T-2-induced nephrotoxicity is related to the inhibition of ROS-mediated apoptosis in mice kidney. Biol Trace Elem Res. 2022;200:206–216. doi: 10.1007/s12011-021-02614-4. [DOI] [PubMed] [Google Scholar]
- Zhang X., Wang Y., Velkov T., Tang S., Dai C. T-2 toxin-induced toxicity in neuroblastoma-2a cells involves the generation of reactive oxygen, mitochondrial dysfunction and inhibition of Nrf2/HO-1 pathway. Food Chem Toxicol. 2018;114:88–97. doi: 10.1016/j.fct.2018.02.010. [DOI] [PubMed] [Google Scholar]
- Zhang X., Wang Y., Yang X., Liu M., Huang W., Zhang J., et al. The nephrotoxicity of T-2 toxin in mice caused by oxidative stress-mediated apoptosis is related to Nrf2 pathway. Food Chem Toxicol. 2021;149 doi: 10.1016/j.fct.2021.112027. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Li Z., He Y., Liu Y., Mi G., Chen J. T-2 toxin induces articular cartilage damage by increasing the expression of MMP-13 via the TGF-beta receptor pathway. Hum Exp Toxicol. 2022;41 doi: 10.1177/09603271221075555. 9603271221075555. [DOI] [PubMed] [Google Scholar]
- Zhang Y.F., Yang J.Y., Meng X.P., Nie N., Tang M.C., Yang X.L. L-Arginine protects mouse Leydig cells against T-2 toxin-induced apoptosis in vitro. Toxicol Ind Health. 2020;36:1031–1038. doi: 10.1177/0748233720964312. [DOI] [PubMed] [Google Scholar]
- Zhang Y.F., Yang J.Y., Meng X.P., Qiao X.L. l-arginine protects against T-2 toxin-induced male reproductive impairments in mice. Theriogenology. 2019;126:249–253. doi: 10.1016/j.theriogenology.2018.12.024. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Xu Y., Wang J., Xie H., Sun X., Zhu X., et al. Protective effect of selenomethionine on T-2 toxin-induced rabbit immunotoxicity. Biol Trace Elem Res. 2022;200:172–182. doi: 10.1007/s12011-021-02625-1. [DOI] [PubMed] [Google Scholar]
- Zhou X., Yang H., Guan F., Xue S., Song D., Chen J., et al. T-2 toxin alters the levels of collagen II and its regulatory enzymes MMPs/TIMP-1 in a low-selenium rat model of kashin-beck disease. Biol Trace Elem Res. 2016;169:237–246. doi: 10.1007/s12011-015-0408-2. [DOI] [PubMed] [Google Scholar]
- Zhu J., Li G., Huang Q., Wen J., Deng Y., Jiang J. TET3-mediated DNA demethylation and chromatin remodeling regulate T-2 toxin-induced human CYP1A1 expression and cytotoxicity in HepG2 cells. Biochem Pharmacol. 2023;211 doi: 10.1016/j.bcp.2023.115506. [DOI] [PubMed] [Google Scholar]
- Zhu L., Yi X., Ma C., Luo C., Kong L., Lin X., et al. Betulinic acid attenuates oxidative stress in the thymus induced by acute exposure to T-2 toxin via regulation of the MAPK/Nrf2 signaling pathway. Toxins. 2020;12:540. doi: 10.3390/toxins12090540. [DOI] [PMC free article] [PubMed] [Google Scholar]