Abstract
Background
Different approaches to the prevention of postoperative ileus have been evaluated in numerous randomized controlled trials. This network meta-analysis aimed to investigate the relative effectiveness of different interventions in preventing postoperative ileus.
Methods
Randomized controlled trials (RCTS) on the prevention of postoperative ileus were screened from Chinese and foreign medical databases and compared. STATA software was used for network meta-analysis using the frequency method. Random-effects network meta-analysis was also used to compare all schemes directly and indirectly.
Results
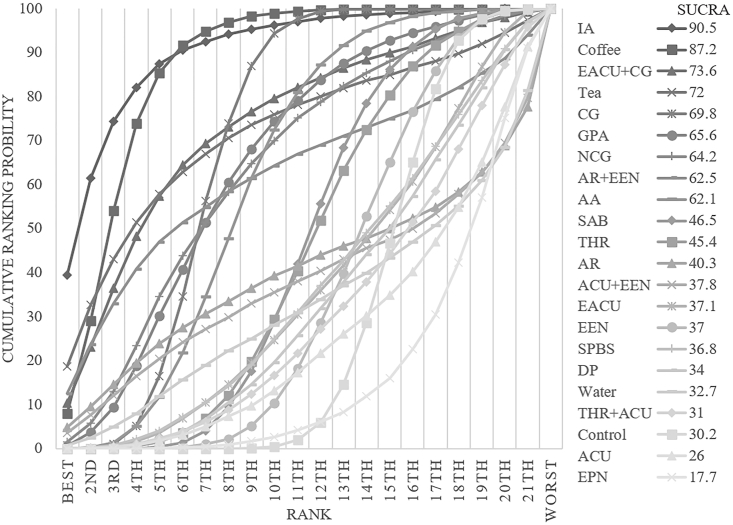
A total of 105 randomized controlled trials with 18,840 participants were included in this report. The results of the network meta-analysis showed that intravenous analgesia was most effective in preventing the incidence of postoperative ileus, the surface under the cumulative ranking curve (SUCRA) is 90.5. The most effective intervention for reducing the first postoperative exhaust time was postoperative abdominal mechanical massage (SUCRA: 97.3), and the most effective intervention for reducing the first postoperative defecation time was high-dose opioid antagonists (SUCRA: 84.3). Additionally, the most effective intervention for reducing the time to initiate a normal diet after surgery was accelerated rehabilitation (SUCRA: 85.4). A comprehensive analysis demonstrated the effectiveness and prominence of oral opioid antagonists and electroacupuncture (EA) combined with gum.
Conclusion
This network meta-analysis determined that oral opioid antagonists and EA combined with chewing gum are the most effective treatments and optimal interventions for reducing the incidence of postoperative ileus. However, methods such as abdominal mechanical massage and coffee require further high-quality research.
Keywords: Abdominal surgery, Ileus, Prevention, Network meta-analysis
1. Introduction
Postoperative ileus (POI), a concerning complication following surgery, continues to play a significant role in patient prognosis [1]. It is characterized by temporary inhibition of gastrointestinal peristalsis and prolonging the time for patients to begin to exhaust and defecate. Complex pharmacological (opioid and anesthetic), neurological, and immune-mediated pathways play a role in its pathogenesis [2]. Despite extensive research focusing on the high-risk population, 10 %–30 % of patients still experience POI after abdominal surgery, potentially due to the traumatic nature of the procedure [1]. Patients who experience different levels of exposure over time lose their peritoneal integrity, resulting in extended hospital stays, increased financial burden, and symptoms related to ileus, such as prolonged exhaustion and defecation times, abdominal distension, abdominal pain, nausea, and vomiting. Although postoperative ileus is typically inevitable, experimental investigations have sought to identify approaches that can reduce its duration and facilitate the restoration of gastrointestinal function soon after surgery [[3], [4], [5]]. Drug and non-drug therapies are primarily used to prevent and cure postoperative gastrointestinal dysfunction (POGD) [6], and prevention is prioritized over treatment for better recovery after surgery. Currently, pharmacological therapies such as opioid receptor antagonists, intravenous analgesia, epidural analgesia, and conventional herbal decoctions are the mainstay of treatment for intestinal blockage [7]. Acupuncture, early diet, fake feeding, stomach massage, and other techniques are examples of non-drug therapies [8,9]. It is currently unclear which approach is the most effective for preventing postoperative intestinal blockage following abdominal surgery, as a significant number of Randomized controlled trials (RCTS) investigations have shown inconsistent experimental results. To offer suggestions for the clinical diagnosis and treatment of postoperative ileus and to identify a more general prevention strategy for clinical practice, the author of this research article screened randomized controlled trials and evaluated the best intervention measures to prevent postoperative ileus after abdominal operation through a network meta-analysis and systematic review.
2. Methods
2.1. Search strategy
A thorough search for related research was undertaken across many databases, including Chinese data from the CNKI, VIP, WANFANG, and CBM databases, and English data from the Cochrane Library, Embase, Web of Science, and PubMed databases. The search terms included prevent, postoperative ileus, ileus, and free words. The search duration is not restricted, and languages such as Chinese and English are available. In addition, references in the initial meta-analyses and review articles were searched for and reviewed.
2.2. Selection of research studies
This study followed the PRISMA guidelines. The inclusion and exclusion of studies for the focused questions mentioned above were guided by the Population, Intervention, Comparison, and Outcomes (PICO) framework:
Population (P): patients who had undergone abdominal surgery without restrictions on age or sex.
Intervention (I): prophylactic administration refers to any therapies that are intended to avoid postoperative ileus.
Comparison (C): every conceivable comparison between the therapies that were included was thoroughly examined.
Outcome (O): the occurrence of postoperative ileus, the duration until the first bowel movement, the duration until the first defecation, and the duration until resuming a regular diet.
Inclusion Criteria.
-
•
Patients undergoing abdominal surgery.
-
•
randomized controlled trial (RCT).
-
•
The clinical outcomes evaluated and documented throughout the follow-up period included the occurrence of ileus, duration until the first expulsion of gas, duration until the first bowel movement, and duration until resumption of a regular diet.
Exclusion Criteria.
-
•
Non-abdominal surgical procedures, administration without preventive intent, animal trials, protocols, absence of a control group, examination, anecdotal accounts, unavailability of data or complete text, and statistical investigation is impractical.
2.3. Data extraction
Two researchers reviewed all the titles and abstracts of the retrieved articles. After the preliminary exclusion and inclusion of the literature, the full texts were downloaded for further evaluation. Any discrepancies between the two reviewers were resolved through consultation with a third researcher to establish the final inclusion criteria. The basic information of the included studies was as follows: first author name, publication date, country, type of surgery, sample size, mean age, interventions, and outcome indicators.
2.4. Quality assessment
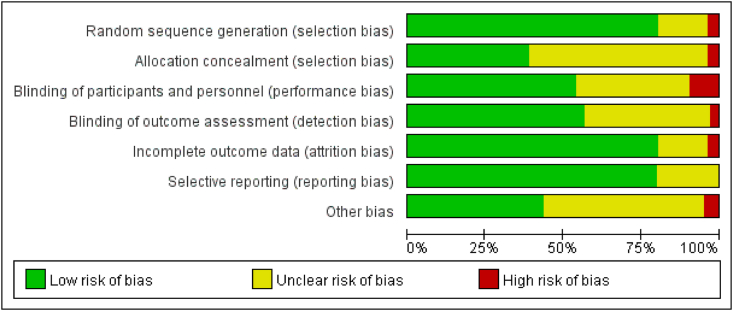
The Cochrane Collaboration tool was used to assess the risk of bias in randomized controlled trials (RCTs), which consisted of random sequence generation, allocation concealment, participant and personnel blinding, outcome assessment blinding, incomplete outcome data, selective reporting, and other biases. Visualization was achieved using the RevMan software (Version 5.4.1; The Cochrane Collaboration, 2020), as presented in the evaluation results in Fig. 2.
Fig. 2.
Figure of risk of bias assessment.
2.5. Statistical analysis
Network meta-analyses are statistical studies that examine more than two treatments by using indirect comparisons or a combination of indirect and direct comparisons. Network meta-analysis, in comparison to classic meta-analysis, has the ability to identify the most effective intervention among several options. Stata software (version 15; STATA Corporation, College Station, TX, USA) was used to draw network evidence maps of different interventions to visually represent the evidence quantity and compare the relationships between different interventions. First, direct comparisons between different interventions were performed. A network meta-analysis was performed using the frequentist framework random-effects model, allowing indirect and direct evidence to be combined into the analysis, and the efficacy of different interventions to be ranked. The inconsistency test was first performed when the test results were P > 0.05, which implied no statistical significance and indicated consistency, and a consistency model was employed for the network meta-analysis. The Surface Under the Cumulative Ranking Curve (SUCRA) measure was used to evaluate and rank the efficacy of each therapy, ultimately determining the most successful treatment. Finally, a funnel plot was constructed to present the publication bias for all available treatments. Statistical significance was set at P < 0.05 for all measurements.
3. Result
3.1. Literature screening and basic data information
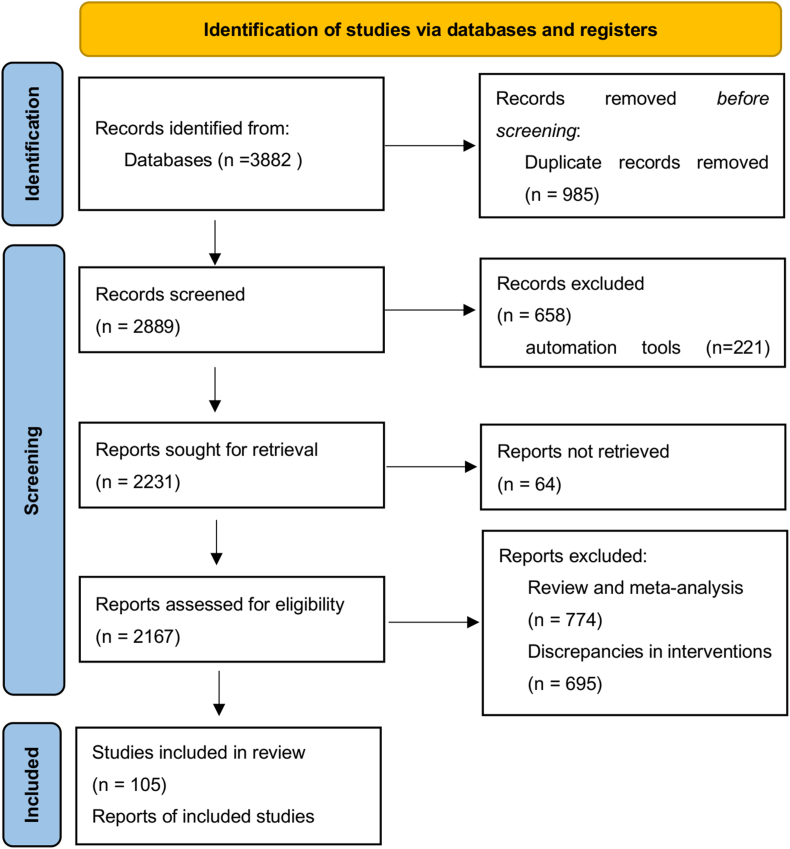
A total of 18,840 participants and 30 different types of therapies were included in 105 randomized controlled trials [[10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106], [107], [108], [109], [110], [111], [112], [113], [114]], which were obtained from various databases. The essential details of these trials are presented in (Supplementary Table S1). Two treatment categories were based on different doses of the same class of medication. Routine postoperative care served as the intervention in the control group. However, the nursing strategy varied depending on the surgical technique used. In Fig. 1, the retrieval procedure and outcomes are displayed.
Fig. 1.
Flow chart of data retrieval and screening.
All 105 studies included in the study were randomized controlled trials, and 85 of these studies provided a detailed explanation of the randomization process. Blinding research participants to acupuncture and moxibustion therapies is challenging owing to the nature of these treatments. The lack of a clear explanation and potential for follow-up bias makes it a high-risk procedure. Fig. 2 shows the risk of bias assessment, with the opinions of the review authors on each risk of bias item presented as a percentage of all included studies.
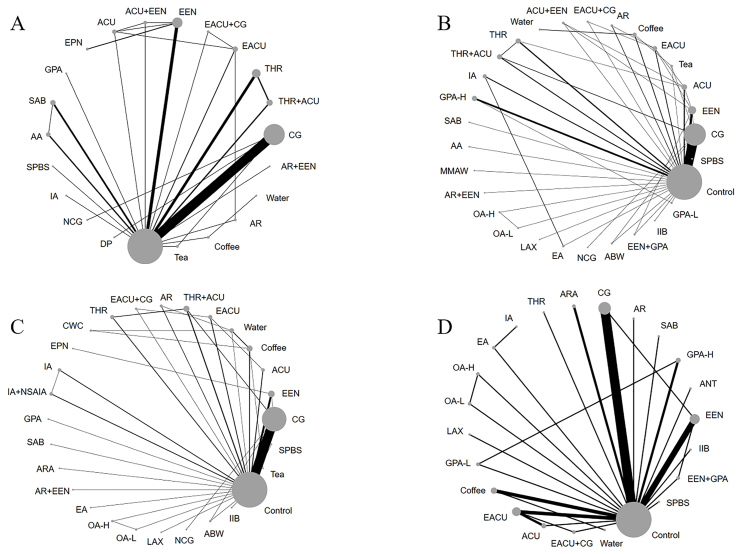
The primary prognostic indicator was the incidence of postoperative intestinal blockage, with the first occurrence of exhaustion, the first occurrence of bowel movement, and normal eating time serving as supplementary outcome indicators. Fig. 3 shows the primary outcome indicators, postoperative ileus intervention measures, and secondary end-index network diagram.
Fig. 3.
Prevention of all interventions network maps after postoperative ileus. Figure A: The incidence of postoperative ileus; Figure B: The first exhaust time; Figure C: The first time of bowel movement; Figure D: The normal eating time. CG: Chewing gum; THR: Traditional herbal remedies; ACU: Acupuncture; EACU: Electroacupuncture; EEN: Early enteral nutrition; EPN: Early parenteral nutrition; GPA: Gastric prokinetic agents; SAB: Seprafilm Adhesion Barrier; AA: Antiadhesive agent; SPBS: Stimulation with Probiotics before Surgery; IA: Intravenous analgesia; NCG: Nicotine chewing gum; DP: Dermal patch; MMAW: Mechanical Massage of the Abdominal Wall; IOA: Infusion of local anesthetic; AR: Accelerated rehabilitation; OA: Opioid antagonist; LAX: Laxative; ABW: Acupressure bracelet worn; IIB: Intestinal isolation bag; CWC: Coffee without caffeine; ARA: Adrenergic receptor agonists; NSAIA: Non-steroidal anti-inflammatory agents; EA: Epidural analgesia; ANT: Antemetic.
3.2. The incidence of postoperative ileus
The consistency model was used for analysis after conducting an inconsistency test, which resulted in a p-value of 0.8544. The inconsistency test was not significant. A comparison network of several therapies employed to prevent postoperative ileus is shown in Fig. 3A. Among these interventions, IA was the first substantially effective intervention, reducing the incidence of ileus by 2.56 % compared to the control group (95 % CI [−4.79,-0.33]). Supplementary Table 2 (S2) compares the interventions. With a cumulative ranking probability of 90.5, IA was the most effective strategy for reducing the risk of postoperative ileus (Fig. 4).
Fig. 4.
Ranking of cumulative probability of bowel obstruction reduction for each intervention measured by incidence of postoperative bowel obstruction.
3.3. The first exhaust time
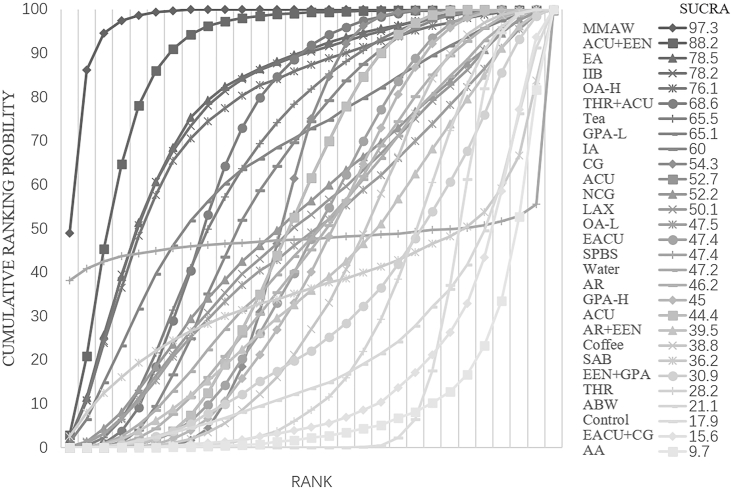
The consistency model was used for analysis after conducting an inconsistency test, which resulted in a p-value of 0.4297. The inconsistency test was not significant. A comparison network of several therapies to reduce the first exhaust time is shown in Fig. 3B. MMAW was the first substantially effective intervention, reducing exhaustion time by 43.20 h on average compared to the control group and by 95 % CI (−61.91, −24.48). For a comparison of the interventions, see Supplementary Table 3 (S3). With a cumulative ranking probability of 97.3, MMAW was the most effective strategy for reducing the first exhaust time after surgery (Fig. 5).
Fig. 5.
Ranking of cumulative probability of a reduction in ileus by each intervention measured as time to first flatus.
3.4. The first time of bowel movement
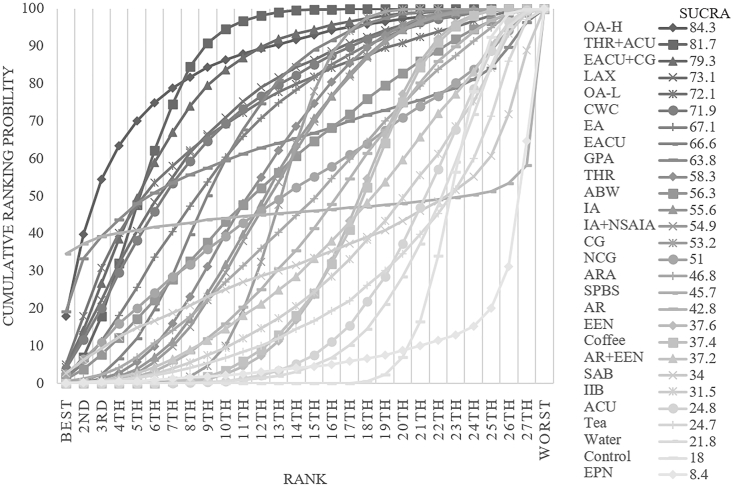
The consistency model was used for analysis after conducting an inconsistency test, which resulted in a p-value of 0.4297. The inconsistency test was not significant. A comparison network of several therapies used to reduce the first bowel movement time is shown in Fig. 3C. OA-H was the first substantially effective intervention, reducing exhaustion time by 41.00 h on average compared to the control group and by 95 % CI (−79.36, −2.64). For a comparison of the interventions, see Supplementary Table 4 (S4). With a cumulative ranking probability of 84.3, OA-H was the most effective strategy to reduce the first exhaust time after surgery (Fig. 6).
Fig. 6.
Ranking of cumulative probability of a reduction in ileus by each intervention measured as time to first bowel movement.
3.5. The return to normal eating time
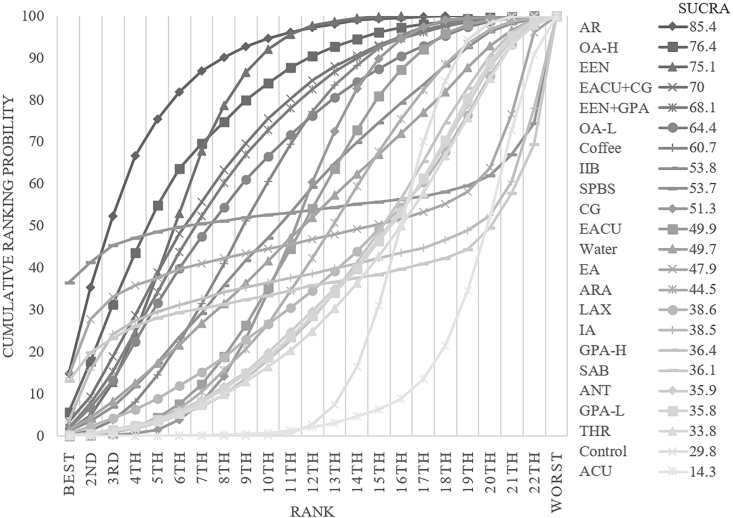
The consistency model was used for analysis after conducting an inconsistency test, which resulted in a p-value of 0.6685. The inconsistency test was not significant. A comparison network of several therapies for reducing the return to normal eating times is shown in Fig. 3D. AR was the first substantially effective intervention, reducing the time to return to normal eating time by 43.00 h on average compared to the control group and by 95 % CI (−76.52, −9.49). For a comparison of the interventions, see Supplementary Table 5 (S5). With a cumulative ranking probability of 85.4, AR was the most effective strategy for reducing the time to return to normal eating after surgery (Fig. 7).
Fig. 7.
Ranking of cumulative probability of a reduction in ileus by each intervention measured as time to normal eating.
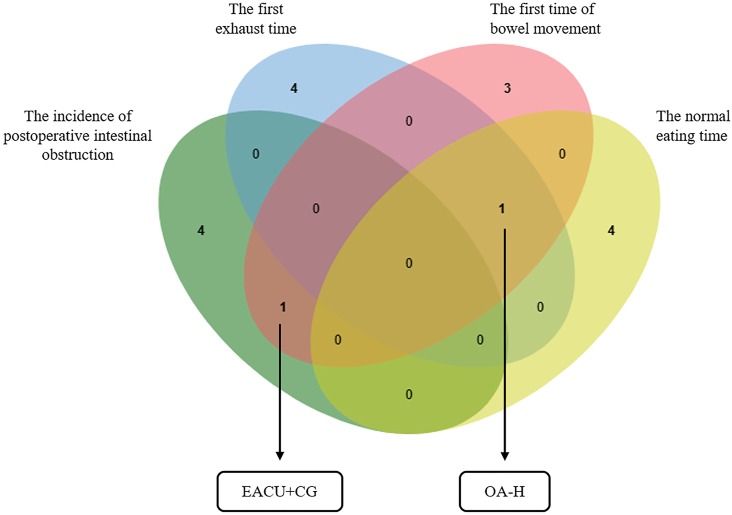
The best intervention among the top five cumulative probabilities of the four outcomes was screened.
Fig. 8 The interventions with good results in different outcome measures, OA-H and EACU + CG, were obtained through a Venn diagram. Table 1 presents the cumulative probabilities of the most effective and the combined best effective interventions for the four outcome measures, respectively.
Fig. 8.
Venn diagram of the top 5 interventions in the prevention of postoperative ileus in different outcome indicators.
Table 1.
Summary of the cumulative probability rankings of the top-ranked interventions and EACU + CG for each outcome measure.
| IA | MMAW | OA-H | AR | EACU + CG | |
|---|---|---|---|---|---|
| The incidence of postoperative ileus | 90.5* | 97.3* | – | 40.3 | 73.6# |
| The first exhaust time | 60 | – | 76.1# | 46.2 | 15.6 |
| The first time of bowel movement | 55.6 | – | 84.3* | 42.8 | 79.3# |
| The normal eating time | 38.5 | – | 76.4# | 85.4* | 70# |
“*“: The cumulative probability of each index ranked first; “#“: The cumulative probability of each index ranked the top 5.
4. Discussion
According to the findings of our network meta-analysis, the OA-H and EACU + CG had a positive effect on restoring postoperative intestinal recovery and preventing ileus, which was essentially consistent with the current clinical medication regimen for the prevention of postoperative ileus. Opioid analgesics are routinely administered during and after surgery to treat intraoperative and postoperative pain [115]. In addition to their cerebral effects on pain receptors, they also have negative GI effects, such as reduced gastric motility and emptying, suppression of intestinal propulsion, and altered fluid and electrolyte balance, which can lead to intestinal blockage [116]. The only licensed drugs in this class are methylnaltrexone (MNTX) and alvimopan, which selectively eliminate or attenuate peripheral opioid-induced side effects without affecting the central analgesic effect, owing to their polarity, large structure, and low lipid solubility, which prevent them from crossing the blood-brain barrier [117]. The peripherally-acting mu-opioid receptor antagonist (PAMORA) activity was restricted to the periphery. According to Tyler's meta-analysis, the use of selective opioid antagonists reduced the incidence of POI in patients undergoing bowel resection and minimized hospitalization expenditure for patients undergoing intestinal resection [118]. The drug used in the clinical trials in the included studies was alvimopan [94]; the total dosage of alvimopan was restricted to 15 doses, with instructions to take a 12-mg capsule 30 min to 5 h before surgery and a 12-mg capsule twice daily from the day of the operation until discharge [116], for up to seven days. GI2 was an objective composite endpoint assessed based on the time the patient first tolerated solid food (a sign of upper GI tract function recovery) and the time when the patient first defecated (a measure of lower GI tract function recovery). The time to the GI2 endpoint was shortened in the alvimopan group compared with the placebo group in most trials, regardless of whether it was a main or secondary endpoint, similar to the results of the network meta-analysis of the current investigation. Sameh et al. investigated many therapeutic approaches targeting the reduction of postoperative ileus incidence and severity after colorectal surgery. These results are consistent with our own studies, suggesting that avimopan is a common and successful method for reducing intestinal obstruction after surgery for colorectal cancer [119]. Electroacupuncture, in conjunction with chewing gum, is identified as an effective prophylactic therapy for postoperative ileus in this research. While this combination is not universally used, it is extensively utilized in China and other Asian nations, such as Korea. Acupoint stimulation, represented by acupuncture, has been used for thousands of years in China and has gradually become an important supplement to the comprehensive prevention and treatment strategy for POGD owing to its advantages of multi-target, precise effect, and no toxic side effects. Transcutaneous electrical acupoint stimulation (TEAS), a mature modern acupoint stimulation method, combines transcutaneous electrical nerve stimulation and acupoint stimulation. It is simpler, more convenient, noninvasive, and has the same therapeutic effect as traditional hand acupuncture [6]. This is more acceptable to patients. Multiple studies have shown that EACU may effectively prevent and cure postoperative ileus by skillfully managing local inflammation. This is accomplished by suppressing the activity of inflammatory molecules, such as TNF-α, IL-6 [120], and JAK2/STAT3 [121] inflammatory signaling pathways, in the gastrointestinal tract and peritoneal cavity. Furthermore, EACU has the capability to modify the movement of the small intestine and the process of emptying the stomach, perhaps due to its ability to increase levels of ghrelin and motilin, as well as the activity of the autonomic nervous system, particularly the vagus nerve [122,123]. Additionally, it may alleviate pain after surgery by influencing cytokines [124].
Gum chewing is a non-pharmacological, affordable, well-tolerated, secure, and efficient method for reducing intestinal blockage following colorectal surgery [125]. The mechanism of action is similar to that of early postoperative feeding [126]. Research has shown that chewing gum increases the levels of ghrelin via the act of chewing or the production of gastrin [81]. Furthermore, engaging in oral stimulation and chewing may activate the vagus nerve, which plays a role in facilitating peristalsis [69]. The efficacy of chewing gum in avoiding postoperative paralytic ileus is still inconclusive due to contradictory findings. For instance, a research shown that chewing gum is linked to the prompt restoration of gastrointestinal function after gynecological cancer surgery. It may serve as an efficient and safe measure to avoid postoperative ileus [127]. In contrast, a separate research indicates that gum chewing does not have a correlation with accelerated gastrointestinal healing after surgery in children [128]. This discrepancy may arise from variations in outcomes attributable to diverse research cohorts. The objective of this research was to examine the overall efficacy of various preventative interventions in all abdominal operations. Postoperative gum chewing was placed in the middle of the range of interventions in our network analysis, lowering the incidence of bowel obstruction prevention and the time to the first bowel movement and flatus, but it had no impact on the time to return to a regular diet. Furthermore, despite the NCG ranking second, there was no distinction between the NCG and control groups. Chewing gum and electroacupuncture ranked in the top five in the prevention of postoperative intestinal blockage, first feces time, and time to resume a regular diet, suggesting that this combination may be a useful and affordable preventative strategy.
While there are numerous common meta-analyses, there are few network meta-analyses on the prevention of postoperative intestinal blockages. Our analysis revealed that early diet consistently ranked in the middle of the four outcome indicators. A previous network meta-analysis revealed that early diet and epidural anesthesia were the most effective interventions for the prevention of postoperative ileus following colorectal surgery [129]. However, the findings of the pairwise comparison revealed that there was no difference in the other three indicators, and that only the time it took to resume a regular diet was superior to that of the control group. Postoperative coffee consumption following colorectal and gynecological surgery can lower the POI and LOS [130], according to a meta-analysis. In this study's analysis, coffee ranked second in preventing postoperative ileus and outperformed EEN, SPBS, DP, THR + ACU, control, ACU, and EPN. However, it did not promote exhaustion or feces, although it may accelerate the iniatiation of a regular diet. Improved recovery programs have evolved into industrial standards of care in several surgical specialties. According to a meta-analysis of gynecological transabdominal surgery, improved recovery successfully reduced the length of hospital stay without increasing the risk of ileus [131]. Our findings demonstrate that AR was superior to other therapies in shortening the interval before normal eating, although the difference was not statistically significant.
5. Limitations
All the RCTS included in this study had high evidence credibility, but there were also some limitations. The included procedures included both laparoscopic and open procedures, and studies have shown that laparoscopic procedures are associated with a reduced risk of postoperative ileus during and after gastric surgery [132]. The following research might individually examine endoscopic surgery and open surgery to ascertain the benefits and drawbacks of various treatments performed via endoscopic or open surgical procedures. Second, differences in the doses of some drugs within the same class were ignored, which may have biased the results, investigating the dose-correlation of various medicines in preventing postoperative ileus is another promising area of inquiry. In addition, to reduce heterogeneity, we excluded studies with a sample size of less than 30, which may have prevented the interventions we included from creating more closed loops that could allow direct comparisons and reduce confidence.
6. Conclusion
Adhesions are a frequent complication after abdominal surgery. However, how to avoid it is debatable. According to the findings of this network meta-analysis, alvimopan and non-pharmacological electroacupuncture combined with chewing gum may be the most effective strategies for enhancing bowel function recovery and minimizing the incidence of intestinal blockage following abdominal surgery. The recommended dose for alvimopan is one 12 mg capsule taken 30 min to 5 h before surgery, and another 12 mg capsule taken twice daily from the first postoperative day until discharge, for a maximum of 7 days. Therapeutic doses of alvimopan should not be used for more than 7 days prior to starting opioids. Electroacupuncture was applied to Neiguan (PC6), the currently recognized standard acupoint for the prevention of post operative nausea and vomiting (PONV); however, the optimal time of acupoint stimulation intervention is controversial in the academic community, and there is no unified standard. Chewing gum 2 h after the surgery, one or two pieces of sugar-free gum were chewed every 2 h for 15 min each time, with a 2-h interval between the chewing, until anal exhaust. Further, the chewing of the gum was stopped at night. These two interventions, as identified by the network meta-analysis, have their own advantages and disadvantages. However, the literature support for these interventions is limited, and further high-quality RCTs are needed to confirm our results.
Funding statement
This study received funding from the National Natural Science Foundation of China (81860850) , Gansu Education Department (2023A-076), and Lanzhou City Science and Technology Bureau (2020-XG-26)
Additional information
No additional information is available for this paper.
Data availability statement
The data that support the findings of this study are available from the corresponding author, [HBP], upon reasonable request.
Ethics declarations
Review and/or approval by an ethics committee was not needed for this study because [This work is a review of the literature and does not address the ethical considerations of animal, cell, and human experimentation.].
CRediT authorship contribution statement
Yan Cui: Writing – original draft, Writing – review & editing. Mei Liu: Data curation. Hui Zhang: Data curation, Formal analysis. Xuan Zhang: Formal analysis, Methodology. Yuan Tang: Methodology, Software. Zhi Hang Wu: Visualization. Ri Cheng Li: Conceptualization. Quan Xin Chen: Writing – review & editing. Ying Meng: Conceptualization, Formal analysis. Bo Wang: Project administration, Supervision. Hai Bang Pan: Funding acquisition, Project administration, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Haibang Pan reports article publishing charges was provided by Haibang.
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e25412.
Contributor Information
Chengzu Zhang, Email: zczgszy@163.com.
Tianming Wang, Email: wtm20202100062@gmail.com.
Bo Wang, Email: wbbphb@gszy.edu.cn.
Jianfeng Yi, Email: yijf02@163.com.
Yuhong Shi, Email: shiyuhong801019@163.com.
Haibang Pan, Email: phbwbb@126.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Venara A., Neunlist M., Slim K., et al. Postoperative ileus: pathophysiology, incidence, and prevention. Journal of visceral surgery. 2016;153(6):439–446. doi: 10.1016/j.jviscsurg.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Stakenborg N., Gomez-Pinilla P.J., Boeckxstaens G.E. Postoperative ileus: pathophysiology, current therapeutic approaches. Handb. Exp. Pharmacol. 2017;239:39–57. doi: 10.1007/164_2016_108. [DOI] [PubMed] [Google Scholar]
- 3.Sammut R., Trapani J., Deguara J., Ravasi V. The effect of gum chewing on postoperative ileus in open colorectal surgery patients: a review. J. Perioperat. Pract. 2021;31(4):132–139. doi: 10.1177/1750458920917015. [DOI] [PubMed] [Google Scholar]
- 4.Hasler-Gehrer S., Linecker M., Keerl A., et al. Does coffee intake reduce postoperative ileus after laparoscopic elective colorectal surgery? A prospective, randomized controlled study: the coffee study. Dis. Colon Rectum. 2019;62(8) doi: 10.1097/DCR.0000000000001405. [DOI] [PubMed] [Google Scholar]
- 5.Bragg D., El-Sharkawy A.M., Psaltis E., Maxwell-Armstrong C.A., Lobo D.N. Postoperative ileus: recent developments in pathophysiology and management. Clin. Nutr. 2015;34(3):367–376. doi: 10.1016/j.clnu.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Si L., Kexuan L., Xiaoming D., et al. Expert consensus on prevention and treatment of postoperative gastrointestinal dysfunction. International Journal of Anesthesiology and Resuscitation. 2021;(11):1133–1142. [Google Scholar]
- 7.Behm B., Stollman N. Postoperative ileus: etiologies and interventions. Clin. Gastroenterol. Hepatol. : the official clinical practice journal of the American Gastroenterological Association. 2003;1(2):71–80. doi: 10.1053/cgh.2003.50012. [DOI] [PubMed] [Google Scholar]
- 8.Buscail E., Deraison C. Postoperative ileus: a pharmacological perspective. Br. J. Pharmacol. 2022;179(13):3283–3305. doi: 10.1111/bph.15800. [DOI] [PubMed] [Google Scholar]
- 9.Drake T.M., Ward A.E. Pharmacological management to prevent ileus in major abdominal surgery: a systematic review and meta-analysis. J. Gastrointest. Surg. : official journal of the Society for Surgery of the Alimentary Tract. 2016;20(6):1253–1264. doi: 10.1007/s11605-016-3140-0. [DOI] [PubMed] [Google Scholar]
- 10.Pan Q., Xianli R. Application of chewing gum in the recovery of gastrointestinal function in gynecological patients after abdominal operation. Mod. Nurs. 2006;12(17):1624–1625. [Google Scholar]
- 11.Han-Geurts I.J., Hop W.C., Kok N.F., Lim A., Brouwer K.J., Jeekel J. Randomized clinical trial of the impact of early enteral feeding on postoperative ileus and recovery. Br. J. Surg. May 2007;94(5):555–561. doi: 10.1002/bjs.5753. [DOI] [PubMed] [Google Scholar]
- 12.Jianqiao S. Clinical observation of the recovery of gastrointestinal function after abdominal surgery. HEBEI JOURNAL OF TRADITIONAL CHINESE MEDICINE. 2009;31(2) 288-288,292. [Google Scholar]
- 13.Zifeng Z., Feng Y., Jianjiang L. Masticatory exercise promotes gastrointestinal motility after radical resection of rectal cance. Chin. J. Gen. Surg. 2009;12(6):632–633. [Google Scholar]
- 14.Meng Z.Q., Garcia M.K., Chiang J.S., et al. Electro-acupuncture to prevent prolonged postoperative ileus: a randomized clinical trial. World J. Gastroenterol. Jan 7 2010;16(1):104–111. doi: 10.3748/wjg.v16.i1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shang H., Yang Y., Tong X., Zhang L., Fang A., Hong L. Gum chewing slightly enhances early recovery from postoperative ileus after cesarean section: results of a prospective, randomized, controlled trial. Am. J. Perinatol. May 2010;27(5):387–391. doi: 10.1055/s-0029-1243313. [DOI] [PubMed] [Google Scholar]
- 16.Wei C., Liang Y. To investigate the effect of chewing gum on gastrointestinal function recovery after abdominal surgery. Shaanxi Medical Journal. 2011;40(10):1381–1382. [Google Scholar]
- 17.Zhi H., Hongli Z., Chunrong L., Qiong J. A randomized controlled study on the effect of chewing gum on the recovery of gastrointestinal function in patients with uterine fibroids after operation. CHINA MEDICAL HERALD. 2011;8(28):37–39. [Google Scholar]
- 18.Shaoyuan W., Yingkui H., Shiping D., Bieying L., Kai Z. A randomized controlled trial of chewing gum to promote gastrointestinal rehabilitation after rectal cancer surgery. Sichuan Medical Journal. 2011;32(12):1956–1958. [Google Scholar]
- 19.Jinlin Z., Zhendong L., Qiwen Y. Effect of Dachengqi decoction combined with acupuncture on intestinal function recovery after abdominal surgery. CHINESE JOURNAL OF INTEGRATED TRADITIONAL AND WESTERN MEDICINE IN INTENSIVE AND CRITICAL CARE. 2011;18(3):152–154. [Google Scholar]
- 20.Marwah S., Singla S., Tinna P. Role of gum chewing on the duration of postoperative ileus following ileostomy closure done for typhoid ileal perforation: a prospective randomized trial. Saudi J. Gastroenterol. : official journal of the Saudi Gastroenterology Association. Mar-Apr 2012;18(2):111–117. doi: 10.4103/1319-3767.93812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Müller S.A., Rahbari N.N., Schneider F., et al. Randomized clinical trial on the effect of coffee on postoperative ileus following elective colectomy. Br. J. Sch. Nurs. 2012;99(11):1530–1538. doi: 10.1002/bjs.8885. [DOI] [PubMed] [Google Scholar]
- 22.Xiang C., Danli S. Effects of chewing gum on recovery of gastrointestinal function after gastrectomy. Medical Journal of West China. 2012;24(10):2003–2004. [Google Scholar]
- 23.Deng G., Wong W.D., Guillem J., et al. A phase II, randomized, controlled trial of acupuncture for reduction of Postcolectomy Ileus. Ann. Surg Oncol. Apr 2013;20(4):1164–1169. doi: 10.1245/s10434-012-2759-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ertas I.E., Gungorduk K., Ozdemir A., Solmaz U., Dogan A., Yildirim Y. Influence of gum chewing on postoperative bowel activity after complete staging surgery for gynecological malignancies: a randomized controlled trial. Gynecol. Oncol. Oct 2013;131(1):118–122. doi: 10.1016/j.ygyno.2013.07.098. [DOI] [PubMed] [Google Scholar]
- 25.Jakkaew B., Charoenkwan K. Effects of gum chewing on recovery of bowel function following cesarean section: a randomized controlled trial. Arch. Gynecol. Obstet. Aug 2013;288(2):255–260. doi: 10.1007/s00404-013-2727-x. [DOI] [PubMed] [Google Scholar]
- 26.Ng S.S., Leung W.W., Hon S.S., Li J.C., Wong C.Y., Lee J.F. Electroacupuncture for ileus after laparoscopic colorectal surgery: a randomised sham-controlled study. Hong Kong medical journal = Xianggang yi xue za zhi. Dec 2013;19(Suppl 9):33–35. [PubMed] [Google Scholar]
- 27.Ng S.S.M., Leung W.W., Mak T.W.C., et al. Electroacupuncture reduces duration of postoperative ileus after laparoscopic surgery for colorectal cancer. Gastroenterology. Feb 2013;144(2):307–313. doi: 10.1053/j.gastro.2012.10.050. e301. [DOI] [PubMed] [Google Scholar]
- 28.Zhiyuan S., Xinxiu G. Effects of chewing gum on recovery of gastrointestinal function after open surgery. Chinese Journal of Clinical Research. 2013;26(3):249–250. [Google Scholar]
- 29.Xidi W., Wanxia W. To evaluate the effect of chewing gum on the recovery of gastrointestinal function after transabdominal radical resection of cervical cancer. THE PRACTICAL JOURNAL OF CANCER. 2013;28(6) 770-770,774. [Google Scholar]
- 30.Boelens P.G., Heesakkers F.F., Luyer M.D., et al. Reduction of postoperative ileus by early enteral nutrition in patients undergoing major rectal surgery: prospective, randomized, controlled trial. Ann. Surg. Apr 2014;259(4):649–655. doi: 10.1097/SLA.0000000000000288. [DOI] [PubMed] [Google Scholar]
- 31.Shuhua H. Effect of Shenghua decoction combined with acupuncture on urinary and digestive system after cesarean section. Journal of Sichuan Traditional Chinese Medicine. 2014;32(7):86–89. [Google Scholar]
- 32.Ziqaing H. Clinical study of gastrointestinal function recovery after abdominal surgery. Guide of China Medicine. 2014;(35):238–239. [Google Scholar]
- 33.Ajuzieogu O.V., Amucheazi A., Ezike H.A., Achi J., Abam D.S. The efficacy of chewing gum on postoperative ileus following cesarean section in Enugu, South East Nigeria: a randomized controlled clinical trial. Nigerian journal of clinical practice. Nov-Dec 2014;17(6):739–742. doi: 10.4103/1119-3077.144388. [DOI] [PubMed] [Google Scholar]
- 34.Jernigan A.M., Chen C.C., Sewell C. A randomized trial of chewing gum to prevent postoperative ileus after laparotomy for benign gynecologic surgery. Int. J. Gynaecol. Obstet.: the official organ of the International Federation of Gynaecology and Obstetrics. Dec 2014;127(3):279–282. doi: 10.1016/j.ijgo.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Wei L., Nian L., Shitai Z., Bo L., Ying B., Yangling O. To observe the effect of chewing gum on gastrointestinal function recovery after transabdominal surgery for benign gynecological diseases. J. Clin. Res. 2015;(11):2161–2163. 2164. [Google Scholar]
- 36.Guankui H., Yuanping S., Qifang L., Xueying X. Effect of chewing gum on intestinal function recovery after gynecological malignant tumor surgery. Jilin Medicine. 2016;37(8):2057–2058. [Google Scholar]
- 37.Wei L., Nian L., Shitai Z., Zhong Y., Bo L., Yangling O. To investigate the effect of chewing gum on the recovery of gastrointestinal function after transabdominal surgery for gynecological malignant tumors. Chin. J. Cancer Prev. Treat. 2016;23(3):198–202. [Google Scholar]
- 38.Xingyu L., Zhengjun F., Xiawan W. Effects of chewing gum on recovery of gastrointestinal function in patients after splenectomy and devascularization. World Chin. J. Dig. 2016;24(29):4110–4114. [Google Scholar]
- 39.Chunjun S., Lina H., Zhitao Z. Application experience of Shenghua decoction combined with acupuncture after cesarean section. Asia-Pacific Traditional Medicine. 2016;12(1):113–114. [Google Scholar]
- 40.Lee H., Cho C.W., Yoon S., Suh K.S., Ryu H.G. Effect of sham feeding with gum chewing on postoperative ileus after liver transplantation-a randomized controlled trial. Clin. Transplant. Nov 2016;30(11):1501–1507. doi: 10.1111/ctr.12849. [DOI] [PubMed] [Google Scholar]
- 41.Dulskas A., Klimovskij M., Vitkauskiene M., Samalavicius N.E. Effect of coffee on the length of postoperative ileus after elective laparoscopic left-sided colectomy: a randomized, prospective single-center study. Diseases of the colon and rectum. Nov 2015;58(11):1064–1069. doi: 10.1097/DCR.0000000000000449. [DOI] [PubMed] [Google Scholar]
- 42.Gong Y., Zhang Q., Qiao L., et al. Xylitol gum chewing to achieve early postoperative restoration of bowel motility after laparoscopic surgery. Surgical laparoscopy, endoscopy & percutaneous techniques. Aug 2015;25(4):303–306. doi: 10.1097/SLE.0000000000000174. [DOI] [PubMed] [Google Scholar]
- 43.You X.M., Mo X.S., Ma L., et al. Randomized clinical trial comparing efficacy of simo decoction and acupuncture or chewing gum alone on postoperative ileus in patients with hepatocellular carcinoma after hepatectomy. Medicine. Nov 2015;94(45) doi: 10.1097/MD.0000000000001968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Atkinson C., Penfold C.M., Ness A.R., et al. Randomized clinical trial of postoperative chewing gum versus standard care after colorectal resection. Br. J. Surg. Jul 2016;103(8):962–970. doi: 10.1002/bjs.10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shum N.F., Choi H.K., Mak J.C., Foo D.C., Li W.C., Law W.L. Randomized clinical trial of chewing gum after laparoscopic colorectal resection. Br. J. Surg. Oct 2016;103(11):1447–1452. doi: 10.1002/bjs.10277. [DOI] [PubMed] [Google Scholar]
- 46.Topcu S.Y., Oztekin S.D. Effect of gum chewing on reducing postoperative ileus and recovery after colorectal surgery: a randomised controlled trial. Compl. Ther. Clin. Pract. May 2016;23:21–25. doi: 10.1016/j.ctcp.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 47.Vergara-Fernandez O., Gonzalez-Vargas A.P., Castellanos-Juarez J.C., Salgado-Nesme N., Sanchez-Garcia Ramos E. Usefulness of gum chewing to decrease postoperative ileus in colorectal surgery with primary anastomosis: a randomized controlled trial. Revista de investigacion clinica; organo del Hospital de Enfermedades de la Nutricion. Nov-Dec 2016;68(6):314–318. [PubMed] [Google Scholar]
- 48.Abadi F., Shahabinejad M., Abadi F., Kazemi M. Effect of acupressure on symptoms of postoperative ileus after cesarean section. Journal of acupuncture and meridian studies. Apr 2017;10(2):114–119. doi: 10.1016/j.jams.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 49.Güngördük K., Özdemir İ., Güngördük Ö., Gülseren V., Gokçü M., Sancı M. Effects of coffee consumption on gut recovery after surgery of gynecological cancer patients: a randomized controlled trial. Am. J. Obstet. Gynecol. 2017;216(2) doi: 10.1016/j.ajog.2016.10.019. 145.e141‐145.e147. [DOI] [PubMed] [Google Scholar]
- 50.Yang Y., Zuo H.Q., Li Z., et al. Comparison of efficacy of simo decoction and acupuncture or chewing gum alone on postoperative ileus in colorectal cancer resection: a randomized trial. Sci. Rep. Jan 19 2017;7 doi: 10.1038/srep37826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suhua L., Xiangjun C. Effects of chewing gum on intestinal function recovery after cesarean section. Psychol. Doc. 2017;23(25):337–338. [Google Scholar]
- 52.Ahmed M.R., Sayed Ahmed W.A., Khamess R.E., Youwakim M.S., El-Nahas K.M. Efficacy of three different regimens in recovery of bowel function following elective cesarean section: a randomized trial. J. Perinat. Med. Sep 25 2018;46(7):786–790. doi: 10.1515/jpm-2017-0389. [DOI] [PubMed] [Google Scholar]
- 53.de Leede E.M., van Leersum N.J., Kroon H.M., van Weel V., van der Sijp J.R.M., Bonsing B.A. Multicentre randomized clinical trial of the effect of chewing gum after abdominal surgery. Br. J. Surg. Jun 2018;105(7):820–828. doi: 10.1002/bjs.10828. [DOI] [PubMed] [Google Scholar]
- 54.Esfehani R.J., Yazd M.M., Esfehani J., Foji S., Kamalimanesh B. Effect of chewing gum on post cesarean ileus in the north East of Iran: a randomized clinical trial. Journal of midwifery & reproductive health. 2018;6(3):1348–1355. [Google Scholar]
- 55.Pattamatta M., Smeets B.J.J., Evers S., Rutten H.J.T., Luyer M.D.P., Hiligsmann M. Health-related quality of life and cost-effectiveness analysis of gum chewing in patients undergoing colorectal surgery: results of a randomized controlled trial. Acta Chir. Belg. Oct 2018;118(5):299–306. doi: 10.1080/00015458.2018.1432742. [DOI] [PubMed] [Google Scholar]
- 56.Rabiepoor S., Yas A., Navaei J., Khalkhali H.R. Does coffee affect the bowel function after caesarean section? Eur. J. Obstet. Gynecol. Reprod. Biol. Jan 2018;220:96–99. doi: 10.1016/j.ejogrb.2017.07.028. [DOI] [PubMed] [Google Scholar]
- 57.Yang P., Long W.J., Wei L. Chewing xylitol gum could accelerate bowel motility recovery after elective open proctectomy for rectal cancer. Revista de investigacion clinica; organo del Hospital de Enfermedades de la Nutricion. 2018;70(1):53–58. doi: 10.24875/RIC.18002428. [DOI] [PubMed] [Google Scholar]
- 58.Zhang D., Lijun L., Jingfeng Y. Effects of chewing gum on recovery of gastrointestinal function after colorectal surgery. Chin. J. Prev. Med. 2018;45(11):91–93. [Google Scholar]
- 59.Hasler-Gehrer S., Linecker M., Keerl A., et al. Does coffee intake reduce postoperative ileus after laparoscopic elective colorectal surgery? A prospective, randomized controlled study: the coffee study. Diseases of the colon and rectum. Aug 2019;62(8):997–1004. doi: 10.1097/DCR.0000000000001405. [DOI] [PubMed] [Google Scholar]
- 60.Huisi L., Xiaochan L., Yaoquan H. Clinical study on the effect of acupuncture and moxibustion on gastrointestinal function after gynecological benign tumor surgery. Journal of Minimally Invasive Medicine. 2019;14(3):290–292. [Google Scholar]
- 61.Qiaoling W., Yonghui P., Hongqun Z., Xianwei M., Riying W., Xia L. To investigate the effect of transcutaneous electrical acupoint stimulation (TEAS) combined with chewing gum on gastrointestinal function in postoperative patients with colorectal cancer. Chinese Journal of Modern Nursing. 2019;25(21):2746–2749. [Google Scholar]
- 62.Bozkurt Koseoglu S., Korkmaz Toker M., Gokbel I., Celikkol O., Gungorduk K. Can coffee consumption be used to accelerate the recovery of bowel function after cesarean section? Randomized prospective trial. Ginekol. Pol. 2020;91(2):85–90. doi: 10.5603/GP.2020.0014. [DOI] [PubMed] [Google Scholar]
- 63.Gungorduk K., Paskal E.K., Demirayak G., Köseoğlu S.B., Akbaba E., Ozdemir I.A. Coffee consumption for recovery of intestinal function after laparoscopic gynecological surgery: a randomized controlled trial. Int. J. Surg. Oct 2020;82:130–135. doi: 10.1016/j.ijsu.2020.08.016. [DOI] [PubMed] [Google Scholar]
- 64.Yuan H.C., Xiang Q., Zhang N., Qin W.J., Cai W. Acupuncture combined with early enteral nutrition on patients with postoperative laparoscopic common bile duct exploration: a prospective randomized trial. Chin. J. Integr. Med. Oct 2020;26(10):769–775. doi: 10.1007/s11655-019-3048-0. [DOI] [PubMed] [Google Scholar]
- 65.Mei W., Yonghui C., Wenjun Z. Effects of chewing gum combined with acupoint massage on gastrointestinal function recovery after abdominal surgery. Journal of Anhui Health Vocational & Technical College. 2020;19(5):135–137. [Google Scholar]
- 66.Bhatti S., Malik Y.J., Changazi S.H., et al. Role of chewing gum in reducing postoperative ileus after reversal of ileostomy: a randomized controlled trial. World J. Surg. Apr 2021;45(4):1066–1070. doi: 10.1007/s00268-020-05897-1. [DOI] [PubMed] [Google Scholar]
- 67.Gao W., Li W., Yan Y., et al. Transcutaneous electrical acupoint stimulation applied in lower limbs decreases the incidence of paralytic ileus after colorectal surgery: a multicenter randomized controlled trial. Surgery. Dec 2021;170(6):1618–1626. doi: 10.1016/j.surg.2021.08.007. [DOI] [PubMed] [Google Scholar]
- 68.Jinping Z., Youzhen Y., Ying L., Ning C. Effect of coffee on postoperative ileus after laparoscopic colorectal cancer surgery. Contemporary nurses. 2021:28. [Google Scholar]
- 69.Hsu Y.C., Szu S.Y. Effects of gum chewing on recovery from postoperative ileus: a randomized clinical trail. J. Nurs. Res. : J. Nurs. Res. Oct 1 2022;30(5):e233. doi: 10.1097/jnr.0000000000000510. [DOI] [PubMed] [Google Scholar]
- 70.Hogan S., Reece L., Solomon M., Rangan A., Carey S. Early enteral feeding is beneficial for patients after pelvic exenteration surgery: a randomized controlled trial. JPEN - J. Parenter. Enter. Nutr. Feb 2022;46(2):411–421. doi: 10.1002/jpen.2120. [DOI] [PubMed] [Google Scholar]
- 71.Akamaru Y., Takahashi T., Nishida T., et al. Effects of daikenchuto, a Japanese herb, on intestinal motility after total gastrectomy: a prospective randomized trial. J. Gastrointest. Surg. : official journal of the Society for Surgery of the Alimentary Tract. Mar 2015;19(3):467–472. doi: 10.1007/s11605-014-2730-y. [DOI] [PubMed] [Google Scholar]
- 72.Beaussier M., Weickmans H., Parc Y., et al. Postoperative analgesia and recovery course after major colorectal surgery in elderly patients: a randomized comparison between intrathecal morphine and intravenous PCA morphine. Reg. Anesth. Pain Med. Nov-Dec 2006;31(6):531–538. doi: 10.1016/j.rapm.2006.06.250. [DOI] [PubMed] [Google Scholar]
- 73.Chantarasorn V., Tannirandorn Y. A comparative study of early postoperative feeding versus conventional feeding for patients undergoing cesarean section; a randomized controlled trial. Journal of the Medical Association of Thailand = Chotmaihet thangphaet. Oct 2006;89(Suppl 4) S11-16. [PubMed] [Google Scholar]
- 74.Charoenkwan K., Palapinyo C. Early solid food after cesarean section and postoperative ileus. Int. J. Gynaecol. Obstet.: the official organ of the International Federation of Gynaecology and Obstetrics. Aug 2005;90(2):144–145. doi: 10.1016/j.ijgo.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 75.Cheape J.D., Wexner S.D., James K., Jagelman D.G. Does metoclopramide reduce the length of ileus after colorectal surgery? A prospective randomized trial. Dis. Colon Rectum. Jun 1991;34(6):437–441. doi: 10.1007/BF02049925. [DOI] [PubMed] [Google Scholar]
- 76.Chen J.Y., Wu G.J., Mok M.S., et al. Effect of adding ketorolac to intravenous morphine patient-controlled analgesia on bowel function in colorectal surgery patients--a prospective, randomized, double-blind study. Acta anaesthesiologica Scandinavica. Apr. 2005;49(4):546–551. doi: 10.1111/j.1399-6576.2005.00674.x. [DOI] [PubMed] [Google Scholar]
- 77.Gong J., Xie Z., Zhang T., et al. Randomised clinical trial: prucalopride, a colonic pro-motility agent, reduces the duration of post-operative ileus after elective gastrointestinal surgery. Aliment Pharmacol. Therapeut. Apr 2016;43(7):778–789. doi: 10.1111/apt.13557. [DOI] [PubMed] [Google Scholar]
- 78.Hayashi S., Takayama T., Masuda H., et al. Bioresorbable membrane to reduce postoperative small bowel obstruction in patients with gastric cancer: a randomized clinical trial. Ann. Surg. May 2008;247(5):766–770. doi: 10.1097/SLA.0b013e3181656d4e. [DOI] [PubMed] [Google Scholar]
- 79.Kang J.G., Kim M.H., Kim E.H., Lee S.H. Intraoperative intravenous lidocaine reduces hospital length of stay following open gastrectomy for stomach cancer in men. J. Clin. Anesth. Sep 2012;24(6):465–470. doi: 10.1016/j.jclinane.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 80.Kim S.G., Song K.Y., Lee H.H., et al. Efficacy of an antiadhesive agent for the prevention of intra-abdominal adhesions after radical gastrectomy: a prospective randomized, multicenter trial. Medicine. May 2019;98(19) doi: 10.1097/MD.0000000000015141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kobayashi T., Masaki T., Kogawa K., Matsuoka H., Sugiyama M. Efficacy of gum chewing on bowel movement after open colectomy for left-sided colorectal cancer: a randomized clinical trial. Dis. Colon Rectum. Nov 2015;58(11):1058–1063. doi: 10.1097/DCR.0000000000000452. [DOI] [PubMed] [Google Scholar]
- 82.Le Blanc-Louvry I., Costaglioli B., Boulon C., Leroi A.M., Ducrotte P. Does mechanical massage of the abdominal wall after colectomy reduce postoperative pain and shorten the duration of ileus? Results of a randomized study. J. Gastrointest. Surg. : official journal of the Society for Surgery of the Alimentary Tract. Jan-Feb 2002;6(1):43–49. doi: 10.1016/s1091-255x(01)00009-9. [DOI] [PubMed] [Google Scholar]
- 83.Lee S.M., Kang S.B., Jang J.H., et al. Early rehabilitation versus conventional care after laparoscopic rectal surgery: a prospective, randomized, controlled trial. Surg. Endosc. Oct 2013;27(10):3902–3909. doi: 10.1007/s00464-013-3006-4. [DOI] [PubMed] [Google Scholar]
- 84.López-Jaimez G., Cuello-García C.A. Use of chewing gum in children undergoing an appendectomy: a randomized clinical controlled trial. Int. J. Surg. Aug 2016;32:38–42. doi: 10.1016/j.ijsu.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 85.Lu Y., Fang P.P., Yu Y.Q., et al. Effect of intraoperative dexmedetomidine on recovery of gastrointestinal function after abdominal surgery in older adults: a randomized clinical trial. JAMA Netw. Open. Oct 1 2021;4(10) doi: 10.1001/jamanetworkopen.2021.28886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.MacMillan S.L., Kammerer-Doak D., Rogers R.G., Parker K.M. Early feeding and the incidence of gastrointestinal symptoms after major gynecologic surgery. Obstet. Gynecol. Oct 2000;96(4):604–608. doi: 10.1016/s0029-7844(00)00957-1. [DOI] [PubMed] [Google Scholar]
- 87.Okada K., Kawai M., Hirono S., et al. Evaluation of the efficacy of daikenchuto (TJ -100) for the prevention of paralytic ileus after pancreaticoduodenectomy: a multicenter, double-blind, randomized, placebo-controlled trial. Surgery. May 2016;159(5):1333–1341. doi: 10.1016/j.surg.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 88.Ortiz M.P., Godoy M.C., Schlosser R.S., et al. Effect of endovenous lidocaine on analgesia and serum cytokines: double-blinded and randomized trial. J. Clin. Anesth. Dec 2016;35:70–77. doi: 10.1016/j.jclinane.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 89.Patolia D.S., Hilliard R.L., Toy E.C., Baker B. Early feeding after cesarean: randomized trial. Obstet. Gynecol. Jul 2001;98(1):113–116. doi: 10.1016/s0029-7844(01)01387-4. [DOI] [PubMed] [Google Scholar]
- 90.Qiu J., Wang Y. Effect of accelerated rehabilitation combined with enteral nutrition on gastrointestinal function recovery after hepatectomy. Support. Care Cancer : official journal of the Multinational Association of Supportive Care in Cancer. Nov 2022;30(11):8927–8933. doi: 10.1007/s00520-022-07290-1. [DOI] [PubMed] [Google Scholar]
- 91.Rodríguez-Padilla Á, Morales-Martín G., Pérez-Quintero R., Gómez-Salgado J., Balongo-García R., Ruiz-Frutos C. Postoperative ileus after stimulation with Probiotics before ileostomy closure. Nutrients. Feb 15 2021;13(2) doi: 10.3390/nu13020626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Popescu I., Fleshner P.R., Pezzullo J.C., Charlton P.A., Kosutic G., Senagore A.J. The Ghrelin agonist TZP-101 for management of postoperative ileus after partial colectomy: a randomized, dose-ranging, placebo-controlled clinical trial. Dis. Colon Rectum. Feb 2010;53(2):126–134. doi: 10.1007/DCR.0b013e3181b54166. [DOI] [PubMed] [Google Scholar]
- 93.Swenson B.R., Gottschalk A., Wells L.T., et al. Intravenous lidocaine is as effective as epidural bupivacaine in reducing ileus duration, hospital stay, and pain after open colon resection: a randomized clinical trial. Reg. Anesth. Pain Med. Jul-Aug 2010;35(4):370–376. doi: 10.1097/AAP.0b013e3181e8d5da. [DOI] [PubMed] [Google Scholar]
- 94.Taguchi A., Sharma N., Saleem R.M., et al. Selective postoperative inhibition of gastrointestinal opioid receptors. N. Engl. J. Med. Sep 27 2001;345(13):935–940. doi: 10.1056/NEJMoa010564. [DOI] [PubMed] [Google Scholar]
- 95.Teoh W.H., Shah M.K., Mah C.L. A randomised controlled trial on beneficial effects of early feeding post-Caesarean delivery under regional anaesthesia. Singap. Med. J. Feb 2007;48(2):152–157. [PubMed] [Google Scholar]
- 96.Tollesson P.O., Cassuto J., Rimbäck G., Faxén A., Bergman L., Mattsson E. Treatment of postoperative paralytic ileus with cisapride. Scand. J. Gastroenterol. May 1991;26(5):477–482. doi: 10.3109/00365529108998569. [DOI] [PubMed] [Google Scholar]
- 97.van den Heijkant T.C., Costes L.M., van der Lee D.G., et al. Randomized clinical trial of the effect of gum chewing on postoperative ileus and inflammation in colorectal surgery. Br. J. Surg. Feb 2015;102(3):202–211. doi: 10.1002/bjs.9691. [DOI] [PubMed] [Google Scholar]
- 98.Yoshikawa K., Shimada M., Wakabayashi G., et al. Effect of daikenchuto, a traditional Japanese herbal medicine, after total gastrectomy for gastric cancer: a multicenter, randomized, double-blind, placebo-controlled, phase II trial. J. Am. Coll. Surg. Aug 2015;221(2):571–578. doi: 10.1016/j.jamcollsurg.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 99.Zingg U., Miskovic D., Pasternak I., Meyer P., Hamel C.T., Metzger U. Effect of bisacodyl on postoperative bowel motility in elective colorectal surgery: a prospective, randomized trial. Int. J. Colorectal Dis. Dec 2008;23(12):1175–1183. doi: 10.1007/s00384-008-0536-7. [DOI] [PubMed] [Google Scholar]
- 100.Agah J., Baghani R., Rakhshani M.H., Rad A. Metoclopramide role in preventing ileus after cesarean, a clinical trial. Eur. J. Clin. Pharmacol. Jun 2015;71(6):657–662. doi: 10.1007/s00228-015-1845-8. [DOI] [PubMed] [Google Scholar]
- 101.Aryaie A.H., Lalezari S., Sergent W.K., et al. Decreased opioid consumption and enhance recovery with the addition of IV Acetaminophen in colorectal patients: a prospective, multi-institutional, randomized, double-blinded, placebo-controlled study (DOCIVA study) Surg. Endosc. Aug 2018;32(8):3432–3438. doi: 10.1007/s00464-018-6062-y. [DOI] [PubMed] [Google Scholar]
- 102.Cho J.S., Kim H.I., Lee K.Y., et al. Effect of intraoperative dexmedetomidine infusion on postoperative bowel movements in patients undergoing laparoscopic gastrectomy: a prospective, randomized, placebo-controlled study. Medicine. Jun 2015;94(24):e959. doi: 10.1097/MD.0000000000000959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cho J.S., Kim H.I., Lee K.Y., et al. Comparison of the effects of patient-controlled epidural and intravenous analgesia on postoperative bowel function after laparoscopic gastrectomy: a prospective randomized study. Surg. Endosc. Nov 2017;31(11):4688–4696. doi: 10.1007/s00464-017-5537-6. [DOI] [PubMed] [Google Scholar]
- 104.Fazio V.W., Cohen Z., Fleshman J.W., et al. Reduction in adhesive small-bowel obstruction by Seprafilm adhesion barrier after intestinal resection. Dis. Colon Rectum. Jan 2006;49(1):1–11. doi: 10.1007/s10350-005-0268-5. [DOI] [PubMed] [Google Scholar]
- 105.Lambrichts D.P.V., Boersema G.S.A., Tas B., et al. Nicotine chewing gum for the prevention of postoperative ileus after colorectal surgery: a multicenter, double-blind, randomised, controlled pilot study. Int. J. Colorectal Dis. Sep 2017;32(9):1267–1275. doi: 10.1007/s00384-017-2839-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee W.K., Park Y.H., Choi S., Lee W.S. Is liquid-based hyaluronic acid equivalent to sodium hyaluronate-based bioresorbable membrane to reduce small bowel obstruction in patients undergoing colorectal surgery. Asian J. Surg. Feb 2019;42(2):443–449. doi: 10.1016/j.asjsur.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 107.Matros E., Rocha F., Zinner M., et al. Does gum chewing ameliorate postoperative ileus? Results of a prospective, randomized, placebo-controlled trial. J. Am. Coll. Surg. May 2006;202(5):773–778. doi: 10.1016/j.jamcollsurg.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 108.Nantasupha C., Ruengkhachorn I., Ruangvutilert P. Effect of conventional diet schedule, early feeding and early feeding plus domperidone on postcesarean diet tolerance: a randomized controlled trial. J. Obstet. Gynaecol. Res. May 2016;42(5):519–525. doi: 10.1111/jog.12942. [DOI] [PubMed] [Google Scholar]
- 109.Narita K., Tsunoda A., Takenaka K., Watanabe M., Nakao K., Kusano M. Effect of mosapride on recovery of intestinal motility after hand-assisted laparoscopic colectomy for carcinoma. Dis. Colon Rectum. Nov 2008;51(11):1692–1695. doi: 10.1007/s10350-008-9407-0. [DOI] [PubMed] [Google Scholar]
- 110.Ngowe M.N., Eyenga V.C., Kengne B.H., Bahebeck J., Sosso A.M. Chewing gum reduces postoperative ileus after open appendectomy. Acta Chir. Belg. Mar-Apr 2010;110(2):195–199. doi: 10.1080/00015458.2010.11680596. [DOI] [PubMed] [Google Scholar]
- 111.Perutelli A., Ferrandina G., Domenici L., et al. Modified intestinal isolation bag as promising tool in promoting bowel resumption after ovarian cancer cytoreductive surgery: a randomized clinical trial. Arch. Gynecol. Obstet. Sep 2021;304(3):733–742. doi: 10.1007/s00404-021-05981-4. [DOI] [PubMed] [Google Scholar]
- 112.Saito G., Sadahiro S., Ogimi T., et al. Preventive effects of a synthetic absorbable antiadhesive film (seprafilm) on small bowel obstruction in patients who underwent elective surgery for colon cancer: a randomized controlled trial. J. Surg. Oncol. Nov 2019;120(6):1038–1043. doi: 10.1002/jso.25664. [DOI] [PubMed] [Google Scholar]
- 113.Stewart B.T., Woods R.J., Collopy B.T., Fink R.J., Mackay J.R., Keck J.O. Early feeding after elective open colorectal resections: a prospective randomized trial. Aust. N. Z. J. Surg. Feb 1998;68(2):125–128. doi: 10.1111/j.1445-2197.1998.tb04721.x. [DOI] [PubMed] [Google Scholar]
- 114.Zingg U., Miskovic D., Hamel C.T., Erni L., Oertli D., Metzger U. Influence of thoracic epidural analgesia on postoperative pain relief and ileus after laparoscopic colorectal resection : benefit with epidural analgesia. Surg. Endosc. Feb 2009;23(2):276–282. doi: 10.1007/s00464-008-9888-x. [DOI] [PubMed] [Google Scholar]
- 115.Camilleri M., Lembo A., Katzka D.A. Opioids in gastroenterology: treating adverse effects and creating therapeutic benefits. Clin. Gastroenterol. Hepatol. : the official clinical practice journal of the American Gastroenterological Association. 2017;15(9):1338–1349. doi: 10.1016/j.cgh.2017.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chamie K., Golla V., Lenis A.T., Lec P.M., Rahman S., Viscusi E.R. Peripherally acting μ-opioid receptor antagonists in the management of postoperative ileus: a clinical review. J. Gastrointest. Surg. : official journal of the Society for Surgery of the Alimentary Tract. 2021;25(1):293–302. doi: 10.1007/s11605-020-04671-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Becker G., Blum H.E. Novel opioid antagonists for opioid-induced bowel dysfunction and postoperative ileus. Lancet (London, England) 2009;373(9670):1198–1206. doi: 10.1016/S0140-6736(09)60139-2. [DOI] [PubMed] [Google Scholar]
- 118.McKechnie T., Anpalagan T., Ichhpuniani S., Lee Y., Ramji K., Eskicioglu C. Selective opioid antagonists following bowel resection for prevention of postoperative ileus: a systematic review and meta-analysis. J. Gastrointest. Surg. : official journal of the Society for Surgery of the Alimentary Tract. 2021;25(6):1601–1624. doi: 10.1007/s11605-021-04973-8. [DOI] [PubMed] [Google Scholar]
- 119.Emile S.H., Horesh N., Garoufalia Z., Gefen R., Ray-Offor E., Wexner S.D. Strategies to reduce ileus after colorectal surgery: a qualitative umbrella review of the collective evidence. Surgery. 2023 doi: 10.1016/j.surg.2023.10.005. [DOI] [PubMed] [Google Scholar]
- 120.Zheng Y., Yang N.-N., Yang J.-W., et al. [Effect of electroacupuncture at "Zusanli" (ST 36) and its matching point at abdomen on intestinal motility in mice with postoperative ileus] Zhongguo zhen jiu = Chinese acupuncture & moxibustion. 2020;40(10):1097–1102. doi: 10.13703/j.0255-2930.20190904-k0003. [DOI] [PubMed] [Google Scholar]
- 121.Yang N.-N., Yang J.-W., Ye Y., et al. Electroacupuncture ameliorates intestinal inflammation by activating α7nAChR-mediated JAK2/STAT3 signaling pathway in postoperative ileus. Theranostics. 2021;11(9):4078–4089. doi: 10.7150/thno.52574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Okada M., Itoh K., Kitakoji H., Imai K. Mechanism of electroacupuncture on postoperative ileus induced by surgical stress in rats. Med. Acupunct. 2019;31(2):109–115. doi: 10.1089/acu.2018.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li J.-J., Zhao W.-S., Shao X.-M., Yang A.-M., Zhang F.-F., Fang J.-Q. [Effect of transcutaneous electrical acupoint stimulation on post-surgical gastrointestinal function, autonomic nerve activities and plasma brain-gut peptide levels in patients undergoing gastrointestinal surgery] Zhen ci yan jiu = Acupuncture research. 2016;41(3):240–246. [PubMed] [Google Scholar]
- 124.Murakami H., Li S., Foreman R., Yin J., Hirai T., Chen J.D.Z. Ameliorating effects of electroacupuncture on dysmotility, inflammation, and pain mediated via the autonomic mechanism in a rat model of postoperative ileus. J Neurogastroenterol Motil. 2019;25(2):286–299. doi: 10.5056/jnm18094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu Q., Jiang H., Xu D., Jin J. Effect of gum chewing on ameliorating ileus following colorectal surgery: a meta-analysis of 18 randomized controlled trials. Int. J. Surg. 2017;47:107–115. doi: 10.1016/j.ijsu.2017.07.107. [DOI] [PubMed] [Google Scholar]
- 126.van Leersum N.J., Bonsing B.A., Kroon H.M., van der Sijp J.R.M., van Weel V. [Chewing gum to prevent postoperative ileus] Ned. Tijdschr. Geneeskd. 2012;156(22):A4794. [PubMed] [Google Scholar]
- 127.Yin Y.-N., Xie H., Ren J.-H., Jiang N.-J., Dai L. The impact of gum-chewing on postoperative ileus following gynecological cancer surgery: a systematic review and meta-analysis of randomized controlled trials. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.1059924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fung A.C.-H., Tsang J.T.-W., Chung P.H.-Y., Kak-Yuen Wong K. Does chewing gum lead to earlier postoperative gastrointestinal recovery in children? A systematic review and meta-analysis. J. Pediatr. Surg. 2023 doi: 10.1016/j.jpedsurg.2023.10.020. [DOI] [PubMed] [Google Scholar]
- 129.Ashcroft J., Singh A.A., Ramachandran B., et al. Reducing ileus after colorectal surgery: a network meta-analysis of therapeutic interventions. Clin. Nutr. 2021;40(7):4772–4782. doi: 10.1016/j.clnu.2021.05.030. [DOI] [PubMed] [Google Scholar]
- 130.Watanabe J., Miki A., Koizumi M., Kotani K., Sata N. Effect of postoperative coffee consumption on postoperative ileus after abdominal surgery: an updated systematic review and meta-analysis. Nutrients. 2021;13(12) doi: 10.3390/nu13124394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.O'Neill A.M., Calpin G.G., Norris L., Beirne J.P. The impact of enhanced recovery after gynaecological surgery: a systematic review and meta-analysis. Gynecol. Oncol. 2023:168. doi: 10.1016/j.ygyno.2022.10.019. [DOI] [PubMed] [Google Scholar]
- 132.Liang W., Li J., Zhang W., et al. Prolonged postoperative ileus in gastric surgery: is there any difference between laparoscopic and open surgery? Cancer Med. 2019;8(12):5515–5523. doi: 10.1002/cam4.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, [HBP], upon reasonable request.