Abstract
Background and Aims
Since pregnancy is considered one of the major risk factors of cerebral venous thrombosis (CVT), the safety of pregnancy in women of childbearing age and a previous history of CVT, is concerning in terms of prevention, family planning, and management. This study aims to estimate the prevalence of pregnancies among women of childbearing age with previous CVT, evaluate the pregnancy‐associated risk of CVT recurrence, and explore the maternal and fetal outcomes among CVT women in comparison with pregnant women without a history of CVT.
Methods
A retrospective, case‐control study was conducted at the Obstetrics Departments of King Fahad Medical City Hospital, Saudi Arabia. It included all women with a history of CVT diagnosed in the last 5 years (cases), as well as CVT history‐free pregnant women (control). The prevalence of pregnancy after CVT was estimated and the prepartum and postpartum parameters of the two groups were compared.
Results
Fifty women with CVT and 100 controls were included. Among the 50 CVT cases, 28 (56.0%) have been pregnant. The incidence of pre‐eclampsia was significantly more frequent in CVT women (7.1% vs. 0.0%, p = 0.047); however, only one case of deep vein thrombosis (3.6%) was reported in CVT patients versus none in controls (p = 0.219). CVT women delivered at a lower gestational age (mean [SD] = 36.9 [3.5] weeks) compared with controls (38.3 [1.4] weeks) (p = 0.006). No significant differences in other pregnancy or delivery outcomes were observed between the two groups.
Conclusion
More than half of women of childbearing age with a history of CVT opt for pregnancy after the CVT episode, with no major additional risk for pre or postpartum complications.
Keywords: cerebral venous thrombosis, delivery, pregnancy, risk, safety
1. INTRODUCTION
Cerebral venous thrombosis (CVT) is a rare condition that represents a major cause of stroke in young and middle‐aged adults. 1 It has a yearly incidence as low as 0.5 per 100,000 individuals among the general population, which may vary in some specific populations. 2 , 3 A hospital‐based study estimated the incidence of CVT among adult patients at 1.32 per 100,000 person‐year, with a 2–3‐time higher incidence among females. 4 , 5 The pathophysiology of CVT consists of the thrombotic occlusion of the cerebral veins or sinuses, resulting in ischemic brain injury and parenchymal edema due to high venular pressure and impaired perfusion of the brain tissue. CVT may secondarily result in cerebral hemorrhage and reduced absorption of the cerebrospinal fluid. 2 , 6
Pregnancy is considered one of the major risk factors of CVT, besides other genetic and nongenetic conditions such as hereditary thrombophilia, chronic inflammatory diseases, trauma, and infections. A risk factor is identified in more than 8 out of 10 cases, and cohort studies show up to an 18‐ or 11‐fold increase in the risk of CVT in individuals having a nongenetic or genetic risk factor, respectively. 7 , 8 , 9 In pregnant women, the incidence risk of CVT increases to 12 cases per 100,000 deliveries 10 ; however, the risk of CVT is higher during the first months of the postpartum period. 11 Up to 60% of CVTs in women are associated with pregnancy or postpartum and CVT accounts for up to 57% of pregnancy‐related strokes. Consequently, the safety of pregnancy in women of childbearing age, who have a positive CVT history, constitutes an issue of big concern, both in terms of prevention and family planning, and management. 12 Thus, the study aimed to estimate the prevalence of pregnancies among women at childbearing age after CVT episodes and to evaluate the pregnancy‐associated risk of CVT recurrence. Additionally, we explored the maternal and fetal outcomes among CVT women in comparison with a concomitant series of pregnant women without a history of CVT.
2. METHODS
2.1. Study design and setting
A retrospective, case‐control study was conducted at the Obstetrics Departments of King Fahad Medical City Hospital (KFMC) in Riyadh, Kingdom of Saudi Arabia.
2.2. Population
The study population included two groups of participants. Group one (cases) included all women with a history of CVT diagnosed in the last 5 years, who were followed for pregnancy in the participating center. Group two (controls) included pregnant women with no history of CVT, who were followed in the participating center.
2.3. Inclusion/exclusion criteria
Only confirmed cases of CVT diagnosed by CT venogram or MR venogram were included in the study. All patients with missing hospital charts, those with only a clinical diagnosis of CVT that was not confirmed through radiology, patients with venous thromboembolism without CVT were excluded from the study. Figure 1 shows the flowchart of participant recruitment in the study.
Figure 1.
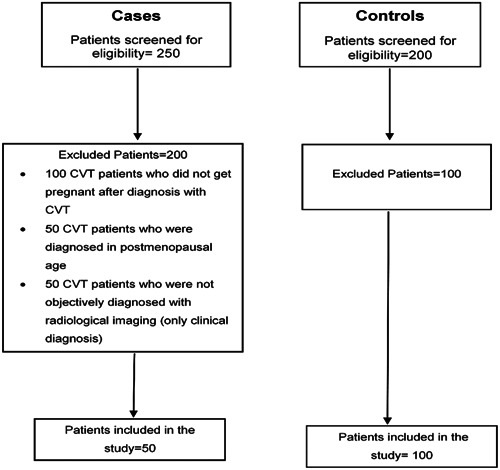
Flowchart of participant recruitment in the study.
2.4. Power analysis and sample size
The estimation of the sample size was determined using PASS ® software version 11.0.10 (1983‐2011.NCSS, LLC). By considering the 60% rate of CVTs in women were associated with pregnancy or postpartum and CVT. 13 Therefore, in a matched case‐control study, the probability of exposure among sampled control patients was 0.60 and the correlation coefficient for exposure between matched case and control patents was 0.1. A sample of 100 case patients was obtained. For each case patient, a matching sample of two control patient(s) was also obtained. This sample of 300 patients achieves 80% power to detect an odds ratio of 2.50 versus the alternative of equal odds using a chi‐square test with a 0.05 significance level.
In the cases group, a convenience sampling method was used to enroll all eligible women who were followed for pregnancy during the period January 2019 to December 2020. In the control group, a simple random sampling technique was used to select two controls for each case, concerning the previously specified inclusion period.
2.5. Data collection
A structured case report form was designed and comprised four parts. Part one included group identification (case or control group) in addition to specific and nonspecific personal and family history before CVT episode, such as deep vein thrombosis (DVT), hereditary thrombophilia, hypertension, ischemic heart disease. Part two included data related to CVT episodes including age at diagnosis, affected artery, associated thrombotic events, ICU admissions and adherence to anticoagulant treatment. Part 3 explored obstetrical history in previous pregnancies, such as gravidity, parity, number of living children, contraception use, previous pregnancy complications. Part 4 explored current pregnant parameters and delivery and maternal and fetal outcomes.
2.6. Ethical considerations
The study received approval from KFMC's institutional review board (IRB log number 20‐542). Since the study is retrospective, informed consent is not required.
2.7. Statistical analysis
Statistical analysis will be performed with the Statistical Package for Social Sciences version 21.0 for Windows (SPSS Inc.). Categorical variables will be presented as frequency and percentage, while continuous variables will be presented as mean ± standard deviation (SD) or median. Comparisons between CVT and control groups used independent t‐test or Mann–Whitney U test for continuous variables as appropriate, and chi‐square or Fisher's exact test for categorical variables, as appropriate. A p‐value of <0.05 will be considered to reject the null hypothesis.
3. RESULTS
3.1. Baseline characteristics of the two study groups
Personal and family medical history data for the two study groups are presented in Table 1, while obstetrical history data are presented in Table 2. Fifty women with CVT (cases) were included, of whom 2 (4.0%) had recurrent CVT; while 100 women with no history of CVT were included as controls. Comparative medical history in cases versus controls showed hereditary thrombophilia (14.3% vs. 0.0%, p < 0.001) and epilepsy (28.0% vs. 11.0%, p = 0.008), respectively, and no significant difference in other personal or family history parameters such as DVT (p = 1.000), hypertension (p = 0.664), or thyroid disease (p = 0.142). Regarding obstetrical history, CVT women had lower gravidity (44.0% vs. 1.0% were nulligravida, p < 0.001) and parity (38.0% vs. 14.0% were nulliparous, p = 0.006), compared with controls respectively. This was associated with higher use of contraception (38.0% vs. 2.0%, p < 0.001), notably oral contraceptives (34.0% vs. 2.0%, p < 0.001) among cases versus controls respectively.
Table 1.
Personal and family medical history in the two study groups.
| Parameter | Total (N = 150) | Cases (N = 50) | Controls (N = 100) | p‐Value | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Personal medical history | |||||||
| Previous cerebral venous thrombosis (CVT) episodes | 3 | 2.0 | 2 | 4.0 | 1 | 1.0 | 0.258 |
| DVT | 12 | 8.0 | 8 | 8.0 | 8 | 8.0 | 1.000 |
| Hereditary thrombophilia | 7 | 4.7 | 7 | 14.3 | 0 | 0.0 | <0.001* |
| Other thrombotic disorder | 140 | 6.7 | 1 | 2.0 | 9 | 9.0 | 0.166 |
| Hypertension | 6 | 4.0 | 1 | 2.0 | 5 | 5.0 | 0.664 |
| Ischemic heart disease | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | – |
| Diabetes | 5 | 3.4 | 0 | 0.0 | 5 | 5.1 | 0.169 |
| Thyroid disease | 22 | 14.7 | 4 | 8.0 | 18 | 18.0 | 0.142 |
| Sickle cell disease | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | – |
| Cancer | 17 | 11.3 | 2 | 4.0 | 15 | 15.0 | 0.056 |
| Epilepsy | 25 | 16.7 | 14 | 28.0 | 11 | 11.0 | 0.008* |
| Rheumatic disease | 10 | 6.7 | 1 | 2.0 | 9 | 9.0 | 0.166 |
| Myasthenia gravis | 4 | 2.7 | 0 | 0.0 | 4 | 4.0 | 0.102 |
| SLE | 13 | 8.7 | 4 | 8.0 | 9 | 9.0 | 1.000 |
| Nephrotic syndrome | 1 | 0.7 | 1 | 2.0 | 0 | 0.0 | 0.333 |
| Head Trauma | 2 | 1.4 | 1 | 2.0 | 1 | 1.0 | 1.000 |
| Other comorbidities | 43 | 28.7 | 12 | 24.0 | 31 | 31.0 | 0.371 |
| Smoking | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | – |
| Family history | |||||||
| CVT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | – |
| Thrombophilia | 2 | 1.3 | 2 | 4.0 | 0 | 0.0 | 0.110 |
Abbreviations: DVT, deep vein thrombosis; SLE, systemic lupus erythematosus.
Significant p‐value.
Table 2.
Obstetrical history in the two study groups.
| Parameter | Level | Total (N = 150) | Cases (N = 50) | Controls (N = 100) | p‐Value | |||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |||
| Gravidity | Null | 23 | 25.3 | 22 | 44.0 | 1 | 1.0 | |
| 1 | 20 | 13.3 | 2 | 4.0 | 18 | 18.0 | ||
| 2 | 34 | 22.7 | 7 | 14.0 | 27 | 27.0 | ||
| 3 | 20 | 13.3 | 7 | 14.0 | 13 | 13.0 | ||
| 4 | 13 | 8.7 | 1 | 2.0 | 12 | 12.0 | ||
| 5+ | 40 | 26.7 | 11 | 22.0 | 29 | 29.0 | <0.001* | |
| Parity | Nulliparous | 33 | 22.0 | 19 | 38.0 | 14 | 14.0 | |
| 1 | 41 | 27.3 | 9 | 18.0 | 32 | 32.0 | ||
| 2 | 22 | 14.7 | 8 | 16.0 | 14 | 14.0 | ||
| 3+ | 54 | 36.0 | 14 | 28.0 | 40 | 40.0 | 0.006* | |
| Complications in previous pregnancies | Yes | 17 | 11.3 | 5 | 10.0 | 12 | 12.0 | 0.792 |
| Abortions | None | 102 | 68.0 | 39 | 78.0 | 63 | 63.0 | |
| One | 29 | 19.3 | 7 | 14.0 | 22 | 22.0 | ||
| Multiple | 19 | 12.7 | 4 | 8.0 | 15 | 15.0 | 0.174 | |
| Pre‐eclampsia | Yes | 8 | 5.4 | 4 | 8.0 | 4 | 4.0 | 0.443 |
| Gestational diabetes | Yes | 6 | 4.0 | 0 | 0.0 | 6 | 6.0 | 0.179 |
| Thrombotic events during pregnancy | Yes | 11 | 7.3 | 5 | 10.0 | 6 | 6.0 | 0.507 |
| Children with congenital anomalies | 0 | 146 | 97.3 | 50 | 100.0 | 96 | 96.0 | |
| 1 | 3 | 2.0 | 0 | 0.0 | 3 | 3.0 | ||
| 2 | 1 | 0.7 | 0 | 0.0 | 1 | 1.0 | 0.358 | |
| Contraception | Yes | 21 | 14.0 | 19 | 38.0 | 2 | 2.0 | <0.001* |
| OCP | Yes | 19 | 12.8 | 17 | 34.0 | 2 | 2.0 | <0.001* |
Abbreviation: OCP, oral contraceptive pills.
Significant p‐value.
3.2. Data related to CVT
The mean (SD) age of women at the studied CVT episode was 29.44 (6.34) years. The majority (86.0%) had one affected artery, while seven (14.0%) had two affected arteries. The most frequently affected artery was the transverse sinus (26.0%), followed by the sigmoid sinus (12.0%) and the superior sagittal sinus (10.0%); while the artery was not documented in 18 (36.0%) of the patients' files. The CVT episode was associated with DVT and pulmonary embolism in 8.0% and 4.0% of the cases. Adherence to anticoagulants was inadequate in two (4.0%) patients (Table 3). Among the 50 CVT cases, 28 (56.0%) have been pregnant (95% confidence interval = 41.3%–70.0%), while 22 (44.0%) others have not been pregnant subsequent to the CVT episode.
Table 3.
Data related to cerebral venous thrombosis (N = 50).
| Parameter | Category | Mean | SD |
|---|---|---|---|
| Age at diagnosis | (years) | 29.44 | 6.34 |
| Parameter | Category | Frequency | Percentage |
| Number of affected arteries | 1 | 43 | 86.0 |
| 2 | 7 | 14.0 | |
| Primary artery affected | Transverse sinus | 13 | 26.0 |
| Sigmoid sinus | 6 | 12.0 | |
| Superior sagittal sinus | 5 | 10.0 | |
| Internal jugular veins | 1 | 2.0 | |
| Internal cerebral vein | 4 | 8.0 | |
| Cortical vein | 2 | 4.0 | |
| Cavernous sinus | 1 | 2.0 | |
| Not specified | 18 | 36.0 | |
| Secondary artery affected | Sigmoid sinus | 4 | 8.0 |
| Internal jugular veins | 1 | 2.0 | |
| Straight sinus | 1 | 2.0 | |
| Cavernous sinus | 1 | 2.0 | |
| Associated venous thrombosis | DVT | 4 | 8.0 |
| PE | 2 | 4.0 | |
| None | 43 | 86.0 | |
| ICU admissions | Yes | 3 | 6.0 |
| Adherence to anticoagulant therapy | Poor | 1 | 2.0 |
| Moderate | 1 | 2.0 | |
| Adequate | 48 | 96.0 |
Abbreviation: DVT, deep vein thrombosis.
3.3. Pregnancy and delivery outcomes
The incidence of pre‐eclampsia was significantly more frequent in CVT women (7.1% vs. 0.0%, p = 0.047); however, only one case of DVT (3.6%) was reported in CVT patients versus none in controls (p = 0.219). There was no significant difference in the delivery mode between cases and controls (p = 0.389). On the other hand, CVT women delivered at a lower gestational age (mean [SD] = 36.9 [3.5] weeks) compared with controls (38.3 [1.4] weeks) and the difference was statistically significant (p = 0.006). Consequently, the birthweights of babies born to CVT women were lower (mean [SD] = 2793.1 [7335] g) than controls (3048.1 [493.9]); however, the difference did not reach the statistical significance level (p = 0.060, independent t‐test). No noticeable differences in other pregnancy or delivery outcomes were observed between the two groups (Table 4). Of note, CVT recurrence during pregnancy was observed in one patient among the 28 CVT women who became pregnant (recurrence rate = 3.6%).
Table 4.
Pregnancy and delivery outcomes (N = 128).
| Parameter | Level | Cases (N = 28) | Controls (N = 100) | p‐Value | ||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Pregnancy outcomes | ||||||
| Infection | Yes | 0 | 0.0 | 0 | 0.0 | – |
| Gestational diabetes | Yes | 1 | 3.6 | 6 | 6.0 | 1.000 |
| Gestational hypertension | Yes | 1 | 3.6 | 0 | 0.0 | 0.219 |
| Pre‐eclampsia | Yes | 2 | 7.1 | 0 | 0.0 | 0.047* |
| Eclampsia | Yes | 0 | 0.0 | 0 | 0.0 | – |
| DVT | Yes | 1 | 3.6 | 0 | 0.0 | 0.219 |
| Prophylaxis anticoagulant | Yes | 25 | 89.3 | 21 | 21.0 | <0.001* |
| Delivery outcomes | ||||||
| Delivery mode | Spontaneous vaginal | 12 | 42.9 | 32 | 32.0 | |
| Induced vaginal | 3 | 10.7 | 4 | 4.0 | ||
| Scheduled cesarean | 4 | 14.3 | 28 | 28.0 | ||
| Unplanned cesarean | 4 | 14.3 | 11 | 11.0 | ||
| Abortion | 0 | 0.0 | 2 | 2.0 | ||
| Not delivered yet | 5 | 17.9 | 23 | 23.0 | 0.389 | |
| GA at delivery | (weeks) | 36.9 | 3.5 | 38.3 | 1.4 | 0.006* |
| Birthweight | (g) | 2793.1 | 733.5 | 3048.1 | 493.9 | 0.060 |
| Apgar at 1 min | (Score) | 8.3 | 1.2 | 8.1 | 1.4 | 0.637 |
| Apgar at 5 min | (Score) | 9.4 | 0.9 | 9.3 | 1.0 | 0.634 |
| IUFD | Yes | 0 | 0.0 | 1 | 1.3 | 1.000 |
| IUGR | Yes | 1 | 4.3 | 2 | 2.7 | 0.556 |
| Postpartum hospital stay | Days (mean, median) | 3.48 | 3 | 3.12 | 3 | 0.912 |
Abbreviations: DVT, deep vein thrombosis; IUFD, intrauterine fetal demise; IUGR, Intrauterine growth restriction.
Significant p‐Value.
4. DISCUSSION
4.1. Summary of findings
The present study showed that 56% of CVT women at childbearing age have attempted pregnancy subsequent to the CVT episode, which exposed them to a higher risk of pre‐eclampsia and earlier delivery with relatively lower birthweight, compared to pregnancies in non‐CVT women. CVT recurrence rate was as low as 3.6% with respect to the 28 concerned pregnancies, while no case of CVT was observed among controls. No further maternal or fetal risks were identified.
It is well established that pregnancy and puerperium considerably increase the incidence risk of VTEs, notably in women with a positive history of thromboembolic events, which is associated with a fourfold risk of recurrence during subsequent pregnancies. This risk is further increased in women having comorbid thrombophilia or a family history of VE. 14 Such observations have raised the particular issue of antepartum monitoring and postpartum management and follow‐up in the concerned women. In the present study, we observed a high prevalence of contraception use among women with CVT history, and a high percentage among these were on oral contraceptives, by comparison to the control group. Additionally, a high percentage of CVT women were null gravida or nulliparous. Unfortunately, no data was found in the files regarding the indication of the contraception or the cause of nulliparity. Although CVT does not constitute a contraindication for pregnancy, contraception using Astro‐progestogens is contra‐indicated, as it represents an additional risk factor for thrombosis. 15
The present study showed one case of CVT recurrence during pregnancy, representing an incidence rate of 3.6%. In the control group (women without CVT history), no case of CVT was observed. However, the data concerned only the prepartum and early postpartum periods, as no further follow‐up data were available in the patient's files. In a systematic review and meta‐analysis published in 2016, Aguiar de Sousa et al. estimated a 0.9% incidence of CVT recurrence among 217 assessed pregnancies. Additionally, the authors compared the incidence of CVT recurrence in pregnant women with CVT history with the incidence of CVT in the general population and found that the risk of recurrence was 80 times higher than the risk of occurrence in the general population. 16 This is to be compared with the threefold increase reported in females versus males, 4 , 5 the 18‐fold increase reported in individuals with nongenetic genetic risk factors, 7 , 8 , 9 and the 4‐fivefold increase reported in pregnant women. 10 Similarly, the occurrence of VTE in pregnant women with CVT history was assessed in the previously mentioned systematic review and was found to be 1.3%, which was 16‐fold higher than the incidence in the general population. 16 This could be considered consistent with the finding from the present study showing one case of DVT in the CVT group, while no case was observed in the controls.
Most remarkably, the present study evidenced a high risk of pre‐eclampsia among pregnant women with a CVT history. pre‐eclampsia occurred in two out of the 28 patients, representing an incidence of 7.1%, while no case of pre‐eclampsia was observed among the 100 non‐CVT controls. Both the concerned patients were gravida 2, had one living child, and had thrombophilia‐free, hypertension‐free and pre‐eclampsia‐free histories. One of the two patients had a history of DVT besides her CVT. By searching the literature, no causation relationship between CVT and pre‐eclampsia could be found. However, it was reported that CVT might mimic pre‐eclampsia, given the high clinical similarity associated with acute‐onset headache, altered mental status, visual impairment, and seizures. 17 Additionally, depending on the location of the thrombus, patients may present variable focal neurological symptoms, in addition to seizures, which remain very frequent and can be life‐threatening. 2 Thus, it is possible that the two patients in our series had recurrence of CVT, but were misdiagnosed pre‐eclampsia.
Another eventuality for the two cases is the coexistence of pre‐eclampsia masking the diagnosis of recurrent CVT. Such concomitant morbidity could be found in the literature. One case was described by Soydinc et al., 13 concerning a 21‐year‐old pregnant woman who was diagnosed with severe pre‐eclampsia during labor, and who presented convulsions during the postpartum period. As she was treated for pre‐eclampsia, she developed other neurological symptoms, leading to the diagnosis of CVT. The authors recognized that the two diagnoses overlapped, which probably lead to inappropriate management of CVT and patient death. Another case was reported by Ilkhchoui et al., 18 of a 26‐year‐old woman with a history of migraine, who had a cesarean section for pre‐eclampsia in her first pregnancy. She developed CVT 6 days after delivery, which was challenging to diagnose due to the clinical picture occulted by the pre‐eclampsia and the history of migraine. These two cases highlight the eventual coexistence of CVT with pre‐eclampsia, which should be prevented in pregnant women with CVT history and even more suspected and investigated in those among them who develop pre‐eclampsia.
In the current study, the mode of delivery was not found to be significantly different between the cases and controls. In contrast, a previous study by Sharma et al. reported cesarean delivery to be strongly and significantly related to CVT. 19 With regard to fetal outcome, in the present study, women with a history of CVT delivered at a significantly lower gestational age (mean [SD] = 36.9 [3.5] weeks) as compared to the control group (38.3 [1.4] weeks) (p = 0.006). Relevantly, Aguiar de Sousa et al. reported preterm birth among 11% women with prior CVT and given antithrombotic therapy during the first trimester. 12
The present study highlights important aspects related to the safety of pregnancy in women of childbearing age in Saudi Arabia and with a previous history of CVT including the relationship of CVT with pre‐eclampsia and lower gestional age. These findings may guide actions related to prevention, family planning, and management.
The authors recognize several limitations that impact the generalizability of the findings. The major limitation is the small sample size, notably pregnant women with CVT history, and this is due to the rarity of the condition and the high percentage of affected women who did not become pregnant during the study period. Additionally, important data were missing due to the retrospective design and the lack of some crucial information, notably regarding the postpartum follow‐up of CVT. Due to the case‐control study design, causality cannot be inferred. Finally, the single‐center design may induce further bias, such as the selectiveness of patients and institution‐related management strategies.
Further studies are warranted to study the association of CVT with pre‐eclampsia and eclampsia, especially in women with CVT or DVT history, or other risk factors of CVT. Due to the paucity of local data in Saudi Arabia, multicentric longitudinal studies should be carried out at the national level to explore the issue of CVT and pregnancy. Such data would enable establishing guidelines regarding family planning, contraceptive use, and pregnancy and delivery monitoring and management for this high‐risk population.
5. CONCLUSION
More than half of women of childbearing age with a history of CVT opt for pregnancy after the CVT episode, with no major additional risk for pre‐ or postpartum complications. Children born to such women are born at a relatively lower gestational age, probably due to the scheduled delivery. The pregnancy‐related risk of CVT recurrence was found to be low; however, CVT may be confused with other pregnancy complications, such as eclampsia. Therefore, pregnant women with CVT history should be systematically and scrupulously screened for recurrence of CVT of DVTs, both in the prepartum and postpartum periods, notably in case of complicated pregnancy and even in the absence of strongly evocative clinical symptomatology.
AUTHOR CONTRIBUTIONS
Amal A. AlSerehi: Conceptualization; data curation; investigation; methodology; writing—original draft; writing—review and editing. Basmah M. Al Mufarrih: Investigation; methodology; resources; writing—original draft; writing—review and editing. Amani Abu‐Shaheen: Conceptualization; methodology; writing—original draft; writing—review and editing. Ahmed Saleh: Data curation; formal analysis; methodology; writing—original draft; writing—review and editing. Mohammed AlSheef: Conceptualization; data curation; formal analysis; methodology; supervision; writing—original draft; writing—review and editing.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
TRANSPARENCY STATEMENT
The lead author Amani Abu‐Shaheen affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
ACKNOWLEDGMENTS
The authors would like to thank the Research Center at King Fahd Medical City, Riyadh Second Health Cluster, Riyadh, Saudi Arabia for their assistance in the preparation of this manuscript.
AlSerehi AA, Al Mufarrih BM, Abu‐Shaheen A, Saleh A, AlSheef M. Safety of pregnancy after cerebral venous thrombosis: a case‐control study. Health Sci Rep. 2024;7:e1872. 10.1002/hsr2.1872
DATA AVAILABILITY STATEMENT
Data are available upon request from the corresponding author.
REFERENCES
- 1. Silvis SM, de Sousa DA, Ferro JM, Coutinho JM. Cerebral venous thrombosis. Nat Rev Neurol. 2017;13(9):555‐565. 10.1038/nrneurol.2017.104 [DOI] [PubMed] [Google Scholar]
- 2. Stam J. Thrombosis of the cerebral veins and sinuses. N Engl J Med. 2005;352(17):1791‐1798. 10.1056/NEJMra042354 [DOI] [PubMed] [Google Scholar]
- 3. Bousser MG, Ferro JM. Cerebral venous thrombosis: an update. Lancet Neurol. 2007;6(2):162‐170. 10.1016/S1474-4422(07)70029-7 [DOI] [PubMed] [Google Scholar]
- 4. Coutinho JM, Zuurbier SM, Aramideh M, Stam J. The incidence of cerebral venous thrombosis: a cross‐sectional study. Stroke. 2012;43(12):3375‐3377. 10.1161/STROKEAHA.112.671453 [DOI] [PubMed] [Google Scholar]
- 5. Coutinho JM, Ferro M, Canhão P, et al. Cerebral venous and sinus thrombosis in women. Stroke. 2009;40(7):2356‐2361. 10.1161/STROKEAHA.108.543884 [DOI] [PubMed] [Google Scholar]
- 6. Piazza G. Cerebral venous thrombosis. Circulation. 2012;125(13):1704‐1709. 10.1161/CIRCULATIONAHA.111.067835 [DOI] [PubMed] [Google Scholar]
- 7. Sidhom Y, Mansour M, Messelmani M, et al. Cerebral venous thrombosis: clinical features, risk factors, and long‐term outcome in a Tunisian cohort. J Stroke Cerebrovasc Dis. 2014;23(6):1291‐1295. 10.1016/j.jstrokecerebrovasdis.2013.10.025 [DOI] [PubMed] [Google Scholar]
- 8. Ferro M, Canhão P, Stam J, Bousser MG, Barinagarrementeria F, ISCVT Investigators . Prognosis of cerebral vein and dural sinus thrombosis: results of the international study on cerebral vein and dural sinus thrombosis (ISCVT). Stroke. 2004;35(3):664‐670. 10.1161/01.STR.0000117571.76197.26 [DOI] [PubMed] [Google Scholar]
- 9. Green M, Styles T, Russell T, et al. Non‐genetic and genetic risk factors for adult cerebral venous thrombosis. Thromb Res. 2018;169:15‐22. 10.1016/j.thromres.2018.07.005 [DOI] [PubMed] [Google Scholar]
- 10. Lanska DJ, Kryscio RJ. Risk factors for peripartum and postpartum stroke and intracranial venous thrombosis. Stroke. 2000;31(6):1274‐1282. 10.1161/01.str.31.6.1274 [DOI] [PubMed] [Google Scholar]
- 11. Ferro JM, Aguiar de Sousa D. Cerebral venous thrombosis: an update. Curr Neurol Neurosci Rep. 2019;19(10):74. 10.1007/s11910-019-0988-x [DOI] [PubMed] [Google Scholar]
- 12. Aguiar de Sousa D, Canhão P, Crassard I, et al. Safety of pregnancy after cerebral venous thrombosis: results of the ISCVT (International Study on Cerebral Vein and Dural Sinus Thrombosis)‐2 pregnancy study. Stroke. 2017;48(11):3130‐3133. 10.1161/STROKEAHA.117.018829 [DOI] [PubMed] [Google Scholar]
- 13. Soydinc HE, Ozler A, Evsen MS, et al. A case of cerebral sinus venous thrombosis resulting in mortality in severe preeclamptic pregnant woman. Case Rep Obstet Gynecol. 2013;2013:402601. 10.1155/2013/402601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Armstrong EM, Bellone JM, Hornsby LB, Treadway S, Phillippe HM. Pregnancy‐related venous thromboembolism. J Pharm Pract. 2014;27(3):243‐252. 10.1177/0897190014530425 [DOI] [PubMed] [Google Scholar]
- 15. Bousser MG, Crassard I. Cerebral venous thrombosis, pregnancy and oral contraceptives. Thromb Res. 2012;130(suppl 1):S19‐S22. 10.1016/j.thromres.2012.08.264 [DOI] [PubMed] [Google Scholar]
- 16. Aguiar de Sousa D, Canhão P, Ferro JM. Safety of pregnancy after cerebral venous thrombosis: a systematic review. Stroke. 2016;47(3):713‐718. 10.1161/STROKEAHA.115.011955 [DOI] [PubMed] [Google Scholar]
- 17. Varner MW. Cerebral vasculopathies masquerading as eclampsia. Obstetr Gynecol. 2006;107(2 pt 2):437‐438. 10.1097/01.AOG.0000199430.46837.10 [DOI] [PubMed] [Google Scholar]
- 18. Ilkhchoui Y, Szabo EE, Gerstein NS, Jaime F. Cerebral venous thrombosis complicating severe preeclampsia in the postpartum period: a diagnostic challenge. J Clin Anesth. 2014;26(2):143‐146. 10.1016/j.jclinane.2013.11.007 [DOI] [PubMed] [Google Scholar]
- 19. Sharma N, Sharma S, Hussain M. An audit of cerebral venous thrombosis associated with pregnancy and puerperium in teaching hospital in North Eastern India. J Family Med Prim Care. 2019;8(3):1054‐1057. 10.4103/jfmpc.jfmpc_366_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request from the corresponding author.


