Abstract
Major surface proteins of Anaplasma marginale are vaccine candidates. We recently demonstrated that immunization of calves with outer membranes of the Florida strain of A. marginale resulted in protective immunity that correlated with a memory CD4+ T-lymphocyte response specific for major surface protein 1 (MSP-1), MSP-2, and MSP-3 (W. C. Brown, V. Shkap, D. Zhu, T. C. McGuire, W. Tuo, T. F. McElwain, and G. H. Palmer, Infect. Immun. 66:5406–5413, 1998). As immunogens, these proteins have been shown to induce complete or partial protection against homologous challenge. To further define the T helper (Th) cell response to these and other A. marginale antigens and to determine conservation of Th cell epitopes among genetically distinct A. marginale strains, Th cell clones obtained prior to challenge from three immunized calves were characterized for antigen-specific responses. Nine distinct antigenic profiles were defined by 11 Th cell clones derived by stimulation with the Florida strain. Several clones responded to MSP-2, MSP-3, or both. All of these MSP-2- or MSP-3-specific clones and the majority of other clones that did not respond to MSPs recognized all bovine blood-passaged strains of A. marginale. These results demonstrate conservation of certain Th cell epitopes between MSP-2 and MSP-3 and show that Th cell epitopes in MSP-2, MSP-3, and undefined antigens are conserved among strains of A. marginale. Of seven clones that responded to the blood-passaged Virginia strain, two did not recognize antigen prepared from this strain cultured in tick cells, suggesting differences in the antigenic composition between these stages. Analysis of the cytokines expressed by the Th cells revealed that all clones expressed gamma interferon and tumor necrosis factor alpha, and most coexpressed interleukin-4. Our results provide a rationale for identifying Th cell epitopes conserved among different strains of A. marginale for inclusion in a nucleic acid or recombinant protein vaccine.
Anaplasmosis in cattle is caused by Anaplasma marginale, a member of the ehrlichial genogroup II within the order Rickettsiales, which is transmitted biologically by ixodid ticks and mechanically by biting flies. A. marginale is genetically very closely related to other tick-borne ehrlichiae, including the human pathogens Ehrlichia chaffeensis and the agent of human granulocytic ehrlichiosis (16, 40). These ehrlichiae induce disease typified by an acute onset which can rapidly progress to death. A. marginale replicates exclusively within bovine erythrocytes (RBC), which results in severe anemia during acute infection.
Immunization of calves with A. marginale outer membranes can induce complete protection from detectable rickettsemia against a virulent homologous challenge (39). Furthermore, several defined surface exposed outer membrane proteins have been evaluated as vaccine antigens. Immunoaffinity-purified native major surface protein 1 (MSP-1), which in the Florida (FL) strain consists as a complex of 100- and 105-kDa peptides, induced protection against homologous and heterologous strain challenge (30, 31). Immunization with native MSP-2, which in the FL strain is a 36- to 40-kDa protein, resulted in protection ranging from partial to complete in cattle challenged with the immunizing (FL) or heterologous strains (36). Cattle immunized with native MSP-3, an 86-kDa outer membrane protein that shares approximately 55% amino acid sequence identity with the amino-terminal half of MSP-2 (2), had a delayed onset of rickettsemia (34). Native 31-kDa MSP-4 protein also induced protection against homologous challenge, whereas native MSP-5, which is a highly conserved 19-kDa protein, did not (34).
We have recently demonstrated that two of three calves immunized with outer membranes of the FL strain of A. marginale were completely protected against developing persistent infection following challenge (13). The antibody response in the completely protected calves was an immunoglobulin G2 (IgG2) response directed against MSP-2, whereas the one calf that developed a mild but persistent infection produced predominantly IgG1 antibody in response to immunization (13). When immune peripheral blood mononuclear cells (PBMC) were evaluated for antigen specificity, MSP-1, MSP-2, and MSP-3 induced recall proliferative responses. T-cell lines stimulated with A. marginale produced high levels of gamma interferon (IFN-γ). Although the mechanisms of protective immunity against Anaplasma and related human pathogens have not been defined, our results are consistent with the hypothesis that protective immunity correlates with the induction of IFN-γ, which can enhance production of the opsonizing IgG2 isotype in cattle (18, 24) and activate macrophages to express potentially toxic molecules, such as NO (1, 38).
Because T helper (Th) cells are believed to be a vital component of protective immunity against intraerythrocytic pathogens and intracellular rickettsia (12, 19, 22, 34), additional experiments were designed to characterize T-cell epitopes on the MSPs of A. marginale that are targets of vaccine development. Identification of conserved T-cell epitopes is especially important, since MSP-1b, MSP-2, and MSP-3 are members of polymorphic, multigene families (1, 4, 33), and polymorphic MSP-2 variants emerge during cyclic rickettsemia in persistent A. marginale infection (20). Furthermore, strain-specific B-cell epitopes have been identified (25). We report that a panel of T-cell clones obtained from outer membrane protein-immunized calves prior to challenge, which were subsequently protected against infection (as described previously [13]), recognize multiple antigens, including MSP-2, MSP-3, and epitopes apparently shared by these two proteins. The T-cell epitopes on MSP-2, MSP-3, and other, unidentified antigens are conserved at the population level within genetically distinct strains of A. marginale. These results provide a basis for identifying the Th cell epitopes on these proteins and determining their conservation and role in induction of protective immunity.
MATERIALS AND METHODS
Anaplasma strains and preparation of homogenate and membrane antigens.
The A. marginale strains used in this study are designated by their original location of isolation and include the FL, South Idaho (ID), Washington C (WAC), Washington O (WAO), and Virginia (VA) strains. These have been described or referenced previously (24). A strain of A. ovis originating from Idaho was also used (28). All Anaplasma strains were maintained as liquid nitrogen-cryopreserved stabilates of infected bovine RBC in dimethyl sulfoxide–phosphate-buffered saline (PBS). Anaplasma organisms were isolated from thawed, infected bovine RBC by sonication and differential ultracentrifugation as previously described (35).
To prepare homogenate antigen for in vitro assays, the organisms were resuspended in PBS containing the protease inhibitors antipain and E-64 (Boehringer Mannheim, Indianapolis, Ind.) at 25 μg/ml and phenylmethylsulfonyl fluoride (Sigma Chemical Co., St. Louis, Mo.) at 300 μg/ml and were homogenized by two passages through a French pressure cell (SLM Instruments, Inc., Urbana, Ill.) at 1,500 lb/in2. Membranes were prepared from A. marginale FL by sucrose density gradient centrifugation and separated into two fractions (at 1.15 and 1.22 g of sucrose/cm3) as described previously (39). Protein concentrations were determined by the Bradford assay (Bio-Rad, Hercules, Calif.).
The VA isolate of A. marginale, passaged 10 times in tick cell culture, was also used as antigen for T-cell stimulation studies (7, 26). The five MSPs found on A. marginale from bovine RBC were found to be present on A. marginale harvested from cell culture, as determined by immunoblotting with specific immune sera and monoclonal antibodies (MAbs) (5, 6). The cell culture-derived A. marginale maintained the same size MSP-1a as found on the VA isolate in bovine RBC. Furthermore, the antigenic composition of the five MSPs, determined by two-dimensional gel electrophoretic patterns, did not appear to vary after continuous passage in culture (5).
Animal immunization and challenge.
Three male, neutered Holstein calves aged 3 months and designated 96BO5, 96BO6, and 96BO9, were immunized four times with outer membranes (consisting of the higher-density sucrose gradient fraction) prepared from the FL strain of A. marginale as described previously (13, 39). Briefly, each calf received four subcutaneous inoculations, at 2-, 4-, and 4-week intervals, of 67 μg of total protein resuspended in PBS containing 6 mg of saponin per inoculation. Three age-matched control calves received saponin alone. Six months following the last antigen inoculation, the calves were challenged by intravenous inoculation of RBC infected with the FL strain of A. marginale. All immunized calves were protected and experienced lower reductions in packed RBC volumes. All control calves and one of the three immunized calves (96BO5) became infected, as evidenced by peripheral blood rickettsemias. However, the level of rickettsemia in the immunized, infected calf (0.15% infected RBC) was significantly less than that in the control calves (3.5 to 7.4%). These four animals, but not immunized calves 96BO6 and 96BO9, were persistently infected at 60 to 128 days following challenge, as evidenced by nested PCR using primers specific for the conserved msp-5 gene of A. marginale (13).
A. marginale-specific T-cell clones.
T-cell clones were established prior to challenge from antigen-stimulated T-cell cultures from all three immune cattle. In the first cloning experiment, T cells cultured from PBMC of calves 96BO5, 96BO6, and 96BO9 and stimulated for 7 days with 5 μg of A. marginale homogenate per ml were cloned. In the second cloning experiment, a T-cell line from calf 96BO9 cultured for 4 weeks with 5 μg of antigen per ml was cloned. This cell line proliferated specifically to A. marginale antigen in a dose-dependent manner (13). Cells were cloned by limiting dilution in 96-well round-bottomed plates (Costar, Cambridge, Mass.) as described (14). Briefly, T cells were diluted to a statistical average of 1 or 0.3 cells per well and stimulated with 1 μg of A. marginale FL homogenate per ml of medium containing 10% bovine T-cell growth factor and 5 × 104 autologous, irradiated (3,000 rads) PBMC as a source of antigen-presenting cells (APC). Proliferating cells were transferred successively to 48- and 24-well plates and tested for A. marginale-specific proliferation. Clones were selected on the basis of good growth and specific proliferation against A. marginale, with no response to uninfected bovine RBC membranes. In the first cloning experiment, the cloning frequencies (percentage of growth-positive wells) of T-cell cultures from all three calves, when plated at 0.3 or 1 cell per well, were less than 10%, and six cloned cell lines were obtained. In the second experiment, with the 96BO9 cell line, the cloning frequency of T cells plated at 0.3 cells per well was 16.6%, and an additional six clones were obtained from these cultures. The designation of the clones indicates the animal origin; thus, clones designated 5.1D3 and 5.1D11 originated from calf 96BO5; clones designated 6.1E3, 6.2D9, and 6.1F11 originated from calf 96BO6, and clones designated 9.3D3, 9.4A11, 9.4E3, 9.4E7, 9.4G4, 9.4G8, and 9.4H4 originated from calf 96BO9. The first six clones are from the first cloning experiment, and the last six are from the second experiment.
Lymphocyte proliferation assays.
Proliferation assays with T-cell clones were carried out in replicate wells of round-bottomed 96-well plates (Costar) for 3 to 4 days, essentially as described previously (14). T-cell clones were assayed 7 days after the last stimulation with antigen and APC. Briefly, 3 × 104 T cells were cultured in duplicate wells in a total volume of 100 μl of complete medium containing 2 × 105 autologous or allogeneic (major histocompatibility complex [MHC]-mismatched) APC and antigen. Antigens consisted of the following, each at 0.016 to 25.0 μg/ml: membranes prepared from normal bovine RBC or the FL strain of A. marginale; homogenates prepared from the FL, ID, WAC, WAO, and VA strains of A. marginale or the ID strain of A. ovis; purified native MSPs, which were immunoaffinity purified with specific MAbs and dialyzed extensively against PBS as described in previous publications, and which included MSP-1, consisting of MSP-1a and MSP-1b (31), MSP-2 (32), and MSP-3 (23); and cell lysates from uninfected Ixodes scapularis-derived IDE8 tick cell culture (27) or IDE8 cell cultures which were infected with the 10th passage of the VA strain of A. marginale (7). The contents of confluent T-25 flasks of uninfected or infected IDE8 cells were recovered by centrifugation and resuspended in PBS. Protein concentrations in all antigen preparations were determined by the Bradford assay. Irradiated PBMC from cattle used in this study and from a Charolais cow (G3) were used to verify MHC restriction of T-cell clones in response to A. marginale. To determine proliferation, cells were radiolabeled for the last 6 to 18 h of culture with 0.25 μCi of [3H]thymidine (Dupont, New England Nuclear, Boston, Mass.), radiolabeled nucleic acids were harvested onto glass filters and radionucleotide incorporation was measured with a Betaplate 1205 liquid scintillation counter (Wallac, Gaithersburg, Md.). Results are presented as mean counts per minute of replicate cultures ± 1 standard error of the mean (SEM) or as the stimulation index (SI), which represents mean counts per minute of replicate cultures of cells plus antigen/mean counts per minute of replicate cultures of cells plus medium. In comparisons of the responses to different strains of A. marginale, MSPs, or antigen derived from cultured, infected tick cells, a response was considered positive if the SI was ≥2.0 and if the response to antigen was at least 25% of the response to a comparable concentration of A. marginale FL homogenate.
Cell surface phenotypic analysis.
Differentiation markers on T-cell clones were analyzed by indirect immunofluorescence and flow cytometry. The MAbs used were specific for bovine CD2 (MAb MUC2A), CD3 (MAb MM1A), CD4 (MAb CACT 138A), CD8 (MAbs CACT 80C and BAT 82B), and the δ chain of the γδ T-cell receptor (TcR) (MAb CACT 61A). These MAbs were kindly provided by William C. Davis, Washington State University, Pullman, Wash. MAb IL-A29, which recognizes the WC1 molecule on a subset of γδ T cells, was obtained from the International Laboratory for Research on Animal Diseases, Nairobi, Kenya.
Stimulation of cells and detection of cytokine mRNA by reverse transcription (RT)-PCR.
T cell clones were used 7 days after the last stimulation with antigen and APC, washed twice in complete medium, and cultured at a concentration of 2 × 106 cells/ml in the presence of an equal number of autologous APC and a 5-μg/ml concentration of homogenate prepared from A. marginale FL. After 6 h of culture, total cellular RNA was isolated by the TRIzol Reagent RNA isolation method as instructed by the manufacturer (GIBCO BRL, Gaithersburg, Md.). RNA purity was assessed by evaluation of the A260/A280 ratio, and integrity was verified by agarose gel electrophoresis. As a positive control for cytokine mRNA expression, RNA was prepared from bovine PBMC stimulated at a concentration of 2 × 106 cells per ml with 2.5 μg of concanavalin A (ConA; Sigma) per ml for 18 h. For negative controls, RNA was prepared from cultures of irradiated APC and antigen.
Total RNA (0.125 μg/reaction) was reverse transcribed to cDNA by adding a master mixture, prepared as instructed by the manufacturer (Perkin-Elmer, Norwalk, Conn.), consisting of (final concentrations) 5 mM MgCl2, 10 mM KCl, 10 mM Tris-HCl (pH 8.3), 1 mM each deoxynucleoside triphosphate (PCR Nucleotide Mix; Boehringer Mannheim), 20 U of RNase inhibitor, 50 U of murine leukemia virus reverse transcriptase, and 2.5 μM oligo(dT)16 in a final volume of 20 μl. The reactions were performed with a GeneAmp PCR 9600/System (Perkin-Elmer) under the following incubation conditions: 25°C for 10 min, 42°C for 15 min, and 99°C for 5 min. Following the RT reaction, 0.05 to 10 ng of cDNA was amplified by PCR with bovine cytokine or β-actin-specific primers (Table 1). The primer sequences for bovine interleukin-2 (IL-2) and IFN-γ were kindly provided by Dante Zarlenga (U.S. Department of Agriculture, Beltsville, Md.), and those for β-actin were kindly provided by Gary Splitter (University of Wisconsin, Madison). Forward and reverse primers (25 μM each) were added to each reaction, and a master mixture consisting of (final concentrations) 2.5 mM MgCl2, 10 mM KCl, 10 mM Tris-HCl, and 1.25 U of AmpliTaq DNA polymerase (Perkin-Elmer) was added, according to the manufacturer’s instructions, in a total volume of 50 μl. The mixtures were heated to 94°C for 10 min and then amplified with a GeneAmp PCR 9600 system for 35 cycles under the following conditions: 94°C for 1 min, 60°C for 1 min, and 72°C for 2 min, with an extension at 72°C for 10 min. The PCR products (25 μl) were electrophoresed on a 1.5% agarose gel containing ethidium bromide. The relative amount of amplified DNA stained with ethidium bromide was quantified by detecting fluorescent signals activated by UV light, using an IS-1000 digital imaging system (Alpha Innotech Corporation, San Leandro, Calif.). To amplify cytokine-specific product, the same amount (either 2 or 10 ng) of cDNA was used for each clone; to amplify actin product, 0.05 ng of cDNA was used for each clone. After 35 cycles, these quantities of input cDNA resulted in amplification of product within the linear portion of the curve plotted as input cDNA concentration versus relative density of the PCR product. The specificities of the PCR products amplified from ConA-activated PBMC were verified by sequencing, and all sequences were identical to the published GenBank sequences. Parallel reactions performed in the absence of RT yielded no detectable PCR products. Irradiated PBMC cultured with A. marginale antigen expressed only β-actin, not cytokine mRNA, as determined by visualization of the ethidium bromide-stained gels (data not shown).
TABLE 1.
Cytokine and actin primer sequences used in this study
| Product | Forward primer (5′ to 3′) | Reverse primer (5′ to 3′) | Size (bp) | GenBank accession no. |
|---|---|---|---|---|
| IL-2 | GTACAAGATACAACTCTTGTCTTGC | TCAAGTCATTGTTGAGATGCTT | 466 | M12791 |
| IL-4 | TGCATTGTTAGCGTCTCCT | GTCTTTCAGCGTACTTGT | 423 | M77120 |
| IL-10 | GTTGCCTGGTCTTCCTGGCTG | TATGTAGTTGATGAAGATGTC | 482 | U00799 |
| IFN-γ | TATGGCCAGGGCCAATTTTTTAGAGAAATAG | TTACGTTGATGCTCTCCGGCCTCGAAAGAG | 438 | M29867 |
| TNF-α | ATGAGCACCAAAAGCATGATCCGG | CCAAAGTAGACCTGCCCAGACTC | 686 | Z14137 |
| TNF-β | TCCGTGGCATTGGCCTCACA | GGGACCAGGAGGGAATTGTTGC | 202 | Z14137 |
| β-Actin | ACCAACTGGGACGACATGGAG | GCATTTGCGGTGGACAATGGA | 890 | K00622/K00623 |
Detection of IFN-γ and TNF in supernatants of Anaplasma-specific T-cell clones.
T-cell clones were cultured for 24 or 48 h at a density of 2.0 × 106 cells per ml with 2.0 × 106 APC per ml and homogenate prepared from the FL strain of A. marginale (5 μg/ml) or with ConA (1 μg/ml; Sigma) and IL-2 (20 U/ml; Boehringer Mannheim). Supernatants were harvested by centrifugation and stored frozen at −70°C. The bovine IFN-γ assay was performed with a commercial enzyme-linked immunosorbent assay (ELISA) kit (IDEXX Laboratories, Westbrook, Maine) according to the manufacturer’s protocol. The amount of IFN-γ in culture supernatants diluted 1:5 to 1:500 was determined by comparison with a standard curve obtained with a supernatant from a Mycobacterium bovis purified protein derivative-specific Th cell clone that contained 440 U of IFN-γ per ml (previously determined by the neutralization of vesicular stomatitis virus [15]). Tumor necrosis factor (TNF) activity in 24- or 48-h supernatants diluted 1:2 to 1:8 was determined by a biological assay using murine L929 cells and recombinant TNF-α as a standard, essentially as described previously (14). Cytopathicity was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide dye reduction assay. TNF titers in the supernatants were compared with a standard human recombinant TNF-α (Upstate Biotechnology Inc., Lake Placid, N.Y.).
RESULTS
A. marginale-specific Th cell clones.
T-cell clones were derived by limiting dilution culture of T cells stimulated for 1 or 4 weeks with homogenate prepared from the FL strain of A. marginale. The cloning frequencies were ≤16.6%, indicating that the clones were derived from a single precursor (17). All clones expressed the surface phenotype CD3+ CD4+ CD8− γδ TcR−, which is characteristic of Th cells stimulated with exogenously presented antigen. Twelve clones that responded to A. marginale but not to RBC were obtained and characterized for MHC-restricted proliferation to rule out any possible mitogenic effect of the crude A. marginale preparation. All clones responded vigorously and in a dose-dependent manner to A. marginale homogenate and outer membrane antigen preparations (data not shown), and the response to antigen was MHC restricted, as evidenced by the inability of allogeneic APC to present antigen. Table 2 presents data from one of two experiments with similar results. It should be noted that although the clones all responded specifically to A. marginale antigen, the level of response varied widely among the individual clones. Even clones which had similar levels of proliferation, determined by counts per minute, had variable SIs, which is reflected by differences in the level of spontaneous proliferation without antigen. The reason for this variability is not known but may reflect the level of endogenous cytokine expression by individual clones.
TABLE 2.
Proliferative responses of Anaplasma-specific Th cell clones are MHC restricted
| T-cell clone | Proliferation and SI of T cells stimulated with A. marginale antigena
|
|||
|---|---|---|---|---|
| Autologous APC
|
Allogeneic APC
|
|||
| Proliferation (mean cpm ± 1 SEM of [3H]thy-midine incorporation) | SIb | Proliferation (mean cpm ± 1 SEM of [3H]thymidine incorporation) | SI | |
| 5.1D3 | 34,817 ± 5,657 | 220.4 | 128 ± 27 | 1.0 |
| 5.1D11 | 42,308 ± 1,330 | 7.8 | 136 ± 9 | 0.7 |
| 6.1E3 | 55,548 ± 7,213 | 35.0 | 222 ± 64 | 2.7 |
| 6.1F11 | 47,600 ± 35 | 101.3 | 118 ± 31 | 1.1 |
| 6.2D9 | 7,319 ± 248 | 6.6 | 368 ± 58 | 1.5 |
| 9.3D3 | 30,854 ± 3,222 | 4.1 | 148 ± 2 | 1.1 |
| 9.4A11 | 15,127 ± 2,654 | 408.8 | 107 ± 58 | 1.4 |
| 9.4E3 | 41,394 ± 7,499 | 559.3 | 73 ± 6 | 1.1 |
| 9.4E7 | 172,391 ± 8,244 | 305.1 | 252 ± 200 | 1.2 |
| 9.4G4 | 40,558 ± 359 | 289.7 | 226 ± 37 | 2.2 |
| 9.4G8 | 42,584 ± 8,244 | 60.1 | 252 ± 200 | 3.2 |
| 9.4H4 | 909 ± 124 | 14.9 | 67 ± 7 | 0.7 |
a T cells (3 × 104) were cultured for 4 days with 10 μg of A. marginale FL protein per ml of homogenate and 2 × 105 autologous or allogeneic APC. Allogeneic APC were derived from calf 96BO9 for clones 5.1D3, 5.1D11, 6.1E3, and 6.2D9, a Charolais cow (G3) for clone 6.1F11, calf 96BO6 for clones 9.3D3 and 9.4H4, and calf 96BO5 for clones 9.4A11, 9.4E3, 9.4E7, 9.4G4, and 9.4G8.
b Calculated as described in Materials and Methods.
Differential response of Th cell clones to different A. marginale strains and A. ovis.
The proliferative responses of 11 T-cell clones to homogenized organisms prepared from the ID, WAO, WAC, or VA strain of A. marginale or the ID strain of A. ovis, and ranging in concentration from 0.08 to 10.0 μg protein per ml, were compared to the response to the immunizing FL strain of A. marginale. A differential pattern of response to the different Anaplasma strains or species became evident, as shown in Table 3 for clones stimulated with 10 μg of protein per ml. An SI of >2.0 was considered a positive response. Four clones did not respond to A. ovis. One of these (9.4E3) responded only to the FL strain, one (5.1D3) responded to all strains of A. marginale except the VA strain, and two (5.1D11 and 6.1E3) responded to all A. marginale strains. The remaining seven clones responded to A. ovis and all of the A. marginale strains tested. These experiments were performed at least two times to verify the differential response patterns. Clone 6.2D9 did not grow well enough to test the response to different strains.
TABLE 3.
Proliferative responses of Anaplasma-specific Th cell clones to genetically distinct strains of A. marginale and to A. ovis
| Th clone | Proliferation (SIa) in response to:
|
||||||
|---|---|---|---|---|---|---|---|
| Expt 1, A. marginale
|
Expt 2
|
||||||
| FL | WAO | WAC | ID | VA | A. marginale FL | A. ovis ID | |
| 5.1D3 | 46.3 | 45.2 | 14.1 | 29.6 | 1.4 | 36.8 | 1.5 |
| 5.1D11 | 12.0 | 21.8 | 10.4 | 23.7 | 13.5 | 16.6 | 1.8 |
| 6.1E3 | 108.5 | 97.2 | 138.7 | 107.2 | 103.1 | 982.1 | 1.9 |
| 6.1F11 | 152.7 | 145.7 | 168.2 | 138.6 | 202.7 | 535.9 | 551.4 |
| 9.3D3 | 15.7 | 12.1 | 16.9 | 14.8 | 16.2 | 18.1 | 15.1 |
| 9.4A11 | 220.5 | 144.7 | 198.9 | 175.7 | 171.1 | 241.3 | 73.4 |
| 9.4E3 | 178.2 | 0.2 | 0.2 | 0.4 | 0.4 | 210.0 | 0.3 |
| 9.4E7 | 8.6 | 7.4 | 10.4 | 7.9 | 8.5 | 10.7 | 6.6 |
| 9.4G4 | 7.4 | 6.2 | 8.5 | 6.4 | 6.5 | 79.3 | 53.5 |
| 9.4G8 | 21.3 | 19.7 | 19.6 | 22.5 | 19.4 | 31.2 | 23.5 |
| 9.4H4 | 64.9 | 35.7 | 67.5 | 53.9 | 40.0 | 22.2 | 13.8 |
a Calculated as described in Materials and Methods. The response to 10 μg of protein per ml of homogenate prepared from A. marginale strains or from A. ovis ID is presented. An SI of ≥2.0 is considered a positive response; responses that are not positive are in boldface.
Differential response of Th cell clones to native MSP proteins of A. marginale.
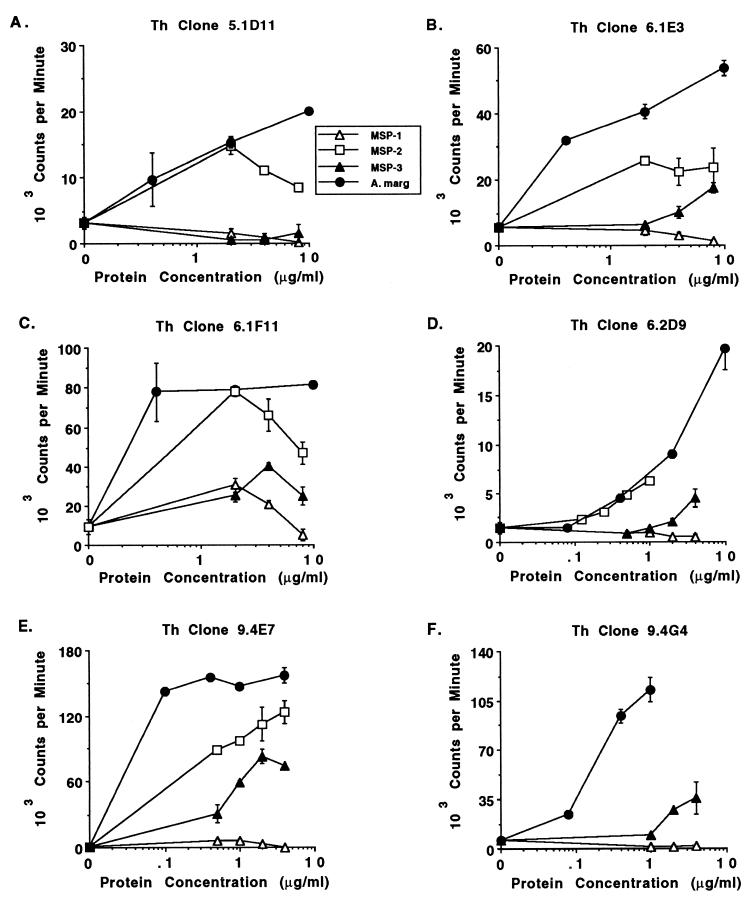
The variation in response patterns became more evident when native MSP-1, MSP-2, and MSP-3, affinity purified from the FL strain, were tested for stimulation of the Th cell clones. Table 4 summarizes the results of several experiments. MSPs were tested at a concentration of 1 to 8 μg/ml; higher concentrations were notably toxic, likely because of residual detergent used to solubilize the proteins. The data presented in Table 4 represent the optimal stimulation of each clone with a given concentration of MSP (1 to 4 μg of MSP-1 per ml, 1 to 8 μg of MSP-2 per ml, or 2 to 8 μg of MSP-3 per ml) and are compared with the response to 2 μg of A. marginale FL outer membrane antigen per ml. A response that has an SI of >2.0 and which is at least 25% of the response to A. marginale is considered positive. Six clones (5.1D11, 6.1E3, 6.1F11, 6.2D9, 9.3D3, and 9.4E7) derived from three calves responded to MSP-2. Of these, four clones (6.1E3, 6.1F11, 6.2D9, and 9.4E7) also responded to MSP-3. In a second experiment testing four of these clones (all but 6.2D9 and 9.3D3), similar results were obtained (data not shown). Clone 6.1F11 also responded to MSP-1, albeit less strongly. Clones 9.4G4 and 9.4G8 responded to MSP-3 but not to MSP-1 or MSP-2. Clone 9.4E3 responded weakly to MSP-2 and somewhat better to MSP-3, but these responses were only 5.5 and 11.9% of the response to A. marginale homogenate. The dose-dependent responses of six clones (5.1D11, 6.1E3, 6.1F11, 6.2D9, 9.4E7, and 9.4G4) to MSP-1, MSP-2, or MSP-3 or to A. marginale antigen are shown in Fig. 1. For clones 5.1D11, 6.1F11, and 6.2D9, similar levels of proliferation were achieved with 1 to 2 μg of MSP-2 or A. marginale antigen per ml. The maximal stimulation of clone 6.1E3 by MSP-2 is approximately 50% of that induced by A. marginale homogenate antigen, the response of clone 9.4E7 to MSP-2 was approximately 79% of that of A. marginale membrane antigen, and the response of clone 9.4G4 to MSP-3 was approximately 40% of that induced by A. marginale outer membrane antigen. Nonspecific stimulation of these T cells by MSPs was ruled out by the lack of response by many of the T-cell clones to individual proteins (Table 4). None of the clones responded to recombinant MSP-5, which was tested as a maltose binding protein fusion protein and as a His6 fusion protein (data not shown). However, potential toxicity of these recombinant fusion proteins cannot be excluded.
TABLE 4.
Proliferative responses of Anaplasma-specific Th cell clones to purified MSP-1, MSP-2, and MSP-3
| Th clone | Proliferation (SIa) in response to:
|
|||
|---|---|---|---|---|
| MSP-1 | MSP-2 | MSP-3 | A. marginale | |
| 5.1D3 | 0.6 | 1.3 | 0.3 | 64.6 |
| 5.1D11 | 1.0 | 4.6 | 0.5 | 4.8 |
| 6.1E3 | 0.8 | 4.5 | 3.1 | 7.1 |
| 6.1F11 | 3.3 | 8.3 | 4.3 | 8.4 |
| 6.2D9 | 0.6 | 4.4 | 3.1 | 6.3 |
| 9.3D3 | 0.6 | 2.3 | 0.1 | 4.0 |
| 9.4A11 | 1.6 | 1.0 | 1.8 | 99.1 |
| 9.4E3 | 0.9 | 6.1 | 13.1 | 109.8 |
| 9.4E7 | 6.8 | 140.0 | 93.6 | 166.9 |
| 9.4G4 | 2.0 | 1.5 | 6.1 | 19.6 |
| 9.4G8 | 1.5 | 0.8 | 5.4 | 21.3 |
| 9.4H4 | 1.8 | 1.3 | 1.5 | 30.7 |
a Calculated as described in Materials and Methods. Data presented represent the maximal stimulation for a given antigen concentration, which ranged from 1 to 8 μg of native MSP-1 ml, MSP-2, or MSP-3 per ml and 2 μg of antigen per ml prepared from A. marginale FL homogenate. Positive responses (SI of ≥2.0 and response that is ≥25% of the response to A. marginale antigen) are in boldface.
FIG. 1.
Dose-dependent proliferative response of selected Anaplasma-specific Th cell clones to purified native MSP-1, MSP-2, and MSP-3. T-cell clones 5.1D11, 6.1E3, 6.1F11, 6.2D9, 9.4E7, and 9.4G4 were cultured for 4 days with 0.5 to 8 μg of MSP-1, MSP-2, or MSP-3 per ml or 0.1 to 10 μg of A. marginale FL outer membranes (A. marg) per ml, radiolabeled, and harvested. The results are means for duplicate cultures ± 1 standard error of the mean.
Differential response of Th cell clones to the VA strain of A. marginale cultured in I. scapularis cells.
The VA strain of A. marginale, originating from infected bovine RBC, was recently propagated in vitro in I. scapularis IDE8 cells (26). This cell line has been passaged and maintained in vitro for several years, with 60 to 100% of the cells infected. To determine whether antigens recognized by our Th cell clones from cattle immunized with outer membranes from the blood-stage FL strain are present in the tick cell-cultured organisms, we performed proliferation assays with 0.08 to 10 μg of antigen per ml prepared from uninfected IDE8 cell lysates or A. marginale VA-infected IDE8 cell lysates. All clones were tested at least twice, with similar results. Optimal stimulation was achieved with 10 μg of protein per ml, and data from representative experiments comparing the proliferative responses to A. marginale FL homogenate and uninfected or infected cultured tick cell lysates are presented in Table 5. Uninfected IDE8 cell lysate was not stimulatory for any clone tested, although PBMC from all cattle responded to uninfected tick cell antigen (data not shown). Five clones did not proliferate in response to the infected tick culture material (5.1D3, 5.1D11, 6.2D9, 9.4E3, and 9.4G8). Two of these clones (5.1D3 and 9.4E3) also failed to respond to the blood-stage homogenate prepared from the VA strain, whereas two clones (5.1D11 and 9.4G8) did respond to blood-stage antigen prepared from the VA strain (clone 6.2D9 was not tested) (Table 3). Thus, Th clones 5.1D11 and 9.4G8 recognize an epitope expressed on all strains of A. marginale organisms, including the VA strain, isolated from infected bovine blood but not expressed on the VA strain passaged in tick cells. The remaining seven clones, which responded to blood-stage A. marginale antigen from the VA strain, also responded to VA strain passaged in tick cell culture.
TABLE 5.
Proliferative responses of Anaplasma-specific Th cell clones to A. marginale VA cultured in I. scapularis IDE8 cells
| Th clone | Proliferation (SIa) in response to:
|
||
|---|---|---|---|
| IDE8 cells | A. marginale-IDE8 | A. marginale | |
| 5.1D3 | 0.5 | 0.6 | 90.3 |
| 5.1D11 | 0.4 | 0.8 | 8.2 |
| 6.1E3 | 0.2 | 8.1 | 14.5 |
| 6.1F11 | 0.2 | 3.8 | 4.0 |
| 6.2D9 | 0.1 | 0.3 | 5.2 |
| 9.3D3 | 0.4 | 6.5 | 5.7 |
| 9.4A11 | 2.5 | 71.1 | 220.5 |
| 9.4E3 | 1.0 | 1.0 | 178.2 |
| 9.4E7 | 1.8 | 172.7 | 177.9 |
| 9.4G4 | 0.4 | 16.6 | 15.1 |
| 9.4G8 | 0.2 | 0.1 | 21.3 |
| 9.4H4 | 0.6 | 21.9 | 64.9 |
a Calculated as described in Materials and Methods. Data are presented for the response to 10 μg of protein per ml in culture lysates of I. scapularis IDE8 cells, either uninfected or infected with the A. marginale VA, or in homogenate prepared from blood-stage FL strain organisms. Positive responses (SI of ≥2.0 and response that is ≥25% of the response to A. marginale antigen) are in boldface.
A summary of the proliferative responses of the T-cell clones, listed by response patterns to the different antigen preparations, is presented in Table 6. When the patterns of response to MSP-1, MSP-2, MSP-3, tick-cultured A. marginale VA, and antigen prepared from five strains of A. marginale and A. ovis are compared, a heterogeneous response pattern is apparent. At least nine distinct patterns of response are manifested by the 12 clones examined, and the clones can accordingly be classified into one of nine groups.
TABLE 6.
Summary of responses of Anaplasma-specific Th cell clones to different antigen preparations
| Th clone | Responsea to:
|
Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Native affinity-purified protein(s)
|
Ixodes culture |
A. marginale
|
A. ovis ID | ||||||||
| MSP-1a + MSP-1b | MSP-2 | MSP-3 | WAO | WAC | FL | ID | VA | ||||
| 9.4E3 | − | w | w | − | − | − | + | − | − | − | I |
| 5.1D3 | − | − | − | − | + | + | + | + | − | − | II |
| 6.1E3 | − | + | + | + | + | + | + | + | + | − | III |
| 5.1D11 | − | + | − | − | + | + | + | + | + | − | IV |
| 9.4G8 | − | − | + | − | + | + | + | + | + | + | V |
| 9.4A11 | − | − | − | + | + | + | + | + | + | + | VI |
| 9.4H4 | − | − | − | + | + | + | + | + | + | + | VI |
| 9.3D3 | − | + | − | + | + | + | + | + | + | + | VII |
| 9.4G4 | − | − | + | + | + | + | + | + | + | + | VIII |
| 9.4E7 | w | + | + | + | + | + | + | + | + | + | IX |
| 6.1F11 | + | + | + | + | + | + | + | + | + | + | IX |
| 6.2D9 | − | + | + | − | NDb | ND | + | ND | ND | ND | ND |
a −, lack of response to an antigen (determined as an SI of <2.0); +, SI of ≥2.0 and response that is ≥25% of the response to A. marginale homogenate; w, SI of ≥2.0 and a response that is <25% of the response to A. marginale homogenate.
b ND, not determined.
Cytokine mRNA expression by Anaplasma-specific Th cell clones.
Oligoclonal T-cell lines from immunized calves 96BO5, 96BO6, and 96BO9 secreted high levels of IFN-γ (13). To determine the nature of the cytokines expressed by cloned Anaplasma-specific memory T cells, IFN-γ protein was measured by ELISA in supernatants of 11 Th cell clones stimulated with antigen and APC, TNF was measured by a biological assay, and transcripts for IL-2, IL-4, IL-10, IFN-γ, TNF-α, and TNF-β were examined in RNA prepared from similarly activated T cells. IFN-γ levels in the supernatants of antigen-stimulated cells ranged from 1 to 56 U per ml (Table 7), with an average of 21 U per ml, which is comparable to the levels of IFN-γ in T-cell lines maintained in vitro for 8 to 10 weeks (13). As previously observed (14), higher levels of IFN-γ (ranging from 126 to 200 U per ml) were secreted by T cells stimulated with ConA and IL-2 (data not shown). TNF levels in the supernatants of antigen-stimulated Th cell clones ranged from 0.5 to 7.3 U per ml, which are comparable to those produced by antigen-stimulated Babesia bovis-specific T-cell clones (14). Examination of steady-state levels of cytokine transcripts revealed that all clones except 5.1D11 coexpressed IL-4, IL-10, and IFN-γ mRNA (Fig. 2). Clone 5.1D11 expressed only IFN-γ and TNF-α transcripts. Interestingly, IL-2 message was expressed only weakly by the majority of Th cell clones, clones 5.1D3 and 6.1E3 being the exceptions. All clones except 9.4A11 expressed strong levels of TNF-α, and TNF-β was not detected. Thus, the majority of Th cell clones expressed a mixed or Th0 cytokine profile, and only one clone expressed IFN-γ in the absence of IL-4 (Th1-like profile).
TABLE 7.
Production of IFN-γ and TNF by A. marginale-specific Th lymphocyte clonesa
| T-cell clone | Cytokine produced (U/ml)
|
|
|---|---|---|
| IFN-γ | TNF-α | |
| 5.1D3 | 2.6 | NDb |
| 5.1D11 | 1.1 | 1.3 |
| 6.1E3 | 55.6 | 3.5 |
| 6.1F11 | 38.1 | 4.0 |
| 9.3D3 | 43.2 | 0.5 |
| 9.4A11 | 10.2 | 2.3 |
| 9.4E3 | 18.4 | 1.2 |
| 9.4E7 | 24.2 | 3.2 |
| 9.4G4 | 24.1 | 1.0 |
| 9.4G8 | 15.9 | 7.3 |
| 9.4H4 | 24.0 | ND |
| APC | 0 | 0.6 |
a T cells (2 × 106 cells per ml) were cultured for 24 or 48 h with irradiated (3,000 rads) autologous PBMC (2 × 106 cells per ml) as a source of APC and 5 μg of A. marginale antigen per ml. Control cultures of APC and antigen were simultaneously set up. Supernatants were collected and assayed in duplicate for IFN-γ by ELISA (24-h supernatants) or for TNF (24- or 48-h supernatants) by cytotoxicity of L929 cells. Results are presented for the values in the culture supernatants of T cells plus APC, and background APC values were not subtracted.
b ND, not determined.
FIG. 2.
Analysis of cytokine transcripts in Anaplasma-specific Th cell clones by RT-PCR. RT-PCR was performed with total cellular RNA prepared from PBMC stimulated with ConA for 18 h (positive control) or from 11 Th cell clones, stimulated with 5 μg of A. marginale antigen per ml and APC for 6 h, as indicated to the left of each gel. Primers specific for bovine IL-2 (lane 1), IL-4 (lane 2), IL-10 (lane 3), IFN-γ (lane 4), TNF-α (lane 5), TNF-β (lane 6), or β-actin (lane 7) were used. The amplified PCR products were electrophoresed on agarose gels, stained with ethidium bromide, and visualized under UV light, and the densitometry images were recorded. The sizes of the amplified PCR products are listed in Table 1. Markers (M), consisting of a 1-kb DNA ladder, were included for each gel but are shown only for ConA-stimulated PBMC.
DISCUSSION
MSP-1, MSP-2, and MSP-3 have been shown to confer protective immunity against A. marginale, as measured by prevention or significant reduction in rickettsemia following homologous challenge (34). However, these proteins vary both antigenically and structurally among strains (2, 3, 29, 32, 33). The problems posed by antigenic variation and the lack of protection against A. marginale challenge in calves administered immune serum (21) emphasize the importance of targeting conserved T-cell epitopes in these candidate vaccine proteins. We recently demonstrated that PBMC from calves protectively immunized with outer membranes against A. marginale challenge responded to all strains of A. marginale tested and had recall Th cell responses against the major surface proteins MSP-1, MSP-2, and MSP-3 (13). The present study extends these findings by showing that 8 of 12 T-cell clones, derived from these animals by stimulation with A. marginale homogenate prior to challenge, recognized MSP-2, MSP-3, or both. Additional undefined specificities were also observed, resulting in a total of at least nine distinct antigenic specificities. Furthermore, the majority of Th cell clones responded to multiple strains of A. marginale, suggesting conservation of Th cell epitopes at the population level among these strains.
One or more T-cell clones from each calf responded to MSP-2, supporting the immunodominant nature of this protein, which was mirrored by the predominant anti-MSP-2 antibody response in these calves (13). Three MSP-2-reactive clones from 96BO6 and one MSP-2-reactive clone from 96BO9 also responded to MSP-3. However, at least two different epitopes appear to be recognized by these clones, as clone 6.1E3 (group III) and clones 6.1F11 and 9.4E7 (group VIII) respond differentially to A. ovis. Comparison of the amino acid sequences of MSP-2 and MSP-3 from the FL strain revealed significant identity (55%) in the amino-terminal, nonpolymorphic region of MSP-2 (amino acids 55 to 176) and the amino-terminal region of MSP-3 (2). Thus, these proteins contain blocks of identical amino acids that could potentially comprise Th cell epitopes and explain the dual reactivity of the cloned T cells. Two clones from calf 96BO9 (9.4G4 and 9.4G8) responded uniquely to MSP-3, but these must recognize distinct MSP-3 epitopes since they responded differentially to A. marginale (VA strain) passaged in tick cells. The apparent preferential response of Th cell clones from animal 96BO9 to MSP-3 was also observed with PBMC, which had the strongest proliferative response to this protein (13). None of the clones responded uniquely to MSP-1. However, in one experiment clone 6.1F11 did proliferate to MSP-1, MSP-2, and MSP-3, and the response to MSP-2 and MSP-3 was verified in a second assay. MSP-1 has no known amino acid sequences conserved with either MSP-2 or MSP-3, so the reason for this result is not known.
The finding that all MSP-2- and MSP-3-specific clones also responded to all strains of A. marginale demonstrates conservation of T-cell epitopes in the different strains. However, MSP-2 and MSP-3 are encoded by multiple genes which display conserved and polymorphic regions. Comparison of multiple cDNA clones encoding MSP-2 variants during persistent, cyclic rickettsemia revealed a polymorphic region (amino acids 185 to 297) within the predicted amino acid sequence (20). Comparison of three MSP-3 genomic clones also revealed polymorphic and conserved areas (2). Our data suggest that MSP-2 and MSP-3 T-cell epitopes recognized by certain T-cell clones in this study are in nonpolymorphic regions of the protein, but precise mapping studies are needed to verify this assumption, since multiple gene copies are expressed within a population and even within a single organism (20). Two exceptional clones, 5.1D11 (MSP-2 specific) and 9.4G8 (MSP-3 specific), responded to homogenate from all A. marginale strains passaged in cattle, but both failed to respond to lysate prepared from the VA strain grown in I. scapularis cells. This finding suggests that the epitopes defined by these two clones are either altered or missing in the organisms grown within tick cells in vitro.
The observation that many of the T-cell epitopes have been conserved on organisms propagated in tick cell culture is of interest. The five MSPs were found to be present on the cell culture-derived VA organisms, and MSP-1a was the same size as the blood-derived VA strain (5). Furthermore, the developmental cycle and morphology of cell culture-derived VA organisms are similar to those of A. marginale in naturally infected ticks (7). Differences in the polymorphic MSP-2 antigen between culture and salivary gland stages were observed, but MSP-2 did not appear to vary during continuous passage in culture (5). Of the clones that responded to blood-passaged VA strain, all but two (5.1D11 and 9.4G8) responded to this strain of A. marginale harvested from tick cell culture. Retention of other T-cell epitopes by the cell culture-derived VA organisms defined by additional T-cell clones suggests the potential use of culture-derived A. marginale to define immunodominant antigenic epitopes, which may be important to induce immunity against both tick and blood challenge.
The patterns of response of the Th cell clones revealed additional antigen specificities which result in a total of nine response patterns defined by the first 11 T-cell clones listed in Table 6. The additional antigen specificities fell into three distinct groups (I, II, and VI). Clone 9.4E3 (group I) recognized an epitope expressed only on the immunizing (FL) strain, and thus it is not conserved. Clone 5.1D3 (group II) recognized an epitope shared by WA, FL, and ID strains but, interestingly, absent from the VA strain. Finally, clones 9.4A11, 9.4H4, and 9.3D3 (group VI) responded to an epitope, apparently not associated with MSP-1, MSP-2, or MSP-3, conserved in all strains of A. marginale and the ID strain of A. ovis. The identity of the proteins recognized by T cells that were not specific for the MSPs tested is not known. MSP-4 was not available for testing, and recombinant MSP-5 was not stimulatory. Additional studies are planned to determine if MSP-4 is the target of any of these T cells. Furthermore, fractionation of A. marginale homogenate by continuous-flow electrophoresis could provide information regarding additional antigen specificities (11).
A. marginale-specific T-cell clones expressed a mixture of cytokines, and all but one coexpressed IL-4 and IFN-γ. These data are consistent with the lack of a polarized cytokine response by Th cells cloned from cattle immune to either Babesia or Fasciola species (9, 10, 14). The expression of both IL-4 and IFN-γ by A. marginale-specific Th cells is consistent with their potential role as helper cells to enhance IgG1 and IgG2 responses (8), but functional helper cell assays with A. marginale-specific clones have not been performed. Furthermore, the expression of both IFN-γ and TNF-α is consistent with the potential for A. marginale-immune CD4+ T cells to activate macrophages to produce molecules, such as NO, that have been shown to inhibit other rickettsiae (37).
In summary, the use of T-cell clones enables characterization of antigen specificity and function of memory T cells from animals shown to be protected against homologous challenge infection. The observation that the majority of T-cell epitopes, including at least two epitopes in MSP-2 and MSP-3, are conserved in different strains of A. marginale, provides a rationale for mapping the T-cell epitopes on these candidate vaccine antigens and for identifying the additional antigens and epitopes defined by the panel of T-cell clones. The antigenic variation known to occur in MSP-2 during cyclic rickettsemia in persistently infected cattle (20) further underscores the importance of defining Th cell epitopes in sequential populations of organisms in these animals. Studies are planned to identify the Th cell epitopes on MSP-2 and MSP-3 and to determine their role in persistent infection. Functional assays with the Th cell clones will be performed to assess their helper cell capacity and ability to inhibit the growth of A. marginale in the presence of macrophages. Together, these studies will provide a basis for vaccine design for this important bovine pathogen.
ACKNOWLEDGMENTS
We thank Sue Ellen Chantler, Beverly Hunter, Emma Karel, and Kimberly Kegerreis for excellent technical assistance.
This research was supported in part by United States-Israel Binational Agricultural Research and Development Fund projects US-2238-92C and US-2799-96C and U.S. Department of Agriculture NRICGP project 95-37204-2348.
REFERENCES
- 1.Adler H, Peterhans E, Nicolet J, Jungi T W. Inducible l-arginine-dependent nitric oxide synthase activity in bone marrow-derived macrophages. Biochem Biophys Res Commun. 1994;198:510–515. doi: 10.1006/bbrc.1994.1075. [DOI] [PubMed] [Google Scholar]
- 2.Alleman A R, Palmer G H, McGuire T C, McElwain T F, Perryman L E, Barbet A F. Anaplasma marginale major surface protein 3 is encoded by a polymorphic, multigene family. Infect Immun. 1997;65:156–163. doi: 10.1128/iai.65.1.156-163.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allred D R, McGuire T C, Palmer G H, Leib S R, Harkins T M, McElwain T F, Barbet A F. Molecular basis for surface antigen size polymorphisms and conservation of a neutralization-sensitive epitope in Anaplasma marginale. Proc Natl Acad Sci USA. 1990;87:3220–3224. doi: 10.1073/pnas.87.8.3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbet A F, Allred D R. The msp1b multigene family of Anaplasma marginale: nucleotide sequence analysis of an expressed copy. Infect Immun. 1991;59:971–976. doi: 10.1128/iai.59.3.971-976.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbet, A. F., R. Bentlinger, Y. I. Jooyoung, A. M. Lundgren, E. F. Blouin, and K. M. Kocan. Comparison of surface proteins of Anaplasma marginale grown in tick cell culture, tick salivary glands and cattle. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 6.Barbet A F, Bentlinger R, Lundgren A M, Blouin E F, Kocan K M. Comparison of surface proteins of Anaplasma marginale grown in tick cell culture and cattle. In: Kocan K M, editor. Program and abstracts for the Society for Tropical Veterinary Medicine 4th Biennial Meeting, Montpellier, France. 1997. p. 26. [Google Scholar]
- 7.Blouin E F, Kocan K M. Morphology and development of Anaplasma marginale (Rickettsiales: Anaplasmataceae) in cultured Ixodes scapularis (Acari: Ixodidae) cells. J Med Entomol. 1998;35:136–155. doi: 10.1093/jmedent/35.5.788. [DOI] [PubMed] [Google Scholar]
- 8.Brown, W. C., T. F. McElwain, G. H. Palmer, S. E. Chantler, and D. M. Estes. Bovine CD4+ T-lymphocyte clones specific for rhoptry-associated protein-1 (RAP-1) of Babesia bigemina provide cognate help for IgG1 and IgG2 synthesis. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 9.Brown W C, Davis W C, Dobbelaere D A E, Rice-Ficht A C. CD4+ T-cell clones obtained from cattle chronically infected with Fasciola hepatica and specific for adult worm antigen express both unrestricted and Th2 cytokine profiles. Infect Immun. 1994;62:818–827. doi: 10.1128/iai.62.3.818-827.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown W C, Estes D M. Type 1 and type 2 responses in cattle and their regulation. In: Schijns VEC J, Horzinek M C, editors. Cytokines in veterinary medicine. Wallingford, England: CAB International; 1997. pp. 15–33. [Google Scholar]
- 11.Brown W C, Logan K S, Zhao S, Bergman D K, Rice-Ficht A C. Identification of Babesia bovis merozoite antigens separated by continuous-flow electrophoresis that stimulate proliferation of helper T-cell clones derived from B. bovis-immune cattle. Infect Immun. 1995;63:3106–3116. doi: 10.1128/iai.63.8.3106-3116.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown W C, Rice-Ficht A C. Use of Th cells to identify potential vaccine antigens of Babesia bovis. Parasitol Today. 1994;11:145–149. doi: 10.1016/0169-4758(94)90265-8. [DOI] [PubMed] [Google Scholar]
- 13.Brown W C, Shkap V, Zhu D, McGuire T C, Tuo W, McElwain T F, Palmer G H. CD4+ T-lymphocyte and immunoglobulin G2 responses in calves immunized with Anaplasma marginale outer membranes and protected against homologous challenge. Infect Immun. 1998;66:5406–5413. doi: 10.1128/iai.66.11.5406-5413.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown W C, Woods V M, Dobbelaere D A E, Logan K S. Heterogeneity in cytokine profiles of Babesia bovis-specific bovine CD4+ T-cell clones activated in vitro. Infect Immun. 1993;61:3273–3281. doi: 10.1128/iai.61.8.3273-3281.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown W C, Zhao S, Rice-Ficht A C, Logan K S, Woods V M. Bovine helper T-cell clones recognize five distinct epitopes on Babesia bovis merozoite antigens. Infect Immun. 1992;60:4364–4372. doi: 10.1128/iai.60.10.4364-4372.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dame J B, Mahan S M, Yowell C A. Phylogenetic relationship of Cowdria ruminantium, agent of heartwater, to Anaplasma marginale and other members of the order Rickettsiales determined on the basis of 16S rRNA sequence. Int J Syst Bacteriol. 1992;42:270–274. doi: 10.1099/00207713-42-2-270. [DOI] [PubMed] [Google Scholar]
- 17.de St. Groth S F. The evaluation of limiting dilution assays. J Immunol Methods. 1982;49:R11–R23. doi: 10.1016/0022-1759(82)90269-1. [DOI] [PubMed] [Google Scholar]
- 18.Estes D M, Closser N M, Allen G K. IFN-γ stimulates IgG2 production from bovine B cells costimulated with anti-μ and mitogen. Cell Immunol. 1994;154:287–295. doi: 10.1006/cimm.1994.1078. [DOI] [PubMed] [Google Scholar]
- 19.Feng H M, Popov V L, Walker D H. Depletion of gamma interferon and tumor necrosis factor alpha in mice with Rickettsia conorii-infected endothelium: impairment of rickettsicidal nitric oxide production resulting in fatal, overwhelming rickettsial disease. Infect Immun. 1994;62:1952–1960. doi: 10.1128/iai.62.5.1952-1960.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.French D F, McElwain T F, McGuire T C, Palmer G H. Expression of Anaplasma marginale major surface protein 2 variants during persistent cyclic rickettsemia. Infect Immun. 1998;66:1200–1207. doi: 10.1128/iai.66.3.1200-1207.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gale K R, Leatch G, Gartside M, Dimmock C M. Anaplasma marginale: failure of sera from immune cattle to confer protection in passive-transfer experiments. Parasitol Res. 1992;78:410–415. doi: 10.1007/BF00931697. [DOI] [PubMed] [Google Scholar]
- 22.Kodama K, Kawamura S, Yasukawa M, Kobayashi Y. Establishment and characterization of a T-cell line specific for Rickettsia tsutsugamushi. Infect Immun. 1987;55:2490–2495. doi: 10.1128/iai.55.10.2490-2495.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGuire T C, Davis W C, Brassfield A L, McElwain T F, Palmer G H. Identification of Anaplasma marginale long-term carrier cattle by detection of serum antibody to isolated MSP-3. J Clin Microbiol. 1991;29:788–793. doi: 10.1128/jcm.29.4.788-793.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGuire T C, Musoke A J, Kurtti T. Functional properties of bovine IgG1 and IgG2: interaction with complement, macrophages, neutrophils, and skin. Immunology. 1979;38:249–256. [PMC free article] [PubMed] [Google Scholar]
- 25.McGuire T C, Palmer G H, Goff W L, Johnson M I, Davis W C. Common and isolate-restricted antigens of Anaplasma marginale detected with monoclonal antibodies. Infect Immun. 1984;45:697–700. doi: 10.1128/iai.45.3.697-700.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Munderloh U G, Blouin E F, Kocan K M, Ge N L, Edwards W E, Kurtti T J. Establishment of the tick (Acari: Ixodidae)-borne cattle pathogen Anaplasma marginale (Rickettsiales: Anaplasmataceae) in tick cell culture. J Med Entomol. 1996;33:656–664. doi: 10.1093/jmedent/33.4.656. [DOI] [PubMed] [Google Scholar]
- 27.Munderloh U G, Wang Y L M, Chen C, Kurtti T J. Establishment, maintenance and description of cell lines from the tick Ixodes scapularis. J Parasitol. 1994;80:533–543. [PubMed] [Google Scholar]
- 28.Ndung’u L W, Aguirre C, Rurangirwa F R, McElwain T F, McGuire T C, Knowles D P, Palmer G H. Detection of Anaplasma ovis infection in goats by major surface protein 5 competitive inhibition enzyme-linked immunosorbent assay. J Clin Microbiol. 1995;33:675–679. doi: 10.1128/jcm.33.3.675-679.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oberle S M, Palmer G H, Barbet A F, McGuire T C. Molecular size variation in an immunoprotective protein complex among isolates of Anaplasma marginale. Infect Immun. 1988;56:1567–1573. doi: 10.1128/iai.56.6.1567-1573.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmer G H, Barbet A F, Cantor G H, McGuire T C. Immunization of cattle with the MSP-1 surface protein complex induces protection against a structurally variant Anaplasma marginale isolate. Infect Immun. 1989;56:1526–1531. doi: 10.1128/iai.57.11.3666-3669.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmer G H, Barbet A F, Davis W C, McGuire T C. Immunization with an isolate-common surface protein protects cattle against anaplasmosis. Science. 1986;231:1299–1302. doi: 10.1126/science.3945825. [DOI] [PubMed] [Google Scholar]
- 32.Palmer G H, Barbet A F, Musoke A J, Katende J M, Rurangirwa F, Shkap V, Pipano E, Davis W C, McGuire T C. Recognition of conserved surface protein epitopes on Anaplasma centrale and Anaplasma marginale isolates from Israel, Kenya and the United States. Int J Parasitol. 1988;18:33–38. doi: 10.1016/0020-7519(88)90033-1. [DOI] [PubMed] [Google Scholar]
- 33.Palmer G H, Eid G, Barbet A F, McGuire T C, McElwain T F. The immunoprotective Anaplasma marginale major surface protein 2 is encoded by a polymorphic multigene family. Infect Immun. 1994;62:3808–3816. doi: 10.1128/iai.62.9.3808-3816.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palmer G H, McElwain T F. Molecular basis for vaccine development against anaplasmosis and babesiosis. Vet Parasitol. 1995;57:233–253. doi: 10.1016/0304-4017(94)03123-e. [DOI] [PubMed] [Google Scholar]
- 35.Palmer G H, McGuire T C. Immune serum against Anaplasma marginale initial bodies neutralizes infectivity for cattle. J Immunol. 1984;133:1010–1015. [PubMed] [Google Scholar]
- 36.Palmer G H, Oberle S M, Barbet A F, Davis W C, Goff W L, McGuire T C. Immunization with a 36-kilodalton surface protein induces protection against homologous and heterologous Anaplasma marginale challenge. Infect Immun. 1988;56:1526–1531. doi: 10.1128/iai.56.6.1526-1531.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park J, Rikihisa Y. l-Arginine-dependent killing of intracellular Ehrlichia risticii by macrophages treated with gamma interferon. Infect Immun. 1992;60:3504–3508. doi: 10.1128/iai.60.9.3504-3508.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stich R W, Shoda L K M, Dreewes M, Adler B, Jungi T W, Brown W C. Stimulation of nitric oxide production in macrophages by Babesia bovis. Infect Immun. 1998;66:4130–4136. doi: 10.1128/iai.66.9.4130-4136.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tebele N, McGuire T C, Palmer G H. Induction of protective immunity by using Anaplasma marginale initial body membranes. Infect Immun. 1991;59:3199–3204. doi: 10.1128/iai.59.9.3199-3204.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walker D H, Dumler J S. Emergence of the ehrlichioses as human health problems. Emerg Infect Dis. 1996;2:18–29. doi: 10.3201/eid0201.960102. [DOI] [PMC free article] [PubMed] [Google Scholar]