Abstract
Purpose of Review
E-cigarettes (e-cigs) release toxic chemicals known to increase blood pressure (BP) levels. The effects of e-cigs on BP, however, remain unknown. Studying BP may help characterize potential cardiovascular risks of short- and long-term e-cig use. We summarized published studies on the association of e-cig use with BP endpoints.
Recent Findings
Thirteen e-cig trials (12 cross-over designs) and 1 observational study evaluated systolic and diastolic blood pressure (SBP and DBP). All trials included at least one e-cig arm with nicotine, 6 a no-nicotine e-cig arm, and 3 a placebo arm. SBP/DBP increased in most nicotine e-cig arms, in some non-nicotine e-cig arms, and in none of the placebo arms. The observational study followed e-cig users and nonsmokers for 3.5 years with inconsistent findings.
Summary
The use of e-cigs with and without nicotine may result in short-term elevations of both SBP and DBP. Prospective studies that investigate the long-term cardiovascular impact of e-cig use are needed.
Keywords: Blood pressure, Electronic-cigarettes, Hypertension, Nicotine
Introduction
Hypertension and cigarette smoking are the two leading causes of premature mortality worldwide. In 2017, high systolic blood pressure (SBP) was responsible for 10.4 million deaths, followed by cigarette smoking, responsible for 7.10 million deaths [1, 2]. Early diagnosis and treatment of hyper-tension play a crucial role in preventing clinical cardiovascular events including myocardial infarction and stroke [3••].
Electronic cigarettes (e-cigs) have been introduced in the market in recent years, promoted in part as a harm reduction strategy for smoking cessation. E-cigs, however, are also being marketed to never smokers and a rapidly increasing number of adolescents and young adults who have never smoked are using e-cigs. In the USA, for instance, e-cigs are the most commonly used tobacco product among youth, with 3.6 million middle and high school students using them in 2020 [4]. It is estimated that around 40% of e-cig users between 18 and 24 years have never smoked cigarettes before [5].
E-cigs are chargeable devices that produce an aerosol from heating an e-liquid with a coil. E-cig aerosols contain aldehydes and other carbonyl derivatives, metals, particulate matter, nicotine, and flavoring compounds [6••]. Chemical components in e-cigs remain unregulated, despite the extension of the FDA regulatory authority to all electronic nicotine delivery systems (ENDS) in 2016 [7]. For the organic and inorganic toxic compounds, there is substantial concern of long-term toxicity derived from the chronic use of these products [8].
Few studies have evaluated the potential effects of e-cigs on hypertension and cardiovascular health, including short-term and long-term effects. To ascertain the evidence and guide future research, in this review, we summarize the available studies on the short-term effects of e-cigs on blood pressure in experimental studies as well as the association between e-cigs and blood pressure endpoints in observational studies. For experimental studies, the comparisons for the short-term effect of e-cig use on blood pressure levels compared to baseline (no use) were reported for tobacco smokers and non-tobacco smokers separately.
Methods
Research Strategy and Data Abstraction We searched PubMed to find all published studies evaluating the relationship between e-cigarettes and hypertension or blood pressure endpoints using the free text and Medical Subject Headings (MeSH) terms “ Electronic Cigarettes”[MeSH], “Vaping”[MeSH], electronic cigarette*, e cig*, ecig*, vaping, nicotine delivery system, nicotine delivery systems, nicotine inhaler, nicotine inhalers, NICOTROL, smokeless cigarette, smokeless cigarettes, electronic nicotine, nicotine inhalator, vapor device, vapor devices, vapor device, vapor devices, alternative cigarettes, digital cigarettes or vapor smoking and “Hypertension”[MeSH], systolic, diastolic, blood pressure, hypertension, hypertensive, or hypertens*. The search period was from 2003 (when the first e-cigs started to be distributed) through April 2020 with no language restrictions.
A total of 69 publications were found (Fig. 1). We included experimental and observational studies with data on the association of e-cig use with blood pressure endpoints. We excluded animal and in vitro studies, systematic and bibliographic reviews (including the reference lists of the published studies), commentaries, and studies without data on blood pressure end-points, on e-cigs, or on the associations between both. We also excluded studies that exclusively evaluated e-cigs as a harm reduction strategy compared to traditional cigarettes without a comparison to no e-cig and no traditional cigarette use.
Fig. 1.
PRISMA flow chart
For each study, we abstracted the following data: first author and study year, study population (age, sex, traditional cigarettes smoking status, e-cig use), e-cig intervention or pattern of use, e-cig device and e-liquid characteristics, sample size, blood pressure assessment and/or hypertension definition, measure of association, effect estimate, and adjustment for potential confounders. The study findings are summarized for experimental and observational studies separately. For experimental studies, findings related to traditional tobacco cigarettes were excluded.
Results
A total of 14 studies published between 2012 and 2020 evaluating the relationship between the use of e-cigs and blood pressure endpoints were identified. All the studies recruited healthy participants with no previous hypertension diagnosis. Hypertension was defined as the consistent elevation of SPB and/or DBP above 140 and 80 mmHg, according to previous guidelines [9]. Thirteen were experimental studies and 1 was an observational study [10•]. Four studies were conducted in the USA [11–14], two studies were conducted in Greece [15, 16], Italy [10•,17], and Poland [18, 19], and one study was conducted in Belgium [20], Germany [21], Sweden [22], and the UK [23].
The sample size ranged from 15 [19, 21] to 76 [15] participants. One study included only women [19], one study included only men [23], and other studies included both men and women. The age range was from 20 to 58 years old.
All the experimental studies were randomized controlled trials; 12 of them had a crossover trial design. Three studies recruited exclusively nonsmokers [11–13]. The remaining 10 studies recruited current cigarette smokers, although 2 of those studies recruited occasional smokers of less than 10 cigarettes per month and the median cumulative pack-years was 0.2 [20, 22].
All the experimental studies included at least one e-cig arm with variable concentrations of nicotine that ranged from 11 mg/mL [15] to 50 mg/mL [12] (Table 1). Six studies included a 0% nicotine e-cig arm, eight included a tobacco cigarette arm, and three included a no intervention or placebo arm. Except for one experimental study [16], all the studies described the e-cig characteristics and e-liquid composition (Table 2).
Table 1.
Experimental studies: population age, biological gender, smoking status at baseline, number of participants, and intervention arms
| Author/year | Country | Age, y Mean (SD) | % Men | % cigarette smokers | % naive e-cig users | N | Cross-over study | Intervention arms |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cigarette | Nicotine e-cig | 0% nicotine e-cig | Placeb | ||||||||
|
| |||||||||||
| Cossio 2020 | United States | 24 (3) | 50% | 0% | 100% | 16 | X | X | X | X | |
| Biondi-Zoccai 2019 | Italy | 35 (13) | 30% | 100% | NR% | 20 | X | X | X | ||
| Kerr 2018 | United Kingdom | 31.6 (10.5) | 100% | 100% | 100% | 20 | X | X | X | ||
| Antoniewicz 2019 | Sweden | 26 (3) | 40% | 100% | NR | 17 | X | X | X | ||
| Chaumont 2018 | Belgium | 23 (0.4) | 72% | 100% | NR | 25 | X | X | X | X | |
| Franzen 2018 | Germany | 22.9 (3.5) | 33% | 100% | 0% | 15 | X | X | X | X | |
| Fogt 2016 | United States | 23.1 (2.5) | 50% | 0% | 100% | 20 | X | X | X | ||
| Vlachaupoulos, 2016 | Greece | 30 (8) | NR | 100% | NR | 24 | X | X | X | X | |
| Cooke 2015 | United States | 23 (1) | 50% | 0% | 100% | 20 | X | X | X | ||
| Yan 2015 | United States | 38.7(11) | 48% | 100% | 0% | 23 | X | X | X | ||
| Szolty 2014 | Poland | 23 (2) | 0% | 100% | 0% | 15 | X | X | |||
| Farsalinos 2014 | Greece | 35 (5) | 90% | 100% | NR | 76 | X | ||||
| Czogala 2012 | Poland | 34.9 (15.3) | 50% | 100% | 100% | 42 | X | X | |||
Table 2.
E-cigarette device and e-liquid characteristics
| Author, year | e-cig devices | e-liquid | Nicotine content in mg/mL |
|---|---|---|---|
|
| |||
| Cossio, 2020 | Cirrus 3, white cloud cigarette | Menthol flavor clear draw | 50 |
| Biondi-Zoccai, 2019 | Blu Pro, Fontem, Netherlands | e-liquid details not described | 16 |
| Kerr, 2019 | 2nd generation e-cig. 1300 mAh variable voltage rechargeable battery, a tank and an atomizer (SmokeMax; Groove Trading Ltd., Glasgow, UK) | Tobacco flavored (Pillbox38 UK Ltd., Totally Wicked, Blackburn, UK) | 18 |
| Antoniewicz, 2019 | 3rd generation e-cigarette (eVic-VT, Shenzhen Joyetech Co., Ltd., China). Temperature 230 °C, effect 32 W, resistance 0.20 ω). dual-coil nickel atomizer | 49.4% propylene glycol, 44.4% vegetable glycerin, and 5% ethanol without any added flavorings (Valeo laboratories GmbH, Germany) | 19 |
| Chaumont, 2018 | Last-generation high-power vaping device with popular and commercially available parts in USA (Smoke©, Shenzen, China). 60 W. 0.4 Ω dual coils | 50% propylene glycol and 50% glycerin pharmaceutical grade (Fagron©, Waregem, Belgium) | 19 (Nicobrand©, Coleraine, UK) |
| Franzen, 2018 | DIPSE, eGo-T CE4 vaporizer third generation, (SSR Produkt GmbH & Co KG, Oldenburg, Germany). 3.3 V, 1.5 Ω, and 7.26 W | 55% propylene glycol and 35% glycerin, tobacco flavor | 24 |
| Fogt, 2016 | Green Smart Living, Salt Lake City, UT) | e-liquid (details not described?) | 18 |
| Vlachaupoulos, 2016 | NR. | NR. | NR |
| Cooke, 2015 | Green Smart Living (Salt Lake City, UT) and Clean Electronic cigarettes (details not described) | e-liquid (details not described) | 18 |
| Yan, 2015 | Blu e-cigs, sold in retail outlets across the USA in both disposable and re-useable forms. | 3 e-liquid formulations: A and C: 75% vegetable glycerin. B and E: 50% vegetable glycerin 20% propylene glycol. D: 75% vegetable glycerin | A, B, C: 24 D: 16 |
| Szoltysek-Boldys, 2014 | e-Go, Clearomizer Crystal 2 with coil, 2.4 Ω, voltage battery 900 mAh, 3.4 V | e-liquid details not described | 24 |
| Farsalinos, 2014 | 2nd generation eGo-T battery (Nobacco Athens, Greece) with an eGo-C atomiser (Alter Ego Athens, Greece). 650-mAh rechargeable lithium battery, 3.5 V | α -propylene glycol or 1,2-propanediol > 60%, linalool (3,7-dimethylocta-1, 6-dien-3-ol) < 5%, tobacco essence (< 5%), and methyl vanillin (4-hydroxy-3-methoxybenzaldehyde) at < 1% | 11 |
| Czogala, 2012 | Mild M2001 (1st generation) | e-liquid details not described | 14 |
| Polosa, 2014 | Use of personal devices. Assorted eGo products. Advanced and standard refillable. Provari, Innokin, Joyetech, eVIC, Avatar Puff | e-liquid details not described | 9–12 (n = 4) 16–18(n = 5) |
NR., not reported
All experimental studies measured systolic and diastolic blood pressure levels at pre-intervention baseline and up to 4 h after the intervention. The washout period between different intervention arms in the crossover studies ranged from 24 h to 1 week.
Three experimental studies were conducted in non-tobacco smokers (Table 3). In the e-cig with nicotine arm, SBP and DBP increased compared to baseline in all studies. Comparing SBP and DBP changes happening in less than an hour post-intervention vs. pre-intervention, the mean SBP/DBP change was + 5/+ 4 mmHg [12] and + 2/+ 4 mmHg [11]. For the no nicotine e-cig arm, the corresponding changes were + 3/+ 2 mmHg [12] and a nonsignificant decline in Cooke et al. Fogt et al. did not provide the baseline SBP/DBP data but reported a significant increase at 40 min after the intervention with a nicotine e-cig arm and no significant changes in either SBP or DBP with the no nicotine e-cig arm. The study by Cossio et al. reported that between 1 and 2 h of the intervention, SBP/DBP declined, but they were still higher compared to baseline both in the nicotine and non-nicotine e-cig arms.
Table 3.
Experimental studies in non-smokers, intervention description, groups and results for SBP and DBP
| Author, year | Intervention Description / Washout | Groups | Results for SBP (group mean (SD)) in mmHg | Results for DBP (group mean (SD)) in mmHg |
|---|---|---|---|---|
|
| ||||
| Cossio, 2020 | 3 cycles of one product use: 18 e-cig puffs at 20s interval / 48 h | Nico, e-cig (50 mg/mL) No nico. e-cig Placebo |
(Baseline/immediately post/1 h/2 h): Nico e-cig:119(10)/124(10)/121(10)/121(9) No nico. e-cig:115(8)/118(10)/120(8)/119(10) Placebo: 117(6)/119(8)/120(7)/120(7) |
(Baseline/immediately post/1 h/2 h): Nico. e-cig: 69(4)/73(5)/71(6)/70(5) No nico. e-cig: 66(4)/68(5)/70(5)/68(5) Placebo: 68(3)/68(6)/71(6)/69(5) |
| Fogt, 2016 | 2 cycles of one product use: 20 e-cig puffs in 10 min/L week | Nico. e-cig (18 mg/mL) No nico. e-cig |
(Baseline/40mins) Nico. e-cig: NR/112.1(6.8) p = 0.04 No nico. e-cig: NR/115.8(8) |
(Baseline/40mins) Nico. e-cig: NR/76.6(6) p = 0.04 No nico. e-eig: NR/73.6(8.3) |
| Cooke, 2015 | 2 cycles of one product use: 20 e-cig puffs in 10 min/L week | Nico. e-cig (18 mg/mL) No nico e-cig |
(Change pre-post 20 mins) Nico. e-cig: 2 p = 0.03 No nico. e-cig: −2 |
(Change pre-post 20 mins) Nico. e-cig: 4 p = 0.001 No nico. e-cig: −2 |
Nico., nicotine
Ten experimental studies were conducted in smokers (Table 4). All of them included an e-cig arm with nicotine that experienced elevations of SBP and DBP after the intervention compared to baseline. Comparing the SBP and DBP change from baseline vs. after the intervention, immediately after the intervention, the mean SBP/DBP change was + 12/+10 mmHg [20] and + 9.9/+ 8.6 mmHg [22]. At 10 min, the mean SBP/DBP change was + 8.0/+ 7.4 mmHg [22], + 8.9/+6.2 mmHg [17], + 6.6/+ 3.0 mmHg [15], no change/+1.9 mmHg [18], and − 1/no change [23]. And at 20 min, the mean SBP/DBP change was + 5.8/+ 6.8 mmHg [14]. Vlachopoulos et al. did not provide numerical results but reported a significant increase of SBP in this group vs. placebo at 30 min. Franzen et al. graphically reported an increase of SBP and DBP at 40 min. Antoniewicz et al. reported a non-significant mean change of + 2 mmHg for SBP and a significant mean change of + 4.9 mmHg for DBP 2 h after the intervention. Chaumont et al. also reported an increase of SBP and DBP at 2 h that was significant for DBP.
Table 4.
Experimental studies in smokers, intervention description, groups and results for SBP and DBP
| Author, year | Intervention | Groups | Results for SBP (group mean (SD)) | Results for DBP (group mean (SD)) |
|---|---|---|---|---|
|
| ||||
| Biondi-Zoccai, 2019 | 6 cycles of one product use: 9 e-cig puffs/1 week washout | Nico. e-cig (16 mg/mL) | (Baseline/10 min): Nico e-cig: 121.7(6.5)/130.6(6.5) p < 0.001 |
(Baseline/10 min): Nico e-cig: 72.2(4.4)/78.4(4.8) p < 0.001 |
| Kerr, 2019 | 2 cycles of 1 product use: 15 e-cig puffs/24 h washout | Nico. e-cig (18 mg/mL) | (Baseline/10 min): Nico. e-cig: 124(12)/123(11) NS |
(Baseline/10 min): Nico. e-cig: 80(11)/80(10) NS |
| Antoniewicz, 2019 | 2 cycles of one product use: 30 e-cig puffs in 30 min/1 week washout | Nico. e-cig (19 mg/mL) No nico. e-cig |
(Baseline/0 min/10 min/20 min/30 min/2 h/4 h): Nico. e-cig: 109.4(9.5)/119.3(9.5)p < 0.05/117.4(13)p < 0.05/113.7(10.3)NS/114.5(12)NS/111.1(10.1)NS/109.1(9.5)NS No nico. e-cig: 109.3(10.3)/114.5(13.2) p < 0.05/111.2(16.1)p < 0.05/109.3(15.5)NS/108.8(15.4)NS/109(10.2)NS/108.8(11.7)NS |
(Baseline/0 min/10 min/20 min/30min/2 h/4 h): Nico. e-cig: 70.3(5.7/78.9(5.9) p < 0.05/77.7(6.6)p < 0.05/76.5(6.6) p < 0.05/74.9(5.8)p < 0.05/72.6(5.4)NS No Nico. e-cig: 70.2(5.8)/74.5(6.9)p < 0.05/72.7(82)p < 0.05/71.1(8.1)p < 0.05/72.2(8)p < 0.05/72(6.5)NS/69.8 (6.6) NS |
| Chaumont, 2018 | 3 cycles of one product use. 25 puffs at 30 s interval: e-cig or placebo (e-cig turned off under supervision)/washout NR | Nico. e-cig (19 mg/mL) No Nico. e-cig Placebo |
(Baseline during exposure/60 min/120 min): Nico. e-cig: 109(1)/121(2)p < 0.001/p < 0.05/NS No Nico. e-cig 111(2)/118(5)p <0.001/NS/NS Placebo: NS |
(Baseline/during exposure/60 min/120 min): Nico. e-cig: 68(1)/78(2)p < 0.001/p < 0.05/p < 0.05 No nico. e-cig: 68(1)/73(2)p < 0.001/NS/NS Placebo: NS |
| Franzen, 2018 | 3 cycles of one product use. 10 e-cig puffs at 30s interval/48 h washout | Nico. e-cig (24 mg/mL) No Nico. e-cig |
(Baseline/ 15 min/30 min/40 min): Nico e-cig Mean (SD)NR/increase p < 0.05/increase p < 0.05/increase p < 005 No nico. e-cig no change NS |
(Baseline/15 min/30 min/40 min): Nico.e-cig: mean (SD)NR/increase NS/increaseNS/increase NS No nico. e-cig: decreased DBP (p < 0.05) at 30 min |
| Vlachaupoulos, 2016 | 4 cycles of one product use. 5 min e-cig. 30 min e-cig. 60 min placebo/washout NR | Nico. e-cig Placebo |
e-cig vs sham at 5 and 30 min p < 0.05 | NR |
| Yan, 2015 | 2 cycles of one product use. 50 puffs at 30s intervals e-cig or free use for 1 h/washout NR | Nico. e-cig (2.4%) A, B, C (1.6%) D, E | Mean (SD) change (baseline/20 min): A-B-C: 1.1 (11.1) NS-2.8 (11.3) NS-4.0 (10.0) NS D-E: 5.8 (10.0) p = 0.02–3.8 (10.7)p = 0.10 |
Mean (SD) change (baseline/20 min): A-B-C: 6.8 (6.7)p < 0.001–6.8 (6.5) p < 0.001–3.2 (7.3) p < 0.05 D-E: 6.8 (3.8)p < 0.001–6.8 (7.1) p < 0.001 |
| Farsalinos, 2014 | one product free use for 7 min/washout NR | Nico. e-cig (11 mg/mL) | Mean (SD) change (baseline/10 min): Nico. e-cig: 0.7 (4.6) NS |
Mean (SD) change (baseline/10 min): Nico. e-cig: 3.0 (3.6) p < 0.001 |
| Czogała, 2012 | cycles of one product use ad lib/1 week washout | Nico. e-cig (14 mg/mL) | (Baseline/post-intervention): Nico. e-cig: 122.6(11.4) / 122.5 (12.6) NS |
(Baseline/post-intervention): Nico. e-cig: 76.7(9.5)/78.6(10.8) NS |
| Szolty, 2014 | Cycles of one product use. 15 puffs e-cigarette/1 day washout | Nico. e-eig (24 mg/mL) | Both cigarette and e-cig showed a small increase in SBP, DBP but it was not significant | |
Nico., nicotine. NS., nonsignificant. NR., non-reported
Three studies conducted in smokers included a no nicotine e-cigarette arm, and all three studies found increases in SBP during the intervention. The findings for DBP were variable. The mean SBP/DBP change immediately after the intervention compared to baseline was + 5.2/+ 4.3 mmHg [22] and + 7/+ 5 mmHg [20]. At 10 min, the mean SBP/DBP change was + 2.0/+ 2.5 mmHg [22]. Antoniewicz et al. reported a significant mean change of + 2 mmHg for DBP 2 h after the intervention. Chaumont et al. graphically reported increases of SBP and DBP compared to baseline for 2 h after the intervention. This study included a placebo arm that experienced no significant changes in BP levels. Franzen et al. graphically reported an increase in SBP at 60 min vs baseline that was not statistically significant, and a significant decrease in DBP at 10 and 30 min. All the studies that included a traditional cigarette arm found elevations of SBP and DBP for this group (data not shown). A graphic visualization of the short-term SBP and DBP effects of e-cigarette use with and without nicotine and placebo device in nonsmokers and smokers in the experimental studies is represented in Fig. 2.
Fig. 2.
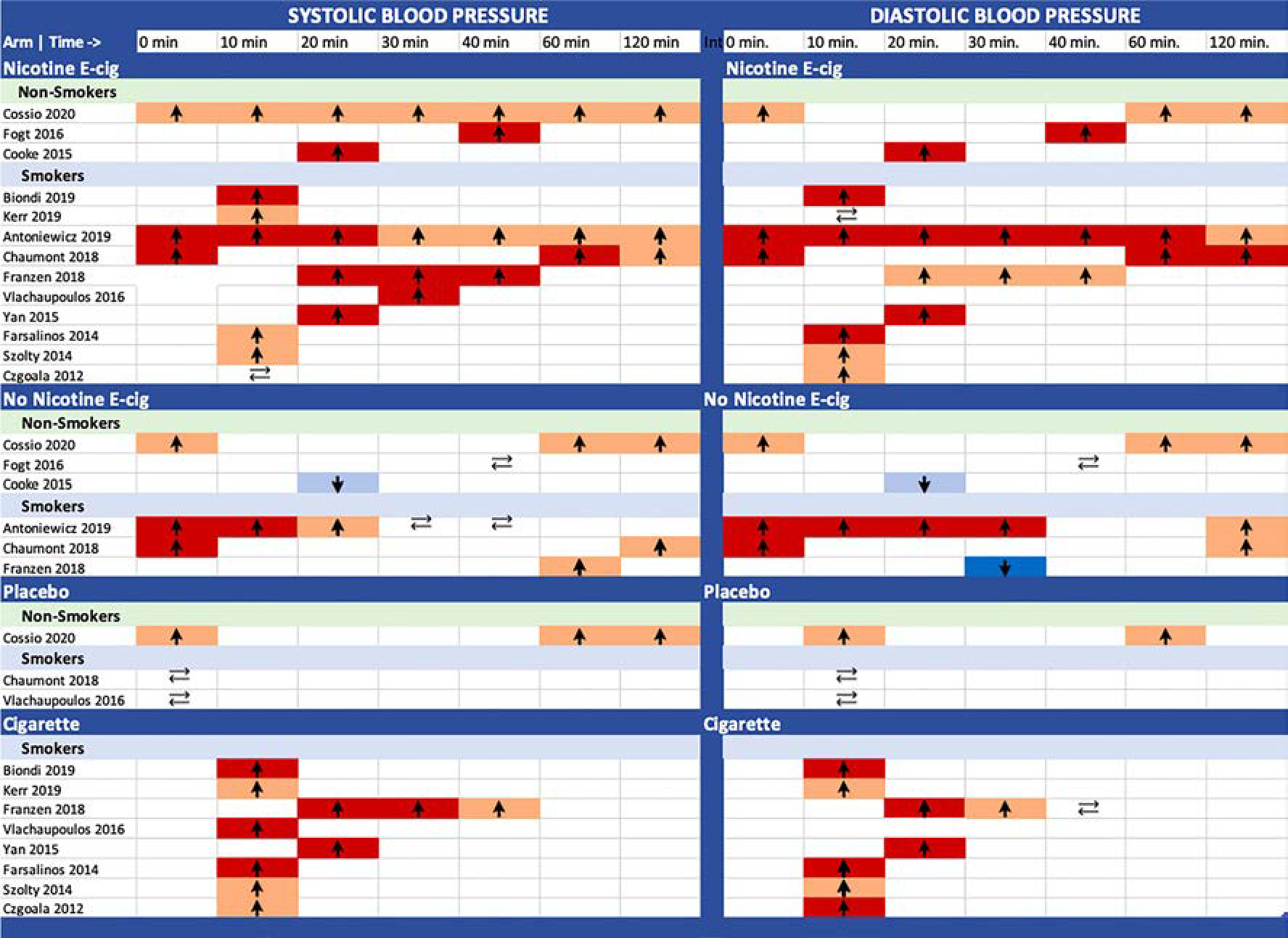
Summary of the short-term SBP and DBP effects (between 0 and 120 min) of e-cigarette use with and without nicotine and placebo device in nonsmokers and smokers. Arrows indicate if blood pressure increased, decreased or remained the same. Light and dark red reflect nonsignificant and significant increases, light and dark blue reflect nonsignificant and significant decreases, respectively.
We identified one observational study reporting the association between e-cigarette use and blood pressure endpoints [10•]. This study was a prospective 3.5 years follow-up study with a cohort of 9 daily exclusively e-cig users for more than 3 months and a control group of 12 people naïve to any tobacco products. E-cig users were recruited from a pool of vape shops customers and the control participants were selected from hospital staff at the “Centro per la Prevenzione e Cura del Tabagismo” of the University of Catania, Italy, and matched by age and sex with the e-cig users. One initial and 3 follow-up visits were scheduled until the end of follow-up at 3.5 years. Participants were asked to use their own e-cig devices and were instructed not to vape or consume any caffeine at least 60 min before the BP measurement. A discrete increase in SBP and a discrete decrease of DBP at the end of the follow up period were reported for the e-cig group. The mean change for SBP/DBP at 3 years was + 3/− 3 mmHg for the e-cig group and + 1/− 1 mmHg for the control group. None of the changes was significant during the follow-up period. The study description and results are summarized in (Table 5).
Table 5.
Polosa et al., epidemiological study description and results
| Author, year | Study design | Population | Mean Age (SD) | %Men | Nicotine levels | Blood pressure measures | Results for SBP (baseline/1/2/3 years) | Results for DBP (baseline/1/2/3 years) |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Polosa, 2017 | Prospective cohort (3.5 years follow-up) |
Daily e-cig users (n = 9) and never smokers (controls) (n = 12) | e-cig users/controls 26.6 (6.0)/27.8 (5.2) years | 66% | 0% (n = 3) 0.9–1.2% (n = 4) 1.6–1.8% (n = 2) |
2 SBP/DBP measures in seated position after 5 min of rest at baseline and follow-up visits at 1, 2, and 3 years | e-cig 115(9)/116(5)/114(9)/118(10) controls: 117(9)/117(10)/116(10)/116(9) p value effect between groups = 0.82 |
e-cig: 79(6)/78(4)/73(9)/76(8) controls: 74(9)/76(6)/75(9)/73(9) p value effect between groups = 0.50 |
Discussion
Intervention studies on the short-term effects of e-cig use on blood pressure endpoints showed a consistent increase of blood pressure immediately to several hours after exposure to e-cigs containing nicotine, variable changes after exposure to non-nicotine e-cigs, including significant increases of SBP and/or DBP, and no changes when using a placebo device. The study populations were heterogeneous including tobacco smokers, nonsmokers, or e-cig users. The intervention arms, protocols, and e-cig devices, including their nicotine content and other e-liquid and coil components, varied among studies, representing potential limitations for their comparability.
The short-term increases in SBP and DBP for the nicotine e-cig groups are consistent with previous research attributing these increases to the activation of the sympathetic nervous system mediated by nicotine in the e-cig aerosol [14, 24]. Nicotine stimulates the sympathetic nervous system, producing an increase in heart rate, blood pressure, and myocardial contractility [2, 25]. The sympathetic activation is dependent on plasma levels of nicotine. Some studies, especially those conducted with first generation e-cigs, have shown a lower concentration of plasma nicotine comparing e-cig use to smoking tobacco cigarettes. However, new generations of e-cig devices have increased the amount of nicotine in the aerosol and have enhanced nicotine delivery, resulting in similar plasma concentrations compared to traditional cigarettes [26]. The short-term effects of nicotine on the sympathetic nervous system have been widely studied in tobacco cigarettes [27, 28].
Higher concentrations of urinary cotinine were reported in nicotine e-cig users compared to non-nicotine e-cig users. Fogt et al. reported significantly higher urine cotinine levels in between the group of e-cig users with (30–100 ng/ml) vs. without (0–10 ng/ml) (p < 0.001) in a study included in our review [13].
The studies that included a non-nicotine e-cig arm also found increases of blood pressure in the short term, although those increases tended to be smaller compared to those found for nicotine e-cigs. These findings suggest the potential existence of blood pressure elevation mechanisms other than nicotine, mediated by other compounds in the e-cig aerosol. Recent human experimental studies with no nicotine e-cigs have documented an increase in endothelial dysfunction parameters in the short term [22, 29]. The studies in our review that included a placebo arm did not report any significant changes in blood pressure, supporting this hypothesis. Franzen et al. reported a decrease in DBP after the use of no nicotine e-cigs. The authors attribute the decrease in blood pressure to the relaxation caused by the use of a device which supports the finger-mouth coupling mechanism. They stated that this mechanism could have influenced all groups, lowering their expected increase in BP [21].
E-cigarettes aerosol contains a variable concentration of carbonyl derivatives, metals, particulate matter, and flavoring [6••]. The concentration of these substances in the aerosol is variable among devices and the mechanisms by which they could potentially impact the cardiovascular and respiratory system in humans when inhaled have yet not been clearly stablished. Nevertheless, the molecular mechanisms of action of these compounds have been studied in laboratory and animal models as well as in epidemiologic studies.
Carbonyl derivatives like formaldehyde, acrolein, or acetaldehyde have been widely documented in exhaled e-cig aerosols in a higher concentration than background breath [30,31•]. These aldehydes are derived from propylene glycol, vegetable glycerin, and some flavorings [32]. Formaldehyde and acrolein cause intracellular oxidative stress and endothelial dysfunction as well as alterations in myocardial contractibility. A recent ex vivo experimental study in cultured endothelial cells has observed that exposure to these aldehydes increases NOX-2 expression which increases the oxidative stress in vascular and brain tissues [33•]. Formaldehyde and acrolein are classified as human carcinogens and long-term effects on coagulation and myocardial remodulation on animal models have been observed after exposure to these toxicants [34, 35].
There are more than 7000 flavorings available in the market; many of them are widely used in e-cigs. These chemicals are safe for ingestion but their inhalation has been associated with respiratory diseases [36]. While their cardiovascular toxicity is not yet well understood, flavoring chemicals have been shown to cause oxidative stress and cytotoxic and proinflammatory effects on endothelial cells in laboratory studies [37]. E-cig aerosol also constitutes a source of variable amount of ultrafine and fine particulate matter [38]. There is a causal association between exposure to particulate matter and hypertension [39]. Ultrafine particulate matter cause direct endothelial dysfunction and increased oxidative stress and prothrombotic activity [38, 40, 41].
Recently published reviews of preclinical and clinical studies assessing cardiovascular risk of e-cigs use cite oxidative stress, inflammation, DNA damage, and abnormal coagulation as potential mechanisms of cardiovascular harm that may be caused by e-cig aerosol compounds. The authors remark the relevance of assessing the role of inhalation of flavoring molecules in cardiovascular health and recommend a cautious use of e-cig until their long term health effects are understood [31•,42••,43].
The endothelium plays a crucial role in vascular homeostasis, regulating the vascular tone, balance between vasodilator and vasoconstrictor factors, smooth muscle proliferation, and inflammatory processes. Vasoactive molecules like nitric oxide (NO), prostacyclins, adenosine, or bradykinin are released by the endothelium [44, 45]. Inflammation, oxidative stress, and ROS production contribute to endothelial dysfunction, which is a precursor of atherosclerosis and cardiovascular risk [45]. Increasing evidence suggests that oxidative stress and endothelial dysfunction also play a role in hypertension development [44, 46]. However, it is still not completely understood whether these processes are causal agents or consequences of hypertension [47, 48].
Besides carbonyl derivatives, flavorings, and particulate matter, the e-liquid and the coil represent sources of exposure to metals [49,50•]. The association between metals and cardiovascular diseases is well known [51–53]. In studies of daily e-cig users, we have estimated that the documented concentrations of metals in the e-cig aerosol is above the recommended guidelines for aerosol samples collected from devices of around half of the participants [50•,54]. The use of e-cigs thus represents an unnecessary source of exposure to metals.
The studies included in our systematic review suggest the short-term effects between e-cig use and elevation of blood pressure endpoints. However, there is a need for prospective studies that assess the long-term consequences on blood pressure elevation and the risk of developing hypertension. There is limited evidence that explain the mechanism of action and long-term outcomes of e-cigarettes use. Moreover, few pro-spective studies are available and additional research is needed to evaluate these long-term effects. In particular, large-scale population studies, which are currently not available, are needed to assess the effects of e-cig use on blood pressure levels outside of the experimental settings.
Conclusion
This systematic review supports that the use of e-cig with and without nicotine results in short-term elevations of both SBP and DBP. The mechanisms that underlie this relationship are incompletely understood, particularly for e-cigs without nicotine. E-cig aerosol contains several toxic components that increase oxidative stress and endothelial dysfunction in preclinical and laboratory studies. Aldehydes, flavoring molecules, metals, and particulate matter could represent an additional source of oxidative-stress inducing compounds, particularly for non-smokers. While prospective research is needed to evaluate the possible long-term effects of e-cigs on hypertension and cardiovascular risk, the short-term vascular effects support the need to further prevent the initiation of e-cig use, especially in never smokers.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Compliance with Ethical Standards
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16(4):223–37. 10.1038/s41581-019-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanaway JD, Afshin A, Gakidou E, Lim SS, Abate D, Abate KH, et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990 2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1923–94. 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The health con-sequences of smoking—50 years of progress: a report of the surgeon general [Internet]. Atlanta (GA): Centers for Disease Control and Prevention (US); 2014. (Reports of the Surgeon General). Available from: http://www.ncbi.nlm.nih.gov/books/NBK179276/. ••.This report summarizes the burden of disease and death caused by tobacco smoke in the United States and the progress in smoking reduction between 1964 and 2014.
- 4.Wang TW, Neff LJ, Park-Lee E, Ren C, Cullen KA, King BA. E-cigarette Use Among Middle and High School Students — United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1310–2. 10.15585/mmwr.mm6937e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.QuickStats: cigarette smoking status among current adult e-cigarette users, by age group — national health interview survey, United States, 2015. MMWR Morb Mortal Wkly Rep 2016;65: 1177. DOI: 10.15585/mmwr.mm6542a7externalicon. [DOI] [PubMed] [Google Scholar]
- 6. National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Population Health and Public Health Practice, Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems. In: Eaton DL, Kwan LY, Stratton K, editors. Public Health Consequences of E-Cigarettes. Washington (DC): National Academies Press (US); 2018. ••.This is a comprehensive review of the emerging evidence assessing the use and health implications of e-cigarettes. This review provides guidance and recommendations for conducting research in this field and highlights research gaps that should be given priority in the future.
- 7.Products C for T. vaporizers, e-cigarettes, and other electronic nicotine delivery systems (ENDS). FDA; [Internet]. 2020. Apr 13. Available from: https://www.fda.gov/tobacco-products/products-ingredients-components/vaporizers-e-cigarettes-and-other-electronic-nicotine-delivery-systems-ends [Google Scholar]
- 8.Hahn J, Monakhova YB, Hengen J, Kohl-Himmelseher M, Schüssler J, Hahn H, et al. Electronic cigarettes: overview of chemical composition and exposure estimation. Tob Induc Dis. 2014;12(1):23. 10.1186/s12971-014-0023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206–52. [DOI] [PubMed] [Google Scholar]
- 10. Polosa R, Cibella F, Caponnetto P, Maglia M, Prosperini U, Russo C, et al. Health impact of E-cigarettes: a prospective 3.5-year study of regular daily users who have never smoked. Sci Rep. 2017. Dec;7(1):13825. Doi: 10.1038/s41598-017-14043-2. •.This study is an observational study included in the present review. It is a 3.5 years prospective cohort study in exclusively e-cigarette users with a control group.
- 11.Cooke WH, Pokhrel A, Dowling C, Fogt DL, Rickards CA. Acute inhalation of vaporized nicotine increases arterial pressure in young non-smokers: a pilot study. Clin Auton Res. 2015;25(4):267–70. 10.1007/s10286-015-0304-z. [DOI] [PubMed] [Google Scholar]
- 12.Cossio R, Cerra ZA, Tanaka H. Vascular effects of a single bout of electronic cigarette use. Clin Exp Pharmacol Physiol. 2020;47(1): 3–6. 10.1111/1440-1681.13180. [DOI] [PubMed] [Google Scholar]
- 13.Fogt DL, Levi MA, Rickards CA, Stelly SP, Cooke WH. Effects of acute vaporized nicotine in non-tobacco users at rest and during exercise. Int J Exerc Sci. 2016;9(5):607–15. [PMC free article] [PubMed] [Google Scholar]
- 14.Yan XS, D’Ruiz C. Effects of using electronic cigarettes on nicotine delivery and cardiovascular function in comparison with regular cigarettes. Regul Toxicol Pharmacol. 2015;71(1):24–34. 10.1016/j.yrtph.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Farsalinos Tsiapras D, Kyrzopoulos S, Savvopoulou M, Voudris V. Acute effects of using an electronic nicotine-delivery device (electronic cigarette) on myocardial function: comparison with the effects of regular cigarettes. BMC Cardiovasc Disord. 2014. Dec;14(1):78. Doi: 10.1186/1471-2261-14-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vlachopoulos C, Ioakeimidis N, Abdelrasoul M, Terentes-Printzios D, Georgakopoulos C, Pietri P, et al. Electronic cigarette smoking increases aortic stiffness and blood pressure in young smokers. J Am Coll Cardiol. 2016;67(23):2802–3. 10.1016/j.jacc.2016.03.569. [DOI] [PubMed] [Google Scholar]
- 17.Biondi-Zoccai G, Sciarretta S, Bullen C, Nocella C, Violi F, Loffredo L, et al. Acute effects of heat-not-burn, electronic vaping, and traditional tobacco combustion cigarettes: the Sapienza University of Rome-Vascular Assessment of Proatherosclerotic Effects of Smoking (SUR-VAPES) 2 Randomized Trial. JAHA. 2019. Mar 19;8(6). Doi: 10.1161/JAHA.118.010455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Czogała J, Mateusz C, Kutek A, Zielińska-Danch W. lekarski Citation Przeglad. Evaluation of changes in hemodynamic parameters after the use of electronic nicotine delivery systems among regular cigarette smokers. Przeglad lekarski. 2012;69(10):841–5. [PubMed] [Google Scholar]
- 19.Szołtysek-Bołdys I, Sobczak Andrzej, Zielińska-Danch Bartoń, Aleksandra Bartosz K, Kośmider Leon. Influence of inhaled nicotine source on arterial stiffness. Przeglad lekarski. 2014;71(11): 572. Doi: 23421044. [PubMed] [Google Scholar]
- 20.Chaumont M, de Becker B, Zaher W, Culié A, Deprez G, Mélot C, et al. Differential effects of E-cigarette on microvascular endothelial function, arterial stiffness and oxidative stress: a randomized crossover trial. Sci Rep. 2018;8(1):10378. 10.1038/s41598-018-28723-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franzen KF, Willig J, Cayo Talavera S, Meusel M, Sayk F, Reppel M, et al. E-cigarettes and cigarettes worsen peripheral and central hemodynamics as well as arterial stiffness: a randomized, double-blinded pilot study. Vasc Med. 2018;23(5):419–25. 10.1177/1358863X18779694. [DOI] [PubMed] [Google Scholar]
- 22.Antoniewicz L, Brynedal A, Hedman L, Lundbäck M, Bosson JA. Acute effects of electronic cigarette inhalation on the vasculature and the conducting airways. Cardiovasc Toxicol. 2019;19(5):441–50. 10.1007/s12012-019-09516-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerr DMI, Brooksbank KJM, Taylor RG, Pinel K, Rios FJ, Touyz RM, et al. Acute effects of electronic and tobacco cigarettes on vascular and respiratory function in healthy volunteers: a crossover study. J Hypertens. 2018;1. 10.1097/HJH.0000000000001890. [DOI] [PubMed] [Google Scholar]
- 24.Moheimani RS, Bhetraratana M, Yin F, Peters KM, Gornbein J, Araujo JA, et al. Increased cardiac sympathetic activity and oxidative stress in habitual electronic cigarette users: implications for cardiovascular risk. JAMA Cardiol. 2017;2(3):278–84. 10.1001/jamacardio.2016.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Middlekauff HR, Park J, Moheimani RS. Adverse effects of cigarette and noncigarette smoke exposure on the autonomic nervous system: mechanisms and implications for cardiovascular risk. J Am Coll Cardiol. 2014;64(16):1740–50. 10.1016/j.jacc.2014.06.1201. [DOI] [PubMed] [Google Scholar]
- 26.St Helen G, Havel C, Dempsey DA, Jacob P, Benowitz NL. Nicotine delivery, retention and pharmacokinetics from various electronic cigarettes. Addiction. 2016;111(3):535–44. 10.1111/add.13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hackshaw A, Morris JK, Boniface S, Tang J-L, Milenković D. Low cigarette consumption and risk of coronary heart disease and stroke: meta-analysis of 141 cohort studies in 55 study reports. BMJ. 2018. 10.1136/bmj.k5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu L, Mackay DF, Pell JP. Meta-analysis of the association between cigarette smoking and peripheral arterial disease. Heart. 2014;100(5):414–23. 10.1136/heartjnl-2013-304082. [DOI] [PubMed] [Google Scholar]
- 29.Caporale A, Langham MC, Guo W, Johncola A, Chatterjee S, Wehrli FW. Acute effects of electronic cigarette aerosol inhalation on vascular function detected at quantitative MRI. Radiology. 2019;293(1):97–106. 10.1148/radiol.2019190562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogunwale MA, Li M, Ramakrishnam Raju MV, Chen Y, Nantz MH, Conklin DJ, et al. Aldehyde detection in electronic cigarette aerosols. ACS Omega. 2017;2(3):1207–14. 10.1021/acsomega.6b00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Skotsimara G, Antonopoulos AS, Oikonomou E, Siasos G, Ioakeimidis N, Tsalamandris S, et al. Cardiovascular effects of electronic cigarettes: a systematic review and meta-analysis. Eur J Prev Cardiol. 2019;26(11):1219–28. Doi: 10.1177/2047487319832975. •.This recent systematic review and meta-analysis of literature published between 2000 and 2017 assessing e-cig use and various cardiovascular disease endpoints.
- 32.Conklin DJ, Ogunwale MA, Chen Y, Theis WS, Nantz MH, Fu X-A, et al. Electronic cigarette-generated aldehydes: the contribution of e-liquid components to their formation and the use of urinary aldehyde metabolites as biomarkers of exposure. Aerosol Sci Technol. 2018;52(11):1219–32. 10.1080/02786826.2018.1500013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kuntic M, Oelze M, Steven S, Kröller-Schön S, Stamm P, Kalinovic S, et al. Short-term e-cigarette vapour exposure causes vascular oxidative stress and dysfunction: evidence for a close connection to brain damage and a key role of the phagocytic NADPH oxidase (NOX-2). European Heart Journal. 2019. Nov 13;ehz772. Doi: 10.1093/eurheartj/ehz772. • This in vitro experimental study addresses in depth the potential role of the phagocytic NADPH oxidase (NOX-2) route as mediator for vascular damage and oxidative stress from e-cig use.
- 34.Lynch J, Jin L, Richardson A, Conklin DJ. Tobacco smoke and endothelial dysfunction: role of aldehydes? Curr Hypertens Rep. 2020;22(9):73. 10.1007/s11906-020-01085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takeshita D, Nakajima-Takenaka C, Shimizu J, Hattori H, Nakashima T, Kikuta A, et al. Effects of formaldehyde on cardiovascular system in in situ rat hearts. Basic Clin Pharmacol Toxicol. 2009;105(4):271–80. 10.1111/j.1742-7843.2009.00442.x. [DOI] [PubMed] [Google Scholar]
- 36.Allen JG, Flanigan SS, LeBlanc M, Vallarino J, MacNaughton P, Stewart JH, et al. Flavoring chemicals in E-cigarettes: diacetyl, 2,3-pentanedione, and acetoin in a sample of 51 products, including fruit-, candy-, and cocktail-flavored E-cigarettes. Environ Health Perspect. 2016;124(6):733–9. 10.1289/ehp.1510185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muthumalage T, Prinz M, Ansah KO, Gerloff J, Sundar IK, Rahman I. Inflammatory and oxidative responses induced by exposure to commonly used e-cigarette flavoring chemicals and flavored e-liquids without nicotine. Front Physiol. 2017;8:1130. 10.3389/fphys.2017.01130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernández E, Ballbè M, Sureda X, Fu M, Saltó E, Martínez-Sánchez JM. Particulate matter from electronic cigarettes and conventional cigarettes: a systematic review and observational study. Curr Environ Health Rep. 2015;2(4):423–9. 10.1007/s40572-015-0072-x. [DOI] [PubMed] [Google Scholar]
- 39.Liang R, Zhang B, Zhao X, Ruan Y, Lian H, Fan Z. Effect of exposure to PM2.5 on blood pressure: a systematic review and meta-analysis. J Hypertens. 2014;32(11):2130–40; discussion 2141. 10.1097/HJH.0000000000000342. [DOI] [PubMed] [Google Scholar]
- 40.Brook RD, Sanjay R, Arden PC, Brook Jeffrey R, Aruni B, Diez-Roux Ana V, et al. Particulate matter air pollution and cardiovascular disease. Circulation. 2010;121(21):2331–78. 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 41.Dai J, Chen W, Lin Y, Wang S, Guo X, Zhang Q-Q. Exposure to concentrated ambient fine particulate matter induces vascular endothelial dysfunction via miR-21. Int J Biol Sci. 2017;13(7):868–77. 10.7150/ijbs.19868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Buchanan ND, Grimmer JA, Tanwar V, Schwieterman N, Mohler PJ, Wold LE. Cardiovascular risk of electronic cigarettes: a review of preclinical and clinical studies. Cardiovasc Res. 2020. Jan 1;116(1):40–50. Doi: 10.1093/cvr/cvz256. ••.This review summarizes clinical and pre-clinical studies assessing the cardiovascular risk and molecular mechanism for several chemicals documented in the e-cig aerosol, including nicotine, aldehydes, particulate matter, carbonyl compounds and metals among others.
- 43.MacDonald A, Middlekauff HR. Electronic cigarettes and cardiovascular health: what do we know so far? Vasc Health Risk Manag. 2019;15:159–74. 10.2147/VHRM.S175970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brandes RP. Endothelial dysfunction and hypertension. Hypertension. 2014;64(5):924–8. 10.1161/HYPERTENSIONAHA.114.03575. [DOI] [PubMed] [Google Scholar]
- 45.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction. Circulation. 2007;115(10):1285–95. 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 46.Schulz E, Gori T, Münzel T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens Res. 2011;34(6):665–73. 10.1038/hr.2011.39. [DOI] [PubMed] [Google Scholar]
- 47.Dharmashankar K, Widlansky ME. Vascular endothelial function and hypertension: insights and directions. Curr Hypertens Rep. 2010;12(6):448–55. 10.1007/s11906-010-0150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galley HF, Webster NR. Physiology of the endothelium. Br J Anaesth. 2004;93(1):105–13. 10.1093/bja/aeh163. [DOI] [PubMed] [Google Scholar]
- 49.Pablo O, Walter G, Stefan T, Maria G-P, Stephanie J, Angela A, et al. Metal concentrations in e-cigarette liquid and aerosol samples: the contribution of metallic coils. Environ Health Perspect. 2018;126(2):027010. 10.1289/EHP2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Di Zhao, Atul Aravindakshan, Markus Hilpert, Pablo Olmedo, Rule Ana M., Navas-Acien Ana, et al. Metal/metalloid levels in electronic cigarette liquids, aerosols, and human biosamples: a systematic review. Environmental Health Perspectives. 2020;128(3):036001. Doi: 10.1289/EHP5686. • This review summarizes the presence of metals and metalloids in e-cig aerosol and e-liquid and its correlation with human biosamples of e-cig users. It concludes that levels of metals in e-cig users were similar or higher than levels found in biosamples of cigarette smokers.
- 51.Chowdhury R, Ramond A, O’Keeffe LM, Shahzad S, Kunutsor SK, Muka T, et al. Environmental toxic metal contaminants and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2018;362:k3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ana N-A, Eliseo G, Silbergeld EK, Rothenberg Stephen J. Lead exposure and cardiovascular disease—a systematic review. Environ Health Perspect. 2007;115(3):472–82. 10.1289/ehp.9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tellez-Plaza M, Guallar E, Navas-Acien A. Environmental metals and cardiovascular disease. BMJ. 2018;362. 10.1136/bmj.k3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao D, Navas-Acien A, Ilievski V, Slavkovich V, Olmedo P, Adria-Mora B, et al. Metal concentrations in electronic cigarette aerosol: effect of open-system and closed-system devices and power settings. Environ Res. 2019;174:125–34. 10.1016/j.envres.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


