Abstract
In the field of stroke thrombectomy, ineffective clinical and angiographic reperfusion after successful recanalization has drawn attention. Partial or complete microcirculatory reperfusion failure after the achievement of full patency of a former obstructed large vessel, known as the “no‐reflow phenomenon” or “microvascular obstruction,” was first reported in the 1960s and was later detected in both experimental models and patients with stroke. The no‐reflow phenomenon (NRP) was reported to result from intraluminal occlusions formed by blood components and extraluminal constriction exerted by the surrounding structures of the vessel wall. More recently, an emerging number of clinical studies have estimated the prevalence of the NRP in stroke patients following reperfusion therapy, ranging from 3.3% to 63% depending on its evaluation methods or study population. Studies also demonstrated its detrimental effects on infarction progress and neurological outcomes. In this review, we discuss the research advances, underlying pathogenesis, diagnostic techniques, and management approaches concerning the no‐reflow phenomenon in the stroke population to provide a comprehensive understanding of this phenomenon and offer references for future investigations.
Keywords: acute ischemic stroke, endovascular thrombectomy, microvascular disturbance, no‐reflow phenomenon, reperfusion therapy
The illustration of the consequences, pathogenesis, diagnostic methods, and promising management of the cerebral no‐reflow phenomenon.

1. INTRODUCTION
Acute ischemic stroke (AIS), especially in patients with intracranial proximal large vessel occlusion, is a major cause of mortality and morbidity worldwide. 1 Timely recanalization with intravenous thrombolysis and endovascular thrombectomy is currently the first choice for AIS management and has effectively improved the clinical outcomes of stroke patients. 2 However, there remains a gap between successful recanalization and a good prognosis, as almost half of the patients do not experience favorable outcomes despite successful thrombectomy and the full patency of the occluded artery being achieved. 3 This discrepancy may be ascribed to many reasons, including established infarction preceding the recanalization, premorbid poor collateral reserve, comorbidities, and complications. It is significant to note that successful recanalization might not be equivalent to successful reperfusion, as recent studies have found that over 30% of patients who achieved a modified Thrombolysis in Cerebral Ischemia (mTICI) score of 3 after thrombectomy, which in general, is considered as complete patency of the affected vessel, still suffered from sustained hypoperfusion in certain areas. 4 , 5 Such perfusion deficiency after successful recanalization is known as the no‐reflow phenomenon (NRP), the clinical manifestation of the functional and structural alterations in the microcirculation during the ischemia–reperfusion process. 6 It is worth noting that the concept of NRP is not identical to concepts like Futile Reperfusion (FR) 7 and Clinical Ineffective Reperfusion (CIR), 8 which are more extensively used and indicate unfavorable functional outcomes despite successful recanalization whether under guideline‐recommended management or not. The NRP specifically refers to the microcirculation reperfusion failure despite recanalization of the occluded large artery, which offers insights into the understanding of possible courses of both FR and CIR. To date, the NRP has been detected in various circulatory systems, including the heart, kidney, and skeletal muscle. Inspiringly, research has developed rapidly regarding the risk factor screening, diagnosis, and management of the NRP in coronary diseases. 9 However, not as much has been realized in stroke research, despite the fact that this phenomenon was first detected in the brain rather than in the heart. Therefore, this review will briefly introduce the research history of the cerebral NRP, summarize its underlying pathogenesis, and provide a contemporary overview of its diagnostic and management strategies to offer a deeper insight into this field and advance future studies.
2. HISTORY OF NRP RESEARCH
In 1968, the NRP was first described by Ames et al. 6 in a rabbit model. In their experiment, with prolongation of ischemia to 5 or 7.5 min, cerebral flow failed to be fully restored after relief of the vessel obstruction due to the blockage of capillaries by erythrocytes. Following this, microcirculation disturbance, with reperfusion deficiency after embolization removal, was discovered in other animal models, ranging from total to focal cerebral ischemia. 10 , 11 , 12 Ames et al. speculated that impaired microcirculation could be attributed to changes in the blood components, vascular lumen, or both. This conjecture was later confirmed. Under the microscope and in vivo two‐photon imaging, it was directly observed that the vascular lumen could be blocked by assorted blood components such as platelets, fibrin, and blood cells. 13 , 14 , 15 , 16 , 17 Moreover, exterior compression by compositions of the capillary wall and the surrounding structure also deteriorates lumen constriction. 12 , 18 , 19
Early research efforts were directed toward the exploration of potential pathophysiological mechanisms, while the incidence and clinical impact of the NRP in stroke patients were not investigated as thoroughly, due to the unavailability of effective revascularization strategies along with the restriction of imaging assessment techniques in clinical settings. 20 , 21 It was not until 1994, with the ubiquity of thrombolytic agents, that the NRP was detected in the human brain for the first time. Through single‐photon emission computed tomography (SPECT), researchers noted a mismatch between arterial recanalization and successful reperfusion in a stroke patient receiving streptokinase thrombolysis. 22
Later in the 2010s, the full patency of the obstructed artery could be realized and validated under angiography. 23 , 24 Some studies discovered that the reperfusion state is superior to the recanalization state in terms of prognosis prediction, indicating the significance of the post‐recanalization reperfusion failure. 4 , 25 , 26 Such a condition has once again set off an upsurge in the NRP research. Recent studies have focused on the investigation of the incidence, prognostic value, correlative risk factors, and management of the NRP in stroke patients who underwent recanalization therapy. 5 , 27
3. PATHOPHYSIOLOGICAL PERSPECTIVE
A thorough interpretation of the pathophysiological mechanisms of the NRP may help to develop evaluation techniques and effective management. In 1968, Ames et al. 6 first demonstrated the entrapped erythrocytes at the capillary level after recanalization, marking the first identification of the NRP. Later, under electron microscopy and erythrocyte autofluorescence, such a phenomenon was detected in other studies in both the ischemic core and the penumbra. 12 , 28 , 29
Microcirculatory disturbance related to the NRP may lead to both inadequate supply of the peripheral energy substrate and insufficient delivery of metabolites. Continuous hypoperfusion in the penumbra may lead to infarction expansion and further neurological impairment despite successful recanalization success. 30 Alternatively, persistent hypoperfusion in the ischemic core might potentially affect the resolution of edema, the clearance of necrotic tissues, and collateral angiogenesis, which would ultimately impact neural plasticity and tissue repair. While it is clear that the abnormal vascular events inducing the NRP occur at the microvasculature level, the underlying mechanisms still remain unclear. 31 According to current research, the NRP is primarily attributed to mechanical obstruction by blood components or clog fragmentation, and compression due to functional or structural change in the vessel wall or the surrounding cells (Figure 1).
FIGURE 1.
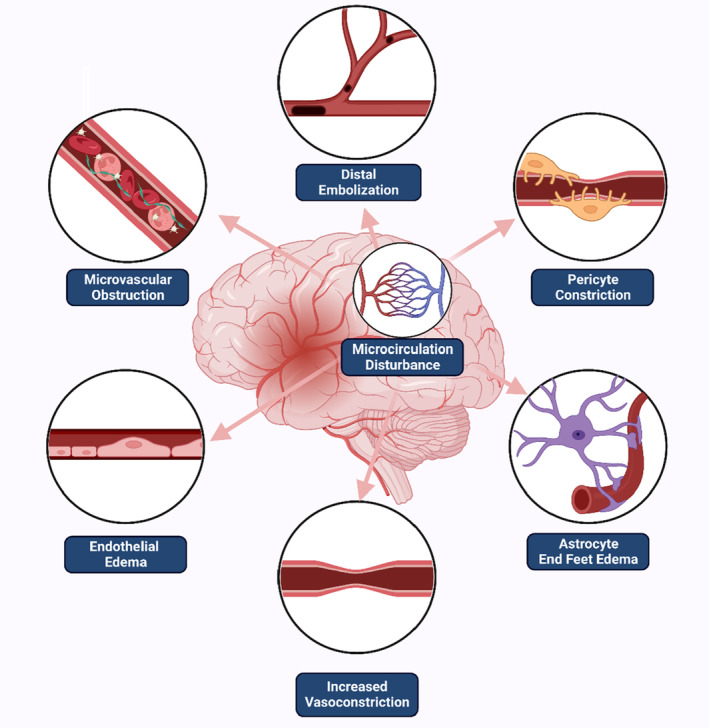
Illustration of the no‐reflow phenomenon pathogenesis (Created with BioRender.com) The no‐reflow phenomenon, which refers to the post‐recanalization reperfusion failure, is the clinical manifestation of microcirculation disturbance. The latter is primarily attributed to mechanical obstruction by blood components or clog fragmentation, and compression due to functional or structural change in the vessel wall or the surrounding cells.
3.1. Potential mechanisms accounting for the obstruction of the microcirculation
The blockage of the microcirculation may be secondary to distal embolization from the proximal cerebral arteries, in‐situ thrombosis, and microcirculatory stasis. During reperfusion therapy, a thrombus may arise from clot fragmentation, which could drift to distal vasculature, resulting in incomplete reperfusion after recanalization. 32 Recently, via high‐resolution MRI, studies have discovered the existence of peripheral emboli within the vascular territory after digital subtraction angiography (DSA)‐validated full patency of proximal large vessels. 33 This validated the role of clot fragments in microvascular circulation disturbance. Considering the amount and location of the fragments, it remains uncertain how such thrombus migration would influence the outcome of successful recanalization. 34 Additionally, the fracture properties and migration tendency may vary among clots of different sizes and compositions or diverse collateral conditions. 35 , 36
During an ischemic attack, the narrowed microvascular lumina may be clogged with platelets, leukocytes, and fibrin, which also contributes to hypoperfusion after vessel recanalization. Platelets play a pivotal role in thrombogenesis and microcirculation blockage after stroke. Thrombi that were abundant in platelets and erythrocytes were observed in the capillaries and precapillary arterioles in a baboon model of 3‐hour middle cerebral artery occlusion (MCAO) and blood flow restoration. 17 In addition to being involved in micro‐thrombi formation, platelets may also be implicated in the NRP, serving as a “bridge” in multifaceted interactions between cells and factors within the vascular system (e.g., neutrophils, P‐selectin), along with the neighboring capillary wall elements (e.g., pericytes, endothelium). 37 , 38 By releasing mediators and granules, activated platelets enhance the above interactions and further promote lesion progress. Consequently, antiplatelet treatment, which includes preventing platelet activation, adhesion, or aggregation, along with therapy that inhibits interactions between platelets and other elements, could improve blood flow after reperfusion in animal models. 39
Fibrin deposition, along with the activation of tissue factor‐mediated coagulation, is also involved in the impairment of microvascular patency. Okada et al. 40 found that in a primate reversible MCAO model, fibrin deposition in the microvasculature accumulated in a time‐dependent manner. More recently, via near‐infrared fluorescence (NIRF) with an FXIIIa‐targeted probe and histopathological analysis, Chen et al. 41 confirmed the existence of fibrin deposition and erythrocyte aggregation in the capillaries of mice after thrombolysis. Thomas et al. 15 detected an increase of reflow in micro‐vessels of all sizes after the inhibition of coagulation via the murine anti‐tissue factor monoclonal antibody TF9‐6B4, suggesting the potential effect of tissue factor‐mediated coagulation in microvascular perfusion deficit. The suppression of fibrinolysis, on the other hand, through the upregulation of the type 1 plasminogen activator inhibitor (PAI‐1) gene, may aggravate the fibrin deposition and further impair the microcirculation. 42
Another proposed mechanism of the NRP is capillary flow stagnation due to polymorphonuclear leukocyte blocking inside the lumen. Early in 1989, Grogaard et al. 43 discovered that intraperitoneal injections of an antineutrophil serum extracted from sheep could facilitate the reperfusion status in a rat model, suggesting the potential role of leukocytes in the NRP. The presence of polymorphonuclear leukocytes was then directly observed in occluded capillaries of baboons by light microscopy. 11 Two subsequent studies found leukocyte trapping in the microcirculation in MCAO models with early reperfusion. 44 , 45 Through two‐photon imaging, investigators directly observed that neutrophils plugged the capillaries in vivo after thrombolysis even with complete clot dissolution, while neutrophil depletion induced by the targeted antibody could facilitate capillary reperfusion and stroke recovery. 29 Different from permanent cellular plugs involved in no‐reflow, Erdener et.al discovered that neutrophils could stall in a more dynamic way in which they briefly and repetitively got stuck and released. 46 By blocking polymorphonuclear leukocyte adherence to the microvascular endothelium through anti‐inflammatory approaches, the NRP was seen to be suppressed post‐focal cerebral ischemia. 31
More recently, Strinitz et al. 47 collected blood samples from the occluded anterior circulation of stroke patients and detected that there was local neutrophil‐dominant immune cell recruitment, suggesting that there may be potential hazards of microvascular plugging and elevation of blood viscosity. This, in turn, may influence the retrograde collateral flow by increasing vascular resistance. 47 , 48 Another form of neutrophil involvement was neutrophil extracellular traps (NETs), a web‐like structure containing DNA released by a neutrophil. These traps play a pivotal role in thrombosis. Emerging evidence suggested the intraluminal occurrence of NETs after prolonged ischemia and the potential impact they may have on the microcirculation. 49 , 50
3.2. Factors affecting the diameter of the microcirculatory lumen
Cerebral capillary walls consist of endothelial cells, pericytes, basal lamina, and astrocyte end feet, which are distributed in a close alignment. Alternations in the microvascular and perivascular structures, such as swelling or constriction of the neighboring cells, can narrow the lumen, increase vascular resistance, and result in incomplete restoration of blood flow.
Pericytes are present at the outer surface of capillaries and the smallest venules. They are elongated cells, whose long cytoplasmic processes are wrapped around the endothelium. As a crucial component of the neurovascular unit, pericytes exert great influence on blood–brain barrier stabilization, blood flow regulation, immunomodulation, angiogenesis, and neurogenesis, thus providing support for other cellular components and maintaining the normal physiological function of the neurovascular unit. 51 , 52 The cytoplasm of pericytes contains actin, myosin, tropomyosin, and desmin, suggesting their capacity for contractile activity. Normally, contractile pericytes can modulate cerebral capillary resistance. 53 However, during ischemia or even after successful recanalization, pericytes might persistently contract due to multifaceted pathways including intracellular calcium overload, excitotoxicity, and oxidative‐nitrative stress. 19 , 53 , 54 , 55 Such contraction, along with subsequent pericyte death in rigor mortis, would cause long‐lasting capillary constriction and incomplete microvascular blood restoration, leading to limited oxygen supply and subsequent infarction progress with time. 19 , 56 On the contrary, one study dissented from the contribution of pericytes in microcirculation regulation. Instead, this study found arteriolar smooth muscle cells as the mediator of the capillary blood flow under both physiological and pathological conditions. 57
Increased vasoconstriction of parenchymal arterioles (PAs), in response to ischemia and reperfusion, also contributes to the microvascular perfusion deficit. PAs are long and relatively unbranched vessels connecting the pial vessels to the capillaries, and they have been shown to bottleneck the flow within the cortex due to their high resistance. During postischemic reperfusion, PAs may remain constricted. This influences the basal tone or myogenic reactivity and limits focal blood restoration in the ischemic region. 18 , 58
Functional and structural changes of other elements in the neurovascular unit have also been found to aggravate a narrowing lumen. Early in 1964, Hills et al. 59 reported astrocytic swelling in ischemic rat models but overlooked its potent impingement on the capillary lumen and perfusion. It was later found that the swelling of the perivascular astrocyte end‐feet would compress the capillaries. 12 , 20 Investigators additionally detected that the paucity of oxygen and substrates, due to blood flow interference, may lead to energy failure of the cellular sodium‐potassium pump and electrolyte disturbances, which are involved in the swelling of endothelial cells and astrocytes. 60 Furthermore, cerebral edema was also observed due to the breakdown of the blood–brain barrier and cerebrospinal fluid influx. 61 In conclusion, both cellular and perivascular swelling could lead to capillary compression, impair the patency of the micro‐vessels, and thus contribute to the NRP.
4. EVALUATION OF THE NRP
Despite abundant imaging and histological analytical methods available in animal models for direct visualization of microcirculation, practical challenges still exist over microcirculatory perfusion status assessment in stroke patients. Therefore, there are limited trials concerning the NRP prevalence in stroke patients with wide variation from 0% to 81% (as shown in Table 1). 26 , 27 , 62 This variation may be attributed to the difference in evaluation techniques and definitions of the NRP across studies, as some studies set a specific cutoff value to define reperfusion deficiency, while others consider a relative change from the baseline or even surrogate indicators of microcirculatory resistance for the NRP diagnosis. 63 , 64 , 65 , 66 At present, no consensus has been reached neither on the definition and diagnostic criteria for the NRP nor the optimal choice of diagnostic imaging techniques. Thus, we will discuss the relative merits and shortcomings of current imaging approaches with regard to the stroke population.
TABLE 1.
Previous studies investigating NRP in stroke patients.
| First author, year | Treatment | Recanalization | Reperfusion | Number of recanalized patients with hypoperfusion | |||||
|---|---|---|---|---|---|---|---|---|---|
| OTR | Image | Assessment time | Definition of recanalization | Image | Assessment time | Definition of hypoperfusion | |||
| Baird, 1994 22 | IA SK | 4–24 h | DSA | 24 h | Partial or complete | SPECT | 24 h | Perfusion level ≤12% of the homologous region | 1/4 (25%) |
| Yasaka, 1998 67 | IV SK or placebo | <4 h | TCD | 24 h | Partial or complete | SPECT | 24 h | Perfusion level ≤12% of the homologous region | 4/8 (50%) |
| Khatri, 2005 62 | IV + IA t‐PA | <3 h | DSA | On DSA completion | Complete (AOL III) | DSA | On DSA completion | TIMI 0–2 | 26/32 (81%) |
| Albers, 2006 77 | IV t‐PA | 4–24 h | MRA | 3–6 h after t‐PA | Partial or complete | PWI | 3‐6 h after t‐PA | Tmax ≥2 s | 4/19 (21%) |
| De Silva, 2009 63 | IV t‐PA | 3‐6 h | MRA | 3–5 days | Partial or complete (TIMI 2–3) | PWI | 3–5 days | Perfusion lesion (Tmax ≥2 s) Reduction ≤90% | 4/13 (31%) |
| Soares, 2010 78 | IV t‐PA ± MT or no treatment | <6 h | CTA | 24 h | Partial or complete (Recanalization Index >50%) | CTP | 24 h | CBV <2.0 mL/100 g, CBF <66%, MTT >145% | 5/13 (38%) |
| Bivard, 2013 69 | IV t‐PA or no treatment | <6 h | MRA | 24 h | Complete (TIMI3) | ASL | 24 h | Mean ± 2SD of normal pixels from the healthy hemisphere | 0% |
| Eilaghi, 2013 79 | IV t‐PA or no treatment | <4.5 h | CTA | ≤24 h | Partial or complete (TIMI 2–3) | CTP | ≤24 h | Absolute Tmax reperfusion index ≤58.7% | 14/58 (24%) |
| Marks, 2014 64 | MT ± t‐PA | <12 h | DSA | On DSA completion |
Complete (TICI 2b‐3) |
PWI | Within 12 h after the procedure | Perfusion lesion (Tmax>6 s) Reduction ≤50% | 7/47 (15%) |
| Horsch, 2015 80 | IV t‐PA or no treatment | <9 h | CTA | 3 days | Complete | CTP | 3 days | MTT >145% | 33 (40%) |
| Cho, 2015 26 | IV t‐PA or no treatment | <6 h | MRA | <6 h after stroke onset | Partial or complete (AOL II‐III) | PWI | <6 h after onset | Reperfusion ratio <50% | 0/13 (0%) |
| Carbone, 2019 81 | IV t‐PA ± MT or no treatment | <8 h | CTA | 24 h | Partial or complete (TIMI 2–3) | CTP | 24 h | Reduction of baseline MTT lesion (MTT>145%) ≤75% | 15/39 (38%) |
| Rubiera, 2020 4 | MT ± t‐PA | 287 min (177–492) | DSA | On DSA completion | Complete (mTICI 2b‐3) | CTP | ≤30 min after recanalization | Tmax ≥6 s |
2b: 29/46 (63%) 3: 40/94 (42.5%) |
| Schiphorst, 2021 27 | MT ± t‐PA | <24 h | DSA | On DSA completion | Complete (mTICI 2c‐3) | ASL | 24 h | Mean CBF Reduction ≥40% | 1/33 (3.3%) |
| Luby, 2021 82 | MT ± t‐PA | ≤12 h | DSA | On DSA completion | Complete (mTICI3) | PWI | 24 h | Tmax volume >10 mL with 6 s delay | 2/33 (6.67%) |
| Ng FC, 2022 5 | MT ± t‐PA/Tenecteplase | ≤4.5 h | DSA | 24 h | Complete (eTICI 2c‐3) | CTP or PWI | 24 h | rCBF or rCBV reduction >15% | 33/130 (25.3%) |
Abbreviations: AOL, arterial occlusion level; ASL, arterial spin labeling; CBF, cerebral blood flow; CBV, cerebral blood volume; CTA, computed tomography angiography; CTP, computed tomography perfusion; DSA, digital subtraction angiography; eTICI, expanded thrombolysis in cerebral infarction; IA, intraarterial; IV, intravenous; MRA, magnetic resonance angiography; MT, mechanical thrombectomy; mTICI, modified thrombolysis in cerebral infarction; MTT, mean transit time; OTT, onset‐to‐recanalization delay; PWI, perfusion‐weighted imaging; rCBF, regional CBF; rCBV, regional CBV; SD, standard deviation; SK, streptokinase; SPECT, single‐photon emission computed tomography; TCD, transcranial Doppler; TICI, thrombolysis in cerebral infarction; TIMI, thrombolysis in myocardial infarction; Tmax, time to max; t‐PA, tissue plasminogen activator.
In early studies, non‐invasive methods, including magnetic resonance angiography (MRA), computed tomography angiography (CTA), and transcranial Doppler ultrasound (TCD), were used to validate successful recanalization in patients receiving intravenous thrombolysis (IV), which only allow evaluation for proximal and relatively large arteries and may cause the overlook of evident distal occlusions. 63 , 67 Later in the era of thrombectomy, evaluation by digital subtraction angiography (DSA), performed instantly after EVT, enables direct observation of both macrovascular patency and dynamic collateral perfusion process with excellent spatial and temporal resolution. Grading scales such as the Thrombolysis in Myocardial Infarction (TIMI), Thrombolysis in Cerebral Infarction (TICI), modified TICI (mTICI), extended modified TICI scale (eTICI), and Arterial Occlusive Lesion (AOL) are applied for assessment. 68 Of note, different definitions of successful recanalization according to these grading scales may influence the estimated frequency of the NRP, as a rigorous standard (like TIMI 3) may lead to a potential risk of the underestimation of the prevalence, 69 while a moderate inclusion criterion (TICI2b‐2c) may include perfusion abnormalities related to persistent vessel occlusion rather than the microcirculatory disturbance and lead to the NRP overestimation. More recently, Jayme C. Kosior et al. demonstrated the feasibility and potential clinical utility of DSA perfusion (DSAP) which derives a quantitative assessment of perfusion status from standard DSA source images according to conventional contrast bolus‐tracking methodology. 66 However, it is of note that angiography‐based techniques are performed right after revascularization when patients are still in the catheterization room, thus it might be too early to detect the consistent hypoperfusion and fail to find the true frequency of NRP. Angiography also has limitations in routine evaluation due to its invasive nature and the potential risk of contrast agents.
Other non‐invasive imaging techniques also help to characterize the presence, extent, and localization of the NRP. In the early days, nuclear imaging approaches were applied to detect the NRP in stroke, which have limited use later due to the cost and radiation exposure, especially with the emergence and widespread use of other imaging techniques with high sensitivity and high resolution. 22 , 67 Parameters calculated from perfusion imaging like perfusion‐weighted imaging (PWI), diffusion‐weighted imaging (DWI), and computed tomographic perfusion (CTP), give a comprehensive and detailed description of perfusion mappings qualitatively and quantitatively, including time‐to‐maximum of the tissue residue function (Tmax), cerebral blood flow (rCBF), cerebral blood volume (rCBV), time to peak (TTP), or mean transit time (MTT). These perfusion imaging techniques are suitable for routine perfusion evaluation and detection of the NRP, while contrast agent injury and CT‐related radiation injury should be considered during utilization. Other studies implied arterial spin labeling (ASL) for perfusion assessment. ASL provides absolute quantification of CBF with high spatial and temporal resolution and is free of contrast medium injury while may endure the risk of motion artifacts. 27 , 69
Moreover, ultrasound‐based methods, such as transcranial Doppler (TCD), allow real‐time bedside monitoring of cerebral hemodynamic status with parameters, such as pulsatility index and resistance index, 70 , 71 which is especially suitable for patients with transportation inconvenience or limitation. TCD combined with servo‐controlled finger photoplethysmography (Finapres) enables the assessment of cerebral autoregulation (CA), which refers to the inherent properties of the cerebral vasculature to maintain relatively constant cerebral perfusion in response to rapid fluctuations in cerebral perfusion pressure. 72 However, TCD measurement refines to large supplying arteries (usually the MCA) while not available for measurement for flow at the brain tissue level. Whether TCD‐related parameters can reflect the perfusion status in microcirculation remains further discussion. More recently, optic‐based techniques, such as diffuse correlation spectroscopy (DCS), functional near‐infrared spectroscopy (fNIRS), functional interferometric diffusing wave spectroscopy (fiDWS), and optical coherence tomography‐based angiography (OCTA), are emerging tools for the dynamic detection of cerebral perfusion status, which are promising for the NRP assessment. 73 , 74 , 75 , 76 Meanwhile, it should be noted that either optic‐based or ultrasound‐based techniques only characterize perfusion status through surrogate indicators of hemodynamic changes instead of direct visualization and quantitation of microcirculation. Besides, such assessment is influenced by multiple variables such as caffeine intake, room temperature, cognitive activation, mental stress, etc., which brings challenges to the consistency of the experiment environment to ascertain the stability of parameter measurement. 72 Therefore, results from these approaches should be considered cautiously during interpretation.
In conclusion, the imaging techniques have their own pros and cons with regard to the specific stroke population and application scenario, it is far from certain which approach might be the best. Therefore, a multi‐phase and multi‐technique measurement of the NRP based on individual conditions, disease stage, and application scenario might be preferable for comprehensive NRP evaluation in future clinical settings.
5. MANAGEMENT OF THE NO‐REFLOW PHENOMENON
Clinical management of the cerebral NRP is still insufficient. However, many possibilities are currently being explored to reduce the incidence and extent of NRP in stroke patients by referring to experiences in preclinical studies and cardiovascular research (Figure 2).
FIGURE 2.
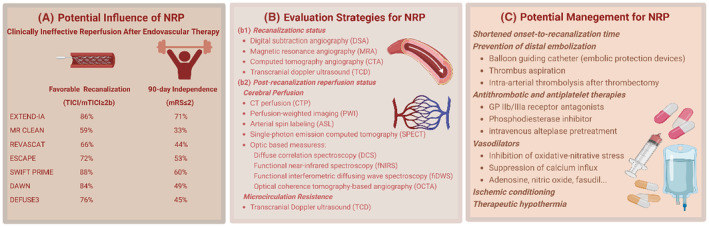
The potential influence, evaluation strategies, and management of the no‐reflow phenomenon in clinical settings (Created with BioRender.com). (A) The potential influence of the NRP. The NRP is involved in clinically ineffective reperfusion after endovascular therapy. The diagram shows favorable recanalization rates (TICI/mTICI ≥2b) and favorable 90‐day clinical outcome proportions (mRS ≤2) in major studies concerning the efficacy of endovascular thrombectomy. It is of note that nearly half of the patients failed to achieve favorable outcomes despite successful recanalization. (B) Evaluation strategies for the NRP. There are currently no available techniques in clinical settings for the monitoring of microcirculation disturbance. After successful recanalization of the proximal large artery based on imaging assessment (b1), strategies for evaluation of cerebral blood flow status or microcirculatory resistance (b2) are applied for detection of the NRP. (C) Potential management for the NRP. Management of the NRP should be emphasized to further improve the functional outcomes following recanalization therapy. Strategies including first‐aid workflow refinement, distal embolization prevention, antiplatelet and antithrombotic therapy, vasodilator, ischemic conditioning, and therapeutic hypothermia, are promising for NRP management.
5.1. Shortened onset‐to‐recanalization time
In animal models, prolonged ischemia was associated with the development of the NRP. 6 In patients with acute myocardial infarction, a shortened door‐to‐balloon time is associated with a lower incidence of no‐reflow. 83 Therefore, efforts should be made to optimize the first‐aid workflow and shorten the onset‐to‐recanalization time in stroke patients. In the meantime, the benefit of any strategy for NRP prevention should be weighed up against the time delay in applying it.
5.2. Prevention of distal embolization
Strategies like embolic protection devices and thrombus aspiration may reduce the occurrence of clot fragments and thus result in better reperfusion and clinical outcomes, as has been demonstrated in patients who underwent percutaneous coronary intervention (PCI). 84 Intra‐arterial thrombolysis during or after thrombectomy was demonstrated to improve angiographic reperfusion. 85
5.3. Antithrombotic and antiplatelet therapies
Antiplatelet and thrombolytic therapies may prevent the NRP. In animal studies, GP IIb/IIIa receptor antagonists could effectively block fibrinogen‐dependent platelet aggregation, thereby preventing cerebral microvascular thrombosis. 37 , 39 , 86 , 87 It was also preliminarily observed in some trials that tirofiban, either through oral, intravenous, or intra‐arterial administration, may improve functional outcomes and reduce mortality in AIS patients undergoing reperfusion therapy. 88 , 89 Other remedies such as adding the phosphodiesterase inhibitor (Cilostazol) or inhibiting Von Willebrand factor‐mediated thrombo‐inflammation, by blocking the platelet GPIbα binding site in the Von Willebrand factor A1 domain or by cleaving the Von Willebrand factor with ADAMTS13, can also affect blood flow and microvascular integrity. 90 This highlights a method for NRP prevention in the future. Apart from the inhibition of platelets, early administration of alteplase, which targets fibrinogen‐dependent downstream microvascular thrombosis, may also optimize the effect of recanalization. 91 , 92 , 93 One study found that intravenous thrombolysis pretreatment might increase the odds of successful reperfusion in patients undergoing endovascular thrombectomy. 94 Recently, a phase 2b randomized clinical trial preliminarily indicated that an auxiliary therapy of intra‐arterial alteplase following recanalization (eTICI 2b50 or above) through endovascular thrombectomy appears to have a greater probability of excellent neurological outcome at 90 days in patients with successful reperfusion via thrombectomy. 95
However, in published trials, controversial results still exist over the safety and efficacy of antiplatelet or thrombolytic therapy adjunct to reperfusion therapy, partially due to the heterogeneity in sample size, medication modality, therapeutic regimen, and trial protocol. The increased risk of intracranial hemorrhage transformation should also be carefully considered in future clinical trials. 96
5.4. Vasodilators
Therapeutic strategies that prevent the sustained constriction of pericytes or swelling of the end‐feet in astrocytes are also considered promising for NRP management, as they may help to reduce the cerebral capillary resistance with subtle influence on the systematic circulation. 97 Persistent contraction of pericytes could be reversed by the inhibition of oxidative‐nitrative stress and suppression of calcium influx. 19 , 98 In addition, adenosine and nitric oxide, with vasodilation, anti‐inflammation, and anti‐thrombus properties, have been demonstrated to restore microcirculation patency and improve reperfusion status, contributing to the overall survival of ischemic cerebral tissue during the ischemia–reperfusion process. 99 , 100 Considering the shared pathogenesis of the NRP in the heart and brain, other vasodilators (like nicardipine, nitroprusside, verapamil, and fasudil), that appear to reduce microvascular obstruction following PCI, also have the potential to ameliorate the NRP in stroke patients, though more studies must be carried out in both experimental models and stroke patients. 101 , 102
5.5. Ischemic conditioning
Ischemic conditioning, which refers to repetitive short episodes of ischemia with intermittent reperfusion, involves a complex network of molecular triggering and signaling pathways and has shown protective effects in both cardiovascular and cerebral vascular diseases. 103 It can be divided into pre‐, per‐, and postconditioning according to the intervention timepoint, or termed as remote ischemic conditioning when applied to an organ away from the protected one. 104 Preclinical studies have suggested its critical role in microcirculation modulation after reperfusion. 105 Small sample trials have demonstrated that ischemic postconditioning attenuates no‐reflow extent in patients with acute myocardial infarction.101,102 More recently, an ongoing phase‐I study (NCT05153655) has been carried out to ascertain the safety and tolerability of ischemic postconditioning in stroke patients receiving mechanical thrombectomy. Future research regarding the efficacy of ischemic conditioning in the management of NRP should be explored.
5.6. Therapeutic hypothermia
Induced cooling, especially selective therapeutic cooling, is a promising technique for reducing the final infarct volume and improving outcomes in ischemic stroke. 106 , 107 It has been demonstrated to protect against the NRP in experimental models of acute myocardial infarction. 108 , 109 More compelling evidence is needed to support the efficacy of hypothermia in clinical settings and its correlation with cerebral reperfusion status after recanalization. 110
6. FUTURE PERSPECTIVE
By now, advances in coronary NRP research have developed vastly and improved the outcomes of STEMI patients following PCI. Therefore, it is essential to take a page from the experience in coronary studies. In this section, we compared the similarities and differences between the coronary and cerebral NRP and discussed future directions for cerebral NRP investigations.
The main pathogenesis of the NRP in both the heart and brain lies in functional and structural damage to the microvasculature after prolonged cessation of artery occlusion and delayed restoration of blood flow. However, it is of note that cerebral blood flow regulation is more complicated compared with other circulation systems, with specific vascular structures, interaction between multiple cell types, and varieties of regulatory mechanisms and responses. 111 Meanwhile, there is still no animal model available to mimic the pathophysiological process of cerebral NRP, which presents challenges for preclinical study.
In patients with acute coronary syndrome, there are abundant imaging modalities to identify and quantify the NRP including coronary angiography, cardiac magnetic resonance (CMR), myocardial contrast echocardiography (MCE), nuclear approaches, catheter‐based coronary physiology measurements, and electrocardiography, with measurements like TIMI flow, TIMI frame count, myocardial blush grade, ST‐segment resolution, index of microcirculatory resistance (IMR). 112 CMR is currently considered the gold standard for coronary NRP assessment, based on which, a dark hypointense core in the areas of hyperenhancement that is identified early (~1 min) or late (15 min) after gadolinium injection is indicative of early or late CMVO, respectively. 113 Certain risk factors have been detected in previous studies, including patient clinical characteristics, procedural variables, duration of ischemia, infarct location and size, and culprit plaque morphology. Modifiable risk factor control like intensive statin therapy and optimization of blood pressure and blood sugar has been used for coronary NRP prevention. In addition, process optimization to shorten onset‐to recanalization time, procedure improvement, intra‐coronary vasodilators, anti‐coagulant agents, anti‐platelet agents, and therapeutic hypothermia are now trialed in patients for the management of coronary NRP. 114
However, our knowledge of the risk factors, prevention, and management of cerebral NRP in stroke patients is still scanty and conflicting due to the absence of consensus on detecting techniques, indicators, scales, and threshold value for post‐thrombectomy hypoperfusion identification and quantification. Thus, the top priority of future study is to establish a standardized definition and assessment criteria for the cerebral no‐reflow phenomenon. What follows is the investigation of risk factors of the cerebral NRP to identify patients at risk before thrombectomy and raise awareness for timely interventions, which may reduce the prevalence and extent of the NRP. Moreover, potential management derived from preclinical studies and coronary NRP experience should be trialed in stroke patients to evaluate the safety, feasibility, and efficacy and translated into a clinical effect on no‐reflow. In all, the future study of the cerebral NRP should mainly focus on pathophysiology, potential therapeutic targets, risk factors, and management translation. An integrated therapeutical management with timely identification, prevention, and multi‐target treatment seems promising to further improve the prognosis of stroke patients.
7. CONCLUSION
Microvascular obstruction after recanalization, which clinically manifests as reperfusion deficiency, namely the no‐reflow phenomenon, is common among ischemic stroke patients receiving thrombolysis or thrombectomy. Due to the significance of the reperfusion status for AIS patients, priority should be given to the in‐depth understanding of the pathogenesis, diagnosis, prevention, and management of the NRP to improve the prognosis of stroke patients receiving recanalization therapy.
FUNDING INFORMATION
This study was supported by Beijing Natural Science Foundation (JQ22020), Beijing Nova Program (No. Z201100006820143), and the National Natural Science Foundation of China (No. 82001257, 81801313, and 81971114).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
ACKNOWLEDGMENTS
We would like to thank Prof. Heng Zhao from Beijing Institute of Brain Disorders, Capital Medical University, Beijing, China, for his intellectual suggestions for the improvement of this review.
Jia M, Jin F, Li S, et al. No‐reflow after stroke reperfusion therapy: An emerging phenomenon to be explored. CNS Neurosci Ther. 2024;30:e14631. doi: 10.1111/cns.14631
Contributor Information
Wenbo Zhao, Email: zhaowb@xwh.ccmu.edu.cn.
Xunming Ji, Email: jixm@ccmu.edu.cn.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Campbell BCV, Khatri P. Stroke. Lancet. 2020;396(10244):129‐142. [DOI] [PubMed] [Google Scholar]
- 2. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early Management of Patients with Acute Ischemic Stroke: 2019 update to the 2018 guidelines for the early Management of Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344‐e418. [DOI] [PubMed] [Google Scholar]
- 3. Suzuki K, Matsumaru Y, Takeuchi M, et al. Effect of mechanical thrombectomy without vs with intravenous thrombolysis on functional outcome among patients with acute ischemic stroke: the SKIP randomized clinical trial. JAMA. 2021;325(3):244‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rubiera M, Garcia‐Tornel A, Olivé‐Gadea M, et al. Computed tomography perfusion after thrombectomy: an immediate surrogate marker of outcome after recanalization in acute stroke. Stroke. 2020;51(6):1736‐1742. [DOI] [PubMed] [Google Scholar]
- 5. Ng FC, Churilov L, Yassi N, et al. Prevalence and significance of impaired microvascular tissue reperfusion despite macrovascular angiographic reperfusion (No‐reflow). Neurology. 2022;98(8):e790‐e801. [DOI] [PubMed] [Google Scholar]
- 6. Ames A 3rd, Wright RL, Kowada M, Thurston JM, Majno G. Cerebral ischemia. II. The no‐reflow phenomenon. Am J Pathol. 1968;52(2):437‐453. [PMC free article] [PubMed] [Google Scholar]
- 7. Deng G, Chu YH, Xiao J, et al. Risk factors, pathophysiologic mechanisms, and potential treatment strategies of futile recanalization after endovascular therapy in acute ischemic stroke. Aging Dis. 2023;14(6):2096‐2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nie X, Leng X, Miao Z, Fisher M, Liu L. Clinically ineffective reperfusion after endovascular therapy in acute ischemic stroke. Stroke. 2023;54(3):873‐881. [DOI] [PubMed] [Google Scholar]
- 9. Heusch G. Coronary microvascular obstruction: the new frontier in cardioprotection. Basic Res Cardiol. 2019;114(6):45. [DOI] [PubMed] [Google Scholar]
- 10. Harrison MJ, Sedal L, Arnold J, Russell RW. No‐reflow phenomenon in the cerebral circulation of the gerbil. J Neurol Neurosurg Psychiatry. 1975;38(12):1190‐1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. del Zoppo GJ, Schmid‐Schönbein GW, Mori E, Copeland BR, Chang CM. Polymorphonuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboons. Stroke. 1991;22(10):1276‐1283. [DOI] [PubMed] [Google Scholar]
- 12. Little JR, Kerr FW, Sundt TM Jr. Microcirculatory obstruction in focal cerebral ischemia: an electron microscopic investigation in monkeys. Stroke. 1976;7(1):25‐30. [DOI] [PubMed] [Google Scholar]
- 13. Fischer EG, Ames A 3d. Studies on mechanisms of impairment of cerebral circulation following ischemia: effect of hemodilution and perfusion pressure. Stroke. 1972;3(5):538‐542. [DOI] [PubMed] [Google Scholar]
- 14. Kochanek PM, Dutka AJ, Kumaroo KK, Hallenbeck JM. Effects of prostacyclin, indomethacin, and heparin on cerebral blood flow and platelet adhesion after multifocal ischemia of canine brain. Stroke. 1988;19(6):693‐699. [DOI] [PubMed] [Google Scholar]
- 15. Thomas WS, Mori E, Copeland BR, Yu JQ, Morrissey JH, del Zoppo GJ. Tissue factor contributes to microvascular defects after focal cerebral ischemia. Stroke. 1993;24(6):847‐853. discussion 847. [DOI] [PubMed] [Google Scholar]
- 16. Hallenbeck JM, Dutka AJ, Tanishima T, et al. Polymorphonuclear leukocyte accumulation in brain regions with low blood flow during the early postischemic period. Stroke. 1986;17(2):246‐253. [DOI] [PubMed] [Google Scholar]
- 17. Del Zoppo GJ, Copeland BR, Harker LA, et al. Experimental acute thrombotic stroke in baboons. Stroke. 1986;17(6):1254‐1265. [DOI] [PubMed] [Google Scholar]
- 18. Cipolla MJ, Bullinger LV. Reactivity of brain parenchymal arterioles after ischemia and reperfusion. Microcirculation. 2008;15(6):495‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yemisci M, Gursoy‐Ozdemir Y, Vural A, Can A, Topalkara K, Dalkara T. Pericyte contraction induced by oxidative‐nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med. 2009;15(9):1031‐1037. [DOI] [PubMed] [Google Scholar]
- 20. Chiang J, Kowada M, Ames A 3rd, Wright RL, Majno G. Cerebral ischemia. III. vascular changes. Am J Pathol. 1968;52(2):455‐476. [PMC free article] [PubMed] [Google Scholar]
- 21. Hossmann KA. Reperfusion of the brain after global ischemia: hemodynamic disturbances. Shock. 1997;8(2):95‐101. discussion 102–3. [DOI] [PubMed] [Google Scholar]
- 22. Baird AE, Donnan GA, Austin MC, Fitt GJ, Davis SM, McKay WJ. Reperfusion after thrombolytic therapy in ischemic stroke measured by single‐photon emission computed tomography. Stroke. 1994;25(1):79‐85. [DOI] [PubMed] [Google Scholar]
- 23. Goyal M, Menon BK, van Zwam W, et al. Endovascular thrombectomy after large‐vessel ischaemic stroke: a meta‐analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723‐1731. [DOI] [PubMed] [Google Scholar]
- 24. Zhao W, Wu C, Dornbos D 3rd, et al. Multiphase adjuvant neuroprotection: a novel paradigm for improving acute ischemic stroke outcomes. Brain Circ. 2020;6(1):11‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shin J, Kim YS, Jang HS, et al. Perfusion recovery on TTP maps after endovascular stroke treatment might predict favorable neurological outcomes. Eur Radiol. 2020;30(12):6421‐6431. [DOI] [PubMed] [Google Scholar]
- 26. Cho TH, Nighoghossian N, Mikkelsen IK, et al. Reperfusion within 6 hours outperforms recanalization in predicting penumbra salvage, lesion growth, final infarct, and clinical outcome. Stroke. 2015;46(6):1582‐1589. [DOI] [PubMed] [Google Scholar]
- 27. Ter Schiphorst A, Charron S, Hassen WB, et al. Tissue no‐reflow despite full recanalization following thrombectomy for anterior circulation stroke with proximal occlusion: a clinical study. J Cereb Blood Flow Metab. 2021;41(2):253‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu S, Connor J, Peterson S, Shuttleworth CW, Liu KJ. Direct visualization of trapped erythrocytes in rat brain after focal ischemia and reperfusion. J Cereb Blood Flow Metab. 2002;22(10):1222‐1230. [DOI] [PubMed] [Google Scholar]
- 29. El Amki M, Glück C, Binder N, et al. Neutrophils obstructing brain capillaries are a major cause of No‐reflow in ischemic stroke. Cell Rep. 2020;33(2):108260. [DOI] [PubMed] [Google Scholar]
- 30. Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med. 2008;14(5):497‐500. [DOI] [PubMed] [Google Scholar]
- 31. Mori E, del Zoppo GJ, Chambers JD, Copeland BR, Arfors KE. Inhibition of polymorphonuclear leukocyte adherence suppresses no‐reflow after focal cerebral ischemia in baboons. Stroke. 1992;23(5):712‐718. [DOI] [PubMed] [Google Scholar]
- 32. Wong GJ, Yoo B, Liebeskind D, et al. Frequency, determinants, and outcomes of emboli to distal and new territories related to mechanical thrombectomy for acute ischemic stroke. Stroke. 2021;52(7):2241‐2249. [DOI] [PubMed] [Google Scholar]
- 33. Schonfeld MH, Kabiri R, Kniep HC, et al. Sub‐angiographic peripheral emboli in high resolution DWI after endovascular recanalization. J Neurol. 2020;267(5):1401‐1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rapp JH, Pan XM, Sharp FR, et al. Atheroemboli to the brain: size threshold for causing acute neuronal cell death. J Vasc Surg. 2000;32(1):68‐76. [DOI] [PubMed] [Google Scholar]
- 35. Sporns PB, Krähling H, Psychogios MN, et al. Small thrombus size, thrombus composition, and poor collaterals predict pre‐interventional thrombus migration. J Neurointerv Surg. 2021;13(5):409‐414. [DOI] [PubMed] [Google Scholar]
- 36. Chatterjee D, Nagarajan K, Narayan SK, Narasimhan RL. Regional leptomeningeal collateral score by computed tomographic angiography correlates with 3‐month clinical outcome in acute ischemic stroke. Brain Circ. 2020;6(2):107‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Choudhri TF, Hoh BL, Zerwes HG, et al. Reduced microvascular thrombosis and improved outcome in acute murine stroke by inhibiting GP IIb/IIIa receptor‐mediated platelet aggregation. J Clin Invest. 1998;102(7):1301‐1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shaik NF, Regan RF, Naik UP. Platelets as drivers of ischemia/reperfusion injury after stroke. Blood Adv. 2021;5(5):1576‐1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Abumiya T, Fitridge R, Mazur C, et al. Integrin alpha(IIb)beta(3) inhibitor preserves microvascular patency in experimental acute focal cerebral ischemia. Stroke. 2000;31(6):1402‐1409. discussion 1409‐10. [DOI] [PubMed] [Google Scholar]
- 40. Okada Y, Copeland BR, Fitridge R, Koziol JA, del Zoppo GJ. Fibrin contributes to microvascular obstructions and parenchymal changes during early focal cerebral ischemia and reperfusion. Stroke. 1994;25(9):1847‐1853. discussion 1853–4. [DOI] [PubMed] [Google Scholar]
- 41. Chen X, Wang J, Ge L, et al. A fibrin targeted molecular imaging evaluation of microvascular no‐reflow in acute ischemic stroke. Brain Behav. 2022;12(2):e2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang ZG, Chopp M, Goussev A, et al. Cerebral microvascular obstruction by fibrin is associated with upregulation of PAI‐1 acutely after onset of focal embolic ischemia in rats. J Neurosci. 1999;19(24):10898‐10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grogaard B, Schürer L, Gerdin B, Arfors KE. Delayed hypoperfusion after incomplete forebrain ischemia in the rat. The role of polymorphonuclear leukocytes. J Cereb Blood Flow Metab. 1989;9(4):500‐505. [DOI] [PubMed] [Google Scholar]
- 44. Ritter LS, Orozco JA, Coull BM, McDonagh P, Rosenblum WI. Leukocyte accumulation and hemodynamic changes in the cerebral microcirculation during early reperfusion after stroke. Stroke. 2000;31(5):1153‐1161. [DOI] [PubMed] [Google Scholar]
- 45. Okada Y, Copeland BR, Mori E, Tung MM, Thomas WS, del Zoppo GJ. P‐selectin and intercellular adhesion molecule‐1 expression after focal brain ischemia and reperfusion. Stroke. 1994;25(1):202‐211. [DOI] [PubMed] [Google Scholar]
- 46. Erdener SE, Tang J, Kılıç K, et al. Dynamic capillary stalls in reperfused ischemic penumbra contribute to injury: a hyperacute role for neutrophils in persistent traffic jams. J Cereb Blood Flow Metab. 2021;41(2):236‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Strinitz M, Pham M, März AG, et al. Immune cells invade the collateral circulation during human stroke: prospective replication and extension. Int J Mol Sci. 2021;22(17):9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kollikowski AM, Schuhmann MK, Nieswandt B, Müllges W, Stoll G, Pham M. Local leukocyte invasion during hyperacute human ischemic stroke. Ann Neurol. 2020;87(3):466‐479. [DOI] [PubMed] [Google Scholar]
- 49. Peña‐Martínez C, Durán‐Laforet V, García‐Culebras A, Cuartero MI, Moro MÁ, Lizasoain I. Neutrophil extracellular trap targeting protects against ischemic damage after fibrin‐rich thrombotic stroke despite non‐reperfusion. Front Immunol. 2022;13:790002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Perez‐de‐Puig I, Miró‐Mur F, Ferrer‐Ferrer M, et al. Neutrophil recruitment to the brain in mouse and human ischemic stroke. Acta Neuropathol. 2015;129(2):239‐257. [DOI] [PubMed] [Google Scholar]
- 51. Zhou SY, Guo ZN, Zhang DH, Qu Y, Jin H. The role of pericytes in ischemic stroke: fom cellular functions to therapeutic targets. Front Mol Neurosci. 2022;15:866700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yang S, Jin H, Zhu Y, et al. Diverse functions and mechanisms of pericytes in ischemic stroke. Curr Neuropharmacol. 2017;15(6):892‐905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hall CN, Reynell C, Gesslein B, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508(7494):55‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nahirney PC, Reeson P, Brown CE. Ultrastructural analysis of blood‐brain barrier breakdown in the peri‐infarct zone in young adult and aged mice. J Cereb Blood Flow Metab. 2016;36(2):413‐425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Korte N, Ilkan Z, Pearson CL, et al. The Ca2+−gated channel TMEM16A amplifies capillary pericyte contraction and reduces cerebral blood flow after ischemia. J Clin Invest. 2022;132(9):e154118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang S, Liao XJ, Wang J, et al. Temporal alterations in pericytes at the acute phase of ischemia/reperfusion in the mouse brain. Neural Regen Res. 2022;17(10):2247‐2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hill RA, Tong L, Yuan P, Murikinati S, Gupta S, Grutzendler J. Regional blood flow in the Normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron. 2015;87(1):95‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cipolla MJ, Chan SL, Sweet J, Tavares MJ, Gokina N, Brayden JE. Postischemic reperfusion causes smooth muscle calcium sensitization and vasoconstriction of parenchymal arterioles. Stroke. 2014;45(8):2425‐2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hills CP. The ultrastructure of anoxic‐Ischaemic lesions in the cerebral cortex of the adult rat brain. Guys Hosp Rep. 1964;113:333‐348. [PubMed] [Google Scholar]
- 60. Ito U, Hakamata Y, Kawakami E, Oyanagi K. Temporary [corrected] cerebral ischemia results in swollen astrocytic end‐feet that compress microvessels and lead to delayed [corrected] focal cortical infarction. J Cereb Blood Flow Metab. 2011;31(1):328‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mestre H, du T, Sweeney AM, et al. Cerebrospinal fluid influx drives acute ischemic tissue swelling. Science. 2020;367(6483):eaax7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Khatri P, Neff J, Broderick JP, Khoury JC, Carrozzella J, Tomsick T. Revascularization end points in stroke interventional trials: recanalization versus reperfusion in IMS‐I. Stroke. 2005;36(11):2400‐2403. [DOI] [PubMed] [Google Scholar]
- 63. De Silva DA, Fink JN, Christensen S, et al. Assessing reperfusion and recanalization as markers of clinical outcomes after intravenous thrombolysis in the echoplanar imaging thrombolytic evaluation trial (EPITHET). Stroke. 2009;40(8):2872‐2874. [DOI] [PubMed] [Google Scholar]
- 64. Marks MP, Lansberg MG, Mlynash M, et al. Angiographic outcome of endovascular stroke therapy correlated with MR findings, infarct growth, and clinical outcome in the DEFUSE 2 trial. Int J Stroke. 2014;9(7):860‐865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wintermark M, Flanders AE, Velthuis B, et al. Perfusion‐CT assessment of infarct core and penumbra: receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke. 2006;37(4):979‐985. [DOI] [PubMed] [Google Scholar]
- 66. Kosior JC, Buck B, Wannamaker R, et al. Exploring reperfusion following endovascular thrombectomy. Stroke. 2019;50(9):2389‐2395. [DOI] [PubMed] [Google Scholar]
- 67. Yasaka M, O'Keefe GJ, Chambers BR, et al. Streptokinase in acute stroke: effect on reperfusion and recanalization. Australian Streptokinase Trial Study Group. Neurology. 1998;50(3):626‐632. [DOI] [PubMed] [Google Scholar]
- 68. Zaidat OO, Yoo AJ, Khatri P, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. 2013;44(9):2650‐2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bivard A, Stanwell P, Levi C, Parsons M. Arterial spin labeling identifies tissue salvage and good clinical recovery after acute ischemic stroke. J Neuroimaging. 2013;23(3):391‐396. [DOI] [PubMed] [Google Scholar]
- 70. Ng FC, Coulton B, Chambers B, Thijs V. Persistently elevated microvascular resistance Postrecanalization. Stroke. 2018;49(10):2512‐2515. [DOI] [PubMed] [Google Scholar]
- 71. Zhao W, Liu R, Yu W, et al. Elevated pulsatility index is associated with poor functional outcome in stroke patients treated with thrombectomy: a retrospective cohort study. CNS Neurosci Ther. 2022;28:1568‐1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Claassen J, Thijssen DHJ, Panerai RB, et al. Regulation of cerebral blood flow in humans: physiology and clinical implications of autoregulation. Physiol Rev. 2021;101(4):1487‐1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chen CL, Wang RK. Optical coherence tomography based angiography [invited]. Biomed Opt Express. 2017;8(2):1056‐1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Forti RM, Favilla CG, Cochran JM, et al. Transcranial optical monitoring of cerebral hemodynamics in acute stroke patients during mechanical thrombectomy. J Stroke Cerebrovasc Dis. 2019;28(6):1483‐1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhou W, Kholiqov O, Zhu J, et al. Functional interferometric diffusing wave spectroscopy of the human brain. Sci Adv. 2021;7(20):eabe0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pham T, Tgavalekos K, Sassaroli A, Blaney G, Fantini S. Quantitative measurements of cerebral blood flow with near‐infrared spectroscopy. Biomed Opt Express. 2019;10(4):2117‐2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Albers GW, Thijs VN, Wechsler L, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006;60(5):508‐517. [DOI] [PubMed] [Google Scholar]
- 78. Soares BP, Tong E, Hom J, et al. Reperfusion is a more accurate predictor of follow‐up infarct volume than recanalization: a proof of concept using CT in acute ischemic stroke patients. Stroke. 2010;41(1):e34‐e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Eilaghi A, Brooks J, d'Esterre C, et al. Reperfusion is a stronger predictor of good clinical outcome than recanalization in ischemic stroke. Radiology. 2013;269(1):240‐248. [DOI] [PubMed] [Google Scholar]
- 80. Horsch AD, Dankbaar JW, Niesten JM, et al. Predictors of reperfusion in patients with acute ischemic stroke. AJNR Am J Neuroradiol. 2015;36(6):1056‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Carbone F, Busto G, Padroni M, et al. Radiologic cerebral reperfusion at 24 h predicts good clinical outcome. Transl Stroke Res. 2019;10(2):178‐188. [DOI] [PubMed] [Google Scholar]
- 82. Luby M, Merino JG, Davis R, et al. Association of Multiple Passes during mechanical thrombectomy with incomplete reperfusion and lesion growth. Cerebrovasc Dis. 2021;51:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Brodie BR, Webb J, Cox DA, et al. Impact of time to treatment on myocardial reperfusion and infarct size with primary percutaneous coronary intervention for acute myocardial infarction (from the EMERALD trial). Am J Cardiol. 2007;99(12):1680‐1686. [DOI] [PubMed] [Google Scholar]
- 84. Svilaas T, Vlaar PJ, van der Horst IC, et al. Thrombus aspiration during primary percutaneous coronary intervention. N Engl J Med. 2008;358(6):557‐567. [DOI] [PubMed] [Google Scholar]
- 85. Kaesmacher J, Bellwald S, Dobrocky T, et al. Safety and efficacy of intra‐arterial urokinase after failed, unsuccessful, or incomplete mechanical thrombectomy in anterior circulation large‐vessel occlusion stroke. JAMA Neurol. 2020;77(3):318‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Moriguchi A, Maeda M, Mihara K, Aoki T, Matsuoka N, Mutoh S. FK419, a novel nonpeptide GPIIb/IIIa antagonist, restores microvascular patency and improves outcome in the Guinea‐pig middle cerebral artery thrombotic occlusion model: comparison with tirofiban. J Cereb Blood Flow Metab. 2005;25(1):75‐86. [DOI] [PubMed] [Google Scholar]
- 87. Yang M, Huo X, Miao Z, Wang Y. Platelet glycoprotein IIb/IIIa receptor inhibitor tirofiban in acute ischemic stroke. Drugs. 2019;79(5):515‐529. [DOI] [PubMed] [Google Scholar]
- 88. Huo X, Raynald R, Jing J, et al. Safety and efficacy of oral antiplatelet for patients who had acute ischaemic stroke undergoing endovascular therapy. Stroke Vasc Neurol. 2020;6(2):230‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhao W, Che R, Shang S, et al. Low‐dose tirofiban improves functional outcome in acute ischemic stroke patients treated with endovascular thrombectomy. Stroke. 2017;48(12):3289‐3294. [DOI] [PubMed] [Google Scholar]
- 90. Hase Y, Okamoto Y, Fujita Y, et al. Cilostazol, a phosphodiesterase inhibitor, prevents no‐reflow and hemorrhage in mice with focal cerebral ischemia. Exp Neurol. 2012;233(1):523‐533. [DOI] [PubMed] [Google Scholar]
- 91. Denorme F, Martinod K, Vandenbulcke A, et al. The von Willebrand factor A1 domain mediates thromboinflammation, aggravating ischemic stroke outcome in mice. Haematologica. 2021;106(3):819‐828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Eerenberg ES, Teunissen PFA, van den Born BJ, et al. The role of ADAMTS13 in acute myocardial infarction: cause or consequence? Cardiovasc Res. 2016;111(3):194‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Desilles JP, Loyau S, Syvannarath V, et al. Alteplase reduces downstream microvascular thrombosis and improves the benefit of large artery recanalization in stroke. Stroke. 2015;46(11):3241‐3248. [DOI] [PubMed] [Google Scholar]
- 94. Goyal N, Tsivgoulis G, Pandhi A, et al. Impact of pretreatment with intravenous thrombolysis on reperfusion status in acute strokes treated with mechanical thrombectomy. J Neurointerv Surg. 2019;11(11):1073‐1079. [DOI] [PubMed] [Google Scholar]
- 95. Renu A, Millán M, San Román L, et al. Effect of intra‐arterial alteplase vs placebo following successful thrombectomy on functional outcomes in patients with large vessel occlusion acute ischemic stroke: the CHOICE randomized clinical trial. JAMA. 2022;327(9):826‐835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zinkstok SM, Roos YB. Early administration of aspirin in patients treated with alteplase for acute ischaemic stroke: a randomised controlled trial. Lancet. 2012;380(9843):731‐737. [DOI] [PubMed] [Google Scholar]
- 97. Hartmann DA, Berthiaume AA, Grant RI, et al. Brain capillary pericytes exert a substantial but slow influence on blood flow. Nat Neurosci. 2021;24(5):633‐645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Guo RB, Dong YF, Yin Z, et al. Iptakalim improves cerebral microcirculation in mice after ischemic stroke by inhibiting pericyte contraction. Acta Pharmacology Sin. 2021;43:1349‐1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gaudin A, Yemisci M, Eroglu H, et al. Squalenoyl adenosine nanoparticles provide neuroprotection after stroke and spinal cord injury. Nat Nanotechnol. 2014;9(12):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Terpolilli NA, Kim SW, Thal SC, et al. Inhalation of nitric oxide prevents ischemic brain damage in experimental stroke by selective dilatation of collateral arterioles. Circ Res. 2012;110(5):727‐738. [DOI] [PubMed] [Google Scholar]
- 101. Jaffe R, Dick A, Strauss BH. Prevention and treatment of microvascular obstruction‐related myocardial injury and coronary no‐reflow following percutaneous coronary intervention: a systematic approach. JACC Cardiovasc Interv. 2010;3(7):695‐704. [DOI] [PubMed] [Google Scholar]
- 102. Fraser JF, Maniskas M, Trout A, et al. Intra‐arterial verapamil post‐thrombectomy is feasible, safe, and neuroprotective in stroke. J Cereb Blood Flow Metab. 2017;37(11):3531‐3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lee H, Yun H, Ding Y. Timing is everything: exercise therapy and remote ischemic conditioning for acute ischemic stroke patients. Brain Circ. 2021;7(3):178‐186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wills M, Ding Y. Mini‐review (part II): a clinical consideration on exercise and ischemic conditioning in stroke rehabilitation. Brain Circ. 2021;7(4):225‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Pico F, Lapergue B, Ferrigno M, et al. Effect of in‐hospital remote ischemic Perconditioning on BRAIN infarction growth and clinical outcomes in patients with acute ischemic stroke: the RESCUE BRAIN randomized clinical trial. JAMA Neurol. 2020;77(6):725‐734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Wu D, Chen J, Zhang X, Ilagan R, Ding Y, Ji X. Selective therapeutic cooling: to maximize benefits and minimize side effects related to hypothermia. J Cereb Blood Flow Metab. 2022;42(1):213‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Eren F, Yilmaz S. Neuroprotective approach in acute ischemic stroke: a systematic review of clinical and experimental studies. Brain Circ. 2022;8(4):172‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Hale SL, Herring MJ, Kloner RA. Delayed treatment with hypothermia protects against the no‐reflow phenomenon despite failure to reduce infarct size. J Am Heart Assoc. 2013;2(1):e004234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Dai W, Hale S, Kloner RA. Delayed therapeutic hypothermia protects against the myocardial no‐reflow phenomenon independently of myocardial infarct size in a rat ischemia/reperfusion model. Int J Cardiol. 2017;236:400‐404. [DOI] [PubMed] [Google Scholar]
- 110. Li F, Gao J, Kohls W, Geng X, Ding Y. Perspectives on benefit of early and prereperfusion hypothermia by pharmacological approach in stroke. Brain Circ. 2022;8(2):69‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Schaeffer S, Iadecola C. Revisiting the neurovascular unit. Nat Neurosci. 2021;24(9):1198‐1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Galli M, Niccoli G, de Maria G, et al. Coronary microvascular obstruction and dysfunction in patients with acute myocardial infarction. Nat Rev Cardiol. 2023. doi: 10.1038/s41569-023-00953-4. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 113. Bulluck H, Dharmakumar R, Arai AE, Berry C, Hausenloy DJ. Cardiovascular magnetic resonance in acute ST‐segment‐elevation myocardial infarction: recent advances, controversies, and future directions. Circulation. 2018;137(18):1949‐1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Rezkalla SH, Stankowski RV, Hanna J, Kloner RA. Management of No‐Reflow Phenomenon in the catheterization laboratory. JACC Cardiovasc Interv. 2017;10(3):215‐223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


