Abstract
Background
Interventions incorporating meditation to address stress, anxiety, and depression, and improve self‐management, are becoming popular for many health conditions. Stress is a risk factor for cardiovascular disease (CVD) and clusters with other modifiable behavioural risk factors, such as smoking. Meditation may therefore be a useful CVD prevention strategy.
Objectives
To determine the effectiveness of meditation, primarily mindfulness‐based interventions (MBIs) and transcendental meditation (TM), for the primary and secondary prevention of CVD.
Search methods
We searched CENTRAL, MEDLINE, Embase, three other databases, and two trials registers on 14 November 2021, together with reference checking, citation searching, and contact with study authors to identify additional studies.
Selection criteria
We included randomised controlled trials (RCTs) of 12 weeks or more in adults at high risk of CVD and those with established CVD. We explored four comparisons: MBIs versus active comparators (alternative interventions); MBIs versus non‐active comparators (no intervention, wait list, usual care); TM versus active comparators; TM versus non‐active comparators.
Data collection and analysis
We used standard Cochrane methods. Our primary outcomes were CVD clinical events (e.g. cardiovascular mortality), blood pressure, measures of psychological distress and well‐being, and adverse events. Secondary outcomes included other CVD risk factors (e.g. blood lipid levels), quality of life, and coping abilities. We used GRADE to assess the certainty of evidence.
Main results
We included 81 RCTs (6971 participants), with most studies at unclear risk of bias.
MBIs versus active comparators (29 RCTs, 2883 participants)
Systolic (SBP) and diastolic (DBP) blood pressure were reported in six trials (388 participants) where heterogeneity was considerable (SBP: MD ‐6.08 mmHg, 95% CI ‐12.79 to 0.63, I2 = 88%; DBP: MD ‐5.18 mmHg, 95% CI ‐10.65 to 0.29, I2 = 91%; both outcomes based on low‐certainty evidence). There was little or no effect of MBIs on anxiety (SMD ‐0.06 units, 95% CI ‐0.25 to 0.13; I2 = 0%; 9 trials, 438 participants; moderate‐certainty evidence), or depression (SMD 0.08 units, 95% CI ‐0.08 to 0.24; I2 = 0%; 11 trials, 595 participants; moderate‐certainty evidence). Perceived stress was reduced with MBIs (SMD ‐0.24 units, 95% CI ‐0.45 to ‐0.03; I2 = 0%; P = 0.03; 6 trials, 357 participants; moderate‐certainty evidence). There was little to no effect on well‐being (SMD ‐0.18 units, 95% CI ‐0.67 to 0.32; 1 trial, 63 participants; low‐certainty evidence). There was little to no effect on smoking cessation (RR 1.45, 95% CI 0.78 to 2.68; I2 = 79%; 6 trials, 1087 participants; low‐certainty evidence). None of the trials reported CVD clinical events or adverse events.
MBIs versus non‐active comparators (38 RCTs, 2905 participants)
Clinical events were reported in one trial (110 participants), providing very low‐certainty evidence (RR 0.94, 95% CI 0.37 to 2.42). SBP and DBP were reduced in nine trials (379 participants) but heterogeneity was substantial (SBP: MD ‐6.62 mmHg, 95% CI ‐13.15 to ‐0.1, I2 = 87%; DBP: MD ‐3.35 mmHg, 95% CI ‐5.86 to ‐0.85, I2 = 61%; both outcomes based on low‐certainty evidence). There was low‐certainty evidence of reductions in anxiety (SMD ‐0.78 units, 95% CI ‐1.09 to ‐0.41; I2 = 61%; 9 trials, 533 participants; low‐certainty evidence), depression (SMD ‐0.66 units, 95% CI ‐0.91 to ‐0.41; I2 = 67%; 15 trials, 912 participants; low‐certainty evidence) and perceived stress (SMD ‐0.59 units, 95% CI ‐0.89 to ‐0.29; I2 = 70%; 11 trials, 708 participants; low‐certainty evidence) but heterogeneity was substantial. Well‐being increased (SMD 0.5 units, 95% CI 0.09 to 0.91; I2 = 47%; 2 trials, 198 participants; moderate‐certainty evidence). There was little to no effect on smoking cessation (RR 1.36, 95% CI 0.86 to 2.13; I2 = 0%; 2 trials, 453 participants; low‐certainty evidence). One small study (18 participants) reported two adverse events in the MBI group, which were not regarded as serious by the study investigators (RR 5.0, 95% CI 0.27 to 91.52; low‐certainty evidence).
No subgroup effects were seen for SBP, DBP, anxiety, depression, or perceived stress by primary and secondary prevention.
TM versus active comparators (8 RCTs, 830 participants)
Clinical events were reported in one trial (201 participants) based on low‐certainty evidence (RR 0.91, 95% CI 0.56 to 1.49). SBP was reduced (MD ‐2.33 mmHg, 95% CI ‐3.99 to ‐0.68; I2 = 2%; 8 trials, 774 participants; moderate‐certainty evidence), with an uncertain effect on DBP (MD ‐1.15 mmHg, 95% CI ‐2.85 to 0.55; I2 = 53%; low‐certainty evidence). There was little or no effect on anxiety (SMD 0.06 units, 95% CI ‐0.22 to 0.33; I2 = 0%; 3 trials, 200 participants; low‐certainty evidence), depression (SMD ‐0.12 units, 95% CI ‐0.31 to 0.07; I2 = 0%; 5 trials, 421 participants; moderate‐certainty evidence), or perceived stress (SMD 0.04 units, 95% CI ‐0.49 to 0.57; I2 = 70%; 3 trials, 194 participants; very low‐certainty evidence). None of the trials reported adverse events or smoking rates.
No subgroup effects were seen for SBP or DBP by primary and secondary prevention.
TM versus non‐active comparators (2 RCTs, 186 participants)
Two trials (139 participants) reported blood pressure, where reductions were seen in SBP (MD ‐6.34 mmHg, 95% CI ‐9.86 to ‐2.81; I2 = 0%; low‐certainty evidence) and DBP (MD ‐5.13 mmHg, 95% CI ‐9.07 to ‐1.19; I2 = 18%; very low‐certainty evidence). One trial (112 participants) reported anxiety and depression and found reductions in both (anxiety SMD ‐0.71 units, 95% CI ‐1.09 to ‐0.32; depression SMD ‐0.48 units, 95% CI ‐0.86 to ‐0.11; low‐certainty evidence). None of the trials reported CVD clinical events, adverse events, or smoking rates.
Authors' conclusions
Despite the large number of studies included in the review, heterogeneity was substantial for many of the outcomes, which reduced the certainty of our findings. We attempted to address this by presenting four main comparisons of MBIs or TM versus active or inactive comparators, and by subgroup analyses according to primary or secondary prevention, where there were sufficient studies. The majority of studies were small and there was unclear risk of bias for most domains. Overall, we found very little information on the effects of meditation on CVD clinical endpoints, and limited information on blood pressure and psychological outcomes, for people at risk of or with established CVD.
This is a very active area of research as shown by the large number of ongoing studies, with some having been completed at the time of writing this review. The status of all ongoing studies will be formally assessed and incorporated in further updates.
Keywords: Adult, Humans, Anxiety, Anxiety/prevention & control, Anxiety Disorders, Cardiovascular Diseases, Meditation, Primary Prevention, Primary Prevention/methods, Secondary Prevention
Plain language summary
Does meditation help prevent people from developing cardiovascular disease or from worsening cardiovascular disease?
Key messages
· We looked primarily at two main types of meditation, mindfulness‐based interventions (MBIs) and transcendental meditation (TM), compared to receiving something else or nothing else (referred to as active and inactive comparison groups, respectively). We found inconsistent results for many of the outcomes of interest.
· Compared to inactive comparators, MBIs probably reduce stress, and may also reduce anxiety and depression and blood pressure. TM may reduce blood pressure when compared to either active or inactive comparators, with few studies reporting psychological outcomes. Results will be more certain with the addition of further well‐conducted studies.
What is cardiovascular disease?
Cardiovascular disease (CVD) includes several different diseases of the heart and blood vessels, some of which are caused by problems like high cholesterol, physical inactivity, stress, poor diet, being overweight, smoking, or drinking alcohol. Overall, CVDs are the world’s leading cause of death.
How can meditation help?
Meditation may help to reduce people’s stress levels, which could benefit them directly (for example, by lowering blood pressure), and indirectly by helping them to avoid unhealthy ways of coping with stress (for example, smoking, drinking alcohol, or making poor food choices).
What types of meditation did we look at?
We looked at two main types of meditation for this study:
· mindfulness‐based interventions (MBI);
· transcendental meditation (TM).
What did we want to find out?
We wanted to find out if meditation helped to:
· reduce the risk of CVD clinical events, such as death, heart attack, stroke, or chest pain;
· reduce blood pressure;
· improve stress, depression, anxiety, and well‐being;
· improve blood measures like cholesterol and blood glucose levels;
· reduce weight;
· reduce smoking;
· improve quality of life and people’s ability to cope.
What did we do? We searched for studies that looked at meditation compared with no intervention (inactive comparators) or another non‐pharmacological intervention (active comparators), in people at high risk of developing CVD and people who already had established CVD. We assessed outcomes for the totality of the participants and separately for these two groups.
We compared and summarised the results of the studies and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We found 81 studies that involved 6971 people either at high risk of or who already had CVD. The studies lasted between 12 weeks and five years.
Only one MBI study and one TM study reported CVD clinical events, and we found that either type of meditation may have little to no effect, but we are very uncertain about the results.
Six studies (388 people) that compared MBIs to active comparators suggest that it may have little to no effect on blood pressure, but we are uncertain about the results. Results from eight studies (774 people) found that TM probably reduces systolic blood pressure compared to active comparators, but the evidence for diastolic blood pressure was less certain.
When compared to inactive comparators, people who practised mindfulness (nine studies, 379 people) may have reductions in blood pressure, but results were inconsistent. When comparing TM to inactive comparators (2 studies, 154 people) we found that TM may reduce blood pressure.
We found that there was probably little or no difference in anxiety, depression, or well‐being between MBIs and active comparators. Six studies (357 people) reported that stress probably improved more in people who practised mindfulness. Five studies (421 people) reported little to no effect on depression among people who practised TM compared with another intervention. We are very uncertain about the effect of TM on anxiety or stress.
When compared to inactive comparators, people who practised mindfulness may have reductions in anxiety (nine studies, 533 people), depression (15 studies, 912 people), and stress (11 studies, 708 people), but results were inconsistent. Two trials (198 people) reported a probable increase in well‐being among people who practised mindfulness, compared to no intervention. We found no differences in the results for blood pressure, anxiety, depression, and stress, where we had enough studies to compare people at risk of CVD with those with established CVD.
One small study reported two unwanted/adverse effects of MBI when compared to inactive comparators. One participant had transitory vertigo during head rolling in mindful movement and in another the MBI caused resurfacing of repressed traumatic memories and depression. This participant received counselling and continued with MBI, which they found beneficial.
What are the limitations of the evidence?
Even though we tried to group studies by the type of meditation intervention and by comparison groups, so they were more similar for the analyses, there was still a lot of inconsistency in the findings that remains unexplained.
Most of the studies were small in size, and we are uncertain as to how well they were carried out, mainly due to poor reporting.
The search cut‐off date of November 2021 is a limitation of the review. However, in May 2023 we revisited the status of the 74 ongoing studies and provided details of these. Nine studies were found to have been completed in this time and will be formally assessed in an update of this review.
How up‐to‐date is this evidence?
The evidence is up‐to‐date to November 2021.
Summary of findings
Summary of findings 1. Mindfulness‐based interventions (MBIs) compared to active comparators for the primary and secondary prevention of cardiovascular disease.
| Mindfulness‐based interventions (MBIs) compared to active comparators for the primary and secondary prevention of cardiovascular disease | ||||||
| Patient or population: people at high risk of or withcardiovascular disease Setting: community Intervention: MBIs Comparison: active comparators | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with active comparators | Risk with MBIs | |||||
| Clinical CVD events | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported. |
| Systolic blood pressure (mmHg), change from baseline Follow‐up (range): 3 to 18 months |
The mean systolic blood pressure change from baseline ranged from ‐7.0 to 0.2 mmHg | MD 6.08 lower (12.79 lower to 0.63 higher) | ‐ | 388 (6 RCTs) | ⊕⊕⊝⊝ LOWa,b | Three small trials reported large beneficial effects of the MBIs compared to active comparators, whereas the remaining three with larger sample size showed little or no effect of the intervention. |
| Diastolic blood pressure (mmHg), change from baseline Follow‐up (range): 3 to 18 months |
The mean diastolic blood pressure change from baseline ranged from ‐3.4 to 3.2 mmHg | MD 5.18 lower (10.65 lower to 0.29 higher) | ‐ | 388 (6 RCTs) | ⊕⊕⊝⊝ LOWa,c | Three small trials reported large beneficial effects of the MBIs compared to active comparators, whereas the remaining three with larger sample size showed little or no effect of the intervention. |
| Anxiety, change from baseline Follow‐up (range): 3 to 9 months |
SMD 0.06 lower (0.25 lower to 0.13 higher) | ‐ | 438 (9 RCTs) | ⊕⊕⊕⊝ MODERATEd | We interpret an SMD of 0.2 to represent a small effect, 0.5 a moderate effect, and 0.8 a large effect. | |
| Depression, change from baseline Follow‐up (range): 3 to 9 months |
SMD 0.08 higher (0.08 lower to 0.24 higher) | ‐ | 595 (11 RCTs) | ⊕⊕⊕⊝ MODERATEd | We interpret an SMD of 0.2 to represent a small effect, 0.5 a moderate effect, and 0.8 a large effect. | |
| Perceived stress, change from baseline Follow‐up (range): 4 to 7 months |
SMD 0.24 lower (0.45 lower to 0.03 lower) | ‐ | 357 (6 RCTs) | ⊕⊕⊕⊝ MODERATEe | We interpret an SMD of 0.2 to represent a small effect, 0.5 a moderate effect, and 0.8 a large effect. | |
| Well‐being Follow‐up: 9 months |
SMD 0.18 lower (0.67 lower to 0.32 higher) | ‐ | 63 (1 RCT) | ⊕⊕⊝⊝ LOWf | We interpret an SMD of 0.2 to represent a small effect, 0.5 a moderate effect, and 0.8 a large effect. | |
| Adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported. |
| Smoking cessation Follow‐up (mean): 6 months |
Study population 151 per 1000 |
218 per 1000 (117 to 403) | RR 1.45 (0.78 to 2.68) | 1087 (6 RCTs) | ⊕⊕⊝⊝ LOWg,h | Two studies showed large beneficial effects of the MBI, and the remaining four showed little or no effect of MBIs on smoking cessation. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CVD: cardiovascular disease; MBI: mindfulness‐based intervention; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by one level for risk of bias: one study was at high risk of attrition bias and all other studies were at unclear risk for this domain; one study was at high risk of selection bias and three were at unclear risk for this domain. bDowngraded by one level for inconsistency: I2 = 88%. cDowngraded by one level for inconsistency: I2 = 91%. dDowngraded by one level for risk of bias: all but two studies at unclear or high risk of attrition bias. eDowngraded by one level for risk of bias: one study was at high risk of attrition bias and three other studies were at unclear risk for this domain; one study was at unclear risk of selection bias. fDowngraded by two levels for imprecision: very small sample size and the CI includes both appreciable benefit and harm. gDowngraded by one level for inconsistency: I2 = 79%. hDowngraded by one level for risk of bias: all but three studies at unclear or high risk of attrition bias and two studies at unclear risk of selection bias.
Summary of findings 2. Mindfulness‐based interventions (MBIs) compared to non‐active comparators for the primary and secondary prevention of cardiovascular disease.
| Mindfulness‐based interventions (MBIs) compared to non‐active comparators for the primary and secondary prevention of cardiovascular disease | ||||||
| Patient or population: people at high risk of or withcardiovascular disease Setting: community Intervention: MBIs Comparison: non‐active comparators | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with non‐active comparators | Risk with MBIs | |||||
| Clinical CVD events (CVD mortality and non‐fatal MI) Follow‐up: 36 months |
Study population | RR 0.94 (0.37 to 2.42) | 110 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b | ‐ | |
| 140 per 1000 | 132 per 1000 (52 to 340) | |||||
| Systolic blood pressure (mmHg), change from baseline Follow‐up (range): 3 to 9 months |
The mean systolic blood pressure change from baseline ranged from ‐9.7 to 9.47 mmHg | MD 6.62 lower (13.15 lower to 0.1 lower) | ‐ | 379 (9 RCTs) | ⊕⊕⊝⊝ LOWc,d | Three trials reported beneficial effects of the MBIs compared to non‐active comparators, whereas the remaining six showed little or no effect of the intervention. |
| Diastolic blood pressure (mmHg), change from baseline Follow‐up (range): 3 to 9 months |
The mean diastolic blood pressure change from baseline ranged from ‐7.7 to 4.1 mmHg | MD 3.35 lower (5.86 lower to 0.85 lower) | ‐ | 379 (9 RCTs) | ⊕⊕⊝⊝ LOWc,e | Three trials reported beneficial effects of the MBIs compared to non‐active comparators, whereas the remaining six showed little or no effect of the intervention. |
| Anxiety, change from baseline Follow‐up (range): 3 to 9 months |
SMD 0.78 lower (1.09 lower to 0.47 lower) | ‐ | 533 (9 RCTs) | ⊕⊕⊝⊝ LOWf,g | Six trials reported beneficial effects of the MBIs compared to non‐active comparators, whereas the remaining three showed little or no effect of the intervention. We interpret an SMD of 0.2 to represent a small effect, 0.5 a moderate effect, and 0.8 a large effect. | |
| Depression, change from baseline Follow‐up (range): 3 to 12 months |
SMD 0.66 lower (0.91 lower to 0.41 lower) | ‐ | 912 (15 RCTs) | ⊕⊕⊝⊝ LOWh,i | Eleven trials reported beneficial effects of the MBIs compared to non‐active comparators, whereas the remaining four showed little or no effect of the intervention. We interpret an SMD of 0.2 to represent a small effect, 0.5 a moderate effect, and 0.8 a large effect. | |
| Perceived stress, change from baseline Follow‐up (range): 3 to 12 months |
SMD 0.59 lower (0.89 lower to 0.29 lower) | ‐ | 708 (11 RCTs) | ⊕⊕⊝⊝ LOWj,k | Six trials reported beneficial effects of the MBIs compared to non‐active comparators, one reported beneficial effects of the non‐active comparator, and the remaining four showed little or no effect of the intervention. We interpret an SMD of 0.2 to represent a small effect, 0.5 a moderate effect, and 0.8 a large effect. | |
| Well‐being Follow‐up (range): 5 to 9 months |
SMD 0.5 higher (0.09 higher to 0.91 higher) | ‐ | 198 (2 RCTs) | ⊕⊕⊕⊝ MODERATEl | We interpret an SMD of 0.2 to represent a small effect, 0.5 a moderate effect, and 0.8 a large effect. | |
| Adverse events | There were 2/9 participants with an adverse event in the MBI group and 0/9 in the control group. | RR 5.0 (0.27 to 91.52) | 18 (1 RCT) | ⊕⊕⊝⊝ LOWm | Calculation of anticipated absolute effects is not possible due to 0 events in the control arm. Adverse events reported were not serious. | |
| Smoking cessation Follow‐up (mean): 6 months |
Study population | RR 1.36 (0.86 to 2.13) | 453 (2 RCTs) | ⊕⊕⊝⊝ LOWn,o | ‐ | |
| 129 per 1000 | 175 per 1000 (111 to 274) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CVD: cardiovascular disease; MBI: mindfulness‐based intervention; MD: mean difference; MI: myocardial infarction; RCT: randomised controlled trial; RR: risk ratio; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by one level for risk of bias: the study is at unclear risk in all domains. bDowngraded by two levels for imprecision: very small sample size, and CI wide enough to include the possibility of no benefit, no difference, and a large benefit. cDowngraded by one level for risk of bias: all but two studies at unclear or high risk of attrition bias. dDowngraded by one level for inconsistency: I2 = 87%. eDowngraded by one level for inconsistency: I2 = 61%. fDowngraded by one level for risk of bias: four studies at unclear or high risk of attrition bias and one study each at high risk or unclear risk of selection bias. gDowngraded by one level for inconsistency: I2 = 61%. hDowngraded by one level for risk of bias: the majority of studies at unclear or high risk of attrition bias and four studies at unclear risk of selection bias. iDowngraded by one level for inconsistency: I2 = 67%. jDowngraded by one level for risk of bias: four studies at unclear risk and one at high risk of attrition bias. kDowngraded by one level for inconsistency: I2 = 70%. lDowngraded by one level for imprecision: small sample size. mDowngraded by two levels for imprecision: very small sample size and CI includes the possibility of no difference as well as harm. nDowngraded by one level for risk of bias: one study at high risk of attrition bias.
Summary of findings 3. Transcendental meditation (TM) compared to active comparators for the primary and secondary prevention of cardiovascular disease.
| Transcendental meditation (TM) compared to active comparators for the primary and secondary prevention of cardiovascular disease | ||||||
| Patient or population: people at high risk of or with cardiovascular disease Setting: community Intervention: TM Comparison: active comparators | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with active comparators | Risk with TM | |||||
| Clinical CVD events (CVD mortality, non‐fatal MI and stroke, revascularisations) Follow‐up: 5.4 years |
Study population | RR 0.91 (0.56 to 1.49) | 201 (1 RCT) | ⊕⊕⊝⊝ LOWa | ‐ | |
| 255 per 1000 | 232 per 1000 (143 to 380) | |||||
| Systolic blood pressure (mmHg), change from baseline Follow‐up (median): 9 months; (range): 3 months to 5.4 years |
The mean systolic blood pressure change from baseline ranged from ‐7.49 to 4.88 mmHg | MD 2.33 mmHg lower (3.99 lower to 0.68 lower) | ‐ | 774 (8 RCTs) | ⊕⊕⊕⊝ MODERATEb | ‐ |
| Diastolic blood pressure (mmHg), change from baseline Follow‐up (median): 9 months; (range): 3 months to 5.4 years |
The mean diastolic blood pressure change from baseline ranged from ‐6.6 to 0.3 mmHg | MD 1.15 mmHg lower (2.85 lower to 0.55 higher) | ‐ | 774 (8 RCTs) | ⊕⊕⊝⊝ LOWb,c | Beneficial effects of TM on diastolic blood pressure were seen in one trial, possible benefits of TM in two, possible benefits of the active comparator in two, and little or no effect of TM in the remaining three trials. |
| Anxiety, change from baseline Follow‐up (range): 4 to 9 months |
SMD 0.06 higher (0.22 lower to 0.33 higher) |
‐ | 200 (3 RCTs) | ⊕⊕⊝⊝ LOWd,e | We interpret an SMD of 0.2 to represent a small effect, 0.5 a moderate effect, and 0.8 a large effect. | |
| Depression, change from baseline Follow‐up (median): 7 months; (range): 4 months to 5.4 years |
SMD 0.12 lower (0.31 lower to 0.07 higher) | ‐ | 421 (5 RCTs) | ⊕⊕⊕⊝ MODERATEf | We interpret an SMD of 0.2 to represent a small effect, 0.5 a moderate effect, and 0.8 a large effect. | |
| Perceived stress, change from baseline Follow‐up (range): 6 to 7 months |
SMD 0.04 higher (0.49 lower to 0.57 higher) | ‐ | 194 (3 RCTs) | ⊕⊝⊝⊝ VERY LOWd,g,h | One study favoured the active comparator and the remaining two showed little or no effect of TM on depression. We interpret an SMD of 0.2 to represent a small effect, 0.5 a moderate effect, and 0.8 a large effect. | |
| Well‐being | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported. |
| Adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported. |
| Smoking cessation | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CVD: cardiovascular disease; MD: mean difference; MI: myocardial infarction; RCT: randomised controlled trial; RR: risk ratio; SMD: standardised mean difference; TM: transcendental meditation | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by two levels for imprecision: CI includes the possibility of a negative effect, no effect, or a positive effect; small sample size. bDowngraded by one level for risk of bias: six studies were at unclear risk of attrition bias and one study was at high risk of attrition bias; one study was at unclear risk of selection bias. cDowngraded by one level for inconsistency: I2 = 53%. dDowngraded by one level for imprecision: small sample size. eDowngraded by one level for risk of bias: one study at unclear risk of selection bias. fDowngraded by one level for risk of bias: three studies were at unclear risk and one study at high risk of attrition bias, and one study at unclear risk of selection bias. gDowngraded by one level for risk of bias: two studies were at unclear risk of attrition bias. hDowngraded by one level for inconsistency: I2 = 70%.
Summary of findings 4. Transcendental meditation (TM) compared to non‐active comparators for the primary and secondary prevention of cardiovascular disease.
| Transcendental meditation (TM) compared to non‐active comparators for the primary and secondary prevention of cardiovascular disease | ||||||
| Patient or population: people at high risk of or withcardiovascular disease Setting: community Intervention: TM Comparison: non‐active comparators | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with non‐active comparators | Risk with TM | |||||
| Clinical CVD events | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported. |
| Systolic blood pressure (mmHg), change from baseline Follow‐up: mean 3 months | The mean systolic blood pressure change from baseline ranged from 1.3 to 1.85 mmHg | MD 6.34 mmHg lower (9.86 lower to 2.81 lower) | ‐ | 139 (2 RCTs) | ⊕⊕⊝⊝ LOWa,b | ‐ |
| Diastolic blood pressure (mmHg), change from baseline Follow‐up: mean 3 months | The mean diastolic blood pressure change from baseline ranged from 1.2 to 2.26 mmHg | MD 5.13 mmHg lower (9.07 lower to 1.19 lower) | ‐ | 139 (2 RCTs) | ⊕⊕⊝⊝ LOWa,b | ‐ |
| Anxiety, change from baseline Follow‐up: 3 months | SMD 0.71 lower (1.09 lower to 0.32 lower) | ‐ | 112 (1 RCT) | ⊕⊕⊝⊝ LOWb,c | We interpret an SMD of 0.2 to represent a small effect, 0.5 a moderate effect, and 0.8 a large effect. | |
| Depression, change from baseline Follow‐up: 3 months | SMD 0.48 lower (0.86 lower to 0.11 lower) | ‐ | 112 (1 RCT) | ⊕⊕⊝⊝ LOWb,c | We interpret an SMD of 0.2 to represent a small effect, 0.5 a moderate effect, and 0.8 a large effect. | |
| Perceived stress | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported. |
| Well‐being | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported. |
| Adverse events | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported. |
| Smoking cessation | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CVD: cardiovascular disease; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; SMD: standardised mean difference; TM: transcendental meditation | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by one level for risk of bias: one study at high risk of attrition bias and one study at unclear risk of selection bias. bDowngraded by one level for imprecision: small sample size. cDowngraded by one level for risk of bias: study at high risk of attrition bias.
Background
Description of the condition
Cardiovascular diseases (CVDs) are a group of disorders of the heart and blood vessels, which include CVDs due to atherosclerosis (coronary heart disease (CHD), cerebrovascular disease, and peripheral vascular disease) and other CVDs (rheumatic heart disease, congenital heart disease, cardiomyopathies, and cardiac arrhythmias). Atherosclerosis is a complex process that occurs in the walls of blood vessels over many years, where fatty material and cholesterol deposit and form plaques, which narrow and stiffen arteries and reduce blood flow. Ruptured plaques can cause the formation of blood clots, which trigger heart attacks if they develop in the coronary arteries and strokes if clots develop in the brain (WHO 2011).
CVDs are the world's leading cause of death and caused 17.9 million deaths in 2019; this represented 32% of all global deaths that year, over three‐quarters of which occurred in low‐ and middle‐income countries (WHO 2021). Of these 17.9 million deaths, 85% were due to heart attack and stroke (WHO 2021).
Many CVDs are preventable by addressing behavioural cardiovascular risk factors, the most important of which are unhealthy diet, physical inactivity, tobacco use, and harmful use of alcohol, which in turn can affect markers of increased CVD risk such as raised blood pressure, raised blood glucose, raised blood lipids, and overweight and obesity (WHO 2021). Population‐wide strategies to address these behavioural risk factors and health policies to create environments where healthy options are available and affordable are recommended (WHO 2021). Other determinants of atherosclerotic CVD include advancing age, hereditary factors, gender, poverty, and psychological factors including stress and depression (WHO 2011). Psychosocial stress has been shown to be a risk factor for CVD (Dimsdale 2008; Merz 2002; O’Donnell 2010; Rosengren 2004; Yusuf 2004), and clusters with other behavioural risk factors such as smoking and increased consumption of alcohol and unhealthy foods. As many of these risk factors are related to lifestyle choices and are modifiable, they have become the focus of CVD prevention strategies. It is estimated that as much as 90% of the population‐attributable risk for CHD (specifically myocardial infarction) and stroke worldwide is accounted for by contributions from nine modifiable risk factors: abnormal cholesterol, raised blood pressure, diabetes mellitus, smoking, excessive alcohol intake, unhealthy diet, psychosocial stress, abdominal obesity, and lack of physical activity (O’Donnell 2010; Yusuf 2004).
CHD, high blood pressure, and diabetes mellitus are also major risk factors for heart failure. Heart failure occurs when there is insufficient oxygen to meet the metabolic demands of the body, resulting from a reduced ability of the heart to pump or fill with blood, or both (DHDSP 2016; Fox 2001). Heart failure is a growing public health burden, where it is estimated that over 26 million people worldwide are affected, and the prevalence is increasing (Savarese 2017). Mortality and morbidity associated with heart failure are high even with advances in treatment and prevention, and quality of life is poor (Savarese 2017). Reducing cardiovascular risk thus also impacts the numbers affected by heart failure.
Description of the intervention
Interventions incorporating meditation to address stress, anxiety, and depression, and self‐management of chronic conditions, are becoming popular for many health complaints and to enhance well‐being, yet the benefits of meditation were understood thousands of years ago. Meditation is a contemplative practice that originates from the world's wisdom traditions, but interventions are now commonly delivered in a secular context. There are many types of meditation practice, but the most researched interventions are mindfulness‐based interventions (MBIs) and transcendental meditation (TM).
MBIs in health care originate from the work of Jon Kabat Zinn in the late 1970s and the development of his eight‐week programme of mindfulness‐based stress reduction (MBSR) (Kabat‐Zinn 1990). This programme has been evaluated for a number of conditions, for example chronic pain (Kabat‐Zinn 1986) and anxiety (Kabat‐Zinn 1992). MBSR has been informed by Buddhist teachings but is taught in a secular context. MBSR has also informed another well‐researched intervention, mindfulness‐based cognitive therapy (MBCT), developed by Segal 2013 for the treatment of depression. Mindfulness is most commonly defined as "paying attention, in a particular way, on purpose, in the present moment and non‐judgementally" (Kabat‐Zinn 1994). Mindfulness has also been described by a UK Mindfulness All Party Parliamentary Group in the following way: “Mindfulness is best considered an inherent human capacity akin to language acquisition; a capacity that enables people to focus on what they experience in the moment, inside themselves as well as in their environment, with an attitude of openness, curiosity and care” and has been defined as "live in the moment, notice what is happening and make choices in how you respond to your experience rather than being driven by habitual reactions" (Breathworks 2019). Simply, it means being aware of our experience moment to moment, and with this awareness comes choice. Other key aspects of many MBIs are compassion practices for self and others, and in this respect mindfulness has also been described as heartfulness: it is as much of the heart as it is of the mind (Kabat‐Zinn 1994; Williams 2007). Mindfulness courses are typically eight weeks long, delivered by trained teachers from their own mindfulness practice, and cover a series of formal meditation practices including body scanning, mindfulness of breathing, compassion practices, and informal practices of mindfulness of daily living, including mindful movement (Burch 2013; Hennessey 2016; Kabat‐Zinn 1990; Segal 2013). The essential components of MBIs, including teachers' training and characteristics, have been reviewed (Crane 2017). The expectation of participants is home practice daily, starting at a minimum of 10 minutes twice a day, which increases over the course of eight‐week programmes, with continued practice thereafter.
TM is described as a technique for inner peace and wellness, an effortless practice that enables the mind and body to access a special quality of rest (Transcendental Meditation® 2018). Transcending means to go beyond the steps of the meditation practice itself to an inner stillness. The technique is delivered by certified teachers, trained in the techniques of the founder Maharishi Mahesh. It is based on ancient Vedic teachings from India, which were popularised and brought to the west by Maharishi Mahesh in the late 1950s. Practitioners receive a personal mantra to repeat silently to settle the mind inward; this is practised for 15 or 20 minutes twice a day. Training in TM takes four days, personal instruction on day one and small group sessions days two to four to consolidate the practice. TM distinguishes itself from other forms of meditation that train the mind in some way, for example, in the case of mindfulness, to be in the present moment. TM liberates rather than trains the mind, allowing it to settle effortlessly into a silence more profound than the present moment (Meditation Trust). There is a considerable volume of research on the potential benefits of TM and its standardised format has allowed comparisons between studies. A meta‐analysis, for example, found beneficial effects of TM for trait anxiety compared to alternative treatments and care as usual, with larger effect sizes for those with higher levels of anxiety at baseline (Orme‐Johnson 2014).
How the intervention might work
There is extensive evidence to show a link between psychosocial stress and CVD. Whilst psychosocial stress clusters with other behavioural risk factors for CVD, independent associations are seen for perceived stress (Richardson 2012), stress at work (general work stress (Rosengren 2004), specifically job strain (Kuper 2003), and an imbalance between effort and reward (Dragano 2017)), stress at home, financial stress, stressful life events, and depression (Rosengren 2004). Rosengren 2004 was a case control study conducted over 52 countries, which found that these differences were consistent across different regions, in different ethnic groups, and in both men and women. It is clear that stress is a trigger for CVD, but less clear are the mechanisms by which this operates and these may be complex and multifactorial (Dimsdale 2008; Merz 2002; Rozanski 1999; Vale 2005).
There is evidence from systematic reviews to show that MBIs are effective at reducing stress, anxiety, and depression (Fjorback 2011; Janssen 2018; Khoury 2013; Khoury 2015), with modest results for studies employing active control groups (Goyal 2014). There is also a developing body of research demonstrating that MBIs could have beneficial effects on other CVD risk factors (Fulwiler 2015). For example, mindfulness training has been used for smoking cessation (Brewer 2011), weight loss (Fulwiler 2015), and to reduce blood pressure (Blom 2014). The possible mechanisms by which MBIs could influence cardiovascular risk include attention control, emotional regulation, and self‐awareness (Fulwiler 2015). Few studies have been conducted in patients with established CVD. The effects of MBSR and MBCT have been examined in a systematic review of patients with vascular disease including hypertension, heart disease, and stroke. Some improvements were seen in psychological outcomes in vascular disease patients, but the effects on physical outcomes were mixed (Abbott 2014). Another review of MBIs in patients following transient ischaemic attack (TIA) or stroke found very few studies and could draw no firm conclusions (Lawrence 2013).
Similarly, there is evidence of beneficial effects of TM on psychological distress (Orme‐Johnson 2014), but positive effects are less apparent in studies using active control groups (Goyal 2014). There are a large number of studies looking at the effects of TM on blood pressure. Overall beneficial effects have been seen in systematic reviews (e.g. Anderson 2008), but positive effects of TM on blood pressure have been attributed to important methodological weaknesses and bias (Canter 2004). An overview of eight systematic reviews and meta‐analyses finds a clear trend of increasing evidence to support reductions in blood pressure with TM but cautions that there are some conflicting findings and potential risk of bias in many of the included RCTs (Ooi 2017). The effects of ambulatory blood pressure monitoring (ABPM) and non‐ABPM have been examined in response to both TM and other forms of meditation (non‐TM) where both interventions showed reductions in blood pressure and smaller effect sizes with ABPM (Shi 2017). Components of the metabolic syndrome, including blood pressure, have been examined in a trial of TM compared to health education as an active control in patients with stable CHD. TM was found to lead to beneficial changes in systolic blood pressure, insulin resistance, and heart rate variability compared to health education (Paul‐Labrador 2006). A trial with long‐term follow‐up has shown reduced clinical events (composite of total mortality, non‐fatal myocardial infarction, and stroke) and improvements in psychological outcomes compared to health education in Black patients with CHD (subjects were identified from the African American Heart Health Registry) (Schneider 2012).
Two recent Cochrane reviews have examined a range of psychological interventions for CHD (Richards 2017) and for diabetes distress in adults with type 2 diabetes (Chew 2017). Richards 2017 looked at psychological interventions compared to usual care, delivered by trained staff and with a minimum follow‐up of six months. Only two of the 35 included studies examined meditation. Chew 2017 looked at a range of psychological interventions, and none of these focused on meditation (Chew 2017). Two further systematic reviews have looked at mixed mind‐body interventions for cardiac disease, including meditation. Younge 2015 found promising results in quality of life measures, psychological measures, and blood pressure but reported overall low‐quality studies. Similarly, in heart failure patients, small to moderate effects were seen in quality of life, exercise capacity, anxiety, depression, blood pressure, and heart rate variability (Gok Metin 2018). Anxiety and depression often co‐exist with long‐term conditions such as CVD, type 2 diabetes, and heart failure. Meditation could be effective in addressing these co‐morbidities, as well as potentially affecting risk factors for CVD.
Why it is important to do this review
The 2017 scientific statement from the American Heart Association (AHA) highlights the potential benefits of meditation on CVD risk factors including psychosocial stress, blood pressure, smoking, insulin resistance and the metabolic syndrome, subclinical atherosclerosis, endothelial function, exercise capacity, and the primary and secondary prevention of CVD (AHA 2017). Their findings suggest possible benefits of meditation, although the overall quality and sometimes quantity of studies is poor. Research recommendations include use of randomised controlled trials (RCTs), blinded outcome assessment, adequate power of studies, longer and more complete follow‐up, and studies performed by investigators without financial or intellectual bias (AHA 2017). This Cochrane review will update these findings and will focus on evidence from RCTs and assess risk of bias and overall quality of contributing research. This is a rapidly expanding field and it is important to synthesise the evidence of these potentially useful interventions in a format that can be updated to further inform guideline development and end‐user choice.
Objectives
To determine the effectiveness of meditation techniques, primarily MBIs and TM, for the primary and secondary prevention of CVD in adults at high risk and with established CVD.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel‐arm and cluster‐RCTs. We also included cross‐over RCTs but analysed only the first phase as a parallel‐group design. We did not include quasi‐RCTs. We included studies reported as full‐text, those published as abstract only, and unpublished data.
Types of participants
We included adults (defined as ≥ 18 years of age) both at high risk of, and with established CVD, to examine both primary and secondary prevention.
Primary prevention
Adults identified as being at increased risk of CVD exhibiting one or more of the following risk factors as defined by the trial authors: hypertension, abnormal cholesterol levels, overweight/obesity, smoking, impaired glucose control/type 2 diabetes.
Secondary prevention
Adults diagnosed with CVD as defined by the trial authors, including the following: experienced a myocardial infarction (MI); undergone a revascularisation procedure (coronary artery bypass graft (CABG) or percutaneous coronary intervention (PCI)); people with angina; people with angiographically defined CHD, cerebrovascular disease including stroke and TIA, or peripheral vascular disease (PVD). We also included patients with heart failure that has developed as a consequence of CVD where these can be identified from other forms of heart failure, or form the majority in mixed populations.
For studies involving only a subset of relevant participants, we included only studies where participants of interest formed the majority of the sample if stratified data were not reported separately.
We explored the effects of primary and secondary prevention on outcomes in subgroup analyses (Subgroup analysis and investigation of heterogeneity).
Types of interventions
We included trials of meditation interventions, predominantly MBIs and TM using methods/elements as illustrated previously (see Description of the intervention), or as defined/described by study investigators. We also included other types of meditation where the methods were adequately described. Multifactorial interventions were only included where meditation was the main focus. We excluded interventions that comprised predominantly physical practices as well as meditation such as yoga, tai chi, and qigong to avoid the confounding effects of physical activity on CVD outcomes. We included trials with any comparison group, for example no intervention/wait list, usual care (inactive comparators), or attention control, or alternative interventions (active comparators). Trials where the comparison was another form of meditation or different levels of intensity of the intervention of interest were excluded. We excluded trials where meditation was delivered alongside co‐interventions unless the comparison group also received the co‐intervention, so the effects of meditation alone could be determined.
We included studies with follow‐up periods of 12 weeks or more, defined as the intervention period plus post‐intervention follow‐up.
We did not combine different meditation interventions and comparators in the main analysis as this would make interpretation of the results difficult due to heterogeneity. Instead, we undertook four main analyses:
MBIs versus active comparators;
MBIs versus non‐active comparators;
TM versus active comparators;
TM versus non‐active comparators.
Other meditation interventions that met the inclusion criteria but did not fit the above categories were described narratively.
Types of outcome measures
Primary outcomes
-
CVD clinical events:
cardiovascular mortality;
myocardial infarction;
coronary artery bypass graft (CABG);
percutaneous coronary intervention (PCI);
angina;
angiographically defined coronary heart disease (CHD);
stroke;
transient ischaemic attack (TIA);
peripheral vascular disease (PVD);
-
Blood pressure:
systolic blood pressure;
diastolic blood pressure.
Validated measures of psychological distress (e.g. Beck Anxiety Inventory (Beck 1988) and Beck Depression Inventory (Beck 1996), Centre for Epidemiological Studies ‐ Depression Scale (CES‐D; Radloff 1977), Patient Health Questionnaire‐9 (PHQ‐9; Spitzer 1999), Hospital Anxiety and Depression Scale (HADS; Zigmond 1983), Generalized Anxiety Disorder 7 (GAD‐7; Spitzer 2006)) and well‐being (e.g. the Warwick‐Edinburgh Mental Well‐being Scale (WEMWBS; Tennant 2007)).
Adverse events (number of patients affected).
Secondary outcomes
-
Individual CVD risk factors other than blood pressure including:
lipid levels (total cholesterol, low‐density lipoprotein (LDL) and high‐density lipoprotein (HDL) cholesterol, triglycerides, measured separately);
glycaemic control (measures such as fasting blood glucose and HbA1c, and the incidence of type 2 diabetes);
body weight or body mass index (BMI), or both;
smoking rates.
Validated measures of quality of life (QoL) (e.g. 36‐item short‐form health survey (SF‐36; Ware 1992)), any validated QoL scale, generic or disease‐specific.
Validated measures of coping, resilience, mastery (e.g. the Self‐Efficacy Scale (Sherer 1982) and the Self‐Management Screening (SeMaS) tool (Eikelenboom 2015)).
Search methods for identification of studies
Electronic searches
We identified trials through systematic searches of the following bibliographic databases on 14 November 2021:
Cochrane Central Register of Controlled Trials (CENTRAL 2021, Issue 11) (Cochrane Library).
Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, MEDLINE Daily and MEDLINE (Ovid, 1946 to 12 November 2021).
Embase (Ovid, 1980 to week 45, 2021).
PsycINFO (Ovid, 1806 to November week 1, 2021).
CINAHL (Cumulative Index to Nursing and Allied Health Literature) (EBSCO, 1937 to 14 October 2021).
AMED (Allied and Complementary Medicine Database) (Ovid, 1985 to November 2021).
The RCT filter for MEDLINE is the Cochrane sensitivity‐maximising RCT filter, and for Embase, terms as recommended in the Cochrane Handbook for Systematic Reviews of Interventions have been applied (Lefebvre 2019). For the other databases, except CENTRAL, an adaptation of the Cochrane RCT filter has been applied. Search strategies for all databases are presented in Appendix 1.
We also searched ClinicalTrials.gov and WHO ICTRP on 15 November 2021 for ongoing or unpublished trials.
We searched all databases from their inception to the present, and we imposed no restriction on language of publication or publication status.
We did not perform a separate search for adverse effects of interventions. We considered adverse effects described in the included studies only.
Searching other resources
We checked reference lists of included studies and any relevant systematic reviews identified for additional references to trials. We also examined any relevant retraction statements and errata for included studies. We contacted authors for missing information and details of ongoing trials where necessary.
Data collection and analysis
Selection of studies
Two review authors (of KR, AT, RC) independently screened titles and abstracts of all the potential studies identified as a result of the search and coded them as either 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We resolved any disagreements through discussion. We retrieved the full‐text study reports/publication and two review authors (of KR, AT, RC, LK) independently screened the full text and identified studies for inclusion, and identified and recorded reasons for exclusion of the ineligible studies. We resolved any disagreements through discussion. We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, is the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and 'Characteristics of excluded studies' table.
Data extraction and management
We used a data collection form for study characteristics and outcome data, which we piloted. Two review authors (of KR, AT, RC, LK, LH) extracted study characteristics from included studies. We extracted the following study characteristics:
Methods: study design, total duration of study, number of study centres and location, study setting, and date of study.
Participants: N randomised, N lost to follow‐up/withdrawn, N analysed, mean age, age range, gender, primary or secondary prevention (at increased risk of CVD, or established CVD), inclusion criteria, and exclusion criteria.
Interventions: intervention, comparison, concomitant treatments/medications.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for trial, and notable conflicts of interest of trial authors.
Two review authors (of KR, AT, RC, LK, LH) independently extracted outcome data from included studies. We resolved disagreements by consensus. One review author (KR) transferred data into the Review Manager 5 file (RevMan 2020). We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the data extraction form. A second review author (AT) spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors (of KR, AT, RC, LK, LH) independently assessed the risk of bias for each included study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We resolved any disagreements by discussion. We assessed the risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We assessed each potential source of bias as either high, low, or unclear and provided a quote from the study report together with a justification for our judgement in the risk of bias table. We summarised the risk of bias judgements across different studies for each of the domains listed. We expected blinding of participants and personnel to be difficult to achieve and unlikely for trials of meditation interventions, and so we have not recorded this as high risk but as unclear. Where information on risk of bias related to unpublished data or correspondence with a trial author, we noted this in the risk of bias table.
Where we found cluster‐randomised trials that met the inclusion criteria, we followed the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017), and explored the following: recruitment bias, baseline imbalance, loss of clusters, incorrect analysis, and comparability with individually randomised trials.
When considering treatment effects, we took into account the risk of bias for the studies that contribute to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol (Rees 2019a), and report any deviations from it in the Differences between protocol and review section of the systematic review.
Measures of treatment effect
We analysed dichotomous data (e.g. CVD clinical events, smoking rates) as risk ratios with 95% confidence intervals (CIs). For continuous variables (e.g. blood pressure, lipid levels, glycaemic control, weight), we compared net changes (i.e. intervention group minus control group differences) and calculated mean differences (MD) and 95% CIs for each study. We used the standardised mean difference (SMD) where different scales have been used to measure the same outcome, e.g. psychological distress, well‐being, quality of life, and tested the robustness of using this and MD using sensitivity analyses. We interpreted the SMD in accordance with Cohen's 'rule of thumb' described in the Cochrane Handbook of Systematic Reviews of Interventions as follows: an SMD of 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect (Schünemann 2022). We planned to, where possible, calculate the number needed to treat for an additional beneficial or harmful outcome (NNTB or NNTH) to aid the interpretation of findings. We entered data presented as a scale with a consistent direction of effect and labelled these clearly.
We narratively described skewed data reported as medians and interquartile ranges.
Unit of analysis issues
Where cluster‐randomised trials met the inclusion criteria of the review, we analysed these in accordance with guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022). For trials with multiple arms, we divided the control group N by the number of intervention arms to avoid double‐counting in meta‐analyses. We analysed outcomes at the longest period of follow‐up where multiple measurements had been taken, unless there was significant (> 30%) attrition. We planned to explore the effects of short‐term (12 to 26 weeks) and longer‐term (greater than 26 to 52 weeks, greater than 52 weeks) follow‐up in subgroup analyses if there were sufficient data.
Dealing with missing data
We planned to contact investigators or study sponsors in order to verify key study characteristics and obtain missing numerical outcome data where necessary (e.g. when a study is identified as abstract only). Where standard deviations (SD) for outcomes were not reported, other variance measures, such as standard errors and CIs, were unavailable to derive SDs from, and we were unable to obtain information from study authors, we imputed these following the methods presented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). Where studies did not report results as change from baseline for continuous outcomes, we calculated this and the SD differences following the methods presented in the Cochrane Handbook for Systematic Reviews of Interventions for imputing these (Higgins 2021), and assumed a correlation of 0.5 between baseline and follow‐up measures, as suggested by Follman 1992.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the trials in each analysis. If we identified substantial heterogeneity (50% to 90%; Deeks 2022), we reported it and explored possible causes by pre‐specified subgroup analysis (Subgroup analysis and investigation of heterogeneity). If we were unable to conduct subgroup analyses to explore this or where heterogeneity remained unexplained, we presented individual studies in forest plots to show the direction of effect of individual studies but described narratively our uncertainty around pooled effect estimates.
Assessment of reporting biases
Where we were able to pool more than 10 trials, we created and examined a funnel plot to explore possible small study biases for the primary outcomes (Primary outcomes).
Data synthesis
We undertook meta‐analyses only where this was meaningful, i.e. if the treatments, participants, and the underlying clinical question were similar enough for pooling to make sense.
We used a random‐effects model as we cannot assume that all studies in the meta‐analyses are estimating the same intervention effect, but rather are estimating intervention effects that follow a distribution across studies.
Subgroup analysis and investigation of heterogeneity
We performed the following subgroup analysis for two comparisons: MBIs versus non‐active comparators and TM versus active comparators.
By types of subpopulation: primary prevention (those at high risk of CVD) and secondary prevention (those with established CVD).
We assessed the following outcomes in subgroup analysis.
Blood pressure.
Validated measures of psychological distress and well‐being.
We used the formal test for subgroup interactions in Review Manager 5 (RevMan 2020).
Where there are sufficient studies in future updates, we will explore heterogeneity for the subgroups below for all comparisons:
Nature and intensity of the meditation intervention.
Nature and intensity of the comparator.
Length of follow‐up (12 to 26 weeks, greater than 26 to 52 weeks, and greater than 52 weeks).
Setting of the intervention (e.g. group, individual, online, community, inpatient).
Sensitivity analysis
At the protocol stage, we planned to carry out the following sensitivity analyses.
Only including studies with a low risk of bias. We defined this as: low risk in at least four domains, but not including blinding of participants and personnel, which is difficult and therefore unlikely in the trials of interest. For most outcomes of interest, important domains are adequate randomisation and concealment, blinding of outcome assessors, attrition, and selective reporting.
Only including studies where there are no conflicts of interest, e.g. funding source of trials.
Restricting the analyses to published peer‐reviewed trials.
Testing the robustness of using SMD or MD where appropriate.
Testing the robustness of the results by repeating the analyses using different statistical models (fixed‐effect and random‐effects models).
Note that we only reported these where there were sufficient studies to do so. We did not perform the sensitivity analyses including studies at low risk of bias, as risk of bias was rated as unclear for most domains for the majority of studies. Similarly, few conflicts of interest were reported, and all but one trial was published in a peer‐reviewed journal.
Where we pooled data and found possible effects of the interventions, we tested the robustness of these by exploring differences between random‐effects and fixed‐effect models and using MD instead of SMD where appropriate.
Reaching conclusions
We based our conclusions only on findings from the quantitative and narrative synthesis of included studies for this review. We avoided making recommendations for practice and our implications for research suggest priorities for future research and outline what the remaining uncertainties are in the area.
Summary of findings and assessment of the certainty of the evidence
We created summary of findings tables using the following outcomes:
CVD clinical events (cardiovascular mortality and non‐fatal endpoints reported separately and described in a narrative synthesis);
systolic and diastolic blood pressure;
validated measures of psychological distress (anxiety, depression, perceived stress) and well‐being;
adverse events;
smoking rates.
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of a body of evidence as it relates to the studies that contribute data to the meta‐analyses for the prespecified outcomes. We used methods and recommendations described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021), and used GRADEpro software (GRADEPro GDT 2015). We created a separate summary of findings table for each comparison (MBI versus active control, MBI versus non‐active control, TM versus active control, TM versus non‐active control). We justified all decisions to downgrade the certainty of the evidence using footnotes and made comments to aid the reader's understanding of the review where necessary.
Two review authors (KR, AT) independently assessed the certainty of the evidence. We resolved any disagreements through discussion. Judgements were justified, documented, and incorporated into the reporting of results for each outcome.
We extracted study data, formatted our comparisons in data tables, and prepared a summary of findings table before writing the results and conclusions of our review.
Results
Description of studies
Results of the search
Searching medical databases and clinical trial registries to November 2021 and other sources, we identified 6993 references, which reduced to 4019 after de‐duplication. Of the 4019 references screened, 644 went forward for formal full‐text assessment based on our pre‐defined inclusion/exclusion criteria. Following full‐text assessment and collation of multiple papers for individual studies, 81 studies (156 references) were included. We also identified 74 ongoing trials and categorised 14 studies as awaiting classification due to insufficient information.
In May 2023, we revisited the status of the 74 ongoing studies. Of these, nine studies that were ongoing back in November 2021 were re‐categorised as awaiting classification because results had become available (Table 5). This brings us to a total of 23 studies awaiting classification (Characteristics of studies awaiting classification) and 65 ongoing studies (Characteristics of ongoing studies). The flow of study selection is presented in the PRISMA diagram in Figure 1.
1. Status updates of ongoing studies identified in November 2021.
| Study ID and citation details | Status updates as of May 2023 |
|
ACTRN12618000844246 ImpleMENTing meditatiOn into heart disease clinical settings (The MENTOR Study). https://anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12618000844246 (first received 18 May 2018). |
Status still recruiting, registry last updated 5 June 2019. |
|
ACTRN12618001247268 Compassion Focused Therapy as a Treatment for Body Weight Shame Associated with Obesity. https://anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12618001247268 (first received 24 July 2018). |
Status still recruiting, registry last updated 13 December 2019. |
|
ACTRN12620000105943 Support After Stroke with group‐based classeS: The SASS study. https://trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12620000105943 (first received 5 February 2020). |
Status still recruiting, registry last updated 21 November 2022. |
|
ACTRN12621000445875 Investigating the effect of cognitive, behavioural and mindfulness‐based interventions on smoking rates in lower socio‐economic groups. https://trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12621000445875 (first received 19 April 2021). |
Status recruiting, registry last updated 19 September 2022. |
|
ACTRN12621000580875 Self‐compassion for weight management: an online intervention for adults seeking to manage weight. https://trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12621000580875 (first received 17 May 2021). |
Status completed, no results, registry last updated 24 January 2022. |
Asfar 2021
|
Status recruiting, registry last updated 27 October 2022. |
|
Chandra 2020 Chandra M, Raveendranathan D, Pradeep RJ, Patra S, Rushi, Prasad K, et al. Managing Depression in Diabetes Mellitus: A Multicentric Randomized Controlled Trial Comparing Effectiveness of Fluoxetine and Mindfulness in Primary Care: Protocol for DIAbetes Mellitus ANd Depression (DIAMAND) Study. Indian Journal of Psychological Medicine 2020;42(6 Suppl):S31‐S38. [DOI: 10.1177/0253717620971200] |
Cannot access registry to check status but described as ongoing in a cross‐sectional analysis of baseline data published in 2023 (Patra, Suravi; Patro, Binod Kumar1; Padhy, Susanta Kumar; Mantri, Jogamaya. Relationship of Mindfulness with Depression, Self‐Management, and Quality of Life in Type 2 Diabetes Mellitus: Mindfulness is a Predictor of Quality of Life. Indian Journal of Social Psychiatry 39(1):p 70‐76, Jan–Mar 2023. | DOI: 10.4103/ijsp.ijsp_436_20) |
Chung 2019
|
Status recruiting, registry last updated 7 July2020. Protocol published in full in 2022. |
|
DRKS00021412 Self‐Compassion, Eating Behavior & Dieting Success. https://trialsearch.who.int/Trial2.aspx?TrialID=DRKS00021412 (first received 15 April 2020). |
Status recruitment suspended, registry last updated on 14 November 2022. |
|
Forman 2021 Forman EM, Chwyl C, Berry MP, Taylor LC, Butryn ML, Coffman DL, et al. Evaluating the efficacy of mindfulness and acceptance‐based treatment components for weight loss: Protocol for a multiphase optimization strategy trial. Contemporary Clinical Trials 2021;110:106573. [DOI: 10.1016/j.cct.2021.106573] Project Activate: Mindfulness and Acceptance Based Behavioral Treatment for Weight Loss. https://clinicaltrials.gov/ct2/show/NCT04337619. |
Status active not recruiting, expected completion 24 May 2024. Registry last updated on 25 March 2022. |
|
Guerrini Usubini 2021 Guerrini Usubini A, Cattivelli R, Giusti EM, Riboni FV, Varallo G, Pietrabissa G, et al. The ACTyourCHANGE study protocol: promoting a healthy lifestyle in patients with obesity with Acceptance and Commitment Therapy‐a randomized controlled trial. Trials 2021;22(1):290. [DOI: 10.1186/s13063‐021‐05191‐y] NCT04474509. ACTyourCHANGE Study Protocol. Promoting Healthy Lifestyle With ACT for Obesity. https://clinicaltrials.gov/ct2/show/NCT04474509 (first received 4 July 2020). |
Status recruiting, expected completion 30 September 2024. Registry last updated 1 March 2023. |
|
Hemenway 2021 NCT03734666. Development of a Mindfulness‐Based Treatment for the Reduction of Alcohol Use and Smoking Cessation. https://clinicaltrials.gov/ct2/show/NCT03734666 (first received 4 July 2020). Hemenway M, Witkiewitz K, Unrod M, Brandon KO, Brandon TH, Wetter DW, et al. Development of a mindfulness‐based treatment for smoking cessation and the modification of alcohol use: A protocol for a randomized controlled trial and pilot study findings. Contemporary clinical trials 2021;100:106218. [DOI: 10.1016/j.cct.2020.106218] |
Status active not recruiting, registry last updated 26 January 2023. Results submitted 27 February 2023 and returned 22 March 2023 after quality control review. |
|
IRCT20150519022320N14 Comparative investigating the effect of relaxation and meditation techniques on quality of life in patients with coronary artery disease. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20150519022320N14 (first received 28 October 2018). |
Same. Status still recruiting, registry last updated 14 January 2019. |
|
IRCT2015122825739N1 Psychological interventions in weight loss. http://www.who.int/trialsearch/trial2.aspx?Trialid=IRCT2015122825739N1 (first received 1 September 2017). |
Same. Status still recruiting, expected end date 3 November 2018, registry last updated February 2018. |
| IRCT2016070126600N1 Effectiveness of mindfulness‐based stress reduction on Perceived Stress and blood pressure in high blood pressure patients. http://www.who.int/trialsearch/trial2.aspx?Trialid=IRCT2016070126600N1 (first received 23 July 2014). |
Results published ‐ re‐categorised as study awaiting classification (Khosravi 2016). |
| IRCT20190410043230N1 The Effect of Mindfulness Meditation, when Compared to routine education on Mental Health in Patients with Hypertension. https://trialsearch.who.int/?TrialID=IRCT20190410043230N1 (first received 23 July 2018). |
Results published ‐ re‐categorised as study awaiting classification (Babak 2022). |
|
IRCT20190804044436N1 Effect of mindfulness‐based stress management therapy on the emotion regulation, anxiety, depression and food addiction in obese people. https://trialsearch.who.int/Trial2.aspx?TrialID=IRCT20190804044436N1 (first received 21 September 2019). |
Same. Status complete, no results posted. Registry last updated February 2020. |
| IRCT20190924044866N1 Effect of an acceptance, mindfulness and compassionate‐based group intervention in overweight and obese women. https://trialsearch.who.int/?TrialID=IRCT20190924044866N1 (first received 22 October 2017). |
Results published ‐ re‐categorised as study awaiting classification (Pirmoradi 2022). |
| IRCT20200210046451N1 The Comparison of the effectiveness of mindfulness cognitive group psychotherapy and schema therapy on weight, body image and self‐esteem of people with obesity. https://trialsearch.who.int/Trial2.aspx?TrialID=IRCT20200210046451N1 (first received 6 March 2020). |
Results published ‐ re‐categorised as study awaiting classification (Bahadori 2022). |
|
IRCT20200225046618N1 Comparing the compassion‐focused therapy and dialectical behavior therapy training on state and trait anxiety symptoms and impulsivity in patients with coronary heart disease. https://trialsearch.who.int/Trial2.aspx?TrialID=IRCT20200225046618N1 (first received 15 December 2019). |
Same. Registry last updated 12 April 2020, recruitment complete. No results posted. |
|
IRCT20200226046625N1 Comparison of the Effectiveness of Cognitive‐Behavioral Therapy Based on Mindfulness and Education on Health Promoting Lifestyle in In treatment of diabetes. https://trialsearch.who.int/Trial2.aspx?TrialID=IRCT20200226046625N1 (first received 15 December 2019). |
Same. Registry last updated 24 March 2020, recruitment complete. No results posted. |
|
IRCT20200305046699N1 The Effectiveness of Group Therapy Based on Mindfulness and cognitive‐behavioral therapy (CBT) in the anxiety, metabolic control and quality of life in type 2 diabetic patients. https://trialsearch.who.int/Trial2.aspx?TrialID=IRCT20200305046699N1 (first received 7 January 2020). |
Registry last updated 18 May 2020, status recruiting. |
| IRCT20200919048767N1 Effect of mindfulness training on weight loss. https://trialsearch.who.int/Trial2.aspx?TrialID=IRCT20200919048767N1 (first received 22 October 2020). |
Results published ‐ re‐categorised as study awaiting classification (Jassemi Zergani 2021). |
|
JPRN‐jRCT1030200197 Online Mindfulness‐Based Eating Awareness Training for obesity. https://trialsearch.who.int/Trial2.aspx?TrialID=JPRN‐Jrct1030200197 (first received 12 November 2020). |
Status recruiting, registry last updated 10 January 2022 |
|
JPRN‐UMIN000030444 Effects of a mindfulness‐based intervention versus cognitive behavioral therapy on weight loss and weight maintenance for women with overweight or obesity: a randomized controlled trial. https://trialsearch.who.int/?TrialID=JPRN‐UMIN000030444 (first received 19 December 2017). |
Status recruiting, registry last updated 10 October 2022 |
|
JPRN‐UMIN000042260 Effects of a Mindfulness‐Based Eating Awareness Training online intervention in adults of obesity: a randomized controlled trial. https://trialsearch.who.int/Trial2.aspx?TrialID=JPRN‐UMIN000042260 (first received 28 October 2020). |
Status recruitment pending, registry last updated 6 April 2022 |
|
JPRN‐UMIN000042626 An exploratory study of the Mindfulness App for Weight loss in metabolic syndrome. https://trialsearch.who.int/Trial2.aspx?TrialID=JPRN‐UMIN000042626 (first received 31 March 2021). |
Status complete, follow‐up continuing. Registry last updated 6 July 2022 |
|
Martorella 2021 Martorella G, Hanley AW, Pickett SM, Gelinas C. Web‐ and Mindfulness‐Based Intervention to Prevent Chronic Pain After Cardiac Surgery: Protocol for a Pilot Randomized Controlled Trial. JMIR Research Protocols 2021;10(8):e30951. [DOI: /10.2196/30951] |
Same. Published protocol only, no trial registration found. Status recruiting (delays due to COVID pandemic). |
|
Mason 2019 Mason AE, Saslow L, Moran PJ, Kim S, Wali PK, Abousleiman H, et al. Examining the Effects of Mindful Eating Training on Adherence to a Carbohydrate‐Restricted Diet in Patients With Type 2 Diabetes (the DELISH Study): Protocol for a Randomized Controlled Trial. JMIR Research Protocols 2019;8(2):e11002. NCT03207711. Delish Study: Diabetes Education to Lower Insulin, Sugars, and Hunger (Delish). https://clinicaltrials.gov/ct2/show/NCT03207711 (first received 5 July 2017). |
Same. Registry last updated 30 June 2021 when status is study complete, no results posted. Citation of analyses of lipid levels combining both groups so not relevant to this review. |
|
NCT00224835 Mindfulness‐Based Stress Reduction and Myocardial Ischemia. https://clinicaltrials.gov/ct2/show/NCT00224835 (first received 23 September 2005). |
Same. Registry last updated 13 May 2016, study complete in 2008, no results posted or publications listed. |
|
NCT01227473 We Can Prevent Diabetes: A Behavioral Intervention to Reduce Diabetes Risk in African Americans. https://clinicaltrials.gov/ct2/show/NCT01227473 (first received 25 October 2010). |
Same. Registry last updated 15 June 2012, status completed, no results posted or publications listed. (Eligible if FBG reported separately). |
|
NCT01375504 Effect of Self Regulation With Mindfulness Training on Body Mass Index and Cardiovascular Risk Markers in Obese Adults. https://clinicaltrials.gov/ct2/show/NCT01375504 (first received 17 June 2011). |
Same. Registry last updated 19 April 2012, status completed, no results posted or publications listed. |
|
NCT01805245 Mindfulness: a Novel Approach for the Management of Diabetes‐related Distress. https://clinicaltrials.gov/ct2/show/NCT01805245 (first received 6 March 2013). |
Same. Registry last updated 19 April 2017, status completed, no results posted or publications listed. |
|
NCT02037360 Mobile Mindfulness Training for Smoking Cessation. https://clinicaltrials.gov/ct2/show/NCT02037360 (first received 15 January 2014). |
Same. Registry last updated 30 January 2018, status completed, no results posted or publications listed. (Cochrane review citing the trial registration was listed where contact with the PI revealed the data had never been analysed) |
|
NCT02165228 Strategies for Inflammation and Cardiovascular Disease (CVD) Prevention (SICVDP). https://clinicaltrials.gov/ct2/show/NCT02165228 (first received 17 June 2014) |
Same. Registry last updated 17 April 2014, status completed, no results posted or publications listed. |
|
NCT02753972 Mindful Eating and Living for Obese Women (MEAL). https://clinicaltrials.gov/ct2/show/NCT02753972 (first received 28 April 2016). |
Same. Registry last updated 28 April 2016, status completed, no results posted or publications listed. |
|
NCT02830529 Mindfulness Attitude to Deliver Dietary Approach to Stop Hypertension (MADDASH). https://clinicaltrials.gov/ct2/show/NCT02830529 (first received 13 July 2016). |
Same. Registry last updated 17.12.20, complete 2017, other findings published but not on relevant outcomes for this review. No results posted. |
|
NCT02928952 Mindful Stress Reduction in Diabetes Self‐management Education for Veterans (Mind‐STRIDE). https://clinicaltrials.gov/ct2/show/NCT02928952 (first received 10 October 2016). |
Results published ‐ re‐categorised as study awaiting classification (DiNardo 2022). |
|
NCT03013907 Emotional Awareness and SElf‐regulation for Depression in Patients With Hypertension (EASE) Study. https://clinicaltrials.gov/ct2/show/NCT03013907 (first received 9 January 2017). |
Same. Registry last updated 13 November 2019, status completed, no results posted or publications listed. |
|
NCT03020316 Peer Mentored Approaches For Men And Women With Coronary Artery Disease ("4Steps"). https://clinicaltrials.gov/ct2/show/NCT03020316 (first received 13 January 2017). |
Same. Registry last updated 26 November 2019, status completed, no results posted or publications listed. |
|
NCT03131128 The Effectiveness of Mindfulness‐based Intervention as a Workplace Health Promotion Program on Weight Loss. https://clinicaltrials.gov/ct2/show/NCT03131128 (first received 27 April 2017). |
Same. Registry last updated 22 March 2018, recruitment status unknown, recruitment status was recruiting. |
|
NCT03256890 Mindfulness‐Based Blood Pressure Reduction: Stage 2a Randomized Controlled Trial. https://clinicaltrials.gov/ct2/show/NCT03256890 (first received 22 August 2017) |
Same. Registry last updated 26 January 2021, status completed, no results posted or relevant publications listed. |
|
NCT03274362 Evaluating the Effectiveness of Headspace Mindfulness Application Versus Standard Care on the HbA1C and Quality of Life in Patients With Diabetes: A Randomized Control Trial. https://clinicaltrials.gov/ct2/show/NCT03274362 (first received 6 September 2017). |
Same. Registry last updated 6 September 2017, recruitment status unknown, recruitment status was active not yet recruiting. |
|
NCT03368950 Online Mindfulness for Stroke Sufferers. https://clinicaltrials.gov/ct2/show/NCT03368950 (first received 11 December 2017). |
Same. Registry last updated 14 February 2018, recruitment status unknown, recruitment status was active not yet recruiting. |
|
NCT03373110 Healthy Hearts Healthy Minds: A PPRN Demonstration Pragmatic Trial. https://clinicaltrials.gov/ct2/show/NCT03373110 (first received 14 December 2017). |
Registry last updated 2 February 2022, results are now posted but no publications listed. Results do not report outcomes of interest separately from number of steps and so are therefore ineligible. |
|
NCT03480113 The Effectiveness of Mindfulness‐based Intervention on Smoking Cessation. https://clinicaltrials.gov/ct2/show/NCT03480113 (first received 29 March 2018). |
Same. Registry last updated 18 May 2018, recruitment status unknown, recruitment status was recruiting. |
|
NCT03488966 Mindfulness Based Eating Awareness Training for Bariatric Surgery Patients (MB‐EAT). https://clinicaltrials.gov/ct2/show/NCT03488966 (first received 5 April 2018). |
Registry last updated 25 May 2022, status active not recruiting. |
|
NCT03659409 Investigating the Impact of Mindfulness on the Physiological and Psychological Well‐being of Stroke Survivors and Their Caregivers. https://clinicaltrials.gov/ct2/show/NCT03659409 (first received 6 September 2018). |
Same. Registry last updated 19 March 2020, status completed, no results posted or publications listed. |
|
NCT03736434 Brain Connections and Blood Pressure. https://clinicaltrials.gov/ct2/show/NCT03736434 (first received 9 November 2018). |
Same. Registry last updated 19 December 2020, status completed, no results posted or publications listed. |
|
NCT03793855 Effectiveness of a Nutritional Strategy for Glycemic Control in Patients With Type 2 Diabetes Mellitus Users of a Public Health System: NUGLIC Study. https://clinicaltrials.gov/ct2/show/NCT03793855 (first received 4 January 2019). |
Registry last updated 1 March 2023, status completed, no results posted or publications listed. |
|
NCT03793881 Nutritional Strategy for Blood Pressure Control in Patients With Hypertension (NUPRESS). https://clinicaltrials.gov/ct2/show/NCT03793881 (first received 4 January 2019). |
Registry last updated 1 March 2023, status completed, no results posted or publications listed. |
|
NCT03826836 Effectiveness of Mindfulness‐based Stress Reduction for Improving Quality of Life in Patients With Cardiovascular Disease: a Randomised Controlled Trial. https://clinicaltrials.gov/ct2/show/NCT03826836 (first received 1 February 2019). |
Same. Registry last updated 1 February 2019, recruitment status not yet recruiting. |
|
NCT03837405 Optimizing Lifestyle Interventions With Mindfulness‐based Strategies in Type 2 Diabetes. https://clinicaltrials.gov/ct2/show/NCT03837405 (first received 12 February 2019). |
Same. Registry last updated 29 June 2021, status completed, no results posted or publications listed. |
|
NCT03859076 UH3 Phase ‐ Mindfulness‐Based Blood Pressure Reduction (MB‐BP) : Stage 2a RCT (MB‐BP). https://clinicaltrials.gov/ct2/show/NCT03859076 (first received 1 March 2019). |
Same. Registry last updated 13 January 2021, status completed, no results posted or publications listed. |
|
NCT03910855 Impact of Mindfulness on Psychological Well‐being of Stroke Survivors and Their Caregivers (SOMII). https://clinicaltrials.gov/ct2/show/NCT03910855 (first received 10 April 2019). |
Same. Registry last updated 15 June 2021, status completed, no results posted or publications listed. |
|
NCT04016415 Decreasing Stress in Diabetes: A Randomized Controlled Trial. https://clinicaltrials.gov/ct2/show/NCT04016415 (first received 11 July 2019). |
Registry last updated 4 August 2022, status recruiting. |
|
NCT04171713 Mindfulness and Compassion‐based Programs on Food Behavior of Patients With Weight Regain After Bariatric Surgery. https://clinicaltrials.gov/ct2/show/NCT04171713 (first received 21 November 2019). |
Same. Registry last updated 3 December 2019, recruitment status unknown, recruitment status was recruiting. |
|
NCT04302493 Mindfulness Based Stress Reduction and Post‐Stroke Cognition. https://clinicaltrials.gov/ct2/show/NCT04302493 (first received 10 March 2020). |
Registry last updated 10 January 2023, status recruiting. |
|
NCT04759950 Exercise the Mind and Brain. A Multimodal Intervention in Stroke. https://clinicaltrials.gov/show/NCT04759950 (first received 18 February 2021). Protocol now published: Bermudo‐Gallaguet A, Ariza M, Dacosta‐Aguayo R, Agudelo D, Camins‐Vila N, Boldó M, Carrera Ò, Vidal S, Ferrer‐Uris B, Busquets A, Via M, Pera G, Cáceres C, Gomis M, García‐Molina A, Tormos JM, Arrabé A, Diez G, Durà Mata MJ, Torán‐Monserrat P, Soriano‐Raya JJ, Domènech S, Perera‐Lluna A, Erickson KI, Mataró M. Effects and mechanisms of mindfulness training and physical exercise on cognition, emotional wellbeing, and brain outcomes in chronic stroke patients: Study protocol of the MindFit project randomized controlled trial. Front Aging Neurosci. 2022 Sep 29;14:936077. doi: 10.3389/fnagi.2022.936077. PMID: 36248000; PMCID: PMC9557300. |
Registry last updated 2 March 2022, status completed, no results posted or publications of results listed. |
|
NCT04799899 MBCT Via Group Videoconferencing for Acute Coronary Syndrome Patients With Depressive Symptoms. https://clinicaltrials.gov/show/NCT04799899 (first received 16 March 2021). |
Registry last updated 10 November 2022, recruitment status active not recruiting. |
|
NCT04847843 Eating Mindfully to Prevent Weight Regain. https://ClinicalTrials.gov/show/NCT04847843 (first received 19 April 2021). |
Registry last updated 14 October 2022, status recruiting. |
|
NCT04965181 Mindfulness‐based Smoking Cessation Enhanced With Mobile Technology. https://clinicaltrials.gov/show/NCT04965181 (first received 16 July 2021). Protocol now published: Spears CA, Mhende J, Hawkins C, Do VV, Hayat MJ, Eriksen MP, Hedeker D, Abroms LC, Wetter DW. Mindfulness‐Based Smoking Cessation Delivered Through Telehealth and Text Messaging for Low‐Income Smokers: Protocol for a Randomized Controlled Trial. JMIR Res Protoc. 2022 Aug 1;11(8):e35688. doi: 10.2196/35688. |
Registry last updated 27 July 2022, status recruiting. |
|
NCT04985838 Helping Ease Anxiety and Depression Following Stroke Stage 3. https://clinicaltrials.gov/show/NCT04985838 (first received 2 August 2021). |
Registry last updated 28 March 2023, status completed, no results posted or publications of results listed. |
|
NCT05035758 Transcendental Meditation and Yoga: short‐ and Long‐term Effects in Cardiac Rehabilitation Patients ‐ a Pilot Study. https://clinicaltrials.gov/show/NCT05035758 (first received 5 September 2021). |
Results (assessment at 4 weeks) published ‐ re‐categorised as study awaiting classification (Rudlof 2022). |
|
NCT05070949 Self‐compassion to Reduce Diabetes Distress in Persons With Type 1 Diabetes. https://clinicaltrials.gov/show/NCT05070949 (first received 7 October 2021). |
Same. Registry last updated 19 October 2021, recruitment status not yet recruiting. |
|
RBR‐458tbcd Effects of an intervention based on Eating with Full Attention in patients who underwent Stomach Reduction Surgery and regained weight: a randomized clinical trial. https://trialsearch.who.int/Trial2.aspx?TrialID=RBR‐458tbcd (first received 5 March 2021). |
Registry last updated 3 April 2023. Recruitment status not yet recruiting. |
|
Ruffault 2016 MindOb: A 12‐month Computerized Mindfulness‐based Intervention for Obese Individuals (MindOb). https://clinicaltrials.gov/ct2/show/NCT02571387 (first received 8 October 2015). Ruffault A, Carette C, Lurbe I Puerto K, Juge N, Beauchet A, Benoliel JJ, et al. Randomized controlled trial of a 12‐month computerized mindfulness‐based intervention for obese patients with binge eating disorder: The MindOb study protocol. Contemp Clin Trials 2016;49:126‐33. [DOI: 10.1016/j.cct.2016.06.012] |
Registry last updated 26 December 2018. Recruitment status unknown, recruitment status was active not recruiting. |
|
NCT02893150 Mindfulness Intervention for Overweight Primary Care Patients (MindEat). https://clinicaltrials.gov/ct2/show/NCT02893150 (first received 8 September 2016). Salvo V, Kristeller J, Montero Marin J, Sanudo A, Lourenço BH, Schveitzer MC, et al. Mindfulness as a complementary intervention in the treatment of overweight and obesity in primary health care: study protocol for a randomised controlled trial. Trials 2018;19:277. [DOI: 10.1186/s13063‐018‐2639‐y] |
Results published ‐ re‐categorised as study awaiting classification (Salvo 2022). |
|
NCT03927534 Efficacy of a Mindful‐eating Program to Reduce Emotional Eating. https://clinicaltrials.gov/ct2/show/NCT03927534 (first received 25 April 2019). Morillo Sarto H, Barcelo‐Soler A, Herrera‐Mercadal P, Pantilie B, Navarro‐Gil M, Garcia‐Campayo J, et al. Efficacy of a mindful‐eating programme to reduce emotional eating in patients suffering from overweight or obesity in primary care settings: a cluster‐randomised trial protocol. BMJ Open 2019;9(11):e031327. [DOI: 10.1136/bmjopen‐2019‐031327] |
Results published ‐ re‐categorised as study awaiting classification (Morillo‐Sarto 2023). |
|
SLCTR/2021/015 Glycemic control in patients with type 2 diabetes mellitus following mindfulness meditation compared to standard therapy. https://trialsearch.who.int/Trial2.aspx?TrialID=SLCTR/2021/015 (first received 1 July 2021). |
Registry last updated 3 April 2023, recruitment status pending. |
|
Spruill 2018 Spruill TM, Reynolds HR, Dickson VV, Shallcross AJ, Visvanathan PD, Park C, et al. Telephone‐based mindfulness training to reduce stress in women with myocardial infarction: Rationale and design of a multicenter randomized controlled trial. American Heart Journal 2018;202:61‐67. [DOI: 10.1016/j.ahj.2018.03.028] NCT02914483. Women's Heart Attack Research Program: Stress Ancillary Study; Telephone‐Based Stress Management for Women With Myocardial Infarction. https://clinicaltrials.gov/ct2/show/NCT02914483 (first received 26 September 2016). |
Registry last updated 19 July 2022, recruitment status active not recruiting. |
|
TCTR20210406004 The effect of walking meditation on peripheral neuropathy in diabetes type 2. https://trialsearch.who.int/Trial2.aspx?TrialID=TCTR20210406004 (first received 31 March 2021). |
Registry last updated 3 April 2023, recruitment status recruiting. |
|
TCTR20210417002 Effects on a mindfulness meditation for decreasing blood pressure. https://trialsearch.who.int/Trial2.aspx?TrialID=TCTR20210417002 (first received 17 April 2021). |
Registry last updated 3 April 2023, recruitment status pending not yet recruiting. |
|
Wheelwright 2017 Wheelwright DS, Dangayach N, Griffiths S, Gordon E, Bederson J, Kellner C, et al. Does Intra‐ICU Initiation of Guided Mindfulness Meditation Decrease Anxiety and Depression in SAH?: A Unique Methodology for the Neurocritical Care Setting. Neurology 2017;88(16 Suppl):P5.069. |
Same. Conference proceeding and no trial registry reported for further details or to check current status. |
BMI: body mass index; FBG: fasting blood glucose; HbA1c: glycated haemoglobin (A1c); MB‐EAT; mindfulness‐based eating programme; PI: primary investigator; TAU: treatment as usual
1.
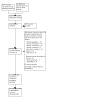
PRISMA study flow diagram
Details of the methods, participants, intervention, comparison groups, and outcome measures for each of the studies included in the review are shown in Characteristics of included studies. We present a summary of the description of included studies below for each comparison group.
Included studies
The total number of included studies was 81 (6971 participants randomised).
Five studies had active and inactive comparison groups for MBIs and have contributed to both active and inactive main comparisons for MBI (Alamout 2020; Brach 1992; Kristeller 2014; Tovote 2014; Vidrine 2016).
1. MBIs versus active comparators
We included 29 trials with 2883 participants randomised. Of these, 26 recruited participants at increased risk of CVD (primary prevention subpopulation) and three trials recruited participants with established CVD (secondary prevention subpopulation).
Populations
Twelve primary‐prevention trials recruited participants who were either overweight or obese (Alamout 2020; Blevins 2009; Brach 1992; Bressan 2020; Carpenter 2019; Chumachenko 2021; Daubenmier 2016; Kristeller 2014; Raja‐Khan 2017; Smith 2018; Spadaro 2018; Zanella 2021); seven trials recruited smokers (Brewer 2011; Davis 2014a; Garrison 2020; Goldenhersch 2020; Vidrine 2016; Weiss de Souza 2020; Weng 2021); four trials recruited participants with type 2 diabetes (Izgu 2020; Miller 2014), diabetes (Tovote 2014), or pre‐diabetes (Woods‐Giscombe 2019); and three trials recruited participants with hypertension (An 2021; McTigue 2020; Ponte Marquez 2019).
The three secondary‐prevention trials recruited stroke survivors (Baldo 2021; Baylan 2019; Beauchamp 2020).
Nine trials recruited only women (Alamout 2020; Blevins 2009; Brach 1992; Bressan 2020; Chumachenko 2021; Raja‐Khan 2017; Smith 2018; Weng 2021; Zanella 2021), and except for Weng 2021 all other trials also recruited women who were overweight or obese. The remaining trials recruited both men and women.
Settings
Nineteen trials were conducted in the US (An 2021; Baldo 2021; Beauchamp 2020; Blevins 2009; Brach 1992; Brewer 2011; Carpenter 2019; Chumachenko 2021; Daubenmier 2016; Davis 2014a; Garrison 2020; Kristeller 2014; McTigue 2020; Miller 2014; Raja‐Khan 2017; Smith 2018; Spadaro 2018; Vidrine 2016; Woods‐Giscombe 2019), three in Brazil (Bressan 2020; Weiss de Souza 2020; Zanella 2021), and one trial in each of Iran (Alamout 2020), the UK (Baylan 2019), Argentina (Goldenhersch 2020), Turkey (Izgu 2020), Spain (Ponte Marquez 2019), the Netherlands (Tovote 2014), and China (Weng 2021).
The duration of the intervention and follow‐up periods varied between 12 weeks and 18 months.
Interventions and comparators
The types of MBIs differ across trials. Several used standard MBSR (Kabat‐Zinn 1990) or MBCT (Segal 2002) protocols (Alamout 2020; Baldo 2021; Blevins 2009; Chumachenko 2021; Ponte Marquez 2019; Raja‐Khan 2017; Tovote 2014). Others adapted these to target specific behaviours such as smoking (Davis 2014a; Garrison 2020; Goldenhersch 2020; Vidrine 2016) or disordered eating (Carpenter 2019; Daubenmier 2016; Spadaro 2018), or for specific conditions such as diabetes (Izgu 2020; Woods‐Giscombe 2019). Mindfulness‐based relapse prevention protocols (Bowen 2011) were developed for smoking cessation interventions in two trials (Brewer 2011; Weiss de Souza 2020). Other standard MBIs were also developed, such as mindfulness‐based eating awareness training (MB‐EAT; Kristeller 2011), which was used in two trials (Kristeller 2014; Miller 2014) and adapted in another (Smith 2018). Other MBIs were based more broadly on mindfulness meditation practices, including mindfulness awareness programmes (An 2021), breath‐based Buddhist meditations along with informal mindfulness practices (Brach 1992), meditation and mindful eating (Bressan 2020; Zanella 2021), breathing meditation (Beauchamp 2020), mindful music listening and meditation (Baylan 2019), mindfulness and behavioural support interventions (McTigue 2020), and brief mindfulness training (Weng 2021).
The types of active comparators also varied widely. For interventions targeting participants who were overweight/obese, comparators were weight management and other dietary interventions (Alamout 2020; Blevins 2009; Bressan 2020; Carpenter 2019; Smith 2018; Spadaro 2018; Zanella 2021), psychological interventions (Brach 1992; Daubenmier 2016; Kristeller 2014) or health education (Chumachenko 2021; Raja‐Khan 2017). For interventions targeting smokers, the active comparators were other forms of smoking cessation interventions (Brewer 2011; Davis 2014a; Garrison 2020; Goldenhersch 2020; Weiss de Souza 2020; Weng 2021), or cognitive behavioural therapy (CBT) (Vidrine 2016). For interventions targeting diabetes and pre‐diabetes, the active comparator was progressive muscle relaxation (PMR) (Izgu 2020), stress management education (Miller 2014), CBT (Tovote 2014), and health education (Woods‐Giscombe 2019). In the trials recruiting those with hypertension, the active comparators were health education (An 2021; Ponte Marquez 2019) and behavioural support (McTigue 2020). For the three secondary‐prevention trials involving stroke survivors, the comparators were health education (Baldo 2021), music/audio listening (Baylan 2019), and expressive writing (Beauchamp 2020).
2. MBIs versus non‐active comparators
We include 38 trials with 2905 participants randomised. Of these, 29 trials recruited participants at increased risk of CVD (primary prevention subpopulation) and nine trials recruited participants with established CVD (secondary prevention subpopulation).
Populations
Fourteen primary prevention trials recruited participants with type 2 diabetes (Armani Kian 2018; Chen 2020; Davoudi 2021; Guo 2021; Kopf 2014; Nikkhah Ravari 2020; Pearson 2018; Razavizadeh Tabadkan 2019) or diabetes (Friis 2016; Nathan 2017; Schroevers 2015; Tovote 2014; van Son 2014; Zarifsanaiey 2020), or type 1 diabetes (Shukla 2021); eight trials recruited participants who were either overweight or obese (Alamout 2020; Brach 1992; Daubenmier 2011; Ingraham 2017; Kristeller 2014; Palmeira 2017), or after gastric surgery (Chacko 2016; Ng 2018); four trials recruited participants with hypertension (Blom 2013; de Fatima Rosas Marchiori 2015; Nejati 2015) or pre‐hypertension (Kalinowski 2021), and two trials recruited smokers (Davis 2014b; Vidrine 2016).
The nine secondary prevention trials recruited patients with coronary artery disease (Alsubaie 2020; Gu 2018; Jalali 2019; Jang 2018; Nasiri 2020; Nijjar 2019; Parswani 2013; Sinha 2018; Turrise 2017).
Eight trials recruited only women (Alamout 2020; Brach 1992; Daubenmier 2011; Ingraham 2017; Kalinowski 2021; Nikkhah Ravari 2020; Palmeira 2017; Razavizadeh Tabadkan 2019), one trial recruited only men (Parswani 2013), and the remaining trials recruited both men and women.
Settings
Nine trials were conducted in the USA (Brach 1992; Chacko 2016; Daubenmier 2011; Davis 2014b; Ingraham 2017; Kristeller 2014; Nijjar 2019; Turrise 2017; Vidrine 2016), nine in Iran (Alamout 2020; Armani Kian 2018; Davoudi 2021; Nasiri 2020; Nejati 2015; Nikkhah Ravari 2020; Razavizadeh Tabadkan 2019; Zarifsanaiey 2020), three in the Netherlands (Schroevers 2015; Tovote 2014; van Son 2014), two in Canada (Blom 2013; Nathan 2017), three in India (Parswani 2013; Sinha 2018; Shukla 2021), two in China (Gu 2018; Guo 2021), and one trial in each of the UK (Alsubaie 2020), Brazil (de Fatima Rosas Marchiori 2015), Taiwan (Chen 2020), Republic of Korea (Jang 2018), Germany (Kopf 2014), Portugal (Palmeira 2017), Australia (Pearson 2018), and New Zealand (Friis 2016), with no country reported in two trials (Kalinowski 2021; Ng 2018).
The duration of the intervention and follow‐up periods varied between 12 weeks and 36 months.
Interventions and comparators
The types of MBIs differed across trials. Many used standard MBSR (Kabat‐Zinn 1990) or MBCT (Segal 2002) protocols (Alamout 2020; Alsubaie 2020; Armani Kian 2018; Blom 2013; Gu 2018; Jalali 2019; Guo 2021; Kalinowski 2021; Kopf 2014; Nathan 2017; Nejati 2015; Nijjar 2019; Parswani 2013; Razavizadeh Tabadkan 2019; Schroevers 2015; Tovote 2014). One trial had two intervention groups and implemented MBSR to examine the effects of MBCT adapted for use in patients with CVD and low mood (Alsubaie 2020). Other studies adapted standard protocols to target specific behaviours, such as smoking (Davis 2014b; Vidrine 2016), disordered eating (Chacko 2016; Daubenmier 2011; Ingraham 2017), or for specific conditions such as diabetes (Kopf 2014; van Son 2014). Mindfulness‐based substance abuse relapse prevention protocols were adapted in one trial of bariatric patients (Ng 2018). Other standard MBIs were also developed, such as mindfulness‐based eating awareness training (MB‐EAT Kristeller 2011), which was used in one trial (Kristeller 2014) and adapted in two others (Chacko 2016; Daubenmier 2011); Mindful Self Compassion (MSC; Neff 2013) was used in one trial (Friis 2016) and adapted in another (Chacko 2016). One trial used art therapy alongside MBSR (Jang 2018), and another used acceptance and commitment therapy alongside mindfulness meditation and self‐compassion (Palmeira 2017).
Other MBs were based more broadly on mindfulness meditation practices. These included breath‐based Buddhist meditations along with informal mindfulness practices (Brach 1992), mindfulness meditation and neuropathic pain awareness (Davoudi 2021), mindfulness and self compassion meditation practices (Sinha 2018; Turrise 2017), mindfulness training (Nasiri 2020; Nikkhah Ravari 2020), mindfulness meditations (Shukla 2021; Zarifsanaiey 2020), self‐directed mindful breath awareness meditation (Pearson 2018), a programme developed from the Taiwan mindfulness association ('Meditation and Mindful Eating'; Chen 2020), and breath‐based Zen meditation and present‐moment awareness (de Fatima Rosas Marchiori 2015).
The types of inactive comparators were treatment as usual (Alsubaie 2020; Armani Kian 2018; Chacko 2016; Chen 2020; Davis 2014b; Gu 2018; Guo 2021; Kalinowski 2021; Kopf 2014; Nasiri 2020; Nijjar 2019; Nikkhah Ravari 2020; Ng 2018; Palmeira 2017; Parswani 2013; Pearson 2018; Sinha 2018; van Son 2014; Vidrine 2016), no treatment (Alamout 2020; Brach 1992; Davoudi 2021; Nejati 2015; Razavizadeh Tabadkan 2019; Shukla 2021; Zarifsanaiey 2020), or wait list control (Blom 2013; Daubenmier 2011; de Fatima Rosas Marchiori 2015; Friis 2016; Ingraham 2017; Jalali 2019; Jang 2018; Kristeller 2014; Nathan 2017; Schroevers 2015; Tovote 2014; Turrise 2017).
3. TM versus active comparators
We include eight trials with 830 participants randomised. Five trials recruited participants at increased risk of CVD (primary prevention subpopulation) and three trials recruited participants with established CVD (secondary prevention subpopulation).
Populations
Primary prevention trials recruited participants with hypertension (Schneider 2005; Schneider 2019), high normal blood pressure (Schneider 2021), increased CVD risk factors (Toomey 2007), and metabolic syndrome (Vaccarino 2013). All secondary prevention trials recruited participants with established CHD (Bokhari 2021; Paul‐Labrador 2006; Schneider 2012). All trials recruited both men and women, six recruited exclusively African Americans (Bokhari 2021; Schneider 2005; Schneider 2012; Schneider 2019; Schneider 2021; Vaccarino 2013), and one native Hawaiians (Toomey 2007).
Settings
All studies were conducted in the USA. The duration of the intervention and follow‐up periods varied between 12 weeks and an average of 5.4 years.
Interventions and comparators
Seven studies followed the standard TM protocol (Roth 1994; Roth 2018), and one used conscious resting meditation (CRM), a sound or mantra‐based meditation similar to TM with a standardised protocol (Vaccarino 2013).
Active comparators were health education in most trials (Paul‐Labrador 2006; Schneider 2005; Schneider 2012; Schneider 2019; Schneider 2021; Toomey 2007; Vaccarino 2013), one secondary prevention trial had two active comparators, health education and progressive muscle relaxation (Schneider 2012), and in another the active comparator was cardiac rehabilitation also received by the intervention group (Bokhari 2021).
4. TM versus non‐active comparators
We included two trials with 186 participants randomised. Both trials recruited participants at increased risk of CVD, one in those with hypertension (Seer 1980), the other in those with increased cardiovascular risk (Nidich 2009). Both trials recruited men and women. One trial was conducted in the USA (Nidich 2009) and the other in New Zealand (Seer 1980). The duration of the intervention and follow‐up periods were 12 weeks (Nidich 2009) and 13 weeks (Seer 1980).
One trial followed the standard TM protocol (Nidich 2009), and the other used an intervention called SRELAX, modelled on TM except that the meditation technique was referred to as self‐relaxation, and one standard mantra or sound as it was referred to was used (Seer 1980). Both trials used wait list controls as the inactive comparator.
Other meditation interventions
We include nine trials with 427 participants randomised. Interventions varied widely and each is described separately with the relative comparator and participants recruited.
The tension tamer breathing awareness mobile application was used in one trial, providing positive feedback from heart rate measurement and motivational texts based on adherence. The comparator was an attention‐control mobile application based on lifestyle education. This trial recruited men and women with hypertension, was conducted in the USA, and the duration of the intervention and follow‐up period was 12 months (Chandler 2020).
Progressive muscle relaxation along with five different meditation practices aimed at control of attentional processes and somatic awareness was studied in one trial of veterans with hypertension and compared to a health education comparator. The trial recruited predominantly men, was conducted in the USA and the intervention and follow‐up period was 20 weeks (Friskey 1984).
One trial recruited female participants with type 2 diabetes to Buddhist walking meditation or traditional walking. The trial was conducted in Thailand and the duration of the intervention and follow‐up period was 12 weeks (Gainey 2016).
Meditation alone and meditation plus biofeedback were two interventions compared to a no treatment control for participants with essential hypertension. The trial was conducted in Australia, sex was not reported, and the duration of the intervention and follow‐up period was 12 weeks (Hafner 1982).
Male and female participants with CHD were recruited in Argentina and underwent pituitary pineal activation (APP) meditation, a technique that differs from TM based on activations and connections of the subject with images related to their cardiovascular health. The comparator group received health education and the duration of the intervention and follow‐up period was 12 weeks (Huerin 2015).
In one trial, CBT along with the HeartMath protocol was used where participants with atrial fibrillation (a risk factor for stroke) were trained to be aware of their breathing, and heart rate variability biofeedback was demonstrated. The comparator group received treatment as usual. The trial was conducted in Sweden and the duration of the intervention and follow‐up period was 12 months (Malm 2018).
Women with type 2 diabetes were recruited in Thailand and assigned to Buddhist group therapy with a focus on dynamic meditation or treatment as usual. The duration of the intervention and follow‐up period was 26 weeks (Rungreangkulkij 2011).
Men and women with diabetes were recruited to IAM® training, which focusses on yoga poses, breathing and meditation, or on standard care. The trial was conducted in India and the duration of intervention and follow‐up period was 12 weeks (Sarika 2020).
One trial focused on healing meditation (Sampaio 2016), involving a series of meditation practices, of balancing polarities, of centring and reflexion, which when associated with breathing and relaxation, make the person’s energy circulate through their chakras (Perret 2012). The trial recruited men and women who were overweight or obese in Brazil, where the comparator was a wait list control and the duration of intervention and follow‐up was 12 weeks (Sampaio 2016).
Ongoing studies and studies awaiting classification
We identified 65 ongoing studies. Details are given in the Characteristics of ongoing studies table. The status of these studies, as of May 2023, is summarised in Table 5. Reassessment of these 65 ongoing studies, as well as the 23 studies awaiting classification (see Characteristics of studies awaiting classification), is underway.
Excluded studies
Of 644 full‐text reports, we excluded 390 following full‐text assessment with reasons documented in Figure 1. We present details and reasons for exclusion for 28 studies (33 reports) that most closely missed the inclusion criteria in the Characteristics of excluded studies table. Most of these studies were excluded on the basis of being ACT interventions, which incorporate mindfulness techniques but not specifically meditation (see Overall completeness and applicability of evidence for more detail) or for being short‐term and less than 12 weeks in duration (defined as intervention period and any post‐intervention follow‐up).
Risk of bias in included studies
Details are provided for each of the included studies in the risk of bias section of Characteristics of included studies and summaries are presented in Figure 2 and Figure 3. We assessed risk of bias as 'low', 'high', or 'unclear'. A summary of the risks of bias in the included studies is presented below for each comparison group.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
MBIs versus active comparators
The methods of random sequence generation were unclear in 12 studies. In the remaining 17 studies where this was clear, we judged the methods used to be at low risk of bias.
The methods of allocation concealment were unclear in 22 of the 29 included studies. In the seven studies where this was clear, we judged the methods used to be at low risk of bias.
MBIs versus non‐active comparators
The methods of random sequence generation were unclear in 17 studies. In the remaining 21 studies where this was clear, we judged the methods used to be at low risk of bias.
The methods of allocation concealment were unclear in 27 of the 38 included studies. In the 11 studies where this was clear, we judged the methods used to be at low risk of bias.
TM versus active comparators
The methods of random sequence generation were clear in seven of eight studies, and we judged these methods to be at low risk of bias. For the remaining trial, we judged the risk of bias as unclear.
The methods of allocation concealment were unclear in four of the eight included studies. In the four studies where this was clear, we judged the methods used to be at low risk of bias.
TM versus non‐active comparators
The methods of random sequence generation were clear in one study, where we judged these methods to be at low risk of bias. For the remaining trial, we judged the risk of bias as unclear.
The methods of allocation concealment were unclear in both of the included studies.
Other meditation interventions
The methods of random sequence generation were clear in four studies, where we judged these methods to be at low risk of bias. For the remaining five trials, we judged the risk of bias as unclear.
The methods of allocation concealment were unclear in all nine of the included studies.
Blinding
The blinding of participants and personnel for behavioural interventions is difficult, if not impossible, in most cases, and so we have not judged this as high risk of bias. This domain was rated as unclear for all trials in all comparison groups as we have done so in similar previous reviews of behavioural interventions (Rees 2013; Rees 2019; Rees 2021).
MBIs versus active comparators
The blinding of participants and personnel was unclear in all 29 trials. Blinding of outcome assessment was unclear in 22 of the 29 trials. In the remaining seven trials, outcome assessments were made blind to the group assignment, and we judged this to be at low risk of bias.
MBIs versus non‐active comparators
The blinding of participants and personnel was unclear in all 38 trials. Blinding of outcome assessment was unclear in 34 of the 38 trials. In the remaining four trials, outcome assessments were made blind to the group assignment, and we judged this to be at low risk of bias.
TM versus active comparators
The blinding of participants and personnel was unclear in all eight trials. Blinding of outcome assessment was clear in all eight trials where outcome assessments were made blind to the group assignment, and we judged this to be at low risk of bias.
TM versus non‐active comparators
The blinding of participants and personnel was unclear in both trials. Blinding of outcome assessment was clear in both trials where outcome assessments were made blind to the group assignment, and we judged this to be at low risk of bias.
Other meditation interventions
The blinding of participants and personnel was unclear in all nine trials. Blinding of outcome assessment was clear in three trials where outcome assessments were made blind to the group assignment, and we judged this to be at low risk of bias. In the remaining six trials we judged blinding of outcome assessment as unclear.
Incomplete outcome data
MBIs versus active comparators
We judged eight of the 29 trials to be at low risk of bias, as loss to follow‐up was low and reasons provided or intention‐to‐treat (ITT) analyses were performed, or both. We judged three studies to be at high risk of bias for attrition due to high losses (> 30%) to follow‐up and high differential loss to follow‐up between the intervention and comparison groups. For the remaining 18 trials, we judged the risk of bias as unclear.
MBIs versus non‐active comparators
We judged 15 of the 38 trials to be at low risk of bias, as loss to follow‐up was low and reasons provided or ITT analyses were performed, or both. We judged two studies to be at high risk of bias for attrition due to high losses (> 30%) to follow‐up and high differential loss to follow‐up between the intervention and comparison groups. For the remaining 21 trials, we judged the risk of bias as unclear.
TM versus active comparators
We judged one of the eight trials to be at low risk of bias, as loss to follow‐up was low and reasons provided or ITT analyses were performed, or both. We judged one study to be at high risk of bias for attrition due to high losses (> 30%) to follow‐up in both the intervention and comparison groups. For the remaining six trials, we judged the risk of bias as unclear.
TM versus non‐active comparators
We judged one of the two trials to be at low risk of bias, as loss to follow‐up was low and reasons provided or ITT analyses were performed, or both. We judged the other study to be at high risk of bias for attrition due to high losses (> 30%) to follow‐up in both the intervention and comparison groups.
Other meditation interventions
We judged three of the nine trials to be at low risk of bias, as loss to follow‐up was low and reasons provided or ITT analyses were performed, or both. For the remaining six trials, we judged the risk of bias as unclear.
Selective reporting
MBIs versus active comparators
For 24 studies, we judged the risk of bias associated with selective reporting as unclear. The remaining five studies clearly stated the outcomes a priori and reported the results for these and were therefore judged to be at low risk of bias in this domain.
MBIs versus non‐active comparators
For 36 studies, we judged the risk of bias associated with selective reporting as unclear. The remaining two studies clearly stated the outcomes a priori and reported the results for these and were therefore judged to be at low risk of bias in this domain.
TM versus active comparators
For all eight studies, we judged the risk of bias associated with selective reporting as unclear.
TM versus non‐active comparators
For both studies, we judged the risk of bias associated with selective reporting as unclear.
Other meditation interventions
For eight studies, we judged the risk of bias associated with selective reporting as unclear. The remaining study clearly stated the outcomes a priori and reported the results for these and was therefore judged to be at low risk of bias in this domain.
Other potential sources of bias
MBIs versus active comparators
There was insufficient information to judge the risk of other sources of bias, and we categorised all 29 studies as unclear.
MBIs versus non‐active comparators
There was insufficient information to judge the risk of other sources of bias, and we categorised all 38 studies as unclear.
TM versus active comparators
There was insufficient information to judge the risk of other sources of bias, and we categorised all eight studies as unclear.
TM versus non‐active comparators
There was insufficient information to judge the risk of other sources of bias, and we categorised both studies as unclear.
Other meditation interventions
There was insufficient information to judge the risk of other sources of bias, and we categorised all nine studies as unclear.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Please see: Table 1; Table 2; Table 3; Table 4.
Data are presented in the analyses by intervention group (MBI or TM) and comparison group (active and non‐active) for primary and secondary outcomes (comparisons 1 to 4). We performed subgroup analyses for primary outcomes by primary and secondary prevention of CVD where there were sufficient studies to do so (MBI versus non‐active comparators and TM versus active comparators, comparison 5). Other meditation interventions are described narratively for primary and secondary outcomes (comparison 6).
1. MBI versus active comparators
See Table 1.
1.1 Primary outcomes
CVD clinical events (cardiovascular mortality and other non‐fatal endpoints)
None of the included trials reported data on CVD clinical events.
Blood pressure
Six trials (388 participants randomised) measured systolic blood pressure and reported data that could be used in meta‐analyses (Alamout 2020; An 2021; Bressan 2020; Daubenmier 2016; Ponte Marquez 2019; Raja‐Khan 2017). Three small trials reported beneficial effects of the MBIs compared to active comparators (Alamout 2020; An 2021; Ponte Marquez 2019), whereas the remaining three trials with larger sample size showed little or no effect of the intervention (Bressan 2020; Daubenmier 2016; Raja‐Khan 2017). We assessed the overall certainty of evidence as low. There was considerable heterogeneity between trials (I2 = 88%) and so the pooled effect estimate should be interpreted with caution (MD ‐6.08 mmHg, 95% CI ‐12.79 to 0.63; Analysis 1.1).
1.1. Analysis.

Comparison 1: MBIs versus active comparators, Outcome 1: Systolic blood pressure (mmHg), change from baseline
For diastolic blood pressure measured in the same six trials, the same three trials reported beneficial effects of the MBIs compared to active comparators (Alamout 2020; An 2021; Ponte Marquez 2019), whereas the remaining three trials with larger sample size showed little or no effect of the intervention (Bressan 2020; Daubenmier 2016; Raja‐Khan 2017). Similarly, we assessed the overall certainty of evidence as low and there was similarly considerable heterogeneity between trials (I2 = 91%) and so the pooled effect estimate should be interpreted cautiously (MD ‐5.18, 95% CI ‐10.65 to 0.29; Analysis 1.2).
1.2. Analysis.

Comparison 1: MBIs versus active comparators, Outcome 2: Diastolic blood pressure (mmHg), change from baseline
One feasibility trial, available as a conference proceeding, did not provide the numbers randomised to each group at baseline or follow‐up, and thus we did not include these data in the meta‐analyses (McTigue 2020). This trial found reductions in both systolic and diastolic blood pressure with both the MBI and active comparator, but there were no differences between the groups.
Anxiety
Nine trials (438 participants randomised) measured anxiety and reported data that could be used in meta‐analyses (Baylan 2019; Beauchamp 2020; Blevins 2009; Carpenter 2019; Miller 2014; Ponte Marquez 2019; Raja‐Khan 2017; Tovote 2014; Weiss de Souza 2020). MBIs likely result in little to no difference in anxiety compared to active comparators (SMD ‐0.06 units, 95% CI ‐0.25 to 0.13; I2 = 0%; moderate‐certainty evidence; Analysis 1.3).
1.3. Analysis.

Comparison 1: MBIs versus active comparators, Outcome 3: Anxiety, change from baseline
One trial, reported as a conference proceeding, provided narrative findings only so there were insufficient details to be included in the meta‐analyses. This pilot study reported that there were no differences in anxiety between groups (Turrise 2017).
Another trial used medians and interquartile ranges and so was not included in the meta‐analysis for this outcome and comparison group. Anxiety was decreased in both the MB‐EAT group and nutrition advice group from baseline to follow‐up, but there was no difference between groups (Bressan 2020).
A further trial reported data on pre‐ and post‐intervention (eight weeks) and on post‐intervention to three‐month follow‐up, but not on pre‐intervention to follow‐up, so we were unable to use these data in meta‐analyses (Baldo 2021).
Depression
Eleven trials (595 participants randomised) measured depression and reported data that could be used in meta‐analyses (Baylan 2019; Beauchamp 2020; Blevins 2009; Brach 1992; Carpenter 2019; Kristeller 2014; Miller 2014; Ponte Marquez 2019; Raja‐Khan 2017; Tovote 2014; Weiss de Souza 2020). MBIs likely result in little to no difference in depression compared to active comparators (SMD 0.08 units, 95% CI ‐0.08 to 0.24; I2 = 0%; moderate‐certainty evidence; Analysis 1.4). We created a funnel plot for this outcome as there were 11 studies included within the meta‐analysis (Figure 4). There does not appear to be any strong sign of funnel plot asymmetry that would indicate either non‐reporting bias or small study effects.
1.4. Analysis.

Comparison 1: MBIs versus active comparators, Outcome 4: Depression, change from baseline
4.

MBIs versus active comparators
One trial used medians and interquartile ranges and so was not included in the meta‐analysis for this outcome and comparison group. Depression was decreased in both the MB‐EAT group and the nutrition advice group from baseline to follow‐up, but there was no difference between groups (Bressan 2020).
A further trial reported pre‐ and post‐intervention (eight weeks) and post‐intervention to three months follow‐up, but not pre‐intervention to follow‐up, so we were unable to use these data in meta‐analyses (Baldo 2021).
Another study did not report follow‐up values but stated there were no "statistically significant" changes in depression from baseline to six months follow‐up (Chumachenko 2021).
Perceived stress
Six trials (357 participants randomised) measured perceived stress and reported data that could be used in meta‐analyses (Bressan 2020; Carpenter 2019; Davis 2014a; Ponte Marquez 2019; Raja‐Khan 2017; Weiss de Souza 2020). There was moderate‐certainty evidence of a reduction in perceived stress with MBIs compared to active comparators (SMD ‐0.24 units, 95% CI ‐0.45 to ‐0.03; I2 = 0%; Analysis 1.5). This result was stable in sensitivity analyses using both mean differences and standardised mean differences, and fixed‐effect and random‐effects models for statistical pooling.
1.5. Analysis.

Comparison 1: MBIs versus active comparators, Outcome 5: Perceived stress, change from baseline
One trial, reported as a conference proceeding, provided narrative findings only so there were insufficient details to be included in the meta‐analyses. This pilot study reported that there were no differences in perceived stress between groups (Turrise 2017).
Another study did not report follow‐up values but stated there were no "statistically significant" changes in perceived stress from baseline to six months follow‐up (Chumachenko 2021).
A further study reported significance levels on a trial registry and found a greater reduction of stress perception in the meditation group after three months of intervention, evaluated by the perceived stress scale, with a 5% significance level (Zanella 2021).
Well‐being
Only one trial involving 63 participants measured well‐being, and showed little or no effect of MBIs compared to active comparators (SMD ‐0.18 units, 95% CI ‐0.67 to 0.32; low‐certainty evidence; Analysis 1.6).
1.6. Analysis.

Comparison 1: MBIs versus active comparators, Outcome 6: Well‐being
Adverse events
None of the included trials reported adverse events.
1.2 Secondary outcomes
Blood lipid levels
One trial (47 participants randomised) measured total cholesterol and reported data that could be used in meta‐analyses (Bressan 2020). There was little or no effect of MBIs on total cholesterol compared to the active comparator (MD 0.37 mmol/L, 95% CI ‐0.1 to 0.84; Analysis 1.7).
1.7. Analysis.

Comparison 1: MBIs versus active comparators, Outcome 7: Total cholesterol (mmol/L), change from baseline
Three trials (247 participants randomised) measured LDL cholesterol and reported data that could be used in meta‐analyses (Bressan 2020; Daubenmier 2016; Raja‐Khan 2017). There was little or no effect of MBIs on LDL cholesterol compared to active comparators (MD 0.04 mmol/L, 95% CI ‐0.13 to 0.22; I2 = 21%; Analysis 1.8).
1.8. Analysis.

Comparison 1: MBIs versus active comparators, Outcome 8: LDL cholesterol (mmol/L), change from baseline
One trial (147 participants randomised) measured HDL cholesterol and reported data that could be used in meta‐analyses (Daubenmier 2016). There was little or no effect of MBIs on total cholesterol compared to the active comparator (MD 0.07 mmol/L, 95% CI ‐0.00 to 0.14; Analysis 1.9).
1.9. Analysis.

Comparison 1: MBIs versus active comparators, Outcome 9: HDL cholesterol (mmol/L), change from baseline
In one study (Bressan 2020), HDL cholesterol was reported using medians and interquartile ranges and so was not included in the meta‐analysis for this outcome and comparison group. There was no change in HDL cholesterol in the MB‐EAT group from baseline to follow‐up, and a significant increase in the nutrition advice group, but there was no difference between groups.
One trial (147 participants randomised) measured triglycerides and reported data that could be used in meta‐analyses (Daubenmier 2016). There was little or no effect of MBIs on total cholesterol compared to the active comparator (MD ‐0.1 mmol/L, 95% CI ‐0.27 to 0.07; Analysis 1.10).
1.10. Analysis.

Comparison 1: MBIs versus active comparators, Outcome 10: Triglycerides (mmol/L), change from baseline
In one study (Bressan 2020), triglycerides were reported using medians and interquartile ranges and so data were not included in the meta‐analysis for this outcome and comparison group. There was no change in triglyceride levels in the MB‐EAT group from baseline to follow‐up, and a significant decrease in the nutrition advice group, but there was no difference between groups.
One trial reported only significance levels on a trial registry (Zanella 2021). The authors found greater reductions in the lipid profile in the meditation group after three months, and differences between the intervention and control groups (P = 0.05).
Fasting blood glucose
Two trials (200 participants randomised) measured fasting blood glucose and reported data that could be used in meta‐analyses (Daubenmier 2016; Raja‐Khan 2017). There was a reduction in fasting blood glucose with MBIs compared to active comparators (MD ‐0.41 mmol/L, 95% CI ‐0.56 to ‐0.25; I2 = 0%; Analysis 1.11).
1.11. Analysis.

Comparison 1: MBIs versus active comparators, Outcome 11: Fasting blood glucose (mmol/L), change from baseline
One trial reported only significance levels on a trial registry (Zanella 2021). The authors found greater reductions in glycaemia in the meditation group after three months, and differences between the intervention and control groups (P = 0.05).
HbA1C
Four trials (331 participants randomised) measured HbA1C and reported data that could be used in meta‐analyses (Daubenmier 2016; Raja‐Khan 2017; Tovote 2014; Woods‐Giscombe 2019). There was little or no effect of MBIs on HbA1C compared to active comparators (MD 0.00%, 95% CI ‐0.09 to 0.1; I2 = 40%; Analysis 1.12).
1.12. Analysis.

Comparison 1: MBIs versus active comparators, Outcome 12: HbA1c (%), change from baseline
Body weight
Ten trials (552 participants randomised) measured weight and reported data that could be used in meta‐analyses (Alamout 2020; Blevins 2009; Brach 1992; Bressan 2020; Carpenter 2019; Daubenmier 2016; Miller 2014; Raja‐Khan 2017; Smith 2018; Spadaro 2018). There was little or no effect of MBIs on weight compared to active comparators (MD 0.33 kg, 95% CI ‐0.78 to 1.44; I2 = 24%; Analysis 1.13).
1.13. Analysis.

Comparison 1: MBIs versus active comparators, Outcome 13: Weight (kg), change from baseline
One trial reported only significance levels on a trial registry (Zanella 2021). The authors found greater reductions in weight in the meditation group after three months, and differences between the intervention and control groups (P = 0.05).
BMI
Seven trials (332 participants randomised) measured BMI and reported data that could be used in meta‐analyses (Alamout 2020; Blevins 2009; Kristeller 2014; Raja‐Khan 2017; Smith 2018; Spadaro 2018; Woods‐Giscombe 2019). There was substantial heterogeneity between trials (I2 = 63%) and so the pooled effect estimate should be interpreted cautiously (MD ‐0.26 units, 95% CI ‐1.22 to 0.7; Analysis 1.14). Six of seven studies showed little or no effect of MBIs on BMI compared to active comparators.
1.14. Analysis.

Comparison 1: MBIs versus active comparators, Outcome 14: BMI (kg/m2), change from baseline
In one study (Bressan 2020), BMI was reported using medians and interquartile ranges and so was not included in the meta‐analysis for this outcome and comparison group. BMI was decreased in both the MB‐EAT group and nutrition advice group from baseline to follow‐up, but there was no difference between groups.
One trial reported only significance levels on a trial registry (Zanella 2021). The authors found greater reductions in BMI in the meditation group after three months, and differences between the intervention and control groups (P = 0.05).
Smoking rates
Six trials (1087 participants randomised) measured smoking cessation and reported data that could be used in meta‐analyses (Brewer 2011; Davis 2014a; Garrison 2020; Vidrine 2016; Weiss de Souza 2020; Weng 2021). Two studies showed large beneficial effects of MBI (Brewer 2011; Davis 2014a), and the remaining four studies showed little or no effect of MBIs on smoking cessation. We assessed the overall certainty of evidence as very low. There was substantial heterogeneity between trials (I2 = 79%) and so the pooled effect estimate should be interpreted cautiously (RR 1.45, 95% CI 0.78 to 2.68; Analysis 1.15).
1.15. Analysis.

Comparison 1: MBIs versus active comparators, Outcome 15: Smoking cessation
One study did not make comparisons between groups due to very high losses to follow‐up in the control group at 90 days (Goldenhersch 2020). In this study they found that the treatment group reported sustained abstinence of 33% (20/60) at 90 days.
Quality of life
One trial (52 participants randomised) measured quality of life and reported data that could be used in meta‐analyses (Raja‐Khan 2017). Composite scores for mental and physical health were pooled for this trial with N numbers correspondingly divided by two in both groups to prevent double‐counting. There was little or no effect of MBI on quality of life compared to the active comparator (Analysis 1.16).
1.16. Analysis.

Comparison 1: MBIs versus active comparators, Outcome 16: Quality of life, change from baseline
In one study (Bressan 2020), HRQoL was reported using the SF‐36 reporting individual domains and using medians and interquartile ranges and so data were not included in the meta‐analysis for this outcome and comparison group. For each domain, quality of life improved in both the MB‐EAT group and nutrition advice group from baseline to follow‐up, but there was no difference between groups.
One trial reported only significance levels on a trial registry (Zanella 2021). The authors found greater increases in quality of life in the meditation group after three months, and differences between the intervention and control groups (P = 0.05).
One trial reported medians and interquartile ranges for the Neuropathic Pain Impact on Quality of Life Questionnaire (NEPIQOL) in patients with type 2 diabetes (Izgu 2020). The authors found no "statistically significant" differences in the median quality of life scores between comparison groups at baseline, week 12, or week 14.
Coping, resilience, mastery
Three trials (208 participants randomised) measured self‐efficacy and reported data that could be used in meta‐analyses (Brach 1992; Kristeller 2014; Miller 2014). Two composite scores were used from the eating self‐efficacy scale in one study where N numbers have been reduced appropriately to prevent double‐counting of participants (Brach 1992). There was an increase in self‐efficacy with MBIs compared to active comparators, but the 95% CI overlaps no effect (SMD 0.22 mmol/L, 95% CI ‐0.05 to 0.5; I2 = 0%; Analysis 1.17).
1.17. Analysis.

Comparison 1: MBIs versus active comparators, Outcome 17: Self‐efficacy, change from baseline
One study measured resilience using the brief resilience scale and is described narratively as no other trials reported this outcome (Smith 2018). The authors found "non‐significant" changes in resilience for both the MBI group and active comparator group.
2. MBI versus non‐active comparators
See Table 2.
2.1 Primary outcomes
CVD clinical events (cardiovascular mortality and other non‐fatal endpoints)
Only one trial involving participants with type 2 diabetes reported CVD clinical events (cardiovascular mortality and non‐fatal MI). Kopf 2014 randomised a total of 110 participants to MBSR or treatment as usual, with follow‐up reported at 36 months. No differences were seen between the two groups (RR 0.94, 95% CI 0.37 to 2.42; very low‐certainty evidence; Analysis 2.1).
2.1. Analysis.

Comparison 2: MBIs versus non‐active comparators, Outcome 1: Clinical CVD events (CVD mortality and non‐fatal MI)
Blood pressure
Nine trials (379 participants randomised) measured systolic blood pressure and reported data that could be used in meta‐analyses (Alamout 2020; Alsubaie 2020; Blom 2013; Nejati 2015; Nijjar 2019; Parswani 2013; Pearson 2018; Shukla 2021; Sinha 2018). Three trials reported beneficial effects of the MBIs compared to non‐active comparators (Alamout 2020; Nejati 2015; Sinha 2018), whereas the remaining six trials showed little or no effect of the intervention (Alsubaie 2020; Blom 2013; Nijjar 2019; Parswani 2013; Pearson 2018; Shukla 2021). We assessed the overall certainty of evidence as low. There was considerable heterogeneity between trials (I2 = 87%) and so the pooled effect estimate should be interpreted cautiously (MD ‐6.62, 95% CI ‐13.15 to ‐0.1; Analysis 2.2).
2.2. Analysis.

Comparison 2: MBIs versus non‐active comparators, Outcome 2: Systolic blood pressure (mmHg), change from baseline
For diastolic blood pressure, measured in the same nine trials, we assessed the overall certainty of evidence as low and there was substantial heterogeneity between trials (I2 = 61%) and so the pooled effect estimate should be interpreted cautiously (MD ‐3.35, 95% CI ‐5.86 to ‐0.85; Analysis 2.3). The same three trials reported beneficial effects of the MBIs compared to active comparators (Alamout 2020; Nejati 2015; Sinha 2018), whereas the remaining six trials showed little or no effect of the intervention (Alsubaie 2020; Blom 2013; Nijjar 2019; Parswani 2013; Pearson 2018; Shukla 2021).
2.3. Analysis.

Comparison 2: MBIs versus non‐active comparators, Outcome 3: Diastolic blood pressure (mmHg), change from baseline
One trial reported as a conference proceeding provided narrative findings only so there were insufficient details to be included in the meta‐analyses (Kalinowski 2021). Overall, 12‐week retention was 89%, but follow‐up blood pressure was missing in approximately 50% of participants due to COVID‐19 research restrictions. In the subgroup with complete follow‐up data, systolic blood pressure declined in both study arms, but there was "no significant between‐group difference" (P = 0.51).
Another trial reported as a conference proceeding reported adjusted systolic blood pressure only and without the number randomised to each group (Gu 2018). They found that patients in the MBSR group had beneficial changes (measured as mean (SD)) in adjusted systolic blood pressure (‐4.5 (1.9) versus 3.6 (2.0) mmHg; P = 0.03).
One trial reported maximum blood pressure and 24‐hour blood pressure, and a further report of this trial lacked baseline data to calculate the change from baseline to follow‐up (Kopf 2014). Diastolic blood pressure was "significantly lower" in the MBSR group at 12 months compared to treatment as usual (P = 0.018), but systolic blood pressure was "not significantly affected".
A further trial reported blood pressure data in graphical form only (de Fatima Rosas Marchiori 2015). They found that the meditation group showed a "significant decrease" in systolic blood pressure levels after two months of meditation practice compared to the wait list control (P = 0.05), but not at the end of the three‐month intervention or follow‐up at four months. No differences between groups were seen in diastolic blood pressure at any time point.
Anxiety
Nine trials (533 participants randomised) measured anxiety and reported data that could be used in meta‐analyses (Alsubaie 2020; Daubenmier 2011; Jang 2018; Nijjar 2019; Nikkhah Ravari 2020; Parswani 2013; Pearson 2018; Tovote 2014; van Son 2014). We assessed the overall certainty of evidence as low. There was substantial heterogeneity between trials (I2 = 61%) and so the pooled effect estimate should be interpreted cautiously (SMD ‐0.78 units, 95% CI ‐1.09 to ‐0.47; Analysis 2.4). Six trials reported beneficial effects of the MBIs compared to non‐active comparators (Alsubaie 2020; Jang 2018; Nikkhah Ravari 2020; Parswani 2013; Tovote 2014; van Son 2014), whereas the remaining three trials showed little or no effect of the intervention (Daubenmier 2011; Nijjar 2019; Pearson 2018).
2.4. Analysis.

Comparison 2: MBIs versus non‐active comparators, Outcome 4: Anxiety, change from baseline
One study did not report follow‐up values but stated that there were "significant differences" in anxiety between groups only at eight weeks and not at three months (Armani Kian 2018).
Depression
Fifteen trials (912 participants randomised) measured depression and reported data that could be used in meta‐analyses (Alsubaie 2020; Brach 1992; Chacko 2016; Friis 2016; Jang 2018; Kopf 2014; Kristeller 2014; Nathan 2017; Nijjar 2019; Nikkhah Ravari 2020; Parswani 2013; Pearson 2018; Schroevers 2015; Tovote 2014; van Son 2014). Eleven trials reported beneficial effects of the MBIs compared to non‐active comparators (Alsubaie 2020; Friis 2016; Jang 2018; Kristeller 2014; Nathan 2017; Nikkhah Ravari 2020; Parswani 2013; Pearson 2018; Schroevers 2015; Tovote 2014; van Son 2014), whereas the remaining four trials showed little or no effect of the intervention (Brach 1992; Chacko 2016; Kopf 2014; Nijjar 2019).
We assessed the overall certainty of evidence as low. There was substantial heterogeneity between trials (I2 = 67%) and so the pooled effect estimate should be interpreted cautiously (SMD ‐0.66 units, 95% CI ‐0.91 to ‐0.41; Analysis 2.5). We created a funnel plot for this outcome as there were 15 studies included within the meta‐analysis (Figure 5). There does not appear to be any strong sign of funnel plot asymmetry that would indicate either non‐reporting bias or small study effects.
2.5. Analysis.

Comparison 2: MBIs versus non‐active comparators, Outcome 5: Depression, change from baseline
5.

MBIs versus non‐active comparators
One study did not report follow‐up values but stated that there were "significant differences" in depression between groups only at eight weeks and not at three months (Armani Kian 2018).
In another study, the numbers randomised to each group were not reported but the authors state that those in the MBSR group had lower depression scores than those in the usual care group (P = 0.01) (Gu 2018).
In a further study, greater reductions in depressive symptoms were seen in the MBCT group compared to usual care (P = 0.008) (Kalinowski 2021).
Perceived stress
Eleven trials (708 participants randomised) measured perceived stress and reported data that could be used in meta‐analyses (Chacko 2016; Daubenmier 2011; Davis 2014a; Kopf 2014; Nasiri 2020; Nathan 2017; Nijjar 2019; Nikkhah Ravari 2020; Parswani 2013; Pearson 2018; van Son 2014). Six trials reported beneficial effects of the MBIs compared to non‐active comparators (Kopf 2014; Nasiri 2020; Nathan 2017; Parswani 2013; Pearson 2018; van Son 2014), and one reported beneficial effects of the non‐active comparator (Chacko 2016), whereas the remaining four trials showed little or no effect of the intervention (Daubenmier 2011; Davis 2014a; Nijjar 2019; Nikkhah Ravari 2020).
We assessed the overall certainty of evidence as low. There was substantial heterogeneity between trials (I2 = 70%) and so the pooled effect estimate should be interpreted cautiously (SMD ‐0.59 units, 95% CI ‐0.89 to ‐0.29; Analysis 2.6). We created a funnel plot for this outcome as there were 11 studies included within the meta‐analysis (Figure 6). There does not appear to be any strong sign of funnel plot asymmetry that would indicate either non‐reporting bias or small study effects.
2.6. Analysis.

Comparison 2: MBIs versus non‐active comparators, Outcome 6: Perceived stress, change from baseline
6.

MBIs versus non‐active comparators
One study reported summary findings in English and stated that MBCT was effective in reducing perceived stress in women with type 2 diabetes (Razavizadeh Tabadkan 2019).
In another study, greater reductions in perceived stress were seen in the MBCT group compared to usual care, but this did not reach "statistical significance" (P = 0.16) (Kalinowski 2021).
Well‐being
Two trials (198 participants randomised) measured well‐being and reported data that could be used in meta‐analyses (Tovote 2014; Zarifsanaiey 2020). There was moderate‐certainty evidence of an increase in well‐being with MBIs compared to non‐active comparators (SMD 0.5 units, 95% CI 0.09 to 0.91; I2 = 47%; Analysis 2.7). This result was stable in sensitivity analyses using fixed‐effect and random‐effects models for statistical pooling, but not when comparing mean differences and standardised mean differences.
2.7. Analysis.

Comparison 2: MBIs versus non‐active comparators, Outcome 7: Well‐being
Adverse events
Two adverse events related to MBI were reported in one small trial (Chacko 2016) (RR 5.0, 95% CI 0.27 to 91.52; 1 trial, 18 participants; low‐certainty evidence; Analysis 2.8). One participant reported transitory vertigo while gentle head rolling during yoga, which she stated subsided after focusing attention on her breath. Another participant experienced surfacing of repressed memories and depression during the MBI and was referred to a social worker for counselling. She continued attending classes and practising meditation and stated the mindfulness helped her to cope with the traumatic memories.
2.8. Analysis.

Comparison 2: MBIs versus non‐active comparators, Outcome 8: Adverse events
2.2 Secondary outcomes
Blood lipid levels
Three trials (228 participants randomised) measured total cholesterol and reported data that could be used in meta‐analyses (Kopf 2014; Nijjar 2019; Palmeira 2017). There was little or no effect of MBIs on total cholesterol compared to non‐active comparators (MD ‐0.06 mmol/L, 95% CI ‐0.27 to 0.14; I2 = 0%; Analysis 2.9).
2.9. Analysis.

Comparison 2: MBIs versus non‐active comparators, Outcome 9: Total cholesterol (mmol/L), change from baseline
Two trials (155 participants randomised) measured LDL cholesterol and reported data that could be used in meta‐analyses (Kopf 2014; Nijjar 2019). There was little or no effect of MBIs on LDL cholesterol compared to non‐active comparators (MD 0.02 mmol/L, 95% CI ‐0.26 to 0.29; I2 = 0%; Analysis 2.10).
2.10. Analysis.

Comparison 2: MBIs versus non‐active comparators, Outcome 10: LDL cholesterol (mmol/L), change from baseline
Two trials (155 participants randomised) measured HDL cholesterol and reported data that could be used in meta‐analyses (Kopf 2014; Nijjar 2019). There was little or no effect of MBIs on HDL cholesterol compared to non‐active comparators (MD ‐0.02 mmol/L, 95% CI ‐0.1 to 0.06; I2 = 0%; Analysis 2.11).
2.11. Analysis.

Comparison 2: MBIs versus non‐active comparators, Outcome 11: HDL cholesterol (mmol/L), change from baseline
Two trials (155 participants randomised) measured triglycerides and reported data that could be used in meta‐analyses (Kopf 2014; Nijjar 2019). There was little or no effect of MBIs on triglycerides compared to non‐active comparators (MD ‐0.08 mmol/L, 95% CI ‐0.53 to 0.38; I2 = 0%; Analysis 2.12).
2.12. Analysis.

Comparison 2: MBIs versus non‐active comparators, Outcome 12: Triglycerides (mmol/L), change from baseline
One study reported medians and ranges as data were not normally distributed (Ingraham 2017). The authors found that comparison of endpoint data between the immediate start WHAM intervention and delayed start showed "significantly lower" levels of total cholesterol in the intervention group (P = 0.05), and lower levels of LDL cholesterol (P = 0.1), but no differences between HDL cholesterol and triglycerides.
Fasting blood glucose
Five trials (459 participants randomised) measured fasting blood glucose and reported data that could be used in meta‐analyses (Davoudi 2021; Kopf 2014; Nikkhah Ravari 2020; Shukla 2021; Zarifsanaiey 2020). There was a reduction in fasting blood glucose with MBIs compared to non‐active comparators, but the 95% CI overlaps no effect (MD ‐0.35 mmol/L, 95% CI ‐0.75 to 0.05; I2 = 0%; Analysis 2.13).
2.13. Analysis.

Comparison 2: MBIs versus non‐active comparators, Outcome 13: Fasting blood glucose (mmol/L), change from baseline
One study did not report follow‐up values but stated that there were "significant differences" in fasting blood glucose between groups at three months (Armani Kian 2018).
HbA1C
Twelve trials (990 participants randomised) measured HbA1C and reported data that could be used in meta‐analyses (Chacko 2016; Chen 2020; Friis 2016; Guo 2021; Kopf 2014; Nathan 2017; Nijjar 2019; Nikkhah Ravari 2020; Pearson 2018; Shukla 2021; van Son 2014; Zarifsanaiey 2020). Five studies showed favourable effects of MBIs (Chen 2020; Friis 2016; Nikkhah Ravari 2020; Pearson 2018; Zarifsanaiey 2020), one showed favourable effects of the non‐active comparator (Chacko 2016), and the remainder showed little or no effect of MBIs on HbA1C compared to non‐active comparators.
There was substantial heterogeneity between trials (I2 = 74%) and so the pooled effect estimate should be interpreted cautiously (MD ‐0.36%, 95% CI ‐0.62 to ‐0.1; Analysis 2.14).
2.14. Analysis.

Comparison 2: MBIs versus non‐active comparators, Outcome 14: HbA1c (%), change from baseline
One study reported medians and ranges as data were not normally distributed (Ingraham 2017). The authors did not find evidence of intervention effects on HbA1C levels.
One study did not report follow‐up values but stated that there were "significant differences" in HbA1C between groups at three months (Armani Kian 2018).
Body weight
Four trials (140 participants randomised) measured weight and reported data that could be used in meta‐analyses (Alamout 2020; Brach 1992; Chacko 2016; Daubenmier 2011). One study showed favourable effects of MBIs (Alamout 2020), and the remainder showed little or no effect of MBIs on weight compared to non‐active comparators. There was substantial heterogeneity between trials (I2 = 59%) and so the pooled effect estimate should be interpreted cautiously (MD ‐0.47 kg, 95% CI ‐3.06 to 2.12; Analysis 2.15).
2.15. Analysis.

Comparison 2: MBIs versus non‐active comparators, Outcome 15: Weight (kg), change from baseline
One trial reported percentage weight loss and weight regain, so data could not be used in meta‐analyses (Ng 2018). Percent total weight loss was calculated at study baseline six months after sleeve gastrectomy and 6 and 18 months follow‐up (12 and 24 months postoperative). No "significant group differences" were found between the MBI and treatment as usual for percent total weight loss at 12 months or for weight regain at six months follow‐up.
BMI
Eight trials (390 participants randomised) measured BMI and reported data that could be used in meta‐analyses (Alamout 2020; Chacko 2016; Kopf 2014; Kristeller 2014; Nijjar 2019; Palmeira 2017; Parswani 2013; Shukla 2021). Three studies showed favourable effects of MBIs (Alamout 2020; Kopf 2014; Palmeira 2017), and the remainder showed little or no effect of MBIs on BMI compared to non‐active comparators. There was substantial heterogeneity between trials (I2 = 63%) and so the pooled effect estimate should be interpreted cautiously (MD ‐0.7 units, 95% CI ‐1.54 to 0.13; Analysis 2.16).
2.16. Analysis.

Comparison 2: MBIs versus non‐active comparators, Outcome 16: BMI (kg/m2), change from baseline
Smoking rates
Two trials (453 participants randomised) measured smoking cessation and reported data that could be used in meta‐analyses (Davis 2014b; Vidrine 2016). We assessed the overall certainty of evidence as low. There was little or no effect of MBIs on smoking cessation compared to non‐active comparators (RR 1.36, 95% CI 0.86 to 2.13; I2 = 0%; Analysis 2.17).
2.17. Analysis.

Comparison 2: MBIs versus non‐active comparators, Outcome 17: Smoking cessation
Quality of life
Ten trials (644 participants randomised) measured quality of life and reported data that could be used in meta‐analyses (Alsubaie 2020; Chacko 2016; Davoudi 2021; Jalali 2019; Kopf 2014; Nathan 2017; Nijjar 2019; Palmeira 2017; Shukla 2021; van Son 2014). There was substantial heterogeneity between trials (I2 = 52%) and so the pooled effect estimate should be interpreted cautiously (SMD 0.53 units, 95% CI 0.28 to 0.77; Analysis 2.18). There were mixed effects of MBIs on quality of life compared to non‐active comparators.
2.18. Analysis.

Comparison 2: MBIs versus non‐active comparators, Outcome 18: Quality of life, change from baseline
One study reported medians and ranges as data were not normally distributed (Ingraham 2017). The authors found no significant differences in mental health quality of life between the intervention and control groups.
One study did not report follow‐up values but stated that there were "significant differences" in quality of life between groups at three months (Armani Kian 2018).
One study reported only endpoint data (de Fatima Rosas Marchiori 2015). The authors report higher scores for some WHOQOL‐100 facets in the meditation group compared to the control group, in particular in facets 5 (thinking, learning, memory, and concentration), 6 (self‐esteem), 20 (opportunity to acquire new information and skills), and 25 (overall quality of life and overall health perception).
Coping, resilience, mastery
Five trials (325 participants randomised) measured self‐efficacy and reported data that could be used in meta‐analyses (Brach 1992; Chacko 2016; Guo 2021; Jalali 2019; Kristeller 2014). Two composite scores were used from the eating self‐efficacy scale in one study where N numbers were reduced appropriately to prevent double‐counting of participants (Brach 1992). Three studies showed favourable effects of MBIs (Guo 2021; Jalali 2019; Kristeller 2014), and the remainder showed little or no effect of MBIs on self‐efficacy compared to non‐active comparators. There was considerable heterogeneity between trials (I2 = 96%) and so the pooled effect estimate should be interpreted cautiously (SMD 1.08 units, 95% CI ‐0.24 to 2.4; Analysis 2.19).
2.19. Analysis.

Comparison 2: MBIs versus non‐active comparators, Outcome 19: Self‐efficacy, change from baseline
One trial (30 participants randomised) measured coping strategies and reported data that could be used in meta‐analyses (Nejati 2015). There was an increase in coping strategies with MBI as compared to a non‐active comparator (Analysis 2.20).
2.20. Analysis.

Comparison 2: MBIs versus non‐active comparators, Outcome 20: Coping strategies, change from baseline
One study reported coping ability using the brief COPE questionnaire and reported individual domains rather than summary scores (Chacko 2016). The authors found no consistent effect on coping ability at 12 weeks or six months in either group.
One trial (100 participants randomised) measured self‐management and reported data that could be used in meta‐analyses (Guo 2021). There was an increase in self‐management with the MBI compared to the non‐active comparator (Analysis 2.21).
2.21. Analysis.

Comparison 2: MBIs versus non‐active comparators, Outcome 21: Self‐management, change from baseline
One study measured self‐management using the summary of diabetes self‐care activities scale and is described narratively as no other trials reported this outcome (Pearson 2018). There was a "non‐significant" time x group interaction for blood glucose monitoring (P = 0.06), but no "significant differences" between the groups in the other domains of self‐management, including diet, exercise, and foot care.
3. TM versus active comparators
See Table 3.
3.1 Primary outcomes
CVD clinical events (cardiovascular mortality and other non‐fatal endpoints)
Only one trial involving African Americans with coronary artery stenosis reported clinical events (cardiovascular mortality and non‐fatal MI, stroke, revascularisation). Schneider 2012 randomised 203 participants to TM or cardiovascular health education with mean follow‐up reported at 5.4 years. No differences were seen between the two groups in this trial (RR 0.91, 95% CI 0.56 to 1.49; low‐certainty evidence; Analysis 3.1).
3.1. Analysis.

Comparison 3: TM versus active comparators, Outcome 1: Clinical CVD events (CVD mortality, non‐fatal MI and stroke, revascularisations)
Blood pressure
Eight trials (379 participants randomised) measured systolic blood pressure and reported data that could be used in meta‐analyses (Bokhari 2021; Paul‐Labrador 2006; Schneider 2005; Schneider 2012; Schneider 2019; Schneider 2021; Toomey 2007; Vaccarino 2013). TM probably results in a reduction in systolic blood pressure (MD ‐2.33 mmHg, 95% CI ‐3.99 to ‐0.68; I2 = 2%; moderate‐certainty evidence; Analysis 3.2). This result was stable in sensitivity analyses using fixed‐effect and random‐effects models for statistical pooling.
3.2. Analysis.

Comparison 3: TM versus active comparators, Outcome 2: Systolic blood pressure (mmHg), change from baseline
For diastolic blood pressure measured in the same eight trials, we assessed the overall quality of evidence as low and there was substantial heterogeneity between trials (I2 = 53%), so results should be interpreted cautiously (MD ‐1.15 mmHg, 95% CI ‐2.85 to 0.55; Analysis 3.3). Beneficial effects of TM on diastolic blood pressure were seen in one trial (Schneider 2021), possible benefits in two (Schneider 2005; Schneider 2012), and possible benefits of the active comparator in two (Bokhari 2021; Schneider 2019), with little or no effect of TM in the remaining three trials (Paul‐Labrador 2006; Toomey 2007; Vaccarino 2013).
3.3. Analysis.

Comparison 3: TM versus active comparators, Outcome 3: Diastolic blood pressure (mmHg), change from baseline
Anxiety
Three trials (200 participants randomised) measured anxiety and reported data that could be used in meta‐analyses (Paul‐Labrador 2006; Toomey 2007; Vaccarino 2013). TM may result in little to no difference in anxiety compared to active comparators (SMD 0.06 units, 95% CI −0.22 to 0.33; I2 = 0%; low‐certainty evidence; Analysis 3.4).
3.4. Analysis.

Comparison 3: TM versus active comparators, Outcome 4: Anxiety, change from baseline
Depression
Five trials (421 participants randomised) measured depression and reported data that could be used in meta‐analyses (Bokhari 2021; Paul‐Labrador 2006; Schneider 2012; Toomey 2007; Vaccarino 2013). TM likely results in little to no difference in depression compared to active comparators (SMD ‐0.12 units, 95% CI ‐0.31 to 0.07; I2 = 0%; moderate‐certainty evidence; Analysis 3.5).
3.5. Analysis.

Comparison 3: TM versus active comparators, Outcome 5: Depression, change from baseline
Perceived stress
Three trials (194 participants randomised) measured perceived stress and reported data that could be used in meta‐analyses (Bokhari 2021; Schneider 2019; Vaccarino 2013). One study favoured the active comparator (Vaccarino 2013) and the remaining two studies showed little or no effect of TM on depression. We assessed the overall certainty of evidence as very low. There was substantial heterogeneity between trials (I2 = 70%) and so the results should be interpreted cautiously (SMD 0.04 units, 95% CI ‐0.49 to 0.57; Analysis 3.6).
3.6. Analysis.

Comparison 3: TM versus active comparators, Outcome 6: Perceived stress, change from baseline
Well‐being
None of the studies in this comparison group measured well‐being.
Adverse events
None of the included trials reported adverse events.
3.2 Secondary outcomes
Blood lipid levels
Three trials (170 participants randomised) measured total cholesterol and reported data that could be used in meta‐analyses (Bokhari 2021; Paul‐Labrador 2006; Toomey 2007). There was little or no effect of TM on total cholesterol compared to active comparators (MD ‐0.03 mmol/L, 95% CI ‐0.28 to 0.22; I2 = 0%; Analysis 3.7).
3.7. Analysis.

Comparison 3: TM versus active comparators, Outcome 7: Total cholesterol (mmol/L), change from baseline
Three trials (169 participants randomised) measured LDL cholesterol and reported data that could be used in meta‐analyses (Bokhari 2021; Paul‐Labrador 2006; Toomey 2007). There was little or no effect of TM on LDL cholesterol compared to active comparators (MD ‐0.05 mmol/L, 95% CI ‐0.26 to 0.17; I2 = 0%; Analysis 3.8).
3.8. Analysis.

Comparison 3: TM versus active comparators, Outcome 8: LDL cholesterol (mmol/L), change from baseline
Three trials (169 participants randomised) measured HDL cholesterol and reported data that could be used in meta‐analyses (Bokhari 2021; Paul‐Labrador 2006; Toomey 2007). There was little or no effect of TM on HDL cholesterol compared to active comparators (MD 0.00 mmol/L, 95% CI ‐0.07 to 0.08; I2 = 0%; Analysis 3.9).
3.9. Analysis.

Comparison 3: TM versus active comparators, Outcome 9: HDL cholesterol (mmol/L), change from baseline
Two trials (122 participants randomised) measured triglycerides and reported data that could be used in meta‐analyses (Bokhari 2021; Paul‐Labrador 2006). There was a reduction in triglycerides with TM compared to active comparators, but the 95% CI overlaps no effect (MD ‐0.22 mmol/L, 95% CI ‐0.5 to 0.06; I2 = 0%; Analysis 3.10).
3.10. Analysis.

Comparison 3: TM versus active comparators, Outcome 10: Triglycerides (mmol/L), change from baseline
Fasting blood glucose
None of the studies in this comparison group measured fasting blood glucose.
HbA1C
None of the studies in this comparison group measured HbA1C.
Body weight
One trial (86 participants randomised) measured body weight and reported data that could be used in meta‐analyses (Schneider 2019). There was little or no effect of TM on body weight compared to the active comparator (Analysis 3.11).
3.11. Analysis.

Comparison 3: TM versus active comparators, Outcome 11: Weight (kg), change from baseline
BMI
Four trials (423 participants randomised) measured BMI and reported data that could be used in meta‐analyses (Bokhari 2021; Schneider 2012; Schneider 2019; Schneider 2021). There was little or no effect of TM on BMI compared to active comparators (MD 0.07 mmol/L, 95% CI ‐0.45 to 0.59; I2 = 0%; Analysis 3.12).
3.12. Analysis.

Comparison 3: TM versus active comparators, Outcome 12: BMI (kg/m2), change from baseline
Smoking rates
None of the studies in this comparison group measured smoking cessation.
Quality of life
One trial (41 participants randomised) measured quality of life and reported data that could be used in meta‐analyses (Bokhari 2021). There was little or no effect of TM on quality of life compared to the active comparator (Analysis 3.13).
3.13. Analysis.

Comparison 3: TM versus active comparators, Outcome 13: Quality of life, change from baseline
Coping, resilience, mastery
None of the studies in this comparison group measured these outcomes.
4. TM versus non‐active comparators
See Table 4.
4.1 Primary outcomes
CVD clinical events (cardiovascular mortality and other non‐fatal endpoints)
None of the included trials reported on clinical events.
Blood pressure
Two trials (139 participants randomised) measured systolic blood pressure and reported data that could be used in meta‐analyses (Nidich 2009; Seer 1980). TM may reduce systolic blood pressure compared to wait list controls (MD ‐6.34 mmHg, 95% CI ‐9.86 to ‐2.81; I2 = 0%; low‐certainty evidence) (Analysis 4.1).
4.1. Analysis.

Comparison 4: TM versus non‐active comparators, Outcome 1: Systolic blood pressure (mmHg), change from baseline
The same two trials measured diastolic blood pressure and reported data that could be used in meta‐analyses (Nidich 2009; Seer 1980). TM may reduce diastolic blood pressure compared to wait list controls (MD ‐5.13 mmHg, 95% CI ‐9.07 to ‐1.19; I2 = 18%; low‐certainty evidence) (Analysis 4.2).
4.2. Analysis.

Comparison 4: TM versus non‐active comparators, Outcome 2: Diastolic blood pressure (mmHg), change from baseline
Anxiety
One trial (112 participants randomised) measured anxiety and reported data that could be used in meta‐analyses (Nidich 2009). TM may have a moderate effect on reducing anxiety levels compared to the non‐active comparator (SMD ‐0.71 units, 95% CI ‐1.09 to ‐0.32; low‐certainty evidence) (Analysis 4.3).
4.3. Analysis.

Comparison 4: TM versus non‐active comparators, Outcome 3: Anxiety, change from baseline
Depression
One trial (112 participants randomised) measured depression and reported data that could be used in meta‐analyses (Nidich 2009). TM may have a moderate effect on reducing depression compared to the non‐active comparator (SMD ‐0.48 units, 95% CI ‐0.86 to ‐0.11; low‐certainty evidence) (Analysis 4.4)
4.4. Analysis.

Comparison 4: TM versus non‐active comparators, Outcome 4: Depression, change from baseline
Perceived stress
None of the studies in this comparison group measured perceived stress.
Well‐being
None of the studies in this comparison group measured well‐being.
Adverse events
None of the included trials reported adverse events.
4.2 Secondary outcomes
Blood lipids
None of the studies in this comparison group measured blood lipids.
Fasting blood glucose
None of the studies in this comparison group measured fasting blood glucose.
HbA1C
None of the studies in this comparison group measured HbA1C.
Body weight
None of the studies in this comparison group measured body weight.
BMI
None of the studies in this comparison group measured BMI.
Smoking rates
None of the studies in this comparison group measured smoking cessation.
Quality of life
None of the studies in this comparison group measured quality of life.
Coping, resilience, mastery
None of the studies in this comparison group measured these outcomes.
5. Subgroup analyses
There were sufficient studies to conduct subgroup analyses by primary and secondary prevention for the two comparisons: MBIs versus non‐active comparators and TM versus active comparators, for 'Blood pressure' and 'Validated measures of psychological distress'.
MBIs versus non‐active comparators
Blood pressure
As mentioned above, nine trials (379 participants randomised) measured systolic blood pressure and data were pooled (Alamout 2020; Alsubaie 2020; Blom 2013; Nejati 2015; Nijjar 2019; Parswani 2013; Pearson 2018; Shukla 2021; Sinha 2018). We assessed the overall certainty of evidence as low, with considerable heterogeneity between trials (I2 = 87%). See Analysis 2.2.
Heterogeneity was partially explained by case mix in primary prevention studies (I2 = 93%) versus in secondary prevention studies (I2 = 0%); however, heterogeneity was driven by two small studies with large effect sizes (Alamout 2020; Nejati 2015). No differences in systolic blood pressure were seen between subgroups (test for subgroup differences: Chi² = 0.06, df = 1 (P = 0.80), I² = 0%; Analysis 5.1).
5.1. Analysis.

Comparison 5: Subgroup analysis by primary and secondary prevention ‐ MBIs versus non‐active comparators, Outcome 1: Systolic blood pressure (mmHg), change from baseline
For diastolic blood pressure measured in the same nine trials, we assessed the overall certainty of evidence as low and there was substantial heterogeneity between trials (I2 = 61%). See Analysis 2.3.
Heterogeneity was not explained by case mix: primary prevention studies, I2 = 74%; secondary prevention studies, I2 = 61%. No differences in diastolic blood pressure were seen between subgroups (test for subgroup differences: Chi² = 0.00, df = 1 (P = 0.97), I² = 0%; Analysis 5.2).
5.2. Analysis.

Comparison 5: Subgroup analysis by primary and secondary prevention ‐ MBIs versus non‐active comparators, Outcome 2: Diastolic blood pressure (mmHg), change from baseline
Anxiety
Nine trials (533 participants randomised) measured anxiety and reported data that could be used in meta‐analyses (Alsubaie 2020; Daubenmier 2011; Jang 2018; Nijjar 2019; Nikkhah Ravari 2020; Parswani 2013; Pearson 2018; Tovote 2014; van Son 2014). We assessed the overall certainty of evidence as low. There was substantial heterogeneity between trials (I2 = 61%) and so the results should be interpreted cautiously (SMD ‐0.78 units, 95% CI ‐1.09 to ‐0.4; Analysis 2.4).
Heterogeneity was partially explained by case mix: primary prevention studies (I2 = 0%); secondary prevention studies (I2 = 79%) (Analysis 5.3). No differences in anxiety were seen between subgroups (test for subgroup differences: Chi² = 1.99, df = 1 (P = 0.16), I² = 49.7%).
5.3. Analysis.

Comparison 5: Subgroup analysis by primary and secondary prevention ‐ MBIs versus non‐active comparators, Outcome 3: Anxiety, change from baseline
Depression
Fifteen trials (912 participants randomised) measured depression and reported data that could be used in meta‐analyses (Alsubaie 2020; Brach 1992; Chacko 2016; Friis 2016; Jang 2018; Kopf 2014; Kristeller 2014; Nathan 2017; Nijjar 2019; Nikkhah Ravari 2020; Parswani 2013; Pearson 2018; Schroevers 2015; Tovote 2014; van Son 2014). We assessed the overall certainty of evidence as low. There was substantial heterogeneity between trials (I2 = 67%) and so the results should be interpreted cautiously (SMD ‐0.66 units, 95% CI ‐0.91 to ‐0.41; Analysis 2.5). Heterogeneity was not explained by case mix: primary prevention studies, I2 = 61%; secondary prevention studies, I2 = 71% (Analysis 5.4). No differences in depression were seen between subgroups (test for subgroup differences: Chi² = 2.44, df = 1 (P = 0.12), I² = 59.0%).
5.4. Analysis.

Comparison 5: Subgroup analysis by primary and secondary prevention ‐ MBIs versus non‐active comparators, Outcome 4: Depression, change from baseline
Perceived stress
Eleven trials (708 participants randomised) measured perceived stress and reported data that could be used in meta‐analyses (Chacko 2016; Daubenmier 2011; Davis 2014a; Kopf 2014; Nasiri 2020; Nathan 2017; Nijjar 2019; Nikkhah Ravari 2020; Parswani 2013; Pearson 2018; van Son 2014). We assessed the overall certainty of evidence as low. There was substantial heterogeneity between trials (I2 = 70%) and so the results should be interpreted cautiously (SMD ‐0.59 units, 95% CI ‐0.89 to ‐0.29; Analysis 2.6). Heterogeneity was not explained by case mix: primary prevention studies, I2 = 55%; secondary prevention studies, I2 = 86%. See Analysis 5.5. However, for primary prevention studies, heterogeneity was driven by one outlier (Chacko 2016), a very small study (nine participants in each group, having undergone bariatric surgery).No differences in perceived stress were seen between subgroups (test for subgroup differences: Chi² = 1.54, df = 1 (P = 0.22), I² = 34.9%)
5.5. Analysis.

Comparison 5: Subgroup analysis by primary and secondary prevention ‐ MBIs versus non‐active comparators, Outcome 5: Perceived stress, change from baseline
TM versus active comparators
Blood pressure
Eight trials (379 participants randomised) measured systolic blood pressure and reported data that could be used in meta‐analyses (Bokhari 2021; Paul‐Labrador 2006; Schneider 2005; Schneider 2012; Schneider 2019; Schneider 2021; Toomey 2007; Vaccarino 2013). TM probably reduces systolic blood pressure compared to active comparators (MD ‐2.33 mmHg, 95% CI ‐3.99 to ‐0.68; I2 = 2%; moderate‐certainty evidence) (Analysis 3.2).
The possible moderate heterogeneity was partially explained by case mix: primary prevention studies (I2 = 0%); secondary prevention studies (I2 = 32%; Analysis 6.1). No differences in systolic blood pressure were seen between subgroups (test for subgroup differences: Chi² = 1.19, df = 1 (P = 0.28), I² = 15.8%).
6.1. Analysis.

Comparison 6: Subgroup analysis by primary and secondary prevention ‐ TM versus active comparators, Outcome 1: Systolic blood pressure (mmHg), change from baseline
For diastolic blood pressure measured in the same eight trials, we assessed the overall certainty of evidence as very low and there was substantial heterogeneity between trials (I2 = 53%). See Analysis 3.3.
Heterogeneity was not explained by case mix: primary prevention studies, I2 = 58%; secondary prevention studies, I2 = 53% (Analysis 6.2). No differences in diastolic blood pressure were seen between subgroups (test for subgroup differences: Chi² = 0.66, df = 1 (P = 0.42), I² = 0%).
6.2. Analysis.

Comparison 6: Subgroup analysis by primary and secondary prevention ‐ TM versus active comparators, Outcome 2: Diastolic blood pressure (mmHg), change from baseline
Anxiety
Three trials (200 participants randomised) measured anxiety and reported data that could be used in meta‐analyses (Paul‐Labrador 2006; Toomey 2007; Vaccarino 2013). TM may result in little to no difference in anxiety compared to active comparators (SMD 0.06 units, 95% CI ‐0.22 to 0.33; I2 = 0%; low‐certainty evidence; Analysis 3.4).
Heterogeneity may not be important for this outcome; in the primary prevention studies I2 = 0% and only one trial reported on secondary prevention (Analysis 6.3). No differences in anxiety were seen between subgroups (test for subgroup differences: Chi² = 0.01, df = 1 (P = 0.92), I² = 0%).
6.3. Analysis.

Comparison 6: Subgroup analysis by primary and secondary prevention ‐ TM versus active comparators, Outcome 3: Anxiety, change from baseline
Depression
Five trials (421 participants randomised) measured depression and reported data that could be used in meta‐analyses (Bokhari 2021; Paul‐Labrador 2006; Schneider 2012; Toomey 2007; Vaccarino 2013). TM likely results in little to no difference in depression compared to active comparators (SMD ‐0.12 units, 95% CI ‐0.31 to 0.07; I2 = 0%; moderate‐certainty evidence; Analysis 3.5).
Heterogeneity may not be important for this outcome; in the primary prevention studies I2 = 0% and in the secondary prevention studies I2 = 0% (Analysis 6.4). No differences in depression were seen between subgroups (test for subgroup differences: Chi² = 0.24, df = 1 (P = 0.63), I² = 0%).
6.4. Analysis.

Comparison 6: Subgroup analysis by primary and secondary prevention ‐ TM versus active comparators, Outcome 4: Depression, change from baseline
Perceived stress
Three trials (194 participants randomised) measured perceived stress and reported data that could be used in meta‐analyses (Bokhari 2021; Schneider 2019; Vaccarino 2013). We assessed the overall certainty of evidence as very low. There was substantial heterogeneity between trials (I2 = 70%) and so the results should be interpreted cautiously (SMD 0.04 units, 95%CI ‐0.49 to 0.57; Analysis 3.6).
Heterogeneity was partially explained by case mix; in the primary prevention studies I2 = 71% and only one trial reported on secondary prevention (Analysis 6.5). No differences in perceived stress were seen between subgroups (test for subgroup differences: Chi² = 2.28, df = 1 (P = 0.13), I² = 56.2%).
6.5. Analysis.

Comparison 6: Subgroup analysis by primary and secondary prevention ‐ TM versus active comparators, Outcome 5: Perceived stress, change from baseline
Further subgroup analyses
It was our intention to explore the impact of the nature of the active comparator where there were sufficient studies to do so. For active comparators, we planned to perform a subgroup analysis by psychological comparator compared to health education/other intervention for psychological outcomes, but there were only two studies reporting psychological comparators for MBIs (Brach 1992; Tovote 2014), and none for TM.
In addition to subgrouping by primary and secondary prevention, we intended to look at differences between participant groups recruited, such as those who are overweight/obese, those with diabetes, and those with hypertension. However, these analyses are made challenging by the different comparators as well as different meditation interventions and outcomes measured. We also intended to explore the impact of different MBIs in terms of focus and intensity, but this was similarly affected by different comparator groups, participant groups and outcomes measured.
The addition of a large number of ongoing studies when completed in future updates of this review should allow some or all of these planned analyses.
6. Other meditation interventions
6.1 Primary outcomes
CVD clinical events (cardiovascular mortality and other non‐fatal endpoints)
None of the included trials reported on clinical events.
Blood pressure
Blood pressure was measured in three trials (84 participants randomised) categorised as other meditation interventions (Chandler 2020; Friskey 1984; Hafner 1982). In one trial, the tension tamer breathing awareness meditation application showed significantly greater systolic blood pressure reductions from baseline compared to an attention control in those with stage 1 systolic hypertension at three, six, and 12 months follow‐up (P = 0.04) (Chandler 2020). In another trial, systolic and diastolic blood pressure decreased in both progressive muscle relaxation (PMR) plus meditation intervention and the health education comparator between baseline and follow‐up, but there were no differences between groups (Friskey 1984). In the third trial, similarly, systolic and diastolic blood pressure decreased in both the meditation and biofeedback group and health education comparator, with no differences between groups (Hafner 1982).
Anxiety
Anxiety was measured in three trials (185 participants randomised) categorised as other meditation interventions (Friskey 1984; Malm 2018; Sampaio 2016). One trial found that trait anxiety was relatively stable over time in the PMR plus meditation and health education groups and that state anxiety was less stable over time and consistently lower in the health education control group (Friskey 1984). In another trial of CBT plus HeartMath protocol in patients with atrial fibrillation, the mean change in anxiety from baseline to 12 months follow‐up in the intervention group was ‐0.22 (SD 3.6) and 0.12 (SD 4.13) in the treatment as usual control group (P = 0.4) (Malm 2018). In a further trial of healing meditation in previously obese participants on weight maintenance programmes, the authors detected a difference in the mean variation between the intervention and control groups in the total anxiety scores of 7.7 (95% CI 6.3 to 9.2; Cohen’s d = 3.41) showing reduced anxiety with the intervention (Sampaio 2016).
Depression
Depression was measured in two trials (175 participants randomised) categorised as other meditation interventions (Malm 2018; Rungreangkulkij 2011). In one trial of CBT plus HeartMath protocol in patients with atrial fibrillation, the mean change in depression from baseline to 12 months follow‐up in the intervention group was ‐0.77 (SD 2.84) and 0.05 (SD 3.03) in the treatment as usual control group (P = 0.04) (Malm 2018). In another trial of Buddhist group therapy in women with type 2 diabetes and depressive symptoms, they found that the number of responders, in terms of those who returned to a normal level, was 93.8% in the intervention group and 65.6% in the treatment as usual group (RR 6.5, 95% CI 1.4 to 30.6; P = 0.02) (Rungreangkulkij 2011).
Perceived stress
Perceived stress was measured in two trials (60 participants randomised) categorised as other meditation interventions (Chandler 2020; Sarika 2020). In one trial there were no significant differences between or within groups at any time points for the tension tamer breathing awareness meditation application compared to an attention control in those with stage 1 systolic hypertension (Chandler 2020). Conversely, the mean change in perceived stress was significantly reduced in the IAM® training group compared to standard care in those with diabetes (P ≤ 0.001; Sarika 2020).
Well‐being
None of the studies in this comparison group measured well‐being.
Adverse events
None of the included trials reported on adverse events.
6.2 Secondary outcomes
Blood lipids
One trial of female participants with type 2 diabetes measured lipid levels and found no changes between baseline and 12 weeks follow‐up for either Buddhist walking meditation or traditional walking in total cholesterol, LDL and HDL cholesterol, or triglycerides levels (Gainey 2016).
Fasting blood glucose
One trial of female participants with type 2 diabetes measured fasting blood glucose and found reductions in both the Buddhist walking meditation group and the traditional walking group from baseline, but no differences between groups (Gainey 2016).
Another trial of IAM® training in men and women with diabetes found an increase in fasting blood glucose in the standard care group, but no differences between groups (Sarika 2020).
HbA1C
One trial of female participants with type 2 diabetes measured HbA1C and found reductions between baseline and 12 week follow‐up in the Buddhist walking meditation group only with no change in the traditional walking group (Gainey 2016).
A trial of IAM® training in men and women with diabetes found a decrease in HbA1C with the intervention and mean difference between groups of ‐0.75% (SD 0.97; P = 0.03) (Sarika 2020).
Body weight
A trial of IAM® training in men and women with diabetes compared to standard care reported a mean difference between groups of ‐0.9 kg (SD 1.6; P = 0.05) favouring the intervention (Sarika 2020).
BMI
A trial of IAM® training in men and women with diabetes compared to standard care reported a mean difference between groups of ‐0.38 units (SD 0.65; P = 0.055) favouring the intervention (Sarika 2020).
Smoking rates
None of the studies in this comparison group measured smoking cessation.
Quality of life
One study of APP (pituitary pineal activation) meditation compared to health education in CHD patients found larger increases from baseline to 12 weeks follow‐up in quality of life using SF‐36 in the intervention group (mean change 5.4 (SD 9.69)) intervention; mean change 0.79 (SD 7.58) control; P = 0.03) (Huerin 2015).
Another trial of CBT plus HeartMath protocol compared to treatment as usual in patients with atrial fibrillation found that at the 12‐month follow‐up, the intervention group experienced a higher health‐related quality of life using EQ‐5D than the control group (mean change in the intervention group 0.062 versus mean change in the control group ‐0.015; P = 0.02) (Malm 2018).
Coping, resilience, mastery
None of the studies in this comparison group measured these outcomes.
Discussion
The aim of this review is to determine the effectiveness of meditation techniques, primarily MBIs and TM, for the primary and secondary prevention of CVD in adults at high risk and with established CVD. As well as seeking clinical endpoints, we also examined the effects of meditation on blood pressure and psychological distress and well‐being as our primary outcomes, and other cardiovascular risk factors such as lipid levels, glycaemic control, weight, smoking, and quality of life and self‐efficacy, coping and self‐management as secondary outcomes.
Summary of main results
In this review, 81 RCTs met our inclusion criteria. We used prespecified comparison groups to analyse the data to address both heterogeneity between participants and comparison groups and to aid interpretation of our findings. The comparison groups and number of trials and participants contributing to each are presented below, as are the narrative interpretations of primary outcomes for each.
MBIs versus active comparators: 29 trials (2883 participants randomised)
None of the trials reported clinical events. MBIs may reduce blood pressure compared to active comparators, but heterogeneity was very high and overall evidence rated as low. Based on moderate‐certainty evidence, MBIs likely result in little to no difference in anxiety or depression, but MBIs probably reduce perceived stress compared to active comparators. Well‐being was measured in only one trial where there was probably little or no effect of the intervention. See Table 1.
MBIs versus non‐active comparators: 38 trials (2905 participants randomised)
CVD mortality and non‐fatal MI as a composite outcome were reported in one trial, but the evidence is very uncertain. There was low‐certainty evidence that MBIs may reduce blood pressure. Heterogeneity for this outcome was high, which was partially explained by stratifying by primary and secondary prevention studies, where there was more certainty in secondary prevention. Compared to non‐active comparators, there was low‐certainty evidence that MBIs may reduce anxiety, depression, and perceived stress. Heterogeneity was high for these outcomes, and we were more certain of the reduction in anxiety in primary prevention studies. Well‐being was measured in only two trials where there was moderate‐certainty evidence that well‐being probably increases with MBIs compared to non‐active comparators. See Table 2.
TM versus active comparators: eight trials (830 participants randomised)
CVD mortality, non‐fatal MI, stroke, and revascularisation, as a composite outcome, were reported in one trial and based on low‐certainty evidence, TM may result in little to no difference in this composite outcome when compared to an active comparator. TM probably reduces systolic blood pressure with less certainty for diastolic blood pressure. There was moderate‐certainty evidence of TM for depression, and low‐certainty evidence for anxiety and very low‐certainty evidence for perceived stress. None of the trials reported on well‐being. See Table 3.
TM versus non‐active comparators: two trials (186 participants randomised)
None of the trials reported clinical events. There may be a reduction in systolic blood pressure with TM, and we are uncertain of the effects on diastolic blood pressure in the two trials that measured this. Anxiety and depression may be reduced with TM compared to a non‐active comparator in the trial that reported this. Neither trial measured perceived stress or well‐being. See Table 4.
Other meditation interventions: nine trials (427 participants randomised)
None of the trials reported clinical events. Blood pressure was measured in three trials, where results were mixed. Beneficial effects were seen for anxiety (three trials) and depression (two trials), with mixed results for perceived stress (two trials).
Only one study reported adverse events across all comparison groups.
Subgroup analyses for the two comparisons with sufficient studies, MBI versus non‐active comparators and TM versus active comparators, showed no subgroup differences in blood pressure or psychological distress between primary and secondary prevention studies.
Secondary outcomes included other CVD risk factors, quality of life and self‐efficacy, coping, and self‐management. Few trials measured lipid levels across comparison groups and, where reported, meta‐analyses showed little or no effect of MBIs compared to active or non‐active comparators or TM compared to active comparators. Favourable effects were seen on fasting blood glucose for MBIs compared to both active and non‐active comparators, with little or no effect on HbA1C for active comparators and mixed results for non‐active comparators. Glycaemic control was not reported in trials of TM. Where reported, meta‐analyses showed little or no effect of MBIs on body weight or BMI compared to active and non‐active comparators. Similarly, in the few trials reporting these outcomes, there was little or no effect of TM compared to active comparators. There was very low‐certainty evidence of mixed results for smoking cessation with MBIs compared to active comparators, and based on low‐certainty evidence, there was little or no effect of MBIs on smoking cessation compared to non‐active comparators. The effects of MBIs on quality of life were mixed compared to non‐active comparators. Few trials reported quality of life for MBIs or TM compared to active comparators and none for TM compared to non‐active comparators. Similarly, few trials reported on self‐efficacy, coping strategies, and self‐management. Overall, where reported, there was an increase with MBIs compared to active and non‐active comparators. Coping was measured in only one trial of TM with favourable effects.
Few trials of other meditation interventions reported secondary outcomes. Where reported, quality of life increased with the intervention.
Overall completeness and applicability of evidence
We have synthesised the available evidence to date on the effects of meditation on our primary outcomes CVD clinical endpoints, blood pressure, psychological distress, and well‐being, and secondary outcomes including other CVD risk factors, quality of life, and coping ability, in those at risk of or with diagnosed CVD. Currently, 81 completed RCTs (6971 participants randomised) met our inclusion criteria. Only two reported on clinical endpoints, as most trials were small and short‐term.
We have stratified our analyses by type of meditation (MBIs or TM) and by comparison group (active and non‐active comparators) in an attempt to address heterogeneity and aid interpretation of findings, to make the review as useful as possible. We have also explored the effects of primary and secondary prevention in subgroup analyses where there were sufficient studies to do so. Other meditation interventions were not excluded but were reported narratively. We were strict in our definition of a meditation intervention in that multifactorial studies were excluded unless co‐interventions were also included in the comparison group to be able to determine the effects of meditation alone, and in that meditation had to be the major component of interventions. Included studies were also limited by our requirements for an intervention and follow‐up duration of 12 weeks or more, which excluded a number of short‐term studies. However, we were interested in longer‐term studies as the sustainability of any behavioural change is challenging, and in terms of the relevance to public health, the impact on longer‐term cardiovascular health is probably due to sustained change.
Whilst we were strict in our definition of meditation interventions, there were significant differences in the interventions tested. We attempted to control for these by grouping studies according to the most commonly studied interventions ‐ mindfulness‐based interventions (MBIs) and transcendental meditation (TM), and other meditation interventions that were well described but did not fit either of these categories. TM is very standardised (Roth 1994; Roth 2018) and most of the included studies testing the effects of TM were conducted within the same research group. There are also standard MBIs, notably MBSR (Kabat‐Zinn 1990; Kabat‐Zinn 2013) and MBCT (Segal 2002; Segal 2013), and adaptations of these such as mindfulness‐based relapse prevention protocols (Bowen 2011), developed for smoking cessation interventions in some studies, and mindfulness‐based eating awareness training (MB‐EAT; Kristeller 2011) used to target overweight and obesity. Other studies used standard MBSR/MBCT protocols and adapted them to particular patient groups such as those with diabetes or cardiovascular disease and included content or practices relevant to these conditions. In some cases, categorisation of interventions between MBIs and other meditation interventions was difficult and required judgement based on the content, for example Brach 1992 and de Fatima Rosas Marchiori 2015 could have been categorised as other interventions instead of MBIs, but they both focus on mindfulness meditation. Conversely, Buddhist group therapy described in Rungreangkulkij 2011, categorised in other meditation interventions, includes mindfulness, but the content is broader. We intended to explore the impact of different MBIs in terms of focus and intensity, but this was made challenging by different comparator groups, participants, and outcomes measured.
We excluded most studies using acceptance and commitment therapy (ACT) as an intervention, as whilst ACT utilises mindfulness practices, these are not generally based on formal meditation but rather a large array of mindfulness tools and techniques (Harris 2021). However, it is possible that some ACT interventions encourage or support the use of mindfulness meditation and formal practice, so we may have underestimated the number of studies utilising mindfulness meditation by taking this decision. ACT, however, is a very specific therapeutic intervention where mindfulness techniques form part of three main components:
Defusion: distancing from, and letting go of, unhelpful thoughts, beliefs, and memories.
Acceptance: making room for painful feelings, urges, and sensations, and allowing them to come and go without a struggle.
Contact with the present moment: engaging fully with your here‐and‐now experience, with an attitude of openness and curiosity (Harris 2021).
If the current review was based on mindfulness interventions per se rather than meditation more broadly, ACT interventions would have been included. We did, however, include one completed trial using ACT along with mindfulness and self‐compassion practices compared to treatment as usual, where we judged the focus of the intervention to be mindfulness meditation (Palmeira 2017), and two ongoing studies which randomise groups to each of the three main components of ACT (Forman 2021; Guerrini Usubini 2021). Overall, we have excluded 26 completed or ongoing studies using ACT interventions.
Interest in meditation for health is significant, and it is likely that many more trials will be conducted in this area to add to the evidence base. We identified 74 ongoing trials in our search of November 2021, a substantial number which, when complete, we will incorporate in a future update of this review.
Of note, the search cut‐off date of November 2021 is a limitation of the review. The ongoing studies are trial registry records or published protocols linking to trial registries (Characteristics of ongoing studies). In an attempt to assure transparency and integrity, we have ascertained the up‐to‐date status of the 74 ongoing trials as of May 2023 and provide details in Table 5 to help address this limitation. Of the 74 ongoing studies identified in the November 2021 search, nine have since released results in full and these have been re‐categorised as studies awaiting classification (Characteristics of studies awaiting classification), so the number of ongoing studies is now 65. Two of these nine studies now awaiting classification have been completed, but they may be ineligible due to short follow‐up durations. Of the remaining seven studies, five had inactive comparators with mixed findings, and for the two studies involving active comparators, there was little evidence of an effect of meditation. These results are broadly in line with the current review findings as a whole, but sample sizes were small, and these nine studies have yet to be formally assessed. The majority of the ongoing studies assessed reported the same findings as those assessed during the November 2021 search. Some records have not been updated for many years and have not completed or reported results, so may not be contributory. Reassuringly, these findings demonstrate that the limitations of the search cut‐off date may not have a detrimental effect on how up‐to‐date the evidence is, as presented in this Cochrane review. It is our intention to update the current review in 2025 when some of the larger ongoing trials identified will be complete.
Quality of the evidence
Heterogeneity of participants, interventions, and comparators was high, and we have attempted to reduce this by conducting the main analyses in four comparison groups for MBIs and TM compared to active and non‐active comparators for meta‐analyses, and a further group of other meditation interventions that we have described narratively. We further explored heterogeneity by primary and secondary prevention where there were sufficient studies to do this (for the comparison of MBI versus non‐active comparators and TM versus active comparators). We intended to explore heterogeneity further in subgroup analyses exploring the nature and intensity of interventions and comparators, but this was limited by the number of studies for specific outcomes in each of the main comparison groups and the heterogeneity of participants. We conducted sensitivity analyses by comparing standardised mean differences to mean differences and random‐effects models to fixed‐effect models to test the robustness of significant findings.
Most studies included in this review were at unclear risk of bias for many of the risk of bias domains, so results should be interpreted cautiously. As blinding of participants and personnel for behavioural interventions is difficult, if not impossible, we judged this to be an unclear risk rather than rate it as high risk. We used the original risk of bias (RoB) tool rather than the new RoB2 tool and did not formally report separately the blinding of outcome assessors according to the nature of the outcomes (objective or subjective). We noted high risk of bias due to high attrition rates in five trials, for differential attrition rates between the intervention and control groups in two trials, and a high risk of other bias in one study where high losses to follow‐up prevented comparison between groups. The summary of findings tables provide our GRADE assessment of overall evidence certainty for each of the four comparison groups:
For MBIs compared to active comparators, GRADE assessment of the outcomes has led to trials being downgraded for imprecision due to wide confidence intervals that include the possibilities of both substantial benefit and a possible negative effect, risk of selection and attrition bias, and inconsistency where heterogeneity was considerable.
For MBIs compared to non‐active comparators, GRADE assessment of the outcomes has led to trials being downgraded for imprecision due to small sample size and wide confidence intervals that include the possibilities of no benefit, no difference, and large benefit, risk of selection and attrition bias, and inconsistency where heterogeneity was either substantial or considerable.
For TM compared to active comparators, GRADE assessment of the outcomes has led to downgrades for risk of selection and attrition bias, and imprecision due to small sample size and wide confidence intervals that include the possibilities of small negative effect, no difference, or positive effect.
For TM compared to non‐active comparators, GRADE assessment of the outcomes has led to downgrades for risk of selection and attrition bias, and imprecision due to small sample size.
Potential biases in the review process
We conducted a comprehensive search across major databases for interventions involving meditation. As mentioned above, we recognise the search cut‐off date of November 2021 as a limitation of the review and we plan to fully update the review in 2025 (Overall completeness and applicability of evidence). Two review authors independently selected and assessed trials for inclusion using prespecified criteria, extracted data, and assessed the risk of bias to minimise potential biases in the review processes.
There was heterogeneity between trials from different sources (participants, nature and duration of intervention, comparison groups, follow‐up, outcome data), which was substantial or considerable for some outcomes. We prespecified four main comparison groups for analysis to address the likely heterogeneity that we would encounter, by intervention type (MBIs or TM) and comparator (active and non‐active), and reported the findings from other meditation interventions separately. We have also explored the effects of primary and secondary prevention where there were sufficient studies. By exploring the effects of meditation in these main comparison groups and subgroups, we have reduced heterogeneity and provided a more comprehensive picture.
We intended to explore heterogeneity further in subgroup analyses exploring the nature and intensity of interventions and comparators, but this was limited by the number of studies for specific outcomes in each of the main comparison groups and the heterogeneity of participants. It is anticipated that further stratified analyses can be conducted with the addition of the large number of ongoing trials to future updates of this review.
Not all data from all studies were reported in a useable format to contribute to meta‐analyses. We have reported the data narratively where we were unable to pool them. We have also contacted authors to provide additional data where these were missing.
Our strict inclusion criteria, including only interventions where meditation was the major component and restrictions on study duration and follow‐up, limited the number of studies available for inclusion. However, these criteria add clarity and allow presentation of sustained rather than short‐term effects.
Agreements and disagreements with other studies or reviews
This review updates and builds on the recommendations of the AHA scientific statement examining the effects of meditation on CVD (AHA 2017). Since this time, many trials have been published, and systematic reviews have been conducted synthesising the findings from these trials, for both the primary and secondary prevention of CVD. We present the most recent systematic reviews below in the context of our own findings to date.
Two recent systematic reviews focused exclusively on trials of MBIs in secondary prevention (Scott‐Sheldon 2020; Zou 2021). Both included studies we excluded on the basis of being short‐term (< 12 weeks duration and follow‐up) and focused analyses on the immediate period post‐intervention, which was typically eight weeks. In one review including 16 trials, they found improved systolic blood pressure and psychological outcomes with MBIs (Scott‐Sheldon 2020). The other systematic review included nine trials and performed analyses by active and inactive comparators. They found reductions in stress and depression compared to inactive comparators, and no effects on psychological outcomes compared to active comparators (Zou 2021). We explored the effects of primary and secondary prevention in trials of MBIs compared with inactive comparators where there were sufficient studies. There were reductions in both systolic and diastolic blood pressure in secondary prevention trials, but heterogeneity remained high for psychological outcomes, so findings are uncertain. There were no subgroup effects between primary and secondary prevention trials for our primary outcomes.
The effects of MBIs in participants with hypertension were reported in two recent systematic reviews (Conversano 2021; Lee 2020). Six trials were reported on in one, including both active and inactive comparators. We have identified the same trials in our review but excluded three on the basis of being short‐term. The authors found a reduction in both systolic and diastolic blood pressure with MBSR (Conversano 2021). The other review included 12 trials of MBIs, five of which we excluded from our review. The authors report sustained effects in a reduction in diastolic blood pressure, and also sustained improvements in anxiety, depression, and stress. Results were reported combining both active and inactive comparators and few trials contributed to psychological outcomes (Lee 2020). We did not specifically look at the effects of MBIs in hypertensive subjects as there was significant heterogeneity in terms of interventions, comparators, participants, and outcomes. We will explore these subgroups with the addition of further studies in future updates of this review.
Mindful eating and other MBIs targeting participants with overweight and obesity were the subject of a further systematic review (Mercado 2021). Twelve studies were included measuring BMI, 10 of which are included in our review. Active and inactive comparators were pooled together. Reductions were seen in BMI, but heterogeneity was considerable (Mercado 2021). This is in line with our own findings where heterogeneity was high and pooled effects should be interpreted cautiously for BMI for active and inactive comparators, and weight for inactive comparators, and for weight compared to active comparators where there was little or no effect of MBIs. We did not specifically look at the effects of MBIs in participants who were overweight or obese as there was significant heterogeneity in terms of interventions, comparators, participants, and outcomes. We will explore these subgroups with the addition of further studies in future updates of this review.
MBIs targeting diabetes were the subject of three recent systematic reviews (Bersch‐Ferreira 2021; Ngan 2021; Ni 2021). One systematic review included four trials, three of which were included in our review; the fourth was excluded as it was short‐term. The authors found very low‐quality evidence of no differences in fasting blood glucose or HbA1c with MBIs (Bersch‐Ferreira 2021). Another review looked at both MBIs and ACT interventions in type 2 diabetes and found reductions in HbA1C, but there was significant heterogeneity (Ngan 2021). The third review included eight trials of MBIs in type 2 diabetes, all of which we have included in our review. They found reductions in HbA1C, depression, and stress with MBIs but did not pool studies separately for active and inactive comparators. Our review found favourable effects on fasting blood glucose with MBIs compared to both active and non‐active comparators, with little or no effect on HbA1C for active comparators and mixed results for non‐active comparators. We did not specifically look at the effects of MBIs in participants with diabetes as there was significant heterogeneity in terms of interventions, comparators, participants, and outcomes. We will explore these subgroups with the addition of further studies in future updates of this review.
The most recent systematic reviews report findings from MBIs. An earlier overview of eight systematic reviews reports on the effects of TM on blood pressure where they find a clear trend of reductions in blood pressure with TM but caution that there are some conflicting findings and potential risk of bias in many of the included RCTs (Ooi 2017). This broadly supports our findings for TM where there is moderate‐certainty evidence of a reduction in systolic blood pressure compared to active comparators and low‐certainty evidence of reductions in both systolic and diastolic blood pressure compared to inactive comparators; this latter finding is, however, confined to only two trials. Few trials reported on psychological outcomes in the current review and, where reported, there was little to no effect. Fewer trials of TM reported on our secondary outcomes. We explored the effects of primary and secondary prevention in trials of TM compared to active comparators and found no subgroup effects for blood pressure.
This is a very active research area as demonstrated by the very large number of ongoing studies we found meeting our inclusion criteria. It will be important to incorporate these as results are reported in future updates of the review to reduce heterogeneity and uncertainty in the findings.
Authors' conclusions
Implications for practice.
Our review found very little information on the effects of meditation on cardiovascular disease (CVD) clinical endpoints, and limited information on blood pressure and psychological outcomes for people at risk of and with established CVD, with substantial heterogeneity between studies, and the body of evidence was generally of low certainty. Available evidence regarding potential adverse effects of meditation is also limited and uncertain, and there is also uncertainty about the effects of meditation on other CVD risk factors, quality of life, and measures of resilience.
Despite the current uncertainty, this is a very active area of research, with a large number of ongoing studies that are potentially eligible to be included in the evolving evidence base. We will endeavour to update this review imminently to better inform healthcare or policy decisions.
Implications for research.
There remains considerable uncertainty about the effects of meditation on CVD clinical endpoints, blood pressure, and psychological outcomes, as well as other CVD risk factors (such as blood lipids and glycaemic control), quality of life, and coping ability for both primary and secondary prevention of CVD due to a lack of current evidence for clinical outcomes and substantial heterogeneity between studies for others. We attempted to reduce the level of uncertainty due to heterogeneity by analysing results according to the nature of the interventions (mindfulness‐based interventions or transcendental meditation) and comparators (active or inactive) and by primary prevention (participants at high risk of CVD) and secondary prevention (participants with established CVD). However, further analyses will be possible and are warranted with the inclusion of the substantial number of ongoing studies in future updates of this review.
Adequately powered, well‐conducted primary and secondary prevention trials assessing patient‐important outcomes are needed to establish the effects on CVD clinical endpoints, where most research is needed, and to confirm initial findings on blood pressure and psychological outcomes.
History
Protocol first published: Issue 6, 2019
Notes
This is a new Cochrane review, which supersedes the Cochrane review on transcendental meditation for the primary prevention of cardiovascular disease (Hartley 2014). The scope of this new review has broadened considerably and includes other meditation interventions, notably mindfulness‐based interventions, and also examines the effects of these interventions in those with established heart disease and those at risk.
Acknowledgements
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Heart, up to its closure in March 2023. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, the NIHR, the NHS or the Department of Health.
We are very grateful for the additional data and further information provided about their study from Maria Edna de Melo and Renata Bressan (Bressan 2020) from the Grupo de Obesidade e Sindrome Metabolica, Hospital das Clinicas HCFMUSP, Faculdade de Medicina, Universidade de São Paulo.
Editorial and peer reviewer contributions
Cochrane Heart supported the authors in the development of this review until its closure in March 2023. Karen Rees was a member of Cochrane Heart but was not involved in the editorial process or decision‐making for this review. The following people conducted the editorial process for this review:
Sign‐off Editor (final editorial decision): Michael Brown, Michigan State University College of Human Medicine
Managing Editor (selected peer reviewers, collated peer reviewer comments, provided editorial comments/guidance to authors, edited the article): Joey Kwong, Cochrane Central Editorial Service
Editorial Assistant (conducted editorial policy checks, collated peer reviewer comments, supported the editorial team): Leticia Rodrigues, Cochrane Central Editorial Service
Copy Editor (copy editing and production): Jenny Bellorini, Cochrane Central Production Service
Peer reviewers (provided comments and recommended an editorial decision)*: Dulce Estêvão, School of Health, University of Algarve, Faro, Portugal (clinical/content review); Ali AlKhabbaz (clinical/content review); Nuala Livingstone, Cochrane Evidence Production & Methods Directorate (methods review); Ina Monsef, Cochrane Haematology, Department of Internal Medicine, Center for Integrated Oncology, Aachen Bonn Cologne Duesseldorf, Faculty of Medicine; University Hospital Cologne, University of Cologne, German (search review). *One additional peer reviewer provided clinical/content peer review, but chose not to be publicly acknowledged.
Appendices
Appendix 1. Search strategies
CENTRAL
#1 MeSH descriptor: [Meditation] this term only
#2 meditat*
#3 MeSH descriptor: [Mindfulness] this term only
#4 mindful*
#5 (MBCT or MBSR)
#6 #1 or #2 or #3 or #4 or #5
#7 MeSH descriptor: [Cardiovascular Diseases] explode all trees
#8 MeSH descriptor: [Pulmonary Embolism] explode all trees
#9 cardio*
#10 cardia*
#11 heart*
#12 coronary*
#13 angina*
#14 ventric*
#15 myocard*
#16 pericard*
#17 (ischaem* or ischem*)
#18 emboli*
#19 arrhythmi*
#20 thrombo*
#21 atrial fibrillat*
#22 tachycardi*
#23 endocardi*
#24 (sick NEAR sinus)
#25 MeSH descriptor: [Stroke] explode all trees
#26 (stroke or strokes)
#27 cerebrovasc*
#28 cerebral vascular
#29 apoplexy
#30 (brain NEAR/2 accident*)
#31 ((brain* or cerebral or lacunar) NEAR/2 infarct*)
#32 MeSH descriptor: [Hypertension] explode all trees
#33 hypertensi*
#34 peripheral arter* disease*
#35 MeSH descriptor: [Vascular Stiffness] explode all trees
#36 MeSH descriptor: [Plaque, Atherosclerotic] explode all trees
#37 ((high or increased or elevated) NEAR/2 blood pressure)
#38 MeSH descriptor: [Hyperlipidemias] explode all trees
#39 hyperlipid*
#40 (hyperlipaemia* or hyperlipemia*)
#41 hypercholesterol*
#42 (hypercholesteraemia* or hypercholesteremia*)
#43 (hyperlipoproteinaemia* or hyperlipoproteinemia*)
#44 (hypertriglyceridaemia* or hypertriglyceridemia*)
#45 MeSH descriptor: [Arteriosclerosis] explode all trees
#46 MeSH descriptor: [Cholesterol] explode all trees
#47 cholesterol
#48 coronary risk factor*
#49 MeSH descriptor: [Blood Pressure] this term only
#50 blood pressure
#51 ((blood or venous or arterial) NEAR/2 (clot* or emboli* or thrombo*))
#52 MeSH descriptor: [Diabetes Mellitus] explode all trees
#53 diabet*
#54 MeSH descriptor: [Hyperglycemia] explode all trees
#55 (hyperglycaemi* or hyperglycemi*)
#56 (glucose NEAR/2 intoleran*)
#57 MeSH descriptor: [Insulin Resistance] this term only
#58 insulin resistan*
#59 (metabolic NEAR/3 syndrome)
#60 (dysmetabolic NEAR/3 syndrome)
#61 MeSH descriptor: [Smoking] explode all trees
#62 (smoking or smoker* or smoke* or tobacco or cigarette*)
#63 MeSH descriptor: [Obesity] explode all trees
#64 (obese or obesity)
#65 MeSH descriptor: [Overweight] this term only
#66 (overweight or over weight)
#67 {OR #7‐#66}
#68 #6 and #67
MEDLINE Ovid
1 Meditation/
2 meditat*.ti,ab,kf.
3 Mindfulness/
4 mindful*.ti,ab,kf.
5 (MBCT or MBSR).ti,ab,kf.
6 1 or 2 or 3 or 4 or 5
7 exp Cardiovascular Diseases/
8 exp Pulmonary Embolism/
9 cardio*.ti,ab,kf.
10 cardia*.ti,ab,kf.
11 heart*.ti,ab,kf.
12 coronary*.ti,ab,kf.
13 angina*.ti,ab,kf.
14 ventric*.ti,ab,kf.
15 myocard*.ti,ab,kf.
16 pericard*.ti,ab,kf.
17 (ischaem* or ischem*).ti,ab,kf.
18 emboli*.ti,ab,kf.
19 arrhythmi*.ti,ab,kf.
20 thrombo*.ti,ab,kf.
21 atrial fibrillat*.ti,ab,kf.
22 tachycardi*.ti,ab,kf.
23 endocardi*.ti,ab,kf.
24 (sick adj sinus).ti,ab,kf.
25 exp Stroke/
26 (stroke or strokes).ti,ab,kf.
27 cerebrovasc*.ti,ab,kf.
28 cerebral vascular.ti,ab,kf.
29 apoplexy.ti,ab,kf.
30 (brain adj2 accident*).ti,ab,kf.
31 ((brain* or cerebral or lacunar) adj2 infarct*).ti,ab,kf.
32 exp Hypertension/
33 hypertensi*.ti,ab,kf.
34 peripheral arter* disease*.ti,ab,kf.
35 exp Vascular Stiffness/
36 exp Plaque, Atherosclerotic/
37 ((high or increased or elevated) adj2 blood pressure).ti,ab,kf.
38 exp Hyperlipidemias/
39 hyperlipid*.ti,ab,kf.
40 (hyperlipaemia* or hyperlipemia*).ti,ab,kf.
41 hypercholesterol*.ti,ab,kf.
42 (hypercholesteraemia* or hypercholesteremia*).ti,ab,kf.
43 (hyperlipoproteinaemia* or hyperlipoproteinemia*).ti,ab,kf.
44 (hypertriglyceridaemia* or hypertriglyceridemia*).ti,ab,kf.
45 exp Arteriosclerosis/
46 exp Cholesterol/
47 cholesterol.ti,ab,kf.
48 coronary risk factor*.ti,ab,kf.
49 Blood Pressure/
50 blood pressure.ti,ab,kf.
51 ((blood or venous or arterial) adj2 (clot* or emboli* or thrombo*)).ti,ab,kf.
52 exp Diabetes Mellitus/
53 diabet*.ti,ab,kf.
54 exp Hyperglycemia/
55 (hyperglycaemi* or hyperglycemi*).ti,ab,kf.
56 (glucose adj2 intoleran*).ti,ab,kf.
57 Insulin Resistance/
58 insulin resistan*.ti,ab,kf.
59 (metabolic adj3 syndrome).ti,ab,kf.
60 (dysmetabolic adj3 syndrome).ti,ab,kf.
61 exp Smoking/
62 (smoking or smoker* or smoke* or tobacco or cigarette*).ti,ab,kf.
63 exp Obesity/
64 (obese or obesity).ti,ab,kf.
65 Overweight/
66 (overweight or over weight).ti,ab,kf.
67 or/7‐66
68 6 and 67
69 randomized controlled trial.pt.
70 controlled clinical trial.pt.
71 randomized.ab.
72 placebo.ab.
73 drug therapy.fs.
74 randomly.ab.
75 trial.ab.
76 groups.ab.
77 69 or 70 or 71 or 72 or 73 or 74 or 75 or 76
78 exp animals/ not humans.sh.
79 77 not 78
80 68 and 79
Embase Ovid
1 meditation/
2 meditat*.tw.
3 mindfulness/
4 mindful*.tw.
5 (MBCT or MBSR).tw.
6 1 or 2 or 3 or 4 or 5
7 exp cardiovascular disease/
8 exp lung embolism/
9 cardio*.tw.
10 cardia*.tw.
11 heart*.tw.
12 coronary*.tw.
13 angina*.tw.
14 ventric*.tw.
15 myocard*.tw.
16 pericard*.tw.
17 (ischaem* or ischem*).tw.
18 emboli*.tw.
19 arrhythmi*.tw.
20 thrombo*.tw.
21 atrial fibrillat*.tw.
22 tachycardi*.tw.
23 endocardi*.tw.
24 (sick adj sinus).tw.
25 exp cerebrovascular accident/
26 (stroke or strokes).tw.
27 cerebrovasc*.tw.
28 cerebral vascular.tw.
29 apoplexy.tw.
30 (brain adj2 accident*).tw.
31 ((brain* or cerebral or lacunar) adj2 infarct*).tw.
32 exp hypertension/
33 hypertensi*.tw.
34 peripheral arter* disease*.tw.
35 exp arterial stiffness/
36 exp atherosclerotic plaque/
37 ((high or increased or elevated) adj2 blood pressure).tw.
38 exp hyperlipidemia/
39 hyperlipid*.tw.
40 (hyperlipaemia* or hyperlipemia*).tw.
41 hypercholesterol*.tw.
42 (hypercholesteraemia* or hypercholesteremia*).tw.
43 (hyperlipoproteinaemia* or hyperlipoproteinemia*).tw.
44 (hypertriglyceridaemia* or hypertriglyceridemia*).tw.
45 exp arteriosclerosis/
46 exp cholesterol/
47 cholesterol.tw.
48 coronary risk factor*.tw.
49 blood pressure/
50 blood pressure.tw.
51 ((blood or venous or arterial) adj2 (clot* or emboli* or thrombo*)).tw.
52 exp diabetes mellitus/
53 diabet*.tw.
54 exp hyperglycemia/
55 (hyperglycaemi* or hyperglycemi*).tw.
56 (glucose adj2 intoleran*).tw.
57 insulin resistance/
58 insulin resistan*.tw.
59 (metabolic adj3 syndrome).tw.
60 (dysmetabolic adj3 syndrome).tw.
61 exp smoking/
62 (smoking or smoker* or smoke* or tobacco or cigarette*).tw.
63 exp obesity/
64 (obese or obesity).tw.
65 (overweight or over weight).tw.
66 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 or 64 or 65
67 6 and 66
68 random$.tw.
69 factorial$.tw.
70 crossover$.tw.
71 cross over$.tw.
72 cross‐over$.tw.
73 placebo$.tw.
74 (doubl$ adj blind$).tw.
75 (singl$ adj blind$).tw.
76 assign$.tw.
77 allocat$.tw.
78 volunteer$.tw.
79 crossover procedure/
80 double blind procedure/
81 randomized controlled trial/
82 single blind procedure/
83 68 or 69 or 70 or 71 or 72 or 73 or 74 or 75 or 76 or 77 or 78 or 79 or 80 or 81 or 82
84 (animal/ or nonhuman/) not human/
85 83 not 84
86 67 and 85
PsycINFO Ovid
1 Meditation/
2 meditat*.tw.
3 Mindfulness/
4 mindful*.tw.
5 (MBCT or MBSR).tw.
6 1 or 2 or 3 or 4 or 5
7 exp Cardiovascular Disorders/
8 embolisms/
9 cardio*.tw.
10 cardia*.tw.
11 heart*.tw.
12 coronary*.tw.
13 angina*.tw.
14 ventric*.tw.
15 myocard*.tw.
16 pericard*.tw.
17 (ischaem* or ischem*).tw.
18 emboli*.tw.
19 arrhythmi*.tw.
20 thrombo*.tw.
21 atrial fibrillat*.tw.
22 tachycardi*.tw.
23 endocardi*.tw.
24 (sick adj sinus).tw.
25 exp Cerebrovascular Accidents/
26 (stroke or strokes).tw.
27 cerebrovasc*.tw.
28 cerebral vascular.tw.
29 apoplexy.tw.
30 (brain adj2 accident*).tw.
31 ((brain* or cerebral or lacunar) adj2 infarct*).tw.
32 exp Hypertension/
33 hypertensi*.tw.
34 peripheral arter* disease*.tw.
35 exp Atherosclerosis/
36 ((high or increased or elevated) adj2 blood pressure).tw.
37 hyperlipid*.tw.
38 (hyperlipaemia* or hyperlipemia*).tw.
39 hypercholesterol*.tw.
40 (hypercholesteraemia* or hypercholesteremia*).tw.
41 (hyperlipoproteinaemia* or hyperlipoproteinemia*).tw.
42 (hypertriglyceridaemia* or hypertriglyceridemia*).tw.
43 exp Arteriosclerosis/
44 exp Cholesterol/
45 cholesterol.tw.
46 coronary risk factor*.tw.
47 Blood Pressure/
48 blood pressure.tw.
49 ((blood or venous or arterial) adj2 (clot* or emboli* or thrombo*)).tw.
50 exp Diabetes Mellitus/
51 diabet*.tw.
52 exp Hyperglycemia/
53 (hyperglycaemi* or hyperglycemi*).tw.
54 (glucose adj2 intoleran*).tw.
55 insulin resistan*.tw.
56 (metabolic adj3 syndrome).tw.
57 (dysmetabolic adj3 syndrome).tw.
58 exp Tobacco Smoking/
59 (smoking or smoker* or smoke* or tobacco or cigarette*).tw.
60 exp Obesity/
61 (obese or obesity).tw.
62 Overweight/
63 (overweight or over weight).tw.
64 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63
65 6 and 64
66 random$.tw.
67 factorial$.tw.
68 crossover$.tw.
69 cross‐over$.tw.
70 placebo$.tw.
71 (doubl$ adj blind$).tw.
72 (singl$ adj blind$).tw.
73 assign$.tw.
74 allocat$.tw.
75 volunteer$.tw.
76 control*.tw.
77 "2000".md.
78 or/66‐77
79 65 and 78
CINAHL
S78 S65 AND S77
S77 S66 OR S67 OR S68 OR S69 OR S70 OR S71 OR S72 OR S73 OR S74 OR S75 OR S76
S76 TX allocat* random*
S75 (MH "Quantitative Studies")
S74 (MH "Placebos")
S73 TX placebo*
S72 TX random* allocat*
S71 (MH "Random Assignment")
S70 TX randomi* control* trial*
S69 TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) )
S68 TX clinic* n1 trial*
S67 PT Clinical trial
S66 (MH "Clinical Trials+")
S65 S6 AND S64
S64 S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55 OR S56 OR S57 OR S58 OR S59 OR S60 OR S61 OR S62 OR S63
S63 TX (overweight or over weight)
S62 TX (obese or obesity)
S61 (MH "Obesity+")
S60 TX (smoking or smoker* or smoke* or tobacco or cigarette*)
S59 (MH "Smoking+")
S58 TX (dysmetabolic N3 syndrome)
S57 TX (metabolic N3 syndrome)
S56 TX insulin resistan*
S55 (MH "Insulin Resistance")
S54 TX (glucose N2 intoleran*)
S53 TX (hyperglycaemi* or hyperglycemi*)
S52 (MH "Hyperglycemia+")
S51 TX diabet*
S50 (MH "Diabetes Mellitus+")
S49 TX ((blood or venous or arterial) N2 (clot* or emboli* or thrombo*))
S48 TX blood pressure
S47 (MH "Blood Pressure")
S46 TX coronary risk factor*
S45 TX cholesterol
S44 (MH "Cholesterol+")
S43 (MH "Arteriosclerosis+")
S42 TX (hypertriglyceridaemia* or hypertriglyceridemia*)
S41 TX (hyperlipoproteinaemia* or hyperlipoproteinemia*)
S40 TX (hypercholesteraemia* or hypercholesteremia*)
S39 TX hypercholesterol*
S38 TX (hyperlipaemia* or hyperlipemia*)
S37 TX hyperlipid*
S36 (MH "Hyperlipidemia+")
S35 TX ((high or increased or elevated) N2 blood pressure)
S34 TX peripheral arter* disease*
S33 TX hypertensi*
S32 (MH "Hypertension+")
S31 TX ((brain* or cerebral or lacunar) N2 infarct*)
S30 TX (brain N2 accident*)
S29 TX apoplexy
S28 TX cerebral vascular
S27 TX cerebrovasc*
S26 TX (stroke or strokes)
S25 (MH "Stroke+")
S24 TX (sick N sinus)
S23 TX endocardi*
S22 TX tachycardi*
S21 TX atrial fibrillat*
S20 TX thrombo*
S19 TX arrhythmi*
S18 TX emboli*
S17 TX (ischaem* or ischem*)
S16 TX pericard*
S15 TX myocard*
S14 TX ventric*
S13 TX angina*
S12 TX coronary*
S11 TX heart*
S10 TX cardia*
S9 TX cardio*
S8 (MH "Pulmonary Embolism")
S7 (MH "Cardiovascular Diseases+")
S6 S1 OR S2 OR S3 OR S4 OR S5
S5 TX (MBCT or MBSR)
S4 TX mindful*
S3 (MH "Mindfulness")
S2 TX meditat*
S1 (MH "Meditation")
AMED
1 Meditation/
2 meditat*.tw.
3 awareness/
4 mindful*.tw.
5 (MBCT or MBSR).tw.
6 1 or 2 or 3 or 4 or 5
7 exp Cardiovascular disease/
8 exp Pulmonary embolism/
9 cardio*.tw.
10 cardia*.tw.
11 heart*.tw.
12 coronary*.tw.
13 angina*.tw.
14 ventric*.tw.
15 myocard*.tw.
16 pericard*.tw.
17 (ischaem* or ischem*).tw.
18 emboli*.tw.
19 arrhythmi*.tw.
20 thrombo*.tw.
21 atrial fibrillat*.tw.
22 tachycardi*.tw.
23 endocardi*.tw.
24 (sick adj sinus).tw.
25 exp stroke/
26 (stroke or strokes).tw.
27 cerebrovasc*.tw.
28 cerebral vascular.tw.
29 apoplexy.tw.
30 (brain adj2 accident*).tw.
31 ((brain* or cerebral or lacunar) adj2 infarct*).tw.
32 exp Hypertension/
33 hypertensi*.tw.
34 peripheral arter* disease*.tw.
35 ((high or increased or elevated) adj2 blood pressure).tw.
36 exp Hyperlipidemia/
37 hyperlipid*.tw.
38 (hyperlipaemia* or hyperlipemia*).tw.
39 hypercholesterol*.tw.
40 (hypercholesteraemia* or hypercholesteremia*).tw.
41 (hyperlipoproteinaemia* or hyperlipoproteinemia*).tw.
42 (hypertriglyceridaemia* or hypertriglyceridemia*).tw.
43 exp Arteriosclerosis/
44 exp Cholesterol/
45 cholesterol.tw.
46 coronary risk factor*.tw.
47 Blood pressure/
48 blood pressure.tw.
49 ((blood or venous or arterial) adj2 (clot* or emboli* or thrombo*)).tw.
50 exp Diabetes mellitus/
51 diabet*.tw.
52 exp Hyperglycemia/
53 (hyperglycaemi* or hyperglycemi*).tw.
54 (glucose adj2 intoleran*).tw.
55 Insulin Resistance/
56 insulin resistan*.tw.
57 (metabolic adj3 syndrome).tw.
58 (dysmetabolic adj3 syndrome).tw.
59 exp Smoking/
60 (smoking or smoker* or smoke* or tobacco or cigarette*).tw.
61 exp Obesity/
62 (obese or obesity).tw.
63 (overweight or over weight).tw.
64 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63
65 6 and 64
66 randomized controlled trial.pt.
67 controlled clinical trial.pt.
68 randomized.ab.
69 placebo.ab.
70 randomly.ab.
71 trial.ab.
72 groups.ab.
73 66 or 67 or 68 or 69 or 70 or 71 or 72
74 exp animals/ not humans.sh.
75 73 not 74
76 65 and 75
ClinicalTrials.gov
Advanced Search
Search #1
Condition or disease: Heart OR Coronary OR Cardiovascular OR Cardiac OR Angina OR Myocardial OR Ventricular OR Atrial OR Pericardial OR Ischemic OR Ischaemic OR Ischemia OR Ischaemia OR Embolism OR Embolic OR Arrhythmia OR Thrombosis OR Tachycardia OR Endocardial
Study type: Interventional Studies (Clinical Trials)
Intervention/treatment: Meditation OR Mindfulness
Search #2
Condition or disease: Stroke OR Cerebrovascular OR Cerebral OR Apoplexy OR Artery OR Arteries OR Infarction OR Hypertension OR Hypertensive OR Vascular Stiffness OR Atherosclerotic OR Atherosclerosis OR Hyperlipidemia OR Hyperlipidaemia OR Hyperlipaemia OR Hyperlipemia
Study type: Interventional Studies (Clinical Trials)
Intervention/treatment: Meditation OR Mindfulness
Search #3
Condition or disease: Hypercholesterolemia OR Hypercholesterolaemia OR Hypercholesteraemia OR Hypercholesteremia OR Hyperlipoproteinaemia OR Hyperlipoproteinemia OR Hypertriglyceridaemia OR Hypertriglyceridemia OR Arteriosclerosis OR Cholesterol OR Blood Pressure
Study type: Interventional Studies (Clinical Trials)
Intervention/treatment: Meditation OR Mindfulness
Search #4
Condition or disease: Diabetes OR Diabetic OR Hyperglycemia OR Hyperglycaemia OR Glucose Intolerance OR Glucose Intolerant OR Insulin Resistance OR Insulin Resistant OR Metabolic Syndrome OR Smoking OR Smokers OR Tobacco OR Obesity OR Obese OR Overweight OR Over Weight
Study type: Interventional Studies (Clinical Trials)
Intervention/treatment: Meditation OR Mindfulness
WHO ICTRP
Advanced Search
Search #1
Heart OR Coronary OR Cardiovascular OR Cardiac OR Angina OR Myocardial OR Ventricular OR Atrial OR Pericardial OR Ischemic OR Ischaemic OR Ischemia OR Ischaemia OR Embolism OR Embolic OR Arrhythmia OR Thrombosis OR Tachycardia OR Endocardial OR Stroke in the Condition
Meditation OR Mindfulness in the Intervention
Recruitment status is ALL
Search #2
Cerebrovascular OR Cerebral OR Apoplexy OR Artery OR Arteries OR Infarction OR Hypertension OR Hypertensive OR Vascular Stiffness OR Atherosclerotic OR Atherosclerosis OR Hyperlipidemia OR Hyperlipidaemia OR Hyperlipaemia OR Hyperlipemia in the Condition
Meditation OR Mindfulness in the Intervention
Recruitment status is ALL
Search #3
Hypercholesterolemia OR Hypercholesterolaemia OR Hypercholesteraemia OR Hypercholesteremia OR Hyperlipoproteinaemia OR Hyperlipoproteinemia OR Hypertriglyceridaemia OR Hypertriglyceridemia OR Arteriosclerosis OR Cholesterol OR Blood Pressure OR Diabetes in the Condition
Meditation OR Mindfulness in the Intervention
Recruitment status is ALL
Search #4
Diabetic OR Hyperglycemia OR Hyperglycaemia OR Glucose Intolerance OR Glucose Intolerant OR Insulin Resistance OR Insulin Resistant OR Metabolic Syndrome OR Smoking OR Smokers OR Tobacco OR Obesity OR Obese OR Overweight OR Over Weight in the Condition
Meditation OR Mindfulness in the Intervention
Recruitment status is ALL
Data and analyses
Comparison 1. MBIs versus active comparators.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Systolic blood pressure (mmHg), change from baseline | 6 | 388 | Mean Difference (IV, Random, 95% CI) | ‐6.08 [‐12.79, 0.63] |
| 1.2 Diastolic blood pressure (mmHg), change from baseline | 6 | 388 | Mean Difference (IV, Random, 95% CI) | ‐5.18 [‐10.65, 0.29] |
| 1.3 Anxiety, change from baseline | 9 | 438 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.25, 0.13] |
| 1.4 Depression, change from baseline | 11 | 595 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.08, 0.24] |
| 1.5 Perceived stress, change from baseline | 6 | 357 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.45, ‐0.03] |
| 1.6 Well‐being | 1 | 63 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.67, 0.32] |
| 1.7 Total cholesterol (mmol/L), change from baseline | 1 | 47 | Mean Difference (IV, Random, 95% CI) | 0.37 [‐0.10, 0.84] |
| 1.8 LDL cholesterol (mmol/L), change from baseline | 3 | 247 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.13, 0.22] |
| 1.9 HDL cholesterol (mmol/L), change from baseline | 1 | 147 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.00, 0.14] |
| 1.10 Triglycerides (mmol/L), change from baseline | 1 | 147 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.27, 0.07] |
| 1.11 Fasting blood glucose (mmol/L), change from baseline | 2 | 200 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.56, ‐0.25] |
| 1.12 HbA1c (%), change from baseline | 4 | 331 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.09, 0.10] |
| 1.13 Weight (kg), change from baseline | 10 | 552 | Mean Difference (IV, Random, 95% CI) | 0.33 [‐0.78, 1.44] |
| 1.14 BMI (kg/m2), change from baseline | 7 | 322 | Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐1.22, 0.70] |
| 1.15 Smoking cessation | 6 | 1087 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.78, 2.68] |
| 1.16 Quality of life, change from baseline | 1 | 52 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.54, 0.56] |
| 1.17 Self‐efficacy, change from baseline | 3 | 208 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.05, 0.50] |
Comparison 2. MBIs versus non‐active comparators.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Clinical CVD events (CVD mortality and non‐fatal MI) | 1 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.37, 2.42] |
| 2.2 Systolic blood pressure (mmHg), change from baseline | 9 | 379 | Mean Difference (IV, Random, 95% CI) | ‐6.62 [‐13.15, ‐0.10] |
| 2.3 Diastolic blood pressure (mmHg), change from baseline | 9 | 379 | Mean Difference (IV, Random, 95% CI) | ‐3.35 [‐5.86, ‐0.85] |
| 2.4 Anxiety, change from baseline | 9 | 533 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.09, ‐0.47] |
| 2.5 Depression, change from baseline | 15 | 912 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐0.91, ‐0.41] |
| 2.6 Perceived stress, change from baseline | 11 | 708 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐0.89, ‐0.29] |
| 2.7 Well‐being | 2 | 198 | Std. Mean Difference (IV, Random, 95% CI) | 0.50 [0.09, 0.91] |
| 2.8 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.9 Total cholesterol (mmol/L), change from baseline | 3 | 228 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.27, 0.14] |
| 2.10 LDL cholesterol (mmol/L), change from baseline | 2 | 155 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.26, 0.29] |
| 2.11 HDL cholesterol (mmol/L), change from baseline | 2 | 155 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.10, 0.06] |
| 2.12 Triglycerides (mmol/L), change from baseline | 2 | 155 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.53, 0.38] |
| 2.13 Fasting blood glucose (mmol/L), change from baseline | 5 | 459 | Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.75, 0.05] |
| 2.14 HbA1c (%), change from baseline | 12 | 990 | Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.62, ‐0.10] |
| 2.15 Weight (kg), change from baseline | 4 | 140 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐3.06, 2.12] |
| 2.16 BMI (kg/m2), change from baseline | 8 | 390 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐1.54, 0.13] |
| 2.17 Smoking cessation | 2 | 453 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.86, 2.13] |
| 2.18 Quality of life, change from baseline | 10 | 644 | Std. Mean Difference (IV, Random, 95% CI) | 0.53 [0.28, 0.77] |
| 2.19 Self‐efficacy, change from baseline | 5 | 325 | Std. Mean Difference (IV, Random, 95% CI) | 1.08 [‐0.24, 2.40] |
| 2.20 Coping strategies, change from baseline | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 1.63 [0.79, 2.47] |
| 2.21 Self‐management, change from baseline | 1 | 100 | Std. Mean Difference (IV, Random, 95% CI) | 3.70 [3.05, 4.36] |
Comparison 3. TM versus active comparators.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Clinical CVD events (CVD mortality, non‐fatal MI and stroke, revascularisations) | 1 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.56, 1.49] |
| 3.2 Systolic blood pressure (mmHg), change from baseline | 8 | 774 | Mean Difference (IV, Random, 95% CI) | ‐2.33 [‐3.99, ‐0.68] |
| 3.3 Diastolic blood pressure (mmHg), change from baseline | 8 | 774 | Mean Difference (IV, Random, 95% CI) | ‐1.15 [‐2.85, 0.55] |
| 3.4 Anxiety, change from baseline | 3 | 200 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.22, 0.33] |
| 3.5 Depression, change from baseline | 5 | 421 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.31, 0.07] |
| 3.6 Perceived stress, change from baseline | 3 | 194 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.49, 0.57] |
| 3.7 Total cholesterol (mmol/L), change from baseline | 3 | 170 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.28, 0.22] |
| 3.8 LDL cholesterol (mmol/L), change from baseline | 3 | 169 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.26, 0.17] |
| 3.9 HDL cholesterol (mmol/L), change from baseline | 3 | 169 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.07, 0.08] |
| 3.10 Triglycerides (mmol/L), change from baseline | 2 | 122 | Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.50, 0.06] |
| 3.11 Weight (kg), change from baseline | 1 | 86 | Mean Difference (IV, Random, 95% CI) | 0.98 [‐1.49, 3.44] |
| 3.12 BMI (kg/m2), change from baseline | 4 | 423 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.45, 0.59] |
| 3.13 Quality of life, change from baseline | 1 | 41 | Std. Mean Difference (IV, Random, 95% CI) | 0.44 [‐0.19, 1.06] |
Comparison 4. TM versus non‐active comparators.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Systolic blood pressure (mmHg), change from baseline | 2 | 139 | Mean Difference (IV, Random, 95% CI) | ‐6.34 [‐9.86, ‐2.81] |
| 4.2 Diastolic blood pressure (mmHg), change from baseline | 2 | 139 | Mean Difference (IV, Random, 95% CI) | ‐5.13 [‐9.07, ‐1.19] |
| 4.3 Anxiety, change from baseline | 1 | 112 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐1.09, ‐0.32] |
| 4.4 Depression, change from baseline | 1 | 112 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐0.86, ‐0.11] |
| 4.5 Coping strategies, change from baseline | 1 | 207 | Std. Mean Difference (IV, Random, 95% CI) | 0.42 [0.14, 0.70] |
4.5. Analysis.

Comparison 4: TM versus non‐active comparators, Outcome 5: Coping strategies, change from baseline
Comparison 5. Subgroup analysis by primary and secondary prevention ‐ MBIs versus non‐active comparators.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Systolic blood pressure (mmHg), change from baseline | 9 | 379 | Mean Difference (IV, Random, 95% CI) | ‐6.62 [‐13.15, ‐0.10] |
| 5.1.1 Primary prevention | 5 | 237 | Mean Difference (IV, Random, 95% CI) | ‐6.90 [‐16.97, 3.17] |
| 5.1.2 Secondary prevention | 4 | 142 | Mean Difference (IV, Random, 95% CI) | ‐5.50 [‐9.82, ‐1.19] |
| 5.2 Diastolic blood pressure (mmHg), change from baseline | 9 | 379 | Mean Difference (IV, Random, 95% CI) | ‐3.35 [‐5.86, ‐0.85] |
| 5.2.1 Primary prevention | 5 | 237 | Mean Difference (IV, Random, 95% CI) | ‐3.38 [‐6.99, 0.22] |
| 5.2.2 Secondary prevention | 4 | 142 | Mean Difference (IV, Random, 95% CI) | ‐3.46 [‐6.84, ‐0.09] |
| 5.3 Anxiety, change from baseline | 9 | 533 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.09, ‐0.47] |
| 5.3.1 Primary prevention | 5 | 407 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐0.81, ‐0.41] |
| 5.3.2 Secondary prevention | 4 | 126 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.32 [‐2.27, ‐0.36] |
| 5.4 Depression, change from baseline | 15 | 912 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐0.91, ‐0.41] |
| 5.4.1 Primary prevention | 11 | 786 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐0.78, ‐0.30] |
| 5.4.2 Secondary prevention | 4 | 126 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐2.00, ‐0.40] |
| 5.5 Perceived stress, change from baseline | 10 | 598 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐0.96, ‐0.27] |
| 5.5.1 Primary prevention | 7 | 470 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.77, ‐0.18] |
| 5.5.2 Secondary prevention | 3 | 128 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.23 [‐2.40, ‐0.07] |
Comparison 6. Subgroup analysis by primary and secondary prevention ‐ TM versus active comparators.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Systolic blood pressure (mmHg), change from baseline | 8 | 774 | Mean Difference (IV, Random, 95% CI) | ‐2.33 [‐3.99, ‐0.68] |
| 6.1.1 Primary prevention | 5 | 464 | Mean Difference (IV, Random, 95% CI) | ‐1.39 [‐3.33, 0.55] |
| 6.1.2 Secondary prevention | 3 | 310 | Mean Difference (IV, Random, 95% CI) | ‐4.06 [‐8.46, 0.33] |
| 6.2 Diastolic blood pressure (mmHg), change from baseline | 8 | 774 | Mean Difference (IV, Random, 95% CI) | ‐1.15 [‐2.85, 0.55] |
| 6.2.1 Primary prevention | 5 | 464 | Mean Difference (IV, Random, 95% CI) | ‐1.64 [‐3.92, 0.64] |
| 6.2.2 Secondary prevention | 3 | 310 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐3.31, 3.38] |
| 6.3 Anxiety, change from baseline | 3 | 200 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.22, 0.33] |
| 6.3.1 Primary prevention | 2 | 116 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.30, 0.43] |
| 6.3.2 Secondary prevention | 1 | 84 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.39, 0.47] |
| 6.4 Depression, change from baseline | 5 | 421 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.31, 0.07] |
| 6.4.1 Primary prevention | 2 | 116 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.56, 0.17] |
| 6.4.2 Secondary prevention | 3 | 305 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.31, 0.14] |
| 6.5 Perceived stress, change from baseline | 3 | 194 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.49, 0.57] |
| 6.5.1 Primary prevention | 2 | 153 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.37, 0.83] |
| 6.5.2 Secondary prevention | 1 | 41 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐1.06, 0.19] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alamout 2020.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Participants were selected from overweight women referring to the Nutrition and Diet Therapy Clinic affiliated to Shahid Beheshti University of Medical Sciences, Iran. Recruitment dates not reported. Inclusion criteria: Women aged 30 to 50 with BMI between 25 and 29.9 Exclusion criteria: Pregnancy, lactating or menopausal, cancer, hepatic, renal, thyroid, gastrointestinal disease, psychiatric disorders, weight‐loss surgery, weight loss over the past 6 months, use of herbs and medications to suppress appetite, consumption of vitamin‐mineral supplements. Participants were also excluded for non‐attendance of 2 or more sessions, divorce and death of one of the relatives during the study, taking medications for certain physical illness, or adhering to a special diet. Number eligible not reported. 15 randomised to the intervention group (mean age not reported, 0% men), 15 to the diet comparison group (mean age not reported, 0% men) and 15 to the no intervention comparison group (mean age not reported, 0% men). Medications at baseline: Not reported Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 8 weeks, setting not reported): MBCT plus diet therapy. Weekly sessions for 2 hours, schedule for each session as follows: 1) Introductions, aim of programme, concepts and usages of MBCT, conscious breathing and scan body, home practice. 2) To practise eating based on mindfulness and conscious breathing. 3) To practise conscious “see” or “hear”, sitting meditation, the benefits of meditation and its effects, walking with awareness. 4) Practising mindfulness‐based eating, sitting meditation, awareness breathing, shared experiences. 5) Mindfulness‐based attention to hard and painful thoughts, conscious breathing and mental imaging. 6) Practising mindfulness‐based walking and eating, sitting meditation. 7) Sitting meditation, conscious breathing, body scanning, exchange of ideas and to express feelings by participants and to prepare for the end of the course. 8) Reviewing past sessions and summarising, focusing on the future, reminding of aims. Diet therapy: delivered by a trained nutritionist. Participants adhered to a diet plan involving eating 800 kcal below the total daily dietary intake, which was assessed on 3 days using 24‐h recall questionnaires (2 week days and 1 weekend). To create a variety in the diet while maintaining its general principles, all the participants were given a dietary exchange list. They were asked to visit the nutritionist every 2 weeks for further control and counselling. Comparison (duration 8 weeks, setting not reported): Comparison 1 ‐ diet therapy (as above) Comparison 2 ‐ no intervention |
|
| Outcomes | Follow‐up at 12 weeks: SBP, DBP, weight, BMI | |
| Notes |
Country: Iran Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors. Declarations of interest: The authors declared no conflict of interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly stratified according to their BMI via a permuted block randomisation using Random Allocation Software (RAS), and then assigned to 3 groups of 15. |
| Allocation concealment (selection bias) | Low risk | Used Random Allocation Software (RAS) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information, but blinding unlikely. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry number identified to check. |
| Other bias | Unclear risk | Insufficient information to judge |
Alsubaie 2020.
| Study characteristics | ||
| Methods | Parallel‐group RCT – feasibility study | |
| Participants | The recruitment process was conducted through 3 resources: primary care (GPs), specialised services (the cardiology department at Royal Devon and Exeter Hospital), and in the community in Exeter, UK, via distributed materials, between July and October 2014. Inclusion criteria: Adults aged 18 and older with a cardiovascular disorder (heart condition, stroke, or hypertension). Participants also needed to have either a history of clinical depression (major depression disorder, minor depression, or dysthymia) and/or current minor depression with or without anxiety symptoms. Exclusion criteria: Excluded those who met the criteria for a current episode of major depression disorder. Other exclusion criteria were comorbid diagnoses of current substance dependence or abuse, organic brain damage, current or past psychosis, persistent antisocial behaviour, persistent self‐injury, and formal concurrent psychotherapy. Number eligible 59; 33 randomised to 2 intervention groups (11 in each group, mean age and % men not reported) and 1 comparison group (N = 11, mean age and % men not reported Medications at baseline: Medication at baseline not reported Medication change during the trial not reported |
|
| Interventions |
Intervention(s) (duration 8 weeks, setting – the AccEPT Clinic/Mood Disorders Centre at the University of Exeter): Adapted‐MBCT (HeLM) MBCT is a programme outlined by Segal et al. (Segal 2013) and developed based on MBSR. It comprises an individual orientation session and eight weekly 2.5‐h group sessions. MBCT entails extensive mindfulness practices as well as cognitive‐behavioural exercises. It is designed to help people become aware of problematic styles of thinking and reacting to decentre from these and respond more adaptively at times of a potential depressive relapse. The MBCT‐HeLM manual maintained the essential structure and content of the original MBCT manual, but the focus and themes were reoriented to those that characterise people with low mood and CVDs. For example, participants were oriented to turning towards bodily experiences associated with CVD, with curiosity, friendliness and care. Throughout the programme, there was a greater emphasis on mental and physical self‐care. Participants were asked to complete a daily home practice diary 6 days per week and they were given mindfulness CDs to guide this practice. They were also invited to a long‐day practice after session 6 to make sure that those in both groups were receiving the same dose of MBCT‐HeLM and MBSR. The participants were asked to continue with treatment as usual (their normal clinical care). Standard MBSR MBSR (Kabat‐Zinn 2013) consists of eight weekly 2.5‐h group sessions and includes a full day of practice. MBSR comprises extensive formal and informal mindfulness‐based exercises (e.g. body scan, breathing awareness, mindful yoga, mindful eating and mindful walking). The intention is to develop awareness and a new relationship with experience characterised by present moment‐focus, approach orientation, compassion, understanding and equanimity. Participants were asked to complete a daily home practice diary 6 days per week, and given mindfulness CDs to guide this practice. They were also asked to continue with treatment as usual (their normal clinical care). Comparison (duration 20 weeks, healthcare settings): Treatment as usual group. Participants were asked to continue their normal clinical care. Treatment as usual could include psychiatric treatment, outpatient consultation, routine visits to the GP and support programmes from the mental health or cardiac nurse. These participants were offered standard MBCT service AccEPT Clinic at the Mood Disorders Centre after completing the follow up assessment. Due to small numbers have compared HeLM with TAU rather than both MBIs versus TAU where the N for TAU would need to be divided by 2 to prevent double counting. |
|
| Outcomes | Follow‐up 20 weeks intervention: SBP, DBP, anxiety, depression, HRQoL. | |
| Notes |
Country: UK Funding: This research was, in part, supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care South West Peninsula. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. Also, the research was carried out as part of a PhD funded by King Saud University, Saudi Arabian Ministry of Higher Education. Declarations of interest: WK is Director of the Oxford Mindfulness Centre and until 2015 was an unpaid Director of the Mindfulness Network Community Interest Company. He is the Principal Investigator of several externally funded projects evaluating the efficacy of MBCT. AE is co‐director of the Mindfulness Network Community Interest Company and teaches nationally on MBCT. The other authors declare that they have no conflict interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Low risk | "Participants were randomised using sealed envelopes to conceal allocation. Blocking was used with randomly permuted block sizes in a non‐systematic sequence. Randomisation of participants was stratified to ensure balance between the three trial arms based on severity of depression (based on PHQ‐9 cutoff) and type of cardiovascular disorder (heart conditions, stroke and hypertension). The randomisation process was conducted by an independent researcher after the baseline assessment." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Participants were informed about their allocation status by post." We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "It was not possible for the lead researcher (MA) to be blind at the post‐intervention and follow up assessments." We have left this as unclear as the outcomes assessed are either objective or self‐reported by participants, so unlikely to be affected by blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No dropouts from the HeLM intervention, 1/11 from MBSR, and 4/11 for treatment as usual |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry found to check |
| Other bias | Unclear risk | "Regarding the quantitative results, it is important to emphasise that we did not set out to establish effectiveness, nor was it sufficiently powered to do so." |
An 2021.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Participants were recruited from the UCLA campus, medical centre, and surrounding communities through flyers and online postings. The study was conducted between 1 August 2017 and 1 August 2018. Inclusion criteria: People with elevated blood pressure, with or without prescribed antihypertensive medications Exclusion criteria: Current pregnancy or lactation, substance abuse, chemotherapy, currently practising meditation or yoga, enrolled in weight loss programmes or other behavioural interventions, unable to commit to the length of the study, uncontrolled psychiatric problems, inability to speak or read English Number eligible 51; 26 randomised to the intervention group (mean age 58, 30% men) and 20 to the comparison group (mean age 64, 19% men) Medications at baseline: Antihypertensive therapy at baseline in 75% of the intervention group and 81% of the comparison group Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 6 weeks, setting ‐ the Mindful Awareness Research Centre (MARC) at UCLA): The Mindful Awareness Programme (MAP) classes are taught by MARC‐certified instructors. The 6‐week, 2‐hour weekly series provides fundamentals of mindfulness practice, including developing a personal practice and applying it during daily activities. Each session is a combination of lecture, practice, group feedback, and class discussion, facilitated by the MAP instructor. The MAP includes mindful walking and mindful eating, so there is minimal physical activity involved. All MAP participants received a book on mindfulness practice. They also received audio recordings containing guided mindfulness practices, ranging from 5 to 20 minutes, for use at home. The MAP instructor encouraged a daily practice of mindfulness, starting with 5 minutes and increasing to 20 minutes by week 5. The MAP group received the same health information that was part of the Health Promotion Programme (HPP). Comparison (duration 6 weeks, setting not reported): The HPP programme is held for 1 hour per week for 6 weeks, consisting of health educational sessions that are publicly available through the Office of Disease Prevention and Health Promotion (https://health.gov/). The sessions are called “Eat Healthy, Be Active Community Workshops”. These educational materials follow the Dietary Guidelines for Americans and the Physical Activity Guidelines for Americans published by the US Department of Health and Human Services. The 1‐hour class includes lectures, group feedback, and discussion, as well as food tasting demonstrations. A registered nurse provided the weekly educational sessions. |
|
| Outcomes | Follow‐up at 12 weeks: SBP, DBP | |
| Notes |
Country: USA Funding: This study was supported by the NIH Ruth L. Kirschstein National Research Service Award (1F31NR017350). Additional support was provided by the STTI/Gamma Tau‐at‐Large Research Award, and the UCLA Women's Cardiovascular Centre. Declarations of interest: The authors declare no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States randomised in title, abstract, and trial registry but no details regarding method and the following statement suggests this may have been inadequate: "Recruitment was conducted in blocks (cap of 15 participants) with participants assigned in sequence to the next upcoming MAP program and Health Promotion Program (HPP). Hence, the group assignments were dependent on the class schedules". |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Analysed only those who attended the programmes, so effective losses were 23% in the intervention group and 15% in the control group. |
| Selective reporting (reporting bias) | Low risk | Trial registry checked (NCT03924531) and all outcomes listed are reported on. |
| Other bias | Unclear risk | Insufficient information to judge |
Armani Kian 2018.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Recruited from an endocrine outpatient clinic at Imam Hospital in Iran between April 2015 and June 2016 Inclusion criteria: Adult patients with T2DM Exclusion criteria: Patients suffering from other chronic diseases, except hypertension, and patients with a history of treatment for psychiatric disorders or substance‐related problems were excluded from this research. Number eligible not reported; 30 randomised to the intervention group (mean age 53.5, 27.5% men) and 30 to the comparison group (mean age 59, 10% men) Medications at baseline: Metformin at baseline in 31% of the intervention group and 50% of the comparison group. Sulfonylureas at baseline in 17% of the intervention group and 10% of the comparison group. Antidiabetic combination at baseline in 51% of the intervention group and 40% of the comparison group. Medication change during the trial not reported |
|
| Interventions |
Intervention (MBSR duration 8 weeks, setting not reported): 8 weekly sessions of MBSR. All mindfulness sessions were supervised by a certified instructor with at least 3 years professional experience. Comparison (duration 8 weeks, setting not reported) The control group continued their treatment as 8‐weekly usual visits. |
|
| Outcomes | Follow‐up at 12 weeks: anxiety, depression, FPG, HbA1c, HRQoL | |
| Notes |
Country: Iran Funding: not reported Declarations of interest: The authors declare that they have no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The sampling method is available through the referral's referral centre to Imam Khomeini Hospital's Endocrine Clinic, which is blind assigned to two experimental and control groups by coding and randomising the patients." |
| Allocation concealment (selection bias) | Unclear risk | "The sampling method is available through the referral's referral centre to Imam Khomeini Hospital's Endocrine Clinic, which is blind assigned to two experimental and control groups by coding and randomising the patients." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Not blinded." We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Not blinded." Reported in trial registry. Unclear if this relates to participants, personnel, or outcome assessors, or all three. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "Sixty patients with type 2 diabetes participated in the study. Thirty patients (27 females and 3 male) were in the control group, and 30 patients (22 females and 8 male) were in the MBSR intervention group. A female patient in the intervention group resigned after the first session." No further details of any dropouts. |
| Selective reporting (reporting bias) | Low risk | Outcomes in trial registry record are all reported (https://en.irct.ir/trial/21551). |
| Other bias | Unclear risk | Insufficient information to judge |
Baldo 2021.
| Study characteristics | ||
| Methods | Parallel‐group RCT ‐ pilot study | |
| Participants | Participants were recruited from a stroke database at VA Northern California Health Care System and included both Veterans and non‐Veterans. Recruitment dates not reported. Inclusion criteria: History of a single, chronic right or left hemisphere stroke (≥ 3 months post‐onset so that residual symptoms had stabilised), age between 20 and 80, and native English proficiency Exclusion criteria: Mini‐Mental State Examination score < 19 (which would suggest moderate‐severe cognitive impairment); pre‐morbid neurologic history (e.g. dementia, Parkinson’s disease); history of severe psychiatric disorder (e.g. schizophrenia, bipolar disorder); recent substance abuse/dependence disorder (within 1 year); acutely suicidal; concurrent involvement in another rehabilitation programme; moderate‐severe aphasia; and significant visual or hearing disabilities that would preclude effective participation Number eligible 77, 42 contacted; 16 randomised to the intervention group (mean age 64.8, 69% men) and 16 to the comparison group (mean age 67.3, 69% men) Medications at baseline: Not reported Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 8 weeks, setting not reported): The MBSR class was a standard University of Massachusetts MBSR course, which includes an introductory session followed by 8 weeks of classes (Kabat‐Zinn 1990). The class was led by a trained and certified MBSR instructor with over 10 years of experience. The MBSR class met once per week for 2.5 hours, with a day‐long retreat in the 6th week of the 8‐week programme. Comparison (duration 8 weeks, setting not reported): Active control intervention, a Brain Health education class. This class was matched to the MBSR intervention with respect to the number of class hours (2.5 hours/week) schedule (8‐week class plus an introductory session and a day‐long retreat), class size (7 to 10 participants), instructor, and homework. The Brain Health class was a modification of an existing VA education class for brain‐injured individuals. The class provided background and education about brain‐behaviour relationships and discussed how brain injuries can disrupt various aspects of cognition, such as memory and attention. There were also units on nutrition, sleep, and strategies for successful ageing. |
|
| Outcomes | Follow‐up at 20 weeks: anxiety and depression | |
| Notes |
Country: USA Funding: This research was supported by VA Rehabilitation R&D Merit Review Project 5I21RX001893 to Dr. Baldo from the United States (U.S.) Department of Veterans Affairs Rehabilitation Research and Development Service. Declarations of interest: The authors declare no competing interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization assignments were generated by the study statistician with age and gender as blocking factors (male vs. female and < 65 vs. ≥ 65)". |
| Allocation concealment (selection bias) | Low risk | "Random assignments were placed in sealed numbered envelopes by the statistician, marked with the combined factor group, and opened in sequence by study staff as participants were recruited". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "It was not possible to have a true double‐blind trial since participants were informed about the course content during the first session of the class". Authors attempted to minimise participant bias by informing them at the outset that they were being randomised to one of two Brain Health and Wellness classes taught previously to compare their usefulness, and instructed participants not to speak to participants in the other class about the course material. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blind to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 19% and 25% loss to follow‐up in the intervention and control groups, respectively. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the protocol are reported on. |
| Other bias | Unclear risk | Insufficient information to judge |
Baylan 2019.
| Study characteristics | ||
| Methods | Parallel‐group RCT – feasibility study | |
| Participants | Participants were recruited from acute stroke units within NHS Greater Glasgow and Clyde to have baseline assessments within 4 weeks of stroke onset. The Scottish Stroke Research Network nurses approached potential participants at the ward who were enrolled between 12 January 2015 and 28 January 2016. Inclusion criteria: Native English‐speaking adults (aged ≥ 18, upper age limit of 80 for the first 11 months of recruitment) in the acute phase (≤ 14 days post‐stroke) following clinically and/or radiologically (CT and/or MRI) confirmed diagnosis of ischaemic stroke Exclusion criteria: Comorbid progressive neurological or neurodegenerative condition, major psychiatric disorder (pre‐stroke history of mood disorder or stable antidepressant medication did not lead to exclusion), history of major substance abuse problems, clinically unstable, unable to give informed consent or unable to co‐operate with the study protocol (e.g. due to severe aphasia, uncorrected impairment of hearing or vision). Co‐recruitment with intervention studies with potential impact on mood/cognition was not allowed. Number eligible not reported, 243 were approached, 89 consented, 72 randomised; 23 randomised to the intervention group (mean age 65.3, 56.5% men), 24 to the music listening comparison group (mean age 61.1, 70.8% men) and 25 to the audiobook listening comparison group (mean age 65.7, 60% men) Medications at baseline: Medications at baseline not reported Medication change during the trial not reported |
|
| Interventions | One intervention mindful music listening, and 2 comparison groups, music listening and audiobook listening. All interventions followed a manualised 8‐week programme developed by the research team and delivered by an assistant psychologist on an individual basis. Participants were given an iPod Nano (7th Generation, Apple Inc.) and asked to listen to their material daily on their own for at least an hour during the intervention phase (target, 56 hours over 8 consecutive weeks). They were also asked to keep a written daily record of listening through which adherence to the interventions was measured. Intervention (duration 8 weeks, setting not reported): The mindful‐music participants were introduced to the concept of mindfulness at the first visit and given a recording containing a brief mindfulness exercise (Body scan) to complete daily prior to music listening (weeks 1 to 3). The brief (5 min) mindfulness exercises focused on key elements of mindfulness (e.g. paying attention to the present moment). If participants were to notice any thoughts or sensations arising either during the brief exercise or during subsequent music listening, they were to allow them to pass and to gently bring their attention back to the exercise/music. Seven more weekly visits followed during which progress was monitored, listening records collected and additional material provided as required. At the fourth visit, another brief exercise (Following the breath) was provided for use over the following 3 weeks (weeks 4 to 6). For the last 2 weeks, participants could choose which exercise to complete. The 2 music groups therefore differed by the virtue of attentional control, with the mindful‐music group asked to return their attention back to the music whenever their mind had wandered, with no specific listening instructions given to the music‐only group. At the final visit, plans for listening post‐intervention were discussed and CD/recording of the mindfulness exercises given to the mindful‐music group. Comparison(s) (duration 8 weeks, setting not reported): Participants selected their preferred music/audiobooks from any genre with the therapist keeping a record of the genres given at each visit. Music listening used as the comparator in analyses. |
|
| Outcomes | Follow‐up at 26 weeks: anxiety, depression | |
| Notes |
Country: Scotland Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by the Dunhill Medical Trust, grant R432/0214. Additional support from Scottish Executive Chief Scientist Office (TQ/BC), Stroke Association (TQ) and The Dr Mortimer and Theresa Sackler Foundation (BC/SB). Declarations of interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Following completion of the baseline assessments, stratified randomization with blocking (block size 3, allocation ratio 1:1:1) was used to allocate participants to groups based on: (i) recruitment location, (ii) type of stroke (cortical vs. subcortical), and (iii) recurrence (first vs. recurrent stroke). Randomization was via an automated telephone system." |
| Allocation concealment (selection bias) | Low risk | "Randomization was via an automated telephone system." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Being a single blind study, participants were not blind to intervention group allocation." We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Follow‐up assessments of mood and cognition using parallel versions where available (Table 1) were completed post‐intervention and at six months post stroke by an assessor blind to group allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Higher losses to follow‐up in the mindfulness group (30.4% versus 8.3% in the music only group and 12% in the audio group). Authors state that this was not statistically significant (P = 0.13) and that "withdrawals due to ill‐health did not appear to be related to the interventions but to other health problems or the effects of stroke (e.g. feeling overwhelmed after returning home from hospital)." |
| Selective reporting (reporting bias) | Low risk | Checked trial register record: https://clinicaltrials.gov/ct2/show/NCT02259062. All outcomes listed are reported in either Baylan 2019 or Baylan 2018 (qualitative results). |
| Other bias | Unclear risk |
|
Beauchamp 2020.
| Study characteristics | ||
| Methods | Parallel‐group RCT, Pilot study | |
| Participants | No details on recruitment or recruitment dates Inclusion criteria: Stroke survivors will be included if they speak English, can provide written informed consent, have a history of ischaemic stroke, haemorrhagic, or transient ischaemic attack within the past 8 weeks, have a Center for Epidemiologic Studies‐Depression (CES‐D) total score of 16 or greater suggestive of clinically significant depressive symptoms, and currently live at home. If patients are taking antidepressant therapy the dose must have been stable for at least 1 month prior to recruitment into the study. Exclusion criteria: Stroke survivors will be excluded if they currently reside outside of the home (e.g. rehabilitation centre). Stroke survivors will be excluded if they have severe cognitive impairment (Montreal Cognitive Assessment (MoCA) cut‐off score of 20), active psychosis, or bipolar disorder; if they currently abuse substances; or if they are acutely suicidal. Those currently receiving formal psychotherapy or currently engaging in self‐identified meditation practices will also be excluded. Number eligible not reported; 27 randomised to the intervention group (mean age 57.9, 36% men) and 14 to the comparison group (mean age 56.3, 38% men) Medications at baseline: Not reported Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 12 weeks, setting not reported): 4 group sessions of breath‐based meditation over 4 weeks, as well as meditation educational materials Comparison (duration 12 weeks, setting not reported): Expressive writing, no further details |
|
| Outcomes | Follow‐up at 12 weeks: anxiety, depression | |
| Notes |
Country: USA Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single blinding (outcome assessor). We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor was blind. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 39% completed the study, which suggests high attrition, but assessment completion rate for the intervention was 96%. |
| Selective reporting (reporting bias) | Unclear risk | Trial registry also reports inflammatory markers. Findings are reported in a conference proceeding. |
| Other bias | Unclear risk | Few details as conference proceeding |
Blevins 2009.
| Study characteristics | ||
| Methods | Parallel‐group RCT ‐ pilot study | |
| Participants | Female students recruited from the University of Florida through advertisements placed around campus and through student website announcements. Recruitment dates not reported. Inclusion criteria: Women eligible for inclusion were between the ages of 18 and 25 and had a BMI greater than 25. Exclusion criteria: Women were excluded from participation if they were currently involved in a commercial diet programme, were planning to relocate out of the area, carried a current or past diagnosis of bulimia nervosa, or had a BMI greater than 35. Number eligible 52; 21 randomised to the intervention group (mean age 21, 0% men) and 20 to the comparison group (mean age 21, 0% men) Medications at baseline: Not reported Medication change during the trial not reported |
|
| Interventions | Standard behavioural weight management strategies were presented to participants in both the intervention group and control group. Participants were taught and encouraged to use goal‐setting, self monitoring, stimulus control, problem‐solving, social support, reinforcement strategies, and relapse prevention skills. A treatment manual was distributed to each participant which contained session‐by‐session plans with specific learning objectives, methods to accomplish the objectives, and appropriate self‐monitoring materials. A recommendation of 30 minutes moderate to high intensity physical activity on at least 5 days per week was made. Intervention (duration 8 weeks, setting not reported): In addition to standard behavioural weight loss strategies, participants were also given structured lessons on MBSR (Kabat‐Zinn 1990). Lessons included instruction on traditional mindfulness meditation techniques and guided meditation exercises designed to address specific issues pertaining to weight, shape, and eating‐related self‐regulatory processes such as appetite and satiety. The meditative process was gradually integrated into daily activity primarily related to food craving and eating. A separate treatment manual was developed and distributed which contained session‐by‐session plans with specific learning objectives, methods to accomplish the objectives, and appropriate self‐monitoring materials. Participants were provided with a recorded meditation CD designed to assist them in their weekly meditation practice between sessions. “Mini‐meditations” were also assigned, in which participants were asked to stop for a few moments at key times during the day, particularly during meal and snack times, to practise non‐judgemental awareness of thoughts and feelings. Comparison (duration 8 weeks, setting not reported): Standard behavioural weight management only (described above). In the weight management group, 30 minutes was allocated for check‐in, review, and general problem‐solving, 30 minutes was allocated for didactic training, and 60 minutes was allocated for group activity, discussion, and homework assignment. In the weight management and MBSR group, 30 minutes was allocated for check‐in, another 30 minutes was allocated for didactic training, 15 minutes was allocated for group activity, discussion, and homework assignment, and 45 minutes was allocated for mindfulness training, group activity, and homework assignment. Sessions were conducted once a week for eight weeks. A group in each treatment condition was run on two consecutive week night evenings to minimise conflict with class schedules. Each session lasted approximately 2 hours. The sessions were led by advanced graduate students in clinical and health psychology with experience in behavioural weight loss treatment and undergone structured training in mindfulness meditation. |
|
| Outcomes | Follow‐up at 20 weeks: anxiety, depression, weight, BMI | |
| Notes |
Country: USA Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation scheme was created using an online random sequence generator. |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information but blinding unlikely. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Thirty‐one participants (86%) completed the treatment phase and entered the follow‐up phase of the study. Reasons for dropout were time constraints, lost interest, relocation, or death in the immediate family. The rate of dropout during the treatment phase was not equivalent across conditions and all participants who failed to complete testing at Time 2 were in the SBT condition. |
| Selective reporting (reporting bias) | Unclear risk | Thesis so no protocol found |
| Other bias | Unclear risk | Insufficient information to judge |
Blom 2013.
| Study characteristics | ||
| Methods | Parallel‐group RCT ‐ The HARMONY study | |
| Participants | Participants were recruited from referring physicians, advertisements in local newspapers, and posters at local hospitals. Recruitment dates not reported. Inclusion criteria: Eligible participants were aged 20 to 75 years with mean awake ambulatory systolic or diastolic BP ≥ 135/85 mmHg or mean 24‐hour ambulatory BP ≥ 130/80 mmHg. BP was required to be < 160/100 mmHg on both office and ambulatory measurements. Participants were naive to antihypertensive medication for at least 6 months before the baseline screening visit. Exclusion criteria: Use of antihypertensive within 6 months of the screening ambulatory blood pressure measurements (ABPM). Screening office BP > 180/100 mmHg and ABPM ≥ 160/100 mmHg. Presence of diabetes, secondary hypertension, renal disease, history of heart attack, stroke or transient Ischaemic attack, revascularisation procedure, active malignant disease (except non‐melanoma skin cancer), epileptic seizure 6 months before the screening visit, congestive heart failure, severe liver disease. Pregnancy or lactation period. Participation in a clinical trial in the 3 months prior to the initial screening visit. Planned elective surgery during the study period except for cataract surgery. Number eligible was 162; 50 randomised to the intervention group (mean age 57, 36% men) and 51 to the comparison group (mean age 55, 37% men) Medications at baseline: Antihypertensive medication at baseline was an exclusion criterion. During the trial, 3 participants in the control group started antihypertensive medication. Ambulatory BP data for those started on antihypertensive therapy were censored from the point of antihypertensive initiation onward. |
|
| Interventions |
Intervention (duration 9 weeks, setting ‐ all MBSR therapy is conducted at the University Health Network’s Toronto General Hospital, Ontario): MBSR was delivered by 2 trained therapists to groups of 25 to 30 individuals. It consisted of 10 sessions over 9 weeks (introduction, 8 weekly 2.5‐hour sessions, and a 6‐hour session/silent retreat). Participants agreed to complete 45 minutes of homework meditation practice per day, which included practising the various techniques learned during formal class. MBSR focuses on 4 major therapeutic elements: formal meditation, informal mindfulness practice, psycho‐education activities, and self‐monitoring/reflection exercises. These therapeutic elements are explored through activities including, but not limited to, gentle stretching and mindful yoga, a meditative body scan, mindful breathing, and mindful walking. Comparison (duration 12 weeks, setting not reported) Wait list control |
|
| Outcomes | Follow‐up at 12 weeks: 24‐hour ambulatory blood pressure, awake blood pressure, night‐time blood pressure, 24‐hour systolic blood pressure | |
| Notes |
Country: Canada Funding: This work was supported by Heart and Stroke Foundation of Ontario (NA 6349). Declarations of interest: The authors declared no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Upon achieving study entry participants were randomised to 1 of 2 study arms by sealed envelope method using a permuted block design. Sealed envelopes were not opened until the participant’s eligibility was confirmed with ambulatory BP results." Method of randomisation not stated. |
| Allocation concealment (selection bias) | Low risk | "Upon achieving study entry participants were randomised to 1 of 2 study arms by sealed envelope method using a permuted block design. Sealed envelopes were not opened until the participant’s eligibility was confirmed with ambulatory BP results." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Patients were not blinded to their randomisation to immediate intervention or wait‐list control status. Staff members who instructed MBSR were not informed of participants’ randomisation status". We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "... study coordinator was not blinded to randomisation status when processing the ambulatory blood pressure monitoring (ABPM)." Unclear rather than high risk as an objective measure. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 8% lost to follow‐up in the intervention group and 19.6% in the control group. Differential loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Results data for some outcomes in the protocol (Blom 2012) are not reported in the main publication or meeting abstracts (e.g. lifestyle or anthropomorphic measurements, psychological questionnaires). |
| Other bias | Unclear risk | Insufficient information to judge |
Bokhari 2021.
| Study characteristics | ||
| Methods | Parallel‐group RCT ‐ pilot study | |
| Participants | Recruited from the outpatient cardiology services of New York Presbyterian Hospital ‐ Columbia University Medical Centre. Recruitment dates not reported. Inclusion criteria: African‐American men and women of any age with documented CHD (previous MI, CABG, PCI or chronic stable angina) Exclusion criteria: Concomitant life‐threatening illness or physical and mental disabilities that would interfere with study participation Number eligible not reported; 19 randomised to the intervention group (mean age 62, 52.6% men) and 18 to the comparison group (mean age 63.7, 66.7% men) Medications at baseline: Statin therapy at baseline in 47.4% of the intervention group and 50% of the comparison group. Antihypertensive therapy at baseline in 84.2% of the intervention group and 100% of the comparison group. Aspirin therapy at baseline in 73.7% of the intervention group and 66.7% of the comparison group. Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 12 weeks, setting ‐ Columbia University Medical Centre): TM plus cardiac rehabilitation or usual care Certified and experienced TM teachers, similar in race and ethnicity to the patient population, delivered the intervention. The core instruction in the TM technique involved a 7‐step course over 6 days, complying with the standard format (Roth 2018). Each session lasted 1 to 1.5 hours. The general format of most sessions was lecture/discussion in both personal one‐on‐one and small‐group formats. Following core instruction in the TM programme, patients attended biweekly follow‐up sessions for the remainder of the 12‐week intervention period. Comparison (duration 12 weeks, setting not reported) Cardiac rehabilitation or usual care |
|
| Outcomes | Follow‐up at 12 weeks: BP, depression, perceived stress, lipids, smoking, BMI, HRQoL | |
| Notes |
Country: USA Funding: This study was supported by a Grant from NIH ‐ National Heart, Lung and Blood Institute, # HL 100386. Declarations of interest: All the authors report that there are no relevant financial conflicts of interest to disclose. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned to treatment groups using stratified randomisation. Stratified variables were gender, enrolment in CR, and whether or not the subject required instructions in Spanish. |
| Allocation concealment (selection bias) | Low risk | The study biostatistician concealed the sequence of allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The investigators, medical providers, CR providers, and data collection staff were all blinded to treatment status. No statement regarding participants or TM teachers. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data collection staff were all blinded to treatment status. Endpoint data were collected at the field site in New York and coded without patient or treatment status identifiers. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Of the 56 participants, 53 completed baseline myocardial blood flow measurements, 43 completed the 12‐week post‐test for resting myocardial blood flow, and 37 completed baseline and post‐test MFR assessment. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry number identified to check. |
| Other bias | Unclear risk | Insufficient information to judge. |
Brach 1992.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Recruited from the Washington DC metropolitan area. The mode of outreach was through local newspapers (advertisements and calendars), and fliers at college campuses, health clinics, employee assistance programmes, and self‐help group meetings (such as Overeaters Anonymous). Dates of recruitment not reported. Inclusion criteria: Female, 18 years and older, have never practised meditation with any regularity (i.e. on a daily basis for more than 6 months), are 20 to 100 lb over ideal weight, binge an average of no less than twice‐weekly, bingeing has occurred for at least 3 months, score at least 25 on the Binge Eating Scale, not seriously bulimic Exclusion criteria: Regularly follow bingeing with vomiting, laxatives or diuretics (more than 10% of bingeing episodes), are also currently actively addicted to alcohol, drugs, etc., have a history of anorexia nervosa, are currently in psychiatric treatment and using anti‐psychotics, lithium, or similar medications; or otherwise exhibit the presence of symptoms suggesting a concurrent diagnosis of psychopathology Number eligible 96; 79 randomised, 28 randomised to the meditation group (mean age not reported, 0% men) and 27 to the comparison group CBT (mean age not reported, 0% men) and 24 to the comparison group no treatment (mean age not reported, 0% men). Mean age overall 40.7 years. Medications at baseline: Not reported Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 8 weeks, setting not reported): Subjects were taught to self‐monitor for high‐risk situations. They were instructed to spend 1 week identifying primary cues (internal or external) that seemed to precede bingeing episodes. They were then guided into the formal meditation, a Buddhist meditation with concentration on sensations and the breath. They were instructed to practise the meditation for at least 15 minutes, twice a day. After this, and immediately following each subsequent meditation during the training classes, they were asked to describe their subjective experiences of the meditation. Subjects were also provided with daily self‐report forms so that they could record meditation times and comment on the quality of their experience. The practice of writing daily descriptions helped to train practitioners to pay closer attention to the nature of their experience. In week 2, the use of "noting" was included as part of the meditation instructions. "Noting", or the labelling of whatever is predominant in the field of awareness, is used to maintain a detached equanimity. After the formal meditation, subjects were introduced to informal meditation, or meeting all activities and experience with the mindful detached awareness of a "compassionate witness". Subjects were then guided through the following contingent meditation sequence: 1) Discrimination of cue when it arises (based largely on what they identified via self‐monitoring during the prior week). 2) Taking several deep diaphragmatic breaths; feeling the body and "releasing tension" with the exhale. 3) Initiation of a 2 to 3 minute mini‐meditation with concentration on the sensations and the breath. 4) Using noting, opening the awareness to whatever arises, and then moving into informal meditation (i.e. mindfully returning to regular activities with the detached positioning of the "compassionate witness"). The process was followed by some group sharing and questions concerning individual experiences. Subjects were encouraged to begin to use contingent meditation whenever they encountered high‐risk situations. Subjects were instructed to continue the regular practice of formal meditation and to complete each sitting by mentally rehearsing contingent meditation. During the second week of the treatment, members of the meditation group met in groups of 4 with their instructor for interviews. During these meetings, the instructor made sure that the subjects had identified high‐risk situations and understood the formal and contingent meditation procedures. Subjects met in group on the third week for practice of formal meditation, more guidance on formal and informal meditation, and rehearsal of contingent meditation. Subjects met in their training groups for the 5 remaining weeks to rehearse contingent meditation and practice formal meditation. There was continued discussion about the practice of formal and informal meditation and its function as a coping strategy in addressing addictive behaviour. Comparison (duration 8 weeks, setting not reported): Cognitive Behaviour Group ‐ Subjects were taught to self‐monitor for high‐risk situations. They were instructed to spend 1 week identifying primary cues (internal or external) that seemed to precede binge episodes. The use of covert verbalisations and images in treating binge‐eating was introduced. Subjects were assisted in selecting phrases and images that they felt would enhance their sense of being in control. Subjects were instructed to covertly practice their phrase(s) and image(s) for a minute or two, twice a day during the first week of training. In week 2, subjects were taught the following process of contingent self‐statements and images: 1) Discrimination of cue when it arises (based largely on what they identified via self‐monitoring during the prior week). 2) Take several deep diaphragmatic breaths. 3) Initiate covert coping self‐statements and images. During the second week, subjects met in groups of 4 with their trainer in order to insure that the instructions were understood and that they had identified their high‐risk situations. When necessary, the trainers helped subjects refine the content of self‐statements and coping imagery. For the remaining weeks, subjects met as a group to rehearse the intervention, ask questions and discuss the particular distorted cognitions that surround binge‐eating. Control ‐ no treatment |
|
| Outcomes | Follow‐up at 14 weeks and 28 weeks: depression, weight, eating self‐efficacy | |
| Notes |
Country: USA Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Subjects were placed in one of three conditions by stratified random assignment. Stratification was for weight, high frequency of bingeing, and use of antidepressants. |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information but blinding unlikely. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | As 17 of the subjects dropped out during the training, the final results are based on a sample of 79 women (78.5%). |
| Selective reporting (reporting bias) | Unclear risk | Thesis so no protocol found to check |
| Other bias | Unclear risk | Insufficient information to judge |
Bressan 2020.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Recruitment by advertisement and study performed from March 2018 to August 2019 at the outpatient clinic of Hospital das Clinicas, São Paulo University Inclusion criteria: Women between the ages of 18 and 50 with a BMI between 30 and 39.9 kg/m2 Exclusion criteria: Male gender; age over 50 or under 18; pregnancy; lactation; menopause; illiterate individuals; individuals not adhering to the project proposal; undergoing bariatric surgery or already on a weight loss programme; endocrine diseases; genetic diseases; heart disease; nephropathy; liver disease; use of medications that cause weight gain; cognitive deficit; active psychiatric illness Number eligible 138; 46 randomised to the intervention group (mean age 37, 0% men) and 49 to the comparison group (mean age 36.6, 0% men). Further intervention group of mindfulness and nutritional guidance not used in this review. Medications at baseline: Not reported Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 7 months, hospital setting): Volunteers participated in 7 monthly group meetings to develop skills for meditation practice and mindful eating techniques (using MB‐EAT). Comparison (duration not reported, hospital setting) Volunteers received traditional nutritional guidance, with a low‐calorie diet plan and guidance on eating behaviour. |
|
| Outcomes | Follow‐up at 7 months: anxiety, depression, stress, SBP, DBP, lipids, weight, BMI, HRQoL | |
| Notes |
Country: Brazil Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Trial registry provided no information. The authors reported by email that randomisation was according to the day of inclusion in the study to prevent contamination between groups, so potential for bias. |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Trial registry provided no information. Authors reported attrition rates by email of 58.7% in the intervention group and 36.7% in the control group (P = 0.030). |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered, but all outcomes listed reported on |
| Other bias | Unclear risk | Insufficient information to judge |
Brewer 2011.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Recruited through flyers and media advertisements offering behavioural treatment for smoking cessation. Recruitment dates not reported. Inclusion criteria: 18 to 60 years of age, smoked 10+ cigarettes/day, had fewer than 3 months of abstinence in the past year, and reported interest in quitting smoking Exclusion criteria: Participants were excluded if they currently used psychoactive medications, had a serious or unstable medical condition in the past 6 months, or met DSM‐IV criteria for other substance dependence in the past year. Number eligible 103; 41 randomised to the intervention group (mean age 46.5, 65.9% men) and 47 to the comparison group (mean age 45.3, 58.7% men) Medications at baseline: Not reported Medication change during the trial ‐ though participants were neither encouraged nor discouraged from using nicotine replacement, 9% in the intervention group and 11% in the comparison group reported some type of nicotine replacement use during the 4‐week treatment period, and this was 9% and 21% respectively during the follow‐up period. |
|
| Interventions | All participants received twice‐weekly group sessions (8 total) that were manualised and delivered by instructors experienced in the intervention Mindfulness Training (MT) or certified in delivering the American Lung Association Freedom From Smoking programme (ALA FFS) for the comparison group. FFS was chosen as an active ‘standard treatment’ comparison condition because it has demonstrated efficacy, is manualised and standards for training and certification of therapists are established, is widely available, and includes components that are well‐matched with MT, but does not include hypothesised mechanism of MT. Both MT and FFS had a quit date at the end of week 2 (session 4) and were matched for length (1.5 h/session). In addition, home practice materials were matched for length (~30 minutes total) and number of tracks (5) on respective CDs. Participants were neither encouraged nor discouraged from using nicotine replacement in either group during active treatment or in the post‐treatment follow‐up phase. Intervention (duration 4 weeks, setting not reported): The MT manual was adapted for active smoking cessation from a previous MT manual for drug relapse prevention. The overarching theme of momentary awareness and acceptance of cravings and affect (e.g. stress, anxiety etc.) was introduced and reinforced in complementary ways throughout the training. The first session introduced participants to the concept of how smoking can become a habituated behaviour triggered by an environmental, physical, or mental stimulus through associative learning. It also explored how cravings feel in the body and how MT can help individuals become more aware of these processes. Session 2 examined how thoughts, emotions, and body sensations become triggers for craving and smoking, and introduced a technique to ‘mindfully’ work with cravings (Recognise, Accept, Investigate, and Note what cravings feel like as they arise, acronym: RAIN). Session 3 introduced how difficult emotions perpetuate smoking as well as loving‐kindness meditation as a way to work with them. Session 4 (quit date) taught participants how cravings thwart long‐term goals, and reinforced mindfulness techniques as a way to help individuals disengage from habitual responding and realign with their goals. Session 5 introduced participants to mindfulness practice in everyday life, including “awareness of breath” meditation and mindful walking. Session 6 explored the automaticity of thought, and how thoughts can lead to habitual behaviours. Session 7 reinforced the concept of acceptance and its role in changing habits. It also explored how both mental and physical actions can “plant seeds” for future actions and habits. Session 8 summarised the course tools and explored ways of maintaining these in the future. Home practice was suggested after each session as a combination of formal MT meditations and informal practices. Each participant received a meditation practice CD. Comparison (duration 4 weeks, setting not reported): FFS was delivered over 4 weeks (twice‐weekly) instead of the standard 8 weeks. The programme covered behaviour modification, stress reduction, and relapse prevention, and was divided into 3 stages: preparation, action, and maintenance. In the preparation stage (sessions 1 to 3), participants examined smoking patterns through self‐monitoring, identified triggers, and developed a personalised quit plan. On quit day (session 4), participants affirmed their decision to quit and identified specific coping strategies. During the maintenance stage, participants identified ways to remain smoke‐free and maintain a healthy lifestyle (e.g. weight management, exercise, relapse prevention), and continued to discuss the importance of social support and relaxation strategies. Home practice was suggested after each session, typically as a combination of formal (e.g. practising guided relaxation techniques) and informal (e.g. “packtracks”) techniques. Each participant received a practice CD of cessation techniques. |
|
| Outcomes | Follow‐up at 17 weeks: 7‐day point prevalence abstinence | |
| Notes |
Country: USA Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated urn randomisation program assigned participants to MT or FFS based on age, sex, race, and cigarettes smoked/day. |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information, but blinding unlikely. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only 1 participant was excluded from ITT analysis because of incarceration. This participant was in the FFS group. In the FFS group, 8 participants were randomised but did not complete baseline assessment or start treatment and 32 completed treatment. In the MT group, 8 participants were randomised but did not complete baseline assessment or start treatment and 29 completed treatment. ITT analysis used for some but not all analyses. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry number identified to check |
| Other bias | Unclear risk | Insufficient information to judge |
Carpenter 2019.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Enrollees from 5 employers of the phone‐based employer‐sponsored behavioural weight loss programme, Weight Talk (WT), between August 2014 and June 2015. The 5 employers represented a range of blue‐ and white‐collar industries, included large and small companies, and included participants living across the United States. Inclusion criteria: 18 years or older, BMI between 25 kg/m2 and 35 kg/m2, high emotional eating scores, had regular access to email and the Internet Exclusion criteria: Pregnancy, diabetes, bariatric surgery in the past 12 months or planned within next 6 months, diagnosis of anorexia or bulimia nervosa, and use of weight loss medications Number eligible 87; 50 randomised to the intervention group (mean age 45.5, 8% men) and 25 to the comparison group (mean age 50.8, 8% men) Medications at baseline: Not reported, although weight loss medication was an exclusion criterion Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 26 weeks, community setting): The Mind Your Weight (MYW) weight loss programme follows the same structure as WT, offering 11 proactive coaching calls, 2 of which were with a dietician. Each MYW call started with a “mindful moment” (a 60‐second mindfulness exercise). The coach would check in with the participant about weight and progress toward the weight loss goal, food tracking, and physical activity, followed by a discussion of the mindfulness topic for the call. Coaches monitored use of the eLessons and mindfulness exercises and encouraged participants to practise mindfulness between calls. 11 sessions as follows:
Comparison (duration 26 weeks, community setting): The intervention Weight Talk (WT) consists of 11 proactive phone‐based counselling sessions as well as unlimited inbound support calls. Two of the calls are conducted by registered dietitians and the other calls are with a health coach. WT calls are scheduled at the participant’s convenience, with most participants choosing to schedule calls weekly or biweekly. The overall programme lasts 6 months and during that time participants can call a coach at any point after their 11 calls are completed. Calls last on average between 20 and 30 minutes and there is an integrated website and printed programme guide. 11 sessions as follows:
|
|
| Outcomes | Follow‐up at 26 weeks: anxiety, depression, perceived stress, weight | |
| Notes |
Country: USA Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation utilised a random numbers table. |
| Allocation concealment (selection bias) | Unclear risk | Study staff were not privy to the randomisation sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information, but blinding unlikely. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | From the patient flow diagram, 10% of the MYW group were lost to follow‐up compared to 4% of the WT group. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry number identified to check |
| Other bias | Unclear risk | Insufficient information to judge |
Chacko 2016.
| Study characteristics | ||
| Methods | Parallel‐group RCT ‐ pilot study | |
| Participants | Eligible bariatric patients were recruited from the Weight Loss Surgery Center at Beth Israel Deaconess Medical Center (BIDMC) through targeted mailings and recruitment fliers. Recruitment dates between January and March 2014. Inclusion criteria: Eligible participants had undergone bariatric surgery 1 to 5 years prior to the start of the intervention, were between the ages of 18 and 65, and reported < 5 lb weight loss in the previous 3 months. Exclusion criteria: Patients with serious psychiatric illness, measured by self‐report of hospitalisation for psychiatric reasons in the past year and medical record review, personality disorders assessed by medical record review, severe depression assessed by an adapted version of the PHQ‐9, current alcohol or substance abuse, > 1 weight loss surgery and prior experience with meditation in the past 6 months or a regular meditation practice After screening eligible, potential participants attended a run‐in session to assess motivation, commitment, and availability. This 1‐hour nutrition class was also intended to balance nutrition knowledge in participants at study start. Number screened 43; 9 randomised to the intervention group (mean age 53.4, 10% men) and 9 to the comparison group (mean age 54.5, 22% men) Medications at baseline: Not reported Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 10 weeks, setting ‐ medical centre): The authors developed a novel mindfulness‐based intervention designed to prevent weight regain after bariatric surgery. The intervention integrated mindfulness with adapted versions of traditional behavioural strategies for obesity (e.g. goal setting, problem‐solving, stimulus control, self‐monitoring, social support). The primary aim of the intervention was to improve coping skills to support long‐term weight maintenance. Coping attitudes of mindfulness including patience, acceptance, and self‐compassion were emphasised to help mitigate life stressors. Formal meditative practices were taught alongside behavioural skills explained through the lens of mindfulness. The structure of the intervention was adapted from the established MBSR (Kabat‐Zinn 1990) course. Elements from Mindfulness‐based eating awareness (MB‐EAT, Kristeller 2011) and the Mindful Self‐Compassion (MSC, Neff 2013) course were also included. Classes were held once a week for 10 weeks, and each session lasted 90 minutes. Sessions began with formal mindfulness practice (sitting meditation, loving‐kindness meditation, body scan, mindful chair yoga, walking meditation), followed by group sharing on the week’s experience, and ended with a didactic portion covering a behavioural concept or skill taught from the perspective of mindfulness. A half‐day retreat (4 hours) of extended silent meditation practice was held mid‐way through the course. Participants were asked to meditate at home at least 6 days/week, and audio recordings of guided meditations were provided for home practice. A qualified mindfulness instructor trained through the Center for Mindfulness at the University of Massachusetts Medical School led the intervention. Comparison (duration 1 hour, setting ‐ medical centre) Participants assigned to the standard intervention received a 1‐hour individualised counselling session with a registered dietician. The dietician provided guidance on nutrition, exercise and lifestyle strategies tailored to postsurgical patients. This intervention was chosen as a control to mirror the usual nutrition standard of care that bariatric patients receive annually post‐surgery. |
|
| Outcomes | Follow‐up at 12 and 26 weeks: depression, perceived stress, adverse events, weight, BMI, HbA1C, HRQoL, coping ability | |
| Notes |
Country: USA Funding: This work was conducted with grant support from the Center for Nutritional Research Charitable Trust as well as support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centres. Declarations of interest: Dr. Chacko has received payment for instructing mindfulness classes at Beth Israel Deaconess Medical CentRE. All other authors have no conflicts of interest to declare. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment assignments for randomisation were generated in SAS by the study statistician using permuted blocks with randomly varying block sizes. Randomisation was stratified by surgery type. |
| Allocation concealment (selection bias) | Low risk | Treatment assignments were sealed in sequentially numbered, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information, but blinding unlikely. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% completed all study follow‐up visits and were included in the intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry number identified to check |
| Other bias | Unclear risk | Insufficient information to judge |
Chandler 2020.
| Study characteristics | ||
| Methods | Parallel‐group RCT (ancillary study of main trial of pre‐hypertensives, results of which are not yet published) | |
| Participants | Recruited using flyers and clinic referrals for pre‐hypertension in Charleston, South Carolina, from November 2016 through November 2018 Inclusion criteria: Non‐Hispanic White or African American men or women, 18 to 90 years old, and systolic blood pressure 121 to 139 mmHg on 3 consecutive visits, each separated by at least 2 days. Of the 84 people meeting these criteria and in the main trial, 30 were analysed in this ancillary study who met the definition of type 1 hypertension. Exclusion criteria: Any chronic illness or medical condition requiring regular pharmacological intervention that may affect BP; unwillingness to be randomised into one of two study arms; inability to use the smartphone applications; participating in another research study; inability to speak, hear, or understand English; or pregnant, lactating, or intention of becoming pregnant during the trial Number eligible not reported; 16 randomised to the intervention group (mean age 46.5, 48.7% men) and 14 to the comparison group (mean age 43.4, 49.3% men) Medications at baseline: Not reported although medication that affected BP was an exclusion criterion Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 52 weeks, setting not reported): Tension Tamer (TT) is a patient‐centred breathing awareness meditation (BAM) mobile application developed by using social cognitive and self determination theories. Positive feedback and social reinforcement (e.g. TT app’s immediate post‐session heart rate feedback charts, tailored motivational and social reinforcement text messages based upon levels of adherence), is thought to increase self‐efficacy and autonomous regulation contributing to consistent utilisation and sustained engagement over time. The TT app utilises a smartphone’s camera lens to acquire continuous measures of heart rate via detection of fingertip pulsatile blood flow changes and the participant keeps the tip of their index finger on the camera lens during meditation sessions. These BAM sessions are led with an audio guide and there is also a video clip of abdominal breathing. The TT app provides a timer on the screen, which displays the duration of each session. Alerts are sent if there is excessive movement. Immediately following completion of a TT session, users receive a feedback graph displaying average heart rate each minute and maximum decrease observed. Users have the option to turn off (or reactivate) the audio BAM instructions, set middle or end of session alerts (chime, gong), and select different background themes of screen shots (e.g. nature, rock garden, beach scenes). A trained team member downloaded the TT app to the participant’s phone and instructed them in navigating each module (e.g. explained BAM, activating and deactivating the audio instruction guide, frequently asked questions, accessing feedback graphs, technology assistance phone line, etc.). Each participant was given additional guidance and feedback as needed, while practising BAM and listening to the audio instructions to acquire their heart rate through using the app. Based upon results from a previous dose–response trial, each TT participant was instructed to complete two 15‐min daily sessions for the first month, decrease to two 10‐min daily sessions for months 2 and 3, and then decrease to 5‐min sessions for the remainder of the 12‐month trial. Comparison (duration 52 weeks, setting not reported) The lifestyle education attention control group received the same twice‐daily dosage schedule for engagement in a walking or running programme using the RunkeeperTM app (month 1: 15 min sessions; months 2 and 3: 10 min sessions; months 4 to 12: 5 min sessions). As with the TT group, if they wished to engage in longer or more frequent daily sessions, this was permitted. This group also received the same frequency of SMS messages as the TT group, however content did not pertain to their level of regimen adherence. They received healthy lifestyle‐behaviour related educational messages associated with their heart‐healthy diet, low sodium intake, non‐smoking, physical activity, sun exposure, and other factors. |
|
| Outcomes | Follow‐up at 52 weeks: SBP, DBP, perceived stress | |
| Notes |
Country: USA Funding: This research was funded by the NIH National, Heart, Lung and Blood Institue, grant number 114957. Declarations of interest: The authors declare no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only states randomised |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information but blinding unlikely. We have left as unclear risk as it is difficult, if not impossible, participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | With regard to overall retention rate, 62 (75.5%) completed the 12‐month trial (31 TT participants, 31 SPCTL participants). |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry number identified to check. Reports only the Institutional Research Board of the Medical University of South Carolina approved number. |
| Other bias | Unclear risk | Insufficient information to judge |
Chen 2020.
| Study characteristics | ||
| Methods | Cluster‐RCT | |
| Participants | The study was undertaken across 6 hospital‐affiliated long‐term care facilities (LTCFs) in Southern Taiwan. Each LTCF provided similar diabetes care programmes and had capacity for 200 to 250 residents of similar ethnicity, age group, and nurse‐resident ratios. Study dates not reported. Inclusion criteria: Resident's age over 65 years old, being diagnosed with type 2 diabetes, moving into the facility within 12 months, no obvious delirium, confusion or current psychiatric illness as determined by the residents' treating physician, and being able to communicate in Chinese Exclusion criteria: Participants were excluded if they were terminally ill. Number eligible 152; 66 (3 LTCFs) randomised to the intervention group (mean age 78.85, 35.4% men) and 74 (3 LTCFs) to the comparison group (mean age 78.95, 34.8% men) Medications at baseline: Not reported Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 9 weeks, setting ‐ LTCF): The content of the intervention was adapted from a mindfulness programme developed by the Taiwan Mindfulness Association and consisted of 9 weekly sessions, each of 1.5‐hr duration in groups of 8 to 10. Each session was held in an indoor social activity space at each LTCF and included 30 min of mindful deep breathing relaxation and 60 min of practising mindfulness activities and the following content:(a) What is mindfulness, (b) Cognitive and creative responses to stress, moving, (c) The happiness and strength of living, (d) How the restrictive effects and cognition shape our experience, (e) Stress reaction/automation/ missing, (f) Communication and interpersonal relationships of mindfulness, (g) Full day of mindfulness exercises, (h) Fully integrated mindfulness into life and (i) Living mindfully in a new “home.” The mindfulness programme focused on strategies to manage diabetes distress through practising body and meditation exercises and the moment‐to‐moment awareness of one's experience without judgement, to be less reactive and how to stay mindful when lifestyle is changed and incorporating the Chinese value of peacefulness. Comparison (duration 9 weeks, setting ‐ LTCF) Routine care |
|
| Outcomes | Follow‐up at 12 weeks: HbA1C (anxiety, depression, and stress reported only at 9 weeks) | |
| Notes |
Country: Taiwan Funding: This study is supported by the Ministry of Science and Technology in Taiwan for funding this study (grant no. MOST 105‐2410‐H‐242‐ 002). Declarations of interest: The authors declare that they have no competing interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only states 6 centres were randomly allocated to intervention and control. |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single‐blind. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A research assistant, blinded to the study protocol, was employed to undertake data collection at baseline (T0), 3 (T1), 6 (T2), and 9 (T3) weeks. HbA1C data taken at baseline and at 12 weeks from patients' medical records. No details regarding blinding but as an objective measure detection bias likely to be low risk. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only 2 lost to follow‐up in the intervention group with reason being that they moved out of the facility, but 6 were lost to follow‐up in the control group because they either moved out or were hospitalised. Sixty participants in each group completed the study (90% of the intervention group and 81% of the control group). |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry number identified to check |
| Other bias | Unclear risk | Insufficient information to judge |
Chumachenko 2021.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Recruitment involved advertising (Internet, flyers and social media), search queries through the UMass Memorial Medical Centre electronic medical record and word of mouth in the County of Worcester, MA. Recruitment dates not reported. Inclusion criteria: Aged 25 to 60, individuals who intentionally lost > 5% body weight during the previous year, those intending to maintain weight loss, a BMI > 25 in the past 2 years and greater than 20.5 at time of study entry, those under the care of a primary care physician for at least the last year prior to screening, those able to communicate by telephone, those who have a healthcare provider, personal trainer, or weight‐loss counsellor who can complete and sign a form indicating the amount and timing of their weight loss or have a dated photograph or weight loss diary. Exclusion criteria: Those weighing greater than 300 lb, prior participation in an MBSR course, regular meditation practice (including yoga, Tai Chi, contemplative prayer), evidence of psychiatric or cognitive medical disorder, evidence of alcohol or substance abuse, inability to safely undergo MRI, structural brain damage as determined by an independent neuroradiologist, history of eating disorders, those currently on weight loss medications or those who have had a weight loss surgery, participation in another weight‐related study, regain of > 3% body weight in 2 months prior to study, childbirth within past 6 months, claustrophobia or other MRI contraindication, and pregnant or planning to become pregnant Number eligible 91; 29 randomised to the intervention group (mean age 44.5, 24% men) and 28 to the comparison group (mean age 44.5, 13% men) Medications at baseline: Not reported Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 8 weeks, setting not reported): The MSBR course (Kabat‐Zinn 1990) was taught by certified teachers from the UMass Center for Mindfulness in 8 weekly classes and one all‐day retreat, with homework including formal meditation practices and informal practices during daily life. Comparison (duration 8 weeks, setting not reported): Healthy Living Course (HLC) attention control condition matched on course format (small group), length (8 weeks), number of sessions (eight 1.5‐hour sessions plus a day‐long retreat), and process (didactic teaching and group discussion, homework). Sessions contained the following topics: healthy living, healthy eating, physical activity and health, sleep and health, stress management, time management and unhealthy behaviours (smoking, drinking). HLC classes were taught by trained health educators using a structured curriculum. |
|
| Outcomes | Follow‐up at 26 weeks: depression, perceived stress, weight, BMI | |
| Notes |
Country: USA Funding: The National Center for Complementary and Integrative Health (NCCIH)) provided funding for this trial (grants R34AT006963 and R34AT006963‐01A1 to manuscript authors/principal investigators CF and JAK). Declarations of interest: The authors have declared that no competing interests exist. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Study participants will be equally randomised to either the MBSR intervention arm or HLC arm based on a permuted blocks randomisation scheme. In this procedure, treatment allocations will be made within blocks so that the numbers assigned to each arm are equal after each block has been filled. Blocks of various sizes (2, 4, 6) will be used in random order, to facilitate allocation concealment, that is, to make it nearly impossible to determine the treatment assignment based on a pattern of previous treatment allocations". |
| Allocation concealment (selection bias) | Low risk | "Randomization and investigators blinding was implemented using sealed envelopes and unique identification numbers by the study coordinator". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were not blind. "MBSR teachers at the Center for Mindfulness were not aware of who in their classes were study participants. HLC teachers were aware that classes were being offered as part of the research study, but were blinded to study hypotheses". We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All members of the research team involved in data analysis will be blind to treatment assignment". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5/57 subjects were either unable to complete initial MRI or dropped out during the intervention, unclear from which group. |
| Selective reporting (reporting bias) | Unclear risk | Anxiety and sleep quality were listed as outcomes in the protocol, but not reported in the primary publication. |
| Other bias | Unclear risk | Insufficient information to judge |
Daubenmier 2011.
| Study characteristics | ||
| Methods | Parallel‐group RCT – pilot study | |
| Participants | Female participants were recruited through media outlets and flyers posted in the San Francisco Bay Area between November 2006 to March 2007. Inclusion criteria: Women with a BMI between 25 and 40 Exclusion criteria: Medical issues such as diabetes or medication use such as hormonal supplements that could affect weight loss, insulin resistance, or abdominal fat. Postmenopausal women, history of a bilateral oophorectomy, total hysterectomy, or polycystic ovary syndrome, active endocrinologic disorder. Pregnancy, less than 1 year postpartum, or breastfeeding. Currently on active diet plan, self‐reported eating disorder, alcohol or drug addiction. Positive urine test for diabetes or opiate use. Taking steroids or antipsychotic medications, though antidepressant medication use was permitted. Prior experience of MBSR or current meditation or yoga practice. 322 assessed for eligibility, 53 enrolled and 47 randomised. Number eligible not reported. 24 randomised to the intervention group (mean age 40.4, 0% men) and 23 to the comparison group (mean age 41.4, 0% men). Medications at baseline: Medication at baseline and medication change during the trial not reported |
|
| Interventions |
Intervention (duration 16 weeks, setting not reported): A preliminary, novel intervention was developed drawing on components from MBSR, MBCT, and MB‐EAT. The intervention programme consisted of nine 2.5‐hour classes and one 7‐hour silent day of guided meditation practice after class 6. Classes were held on a weekly basis on the weekend. Participants were instructed in the body scan, mindful yoga stretches, sitting and loving kindness meditations as taught in MBSR, and the “3 minute breathing space” as taught in MBCT. Participants were also led through guided meditations as a way to introduce mindful eating practices of paying attention to physical sensations of hunger, stomach fullness, taste satisfaction, and food cravings; identification of emotional and eating triggers; self‐acceptance; and inner wisdom as taught in MB‐EAT. Participants were encouraged to engage in daily home assignments that included up to 30 minutes per day of formal mindfulness practices 6 days per week and mindful practices before and during meals. Comparison (duration 16 weeks, setting not reported): Wait list control To provide guidelines for healthy eating and exercise during the intervention and to control the effects of such information on study outcomes, both groups participated in a 2‐hour nutrition and exercise information session aimed at moderate weight loss midway through the intervention, in which mindfulness was not discussed. |
|
| Outcomes | Follow‐up at 16 weeks: anxiety, weight | |
| Notes |
Country: USA Funding: This paper was supported by the Mount Zion Health Fund; The William Bowes, Jr., Fund; the Robert Deidrick Fund; Robert Wood Johnson Foundation, and NIH Grant K01AT004199 awarded to J. Daubenmier from the National Centre For Complementary & Alternative Medicine, and the National Institutes of Health/National Centre for Research Resources (NIH/NCRR) UCSF‐CTSI Grant no. UL1 RR024131 Declarations of interest: The authors declare no conflict of interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised to the treatment or control group in a 1:1 ratio and stratified on BMI category, age, and current antidepressant medication use. Computer‐generated random numbers were used by the site statistician at the UCSF General Clinical Research Center (GCRC) to assign group condition. |
| Allocation concealment (selection bias) | Low risk | Computer‐generated random numbers were used by the site statistician at the UCSF General Clinical Research Center (GCRC) to assign group condition. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | After all participants had completed baseline assessments, this information was given to study staff who informed participants of their group condition. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study nurses, blind to participant condition, performed the anthropometric and body composition assessments and blood draws. Research assistants administered the computerised questionnaires and provided instructions for the home saliva sampling procedure, but were not blind to participant condition at post‐treatment assessments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Used ITT analysis. In the intervention group, 5 participants were lost to follow‐up (became ill, too busy) and 2 did not receive minimum treatment of 4/10 classes (too busy, disliked classes). In the comparator group, only 2 participants were lost to follow‐up due to death of a close friend and being non‐responsive. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry number identified to check |
| Other bias | Unclear risk | Insufficient information to judge |
Daubenmier 2016.
| Study characteristics | ||
| Methods | Parallel‐group RCT ‐ The SHINE trial | |
| Participants | Recruited participants from the community using fliers, newspaper advertisements, online postings, and referrals at The University of California, San Francisco (UCSF) clinics. Participants were enrolled in 6 rounds from July 2009 to February 2012. Inclusion criteria: Body mass index (BMI) between 30 and 45.9, abdominal obesity (waist circumference > 102 cm for men; > 88 cm for women), and age 18 or older. Exclusion criteria: Diabetes mellitus, fasting glucose 126 mg/dL, or haemoglobin A1c (HbA1c) between 6.0% and 6.5% with an abnormal oral glucose tolerance test and current weight loss diet or taking medications that may affect weight 465 passed initial eligibility, 257 consented and fully screened, 194 randomised; 100 randomised to the intervention group (mean age 47.2, 21% men) and 94 to the comparison group (mean age 47.8, 14% men) Medications at baseline: Lipid lowering therapy at baseline in 11% of the intervention group and 9.6% of the comparison group. Blood pressure therapy at baseline in 16% of the intervention group and 22.3% of the comparison group. Antidepressant therapy at baseline in 17% of the intervention group and 17% of the comparison group. Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 24 weeks, setting UCSF): The mindfulness intervention added mindfulness training for stress management, eating, and exercise. Meditation practices modelled on MBSR, included sitting meditation, loving kindness, and yoga postures. Mindful eating practices, modelled on the Mindfulness‐Based Eating Awareness Training programme, were designed to enhance awareness and self‐regulation of physical hunger, stomach fullness, taste satisfaction, food cravings, emotions, and other eating triggers in the context of reduced caloric intake. Mindful walking included awareness of sensory experience, posture, and alignment. Home practice guidelines included meditation practice for up to 30 min a day/6 days a week, eating meals mindfully, and use of mini‐meditations. The mindfulness intervention was led by one of three mindfulness meditation instructors and co‐led by the same registered dietitian (except for one cohort). Comparison (duration 24 weeks, setting UCSF) To control for attention, social support, expectations of benefit, food provided during the mindful eating exercises, and home practice time in the mindfulness intervention, the control intervention included additional nutrition and physical activity information, strength training with exercise bands, discussion of societal issues concerning weight loss, snacks, and home activities. To control for a mindfulness approach to stress management in the intervention group, progressive muscle relaxation and cognitive‐behavioural training were provided in the control group. The control intervention was led by one of three registered dietitians masked to study hypotheses. Participants had three individual consultations with instructors. Both interventions included identical diet‐exercise guidelines presented in 45‐min segments per session. The dietary component recommended healthy food choices that emphasised modest calorie reduction (typically 500 kcal/day), including decreasing calorie‐dense nutrient‐poor foods, decreasing simple carbohydrates and substituting whole grains, and increasing consumption of fresh fruits and vegetables, healthy oils, and proteins. The exercise component emphasised increasing daily activity and moderate‐intensity exercise, primarily through walking, and strength training. Both interventions included 16 sessions lasting 2 to 2.5 hours (12 weekly, 3 biweekly, and 1 monthly) and one all‐day session (6.5 and 5 hours in the mindfulness and control interventions, respectively) over 5.5 months. |
|
| Outcomes | Follow‐up at 3, 6, 12, 18 months: SBP, DBP, weight, LDL and HDL cholesterol, triglycerides, FBG, HbA1c | |
| Notes |
Country: USA Funding: This study was supported by NIH grants from the National Center for Complementary and Alternative Medicine (NCCAM) P01AT005013 (Hecht), K24AT007827 (Hecht), and K01AT004199 (Daubenmier), as well as the National Center for Advancing Translational Sciences, UCSF‐CTSI Grant Number UL1 TR000004. Declarations of interest: Dr. Kristeller participated in a paid webinar on “mindful snacking” for Allidura Consumer; the other authors declared no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer‐generated random allocation sequence using random block sizes of four to eight was programmed by a database manager not involved in enrolment. No other staff had access to the randomisation sequence. The project director (PM) accessed the allocation sequence using a programmed database that could not be altered once randomised condition was revealed." |
| Allocation concealment (selection bias) | Low risk | "A computer‐generated random allocation sequence using random block sizes of four to eight was programmed by a database manager not involved in enrolment. No other staff had access to the randomisation sequence. The project director (PM) accessed the allocation sequence using a programmed database that could not be altered once randomised condition was revealed." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "In behavioral intervention trials, it is essentially impossible to mask participants to the intervention they receive. We made an effort, however, to mask participants to the fact that we were specifically testing effects of a mindfulness‐enhanced intervention." We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Weight, height, blood pressure, and waist circumference were measured (see Supporting Information, Methods). Staff was not masked to group assignment; as feasible, staff either conducted assessments, or coordinated intervention sessions to minimize unmasking. A blood specimen was obtained for glucose, lipids, HbA1c, insulin, and C‐reactive protein. Staff performing assays was masked to group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk |
|
| Selective reporting (reporting bias) | Unclear risk | Checked trial register record: https://clinicaltrials.gov/ct2/show/NCT00960414 Some secondary outcomes listed here are not reported in Daubenmier 2016, Mason 2016, or other publications related to this trial listed in the trial record. These listed outcomes are: mood, stress hormones, adipocyte activity, influenza vaccine response. |
| Other bias | Unclear risk | Insufficient information to judge |
Davis 2014a.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Participants living in low SES areas of a mid‐sized Midwestern city were targeted for recruitment via advertisements placed on television, newspaper, and flyers. Study dates between 2011 and 2012. Inclusion criteria: Participants at least 18 years of age, smoke 5 or more cigarettes per day, use no other tobacco products, claim high motivation to quit Exclusion criteria: Consume more than 4 alcoholic drinks on more than 4 days per week Number eligible 420; 68 randomised to the intervention group (mean age 43.2, 57.4% men) and 67 to the comparison group (mean age 45.8, 49.3% men) Medications at baseline: Not reported Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 7 weeks, setting not reported): Mindfulness Training for Smoking (MTS). The MTS course for smokers lasted 7 weeks, and was comprised of seven 2.5‐hour classes and a 6.5‐hour Quit Day Retreat (total = 24 hours). During each class, instructors would play approximately 20 minutes of the MTS Instructional Video, which provided instruction in mindful meditation, walking, smoking, eating, and mindful management of smoking triggers, urges, addictive thoughts and emotions. After playing the DVD, instructors would lead exercises and provide more nuance and individualised instruction. The final hour of class was a “Meditation Group,” consisting of guided meditation and group‐support practice called “mindful talking and listening.” On the Quit Day Retreat, smokers attempted smoking cessation and initiated a 2‐week course of nicotine patches. After the intervention, participants were invited to continue to attend the Meditation Group at any time. Throughout the MTS intervention, participants were asked to practise 15 to 30 minutes of meditation per day at home with a guided meditation CD. Comparison (duration 7 weeks, setting not reported): American Lung Association's Freedom from Smoking (FFS), which was enhanced to better match the mindfulness intervention in time, intensity and pharmacotherapy (2 weeks of nicotine patches), to test the effects of mindfulness per se (FFS‐E). MTS provided training in mindfulness and meditation, whereas FFS provided training in a variety of cognitive skills and relaxation. Matching of FFS‐E to MTS included the following “enhancements”: 1) To match MTS, total time in FFS‐E was increased to 24 hours (FFS typically provides eight 90‐ to 120‐minute sessions (12 to 16 hours), 2) like MTS, FFS‐E classes provided an additional 30 minutes per class for group sharing and support, 3) to match meditation practice in MTS, FFS‐E emphasised relaxation, by including a 15‐ to 30‐minute relaxation practice in each class, 15 to 30 minute assigned daily guided relaxation with a CD, 4) like MTS, FFS‐E was provided with an optional long‐term weekly support group, 5) materials were matched with 90‐page manuals and CDs of similar appearance provided to each group, 6) instructor qualifications and training between the 2 groups were matched. |
|
| Outcomes | Follow‐up at 24 weeks: 7‐day point‐prevalence smoking abstinence, perceived stress | |
| Notes |
Country: USA Funding: This study was supported by funding from NIDA Grant K23 DA022471 Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were not blinded to their respective treatment conditions. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intervention attrition was not significantly associated with any baseline variable and did not differ significantly between groups (MTS = 32.4%, FFS‐E = 26.9%). Attrition rates at assessment visits were not significantly different between groups (4‐week assessment visit: MTS = 39.7%, FFS‐E 32.8%) and (24‐week assessment visit: MTS = 57.4%, FFS‐E = 55.2%). ITT analysis used. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry number identified to check |
| Other bias | Unclear risk | Insufficient information to judge |
Davis 2014b.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Subjects were recruited via advertisements placed on television, newspaper, and flyers in the Madison Dane County Area, with ad placement in low SES neighbourhoods. Data were collected between 2010 and 2011. Inclusion criteria: Be 18 or more years of age, smoke 5 or more cigarettes per day, use no other tobacco products, claim “high motivation to quit”, and state “a willingness to attend ten meetings over a two‐month period.” Exclusion criteria: Consumed 4 or more alcoholic drinks on 4 or more nights per week. Scores of 9 or above or statement of suicidal intentions excluded individuals from the study and triggered referral to a medical practitioner. Number eligible 403; 105 randomised to the intervention group and 91 to the comparison group. Demographics not reported by group but overall (mean age 41.7, 50% men). Medications at baseline: Not reported Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 8 weeks, setting not reported): Mindfulness Training for Smoking (MTS). The MTS course schedule began with a 7‐hour introductory class and was followed by 4 weekday classes, each with 90 minutes of instruction. During each class, instructors would play 15 to 20 minutes of the MTS Instructional DVD with instruction provided by addiction experts, mindfulness instructors, physicians, and past participants. The MTS Instructional DVD provided basic instruction in mindfulness practices such as meditation, mindful walking, and mindful talking and listening and targeted mindfulness skills on the use of mindfulness to manage smoking triggers, urges, withdrawal symptoms, addictive thoughts, and negative emotions. After playing each section of the DVD, MTS instructors would have approximately an hour to provide personalised instruction, answer questions and conduct exercises described on the DVD. After each 90‐minute weekly class, there was an hour‐long meeting of the Meditation Group, a long‐term support group open to current and past MTS participants. The Meditation Group consisted of a 30‐minute guided meditation and a 30‐minute support group practice called “mindful talking and listening.” Throughout the MTS intervention, participants were asked to practise 30 minutes of guided meditation per day at home with a 30‐minute guided meditation CD. All participants received a copy of the MTS Manual, which was developed to provide an expanded version of the concepts and exercises provided on the DVD. After 4 weeks of classes participants attended the “Quit Day Retreat,” attempted smoking cessation, and initiated a 4‐week course of 21 mg nicotine patches. The Quit Day Retreat was comprised of 7 hours of instructor‐guided mindfulness practice and was conducted mostly in silence. Including the Quit Day Retreat and Meditation Group, there was a total of 24 hours of class time. After the Quit Day Retreat, graduating participants were invited to continue to attend the Meditation Group for an additional 4 weeks and return to the group for support at any time. Comparison (duration and setting not reported) Controls received smoking cessation counselling through the Wisconsin Tobacco Quit Line, run by Alere, America’s largest telephonic smoking cessation corporation. Controls were encouraged to make repeated calls to the Quit Line, were assigned a quit day on a weekend approximately 2 weeks from their orientation and were asked to return to the study centre to pick up 4 weeks of 21 mg nicotine patches. |
|
| Outcomes | Follow‐up at 24 weeks: 7‐day point‐prevalence smoking abstinence | |
| Notes |
Country: USA Funding: Funding was provided through NIDA grant K23DA022471 Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation via random draws |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding was not performed due to the functional necessity of providing participants information about MTS scheduling before considering enrolment and the ethical preference of allowing participants to have ample information about their intervention prior to put time and effort into a smoking cessation attempt. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Pre‐intervention attrition rates (dropout immediately after randomisation) were 43% for MTS and 37.2% for controls (P = 0.22). |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry number identified to check |
| Other bias | Unclear risk | Insufficient information to judge |
Davoudi 2021.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Participants include all patients with vitamin D deficiency referred to Beheshti hospital, Kermanshah, Iran, between 1 September 2019 and 18 October 2019. Those with type 2 diabetes with neuropathy were identified. Inclusion criteria:
Exclusion criteria:
Number eligible 279; randomisation to 5 groups, 2 relevant to the current review (mindfulness plus placebo versus placebo); 45 randomised to the intervention group (mean age 53.3, 57.5% men) and 45 to the comparison group (mean age 56.3, 60% men) Medications at baseline: Not reported Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 12 weeks, setting not reported): Mindfulness plus placebo group: 12 weeks (90 min per session) of mindfulness including pain relief delivered by trained psychotherapists. Sessions included:
Comparison (duration 12 weeks, setting not reported) Placebo group: oily drops identical in shape to vitamin D supplements |
|
| Outcomes | Follow‐up at 12 weeks: FBG, QoL | |
| Notes |
Country: Iran Funding: not reported Declarations of interest: The authors certify that they have no competing interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomly allocated into groups using a random table". |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "All patients were blinded to receive vitamin D or placebo groups". No information about mindfulness intervention, but blinding unlikely. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11% loss to follow‐up in both the placebo group and mindfulness plus placebo group |
| Selective reporting (reporting bias) | Unclear risk | Checked trial registry and cognitive function and HOMA‐IR listed as outcomes but not reported; however, FBS and physical activity reported in paper but not listed in registry, and BMI similarly not listed but listed as an outcome in the paper but no outcome data provided. |
| Other bias | Unclear risk | Insufficient information to judge |
de Fatima Rosas Marchiori 2015.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Volunteers aged > 60 years were recruited through media advertising. Recruitment dates not reported. Inclusion criteria: Volunteers who did not practise any form of meditation and who had ≥ 4 years of formal school education were eligible to enrol in the study. The volunteers’ BP levels had to meet the following criteria: systolic pressure between 130 and 159 mmHg, and diastolic pressure between 85 and 99 mmHg (considered the upper and lower limit BP levels for mild hypertension stage I), as established by the European Society of Hypertension. Volunteers could be undergoing treatment with antihypertensive drugs as long as the dose was stable for at least 3 months and remained stable until the end of the study. Exclusion criteria: Severe heart disease, kidney failure, secondary hypertension; illicit drug use and alcohol abuse; psychiatric disorders; and severe cognitive impairment. During the study period, participants were excluded if their BP level exceeded the established limits and showed clinical evidence of risk to the patient, if the dose of antihypertensive drugs was changed or the participants used medications known to interfere with BP, including anti‐inflammatory and anorectic drugs, and if those belonging to the meditation group (MG) had 3 consecutive absences. Participants had to maintain regular eating habits, physical activity, and sleep patterns. Number eligible not reported. 347 volunteers responded, 65 selected and randomised and 6 excluded post randomisation; 28 randomised to the intervention group (mean age 67.2, 36% men) and 31 to the comparison group (mean age 67, 35% men). Medications at baseline: All intervention participants used antihypertensive drugs, and 29/31 in the control group, with no change reported throughout the trial. |
|
| Interventions |
Intervention (duration 12 weeks, setting ‐ Department of Psychobiology Universidade Federal de São Paulo): The 3‐month Zen meditation programme adapted for beginners consisted of weekly 1‐h group meetings and daily home meditation practices. The practitioners must remain in a seated position for 20 min, with the spine straight, shoulders relaxed, hands resting on the thighs, eyes closed and mentally counting the breaths in successive cycles of one to 10, for 20 min. During meditation, the focus should be kept on breathing in the present moment. The participants were encouraged to let their thoughts flow without attachment, judgement or criticism, without disturbances from any internal or external factors. Participants were instructed to practice the method at home twice a day, and to resume the relaxation achieved throughout the day. A member of the research team and an invited monk from the Zen Buddhist tradition instructed and monitored the weekly meditation practice. Participants received a diary to record the frequency of meditation practice and other relevant observations. Comparison (duration 12 weeks, setting ‐ Department of Psychobiology Universidade Federal de São Paulo): Wait list control |
|
| Outcomes | Follow‐up at 12 weeks: blood pressure, HRQoL | |
| Notes |
Country: Brazil Funding: Associação Fundo de Incentivo à Pesquisa (AFIP) for financial support. Declarations of interest: The authors declare no conflicts of interest related to the study and preparation of this manuscript. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "were randomly distributed into the MG and the control group" |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information but blinding unlikely. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Of the 65 volunteers who were selected for the study, 59 participated in the BP analysis, with 31 in the control group and 28 in the MG group. Six volunteers were excluded from the study because of changes in their antihypertensive medication or due to a lack of adherence to the meditation programme. Does not say which groups the 6 excluded participants were from. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry number identified to check |
| Other bias | Unclear risk | Insufficient information to judge |
Friis 2016.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Participants were recruited between July 2014 and September 2014 at 3 hospital sites in Auckland, New Zealand. Recruitment was through self‐referral to the trial or following recommendations from a patient’s physician or diabetes nurse; the study was widely advertised through numerous local diabetes centres. Inclusion criteria: Participants were aged 18 to 70 years with either type 1 or type 2 diabetes, fluent in English, and able to attend a minimum of 6 of 8 scheduled treatment sessions. Exclusion criteria: Exclusion criteria were self‐reported inability to read and write English. Number eligible not reported. 35 randomised to the intervention group (mean age 42.2, 37.5% men) and 36 to the comparison group (mean age 46.7, 25.8% men). Medications at baseline: Not reported Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 8 weeks, setting not reported): Mindful Self Compassion (MSC) is a protocol‐standardised intervention aimed at increasing mindfulness and self‐compassion and reducing the suffering associated with experiential avoidance (Neff 2013). Sessions were delivered to groups of 8 to 12 people during 8 weekly sessions, each lasting 2.5 h. The intervention was delivered by registered health psychologist trained to teach the programme according to manualised MSC protocols. Clinical supervision was conducted weekly through Skype conference with MSC trainers across the intervention period. All participants received a standardised e‐mail 2 days after each weekly session that summarised the week’s teachings and encouraged them to practise what they had learned during the previous session. The central components of MSC are formal meditation together with formal and informal self‐compassion practices aimed at developing the cognitive, behavioural, and physical capacities to soothe and comfort oneself when distressed. Comparison (duration 8 weeks, setting not reported) Participants in the wait list control condition received medical treatment as usual. |
|
| Outcomes | Follow‐up at 12 weeks: depression, HbA1C | |
| Notes |
Country: New Zealand Funding: This study was made possible with the support of the New Zealand Society for the Study of Diabetes and the New Zealand Diabetes Foundation. Declarations of interest: No potential conflicts of interest relevant to this article were reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A trained researcher blind to the hypotheses and study design and without participant contact used randomisation software to allocate treatment assignments. |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Patients were told that they would be participating in a programme based on evidence that learning to treat oneself with kindness and understanding when faced with difficult feelings and circumstances could be good for mental and physical health. They were told that they might be allocated to either a skills training workshop or a wait list control. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Eight patients withdrew before baseline assessments. ITT used for all participants where there was any available data. Individual missing values were imputed by using the total scale mean for the PHQ‐9; for missing HbA1c values the most recently recorded value was carried forward. For the 4 participants who withdrew from the study, measurements from the time point before withdrawal were carried forward for analysis, and all multivariate assumptions were met. |
| Selective reporting (reporting bias) | Unclear risk | Trial retrospectively registered |
| Other bias | Unclear risk | Insufficient information to judge |
Friskey 1984.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Some of the volunteers were being seen regularly in various outpatient clinics of the Tucson Veterans Administration Medical Centre; others were being seen by private physicians in the community. Inclusion criteria: Veterans who demonstrated mean diastolic pressures above 90 mmHg, but not above 105 mmHg, on each of 2 successive weeks prior to initiation of training were eligible for inclusion. Exclusion criteria: Patients on glucocorticoids or sympathomimetics were excluded, because of the hypertensive effects of these medications on blood pressure. Other exclusions included patients on major tranquillisers or with a history of hospitalisation for psychiatric problems, patients with renal failure or any disease likely to result in death within a year, patients with severe chronic alcoholism or known history of failure to keep appointments, patients who were not permanent residents of the Tucson area, and those whose antihypertensive medications had been initiated or increased within the previous 6 months. Number eligible 43; 33 randomised, 11 to each of 3 groups. Overall age was between 26 and 72, 94% were men. Medications at baseline: Antihypertensive therapy at baseline in 48% overall Medication change reported for completers where there was an increase or initiation of antihypertensive treatment in 3/8 in the comparison group and no change in the 2 intervention groups. |
|
| Interventions |
Intervention (duration 6 weeks, setting ‐ the Tucson Veterans Administration Medical Centre): Two intervention groups: a 3‐treatment relaxation and meditation group and 6‐treatment relaxation and meditation group. Both groups learned the same skills; only the schedule of training was different, to test the minimal training necessary for significant decrease in blood pressure. Both groups were taught Progressive Muscle Relaxation (PMR) in the first week of the training where various muscle groups throughout the body are in turn tensed and relaxed. Both groups were asked to practice PMR for 20 minutes, twice a day and encouraged to incorporate relaxation into daily living. Five different meditation practices were taught ‐ just listening, counting breaths, repeating a single word with each breath, following the breath through their nose and a philosophic meditation which incorporates relaxation and breathing exercises with visual imagery, in an exercise which directs attention to the major sensory modalities and to the biological symbiosis of all living things. Treatment in these 2 groups was aimed at control of attentional processes, and at somatic awareness and control. Comparison (duration 6 weeks, setting ‐ the Tucson Veterans Administration Medical Centre): Education/cognition group ‐ presented with a series of 6 hypertension information lectures covering hypertension, antihypertensive medications, salt restriction, exercise, weight reduction, and little emphasis on stress management. Didactic training with questions and discussion. All interventions were offered through the Psychology Service of the Tucson Veterans Administration Medical Centre, under the supervision of a staff physician and a staff psychologist. Follow‐up continued for up to 10 months after training. Brief "refresher" courses were offered in about the 2nd, 5th, and 8th months of follow‐up in all of the groups. |
|
| Outcomes | Follow‐up at 20 weeks: blood pressure, anxiety (blood pressure measurements only available to 5 months due to technical problems) | |
| Notes |
Country: USA Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Subjects were randomly assigned to groups according to a previously drawn list from a random numbers table. |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Originally single‐blind. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Although the original plan was to have blood pressure read and recorded by an observer "blind" to group assignments, this plan had to be altered when the technician was not permitted to work evenings or Saturdays, and when hospital volunteers proved unreliable. Group members, instead, then read and recorded each other's blood pressures, which were automatically locked into the machine. Objective measure, so judged as unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only 1 participant dropped out of the study and they were in the REMIN group. 7 participants had incomplete data and these were equally spread across the 3 treatment groups. |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified to check |
| Other bias | Unclear risk | Insufficient information to judge |
Gainey 2016.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Adults with type 2 diabetes were recruited from one of the Primary Health Promoting Hospitals in Samut Prakan Province, Thailand by invitation using the mailing list of the clinic. Recruitment dates not reported. Inclusion criteria: Aged 40 to 75 years; HbA1c 7% to 9%; no diabetic complications; no musculoskeletal disorders that could limit walking; on oral diabetic medications but not yet treated with insulin injections; have not attended any exercise programme (e.g. structured aerobic exercise, resistance exercise, or other modes of exercise including yoga) within the last 6 months and normal resting electrocardiogram. Exclusion criteria: None specifically reported Number eligible not reported; 14 randomised to the intervention group (mean age 58, 16.7% men) and 13 to the comparison group (mean age 63, 18.2% men) Medications at baseline: Not reported Medication change during the trial not reported |
|
| Interventions | Both the intervention and comparison training programmes were divided into 2 phases. In the phase 1 (weeks 1 to 6), the training programmes were conducted at mild to moderate intensity (50% to 60% maximum heart rate) and in the phase 2 (weeks 7 to 12), the training intensity was increased to moderate intensity (60% to 70% maximum heart rate). In both phases, the exercise sessions consisted of a 10‐min warm‐up and general static stretching, followed by a 30‐min workout and 10‐min cool‐down and general static stretching, giving a total session time of 50 min, 3 times per week. The prescribed exercise intensity was confirmed by using the heart rate monitors. All the walking exercise training was performed on the treadmill and was closely supervised. Intervention (duration 12 weeks, setting not reported): Buddhist walking meditation ‐ aerobic walking exercise combined with Buddhist meditation. The subjects performed walking on the treadmill while concentrating on foot stepping. The subjects had to voice “Budd” and “Dha” while setting each foot on the floor. The goal was to practise mindfulness while walking. Comparison (duration 12 weeks, setting not reported): Traditional aerobic walking programme |
|
| Outcomes | Follow‐up at 12 weeks: blood pressure, weight, BMI, FBG, HbA1C, total, LDL and HDL cholesterol, triglycerides | |
| Notes |
Country: Thailand Funding: This study was supported by the Faculty of Sports Science and the 90th Anniversary of Chulalongkorn University Funds. Declarations of interest: All authors declare to have no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The eligible participants were first stratified by gender and the duration of diabetes and then randomised into one of the two groups using a random allocation sequence. |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information but blinding unlikely. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Four subjects dropped out because of illness and/or a lack of compliance (less than 80% of the total training time) and these subjects were eliminated from the analyses. 15% lost to follow‐up, ITT not used. |
| Selective reporting (reporting bias) | Unclear risk | No trial registry or protocol identified to check |
| Other bias | Unclear risk | Insufficient information to judge |
Garrison 2020.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Recruitment took place online from November 2014 to August 2015. Recruitment sources were as follows: 46% Google ads, 23% word of mouth/other, 14% Facebook posts, 11% https://smokefree.gov, 2% Twitter, 2% Reddit ads, 1% www. clinicaltrials.gov, 0.4% Huffington Post. Inclusion criteria: Age 18 to 65 years, smoked ≥ 5 cigarettes/day, had ≤ 3 months past‐year abstinence, owned an iPhone/Android, and were motivated to quit, indicated by ≥ 8/10 on the Contemplation Ladder and ≥ 4/5 on an Action item of the Readiness to Change Questionnaire: “I am trying to smoke less than I used to,” 1 = strongly disagree, 5 = strongly agree. Exclusion criteria: None explicitly reported Number eligible 2200; N = 518 consented and N = 13 were excluded for enrolling more than once, based on contact information and IP address; N = 505 were emailed a link to the baseline survey, at which point quit‐smoking medications were also recommended. At the end of the baseline survey, participants were randomised to receive mobile mindfulness training with experience sampling (MMT‐ES) or only ES. 245 randomised to the intervention group (mean age 43.3, 28% men) and 260 to the comparison group (mean age 39.7, 28.6% men). Medications at baseline: Not reported Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 22 days, setting ‐ online): The intervention group received mindfulness training with experience sampling (MMT‐ES, Craving to Quit) for 22 days of training modules (5 to 15 minutes/day) teaching mindfulness for smoking cessation. The app teaches mindfulness and 3 standard meditation practices: body scan, loving kindness, and breath awareness. Body scan is practised by bringing awareness to different parts of the body, to foster awareness of body sensations that constitute cravings and affective states. Loving kindness is practised by directed well‐wishing by repeating phrases such as “may X be happy,” to foster acceptance of oneself and others. Breath awareness is practised by paying attention to the breath wherever one feels it most strongly in the body, to help retrain the mind away from habitual self‐related thinking toward a more present‐centred awareness. The app also teaches an informal practice to work mindfully with cravings, RAIN: Recognize, Accept, Investigate, and Note what cravings feel like as they arise and pass away. ES is another feature of the app delivered as “check‐ins”. Two check‐in questions were utilised: 1) “When you started this check‐in, how much were you craving a cigarette?” (visual analogue scale from “Not at all” to “Very much”); and 2) The tracker says you have smoked [#] cigarettes today. Adjust your tracker below if needed. “Today I have smoked: [#].” Users were asked to check in 6 times per day for 22 days. They set daily start/end times, their day was divided into 6 intervals, they were notified to check in randomly in each interval and they were manually sent a text message if their response rate dropped below 3 check‐ins/day, monitored daily across treatment by a blinded researcher. Comparison (duration 22 days, setting ‐ online): The control group received a smartphone app with the same look and feel as MMT‐ES, delivering only ES for 22 days, to control for potential effects of ES, expectancy effects and non‐specific effects of using a smartphone for smoking cessation. |
|
| Outcomes | Follow‐up at 26 weeks: 1‐week point‐prevalence abstinence from smoking | |
| Notes |
Country: USA Funding: This study was supported by grants from the American Heart Association [14CRP18200010] and the National Institute on Drug Abuse grant [K12DA00167]. Declarations of interest: Judson A. Brewer and Prasanta Pal own stock in Claritas Mindsciences, the company that developed the apps used in this study. All other authors declare that they have no competing interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Basic randomisation, using Yale Qualtrics Survey Tool (http://www.qualtrics.com/) |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "All randomised participants were followed up by a blinded researcher." Nothing specifying blinding of participants or those delivering the intervention. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All randomised participants were followed up by a blinded researcher." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All analyses were performed on a modified ITT basis. "Multiple imputation of missing 6 months prevalence data was also considered." |
| Selective reporting (reporting bias) | Unclear risk | Mood was mentioned in protocol but missing from the published paper. |
| Other bias | Unclear risk | Insufficient information to judge |
Goldenhersch 2020.
| Study characteristics | ||
| Methods | Parallel‐group RCT ‐ pilot study | |
| Participants | Recruited via unpaid advertisements posted for 75 days on the Mindcotine page on Facebook, and a 13‐minute segment was aired on Argentine national public television (channel C5N). The social network advertisements linked invitations directly to a screening questionnaire. Recruitment dates not reported. Inclusion criteria: 1) Aged between 24 and 65 years, representing smokers with a high prevalence of daily smoking; 2) consume a minimum of 5 cigarettes per day, with a score of 4 to 9 on the Contemplation Ladder; 3) be residents in the city of Buenos Aires; 4) own an Android mobile phone with gyroscope; 5) have a data plan or Wi‐Fi access; and 6) have an interest in using VR as a method to quit smoking Exclusion criteria: None explicitly reported Number eligible 234; 60 randomised to the intervention group (mean age 44.1, 45% men) and 60 to the comparison group (mean age 42.4, 59% men) Medications at baseline: Not reported Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 21 days, setting ‐ online): Received a self‐assisted 21‐day programme based on virtual reality mindful exposure therapy (VR‐MET) sessions, daily surveys, and online peer‐to‐peer support moderated by psychologists. The elements of the programme were 1) practice sessions in formal mindfulness, 2) practice sessions in informal mindfulness using virtual reality mindful exposure therapy (VR‐MET), 3) daily self‐reports, 4) peer‐to‐peer support, and 5) Mindcotine support (Mindcotine is the name of the digital intervention developed by MindCotine Inc). First, practice sessions in formal mindfulness included 6 sessions of mindfulness in video format of up to 10 minutes each and 7 sessions in audio format of 3 to 10 minutes each. The user is given an initial introduction to mindfulness involving the recognition of bodily sensations and the ability to practise non‐reactivity to emotions and thoughts related to smoking, from a compassionate and non‐judgemental position. Second, practice sessions in informal mindfulness using VR included 2 sessions of VR‐MET, each lasting 10 minutes. These sessions included a selection of 2 virtual environments that combined the awareness of the act of smoking and the recognition of craving from a perspective of acceptance and commitment. The mindfulness audio from both environments was chosen to work consciously with craving‐related acts (“RAIN”: Recognise, Accept, Investigate and Nourish, and “Act of Smoking”: consciously review each moment of the act itself). Each video was repeated over a period of 14 consecutive days. Third, daily self‐reports were included. At the end of the day, each user reported on the app their total daily number of cigarettes, reasons that any cravings were triggered, and a written answer to the question “What do you think has changed in your relationship to smoking as of today?” (nightly reflections). Fourth, peer‐to‐peer support was provided via the app’s group chat feature for interacting with all other participants. The group chat was moderated by a psychologist and a mindfulness facilitator to promote engagement and respond to participant questions. Fifth, Mindcotine support could be prompted. If participants were inactive for a certain amount of time, they received a text message (after 2 days) and a phone call (after 4 days) to encourage engagement within the programme. Comparison (duration not reported, setting ‐ online) The control group received the online version of the smoking cessation manual from the Argentine Ministry of Health. |
|
| Outcomes | Follow‐up at 12 weeks: self‐reported smoking abstinence | |
| Notes |
Country: Argentina Funding: This research was supported financially by the University of Flores (internal protocol number 17EX04), by a grant received from the Argentine Ministry of Production (Seed Fund project number 3291022226), and through sponsorship by MindCotine Inc. Declarations of interest: JT’s contribution to this publication was as a member of the Advisory Board of MindCotine Inc. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflicts of interest policies. EG, NR, and CW have shares in MindCotine Inc, the company that developed the VR environments and the app for this study. JU and MRC have no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Participants were randomised into the treatment group (TG) (N=60) or control group (CG) (N=60) 1:1 using a blocked random assignment sequence" (but does not say how randomisation was done) |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "The treatment group was invited to the research site (University of Flores) and all participants signed an informed consent form, underwent the face‐to‐face on boarding process with one member of the study team, and were given the Mindcotine Kit to begin the study. The control group was contacted through email". No further details. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "All assessment invitations were sent via email and assessments were completed online using the Typeform (Typeform SL) platform." No further details. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Participants with missing data at follow‐up were assumed to be smoking." High proportion of missing data at follow‐up (48/60 in the control group). |
| Selective reporting (reporting bias) | Unclear risk | Trial retrospectively registered; 5 facet mindfulness scale outcomes not fully reported. |
| Other bias | High risk | High losses to follow‐up in the control group meant that these data were not analysed at 90 days, only 1 day abstinence data compared between groups. Self‐reported smoking cessation not biochemically confirmed. |
Gu 2018.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Study conducted between January 2015 and March 2017 Inclusion criteria: Stable CHD Exclusion criteria: Not reported Number eligible not reported; 146 randomised overall, aged 45 to 79 years, 59% men Medications at baseline: Medication at baseline and medication change during the trial not reported |
|
| Interventions |
Intervention (duration 12 weeks, setting not reported): Usual care plus mindfulness‐based stress reduction programme for 2.5‐h 2 times per week for 12 weeks Comparison (duration 12 weeks, setting not reported): Usual medical care |
|
| Outcomes | Follow‐up at 12 weeks: depression and QoL in cohort of 146, blood pressure, depression, lipids, FBG and QoL reported in cohort of 112 | |
| Notes |
Country: China Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Abstract so few details and only states randomised. |
| Allocation concealment (selection bias) | Unclear risk | No information ‐ abstract so few details reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information but blinding unlikely. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information ‐ abstract so few details reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information ‐ abstract so few details reported |
| Selective reporting (reporting bias) | Unclear risk | Abstract so few details reported. Lipoprotein levels measured but not reported on. |
| Other bias | Unclear risk | Data from 2 abstracts, little detail provided, selective reporting and N numbers per group not provided so data could not be used in meta‐analyses. |
Guo 2021.
| Study characteristics | ||
| Methods | Parallel‐group RCT ‐ pilot study | |
| Participants | Recruitment took place from March to May 2020 in the endocrinology ward of a tertiary hospital in Changsha, the capital city of Hunan Province, China. Inclusion criteria: Adults (aged over 18 years) who (a) were diagnosed with T2DM, (b) were screened by the Diabetes Distress Scale with diabetes distress score ≥ 3, (c) could access a smart phone with WeChat App useable, (d) could read, write, and speak Chinese, and (e) were assessed by physicians as needing to stay in hospital for treatment at least 8 days to make sure there was time enough to complete the in‐hospital sessions of nurse‐led MBSR Exclusion criteria: (a) A history of mental disorders (e.g. history of depression confirmed by the previous diagnosis) limiting their ability to read and comprehend the consent form and fill out the questionnaires, (b) experiencing difficulty or pain in attending sessions due to comorbid conditions, such as cancer, cardiovascular disease, (c) severe complications (e.g. diabetic cardiomyopathy, nephropathy, retinopathy,neuropathy), which would compromise participation, and (d) had previously participated in mindfulness interventions, cognitive therapy, or structured psychosocial intervention Number eligible not reported; 50 randomised to the intervention group (mean age 61.1, 42% men) and 50 to the comparison group (mean age 63.7, 52% men) Medications at baseline: Diabetes treatment at baseline: oral medication in 44% of the intervention group and 44% of the comparison group, insulin in 24% of the intervention group and 26% of the comparison group, both oral medication and insulin in 20% of the intervention group and 20% of the comparison group, no medication in 12% of the intervention group and 10% of the comparison group Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 12 weeks, hospital setting and via WeChat): Nurse‐led MBSR and diabetes education The intervention group received the nurse‐led MBSR therapy which consisted of 8 daily in‐person group sessions of 120 min plus an 8‐week technology‐based maintenance practice component using WeChat (a popular social media application in China) through a smartphone with Internet access. The nurse‐led MBSR therapy protocol was used to guide each session and ensure standardisation in delivering the intervention across groups. The protocol was modified based on the conventional MBSR therapy which was conducted among Chinese people with T2DM (Ten 2018), but delivered in different formats and dosages. The themes of the 8 sessions of both formats of MBSR therapy are the same: mindfulness training, non‐judging, patience, the beginner's mind, trust, non‐striving, acceptance, and letting go. The protocol was reviewed and approved by an expert panel, including 2 Chinese nurses experienced in diabetes care, 3 Chinese clinical psychologists with expertise in the conventional 8‐week MBSR therapy, and 3 adults with T2DM. Eight to 12 participants per group received the intervention in hospital. To improve attendance, the training was conducted when there was less disruption from unit assessments and treatments. The intervention was supervised by an MBSR‐certified supervisor (Kabat‐Zinn 1990) who was also a diabetes educator. Intervention maintenance for mindfulness activities by a well‐developed and free accessible WeChat mini programme (http://www.inmapp.cn/app/6587) was recommended for the participants after they completed the in‐person sessions. The dosage was 30 min/day, 6 days/week for 8 consecutive weeks. At the end of the 8th in‐person session, the MBSR‐certified supervisor taught participants how to use the WeChat mini programme to practise, reminded them by text to start and tracked adherence by asking for daily screenshots when they logged into the programme. Comparison (duration 12 weeks, hospital setting): Diabetes education Both groups received the same regular diabetes education which was routinely provided in an endocrinology ward. The diabetes education (10 to 30 min/day in the sickroom, in Q & A format, during the hospitalised days) was conducted by a diabetes educator and covered self‐management strategies, such as skilful integration of healthy diet, regular exercise, optimum weight control, self‐monitoring of blood glucose, and medication adjustment into the daily routine over long periods. The regular diabetes education protocol was used to guide the whole process and ensure the quality of delivery across groups. The diabetes educator who provided regular diabetes education did not know the content of the nurse‐led MBSR therapy programme or receive mindfulness‐based training. To avoid contamination, the diabetes educator and participants were asked to sign a non‐disclosure agreement not to share the treatment materials or protocol with others before the completion of the study. |
|
| Outcomes | Follow‐up at 12 weeks: HbA1c, diabetes self‐efficacy, diabetes self‐management | |
| Notes |
Country: China Funding: This study was supported by the Youth science and technology of Hunan province (2019RS2006); and the Innovation Project of Graduate students of Central South University in 2020 (Grant No.2020zzts848). Declarations of interest: The authors declare that there are no conflict of interests. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomized in a 1:1 ratio into two groups by random numbers until 100 participants were included. The random numbers were generated and prepared by the statistician through PASW Statisitics 17, which were uneditable and concealed for others". |
| Allocation concealment (selection bias) | Low risk | "The random numbers were generated and prepared by the statistician through PASW Statisitics 17, which were uneditable and concealed for others. The statistician was not involved in the trial and only the first author could refer to the random numbers. To follow the allocation concealment mechanism, the participants were informed of their group allocation via a sealed opaque envelope". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Due to the nature of the psychological intervention, it did not allow blinding of diabetes educators or participants". We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The statistician who was involved in data analyses and the research assistant who collected the data were blinded for treatment allocation". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "An intention‐to‐treat principle was used in analyzing the follow‐up data on the participants who did not complete their tasks. We used the carry forward method to fill in the missing data". 13/50 (26%) lost to follow‐up in both groups. |
| Selective reporting (reporting bias) | Unclear risk | Paper states that "In this paper, the efficacy of the nurse‐led MBSR to improve primary outcomes of diabetes distress, diabetes self‐efficacy, diabetes self‐management, and HbA1c were reported. The secondary outcomes (depressive symptoms, anxiety, perceived stress, and quality of life) will be reported in a separate paper". No further outcomes listed in the trial registration. No citation for a further paper reporting secondary outcomes. |
| Other bias | Unclear risk | Insufficient information to judge |
Hafner 1982.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Referred from hospital colleagues and local GPs. Recruitment dates not reported. Inclusion criteria: Essential hypertension Exclusion criteria: Not reported Number eligible not reported; 21 randomised overall, mean age 48.9 years, sex not reported Medications at baseline: 19/21 were taking antihypertensive medication at baseline and this remained unchanged during the study. |
|
| Interventions |
Intervention (duration 8 weeks, setting not reported): Two interventions: meditation alone and meditation plus biofeedback Meditation alone group – after 2 introductory sessions, patients were given 8 x 1‐hour training sessions weekly. Each session focused on achieving physical relaxation using instructions from a therapist and patients were then asked to focus on their breathing, or on a mental image or to repeat mentally a relaxing phrase depending on patient preference. Patients were asked to repeat the procedure twice daily. Meditation plus biofeedback was identical except during therapist sessions; biofeedback was given using electrical skin resistance. Comparison (duration 8 weeks, setting not reported): No treatment control |
|
| Outcomes | Follow‐up at 12 weeks: blood pressure | |
| Notes |
Country: Australia Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only states randomly allocated |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information but blinding unlikely. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were followed up |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified to check |
| Other bias | Unclear risk | Insufficient information to judge |
Huerin 2015.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Recruited from different centres of the Autonomous City of Buenos Aires and the Buenos Aires suburbs. Trial dates between January and October 2013. Inclusion criteria: Men and women over 21 years of age, with a history (3 months or more) of acute myocardial infarction, coronary angioplasty, myocardial revascularisation surgery, stable chronic angina or dilated cardiomyopathy/insufficiency stable heart rate. Exclusion criteria: Not reported Number eligible not reported; 35 randomised to the intervention group (mean age 60.2, 75% men) and 35 to the comparison group (mean age 62.4, 66.7% men) Medications at baseline: Angiotensin receptor antagonist therapy at baseline in 63.9% of the intervention group and 66.7% of the comparison group. Aspirin therapy at baseline in 94.4% of the intervention group and 90.9% of the comparison group. Beta‐blocker therapy at baseline in 88.9% of the intervention group and 84.9% of the comparison group. Statin therapy at baseline in 94.4% of the intervention group and 81.9% of the comparison group. Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 12 weeks): Meditation group ‐ patients assigned to this group were transferred to a property in the town of Capilla del Señor, Buenos Aires, to the company Alkymia Global specialists in APP (pituitary pineal activation) meditation where they were trained in this practice during 2 days. APP meditation is a technique of intervention other than TM, based on activations and connections of the subject with images related to their cardiovascular health. The patients had to perform the meditation practice twice a day for 20 minutes. Following this, patients received a CD with a guide to exercises to perform during the 12 weeks. Six fortnightly group sessions were held for monitoring, by the Alkymia team, to reinforce and review the techniques learned. The intervention did not involve any type of renunciation of beliefs, creeds or religion that patients had. Comparison (duration 12 weeks, setting not reported) Cardiovascular education group ‐ patients assigned to this group had to attend 2‐hour workshops on cardiovascular prevention and health education, in which they received information on cardiovascular risk factors and cardiovascular prevention measures. Six biweekly educational talks were given by doctors on healthy lifestyles. |
|
| Outcomes | Follow‐up at 12 weeks: HRQoL | |
| Notes |
Country: Argentina Funding: The study received as its only financial support the cost of the lodging, meals and transfer to the premises for training of patients assigned to the meditation group and the salary of a doctor trained in data entry, evaluating patients for their inclusion and performance of measurements at the beginning and at 12 weeks. These costs were financed by Alkymia Global, entity which remained outside the results of the investigation. Declarations of interest: The authors developed this work with funding part of the Alkymia Global company, in charge of the meditation programme. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was simple 1:1. No further details. |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Statistical analysis was blind. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 70 (91%) completed the study. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry number identified to check |
| Other bias | Unclear risk | Insufficient information to judge |
Ingraham 2017.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Women were recruited in community settings as well as through Lyon‐Martin Health Services (LMHS). Community recruitment included LB‐focused listservs, social networking groups, and Pride festivals. Dates of recruitment not reported, but intervention delivered between 2013 and 2014. Inclusion criteria: Eligible participants were women aged 40 years and older who identified as lesbian or bisexual, had a BMI of ≥ 27, and intended to remain in the San Francisco Bay Area for the intervention period. Transgender women and gender‐queer individuals were eligible to participate if they self‐identified as lesbian or bisexual. Exclusion criteria: Not reported Number eligible not reported; 41 randomised to the intervention group (median age 52, 0% men) and 39 to the comparison group (median age 55, 0% men) Medications at baseline: Not reported Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 12 weeks, setting not reported): Community and academic leaders in LB health contributed to intervention development, which was also guided by formative focus group research. Curriculum content was adapted from existing MBSR. The WHAM intervention was clinician‐delivered with weekly sessions facilitated by a licensed social worker or clinical psychologist. Nutrition and physical activity components were led by a registered dietician and a certified personal trainer, respectively, who met individually with women at least once during the 12 weeks to set goals and provide tailored guidance, and they also made monthly presentations during the group meetings. The facilitator delivered the remaining physical activity and nutrition components during each session. The 12‐week WHAM program consisted of weekly support group sessions where each session included MBSR, nutrition and exercise advice, and health education, and each of the components incorporated discussion regarding the lived experiences of LB women. Each session started and ended with brief meditation and relaxation to ground participants in the group. Mind/body approaches were used to improve stress management, restructuring thoughts about body image and relationships to food, and learning relaxation exercises. Activities include learning mindful eating; practising relaxation exercises, including breathing and yoga; and understanding emotional eating patterns through food and emotion journaling. The curriculum for nutrition concentrated on strategies for increasing vegetable and fruit consumption, meal preparation at home, food insecurity and healthy eating with limited income, and more nutritious choices when eating out. In addition to meeting with a personal trainer, guidance on integrating physical activity into the activities of daily living (e.g. housework, walking, climbing stairs, and running errands) was emphasised in group guest lectures. Didactic sessions were designed to build participant knowledge and understanding of relevant health topics that complemented the practical mindfulness, nutrition, and exercise work. Comparison (duration 12 weeks, setting not reported): Delayed start intervention group |
|
| Outcomes | Follow‐up at 12 weeks: total, LDL, HDL cholesterol and triglycerides, HbA1C, HRQoL | |
| Notes |
Country: USA Funding: This study was supported by the Contract No. HHSP23320095629WC/HHSP23337005T‐01 from the Department of Health and Human Service’s Office on Women’s Health. Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | A stepped‐wedge design was used where the order to which the participants received the intervention was randomly assigned. |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information, but blinding unlikely. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Achieved 88% retention at visit 2 with no differences in attrition by study arm. Overall, 84% of women participated in at least one intervention session; however, attendance varied widely. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry number identified to check |
| Other bias | Unclear risk | Baseline data not reported, so change from baseline could not be reported and data were not included in meta‐analyses. |
Izgu 2020.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Potential participants were contacted through the hospital registration system. Also, a clinical nurse who was actively working in the endocrine outpatient unit listed potential participants every week and informed the principal investigator. Evaluated for eligibility between June 2018 and September 2019. Inclusion criteria: The inclusion criteria of patients with type 2 diabetes included those who were (a) diagnosed with diabetic peripheral neuropathic pain (DPNP), (b) at least primary school graduates, and (c) not using any other complementary or integrative therapy during the study period. Exclusion criteria: (a) Neuropathy history due to any other causes such as megaloblastic anaemia, fibromyalgia, autoimmune diseases, hypothyroidism, and lumbar disc hernia; (b) having end‐stage renal failure, chronic obstructive pulmonary disease, advanced cardiac failure, musculoskeletal disorders, or depression; and (c) having a diabetic foot ulcer or amputation Number eligible 102; 77 patients were stratified in terms of current medication use for DPNP and then randomised to 3 groups; 25 randomised to the mindfulness intervention group (mean age 61.6, 43.5% men), 28 to the progressive muscle relaxation (PMR) comparison group (mean age 64.2, 36% men), and 24 to the diabetes education attention control comparison group (mean age 64.1, 50% men) Medications at baseline: More than half of the participants in all 3 groups were using both oral antidiabetic medications and insulin (mindfulness group = 60.9%, PMR group = 68.0%, attention control group = 55.0%). All 3 groups had similar use of medication for neuropathic pain. Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 12 weeks, setting ‐ home‐based): Patients were taught about mindfulness from an experienced teacher and training booklet for 30 minutes in the endocrine unit and led through a practice for 20 minutes. Audio files were recorded for patients to use at home. A sitting mindfulness meditation, from the MBSR programme (Kabat‐Zinn 2013) was used. Each daily session lasted for 20 min, with thoughts focusing on deep breathing and the present moment. Comparison(s) (duration 12 weeks, setting ‐ home based): Patients were taught about PMR from an experienced teacher and training booklet for 30 minutes in the endocrine unit and led through a practice for 20 minutes. Audio files were recorded for patients to use at home. Each daily session lasted for 20 min, during which participants were instructed about tensing and relaxing the body muscles, along with deep breathing. The patients practised daily progressive muscle relaxation, beginning with the muscles of the face and head, followed by those of the neck, shoulders, hands, chest, abdomen, legs, and feet. The participants of the attention control group were invited to the endocrine unit and received only 1 session of diabetes education. The sessions lasted for 30 min and were performed based on training booklets designed by the research team, focusing on the anatomy and physiological functions of the pancreas and general information about type 2 diabetes, including signs, complications, and treatment methods. At the end of the session, training booklets were given to the participants. Participants in the mindfulness and PMR groups were asked to finalise progressive home practice at the end of the 12th week to the follow‐up assessment at week 14. |
|
| Outcomes | Follow‐up at 14 weeks: HRQoL (neuropathic pain impact on quality of life questionnaire) | |
| Notes |
Country: Turkey Funding: This study was funded by the Scientific Research Projects Coordination Unit of Hacettepe University, Turkey (THD‐2018‐17087). Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly allocated into study groups A (RG), B (MG), or C (CG), with 28, 25, and 24 participants in the groups, respectively, through a random number assignment list that consisted of 6 combinations (ABC, ACB, BCA, BAC, CAB, CBA), generated using an online randomiser (www.randomizer.org). |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Following allocation, the PI informed the participants about randomisation results and asked them to not inform the data collector". We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Data collection was performed by the second co‐author, who was unaware of allocation". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 77 randomised but only "69 analysed"; not clear if health‐related reasons for discontinuing evenly balanced across groups. |
| Selective reporting (reporting bias) | Unclear risk | Trial retrospectively registered |
| Other bias | Unclear risk | Insufficient information to judge |
Jalali 2019.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Patients with CVD referring to the clinic were selected using convenience sampling in 2016. Inclusion criteria: Ensuring development of vascular disease, which included coronary heart disease, angina, myocardial infarction, stroke, and peripheral vascular disease, diagnosed by a cardiologist; having at least completed secondary education to be able to participate in sessions and to perform assignments. Exclusion criteria: Having a history of psychiatric drug use, having received psychological treatment in the past 6 months, and not attending more than 2 consecutive sessions or more than 3 non‐consecutive sessions. Number eligible not reported; 30 randomised to the intervention group (50% men) and 30 to the comparison group (50% men), overall mean age 52 years Medications at baseline: Not reported Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 8 weeks, setting not reported): Received routine medical care and also attended 8 sessions of the MBSR programme in accordance with the proposed protocol of Kabat‐Zinn (Kabat‐Zinn 1990). Two groups of 15 men and 15 women. Comparison (duration 8 weeks, setting not reported) Received routine medical care and the MBSR intervention at study completion. |
|
| Outcomes | Follow‐up at 12 weeks: HRQoL, self‐efficacy | |
| Notes |
Country: Iran Funding: This study was supported by the Research and Technology Deputy of the Islamic Azad University, Shahrekord Branch, Shahrekord, Iran with grant number 13320705939042. Declarations of interest: There is no conflict of interest regarding this work. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only states randomised, no further details |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The MBSR intervention was performed by a psychologist and data collection and analysis by the researcher so that the study was single‐blind. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Data collection and analysis were performed by the researcher, not the psychologist delivering the intervention, so that the study was single‐blind. Outcomes reported are subjective and participants were not blind to group allocation, so left as unclear risk. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry number identified to check |
| Other bias | Unclear risk | Insufficient information to judge |
Jang 2018.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | CAD patients who were regularly visiting the outpatient clinic and receiving medication. Recruitment dates not reported. Inclusion criteria: CAD patients with increased risk of depression, anxiety and anger who scored over 70 points in the T‐scores on the sub‐scales measure of depression, anxiety, and aggression on the Personality Assessment Inventory Exclusion criteria: Not reported Number eligible 70; 21 randomised to the intervention group (mean age 64.8, 40% men) and 23 to the comparison group (mean age 65.3, 60% men) Medications at baseline: Not reported Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 12 weeks, setting not reported): Subjects in the mindfulness‐based art therapy (MBAT) group received 12 sessions lasting 45 minutes each. MBAT is based on Kabat‐Zinn's MBSR (Kabat‐Zinn 1990) and Monti's MBAT (Monti 2006). The MBAT group was divided into A (N = 11) and B (N = 12), and the same type of treatment was given to both of them. The therapist encouraged the patients to express their inner pain or feelings sufficiently. The therapist was a specialist in mental health medicine and had a doctorate in expressive arts therapy with more than 10 years' experience in the field. A senior researcher who is a mindfulness meditation‐based group art therapy instructor provided clinical supervision. Comparison (duration 12 weeks, setting not reported): Wait list control |
|
| Outcomes | Follow‐up at 12 weeks: anxiety, depression | |
| Notes |
Country: Republic of Korea Funding: This study was supported by Wonkwang University, 2017. Declarations of interest: The authors have no potential conflicts of interest to disclose. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by flipping a coin. |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information, but blinding unlikely. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 3 participants lost to follow‐up. Three discontinued treatment in the control arm compared to 0 in the intervention arm. Only 1 participant did not receive allocated intervention in the intervention arm compared to NA in the control arm. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry number identified to check |
| Other bias | Unclear risk | Insufficient information to judge |
Kalinowski 2021.
| Study characteristics | ||
| Methods | Parallel‐group RCT ‐ pilot study | |
| Participants |
Inclusion criteria: Women meeting JNC 7 criteria for pre‐hypertension (SBP 120 to 139 mmHg or DBP 80 to 89 mmHg) and not taking antihypertensive medication were recruited from outpatient clinics or via the EHR. Exclusion criteria: Not explicitly reported Number eligible not reported; 37 randomised overall (mean age 50.4, 0% men) Medications at baseline: Medication for hypertension was an exclusion criteria Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 8 weeks, setting ‐ intervention delivered by telephone): MBCT‐T (MBCT delivered by telephone), which involved 8 weekly 1‐hour phone sessions delivered to small groups by a trained instructor. Comparison (duration 8 weeks, setting not reported) Usual care |
|
| Outcomes | Follow‐up at 12 weeks: SBP, depression, perceived stress | |
| Notes |
Country: not reported Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information: conference proceeding so few details |
| Allocation concealment (selection bias) | Unclear risk | No information: conference proceeding so few details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information: conference proceeding so few details, but blinding unlikely. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information: conference proceeding so few details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 89% retention overall, but approximately 50% had missing blood pressure measurements due to COVID‐19 research restrictions. |
| Selective reporting (reporting bias) | Unclear risk | No information: conference proceeding so few details |
| Other bias | Unclear risk | Insufficient information to judge |
Kopf 2014.
| Study characteristics | ||
| Methods | Parallel‐group RCT, The Heidelberger Diabetes and Stress‐Study (HEIDIS‐Study) | |
| Participants | Recruited from the Diabetes Outpatient Clinic at the University of Heidelberg. Recruitment dates not reported. Inclusion criteria: Diabetes type 2, albuminuria (> 20 mg/L in 2 separated spot urines), age 30 to 70 Exclusion criteria: Diabetes duration < 3 years, pre‐existing non‐diabetic kidney disease, psychiatric disorders, alcohol or drug abuse, malignant tumours or haematologic disorders, heart failure NYHA III‐IV, acute coronary syndrome Number eligible not reported. 694 patients were evaluated in the Diabetes Outpatient Clinic at the University of Heidelberg. A total of 110 patients fulfilled the inclusion criteria and provided written informed consent; 53 randomised to the intervention group (mean age 58.7, 70.2% men) and 57 to the comparison group (mean age 59.3, 80.7% men). Medications at baseline: Insulin therapy at baseline in 60% of the intervention group and 56.6% of the comparison group. Metformin therapy at baseline in 66% of the intervention group and 64.9% of the comparison group. Sulfonylurea therapy at baseline in 26.4% of the intervention group and 26.3% of the comparison group. Meglitinide therapy at baseline in 3.8% of the intervention group and 1.8% of the comparison group. Glitazone therapy at baseline in 14% of the intervention group and 15.1% of the comparison group. ACE/AT1‐inhibitor therapy at baseline in 59.7% of the intervention group and 94.3% of the comparison group. Beta‐blocker therapy at baseline in 52.6% of the intervention group and 52.8% of the comparison group. Calcium antagonist therapy at baseline in 29.8% of the intervention group and 50.9% of the comparison group. Statin therapy at baseline in 56.6% of the intervention group and 54.4% of the comparison group. Acetylsalicylic acid therapy at baseline in 58.5% of the intervention group and 54.4% of the comparison group. Loop diuretic therapy at baseline in 19.3% of the intervention group and 24.5% of the comparison group. Thiazide diuretic therapy at baseline in 41.5% of the intervention group and 33.4% of the comparison group. Medication changes during the trial not reported |
|
| Interventions |
Intervention (duration 8 weeks, setting not reported): Standard 8‐week MBSR protocol (Kabat‐Zinn 1990), adapted for patients with type 2 diabetes (Faude‐Lang 2010), specifically by including practices for difficult thoughts and feelings related to diabetes. Participants met once weekly in groups of 6 to 10 and for a booster session after 6 months. The groups were led by a psychologist and a resident in internal medicine. Comparison (duration 8 weeks, setting – diabetes outpatient clinic): Treatment as usual control group To guarantee standardised medical treatment as usual according to diabetes guidelines in both arms, all patients were seen on a regular basis by a physician in the outpatient clinic. |
|
| Outcomes | Follow‐up at 12 months, 24 and 36 months (scheduled yearly for 5 years). Used 12‐month data for ambulatory and maximum SBP and DBP, depression, stress, lipids, FBG, HbA1C, BMI, HRQoL. Clinical events (cardiac death, non‐fatal MI, stroke) reported at 36 months. | |
| Notes |
Country: Germany Funding: This study was supported by the Lautenschläger Stiftung (to P.P.N.) and the European Foundation for the Study of Diabetes (to A.B.) Declarations of interest: No potential conflicts of interest relevant to this article were reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information in paper, and citation to other paper does not give any more detail. |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label, but as it is difficult or impossible in most cases to blind participants to lifestyle interventions, this is left as unclear risk. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition low but unbalanced. Intervention 2% at 1 year and 11 % at 3‐year follow‐up; control: 11% at year 1 and 26% lost at year 3. ITT used for all outcomes. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes mostly match those in trial registry. QoL does not appear to be reported in the paper, despite being listed on registry and mentioned in the paper as having been measured. |
| Other bias | Unclear risk | Insufficient information to judge |
Kristeller 2014.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Participants were recruited through local advertisements that requested participation from individuals who binge‐eat and were concerned about their weight in Durham, NC and Terre Haute, IN. Recruitment dates not reported. Inclusion criteria: Participants were overweight or obese and reported binge‐eating, which was further assessed using the Eating Disorder Examination. Two thirds (N = 100) met the full DSM‐IV criteria for Binge Eating Disorder (BED) at baseline; 11 reported 5 to 7 binges per month but met all other BED criteria; and 39 met the behavioural criteria (2 binges per week) for BED, but reported subclinical levels of distress generally due to a sense of having “given up” struggling or to viewing their bingeing behaviour as socially acceptable, even if not controllable. Exclusion criteria: Individuals who reported any suicidal symptomology of concern or other psychiatric symptoms potentially likely to interfere with group participation or follow‐up (e.g. psychotic symptoms; drug/alcohol abuse; or unstable medication use) were screened out. Exclusion criteria included previous regular meditation practice; relevant unstable medical conditions (e.g. diabetes likely to require medication changes); concurrent participation in a weight loss programme or psychotherapy focused on weight or eating issues; pregnant or breast‐feeding (due to weight fluctuation); or purging or laxative abuse within 6 months that would meet the criteria for purging bulimia. Number eligible not reported. 150 participants overall, mean age 46.6, mean BMI 40.3, 12% were male and randomised to 3 groups: 53 randomised to the MB‐EAT group, 50 to the psychoeducation group and 47 to a wait list control group. Medications at baseline: Not reported Medication change during the trial not reported |
|
| Interventions | Participants in both interventions received a manualised 12‐session intervention (9 weekly sessions with 3‐monthly booster sessions). In both conditions, sessions were 1.5 h, except for 2 h for session 1 and for Session 6 as it included a pot luck meal. Rather than focusing on weight loss per se, both interventions focused on becoming more aware of patterns of inappropriate eating and on providing appropriate tools and group support for making sustainable change in these patterns. Intervention (duration 21 weeks, setting not reported): The MB‐EAT programme (Kristeller 2011) is designed to increase mindful awareness of experiences related to eating and to decrease mindless or habitual reactivity. In particular, mindful awareness exercises focus on physical hunger and satiety cues, overall food intake, and physical, cognitive, social‐environmental, and emotional triggers of bingeing. Three forms of meditation are used: general (breath/open awareness) mindfulness meditation, guided eating meditations, and “mini‐meditations” to be used at mealtime and throughout the day. Comparison(s) (duration 21 weeks, setting not reported): The psychoeducational cognitive behavioural (PECB) treatment was closely modelled on the programme developed at the Duke Diet and Fitness Center for clients with BED. PECB was designed to provide a comparison treatment known to be clinically effective and to control for non‐specific factors including expectancy, group support, instructor attention, and time spent on homework. Content includes education on obesity and binge‐eating and on basic concepts drawn from cognitive‐behavioural models, such as emotional and cognitive triggers for eating and cultivating alternative coping strategies. It also contains education and skill‐building exercises on nutrition, portion control, fitness, principles for making lifestyles changes, stress management (including problem‐solving, progressive muscle relaxation, and assertiveness training), and psychosocial recommendations for managing binge‐eating and building a support network. Homework was specific to weekly lessons, such as creating a meal plan using guidelines presented in that session. A pot luck meal also occurred, with a focus on nutrients and portion sizes, rather than on mindful choice and mindful eating. While much of the group was structured around educational and skill building materials, time was allowed for discussion of the personal relevance of the material, as in MB‐EAT. The wait list control participants received no treatment during the course of the active cohort in which they were enrolled, but were offered access to either mindful eating or PECB training subsequently. They were contacted midway through the active treatment period for assessment and to encourage retention. |
|
| Outcomes | Follow‐up at 1 and 4 months post intervention: depression, BMI, eating self‐efficacy | |
| Notes |
Country: USA Funding: This research was funded by a grant from the National Institutes of Health, National Center for Complementary and Alternative Medicine “Meditation‐Based Treatment for Binge Eating Disorder” NIH Grant: R21 AT00416‐01. Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "assigned to treatment by random number drawing" and "Ten other participants (N=3, 2, and 5 per condition) were assigned to conditions based on logistic constraints (i.e., the only night they could attend meetings); all such assignments occurred prior to the individual knowing which group met on a given night and after all baseline assessments were completed". 140 randomised overall, 10 assigned. |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Participants were provided a brief individual orientation to their assigned treatments". We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information. Many of the measures used were self‐reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "missing data on active participants were imputed from the most proximal measurement point."; "Intention‐to‐treat” (ITT) analyses were conducted to assess the robustness of the treatment (i.e. whether treatment effects would be evident when non‐completers were assumed to have been unaffected by the treatment)." High losses to follow‐up: 26.4% MB‐EAT, 46% PECB, 44.7% WL. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry number identified to check. |
| Other bias | Unclear risk | Insufficient information to judge. |
Malm 2018.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Recruited patients and spouses at the Department of Cardiac Diseases, at a county hospital in southern Sweden, between September 2011 and December 2015. Inclusion criteria: (a) Age 18 years or above; (b) atrial fibrillation (AF) diagnosis, and (c) accompanied by a spouse Exclusion criteria: Patients were excluded if they had severe complications due to their current disease, unstable coronary artery disease, sepsis or other severe infection, AF early after thoracic surgery, acute pulmonary embolism, known hyperthyroidism, malignant disease with expected survival of less than one year, dementia, residence outside the hospital’s catchment area, current participation in another study and difficulties with the Swedish language preventing them from completing questionnaires. Number eligible 122; 56 randomised to the intervention group (mean age 67.5, 53.7% men) and 55 to the comparison group (mean age 66.9, 54.1% men) Medications at baseline: Medication at baseline in each group not reported Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 9 weeks, setting not reported): The CBT programme consisted of three 2.5‐hour group sessions over a period of 9 weeks, with 4 to 6 AF patients, including spouses. The sessions were conducted by 3 therapists (a cardiac nurse, a cardiologist, and an educationalist), who were trained by an educator with certification from HeartMath Scandinavia in CBT, with a focus on mindfulness practices. In these sessions, participants were trained to be aware of their breathing, and heart rate variability biofeedback was demonstrated during these training sessions. HeartMath comprises a series of self‐regulation techniques that facilitate, through the regulation of emotions and behaviours, psychological coherence. A detailed tutorial manual was developed for the therapists, which outlined the contents of how to teach the exercises to the patients and their spouses, both at the meetings and at home. A corresponding participant manual was provided to each patient that contained the educational information, as well as instructions on mindfulness practice, to be followed at home. Each session included at least a 15‐ to 20‐minute mindfulness practice guided by the therapist. This was followed by an enquiry about the participants’ experiences during practice, as well as encouragement to practise at home on a daily basis. Comparison (duration 52 weeks, hospital setting) Treatment as usual comparison group. Patients in both groups received standard treatment at the hospital. |
|
| Outcomes | Follow‐up at 52 weeks: anxiety, depression, HRQoL | |
| Notes |
Country: Sweden Funding: This work was supported by the Medical Research Council of Southeast Sweden (FORSS, 464211), Jönköping County Council, Sweden Declarations of interest: None declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A 1:1 randomisation was performed by an independent statistician, with a computer‐generated sequence of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information, but blinding unlikely. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The statistician and outcome assessors were blinded to the study groups. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only 41/56 (73%) of CBT group and 37/55 (67%) of treatment as usual group analysed. |
| Selective reporting (reporting bias) | Unclear risk | Trial registry says SF‐36 will be used for HRQoL as primary outcome, and EQ‐5D as a secondary outcome, but paper only reports EQ‐5D (with P = 0.03). Paper also reports anxiety and depression, which were not mentioned in the trial registration. |
| Other bias | Unclear risk | Insufficient information to judge |
McTigue 2020.
| Study characteristics | ||
| Methods | Parallel‐group RCT ‐ feasibility | |
| Participants |
Inclusion criteria: Adult volunteers with uncontrolled hypertension from UPMC primary care Exclusion criteria: Not reported Number eligible not reported, 644 assessed; 37 randomised to the intervention group and 39 to the comparison group, mean age and % men not reported Medications at baseline: On average took 2 medications daily for hypertension. Reported for overall population and not by group. Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 17 weeks, online setting): 17‐week online behavioural support intervention for BP that includes interactive lessons to support healthier diet, physical activity choices, and medication adherence, self‐monitoring tools and e‐coaching. The intervention can be delivered with or without a mindfulness curriculum for stress reduction (Minding GOALS‐BP (MGBP) and GOALS‐BP (GBP)). Comparison (duration 17 weeks, online setting): GOALS‐BP (GBP) |
|
| Outcomes | Follow‐up at 12 months: SBP and DBP | |
| Notes |
Country: USA Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information: conference proceeding so few details |
| Allocation concealment (selection bias) | Unclear risk | No information: conference proceeding so few details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information: conference proceeding so few details, but blinding unlikely. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information: conference proceeding so few details. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 62/76 (81.6%) retained at 12 months follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No information: conference proceeding so few details. |
| Other bias | Unclear risk | Insufficient information to judge |
Miller 2014.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Recruited through local medical practices, the university newswire, radio and electronic advertisements, and community flyers. Dates of recruitment not reported. Inclusion criteria: Eligibility criteria for participation included being age 35 to 65 years with physician‐diagnosed T2DM for ≥ 1 year, body mass index ≥ 27.0, glycosylated haemoglobin ≥ 7.0%, and not requiring insulin therapy. Exclusion criteria: Individuals concurrently participating in a structured weight loss programme or women who were pregnant or lactating were ineligible. 406 were assessed for eligibility, 245 did not meet inclusion criteria and 93 declined to participate; 32 randomised to the intervention group (mean age 53.9, 37% men) and 36 to the comparison group (mean age 54, 36% men). Medications at baseline: Not reported Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 12 weeks, setting not reported): MB‐EAT for Diabetes (MB‐EAT‐D) is a variation of the intervention developed originally for binge‐eating disorder and obesity (Kristeller 2011). Mindful eating is a tool to cultivate attention to increase non‐judgmental awareness of internal experience and automatic patterns related to eating. The training is designed to help individuals interrupt “mindless” and stress‐related eating and re‐engage the natural physiological processes of eating regulation. A primary component was mindfulness meditation and its application to eating. Every session included guided meditations oriented toward the experiences, thoughts, and feelings associated with food intake. Other elements included cultivating awareness between physical and emotional hunger cues, social pressures to eat, and preferences regarding food choices. Participants were encouraged to meditate 6 days/week and to practise mini‐mediations at other times to cultivate awareness of various experiences (e.g. hunger or stress). Components were presented as ways to cultivate “inner wisdom” (i.e. mindful awareness of inner experiences related to eating) and “outer wisdom” (i.e. personal use of knowledge of food/diabetes needs) as MB‐EAT‐D also included basic information regarding diet, physical activity, weight regulation, and glycaemia; however, no specific diet or activity goals were provided. Comparison (duration 12 weeks, setting not reported): The Smart Choices (SC) intervention is based on a behavioural diabetes self‐management education (DSME) programme. The SC intervention was based on social cognitive theory and the theory of meaningful learning and designed to improve diabetes‐related knowledge, outcome expectations, and self‐efficacy for effective self‐care. SC provided in‐depth information regarding the effect of the type and quantity of carbohydrates and fats on blood glucose and lipid parameters; all participants received calorie, carbohydrate, and total fat goals. One session on physical activity was included and several sessions included a 15‐ to 20‐minute walk. However, the study design intentionally de‐emphasised physical activity to maintain the focus on food intake across both conditions. Participants established self‐set goals at the end of each session. Progress in meeting weekly goals and barriers to goal attainment were discussed during the following session. No information regarding mindful eating or meditation was presented. In both groups, each followed a manualised intervention and included 8 weekly and 2 biweekly 2.5‐hour group sessions led by trained facilitators. A dietitian led all cohorts of the DSME intervention, and the same dietitian and a social worker with extensive training in mindful meditation co‐led all cohorts of the MB‐EAT intervention. Participant attendance was tracked, and if individuals missed a group session, they were encouraged to attend a make‐up session. |
|
| Outcomes | Follow‐up at 24 weeks: anxiety, depression, weight, self‐efficacy | |
| Notes |
Country: USA Funding: The research was supported by NIH, including salary support during the course of the study, as well as a grant from the U.S. National Institute of Diabetes and Digestive and Kidney Diseases awarded to Carla Miller (PI) for the proposal R21DK084330, “A Mindfulness‐Based Approach to the Treatment of Obesity and Diabetes.” All co‐authors received support. Declarations of interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to treatment group, stratified by race. Computer randomisation occurred after the collection of baseline data. |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not possible. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 27/32 MB‐EAT participants completed; 25/36 standard care completed data collection. So withdrawals appear unbalanced (16% vs 31%) although the authors state that there was no significant difference in attrition between treatment groups (P > 0.05). |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry identified |
| Other bias | Unclear risk | Insufficient information to judge |
Nasiri 2020.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | The study population comprised patients with acute coronary syndrome who were admitted, being discharged, or discharged (within a week before) the internal wards of Chamran Medical Center affiliated with Isfahan University of Medical Sciences (IUMS), Iran. Trial conducted from September 2018 to July 2019. Inclusion criteria: Ability to attend interventional sessions, minimum reading and writing literacy, age between 35 and 70 years, no attendance in other training and treatment courses over the past year, having coronary artery disease diagnosed (based on clinical and paraclinical diagnosis and file summary), being fully alert as required to attend in the intervention sessions, having ability to complete the research instruments, and living in Isfahan city Exclusion criteria: Patients who did not have these conditions, had other concomitant diseases, had a medical history of psychiatric diagnosis, or had mental retardation were not included in the study Number eligible 73; 32 randomised to the intervention group (mean age 52.7, 62.5% men) and 32 to the comparison group (mean age 52.2, 56.2% men) Medications at baseline: Not reported Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 8 weeks, hospital setting): Mindfulness training, 2 hours each week. Sessions as follows: Session 1: Recognition of excitements, compositional excitements, describe excitement, enjoy the beauty of nature. Session 2: Skill one: Focus on a particular object, skill two: Focus on a single moment, familiarity with the concept of mindfulness at the moment, see the wonders of the present moment. Session 3: Skill three: Inner and outer experience, The concept of physical mindfulness, eating raisins, the great taste of mindfulness, enjoy the journey. Session 4: What is the excitement? What is the feeling? Recognition of excitements, familiarity with some types of excitement, awareness of feelings and excitements, providing a list of main feelings and sub‐feelings, listening to unpleasant excitements. Session 5: What is the excitement? What is the feeling? Recognition of excitements, familiarity with some types of excitement, awareness of feelings and excitements, providing a list of main feelings and sub‐feelings, listening to unpleasant excitements. Session 6: Recognition of thoughts, necessity of training, identifying the difference between thought and feeling, and the concept of thought in mindfulness, Skill five: The fault of thought, Skill six: Record 30 min of thought, keep in your mind that you are different from your thoughts. Session 7: Understanding the concepts of body, thought, and feeling mindfulness, skill seven: mindful breathing. Session 8: A few key methods for a mindful life, Skill eight: A mindful diet, mindfulness in mindful activities. Comparison (duration 12 weeks, hospital setting): Usual care. In order to appreciate the control group’s participation, after the follow‐up stage, 4 similar compressed sessions of intervention were held for them. |
|
| Outcomes | Follow‐up at 12 weeks: perceived stress | |
| Notes |
Country: Iran Funding: This study was financially supported by IUMS, Isfahan, Iran. Declarations of interest: There are no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information ‐ just states randomly allocated |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information but blinding unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information. We have left as unclear risk as iit is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up reported |
| Selective reporting (reporting bias) | Unclear risk | Locus of control listed as an outcome in the trial registry but not reported on |
| Other bias | Unclear risk | Insufficient information to judge |
Nathan 2017.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Most patients were recruited by telephone from a database of patients attending The Ottawa Hospital’s Endocrine and Diabetes Centre who had consented to be contacted regarding research participation. Others were referred from the community. Dates of recruitment not reported, but trial conducted between 5 July 2013 and 4 September 2015. Inclusion criteria: Men and women who were ≥ 18 years of age, had type 1 or type 2 diabetes and symptoms of painful diabetic peripheral neuropathy (PDPN) for > 6 months. Patients responding “yes” to ≥ 3 of the 7 subjective items on the Douleur Neuropathique 4 (DN4) neuropathic pain scale were then asked to rate their pain on 2 visual analogue scales rating from 0 to 10 their pain at rest and with activity at the same time of day for 7 consecutive days. Patients were included if their mean score for either scale was ≥ 4. Exclusion criteria: Patients were excluded if they had previously taken an MBSR or similar course. Number eligible not reported, 255 assessed for eligibility; 33 randomised to the intervention group (mean age 59.7, 50% men) and 33 to the comparison group (mean age 59.8, 37.5% men) Medications at baseline: Not reported Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 8 weeks, community setting): Patients were enrolled in MBSR courses offered at multiple sites in the community by practitioners who had formal training in MBSR and ≥ 5 years of experience as workshop leaders. The methods and materials described in the teacher training course given by the Center for Mindfulness at the University of Massachusetts, where this method was developed, were used (Blacker 2009). The workshops consisted of 9 sessions: 8 weekly, 2.5‐ hour sessions and one 6‐hour session on a weekend day midway through the course. Typically, 2 to 3 study patients would join a group of 12 to 20 MBSR participants with a variety of complaints such as pain, anxiety, or depression. There was no modification of the MBSR course for the purposes of this study. Comparison (duration 8 weeks, community setting): Patients in the control group were offered enrolment in an MBSR course after the study was complete. Patients in both the control and MBSR groups were discouraged from making any changes in medication from the time of randomisation until after the final assessment. |
|
| Outcomes | Follow‐up at 12 weeks: depression, perceived stress, HbA1C, HRQoL | |
| Notes |
Country: Canada Funding: This study was supported by grants from the Canadian Diabetes Association (CDA OG‐2‐12‐3722‐HN) and the University of Ottawa Anesthesiology Research Fund. Declarations of interest: No potential conflicts of interest relevant to this article were reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly allocated to a waiting list or MBSR using computer‐generated random numbers in a permuted block design with randomly varying block lengths. Allocations were stratified by type 1 or type 2 diabetes and by pain severity. |
| Allocation concealment (selection bias) | Low risk | Allocations were generated by an independent statistician and concealed from investigators and treating physicians. Treatment allocation occurred as close as possible to the start of the MBSR course when the next consecutively numbered opaque envelope for the appropriate strata was opened. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not possible. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | After randomisation, participants had had sufficient experience with the instruments that they were able to complete them without any contact from study staff who were aware of the treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 30/33 analysed in the intervention group, 32/33 analysed in the control group. |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes reported in accordance with NCT record |
| Other bias | Unclear risk | Insufficient information to judge |
Nejati 2015.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Recruited when referring to the Hypertension Clinic of Imam Hossein Hospital in 2013. Inclusion criteria: Patients who referred to Imam Hossein Hospital in Tehran in 2013 with a diagnosis of hypertension. Not receiving psychological treatment at the time of diagnosis, having blood pressure > 130/80 mmHg, having high school diploma or higher qualifications, aged between 30 and 55 years, and having similar drug regimen. Exclusion criteria: Patients with mental illnesses and physical diseases such as diabetes, renal disease, liver disease, and a history of myocardial infarction were excluded. Also excluded were pregnant patients and those missing more than 2 intervention sessions. Number eligible not reported; 15 randomised to the intervention group (mean age 43.7, 60% men) and 15 to the comparison group (mean age 43.1, 46% men) Medications at baseline: Medication at baseline in both groups not reported Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 8 weeks, hospital setting): MBSR (Kabat‐Zinn 1990) delivered by 2 holders of master’s degree in psychology familiar enough with the planned intervention. Group treatment was implemented for 8 sessions, once a week for 90 minutes. Comparison (duration 16 weeks, setting not reported) The control group received no treatment. Due to ethical issues, the participants in the control group were given CDs about yoga at the termination of the study. |
|
| Outcomes | Follow‐up at 16 weeks: blood pressure, coping strategies | |
| Notes |
Country: Iran Funding: This study was approved and supported by the Behavioral Sciences Research Center of Imam Hossein Hospital, Shahid Beheshti University of Medical Sciences. Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study population was randomly assigned into experimental and control groups, each consisting of 15 individuals. |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information but blinding unlikely. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding, but only relevant outcome is objective (BP). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Looks like all participants completed and were analysed |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration identified to check |
| Other bias | Unclear risk | Insufficient information to judge |
Ng 2018.
| Study characteristics | ||
| Methods | Parallel‐group RCT ‐ pilot study | |
| Participants | Few details as conference proceeding Inclusion criteria: Patients recruited 5 to 8 months following sleeve gastrectomy. No further details. Exclusion criteria: No details Number eligible not reported; 40 randomised to the intervention and 39 to the comparison group, mean age and % men not reported Medications at baseline: Not reported Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 6 weeks, setting not reported): Six weekly session of mindfulness intervention originally developed to prevent substance abuse relapse Comparison (duration 6 weeks, setting not reported) Treatment as usual |
|
| Outcomes | Follow‐up at 6 and 18 months (12 and 24 months postop): % weight loss and weight regain | |
| Notes |
Country: no information Funding: no information Declarations of interest: no information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States randomly assigned, no further details |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | No information to assess risk of bias as reported only as a conference proceeding so few details. |
Nidich 2009.
| Study characteristics | ||
| Methods | Parallel‐group RCT (data extracted for subgroup only of those at risk of hypertension) | |
| Participants | Students from American University and other surrounding colleges, including Georgetown and University of District of Columbia. Study dates between January 2006 and May 2007. Inclusion criteria: Inclusion criteria included enrolment in an undergraduate or graduate programme through August 2006. Subgroup of interest ‐ Subjects were included in a high‐risk subgroup if they had one or more of the following risk factors for hypertension: systolic BP (SBP) >130, diastolic BP (DBP) > 85, family history of hypertension, overweight, or obesity defined as body mass index ≥ 25. In terms of inclusion criteria for this subgroup, 45% had BP > 130/85, 46% had at least one family member with hypertension, and 50% had a body mass index ≥ 25. Exclusion criteria: Students were excluded if they had a history of hypertension, hypoglycaemia, chronic fainting, coronary heart disease, or current BP above 140/90 mmHg or below 90/60 mmHg. Number eligible for the high‐risk subgroup was 159. Number randomised to each group not reported. 48 completers in the intervention group (mean age 27.7, 37.5% men) and 64 completers in the comparison group (mean age 29.4, 40.6% men). Medications at baseline: Not reported Medication change during the trial not reported |
|
| Interventions |
Intervention (duration and setting not reported): The TM technique was taught in a 7‐step course (Roth 1994) as follows: (i) group introductory lecture on potential benefits and previous research (90 min); (ii) group preparatory lecture, discussing the mechanics and origin of the TM technique (90 min); (iii) personal interview (10 min); (iv) personal instruction session (90 min); (v–vii) group “verification and validation of the practice” sessions on 3 consecutive days following personal instruction, verifying correctness of TM practice, and providing the understanding of the mechanics of the TM technique. After the 7‐step course, students were invited to attend individual meetings with the TM instructor to verify the mechanics of practice and maintain regularity. These meetings were held weekly for the first month, then monthly thereafter (30 min each). Weekly (group) knowledge meetings were also available. Comparison Wait list control group |
|
| Outcomes | Follow‐up at 12 weeks: SBP, DBP, anxiety, depression, coping ability | |
| Notes |
Country: USA Funding: This study was supported, in part, by a Specialized Center of Research Grant from the National Institutes of Health ‐National Center for Complementary and Alternative Medicine (1P50AT00082). Declarations of interest: R.H.S. is a consultant to Maharishi Health Technologies, LLC. Other authors declared no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Students were randomised to experimental or control groups, using the random blocks method, stratifying on gender. |
| Allocation concealment (selection bias) | Unclear risk | The treatment group allocations were concealed by the study statistician, with individual treatment group assignments revealed to the project manager only when study participants completed baseline testing. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single‐blind. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All assessments were administered by research staff who were masked to treatment condition. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | For relevant subgroup, 159 students were randomised and 112 completed 3‐month follow‐up (41.9% loss to follow‐up). |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry identified to check |
| Other bias | Unclear risk | Multiple reports on the effects of TM from the same institution |
Nijjar 2019.
| Study characteristics | ||
| Methods | Parallel‐group RCT – pilot study | |
| Participants | Patients were recruited from a large university‐affiliated hospital system at different points of contact with the care system, including via letters to eligible patients, on‐site at the cardiac rehabilitation settings, and through advertising in cardiology clinic locations. Study conducted between May 2016 and August 2017. Inclusion criteria: Men and women age 21 and older that are medically eligible and been referred for traditional exercise‐based cardiac rehabilitation (heart attack within the past 12 months, open heart surgery such as coronary bypass/valve/heart transplant, coronary angioplasty or stent placement, current stable angina, or heart failure). Because attendance at cardiac rehabilitation (CR) is variable and typically low among referred patients, CR enrolment was not a requirement. Exclusion criteria: Has a cardiac pacemaker and is pacemaker‐dependent or has an untreated atrial arrhythmia; previously completed an MBSR course; unable to read and write in English Number eligible 51; 47 randomised overall, 31 to the MBSR intervention group (mean age 57.5, 54.8% men) and 16 to the usual care group (mean age 58.6, 61.7% men) Medications at baseline: Beta‐blockers 71.0% in MBSR group, 93.8% in usual care group, aspirin 87.1% in MBSR group, 93.8% in usual care group, statin 80.6% in MBSR group, 87.5% in usual care group and ACE‐I/ARB 48.4% in MBSR group, 68.8% in usual care group Medication change during the trial not reported |
|
| Interventions | The study recruited patients eligible for exercise‐based cardiac rehabilitation (CR). Randomisation to either MBSR or control (no MBSR) condition occurred within two strata (CR; no CR) based on current enrolment in CR at time of study enrolment. Intervention (duration 8 weeks, setting not reported): The study intervention was the 8‐week MBSR course that was developed by Jon Kabat‐Zinn (Kabat‐Zinn 1990). It consists of 8 weekly 2.5‐hour group sessions, and 1 all‐day (6.5 hours) retreat, taught per standard protocol in a group setting buy a trained instructor. The course includes instruction and practice of meditation, breathing techniques, gentle yoga and Tai Chi poses, with shared discussion, brief readings, and home practice between sessions. Participants continued with usual care and receive standard educational materials on healthy lifestyles and stress management. Comparison (duration 8 weeks, setting not reported): The control condition continued with usual care and received standard educational materials on healthy lifestyles and stress management. At the end of the study control participants received a compact disc and workbook on MBSR. |
|
| Outcomes | Follow‐up at 3 and 9 months: anxiety, depression, blood pressure, lipids, BMI, HbA1c, HRQoL | |
| Notes |
Country: USA Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was implemented using a REDCap program, which permits web‐based data entry and randomisation assignments" |
| Allocation concealment (selection bias) | Low risk | "... participants were stratified based on self‐reported current CR enrolment (yes/no), and then randomly allocated to an 8‐week MBSR group intervention or usual care control group using a 2:1 (intervention:control) randomisation scheme by the study coordinator, blinded to study investigators." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information, but blinding unlikely. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Retention throughout the study exceeded 95% |
| Selective reporting (reporting bias) | Unclear risk | The Short Physical Performance Battery (SPPB) identified in clinical trial registry was not reported in the paper. |
| Other bias | Unclear risk | Insufficient information to judge |
Nikkhah Ravari 2020.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Conducted in diabetic patients referred to Imam Ali Comprehensive Urban Health Centre in Isfahan, a central city in Iran, in 2019. Inclusion criteria: Adult women (age ranged 30 to 59 years) with type 2 diabetes mellitus diagnosed by a doctor, history of diabetes for at least 6 months, having a health record at the health centre, and haemoglobin A1C (HbA1c) between 7% and 9% Exclusion criteria: Patients with acute mental diseases, chronic diseases such as cancer or any other serious medical condition, pregnancy and lactation, alcohol and substance abuse, serious complications caused by diabetes, undergoing psychological treatments in the past year were excluded. Number eligible 120; 54 randomised to the intervention group (mean age 56.4, 0% men) and 54 to the comparison group (mean age 57.7, 0% men) Medications at baseline: Not reported Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 12 weeks, setting not reported): In addition to routine care, the intervention group received 8 sessions (2 hours each) of group mindfulness training once a week by a certified instructor and then practised at home for 4 weeks and weekly self‐reports were presented on the home practices. The following topics were discussed in the 8 training sessions: explanations about mindfulness, doing yoga exercises, meditation, receiving feedback, and answering questions, breathing exercises, and sleep hygiene training. Comparison (duration 12 weeks, setting not reported): Throughout the study, the control group was routinely cared for by the healthcare system according to national guidelines for diabetes care. |
|
| Outcomes | Follow‐up at 13 weeks: anxiety, depression, stress, FBG, HbA1c | |
| Notes |
Country: Iran Funding: This study was granted by Isfahan University of Medical Sciences. Declarations of interest: There are no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Imam Ali Comprehensive Urban Health Center has 5 population blocks based on geographical area. In order to prevent contamination, using random number table, blocks 1 and 5 were randomly allocated to the intervention and control groups, respectively. Then, based on the electronic health records, the diabetic patients were randomly selected and were replaced with the next record if they did not meet the inclusion criteria". |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information, but blinding unlikely. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition was balanced between groups and low, being 7.4% in the intervention group and 5.6% in the control group. |
| Selective reporting (reporting bias) | Unclear risk | Trial registry checked (IRCT20190813044527N1) and all primary outcomes are reported on. Sleep quality listed as a secondary outcome is not reported on. |
| Other bias | Unclear risk | Insufficient information to judge |
Palmeira 2017.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Recruited when attending appointments for nutritional treatment for weight loss in primary care units and hospitals from Coimbra’s district, Portugal. Recruitment dates not reported. Inclusion criteria: Participants were adult women, aged between 18 and 55 years old, with overweight and obesity (BMI ≥ 25) without binge‐eating. Exclusion criteria: Not reported 108 assessed for eligibility, 92 eligible; 36 randomised to the intervention group (mean age 42, 0% men) and 37 to the comparison group (mean age 42.7, 0% men) Medications at baseline: Not reported Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 14 weeks, setting not reported): Kg‐free is a manualised group intervention based on mindfulness, acceptance and commitment therapy (ACT) and compassion‐based approaches for women with overweight and obesity developed by the authors. It comprises 10 weekly group sessions plus 2 booster fortnightly sessions, 2.5 hours each, run in small groups (from 10 to 12 participants). The intervention was designed to reduce weight self‐stigma and unhealthy eating behaviours and promote quality of life by targeting weight‐related experiential avoidance and self‐criticism. A clinical psychologist with previous training in contextual‐behavioural therapies and 1 clinical psychology master student delivered the sessions for all groups. All sessions shared the same basic structure, starting with 30 minutes of shared experience, followed by a 5‐minute mindfulness practice (e.g. eating a raising meditation, mindfulness of breathing, physical sensations). The session content was delivered, followed by a mindful eating practice to train the ability to pay attention to food and eating physical sensations. Finally, the session content was briefly revised and practices for the week were established (e.g. audio mindfulness and self‐compassion practices). Participants received a manual that included the targeted constructs, examples, and exercise sheets. Audio files were provided to ensure the practice of mindfulness and compassion exercises between sessions. Comparison (duration 14 weeks, setting ‐ medical centre): Treatment as usual (TAU) only (see below) Both groups received treatment as usual (TAU), which includes medical and nutritional appointments. The medical appointment in TAU includes a physical examination and addressing co‐morbidities. In nutritional appointments, individuals are weighed, receive tailored dietary recommendations (according to one’s needs and food preferences) and physical activity prescriptions (at least 3 times per week of moderate to high intensity physical exercise is usually recommended). Difficulties regarding weight loss plans are also addressed in both appointments. TAU does not include any psychological intervention. |
|
| Outcomes | Follow‐up at 14 weeks: BMI, total cholesterol, HRQoL | |
| Notes |
Country: Portugal Funding: This research was supported by the first author's Ph.D. grant (SFRH/BD/84452/2012), sponsored by FCT (Portuguese Foundation for Science and Technology). Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned to an experimental or to control conditions by a member of the research team, using a computer‐based random allocation. |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information, but blinding unlikely. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data collection was carried out by clinical psychologists (blinded to participants’ treatment condition). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition: 9/36 (25%) in the intervention group, 5/37 (14%) in the control group |
| Selective reporting (reporting bias) | Unclear risk | "This trial was registered at clinicaltrial.gov with the Identifier code: NCT02850796 following data collection and analysis." |
| Other bias | Unclear risk | Insufficient information to judge |
Parswani 2013.
| Study characteristics | ||
| Methods | Parallel‐group RCT ‐ pilot study | |
| Participants | Recruited from the inpatient and outpatient services of St. Johns Medical College and Hospital, Bangalore. Recruitment dates not reported. Inclusion criteria: Patients who had been hospitalised or had had symptoms of heart disease within the last 1 year and their echocardiography test showing ejection fraction > 35% with ability to read, write, and speak English were included in the study. Exclusion criteria: Patients with a clinical history suggestive of psychoses, obsessive compulsive disorder, mental retardation, mania, severe depression, neurological or serious medical conditions and those with previous exposure or currently receiving any psychological intervention were excluded from the study. Only males were recruited for this pilot study as the majority of women declined to participate due to transportation problems, child care, and other responsibilities. Number eligible 131; 15 randomised to the intervention group (mean age 47.3, 100% men) and 15 to the comparison group (mean age 50.6, 100% men) Medications at baseline: Not reported Medication change during the trial not reported |
|
| Interventions | Pre‐assessment was carried out for both the groups at least 3 months after the occurrence of a cardiac event, to allow them to stabilise medically in terms of their cardiac status. During the pre‐assessment session, patients in both groups were given health education about CHD and its management. All the patients in both groups followed suggestions from the treating team regarding their health behaviours, i.e. regular exercise for at least 30 min and maintaining the suggested diet. Routine cardiac care continued for both groups, i.e. medical management and once a month follow‐up visit with their cardiologist. Intervention (duration 8 weeks, setting not reported): MBSR intervention was based on Kabat–Zinn (Kabat‐Zinn 1990) and Segal et al (Segal 2002). The therapeutic programme included training in different variants of mindfulness meditation such as body scan meditation, sitting meditation, mindful walking, mindful eating, 3‐min breathing space, mastery and pleasure activities, and cognitive restructuring. Sessions were held once a week, with each session lasting for 1 to 1.5 h. Each participant was provided with an audio cassette with recorded instructions of mindfulness meditation and body scan meditation to practise 30 min of meditation at home. Comparison (duration 8 weeks, setting not reported) Patients in the TAU group did not receive any further sessions after the health education session. |
|
| Outcomes | Follow‐up at 12 weeks: blood pressure, anxiety, depression, perceived stress, BMI | |
| Notes |
Country: India Funding: nil Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned to either groups, MBSR group or treatment as usual group, using computer‐generated random tables. |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information, although blinding is unlikely. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Of the 30 patients, there were a total of 17 available at 3‐month follow‐up (12 in the MBSR and five in the treatment as usual group). For each group, baseline mean scores on outcome measures for patients available for the follow‐up were compared with the baseline mean scores of patients who could not come for the follow‐up using an independent sample t‐test. The results did not show any significant differences, which indicates that the patients who were available for the follow‐up in both the groups were true representatives of their respective groups. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry found to check |
| Other bias | Unclear risk | Insufficient information to judge |
Paul‐Labrador 2006.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Recruited from a supervised cardiac exercise and rehabilitation programme at Cedars‐Sinai Medical Center and the surrounding community. Recruitment dates not reported. Inclusion criteria: Women and men older than 18 years, with CHD documented by prior myocardial infarction, coronary artery bypass surgery, coronary angiography, or angioplasty. Exclusion criteria: Unstable coronary syndromes, congestive heart failure greater than New York Heart Association class III, renal failure, acute myocardial infarction in the preceding 3 months, atrial fibrillation or a predominantly paced rhythm, prior TM, or current stress management practice Number eligible not reported; 52 randomised to the intervention group (mean age 67.7, 79% men) and 51 to the comparison group (mean age 67.1, 84% men) Medications at baseline: Statin therapy at baseline in 83% of the intervention group and 84% of the comparison group. ACE/AII inhibitor therapy at baseline in 42% of the intervention group and 45% of the comparison group. Calcium channel blocker therapy at baseline in 19% of the intervention group and 31% of the comparison group. Beta‐blocker therapy at baseline in 46% of the intervention group and 39% of the comparison group. Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 16 weeks, setting ‐ at an academic medical centre): The highly standardised and reproducible TM teaching materials were used to ensure quality control and consistency. The format of TM includes 2 introductory lectures (1.5 hours each), personal interview (usually 10 to 15 minutes), personal instruction (1 to 1.5 hours), 3 group meetings (1.5 hours each), and follow‐up and maintenance meetings (1.5 hours) twice per week for the first 4 weeks and weekly thereafter, and home TM practice time. Comparison (duration 16 weeks, setting ‐ at an academic medical centre): Subjects randomised to health education (HE) attended the same number, size, and frequency of group meetings led by professional health educators as the TM group. The lectures and discussions included CHD risk factors and the impact of stress, diet, and exercise on CHD. Daily home assignments were given to control for home TM practice time. |
|
| Outcomes | Follow‐up at 16 weeks: SBP, DBP, anxiety, depression, total LDL and HDL cholesterol, triglycerides | |
| Notes |
Country: USA Funding: This study was supported by grants R01 AT00226, 1‐P50‐AA0082‐02, 1‐R15‐HL660242‐ 01, and R01‐HL51519‐08 from the National Center for Alternative and Complementary Medicine, National Institutes of Health; and General Clinical Research Centers grant MO1‐RR00425 from the National Center for Research Resources. Declarations of interest: Financial disclosures ‐ none. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation to TM or to HE was performed via a computerised program with blocking, whereby eligible patients were grouped according to age (65 years) and low‐density lipoprotein cholesterol levels (120 mg/dL (6.66 mmol/L)) and assigned to treatment group accordingly. Once a group of 10 to 16 patients was randomised (i.e. 5 to 8 per treatment group), a new cohort would be formed and begin the respective interventions concomitantly. |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information, although blinding is unlikely. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome data were collected and analysed by personnel blinded to patient treatment status. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall, 103 patients enrolled and 84 (82%) completed the study. Some imbalance in losses to follow‐up with 6.8% in the TM group and 11.7% in the HE group. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry found to check |
| Other bias | Unclear risk | Insufficient information to judge |
Pearson 2018.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Recruited from outpatient diabetes clinics at the Royal Hobart Hospital (RHH), Tasmania, Australia, between August 2013 and November 2013. Inclusion criteria: Adult patients (18 years or more) with T2DM attending the diabetes clinics, which are recognised as national specialist centres. Criteria for referral include T2DM not meeting target glycaemic control despite diabetes education and appropriate glucose‐lowering therapy, T2DM with vascular complications (diabetic nephropathy or microalbuminuria, eGFR < 60 mL/mom/1,73m2 regardless of HbA1c, peripheral neuropathy, dyslipidaemia unresponsive to standard therapy, peripheral vascular disease, active ulcer or recent amputation, suspected Charcot’s joint, suspected or recent cellulitis), type 1 diabetes and/or unstable diabetes management putting patients at risk or recently experienced a severe hypoglycaemia episode. Exclusion criteria: A recent history of severe psychopathology (i.e. a severe mental health disorder such as schizophrenia or bipolar disorder), a severe physical comorbidity and/or cognitive impairment limiting their reading and comprehension skills and/or ability to sign the consent form. Patients were also excluded if they had a current mindfulness practice or had significant psychological distress on screening (scored > 30 following completion of the Kessler 10). 227 screened for eligibility, 170 were eligible and 74 randomised; 38 randomised to the intervention group (mean age 57.5, 38.7% men) and 36 to the comparison group (mean age 61.1, 66.7% men) Medications at baseline: Not reported Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 8 weeks, setting not reported): The self‐directed mindfulness intervention was an audio CD of guided breath awareness that participants were asked to listen to for 30 min a day. The CD was composed by one of the study investigators (EW) who is a mindfulness trainer with over 25 years of personal mindfulness practice, an academic, and a practising medical doctor who specialises in mental health. The CD had been used in previous RCT of medical students. The CD was designed to be self‐directed so participants could undertake the mindfulness practice at home at a time of their choosing. Participants were asked to listen to the CD daily for 8 weeks. An instruction sheet was also included with the CD. Comparison (duration 8 weeks, setting not reported): The control group received a blank CD and otherwise received usual care. |
|
| Outcomes | Follow‐up at 12 weeks: anxiety, depression, stress, SBP, DBP, HbA1c | |
| Notes |
Country: Australia Funding: This research was supported by funding from the University of Tasmania, School of Medicine, Research and Development grant. Declarations of interest: The authors declare that they have no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The random list of numbers was generated using IBM SPSS. |
| Allocation concealment (selection bias) | Low risk | The research nurse consenting the participants and responsible for the data collection was not aware of the group allocation. To ensure allocation concealment, identical sealed envelopes containing a CD and instruction sheet were used. The CD for the treatment group contained the mindfulness intervention, while the CD for the control group was blank. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information, although blinding is unlikely. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Low for objective measures (as research nurse blinded), but could be high for self‐reported outcomes such as depression. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Differential loss to follow‐up ‐ 36.8% in the intervention group, 13.8% in the control group. |
| Selective reporting (reporting bias) | Unclear risk | The trial was retrospectively registered on anzctr.org.au. |
| Other bias | Unclear risk | Insufficient information to judge |
Ponte Marquez 2019.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Recruited between July 2014 and March 2015 from among hospital employees and patients from a hypertension unit. Inclusion criteria: Adults aged 18 to 70 with high‐normal BP or grade 1 hypertension Exclusion criteria: Patients with a medical history of symptomatic heart failure (New York Heart Association (NYHA) class II–IV) or left ventricular ejection fraction (LVEF) < 60%, patients with coronary heart disease cerebrovascular disease or any other condition that might result in death before study completion; patients concomitantly using BP‐modifying drugs (cyclosporine, nonsteroidal anti‐inflammatory drugs (NSAIDs), steroids, vasoconstrictors, etc.); pregnant women; patients participating in another clinical trial; and patients with previous experience of mindfulness, meditation, yoga, tai chi, chi kung, or similar techniques. Number eligible not reported; 24 randomised to the intervention group (mean age 57.1, 45.8% men) and 18 to the comparison group (mean age 55.7, 38.9% men) Medications at baseline: Antihypertensive therapy at baseline in 70.8% of the intervention group and 66.7% of the comparison group. Medication remained unchanged during the trial. |
|
| Interventions |
Intervention (duration 8 weeks, setting not reported): In weekly 2‐hour sessions over 8 weeks, the intervention group received group‐based stress‐reduction therapy based on mindfulness skills, taught by a psychiatrist trained in this technique, drawn from a range of formal and informal mindfulness‐based cognitive therapy practices (Segal 2002), which included mindfulness of breath, thoughts, bodily sensations, sounds, and everyday activities. Intervention group patients were also encouraged to practise meditation at home for 45 min a day. Comparison (duration 8 weeks, setting not reported): The control group received weekly health education over the same period. For both groups, therapeutic regimens and pharmacological doses were maintained unchanged until follow‐up was concluded. |
|
| Outcomes | Follow‐up at 20 weeks: SBP, DBP, depression, anxiety, perceived stress | |
| Notes |
Country: Spain Funding: not reported Declarations of interest: The authors declare that they have no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information, although blinding is unlikely. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Potentially high for self‐reported outcomes such as depression, low for objective clinical outcomes such as blood pressure. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 8 people left after randomisation and were not included. Only 4/24 vs 2/18 lost to follow‐up, so fairly low attrition. States that ITT data presented, but stress/anxiety measures only presented for 16 and 14 for the intervention and control groups respectively. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry found to check |
| Other bias | Unclear risk | Insufficient information to judge |
Raja‐Khan 2017.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Participants were recruited through Penn State Health Hershey Medical Center clinics and advertisements from November 2011 to December 2013. Inclusion criteria: Women, age ≥ 18 years, and BMI ≥ 25 kg/m2 Exclusion criteria: Current pregnancy, untreated hyperthyroidism or hypothyroidism, type 1 diabetes, androgen secreting tumour, Cushing syndrome, prolactin > 30 ng/mL, severe active neuropsychological disorder, psychosis or suicidal ideation, severe untreated depression or anxiety, inpatient admission for psychiatric disorder within the past 2 years, active alcohol or drug abuse, inability to read, speak, or write English, inability to commit to the intervention and follow‐up, current enrolment in a stress reduction programme, mindfulness practice within the past 6 months (regular formal practice at least once a week), and current enrolment in other studies. 93 assessed for eligibility, 89 eligible and 86 randomised; 42 randomised to the intervention group (mean age 47, 0% men) and 44 to the comparison group (mean age 42.2, 0% men) Medications at baseline: Not reported Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 8 weeks, setting not reported): Participants randomised to MBSR received the standard MBSR programme consisting of instructor‐led weekly 2.5‐hour sessions for 8 weeks and a 6‐hour retreat session (Kabat‐Zinn 1990), but were asked to do only 25 to 30 minutes of daily home practice instead of the standard 45 minutes. There were no other changes to the standard MBSR curriculum. The instructor had completed professional MBSR training and had 9 years of experience training others in mindfulness and was in regular supervision. The MBSR intervention lasted 8 weeks. Between 8 and 16 weeks, participants were encouraged to continue with the daily home practice, but there was no contact from intervention staff. Comparison (duration 8 weeks, setting not reported): The health education group was taught by a registered dietitian who delivered additional diet and exercise information. To control for instructor attention and group support, the health education group also received instructor‐led, weekly, 2.5‐hour sessions for 8 weeks and a 6‐hour retreat. During sessions, the health education group received lectures and participated in learning activities about diet, exercise, general stress management, and the diagnosis, symptoms, complications, and treatments for obesity. General stress management was included in the health education group to minimise the bias of subject expectations. The health education group did not receive any mindfulness. Both groups were given the same written guidelines on diet and exercise, which consisted of the American Academy of Nutrition and Dietetics’ “General, Healthful Nutrition” handout and the Centers for Disease Control and Prevention’s “Physical Activity and Health” web page. All subjects were informed at enrolment that the primary focus of the study was stress reduction, not weight reduction. They were informed that the study was being done to determine the effects of stress reduction on glucose, blood pressure, and overall health. |
|
| Outcomes | Follow‐up at 16 weeks: SBP, DBP, anxiety, depression, perceived stress, FBG, HbA1c, weight, BMI, HRQoL | |
| Notes |
Country: USA Funding: This work was supported by a grant from the National Institutes of Health (NIH), National Center for Complementary and Alternative Medicine (NCCAM) (K23AT006340 to NR). This work was also supported by a grant from the National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH (UL1TR000127). Declarations of interest: RSL reports consulting fees from Euroscreen, Astra Zeneca, Clarus Therapeutics, Takeda, and Kindex and research funding from Ferring and Astra Zeneca. ARK reports owning stock in Merck. The other authors declared no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a random number generator. SAS 9.2 proc plan (SAS Corp., Cary, North Carolina) was used to create a list based on a permuted blocks randomisation scheme, having variable block sizes of 2 and 4, with equal allocation to the two arms. Randomisation was stratified based on the presence or absence of polycystic ovarian syndrome (PCOS). |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The principal investigator, study co‐ordinator, and all study personnel involved in the collection and review of outcome data were blinded to the block size and group assignments. The Class Schedulers, participants, and instructors were directed to keep the group assignments concealed from the blinded study personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | All study personnel involved in the collection and review of outcome data were blinded to the block size and group assignments. Low risk for objective measures, but unclear risk for self‐reported measures. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Fifty‐three participants (62%) completed the 16‐week follow‐up visit (MBSR 31/42 = 73.8%; health education 22/44 = 50.0%). High and differential attrition but ITT used. |
| Selective reporting (reporting bias) | Low risk | The trial was registered at clinicaltrials.gov (NCT01464398) prior to enrolment and all relevant outcomes were reported. |
| Other bias | Unclear risk | Insufficient information to judge |
Razavizadeh Tabadkan 2019.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Recruited women with type 2 diabetes from the public sports organisation of North Khorasan Province, Iran in 2017. Inclusion criteria: Women with type 2 diabetes. No further details. Exclusion criteria: Not reported Number eligible not reported; 15 randomised to the intervention group (mean age not reported, 0% men) and 15 to the comparison group (mean age not reported, 0% men) Medications at baseline: Medication at baseline not reported Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 8 weeks, setting not reported): MBCT, 8 x 120‐minute weekly sessions Comparison (duration 8 weeks, setting not reported): No details ‐ just states control group |
|
| Outcomes | Follow‐up at 12 weeks: perceived stress | |
| Notes |
Country: Iran Funding: This paper was extracted from a PhD thesis by the first author and approved by the Department of Psychology at Islamic University of Bojnurd branch. Declarations of interest: The authors declared no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Using random numbers table |
| Allocation concealment (selection bias) | Unclear risk | No information (main paper in Persian, so few details in English summary) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information, although blinding is unlikely. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information (main paper in Persian, so few details in English summary) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information (main paper in Persian, so few details in English summary) |
| Selective reporting (reporting bias) | Unclear risk | No information (main paper in Persian, so few details in English summary) |
| Other bias | Unclear risk | Insufficient information to judge |
Rungreangkulkij 2011.
| Study characteristics | ||
| Methods | Cluster‐RCT (2 villages randomised, individuals matched for gender, age, and scores on the 9 Q (according to level of depression) for the experimental and control villages) | |
| Participants |
Participants Recruited patients with type 2 diabetes who attended the diabetes clinic at Nakae Hospital, Thailand, in January 2009. Inclusion criteria: Eligible patients included residents of the 2 selected villages located within 15 km of the hospital, aged between 30 and 70 years old with a score of 7 or greater on the 9 Q. The patients who met the inclusion criteria were recruited as a sampling frame. Exclusion criteria: Exclusion criteria included 1) admission to a hospital during the course of the study, 2) having suicidal ideation, 3) without comorbid diseases (except hypertension), and 4) of other religion than Buddhism (to prevent religious conflict) Number eligible 89 in the intervention village, 115 in the control village; 32 randomised to the intervention group (mean age 50, 6.3% men) and 32 to the comparison group (mean age 47, 6.3% men) Medications at baseline: Current treatment with antidepressant/antianxiety, 56.2% in the intervention group, 84.4% in the control group, Fisher's exact test P = 0.05. Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 6 weeks, setting not reported): Buddhist group therapy The group leaders are psychiatric nurses who have a Buddhist belief system. Ahead of delivering the intervention, they attended a 5‐day Buddhist counselling workshop led by an Eastern‐based Buddhist psychologist and learnt and practised “Dynamic Meditation” under the supervision of an experienced monk for 7 days. This practice is designed to focus the mind so the mind does not wander during meditation by associating mindfulness with the ongoing, rhythmic movement of the hands whilst aware of the body's motions. With mindfulness thus attached to the body's movements, the subject is able to gain self awareness through meditation. The Buddhist group therapy consisted of a closed group session with 2 nurse leaders and 8 members per group (4 groups). Each weekly session lasted 120 minutes and was organised into 4 phases. First ‐ purpose was to develop rapport between leaders and members, sharing members' personal stories to air their feelings and thoughts to become relaxed. Second ‐ purpose was for members to understand causes of symptoms (craving) and connection between symptoms, feelings, and ruminative thoughts (based on the Universal Natural Laws ‐ Suffering, Impermanence, and Selflessness). Third ‐ to learn how to practise dynamic meditation and other mindfulness strategies. Fourth ‐ to explain causes of symptoms based on craving and self‐attachment, and to understand members' opinions about causes of symptoms based on craving and self, and to hear members' opinions about the group content and mindfulness practice to reduce suffering and experience calmness. Group leaders also recommended members to practise mindfulness consistently at home. Comparison (duration 26 weeks, setting ‐ diabetes clinic): Treatment as usual. Patients usually attended follow‐up appointments at the diabetes clinic once every month or two. If patients had a persistently high HbA1c level, nurses would provide information on diet and exercise and other relevant information as necessary. If patients complained about health problems, nurses would provide basic counselling and refer to a physician at the hospital if required. Usually, physicians prescribed antianxiety or antidepressant medication if the patient complained about sleeping problems. |
|
| Outcomes | Follow‐up at 26 weeks: depression | |
| Notes |
Country: Thailand Funding: This study was supported by a grant from the Department of Mental Health, Ministry of Public Health, Thailand. Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States quasi‐experimental but they randomised the 2 villages and randomly selected eligible participants in the intervention village to take part using the lottery method. The eligible participants in the control village were then matched to those in the intervention village on age, gender, and baseline depression, so this really is a cluster‐RCT with matching at the individual level even though there are just 2 clusters. |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information, but blinding unlikely. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A nurse from the diabetes clinic of Nakae Hospital assessed depressive symptoms for diabetes patients at baseline and 6 months after implementation. She was blind to group conditions. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two participants from the experimental group withdrew from the study due to work commitments in another province. This study analysed the data as an intention‐to‐treat analysis of treatment responses and included the two participants who withdrew from the study. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry number identified to check. |
| Other bias | Unclear risk | Insufficient information to judge. |
Sampaio 2016.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | The study was conducted from 1 January to 31 October 2014, at the Maximo Ravenna Therapeutic Center (CTMR), in Salvador‐Brazil, an institution for the treatment of chronic or recurrent obesity and eating disorders. During this period, all the adults enrolled at the institution who had entered the weight loss maintenance phase were invited to participate in the research, by posters and disclosures made in the therapeutic support groups. Inclusion criteria: Age ≥ 18 years, being part of the weight loss programme of CTMR in the city of Salvador, in the post weight loss maintenance phase Exclusion criteria: Diagnosis of psychosis or borderline personality disorder; not accepting to participate in the study; or not signing the Term of Free and Informed Consent Number eligible not reported; 20 randomised to the intervention group (mean age 49.8, 15% men) and 21 to the comparison group (mean age 52.3, 11% men) Medications at baseline: Anxiolytic agents 5% in the intervention group, 9.5% in the comparison group. Antidepressants 20% in the intervention group, 4.8% in the comparison group. Medication change during the trial not reported |
|
| Interventions | During the study all the participants remained in the standard CTMR programme that included diet, weekly participation in therapeutic support groups, regular physical activity, and a monthly meeting with a doctor and a nutritionist. Intervention (duration 8 weeks, setting CTMR): In addition, once a week for 8 weeks, the intervention group participated in Healing Meditation conducted by one of the researchers (CS), who has a 25 years’ experience in meditative practice. The 8 weekly meetings had a duration of 1 hour, and consisted of: recording the participants’ presence; verifying their adherence to meditation practice at home; explaining any doubts; and conducting Healing Meditation. The participants were oriented to practise meditation daily at home. All received a chart to record the days when they practised meditation at home, and had to return it at the end of the study. The Healing Meditation involves a series of meditation practices, of balancing polarities, of centring and reflexion, which when associated with breathing and relaxation, make the person’s energy circulate through their chakras, energy points and areas in their physical bodies and subtle dimensions, in order to harmonise the energy flow between them. The approach is based on Robert Samuel Moore’s work (Perret 2012). A 40‐minute mediation session consisting of 3 steps: the subject sat silent on a chair with his posture straight, eyes closed, focusing on specific area of the body, aware and open to embrace emerging feelings, sensations and thoughts. First step: balancing of the subject’s energetic polarities. First position: placing both hands over the stomach area, related to the Solar Plexus Chakra. Second position: crossing both arms over the chest, placing the 3rd and 4th fingers of the left hand over the right shoulder and the same fingers of the right hand over the left shoulder. Third position: crossing both arms over the lower abdomen, placing the 3rd and 4th fingers of the left hand over the right iliac spine, and the same fingers of the right hand over the left iliac spine. Fourth position: placing the right hand over a spot approximately 5 cm below the navel (corresponding to the Hara Chakra) and the left hand over the sternum (the Cardiac Chakra). The patient stays 5 minutes in each position, directing his breath to the target area of each exercise, focusing on the inspiration and expiration. Then the subject relaxes his arms, focusing on the target area, noticing the effects of meditation. Second step, contact with the Cardiac Chakra. It consists of placing both hands over the sternum, breathing normally, focusing on this area for 5 minutes. Third step: expanding consciousness. It consists of the subject laying both hands over the legs and imagining a circle around his head, which goes from the centre of the chest to both shoulders, up to a region 40 cm above the subject’s head. The subject focuses on this area for 5 minutes, then for 10 minutes he imagines a golden light filling this region, while he rests with an attitude of surrender, openness and mindfulness, accepting whatever may occur without prejudice. Comparison (duration 8 weeks, setting CTMR): Wait list control with standard CTMR programme |
|
| Outcomes | Follow‐up at 12 weeks: anxiety | |
| Notes |
Country: Brazil Funding: The trial was funded by the authors. This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors. Declarations of interest: All authors declare having no conflicts of interest with the research. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised ‐ no further details. |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information, but blinding unlikely. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Evaluators were blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses reported |
| Selective reporting (reporting bias) | Low risk | Trial registry reports outcomes reported in the trial |
| Other bias | Unclear risk | Insufficient information to judge |
Sarika 2020.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | All the patients were from the department of Endocrinology, Amrita Institute of Medical Sciences and Research Centre, Kochi, India. Recruitment dates not reported. Inclusion criteria: Age range 30 to 70 years with diabetes of 1 to 10 years duration and whose glycated haemoglobin (HbA1c) level ranged between 7% and 10%. These patients had never undergone any specialised relaxation training. Exclusion criteria: Patients diagnosed with advanced diabetic complications – ongoing treatment for retinopathy/renal impairment/symptomatic or unstable heart disease/uncontrolled blood pressure were excluded from the study. Number eligible not reported; 15 randomised to the intervention group (mean age 54.9, 40% men) and 15 to the comparison group (mean age 48.5, 40% men). Medications at baseline: Not reported There was no change in medication during the 3‐month study period in either group. |
|
| Interventions |
Intervention (duration 12 weeks, setting not reported): Standard care plus IAM® training under the guidance of certified IAM® teachers approved by Mata Amritanandamayi Math. IAM® is a simple combination of yoga, pranayama, and meditation. It includes 8 min of energising exercises (yogic postures), a brief period of relaxation for 2 min and meditation for 13 min. Towards the end of the technique, the participants are asked to remain in silence for 5 min. There were periodic refresher courses under the guidance of a certified trainer to help patients adhere to the intervention. The patients practised the technique once daily at home, and for a minimum of 4 times a week for standard of compliance. A self‐maintained diary would assess their daily compliance with practice. Compliance was also assured telephonically. Comparison (duration 12 weeks, setting not reported): Standard care. Telephonic conversation with the dietitian and social worker also motivated them to continue with their routine medication along with healthy diet and exercise. Both groups received advice from a physician, a dietitian and a social worker on maintaining a healthy lifestyle along with the conventional medications for hyperglycaemia. In addition, every 3 months, the participants had visits with the physician and social worker who reviewed their blood glucose levels to identify hypoglycaemic risk and to confirm the adherence to lifestyle modification. |
|
| Outcomes | Follow‐up at 12 weeks: perceived stress, weight, BMI, FBG, HbA1c | |
| Notes |
Country: India Funding: This work was funded by Seed grant support from Amrita Institute of Medical Sciences, Amrita Vishwa Vidyapeetham, Kochi, Kerala, India Declarations of interest: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization of the study participants was done through a computer‐generated sequence". |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information, but blinding unlikely. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No dropouts reported |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry found to check |
| Other bias | Unclear risk | Insufficient information to judge |
Schneider 2005.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Recruited from the adult patient populations of local health centres, senior citizens’ centres, churches, and the Department of Aging programmes. Dates of recruitment not reported. Inclusion criteria: 1) Self‐identified as African American; 2) residents of West Oakland, California, or surrounding communities; and 3) had a systolic BP of 140 to 179 mmHg or a diastolic BP of 90 to 109 mmHg averaged over 3 successive BP measurements. Exclusion criteria: Psychiatric disorder (psychosis, substance abuse disorder, dementia), and life‐threatening medical illness Number randomised 234; 37 subsequently excluded as they did not meet the inclusion criteria. 197 started the trial and 150 completed. Demographic data are only available for the 150 completers divided into the TM intervention group and two comparison groups, PMR and HE. 54 randomised to the TM intervention group (mean age 49.3, 53.7% men), 52 to the PMR comparison group (mean age 49, 44.2% men), and 44 to the HE comparison group (mean age 47.1, 43.2% men). Medications at baseline: Antihypertensive therapy at baseline in 70.4% of the TM intervention group, 69.2% of the PMR comparison group, and 59.1% of the HE comparison group Medication change during the trial ‐ compared to the TM group, the other groups significantly increased their use of antihypertensive therapy: PMR (P = 0.015) and HE (P = 0.006) during the 12‐month period. |
|
| Interventions | Each of the 3 interventions was led by trained and experienced African‐American instructors drawn from the local community. Intervention (duration 52 weeks, setting ‐ urban community health centre): The TM technique is described as a simple, natural, and effortless procedure, practised twice a day for 20 minutes while sitting comfortably with the eyes closed (Roth 1994). During the TM technique, it has been reported that the ordinary thinking process settles down and a distinctive “wakeful hypometabolic state” is gained. The general format of instruction in the standard TM course includes an introductory and preparatory lecture meeting to discuss the benefits and mechanics of the technique, a brief personal interview, a 1‐ to 1.5‐h session of personal instruction, and 3 follow‐up sessions taking place during 3 consecutive days, lasting about 1.5 h per day. After personal instruction, subjects practise on their own at home. Comparison(s) (duration 52 weeks, setting ‐ urban community health centre): PMR ‐ PMR served as a standardised physical relaxation technique and also provided an active control for attention, expectancy, participation in a novel activity, and time spent with the eyes closed. The general format of instruction in PMR was modelled after the standard TM course. The technique involves directing the participants’ attention to tensing and relaxing the various muscle groups throughout the body systematically to achieve deep relaxation. Subjects practised PMR at home for 15 to 20 min, twice a day. HE ‐ This group received written materials, instruction in, and group support for modifying the major cardiovascular risk factors with conventional behavioral approaches. Subjects learned the importance of diet, salt restriction, weight reduction, regular aerobic exercise, smoking cessation, and the effects of these factors on controlling BP. The topic of stress was covered, but subjects did not learn a specific stress reduction or relaxation technique. Subjects were encouraged to practise class recommendations that included exercise, restful activities, and healthful cooking at home for about 20 min twice a day. All three groups had an equal number of follow‐up meetings every month with individual attention from their respective African‐American instructors as needed. All subjects in the TM and the PMR groups received brief instructions and written educational materials on CVD risk factor reduction. |
|
| Outcomes | Follow‐up at 52 weeks: SBP and DBP | |
| Notes |
Country: USA Funding: This study was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH) (1 RO1 HL48107), Bethesda, MD, and by a specialized centre of research (SCOR) grant from the NIH–National Center for Complementary and Alternative Medicine (1P50AT00082). Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | After baseline measurements were completed, subjects were randomly allocated by computer program with stratification by age, gender, and antihypertensive medication status into one of the three treatment groups, TM, PMR, or HE. |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information, although blinding is unlikely. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blood pressure and heart rate measurements were performed by trained clinic staff who were blinded to the treatment status of subjects. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 150/197 participants completed with loss to follow‐up at 23.8% overall. Losses not reported separately by group. Intention‐to‐treat analyses were performed on the 197 subjects randomised to treatment who met the inclusion/exclusion criteria. In this analysis, for missing data for subjects with no post‐test data, the BP recorded at their last clinic visit was used to fill in subsequent missing data. However, BP analysis seems to be presented for only the 150 completers. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry found to check |
| Other bias | Unclear risk | Multiple reports on the effects of TM from the same institution |
Schneider 2012.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Recruited from the African American Heart Health Registry of the Medical College of Wisconsin and other databases of Milwaukee area hospitals. The study ran in 2 phases due to funding difficulties: phase 1 from March 1998 to April 2003 and phase 2 from March 2004 to July 2007. All phase 1 subjects were invited to participate in phase 2. Subjects provided written informed consent separately for each of the 2 phases. Inclusion criteria: Eligible patients were black men and women with angiographic evidence of at least 1 coronary artery with > 50% stenosis. Exclusion criteria: Acute myocardial infarction (MI), stroke, or coronary revascularisation within the previous 3 months, chronic heart failure with ejection fraction < 20%, cognitive impairment, and non‐cardiac life‐threatening illness 222 were eligible, 213 randomised, and 12 subsequently excluded; 99 randomised to the intervention group (mean age 59.9, 58.6% men) and 102 to the comparison group (mean age 58.4, 55.9% men) Medications at baseline: Lipid‐lowering therapy at baseline in 59.6% of the intervention group and 60.8% of the comparison group. ACE inhibitors at baseline in 43.4% of the intervention group and 45.1% of the comparison group. Angiotensin receptor agonists at baseline in 7.1% of the intervention group and 6.9% of the comparison group. β‐blockers at baseline in 2% of the intervention group and 2% of the comparison group. Calcium channel blockers at baseline in 34.3% of the intervention group and 36.3% of the comparison group. Diuretics at baseline in 43.4% of the intervention group and 46.1% of the comparison group. Aspirin at baseline in 41.4% of the intervention group and 31.4% of the comparison group. Medication change during the trial not reported. |
|
| Interventions |
Intervention (duration and setting not reported): The TM technique is described as a simple, natural, effortless procedure that is practised 20 minutes twice a day while sitting comfortably with eyes closed (Roth 1994). Standard teaching materials and format were used. The TM technique was taught in a 7‐step course of instruction comprising six 1.5‐ to 2‐hour individual and group meetings taught by an instructor certified by Maharishi Foundation USA. Thereafter, follow‐up and maintenance meetings were held weekly for the first month, biweekly for the following 2 months, and monthly thereafter for the remainder of phases 1 and 2. Comparison (duration and setting not reported): The control intervention was a cardiovascular health education programme designed to match the format of the experimental intervention for instructional time, instructor attention, participant expectancy, social support, and other non‐specific factors. The content was based on standard, published materials. The instructors were professional health educators. The HE subjects were advised to spend at least 20 minutes a day at home practising heart‐healthy behaviours, e.g. exercise, healthy meal preparation, and non‐specific relaxation. Care was taken to separate both intervention groups to minimise contact and communication. |
|
| Outcomes | Follow‐up at 5.4‐year average follow‐up: composite of all‐cause mortality, nonfatal MI, or nonfatal stroke (primary outcome), composite of cardiovascular mortality, nonfatal MI, nonfatal stroke, coronary revascularisation, or hospitalisation for IHD (non‐MI) or heart failure (secondary outcome), SBP, DBP, depression, BMI, smoking | |
| Notes |
Country: USA Funding: This study was funded by grant RO1HL48107 from the National Institutes of Health‐National Heart, Lung and Blood Institute. Declarations of interest: Dr Schneider has served as an investigator on research grants from the National Institutes of Health and US Department of Defense and is a consultant to Maharishi Foundation USA, a nonprofit educational organization. Dr Grim’s spouse is president and sole owner of Shared Care Research and Education Consulting. Dr Rainforth has served as an investigator on research grants from the National Institutes of Health and US Department of Defense and his spouse is an independent contractor to Maharishi Foundation, USA. Dr Nidich has served as an investigator on research grants from the National Institutes of Health, US Department of Defense and David Lynch Foundation and his spouse is an independent contractor to Maharishi Foundation, USA. Dr Gaylord‐King has served as an investigator on research grants from the National Institutes of Health, US Department of Defense and GMDO, a nonprofit organization. Dr Salerno has served as an investigator on research grants from the National Institutes of Health and US Department of Defense. The other authors report no conflicts. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Subjects were randomly assigned to either the TM or health education (HE) arms using a stratified block design. The strata were sex (male/female), age (above and below median for each cohort), and lipid‐lowering medication (yes/no). |
| Allocation concealment (selection bias) | Low risk | Random allocation was performed by the study biostatistician who concealed the allocation schedule and conveyed the assignments to the study co‐ordinator. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single‐blinded trial. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators, data collectors, and data management staff were blinded to group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20/99 and 21/102 lost to follow‐up and a further 19 and 10 participants did not participate in the course in the TM and HE groups respectively. ITT analysis used. |
| Selective reporting (reporting bias) | Unclear risk | Trial registered in 2011 (NCT01299935), but final data were collected in June 2007. So not possible to check a priori outcomes. |
| Other bias | Unclear risk | Multiple reports on the effects of TM from the same institution The trial was conducted between March 1998 and July 2007 in 2 phases. The first phase was from March 1998 to April 2003. After a hiatus in funding, the second phase was conducted from March 2004 to July 2007. All phase 1 subjects were invited to participate in phase 2. |
Schneider 2019.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | The trial was conducted between 1994 and 1999 at the Martin Luther King Jr. Medical Center, Charles R. Drew University of Medicine and Science clinical site in Los Angeles, CA. Participants were recruited through local radio and press advertising and from community organisations. Inclusion criteria: The study included self‐identified African American men and women aged 20 to 75 years with systolic blood pressure (SBP) of 120 to 179 mmHg and/or diastolic blood pressure (DBP) of 80 to 109 mmHg, with or without antihypertensive medications. Exclusion criteria: Patients with a history of stroke, transient ischaemic attack, heart failure, myocardial infarction, angina pectoris or with significant ECG abnormalities, major psychiatric or behavioural disorders such as alcoholism (> 28 drinks per week) were excluded. Patients were not assessed for hypertrophic obstructive cardiomyopathy or aortic stenosis. Number eligible was 180; 171 randomised; 85 randomised to the intervention group (mean age 53.3, 29.3% men) and 86 to the comparison group (mean age 52.3, 40.9% men) Medications at baseline: Antihypertensive therapy at baseline in 70.7% of the intervention group and 68.2% of the comparison group Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 26 weeks, setting ‐ medical centre): The Transcendental Meditation (TM) technique is a standardised, simple mental procedure, practised twice a day for 20 minutes while sitting comfortably with eyes closed (Roth 2018). Patients’ instruction in the TM programme involved a 7‐step course over 5 sessions (90‐minute meetings). This included the presentation of principles, TM benefits, discussion of mechanics of the technique, and a brief personal interview (session 1), personal instruction (session 2), and 3 follow‐up meetings in small groups (sessions 3, 4, and 5). The last 4 sessions were conducted over four consecutive days. TM requires 20 minutes of practice twice daily for its maximum physiological benefits. Patients were advised to practise the technique for 20 minutes twice daily at home, 7 days/week (14 sessions/week) for the duration of the study. Follow‐up refresher meetings were conducted 1 week later, every 2 weeks for 2 months, and once a month for 3 months. A trained and certified African American TM instructor taught the TM course. The TM group patients did not receive any specific nutritional recommendations, as did the HE group. Comparison (duration 26 weeks, setting ‐ medical centre): The Health Education intervention provided behavioural instructions for CVD risk factor prevention that included nutritional hygiene recommendations from the Trials of Hypertension Prevention (Whelton 1997). This group received written materials, structured presentations, didactic instructions and group support for modifying the major cardiovascular risk factors, including salt restriction, weight reduction, aerobic exercise, alcohol, and smoking cessation. The topic of stress was covered, but patients did not learn a specific stress reduction technique. The HE group participants attended a 90‐minute meeting once a week for the first 5 weeks, followed by 2‐week sessions for 2 months, and once a month for the last 3 months. |
|
| Outcomes | Follow‐up at 26 weeks: SBP, DBP, perceived stress, weight, BMI | |
| Notes |
Country: USA Funding: This work was supported by National Heart, Lung, and Blood Institute of the National Institutes of Health, US #R01 HL5159. Declarations of interest: No conflicts of interest to report. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated list of random sequence numbers was used to randomise patients to either TM or HE (allocation ratio 1:1) group. Randomisation was stratified by age, gender, and lipid‐lowering medication. |
| Allocation concealment (selection bias) | Unclear risk | Investigators, data collectors, and data management staff were blinded to group assignment. No further information. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single‐blinded. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators, data collectors, and data management staff were blinded to group assignment. The biostatistician conveyed the group allocation to the project manager, who then informed each participant and instructed them not to reveal it to the research and data collection staff during the course of the study. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High losses to follow‐up ‐ 50.6% in the TM group, 48.8% in the HE group. |
| Selective reporting (reporting bias) | Unclear risk | The trial was conducted between 1994 and 1999 and this full report was published in 2019. No trial registry. Protocol appears to have been published in 2001 and several ancillary or sub‐studies were reported before the main trial. |
| Other bias | Unclear risk | Multiple reports on the effects of TM from the same institution. Trial conducted many years before publication. |
Schneider 2021.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Trial conducted between January 1999 and April 2005. Recruitment details not reported. Participants with high normal blood pressure are relevant to this review, the paper reports also on participants with normal blood pressure. Inclusion criteria: The sample included self‐identified Black women and men, aged 21 to 75 years with high normal BP (130 to 139 mmHg and/or DBP 85 to 89 mmHg) without antihypertensive medications. Exclusion criteria: History of cardiovascular disease: myocardial infarction, angina, peripheral artery disease, heart failure, stroke, renal failure, diabetes mellitus, major psychiatric disorder or substance abuse disorder or other life‐threatening disease. Excluded also those planning to move out of area and those unwilling to consent or be randomised. Number eligible not reported; 69 randomised to the intervention group (mean age 43.4, 47.3% men) and 70 to the comparison group (mean age 44.5, 43.9% men) Medications at baseline: Antihypertensive medication was an exclusion criterion. Medication change during the trial not reported |
|
| Interventions | The two study interventions were matched for attention, duration, expectancy, and other nonspecific factors. All participants continued their usual medical care during the trial. Intervention (duration and setting not reported): The Transcendental Meditation (TM) technique was a standardised and validated meditation technique that is used for stress reduction that is practiced twice a day for 20 min while sitting comfortably with eyes closed (Roth 2018). Instruction in the TM technique involved a seven‐step course over five sessions of 1.5 hours each (Schneider 2012). This included an introduction and brief personal interview, personal instruction, and three follow‐up meetings in small groups. The last four sessions were conducted over four consecutive days. Follow‐up refresher meetings were conducted one week later, every 2 weeks for 2 months and once a month for the duration of the study. There were no instructions to modify lifestyle in the meditation course. A trained and certified instructor of TM taught the course. Comparison (duration and setting not reported): The health education (HE) intervention comprised a series of classes and group discussions on lifestyle modification for high blood pressure and CVD prevention. This group received written materials, structured presentations, didactic instructions and group support on major CVD risk factors including salt restriction, weight reduction, aerobic exercise, alcohol and smoking cessation. The topic of stress and BP was described but participants did not learn a specific stress reduction technique. Participants attended a 90 minute meeting once a week for the first 5 weeks, followed by bi‐weekly sessions for two months and then once a month for the duration of the study. The HE intervention was taught by a qualified health educator. |
|
| Outcomes | Follow‐up average 19.9 (11.1) months: SBP, DBP. | |
| Notes |
Country: USA Funding: This study was supported by a grant from the National Institutes of Health ‐ National Heart, Lung and Blood Institute, #R01HL060703. Declarations of interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Stratified block randomisation (with stratification for gender, age, and baseline levels of SBP and DBP) was used to assign participants in a 1:1 ratio. The random allocation sequence was based on computer generated random numbers". |
| Allocation concealment (selection bias) | Low risk | "Allocation concealment was maintained by the study biostatistician, who allocated each participant to treatment intervention and informed the study coordinator of treatment assignments, who then notified the participants". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single‐blinded. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Investigators, staff assessing outcomes, and data management staff were blinded to treatment group assignment". |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "All participants, regardless of their adherence to the intervention protocols, who completed at least one posttest were analyzed according to the modified intention‐to‐treat (ITT) principle". Attrition was balanced across groups, being 18.8% in the TM group and 17.1% in the HE group. |
| Selective reporting (reporting bias) | Unclear risk | The trial was conducted between 1999 and 2005 and this full report was published in 2021. Trial registry number reported in the paper does not appear to be valid (NCT#0482150), so unable to check selective reporting as no protocol identified either. |
| Other bias | Unclear risk | Multiple reports on the effects of TM from the same institution. Trial conducted many years before publication. |
Schroevers 2015.
| Study characteristics | ||
| Methods | Parallel‐group RCT ‐ pilot study | |
| Participants | Recruited through the diabetes outpatient clinic of the University Medical Center Groningen (UMCG), the Netherlands. Recruitment dates not reported. Patients were screened in the period between 16 March to 3 April 2009 and 4 May to 12 June 2009. Inclusion criteria: A diagnosis of diabetes, age between 18 and 70 years old and having elevated levels of elevated levels of depressive symptoms and/or diabetes‐related distress (= CES‐D ≥ 16 and/or PAID ≥ 40, required for study inclusion). Exclusion criteria: Serious psychiatric problems (e.g. schizophrenia, autism), severe visual problems, not being able to read and/or write Dutch, pregnancy Number eligible 104; 45 accepted; 21 patients were excluded on interview and 24 randomised; 12 randomised to the intervention group (mean age 54.9, 58% men) and 12 to the comparison group (mean age 55.9, 58% men) Medications at baseline: Not reported Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 8 weeks, setting not reported): The intervention was based on the standardised 8‐week MBCT (Segal 2002). This was modified in the following ways: the group protocol consisting of 8 weekly sessions of 120 to 150 min was adapted to 8 weekly individual sessions of 60 min. Exercises were shortened and 2 were removed (the cognitive exercise in session 2 and relapse prevention within session 7). The psycho‐educational component in sessions 4 and 5 was also focused on a broader range of stress‐ and depression‐related symptoms. Five therapists delivered the intervention, all had a degree in clinical psychology, experience in delivering psychological treatment, and experience with the MBCT group programme. During the first session, the participants received an informational booklet and CDs with guided exercises. The CDs were based on the Dutch version of the guided exercises that accompanied the standard MBCT programme (Segal 2002). Participants were asked to practise every day, at least 30 min, the guided mindfulness exercises on the CD, together with informal exercises such as mindful eating. Comparison (duration 8 weeks, setting not reported): Wait list control group |
|
| Outcomes | Follow‐up at 12 weeks: depression | |
| Notes |
Country: The Netherlands Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random selection of the patients was performed using a computerised program, with stratification on baseline levels of depressive symptoms, diabetes‐related distress, and gender. |
| Allocation concealment (selection bias) | Low risk | After randomisation, patients were notified by a telephone call about the condition (i.e. I‐MBCT or wait list control group). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information, although blinding is unlikely. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Potentially high as self‐reported outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Ten (83 %) of the 12 patients randomised to I‐MBCT successfully finished the 8‐week intervention. The other 2 patients dropped out of the intervention after two and three sessions, respectively, and did not complete follow‐up. They said that they did not experience sufficient benefit from the intervention." "Missing values were imputed for two participants who dropped out of the intervention, using last‐observed response carried forward. Results from the intention‐to treat analyses were not significantly different from completer analyses, and therefore, we present the results of the intention‐to‐treat analyses" |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration identified to check |
| Other bias | Unclear risk | Insufficient information to judge |
Seer 1980.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Referred by General Practitioners, recruitment dates not reported. Inclusion criteria: Established diagnosis of essential hypertension based on a routine hypertension workup, aged between 20 and 62, willingness to attend all assessment and training sessions Exclusion criteria: Patients with diabetes, renal disease, angina pectoris, or any other form of heart disease, concurrent antihypertensive and/or psychotropic drug treatment, previous meditation training 65 patients referred, 24 excluded, and 41 randomised; 14 randomised to the SRELAX intervention group (mean age 44.6, 57% men), 14 randomised to the NSRELAX intervention group (mean age 41.6, 57% men) and 13 to the comparison group (mean age 43.6, 54% men). SRELAX modelled on TM using a mantra or sound, whereas NSRELAX does not use mantras or sounds. We have therefore just used the SRELAX group and comparison group in our analyses. Medications at baseline: Medication for hypertension or psychotropic treatment were exclusion criteria. Medication change during the trial ‐ at the time of follow‐up, 1 SRELAX and 2 NSRELAX subjects were excluded because they had started taking prescribed antihypertensive medication. In addition, 1 NSRELAX subject had to be excluded because of other drug complications. |
|
| Interventions |
Intervention (duration 13 weeks, setting ‐ Auckland University Medical School): SRELAX intervention ‐ modelled after TM and required the subject to sit twice daily for 15 to 20 min in a comfortable position and quiet environment, to turn their attention inward toward the effortless mental repetition of a mantra, and to return gently to this mantra when distracted by unrelated thoughts, images, physical sensations, and emotions. The subject was instructed to do this simple exercise with a passive, "let‐it‐happen," non‐analytical attitude. The teaching procedure took 5 sessions over a 5‐week period. It closely resembled the orthodox TM course of instruction but differed in the following ways: 1) The meditation technique was referred to as "self‐relaxation" and all mystical elements such as the customary TM initiation ceremony and esoteric vocabulary were removed and the term TM was never used. 2) One standard mantra ("shyam") was used for all subjects regardless of sex. 3) The lectures accompanying the course were completely rewritten and given to all subjects in the form of a 23‐page manual. This stressed relaxation and coping rather than "cosmic consciousness" and "pure awareness." 4) There was no instruction fee. 5) Instead of "mantra," the term "sound" was used throughout hence the label "SRELAX" for this group. Overall guidance in the design of this meditation technique was obtained from a highly experienced TM instructor. Comparison (duration 13 weeks, setting ‐ Auckland University Medical School): The control condition was a "waiting list control." For the first 13 weeks, subjects participated in the same type and number of assessment sessions as the other 2 groups, but otherwise received no instruction. |
|
| Outcomes | Follow‐up at 13 weeks: SBP, DBP | |
| Notes |
Country: New Zealand Funding: This research was funded by a grant from the Auckland Medical Research Foundation. Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Subjects were randomly assigned to one of three groups; no further details. |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information, although blinding is unlikely. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Subjects were seen individually by a medically trained psychologist who was blind to all experimental conditions.This independent observer conducted all the assessments while the experimenter, who in turn was blind to the results of the assessment sessions, was exclusively responsible for the meditation training." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "At the time of follow‐up, one SRELAX and two NSRELAX subjects were excluded because they had started taking prescribed antihypertensive medication and one SRELAX subject declined participation. In addition, one NSRELAX subject had to be excluded because of other drug complications." All of the control participants were available for follow‐up. The two intervention groups had further follow‐up sessions but not the control group, so outcomes are taken post intervention at 13 weeks, where there were no losses. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available to check |
| Other bias | Unclear risk | Insufficient information to judge |
Shukla 2021.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Study conducted in the Endocrine Outpatient Department of a tertiary care hospital in North India. No information on recruitment methods or dates. Inclusion criteria: Adult patients with type 1 diabetes Exclusion criteria: Patients suffering from diabetes‐related complications such as proliferative retinopathy, autonomic neuropathy, diabetic nephropathy, established or recent onset coronary heart diseases, and pregnancy Number eligible not reported; 16 randomised to the intervention group (mean age 24.8) and 16 to the comparison group (mean age 22.8), overall 46.9% men Medications at baseline: In addition to insulin therapy, 1 patient in each group was also receiving metformin for diabetes treatment. Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 26 weeks, hospital setting): Participants in the intervention group were formally educated about mindfulness meditation (MM) by a skilled meditation coach. The training programme was divided into 2 components, breathing exercises and meditation. All the subjects were asked to sit in a comfortable posture, preferably with a straight spine in a relaxed state with their eyes closed. Step 1 (Breathing): they were asked to inhale and exhale and breathe deeply 6 times. Step 2 (Breathing): the subjects were asked to inhale while mentally counting up to 6, then hold their breath up to 6 and, finally, exhale for 6. They were asked to do this 6 times. Step 3 (Breathing): they were asked to do deep and rapid inhalations and exhalations 6 times. Step 4 (Meditation): subjects were asked to visualise their thoughts without entertaining any thoughts. They had to relax their minds and let their thoughts move at their own pace. This was observed for 2 min. Step 5 (Meditation): they were asked to visualise their breathing and feel that, with each breath, peace was imbued within them. They were asked to breathe in and out and, with each breath, the peaceful feeling increased and reached its peak. This was observed for 3 min. Step 6 (Meditation): now, they were asked to feel happiness with each breath, which also increased gradually. At this point, they had a feeling of both happiness and peace. This was observed for 2 min. Step 7 (Meditation): then, they were asked to sit in the same state and posture for the next 10 min. They were advised to practise MM for at least 20 min daily. Compliance and adherence were ensured by weekly telephone calls and in‐person monthly follow‐ups. Comparison (duration 26 weeks, setting not reported): Control ‐ no further details |
|
| Outcomes | Follow‐up at 26 weeks: SBP, DBP, FBG, HbA1c, BMI, HRQoL | |
| Notes |
Country: India Funding: This research received no external funding. Declarations of interest: The authors declare no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised via the chit method. |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information, but blinding unlikely. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information |
| Selective reporting (reporting bias) | Unclear risk | No trial registry or protocol available to check |
| Other bias | Unclear risk | Insufficient information to judge |
Sinha 2018.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | The study was conducted in the department of Physiology and Cardiology, Maulana Azad Medical College and associated G.B. Pant Hospital between June 2011 and January 2012. Inclusion criteria:
Exclusion criteria:
Number eligible not reported; 60 randomised overall, 93.3% men; 30 randomised to the intervention group (mean age 53.9, % men not reported) and 30 to the comparison group (mean age 56.2, % men not reported) Medications at baseline: Medications at baseline not reported Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 26 weeks, setting hospital and home): Meditation and medication and dietary changes. Patients attended twice a week in groups of 10 at the hospital. Meditation technique was demonstrated each day for the first few days until they had learned the technique perfectly; subsequently, they followed the procedure themselves. Special emphasis was laid on breathing technique practised by each patient and was checked at each subsequent visit. The following practices were included: concentration on body – body scan being aware of body sensations and any tension and to avoid controlling or resisting any tension. Concentration on breathing ‐ patients were taught to allow their body to breathe naturally and focus their attention wherever they feel the sensation of the breath in the body, being aware of the in‐breath and out‐breath and using the breath as an anchor. Distress to de‐stress ‐ patients were taught that whenever they experience tension, stress or anxiety, to focus their attention on other things such as sounds, sensations in the body, movement in the body, their heart beat and movement in the abdomen with the breath, so they were aware of other things whilst they are experiencing stress. Forgiveness ‐ patients were taught to gently soften their thoughts towards themselves, accept themselves as they are, making friends with themselves as they are and really feel that friendship and kindness. Then they can extend that friendship and kindness to those who have hurt them, learning to accept common humanity. To ensure patients were doing meditation properly, heart rate and blood pressure were recorded before (after 5 minutes of rest) and after doing meditation. Patients in the meditation group were asked to maintain a Record Diary in which they entered days on which they did meditation and for how long. To ensure their compliance to the programme at home, they were subjected to a stress management intake questionnaire. Any patient found not following instructions properly or doing meditation for less than 5 times in a week was not included in the study. Comparison (duration 26 weeks, setting hospital and home): Medication and dietary changes. Patients asked to report for follow‐up every 15 days. |
|
| Outcomes | Follow‐up at 26 weeks: systolic and diastolic blood pressure | |
| Notes |
Country: India Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation to control and meditation group was done with the help of the chit method. |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information, but blinding unlikely. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information |
| Selective reporting (reporting bias) | Unclear risk | No trial registration reported or protocol referred to. Two papers reporting different outcomes. |
| Other bias | Unclear risk | Insufficient information to judge |
Smith 2018.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | The participants were recruited by fliers and email announcements, advertised as a weight loss study for women who struggle with obesity, through the University of New Mexico Health Sciences Center (UNM‐HSC). Mindfulness was not mentioned in recruitment materials due to concern about introducing this concept to members of the control group. Recruitment began and ended in September 2006. Inclusion criteria: Age 50 to 70, postmenopausal status, a BMI of more than 30, ability to participate in the study for 1 year, fluency in English, and ability to walk at least 10 min without stopping Exclusion criteria: Significant untreated medical illness, obesogenous medications, severe psychiatric conditions including treatment for an eating disorder within the past 5 years, loss or gain of greater than or equal to 4.5 kg in the past 6 months, and treatment with a low‐calorie diet within 6 months Number assessed for eligibility 50; number eligible 40; 20 women randomised to the intervention group and 20 to the comparison group (mean age overall 58.5, 0% men) Medications at baseline: Not reported Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 6 weeks plus 10 monthly refresher sessions, setting not reported): The intention of the mindful eating and living (MEAL) intervention was to apply mindfulness to eating behaviour and provide participants with greater control over eating, resulting in decreased binge type eating, loss of weight, and reduced health risks. The MEAL intervention consisted of a 6‐week curriculum taught in weekly sessions of 2 hours each to a group of up to 20 people. Participants used written materials and recorded meditation exercises at home. At the completion of the course, treatment participants attended 10 monthly 1‐hour refresher sessions. The content for the MEAL sessions included group discussion, mindfulness meditation, and group eating exercises. The course, based on MB‐EAT (Kristeller 2011), emphasised brief daily meditation and pairing meditation with eating but was more streamlined in terms of didactic content and course length. The MEAL group was led by a medical doctor and a professionally‐trained mindfulness‐based stress reduction instructor. Comparison (duration 6 weeks plus 10 monthly refresher sessions, setting not reported): The control intervention was created to match the MEAL group on attention from healthcare professionals and expectations of personal benefit. In order to provide a schedule similar to that experienced by the treatment participants, but without mindfulness training, weight loss group sessions were conducted according to the same schedule as the MEAL group. In order to provide attention from knowledgeable healthcare professionals, the group was led by a medical doctor and co‐led by both a registered dietician and a clinical psychology graduate student. The agenda for the sessions involved giving each participant the opportunity to discuss issues such as food choices, activity levels, and caloric goals. The sessions began by having the participants’ check‐in about their experiences with eating. They were encouraged to share both challenging and successful experiences. Next, the clinical psychology graduate student led the participants in goal setting, and finally, there was a question and answer period with the registered dietician regarding food selection. The monthly refresher sessions lasted 1 hour and had the same agenda as the initial weekly sessions. |
|
| Outcomes | Follow‐up at 4 months, 9 months and 1 year: weight, BMI | |
| Notes |
Country: USA Funding: This project received financial support from the La Tierra Sagrada Society of the University of New Mexico (UNM). Declarations of interest: All authors for this manuscript, listed above, do not have any conflicts of interests that might be interpreted as influencing the research. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was determined based on a randomisation code devised by the General Clinical Research Center (GCRC) of the University of New Mexico Health Sciences Center (UNM‐HSC) statistician". |
| Allocation concealment (selection bias) | Low risk | "... this randomisation process was administered by a GCRC staff person such that the investigators involved in the biological aspects of the study were blinded to the randomisation." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Participants were not blinded nor were study investigators and instructors". We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "GCRC staff members were blinded to group assignment when participants would come in for blood draws and nutritional consultations." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 2 lost to follow‐up in each group for the same reasons. |
| Selective reporting (reporting bias) | Unclear risk | Trial retrospectively registered |
| Other bias | Unclear risk | Insufficient information to judge |
Spadaro 2018.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Sedentary, overweight and obese adult men and women who were recruited to participate in a behavioural weight loss study at the University of Pittsburgh Physical Activity and Weight Management Research Centre. Recruitment dates not reported. Inclusion criteria: Male or female, 18 to 55 years of age, BMI between 25.0 to 39.9 kg/m2 Exclusion criteria: Regular exercise participation of at least 20 minutes per day on at least 3 days per week during the previous 6 months. Participation in a previous physical activity or weight management research project in the previous 6 months. Weight loss of > 5% of current body weight in the previous 6 months. For women, those currently pregnant, pregnant during the previous 6 months, or plan on becoming pregnant in the next 6 months. History of myocardial infarction, heart bypass surgery, angioplasty, or other heart‐related surgeries. History of orthopaedic complications that would prevent optimal participation in the exercise component of the intervention. Currently taking any prescription medication that may affect metabolism and/or body weight. Currently being treated for any condition that could affect body weight, such as coronary heart disease, diabetes mellitus, hypertension, cancer, depression, and anxiety. Currently being treated for any psychological issues or problems, taking any psychotropic medications, or receiving treatment with psychotropic medications within the previous 6 months. Non‐medicated resting systolic blood pressure > 160 mmHg or non‐medicated diastolic blood pressure > 100 mmHg, or taking medication that would affect blood pressure or heart rate at rest or in response to exercise. Number eligible 53; 24 randomised to the intervention group (mean age 45.8, 9.1% men) and 25 to the comparison group (mean age 44.8, 16.7% men) Medications at baseline: Not reported although taking medication was an exclusion criterion. Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 24 weeks, setting ‐ University of Pittsburgh Physical Activity and Weight Management Research Centre): Standard Behavioural Weight Loss (SBWL) plus mindfulness meditation (MD). SBWL as outlined below and MD consisted of mindfulness training using meditation, yoga, and mindful eating techniques. Subjects in the SBWL + MD group attended an additional 15‐ to 30‐minute group session immediately following the standard behavioural group session each week of the intervention. These sessions were taught by a graduate student with expertise in mindfulness mediation practice and techniques, and were geared specifically toward improving eating and physical activity behaviours. The lessons taught included topics such as breathing exercises, visualisation, focused eating and walking meditations, progressive relaxation, yoga, and personal awareness. The schedule was as follows: 1) Introduction to Group Assignment and Plan; 2) Introduction to Mindfulness & Steps to Mindful Eating/Raisin Meditation; 3) Sitting Medication – Mastering the Mindful Meal & Mindful Eating; 4) Body Scan Meditation – Stress and the “Monkey Mind”; 5) Conscious Eating & Hunger Meditation; 6) Yoga Standing Meditation (CD); 7) Awareness of Satiety/Fullness Meditation & Hunger/Taste Meditation; 8) Review Mediation Progress‐ Recommitment; 9) Mindful Lying Yoga Session (CD); 10) Lake Meditation and Eating Triggers; 11) Mindful Yoga for Weight Management I – Yoga DVD; 12) Managing Emotional Eating – Taste, Portions, and Emotions; 13) Health‐e‐Weight: Understanding Your Inner Voice/Negative Thinking; 14) Mindful Yoga for Weight Management II – Yoga DVD; 15) The Principles of Mindful Eating Review and Mindfulness Scales; 16) Principles of Mindful Exercise; 17) Mindful Yoga for Weight Management III – Yoga DVD; 18) Seven Mindfulness Qualities; 19) Meditation on Forgiveness; 20) Practicing Walking Meditations; 21) Mindful Walking Meditation‐ 2 mile walk; 22) Inner Wisdom Meditation; 23) What is Normal Eating? Mindful Relapse Prevention; 24) Integrating Mindfulness Into Everyday Life Comparison (duration 24 weeks, setting ‐ University of Pittsburgh Physical Activity and Weight Management Research Centre) SBWL only. SBWL included diet, exercise, and behaviour modifications. Subjects attended 30‐minute group meetings weekly for 6 months, which consisted of structured didactic behavioural lessons and facilitated group discussions led by a qualified nutritionist, exercise physiologist, or health educator with experience conducting weight loss intervention groups. The behavioural lesson topics were based primarily on social cognitive theory and included strategies for adopting and maintaining positive eating and exercise behaviours. All participants were instructed to decrease energy intake to 1200 to 1500 kcal/d and dietary fat intake to 20% to 30% of total energy intake, increase physical activity to 300 min/wk, and attend weekly group meetings. |
|
| Outcomes | Follow‐up at 24 weeks: weight, BMI | |
| Notes |
Country: USA Funding: University of Pittsburgh, Department of Health and Physical Activity, Physical Activity and Weight Management Research Center, Pittsburgh, Pennsylvania, USA. Author John M. Jakicic, PhD has received research grant funding from the National Institutes of Health and the American Heart Association in the past. Declarations of interest: The funding organisation(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information, but blinding unlikely. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "A modified intention to treat approach was applied to investigate treatment efficacy, as three participants were withdrawn from the study. To account for the missing data of non‐completers in the remaining 46 study participants, missing values were imputed with baseline data, using baseline observation carried forward (BOCF) for all participants who did not participate in month 3 or month 6 assessments" |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry number identified to check |
| Other bias | Unclear risk | Insufficient information to judge |
Toomey 2007.
| Study characteristics | ||
| Methods | Parallel‐group RCT ‐ pilot study | |
| Participants | Native Hawaiian subjects were recruited from the rural community of North Kohala on the Big Island of Hawaii. Recruitment dates not reported. Inclusion criteria:
Exclusion criteria:
Number eligible 61; 30 randomised to the intervention group (mean age 64.6, 36% men), 31 to the comparison group (mean age 64.5, 39% men) Medications at baseline: In the Transcendental Meditation programme group, 37.5% were taking antihypertensive medications and 25% in the health education group (P < 0.16). Statin use was 14.5% for the Transcendental Meditation group and 12.5% for the health education group. Medication change during the trial ‐ at post‐test, 6 subjects in the Transcendental Meditation programme group reported changes in medication at 9 months, with 4 subjects increasing medication and 2 decreasing. 12 subjects in the health education group reported changes in their medication status at 9 months, with 10 subjects increasing medication and 2 decreasing medication. |
|
| Interventions |
Intervention (duration 9 months, setting not reported): The core instruction in the Transcendental Meditation programmeme involved a 7‐step course over 5 days, which followed the standard format offered by Maharishi Vedic Universities (Roth 1994). Most sessions lasted 1 to 1.5 hours, with the exception of the personal interview (about 10 minutes). The general format of most sessions was a lecture/discussion according to the following schedule.
Follow‐up programme: After this phase of the intensive intervention, the follow‐up programme for the duration of the intervention period included a) checking of correct practice of the Transcendental Meditation programme and b) advanced lectures and seminars to ensure understanding of the benefits of the practice and its physiological, psychological, and behavioural health effects. Follow‐up meeting began one week after completion of the initial Transcendental Meditation programme course, then once every 2 weeks for the first 3 months (intensive stage), then once per month for the remainder of the study (extended stage, 6 months). Comparison (duration 9 months, setting not reported): The control was the standard cardiac risk reduction programme in the clinical practice guidelines of the American Heart Association and American College of Cardiology. The time and attention given to the health education group matched that of the Transcendental Meditation programme in order to control for these factors. The structure of the experimental and control treatment condition included 2 stages of longitudinal intervention: 1) intensive and 2) extended phases. This consisted of group meetings and individual contacts during each stage. Follow‐up meetings included small group educational sessions. Initial Instruction Stage (7 days) ‐ this was the stage with the most frequent contact and focused on core knowledge and behaviour in the respective programmes. During this phase, groups of approximately 10 participants in each intervention met for a series of 5 sessions during their first week. Extended Stage (Remainder of 9 months intervention). The goal of the extended stage of the intervention was to maintain active participation in each intervention. It included a follow‐up session (post 7‐step initial instruction) after 1 week, and then fortnightly follow‐up sessions for 2.5 months. Subjects met individually with their respective course instructors for programme adjustment, if necessary. This stage also included monthly group follow‐up meetings for the remainder of the 9 months of the study. Attempts were made to re‐engage inactive participants. These sessions fostered contact and promoted adherence with the interventions. |
|
| Outcomes | Follow‐up at 9 months: blood pressure, anxiety, depression, cholesterol | |
| Notes |
Country: Hawaii Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single‐blind. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data collection staff were masked to treatment assignment, except for the project manager, who notified subjects of their treatment assignment. Participants were made aware of their treatment status. Endpoint data collected at the field sites was coded without subject or treatment status identifiers and sent to the Bio‐statistical Core Facility at the Center for Natural Medicine and Prevention (NIH‐NCCAM SCOR) for blinded data processing. Data collection staff did not participate in the data analysis. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 21.3% of participants lost to follow‐up. ITT analysis for the primary outcome but not for the outcomes relevant for this review where completers only were analysed. |
| Selective reporting (reporting bias) | Unclear risk | Thesis and no trial registry or protocol to check. |
| Other bias | Unclear risk | From the same institution as Schneider and others who make up the majority of included studies using TM. Different study population. |
Tovote 2014.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Patients were recruited from June 2011 to February 2013 at 4 hospitals primarily in the northern part of the Netherlands. Inclusion criteria: Patients with type 1 or 2 diabetes diagnosed at least 3 months prior to inclusion, aged between 18 and 70 years, and having symptoms of depression as indicated by a Beck Depression Inventory‐II (BDI‐II) score of ≥ 14 Exclusion criteria: Not being able to read and write Dutch, pregnancy, severe psychiatric comorbidity, acute suicidal ideations, receiving an alternative psychological treatment during or < 2 months prior to starting participation in the study, and unstable treatment with an antidepressant in the last 2 months prior to inclusion in the study Number eligible 183; 94 randomised to 3 groups; 31 randomised to the MBCT intervention group (mean 49.8 age, 55% men), 32 to the CBT comparison group (mean age 54.6, 50% men) and 31 to the wait list group (mean age 54.7, 48% men) Medications at baseline: Oral medication for diabetes at baseline in 13% of the MBCT group, 12% for the CBT group, and 13% of the wait list group. Oral medication and insulin for diabetes at baseline in 32% of the MBCT group, 44% for the CBT group, and 36% of the wait list group. Insulin at baseline in 55% of the MBCT group, 44% for the CBT group, and 51% of the wait list group. Medication change during the trial not reported. |
|
| Interventions |
Intervention (duration 8 weeks, setting not reported): MBCT was delivered individually in 8 weekly sessions of 45 to 60 min. Patients were also instructed to do daily homework for 30 min. MBCT was delivered by trained therapists who received supervision every 3 weeks throughout the intervention period. MBCT was based on the protocol as developed by Segal et al (Segal 2002). The central components of MBCT were formal meditation, yoga exercises, and informal daily mindfulness practices. Comparison (duration 8 weeks for CBT, 3 months for wait list control, setting not reported): Two comparison groups, CBT and wait list control CBT was delivered individually in 8 weekly sessions of 45 to 60 min. Patients were also instructed to do daily homework for 30 min. CBT was delivered by trained therapists who received supervision every 3 weeks throughout the intervention period. The main components of CBT were behavioural activation and cognitive restructuring. Participants in the waiting list condition received no psychological intervention for 3 months. |
|
| Outcomes | Follow‐up at 3 months: depression, anxiety, well‐being, HbA1c | |
| Notes |
Country: The Netherlands. Funding: This study was financed by the University of Groningen. Declarations of interest: No potential conflicts of interest relevant to this article were reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation was carried out stratified by sex, use of antidepressant medication, and baseline BDI‐II score. |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Before randomisation, patients were blinded for the treatment condition. Accordingly, patients did not receive any specific information about the type of intervention or the waiting list condition. They were only told that they were to be randomised to a psychological treatment that focuses on reducing depression and that treatment was to start within 3 months after randomisation." Protocol mentions: "After randomisation the patients are given more precise information about the treatment they are going to receive." We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | At pre‐measurement, the assessors of the HAM‐D7 were blinded to the treatment condition. However, at post‐measurement, the HAM‐D7 was administered together with an evaluation of the treatment for individuals randomised to MBCT or CBT, and therefore, the assessors were not blinded. This measure was not used in the meta‐analyses for depression as other studies use self‐reported measures. All other measures were self‐reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar loss to follow‐up in the MBCT and CBT groups. ITT analysis used. |
| Selective reporting (reporting bias) | Unclear risk | Relevant outcomes seem to be reported as per published protocol, but it is not clear why HbA1c was not presented for the control group. |
| Other bias | Unclear risk | Insufficient information to judge |
Turrise 2017.
| Study characteristics | ||
| Methods | Parallel‐group RCT ‐ Pilot study | |
| Participants |
Participants Participants were recruited from two sites with a phase II cardiac rehabilitation program in Southeastern North Carolina. Recruitment dates not reported but study conducted between April 2016 and July 2017. Inclusion criteria: Attending cardiac rehabilitation, aged 18 or older, read, write and speak English and able to give consent. Exclusion criteria: Diagnosis of thought disorder, bipolar disorder, borderline personality disorder, or illicit substance abuse/ dependence; already practice mindfulness or other types of meditation, or who attended 4 classes in such practices within the past; visual, hearing or cognitive impairments. Number eligible not reported. 28 patients randomised but reported on 10 randomised to the intervention group (mean age 67.6, 40% men) and 10 to the comparison group (mean age 70, 50% men). Medications at baseline: Not reported. Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 8 weeks, setting not reported): Participants in the mindfulness intervention group received eight weeks of assigned interventions, in a group, by a doctoral psychiatric clinical nurse specialist who is also board certified as an advanced holistic nurse. Participants were asked to practise their mindfulness intervention for 20 minutes each day. They also receive a book on mindfulness and an MP3 player with soft music and audio‐guided meditations on mindfulness and self‐compassion. . Comparison (duration 8 weeks, setting not reported) Participants in the control group received treatment as usual, which included general information on stress management and deep breathing exercises. All participants were given a journal to document their daily home practice and any important event related to their daily life and emotional state. |
|
| Outcomes | Follow‐up planned at 4 weeks, 8 weeks, 12 and 24 weeks. Reported preliminary findings on perceived stress and anxiety. In conference proceeding mention also measured depression, blood pressure, BMI, HRQoL with final results are pending completion of the study. No full paper identified, authors describe lessons learnt for a future larger trial. | |
| Notes |
Country: USA Funding: This study was funded by a Charles Cahill Award. Declarations of interest: Not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated by cohorts |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information but blinding unlikely. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Few details as reported in abstract and poster. Mention attrition being a factor. |
| Selective reporting (reporting bias) | Unclear risk | Authors mention outcomes not reported on, but at the time of reporting the study was not yet complete and they reported only preliminary findings for some outcomes. |
| Other bias | Unclear risk | Insufficient information to judge |
Vaccarino 2013.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Participants were recruited from the community in metropolitan Atlanta through flyers and at health fairs, churches, university campuses, and other community locations, as well as through direct referrals. Recruitment dates not reported. Inclusion criteria: Between the ages of 30 and 65, self‐identified as Black, and met specific criteria for metabolic syndrome. Participants were eligible if they met a modified definition which did not include the standard lipid criteria, but they met 2 out of the following 3 criteria: abdominal obesity (waist circumference > 102 cm in men and > 88 cm in women); BP ≥ 130/≥ 85 mmHg; and fasting glucose ≥ 100 mg/dL. Exclusion criteria: Known CVD or renovascular disease; if they had uncontrolled hypertension (systolic BP > 160 mmHg on two or more occasions or diastolic BP > 105 mmHg); if they were current smokers or had been taking over the counter vitamins (affects endothelial function assessments); if they were pregnant; and if they had documented history of alcohol or drug abuse or other psychiatric or medical diagnoses Number eligible 75; 33 randomised to the intervention group (mean age 51.5, 25% men) and 35 to the comparison group (mean age 52.1, 17.1% men) Medications at baseline: Aspirin therapy at baseline in 21.2% of the intervention group and 14.3% of the comparison group. Statin therapy at baseline in 45.5% of the intervention group and 25.7% of the comparison group. Beta blocker therapy at baseline in 18.2% of the intervention group and 20% of the comparison group. ACE Inhibitor therapy at baseline in 27.3% of the intervention group and 28.6% of the comparison group. Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 12 months, setting ‐ National Center for Primary Care at Morehouse School of Medicine): Consciously Resting Meditation (CRM) is a sound or mantra‐based meditation. This meditation approach was chosen because of its standardised protocol and its similarity to the TM programme, which has been previously successfully implemented in Black American samples. Both TM and CRM use sounds that have no meaning, but their quieting effects have been known for thousands of years. These sounds, when used properly, settle the mind and body down to a state of restful alertness. As with many TM studies, the CRM programme included 21 sessions over a 1‐year intervention period where participants learned the technique of consciously resting their mind and body. The core instruction involves a 4‐step course over 4 consecutive days (sessions 1 to 4) and a follow‐up programme over 12 months (sessions 5 to 21). Most sessions last 1 to 1.5 hours, and the general format is group meditation plus a lecture/discussion or videotape. In contrast to TM, CRM does not include a private Sanskrit ceremony, which has been a major objection for inner‐city meditation projects. In contrast to TM, CRM can be taught in groups, is less time‐consuming for participants, and is potentially more affordable, making it more accessible and easier to disseminate among minority groups. All sessions were taught by the same experienced teacher. Subjects were instructed to practise CRM twice a day for 20 minutes. Participants were also given the same health education reading materials given to the control group. Comparison (duration 12 months, setting ‐ National Center for Primary Care at Morehouse School of Medicine): Subjects randomised to Health Education (HE) attended the same number, size and frequency of group meetings lead by a professional health educator. The programme included information on prevention of CVD through lifestyle modification and was modelled on educational material disseminated by the American Heart Association. Topics included the value of a healthy diet, exercise, and weight management. The impact of stress was discussed as it relates to weight management and physical exercise. However, to avoid contamination in the experimental design, the HE sessions did not include instructions on stress reduction or relaxation techniques. To match the 20‐minute twice‐a‐day CRM practice, participants were instructed to undertake a 20‐minute twice‐a‐day home practice session applying the recommendations given in this course ‐ diet, exercise, or other lifestyle habits. |
|
| Outcomes | Follow‐up at 52 weeks: SBP, DBP, anxiety, depression, psychosocial stress, weight, BMI, HDL cholesterol, glucose | |
| Notes |
Country: USA Funding: This study was supported by funding from the National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute U01 HL079156 and U01 HL79214; NIH, National Center for Research Resources (NCRR) Grant M01‐RR00039 for the Emory General Clinical Research Center; NIH/NCRR 5P20RR11104 for the Morehouse Clinical Research Center; and NIH K24HL077506. Declarations of interest: Kofi A. Kondwani, Ph.D., is the founder and CEO of Consciously Resting Meditation (CRM), Inc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | After completion of baseline assessments, participants were randomised to one of two treatment groups by blocked randomisation with stratification on gender. The random allocation sequence was provided in sealed envelopes by the study statistician (who had no contact with participants) to a staff member not involved in data collection. Patients were randomised in subsequent cohorts of participants, with a minimum of 10 and a maximum of 20 in each randomisation cohort (yielding no more than 10 participants per group who would start the program at any one time), for a total of 5 cohorts. |
| Allocation concealment (selection bias) | Low risk | The random allocation sequence was provided in sealed envelopes by the study statistician (who had no contact with participants) to a staff member not involved in data collection. Does not specifically say 'opaque' envelopes but judged as low risk. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The trial was single‐blinded as patients were aware of which intervention they were assigned to (CRM or HE). However, participants were masked to the research hypothesis and both interventions were presented to participants as health‐promoting, which should minimise expectation bias. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators and the staff collecting the data were blinded to the treatment status of the participants. The two treatment providers were not involved with participant recruitment, data collection, analysis or interpretation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data were analysed following the intention‐to‐treat principle. All outcomes (primary and secondary outcome measures) were analysed as continuous variables using repeated measures mixed‐effects models with treatment group, time point, and other study factors treated as fixed‐effect. The model‐based means are unbiased with unbalanced and missing data, so long as the missing data are non‐informative (missing at random). High attrition at 12 months, so rated as unclear as it is possible data are not missing at random (14/33 (42%) CRM, 16/35 (46%) HE lost to follow‐up at 12 months). |
| Selective reporting (reporting bias) | Unclear risk | No trial registry or published protocol identified, but report includes all specified outcomes reported in the methods. |
| Other bias | Unclear risk | Insufficient information to judge |
van Son 2014.
| Study characteristics | ||
| Methods | Parallel‐group RCT. The DiaMind trial | |
| Participants | Recruited from outpatient diabetes clinics between May 2010 and November 2011. Inclusion criteria: Dutch‐speaking adult patients with diabetes (type 1 or type 2) with low levels of emotional well‐being (as evidenced by a score of < 13 on the World Health Organization‐5 Well‐Being Index). Exclusion criteria: Recent history of severe psychopathology (i.e. psychosis, risk of suicide attempts); or alcohol/drugs abuse; have a severe physical co‐morbidity (i.e. severe forms of cancer or heart failure); when they have insufficient reading and comprehension skills of the Dutch language; when they are already in an (extensive) psychological treatment which started within a period of 6 weeks before the start of the training; and when they already have meditation experience (with Vipassana, Zen, or Dzogchen) Number eligible not reported; 70 randomised to the intervention group (mean age 56, 47% men) and 69 to the comparison group (mean age 57, 54% men) Medications at baseline: Use of psychotropic medication at baseline in 26% of the intervention group and 17% of the comparison group. Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 8 weeks, setting not reported): Mindfulness training is based on the MBSR and MBCT programmes as described in Kabat‐Zinn (Kabat‐Zinn 1990) and Segal et al (Segal 2002), consisting of 8 weekly 2‐hour sessions. A few modifications were made to the original protocol and the workbook in order to make the intervention suitable for patients with diabetes. For example, the trainers explained the potential associations between emotional problems and diabetes management and diabetes outcomes (e.g. the associations between emotional stress and eating behaviours were discussed). Instead of the silent day that is part of the original programme, a 2‐hour booster session was added 3 months after the end of the intervention. At each session, the participants received homework assignments that take about 30 minutes 5 days per week. All the sessions were supervised by certified psychologists who have at least 4 years practical experience with mindfulness, and also completed a mindfulness instructors training of 8 days in The Netherlands. Comparison (duration 8 weeks, setting not reported) Treatment as usual |
|
| Outcomes | Follow‐up at 8 months (6 months post intervention): anxiety, depression, HbA1c | |
| Notes |
Country: The Netherlands Funding: The study is supported by a grant from the Dutch Diabetes Research Foundation (Diabetesfonds) (project number 2008.13.005, awarded to Dr I. Nyklíček). Declarations of interest: The authors have no competing interests to report. It may be noted that the second author (IN) also works as mindfulness teacher for people from the general population at Aandachtcentrum Dit Moment, a private mindfulness practice in Tilburg, Netherlands. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomisation will be done as follows. After the interview and upon receipt of a signed informed consent form, the patient will be assigned to a participant number. Subsequently the participant will receive the baseline questionnaire by mail or email. When the first author receives the baseline assessment, she passes on the corresponding participant number to the second author who has no further involvement in the practical recruitment, enrolment, and assessment of the patients. The second author will refer to the computer generated (through PASW Statisitics 17) random list (un editable and concealed for others) prepared by a statistician with no involvement in the trial. The second author will inform the first author about the allocation by email and will archive the allocations in a secured document on his computer. The first author will document the allocation in the general inclusion database, which will be checked by the second author. The first author will inform the patients both by telephone and letter about their allocation." |
| Allocation concealment (selection bias) | Low risk | "When the first author receives the baseline assessment, she passes on the corresponding participant number to the second author who has no further involvement in the practical recruitment, enrolment, and assessment of the patients. The second author will refer to the computer generated (through PASW Statistics 17) random list (un editable and concealed for others) prepared by a statistician with no involvement in the trial." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "The nature of this psychological intervention does not allow “masking” or blinding of patients, and trainers." We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The statistician who will be involved in data analyses will be blinded for treatment allocation. All the questionnaires and homework forms will only be marked with a participant number, which is unknown to the trainer and researcher." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT used, but high losses to follow‐up: at T2 lost to follow‐up: MCBT: 6, treatment as usual 9; at T3 lost to follow‐up: MCBT: 19, treatment as usual 14. |
| Selective reporting (reporting bias) | Unclear risk | https://www.trialregister.nl/trial/2028 also reports BP and heart rate. Protocol reports 24‐hour ambulatory blood pressure. All other outcome reported across different papers. |
| Other bias | Unclear risk | Insufficient information to judge |
Vidrine 2016.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Participants were recruited from the Houston metropolitan area via local print media. Study advertisements asked if individuals wanted help with quitting smoking, indicated that counselling and nicotine patches would be provided, and stated that participants would be compensated for their time. All data were collected between January 2007 and February 2010. Inclusion criteria: ≥ 18 years of age, current smoker with an average of at least 5 cigarettes per day for the past year, motivated to quit smoking within the next 30 days, had a viable home address and phone number, able to read and write in English, an expired air CO level of ≥ 8 ppm, and provided collateral contact information Exclusion criteria: Contraindication for nicotine patch use, regular use of tobacco products other than cigarettes, use of bupropion or nicotine replacement products other than the study patches, pregnancy or lactation, another household member enrolled in the study, active substance dependence, current psychiatric disorder or use of psychotropic medications, and participation in a smoking cessation treatment programme in the previous 90 days Number eligible 1005. Three comparison groups: mindfulness‐based addiction treatment (MBAT), CBT, and usual care. 154 randomised to the MBAT group (mean age 48.4, 45.4% men), 155 randomised to the CBT group (mean age 48.8, 45.8% men), and 103 to the usual care group (mean age 49, 43.7% men). Medications at baseline: Not reported Medication change during the trial not reported |
|
| Interventions | All groups were given self‐help materials and nicotine patch therapy. Patch therapy for participants who smoked > 10 cigarettes per day consisted of 4 weeks of 21 mg patches, 1 week of 14 mg patches, and 1 week of 7 mg patches. Patch therapy for participants who smoked 5 to 10 cigarettes per day consisted of 4 weeks of 14 mg patches and 2 weeks of 7 mg patches. Self‐help materials consisted of the consumer products developed for the 2008 update of the Treating Tobacco Use and Dependence Clinical Practice Guideline. The CBT and MBAT groups received 8 x 2‐hour in‐person group counselling sessions. Usual care participants received 4 x 5‐ to‐10 minute guideline‐based individual counselling sesions. Intervention (duration 8 weeks, setting not reported): MBAT: MBAT closely follows the MBCT (Segal 2002) treatment procedures, but replaces the depression‐related material with nicotine dependence‐related material. The core aims of MBAT are derived from MBCT. Those aims are to help individuals: 1) become more aware of thoughts, feelings, and sensations from moment to moment, 2) develop a different way of relating to thoughts, feelings, and sensations, and 3) increase the ability to disengage attention and choose skilful responses to any thoughts, feelings, or situations that arise. For example, one could note that the craving is a sensation or mental event (as opposed to an imperative) and simply notice the sensations non‐judgementally until they pass, choose to engage in a coping behaviour, or bring one's attention back to the breath, which is designed to refocus attention on the present moment. The scheduled quit day was on session 5 for participants randomised to MBAT. Sessions 5 to 8 focused on continuing to develop awareness of the present moment, along with an expansion of techniques for dealing with problematic thoughts, feelings, and situations. Comparison(s) (duration 8 weeks CBT, 4 weeks usual care, setting not reported): CBT: CBT utilised a fairly standard problem‐solving/coping skills training approach based on relapse prevention theory of the guideline. The treatment is manualised and all activities are geared toward promoting smoking cessation and the maintenance of abstinence. Salient issues covered include nicotine replacement therapy, commitment to abstinence, social/peer pressure, health issues, motivation to change, commitment to change, and coping with stress. The scheduled quit day was session 5 for participants randomised to CBT in order to match MBAT. CBT was chosen as the control condition because it is an empirically supported and recommended treatment for smoking cessation. Treatment contact time, modality, therapists, and assessments were identical to MBAT. Usual care: UC participants received 4 x 5‐ to‐10 minute individual counselling sessions based on the guideline. Usual care was intended to be equivalent to the intervention a smoker might receive when asking a healthcare provider for help. The content of the sessions emphasised problem‐solving and coping skills training. The scheduled quit day was session 3 for participants randomised to usual care. |
|
| Outcomes | Follow‐up at 26 weeks: 7‐day point prevalence smoking abstinence. Anxiety, perceived stress, and self‐efficacy were measured to examine predictors of smoking cessation. | |
| Notes |
Country: USA Funding: This research and preparation of this manuscript were supported by grants from the National Institute on Drug Abuse, the Centers for Disease Control and Prevention, the National Cancer Institute, the National Center for Complementary and Integrative Health, and the Oklahoma Tobacco Settlement Endowment Trust. Declarations of interest: Not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Following the baseline visit, participants were randomised into UC (N = 103), CBT (N = 155), or MBAT (N = 154) using a form of adaptive randomisation called minimization" |
| Allocation concealment (selection bias) | Unclear risk | Participants and research personnel were not blinded to treatment condition following randomisation. No further details. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and research personnel were not blinded to treatment condition following randomisation. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Participants and research personnel were not blinded to treatment condition following randomisation. Self‐reported smoking cessation but biochemically verified. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis. "... sensitivity analyses were conducted to examine the effect of varying missing data assumptions using a multiple imputation approach" |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry number identified to check |
| Other bias | Unclear risk | Insufficient information to judge |
Weiss de Souza 2020.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Recruited by phone from a waiting list of an outpatient public health tobacco treatment service in a single city in Brazil. Recruitment dates not reported. Inclusion criteria: 1) No previous treatment for smoking cessation; 2) literacy (ability to complete written questionnaires); 3) not being pregnant; 4) physical and mental health adequate for participation (attending sessions, medical appointments); 5) no serious health problems or addiction to other drugs per self‐report; 6) smoke 10 or more cigarettes a day Exclusion criteria: Not explicitly stated Number eligible 103; 44 randomised to the intervention group (mean age 50.6, 18.2% men) and 42 to the comparison group (mean age 50.1, 21.4% men) Medications at baseline: Not reported Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 8 weeks MBRP, plus 4 weekly standard treatment sessions and 6 maintenance sessions (at weeks 6, 8, 10, 12, 24, and 48), setting not reported): Mindfulness‐based relapse prevention (MBRP) was delivered by a certified MBRP instructor and was delivered in 8, 2‐hour weekly group sessions with up to 6 participants per cohort. Each session had a primary topic, and all sessions included guided meditation, exercises, discussions, and homework review (Bowen 2011). Participants were instructed to keep a log of lapses and relapses, and to practise daily between the sessions using an audio CD provided for support. Comparison (duration 4 weekly standard treatment sessions and 6 maintenance sessions (at weeks 6, 8, 10, 12, 24, and 48), setting not reported): standard treatment (see below) Both groups received standard treatment. The Brazilian Ministry of Health tobacco cessation protocol for standard treatment is cognitive behavioural treatment delivered in 4 x 90‐minute structured weekly sessions and 6 maintenance follow‐up sessions. Sessions are designed to provide information on risks of smoking and benefits of quitting, stimulate self‐control and self‐management to disrupt the cycle of dependence, and support smokers to become agents of change with respect to their own behaviour. Sessions were conducted by a physician with experience treating smoking and with training in the Ministry of Health ST approach. Prescriptions for nicotine replacement therapy (transdermal nicotine patches and nicotine gum or tablet) or bupropion hydrochloride were provided. |
|
| Outcomes | Follow‐up at 12 and 24 weeks: biochemically confirmed smoking abstinence, at 12 weeks anxiety, depression | |
| Notes |
Country: Brazil Funding: This research was supported by grant number 2013/02316‐5 from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), grant number 870470/1997‐3 from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), grant number APQ‐04279‐10 from Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) and grant number 552452/2011 from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). Declarations of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Says randomly allocated and stratified but not how they were randomly allocated. |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information, but blinding unlikely. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis for smoking cessation. Participants who were lost to follow‐up were counted as smokers. Very high losses to follow‐up for both groups (> 75%). |
| Selective reporting (reporting bias) | Unclear risk | Trial retrospectively registered |
| Other bias | Unclear risk | Insufficient information to judge |
Weng 2021.
| Study characteristics | ||
| Methods | Parallel‐group RCT ‐ pilot study | |
| Participants | From February to November 2015, participants were recruited from companies or individually through outreach recruitment sessions. Inclusion criteria: Eligible participants were adult female smokers (aged ≥ 18 years) who smoked at least 1 cigarette per day in the past 3 months Exclusion criteria: Currently participating in other smoking cessation programmes or could not communicate in Chinese Number eligible 244; 114 randomised to the intervention group and 99 to the comparison group, all female and mean age overall 33.6 years Medications at baseline: Not reported Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 2 weeks, setting not reported): Self‐help booklet and brief mindfulness training (MT). The brief‐MT workshop with 2 sessions was held within 2 weeks and was led by a certified clinical therapist. Each session lasted for 2 hours with 8 to 20 participants. Mindfulness meditation was achieved through training of attention regulation, body awareness, and emotion regulation. The brief‐MT workshop was designed to 1) increase self‐awareness on the emotions and body sensations that trigger craving and smoking; 2) maintain an attitude of acceptance towards craving and nicotine withdrawal symptoms; 3) sit with the negative affections and alleviate stress through mindfulness rather than to habitually react to cues of smoking; and 4) practise meditation techniques including body scan, breath awareness, and mindful yoga to ride out craving Comparison (duration and setting not reported) Self‐help booklet A 50‐page self‐help smoking cessation booklet (about 9000 words) was given to participants in both groups. Trained counsellors provided individual face‐to‐face briefing to the control group on motivating the participants to follow the booklet's advice. The booklet was tailored for female smokers in workplaces, which included 1) knowledge about the impacts of smoking on women's health, appearance, pregnancy and child; 2) career life planning; 3) smoking cessation and its methods along with steps of mindful eating; and 4) stories of successful quitting. A few participants in the intervention group (n = 10) and in the control group (n = 9) joined an optional 1‐hour health talk (including hazards of smoking and benefits and methods of quitting) designed to increase the participation of the study, the content of which was more general and shorter than that of the self‐help booklet. |
|
| Outcomes | Follow‐up at 26 weeks: smoking cessation | |
| Notes |
Country: China Funding: The Smoking Cessation Program for Women in Workplace was funded by the Lok Sin Tong Benevolent Society Kowloon. Declarations of interest: The authors declared that there is no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Low risk | "The allocation sequence was generated by a researcher who was not involved in participant recruitment". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Participants within the same company received the same treatment to avoid intervention contamination". "Blinding of the interventionists and participants were not possible". We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Blinding of the interventionists and participants were not possible, although all outcome assessors and statistical analysts were blinded from the group allocation". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "The main analyses were by intention‐to‐treat (ITT) assuming those who were lost from contact and dropped out as failures to achieve any cessation outcome". |
| Selective reporting (reporting bias) | Low risk | Trial registry checked (NCT02497339) and all outcomes listed are reported on. |
| Other bias | Unclear risk | Insufficient information to judge |
Woods‐Giscombe 2019.
| Study characteristics | ||
| Methods | Parallel‐group RCT ‐ feasibility study | |
| Participants | Recruited from healthcare agencies and community screening events (e.g. community organisational events, health fairs, and churches). Recruitment dates not reported. Inclusion criteria: 1) African American; 2) aged 25 and 65 years; 3) meets ADA criteria for prediabetes either by HbA1C of 5.7% to 6.4%, FPG of 5.6 to 6.9 mmol/L, or glucose of 7.8 to 11.1 mmol/L at 2 hours in an oral glucose tolerance test; 4) Perceived Stress Scale‐14 score > 5 or self‐report of at least “some” general life stress Exclusion criteria: 1) Diabetes diagnosed by a healthcare provider; 2) past or current use of hypoglycaemic medication (except for gestational diabetes); 3) disease associated with disordered glucose metabolism (e.g. Cushing’s Syndrome); 4) regular use of medications associated with impaired glucose metabolism (e.g. oral or parenteral steroids); 5) active treatment for or history of a major medical illness such as coronary heart disease; 6) previous formal training in meditation and other mind/body practices including yoga, tai chi, or qi gong; 7) psychosis or significant depression, anxiety, or substance abuse under active care (> 2 mental healthcare visits per month) or requiring more than 2 psychotropic medicines daily or hospitalisation within the past 2 years; 8) pregnancy or anticipated pregnancy; 9) impaired cognition; 10) lack of self‐reported life stress Number eligible 86; 38 randomised to the intervention group (mean age 52.5, 40% men) and 30 to the comparison group (mean age 52.7, 26.3% men) Medications at baseline: Not reported, although diabetes medications were an exclusion criteria. Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 8 weeks plus booster sessions, setting ‐ local public school): A mindfulness‐based diabetes risk‐reduction education programme (MPD): included diabetes risk reduction education (as in the CPD intervention) as well as training in mindfulness‐based stress management adapted to include information and behavioural‐change content to promote diabetes risk reduction. Taught by an experienced mindfulness instructor who was also knowledgeable about diabetes prevention and followed the basic format outlined in the Kabat‐Zinn’s MBSR programme (Kabat‐Zinn 1990), but was adapted by shortening the amount of training time to 1 hour and including discussions on relevance to diabetes risk reduction. This segment was followed by a 1‐hour diabetes risk‐reduction education segment, facilitated by a certified diabetes educator, which included a question and answer session and discussions about barriers to and facilitators of behaviour‐change strategies. Time during each session also included discussions on the previous week’s homework, strategies for practically applying the lesson of the week at home. Homework assignments included mindfulness skill practices and diabetes‐prevention activities. Booster sessions involved review and practice of mindfulness skills, review of strategies for overcoming barriers and sustaining lifestyle behaviour changes to reduce risk factors for diabetes. Comparison (duration 8 weeks plus booster sessions, setting ‐ local public school): A conventional diabetes risk‐reduction education programme (CPD): The CPD attention‐control group provided experiences that were similar in time to the MPD, including amount of diabetes‐prevention educational content, amount of regular participant contact with instructors, and amount of homework assigned. However, instead of content on mindfulness for 1 hour of the session, the CPD participants engaged in activities and games directly related to the content delivered by the CPD facilitators. CPD was facilitated by health educators experienced in working with small groups, knowledgeable about diabetes prevention, and trained to involve participants in discussion of diabetes risk reduction. CPD homework assignments and booster sessions matched those of the MPD group in terms of time commitment and relevance to diabetes prevention. For both interventions, each weekly session lasted for 2.5 hours, in addition to a 4‐hour half‐day retreat held on a Saturday, plus 6 x 1.5‐hour booster sessions at 1‐month intervals following the 8‐week programme. |
|
| Outcomes | Follow‐up at 26 weeks: perceived stress, HbA1C, BMI | |
| Notes |
Country: USA Funding: This study was supported with a grant from the United States National Institutes of Health, NCCAM R21 AT004276‐03. Postdoctoral support was provided by the NIH NCCIH Research Fellowship in Complementary and Integrative Healthcare (T32 AT003378). Services were provided through the North Carolina Translational and Clinical Sciences Institute supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number UL1TR001111. Dr. Woods‐Giscombe’s time was also supported by the Robert Wood Johnson Foundation Nurse Faculty Scholars Program and the Josiah Macy Foundation (Macy Faculty Scholars Program). Declarations of interest: The authors declare that there are no conflicts of interest regarding the publication of this paper. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random number generator program was used to randomise participants and to ensure equal numbers of MPD and CPD assignments within a permuted variable block size of 4 to 8 subjects. |
| Allocation concealment (selection bias) | Low risk | System used sequential sealed envelopes to ensure allocation concealment. The study co‐ordinator chose each envelope by a sequential study identification number and notified each individual of their allocation the day before the classes were to begin. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although the nature of the interventions did not allow for blinding of the instructors or participants, steps were taken to minimise differences in participant expectancy ‐ the experimental interventions were advertised under the umbrella title of “We Can Prevent Diabetes” and described to participants as two group‐based diabetes prevention educational interventions. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The statistician and data manager were blinded with respect to group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/38 intervention and 3/30 control lost to follow‐up. ITT used. |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registration identified to check |
| Other bias | Unclear risk | Insufficient information to judge |
Zanella 2021.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Recruitment methods and dates not reported. Inclusion criteria: Female patients; with an age range between 21 and 59 years; BMI ≥ 30 kg/m²; who accepted to participate the study Exclusion criteria: Diagnosed diabetes; diagnosis of any psychiatric disorder; being pregnant or suspicious of being pregnant; having a pacemaker or any other electronic devices inside of the body; having a metallic prosthesis inside of the body; or not agreeing to participate in the study Number eligible not reported; 16 randomised to the intervention group and 16 to the comparison group, all female, mean age not reported Medications at baseline: Not reported Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 3 months, setting not reported): Meditation and mindful eating group: received individualised nutritional orientation associated with guided meditation, with meal plans that were hypo‐energetic, with a calorie reduction between 500 and 1000 kcal/day, and a macronutrient distribution of 50% to 60% carbohydrates, 10% to 15% protein, and 30% to 35% fat. Meal plans with a calorific value lower than 1200 kcal/day were not employed. The guided meditations were delivered to the patients in MP3 format, and included: 1st month – 3 x 10‐minute meditations (each one for 10 days); 2nd month – 3 x 15‐minute meditations (each for 10 days); 3rd month – 3 x 20‐minute meditations (each for 10 days), amounting to 90 days of guided meditation. Comparison (duration 3 months, setting not reported) Same nutritional advice as the intervention group with no meditation or mindful eating |
|
| Outcomes | Follow‐up at 3 months: perceived stress, weight, BMI, FBG, lipids, HRQoL | |
| Notes |
Country: Brazil Funding: not reported Declarations of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information. Trial registry with results reported. Few details. |
| Allocation concealment (selection bias) | Unclear risk | No information. Trial registry with results reported. Few details. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information. Trial registry with results reported. Few details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information. Trial registry with results reported. Few details. |
| Selective reporting (reporting bias) | Unclear risk | Retrospectively registered, but all primary outcomes listed reported on. Author reports that they did not determine secondary outcomes. |
| Other bias | Unclear risk | Insufficient information to judge |
Zarifsanaiey 2020.
| Study characteristics | ||
| Methods | Parallel‐group RCT | |
| Participants | Patients who referred to the clinic from November 2018 till July 2019 were selected through a convenience sampling method. Inclusion criteria: Diabetic patients who have had diabetes for more than 1 year, older than 18 years, willingness to participate in the research and reside in Zarqan Exclusion criteria: Severe psychological illness alongside diabetes, physical defect or deformity, experience of diabetic coma, engagement in other training programmes while the mindfulness intervention is in effect Number eligible not reported; 68 randomised to the intervention group (mean age 48.3) and 68 to the comparison group (mean age 49.5). 33.8% male overall Medications at baseline: Not reported Medication change during the trial not reported |
|
| Interventions |
Intervention (duration 8 weeks, setting not reported): Eight 2‐hour sessions of mindfulness training. Each session included mindfulness meditations (of mindfulness of breathing, body scan, mindful movement), group discussions and reviewing home practice. Covered both formal meditations and applying mindfulness to usual daily activities. Topics covered present moment awareness and mindfulness of thoughts. Comparison No intervention |
|
| Outcomes | Follow‐up at 20 weeks: well‐being, FBG, HbA1c | |
| Notes |
Country: Iran Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article. Declarations of interest: On behalf of all authors, the corresponding author states that there is no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random allocation was performed using random allocation software". |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Both of the study samples and statistical analyser were blinded to intervention group (mindfulness training) and control group (without intervention)." Unclear how blinding was achieved as the control group received no intervention. We have left as unclear risk as it is difficult, if not impossible, to blind participants and personnel to lifestyle interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Both of the study samples and statistical analyser were blinded to intervention group (mindfulness training) and control group (without intervention)." Does not specifically state outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All 136 patients completed the study and the follow‐up assessment". |
| Selective reporting (reporting bias) | Unclear risk | No protocol or trial registry number identified to check |
| Other bias | Unclear risk | Insufficient information to judge |
ACE: angiotensin‐converting‐enzyme (inhibitor); ADA: American Diabetes Association; ARB: angiotensin‐II receptor blocker; BMI: body mass index; BP: blood pressure; CABG: coronary artery bypass graft; CAD: coronary artery disease; CBT: cognitive behavioural therapy; CES‐D: Centre for Epidemiological Studies ‐ Depression scale; CHD: coronary heart disease; CHF: congestive heart failure; CR: cardiac rehabilitation; CVD: cardiovascular disease; DBP: diastolic blood pressure; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders IV; ECG: electrocardiogram; EGFR: estimated glomerular filtration rate; FBG: fasting blood glucose; FPG: fasting plasma glucose; h: hours; HbA1c: glycated haemoglobin (A1c); HDL: high‐density lipoprotein; HE: health education; HRQoL: health‐related quality of life; IHD: ischaemic heart disease; ITT: intention‐to‐treat; LDL: low‐density lipoprotein; LTCF: long‐term care facilities; MB‐EAT: mindfulness‐based eating awareness therapy; MBAT: mindfulness‐based addiction therapy; MBCT: mindfulness‐based cognitive therapy; MBSR: mindfulness‐based stress reduction; MI: myocardial infarction; min: minutes; MM: mindfulness meditation; NYHA: New York Heart Association; PAID: Problem Areas in Diabetes; PCI: percutaneous coronary intervention; PMR: progressive muscle relaxation; QoL: quality of life; RCT: randomised controlled trial; SBP: systolic blood pressure; SES: socioeconomic status; T2DM: type 2 diabetes mellitus; TM: Transcendental Meditation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Delui 2013 | Short term as < 12 weeks follow‐up (defined as duration of intervention and any post‐intervention follow‐up) |
| Gentile 2021 | Short term as < 12 weeks follow‐up (defined as duration of intervention and any post‐intervention follow‐up) |
| Gotink 2017 | Wrong population ‐ eligible patients had heart disease including ischaemic heart disease, but those recruited had either congenital heart disease, cardiomyopathies or valvular heart disease |
| Gregg 2007 | Wrong intervention ‐ we excluded studies using acceptance and commitment therapy (ACT) as an intervention, as whilst ACT utilises mindfulness practices, these are not generally based on formal meditation but rather a large array of mindfulness tools and techniques (Harris 2021). |
| Hosseini 2021 | Short term as < 12 weeks follow‐up (defined as duration of intervention and any post‐intervention follow‐up) |
| IRCT20090716002195N3 | Short term as < 12 weeks follow‐up (defined as duration of intervention and any post‐intervention follow‐up) |
| IRCT20160927030002N2 | Short term as < 12 weeks follow‐up (defined as duration of intervention and any post‐intervention follow‐up) |
| Iturbe 2019 | Wrong intervention ‐ we excluded studies using acceptance and commitment therapy (ACT) as an intervention, as whilst ACT utilises mindfulness practices, these are not generally based on formal meditation but rather a large array of mindfulness tools and techniques (Harris 2021). |
| Jayadevappa 2007 | Wrong patients ‐ recruited patients with congestive heart failure (CHF) but unclear how many had CHF as a consequence of CVD. |
| Kumar 2017 | Short term as < 12 weeks follow‐up (defined as duration of intervention and any post‐intervention follow‐up) |
| Lappalainen 2014 | Wrong intervention ‐ we excluded studies using acceptance and commitment therapy (ACT) as an intervention, as whilst ACT utilises mindfulness practices, these are not generally based on formal meditation but rather a large array of mindfulness tools and techniques (Harris 2021). |
| Manikonda 2008 | Short term as < 12 weeks follow‐up (defined as duration of intervention and any post‐intervention follow‐up) |
| Momeni 2016 | Short term as < 12 weeks follow‐up (defined as duration of intervention and any post‐intervention follow‐up) |
| Monin 2020 | Wrong patients ‐ couples were randomised where one or both had metabolic syndrome. Data not analysed separately and analysis included people without cardiovascular risk factors. |
| Nyklíček 2014 | Wrong comparator ‐ the comparison group was a brief mindfulness intervention, so a different level of intensity of the intervention of interest. |
| Packiasabapathy 2019 | Short term as < 12 weeks follow‐up (defined as duration of intervention and any post‐intervention follow‐up) |
| Palta 2012 | Short term as < 12 weeks follow‐up (defined as duration of intervention and any post‐intervention follow‐up |
| Patarathipakorn 2021 | Short term as < 12 weeks follow‐up (defined as duration of intervention and any post‐intervention follow‐up) |
| Pool 1996 | Short term as < 12 weeks follow‐up (defined as duration of intervention and any post‐intervention follow‐up) |
| Potts 2020 | Wrong intervention ‐ we excluded studies using acceptance and commitment therapy (ACT) as an intervention, as whilst ACT utilises mindfulness practices, these are not generally based on formal meditation but rather a large array of mindfulness tools and techniques (Harris 2021). |
| Sampaio 2019 | Short term as < 12 weeks follow‐up (defined as duration of intervention and any post‐intervention follow‐up) in the control group (after completion of the post‐test phase of the study at 8 weeks, the Healing Meditation programme was offered to control participants whereas those in the intervention group were followed for 16 weeks). |
| Spatola 2014 | Wrong intervention ‐ we excluded studies using acceptance and commitment therapy (ACT) as an intervention, as whilst ACT utilises mindfulness practices, these are not generally based on formal meditation but rather a large array of mindfulness tools and techniques (Harris 2021). |
| Spears 2019 | Wrong comparator ‐ the comparison group was MBI compared to MBI plus text messaging, so a different level of intensity of the intervention of interest. |
| Tacon 2003 | Short term as < 12 weeks follow‐up (defined as duration of intervention and any post‐intervention follow‐up) |
| Tapper 2009 | Wrong intervention ‐ we excluded studies using acceptance and commitment therapy (ACT) as an intervention, as whilst ACT utilises mindfulness practices, these are not generally based on formal meditation but rather a large array of mindfulness tools and techniques (Harris 2021). |
| Tedder 2015 | Short term as < 12 weeks follow‐up (defined as duration of intervention and any post‐intervention follow‐up) |
| Vala 2016 | Short term as < 12 weeks follow‐up (defined as duration of intervention and any post‐intervention follow‐up) |
| Zervos 2021 | Short term as < 12 weeks follow‐up (defined as duration of intervention and any post‐intervention follow‐up); only intervention participants in follow‐up study |
Characteristics of studies awaiting classification [ordered by study ID]
Araujo 2021.
| Methods | RCT |
| Participants | Smokers |
| Interventions | Mindfulness |
| Outcomes | Smoking cessation |
| Notes | See also https://ensaiosclinicos.gov.br/rg/RBR-3w2scz for trial registration details |
Babak 2022.
| Methods | RCT |
| Participants | Women with hypertension |
| Interventions | MBSR |
| Outcomes | Blood pressure, perceived stress |
| Notes | Identified when updating current status of the ongoing studies table from November 2021 to May 2023 (see Table 5). Trial registration IRCT20190410043230N1. Briefly, they found reductions in blood pressure and increased quality of life with MBSR (N = 37) versus routine care (N = 39) in women with hypertension. |
Bahadori 2022.
| Methods | RCT |
| Participants | People with obesity |
| Interventions | Schema therapy |
| Outcomes | Weight, self‐esteem |
| Notes | Identified when updating current status of the ongoing studies table from November 2021 to May 2023 (see Table 5). Trial registration IRCT20200210046451N1. Briefly, they found that schema therapy (N = 16) had a positive effect on weight and self‐esteem compared to control (N = 16) in people with obesity. |
Bynum 1980.
| Methods | RCT |
| Participants | Essential hypertension |
| Interventions | Christian meditation |
| Outcomes | Blood pressure |
| Notes | Library unable to obtain full text ‐ need to check intervention details and follow‐up period to determine eligibility |
Davazdah Emamy 2018.
| Methods | RCT |
| Participants | Type 2 diabetes |
| Interventions | Mindfulness‐based stress reduction |
| Outcomes | Quality of life |
| Notes | English abstract looks eligible; needs translation for full‐text review and data extraction |
de la Fuente 2010.
| Methods | RCT |
| Participants | Secondary education teachers suffering essential hypertension, grade 1 and grade 2 |
| Interventions | Training programme in mindfulness meditation |
| Outcomes | Blood pressure |
| Notes | English abstract looks eligible; needs translation for full‐text review and data extraction |
DiNardo 2022.
| Methods | RCT |
| Participants | Diabetes |
| Interventions | Diabetes self‐management and mind‐STRIDE intervention |
| Outcomes | HbA1C, weight, blood pressure, depression |
| Notes | Identified when updating current status of the ongoing studies table from November 2021 to May 2023 (see Table 5). Trial registration NCT02928952. Briefly, one session of mindfulness integrated in a diabetes self‐management education and support programme, and a booster mindfulness session (N = 65), showed similar improvements in depression and HbA1C to the programme without mindfulness (N = 67). |
DRKS00014929.
| Methods | RCT |
| Participants | Smokers |
| Interventions | Mindfulness‐based stress reduction |
| Outcomes | Perceived stress, mental well‐being |
| Notes | Results reported in German: http://drks.de/en/trial/DRKS00014929#studyresults. Need to check follow‐up period for eligibility. |
Duraimani 2015.
| Methods | RCT |
| Participants | African American men and women with stage I hypertension |
| Interventions | Transcendental meditation |
| Outcomes | Blood pressure |
| Notes | This is a sub‐study to a larger trial of TM which does not appear to have been published and the results have not been posted on the trial registration page. Authors include Schneider et al, but have checked the other included studies from this group and although similar the numbers are different ‐ this study has 152 participants randomised. The methods are discussed in this sub‐study but not referenced to any other publication. Concerning given potential overlap with other studies and possible publication bias. NCT00681200. |
IRCT20180205038630N5.
| Methods | RCT |
| Participants | Cardiovascular disease |
| Interventions | Mindful self‐compassion |
| Outcomes | Anxiety, anger |
| Notes | Follow‐up time not stated, intervention 8 weeks but unclear when outcomes were measured. Needs to be 12 weeks or more to be eligible. |
Jassemi Zergani 2021.
| Methods | RCT |
| Participants | Obese women |
| Interventions | MB‐EAT |
| Outcomes | Weight |
| Notes | Identified when updating current status of the ongoing studies table from November 2021 to May 2023 (see Table 5). Trial registration IRCT20200919048767N1. Briefly, the English abstract states that MB‐EAT increased the ability to self‐regulate and be aware of the body sensations and emotional symptoms of eating and doing physical activity on a daily basis, which eventually led to weight loss (41 participants randomised). |
Khosravi 2016.
| Methods | RCT |
| Participants | Women with hypertension |
| Interventions | MBSR |
| Outcomes | Blood pressure, perceived stress |
| Notes | Identified when updating current status of the ongoing studies table from November 2021 to May 2023 (see Table 5). Trial registration IRCT2016070126600N1. English abstract reports relevant outcomes at 8 weeks, unclear if longer follow‐up available to meet eligibility of 12 weeks. 30 participants randomised. Needs translation. |
Li 2018.
| Methods | Unclear |
| Participants | Stroke patients |
| Interventions | Mindfulness |
| Outcomes | Unclear |
| Notes | Translation needed to assess eligibility, no English abstract |
Marwaha 2020.
| Methods | RCT |
| Participants | Hypertension |
| Interventions | Transcendental meditation |
| Outcomes | Blood pressure |
| Notes | Library unable to obtain full text. Abstract describes 3 separate studies. Need full text to check eligibility. |
Morillo‐Sarto 2023.
| Methods | Cluster RCT |
| Participants | Overweight/obese |
| Interventions | Mindful eating programme |
| Outcomes | Anxiety, depression, blood pressure, weight, lipid levels, glucose, HbA1C |
| Notes | Identified when updating current status of the ongoing studies table from November 2021 to May 2023 (see Table 5). Trial registration NCT03927534. Briefly, 76 overweight or obese participants were randomised to mindful eating + TAU or TAU alone. Weight and other physiological parameters were not significantly affected by mindful eating + TAU. |
NCT00010738.
| Methods | RCT |
| Participants | Patients with CHD |
| Interventions | Transcendental meditation |
| Outcomes | Psychological stress and quality of life |
| Notes | Trial completed in 2002, last updated 2006. No results or publications. Cannot find name and few details reported so not clear if follow‐up is long enough for the study to be eligible. |
NTR4968.
| Methods | RCT |
| Participants | Normal weight/overweight/obese |
| Interventions | Mindfulness |
| Outcomes | Anthropometric measurements |
| Notes | — |
Pirmoradi 2022.
| Methods | RCT |
| Participants | Overweight and obese women |
| Interventions | Acceptance mindfulness and compassion |
| Outcomes | BMI |
| Notes | Identified when updating current status of the ongoing studies table from November 2021 to May 2023 (see Table 5). Trial registration IRCT20190924044866N1. Briefly, they found a reduction in BMI with the acceptance mindfulness and compassion intervention (N = 26) compared to treatment as usual (N = 26) in overweight and obese women. |
Rudlof 2022.
| Methods | RCT |
| Participants | CHD |
| Interventions | Transcendental meditation |
| Outcomes | Blood pressure, quality of life, anxiety, perceived stress |
| Notes | Results reported for 4 weeks follow‐up. Unclear if longer‐term assessment will be reported on. To be eligible for inclusion in the review 12 weeks follow‐up (including intervention period) is required. Identified when updating current status of the ongoing studies table from November 2021 to May 2023 (see Table 5). Trial registration NCT05035758. |
Salvo 2022.
| Methods | RCT |
| Participants | Overweight/obesity |
| Interventions | Treatment as usual and MB‐EAT |
| Outcomes | Anxiety, depression, fasting blood glucose, HbA1C, lipid levels |
| Notes | Identified when updating current status of the ongoing studies table from November 2021 to May 2023 (see Table 5). Trial registration NCT02893150. Briefly, overweight and obese women on low incomes were randomised to treatment as usual (N = 48), mindfulness‐based health promotion (N = 40) and MB‐EAT (N = 45). Overall, there were no significant robust intervention effects on weight, HbA1C, lipids, or anxiety and depression. |
Sogol 2020.
| Methods | Unclear |
| Participants | Type 2 diabetes |
| Interventions | Mindfulness |
| Outcomes | Anxiety and self‐care |
| Notes | English abstract looks potentially eligible. Needs translation for full‐text review and data extraction |
Yanhong 2020.
| Methods | RCT |
| Participants | Diabetes |
| Interventions | Mindfulness |
| Outcomes | FBG, HbA1C |
| Notes | English abstract looks eligible. Needs translation for full text review and data extraction |
Zhang 2018.
| Methods | Unclear |
| Participants | Hypertension |
| Interventions | Focussed meditation |
| Outcomes | Anxiety |
| Notes | Translation needed to assess eligibility; no English abstract |
BMI: body mass index; CHD: coronary heart disease; FBG: fasting blood glucose; HbA1c: glycated haemoglobin (A1c); MB‐EAT: mindfulness‐based eating awareness therapy; MBSR: mindfulness‐based stress reduction; RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
ACTRN12618000844246.
| Study name | MENTOR study |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria: Adults with congenital heart disease |
| Interventions | Intervention: An audio CD and a CD player will be used by the facilitator to guide participants through a meditation intervention. Participants will be given a copy of the audio CD, tape, USB stick or will be given access to a free online platform of the meditation intervention sent via an email link for home practice. Procedure: This meditation intervention will consist of 6 consecutive weekly group sessions of 16 minutes duration in addition to conventional cardiac rehabilitation. At the group meetings, before each meditation session, the facilitator will lead a short 1‐ to 2‐minute per person check‐in to identify any issues or challenges participants are having with the practice. Control: The standard care control group will be able to participate in cardiac rehabilitation. There will be no alteration to the cardiologist or other outpatient follow‐up appointments or medications unless advised by the relevant health professional, nor restrictions to use of other complementary and alternative therapies. |
| Outcomes | Anxiety, depression, stress, self‐effectiveness, hospitalisation at 3 months |
| Starting date | Start date: May 2018 |
| Contact information | A/Prof Louise Hickman, angela.rao@uts.edu.au |
| Notes | Last update June 2019, recruiting |
ACTRN12618001247268.
| Study name | Compassion focused therapy as a treatment for body weight shame associated with obesity |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention: Compassion focused therapy will be delivered to participants across 12 x 2‐hour sessions. The intervention will be co‐facilitated by an experienced registered clinical psychologist and a provisional health psychologist at the University of Queensland, Brisbane. The procedures, activities and processes used will follow the 12‐week therapy protocol of Compassion Focused Therapy by Gilbert, Kirby and Petrocchi. It will be delivered face to face in a group therapy format. Covering understanding the mind, mindfulness, compassion, and compassion cultivation.
The programme will focus on practices including: 1) a soothing rhythm breathing practice; 2) a practice focused on creating friendly facial expressions and voice tones as part of compassion; 3) a practice aimed to develop mindfulness and increase attention to one’s current mental state; 4) a practice aimed to develop the sense of a compassionate self that is based upon feelings of wisdom, strength and commitment to be supportive and helpful to self and others; 5) an imagery practice aimed to develop a compassionate image of another mind that has caring intent towards the self; and 6) a practice aimed to develop a compassionate self that has caring intent towards the self and how to use compassion focusing to work with self‐criticism and life difficulties. Control: treatment as usual ‐ wait list control At the end of the study, all participants will be offered treatment. |
| Outcomes | Primary: body weight shame Secondary: anxiety, depression, stress, self‐compassion, eating attitudes, well‐being, shame, self‐criticism and reassurance, fears of compassion, social identification as obese, social comparison, defeat scale Time frame: 12 weeks, 6 months and 12 months |
| Starting date | Start date: February 2019 |
| Contact information | Dr James Kirby, j.kirby@psy.uq.edu.au |
| Notes | Last updated December 2019, status recruiting |
ACTRN12620000105943.
| Study name | SASS study |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Treatment arm: Weekly 60‐minute group movement‐based yoga and mindfulness classes for 12 weeks (~5 to 6 participants per class). The programme will teach mindfulness meditation through breathing and a set of postures that are appropriate for a beginner. The classes will allow for progression, but participants are always able to adjust to the level that best suits them on the day. Intervention will be delivered by trained and experienced yoga teachers (one lead and one assistant per class). Adherence will be monitored using attendance logs. 20 minutes home practice of mindfulness meditation daily (provided on CD/audio link). Adherence will be monitored using participant diary. Attention control arm: Weekly facilitated social group classes (~5 to 6 per class; 60 minutes) on lifestyle strategies for 12 weeks. Practical education on prevention of stroke, and generic topics such as using technology, managing finances, nutritional cooking, tips for driving, getting back to work, travel experiences, internet for beginners, pastimes and hobbies for people with a disability or cognitive improvement. Getting out and about the community with disability aids, and relationship with family and friends will also be discussed. Each 60‐minute class will include 15 to 20 minutes of lectures, followed by discussion time. Participants will not be asked to do home practice. |
| Outcomes | Primary: changes in brain structure Secondary: cholesterol, glucose, blood pressure, cognitive function, fatigue, anxiety, depression, stroke impact, quality of life, cortisol Time point: 12 weeks |
| Starting date | Start date: March 2020 |
| Contact information | Prof Dominique Cadilhac, dominique.cadilhac@monash.edu |
| Notes | Last updated February 2020, status not yet recruiting |
ACTRN12621000445875.
| Study name | RISC‐Resilience Interventions for Smoking Cessation |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria: Children and adolescents (under the age of 18 years of age) |
| Interventions | This study will primarily undertake a quantitative methodological approach with a parallel RCT design. Once consented, participants will be randomised to one of four groups: 1) Arm 1‐Mindfulness‐integrated CBT (MiCBT) plus peer‐support; 2) Arm 2‐Mindfulness Training (MT) plus peer‐support, 3) Arm 3‐Setting Realistic Goals (SRG) plus peer‐support, 4) Control.
Arm 1 (MiCBT)‐In Phase 1 (Months 0‐6), participants will be involved in 8 x 1‐hour online small group sessions with a maximum of 5 other participants. The content of these sessions will cover the MiCBT method and how to apply this method to smoking cessation. These sessions will be delivered at weeks: 1, 3, 5, 7, 9, 13, 17, and 20. During this phase, participants will also be encouraged to complete 2 x daily practice of mindfulness sessions on their own which will take a total duration of approximately 30 to 60 minutes/day. To facilitate this, participants in Arm 1 will be provided with a mobile phone app which provides guided mindfulness exercises. Arm 2 (MT)‐In Phase 1 (Months 0 to 6), participants will be involved in 8 x 1‐hour online small group sessions with a maximum of 5 other participants. The content of these sessions will cover the MT method and how to apply this method to smoking cessation. These sessions will be delivered at weeks: 1, 3, 5, 7, 9, 13, 17, and 20. During this phase, participants will also be encouraged to complete 2 x daily practice of mindfulness sessions on their own which will take a total duration of approximately 30 to 60 minutes/day. To facilitate this, participants in Arm 2 will be provided with a mobile phone app which provides guided mindfulness exercises. Arm 3 (SRG)‐In Phase 1 (Months 0 to 6), participants will be involved in 8 x 1‐hour online small group sessions with a maximum of 5 other participants. The content of these sessions will cover the SRG method and how to apply this method to smoking cessation. These sessions will be delivered at weeks: 1, 3, 5, 7, 9, 13, 17, and 20. In Phase 2 (Months 7 to 12), for all three intervention groups, participants will join an online peer mentoring forum with other participants in their respective groups via a blog which will be facilitated by ex‐smoker peer mentors. Participants will be encouraged to interact with the blog weekly. As a minimum, participants will be asked to react to posts or post their own comment at least once/week. Peer mentors will promote discussion of specified topics and content including: the lived experience of quitting cigarettes; managing triggers and relapses; and how to translate learning from Phase 1 into everyday lives. During Phase 2, participants in arms 1 and 2 will also be encouraged to use the mobile phone app to continue with the 2 x daily mindfulness sessions on their own. The intervention period will be completed at the end of Month 12. In Phase 3, the maintenance phase, participants will not be required to participate in any specific activities but will be able to use or not use the strategies/support provided during Phases 1 and 2 as they desire. Final data collection will then occur at the end of Phase 3, the maintenance phase (Months 13 to 18). Arm 4 Active control group: Participants in this group will be invited to opt in to be referred to their local Quitline service as part of the Royal Australian College of General Practitioner’s 'Ask, Advise, Help' model for smoking cessation. Participants in this group will also be provided with a link to the written materials contained in the National Quitline Quitpack which includes information on accessing Nicotine Replacement Therapy and stop‐smoking medications. |
| Outcomes | Primary: smoking abstinence Secondary: resilience, cost‐effectiveness, self‐esteem, motivation to quit, nicotine dependence, equanimity, nicotine levels, perceived stress Time frame: 3, 6, 9, 12, 18 months |
| Starting date | Start date: April 2021 |
| Contact information | Ms Elissa Mortimer, elissa.mortimer@flinders.edu.au |
| Notes | Last updated April 2021, status not yet recruiting |
ACTRN12621000580875.
| Study name | SC4WM |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Self‐compassion for Weight Management (SC4WM) intervention
The 4‐week SC4WM intervention is delivered online through a mobile‐friendly website. Participants in the intervention group will be asked to follow the digital WW™ program+ SC4WM program. Participants will need to use data or internet connectivity and an SC4WM access code to log into the website content. The SC4WM intervention incorporates simple, evidence‐based techniques (e.g. journaling, letter writing, reflections) to deliver a SC intervention tailored to weight management outcomes (i.e. eating behaviour, physical activity, and weight monitoring). The SC4WM intervention landing page (website) provides an initial definition and background on SC and quick access to each module. The SC4WM has four weekly modules designed to specifically target participants' relationship with each weight management outcome, including SC for eating behaviour, SC for physical activity, and SC for body weight. Each module includes a journal, meditation, and reflection activity incorporating the principles of SC to eating behaviour, physical activity behaviour, and body weight. The activities can be completed in approximately 20 to 30 minutes. It is recommended that participants complete one module in sequence per week, with the last week focused on bringing all of the modules together and incorporating SC4WM into their entire weight management journey.
Adherence to the SC4WM is measured by asking participants at 4 and 12 weeks how many modules they completed and how many days they engaged in the SC4WM activities.
Participants in the intervention group have access to the WW™ program for a total of 12 weeks (4 weeks with the SC4WM program and 8‐weeks on their own until the 3‐month follow‐up). Control group Participants randomised to the control group will have 12‐week access to the digital (100% online) WW™‐ Weight Watchers re‐imagined program. The WW™ program is an evidence‐based behavioural weight management program that aims to support its members with healthier habits. The WW™ program uses the Smartpoints™ food tracking system, which nudges members to make healthier food choices by assigning point values to foods requiring moderation (e.g. higher fat and higher sugar foods). In addition, the WW™ program promotes exercising, cultivating a positive mind set, and tracking body weight with the objective of weight management. The myWW™ program may contain elements of SC within its content, however the SC4WM intervention is designed to have a more concentrated dose of SC. |
| Outcomes | Primary: self‐compassion Secondary: eating behaviour, eating restraint, emotional wellbeing, perceived stress, BMI, coping, weight self‐stigma Time frame: 12 weeks |
| Starting date | Start date: June 2021 |
| Contact information | Ms Jennifer Brenton‐Peters, jbre092@aucklanduni.ac.nz |
| Notes | Last update October 2021, completed |
Asfar 2021.
| Study name | Mindfulness based smoking cessation among cancer survivors |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Experimental: Craving‐to‐Quit app Participants in this group will receive one in‐person orientation session, 6‐week supply of NRT, the "Craving‐to‐Quit" app, and 2 brief follow‐up phone calls. Drug: Nicoderm C‐Q Transdermal Product 6 weeks of nicotine replacement therapy patches Behavioral: Orientation session This session will occur 2 weeks before quit date. It will be moderated by a certified instructor in MT, and will last approximately 90 min. During the session, participants will learn the purpose, format, and procedures of the study, provide written informed consent, and complete the baseline assessment. Behavioural: Craving‐to‐Quit app. The app is comprised of 22 modules for 22 days, 5 to 15 minutes each, designed to teach MT using audio, video, and animation. The app also includes other features such as social support (quit friend sign‐ups, the tip of the week), activity feed (to track interaction with the app), and my morning stats (to track smoking). Behavioural: 2 brief follow‐up phone calls The first phone call will occur one day before the quit date to remind participants about their quit date and provide support. The second will occur at the end of treatment (around day 60 after quit date) to review progress, provide support, and schedule the 3‐month follow‐up visit. Each phone call will last approximately 15 minutes. Experimental: in‐person mindfulness training Participants in this group will receive twice‐weekly group sessions (8 total during 4 weeks) that were manualised and delivered by instructors experienced in Mindfulness Training (MT) (a single therapist with > 4 years of training in MT). Drug: Nicoderm C‐Q Transdermal Product 6 weeks of nicotine replacement therapy patches Behavioural: orientation session. This session will occur 2 weeks before quit date. It will be moderated by a certified instructor in MT, and will last approximately 90 min. During the session, participants will learn the purpose, format, and procedures of the study, provide written informed consent, and complete the baseline assessment. Behavioral: Group MT sessions. Total of 8 group sessions (twice a week) during 4 weeks. The overarching theme of momentary awareness and acceptance of cravings and affect (e.g. stress, anxiety etc.) will be introduced and reinforced in complementary ways throughout the training. Each session will last 45 to 60 minutes. Active comparator: usual care Participants in this group will receive a brief advice to quit smoking, 6‐week supplies of nicotine replacement therapy (NRT), and self‐help materials to quit smoking. Drug: Nicoderm C‐Q Transdermal Product 6 weeks of nicotine replacement therapy patches Behavioural: brief advice on quitting smoking. Standard advice on how to quit smoking. Behavioural: self‐help smoking cessation materials. Written materials on how to quit smoking including contact info for state tobacco quit line. |
| Outcomes | Primary: smoking abstinence Secondary: cigarettes smoked per day, number with reported relapse, usability of the app, acceptability, feasibility of recruitment, attrition Time frame: 3 months |
| Starting date | Start date: September 2019 |
| Contact information | Taghrid Asfar, University of Miami |
| Notes | Last updated March 2021, status recruiting |
Chandra 2020.
| Study name | DIAMAND study |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria: 1) T2DM diagnosed as per American Diabetes Association guidelines, 2) both genders, 3) age 20 to 60 years, 4) on standard of care for management of diabetes for past 3 months, 5) mild to moderate depressive episode diagnosed as per ICD‐10 Diagnostic Criteria for Research (DCR), and severity of depression as assessed by Hamilton Depression Rating Scale (HAM‐D) score between 8 and 24 Exclusion criteria: 1) Antidepressant use for any indication in last 3 months; 2) major complications of diabetes mellitus such as retinopathy, nephropathy, etc.; 3) poorly controlled hypertension; 4) major psychiatric disorders (schizophrenia, bipolar affective disorder); 5) substance use disorders except nicotine; 6) suicidal ideation, suicidal gestures, psychotic symptoms, or agitation; 7) neurological/neurosurgical disorders, cerebrovascular disorders, seizure disorder, and head injury; 8) disorders of cognitive impairment like dementia, mental retardation; 9) recent myocardial infarction within past 6 months; and 10) history of allergy to any antidepressants. |
| Interventions | Four arms: fluoxetine, mindfulness, fluoxetine plus mindfulness, treatment as usual Fluoxetine will be initiated at 20 mg/day in participants in fluoxetine and combination arms by the primary care physician and titrated up to a maximum of 60 mg/day for optimal management. We shall be using a brief mindfulness‐based intervention designed for patients with diabetes and depression based on evidence‐based mindfulness interventions provided in primary care settings. The sessions will include introduction and understanding of the concept of mindfulness, its applicability to patients with diabetes and depression, and general principles of practice of mindfulness. The module will include mindful breathing, body scan, gratitude, and compassion exercise. In addition to formal mindfulness techniques, informal mindfulness procedures like practising mindfulness in daily activities like cooking, cleaning, showering, etc. will be discussed. The participants will be taught the techniques of slowly disengaging from personal thoughts, emotions, and bodily symptoms, gain a sense of personal balance, and reach a state of self‐compassion. |
| Outcomes | Primary: depression Secondary: BMI, WHR, FBG, HbA1C, adherence to medication, diabetes self‐management, mindfulness, quality of life Time frame: 16 weeks |
| Starting date | Not reported |
| Contact information | Suravi Patra, Dept.of Psychiatry, AIIMS Bhubaneswar, Bhubaneswar, Odisha 751019, India. Email: psych_suravi@aiimsbhubaneswar.edu.in |
| Notes | Published protocol, cannot access trial registry |
Chung 2019.
| Study name | Electroacupuncture combined with mindfulness meditation for weight management |
| Methods | Parallel‐group RCT |
| Participants | Target sample size of 165 adults aged 18 to 60, with BMI between 25 and 39.99 and no severe medical complications |
| Interventions | This trial consists of a 2‐week run‐in period, 12 weekly treatment sessions and an 8‐week follow‐up period. Participants will be randomised into one of the three groups: 1) electroacupuncture (EA) + mindfulness meditation (MM) group, 2) sham EA + MM group, and 3) EA only group. EA treatment involves needling 8 acupuncture points and allowing for 30 min electrostimulation with dense‐disperse wave at a frequency of 33 to 100 Hz and 48 mA peak current intensity. Sham EA represents needling to 8 non‐acupuncture points which are approximately 1 cm away from the acupuncture points, with sham electrostimulation that has no actual current going through the body but presents the same as the real electrostimulation. MM involves self‐practising a 10‐minute pre‐recorded MM instruction after EA or sham EA treatment; daily practice is also required. |
| Outcomes | Outcome measurements, including body weight, BMI, waist and hip ratio, psychological influence on eating behaviour and weight‐related quality of life, will be measured at baseline, every 3 weeks during the treatment period and 3 more during the follow‐up period. |
| Starting date | Not reported |
| Contact information | Not reported |
| Notes | Conference proceeding and no trial registry reported for further details. |
DRKS00021412.
| Study name | Self‐compassion, eating behavior & dieting success |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention: Intervention group: daily self‐compassion exercise (either a 16‐minute meditation or a writing exercise, e.g. a letter to oneself) + ecological momentary assessment (EMA) questionnaire on mood, diet success, and mindfulness each evening Control: wait list control group: no exercise or questionnaire during the 14 days of intervention, only 5 days pre‐EMA and 5 days post‐EMA questionnaires |
| Outcomes | Primary: The relationship between increased self‐compassion and an improvement in eating behaviour (more mindful eating, less emotional eating), body weight, and the achievement of a personal weight loss goal will be analysed. We expect that participants in the intervention group will be more self‐compassionate after the intervention phase, compared to before and compared to the wait list control group (questionnaire: SCS‐D). We hypothesise that this will go along with a reduction of BMI and emotional eating (questionnaire: DEBQ) and an increase in mindful eating (questionnaires: IES‐2, FFaMES), and a higher perceived diet success (questionnaire: PSRS). Further, we will investigate the daily correlations between levels of self‐compassion, eating behaviour, and diet success. EMA baseline and post‐EMA assessment will be investigated regarding changes in daily self‐compassion. We expect an improvement in the intervention group. Secondary: Secondary outcomes are an improvement in self‐esteem (1 item), better emotion regulation skills (questionnaire: CERQ), less craving (Questionnaire: FCQT‐R), less stress eating (questionnaire: SSES) and a preference for healthier food (rating of food pictures regarding the desire to eat this food now). The correlation between daily variations in self compassion, mood, stress, mindfulness, and craving will be analysed using EMA data. Time frame: 3 months |
| Starting date | Start date: May 2020 |
| Contact information | Jens Blechert, jens.blechert@sbg.ac.at |
| Notes | Last updated November 2021, status suspended recruitment |
Forman 2021.
| Study name | Project activate: mindfulness and acceptance based behavioral treatment for weight loss |
| Methods | Parallel‐group RCT (factorial) |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Eight comparison groups: Experimental: standard behavioural weight loss treatment (remotely delivered) Experimental: standard behavioural weight loss treatment + mindful acceptance ‐ integration of acceptance and willingness skills into standard behavioural weight loss treatment (remotely delivered). Experimental: standard behavioural weight loss treatment + values ‐ integration of values clarification and awareness skills into standard behavioural weight loss treatment (remotely delivered). Experimental: standard behavioural weight loss treatment + mindful awareness ‐ integration of mindfulness and present‐moment awareness skills into standard behavioural weight loss treatment (remotely delivered). Experimental: standard behavioural weight loss treatment + mindful acceptance + values ‐ integration of acceptance and willingness skills and values clarification and awareness skills into standard behavioural weight loss treatment (remotely delivered). Experimental: standard behavioural weight loss treatment + mindful acceptance + awareness ‐ integration of acceptance and willingness skills and present moment awareness skills into standard behavioural weight loss treatment (remotely delivered). Experimental: standard behavioural weight loss treatment + values + mindful awareness ‐ integration of values clarification and awareness skills and present moment awareness skills into standard behavioural weight loss treatment (remotely delivered). Experimental: standard behavioural weight loss treatment + mindful acceptance + values + mindful awareness ‐ integration of acceptance and willingness skills and values clarification and awareness skills and present moment awareness skills into standard behavioural weight loss treatment (remotely delivered). |
| Outcomes | Primary: weight Secondary: dietary intake, physical activity, quality of life Time frame: 6, 12, 18, 24, 36 months |
| Starting date | Start date: August 2019 |
| Contact information | Contacts: Lauren Taylor, lct42@drexel.edu and Olivia Horgan, oh65@drexel.edu |
| Notes | Last updated June 2021, status recruiting, expected completion May 2024 |
Guerrini Usubini 2021.
| Study name | ACTyourCHANGE |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Focused acceptance and commitment therapy (FACT) Experimental: FACT Module Engagement During this module, patients will have the opportunity to increase their motivation to change and encourage the engagement in committed actions, consistent with their life values. Patients are invited to reflect on what is important in their lives, which values make their life worth living, and which actions they could take to live a meaningful life, in accordance with personal values. The use of metaphors and experiential exercises will facilitate the process of exploring personal values, identifying life directions and related behaviours. For example, the 80th Birthday Party metaphor requires participants to imagine there is a party in honour of their birthday and the time comes when people are starting to give speeches and try to answer the question about what they want to hear people at the party say. This exercise help patients in wondering what person they want to be with themselves and others. Experimental: FACT Module Openness Participants attending this module are guided to recognise and distancing themselves to stressful thoughts, feelings, and sensations. They will learn to read suffering as part of human experience, without self‐judgment and self‐condemnation. Rather, therapist will encourage the patient's assumption of an open and acceptable approach to internal experiences. Throughout the module, therapist will help patients to reflect on their usual, but ineffective efforts to solve personal problems, and encourage the adoption of new responsive strategies based on acceptance and defusion from personal distress. An example of metaphor used during the Module is The Passenger on a bus. In this metaphor patients have to imagine being a bus driver and his every thought is a passenger that gets on and off the bus. This exercise helps patients to accept, defuse from, and reduce the power of their thoughts. Experimental: FACT Module Awareness The module comprises meditation exercises and experiences aimed at learning how to act intentionally with awareness about personal thoughts and sensations without automatically reacting. Participants are supported to recognise their actions and the context where they occur and learn to choose to respond with action consistent with their values and not automatically. Therapist will propose breathing exercises, body scan and other mindfulness experiences. Participants will be encouraged to sit comfortably, close their eyes, feel themselves in contact with the present moment they are living, pay attention to their breath, notice the rhythm and any other aspect of the experience of breathing. Then, the therapist guides the participant's attention to the body, noting any part of their body from the head to feet. Then, the sounds around, any noises that could distract their attention from themselves. |
| Outcomes | Primary: weight, well‐being, psychological treatment Secondary: values, committed actions, cognitive fusion, acceptance, awareness, psychological inflexibility and experiential avoidance Time frame: 6 and 12 months |
| Starting date | Start date: February 2021 |
| Contact information | Gianluca Castelnuovo, Istituto Auxologico Italiano, gianluca.castelnuovo@auxologico.it |
| Notes | Last updated March 2021, status recruiting, expected completion September 2022 |
Hemenway 2021.
| Study name | Development of a mindfulness‐based treatment for the reduction of alcohol use and smoking cessation |
| Methods | Parallel‐group RCT Aim 1: Modify an existing mindfulness‐based treatment to include a focus on smoking cessation and reduced alcohol use Aim 2: Evaluate benchmarks regarding the feasibility and acceptability of Mindfulness Based Relapse Prevention ‐Smoking and Alcohol Use Aim 3: Collect and examine descriptive data on proximal and distal variables associated with increased smoking abstinence and reduced drinking |
| Participants | Inclusion criteria aim 1:
Inclusion Criteria Aim 2:
Exclusion criteria:
|
| Interventions | Experimental: mindfulness based relapse prevention (MBRP) is a treatment for preventing relapse in addictive disorders that integrates mindfulness meditation with standard relapse prevention practices. Participants will receive MBRP which has been modified to focus explicitly on smoking cessation and reduced alcohol use, creating Mindfulness Based Relapse Prevention ‐ Smoking and Alcohol (MBRP‐SA). Active comparator: cognitive behavioural therapy. Participants will receive cognitive behavioural therapy (CBT), a well‐established and commonly used treatment for substance abuse behaviours that utilises problem‐solving and coping skills. |
| Outcomes | Primary: Aim 2 ‐ patient satisfaction, rate of recruitment, participant retention, questionnaire completion (end of treatment at 21 weeks) Secondary: Aim 3 ‐ smoking abstinence (end of treatment and 16 weeks follow‐up), rate of alcohol use (end of treatment and 16 weeks follow‐up) |
| Starting date | Start date January 2019 |
| Contact information | Christine Vinci, PhD, H. Lee Moffitt Cancer Center and Research Institute |
| Notes | Last updated December 2021, status active not recruiting, expected completion November 2023 |
IRCT20150519022320N14.
| Study name | Comparative investigating the effect of relaxation and meditation techniques on quality of life in patients with coronary artery disease |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria: diagnosis of coronary artery disease (based on angiography), complete consciousness, history of admission to the CCU, ability to understand and speak Persian, informed consent to participate in study Exclusion criteria: history of mental illness, history of using psychiatric drugs, heart failure in the last 6 weeks |
| Interventions | Intervention 1: Relaxation: In this group, patients undergo relaxation exercises twice a day (morning and night), each time for 15 minutes Intervention 2: Meditation: In this group, patients undergo meditation exercises twice a day (morning and night), each time for 15 minutes |
| Outcomes | Mean score of quality of life Time point: before the intervention, 1 month after the intervention, 2 months after the intervention |
| Starting date | Start date: 23 October 2018 |
| Contact information | Ravary4776@yahoo.com |
| Notes | Last updated January 2019 when status was still recruiting |
IRCT2015122825739N1.
| Study name | Psychological interventions in weight loss |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria: 1) female, 2) age range 18 to 45 years old, 3) high school degree or above, 4) not receiving current treatment (psychological or medical) for weight, 5) agreed to participate, 6) have enough time to participate, 7) agreed to not engage in another weight loss treatment (whether medication or other), 8) BMI between 25 and 40 Exclusion criteria: 1) current attendance in weight loss programmes, use of appetite suppressant medications, weight loss medications or hormone replacement therapy currently; 2) diagnosis of major depressive disorder, bipolar disorder, psychotic disorder, alcohol or drug dependency, or epilepsy; 3) medical illnesses that effect eating behaviour, weight or metabolism (hypertension, diabetes, thyroid problems); 4) current psychological treatment or taking psychiatric medications; 5) taking psychoactive drugs; 6) history of eating disorder, weight loss‐related surgeries; 7) pregnant or lactating; 8) prior experience with MBSR or current meditation or yoga practice |
| Interventions | Intervention group 1: Mindfulness‐based eating awareness training (MB‐EAT), 8 weekly sessions, 2 hours each session, 8 to 15 in each group Intervention group 2: Cognitive reappraisal training, 8 weekly sessions, 2 hours each session, 8 to 15 in each group Control group: No intervention during study, and after end of the study will receive same intervention |
| Outcomes | Primary: binge‐eating, external eating, emotional eating, food craving, mindful eating, BMI Secondary: Mindfulness, Emotion Regulation Measurement at baseline, mid‐treatment, post‐treatment, follow up (1, 3, 6 months after end of treatment) |
| Starting date | Date of first enrolment:1 September 2017 |
| Contact information | kachooei.m@gmail.com (PhD candidate) |
| Notes | Last updated in February 2018 when status was still recruiting. |
IRCT20190804044436N1.
| Study name | Effect of mindfulness‐based stress management therapy on the emotion regulation, anxiety, depression and food addiction in obese people |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention 1: Intervention group: mindfulness‐based stress reduction is an 8‐session, 90‐minute intervention that teaches participants to communicate with their inner and outer world in the present moment, without judgement, with full awareness and awareness. Intervention 2: Control group: includes 8 x 1‐hour sessions once a week with topics in general psychology and no emotion‐related training including: types of conditioning, types of memory, types of learning, theory of forgetfulness, social psychology, adult developmental theories, personality types, review of tutorials |
| Outcomes | Primary: anxiety, depression, food addiction Secondary: emotion regulation Time frame: 3 months after the intervention |
| Starting date | Start date: September 2019 |
| Contact information | Hanieh Kebriti, m.kebriti20@gmail.com |
| Notes | Last updated February 2020, status complete |
IRCT20200225046618N1.
| Study name | Comparing the compassion‐focused therapy and dialectical behavior therapy training on state and trait anxiety symptoms and impulsivity in patients with coronary heart disease |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention 1: Intervention group one: participants participate in 12 group therapy sessions developed and implemented based on the Theoretical Guide to Compassionate Development. This therapy is held in 12 sessions, each session for 90 minutes, once a week (for 2.5 months) in groups, and during these sessions they are exposed to the basics of self‐compassion, mental imagery, mindfulness, and so on. Intervention 2: Intervention group II: dialectical behavioural therapy in this study is 12 sessions that are held weekly for 90 minutes (for 2.5 months) Intervention 3: Control group: no intervention |
| Outcomes | Primary: impulsivity, anxiety Time frame: 3 months after the intervention |
| Starting date | Start date: December 2019 |
| Contact information | Solmaz Rafieyan, s.rafieyan@iaukishint.ac.ir |
| Notes | Last updated May 2020, status complete |
IRCT20200226046625N1.
| Study name | Comparison of the effectiveness of cognitive‐behavioral therapy based on mindfulness and education on health promoting lifestyle in in treatment of diabetes |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention 1: Intervention group 1: In the present study, the goal of mindfulness‐based cognitive behavioural therapy is 8 sessions of mindfulness‐based cognitive behavioural therapy that were held 2 sessions per week (1 month) and each session 60 minutes. Intervention 2: Intervention group 2: In this study, the purpose of health promoting lifestyle training is 10 sessions of health promoting lifestyle training that were held 2 sessions per week (1.5 months) and each session 60 minutes. Intervention 3: Control group: no intervention |
| Outcomes | Primary: hopefulness, well‐being, quality of life Time frame: 3 months post intervention |
| Starting date | Start date: December 2019 |
| Contact information | Maryam Ghorbani, m.ghorbani@iaukishint.ac.ir |
| Notes | Last updated March 2020, status complete |
IRCT20200305046699N1.
| Study name | The effectiveness of group therapy based on mindfulness and cognitive‐behavioral therapy (CBT) in the anxiety, metabolic control and quality of life in type 2 diabetic patients |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention 1: Intervention group 1: mindfulness‐based stress reduction as a collection of training and treatment instructions and homework will be presented to people during 8 sessions of 1.5 hours over 1 month (2 sessions per week). Intervention 2: Intervention group 2: cognitive behavioural therapy as a collection of training and treatment instructions and homework will be presented to people during 8 sessions of 1.5 hours over 1.5 months. Intervention 3: Control group: no intervention |
| Outcomes | Primary: anxiety, blood glucose, quality of life Time frame: 3 months post intervention |
| Starting date | Start date: January 2020 |
| Contact information | Fatemeh Rezaei kookhdan, f.rezaeikookhdan@iaukishint.ac.ir |
| Notes | Last updated May 2020, status recruiting |
JPRN‐jRCT1030200197.
| Study name | Online mindfulness‐based eating awareness training for obesity |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Mindfulness‐based eating awareness training No treatment control |
| Outcomes | Primary:
Secondary:
|
| Starting date | Start date September 2020 |
| Contact information | Junko Matsumoto, matsujun@chiba‐u.jp |
| Notes | Last updated December 2020, status recruiting |
JPRN‐UMIN000030444.
| Study name | Effects of mindfulness compared with cognitive behavioral therapy for obesity: a randomized controlled trial |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria: missing in trial registry Exclusion criteria: subjects who have lost more than 5 kg within recent half year, pregnant or breastfeeding women, have schedule of pregnancy within 2 years, were taking any medication or supplement that might affect weight or taste, diagnosed as bulimia nervosa, a history of anorexia nervosa, taste disorders, a history of sinusitis and presenting sinus problem, presented such illness that may affect weight as malignancy or endocrine disease, have been during obstructive sleep apnoea syndrome (OSAS) or psychiatric disease treatment, have schedule of move during the research period (8 months), and those who are judged inadequate by responsible doctor for any reason Age minimum: 25 years Age maximum: 65 years Gender: female |
| Interventions | 34‐week weight loss intervention including mindfulness‐based stress management 34‐week weight loss intervention including cognitive behavioural therapy‐based stress management |
| Outcomes | To examine the superiority of the effect of mindfulness over cognitive behavioural therapy in 2‐year weight maintenance after weight loss intervention |
| Starting date | Start date: December 2017 |
| Contact information | Takehiro Nozaki, h‐takano@cephal.med.kyushu‐u.ac.jp |
| Notes | Last updated January 2020, status recruiting |
JPRN‐UMIN000042260.
| Study name | Effects of a mindfulness‐based eating awareness training online intervention in adults of obesity: a randomized controlled trial |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention: mindfulness‐based eating awareness training ‐ 90 min per visit, 14 weeks Comparator: health education ‐ email delivery once every 2 weeks, 7 times |
| Outcomes | Primary:
Secondary:
|
| Starting date | Start date: October 2020 |
| Contact information | Junko Matsumoto, matsujun@chiba‐u.jp |
| Notes | Last update November 2020, status pending |
JPRN‐UMIN000042626.
| Study name | An exploratory study of the mindfulness app for weight loss in metabolic syndrome |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention: participation in a 3‐month diet and exercise therapy health class
In addition to the above, practice daily mobile app mindfulness for 3 months
From month 4 to month 6 after the start of the study, check lifestyle by phone every 1 month Comparator: participation in a 3‐month diet and exercise therapy health class From month 4 to month 6 after the start of the study, check lifestyle by phone every 1 month |
| Outcomes | Primary: difference in weight change at 3 and 6 months after the intervention Secondary: changes in abdominal circumference, body composition (body mass index, body fat percentage, and muscle mass), systolic blood pressure change, diastolic blood pressure change, HDL change, LDL change, HbA1c change, and the results of the questionnaire survey (DEBQ, Motivation to Eat Healthy Scale, IPAQ Japanese version, Behavioral Change Stage Model) at 3 and 6 months |
| Starting date | Start date: April 2021 |
| Contact information | Takaharu Matsuhisa, matsukyuu413@med.nagoya‐u.ac.jp |
| Notes | Last updated May 2021, status complete, follow up continuing |
Martorella 2021.
| Study name | Web‐ and mindfulness‐based intervention to prevent chronic pain after cardiac surgery: protocol for a pilot randomized controlled trial |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention: brief 4 week MBCT (online), sessions 15 to 30 minutes. Each session is structured as follows: an introductory video of the clinician, mindfulness exercise audio recording, wrap‐up and weekly homework, or maintenance instructions from the clinician. Sessions’ Main Themes and Mindfulness Strategies: Session 1: persistent postoperative pain Body scan (20 minutes) Session 2: stepping out of automatic thoughts Mindful breathing (10 minutes) 3‐minute breathing space Session 3: acceptance and self‐care activities Mindful breathing (10 minutes) Session 4: wrap‐up and maintenance plan 3‐minute breathing space Body scan (20 minutes) Comparator: attention control ‐ one educational session online and 3 weekly reminders |
| Outcomes | Acceptability and feasibility, anxiety, depression, pain, opioid use, mindfulness, pain acceptance, pain catastrophising Time frame: 3 and 6 months post surgery |
| Starting date | Not reported |
| Contact information | Geraldine Martorella, PhD, gmartorella@fsu.edu |
| Notes | Published protocol only, no trial registration found. Status recruiting (delays due to COVID pandemic). |
Mason 2019.
| Study name | The DELISH study |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria: History of type 2 diabetes, 6.5% ≤ glycosylated haemoglobin or HbA1c < 12.0%, age ≥ 18 years, experience food‐related cravings most days of the week and/or eat in response to food cravings more than they wish they did, on screening ecological momentary assessment (EMA), report eating in response to cravings at least twice over the course of 3 days, able to engage in light physical activity, have a smartphone and respond to at least 7 of 9 short message service text messages delivered over the course of 3 days during pre‐enrolment, English speaking, willing to participate in diet and mindfulness interventions Exclusion criteria: Unable to provide informed consent, substance abuse, mental health, or medical condition that will make it difficult to participate in the intervention or may alter key outcomes or require important diet modifications, pregnant or planning to get pregnant in the next 6 months, breastfeeding, or < 6 months postpartum, current use of weight loss medications or supplements, history of weight loss (bariatric) surgery in the past 18 months or current plans for bariatric surgery, currently enrolled in a weight loss programme or have unalterable plans to enrol in one of these programmes in the next year, vegan or vegetarian, unwilling to do home blood ketone monitoring. |
| Interventions | Active comparator: diet education ‐ all participants will receive instruction in the carbohydrate‐restricted diet. The study diet has approximately 10% of kcal coming from carbohydrate, typically 50 grams/day or fewer, not including fibre. Experimental: diet education + mindfulness. In addition to the carbohydrate‐restricted diet described above, the experimental group will receive mindfulness training consisting of two integrated components: 1) use of a mindful eating app at home to learn and practice mindfulness skills for food‐cravings and eating, and 2) in‐person group‐based meetings to discuss and troubleshoot how the mindfulness practices are working. Key mindfulness content includes helping people improve their relationship with food and control food cravings and using mindful eating approaches including paying attention, noticing habit loops, understanding brain science and food/sugar addiction, disrupting emotional and stress eating, cultivating acceptance and curiosity, loving kindness, detaching from thoughts, using healthy restraint, and maintaining motivation. |
| Outcomes | Primary outcome: change in craving‐related eating, as assessed using an ecological momentary assessment mobile phone‐based platform Secondary outcomes: include changes in stress‐related eating, impulsivity, glycaemic control, weight change, dietary adherence, and resumption of dietary adherence after dietary non‐adherence |
| Starting date | Start date: 1 January 2017 |
| Contact information | Ashley.Mason@ucsf.edu |
| Notes | NCT record last updated June 2021 when status was study complete, no results posted. Citation of analyses of lipid levels combining both groups so not relevant to this review. |
NCT00224835.
| Study name | Mindfulness‐based stress reduction and myocardial ischemia |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention: MBSR. Subjects randomised to this condition will attend 120‐minute weekly sessions, plus a 7‐hour retreat, for training in mindfulness meditation methods. Control: cardiac education. Subjects in the disease education control condition will attend 8 weekly 60‐minute sessions, plus a 7‐hour "special experience" session, all of which will provide information about CAD in a didactic format. |
| Outcomes | Primary outcomes: psychological stress‐induced ischaemia, heart rate variability, peripheral artery response measured at 9 weeks. Psychological functioning ‐ anxiety, depression and quality of life measured at 9 weeks and 20 weeks. |
| Starting date | Start date: May 2003 |
| Contact information | PI: David S Sheps, MD, University of Florida |
| Notes | Completed 2008, last post 2016, no results or publications |
NCT01227473.
| Study name | We can prevent diabetes: a behavioral intervention to reduce diabetes risk in African Americans |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Behavioural: mindfulness‐based diabetes prevention education group. The mindfulness‐based diabetes prevention group meets for 2.5 hours per week for 8 weeks, with one 4‐hour retreat between the 6th and 7th weeks, and monthly booster sessions for 6 months. During the 8‐week interventions, this group receives a 30‐minute health behaviour presentation (based on the landmark Diabetes Prevention Program). In the mindfulness‐based diabetes prevention group, the instruction will be enhanced with a modified mindfulness meditation training designed to support the behavioural‐change programming. Behavioural: conventional diabetes prevention education group. The conventional diabetes prevention education group meets for 2.5 hours per week for 8 weeks, with one 4‐hour retreat between the 6th and 7th weeks, and monthly booster sessions for 6 months. During the 8‐week interventions, the group receives a 30‐minute health behaviour presentation (based on the landmark Diabetes Prevention Program). In the conventional diabetes prevention group, the instruction will be enhanced with group exercises and discussions. |
| Outcomes | Primary: insulin resistance (calculated from FBG and fasting insulin levels) at 3 months Secondary: salivary cortisol, insulin resistance at 6 months |
| Starting date | Start date: February 2010 |
| Contact information | Susan Gaylord, University of North Carolina, Chapel Hill |
| Notes | Last updated June 2012, status completed, no results posted or publications. Eligible if FBG reported separately. |
NCT01375504.
| Study name | Effect of self regulation with mindfulness training on body mass index and cardiovascular risk markers in obese adults |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Behavioural: mindfulness training program. The mindfulness program will be administered over 3 x 90‐minute sessions by a physician and clinical dietician with expertise in mind‐body medicine and nutrition. Behavioural: dietary counselling. The control group will receive 2 sessions of dietary counselling (with instruction to follow a weight loss programme) provided by a clinical dietitian, consistent with current routine care for adults with obesity. |
| Outcomes | Primary: improvement in general health Secondary: BMI, weight loss, telomere length and telomerase levels, CVD risk factors, stress, anxiety, quality of life, mindful eating, self‐efficacy Time frame: 6 months |
| Starting date | Start date: July 2011 |
| Contact information | Amit Sood, Mayo Clinic |
| Notes | Last updated April 2012, status complete, no results posted or publications |
NCT01805245.
| Study name | Mindfulness: a novel approach for the management of diabetes‐related distress |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention: mindfulness based stress reduction standard 8‐week MBSR program; classes meet for 2.5 hours once weekly Control: the health education control group meets at the same time and for the same amount of time |
| Outcomes | Primary: HbA1C, diabetes distress Secondary: HRQoL, blood pressure, insulin resistance, anxiety, depression, social support, coping, cortisol, interleukin 6, diabetes self‐care, glucose Other: mindfulness Time frame: 24 weeks |
| Starting date | Start date: January 2012 |
| Contact information | Laura A Young, MD, PhD, University of North Carolina |
| Notes | Last update April 2017, status complete, no results posted or publications |
NCT02037360.
| Study name | Mobile mindfulness training for smoking cessation |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria: Participants must be a) 18 to 65 years of age; b) smoke 5+ cigarettes/day, c) < 3 months abstinence in the past year; d) have a smartphone; and motivated to quit smoking Exclusion criteria: There are no exclusion criteria. Aims to recruit a heterogeneous group, representative of the general population. |
| Interventions | Experimental: experimental app/training. This is a 3‐week smart phone‐based training programme that trains mindfulness for smoking cessation by helping smokers self‐monitor their smoking habits, recognise when and how often they smoke, identify triggers for smoking, and learn methods to become more mindful of triggers, to quit smoking with a target quit date of 3 weeks. It is comprised of 22 modules of 10 to 15 minutes each, designed to teach mindfulness for smoking cessation using psychoeducation based audio and videos, animations to reinforce key concepts, and in vivo exercises. In addition, 5 bonus modules become available upon completion of earlier modules; these may be accessed for additional practices to bolster other modules. Active comparator: active comparator app/training. This is a standard free smoking cessation smartphone app using the latest evidence‐based smoking cessation methods and behaviour change theory. Subjects will be encouraged to set a quit date of 3 weeks, to allow comparison to experimental arm quit date. The app allows users to set a quit date, financial goals, and reminders, track daily smoking habits with an easy‐to‐use calendar, see graphs tracking money saved and number of packs not smoked, receive health milestones and craving tips to stay motivated, connect with social networks to give milestone updates, create a video diary, and watch personalised video messages from loved ones. |
| Outcomes | Primary: point prevalence abstinence at 6 months Secondary: point prevalence abstinence at 3 months |
| Starting date | Start date: August 2015 |
| Contact information | Judson Brewer, University of Massachusetts, Worcester |
| Notes | Last updated January 2018, status complete, no results posted or publications |
NCT02165228.
| Study name | Strategies for inflammation and cardiovascular disease (CVD) prevention (SICVDP) |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Behavioural: mindfulness‐based stress reduction. Stress reduction class and behavioural intervention. Behavioural: nutrition enhancement. Nutrition education class and behavioural intervention. |
| Outcomes | Primary: inflammation biomarkers, metabolic syndrome extent (blood pressure, waist circumference, fasting glucose, HDL and triglycerides) Secondary: BMI, waist hip ratio, cholesterol, insulin resistance, cortisol, perceived stress, depression, mindfulness Time frame: 16 weeks |
| Starting date | Start date: January 2010 |
| Contact information | Mary Miles, Montana State University |
| Notes | Last updated June 2014, status complete, no results posted or publications |
NCT02753972.
| Study name | MEAL |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: Mindful Eating and Living. The goal of the Mindful Eating and Living (MEAL) intervention was to apply mindfulness to apply mindfulness to eating behaviour. The content for the MEAL sessions included group discussion, mindfulness meditation, and group eating exercises. The course based on MB‐EAT emphasised brief daily meditation and pairing meditation with eating, but was more streamlined in terms of didactic content and course length. Participants examined hunger and satiety cues, the qualities of foods they crave, and emotional and cognitive states associated with eating. Each session included an eating exercise with a variety of foods. The monthly refresher sessions included a brief meditation, a brief eating exercise, and group discussion. (The MEAL curriculum is available from the authors.) Active Comparator: Active Control The Active Control (CONT) group matched the MEAL group regarding schedule. The agenda for the CONT sessions involved giving each participant the opportunity to discuss issues such as food choices, activity levels, and caloric goals. The sessions began by having the participants check‐in about their experiences with eating. Next, the clinical psychology graduate student led the participants in goal setting and finally there was a question and answer period when the registered dietician answered questions about food selection. The monthly refresher sessions had the same agenda as the initial weekly sessions. |
| Outcomes | Decreases in body mass index (time frame: baseline, 6 months, 9 months, 1 year) |
| Starting date | Start date: August 2006 |
| Contact information | Brian Shelley, University of New Mexico |
| Notes | Last updated April 2016, status complete, no results posted or publications |
NCT02830529.
| Study name | MADDASH |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: MAD DASH. Mindfulness‐based stress reduction and DASH diet education delivered in 8 sessions lasting 2.5 hours each. Mindfulness conducted by a certified trainer. Participants were taught mindfulness meditation, including body scan, loving kindness meditation and breathing exercises. Participants were given homework and meditation CD. Dietitian delivered diet education and conducted interactive food demonstrations. Participants were given the option to complete a weekly diet diary for the dietitian to provide feedback. The diet education component included a lecture on reading labels, low cost healthy meal preparation, and dietary consultation regarding personal strengths and self‐identified areas of improvement. Experimental: DASH diet education. Dietary approaches to stop hypertension sessions were delivered by a registered dietitian in 8 sessions lasting 1 hour each. Dietitian delivered diet education and conducted interactive food demonstrations. Participants were given option to complete weekly diet diary for the dietitian to provide feedback. The diet education component included a lecture on reading labels, low cost healthy meal preparation, and dietary consultation regarding personal strengths and self‐identified areas of improvement. No Intervention: usual care ‐ DASH pamphlet only. A dietary approaches to stop hypertension pamphlet was mailed to each participant. They continued receiving usual care from their healthcare provider. |
| Outcomes | Primary: change in blood pressure Secondary: change in nutrition intake, change in physical activity, change in quality of life, change in neuroprocessing Time frame: 3 and 9 months |
| Starting date | Start date: June 2015 |
| Contact information | PI: Kathy D Wright, Case Western Reserve University |
| Notes | Last updated December 2020, complete 2017, other findings published but not on relevant outcomes for this review. |
NCT03013907.
| Study name | The EASE study |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: UPLIFT. Eligible participants will complete 8 weekly group sessions by phone. The intervention builds cognitive behavioural and mindfulness skills to help reduce depressive symptoms. Each hour‐long weekly session consists of: check‐in, instruction, skill building, discussion, and a home‐based practice assignment. Session 1: Introduction to UPLIFT/Noticing Thoughts. Session 2: Checking and Changing Thoughts. Session 3: Coping & Relaxing Session. 4: Attention & Mindfulness Session. 5: The Present as a Calm Place Session. 6: Thoughts as Changeable, Thoughts as Not Fixed Session. 7: Focus on Pleasure & the Importance of Reinforcement Session. 8: Preventing Lapses and Giving Thanks Active Comparator: usual care (UC). Subjects randomised to UC will be advised to seek help from their primary care physician (PCP) or other sources as they normally would if they encountered symptomatic deterioration or other difficulties over the course of the study. All treatments received over the course of the study for the UC group and outside of the study (for the intervention group) will be assessed at each time point. |
| Outcomes | Primary: feasibility and acceptability, depression and depressive symptomatology Time frame: 6 months |
| Starting date | Start date: April 2017 |
| Contact information | Amanda Shallcross, MD, NYU Langone Health |
| Notes | Last updated November 2019, status complete, no results posted or publications |
NCT03020316.
| Study name | Peer mentored approaches for men and women with coronary artery disease ("4Steps") |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria: For participants:
For mentors: (Male mentors will be paired with male participants, and female mentors will be paired with female participants)
Exclusion criteria: For participants:
For mentors:
|
| Interventions | Active comparator: peer mentor & Transcendental Meditation subjects will be assigned a peer mentor (a volunteer with CAD). After initial contact, the peer mentor and subject will be encouraged to communicate at whatever frequency or medium they deem most appropriate. This may include speaking by telephone, personal email or meeting in person. Mentors (and subjects if willing) will be asked to keep a log of such contacts, which will be provided to the study staff at interval reassessments. In addition to the peer mentor, subjects will be instructed in transcendental meditation (TM) in the standard manner by a trained TM instructor. Active comparator: peer mentor only. No longer recruiting in this arm. No intervention: usual care. Subjects will be encouraged to follow‐up with their primary physicians. Subjects will be informed that they will be periodically contacted by telephone and/or email by the research team for future assessments. |
| Outcomes | Primary: perceived stress Secondary: medication adherence, depression, blood pressure, weight, lipid levels, FPG, hospital admissions, physical activity, number of contacts, knowledge and feedback about mentors Time frame: 52 weeks |
| Starting date | Start date: April 2016 |
| Contact information | Jessica M Peña, MD, MPH, Weill Medical College of Cornell University |
| Notes | Last updated November 2019, status complete, no results posted or publications |
NCT03131128.
| Study name | The effectiveness of mindfulness‐based intervention as a workplace health promotion program on weight loss |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: mindfulness‐based intervention. Two sessions of diet nutrition education and 6 sessions of mindfulness‐based intervention, 1.5 hours each session. Mindfulness‐based intervention: keeping mindful when selecting food. Be aware of hunger and satiety by body clues. Understanding how stress affects diet and life, and learn how to cope with stress in life. Learning to identify overeating caused by mood, social stress, and specific food. Active comparator: health education intervention. Two sessions of diet nutrition education and 6 sessions of health education, 1.5 hours each session. Health education: identifying improper diet patterns and attitudes. Understanding the impact of irrational cognition and attitudes on dietary behaviour. Training coping skills for stresses in real life and preventing the recurrence of bulimia. |
| Outcomes | Primary: body weight Secondary: dietary behaviour, food cravings, perceived stress, mindfulness Time frame: up to 10 months |
| Starting date | Start date: May 2017 |
| Contact information | Professor Yeh, Chung Shan Medical University |
| Notes | Last updated March 2018, status recuiting |
NCT03256890.
| Study name | Mindfulness‐based blood pressure reduction: stage 2a randomized controlled trial |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: MB‐BP Intervention. MB‐BP customises Mindfulness‐Based Stress Reduction (MBSR) to participants with hypertension. It consists of nine 2.5‐hour weekly group sessions and a 7.5‐hour one‐day session. Content includes education on hypertension risk factors, hypertension health effects, and specific mindfulness modules focused on awareness of BP determinants such as diet, physical activity, antihypertensive medication adherence, alcohol consumption, and stress reactivity. Students learn a range of mindfulness skills, including body scan exercises, meditation and yoga. Participants are given a home BP monitor. Participants with uncontrolled hypertension are offered to have their physicians notified; for those without a physician, we work to provide access within health insurance constraints. Active comparator: enhanced usual care control. Control group participants receive an educational brochure from American Heart Association entitled "Understanding and Controlling Your High Blood Pressure Brochure". Every participant is provided with a validated home blood pressure monitor, that as an evidence‐based approach to lower blood pressure, would be considered "enhanced usual care" at this time. All participants who have uncontrolled hypertension (blood pressure > 140/90 mmHg) will be offered to have their physicians notified, if not already being overseen for uncontrolled hypertension. For participants with uncontrolled hypertension who do not have a physician, participants worked with to provide access within the constraints of their health insurance. |
| Outcomes | Primary: SBP, DASH consistent diet Secondary: attention control, mindfulness, stress and perceived stress, self‐awareness, physical activity, step count, alcohol consumption, BMI, antihypertensive medication use and adherence, DBP |
| Starting date | Start date: June 2017 |
| Contact information | PI: Eric B Loucks, PhD, Brown University |
| Notes | Last updated January 2021, complete July 2019, no results posted or publications |
NCT03274362.
| Study name | Evaluating the effectiveness of headspace mindfulness application versus standard care on the HbA1C and quality of life in patients with diabetes: a randomized control trial |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Behavioral: Headspace mindfulness app. Participants will be given instructions on how to download Headspace on an electronic device and will be provided a free 3‐month access code. The app contains a series of 10 step‐wise 10‐minute video sessions that guide the user through mindfulness training. Upon completion of these sessions, additional content will be accessible, including sessions on health (depression, self‐esteem, anxiety, sleep, and pregnancy), relationships (kindness, generosity, relationships, change, appreciation, and acceptance) and performance (creativity, focus, happiness, and balance). The frequency and duration of usage will be in accordance to the instructions of the app. No intervention: standard care control group. Participants in the control group will be provided a list of resources that provides guidance on mindfulness and general health. |
| Outcomes | Primary: HbA1C, HRQoL Secondary: medication adherence, social support, diabetes empowerment Time frame: 3 months |
| Starting date | Estimated: September 2017 |
| Contact information | Tom G Elliott, MBBS. telliott@bcdiabetes.ca |
| Notes | Last update September 2017, status not yet recruiting. Relevant data would only be for adults when results are reported. |
NCT03368950.
| Study name | Online mindfulness for stroke sufferers |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention: an online mindfulness course guiding participants through all elements of mindfulness‐based cognitive therapy (MBCT) and mindfulness‐based stress reduction (MBSR). Ten easy‐to‐follow sessions with videos and interactive exercises led by leading mindfulness trainers. Control: wait list |
| Outcomes | Primary: perceived stress Secondary: anxiety, depression, mindfulness, rumination, HRQoL general and disease‐specific Time frame: 3 and 6 months |
| Starting date | Start date: June 2017 |
| Contact information | Chris Fife‐Schaw, Professor, University of Surrey |
| Notes | Last updated February 2018, recruitment status unknown, was active not recruiting |
NCT03373110.
| Study name | Healthy Hearts Healthy Minds (H3M) |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: online mindfulness based cognitive therapy +Fitbit. A central aspect of MBCT is the concept of awareness. Participants practise a variety of meditation types (e.g. breath awareness) and learn to bring mindfulness to everyday situations. Awareness will be directed to elements in participants' lives that interfere with living a more productive, physically active life (e.g. thoughts and feelings that interfere with becoming more physically active; stressful situations and circumstances that prevent them from engaging in exercise). Two hundred participants will be randomised into this group. Experimental: online cognitive behavioural therapy +Fitbit. 1) Identifying and setting realistic exercise‐based goals and intermediate goals (to maximise success to increase motivation); (2) behavioural scheduling to optimise when to exercise, identify rewards for exercising, and problem solve obstacles to exercising; and (3) identify dysfunctional, maladaptive thoughts about exercise (which decrease motivation) and skills to identify more adaptive, positive thoughts (to overcome thoughts of being too tired or too stressed to exercise). Two hundred participants will be randomised into this group. Active comparator: Fitbit alone participants assigned to the Fitbit only. Control study group will not be receiving therapy. However, they will receive a Fitbit, which they will be asked to wear over the course of 16 weeks, as well as to complete the same schedule of assessments as the therapy arms. One hundred participants will be randomised into this group. |
| Outcomes | Primary: change (per day) in average daily steps from baseline to 16 weeks Secondary: change (per day) in average daily steps from baseline to 16 weeks stratified by depression, anxiety, perceived stress, well‐being, activity, self‐efficacy for activity, education, employment, age, sex |
| Starting date | Start date: February 2018 |
| Contact information | Andrew A. Nierenberg, MD, Director, Dauten Family Center for Bipolar Treatment Innovation, Massachusetts General Hospital |
| Notes | Last updated April 2021, status completed, no results posted or publications Eligible if reports anxiety, depression, perceived stress, well‐being separately and not connected with number of steps |
NCT03480113.
| Study name | The effectiveness of mindfulness‐based intervention on smoking cessation |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: mindfulness‐based intervention. 1 session of health education regarding the harms from smoking and 6 sessions of mindfulness‐based intervention, 1 hour each session. Mindful breath, body scan, sitting meditation and walking meditation and so on. Active comparator: physical fitness intervention. 1 session of health education regarding the harms from smoking and 6 sessions of physical fitness and stretch exercise, 1 hour each session. |
| Outcomes | Primary: nicotine dependence Secondary: perceived stress, psychological distress, mindfulness, level of CO Time frame: up to 8 months |
| Starting date | Start date: March 2018 |
| Contact information | Shu‐Ling Huang, PhD Department of Psychology, Chung Shan Medical University |
| Notes | Last updated May 2018, status recruiting |
NCT03488966.
| Study name | Mindfulness based eating awareness training for bariatric surgery patients (MB‐EAT) |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: MB‐EAT behavioural: group psychotherapy. Eight weekly sessions, each session is 2 hours in duration. Eight sessions of mindfulness based eating and awareness training (MB‐EAT) will be delivered once per week over the course of 8 weeks, following an introductory session. The treatment uses general mindfulness meditation and eating meditation to help participants bring greater awareness and understanding to their relationship with food. Homework consists of weekly mindfulness exercises. Control: wait list control |
| Outcomes | Primary: weight, BMI Secondary: anxiety, depression, eating behaviour, self‐compassion, emotional regulation, body satisfaction, obesity cognitions, gastrointestinal symptoms Time frame: 6 and 12 months |
| Starting date | Start date: March 2017 |
| Contact information | Susan Wnuk, Ph.D, University Health Network, Toronto |
| Notes | Last updated February 2021, status active not recruiting |
NCT03659409.
| Study name | Investigating the impact of mindfulness on the physiological and psychological well‐being of stroke survivors and their caregivers |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: mindfulness‐based intervention, 4 weekly 2‐hour sessions. Participants will be introduced, taught and guided in practice of mindfulness‐based interventions that are focused on their 1) breath, 2) senses, 3) body and bodily movements, 4) feelings of empathy and compassion. Treatment wait list group: participants will receive no intervention, except for a baseline follow‐up at the start and again at the end of the first phase. 2 months after the end of the first intervention phase, participants in this group will receive the same mindfulness‐based intervention for 4 weeks. |
| Outcomes | Primary: perceived stress, depression, stroke‐specific quality of life, stroke impact, general quality of life Secondary: carer burden, mindfulness, personality Other: mental fatigue Time frame: 3 months |
| Starting date | Start date: May 2016 |
| Contact information | PI: Kinjal Doshi, PhD, Singapore General Hospital |
| Notes | Last updated March 2020, complete December 2018, no results posted or publications |
NCT03736434.
| Study name | Brain connections and blood pressure |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Behavioural: mindfulness and DASH diet education. Mindfulness and DASH diet education will include a didactic presentation on stress, theoretical material related to mindfulness, the somatic mind/body connection, relaxation, yoga, meditation, self‐awareness, and bodily cues relating to emotional reactivity. Practices will include body scan, gentle yoga movement, walking/sitting meditation, breathing, relaxation. Additionally, daily 20 min individual practices are done at least 5x/week delivered via a compact disc player or on a preloaded device. Each participant is asked to track his/her daily meditation practice and given a diary for adherence records. A combination of didactic, experiential, and "hands‐on" activities will be done to deliver DASH diet guidelines.
Behavioural: education group. The attention control group will attend 8, 2.5‐hour sessions on non‐health topics such as personal safety, fire prevention, cold weather protection, disaster preparation, internet safety, aging in place, how to know when it's time to move and managing your money. Control: no intervention, usual care |
| Outcomes | Primary outcomes: change in resting state network, change in systolic and diastolic blood pressure Time frame: 3 months |
| Starting date | — |
| Contact information | Kathy D. Wright, Ohio State University |
| Notes | Last updated December 2020, status complete, no results posted or publications |
NCT03793855.
| Study name | NUGLIC Study |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria: > 30 years old with medical diagnosis of T2DM, glycated haemoglobin (HbA1C) ≥ 7% at the moment of the screening and who have not received or received nutritional counselling for at least 6 months Exclusion criteria:
|
| Interventions | Experimental: nutritional strategy. Nutritional counselling based on the quality of the diet, the Food Guide for the Brazilian Population and concepts of mindfulness and mindful eating; dietary guidance. Counselling based on dietary goals and mindfulness techniques. Active comparator: dietary prescription. Individualised dietary prescription according to the guidelines of the Brazilian Society of Cardiology, based on feasible goals built together (patient and nutritionist). |
| Outcomes | Primary: HbA1C Secondary: SBP, DBP, HRQoL, self‐care, FBG, weight, waist circumference, BMI, lipid levels Time frame: 6 months |
| Starting date | Start date: May 2019 |
| Contact information | Aline Marcadenti, PhD, Hospital do Coracao, amarcaden@hcor.com.br |
| Notes | Last updated November 2020, status recruiting |
NCT03793881.
| Study name | NUPRESS study |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria: ≥ 21 years old with medical diagnosis of hypertension, systolic blood pressure (SBP) ≥ 140 mmHg at the moment of the screening and who have not received or received nutritional counselling for at least 6 months. Exclusion criteria:
|
| Interventions | Experimental: nutritional strategy. Nutritional counselling based on the quality of the diet, the Food Guide for the Brazilian Population and concepts of mindfulness and mindful eating; dietary guidance. Counselling based on dietary goals and mindfulness techniques. Active comparator: dietary prescription. Individualised dietary prescription according to the guidelines of the Brazilian Society of Cardiology, based on feasible goals built together (patient and nutritionist). |
| Outcomes | Primary: SBP Secondary: DBP, HRQoL, self‐care, FBG, HbA1C, weight, waist circumference, BMI, lipid levels Time frame: 6 months |
| Starting date | Start date: April 2019 |
| Contact information | Aline Marcadenti, PhD, Hospital do Coracao, amarcaden@hcor.com.br |
| Notes | Last updated November 2020, status recruiting |
NCT03826836.
| Study name | Mind our heart study |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: mindfulness‐based stress reduction. Treatment as usual (i.e. best medical treatment) in combination with an 8‐week mindfulness‐based stress reduction programme. Mindfulness‐based stress reduction (MBSR) is a structured programme of 8 weekly sessions of 2 to 2.5 hours in groups of 8 to 16 participants organised at the hospital sites. Additionally, participants are encouraged to practise each day for 30 to 45 minutes using audio‐recordings of the guided exercises. The programme is provided by experienced mindfulness teachers according to the standard MBSR protocol, except for the all‐day session. No intervention: control group. Treatment as usual (i.e. best medical treatment). |
| Outcomes | Primary: HRQoL Secondary: anxiety, depression, perceived stress, cortisol, mindfulness, smoking. alcohol, BMI, blood pressure, heart rate, lipid levels, HbA1C, cost effectiveness Time frame: 3, 6, 12, 24, 60 months |
| Starting date | Start date January 2019 |
| Contact information | Joep Teijink, Catharina Ziekenhuis Eindhoven |
| Notes | Latest update February 2019, not yet recruiting, estimated completion December 2025 |
NCT03837405.
| Study name | (R33 Phase) Delish Study |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Active comparator: diet education. All participants will receive instruction in the carbohydrate‐restricted (CR) diet and basic behavioural strategies in weekly, in‐person, group sessions for 3 months. The study diet has approximately 10% of kcal coming from carbohydrate, typically 50 grams/day or fewer, not including fibre. Participants will be encouraged to eat a normal amount of protein, typically about 80 to 100 grams/day (about 20% to 25% of calories), and the rest of their calories from fat. Experimental: diet education + mindfulness. In addition to the diet components described above, participants randomised to the education + mindfulness (Ed + MBI) group will receive MBI components using the Eat Right Now (ERN) platform. This will consist of two integrated components: 1) use of the ERN app at home, during the week, to learn and practice mindfulness skills for food‐cravings and eating, and 2) in‐person group‐based discussions of how the mindful eating practices are going, trouble‐shooting obstacles/pain points, and doing group exercises and reflecting on them. |
| Outcomes | Primary: diet adherence as measured by ketones, diet adherence as measured by 24‐hour diet recall Secondary: executive functioning/decreased impulsivity‐delayed discounting, emotion‐related eating, HbA1C, frequency of eating in response to cravings, diet adherence, executive functioning /decreased impulsivity‐‐relative reinforcing value of food, stress‐related eating, dietary resilience, weight change Other outcome measures: stress‐related eating, perceived stress, insulin resistance, fasting blood glucose, mindfulness Time frame: change from baseline to 12 months |
| Starting date | Start date: December 2018 |
| Contact information | PIs: Rick Hecht, MD, Elissa Epel, PhD, University of California, San Francisco |
| Notes | Last update June 2021 when, study was complete, no results posted or publications Follow‐on study from DELISH |
NCT03859076.
| Study name | UH3 Phase ‐ mindfulness‐based blood pressure reduction (MB‐BP): stage 2a RCT (MB‐BP) |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria: Exclusion criteria follow standard guidelines and recommendations:
|
| Interventions | Experimental: MB‐BP Intervention MB‐BP customises mindfulness‐based stress reduction (MBSR) to participants with hypertension. It consists of nine 2.5‐hour weekly group sessions and a 7.5‐hour one‐day session. Content includes education on hypertension risk factors, hypertension health effects, and specific mindfulness modules focused on awareness of BP determinants such as diet, physical activity, anti‐hypertensive medication adherence, alcohol consumption, and stress reactivity. Students learn a range of mindfulness skills (body scan exercises, meditation and yoga). Participants are given a home BP monitor. Participants with uncontrolled hypertension are offered to have their physicians notified; for those without a physician, the investigator works to provide access within health insurance constraints. Active comparator: enhanced usual care control. Those in the control group receive an educational brochure from the American Heart Association (product code 50‐1731) and a validated home blood pressure monitor (Omron, Model PB786N), that has an evidence‐based approach to lower blood pressure. All participants who have uncontrolled hypertension (blood pressure > 140/90 mmHg) will be offered to have their physicians notified, if not already being overseen for it. For participants with uncontrolled hypertension who do not have a physician, the investigator works to provide access within the constraints of their health insurance. Additionally, participants randomised to the control group are asked to refrain from engaging in any type of formal mindfulness practice more than weekly during the first 6 months of study involvement. |
| Outcomes | Primary: self‐regulation Secondary: heartbeat detection task, interoceptive awareness fMRI task, Difficulties in Emotion Regulation Scale, Pittsburgh Stress Battery, Anxiety Symptoms, depressive symptoms, sustained attention to response task, self‐compassion, self‐efficacy, Dietary Approaches to Stop Hypertension (DASH)‐consistent diet, alcohol consumption, electronically measured antihypertensive medication adherence, body mass index, physical activity Time frame: 10 weeks, 6 months |
| Starting date | Start date: December 2018 |
| Contact information | Eric B Loucks, PhD, Brown University |
| Notes | Last update January 2021, status complete, no results posted or publications |
NCT03910855.
| Study name | Impact of mindfulness on psychological well‐being of stroke survivors and their caregivers (SOMII) |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Behavioural: mindfulness based intervention. The mindfulness‐based intervention consists of four 2‐hour sessions covering various mindfulness techniques (e.g. mindfulness of breath, body and movement, senses and informal practice, and empathy and compassion) that pertain to stroke survivors and their family caregivers. Participants will be provided handouts for the information covered during these talks and discussions. Other: health education programme. The health education programme consists of four 2‐hour sessions covering various health topics (e.g. diet, nutrition, and exercise) that pertain to stroke survivors. Participants will be provided handouts for the information covered during these talks and discussions. |
| Outcomes | Primary: perceived stress, anxiety, depression, quality of life (general and disease specific), stroke impact Secondary: caregiver burden, personality, mindfulness |
| Starting date | Start date: September 2018 |
| Contact information | Kinjal Doshi, PhD, Singapore General Hospital |
| Notes | Last updated June 2021, status complete, no results posted or publications |
NCT04016415.
| Study name | Decreasing stress in diabetes: a randomized controlled trial |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Behavioural: mindfulness based stress reduction. Subjects randomised to mindfulness‐based stress reduction (MBSR) will receive the 8‐week University of Massachusetts Authorized MBSR curriculum followed by monthly mindfulness boosters in Months 3 to 6. The University of Massachusetts MBSR curriculum was selected for the intervention, as it is the most standardised and researched mindfulness programme that has been shown to reduce psychological distress in various patient populations. Behavioural: stress management education. Subjects randomised to stress management education (SME) will receive health education on nutrition (adapted for the type 2 diabetes population), exercise as gentle stretching to match yoga in MBSR, and other general health topics that may be relevant to the type 2 diabetes population such as sleep, time management, etc. Stress management education does not have any mindfulness in it. Stress management education was specifically created as a control condition for MBSR studies so it matches MBSR for time, social support, homework, etc. |
| Outcomes | Primary: change in HbA1C from baseline to 6 months Secondary: change in HbA1c from baseline to 2 months, change in diabetes distress from baseline to 2 months and 6 months, change in perceived stress from baseline to 2 months and 6 months |
| Starting date | Start date: July 2020 |
| Contact information | PI: Nazia Raja‐Khan, Milton S. Hershey Medical Center |
| Notes | Last update February 2021, status recruiting, estimated completion date October 2024 |
NCT04171713.
| Study name | Mindfulness and compassion‐based programs on food behavior of patients with weight regain after bariatric surgery |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Behavioural: mindfulness‐based health promotion + treatment as usual. Structured and developed over 8 weeks (8 sessions), participants meet weekly for an average of 2 hours to experience the techniques and conceptual learning about mindfulness and can be adapted to 1‐ or 1.5‐hour sessions to be applicable within the contexts of health, education, and organizations. Plus participation in support groups offered by the bariatric surgery clinic with capacity for 40 participants, monthly attendance and duration of 2 hours. In these meetings, there is always the presence of a surgeon, a psychologist, and another invited professional (endocrinologist, nutritionist, speech therapist, or dentist), who gives a thematic lecture and then there is an open space for doubts and exchange of experiences. Behavioural: attachment‐based compassion therapy + treatment as usual. It is a programme based on one of the fundamental psychological constructs that explains individuals' interpersonal relationships and attachment styles. It lasts 8 weeks, in which participants meet for 2 or 2.5 hours at each meeting. Behavioural: treatment as usual participation in support groups offered by the bariatric surgery clinic with capacity for 40 participants, monthly attendance and duration of 2 hours. In these meetings, there is always the presence of a surgeon, a psychologist, and another invited professional (endocrinologist, nutritionist, speech therapist, or dentist), who gives a thematic lecture and then there is an open space for doubts and exchange of experiences. |
| Outcomes | Primary: food behaviour, body image, weight Secondary: mindfulness, self‐compassion, anxiety Time frame: 6 months after end of intervention |
| Starting date | Start date: November 2019 |
| Contact information | Marcelo Demarzo, MD, PhD, Centro Mente Aberta de Mindfulness |
| Notes | Last update December 2019, status recruiting |
NCT04302493.
| Study name | Mindfulness based stress reduction and post‐stroke cognition |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: mindfulness‐based stress reduction (MBSR). Participants randomised to the MBSR arm will undergo a standard 8‐week course of MBSR taught by a psychologist trained in the MBSR protocol and encouraged to engage in additional individual mindfulness sessions using a cell phone application. Active comparator: Stroke Support Group (SSG). As a control group, participants will participate in 8 weeks of weekly Stroke Support Group sessions to experience activity and socialisation without additional mindfulness training. |
| Outcomes | Primary: change in cognition, cerebral activity, quality of life, depression Secondary: change in anxiety, depression, fatigue, cerebral connectivity patterns, return to work Time frame: 6 months |
| Starting date | Start date: January 2021 |
| Contact information | Elisabeth B Marsh, MD, Johns Hopkins University |
| Notes | Last update January 2022, status recruiting |
NCT04759950.
| Study name | Exercise the mind and brain. A multimodal intervention in stroke |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
Exclusion criteria only for MRI examination:
|
| Interventions | Experimental: mindfulness and cognitive training group. The mindfulness and cognitive training group receives mindfulness‐based stress reduction therapy combined with computer‐based cognitive training. Behavioural: mindfulness‐based stress reduction programme. This intervention follows an adaptation of the scheme and instructions of the official mindfulness‐based stress reduction (MBSR) programme designed by Jon Kabat‐Zinn. It includes body scanning, sitting meditation, and gentle hatha yoga techniques. An accredited mindfulness instructor leads the 12‐week online programme, which comprises a presentation session, followed by 8 intervention sessions (once a week, lasting 2.5 hours) and an intensive practice session interspersed between sessions 6 and 7. Apart from the supervised session, the therapist asks participants to carry out some independent practice daily. Behavioural: computerised cognitive training. An accredited neuropsychologist programs and supervises the computerised cognitive training through the Guttmann Neuropersonal Trainer (GNPT®) platform. The 12‐week programme consists of 5 sessions a week (45 minutes each) that include personalised activities planned to stimulate executive functioning, attention, processing speed, and memory. Experimental: physical exercise and cognitive training group. The physical exercise and cognitive training group receives a multi‐component physical exercise programme combined with computer‐based cognitive training. Behavioural: multicomponent physical activity programme. The proposed physical exercise intervention follows the American College of Sports Medicine (ACSM, 2017) and the American Stroke Association (Billinger et al 2014) recommendations for stroke patients. The 5 weekly 45‐minute sessions are divided into 1) 3 sessions guided and supervised telemetrically by a physical exercise specialist and a physiotherapist; and 2) 2 autonomous exercise sessions. The supervised sessions include exercises to work aerobic capacity, muscle strength and endurance, flexibility, agility, and balance. The intensity of these exercises is prescribed individually according to each participant's initial level and are gradually increased as the programme progresses. Participants are encouraged to walk at a similar intensity on autonomous physical exercise days as performed in the guided sessions (moderate intensity), based on the Borg scale of perceived exertion. Behavioural: computerised cognitive training ‐ as described above. Active comparator: cognitive training group. The cognitive training group, as an active control group, receives only computer‐based cognitive training ‐ as described above. |
| Outcomes | Primary: changes in immediate verbal attention after receiving treatment, in verbal digit working memory after receiving treatment, in verbal memory after receiving treatment, in visual memory after receiving treatment, in executive function, verbal fluency after receiving treatment, in executive function, inhibition after receiving treatment, in executive function, set‐switching task after receiving treatment, in language, naming after receiving treatment Secondary: changes in psychological distress, anxiety and depression, well‐being, mindfulness, white matter integrity, resting state connectivity, brain volumetry, growth factors, microbiota, quality of life and stroke specific quality of life, sleep, fitness, mental fatigue, physical activity Time frame: 3 months |
| Starting date | Start date: October 2020 |
| Contact information | Maria Mataro, PhD, University of Barcelona |
| Notes | Last updated October 2021, status active not recruiting |
NCT04799899.
| Study name | MBCT via group videoconferencing for acute coronary syndrome patients with depressive symptoms |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: virtual MBCT intervention. Participants will participate in 8 weekly virtual group sessions of MBCT. Participants will be asked to complete a brief survey following each session. Within 1 week before and after the intervention and 3 months post‐intervention, participants will be asked to complete a series of questionnaires and provide self‐collected blood samples. Upon completion of the intervention, participants will complete an audio‐ or video recorded exit interview (approximately 30 to 60 minutes). The adapted MBCT intervention will involve 8 virtually delivered MBCT sessions (approximately 1.5 hours each), during which participants will be taught how to use evidence‐based mindfulness skills to regulate distress and choose healthy behaviours, as well as learn about cardiac health. Experimental: virtual health enhancement control. Participants will participate in 8 weekly virtual group sessions that focus on cardiac health and depression education. Participants will be asked to complete a brief survey following each session. Within 1 week before and after the intervention and 3 months post‐intervention, participants will be asked to complete a series of questionnaires and provide self‐collected blood samples. Upon completion of the intervention, participants will complete an audio‐ or video recorded exit interview (approximately 30 to 60 minutes). The cardiac health enhancement control group will involve 8 virtually delivered MBCT sessions (approximately 1.5 hours each), during which participants will learn about depression and cardiac health. |
| Outcomes | Primary: acceptability and feasibility measures Secondary: survivor concerns, mindfulness, experiences, anxiety, depression, reactivity, interoceptive awareness, quality of life, positive and negative affect, physical function, inflammatory biomarkers Time frame: 3 and 6 months |
| Starting date | Start date: April 2021 |
| Contact information | Christina Luberto, Massachusetts General Hospital |
| Notes | Last updated September 2021, status recruiting |
NCT04847843.
| Study name | Eating mindfully to prevent weight regain (EMPWR) |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: mindfulness orientated recovery enhancement (MORE). Intervention 8‐week MORE intervention adapted for preventing weight regain. The MORE curriculum has been adapted for this intervention to address food intake behaviours and will provide training in mindfulness techniques to increase awareness of, and self‐control over, cravings; reappraisal skills to promote emotion regulation and restructure motivations for highly palatable food intake; and savouring pleasant events and emotions to overcome defects in natural reward processing. Active comparator: control intervention. Eight‐week control intervention based on the Diabetes Prevention Program's Prevent T2 for Life programme. The curriculum will be based on the Diabetes Prevention Program's Prevent T2 for Life programme, which is an evidence‐based national healthful lifestyle maintenance intervention. This programme includes training in healthful eating, meal planning, and recipe modification; time and stress management; adapting lifestyle habits for continued success during holidays, vacations, and other special situations; and relapse prevention. |
| Outcomes | Primary: weight, body composition Secondary: food related behaviours, energy intake Time frame: 8 weeks and 6 months |
| Starting date | Start date: June 2021 |
| Contact information | Tanya Halliday, University of Utah |
| Notes | Last updated August 2021, status recruiting, expected completion April 2023 |
NCT04965181.
| Study name | Mindfulness‐based smoking cessation enhanced with mobile technology |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: mindfulness‐based addiction treatment (MBAT) + iQuit Mindfully. Participants will receive virtual group counselling based on the mindfulness‐based addiction treatment (MBAT) group protocol, iQuit Mindfully text messages, and nicotine replacement therapy (NRT). MBAT consists of 8 weekly 2‐hour sessions. NRT consists of 8 weeks of generic nicotine patches and nicotine lozenges. Participants who smoke > 10 cigarettes/day will receive 4 weeks of 21 mg patches, 2 weeks of 14 mg patches, and 2 weeks of 7 mg patches. Those who smoke fewer than 10 cigarettes/day will receive 4 weeks of 14 mg patches and 4 weeks of 7 mg patches. Participants who smoke their first cigarette within 30 minutes of waking will receive 8 weeks of 4 mg mini lozenges (6 to 9 4 mg mini lozenges per day). Those who smoke their first cigarette over 30 minutes of waking will receive 8 weeks of 2 mg mini lozenges (6 to 9 2 mg mini lozenges per day). Participants will also be given the National Cancer Institute "Clearing the Air" booklet and a referral to the Tobacco Cessation Quitline. Experimental: iQuit Mindfully. Participants will receive iQuit Mindfully text messages and nicotine replacement therapy (NRT). NRT consists of 8 weeks of generic nicotine patches and nicotine lozenges. Participants who smoke > 10 cigarettes/day will receive 4 weeks of 21 mg patches, 2 weeks of 14 mg patches, and 2 weeks of 7 mg patches. Those who smoke fewer than 10 cigarettes/day will receive 4 weeks of 14 mg patches and 4 weeks of 7 mg patches. Participants who smoke their first cigarette within 30 minutes of waking will receive 8 weeks of 4 mg mini lozenges (6 to 9 4 mg mini lozenges per day). Those who smoke their first cigarette over 30 minutes of waking will receive 8 weeks of 2 mg mini lozenges (6 to 9 2 mg mini lozenges per day). Participants will also be given the National Cancer Institute "Clearing the Air" booklet and a referral to the Tobacco Cessation Quitline. Experimental: mindfulness‐based addiction treatment (MBAT). Participants will receive virtual group counselling based on the mindfulness‐based addiction treatment (MBAT) group protocol and nicotine replacement therapy (NRT). MBAT consists of 8 weekly 2‐hour sessions. NRT consists of 8 weeks of generic nicotine patches and nicotine lozenges. Participants who smoke > 10 cigarettes/day will receive 4 weeks of 21 mg patches, 2 weeks of 14 mg patches, and 2 weeks of 7 mg patches. Those who smoke fewer than 10 cigarettes/day will receive 4 weeks of 14 mg patches and 4 weeks of 7 mg patches. Participants who smoke their first cigarette within 30 minutes of waking will receive 8 weeks of 4 mg mini lozenges (6 to 9 4 mg mini lozenges per day). Those who smoke their first cigarette over 30 minutes of waking will receive 8 weeks of 2 mg mini lozenges (6 to 9 2 mg mini lozenges per day). Participants will also be given the National Cancer Institute "Clearing the Air" booklet and a referral to the Tobacco Cessation Quitline. Active comparator: usual care. Participants in the usual care condition are provided with nicotine replacement therapy (NRT). NRT consists of 8 weeks of generic nicotine patches and nicotine lozenges. Participants who smoke > 10 cigarettes/day will receive 4 weeks of 21 mg patches, 2 weeks of 14 mg patches, and 2 weeks of 7 mg patches. Those who smoke fewer than 10 cigarettes/day will receive 4 weeks of 14 mg patches and 4 weeks of 7 mg patches. Participants who smoke their first cigarette within 30 minutes of waking will receive 8 weeks of 4 mg mini lozenges (6 to 9 4 mg mini lozenges per day). Those who smoke their first cigarette over 30 minutes of waking will receive 8 weeks of 2 mg mini lozenges (6 to 9 2 mg mini lozenges per day). Participants will also be given the National Cancer Institute "Clearing the Air" booklet and a referral to the Tobacco Cessation Quitline. |
| Outcomes | Primary: smoking abstinence Secondary: mindfulness and home mindfulness practice, self compassion, perceived stress, positive and negative affect, craving and withdrawal, nicotine dependence, self‐efficacy, social support Time frame: 8, 12, 24 weeks |
| Starting date | Start date: July 2021 |
| Contact information | Claire Spears, Georgia State University |
| Notes | Last updated July 2021, status recruiting, expected completion April 2024 |
NCT04985838.
| Study name | HEADS:UP |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention: HEADS: UP (Helping Ease Anxiety and Depression). A group‐based mindfulness based stress reduction (MBSR) course adapted for people affected by stroke and delivered online using a video communication platform. An introductory session in the first week is followed by 8 weekly sessions (2.5 hours, incorporating 30‐minute comfort breaks). A 6‐hour silent retreat is offered in week 7. An optional follow‐up session is offered 6 to 8 weeks after completion of the 9‐week course. Course materials include a manual (provided after the introductory session), weekly pre‐/post‐session emails with instructions and information about joining the sessions and engaging in personal practice, including links to audio files to complement class‐based sessions. Comparator: no intervention control |
| Outcomes | Primary: anxiety and depression Secondary: stroke impact, quality of life Time frame: 8, 20, 32 weeks |
| Starting date | Start date: June 2021 |
| Contact information | Maggie Lawrence, PhD, Glasgow Caledonian University |
| Notes | Last updated December 2021, status active not recruiting, expected completion September 2022 |
NCT05070949.
| Study name | Self‐compassion to reduce diabetes distress in persons with type 1 diabetes |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: mindful self‐compassion (MSC). Participants will meet every 2 weeks via zoom application or equivalent online meeting platforms, for 12 weeks. The sessions will be led by a clinical psychologist. The curriculum will follow a mindful compassion programme by Neff KD No intervention: wait list control. The wait list control group will not participate in the MSC programme during the first 12 weeks of the protocol but will be given an opportunity to participate after 12 weeks, using the same curriculum. |
| Outcomes | Primary: diabetes distress, self compassion Secondary: diabetes self‐efficacy, HbA1C, sleep quality, stress, and depressive symptoms Time frame: 12 weeks |
| Starting date | Estimated start date: November 2021 |
| Contact information | Ratanaporn Jerawatana, Mahidol University, ratanaporn.jer@gmail.com |
| Notes | Last updated October 2021, not yet recruiting, estimated completion September 2024 |
RBR‐458tbcd.
| Study name | Effects of an intervention based on eating with full attention in patients who underwent stomach reduction surgery and regained weight: a randomized clinical trial |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria: present weight regain after bariatric surgery; bariatric surgery was performed in the last 5 years (minimum 2 years after surgery), reported binge‐eating episodes and accepted to participate in the study. Weight gain will be considered for participants who recover 50% of the lost weight achieved in the long term or recovery of 20% of the weight associated with the reappearance of comorbidities (SBCBM, 2015). Binge‐eating episodes are those participants who meet the criteria established by DSM‐V (2014): eating within a limited period of time (for example, within a period of 2 hours), a quantity of food definitely greater than most people would consume at a similar time, under similar circumstances and feeling of lack of control over the episode (a feeling of not being able to stop or control what or how much one eats). Exclusion criteria: current diet for weight loss or use of medications that can affect weight; pregnancy; breastfeeding; presence of psychiatric disorders; personality disorders; severe depression; alcohol abuse and/or being on psychiatric drugs |
| Interventions | Intervention group 1) being composed of 30 individuals who will receive the intervention based on mindfulness, through the MB‐EAT programme, whose main focus is to develop self‐regulatory processes related to appetite, emotional balance, and behaviours. The programme contains 12 sessions, 10 weekly, 1 biweekly, and 1 monthly. The active control group 2) will consist of 30 individuals who will receive the intervention based on the food guide for the Brazilian population using the instructional material published by the Ministry of Health. The programme contains the same frequency as the intervention group. |
| Outcomes | Primary: weight, blood pressure, waist circumference, FBG, insulin, lipid levels, C‐reactive protein, binge‐eating, mindfulness and satiety, and reward mechanism gene expression Time frame: 16 weeks |
| Starting date | Start date: February 2021 |
| Contact information | Camila Cremonezi Japur, camilajapur@usp.br |
| Notes | Last updated December 2021, not yet recruiting |
Ruffault 2016.
| Study name | MindOb |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: mindfulness. Computerised mindfulness‐based intervention, 10 minutes per day, every day for 12 months of MP3 listening. Guidelines are in line with main mindfulness‐based interventions (MBSR, MBCT, ACT). Participants can choose between 4 audio recordings (sessions): awareness of the breathing, awareness of postures and bodily sensations, acceptance of thoughts and emotions, and awareness of bodily sensations and related thoughts and emotions while executing 5 squats. Sham comparator: sham meditation. Computerised sham meditation intervention, 10 minutes per day, every day for 12 months of MP3 listening. The unique guideline is to "meditate" at the beginning of each session. Participants can choose between 4 audio backgrounds: forest, night, beach, and river. No intervention: treatment as usual. Usual care in a nutrition pole in France: nutrition, diet, exercise. |
| Outcomes | Primary: impulsive eating Secondary: motivation towards exercise, physical activity, anxiety, depression, mindfulness, food intake, BMI, leptin, adiponectin Other: compliance with the intervention Time frame: 6 and 12 months |
| Starting date | Start date: May 2016 |
| Contact information | Ruffault Alexis, University of Paris 5 ‐ Rene Descartes |
| Notes | Last updated December 2018, status active not recruiting. |
SLCTR/2021/015.
| Study name | Glycemic control in patients with type 2 diabetes mellitus following mindfulness meditation compared to standard therapy |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention: patients in the intervention group will continue to receive standard therapy in addition to following the mindfulness meditation programme. Standard therapy includes monthly clinic visits, review of fasting blood glucose (FBS)/HbA1C, continuation of oral hypoglycaemic drugs and medication review, dietary and lifestyle advice, annual screening for retinopathy and neuropathy if it falls during the period of study. Patients will undergo other investigations according to the need.
The mindfulness meditation program is for a period of 3 months. This programme is based on Buddhist teaching, but the actual programme does not have any content of the Buddhist preaching nor will they be asked to restrict normal patterns of behaviour. The aim is for the participants to practise mindfulness (keeping attention in the present moment) techniques and achieve a state of calmness and relaxation of the mind. Patients will be expected to attend initially once a week 45‐ to 60‐minute sessions for 12 weeks where mindfulness meditation will be taught.
The techniques used in this mindfulness meditation programme are not complex techniques. Participants will be asked to first walk for 5 minutes, keeping the attention only to the feet/sensation of the feet. If they get distracted, they are expected to focus again. Comparator: wait list control group |
| Outcomes | Primary: glycaemic control, FBS, HbA1C, fructosamine Secondary: lipid profile, blood pressure, insulin resistance, autonomic functions assessed by cardiac autonomic reflex testing, gut transit time, 24‐hour urinary cortisol Time frame: 12 weeks |
| Starting date | Start date: July 2021 |
| Contact information | Dr. K.P. Chamila Dalpatadu, chamila@physiol.cmb.ac.lk |
| Notes | Last updated November 2021, status pending |
Spruill 2018.
| Study name | Womens HARP study |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Behavioural: stress management. Participants randomised into the stress management group will complete 8 weekly group sessions by phone. The intervention is a telephone adaptation of mindfulness‐based cognitive therapy (MBCT) and focuses on building cognitive‐behavioural and mindfulness skills to help manage and cope with stress. Each hour‐long weekly session consists of: check‐in, instruction, skill building, discussion, and home‐based practice assignment. Behavioural: enhanced usual care (EUC). Participants randomised into the EUC group will complete 8 weekly individual sessions by phone. Each weekly session consists of: brief check‐in and review of AHA brochure ‐ "Women, Heart Disease and Stroke". |
| Outcomes | Primary: perceived stress Secondary: HRQoL general and disease‐specific, depression, sleep quality Time frame: 6 months |
| Starting date | Start date: August 2016 |
| Contact information | Harmony R Reynolds, MD, NYU Langone Medical Centre |
| Notes | Last updated August 2021, status active not recruiting, estimated completion date 2025 |
TCTR20210406004.
| Study name | The effect of walking meditation on peripheral neuropathy in diabetes type 2 |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Diabetes with peripheral neuropathy with walking meditation training Diabetes with peripheral neuropathy with walking training Diabetes with peripheral neuropathy without training but education about diet control |
| Outcomes | Primary: FBG, HbA1C Secondary: pulse wave velocity, cortisol Time frame: 12 weeks |
| Starting date | Start date: March 2021 |
| Contact information | Piyadee Jintaruethai, nammy_n23‐kate@hotmail.com |
| Notes | Last updated November 2021, status recruiting |
TCTR20210417002.
| Study name | Effects on a mindfulness meditation for decreasing blood pressure |
| Methods | Parallel‐group RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention: mindfulness based brief intervention, practice mindfulness meditation skills and homework assignments Comparator: health education about exercise, foods and mental health and lifestyle modification |
| Outcomes | Primary: blood pressure Secondary: mental health, mindfulness Time frame: 12 and 20 weeks |
| Starting date | Start date: April 2021 |
| Contact information | Nirun Intarut, nirun.i@msu.ac.th |
| Notes | Last updated November 2021, status pending (not yet recruiting) |
Wheelwright 2017.
| Study name | Does intra‐ICU initiation of guided mindfulness meditation decrease anxiety and depression in SAH?: A unique methodology for the neurocritical care setting |
| Methods | Parallel‐group RCT |
| Participants | Patients ≥ 18 with good grade subarachnoid haemorrhage defined as Hunt‐Hess score 1 to 2 will be included. Subarachnoid haemorrhage will be confirmed by CT scan or by finding of xanthochromia on cerebrospinal fluid. Patients with subarachnoid haemorrhage due to trauma, ruptured arteriovenous formation or other secondary causes or who are admitted > 14 days after onset will be excluded. Non‐English‐speaking patients, or those with a confounding cognitive deficit will not be eligible. |
| Interventions | Intervention arm: daily guided mindfulness meditation; N = 20 Control arm: daily session of supervised TV watching; N = 20 |
| Outcomes | The EQ5D‐5L, State Trait Anxiety Inventory (STAI) and modified Rankin scale (mRS) score will be collected at day 14 and 3 months. Cortisol levels will be collected at day 0, 5, 7, and 10. |
| Starting date | Not reported |
| Contact information | Not reported |
| Notes | Conference proceeding and no trial registry reported for further details |
ACS: acute coronary syndrome; ACT: acceptance and commitment therapy; ADA: American Diabetes Association; BDI: Beck Depression Inventory; BMI: body mass index; BMR: basal metabolic rate; BP: blood pressure; CAD: coronary artery disease; CO: carbon monoxide; CT: computed tomography; CVD: cardiovascular disease; DASH: Dietary Approaches to Stop Hypertension; DASI: Duke Activity Status Index; DASS: Depression and Anxiety Stress Scale; DBP: diastolic blood pressure; DM: diabetes mellitus; DSM: Diagnostic and Statistical Manual of Mental Disorders; ER: emergency room; FBG: fasting blood glucose; FBS: fasting blood sugar; GFR: glomerular filtration rate; HbA1c: glycated haemoglobin (A1c); HDL: high‐density lipoprotein; HRQoL: health‐related quality of life; ICD: International Classification of Diseases; LDL: low‐density lipoprotein; MBCT: mindfulness‐based cognitive therapy; MBSR: mindfulness‐based stress reduction; MEG: magnetoencephalography; MMSE: Mini Mental State Examination; MoCA: Montreal Cognitive Assessment; MRI: magnetic resonance imaging; mRS: modified Rankin scale; NIHSS: NIH Stroke Scale; PHQ‐9: Patient Health Questionnaire‐9; PI: primary investigator; PTSD: post‐traumatic stress disorder; RCT: randomised controlled trial; SBP: systolic blood pressure; T2DM: type 2 diabetes mellitus; TAU: treatment as usual; TM: Transcendental Meditation; WHR: waist hip ratio
Differences between protocol and review
Following the publication of the protocol, it became apparent that some search terms were missing for primary prevention, specifically those for smoking and overweight/obesity. These have now been added in and the searches re‐run and de‐duplicated against the original searches.
In the protocol, no minimum time periods for the duration of intervention and follow‐up were specified. We are interested in sustained behavioural changes in CVD risk factors to reduce the risk of developing CVD for primary prevention and in its management for secondary prevention. Very short‐term studies are therefore not informative. As with other reviews of lifestyle interventions to reduce CVD risk, we have added a cut‐point of 12 weeks (including intervention duration and post‐intervention follow‐up) as a minimum for inclusion.
Contributions of authors
Karen Rees screened titles and abstracts, undertook full‐text review, abstracted data, conducted risk of bias assessments, carried out GRADE assessments, analysed data, and wrote the review.
Andrea Takeda screened titles and abstracts, conducted risk of bias assessments, constructed summary of findings tables, carried out GRADE assessments, and read and approved the final version of the review.
Rachel Court screened titles and abstracts, undertook full‐text review, abstracted data, conducted risk of bias assessments, and read and approved the final version of the review.
Laura Kudrna undertook full‐text review, abstracted data, conducted risk of bias assessments, and read and approved the final version of the review.
Louise Hartley abstracted data, conducted risk of bias assessments, and read and approved the final version of the review.
Edzard Ernst provided critical comments and read and approved the final version of the review.
Sources of support
Internal sources
Warwick Medical School, University of Warwick, UK
Complementary Medicine, Peninsula Medical School, Exeter, UK
External sources
-
National Institute for Health Research (NIHR), UK
Cochrane Infrastructure funding to Cochrane Heart (closed in March 2023).
-
National Institute for Health Research (NIHR), UK
Laura Kudrna was supported by the National Institute for Health Research (NIHR) Applied Research Centre (ARC) West Midlands, grant number NIHR200165.
Declarations of interest
Karen Rees: no relevant interests; accredited Breathworks mindfulness teacher as well as a methodologist (this provides additional subject expertise and is not regarded as a conflict of interest ‐ no benefits in cash or kind but declared here in the interests of transparency); former editor for Cochrane Heart (closed March 2023) and was not involved in the editorial process or decision‐making for this review.
Andrea Takeda: no relevant interests; works for Cochrane Central Production Service as a freelance copy‐editor, but was not involved in the editorial process or decision‐making for this or any other review.
Rachel Court: none known.
Laura Kudrna: none known.
Louise Hartley: none known.
Edzard Ernst: none known.
New
References
References to studies included in this review
Alamout 2020 {published data only}
- Alamout MM, Rahmanian M, Aghamohammadi V, Mohammadi E, Nasiri K. Effectiveness of mindfulness based cognitive therapy on weight loss, improvement of hypertension and attentional bias to eating cues in overweight people. International Journal of Nursing Sciences 2019;7(1):35-40. [DOI: 10.1016/j.ijnss.2019.12.010] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alsubaie 2020 {published data only}
- Alsubaie M, Dickens C, Dunn BD, Gibson A, Ukoumunne OC, Evans A, et al. Feasibility and acceptability of mindfulness-based cognitive therapy compared with mindfulness-based stress reduction and treatment as usual in people with depression and cardiovascular disorders: a three-arm randomised controlled trial. Mindfulness 2020;11:30–50. [DOI: 10.1007/s12671-018-0999-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
An 2021 {published data only}
- An E, Irwin MR, Doering LV, Brecht ML, Watson KE, Corwin E, et al. Mindfulness effects on lifestyle behavior and blood pressure: a randomized controlled trial. Health Science Reports 2021;4(2):e296. [DOI: 10.1002/hsr2.296] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03924531. Promoting adherence to anti-hypertensive medications and lifestyle guidelines through mindfulness practice. https://clinicaltrials.gov/show/NCT03924531 2019.
Armani Kian 2018 {published data only}
- Armani Kian A, Vahdani B, Noorbala AA, Nejatisafa A, Arbabi M, Zenoozian S, et al. The impact of mindfulness-based stress reduction on emotional wellbeing and glycemic control of patients with type 2 diabetes mellitus. Journal of Diabetes Research 2018;2018:1986820. [DOI: 10.1155/2018/1986820] [DOI] [PMC free article] [PubMed] [Google Scholar]
- IRCT2017050925812N2. The impact of mindfulness-based stress reduction in emotional and glycemic regulation of type-2 diabetes mellitus outpatients. https://trialsearch.who.int/Trial2.aspx?TrialID=IRCT2017050925812N2 (first received 17 July 2017).
Baldo 2021 {published data only}
- Baldo JV, Schendel K, Lwi SJ, Herron TJ, Dempsey DG, Muir J, et al. Mindfulness-based stress reduction intervention in chronic stroke: a randomized, controlled pilot study. Mindfulness 2021;12:2908–19. [DOI: 10.1007/s12671-021-01751-0] [DOI] [Google Scholar]
Baylan 2019 {published data only}
- Baylan S, Haig C, MacDonald M, Stiles C, Easto J, Thomson M, et al. Measuring the effects of listening for leisure on outcome after stroke (MELLO): a pilot randomized controlled trial of mindful music listening. International Journal of Stroke 2020;15(2):149-58. [DOI: 10.1177/1747493019841250] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylan S, McGinlay M, MacDonald M, Easto J, Cullen B, Haig C, et al. The effects of music listening on mood and cognition post-stroke. International Journal of Stroke 2016;11(4 Suppl 1):29. [Google Scholar]
- Baylan S, Stiles C, MacDonald M, Easto J, McGinlay M, Cullen B, et al. A single-blind randomised controlled trial of mindful music listening to enhance cognitive recovery and mood after stroke (MELLO): feasibility and acceptability. European Stroke Journal 2017;2(1 Suppl 1):177. [Google Scholar]
- NCT02259062. Listening for leisure after stroke. https://clinicaltrials.gov/ct2/show/NCT02259062 (first received 8 October 2014).
Beauchamp 2020 {published data only}
- Beauchamp JE, Chaoul A, Cron S, Montiel TC, Payen S, Prossin A, et al. Meditation for post-stroke depression: a pilot randomized controlled trial. Stroke 2020;51(Suppl 1):Abstract TMP103. [Google Scholar]
- NCT03239132. Meditation for post stroke depression (MEND). https://clinicaltrials.gov/ct2/show/NCT03239132 (first received 3 August 2017).
Blevins 2009 {published data only}
- Blevins NC. Mindfulness meditation as an intervention for body image and weight management in college women: a pilot study. Dissertation Abstracts International: Section B: The Sciences and Engineering 2009;10-B:6400.
Blom 2013 {published data only}
- Baker B, Irvine J, Abbey S, Myers M, Tobe SW, Blom K, et al. The effects of a mindfulness program on sustained blood pressure: the harmony study (hypertension analysis of stress reduction using mindfulness meditation and yoga). Psychosomatic Medicine 2013;75(3):A-38. [Google Scholar]
- Baker B, Tobe S, Kiss A, Abramson B, Irvine J, Phil D, et al. The effect of mindfulness-based stress reduction on ambulatory blood pressure (the harmony study). Psychosomatic Medicine 2012;74(3):A20. [Google Scholar]
- Blom K, Baker B, How M, Dai M, Abbey S, Myers M, et al. Hypertension analysis of stress reduction using mindfulness meditation and yoga: results from a randomized controlled trial. Canadian Journal of Cardiology 2012;1:S418-9. [DOI] [PubMed] [Google Scholar]
- Blom K, Baker B, How M, Dai M, Irvine J, Abbey S, et al. Hypertension analysis of stress reduction using mindfulness meditation and yoga: results from the HARMONY randomized controlled trial. American Journal of Hypertension 2014;27(1):122-9. [DOI] [PubMed] [Google Scholar]
- Blom K, Baker B, Irvine J, Abbey S, Abramson B, Myers M, et al. The effect of mindfulness-based stress reduction on ambulatory blood pressure (the Harmony study). Journal of Clinical Hypertension 2012;14(Suppl 1):A54. [Google Scholar]
- Blom K, How M, Dai M, Baker B, Irvine J, Abbey S, et al. Hypertension Analysis of stress Reduction using Mindfulness meditatiON and Yoga (The HARMONY Study): study protocol of a randomised control trial. BMJ Open 2012;2(2):e000848. [DOI: 10.1136/bmjopen-2012-000848] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom KC, Baker B, Irvine J, Abbey S, Abramson B, Myers M, et al. The harmony study: hypertension analysis of stress reduction using mindfulness meditation and yoga. Journal of Clinical Hypertension 2011;1:A141. [Google Scholar]
- NCT00825526. HARMONY Study (hypertension analysis of stress reduction using mindfulness meditation and yoga). https://clinicaltrials.gov/ct2/show/NCT00825526 (first received 21 January 2009).
Bokhari 2021 {published data only}
- Bokhari S, Schneider RH, Salerno JW, Rainforth MV, Gaylord-King C, Nidich SI. Effects of cardiac rehabilitation with and without meditation on myocardial blood flow using quantitative positron emission tomography: a pilot study. Journal of Nucleal Cardiology 2021;28:1596–607. [DOI: 10.1007/s12350-019-01884-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01810029. A trial of stress reduction in the secondary prevention of coronary heart disease in blacks. https://www.clinicaltrials.gov/ct2/show/NCT01810029 (first received 13 March 2013).
Brach 1992 {published data only}
- Brach AW. Clinical applications of meditation: a treatment outcome evaluation study of an intervention for binge eating among the obese that combines formal meditation and contingent formal and informal meditation. Dissertation Abstracts International 1992;52(7-B):3898.
Bressan 2020 {published and unpublished data}
- Bressen R. Effect of mindful eating weight loss intervention in women with obesity: ATENTO study [Efeito de orientação nutricional baseada em comer com atenção plena na perda de peso em mulheres com obesidade: estudo ATENTO]. https://www.teses.usp.br/teses/disponiveis/5/5135/tde-22072021-181915/pt-br.php (first received 22 July 2021). [DOI: ]
- Mindful eating in the treatment of obesity. https://trialsearch.who.int/Trial2.aspx?TrialID=RBR-22p3nn2.
Brewer 2011 {published data only}
- Brewer J. Mindfulness as an emerging treatment for smoking and other addictions? Journal of Alternative and Complementary Medicine 2016;22:A70. [Google Scholar]
- Brewer JA, Mallik S, Babuscio TA, Nich C, Johnson HE, Deleone CM, et al. Mindfulness training for smoking cessation: results from a randomized controlled trial. Drug and Alcohol Dependence 2011;119:72-80. [DOI: 10.1016/j.drugalcdep.2011.05.027] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01144689. Mindfulness training for smoking cessation. https://clinicaltrials.gov/show/NCT01144689 (first received 15 June 2010).
Carpenter 2019 {published data only}
- Carpenter KM, Vickerman KA, Salmon EE, Javitz HS, Epel ES, Lovejoy JC. A randomized pilot study of a phone-based mindfulness and weight loss program. Behavioral Medicine 2019;45(4):271-81. [DOI: 10.1080/08964289.2017.1384359] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chacko 2016 {published data only}
- Chacko SA, Yeh GY, Davis RB, Wee CC. A mindfulness-based intervention to control weight after bariatric surgery: preliminary results from a randomized controlled pilot trial. Complementary Therapies in Medicine 2016;28:13-21. [DOI: 10.1016/j.ctim.2016.07.001] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chandler 2020 {published data only}
- Chandler J, Sox L, Diaz V, Kellam K, Neely A, Nemeth L, et al. Impact of 12-month smartphone breathing meditation program upon systolic blood pressure among non-medicated stage 1 hypertensive adults. International Journal of Environmental Research and Public Health 2020;17(6):1955. [DOI: 10.3390/ijerph17061955] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2020 {published data only}
- Chen SM, Lin HS, Atherton JJ, MacIsaac RJ, Wu CJ. Effect of a mindfulness programme for long-term care residents with type 2 diabetes: a cluster randomised controlled trial measuring outcomes of glycaemic control, relocation stress and depression. International Journal of Older People Nursing 2020;15(3):e12312. [EMBASE: 10.1111/opn.12312] [DOI] [PubMed] [Google Scholar]
- NCT03950713. Mindfulness program for older-diabetes. https://clinicaltrials.gov/ct2/show/NCT03950713 (first received 15 May 2019).
Chumachenko 2021 {published data only}
- Chumachenko SY, Cali RJ, Rosal MC, Allison JJ, Person SJ, Ziedonis D, et al. Keeping weight off: mindfulness-based stress reduction alters amygdala functional connectivity during weight loss maintenance in a randomized control trial. PLOS One 2021;16(1):e0244847. [DOI: 10.1371/journal.pone.0244847] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulwiler C, Siegel JA, Allison J, Rosal MC, Brewer J, King JA. Keeping Weight Off: study protocol of an RCT to investigate brain changes associated with mindfulness-based stress reduction. BMJ Open 2016;6:e012573. [DOI: 10.1136/bmjopen-2016-012573] [DOI] [PMC free article] [PubMed] [Google Scholar]
Daubenmier 2011 {published data only}
- Daubenmier J, Kristeller J, Hecht FM, Maninger N, Kuwata M, Jhaveri K, et al. Mindfulness intervention for stress eating to reduce cortisol and abdominal fat among overweight and obese women: an exploratory randomized controlled study. Journal of Obesity 2011;2011:651936. [DOI: 10.1155/2011/651936] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubenmier J, Lin J, Blackburn E, Hecht FM, Kristeller J, Maninger N, et al. Changes in stress, eating, and metabolic factors are related to changes in telomerase activity in a randomized mindfulness intervention pilot study. Psychoneuroendocrinology 2012;37(7):917-28. [DOI: 10.1016/j.psyneuen.2011.10.008] [DOI] [PMC free article] [PubMed] [Google Scholar]
Daubenmier 2016 {published data only}
- Daubenmier J, Epel E, Moran P, Kristeller J, Acree M, Bacchetti P, et al. A randomized controlled trial of a mindfulness-based intervention for metabolic health in obese adults. Journal of Alternative and Complementary Medicine 2014;20(5):A15. [Google Scholar]
- Daubenmier J, Moran PJ, Kristeller J, Acree M, Bacchetti P, Kemeny ME, et al. Effects of a mindfulness-based weight loss intervention in adults with obesity: a randomized clinical trial. Obesity 2016;24(4):794-804. [DOI: 10.1002/oby.21396] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason AE, Hecht FM, Daubenmier JJ, Sbarra DA, Lin J, Moran PJ, et al. Weight Loss Maintenance and Cellular Aging in the Supporting Health Through Nutrition and Exercise Study. Psychosomatic Medicine 2018;80(7):609-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davis 2014a {published data only}
- Davis JM, Manley AR, Goldberg SB, Smith SS, Jorenby DE. Randomized trial comparing mindfulness training for smokers to a matched control. Journal of Substance Abuse Treatment 2014;47(3):213-21. [DOI: 10.1016/j.jsat.2014.04.005] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01299909. Study two on the effectiveness of mindfulness training for smokers. https://clinicaltrials.gov/show/NCT01299909 (first received 1 February 2017).
Davis 2014b {published data only}
- Davis JM, Goldberg SB, Anderson MC, Manley AR, Smith SS, Baker TB. Randomized trial on mindfulness training for smokers targeted to a disadvantaged population. Substance Use & Misuse 2014;49(5):571-85. [DOI: 10.3109/10826084.2013.770025] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01093599. Phase 2 randomized trial on the effectiveness of mindfulness training for smokers, a novel intervention designed to help smokers quit smoking. http://clinicaltrials.gov/show/NCT01093599 (first received 16 February 2015).
Davoudi 2021 {published data only}
- Davoudi M, Allame Z, Niya R, Taghadoosi T, Amir A, Ahmadi SM. The synergistic effect of vitamin D supplement and mindfulness training on pain severity, pain-related disability and neuropathy-specific quality of life dimensions in painful diabetic neuropathy: a randomized clinical trial with placebo-controlled. Journal of Diabetes & Metabolic Disorders 2021;20:49–58. [DOI: 10.1007/s40200-020-00700-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- TCTR20200629004. Vitamin D and mindfulness on diabetes. https://trialsearch.who.int/Trial2.aspx?TrialID=TCTR20200629004 (first received 29 June 2020).
de Fatima Rosas Marchiori 2015 {published data only}
- Fátima Rosas Marchiori M, Kozasa EH, Miranda RD, Monezi Andrade AL, Perrotti TC, Leite JR. Decrease in blood pressure and improved psychological aspects through meditation training in hypertensive older adults: a randomized control study. Geriatrics & Gerontology International 2015;15(10):1158-64. [DOI: 10.1111/ggi.12414] [DOI] [PubMed] [Google Scholar]
Friis 2016 {published data only}
- Friis AM, Johnson MH, Cutfield RG, Consedine NS. Kindness matters: a randomized controlled trial of a mindful self-compassion intervention improves depression, distress, and HbA1c among patients with diabetes. Diabetes Care 2016;39(11):1963-71. [DOI: 10.2337/dc16-0416] [DOI] [PubMed] [Google Scholar]
Friskey 1984 {published data only}
- Friskey LM. Effects of a Combined Relaxation and Meditation Training Program on Hypertensive Patients [PhD Dissertation]. Tucson, AZ: University of Arizona, 1984. [Google Scholar]
Gainey 2016 {published data only}
- Gainey A, Himathongkam T, Tanaka H, Suksom D. Effects of Buddhist walking meditation on glycemic control and vascular function in patients with type 2 diabetes. Complementary Therapies in Medicine 2016;26:92-7. [DOI: 10.1016/j.ctim.2016.03.009] [DOI] [PubMed] [Google Scholar]
- NCT02123901. The effect of walking meditation training on glycemic control and vascular function in patients with type 2 diabetes. https://clinicaltrials.gov/show/nct02123901. [DOI] [PubMed]
Garrison 2020 {published data only}
- Garrison KA, Pal P, O'Malley SS, Pittman BP, Gueorguieva R, Rojiani R, et al. Craving to quit: a randomized controlled trial of smartphone app-based mindfulness training for smoking cessation. Nicotine & Tobacco Research 2020;22(3):324-31. [DOI: 10.1093/ntr/nty126] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison KA, Pal P, Rojiani R, Dallery J, O'Malley SS, Brewer JA. A randomized controlled trial of smartphone-based mindfulness training for smoking cessation: a study protocol. BMC Psychiatry 2015;15:83. [DOI: 10.1186/s12888-015-0468-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldenhersch 2020 {published data only}
- Goldenhersch E, Thrul J, Ungaretti J, Rosencovich N, Waitman C, Ceberio MR. Virtual reality smartphone-based intervention for smoking cessation: pilot randomized controlled trial on initial clinical efficacy and adherence. Journal of Medical Internet Research 2020;22(7):e17571. [DOI: 10.2196/17571] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN50586181. Investigating the use of the Mindcotine® virtual reality smartphone app to help people to quit smoking. https://www.isrctn.com/ISRCTN50586181 (first received 3 October 2019).
Gu 2018 {published data only}
- Gu Y, Zhu Y. Effects of mindfulness-based stress reduction on components of the metabolic syndrome in patients with coronary heart disease: a randomized controlled trial. Journal of the American College of Cardiology 2018;71(16 Suppl 1):S38. [Google Scholar]
- Gu Y, Zhu Y. Effects of mindfulness-based stress reduction on markers of cardiovascular risk in patients with ischemic heart disease a randomized controlled trial. Journal of the American College of Cardiology 2017;70(16 Suppl 1):C166. [Google Scholar]
Guo 2021 {published data only}
- ChiCTR2000030345. The effectiveness of the nurse-led mindfulness-based stress reduction therapy among hospitalized people with type 2 diabetes. https://trialsearch.who.int/Trial2.aspx?TrialID=ChiCTR2000030345 (first received 29 February 2020).
- Guo J, Wang H, Ge L, Valimaki M, Wiley J, Whittemore R. Effectiveness of a nurse-led mindfulness stress-reduction intervention on diabetes distress, diabetes self-management, and HbA1c levels among people with type 2 diabetes: a pilot randomized controlled trial. Research in Nursing & Health 2021;45:46-58. [DOI: 10.1002/nur.22195] [DOI] [PubMed] [Google Scholar]
Hafner 1982 {published data only}
- Hafner RJ. Psychological treatment of essential hypertension: a controlled comparison of meditation and meditation plus biofeedback. Biofeedback & Self Regulation 1982;7(3):305-16. [DOI] [PubMed] [Google Scholar]
Huerin 2015 {published data only}
- Huerin M, Juarez WM, Lobo M, Rodriguez J, Lago N, Rostan M, et al. Impact of a meditation program on pulse-wave velocity, C-reactive protein and quality of life. Revista Argentina de Cardiologia 2015;83(3):198-203. [Google Scholar]
Ingraham 2017 {published data only}
- Ingraham N, Harbatkin D, Lorvick J, Plumb M, Minnis AM. Women's Health and Mindfulness (WHAM): a randomized intervention among older lesbian/bisexual women. Health Promotion Practice 2017;18(3):348-57. [DOI] [PubMed] [Google Scholar]
Izgu 2020 {published data only}
- Izgu N, Gok Metin Z, Karadas C, Ozdemir L, Metinarikan N, Corapcıoglu D. Progressive muscle relaxation and mindfulness meditation on neuropathic pain, fatigue, and quality of life in patients with type 2 diabetes: a randomized clinical trial. Journal of Nursing Scholarship 2020;52(5):476-87. [DOI: 10.1111/jnu.12580] [DOI] [PubMed] [Google Scholar]
Jalali 2019 {published data only}
- Jalali D, Abdolazimi M, Alaei Z, Solati K. Effectiveness of mindfulness-based stress reduction program on quality of life in cardiovascular disease patients. International Journal of Cardiology. Heart & Vasculature 2019;23:100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jang 2018 {published data only}
- Jang SH, Lee JH, Lee HJ, Lee SY. Effects of mindfulness-based art therapy on psychological symptoms in patients with coronary artery disease. Journal of Korean Medical Science 2018;33(12):e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kalinowski 2021 {published data only}
- Kalinowski J, Castaneda SF, Allison MA, Duberstein ZT, Kaur K, Rutigliano M, et al. Telephone-based mindfulness training in diverse prehypertensive women: results of a pilot randomized controlled trial. Circulation 2021;143(Suppl 1):P191. [DOI: 10.1161/circ.143.suppl_1.P191] [DOI] [Google Scholar]
Kopf 2014 {published data only}
- DRKS00009574. Psychosoziale Intervention Zur Reduktion Diabetischer Spätschäden Bei Diabetes Mellitus Typ 2. https://www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00009574 (first received 23 October 2015).
- Hartmann M, Kopf S, Kircher C, Faude-Lang V, Djuric Z, Augstein F, et al. Sustained effects of a mindfulness-based stress-reduction intervention in type 2 diabetic patients: design and first results of a randomized controlled trial (the Heidelberger Diabetes and Stress-study). Diabetes Care 2012;35(5):945-7. [DOI: 10.2337/dc11-1343] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf S, Oikonomou D, Hartmann M, Feier F, Faude-Lang V, Morcos M, et al. Effects of stress reduction on cardiovascular risk factors in type 2 diabetes patients with early kidney disease - results of a randomized controlled trial (HEIDIS). Experimental and Clinical Endocrinology & Diabetes 2014;122(6):341-9. [DOI: 10.1055/s-0034-1372583] [DOI] [PubMed] [Google Scholar]
- Kopf S, Oikonomou D, Hartmann M, Humpert P, Herzog W, Nawroth PP. Mindfulness based stress reduction training was able to improve stress, depression and health status in patients with type 2 diabetes over 3 years-Results of the Heidelberger Diabetes and Stress Study (HEIDIS). Experimental and Clinical Endocrinology and Diabetes 2014;122(3):P164. [DOI] [PubMed] [Google Scholar]
Kristeller 2014 {published data only}
- Kristeller J, Wolever RQ, Sheets V. Mindfulness-based eating awareness training (MB-EAT) for binge eating: a randomized clinical trial. Mindfulness 2014;5(3):282–97. [DOI: 10.1007/s12671-012-0179-1] [DOI] [Google Scholar]
Malm 2018 {published data only}
- Malm D, Fridlund B, Ekblad H, Karlström P, Hag E, Pakpour AH. Effects of brief mindfulness-based cognitive behavioural therapy on health-related quality of life and sense of coherence in atrial fibrillation patients. European Journal of Cardiovascular Nursing 2018;7:589-97. [DOI: 10.1177/1474515118762796] [DOI] [PubMed] [Google Scholar]
McTigue 2020 {published data only}
- McTigue KM, Yabes J, Greco C, Marcum Z, Wolever R, Morone N. Feasibility of online self-management support with mindfulness for treating blood pressure. Journal of General Internal Medicine 2020;35(Suppl 1):S132. [DOI: 10.1007/s11606-020-05890-3] [DOI] [Google Scholar]
- Rajendran I, Morone N, McTigue K, Greco C, Yabes J, Marcum Z, et al. Minding-goals: effects of a web-based, interactive, mind-body intervention in the management of hypertension. Global Advances in Health and Medicine 2020;9:102. [DOI: 10.1177/2164956120912849] [DOI] [Google Scholar]
Miller 2014 {published data only}
- Miller CK, Kristeller J, Nagaraja H, Miser F. Comparative effectiveness of a mindful eating intervention to a targeted goals intervention in adults with type 2 diabetes. Diabetes 2012;1:A207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CK, Kristeller JL, Headings A, Nagaraja H, Miser WF. Comparative effectiveness of a mindful eating intervention to a diabetes self-management intervention among adults with type 2 diabetes: a pilot study. Journal of the Academy of Nutrition & Dietetics 2012;112(11):1835-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CK, Kristeller JL, Headings A, Nagaraja H. Comparison of a mindful eating intervention to a diabetes self-management intervention among adults with type 2 diabetes: a randomized controlled trial. Health Education & Behavior 2014;41(2):145-54. [DOI: 10.1177/1090198113493092] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nasiri 2020 {published data only}
- Nasiri Z, Alavi M, Ghazavi Z, Rabiei K. The effectiveness of mindfulness-based intervention on perceived stress and perception of disease in patients with acute coronary syndrome. Journal of Education and Health Promotion 2020;9:130. [DOI: 10.4103/jehp.jehp_660_19] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nathan 2017 {published data only}
- NCT02127762. The effect of mindfulness based stress reduction in patients with painful diabetic peripheral neuropathy. https://clinicaltrials.gov/ct2/show/NCT02127762 (first received 1 May 2014).
- Nathan HJ, Poulin P, Wozny D, Taljaard M, Smyth C, Gilron I, et al. Randomized trial of the effect of mindfulness-based stress reduction on pain-related disability, pain intensity, health-related quality of life, and A1c in patients with painful diabetic peripheral neuropathy. Clinical Diabetes 2017;35(5):294-304. [DOI: 10.2337/cd17-0077] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nejati 2015 {published data only}
- Nejati S, Zahiroddin A, Afrookhteh G, Rahmani S, Hoveida S. Effect of group mindfulness-based stress-reduction program and conscious yoga on lifestyle, coping strategies, and systolic and diastolic blood pressures in patients with hypertension. Journal of Tehran Heart Center 2015;10(3):140-8. [PMC free article] [PubMed] [Google Scholar]
Ng 2018 {published data only}
- Ng J, Stone A, Papasavas PK, Tishler D, Pearlson G, Staff I, et al. Pilot testing a mindfulness-based weight loss maintenance intervention to enhance outcomes after bariatric surgery. Surgery for Obesity and Related Diseases 2018;14(11 Suppl):S123‐4. [Google Scholar]
Nidich 2009 {published data only}
- Nidich SI, Rainforth MV, Haaga DA, Hagelin J, Salerno JW, Travis F, et al. A randomized controlled trial on effects of the Transcendental Meditation program on blood pressure, psychological distress, and coping in young adults. American Journal of Hypertension 2009;22(12):1326-31. [DOI: 10.1038/ajh.2009.184] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nijjar 2019 {published data only}
- Everson-Rose SA, Nijjar PS, Lindquist R, Kreitzer MJ, Connett JE, Brown RZ, et al. The mindful heart study: results from a pilot randomized controlled trial of mindfulness-based stress reduction for cardiac patients. Psychosomatic Medicine 2019;81(4):A168. [Google Scholar]
- NCT02722213. Mindfulness & stress management study for cardiac patients. https://clinicaltrials.gov/ct2/show/NCT02722213 (first received 29 March 2016).
- Nijjar PS, Connett JE, Lindquist R, Brown R, Burt M, Pergolski A, et al. Randomized trial of mindfulness-based stress reduction in cardiac patients eligible for cardiac rehabilitation. Scientific Reports 2019;9(1):18415. [DOI: 10.1038/s41598-019-54932-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nikkhah Ravari 2020 {published data only}
- IRCT20190813044527N1. The effect of mindfulness based stress management on glycemic control and mental health in patients with diabetes mellitus. https://trialsearch.who.int/Trial2.aspx?TrialID=IRCT20190813044527N1 (first received 29 January 2020).
- Nikkhah Ravari O, Mousavi SZ, Babak A. Evaluation of the effects of 12 weeks mindfulness-based stress reduction on glycemic control and mental health indices in women with diabetes mellitus type 2. Advanced Biomedical Research 2020;9:61. [DOI: 10.4103/abr.abr_133_20] [DOI] [PMC free article] [PubMed] [Google Scholar]
Palmeira 2017 {published data only}
- Palmeira L, Pinto-Gouveia J, Cunha M. Exploring the efficacy of an acceptance, mindfulness & compassionate-based group intervention for women struggling with their weight (Kg-Free): a randomized controlled trial. Appetite 2017;112:107-16. [DOI: 10.1016/j.appet.2017.01.027] [DOI] [PubMed] [Google Scholar]
Parswani 2013 {published data only}
- Parswani MJ, Sharma MP, Iyengar S. Mindfulness-based stress reduction program in coronary heart disease: a randomized control trial. International Journal of Yoga 2013;6(2):111-17. [DOI: 10.4103/0973-6131.113405] [DOI] [PMC free article] [PubMed] [Google Scholar]
Paul‐Labrador 2006 {published data only}
- Paul-Labrador M, Polk D, Dwyer JH, Velasquez I, Nidich S, Rainforth M, et al. Effects of a randomized controlled trial of transcendental meditation on components of the metabolic syndrome in subjects with coronary heart disease. Archives of Internal Medicine 2006;166(11):1218-24. [DOI: 10.1001/archinte.166.11.1218] [DOI] [PubMed] [Google Scholar]
Pearson 2018 {published data only}
- ACTRN12613000898752. Testing the effectiveness of a mindfulness-based intervention to reduce distress in people with diabetes: a pilot study. https://anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12613000898752 (first received 12 August 2013).
- Pearson S, Wills K, Woods M, Warnecke E. Effects of mindfulness on psychological distress and HbA1c in people with diabetes. Mindfulness 2018;9(5):1615-26. [Google Scholar]
Ponte Marquez 2019 {published data only}
- Ponte Márquez PH, Feliu-Soler A, Solé-Villa MJ, Matas-Pericas L, Filella-Agullo D, Ruiz-Herrerias M, et al. Benefits of mindfulness meditation in reducing blood pressure and stress in patients with arterial hypertension. Journal of Human Hypertension 2019;33(3):237-47. [DOI: 10.1038/s41371-018-0130-6] [DOI] [PubMed] [Google Scholar]
- Ponte P, Castella M, Filella D, Feliu A, L Matas L, Soler J, et al. Benefits of mindfulness meditation in reducing blood pressure and stress in patients with arterial hypertension. Journal of Hypertension 2018;36(Suppl 1):e294-5. [DOI] [PubMed] [Google Scholar]
Raja‐Khan 2017 {published data only}
- NCT01464398. Stress reduction for overweight or obese women either with polycystic ovary syndrome (PCOS) or without PCOS (non-PCOS). https://clinicaltrials.gov/show/NCT01464398 (first received 3 November 2011).
- Raja-Khan N, Agito K, Shah J, Stetter CM, Gustafson TS, Socolow H, et al. Mindfulness-based stress reduction decreases fasting glucose in overweight and obese women. Endocrine Reviews. Conference: 97th Annual Meeting and Expo of the Endocrine Society, ENDO 2015;36(Suppl):FRI-550. [Google Scholar]
- Raja-Khan N, Agito K, Shah J, Stetter CM, Gustafson TS, Socolow H, et al. Mindfulness-based stress reduction for overweight/obese women with and without polycystic ovary syndrome: design and methods of a pilot randomized controlled trial. Contemporary Clinical Trials 2015;41:287-97. [DOI: 10.1016/j.cct.2015.01.021] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja-Khan N, Agito K, Shah J, Stetter CM, Gustafson TS, Socolow H, et al. Mindfulness-based stress reduction in women with overweight or obesity: a randomized clinical trial. Obesity (Silver Spring) 2017;25(8):1349-59. [DOI: 10.1002/oby.21910] [DOI] [PMC free article] [PubMed] [Google Scholar]
Razavizadeh Tabadkan 2019 {published data only}
- IRCT2017071515754N2. Clinical trials of comparison of meta cognitive therapy and mindfulness-based cognitive therapy in, ruminative responses, emotion regulation difficulties and perceived stress of diabetic women. http://www.who.int/trialsearch/trial2.aspx?Trialid=IRCT2017071515754N2 (first received 16 August 2017).
- Razavizadeh Tabadkan BZ, Jajarmi M, Vakili Y. The effectiveness of mindfulness-based cognitive therapy on ruminative thoughts, perceived stress and difficulties in emotion regulation of women with type 2 diabetes. Iranian Journal of Psychiatry and Clinical Psychology 2019;24(4):370-83. [Google Scholar]
Rungreangkulkij 2011 {published data only}
- Rungreangkulkij S, Wongtakee W, Thongyot S. Buddhist group therapy for diabetes patients with depressive symptoms. Archives of Psychiatric Nursing 2011;25(3):195-205. [DOI] [PubMed] [Google Scholar]
Sampaio 2016 {published data only}
- Sampaio CV, Lima MG, Ladeia AM. Efficacy of healing meditation in reducing anxiety of individuals at the phase of weight loss maintenance: a randomized blinded clinical trial. Complementary Therapies in Medicine 2016;29:1-8. [DOI: 10.1016/j.ctim.2016.08.005] [DOI] [PubMed] [Google Scholar]
Sarika 2020 {published data only}
- Sarika KS, Kumar H, Balakrishnan V, Sundaram KR. Impact of Integrated Amrita Meditation R technique on stress in type 2 diabetic patients. Indian Journal of Medical Research 2020;152(5):508-14. [DOI: 10.4103/ijmr.IJMR_2109_18] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schneider 2005 {published data only}
- Alexander CN, Schneider RH, Staggers F, Sheppard W, Clayborne BM, Rainforth M, et al. Trial of stress reduction for hypertension in older African Americans. II. Sex and risk subgroup analysis. Hypertension 1996;28(2):228-37. [DOI] [PubMed] [Google Scholar]
- Schneider RH, Alexander CN, Staggers F, Orme-Johnson DW, Rainforth M, Salerno JW, et al. A randomized controlled trial of stress reduction in African Americans treated for hypertension for over one year. American Journal of Hypertension 2005;18(1):88-98. [DOI: 10.1016/j.amjhyper.2004.08.027] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider RH, Staggers F, Alexander CN, Sheppard W, Rainforth M, Kondwani K, et al. A randomized controlled trial of stress reduction for hypertension in older African Americans. Hypertension 1995;26(5):820-7. [DOI] [PubMed] [Google Scholar]
Schneider 2012 {published data only}
- NCT01299935. A randomized controlled trial of stress reduction on cardiovascular morbidity and mortality in African Americans. https://clinicaltrials.gov/show/NCT01299935 (first received 21 February 2011).
- Schneider RH, Grim CE, Rainforth MV, Kotchen T, Nidich SI, Gaylord-King C, et al. Stress reduction in the secondary prevention of cardiovascular disease: randomized, controlled trial of transcendental meditation and health education in Blacks. Circulation. Cardiovascular Quality and Outcomes 2012;5(6):750-8. [DOI: 10.1161/CIRCOUTCOMES.112.967406] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schneider 2019 {published data only}
- Calderon R Jr. Effects of nonpharmacological approaches on cholesterol levels in mild hypertensive African Americans: a pilot study of the transcendental meditation program and a health education program. Dissertation Abstracts International: Section B: The Sciences and Engineering 2000;61(3-B):1619.
- Castillo-Richmond A, Schneider RH, Alexander CN, Cook R, Myers H, Nidich S, et al. Effects of stress reduction on carotid atherosclerosis in hypertensive African Americans. Stroke 2000;31(3):568-73. [DOI: 10.1161/01.str.31.3.568] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondwani KA. Nonpharmacologic treatment of hypertensive heart disease in African-Americans: a trial of the transcendental meditation program and a health education program. Dissertation Abstracts International: Section B: The Sciences and Engineering 1998;59(6-B):3114.
- NCT00000546. Stress reduction and atherosclerotic CVD in blacks. https://clinicaltrials.gov/ct2/show/NCT00000546 (first received 28 October 1999).
- NCT00010608. Clinical trial of meditation for cardiovascular disease in older black women. https://clinicaltrials.gov/ct2/show/NCT00010608 (first received 5 February 2001).
- Schneider RH, Castillo-Richmond A, Alexander CN, Myers H, Kaushik V, Aranguri C, et al. Behavioral treatment of hypertensive heart disease in African Americans: rationale and design of a randomized controlled trial. Behavioral Medicine 2001;27(2):83-95. [DOI] [PubMed] [Google Scholar]
- Schneider RH, Myers HF, Marwaha K, Rainforth MA, Salerno JW, Nidich SI, et al. Stress reduction in the prevention of left ventricular hypertrophy: a randomized controlled trial of transcendental meditation and health education in hypertensive African Americans. Ethnicity & Disease 2019;29(4):577-86. [DOI: 10.18865/ed.29.4.577] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schneider 2021 {published data only}
- NCT04821505. Stress reduction and hypertension prevention in African Americans. https://clinicaltrials.gov/show/NCT04821505 (first received 29 March 2021).
- Schneider RH, Grim C, Kotchen T, Marwaha K, Kotchen J, Salerno JW, et al. Randomized controlled trial of stress reduction with meditation and health education in black men and women with high normal and normal blood pressure. American Journal of Preventive Cardiology 2021;8:100279. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schroevers 2015 {published data only}
- Schroevers MJ, Tovote KA, Keers JC, Links TP, Sanderman R, Fleer J. Individual mindfulness-based cognitive therapy for people with diabetes: a pilot randomized controlled trial. Mindfulness 2015;6:99–110. [DOI: 10.1007/s12671-013-0235-5] [DOI] [Google Scholar]
Seer 1980 {published data only}
- Seer P, Raeburn JM. Meditation training and essential hypertension: a methodological study. Journal of Behavioral Medicine 1980;3(1):59-71. [DOI] [PubMed] [Google Scholar]
Shukla 2021 {published data only}
- Shukla R, Gupta M, Agarwal N, Bajpai A. Mindfulness meditation as adjunctive therapy to improve the glycemic care and quality of life in patients with type 1 diabetes. Medical Sciences (Basel, Switzerland) 2021;9(2):33. [DOI: 10.3390/medsci9020033] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sinha 2018 {published data only}
- Sinha SS, Ajay Kumar Jain AK, Tyagi S, Gupta SK, Mahajan AS. Effect of 6 months of meditation on blood sugar, glycosylated hemoglobin, and insulin levels in patients of coronary artery disease. International Journal of Yoga 2018;11(2):122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha SS, Ajay Kumar Jain AK, Tyagi S, Gupta SK, Mahajan AS. Effect of meditation on heart rate, blood pressure and exercise performance in coronary artery disease patients. Indian Journal of Physiology and Pharmacology 2018;62(2):209‐16. [Google Scholar]
Smith 2018 {published data only}
- Smith BW, Shelley BM, Sloan AL, Colleran K, Erickson K. A preliminary randomized controlled trial of a mindful eating intervention for post-menopausal obese women. Mindfulness 2018;9(3):836–49. [DOI: 10.1007/s12671-017-0824-9] [DOI] [Google Scholar]
Spadaro 2018 {published data only}
- Davis KK. Effect of mindfulness meditation and home-based resistance exercise on weight loss, weight loss behaviors, and psychosocial correlates in overweight adults. Dissertation Abstracts International Section A: Humanities and Social Sciences 2009;69(11-A):4277.
- Spadaro KC, Davis KK, Sereika SM, Gibbs BB, Jakicic JM, Cohen SM. Effect of mindfulness meditation on short-term weight loss and eating behaviors in overweight and obese adults: a randomized controlled trial. Journal of Complementary and Integrative Medicine 2018;15(2):20160048. [DOI: 10.1515/jcim-2016-0048] [DOI] [PubMed] [Google Scholar]
- Spadaro KC. Weight loss: Exploring self-regulation through mindfulness meditation. Dissertation Abstracts International: Section B: The Sciences and Engineering 2021;82(11-B).
Toomey 2007 {published data only}
- Grandinetti NS, Schneider R, Chang H, Ricketts L, Toomey M. The transcendental meditation program and cardiovascular disease in native Hawaiins. Journal of Psychosomatic Research 2003;55:144. [Google Scholar]
- Nidich S, Grandinetti A, Schneider R, Chang H, Ricketts L, Toomey M. The transcendental meditation program and cardiovascular disease in native Hawaiians. Journal of Psychosomatic Research 2003;55:133–45. [Google Scholar]
- Toomey M. The effects of the transcendental meditation program on carotid atherosclerosis and cardiovascular disease risk factors in Native Hawaiians. Dissertation Abstracts International: Section B: The Sciences and Engineering 2007;68(6-B):4169.
Tovote 2014 {published data only}
- NCT01630512. Mindfulness-based cognitive therapy (MBCT) and cognitive behavioral therapy (CBT) for depression in diabetes patients. https://clinicaltrials.gov/ct2/show/NCT01630512 (first received 28 June 2012).
- Tovote KA, Fleer J, Snippe E, Bas IV, Links TP, Emmelkamp PM, et al. Cognitive behavioral therapy and mindfulness-based cognitive therapy for depressive symptoms in patients with diabetes: design of a randomized controlled trial. BMC Psychology 2013;1(1):17. [DOI: 10.1186/2050-7283-1-17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovote KA, Fleer J, Snippe E, Peeters AC, Emmelkamp PM, Sanderman R, et al. Individual mindfulness-based cognitive therapy and cognitive behavior therapy for treating depressive symptoms in patients with diabetes: results of a randomized controlled trial. Diabetes Care 2014;37(9):2427-34. [DOI: 10.2337/dc13-2918] [DOI] [PubMed] [Google Scholar]
- Tovote KA, Schroevers MJ, Snippe E, Emmelkamp PMG, Links TP, Sanderman R, et al. What works best for whom? Cognitive behavior therapy and mindfulness-based cognitive therapy for depressive symptoms in patients with diabetes. PLoS ONE 2017;12(6):e0179941. [DOI: 10.1371/journal.pone.0179941] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovote KA, Schroevers MJ, Snippe E, Sanderman R, Links TP, Emmelkamp PMG, et al. Long-Term effects of individual mindfulness-based cognitive therapy and cognitive behavior therapy for depressive symptoms in patients with diabetes: a randomized trial. Psychotherapy and psychosomatics 2015;84(3):186-7. [DOI: 10.1159/000375453] [DOI] [PubMed] [Google Scholar]
Turrise 2017 {published data only}
- Effects of mindfulness on outcomes in cardiac rehabilitation participants: a pilot study. https://sigma.nursingrepository.org/bitstream/handle/10755/622008/Turrise_83918.pdf?sequence=1&isAllowed=y.
- Turrise SL, Falsafi, Pond RS, Swiger N. Mindfulness in cardiac rehabilitation: A pilot study. Journal of Cardiopulmonary Rehabilitation and Prevention 2017;37:383-4. [Google Scholar]
Vaccarino 2013 {published data only}
- Kondwani KA, Kelley ME, Meng YX, Quyyumi AA, Gibbons GH, Vaccarino V. Effects of a meditation intervention on endothelial function in African Americans with metabolic syndrome: a randomized trial. Psychosomatic Medicine 2011;73(3):A112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Kondwani KA, Kelley ME, Murrah NV, Boyd L, Ahmed Y et al. Effect of meditation on endothelial function in Black Americans with metabolic syndrome: a randomized trial. Psychosomatic Medicine 2013;75(6):591-9. [DOI: 10.1097/PSY.0b013e31829ac4f4] [DOI] [PMC free article] [PubMed] [Google Scholar]
van Son 2014 {published data only}
- Haenen S, Nyklíček I, Son J, Pop V, Pouwer F. Mindfulness facets as differential mediators of short and long-term effects of mindfulness-Based Cognitive Therapy in diabetes outpatients: Findings from the DiaMind randomized trial. Journal of Psychosomatic Research 2016;85:44-50. [DOI: 10.1016/j.jpsychores.2016.04.006] [DOI] [PubMed] [Google Scholar]
- NTR2145. The evaluation of a mindfulness-based psychological intervention for patients with diabetes type 2 and emotional problems. http://www.who.int/trialsearch/trial2.aspx?Trialid=NTR2145 (first received 18 December 2009).
- Van Son J, Nyklicek I, Pouwer F, Pop VJ. Effects of a mindfulness-based psychological intervention in patients with diabetes having emotional distress: the diamind randomized controlled trial. Psychosomatic Medicine 2012;74(3):A18. [Google Scholar]
- Son J, Nyklicek I, Pop VJ, Blonk MC, Erdtsieck RJ, Spooren PF, et al. The effects of a mindfulness-based intervention on emotional distress, quality of life, and HbA(1c) in outpatients with diabetes (DiaMind): a randomized controlled trial. Diabetes Care 2013;36(4):823-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son J, Nyklicek I, Pop VJ, Pouwer F. Testing the effectiveness of a mindfulness-based intervention to reduce emotional distress in outpatients with diabetes (DiaMind): design of a randomized controlled trial. BMC Public Health 2011;11:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son J, Nyklíček I, Pop VJ, Blonk MC, Erdtsieck RJ, Pouwer F. Mindfulness-based cognitive therapy for people with diabetes and emotional problems: long-term follow-up findings from the DiaMind randomized controlled trial. Journal of Psychosomatic Research 2014;77(1):81-4. [DOI: 10.1016/j.jpsychores.2014.03.013] [DOI] [PubMed] [Google Scholar]
Vidrine 2016 {published data only}
- Spears CA, Hedeker D, Li L, Wu C, Anderson NK, Houchins SC, et al. Mechanisms underlying mindfulness-based addiction treatment versus cognitive behavioral therapy and usual care for smoking cessation. Journal of Consulting and Clinical Psychology 2017;85(11):1029-40. [DOI: 10.1037/ccp0000229] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidrine JI, Spears CA, Heppner WL, Reitzel LR, Marcus MT, Cinciripini PM, et al. Efficacy of mindfulness-based addiction treatment (MBAT) for smoking cessation and lapse recovery: a randomized clinical trial. Journal of Consulting and Clinical Psychology 2016;84(9):824-38. [DOI: 10.1037/ccp0000117] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weiss de Souza 2020 {published data only}
- NCT02327104. Effectiveness of mindfulness based relapse prevention for tobacco dependents. https://clinicaltrials.gov/show/NCT02327104 (first received 30 December 2014).
- Weiss de Souza IC, Kozasa EH, Bowen S, Richter KP, Sartes LMA, Colugnati FAB, et al. Effectiveness of mindfulness-based relapse prevention program as an adjunct to the standard treatment for smoking: a pragmatic design pilot study. Nicotine & Tobacco Research 2020;22(9):1605-13. [DOI: 10.1093/ntr/ntaa057] [DOI] [PubMed] [Google Scholar]
Weng 2021 {published data only}
- NCT02497339. Mindfulness training for smoking cessation in women in workplaces. https://clinicaltrials.gov/ct2/show/NCT02497339 (first received 14 July 2015).
- Weng X, Luk TT, Lau OS, Suen YN, Lee JJ, Li WHC, et al. Brief mindfulness training for smoking cessation in Chinese women in workplaces: a pilot randomized controlled trial. Addictive Behaviors 2021;113:106677. [DOI: 10.1016/j.addbeh.2020.106677] [DOI] [PubMed] [Google Scholar]
Woods‐Giscombe 2019 {published data only}
- Woods-Giscombe CL, Gaylord SA, Li Y, Brintz CE, Bangdiwala SI, Buse JB, et al. A mixed-methods, randomized clinical trial to examine feasibility of a mindfulness-based stress management and diabetes risk reduction intervention for african americans with prediabetes. Evidence-Based Complementary and Alternative Medicine 2019;2019:3962623. [DOI: 10.1155/2019/3962623] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zanella 2021 {published data only}
- RBR-9s93d9. Is mindful breathing meditation worth it for obese patients under nutritional orientation? Evaluation of stress perception, anthropometry, and autonomous balance in a Brazilian sample. https://trialsearch.who.int/Trial2.aspx?TrialID=RBR-9s93d9 (first received 8 March 2020).
Zarifsanaiey 2020 {published data only}
- Zarifsanaiey N, Jamalian K, Bazrafcan L, Keshavarzy F, Shahraki HR. The effects of mindfulness training on the level of happiness and blood sugar in diabetes patients. Journal of Diabetes and Metabolic Disorders 2020;19(1):311-7. [DOI: 10.1007/s40200-020-00510-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Delui 2013 {published data only}
- Delui MH, Yari M, Khouyinezhad G, Amini M, Bayazi MH. Comparison of cardiac rehabilitation programs combined with relaxation and meditation techniques on reduction of depression and anxiety of cardiovascular patients. The Open Cardiovascular Medicine Journal 2013;7:99-103. [DOI: 10.2174/1874192401307010099] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gentile 2021 {published data only}
- Gentile C, Starnino L, Dupuis G, D'Antono B. Mindfulness-based stress reduction in older adults at risk for coronary artery disease: a pilot randomized trial. Gerontologist 2021;45(2):272-86. [DOI: 10.1080/07317115.2021.1887421] [DOI] [PubMed] [Google Scholar]
Gotink 2017 {published data only}
- Gotink RA, Younge JO, Wery MF, Utens EMWJ, Michels M, Rizopoulos D, et al. Online mindfulness as a promising method to improve exercise capacity in heart disease: 12-month follow-up of a randomized controlled trial. PLOS One 2017;12(5):e0175923. [DOI: 10.1371/journal.pone.0175923] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gregg 2007 {published data only}
- Gregg JA, Callaghan GM, Hayes SC, Glenn-Lawson JL. Improving diabetes self-management through acceptance, mindfulness, and values: a randomized controlled trial. Journal of Consulting and Clinical Psychology 2007;75(2):336-43. [DOI: 10.1037/0022-006X.75.2.336] [DOI] [PubMed] [Google Scholar]
Hosseini 2021 {published data only}
- Hosseini SS, Ahadi M, Hatami M, Khalatbari J. Effectiveness of mindfulness-based therapy on resilience, psychological well-being, and blood sugar levels in patients with type II diabetes. Razavi International Journal of Medicine 2021;9(2):74-80. [DOI: 10.30483/rijm.2021.254153.1008] [DOI] [Google Scholar]
IRCT20090716002195N3 {published data only}
- IRCT20090716002195N3. Efficacy of mindfulness and TDCS on patients with stroke. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20090716002195N3 (first received 31 July 2019).
IRCT20160927030002N2 {published data only}
- IRCT20160927030002N2. Effectiveness of mindfulness-based intervention on mental health in patients with acute coronary syndrome. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20160927030002N2 (first received 30 April 2019).
Iturbe 2019 {published data only}
- Iturbe I, Echeburua E, Pereda Pereda E, Urkia I, Maiz E. Effect of a group intervention program based on mindfulness and acceptance and commitment therapy on the physical and psychological well-being of overweight and obese individuals (Mind&Life Program). Obesity Facts 2019;12(Suppl 1):170-1. [Google Scholar]
- Iturbe I, Pereda Pereda E, Echeburua E, Maiz E, Urkia I. ACT and mindfulness group intervention for enhancing psychological and physical well-being of adults struggling with overweight: The Mind&Life study protocol. Obesity Facts 221;14(Suppl 1):55. [DOI: 10.1159/000515911] [DOI] [Google Scholar]
- Iturbe I, Pereda Pereda E, Echeburua E, Maiz E, Urkia I. The Effectiveness of an acceptance and commitment therapy and mindfulness group intervention for enhancing the psychological and physical well-being of adults with overweight or obesity seeking treatment: the Mind&Life randomized control trial study protocol. International Journal of Environmental Research and Public Health 2021;18(9):4396. [DOI: 10.3390/ijerph18094396] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturbe I, Pereda Pereda E, Echeburua E, Maiz E, Urkia I. The effect of an ACT and mindfulness group intervention on psychological and physical well-being of adults struggling with overweight: a preliminary study. Obesity Facts 2021;14(Suppl 1):126. [DOI: 10.1159/000515911] [DOI] [Google Scholar]
- NCT03718728. Effect of a group intervention program based on acceptance and mindfulness on the physical and emotional well-being of overweight and obese individuals. https://ClinicalTrials.gov/show/NCT03718728 (first received 24 October 2018).
Jayadevappa 2007 {published data only}
- Jayadevappa R, Johnson JC, Bloom BS, Nidich S, Desai S, Chhatre S, et al. Effectiveness of transcendental meditation on functional capacity and quality of life of African Americans with congestive heart failure: a randomized control study. Ethnicity & Disease 2007;17(1):72-7. [PMC free article] [PubMed] [Google Scholar]
Kumar 2017 {published data only}
- Kumar S, Lathif F, Raghavan V. Effects of mindfulness-based stress reduction on blood pressure (MBSR) among patients with type-2 diabetes - a randomised pilot study. Nursing Journal of India 2017;108(2):61-3. [Google Scholar]
Lappalainen 2014 {published data only}
- Lappalainen R, Sairanen E, Järvelä E, Rantala S, Korpela R, Puttonen S, et al. The effectiveness and applicability of different lifestyle interventions for enhancing wellbeing: the study design for a randomized controlled trial for persons with metabolic syndrome risk factors and psychological distress. BMC Public Health 2014;14:310. [DOI: 10.1186/1471-2458-14-310.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Manikonda 2008 {published data only}
- Manikonda J, Störk S, Tögel S, Lobmüller A, Grünberg I, Bedel S, et al. Contemplative meditation reduces ambulatory blood pressure and stress-induced hypertension: a randomized pilot trial. Journal of Human Hypertension 2008;22:138–40. [DOI: 10.1038/sj.jhh.1002275] [DOI] [PubMed] [Google Scholar]
Momeni 2016 {published data only}
- Momeni J, Omidi A, Raygan F, Akbari H. The effects of mindfulness-based stress reduction on cardiac patients' blood pressure, perceived stress, and anger: a single-blind randomized controlled trial. Journal of the American Society of Hypertension 2016;10(10):763-71. [DOI: 10.1016/j.jash.2016.07.007] [DOI] [PubMed] [Google Scholar]
Monin 2020 {published data only}
- Monin JK, Sperduto CM, Manigault AW, Dutton A, Clark MS, Jastreboff AM. Mindfulnes-based stress reduction for older couples with metabolic syndrome: a pilot randomized controlled trial. Mindfulness 2020;11(4):917-27. [DOI: 10.1007/s12671-019-01301-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nyklíček 2014 {published data only}
- Nyklíček I, Dijksman SC, Lenders PJ, Fonteijn WA, Koolen JJ. A brief mindfulness based intervention for increase in emotional well-being and quality of life in percutaneous coronary intervention (PCI) patients: The MindfulHeart randomized controlled trial. Journal of Behavioral Medicine 2014;37(1):135-44. [DOI: 10.1007/s10865-012-9475-4] [DOI] [PubMed] [Google Scholar]
Packiasabapathy 2019 {published data only}
- Packiasabapathy S, Susheela AT, Mueller A, Patxot M, Gasangwa DV, O'Gara B, et al. Guided meditation as an adjunct to enhance postoperative recovery after cardiac surgery: study protocol for a prospective randomized controlled feasibility trial. Trials 2019;20(1):39. [DOI: 10.1186/s13063-018-3103-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Palta 2012 {published data only}
- Palta P, Page G, Piferi RL, Gill JM, Hayat MJ, Connolly AB et al. Evaluation of a mindfulness-based intervention program to decrease blood pressure in low-income African-American older adults. Journal of Urban Health 2012;89(2):308-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patarathipakorn 2021 {published data only}
- Patarathipakorn O, Ruchiwit M, Smith M. The effects of meditation with a biofeedback program on stress and depression levels among people with mild depression diabetes. Open Public Health Journal 2021;14(1):104-15. [Google Scholar]
Pool 1996 {published data only}
- Pool JI. Cognitive restructuring and meditation training as stress management intervention in post-cardiac adjustment. Dissertation Abstracts International: Section B: The Sciences and Engineering 1996;56(10-B):5779.
Potts 2020 {published data only}
- Potts S, Krafft J, Levin ME. A pilot randomized controlled trial of acceptance and commitment therapy guided self-help for overweight and obese adults high in weight self-stigma. Behavior Modification 2022;46(1):178-201. [DOI: 10.1177/0145445520975112] [DOI] [PubMed] [Google Scholar]
Sampaio 2019 {published data only}
- Sampaio C, Magnavita G, Ladeia AM. Effect of healing meditation on weight loss and waist circumference of overweight and obese women: randomized blinded clinical trial. Journal of Alternative and Complementary Medicine 2019;25(9):930-7. [DOI: 10.1089/acm.2019.0092] [DOI] [PubMed] [Google Scholar]
- Sampaio CVS, Magnavita G, Ladeia AM. Effect of healing meditation on stress and eating behavior in overweight and obese women: a randomized clinical trial. Complementary Therapies in Clinical Practice 2021;45:101468. [DOI: 10.1016/j.ctcp.2021.101468] [DOI] [PubMed] [Google Scholar]
Spatola 2014 {published data only}
- Spatola CA, Manzoni GM, Castelnuovo G, Malfatto G, Facchini M, Goodwin CL, et al. The ACTonHEART study: rationale and design of a randomized controlled clinical trial comparing a brief intervention based on acceptance and commitment therapy to usual secondary prevention care of coronary heart disease. Health and Quality of Life Outcomes 2014;12:22. [DOI: 10.1186/1477-7525-12-22] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spears 2019 {published data only}
- Spears CA, Abroms LC, Glass CR, Hedeker D, Eriksen MP, Cottrell-Daniels C, et al. Mindfulness-based smoking cessation enhanced with mobile technology (iQuit Mindfully): pilot randomized controlled trial. JMIR Mhealth Uhealth 2019;7(6):e13059. [DOI: 10.2196/13059] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tacon 2003 {published data only}
- Tacón AM, McComb J, Caldera Y, Randolph P. Mindfulness meditation, anxiety reduction, and heart disease: a pilot study. Fam Community Health 2003;26(1):25-33. [DOI: 10.1097/00003727-200301000-00004] [DOI] [PubMed] [Google Scholar]
Tapper 2009 {published data only}
- Tapper K, Shaw C, Ilsley J, Hill AJ, Bond FW, Moore L. Exploratory randomised controlled trial of a mindfulness-based weight loss intervention for women. Appetite 2009;52(2):396-404. [DOI: 10.1016/j.appet.2008.11.012] [DOI] [PubMed] [Google Scholar]
Tedder 2015 {published data only}
- Tedder M, Shi L, Si M, Franco R, Chen L. eMindfulness therapy—a study on efficacy of blood pressure and stress control using mindful meditation and eating apps among people with high blood pressure. Medicines 2015;2:298-309. [DOI: 10.3390/medicines2040298] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vala 2016 {published data only}
- Vala M, Razmandeh R, Rambod K, Nasli Esfahani E, Ghodsi Ghasemabadi R. Mindfulness-based stress reduction group training on depression, anxiety, stress, self-confidence and hemoglobin A1C in young women with type 2 diabetes. Iranian Journal of Endocrinology and Metabolism 2016;17(5):382-90. [Google Scholar]
Zervos 2021 {published data only}
- Zervos K, Skopeliti N, Koletsi M, Mantzios M, Tsitsas G, Naska A. An eight-week mindful eating program applied in a mediterranean population with overweight or obesity: The EATT Intervention Study. Psychological Reports 2022;125(2):1011-40. [DOI: 10.1177/0033294120988104] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Araujo 2021 {published data only}
- Araujo MS, Silva LG, Pereira GMA, Pinto NF, Costa FM, Moreira L, et al. Mindfulness-based treatment for smoking cessation: a randomized controlled trial. Jornal Brasileiro de Pneumologia 2021;47(6):e20210254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Babak 2022 {published data only}
- Babak A, Motamedi N, Mousavi SZ, Ghasemi Darestani N. Effects of mindfulness-based stress reduction on blood pressure, mental health, and quality of life in hypertensive adult women: a randomized clinical trial study. Journal of Tehran Heart Center 2022;17(3):127-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bahadori 2022 {published data only}
- Bahadori A, Golkar MK, Pashang S. The effectiveness of schema therapy intervention in weight, body image and self-esteem of obese people: a randomized controlled trial. Hormozgan Medical Journal 2022;26(2):82-7. [Google Scholar]
Bynum 1980 {published data only}
- Bynum JL. Christian Meditation and Biofeedback Training as Psychotherapeutic Agents in the Treatment of Essential Hypertension [Education Doctorate). Fort Worth, Texas: Southwestern Baptist Theological Seminary, 1980. [Google Scholar]
Davazdah Emamy 2018 {published data only}
- Davazdah Emamy MH, Kharatzadeh H, Bakhtiari M, Mahaki B. Effectiveness of mindfulness-based stress reduction on the quality of life of patients with type II diabetes mellitus. Journal of Diabetic Nursing 2018;6(4):607-17. [Google Scholar]
de la Fuente 2010 {published data only}
- la Fuente M, Franco C, Salvador M. Reduction of blood pressure in a group of hypertensive teachers through a program of mindfulness meditation [Reducción de la presión arterial en un grupo de docentes hipertensos mediante un programa de entrenamiento en conciencia plena (mindfulness)]. Behavioral Psychology 2010;18(3):533–52. [Google Scholar]
DiNardo 2022 {published data only}
- DiNardo MM, Greco C, Phares AD, Beyer NM, Youk AO, Obrosky DS, et al. Effects of an integrated mindfulness intervention for veterans with diabetes distress: a randomized controlled trial. BMJ Open Diabetes Research & Care 2022;10(2):e002631. [DOI: 10.1136/bmjdrc-2021-002631] [DOI] [PMC free article] [PubMed] [Google Scholar]
DRKS00014929 {published data only}
- DRKS00014929. Mindfulness-based intervention and smoking in prisons: a randomized controlled trial. https://trialsearch.who.int/Trial2.aspx?TrialID=DRKS00014929 (first received 4 December 2018).
Duraimani 2015 {published data only}
- Duraimani S, Schneider RH, Randall OS, Nidich SI, Xu S, Ketete M, et al. Effects of lifestyle modification on telomerase gene expression in hypertensive patients: a pilot trial of stress reduction and health education programs in African Americans. PLOS One 2015;10(11):e0142689. [DOI: 10.1371/journal.pone.0142689] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00681200. Mechanisms of meditation in hypertension in blacks. http://clinicaltrials.gov/NCT00681200 (first received 21 May 2008).
IRCT20180205038630N5 {published data only}
- IRCT20180205038630N5. The effectiveness of the mindful self-compassion therapy on psychological symptoms of cardiovascular diseases. https://trialsearch.who.int/Trial2.aspx?TrialID=IRCT20180205038630N5 (first received 18 November 2020).
Jassemi Zergani 2021 {published data only}
- Jassemi Zergani M, Seirafi M, Taghdisi M, Malihi Zuckerini S, Taghavi-Kojeidi H. Evaluation of effectiveness of integration of mindfulness-based-eating awareness training and implementation intention model on body mass index, waist circumference, mindfulness eating, and physical activity in obese women [Persian]. Iranian Journal of Health Education and Health Promotion 2021;9(1):94-109. [Google Scholar]
Khosravi 2016 {published data only}
- Khosravi E, Ghorbani M. Effectiveness of mindfulness based stress reduction on perceived stress and blood pressure among the hypertensive women. Feyz, Journal of the Kashan University of Medical Sciences 2016;20(4):361-8. [Google Scholar]
Li 2018 {published data only}
- Li H, Zhao L, Wang Q. Influence of mindfulness behavior combined with virtual environment training on rehabilitation of hemiplegia in stroke patients. Chinese Nursing Research 2018;32(15):2438-40. [Google Scholar]
Marwaha 2020 {published data only}
- Marwaha K. Mind-body medicine in cardiovascular health: mechanisms and clinical outcomes of transcendental meditation in primary and secondary prevention of cardiovascular disease. Dissertation Abstracts International: Section B: The Sciences and Engineering 2020;81(3-B).
Morillo‐Sarto 2023 {published data only}
- Morillo Sarto H, Barcelo-Soler A, Herrera-Mercadal P, Pantilie B, Navarro-Gil M, Garcia-Campayo J, et al. Efficacy of a mindful-eating programme to reduce emotional eating in patients suffering from overweight or obesity in primary care settings: a cluster-randomised trial protocol. BMJ Open 2019;9(11):e031327. [DOI: 10.1136/bmjopen-2019-031327; CRS ID: 23512639] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morillo-Sarto H, López-Del-Hoyo Y, Pérez-Aranda A, Modrego-Alarcón M, Barceló-Soler A, Borao L, et al. 'Mindful eating' for reducing emotional eating in patients with overweight or obesity in primary care settings: a randomized controlled trial. European Eating Disorders Review 2023;31(2):303-19. [DOI: 10.1002/erv.2958] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03927534. Efficacy of a mindful-eating program to reduce emotional eating. https://clinicaltrials.gov/ct2/show/NCT03927534 (first received 25 April 2019). 23512640
NCT00010738 {published data only}
- NCT00010738. Effects of meditation on mechanism of coronary heart disease. https://clinicaltrials.gov/ct2/show/NCT00010738 (first received 5 February 2001).
NTR4968 {published data only}
- NTR4968. Breaking the habit: the effect of mindful eating on the processing of rewards. https://trialsearch.who.int/Trial2.aspx?TrialID=NTR4968 2015;(first received 20 January 2015).
Pirmoradi 2022 {published data only}
- Pirmoradi MR, Asgharzadeh A, Birashk B, Gharraee B, Salehian R, Reza Ostadrahimi A. Acceptance, mindfulness, and compassionate-based intervention in overweight and obese women and its effect on metabolic syndrome components: a randomized controlled trial. Crescent Journal of Medical and Biological Sciences 2022 Dec 7 [Epub ahead of print]. [DOI: 10.34172/cjmb.2023.25] [DOI]
Rudlof 2022 {published data only}
- Rudlof ME, Šimunić B, Steuber B, Bartel TO, Neshev R, Mächler P, et al. Effects of meditation on cardiovascular and muscular responses in patients during cardiac rehabilitation: a randomized pilot study. Journal of Clinical Medicine 2022;11(20):6143. [DOI: 10.3390/jcm11206143] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salvo 2022 {published data only}
- NCT02893150. Mindfulness intervention for overweight primary care patients (MindEat). https://clinicaltrials.gov/ct2/show/NCT02893150 (first received 8 September 2016). 19985602
- Salvo V, Curado DF, Sanudo A, Kristeller J, Schveitzer MC, Favarato ML, et al. Comparative effectiveness of mindfulness and mindful eating programmes among low-income overweight women in primary health care: A randomised controlled pragmatic study with psychological, biochemical, and anthropometric outcomes. Appetite 2022;177:106131. [DOI: 10.1016/j.appet.2022.106131] [DOI] [PubMed] [Google Scholar]
Sogol 2020 {published data only}
- Sogol S, Shaban H, Kazem FM. Comparison of the effectiveness of mindfulness and psychological well-being education on anxiety and self-care behaviors in patients with type II diabetes. Journal of Diabetic Nursing 2020;8(3):1137-49. [Google Scholar]
Yanhong 2020 {published data only}
- Yanhong GE, Lihong YU, Yue Z, Xuan LI, Lihui Z, Shuo K, et al. Curative effects of mindfulness training based on "meta-awareness" combined with intermittent pneumatic compression therapy on TcPO2,distress, mindful attention awareness and blood glucose in diabetic patients with lower extremity arterial disease. Chinese Nursing Research 2020;34(7):574-9. [DOI: 10.12102/j.issn.1009-6493.2020.04.002] [DOI] [Google Scholar]
Zhang 2018 {published data only}
- Zhang Y, Xu L, Zhao Y. Impact of focused meditation on anxiety levels and sleep quality in elderly patients with hypertension. Chinese Nursing Research 2018;32(13):2137-40. [Google Scholar]
References to ongoing studies
ACTRN12618000844246 {published data only}
- ACTRN12618000844246. ImpleMENTing meditatiOn into heart disease clinical settings (The MENTOR Study). https://anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12618000844246 (first received 18 May 2018).
ACTRN12618001247268 {published data only}
- ACTRN12618001247268. Compassion focused therapy as a treatment for body weight shame associated with obesity. https://anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12618001247268 (first received 24 July 2018).
ACTRN12620000105943 {published data only}
- ACTRN12620000105943. Support After Stroke with group-based classeS: The SASS study. https://trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12620000105943 (first received 5 February 2020).
ACTRN12621000445875 {published data only}
- ACTRN12621000445875. Investigating the effect of cognitive, behavioural and mindfulness-based interventions on smoking rates in lower socio-economic groups. https://trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12621000445875 (first received 19 April 2021).
ACTRN12621000580875 {published data only}
- ACTRN12621000580875. Self-compassion for weight management: an online intervention for adults seeking to manage weight. https://trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12621000580875 (first received 17 May 2021).
Asfar 2021 {published data only}
- Asfar T, Koru-Sengul T, Annane D, McClure LA, Perez A, Antoni MA, et al. Reach versus effectiveness: the design and protocol of randomized clinical trial testing a smartphone application versus in-person mindfulness-based smoking cessation intervention among young cancer survivors. Contemporary Clinical Trials Communications 2021;22:100784. [DOI: 10.1016/j.conctc.2021.100784] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04038255. Mindfulness based smoking cessation among cancer survivors. https://clinicaltrials.gov/ct2/show/NCT04038255 (first received 26 July 2019).
Chandra 2020 {published data only}
- Chandra M, Raveendranathan D, Pradeep RJ, Patra S, Rushi, Prasad K, et al. Managing depression in diabetes mellitus: a multicentric randomized controlled trial comparing effectiveness of fluoxetine and mindfulness in primary care: protocol for DIAbetes Mellitus ANd Depression (DIAMAND) Study. Indian Journal of Psychological Medicine 2020;42(6 Suppl):S31-8. [DOI: 10.1177/0253717620971200] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chung 2019 {published data only}
- Chung CY, Lenon G, Yang A, De Foe A. Electroacupuncture combined with mindfulness meditation for weight management: a randomised sham controlled trial protocol. Obesity Research & Clinical Practice 2019;13(3):304-5. [DOI: 10.1016/j.orcp.2018.11.194] [DOI] [Google Scholar]
DRKS00021412 {published data only}
- DRKS00021412. Self-compassion, eating behavior & dieting success. https://trialsearch.who.int/Trial2.aspx?TrialID=DRKS00021412 (first received 15 April 2020).
Forman 2021 {published data only}
- Forman EM, Chwyl C, Berry MP, Taylor LC, Butryn ML, Coffman DL, et al. Evaluating the efficacy of mindfulness and acceptance-based treatment components for weight loss: protocol for a multiphase optimization strategy trial. Contemporary Clinical Trials 2021;110:106573. [DOI: 10.1016/j.cct.2021.106573] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04337619. Project activate: mindfulness and acceptance based behavioral treatment for weight loss. https://clinicaltrials.gov/ct2/show/NCT04337619.
Guerrini Usubini 2021 {published data only}
- Guerrini Usubini A, Cattivelli R, Giusti EM, Riboni FV, Varallo G, Pietrabissa G, et al. The ACTyourCHANGE study protocol: promoting a healthy lifestyle in patients with obesity with acceptance and commitment therapy-a randomized controlled trial. Trials 2021;22(1):290. [DOI: 10.1186/s13063-021-05191-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04474509. ACTyourCHANGE study protocol. Promoting healthy lifestyle with ACT for obesity. https://clinicaltrials.gov/ct2/show/NCT04474509 (first received 4 July 2020).
Hemenway 2021 {published data only}
- Hemenway M, Witkiewitz K, Unrod M, Brandon KO, Brandon TH, Wetter DW, et al. Development of a mindfulness-based treatment for smoking cessation and the modification of alcohol use: a protocol for a randomized controlled trial and pilot study findings. Contemporary Clinical Trials 2021;100:106218. [DOI: 10.1016/j.cct.2020.106218] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03734666. Development of a mindfulness-based treatment for the reduction of alcohol use and smoking cessation. https://clinicaltrials.gov/ct2/show/NCT03734666 (first received 4 July 2020).
IRCT20150519022320N14 {published data only}
- IRCT20150519022320N14. Comparative investigating the effect of relaxation and meditation techniques on quality of life in patients with coronary artery disease. http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20150519022320N14 (first received 28 October 2018).
IRCT2015122825739N1 {published data only}
- IRCT2015122825739N1. Psychological interventions in weight loss. http://www.who.int/trialsearch/trial2.aspx?Trialid=IRCT2015122825739N1 (first received 1 September 2017).
IRCT20190804044436N1 {published data only}
- IRCT20190804044436N1. Effect of mindfulness-based stress management therapy on the emotion regulation, anxiety, depression and food addiction in obese people. https://trialsearch.who.int/Trial2.aspx?TrialID=IRCT20190804044436N1 (first received 21 September 2019).
IRCT20200225046618N1 {published data only}
- IRCT20200225046618N1. Comparing the compassion-focused therapy and dialectical behavior therapy training on state and trait anxiety symptoms and impulsivity in patients with coronary heart disease. https://trialsearch.who.int/Trial2.aspx?TrialID=IRCT20200225046618N1 (first received 15 December 2019).
IRCT20200226046625N1 {published data only}
- IRCT20200226046625N1. Comparison of the effectiveness of cognitive-behavioral therapy based on mindfulness and education on health promoting lifestyle in in treatment of diabetes. https://trialsearch.who.int/Trial2.aspx?TrialID=IRCT20200226046625N1 (first received 15 December 2019).
IRCT20200305046699N1 {published data only}
- IRCT20200305046699N1. The effectiveness of group therapy based on mindfulness and cognitive-behavioral therapy (CBT) in the anxiety, metabolic control and quality of life in type 2 diabetic patients. https://trialsearch.who.int/Trial2.aspx?TrialID=IRCT20200305046699N1 (first received 7 January 2020).
JPRN‐jRCT1030200197 {published data only}
- JPRN-jRCT1030200197. Online mindfulness-based eating awareness training for obesity. https://trialsearch.who.int/Trial2.aspx?TrialID=JPRN-Jrct1030200197 (first received 12 November 2020).
JPRN‐UMIN000030444 {published data only}
- JPRN-UMIN000030444. Effects of a mindfulness-based intervention versus cognitive behavioral therapy on weight loss and weight maintenance for women with overweight or obesity: a randomized controlled trial. https://trialsearch.who.int/?TrialID=JPRN-UMIN000030444 (first received 19 December 2017).
JPRN‐UMIN000042260 {published data only}
- JPRN-UMIN000042260. Effects of a mindfulness-based eating awareness training online intervention in adults of obesity: a randomized controlled trial. https://trialsearch.who.int/Trial2.aspx?TrialID=JPRN-UMIN000042260 (first received 28 October 2020).
JPRN‐UMIN000042626 {published data only}
- JPRN-UMIN000042626. An exploratory study of the mindfulness app for weight loss in metabolic syndrome. https://trialsearch.who.int/Trial2.aspx?TrialID=JPRN-UMIN000042626 (first received 31 March 2021).
Martorella 2021 {published data only}
- Martorella G, Hanley AW, Pickett SM, Gelinas C. Web- and mindfulness-based intervention to prevent chronic pain after cardiac surgery: protocol for a pilot randomized controlled trial. JMIR Research Protocols 2021;10(8):e30951. [DOI: 10.2196/30951] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mason 2019 {published data only}
- Mason AE, Saslow L, Moran PJ, Kim S, Wali PK, Abousleiman H, et al. Examining the effects of mindful eating training on adherence to a carbohydrate-restricted diet in patients with type 2 diabetes (the DELISH Study): protocol for a randomized controlled trial. JMIR Research Protocols 2019;8(2):e11002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03207711. Delish study: diabetes education to lower insulin, sugars, and hunger (Delish). https://clinicaltrials.gov/ct2/show/NCT03207711 (first received 5 July 2017).
NCT00224835 {published data only}
- NCT00224835. Mindfulness-based stress reduction and myocardial ischemia. https://clinicaltrials.gov/ct2/show/NCT00224835 (first received 23 September 2005).
NCT01227473 {published data only}
- NCT01227473. We can prevent diabetes: a behavioral intervention to reduce diabetes risk in African Americans. https://clinicaltrials.gov/ct2/show/NCT01227473 (first received 25 October 2010).
NCT01375504 {published data only}
- NCT01375504. Effect of self regulation with mindfulness training on body mass index and cardiovascular risk markers in obese adults. https://clinicaltrials.gov/ct2/show/NCT01375504 (first received 17 June 2011).
NCT01805245 {published data only}
- NCT01805245. Mindfulness: a novel approach for the management of diabetes-related distress. https://clinicaltrials.gov/ct2/show/NCT01805245 (first received 6 March 2013).
NCT02037360 {published data only}
- NCT02037360. Mobile mindfulness training for smoking cessation. https://clinicaltrials.gov/ct2/show/NCT02037360 (first received 15 January 2014).
NCT02165228 {published data only}
- NCT02165228. Strategies for inflammation and cardiovascular disease (CVD) prevention (SICVDP). https://clinicaltrials.gov/ct2/show/NCT02165228 (first received 17 June 2014).
NCT02753972 {published data only}
- NCT02753972. Mindful eating and living for obese women (MEAL). https://clinicaltrials.gov/ct2/show/NCT02753972 (first received 28 April 2016).
NCT02830529 {published data only}
- NCT02830529. Mindfulness Attitude to Deliver Dietary Approach to Stop Hypertension (MADDASH). https://clinicaltrials.gov/ct2/show/NCT02830529 (first received 13 July 2016).
NCT03013907 {published data only}
- NCT03013907. Emotional Awareness and SElf-regulation for depression in patients with hypertension (EASE) study. https://clinicaltrials.gov/ct2/show/NCT03013907 (first received 9 January 2017).
NCT03020316 {published data only}
- NCT03020316. Peer mentored approaches for men and women with coronary artery disease ("4Steps"). https://clinicaltrials.gov/ct2/show/NCT03020316 (first received 13 January 2017).
NCT03131128 {published data only}
- NCT03131128. The effectiveness of mindfulness-based intervention as a workplace health promotion program on weight loss. https://clinicaltrials.gov/ct2/show/NCT03131128 (first received 27 April 2017).
NCT03256890 {published data only}
- NCT03256890. Mindfulness-based blood pressure reduction: stage 2a randomized controlled trial. https://clinicaltrials.gov/ct2/show/NCT03256890 (first received 22 August 2017).
NCT03274362 {published data only}
- NCT03274362. Evaluating the effectiveness of headspace mindfulness application versus standard care on the HbA1C and quality of life in patients with diabetes: a randomized control trial. https://clinicaltrials.gov/ct2/show/NCT03274362 (first received 6 September 2017).
NCT03368950 {published data only}
- NCT03368950. Online mindfulness for stroke sufferers. https://clinicaltrials.gov/ct2/show/NCT03368950 (first received 11 December 2017).
NCT03373110 {published data only}
- NCT03373110. Healthy hearts healthy minds: a PPRN demonstration pragmatic trial. https://clinicaltrials.gov/ct2/show/NCT03373110 (first received 14 December 2017).
NCT03480113 {published data only}
- NCT03480113. The effectiveness of mindfulness-based intervention on smoking cessation. https://clinicaltrials.gov/ct2/show/NCT03480113 (first received 29 March 2018).
NCT03488966 {published data only}
- NCT03488966. Mindfulness based eating awareness training for bariatric surgery patients (MB-EAT). https://clinicaltrials.gov/ct2/show/NCT03488966 (first received 5 April 2018).
NCT03659409 {published data only}
- NCT03659409. Investigating the impact of mindfulness on the physiological and psychological well-being of stroke survivors and their caregivers. https://clinicaltrials.gov/ct2/show/NCT03659409 (first received 6 September 2018).
NCT03736434 {published data only}
- NCT03736434. Brain connections and blood pressure. https://clinicaltrials.gov/ct2/show/NCT03736434 (first received 9 November 2018).
NCT03793855 {published data only}
- NCT03793855. Effectiveness of a nutritional strategy for glycemic control in patients with type 2 diabetes mellitus users of a public health system: NUGLIC study. https://clinicaltrials.gov/ct2/show/NCT03793855 (first received 4 January 2019).
NCT03793881 {published data only}
- NCT03793881. Nutritional strategy for blood pressure control in patients with hypertension (NUPRESS). https://clinicaltrials.gov/ct2/show/NCT03793881 (first received 4 January 2019).
NCT03826836 {published data only}
- NCT03826836. Effectiveness of mindfulness-based stress reduction for improving quality of life in patients with cardiovascular disease: a randomised controlled trial. https://clinicaltrials.gov/ct2/show/NCT03826836 (first received 1 February 2019).
NCT03837405 {published data only}
- NCT03837405. Optimizing lifestyle interventions with mindfulness-based strategies in type 2 diabetes. https://clinicaltrials.gov/ct2/show/NCT03837405 (first received 12 February 2019).
NCT03859076 {published data only}
- NCT03859076. UH3 Phase - mindfulness-based blood pressure reduction (MB-BP): stage 2a RCT (MB-BP). https://clinicaltrials.gov/ct2/show/NCT03859076 (first received 1 March 2019).
NCT03910855 {published data only}
- NCT03910855. Impact of mindfulness on psychological well-being of stroke survivors and their caregivers (SOMII). https://clinicaltrials.gov/ct2/show/NCT03910855 (first received 10 April 2019).
NCT04016415 {published data only}
- NCT04016415. Decreasing stress in diabetes: a randomized controlled trial. https://clinicaltrials.gov/ct2/show/NCT04016415 (first received 11 July 2019).
NCT04171713 {published data only}
- NCT04171713. Mindfulness and compassion-based programs on food behavior of patients with weight regain after bariatric surgery. https://clinicaltrials.gov/ct2/show/NCT04171713 (first received 21 November 2019).
NCT04302493 {published data only}
- NCT04302493. Mindfulness based stress reduction and post-stroke cognition. https://clinicaltrials.gov/ct2/show/NCT04302493 (first received 10 March 2020).
NCT04759950 {published data only}
- NCT04759950. Exercise the mind and brain. A multimodal intervention in stroke. https://clinicaltrials.gov/show/NCT04759950 (first received 18 February 2021).
NCT04799899 {published data only}
- NCT04799899. MBCT via group videoconferencing for acute coronary syndrome patients with depressive symptoms. https://clinicaltrials.gov/show/NCT04799899 (first received 16 March 2021).
NCT04847843 {published data only}
- NCT04847843. Eating mindfully to prevent weight regain. https://ClinicalTrials.gov/show/NCT04847843 (first received 19 April 2021).
NCT04965181 {published data only}
- NCT04965181. Mindfulness-based smoking cessation enhanced with mobile technology. https://clinicaltrials.gov/show/NCT04965181 (first received 16 July 2021).
NCT04985838 {published data only}
- NCT04985838. Helping ease anxiety and depression following stroke stage 3. https://clinicaltrials.gov/show/NCT04985838 (first received 2 August 2021).
NCT05070949 {published data only}
- NCT05070949. Self-compassion to reduce diabetes distress in persons with type 1 diabetes. https://clinicaltrials.gov/show/NCT05070949 (first received 7 October 2021).
RBR‐458tbcd {published data only}
- RBR-458tbcd. Effects of an intervention based on eating with full attention in patients who underwent stomach reduction surgery and regained weight: a randomized clinical trial. https://trialsearch.who.int/Trial2.aspx?TrialID=RBR-458tbcd (first received 5 March 2021).
Ruffault 2016 {published data only}
- NCT02571387. MindOb: a 12-month computerized mindfulness-based intervention for obese individuals (MindOb). https://clinicaltrials.gov/ct2/show/NCT02571387 (first received 8 October 2015).
- Ruffault A, Carette C, Lurbe I, Puerto K, Juge N, Beauchet A, et al. Randomized controlled trial of a 12-month computerized mindfulness-based intervention for obese patients with binge eating disorder: the MindOb study protocol. Contemporary Clinical Trials 2016;49:126-33. [DOI: 10.1016/j.cct.2016.06.012] [DOI] [PubMed] [Google Scholar]
SLCTR/2021/015 {published data only}
- SLCTR/2021/015. Glycemic control in patients with type 2 diabetes mellitus following mindfulness meditation compared to standard therapy. https://trialsearch.who.int/Trial2.aspx?TrialID=SLCTR/2021/015 (first received 1 July 2021).
Spruill 2018 {published data only}
- NCT02914483. Women's heart attack research program: stress ancillary study; telephone-based stress management for women with myocardial infarction. https://clinicaltrials.gov/ct2/show/NCT02914483 (first received 26 September 2016).
- Spruill TM, Reynolds HR, Dickson VV, Shallcross AJ, Visvanathan PD, Park C, et al. Telephone-based mindfulness training to reduce stress in women with myocardial infarction: rationale and design of a multicenter randomized controlled trial. American Heart Journal 2018;202:61-7. [DOI: 10.1016/j.ahj.2018.03.028] [DOI] [PMC free article] [PubMed] [Google Scholar]
TCTR20210406004 {published data only}
- TCTR20210406004. The effect of walking meditation on peripheral neuropathy in diabetes type 2. https://trialsearch.who.int/Trial2.aspx?TrialID=TCTR20210406004 (first received 31 March 2021).
TCTR20210417002 {published data only}
- TCTR20210417002. Effects on a mindfulness meditation for decreasing blood pressure. https://trialsearch.who.int/Trial2.aspx?TrialID=TCTR20210417002 (first received 17 April 2021).
Wheelwright 2017 {published data only}
- Wheelwright DS, Dangayach N, Griffiths S, Gordon E, Bederson J, Kellner C, et al. Does intra-ICU initiation of guided mindfulness meditation decrease anxiety and depression in SAH?: A unique methodology for the neurocritical care setting. Neurology 2017;88(16 Suppl):P5.069. [Google Scholar]
Additional references
Abbott 2014
- Abbott RA, Whear R, Rodgers LR, Bethel A, Thompson CJ, Kuyken W, et al. Effectiveness of mindfulness-based stress reduction and mindfulness based cognitive therapy in vascular disease: A systematic review and meta-analysis of randomised controlled trials. Journal of Psychosomatic Research 2014;76(5):341-51. [DOI] [PubMed] [Google Scholar]
AHA 2017
- Levine GN, Lange RA, Bairey-Merz CN, Davidson RJ, Jamerson K, Mehta PK, et al. Meditation and cardiovascular risk reduction:a scientific statement from the American Heart Association. Journal of the American Heart Association 2017;6(10):e002218. [DOI: 10.1161/JAHA.117.002218] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 2008
- Anderson JW, Liu C, Kryscio RJ. Blood pressure response to transcendental meditation: a meta-analysis. American Journal of Hypertension 2008;21(3):310–6. [DOI] [PubMed] [Google Scholar]
Beck 1988
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. Journal of Consultulting and Clinical Psychology 1988;56(6):893–7. [DOI] [PubMed] [Google Scholar]
Beck 1996
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory - Second Edition Manual. San Antonio (TX): The Psychological Corporation, 1996. [Google Scholar]
Bersch‐Ferreira 2021
- Bersch-Ferreira AC, Weber B, da Silva LR, Torreglosa CR, Bueno PRT, da Silva JGST, et al. Mindfulness practice for glycemic control: Could it be a new strategy for an old problem? A systematic review and meta-analysis. Current Diabetes Reviews 2021;17(7):e072620184137. [DOI: 10.2174/1573399816666200810131055] [DOI] [PubMed] [Google Scholar]
Blacker 2009
- Blacker M, Meleo-Meyer F, Kabat-Zinn J, Santorelli SF. Mindfulness-Based Stress Reduction (MBSR) Curriculum Guide. Worcester, Mass: Center for Mindfulness in Medicine, Health Care, and Society, University of Massachusetts Medical School, 2009. [Google Scholar]
Blom 2014
- Blom K, Baker B, How M, Dai M, Irvine J, Abbey S, et al. Hypertension analysis of stress reduction using mindfulness meditation and yoga: results from the HARMONY randomized controlled trial. American Journal of Hypertension 2014;27(1):122-9. [DOI] [PubMed] [Google Scholar]
Bowen 2011
- Bowen S, Chawla N, Marlatt GA. Mindfulness-Based Relapse Prevention for Addictive Behaviors: A Clinician’s Guide. New York, NY: Guilford Press, 2011. [Google Scholar]
Breathworks 2019
- Breathworks. What is mindfulness? https://www.breathworks-mindfulness.org.uk/what-is-mindfulness (accessed 12 June 2019).
Burch 2013
- Burch V, Penman D. Mindfulness for Health: a practical guide to relieving pain, reducing stress and restoring wellbeing. London: Piatkus, 2013. [Google Scholar]
Canter 2004
- Canter PH, Ernst E. Insufficient evidence to conclude whether or not transcendental meditation decreases blood pressure: results of a systematic review of randomized clinical trials. Journal of Hypertension 2004;22(11):2049-54. [DOI] [PubMed] [Google Scholar]
Chew 2017
- Chew BH, Vos RC, Metzendorf MI, Scholten RJPM, Rutten GEHM. Psychological interventions for diabetes-related distress in adults with type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2017, Issue 9. Art. No: CD011469. [DOI: 10.1002/14651858.CD011469.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Conversano 2021
- Conversano C, Orru G, Pozza A, Miccoli M, Ciacchini R, Marchi L, et al. Is mindfulness-based stress reduction effective for people with hypertension? A systematic review and meta-analysis of 30 years of evidence. International Journal of Environmental Research and Public Health 2021;18(6):2882. [DOI: 10.3390/ijerph18062882] [DOI] [PMC free article] [PubMed] [Google Scholar]
Crane 2017
- Crane RS, Brewer J, Feldman C, Kabat-Zinn J, Santorelli S, Williams JMG, et al. What defines mindfulness-based programs? The warp and the weft. Psychological Medicine 2017;47(6):990–9. [DOI] [PubMed] [Google Scholar]
Deeks 2022
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook 2022.
DHDSP 2016
- Division for Heart Disease and Stroke Prevention (DHDSP). Heart failure fact sheet. Last updated June 2016. www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_heart_failure.htm (accessed 26 April 2018).
Dimsdale 2008
- Dimsdale JE. Psychological stress and cardiovascular disease. Journal of the American College of Cardiology 2008;51(13):1237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dragano 2017
- Dragano N, Siegrist J, Nyberg ST, Lunau T, Fransson EI, Alfredsson L, et al. Effort–reward imbalance at work and incident coronary heart disease a multicohort study of 90,164 individuals. Epidemiology 2017;28(4):619–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eikelenboom 2015
- Eikelenboom N, Smeele I, Faber M, Jacobs A, Verhulst F, Lacroix J, et al. Validation of Self-Management Screening (SeMaS) a tool to facilitate personalised counselling and support of patients with chronic diseases. BMC Family Practice 2015;16:165. [DOI: 10.1186/s12875-015-0381-z.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Faude‐Lang 2010
- Faude-Lang V, Hartmann M, Schmidt EM, Humpert P, Nawroth P, Herzog W. Acceptance- and mindfulness-based group intervention in advanced type 2 diabetes patients: therapeutic concept and practical experiences [in German]. Psychotherapie, Psychosomatik, Medizinische Psychologie 2010;60:185–9. [DOI] [PubMed] [Google Scholar]
Fjorback 2011
- Fjorback LO, Arendt M, Ørnbøl E, Fink P, Walach H. Mindfulness-based stress reduction and mindfulness-based cognitive therapy: a systematic review of randomized controlled trials. Acta Psychiatrica Scandinavica 2011;124(2):102–19. [DOI] [PubMed] [Google Scholar]
Follman 1992
- Follmann D, Elliot P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45(7):769-73. [DOI] [PubMed] [Google Scholar]
Fox 2001
- Fox KF, Cowie MR, Wood DA, Coats AJ, Gibbs JS, Underwood SR, et al. Coronary artery disease as the cause of incident heart failure in the population. European Heart Journal 2001;22(3):228–36. [DOI] [PubMed] [Google Scholar]
Fulwiler 2015
- Fulwiler C, Brewer JA, Sinnott S, Loucks EB. Mindfulness-based interventions for weight loss and CVD risk management. Current Cardiovascular Risk Reports 2015;9(10):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gok Metin 2018
- Gok Metin Z, Ejem D, Dionne-Odom JN, Turkman Y, Salvador C, Pamboukian S, et al. Mind-body interventions for individuals with heart failure: a systematic review of randomized trials. Journal of Cardiac Failure 2018;24(3):186-201. [DOI] [PubMed] [Google Scholar]
Goyal 2014
- Goyal M, Singh S, Sibinga EMS, Gould NF, Rowland-Seymour A, Sharma R, et al. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Internal Medicine 2014;174(3):357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEPro GDT 2015 [Computer program]
- GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Harris 2021
- Harris R. Acceptance and Commitment Therapy. https://www.actmindfully.com.au/about-act/ (accessed 12 July 2021).
Hennessey 2016
- Hennessey G. The little mindfulness workbook: everyday techniques to help you combat stress and enhance your life. Bath: Crimson Publishing, 2016. [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook. [DOI] [PMC free article] [PubMed]
Higgins 2022
- Higgins JP, Eldridge S, Li T, editor(s). Chapter 23: Including variants on randomized trials. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Janssen 2018
- Janssen M, Heerkens Y, Kuijer W, Heijden B, Engels J. Effects of mindfulness-based stress reduction on employees’ mental health: a systematic review. PLOS One 2018;13(1):e0191332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kabat‐Zinn 1986
- Kabat-Zinn J, Lipworth L, Burney R, Sellers W. Four year follow up of a meditation based program for self regulation of chronic pain: treatment outcomes and compliance. Clinical Journal of Pain 1986;2(3):159-73. [Google Scholar]
Kabat‐Zinn 1990
- Kabat-Zinn J. Full catastrophe living: how to cope with stress, pain and illness using mindfulness meditation. London: Piatkus, 1990. [Google Scholar]
Kabat‐Zinn 1992
- Kabat-Zinn J, Massion AO, Kristeller J, Peterson LG, Fletcher K, Pbert L, et al. Effectiveness of a meditation-based stress reduction program in the treatment of anxiety disorders. American Journal of Psychiatry 1992;149(7):936-43. [DOI] [PubMed] [Google Scholar]
Kabat‐Zinn 1994
- Kabat-Zinn J. Wherever You Go There You Are: Mindfulness Meditation in Everyday Life. New York: Hyperion, 1994. [Google Scholar]
Kabat‐Zinn 2013
- Kabat-Zinn J. Full Catastrophe Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain, and Illness. Random House Publishing Group, 2013. [Google Scholar]
Khoury 2013
- Khoury B, Lecomte T, Fortin G, Masse M, Therien P, Bouchard V, et al. Mindfulness-based therapy: a comprehensive meta-analysis. Clinical Psychology Review 2013;33(6):763–71. [DOI] [PubMed] [Google Scholar]
Khoury 2015
- Khoury B, Sharma M, Rush SE, Fournier C. Mindfuless-based stress reduction for healthy individuals: a meta-analysis. Journal of Psychosomatic Research 2015;78(6):519–28. [DOI] [PubMed] [Google Scholar]
Kristeller 2011
- Kristeller JL, Wolever RQ. Mindfulness-based eating awareness training for treating binge eating disorder: the conceptual foundation. Eating Disorders 2011;19(1):49-61. [DOI: 10.1080/10640266.2011.533605] [DOI] [PubMed] [Google Scholar]
Kuper 2003
- Kuper H, Marmot M. Job strain, job demands, decision latitude, and risk of coronary heart disease within the Whitehall II study. Journal of Epidemiology and Community Health 2003;57(2):147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lawrence 2013
- Lawrence M, Booth J, Mercer S, Crawford E. A systematic review of the benefits of mindfulness-based interventions following transient ischemic attack and stroke. International Journal of Stroke 2013;8(6):465-74. [DOI] [PubMed] [Google Scholar]
Lee 2020
- Lee EKP, Yeung NCY, Xu Z, Zhang D, Yu CP, Wong SYS. Effect and acceptability of mindfulness-based stress reduction program on patients with elevated blood pressure or hypertension: a meta-analysis of randomized controlled trials. Hypertension 2020;76(6):1992-2001. [DOI: 10.1161/HYPERTENSIONAHA.120.16160] [DOI] [PubMed] [Google Scholar]
Lefebvre 2019
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019.. Available from www.training.cochrane.org/handbook..
Meditation Trust
- Meditation Trust. The difference between mindfulness and transcendental meditation. www.meditationtrust.com/the-difference-between-mindfulness-and-transcendental-meditation/ (accessed 11 May 2018).
Mercado 2021
- Mercado D, Robinson L, Gordon G, Werthmann J, Campbell IC, Schmidt U. The outcomes of mindfulness-based interventions for obesity and binge eating disorder: a meta-analysis of randomised controlled trials. Appetite 2021;166:105464. [DOI: 10.1016/j.appet.2021.105464] [DOI] [PubMed] [Google Scholar]
Merz 2002
- Merz CNB, Dwyer J, Nordstrom CK, Walton KG, Salerno JW, Schneider RH. Psychosocial stress and cardiovascular disease: pathophysiological links. Behavioural Medicine 2002;27(4):141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Monti 2006
- Monti DA, Peterson C, Kunkel EJ, Hauck WW, Pequignot E, Rhodes L, et al. A randomized, controlled trial of mindfulness-based art therapy (MBAT) for women with cancer. Psychooncology 2006;15(5):363-73. [DOI] [PubMed] [Google Scholar]
Neff 2013
- Neff KD, Germer CK. A pilot study and randomized controlled trial of the mindful self compassion program. Journal of Clinical Psychology 2013;69:28–44. [DOI] [PubMed] [Google Scholar]
Ngan 2021
- Ngan HY, Chong YY, Chien WT. Effects of mindfulness- and acceptance-based interventions on diabetes distress and glycaemic level in people with type 2 diabetes: systematic review and meta-analysis. Diabetic Medicine 2021;38(4):e14525. [DOI: 10.1111/dme.14525] [DOI] [PubMed] [Google Scholar]
Ni 2021
- Ni YX, Ma L, Li JP. Effects of mindfulness-based intervention on glycemic control and psychological outcomes in people with diabetes: a systematic review and meta-analysis. Journal of Diabetes Investigation 2021;12(6):1092-103. [DOI: 10.1111/jdi.13439] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ooi 2017
- Ooi SL, Giovino M, Pak SC. Transcendental meditation for lowering blood pressure: an overview of systematic reviews and meta-analyses. Complementary Therapies in Medicine 2017;34:26-34. [DOI] [PubMed] [Google Scholar]
Orme‐Johnson 2014
- Orme-Johnson DW, Barnes VA. Effects of the transcendental meditation technique on trait anxiety: a meta-analysis of randomized controlled trials. Journal of Alternative and Complementary Medicine 2014;20(5):330-41. [DOI] [PubMed] [Google Scholar]
O’Donnell 2010
- O’Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet 2010;376(9735):112–23. [DOI] [PubMed] [Google Scholar]
Perret 2012
- Perret D. Feelings Are the Pathway to Your Soul: A Selection of Teachings from Bob Moore. Paris: Books on Demand GmBH, 2012. [Google Scholar]
Radloff 1977
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement 1977;1:385–401. [Google Scholar]
Rees 2013
- Rees K, Dyakova M, Ward K, Burke M, Thorogood M, Brunner EJ. Dietary advice for reducing cardiovascular risk. Cochrane Database of Systematic Reviews 2013, Issue 3. Art. No: CD002128. [DOI: 10.1002/14651858.CD002128.pub4] [DOI] [PubMed] [Google Scholar]
Rees 2019
- Rees K, Takeda A, Martin N, Ellis L, Wijesekara D, Vepa A, et al. Mediterranean-style diet for the primary and secondary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews 2019, Issue 3. Art. No: CD009825. [DOI: 10.1002/14651858.CD009825.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rees 2021
- Rees K, Al-Khudairy L, Takeda A, Stranges, S. Vegan dietary pattern for the primary and secondary prevention of cardiovascular diseases. Cochrane Database of Systematic Reviews 2021, Issue 2. Art. No: CD013501. [DOI: 10.1002/14651858.CD013501.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Richards 2017
- Richards SH, Anderson L, Jenkinson CE, Whalley B, Rees K, Davies P, et al. Psychological interventions for coronary heart disease. Cochrane Database of Systematic Reviews 2017, Issue 4. Art. No: CD002902. [DOI: 10.1002/14651858.CD002902.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Richardson 2012
- Richardson S, Shaffer JA, Falzon L, Krupka D, Davidson KW, Edmondson D. Meta-analysis of perceived stress and its association with incident coronary heart disease. American Journal of Cardiology 2012;110(12):1711–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosengren 2004
- Rosengren A, Hawken S, Ounpuu S, Sliwa K, Zubaid M, Almahmeed WA, et al. Association of psychosocial risk factors with risk of acute myocardial infarction in 11,119 cases and 13,646 controls from 52 countries (the INTERHEART study): case-control study. Lancet 2004;364(9438):953–62. [DOI] [PubMed] [Google Scholar]
Roth 1994
- Roth R. Maharishi Mahesh Yogi’s Transcendental Meditation. Washington DC: Primus, 1994. [Google Scholar]
Roth 2018
- Roth B. Strength in Stillness: The Power of Transcendental Meditation. New York: Simon, 2018. [Google Scholar]
Rozanski 1999
- Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation 1999;99(16):2192-217. [DOI] [PubMed] [Google Scholar]
Savarese 2017
- Savarese G, Lund LH. Global public health burden of heart failure. Cardiac Failure Review 2017;3(1):7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2022
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook 2022.
Scott‐Sheldon 2020
- Scott-Sheldon LAJ, Gathright EC, Donahue ML, Balletto B, Feulner MM, DeCosta J, et al. Mindfulness-based interventions for adults with cardiovascular disease: a systematic review and meta-analysis. Annals of Behavioral Medicine 2020;54(1):67-73. [DOI: 10.1093/abm/kaz020] [DOI] [PMC free article] [PubMed] [Google Scholar]
Segal 2002
- Segal ZV, Williams JMG, Teasdale JD. Mindfulness-Based Cognitive Therapy for Depression: A New Approach to Preventing Relapse. New York: Guilford Press, 2002. [Google Scholar]
Segal 2013
- Segal ZV, Williams JMG, Teasdale JD. Mindfulness-Based Cognitive Therapy for Depression. 2nd edition. New York: Guilford Press, 2013. [Google Scholar]
Sherer 1982
- Sherer M, Maddux JE, Mercandante B, Prentice-Dunn S, Jacobs B, Rogers RW. The Self-Efficacy Scale: construction and validation. Psychological Reports 1982;51(2):663–71. [Google Scholar]
Shi 2017
- Shi L, Zhang D, Wang L, Zhuang J, Cook R, Chen L. Meditation and blood pressure: a meta-analysis of randomized clinical trials. Journal of Hypertension 2017;35(4):696-706. [DOI] [PubMed] [Google Scholar]
Spitzer 1999
- Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary care evaluation of mental disorders. Patient Health Questionnaire. JAMA 1999;282(18):1737-44. [DOI] [PubMed] [Google Scholar]
Spitzer 2006
- Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Archives of Internal Medicine 2006;166(10):1092-7. [DOI] [PubMed] [Google Scholar]
Ten 2018
- Ten L, Zhao Q. Effects of mindfulness therapy on psychological distress and quality of life in II type diabetes patients. Chinese Journal of Health Psychology 2018;26:197–200. [Google Scholar]
Tennant 2007
- Tennant R, Hiller L, Fishwick R, Platt S, Joseph S, Weich S, et al. The Warwick-Edinburgh Mental Well-being Scale (WEMWBS): development and UK validation. Health Quality of Life Outcomes 2007;5:63. [DOI: 10.1186/1477-7525-5-63] [DOI] [PMC free article] [PubMed] [Google Scholar]
Transcendental Meditation® 2018
- Trancendental Meditation® official website. http://uk.tm.org (accessed 11 May 2018).
Vale 2005
- Vale S. Psychosocial stress and cardiovascular diseases. Postgraduate Medical Journal 2005;81(957):429–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473-83. [PubMed] [Google Scholar]
Whelton 1997
- Whelton PK, Kumanyika SK, Cook NR, Cutler JA, Borhani NO, Hennekens CH, et al. Efficacy of nonpharmacologic interventions in adults with high-normal blood pressure: results from phase 1 of the Trials of Hypertension Prevention. Trials of Hypertension Prevention Collaborative Research Group. American Journal of Clinical Nutrition 1997;65(2 Suppl):652S-60S. [DOI: 10.1093/ajcn/65.2.652S] [DOI] [PubMed] [Google Scholar]
WHO 2011
- World Health Organization (WHO). Global atlas on cardiovascular disease prevention and control. Policies, strategies and interventions. 2011. Available at www.who.int/cardiovascular_diseases/publications/atlas_cvd/en/ (accessed 5 April 2018).
WHO 2021
- World Health Organization (WHO). Cardiovascular diseases (CVDs). Updated 11 June 2021. www.who.int/mediacentre/factsheets/fs317/en/ (accessed 30 May 2023).
Williams 2007
- Williams M, Teasdale J, Segal Z, Kabat-Zinn J. The Mindful Way Through Depression: Freeing Yourself from Chronic Unhappiness. New York: Guilford Press, 2007. [Google Scholar]
Younge 2015
- Younge JO, Gotlink RA, Baena CP, Roos-Hesselink JW, Hunink MGM. Mind–body practices for patients with cardiac disease: a systematic review and meta-analysis. European Journal of Preventive Cardiology 2015;22(11):1385-98. [DOI] [PubMed] [Google Scholar]
Yusuf 2004
- Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364(9438):937-52. [DOI] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 1983;67(6):361-70. [DOI] [PubMed] [Google Scholar]
Zou 2021
- Zou H, Cao X, Chair SY. A systematic review and meta-analysis of mindfulness-based interventions for patients with coronary heart disease. Journal of Advanced Nursing 2021;77(5):2197-213. [DOI: 10.1111/jan.14738] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hartley 2014
- Hartley L, Mavrodaris A, Flowers N, Ernst E, Rees K. Transcendental meditation for the primary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews 2014, Issue 12. Art. No: CD010359. [DOI: 10.1002/14651858.CD010359.pub2] [DOI] [PubMed] [Google Scholar]
Rees 2019a
- Rees K, Court R, Takeda A, Ernst E. Meditation for the primary and secondary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews 2019, Issue 6. Art. No: CD013358. [DOI: 10.1002/14651858.CD013358] [DOI] [PMC free article] [PubMed] [Google Scholar]


