Abstract
Rationale
Cardiopulmonary exercise testing (CPET) is the gold standard to evaluate exertional breathlessness, a common and disabling symptom. However, the interpretation of breathlessness responses to CPET is limited by a scarcity of normative data.
Objectives
We aimed to develop normative reference equations for breathlessness intensity (Borg 0–10 category ratio) response in men and women aged ⩾40 years during CPET, in relation to power output (watts), oxygen uptake, and minute ventilation.
Methods
Analysis of ostensibly healthy people aged ⩾40 years undergoing symptom-limited incremental cycle CPET (10 W/min) in the CanCOLD (Canadian Cohort Obstructive Lung Disease) study. Participants had smoking histories <5 pack-years and normal lung function and exercise capacity. The probability of each Borg 0–10 category ratio breathlessness intensity rating by power output, oxygen uptake, and minute ventilation (as an absolute or a relative value [percentage of predicted maximum]) was predicted using ordinal multinomial logistic regression. Model performance was evaluated by fit, calibration, and discrimination (C statistic) and externally validated in an independent sample (n = 86) of healthy Canadian adults.
Results
We included 156 participants (43% women) from CanCOLD; the mean age was 65 (range, 42–91) years, and the mean body mass index was 26.3 (standard deviation, 3.8) kg/m2. Reference equations were developed for women and men separately, accounting for age and/or body mass. Model performance was high across all equations, including in the validation sample (C statistic for men = 0.81–0.92, C statistic for women = 0.81–0.96).
Conclusions
Normative reference equations are provided to compare exertional breathlessness intensity ratings among individuals or groups and to identify and quantify abnormal breathlessness responses (scores greater than the upper limit of normal) during CPET.
Keywords: dyspnea, exercise capacity, normal values
Breathlessness on exertion (1, 2) is one of the leading causes of chronic suffering and disability and the cardinal symptom in people with cardiorespiratory disease (3). The symptom trajectory is often progressive, leading to a vicious cycle of impaired activity, deconditioning, and worsening of breathlessness at progressively lower degrees of exertion (4). As people reduce their physical activity to avoid the symptom, exertional breathlessness should be measured in relation to a given symptom stimulus, such as at a standardized degree of exertion or ventilation (5).
Cardiopulmonary exercise testing (CPET) is valuable for assessing exertional breathlessness in clinical care and research (6–8), including symptom intensity (measured on the Borg 0–10 category ratio [CR10] scale) (9) and its relation to physiological responses such as power output (watts), rate of oxygen uptake (o2), and minute ventilation (e). This enables evaluation of 1) underlying pathophysiological mechanisms that may be contributing to breathlessness and 2) interventional efficacy in clinical trials (8, 10, 11).
However, interpretation of breathlessness responses to CPET is limited by the scarcity of normative reference equations. The ability to predict the normal breathlessness response to any given submaximal or maximal power output, o2, and/or e for an individual is important; it would improve the ability to identify the presence and degree of an abnormal exertional breathlessness response. Reference equations for breathlessness intensity during incremental cycle testing were recently reported by Elmberg and colleagues (12). However, that study pertained to people referred for exercise testing in clinical practice, who did not constitute a population-based sample of healthy people, and the study did not include any measurements of gas exchange (such as o2) or e during the test. Two studies provided data on the normative breathlessness response to symptom-limited incremental CPET on a stationary cycle ergometer. Killian and colleagues reported reference equations for breathlessness intensity in 460 healthy individuals aged 20–70 years (13). However, those equations were limited, as they assumed normally distributed residuals and used linear regression, which can yield predicted scores outside the CR10 scale. In addition, the reference values of Killian and colleagues were calculated in relation to the percentage of a person’s achieved peak power output, which is problematic, as 1) in a symptom-limited test, people will stop exercise at similar degrees of breathlessness across health and disease, and 2) a given percentage (such as 75%) of the achieved peak power output can correspond to widely different absolute power outputs, for example, when comparing a person with severe respiratory disease with a healthy athlete. Therefore, those equations have not been adopted for use in clinical care or research (7, 13). Neder and colleagues reported the distribution of breathlessness intensities during CPET in 275 healthy people (14), including the 95th percentile, which could be used for defining the upper limit of normal (ULN) and abnormal values (greater than the ULN). Breathlessness responses were tabulated in relation to absolute power output and e but not o2, and, importantly, reference equations were not developed.
Reference equations to predict the normal breathlessness intensity response during CPET are crucial, as they would enable clinicians and researchers to identify an abnormal exertional breathlessness (score greater than or equal to the ULN) response in individual subjects. Reference equations would further quantify the severity of the breathlessness experienced and compare symptom intensity among individuals and groups. The aim of this study was to develop normative reference equations for breathlessness intensity in healthy women and men aged ⩾40 years during symptom-limited incremental cycle CPET, in relation to absolute and relative (percentage predicted peak) values of power output, o2, and e.
Methods
Study Design and Development Sample
This was an analysis of the CanCOLD (Canadian Cohort Obstructive Lung Disease) study (15). CanCOLD is a prospective, population-based study conducted across nine communities in Canada (NCT 00920348). Participants were noninstitutionalized male or female adults aged ⩾40 years identified using random telephone digit dialing (15).
The inclusion criterion for this analysis was available CPET data from the CanCOLD baseline visit. Exclusion criteria were as follows (Figure 1): known respiratory, cardiovascular, or metabolic disease (self-report of physician-diagnosed asthma, chronic bronchitis, chronic obstructive pulmonary disease, angina pectoris, myocardial infection, any other cardiovascular or cerebrovascular disease, or diabetes mellitus); treatment with a β-blocker; ⩾5 pack-years of cigarette smoke exposure; abnormally low or high exercise capacity, defined as peak o2 below the lower limit of normal [LLN] or greater than the ULN, respectively (16); impaired lung function at rest, defined as a postbronchodilator value less than the LLN for any of the following: forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC) (17), FEV1:FVC ratio, total lung capacity (18), or diffusing capacity of the lungs for carbon monoxide (19); or an increase in FEV1 or FVC of >12% and >200 ml from baseline 10–15 minutes after the inhalation of 200 μg salbutamol administered using a spacer. Further exclusion criteria were a body mass index (BMI) <18 or >35 kg/m2, inability to reach peak exercise criteria (see Appendix E1 in the data supplement), exercise time < 4 minutes, abnormal response during CPET as judged by the supervising physician, missing peak breathlessness intensity, or termination of CPET by the supervising physician for medical or technical reasons (e.g., a participant reached the end of a predetermined exercise period before reaching a symptom limitation).
Figure 1.
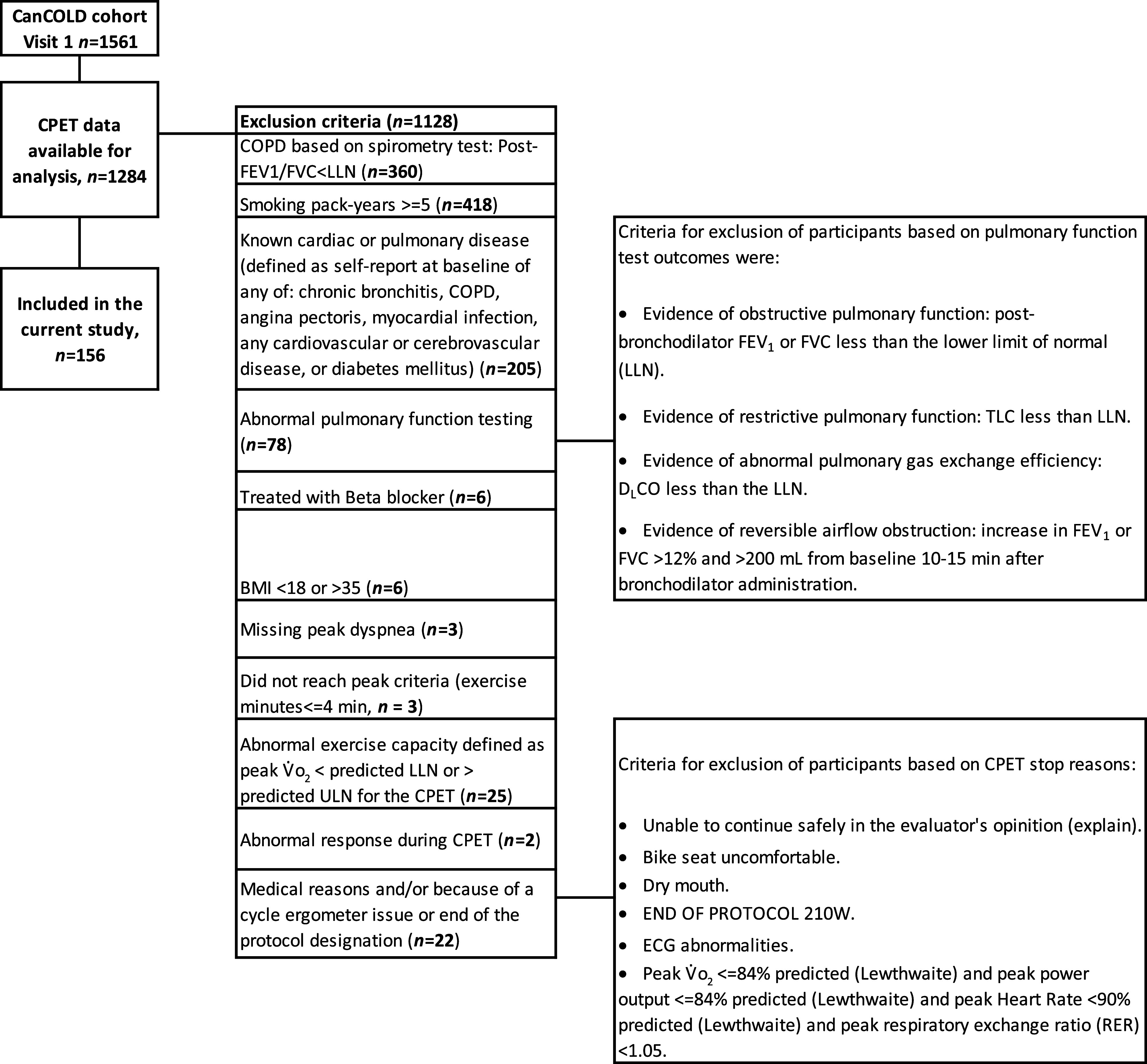
Participant flowchart in the CanCOLD development sample. BMI = body mass index; CanCOLD = Canadian Cohort Obstructive Lung Disease; COPD = chronic obstructive pulmonary disease; CPET = cardiopulmonary exercise testing; DlCO = diffusing capacity of the lungs for carbon monoxide; ECG = electrocardiogram; FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; LLN = lower limit of normal; TLC = total lung capacity; ULN = upper limit of normal; o2 = oxygen uptake.
All participants provided written informed consent before completing study assessments. The research ethics board for each participating institution approved the study protocol. The present CanCOLD substudy is reported in accordance with the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis statement (20).
Procedures
Participants in CanCOLD self-reported data on sociodemographics and health (e.g., smoking history, self-reported health conditions) via structured interviews with trained researchers. Body height and mass were measured. Assessments included pre- and postbronchodilator spirometry, diffusing capacity of the lungs for carbon monoxide, and lung volumes measured on body plethysmography using automated equipment in accordance with American Thoracic Society and European Respiratory Society recommendations (15, 21, 22). Predicted lung function values were calculated using Global Lung Function Initiative references (17–19).
CPET
CPET was performed in accordance with recognized guidelines (23) on an electronically braked cycle ergometer using a computerized CPET system (Vmax, SensorMedics [seven sites], n = 138 [88.5%]; TrueOne, Parvo Medics [one site], and Ergocard, Medisoft [one site], n = 18 [11.5%]). The CPET protocol was standardized across sites, consisting of a steady-state rest period of 3–10 minutes, 1 minute of unloaded pedaling, and then a 10-W increase in power output every minute (starting at 10 W) until symptom limitation. Participants were encouraged to maintain a pedal cadence of 50–70 rpm, and testing was stopped if pedal cadence fell below 40 rpm.
Gas exchange and breathing pattern parameters were collected breath by breath with participants breathing through a mouthpiece and flow transducer while wearing a nose clip. The 12-lead electrocardiogram was monitored to assess heart rate and rhythm; peripheral oxyhemoglobin saturation was monitored using finger pulse oximetry.
Before CPET, breathlessness was defined for each participant as “breathing discomfort” and leg discomfort as “the level of discomfort experienced during pedaling,” and participants were familiarized with the CR10 scale such that 0 represented “no breathing [leg] discomfort” and 10 represented “the most severe breathing [leg] discomfort that you have ever experienced or can imagine experiencing.” Every two minutes during exercise and at peak exercise, blood pressure was assessed, and participants rated their breathlessness and leg discomfort on the CR10 scale. All procedures were the same across the study sites (9).
Physiological variables were averaged over the first 30-second period of every 2-minute interval during CPET and linked with symptom intensity ratings collected over the latter 30 seconds of the same minute. Peak o2 and e were taken as averages of the last 30 seconds of loaded pedaling, whereas peak power output was taken as the highest power output a participant was able to sustain for at least 30 seconds. Predicted values for peak CPET parameters were calculated using published CanCOLD references (16).
External Validation Sample
Validation was performed on a convenience sample of 86 (49% women) ostensibly healthy participants (i.e., without self-reported conditions or clinical evidence of disease) aged ⩾40 years, who performed incremental cycle CPET to symptom limitation as part of studies independent from CanCOLD at the institutions of M.K.S. (n = 27 from previous studies [24, 25]) and D.J. (n = 59; not included in previous studies). Exclusion criteria were abnormal lung function at rest (postbronchodilator FEV1:FVC ratio or FEV1 less than the LLN), BMI <18 or >35 kg/m2, peak o2 less than the LLN (16), or missing data on peak breathlessness intensity. Symptom-limited incremental CPET was performed on an electronically braked cycle ergometer using a Vmax SensorMedics metabolic cart and included increments in power output of 15 W/2 min (n = 1), 20 W/2 min (n = 50), 20 W/3 min (n = 32), and 25 W/2 min (n = 3), depending on the original study designs. Standardized physiological and symptom assessments were performed similarly as in the CanCOLD development sample.
Statistical Analyses
Baseline participant characteristics are summarized using mean with standard deviation (SD) and median with range or interquartile range (IQR) for continuous variables, as appropriate. Categorical variables are expressed as frequencies and percentages. No data were imputed.
Breathlessness intensity ratings (CR10) were analyzed separately for women and men and by the three CPET parameters (power output, o2 and e), each evaluated as absolute values or as a percentage of each participant’s predicted maximal value (%predmax) in separate models (16).
Normative reference equations were developed using CanCOLD data and marginal ordinal multinomial logistic regression. The models were fitted using a generalized estimating equation procedure with cumulative logits link and multinomial distribution, to obtain population-average (marginal) predictions. This method predicts the cumulative probability of reporting an equal or lower score for each of the CR10 scores (0, 0.5, 1, 2, . . . 10). The ULN was calculated using linear interpolation of the linear predictor of the responses closest to below and above a probability of 0.95. The prediction equation was based on the CPET parameter and covariates (specified below) and accounted for the correlation between repeated measurements on the same participant over the exercise time. In this way, no predictions fall outside the CR10 scale range. We used locally estimated scatterplot smoothing plots to check the patterns between the CR10 breathlessness intensity ratings and each of the three CPET parameters. If the trend indicated nonlinearity, restricted cubic splines (26) were applied with four knots, selected on the basis of the distribution of the variables located at the 5th, 35th, 65th, and 95th percentile for men and women separately, constructed using the SAS macro %RCSPLINE (27) Details on how to construct splines are given in the data supplement.
The models were specified, and variables to include were selected using the independence model criterion (QIC), including comparing models with linear variables and cubic splines with four knots. Models with the lowest QICs were preferred. Results indicated that the models with four knots had better fit for most of the variables (see Table E1). Additional factors that may influence the breathlessness response (12) (age, height, body mass, and their interaction terms with the CPET parameter [power output, o2, or e]) with P values <0.05 were also included in the final multivariate reference equations. For use in future validation studies, the distribution of each included variable according to the four knot cut points is shown in Table E2.
Model performance in the development and validation samples was evaluated as calibration (agreement between predicted and observed probabilities for the different breathlessness scores) and discrimination. Calibration plots were created using the predicted probability by deciles on the x-axis and the observed rates by deciles on the y-axis. A good calibration should lie close to the diagonal line of identify. The models were also validated by calculating average absolute difference (observed minus predicted, as a percentage) between the predicted probabilities and observed frequencies. The discriminative ability of the model was assessed as the area under the curve (C statistic) of receiver operating characteristic analysis, indicating the probability of correct prediction of the different breathlessness intensity ratings. Statistical significance was defined as a two-sided P value <0.05. Statistical analyses were conducted using SAS version 9.4 (TS1M5) (SAS Institute Inc.).
Results
Development of the Reference Equations
Data from 156 CanCOLD participants (43% women) were used to develop the normative reference equations (Figure 1). Participant characteristics are shown in Table 1. The mean age was 65 years (range, 42–91 yr), the mean BMI was 26.3 kg/m2 (SD, 3.8 kg/m2), and lung function and peak physiological responses during CPET were within normal ranges (Table 1). Breathlessness intensity ratings at peak exercise were similar between men (median, 5 [IQR, 3–7]) and women (median, 5 [IQR, 4–7]).
Table 1.
Characteristics of ostensibly healthy participants in the development (Canadian Cohort Obstructive Lung Disease) sample
| Characteristic | All | Male | Female |
|---|---|---|---|
| Participants, n (%) | 156 (100) | 89 (57) | 67 (43) |
| Age, yr, mean (SD) | 64.8 (9.5) | 65.8 (9.5) | 63.6 (9.3) |
| Range | 42.0–91.0 | 47.0–91.0 | 42.0–81.0 |
| Height, cm | 168.3 (9.5) | 173.8 (7.4) | 161.0 (6.5) |
| Body mass, kg | 74.7 (14.1) | 81.8 (12.3) | 65.2 (10.3) |
| Body mass index, kg/m2 | 26.3 (3.8) | 27.1 (3.8) | 25.1 (3.6) |
| Cigarette ever-smoker, n (%) | 26 (16.7) | 13 (14.6) | 13 (19.4) |
| Cigarette smoker pack-years | 0.4 (1.1) | 0.3 (1.1) | 0.4 (1.1) |
| Hypertension, n (%) | 33 (21.2) | 20 (22.5) | 13 (19.4) |
| Lung function | |||
| FEV1, %pred | 102.9 (13.4) | 101.4 (12.0) | 104.9 (14.9) |
| FVC, %pred | 106.6 (14.2) | 106.0 (13.5) | 107.3 (15.1) |
| FEV1:FVC ratio, % | 75.1 (6.7) | 73.8 (7.2) | 76.9 (5.6) |
| TLC, %pred | 105.5 (13.1) | 102.0 (11.5) | 110.1 (13.6) |
| RV, %pred | 111.0 (26.8) | 104.5 (26.6) | 119.7 (24.7) |
| RV:TLC ratio, % predicted | 104.5 (18.4) | 101.9 (19.9) | 107.9 (15.7) |
| DlCO, %pred | 102.7 (16.6) | 104.5 (17.3) | 100.3 (15.5) |
| CPET values at peak exercise | |||
| Work rate, W | 131.0 (40.8) | 150.4 (37.1) | 105.2 (29.7) |
| W, %pred | 102.2 (19.2) | 101.8 (17.6) | 102.7 (21.2) |
| HR, beats/min | 148 (20.4) | 146 (21.8) | 150 (18.4) |
| HR, %pred | 100.6 (12.1) | 99.9 (13.2) | 101.6 (10.3) |
| o2, L/min | 1.9 (0.6) | 2.2 (0.5) | 1.5 (0.4) |
| o2, %pred | 100.3 (18.5) | 98.1 (16.0) | 103.2 (21.2) |
| o2, ml/kg/min | 25.4 (6.2) | 27.2 (5.7) | 22.9 (6.0) |
| e, L/min | 66.9 (19.8) | 77.0 (18.2) | 53.4 (12.4) |
| e, %pred | 99.1 (23.2) | 102.7 (23.8) | 94.2 (21.6) |
| SBP, mm Hg | 185.9 (27.1) | 193.3 (24.4) | 176.4 (27.7) |
| DBP, mm Hg | 81 (12.1) | 82 (12.2) | 81 (12.1) |
| SpO2, % | 96.8 (3.1) | 96.3 (2.8) | 97.4 (3.2) |
| RER | 1.1 (0.1) | 1.1 (0.1) | 1.2 (0.1) |
| Breathlessness (CR10), median (IQR) | 5.0 (3.5–7.0) | 5.0 (3.0–7.0) | 5.0 (4.0–7.0) |
| 0, n (%) | 3 (1.9) | 1 (1.1) | 2 (3.0) |
| 0.5, n (%) | 4 (2.6) | 0 (0.0) | 4 (6.0) |
| 1, n (%) | 4 (2.6) | 4 (4.5) | 0 (0.0) |
| 2, n (%) | 9 (5.8) | 6 (6.7) | 3 (4.5) |
| 3, n (%) | 19 (12.2) | 12 (13.5) | 7 (10.4) |
| 4, n (%) | 22 (14.1) | 12 (13.5) | 10 (14.9) |
| 5, n (%) | 31 (19.9) | 18 (20.2) | 13 (19.4) |
| 6, n (%) | 8 (5.1) | 3 (3.4) | 5 (7.5) |
| 7, n (%) | 22 (14.1) | 11 (12.4) | 11 (16.4) |
| 8, n (%) | 5 (3.2) | 5 (5.6) | 0 (0.0) |
| 9, n (%) | 23 (14.7) | 12 (13.5) | 11 (16.4) |
| 10, n (%) | 6 (3.8) | 5 (5.6) | 1 (1.5) |
| Leg discomfort (CR10), median (IQR) | 6.0 (4.0–9.0) | 6.0 (5.0–9.0) | 6.0 (4.0–9.0) |
| 0, n (%) | 1 (0.6) | 0 (0.0) | 1 (1.5) |
| 0.5, n (%) | 1 (0.6) | 1 (1.1) | 0 (0.0) |
| 1, n (%) | 5 (3.2) | 4 (4.5) | 1 (1.5) |
| 2, n (%) | 4 (2.6) | 1 (1.1) | 3 (4.5) |
| 3, n (%) | 14 (9.0) | 7 (7.9) | 7 (10.4) |
| 4, n (%) | 18 (11.5) | 6 (6.7) | 12 (17.9) |
| 5, n (%) | 28 (17.9) | 21 (23.6) | 7 (10.4) |
| 6, n (%) | 8 (5.1) | 5 (5.6) | 3 (4.5) |
| 7, n (%) | 25 (16.0) | 14 (15.7) | 11 (16.4) |
| 8, n (%) | 6 (3.8) | 3 (3.4) | 3 (4.5) |
| 9, n (%) | 24 (15.4) | 15 (16.9) | 9 (13.4) |
| 10, n (%) | 22 (14.1) | 12 (13.5) | 10 (14.9) |
Definition of abbreviations: CPET = cardiopulmonary exercise testing; CR10 = Borg 0–10 category ratio; DBP = diastolic blood pressure; DlCO = diffusing capacity of the lungs for carbon monoxide; FEV1 = forced expired volume in 1 second; FVC = forced vital capacity; HR = heart rate; IQR = interquartile range; %pred = percentage predicted; RER = respiratory exchange ratio; RV = residual volume; SBP = systolic blood pressure; SD = standard deviation; SpO2 = oxygen saturation as measured by pulse oximetry; TLC = total lung capacity; e = minute ventilation; o2 = volume of oxygen uptake.
Data are presented as mean (standard deviation) unless otherwise specified.
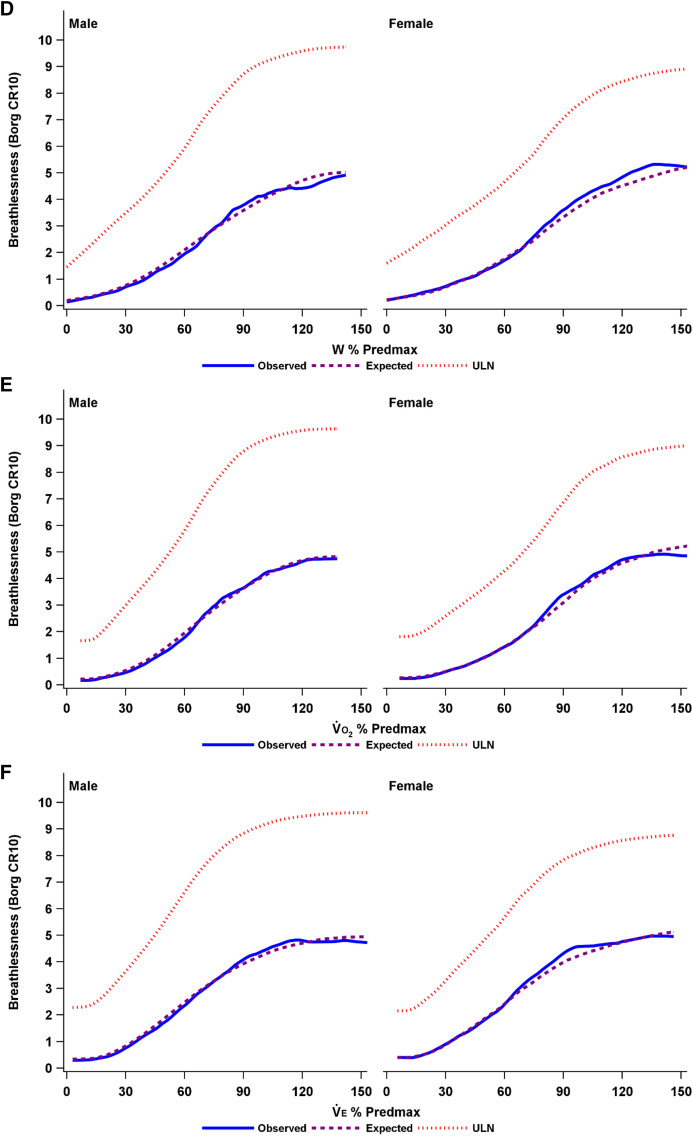
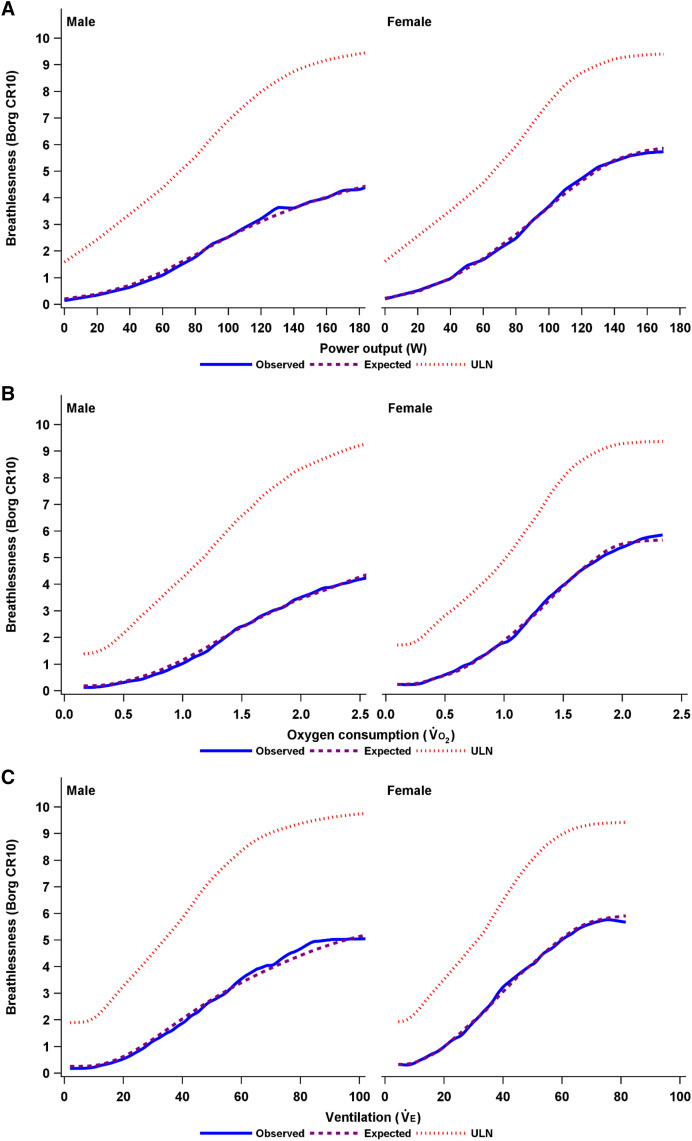
A penalized B-spline was used to fit a smooth curve for the observed and expected breathlessness intensity ratings, as well as the ULN in men and women by each relative CPET parameter in Figure 2. The distribution of breathlessness intensity responses across each CPET parameter is shown in Figure E1.
Figure 2.
(A–F) Observed and expected breathlessness intensity and the ULN during incremental cycle cardiopulmonary exercise testing in men and women, plotted using penalized B-spline by (A) power output (watts), (B) oxygen uptake (o2), (C) minute ventilation (e), (D) W % Predmax, (E) o2 % Predmax, and (F) e % Predmax. The expected breathlessness intensity is an anticipated average breathlessness intensity, calculated as the sum of all possible Borg scores, each multiplied by its predicted probability. CR10 = Borg 0–10 category ratio; ULN = upper limit of normal; e % Predmax = e expressed as a percentage of the predicted maximal value; o2 % Predmax = o2 expressed as a percentage of the predicted maximal value; W % Predmax = power output expressed as a percentage of the predicted maximal value.
In the multivariable modeling, factors that improved the prediction of breathlessness intensity (and thus were included in the final equations) were age, and/or body mass, and/or significant interactions between age and the three CPET parameters (power output, o2, and e). The estimates for each factor are shown in Table E3, and the goodness of fit for each model (assessed using the QIC) is shown in Table E1.
The final normative reference equations, with the highest fit for men and women, are provided in Table E4. These equations can be used to predict, for a given absolute or relative (%predmax) value of power output, o2, or e, the 1) probability (p) of reporting each CR10 breathlessness intensity rating among healthy people; 2) probability of breathlessness normality (the predicted probability of having an equal or greater CR10 rating among healthy people); 3) the expected normal breathlessness intensity (which is an anticipated average breathlessness intensity, calculated as the sum of all possible Borg scores, each multiplied by its predicted probability); and 4) the ULN for breathlessness intensity (corresponding to the 95th percentile among healthy people). A spreadsheet for obtaining the calculations is provided in the data supplement.
Internal Validation
The prediction equations showed excellent performance in terms of agreement (calibration) between predicted and observed probability (see Table E5 and Figure E2) and discriminative ability of the models (receiver operating characteristic curves are shown in Figure E3), with C statistics ranging from 0.84 to 0.92 for men and from 0.87 to 0.98 for women. The models performed similarly well in men and women and when using the different CPET parameters (power output, o2, and e) as either the absolute value or %predmax.
External Validation
The normative reference equations were applied to the validation sample of 86 healthy adults (see Figure E4): mean age of 68 (SD, 9.9) years, 49% woman, mean BMI of 26.0 (SD, 3.3) kg/m2, and lung function and exercise capacity within normal ranges (see Table E6).
Performance of the normative reference equations in the validation sample was high and similar to that observed in the CanCOLD development sample for all the equations (see Table E7 and Figures E5 and E6): the model fit was high, with most differences between observed and predicted probabilities within ±5% (see Table E7). The normal reference values were also well calibrated (see Figure E5), with high discriminative ability to predict the breathlessness intensity ratings (Figure E6): C statistics ranged from 0.81 to 0.92 for men and from 0.81 to 0.96 for women.
Discussion
This study presents normative reference equations for the breathlessness intensity (CR10) response during symptom-limited incremental cycle CPET. The equations were developed and internally validated in healthy Canadian men and women aged ⩾40 years and externally validated in an independent sample. The equations can be used to predict 1) the normative breathlessness intensity response during incremental CPET; 2) the breathlessness intensity ULN for a given individual in relation to absolute and relative power output, o2, and e, accounting for sex, age, and/or body mass; and 3) the presence of abnormal exertional breathlessness intensity, which can be defined as a CR10 rating greater than the ULN. These parameters enable clinicians and researchers to quantify the normality of breathlessness responses to exercise provocation in individuals and to compare the exertional breathlessness response among individuals and groups. All the normative reference equations showed very high performance in internal and external validation.
Importantly, the normative reference equations can be used to evaluate breathlessness at any point of measurement during CPET, throughout submaximal and peak values for power output, o2, and/or e. This enables the evaluation of the exertional breathlessness response in people unwilling or unable to perform a maximal exercise test to the point of symptom limitation.
For the equations using relative power output, o2, or e (%predmax), the predicted maximum should be based on the best representative reference material for the underlying population, similarly to the practice for spirometry (22). Expressing breathlessness intensity in relation to %predmax, which accounts for individual differences in age, sex, and height, can simplify visualization of comparisons among individuals or groups.
How the Normative Reference Equations Can Be Used
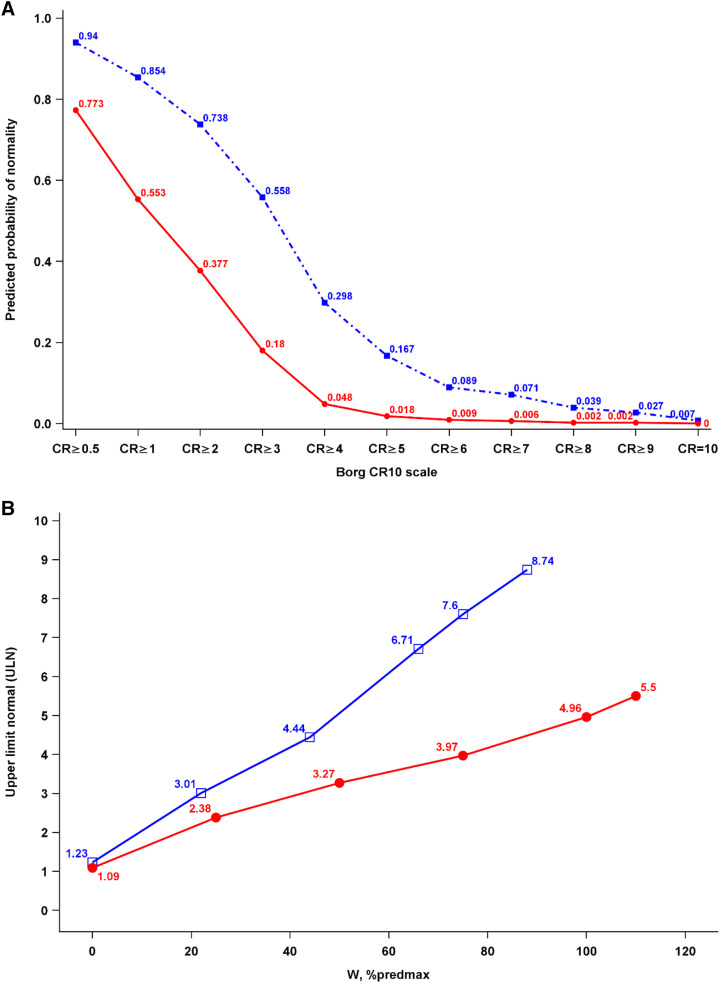
The normative reference equations developed in this study enable the evaluation and comparison of breathlessness intensity ratings at a standardized degree of exertion or e during incremental CPET (5). An example of how they can be used to compare breathlessness between a 50-year-old man and a 75-year-old woman is given in Figure 3.
Figure 3.
(A and B) Example of the predicted normal breathlessness response to incremental cycle cardiopulmonary exercise testing in terms of (A) probability of normality (defined as the probability of having an equal or greater score among healthy people) for each possible Borg 0–10 category ratio (CR10) score at a power output (watts) of 75% predmax for the individual and (B) the ULN for breathlessness (CR10) intensity at different power outputs. Blue lines are values for a man (age 50 years, body mass 80 kg, height 180 cm) and red lines for a woman (age 75 years, body mass 60 kg, height 170 cm). Both reported a breathlessness intensity of 6 of 10 at power output 75% predmax. That breathlessness intensity had a probability of normality of 8.9% for the man and 0.9% for the woman (A), which was within normal predicted ranges (less than or equal to the ULN) for the man but abnormal (greater than the ULN) for the woman (B). CR = category ratio; % predmax = percentage of the predicted maximal value. ULN = upper limit of normal.
The equations enable the evaluation of a number of important clinical and research questions:
-
1.
How breathless is a “normal” healthy person? The normal breathlessness intensity response can be predicted in terms of the probability of each CR10 score among healthy people at any absolute or relative power output, o2, and e during CPET.
-
2.
How breathless is an individual compared with normal? The intensity of breathlessness compared with the normal reference is given by a score’s probability of normality, which can be interpreted as the predicted percentage of people having equal or greater scores among healthy individuals. In studies without healthy control populations, the reference equations can also be used to create breathlessness intensity ratings for a “healthy comparison group.”
-
3.
Is an individual’s exertional breathlessness response abnormal? An abnormal exertional breathlessness intensity can be defined as a score greater than the ULN (95th percentile or scores, corresponding to a probability of normality of <0.05), similarly to current recommendations for interpreting spirometry values and physiological responses during CPET (16, 22, 28). Of note, the cutoff used to define abnormality can be determined by the user as needed, for example, as a probability of normality <0.90 or <0.99. The presence of abnormal exertional breathlessness, or the degree of breathlessness severity (probability of normality), can be used to select and characterize participants in clinical breathlessness trials.
-
4.
Is there a difference in breathlessness severity when expressed in relation to power output, o2, and/or e? Differences in breathlessness intensity ratings relative to power output, o2, and e may indicate different underlying pathophysiological mechanisms of abnormally high exertional breathlessness, where abnormality in relation to e might indicate greater critical inspiratory constraints that warrant further investigation and may be amenable to targeted intervention (8, 29).
Strengths and Limitations
CanCOLD is a well-characterized, population-based sample of men and women undergoing standardized symptom-limited incremental CPET (15). The dataset is unique in its combination of a large-scale population design and detailed physiological assessments, including lung function and CPET performed in accordance with American Thoracic Society and European Respiratory Society standards (21, 22). An extensive set of eligibility criteria were applied to identify a healthy reference sample.
A limitation is the relatively small study sample size. However, the performance of the normative reference equations was also very high in the independent validation sample, which supports the internal and external validity of the current references. The findings pertain to breathlessness intensity measured during incremental CPET on a cycle ergometer in people aged ⩾40 years, using standardized instructions on the symptom and the CR10 scale.
Next Steps
We suggest that the present normative reference equations be used to evaluate the exertional breathlessness intensity response to CPET. They enable a range of novel studies on validation in clinical populations such as cardiopulmonary diseases and obesity; the development of reference equations for other populations (pediatrics, non-Canadian adults) and breathlessness dimensions (30) such as the degree of unpleasantness and qualities such as “work or effort” or “unsatisfied inspiration or air hunger” (7, 31, 32); the prevalence, degree, and predictors of abnormally high exertional breathlessness in different populations and patient groups; comparing the classification of exertional breathlessness with questionnaires (e.g., the modified Medical Research Council dyspnea scale) commonly used to categorize symptom severity (5) and to select participants for inclusion in clinical trials (33); and the prognostic utility of abnormal breathlessness during CPET for predicting clinical outcomes such as incident disease, hospitalization, and premature death.
Conclusions
This study provides the first reference equations to predict the normal breathlessness intensity response at any standardized relative or absolute power output, o2, and e during symptom-limited incremental cycle CPET, developed and validated for men and women aged ⩾40 years. The equations can be used to predict the normal exertional breathlessness intensity rating(s) for a given individual, categorize the presence and degree of abnormal exertional breathlessness, and compare the intensity of exertional breathlessness among individuals or groups.
Acknowledgments
Acknowledgment
The authors thank the people who participated in the study and the many members of the CanCOLD Collaborative Research Group.
CanCOLD Collaborative Research Group members: Executive Committee: Jean Bourbeau (McGill University, Montreal, QC, Canada); Wan C. Tan, J. Mark FitzGerald, Don D. Sin; Darcy D. Marciniuk (University of Saskatoon, Saskatoon, SK, Canada); Denis E. O’Donnell (Queen’s University, Kingston, ON, Canada); Paul Hernandez (Dalhousie University, Halifax, NS, Canada); Kenneth R. Chapman,; Brandie Walker (University of Calgary, Calgary, AB, Canada); Shawn Aaron (University of Ottawa, Ottawa, ON, Canada); François Maltais (University of Laval, Quebec City, QC, Canada). International Advisory Board: Jonathon Samet (the Keck School of Medicine of USC, Los Angeles, California); Milo Puhan (John Hopkins School of Public Health, Baltimore, Maryland); Qutayba Hamid (McGill University, Montreal, QC, Canada); James C. Hogg. Operations Center: Jean Bourbeau (Principal Investigator), Dany Doiron, Palmina Mancino, Pei Zhi Li, Dennis Jensen, Carolyn Baglole (McGill University, Montreal, QC, Canada); Yvan Fortier (Laboratoire telematique, Quebec Respiratory Health Network, Fonds de la recherche en santé du Québec [FRQS]); Wan C. Tan (co–Principal Investigator), Don Sin, Julia Yang, Jeremy Road, Joe Comeau, Adrian Png, Kyle Johnson, Harvey Coxson, Jonathon Leipsic, Cameron Hague, Miranda Kirby, Economic Core: Mohsen Sadatsafavi. Public Health Core: Teresa To, Andrea Gershon. Data Management and Quality Control: Wan C. Tan, Harvey Coxson; Jean Bourbeau, Pei-Zhi Li, Zhi Song, Andrea Benedetti, Dennis Jensen (McGill University, Montreal, QC, Canada); Yvan Fortier (Laboratoire telematique, Quebec Respiratory Health Network, FRQS); Miranda Kirby. Field Centers: Wan C. Tan (Principal Investigator), Christine Lo, Sarah Cheng, Elena Un, Cynthia Fung, Wen Tiang Wang, Liyun Zheng, Faize Faroon, Olga Radivojevic, Sally Chung, Carl Zou; Jean Bourbeau (Principal Investigator), Palmina Mancino, Jacinthe Baril, Laura Labonte (McGill University, Montreal, QC, Canada); Kenneth Chapman (Principal Investigator), Patricia McClean, Nadeen Audisho,; Brandie Walker (Principal Investigator), Curtis Dumonceaux, Lisette Machado (University of Calgary, Calgary, AB, Canada); Paul Hernandez (Principal Investigator), Scott Fulton, Kristen Osterling, Denise Wigerius (University of Halifax, Halifax, NS, Canada); Shawn Aaron (Principal Investigator), Kathy Vandemheen, Gay Pratt, Amanda Bergeron (University of Ottawa, Ottawa, ON, Canada); Denis O’Donnell (Principal Investigator), Matthew McNeil, Kate Whelan (Queen’s University, Kingston, ON, Canada); François Maltais (Principal Investigator), Cynthia Brouillard (University of Laval, Quebec City, QC, Canada); Darcy Marciniuk (Principal Investigator), Ron Clemens, Janet Baran, Candice Leuschen (University of Saskatoon, Saskatoon, SK, Canada).
A complete list of CanCOLD Collaborative Research Group members may be found before the beginning of the References.
Footnotes
The CanCOLD study (NCT 00920348) has received support from the Canadian Respiratory Research Network, the Canadian Institutes of Health Research (CIHR/Rx&D Collaborative Research Program Operating Grant 93326), the Respiratory Health Research Network of Fonds de la Recherche en Santé du Québec, the Foundation of the McGill University Health Centre, and industry partners, including AstraZeneca Canada Ltd., Boehringer Ingelheim Canada Ltd., GlaxoSmithKline Canada Ltd., Novartis, Almirall, Merck, Nycomed, Pfizer Canada Ltd., and Theratechnologies. M.E. was supported by an unrestricted grant from the Swedish Research Council (Dnr: 2019-02081). D.J. holds a Canada Research Chair, Tier II, in Clinical Exercise & Respiratory Physiology from the Canadian Institutes of Health Research. The funders had no role in any aspect of the manuscript.
Author Contributions: Study conception and design, M.E., H.L., and D.J.; data collection, J.B., W.C.T., and D.J.; statistical analysis, P.Z.L.; first draft, M.E.; data acquisition, M.K.S.; interpretation, revision of the manuscript for intellectual content, and approval of the final version to submit, all authors.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
on behalf of the CanCOLD Collaborative Research Group:
Jean Bourbeau, Wan C. Tan, J. Mark FitzGerald, Don D. Sin, Darcy D. Marciniuk, Denis E. O’Donnell, Paul Hernandez, Kenneth R. Chapman, Brandie Walker, Shawn Aaron, François Maltais, Jonathon Samet, Milo Puhan, Qutayba Hamid, James C. Hogg, Dany Doiron, Palmina Mancino, Pei Zhi Li, Dennis Jensen, Carolyn Baglole, Yvan Fortier, Julia Yang, Jeremy Road, Joe Comeau, Adrian Png, Kyle Johnson, Harvey Coxson, Jonathon Leipsic, Cameron Hague, Miranda Kirby, Mohsen Sadatsafavi, Teresa To, Andrea Gershon, Zhi Song, Andrea Benedetti, Christine Lo, Sarah Cheng, Elena Un, Cynthia Fung, Wen Tiang Wang, Liyun Zheng, Faize Faroon, Olga Radivojevic, Sally Chung, Carl Zou, Jacinthe Baril, Laura Labonte, Patricia McClean, Nadeen Audisho, Curtis Dumonceaux, Lisette Machado, Scott Fulton, Kristen Osterling, Denise Wigerius, Kathy Vandemheen, Gay Pratt, Amanda Bergeron, Matthew McNeil, Kate Whelan, Cynthia Brouillard, Ron Clemens, Janet Baran, and Candice Leuschen
References
- 1. Johnson MJ, Yorke J, Hansen-Flaschen J, Lansing R, Ekström M, Similowski T, et al. Towards an expert consensus to delineate a clinical syndrome of chronic breathlessness. Eur Respir J . 2017;49:1602277. doi: 10.1183/13993003.02277-2016. [DOI] [PubMed] [Google Scholar]
- 2. Parshall MB, Schwartzstein RM, Adams L, Banzett RB, Manning HL, Bourbeau J, et al. American Thoracic Society Committee on Dyspnea An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med . 2012;185:435–452. doi: 10.1164/rccm.201111-2042ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moens K, Higginson IJ, Harding R, EURO IMPACT Are there differences in the prevalence of palliative care-related problems in people living with advanced cancer and eight non-cancer conditions? A systematic review. J Pain Symptom Manage . 2014;48:660–677. doi: 10.1016/j.jpainsymman.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 4. Ramon MA, Ter Riet G, Carsin AE, Gimeno-Santos E, Agustí A, Antó JM, et al. PAC-COPD Study Group The dyspnoea-inactivity vicious circle in COPD: development and external validation of a conceptual model. Eur Respir J . 2018;52:1800079. doi: 10.1183/13993003.00079-2018. [DOI] [PubMed] [Google Scholar]
- 5. Ekström M, Elmberg V, Lindow T, Wollmer P. Breathlessness measurement should be standardised for the level of exertion. Eur Respir J . 2018;51:1800486. doi: 10.1183/13993003.00486-2018. [DOI] [PubMed] [Google Scholar]
- 6. Bonini M, Fiorenzano G. Exertional dyspnoea in interstitial lung diseases: the clinical utility of cardiopulmonary exercise testing. Eur Respir Rev . 2017;26:160099. doi: 10.1183/16000617.0099-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lewthwaite H, Jensen D, Ekström M. How to assess breathlessness in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis . 2021;16:1581–1598. doi: 10.2147/COPD.S277523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stickland MK, Neder JA, Guenette JA, O’Donnell DE, Jensen D. Using cardiopulmonary exercise testing to understand dyspnea and exercise intolerance in respiratory disease. Chest . 2022;161:1505–1516. doi: 10.1016/j.chest.2022.01.021. [DOI] [PubMed] [Google Scholar]
- 9. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc . 1982;14:377–381. [PubMed] [Google Scholar]
- 10. Puente-Maestu L, Palange P, Casaburi R, Laveneziana P, Maltais F, Neder JA, et al. Use of exercise testing in the evaluation of interventional efficacy: an official ERS statement. Eur Respir J . 2016;47:429–460. doi: 10.1183/13993003.00745-2015. [DOI] [PubMed] [Google Scholar]
- 11. Ekström M. Why treatment efficacy on breathlessness in laboratory but not daily life trials? The importance of standardized exertion. Curr Opin Support Palliat Care . 2019;13:179–183. doi: 10.1097/SPC.0000000000000444. [DOI] [PubMed] [Google Scholar]
- 12. Elmberg V, Schiöler L, Lindow T, Hedman K, Malinovschi A, Lewthwaite H, et al. Reference equations for breathlessness during incremental cycle exercise testing. ERJ Open Res . 2023;9:00566-2022. doi: 10.1183/23120541.00566-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Killian KJ, Summers E, Jones NL, Campbell EJ. Dyspnea and leg effort during incremental cycle ergometry. Am Rev Respir Dis . 1992;145:1339–1345. doi: 10.1164/ajrccm/145.6.1339. [DOI] [PubMed] [Google Scholar]
- 14. Neder JA, Berton DC, Nery LE, Tan WC, Bourbeau J, O’Donnell DE, Canadian Cohort of Obstructive Lung Disease (CanCOLD) Collaborative Research Group, Canadian Respiratory Research Network (CRRN) A frame of reference for assessing the intensity of exertional dyspnoea during incremental cycle ergometry. Eur Respir J . 2020;56:2000191. doi: 10.1183/13993003.00191-2020. [DOI] [PubMed] [Google Scholar]
- 15. Bourbeau J, Tan WC, Benedetti A, Aaron SD, Chapman KR, Coxson HO, et al. CanCOLD Study Group Canadian Cohort Obstructive Lung Disease (CanCOLD): fulfilling the need for longitudinal observational studies in COPD. COPD . 2014;11:125–132. doi: 10.3109/15412555.2012.665520. [DOI] [PubMed] [Google Scholar]
- 16. Lewthwaite H, Benedetti A, Stickland MK, Bourbeau J, Guenette JA, Maltais F, et al. CanCOLD Collaborative Research Group and the Canadian Respiratory Research Network Normative peak cardiopulmonary exercise test responses in Canadian adults aged >/=40 years. Chest . 2020;158:2532–2545. doi: 10.1016/j.chest.2020.06.074. [DOI] [PubMed] [Google Scholar]
- 17. Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. ERS Global Lung Function Initiative Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J . 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hall GL, Filipow N, Ruppel G, Okitika T, Thompson B, Kirkby J, et al. Contributing GLI Network Members Official ERS technical standard: global lung function initiative reference values for static lung volumes in individuals of European ancestry. Eur Respir J . 2021;57:2000289. doi: 10.1183/13993003.00289-2020. [DOI] [PubMed] [Google Scholar]
- 19. Stanojevic S, Graham BL, Cooper BG, Thompson BR, Carter KW, Francis RW, et al. Global Lung Function Initiative TLCO Working Group, Global Lung Function Initiative (GLI) TLCO Official ERS technical standards: global lung function initiative reference values for the carbon monoxide transfer factor for Caucasians. Eur Respir J . 2017;50:1700010. doi: 10.1183/13993003.00010-2017. [DOI] [PubMed] [Google Scholar]
- 20. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ . 2015;350:g7594. doi: 10.1136/bmj.g7594. [DOI] [PubMed] [Google Scholar]
- 21. American Thoracic Society; American College of Chest Physicians. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med . 2003;167:211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 22. Stanojevic S, Kaminsky DA, Miller MR, Thompson B, Aliverti A, Barjaktarevic I, et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J . 2022;60:2101499. doi: 10.1183/13993003.01499-2021. [DOI] [PubMed] [Google Scholar]
- 23. American Thoracic Society; American College of Chest Physicians. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med . 2003;167:211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 24. Ross BA, Brotto AR, Fuhr DP, Phillips DB, van Diepen S, Bryan TL, et al. The supine position improves but does not normalize the blunted pulmonary capillary blood volume response to exercise in mild COPD. J Appl Physiol . 2020;128:925–933. doi: 10.1152/japplphysiol.00890.2019. [DOI] [PubMed] [Google Scholar]
- 25. Phillips DB, Brotto AR, Ross BA, Bryan TL, Wong EYL, Meah VL, et al. Canadian Respiratory Research Network Inhaled nitric oxide improves ventilatory efficiency and exercise capacity in patients with mild COPD: a randomized-control cross-over trial. J Physiol . 2021;599:1665–1683. doi: 10.1113/JP280913. [DOI] [PubMed] [Google Scholar]
- 26.Harrell FE., Jr. Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis. New York: Springer; 2015. [Google Scholar]
- 27.Harrell E.2004. https://biostat.app.vumc.org/wiki/Main/SasMacros
- 28. Lewthwaite H, Elsewify O, Niro F, Bourbeau J, Guenette JA, Maltais F, et al. CanCOLD Collaborative Research Group; Canadian Respiratory Research Network Normative cardiopulmonary exercise test responses at the ventilatory threshold in Canadian adults 40 to 80 years of age. Chest . 2021;159:1922–1933. doi: 10.1016/j.chest.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jensen D, Schaeffer MR, Guenette JA. Pathophysiological mechanisms of exertional breathlessness in chronic obstructive pulmonary disease and interstitial lung disease. Curr Opin Support Palliat Care . 2018;12:237–245. doi: 10.1097/SPC.0000000000000377. [DOI] [PubMed] [Google Scholar]
- 30. Laviolette L, Laveneziana P, ERS Research Seminar Faculty Dyspnoea: a multidimensional and multidisciplinary approach. Eur Respir J . 2014;43:1750–1762. doi: 10.1183/09031936.00092613. [DOI] [PubMed] [Google Scholar]
- 31. Lewthwaite H, Jensen D. Multidimensional breathlessness assessment during cardiopulmonary exercise testing in healthy adults. Eur J Appl Physiol . 2021;121:499–511. doi: 10.1007/s00421-020-04537-9. [DOI] [PubMed] [Google Scholar]
- 32. Lansing RW, Im BS, Thwing JI, Legedza AT, Banzett RB. The perception of respiratory work and effort can be independent of the perception of air hunger. Am J Respir Crit Care Med . 2000;162:1690–1696. doi: 10.1164/ajrccm.162.5.9907096. [DOI] [PubMed] [Google Scholar]
- 33. Ekström M, Ferreira D, Chang S, Louw S, Johnson MJ, Eckert DJ, et al. Australian National Palliative Care Clinical Studies Collaborative Effect of regular, low-dose, extended-release morphine on chronic breathlessness in chronic obstructive pulmonary disease: the beams randomized clinical trial. JAMA . 2022;328:2022–2032. doi: 10.1001/jama.2022.20206. [DOI] [PMC free article] [PubMed] [Google Scholar]