Chronic obstructive pulmonary disease (COPD), a disease of lung airways and alveoli, is the third leading cause of death worldwide (1, 2). Once the disease is established, COPD cannot be reversed, though in many cases its progression can be slowed by avoiding certain environmental toxins. Risk factors that contribute to the airway remodeling and emphysema seen in COPD include tobacco smoke, air pollutants, genetics, age, infections, sex, and adverse socioeconomic factors (3). Cigarette smoking (CS) is the greatest risk factor among all, and new studies suggest that most chronic smokers will develop some form of airway impairment caused by COPD, much more than the initially estimated 15% of smokers (4). Prior research has uncovered that extracellular vesicles (EVs) are impacted by CS and play an important role in COPD pathophysiology (5).
EVs are nanosized particles enclosed in a membrane of lipid bilayers and serve as biochemical messengers in the extracellular space (6). They may be generated and secreted by nearly any cell type in the body and alter the function of the neighboring or far-off recipient cells (5). EV cargo can include nucleic acids, proteins, lipids, and other biomolecules. In this issue of the Journal, Madison and colleagues (pp. 1115–1125) report the link between CS and the production of EVs armed with surface-bound proteases: neutrophil elastase (NE) and macrophage metalloelastase, also known as matrix metalloproteinase-12 (MMP-12), that are well known to cause alveolar destruction (emphysema), inflammation, and increased airway mucin production in COPD (7, 8, 9). This research comes from an outstanding team of scientists who have made significant contributions to the study of NE and EVs in COPD and CS-induced pulmonary conditions. The study expands on their previous findings that activated polymorphonuclear neutrophils release NE-positive EVs into the airways of individuals with COPD, which cause significant alveolar tissue damage when introduced to the lungs of naive mice (10). In the present study, the authors compared EVs from human BAL fluid (BALF) of patients without COPD (n = 24), defined as FEV1/FVC >0.7 on pulmonary function tests, who were stratified by smoking status (current, former, and never). EVs from BALF were isolated using differential ultracentrifugation and injected intratracheally into mice to see the effects on the lungs. The authors observed that airway EVs from smokers without COPD can cause alveolar damage, and this is caused by the additive effect of NE and MMP-12 present on these EVs. Interestingly, EVs from former smokers also had the capacity to elicit lung pathology in naive mice, although at a relatively lower level.
The authors also exposed mice to cigarette smoke for increasing durations from 1 week to 6 months and found that just 2 weeks of CS exposure in mice was enough to induce increased production of protease-containing small-sized EVs in their airways. These protease-rich airway EVs from CS-exposed mice had the ability to cause emphysematous changes in the lungs of naive syngeneic mice. This pathogenic capacity of EVs increased with a longer duration of CS exposure of donor mice, as observed by an increase in airway resistance and development of right ventricular hypertrophy in mice receiving airway EVs from mice exposed to CS for 3 months. The gene knockout and pharmacological inhibitor studies suggested that the major emphysematous effects were caused by NE and MMP-12 proteases. It remains to be seen whether blocking NE and MMP-12 signaling would also reverse the CS EV-mediated right ventricular hypertrophy and airway resistance.
Fascinatingly, after 2 weeks of CS exposure, BALF EVs from CS-exposed mice caused considerably more alveolar damage in naive mice than when given to CS-exposed mice. The authors suggest that there is a protective mechanism(s) against alveolar damage caused by protease-containing EVs in the CS-exposed airways that were not present in the CS-naive airways. This new insight into the protective response pathway of CS EVs remains up for investigation. Furthermore, the authors showed that the EV preparation from CS-exposed mice mostly comprised macrophage- and neutrophil-derived EVs, with both types contributing roughly equally to the total EV pool. However, the involvement of other MMPs and EVs from other cell types, such as epithelial cells, in CS-induced emphysematous changes as reported earlier (11) cannot be ruled out. Notably, they also observed a decrease in the percentage of macrophages in BALF from CS-exposed mice compared with air-exposed mice, whereas neutrophils increased. To better understand the relative contribution of macrophage- and neutrophil-derived EVs, studies comparing cell-specific EVs from mice exposed to varying lengths of CS exposure will be needed.
A logistical challenge with conventional CS exposure preclinical models is that it can take weeks to months of CS exposure before emphysematous changes are observed in the alveoli of mouse lungs. The ability of EVs from CS-exposed mice to recapitulate the matrix degradation associated with COPD in naive mice within weeks has potentially created a novel model to study CS-induced lung damage, although conventional CS exposure models are still required to isolate EVs. The solution presented to this by the authors is cryopreserving EVs for future use with EVs from one CS mouse that are enough to cause disease in numerous naïve mice. Although EVs can be preserved at −80°C, there remain questions regarding the long-term integrity of NE and MMP-12 activity linked to EVs during cryopreservation. Studies suggest that the integrity of EV surface and membrane proteins, EV size, and EV zeta potential are all affected by cryopreservation over time (12, 13). This is especially important to consider here, because NE and MMP-12 are surface bound, and, in addition to protein function being affected, changes in EV size and electrostatic potential may impact the function of these proteases and consequently their ability to cause pathological changes in mice.
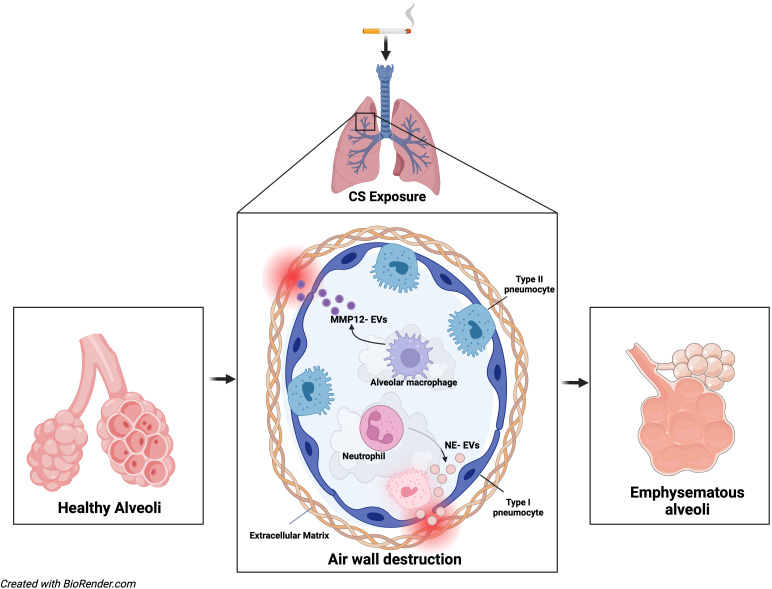
Overall, the study by Madison and colleagues suggests that CS alone has the capacity to generate airway EVs rich in NE and MMP-12 proteases that can initiate phenotypic changes in lungs similar to COPD (Figure 1). This provides us with a strong leap forward not only in our understanding of COPD pathophysiology but also in our understanding of EV contributions to chronic lung disease. Although generally safe, bronchoscopy may cause mechanical trauma to the airway (14). A follow-up study may include investigating if protease-containing EVs derived from polymorphonuclear neutrophils or macrophages can be captured from the bloodstream or respiratory secretions and correlate with COPD symptoms and disease severity, because this would be a much less invasive and translatable way to conduct future EV-based diagnostic studies.
Figure 1.
Potential cigarette smoke (CS) exposure–mediated alveolar changes. CS disrupts the normal function of alveolar macrophages and neutrophils and releases NE- and MMP-12–loaded EVs in the airways, which can damage the airway wall, resulting in chronic obstructive pulmonary disease–like emphysematous changes. EVs = extracellular vesicles; MMP-12 = matrix metalloproteinase-12; NE = neutrophil elastase.
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.202309-1630ED on September 29, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Varmaghani M, Dehghani M, Heidari E, Sharifi F, Moghaddam SS, Farzadfar F. Global prevalence of chronic obstructive pulmonary disease: systematic review and meta-analysis. East Mediterr Health J . 2019;25:47–57. doi: 10.26719/emhj.18.014. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd)
- 3. Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet . 2007;370:765–773. doi: 10.1016/S0140-6736(07)61380-4. [DOI] [PubMed] [Google Scholar]
- 4. Rennard SI, Vestbo J. COPD: the dangerous underestimate of 15% Lancet . 2006;367:1216–1219. doi: 10.1016/S0140-6736(06)68516-4. [DOI] [PubMed] [Google Scholar]
- 5. Mohan A, Agarwal S, Clauss M, Britt NS, Dhillon NK. Extracellular vesicles: novel communicators in lung diseases. Respir Res . 2020;21:175. doi: 10.1186/s12931-020-01423-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells . 2019;8:727. doi: 10.3390/cells8070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Madison MC, Margaroli C, Genschmer KR, Russell DW, Wells JM, Sari E, et al. Protease-armed, pathogenic extracellular vesicles link smoking and COPD. Am J Respir Crit Care Med . 2023;208:1115–1125. doi: 10.1164/rccm.202303-0471OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Voynow JA, Shinbashi M. Neutrophil elastase and chronic lung disease. Biomolecules . 2021;11:1065. doi: 10.3390/biom11081065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gilowska I, Majorczyk E, Kasper Ł, Bogacz K, Szczegielniak J, Kasper M, et al. The role of MMP-12 gene polymorphism - 82 A-to-G (rs2276109) in immunopathology of COPD in polish patients: a case control study. BMC Med Genet . 2019;20:19. doi: 10.1186/s12881-019-0751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Genschmer KR, Russell DW, Lal C, Szul T, Bratcher PE, Noerager BD, et al. Activated PMN exosomes: pathogenic entities causing matrix destruction and disease in the lung. Cell . 2019;176:113–126.e15. doi: 10.1016/j.cell.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moon HG, Kim SH, Gao J, Quan T, Qin Z, Osorio JC, et al. CCN1 secretion and cleavage regulate the lung epithelial cell functions after cigarette smoke. Am J Physiol Lung Cell Mol Physiol . 2014;307:L326–L337. doi: 10.1152/ajplung.00102.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maroto R, Zhao Y, Jamaluddin M, Popov VL, Wang H, Kalubowilage M, et al. Effects of storage temperature on airway exosome integrity for diagnostic and functional analyses. J Extracell Vesicles . 2017;6:1359478. doi: 10.1080/20013078.2017.1359478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gelibter S, Marostica G, Mandelli A, Siciliani S, Podini P, Finardi A, et al. The impact of storage on extracellular vesicles: a systematic study. J Extracell Vesicles . 2022;11:e12162. doi: 10.1002/jev2.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stahl DL, Richard KM, Papadimos TJ. Complications of bronchoscopy: a concise synopsis. Int J Crit Illn Inj Sci . 2015;5:189–195. doi: 10.4103/2229-5151.164995. [DOI] [PMC free article] [PubMed] [Google Scholar]