Abstract
Rationale
Patients with chronic obstructive pulmonary disease (COPD) and type 2 diabetes (T2D) have worse clinical outcomes compared with patients without metabolic dysregulation. GLP-1 (glucagon-like peptide 1) receptor agonists (GLP-1RAs) reduce asthma exacerbation risk and improve FVC in patients with COPD.
Objectives
To determine whether GLP-1RA use is associated with reduced COPD exacerbation rates, and severe and moderate exacerbation risk, compared with other T2D therapies.
Methods
A retrospective, observational, electronic health records–based study was conducted using an active comparator, new-user design of 1,642 patients with COPD in a U.S. health system from 2012 to 2022. The COPD cohort was identified using a previously validated machine learning algorithm that includes a natural language processing tool. Exposures were defined as prescriptions for GLP-1RAs (reference group), DPP-4 (dipeptidyl peptidase 4) inhibitors (DPP-4is), SGLT2 (sodium-glucose cotransporter 2) inhibitors, or sulfonylureas.
Measurements and Main Results
Unadjusted COPD exacerbation counts were lower in GLP-1RA users. Adjusted exacerbation rates were significantly higher in DPP-4i (incidence rate ratio, 1.48 [95% confidence interval, 1.08–2.04]; P = 0.02) and sulfonylurea (incidence rate ratio, 2.09 [95% confidence interval, 1.62–2.69]; P < 0.0001) users compared with GLP-1RA users. GLP-1RA use was also associated with significantly reduced risk of severe exacerbations compared with DPP-4i and sulfonylurea use, and of moderate exacerbations compared with sulfonylurea use. After adjustment for clinical covariates, moderate exacerbation risk was also lower in GLP-1RA users compared with DPP-4i users. No statistically significant difference in exacerbation outcomes was seen between GLP-1RA and SGLT2 inhibitor users.
Conclusions
Prospective studies of COPD exacerbations in patients with comorbid T2D are warranted. Additional research may elucidate the mechanisms underlying these observed associations with T2D medications.
Keywords: type 2 diabetes mellitus, obesity, obstructive lung diseases, health services research
At a Glance Commentary
Scientific Knowledge on the Subject
Metabolic dysregulation is common in patients with chronic obstructive pulmonary disease (COPD). Patients with type 2 diabetes (T2D) and COPD are at elevated risk of poor COPD-related outcomes, including acute exacerbations of COPD. GLP-1 (glucagon-like peptide 1) receptor agonists (GLP-1RAs) are an increasingly used U.S. Food and Drug Administration–approved drug class for T2D with pleotropic effects, including on the airway, yet understanding of their impact on patients with comorbid COPD is limited.
What This Study Adds to the Field
Using electronic health records from patients with COPD treated with GLP-1RAs and other T2D medications in the context of routine care, this study identified an association between GLP-1RA use and reduced COPD exacerbations compared with several alternative T2D therapies. These findings may inform treatment choices for patients with comorbid COPD and T2D, reducing morbidity from exacerbations and from corticosteroids used to treat exacerbations. These findings also highlight the potential relevance of metabolic pathways in COPD, warranting mechanistic and prospective clinical study.
Chronic obstructive pulmonary disease (COPD) exacerbations drive morbidity and cost for patients with COPD (1). Globally, rates of type 2 diabetes (T2D) and the metabolic syndrome are rising (2), increasing multimorbidity in patients with COPD (3, 4). Patients with T2D and COPD are at elevated risk of poor COPD-related outcomes, including severe acute exacerbations of COPD, higher healthcare resource use, and nonrespiratory complications (4).
GLP-1 (glucagon-like peptide 1) receptor agonists (GLP-1RAs) are U.S. Food and Drug Administration (FDA)–approved drugs for T2D with pleotropic effects, including on the airway (5, 6). Preclinical studies of the effects of GLP-1RAs on airway inflammation in lean and obese asthma models (7, 8) include decreased airway hyperreactivity, mucous metaplasia, and lung IL-33 expression (7), all features also associated with COPD (9–11).
In humans, GLP-1RAs increase FVC in patients with T2D without diagnosed respiratory disease (12). GLP-1RA therapy was associated with fewer asthma exacerbations compared with other T2D treatments in a retrospective study (13). Recent observational studies in COPD and populations with combined COPD and asthma suggest that GLP-1RAs may similarly decrease hospitalization and respiratory exacerbation risk (14, 15). However, a randomized controlled trial of a GLP-1RA in COPD demonstrated no effect on FEV1; exacerbations were not reported (16). Other T2D therapies, including SGLT2 (sodium-glucose cotransporter 2) inhibitors (SGLT2is) (17) and metformin (18), may also benefit COPD outcomes.
COPD presents unique challenges for large-scale data phenotyping that can be addressed with precise algorithm development (19). Electronic health records (EHRs) include pulmonary function testing, smoking history, and laboratory results, which administrative databases often lack. Prior observational studies of T2D medications and COPD exacerbations are also limited by a high prevalence of comorbid asthma diagnoses and potential mediation by change in body mass index (BMI) and glycemic control (14, 17). These gaps merit clarification to improve care for patients with COPD and T2D.
We hypothesized that patients with T2D and COPD initiating GLP-1RAs would have fewer COPD exacerbations than patients initiating alternative diabetes therapy in an EHR database. Our prespecified objectives were to test the association between GLP-1RA initiation and COPD exacerbation rates as well as moderate and severe exacerbation risk.
Some of the results of these studies have been previously reported in the form of an abstract (20).
Methods
Study Design and Data Source
We conducted an observational, retrospective analysis of EHR data from an integrated healthcare system in the United States (Mass General Brigham [MGB]) serving 1.5 million people annually across 11 inpatient hospitals, a rehabilitation network, 20 community health centers, a home-based service network, and hundreds of outpatient clinics. EHR data are stored for research in the MGB Research Patient Data Registry. Numerous real-world studies have been published that leverage the Research Patient Data Registry, including in COPD (21), T2D (22), and obesity (23). This study and a waiver of the requirement to obtain informed consent were approved by the MGB Institutional Review Board (protocol 2017P001730), and the study follows the Strengthening the Reporting of Observational Studies in Epidemiology reporting guideline for cohort studies (24).
Study Population
To identify the COPD population in the MGB EHR, we applied an internally and externally validated COPD phenotyping algorithm (CRTPRT+) (19). Patients with COPD-specific diagnosis codes that met a data floor threshold, requiring at least three diagnosis codes related to COPD on distinct dates and at least one unstructured medical note, were selected. A combination of extracted diagnosis codes, medication orders, and a natural language processing (NLP) component for smoking and FEV1 in a linear model classified COPD cases (positive predictive value 91.7%) (19).
We then selected all patients with COPD with new prescriptions for DPP-4 (dipeptidyl peptidase 4) inhibitors (DPP-4is), GLP-1RAs, SGLT2is, or sulfonylureas (see Table E1 in the online supplement) between January 1, 2012, and May 27, 2022 (Figure 1), with study index date (exposure) defined as the date of prescription. Thiazolidinediones were not included as a comparator, because of cardiovascular safety concerns, leading to significantly decreased use of the class compared with other available agents. Prior studies have examined thiazolidinediones in the context of COPD (25–27). The study period was defined as 6 months from the index date on the basis of adherence patterns to T2D therapies (28, 29). Patient demographic and clinical characteristics at baseline and after the study period were extracted for analysis, detailed below in Covariate Selection.
Figure 1.
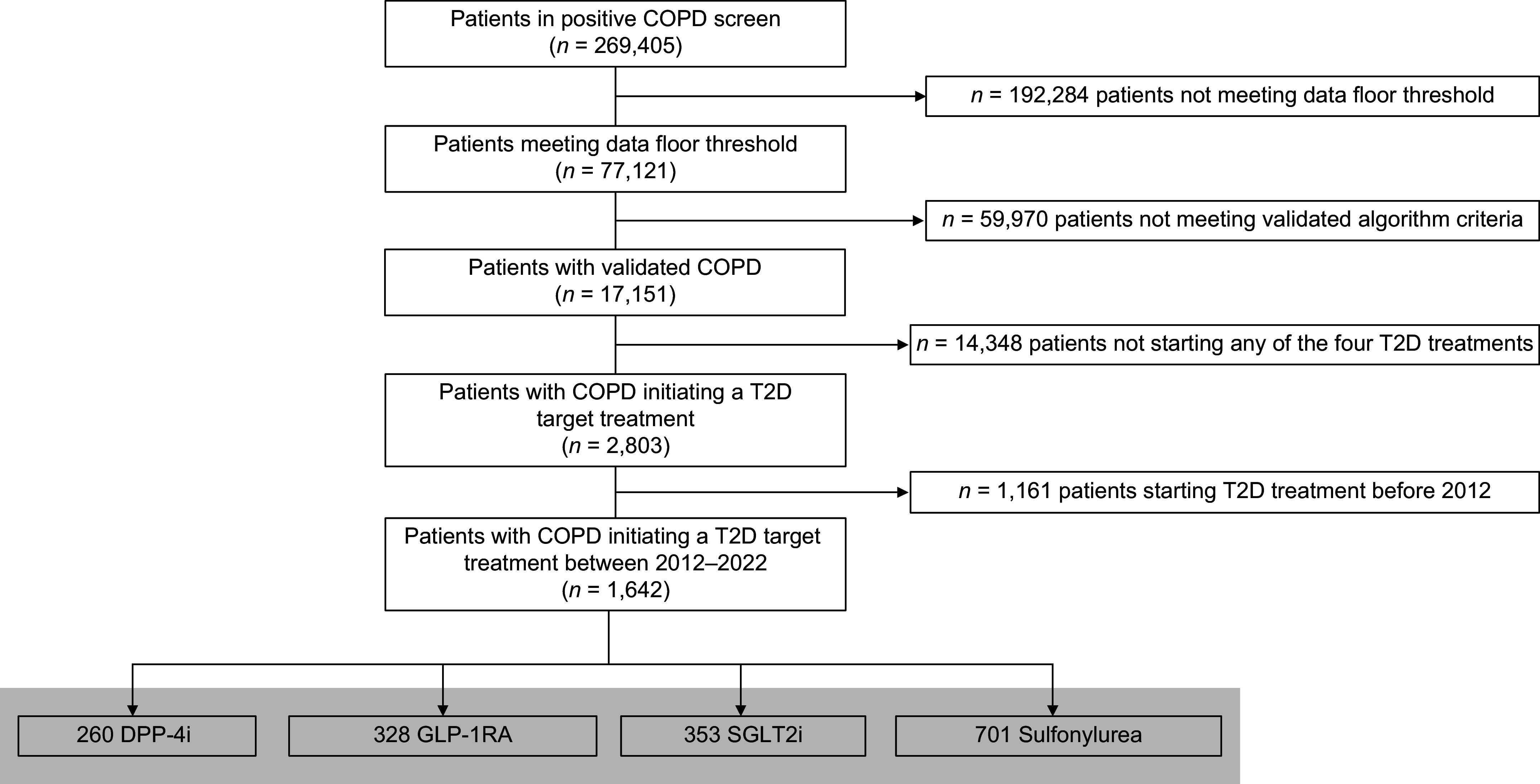
Electronic health record patient cohort selection. COPD = chronic obstructive pulmonary disease; DPP-4i = dipeptidyl peptidase 4 inhibitor; GLP-1RA = glucagon-like peptide 1 receptor agonist; SGLT2i = sodium-glucose cotransporter 2 inhibitor; T2D = type 2 diabetes.
Outcome Measures and Validation
The primary outcome was the combined count of moderate and severe exacerbations in six months after drug initiation. A 7-day rolling window was applied so that more than one episode in a 7-day period was considered a single event. On the basis of a review of the literature, a severe exacerbation was defined as an inpatient encounter with a COPD code as the primary and/or principal diagnosis or admitting diagnosis (see Table E2) (30–32). A moderate exacerbation was defined as a prednisone prescription in the six-month period from the index date, consistent with prior studies that have distinguished moderate from severe exacerbations in a real-world clinical practice database (30). This is also consistent with clinical guidelines that recommend oral glucocorticoids for all moderate COPD exacerbations, whereas antibiotic use is variable and indicated only if specific patient criteria are met (1).
As the prednisone-based outcome has not previously been validated in a U.S.-based EHR database, we conducted an internal validation study of the moderate exacerbation definition. The definition had high sensitivity (97.2%) and specificity (88.6%) with a positive predictive value of 87.5% and a negative predictive value of 97.5%. Additional details on the study methods are provided in the online supplement.
The secondary outcome was exacerbation risk, assessed as time (days) to COPD exacerbation from the index data. Moderate and severe exacerbation types were examined separately.
Covariate Selection
On the basis of prior literature and using a directed acyclic graph (see Figure E1), we identified causal paths between the exposure and outcome. These included demographics (EHR-recorded age, sex [legal and/or administrative sex], race [Asian, Black, White, or other], ethnicity [Hispanic or non-Hispanic]) and index year of medication initiation; season of initiation (flu season, defined as October 1 to April 31 by CDC criteria) was included and considered a precision variable (33, 34). The following variables were captured ⩾14 to ⩽730 days before the index date: baseline comorbidity status as determined using International Statistical Classification of Diseases and Related Health Problems (ICD) codes and calculated using the Elixhauser Comorbidity Index (ECI); total healthcare encounters, as a proxy for healthcare system use; medication orders for metformin, given its potential protective effects in emphysema progression in patients with smoking histories (18) and its antiinflammatory effects in preclinical models of airway inflammation (35), and commonly used COPD maintenance medications including short-acting β-agonists, short-acting muscarinic antagonists, long-acting β-agonists (LABAs) without and with inhaled corticosteroids, long-acting muscarinic antagonists, and triple-therapy inhalers (see Table E1) that contribute to COPD control. Although variable year to year, recent exacerbations are a predictor of future exacerbation risk; exacerbation counts ⩽12 months of the index date were identified (36). Baseline metabolic covariates including BMI, weight, and HbA1c as a proxy for glucose control (homeostatic model assessment for insulin resistance and other direct measures of insulin resistance are not available in routine care) (37) were captured within 60 days of the index date. Changes in BMI and HbA1c (the differences between baseline and final values during the study period) were defined by values within 60 days of the patient-level study end date. If BMI was unrecorded, it was calculated using the baseline weight variable and height carried forward from closest entry before the index date. Smoking history and FEV1 (percentage of predicted) closest to the index date were extracted using NLP as described earlier (19). FEV1 was used to calculate Global Initiative for Chronic Obstructive Lung Disease (GOLD) grades.
Statistical Analysis
Patient baseline demographics are described using proportions for categorical variables and mean (SD) or median (interquartile range) for continuous variables. The study design is detailed in the online supplement (see Figure E2). Multicollinearity was assessed in the models that are estimated and reported if significant. Statistical analyses were conducted using SAS version 9.4 (SAS Institute, Inc.) and R Statistical Software (version 4.1.3; www.r-project.org). ECI was calculated using the R package comorbidity (38). P values <0.05 were considered to indicate statistical significance.
For the primary outcome, negative binomial regression models were used to test incident rate differences among treatment groups, with GLP-1RA users as the reference. We adjusted our models to control for confounding as follows: age, sex, race, index year, ECI, total health system encounter history, season of initiation, current smoking history, concurrent metformin use, and COPD medications were included in the “clinical” model. Baseline HbA1c and baseline BMI, treated as continuous variables, were added to generate a “metabolic” model. Results are reported as unadjusted and adjusted incidence rate ratios (IRRs).
For the secondary outcome, Kaplan-Meier curves were used to demonstrate the unadjusted proportion of patients who attained the event (moderate or severe exacerbation) at each time point. Hazard ratios (HRs) and their 95% confidence intervals (CIs) were computed to estimate the unadjusted and adjusted associations (a weighted average of the true HR) between time of exacerbation and treatment groups at each time point over the follow-up period. Clinical and metabolic models are presented.
We conducted two sensitivity analyses of the primary and secondary outcomes: 1) recent exacerbation history was included as an additional predictor, and 2) the moderate exacerbation outcome definition was expanded to include a prednisone and/or antibiotic prescription associated with a clinician outpatient encounter for COPD exacerbation (39). As with the original moderate exacerbation definition, a 7-day rolling window was applied so that coded encounters and prescriptions within 7 days were considered a single event. Antibiotics were defined by classes specified in the GOLD guidelines for exacerbation treatment and cross-referenced for a labeled indication (United States) for respiratory infection (see Table E1) (1).
To explore potential effect modification by baseline BMI and baseline COPD severity on exacerbations, we performed two subgroup analyses: 1) by baseline BMI category (underweight [<18.5 kg/m2], healthy weight [⩾18.5 to ⩽24.99 kg/m2], overweight [⩾25 to ⩽29.99 kg/m2], and obese [⩾30 kg/m2]) on the basis of categories that reflect GLP-1RA treatment criteria; and 2) by COPD severity using GOLD grades on the basis of baseline FEV1: mild (GOLD grade 1; ⩾80% predicted), moderate (GOLD grade 2; ⩾50% to <80%), and severe and very severe (GOLD grades 3 and 4; <50%) (1). Both subgroup analyses included the clinical and metabolic models.
Two exploratory analyses were conducted: 1) primary and secondary outcomes were assessed at 12 months from the index date, and 2) to estimate the controlled direct effect of change in BMI and glycemic control on exacerbation rates, we added these variables to the metabolic model.
Results
Baseline Characteristics of the Study Population
Of 17,151 patients meeting COPD criteria (Figure 1), a total of 1,642 patients initiated GLP-1RAs (n = 328 [19.98% of the total cohort]), DPP-4is (n = 260 [15.83%]), SGLT2is (n = 353 [21.50%]), or sulfonylureas (n = 701 [42.69%]) in the study period, summarized in Tables 1 and E3. The median age of the total cohort was 70.11 (interquartile range, 63.31–77.32) years. GLP-1RA users included more younger and female patients (Table 1). Race and ethnicity did not differ across the groups; the cohort was predominantly White, consistent with the demographics of the health system. There was no difference in current smoking compared with prior smoking history or never-smokers by T2D treatment; across groups, ⩾90% of patients had histories of smoking. The median baseline BMI was overweight or obese across groups, with GLP-1RA users having a significantly higher median BMI (34.9 kg/m2). Only 10 patients had BMIs <18.5 kg/m2 (underweight)—2 in the DPP-4i group, none in the GLP-1RA group, 2 in the SGLT2i group, and 6 in the sulfonylurea groups—precluding further analysis of this single subgroup. Rates of BMI missingness (see Table E4) ranged from 1.42% (SGLT2is) to 14.2% (sulfonylureas). Fewer BMI values were available at the end of the study period, resulting in lower counts of the BMI change variable. Sulfonylurea users lost the least weight and GLP-1RA users lost the most weight during the study period (see Table E3).
Table 1.
Patient Characteristics
| Treatment Group |
P Value | ||||
|---|---|---|---|---|---|
| DPP-4is | GLP-1RAs | SGLT2is | Sulfonylureas | ||
| Patient count, n | 260 | 328 | 353 | 701 | — |
| Age, yr, median (IQR) | 72.9 (64.0 to 79.9) | 67.2 (60.8 to 73.7) | 72.0 (64.9 to 78.4) | 69.8 (63.8 to 76.6) | <0.0001 |
| Female sex, n (%) | 101 (38.9) | 139 (42.4) | 116 (32.9) | 285 (40.7) | 0.047 |
| Race, n (%)* | |||||
| Asian | 2 (0.77) | 1 (0.30) | 2 (0.57) | 9 (1.28) | 0.22 |
| Black | 17 (6.54) | 21 (6.4) | 27 (7.65) | 30 (4.28) | |
| White | 226 (86.92) | 283 (86.28) | 297 (84.14) | 629 (89.73) | |
| Other | 9 (3.46) | 15 (4.57) | 18 (4.25) | 21 (2.00) | |
| Unknown | 6 (2.31) | 8 (2.44) | 9 (2.55) | 12 (1.71) | |
| Ethnicity, n (%) | |||||
| Hispanic | 7 (2.69) | 4 (1.22) | 10 (2.83) | 11 (1.57) | 0.78 |
| Unknown | 20 (7.69) | 30 (9.15) | 31 (8.78) | 67 (9.56) | |
| Current smoking, n (%) | 69 (26.6) | 117 (35.7) | 134 (38.0) | 225 (32.1) | 0.09 |
| Past | 164 (63.3) | 178 (54.3) | 191 (54.1) | 415 (29.2) | |
| Never | 26 (10) | 33 (10) | 28 (7.9) | 61 (8.7) | |
| BMI, kg/m2, median (IQR) | |||||
| Baseline | 29.7 (25.9 to 34.5) | 34.9 (30.5 to 40.3) | 30.8 (27.2 to 35.3) | 30.7 (26.6 to 34.8) | <0.0001 |
| BMI change | −0.21 (−1.2 to 0.73) | −0.73 (−2.1 to 0.3) | −0.69 (−1.7 to 0.089) | 0 (−0.96 to 0.92) | <0.0001 |
| Elixhauser Comorbidity Index, median (IQR) | 6 (2 to 8) | 6 (4 to 9) | 8 (5 to 10) | 5 (2 to 7) | <0.0001 |
| Rheumatologic disease, n (%) | 12 (4.62) | 21 (6.40) | 27 (7.65) | 32 (4.56) | 0.16 |
| Health system encounters within 2 yr of index date, median (IQR) | 54 (9.5 to 132) | 106.5 (38 to 178.5) | 124 (59 to 205) | 29 (4 to 88) | <0.0001 |
| Year of drug initiation, median year (IQR) | 2017 (2015 to 2019) | 2019 (2017 to 2021) | 2021 (2019 to 2021) | 2016 (2013 to 2018) | <0.0001 |
| Season of initiation, flu season,† yes, n (%) | 141 (54.23) | 199 (60.67) | 222 (62.89) | 403 (57.49) | 0.13 |
| Exacerbations ⩽12 mo before index date, mean (SD) | 1.10 (2.15) | 1.13 (2.04) | 1.36 (2.32) | 1.24 (2.21) | 0.35 |
| FEV1 (% predicted), baseline, median (IQR) | 56 (45 to 69.5) | 57 (44 to 71) | 56 (45 to 70) | 55 (42 to 68) | 0.06 |
| GOLD grade, baseline, n, (%) | |||||
| 1 | 30 (12.3) | 43 (13.7) | 43 (12.9) | 79 (11.8) | 0.07 |
| 2 | 131 (53.7) | 165 (52.6) | 175 (52.6) | 334 (49.9) | — |
| 3 | 70 (28.7) | 94 (30) | 104 (31.2) | 199 (29.8) | — |
| 4 | 13 (5.3) | 12 (3.8) | 11 (3.3) | 57 (8.5) | — |
| COPD medications, baseline use, n (%) | |||||
| SABA | 175 (67.31) | 268 (81.71) | 285 (80.74) | 415 (59.20) | <0.0001 |
| SAMA | 103 (39.62) | 167 (50.91) | 179 (50.71) | 242 (34.52) | <0.0001 |
| LABA | 122 (46.92) | 184 (56.10) | 205 (58.07) | 275 (39.23) | <0.0001 |
| ICS–LABA | 114 (43.9) | 156 (47.6) | 178 (50.4) | 260 (37.1) | <0.0001 |
| LAMA | 128 (49.23) | 183 (55.79) | 222 (62.89) | 26 (37.23) | <0.0001 |
| ICS–LABA–LAMA | 5 (1.92) | 12 (3.66) | 20 (5.67) | 9 (1.28) | 0.0004 |
| Metformin, baseline use, n (%) | 124 (47.69) | 179 (54.57) | 179 (50.71) | 244 (34.81) | <0.0001 |
| HbA1c, mmol/mol, median (IQR) | |||||
| Baseline | 7.6 (6.6 to 8.7) | 7.6 (6.3 to 9.0) | 7.1 (6.2 to 8.4) | 7.4 (6.5 to 8.3) | 0.07 |
| Change | −0.4 (−1.3 to 0.2) | −0.5 (−1.3 to 0.1) | −0.2 (−1 to 0.3) | −0.6 (−1.5 to 0.2) | 0.11 |
| One or more severe exacerbations during the study period, n patients (%) | 39 (15) | 29 (8.84) | 43 (12.18) | 125 (17.83) | 0.0009 |
| One or more moderate exacerbation during the study period, n patients (%) | 71 (27.31) | 76 (23.17) | 85 (24.08) | 220 (31.38) | 0.015 |
Definition of abbreviations: BMI = body mass index; COPD = chronic obstructive pulmonary disease; DPP-4i = dipeptidyl peptidase 4 inhibitors; GLP-1RA = glucagon-like peptide 1 receptor agonist; GOLD = Global Initiative for Chronic Obstructive Lung Disease; IQR = interquartile range; LABA = long-acting β-agonist; LAMA = long-acting muscarinic antagonist; SABA = short-acting muscarinic antagonist; SABA = short-acting β-agonist; SGLT2i = sodium-glucose cotransporter 2 inhibitor.
Race category “other” includes the following electronic health record–defined options: other, American Indian or Alaska native (n = 1; SGLT2i), and Native Hawaiian or other Pacific Islander (n = 1; GLP-1RA). Race category “unknown” includes electronic health record–defined categories of unknown, declined, and Missing.
Defined as October 1 to April 31 on the basis of CDC data (33).
SGLT2i users had a significantly higher median ECI compared with DPP-4i and sulfonylurea users. Congestive heart failure, hypertension, and renal failure were more common in SGLT2i users. Depression was highest in the SGLT2i and GLP-1RA groups. There were no differences in rates of rheumatologic disease. Sulfonylurea users had the lowest ECI and the fewest encounters with the health system within 2 years of the index date. DPP-4i and sulfonylurea users had the fewest inpatient encounters within 2 years of the index date (see Table E3). Flu season initiation of drugs did not differ (Table 1).
Mean exacerbation count in the year before study index date in all treatment groups was less than two and did not differ across groups (P = 0.35) (Tables 1 and E3). Baseline FEV1 and GOLD grade also did not significantly differ across groups. GLP-1RA users had the highest rates of short-acting β-agonist and short-acting muscarinic antagonist prescriptions; SGLT2i users had the highest rates of LABA, LABA with inhaled corticosteroid, long-acting muscarinic antagonist, and triple-therapy inhaler prescriptions; rates of use of these medications were higher among GLP-1RA users than DPP-4i and sulfonylurea users. Concurrent metformin use was highest among GLP-1RA and SGLT2i users. HbA1c was in the diabetic range for all groups, without significant differences at baseline. Missingness of HbA1c values was low, ranging from 2.69% (DPP-4is) to 6.13% (sulfonylureas) (see Table E4). All treatment groups demonstrated decreases in HbA1c during the study period, with no significant difference across groups (Table 1). Unadjusted counts of severe and moderate COPD exacerbations were significantly lower for GLP-1RA users during the study period.
Primary Outcome: COPD Exacerbation Rates
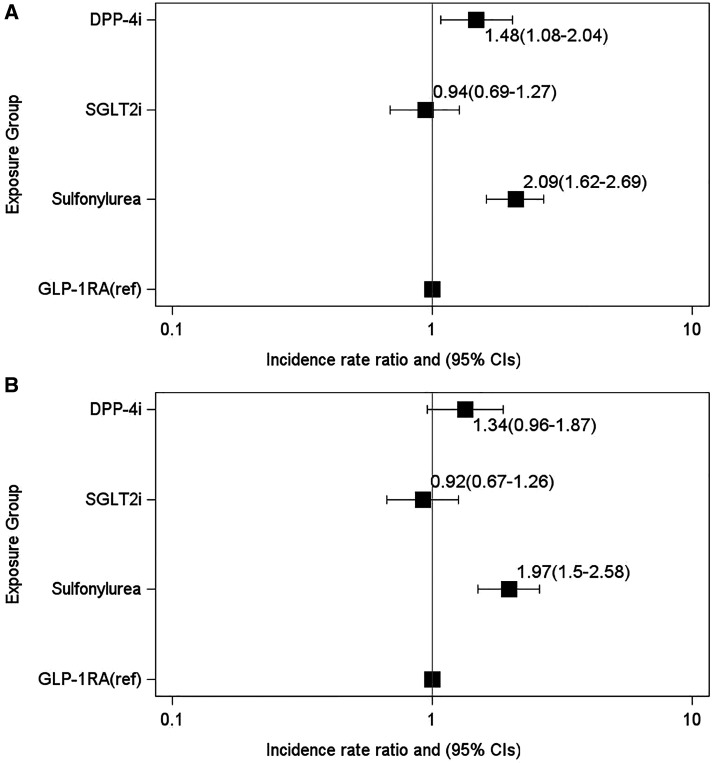
Unadjusted counts of COPD exacerbations were lowest among GLP-1RA users (Table 1). In the clinical model, exacerbation rates remained elevated among DPP-4i (IRR, 1.48 [95% CI, 1.08–2.04]; P = 0.02) and sulfonylurea (IRR, 2.09 [95% CI, 1.62–2.69]; P < 0.0001) users compared with GLP-1RA users (Figure 2A). In the metabolic model, rates among sulfonylurea users remained elevated (IRR, 1.97 [95% CI, 1.50–2.58]; P < .0001) compared with GLP-1RA users (Figure 2B; see Table E5, clinical and metabolic models). Across both models, rates among SGLT2i users were not significantly different.
Figure 2.
Incidence rate ratios of chronic obstructive pulmonary disease exacerbations and the association with GLP-1RAs or comparator treatments by six months after treatment initiation. (A and B) Negative binomial regression models adjusted for (A) clinical covariates (clinical model) and (B) metabolic covariates at baseline (metabolic model). The x-axes for both models are plotted on a log scale. CI = confidence interval; DPP-4i = dipeptidyl peptidase 4 inhibitor; GLP-1RA = glucagon-like peptide 1 receptor agonist; ref = reference group; SGLT2i = sodium-glucose cotransporter 2 inhibitor.
Secondary Outcome: Time to COPD Exacerbation Event
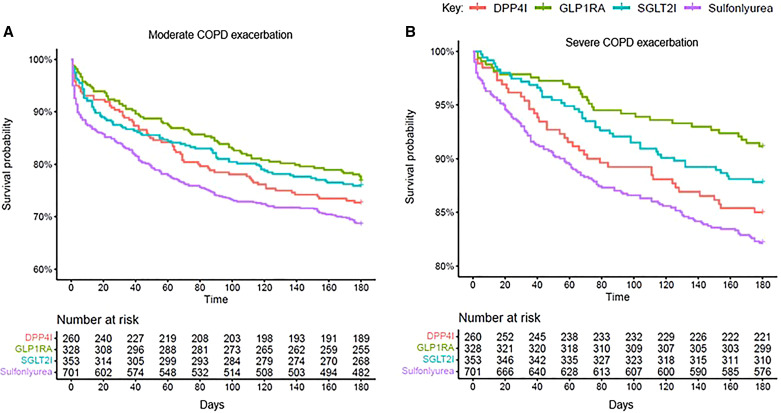
Compared with new GLP-1RA users, sulfonylurea users (HR, 1.48 [95% CI, 1.14–1.92]; P = 0.003) had a higher risk of moderate exacerbations, but not compared with DPP-4i and SGLT2i users (Figure 3A; Table 2, unadjusted). Both DPP-4i (HR, 1.77 [95% CI, 1.09–2.85]; P = 0.02) and sulfonylurea (HR, 2.15 [95% CI, 1.43–3.22]; P = 0.002) users had higher risk for severe exacerbations (Figure 3B; Table 2, unadjusted).
Figure 3.
(A and B) Unadjusted Kaplan-Meier survival curves indicating the time to onset of (A) moderate COPD exacerbations and (B) severe exacerbations. COPD = chronic obstructive pulmonary disease; DPP-4i = dipeptidyl peptidase 4 inhibitor; GLP-1RA = glucagon-like peptide 1 receptor agonist; SGLT2i = sodium-glucose cotransporter 2 inhibitor.
Table 2.
Cox Proportional Hazards Models Estimating the Association of Time to First COPD Exacerbation in Patients Initiating GLP-1RAs or Comparator Medications
| COPD Exacerbation Type | Drug Exposure | Unadjusted |
Clinical Model* |
Metabolic Model† |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | ||
| Moderate | DPP-4is | 1.48 | 0.88–1.69 | 0.22 | 1.52 | 1.09–2.14 | 0.01 | 1.30 | 0.92–1.82 | 0.14 |
| SGLT2is | 1.07 | 0.78–1.45 | 0.68 | 1.01 | 0.73–1.39 | 0.96 | 0.94 | 0.69–1.30 | 0.72 | |
| Sulfonylureas | 1.48 | 1.14–1.92 | 0.003 | 2.09 | 1.56–2.79 | <0.0001 | 1.92 | 1.45–2.54 | <0.0001 | |
| GLP-1RAs (ref) | — | — | — | — | — | — | — | — | — | |
| Severe | DPP-4is | 1.77 | 1.09–2.85 | 0.02 | 1.85 | 1.12–3.05 | 0.02 | 2.04 | 1.23–3.36 | 0.005 |
| SGLT2is | 1.40 | 0.87–2.24 | 0.16 | 1.41 | 0.87–2.29 | 0.16 | 1.28 | 0.79–2.08 | 0.31 | |
| Sulfonylureas | 2.15 | 1.43–3.22 | 0.002 | 2.21 | 1.42–3.44 | 0.0004 | 2.63 | 1.71–4.04 | <0.0001 | |
| GLP-1RAs (ref) | — | — | — | — | — | — | — | — | — | |
Definition of abbreviations: CI = confidence interval; COPD = chronic obstructive pulmonary disease; DPP-4i = dipeptidyl peptidase4 inhibitor; GLP-1RA = glucagon-like peptide 1 receptor agonist; HR = hazard ratio; ref = reference group; SGLT2i = sodium-glucose cotransporter 2 inhibitor.
Adjusted for age; sex; race; Elixhauser Comorbidity Index; two-year health system encounter history; season of initiation; smoking history; concurrent metformin use; and concurrent short-acting β-agonist, short-acting muscarinic antagonist, long-acting β-agonist, long-acting muscarinic antagonist, or triple-therapy use.
Also adjusted for baseline HbA1c and baseline body mass index.
In the clinical model, the adjusted risk of moderate exacerbation was elevated in both DPP-4i (HR, 1.52 [95% CI, 1.09–2.14]; P = 0.01) and sulfonylurea (HR, 2.09 [95% CI, 1.56–2.79; P < 0.0001) users but not SGLT2i users (Table 2). Similarly, risk of severe exacerbation was elevated for DPP-4i (HR, 1.85 [95% CI, 1.12–3.05]; P = 0.02) and sulfonylurea (HR, 2.21 [95% CI, 1.42–3.44; P = 0.0004) users, but not SGLT2i users compared with GLP-1RA users.
In the metabolic model, risk of moderate exacerbation remained increased for sulfonylurea users only (HR, 1.92 [95% CI, 1.45–2.54]; P < 0.0001) compared with GLP-1RA users but remained elevated for both DPP-4i (HR, 2.04 [95% CI, 1.23–3.36]; P = 0.005) and sulfonylurea (HR, 2.63 [95% CI, 1.71–4.04]; P < 0.0001) users for severe exacerbations (Table 2).
Sensitivity Analyses
Recent exacerbation history
After the inclusion of recent exacerbation history in the clinical and metabolic models, IRRs for COPD exacerbations for sulfonylurea users remained significantly different compared with those for GLP-1RA users. DPP-4i users still had numerically more exacerbations compared with GLP-1RA users, but this did not reach statistical significance (IRR, 1.22 [95% CI, 0.90–1.65]; P = 0.21; Table 3, clinical model). After also accounting for the direct effects of changes in weight and glucose control on exacerbations, GLP-1RA users had significantly fewer exacerbations compared with both sulfonylurea and DPP-4i users (Table 3, exploratory analysis).
Table 3.
IRRs of COPD Exacerbations and the Association with Glucagon-like Peptide 1 Receptor Agonists or Comparator Treatments, Inclusive of Recent Exacerbation* History
| Drug Exposure | Clinical Model† |
Metabolic Model† |
Exploratory Analysis, Changes in HbA1c and BMI‡ |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IRR | 95% CI | P Value | IRR | 95% CI | P Value | IRR | 95% CI | P Value | ||
| Moderate and severe COPD exacerbations | DPP-4is | 1.22 | 0.90–1.65 | 0.21 | 1.13 | 0.83–1.55 | 0.44 | 2.01 | 1.00–4.15 | 0.057 |
| SGLT2is | 0.94 | 0.70–1.26 | 0.67 | 0.89 | 0.66–1.20 | 0.44 | 1.67 | 0.85–3.27 | 0.13 | |
| Sulfonylureas | 1.59 | 1.25–2.03 | 0.0002 | 1.49 | 1.16–1.93 | 0.002 | 2.57 | 1.34–4.94 | 0.005 | |
| GLP-1RAs (ref) | — | — | — | — | — | — | — | — | — | |
Definition of abbreviations: BMI = body mass index; CI = confidence interval; COPD = chronic obstructive pulmonary disease; DPP-4i = dipeptidyl peptidase 4 inhibitor; GLP-1RA = glucagon-like peptide 1 receptor agonists; IRR = incidence rate ratio; ref = reference group; SGLT2i = sodium-glucose cotransporter 2 inhibitor.
Defined as ⩽12 months from index date.
The clinical model was adjusted for age; sex; race; Elixhauser Comorbidity Index; two-year health system encounter history; season of initiation; smoking history; number of exacerbations in the prior year; concurrent metformin use; and concurrent short-acting β-agonist, short-acting muscarinic antagonist, long-acting β-agonist, long-acting muscarinic antagonist, or triple-therapy use. The metabolic model was also adjusted for baseline HbA1c and baseline BMI.
To estimate the direct controlled effect, the exploratory analysis was also adjusted for change in BMI and change in HbA1c during the six-month study period.
Regarding COPD exacerbation risk (secondary outcome), the adjusted findings for sulfonylureas and SGLT2is were unchanged by the addition of recent exacerbation history; as with the primary outcome, the moderate exacerbation HR for DPP-4i users remained numerically higher but did not reach statistical significance (HR, 1.35 [95% CI, 0.96–1.90]; P = 0.08; Table E6, clinical model). In contrast, the findings for severe exacerbation were robust to recent exacerbation adjustment, with GLP-1RA users having lower exacerbation risk compared with both DPP-4i and sulfonylurea users in both clinical and metabolic models (see Table E6).
Expanded moderate exacerbation definition outcomes
Findings regarding higher total exacerbation rates in sulfonylurea and DPP-4i users compared with GLP-1RA users were robust in the clinical and metabolic models regardless of moderate exacerbation outcome definition. Regarding the secondary time to event moderate exacerbation outcome, sulfonylurea users had consistently higher risk; DPP-4i user trends for higher risk did not reach statistical significance (see Table E7).
Subgroup Analyses
After stratification by BMI, exacerbation rates were significantly higher only among sulfonylurea users with obesity (IRR, 2.04 [95% CI, 1.49–2.80]; P < 0.0001), but not with healthy weight or overweight BMI in the clinical model (Table 4); these results were consistent in the metabolic model (see Table E8). In contrast, SGLT2i users with overweight had lower exacerbation rates in both clinical (IRR, 0.53 [95% CI, 0.28–1.03]; P = 0.06) and metabolic (IRR, 0.50 [95% CI, 0.26–0.96]; P = 0.04) models.
Table 4.
Subgroup Analysis of COPD Exacerbation Counts according to Baseline BMI Categorization
| Baseline BMI (kg/m2) | Exposure | Patients* (n, % BMI Subgroup) | Total Exacerbation Count, Unadjusted | IRR† | 95% CI | P Value |
|---|---|---|---|---|---|---|
| ⩾18.5 to ⩽24.99 (n = 210 patients) | DPP-4is | 49 (23.3) | 34 | 1.07 | 0.39–2.91 | 0.90 |
| SGLT2is | 50 (23.8) | 31 | 0.85 | 0.31–2.30 | 0.75 | |
| Sulfonylureas | 97 (46.2) | 93 | 1.57 | 0.62–3.95 | 0.34 | |
| GLP-1RAs (ref) | 14 (6.7) | 10 | — | — | — | |
| ⩾25 to ⩽29.9 (n = 447 patients) | DPP-4is | 83 (18.6) | 44 | 0.89 | 0.45–1.80 | 0.78 |
| SGLT2is | 109 (24.4) | 42 | 0.53 | 0.28–1.03 | 0.06 | |
| Sulfonylureas | 200 (44.7) | 169 | 1.36 | 0.74–2.46 | 0.31 | |
| GLP-1RAs (ref) | 55 (12.3) | 32 | — | — | — | |
| ⩾30 (n = 918 patients) | DPP-4is | 116 (12.6) | 79 | 1.51 | 0.97–2.33 | 0.06 |
| SGLT2is | 189 (20.6) | 96 | 1.15 | 0.78–1.71 | 0.47 | |
| Sulfonylureas | 354 (38.6) | 305 | 2.04 | 1.49–2.80 | <0.0001 | |
| GLP-1RAs (ref) | 259 (28.2) | 129 | — | — | — |
Definition of abbreviations: BMI = body mass index; CI = confidence interval; COPD = chronic obstructive pulmonary disease; DPP-4i = dipeptidyl peptidase 4 inhibitor; GLP-1RA = glucagon-like peptide 1 receptor agonist; IRR = incidence rate ratio; ref = reference group; SGLT2i = sodium-glucose cotransporter 2 inhibitor.
Total patients in subgroup analysis: N = 1,575.
Adjusted for age; sex; race; Elixhauser Comorbidity Index; two-year health system encounter history; season of initiation; smoking history; concurrent metformin use; and concurrent short-acting β-agonist, short-acting muscarinic antagonist, long-acting β-agonist, long-acting muscarinic antagonist, or triple-therapy use.
Among 1,560 patients with available FEV1 data for the subgroup analysis by GOLD grade, SGLT2i users had a significantly reduced exacerbation rate compared with GLP-1RA users (IRR, 0.24 [95% CI, 0.09–0.65]; P = 0.005) in the grade 1 group but not in the grades 2–4 group. Sulfonylurea users had higher exacerbation rates in the grade 2 (IRR, 1.86 [95% CI, 1.25–2.75; P = 0.002) and grades 3 and 4 (IRR, 2.05 [95% CI, 1.36–3.07]; P = 0.0005) groups (Table 5). These results were consistent in the metabolic model (see Table E9).
Table 5.
Subgroup Analysis of COPD Exacerbation Counts according to Baseline GOLD Grade
| Baseline GOLD Grade* | Exposure | Patients† (n, % GOLD Subgroup) | Total Exacerbation Count, Unadjusted | IRR‡ | 95% CI | P Value |
|---|---|---|---|---|---|---|
| 1 (n = 195 patients) | DPP-4is | 30 (15.38) | 7 | 0.73 | 0.26–2.01 | 0.54 |
| SGLT2is | 43 (22.05) | 7 | 0.24 | 0.09–0.65 | 0.005 | |
| Sulfonylureas | 79 (40.51) | 47 | 1.19 | 0.59–2.38 | 0.63 | |
| GLP-1RAs (ref) | 43 (22.05) | 29 | — | — | — | |
| 2 (n = 805 patients) | DPP-4is | 131 (16.27) | 72 | 1.30 | 0.81–2.11 | 0.28 |
| SGLT2is | 175 (21.73) | 56 | 0.76 | 0.47–1.24 | 0.27 | |
| Sulfonylureas | 334 (41.49) | 244 | 1.86 | 1.25–2.75 | 0.002 | |
| GLP-1RAs (ref) | 165 (20.49) | 72 | — | — | — | |
| 3 and 4 (n = 560 patients) | DPP-4is | 83 (14.82) | 75 | 1.55 | 0.93–2.56 | 0.09 |
| SGLT2is | 115 (20.54) | 82 | 1.27 | 0.78–2.06 | 0.33 | |
| Sulfonylureas | 256 (45.71) | 269 | 2.05 | 1.36–3.07 | 0.0005 | |
| GLP-1RAs (ref) | 106 (18.93) | 63 | — | — | — |
Definition of abbreviations: CI = confidence interval; COPD = chronic obstructive pulmonary disease; DPP-4i = dipeptidyl peptidase 4 inhibitor; GOLD = Global Initiative for Chronic Obstructive Lung Disease; GLP-1RA = glucagon-like peptide 1 receptor agonist; IRR = incidence rate ratio; ref = reference group; SGLT2i = sodium-glucose cotransporter 2 inhibitor.
The GOLD classification is based on baseline FEV1 values: 1 = mild (⩾80% predicted), 2 = moderate (50% ⩽ FEV1 < 80% predicted), 3 = severe (30% ⩽ FEV1 < 50% predicted), and 4 = very severe (FEV1 < 30%).
Total patients in subgroup analysis: N = 1,560.
Adjusted for age; sex; race; Elixhauser Comorbidity Index; two-year health system encounter history; season of initiation; smoking history; concurrent metformin use; concurrent short-acting β-agonist, short-acting muscarinic antagonist, long-acting β-agonist, long-acting muscarinic antagonist, or triple-therapy use.
Exploratory Analyses
Exacerbations at 12 months from index date
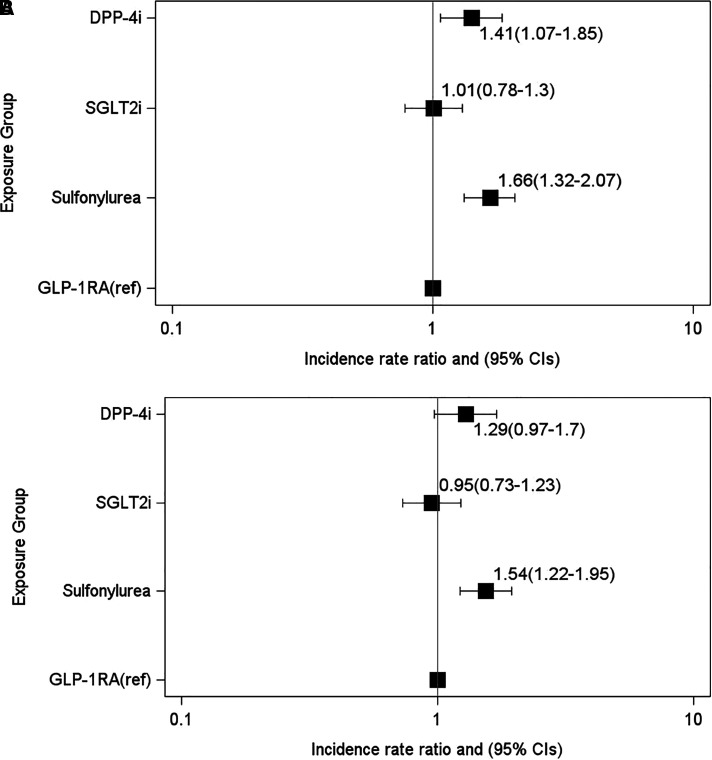
Exacerbation rates across groups were similar at 12 months as they were at 6 months, with higher rates among DPP-4i and sulfonylurea users compared with GLP-1RA users, but not for SGLT2i users (Figure 4).
Figure 4.
Incidence rate ratios of chronic obstructive pulmonary disease exacerbations and the association with GLP-1RAs or comparator treatments by 12 months after treatment initiation. (A and B) Negative binomial regression models adjusted for (A) clinical covariates (clinical model) and (B) metabolic covariates at baseline (metabolic model). The x-axes are plotted on a log scale. For definition of abbreviations, see Figure 2.
Regarding moderate and severe exacerbation risk, results were consistent or more strongly significant at 12 months in the clinical and metabolic models across drug groups (see Table E10). Unadjusted (HR, 1.46 [95% CI, 1.01–2.12; P = 0.043) and adjusted (clinical model; HR, 1.46 [95% CI, 1.0–2.13]; P = 0.052) severe exacerbation risk was consistently higher among SGLT2i users compared with GLP-1RA users at 12 months (see Table E10).
Changes in BMI and HbA1c during the study period
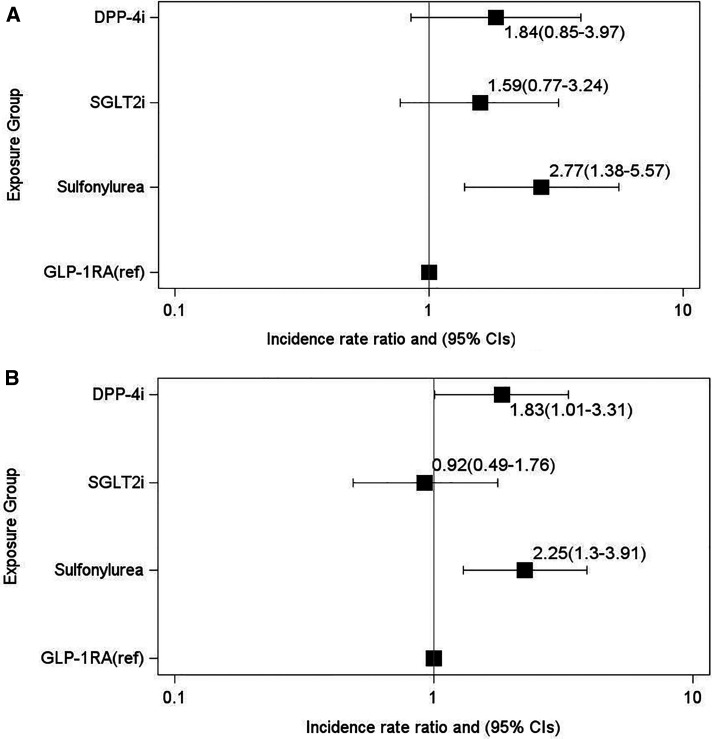
The association of GLP-1RA initiation with a lower exacerbation rate, holding BMI or HbA1c constant, remained significant compared with sulfonylurea users (Figure 5; see Table E11). Rates among DPP-4i and SGLT2i users compared with GLP-1RA users were not significantly different.
Figure 5.
(A and B) Exploratory analysis of changes in BMI and HbA1c on incidence rate ratios of chronic obstructive pulmonary disease exacerbations and the association with GLP-1RAs or comparator treatments by (A) 6 months and (B) 12 months after treatment initiation. Negative binomial regression models were adjusted for all covariates in the metabolic model and change in BMI and change in HbA1c over the study period to generate estimates of the direct controlled effect of the changes in those parameters on the outcome. The x-axes are plotted on a log scale. BMI = body mass index; CI = confidence interval; DPP-4i = dipeptidyl peptidase 4 inhibitor; GLP-1RA = glucagon-like peptide 1 receptor agonist; ref = reference group; SGLT2i = sodium-glucose cotransporter 2 inhibitor.
Discussion
T2D contributes to metabolic multimorbidity for patients with COPD worldwide (1). Among U.S. adults, more than 11% have T2D, with an estimated increase of 54% by 2030 (2). Currently, 10% of patients with COPD have diabetes; COPD guidelines acknowledge the increased morbidity from metabolic syndrome and diabetes but do not address treatment implications (1). These epidemiologic forecasts underscore the gap in clinical guidance for this growing patient population.
In this retrospective, EHR-based study of 1,642 patients with COPD, we examined associations between GLP-1RA initiation and COPD exacerbations compared with other T2D medications. Risks of both moderate and severe exacerbations were decreased in GLP-1RA users compared with sulfonylurea and DPP-4i users but were not different compared with SGLT2i users. When accounting for baseline glucose control and BMI, severe exacerbation risk increased to more than twofold for DPP-4i and sulfonylurea users compared with GLP-1RA users. Similar trends were observed for exacerbation rates. Rates remained higher for sulfonylurea users after adjusting for baseline metabolic covariates and differences in weight loss and HbA1c improvement across treatment groups. Regarding the inclusion of recent exacerbation history, a known predictor of exacerbation risk and criterion for clinical trial eligibility, we observed a decrease in the effect size comparing GLP-1RA with DPP-4i users in the clinical model, with CIs including the null value. This may be attributable to unmeasured features’ affecting exacerbation risk in prior years, which has been shown to be variable and potentially dependent on external factors (36). Finally, among patients with obesity, GLP-1RA users had the fewest exacerbations compared with DPP-4i and sulfonylurea users; no difference was seen between those users in the normal and overweight categories, though there was limited statistical power to detect a difference.
The observed differences in COPD outcomes between GLP-1RA and DPP-4i users in our study are consistent with clinical trial outcomes in which GLP-1RAs demonstrate substantial differences in their glucose-lowering and cardiovascular benefits despite a convergent pathway. This is likely due to the markedly increased pharmacologic potency of GLP-1RAs compared with the relatively weak endogenous effects of DPP-4 on GLP-1 (40). After accounting for recent exacerbation history and the effects of these drugs on metabolic variables, our finding that GLP-1RA users still have fewer exacerbations compared with DPP-4i users (Table 3) may signal a distinct effect of GLP-1RAs on airway inflammation despite the shared pathway. From a clinical standpoint, our findings in this study could be used to support T2D treatment choice in comorbid COPD populations, analogous to the selection of GLP-1RAs in patients with comorbid atherosclerotic cardiovascular disease risk or SGLT2is in the context of comorbid heart failure. Future prospective clinical, mechanistic, and genetic studies are needed to guide personalized T2D treatment selection in the COPD population.
Our study supports recent evidence from a primary care–based population study in the United Kingdom that demonstrated a statistically significant decreased risk of severe and moderate COPD exacerbations among GLP-1RA users compared with sulfonylurea users, and among SGLT2i users compared with sulfonylurea users with severe exacerbations, but not among DPP-4i users compared with sulfonylurea users (15). In a claims-based study of patients with chronic lower respiratory diseases, GLP-1RA users stratified by COPD diagnosis (ICD code based) had lower risk of respiratory disease–related hospitalizations and exacerbation count compared with DPP-4i users (14).
A strength of the present work compared with previous studies is the robust COPD phenotype, supporting generalizability, though this decreased the sample size. Our definition leverages the EHR’s rich clinical data by incorporating NLP to extract key data (e.g., smoking, pulmonary function testing) that may otherwise be missing from structured data types or administrative datasets, outperforming COPD definitions that rely exclusively on diagnosis codes (19). A validated, ICD code–only phenotype was used in the recent United Kingdom–based study of associations between T2D medications and respiratory exacerbations, which may have influenced the high rate of comorbid, ICD-diagnosed asthma in the population, influencing the results when stratified by comorbid asthma history (15).
Our findings among patients with COPD differed in several key respects from those of similar retrospective studies of asthma in which GLP-1RAs are associated with decreased exacerbation rates compared with SGLT2is (13); we found no difference between those two groups regarding COPD exacerbations. In patients with mild COPD, our results suggest that SGLT2i therapy may be protective compared with GLP-1RAs, though the relationship to the degree of airflow obstruction severity should be interpreted cautiously because of small sample sizes. SGLT2i users with overweight (but not healthy weight or obesity) also had fewer exacerbations than GLP-1RA users. These observations can inform patient selection criteria for prospective studies, underscore the methodologic value of distinguishing between asthma and COPD in EHR studies, and may inform T2D treatment selection in patients with comorbid COPD. Overweight status may be advantageous for COPD morbidity and mortality, whereas obesity may be harmful (41); therefore, weight loss effects from GLP-1RAs may not be favorable in certain weight categories compared with drug classes with lesser effects on weight. Whether SGLT2is influence airway inflammation or lung injury is unknown.
To our knowledge, no prior study has considered the change in metabolic covariates with treatment on COPD exacerbations, though the effects of antihyperglycemic drugs on weight and glucose control may confound respiratory outcomes. GLP-1RA users had the highest baseline median HbA1c, highest baseline BMI, and greatest weight loss. After accounting for changes in metabolic parameters, sulfonylurea users remained at increased risk compared with GLP-1RA users. This exploratory analysis suggests that GLP-1RA has a direct effect on exacerbations, not attributable to BMI or glucose control. However, this model cannot account for the indirect effect of GLP-1RA use on exacerbations, for example, the proportion of the drug’s effect on exacerbations that is related to the effect on BMI or HbA1c. Although the lower sample size available for this exploratory analysis may have reduced power to detect differences, prospective study is needed to determine whether the benefits of GLP-1RAs in COPD are independent of weight change and glucose control (8, 13).
A vital question arising from the results of this study is how GLP-1RAs might reduce COPD exacerbation risk in the T2D population. In a placebo-controlled, double-blind trial of liraglutide (a GLP-1RA) in patients with obesity and COPD, liraglutide did not improve FEV1 at Week 40 but did marginally increase FVC (0.33 L, equal to 7.69% of FVC predicted) (16). GLP-1RA treatment also increased FVC (by 5.2% of predicted) in a population with obesity but without diagnosed COPD (12), raising questions as to a direct or an indirect effect on airway inflammation or residual confounding from weight loss. In our study, baseline FEV1 data were available for approximately 95% of each treatment group, though fewer patients had repeat values available at the six-month endpoint, precluding an analysis of FEV1 change. Notably, data from studies of GLP-1RAs in murine models of COPD are limited (42). Lean and obese murine models of asthma treated with GLP-1RAs implicate IL-33 and its downstream pathways, including type 2 cytokines (8). Periostin, a serum biomarker of the IL-13 pathway, is lower in patients on GLP-1RAs than on other T2D therapies (43). Generally, murine models of COPD support a role for an IL-33–driven immune mechanism and suggest that cigarette smoke exposure may mediate increased IL-33 concentrations (44). In human studies, IL-33 concentrations are increased in bronchial biopsy samples, sputum, and serum from patients with COPD (45) and are associated with a history of exacerbations (46); loss-of-function IL-33 mutations reduce COPD risk, whereas gain-of-function mutations increase risk (47). A recent phase 2a trial of an anti–IL-33 biologic showed reduced exacerbation rates and improved lung function in former smokers with COPD (47). Future studies are required to elucidate the mechanisms of action of GLP-1RAs, particularly in the context of an evolving recognition of the pathobiology of epithelial alarmins and type 2 cytokines in COPD (48).
Limitations
This study has several limitations. First, exacerbation events outside the healthcare system may have been missed. Our COPD algorithm may reduce this bias, as it uses a data floor threshold that requires longitudinal system data (49). Exacerbations were identified using externally supported (e.g., admission diagnosis codes) and internally validated (e.g., prednisone prescription) definitions; although broadly used in research, these definitions may lack generalizability to other EHRs or individual practice patterns favoring antibiotic treatment without prednisone. Current treatment guidelines do not recommend treating exacerbations in patients with T2D differently than in patients without T2D, despite potential clinical concern for greater steroid-induced risks in this population (1). However, a sensitivity analysis for the moderate exacerbation outcome inclusive of antibiotics with or without a concurrent prednisone prescription was robust regarding exacerbation rates, supporting our conclusions. Moderate exacerbation risk also remained consistently elevated for sulfonylurea compared with GLP-1RA users. Compared with clinical trials, our outcome definitions lacked patient symptom ascertainment. Prospective studies use standardized methodologies to capture patients’ symptoms, which are lacking in the EHR; machine learning methods to detect exacerbation symptom patterns from free-text notes may also improve the capture of exacerbation outcomes in future studies, as has been done with other domains in COPD (50).
The EHR’s lack of prescription fill data may also lead to exposure misclassification bias and more so for GLP-1RAs, DPP-4is, and SGLT2is, which are newer and more expensive than sulfonylureas, challenging prescription fulfillment. However, this bias would have potentially favored sulfonylureas, as the other (nontreated) patients would be more likely to have higher BMI and HbA1c, which may worsen COPD outcomes. We defined exposure by prescriptions; the “as treated” time could be less than 6 months because of discontinuation. However, within the 6-month period, there was no significant difference in repeat (one or more) prescription rates across treatment groups, minimizing potential bias; all groups also exhibited decreased HbA1c by the end of the study period. The 6-month period reflects real-world patterns in treatment adherence to the newer T2D therapies. Notably, an exploratory analysis at 12 months was consistent with the 6-month results and also showed that severe exacerbation risk was higher in SGLT2i users compared with GLP-1RA users, highlighting the need for prospective longitudinal study. Our inclusion criteria used T2D medications as a proxy for T2D, consistent with FDA approvals for these drugs. Liraglutide and semaglutide are also FDA approved for the treatment of obesity. This could introduce misclassification of some GLP-1RA users with obesity as having T2D; however, the median HbA1c value was equivalent or higher for GLP-1RA users compared with the comparators. In addition, the study dates overlap with the coronavirus disease (COVID-19) pandemic, when COPD and T2D were leading risk factors for morbidity from infection. We accounted for year of drug initiation in our models, though unmeasured confounders secondary to the COVID-19 pandemic may remain. Finally, to minimize model constraints, we did not include less commonly used medications for COPD as covariates, such as methylxanthines, as rates of use have been shown to be extremely low in prior cohorts (30), including cohorts with T2D (15).
Conclusions
Our findings demonstrate an association between GLP-1RA use and reduced COPD exacerbations. These associations are influenced by higher BMI and more severe COPD. The use of a rigorous COPD phenotype directly supports prospective, interventional studies of GLP-1RAs in COPD populations with comorbid T2D, which may potentially inform T2D treatment pathways, as currently established for cardiovascular and renal comorbidities. Mechanistic studies are needed to elucidate pathways underlying these observed associations, as part of a broader effort to improve the health of patients with COPD and multimorbidity related to T2D and the metabolic syndrome.
Acknowledgments
Acknowledgment
The authors gratefully acknowledge Deborah J. Wexler, M.D., M.Sc., for her mentorship and early discussions related to the study design and diabetes clinical care. The authors also thank Sergey Goryachev, M.S., and Vivian Gainer, M.S., for the development and implementation of the smoking NLP algorithm and Noah Greifer, Ph.D., of the Institute of Quantitative Social Science at Harvard University, for biostatistical consultation.
Footnotes
Supported by NHLBI grant K23HL161332 (D.F.); National Human Genome Research Institute grant U01HG008685 (S.N.M.); NIH grant U01 AI1155299 (K.N.C.); National Institute of Allergy and Infectious Diseases grant R01AI078908 (J.A.B.); NIH grants R01HL117945, R37AI052353, R01AI136041, R01HL136209, and U19AI095219 (J.A.B.); National Institute of Arthritis and Musculoskeletal and Skin Diseases grant P30 AR070253 (E.W.K.); and NIH grant U01 HG008685 (E.W.K.); and by the Vinik and Kaye Families (J.A.B.). The funders had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Author Contributions: D.F. and Z.H.S. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: D.F., Z.H.S., K.N.C, and E.W.K. Acquisition, analysis, or interpretation of data: all authors. Drafting of the manuscript: D.F. and Z.H.S. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: J.C. Funding acquisition: D.F. and J.A.B. Administrative, technical, or material support: S.N.M. and J.A.B. Supervision: S.N.M. and E.W.K.
Data sharing statement: The data were extracted from Mass General Brigham’s Research Data Repository. Because of privacy regulations and per institutional and institutional review board approvals for this study, the patient-level data cannot be shared.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202303-0491OC on August 30, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Global Initiative for Chronic Obstructive Lung Disease. 2023. https://www.goldcopd.org/wp-content/uploads/2022/12/GOLD-2023-ver-1.1-2Dec2022_WMV.pdf
- 2. Rowley WR, Bezold C, Arikan Y, Byrne E, Krohe S. Diabetes 2030: insights from yesterday, today, and future trends. Popul Health Manag . 2017;20:6–12. doi: 10.1089/pop.2015.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Agusti A, Calverley PM, Celli B, Coxson HO, Edwards LD, Lomas DA, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators Characterisation of COPD heterogeneity in the ECLIPSE cohort Respir Res 2010. 11 122 20831787 [Google Scholar]
- 4. Belligund P, Attaway A, Lopez R, Damania D, Hatipoğlu U, Zein JG. Diabetes associated with higher health care utilization and poor outcomes after COPD-related hospitalizations. Am J Manag Care . 2022;28:e325–e332. doi: 10.37765/ajmc.2022.89225. [DOI] [PubMed] [Google Scholar]
- 5. Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab . 2018;27:740–756. doi: 10.1016/j.cmet.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 6. Rogliani P, Calzetta L, Capuani B, Facciolo F, Cazzola M, Lauro D, et al. Glucagon-like peptide 1 receptor: a novel pharmacological target for treating human bronchial hyperresponsiveness. Am J Respir Cell Mol Biol . 2016;55:804–814. doi: 10.1165/rcmb.2015-0311OC. [DOI] [PubMed] [Google Scholar]
- 7. Toki S, Goleniewska K, Reiss S, Zhang J, Bloodworth MH, Stier MT, et al. Glucagon-like peptide 1 signaling inhibits allergen-induced lung IL-33 release and reduces group 2 innate lymphoid cell cytokine production in vivo. J Allergy Clin Immunol . 2018;142:1515–1528.e8. doi: 10.1016/j.jaci.2017.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Toki S, Newcomb DC, Printz RL, Cahill KN, Boyd KL, Niswender KD, et al. Glucagon-like peptide-1 receptor agonist inhibits aeroallergen-induced activation of ILC2 and neutrophilic airway inflammation in obese mice. Allergy . 2021;76:3433–3445. doi: 10.1111/all.14879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alshabanat A, Zafari Z, Albanyan O, Dairi M, FitzGerald JM. Asthma and COPD overlap syndrome (ACOS): a systematic review and meta analysis. PLoS One . 2015;10:e0136065. doi: 10.1371/journal.pone.0136065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tkacova R, Dai DLY, Vonk JM, Leung JM, Hiemstra PS, van den Berge M, et al. Airway hyperresponsiveness in chronic obstructive pulmonary disease: a marker of asthma-chronic obstructive pulmonary disease overlap syndrome? J Allergy Clin Immunol . 2016;138:1571–1579.e10. doi: 10.1016/j.jaci.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 11. Huang X, Guan W, Xiang B, Wang W, Xie Y, Zheng J. MUC5B regulates goblet cell differentiation and reduces inflammation in a murine COPD model. Respir Res . 2022;23:11. doi: 10.1186/s12931-021-01920-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. López-Cano C, Ciudin A, Sánchez E, Tinahones FJ, Barbé F, Dalmases M, et al. Liraglutide improves forced vital capacity in individuals with type 2 diabetes: data from the randomized crossover LIRALUNG study. Diabetes . 2022;71:315–320. doi: 10.2337/db21-0688. [DOI] [PubMed] [Google Scholar]
- 13. Foer D, Beeler PE, Cui J, Karlson EW, Bates DW, Cahill KN. Asthma exacerbations in type 2 diabetics with asthma on glucagon-like peptide-1 receptor agonists. Am J Respir Crit Care Med . 2021;203:831–840. doi: 10.1164/rccm.202004-0993OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Albogami Y, Cusi K, Daniels MJ, Wei YJ, Winterstein AG. Glucagon-like peptide 1 receptor agonists and chronic lower respiratory disease exacerbations among patients with type 2 diabetes. Diabetes Care . 2021;44:1344–1352. doi: 10.2337/dc20-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pradhan R, Lu S, Yin H, Yu OHY, Ernst P, Suissa S, et al. Novel antihyperglycaemic drugs and prevention of chronic obstructive pulmonary disease exacerbations among patients with type 2 diabetes: population based cohort study. BMJ . 2022;379:e071380. doi: 10.1136/bmj-2022-071380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Altintas Dogan AD, Hilberg O, Hess S, Jensen TT, Bladbjerg EM, Juhl CB. Respiratory effects of treatment with a glucagon-like peptide-1 receptor agonist in patients suffering from obesity and chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis . 2022;17:405–414. doi: 10.2147/COPD.S350133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Au PCM, Tan KCB, Lam DCL, Cheung BMY, Wong ICK, Kwok WC, et al. Association of sodium-glucose cotransporter 2 inhibitor vs dipeptidyl peptidase-4 inhibitor use with risk of incident obstructive airway disease and exacerbation events among patients with type 2 diabetes in Hong Kong. JAMA Netw Open . 2023;6:e2251177. doi: 10.1001/jamanetworkopen.2022.51177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Polverino F, Wu TD, Rojas-Quintero J, Wang X, Mayo J, Tomchaney M, et al. Metformin: experimental and clinical evidence for a potential role in emphysema treatment. Am J Respir Crit Care Med . 2021;204:651–666. doi: 10.1164/rccm.202012-4510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chu SH, Wan ES, Cho MH, Goryachev S, Gainer V, Linneman J, et al. An independently validated, portable algorithm for the rapid identification of COPD patients using electronic health records. Sci Rep . 2021;11:19959. doi: 10.1038/s41598-021-98719-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Foer D, Strasser ZS, Cui J, Cahill KN, Murphy SN, Karlson EW. COPD exacerbations in patients with type 2 diabetes on glucagon-like peptide-1 receptor agonists [abstract] Am J Respir Crit Care Med . 2023;207:A5920. [Google Scholar]
- 21. Himes BE, Dai Y, Kohane IS, Weiss ST, Ramoni MF. Prediction of chronic obstructive pulmonary disease (COPD) in asthma patients using electronic medical records. J Am Med Inform Assoc . 2009;16:371–379. doi: 10.1197/jamia.M2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cromer SJ, Chen V, Han C, Marshall W, Emongo S, Greaux E, et al. Algorithmic identification of atypical diabetes in electronic health record (EHR) systems. PLoS One . 2022;17:e0278759. doi: 10.1371/journal.pone.0278759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dashti HS, Miranda N, Cade BE, Huang T, Redline S, Karlson EW, et al. Interaction of obesity polygenic score with lifestyle risk factors in an electronic health record biobank. BMC Med . 2022;20:5. doi: 10.1186/s12916-021-02198-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol . 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 25. Yen FS, Wei JC, Yang YC, Hsu CC, Hwu CM. Thiazolidinedione use in individuals with type 2 diabetes and chronic obstructive pulmonary disease. Front Med (Lausanne) . 2021;8:729518. doi: 10.3389/fmed.2021.729518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rinne ST, Liu CF, Feemster LC, Collins BF, Bryson CL, O’Riordan TG, et al. Thiazolidinediones are associated with a reduced risk of COPD exacerbations. Int J Chron Obstruct Pulmon Dis . 2015;10:1591–1597. doi: 10.2147/COPD.S82643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang MT, Lai JH, Huang YL, Kuo FC, Wang YH, Tsai CL, et al. Use of antidiabetic medications and risk of chronic obstructive pulmonary disease exacerbation requiring hospitalization: a disease risk score-matched nested case-control study. Respir Res . 2020;21:319. doi: 10.1186/s12931-020-01547-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Divino V, DeKoven M, Hallinan S, Varol N, Wirta SB, Lee WC, et al. Glucagon-like peptide-1 receptor agonist treatment patterns among type 2 diabetes patients in six European countries. Diabetes Ther . 2014;5:499–520. doi: 10.1007/s13300-014-0087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Essien UR, Singh B, Swabe G, Johnson AE, Eberly LA, Wadhera RK, et al. Association of prescription co-payment with adherence to glucagon-like peptide-1 receptor agonist and sodium-glucose cotransporter-2 inhibitor therapies in patients with heart failure and diabetes. JAMA Netw Open . 2023;6:e2316290. doi: 10.1001/jamanetworkopen.2023.16290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Suissa S, Dell’Aniello S, Ernst P. Comparative effects of LAMA-LABA-ICS vs LAMA-LABA for COPD: cohort study in real-world clinical practice. Chest . 2020;157:846–855. doi: 10.1016/j.chest.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 31. Bade BC, DeRycke EC, Ramsey C, Skanderson M, Crothers K, Haskell S, et al. Sex differences in veterans admitted to the hospital for chronic obstructive pulmonary disease exacerbation. Ann Am Thorac Soc . 2019;16:707–714. doi: 10.1513/AnnalsATS.201809-615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rothnie KJ, Müllerová H, Thomas SL, Chandan JS, Smeeth L, Hurst JR, et al. Recording of hospitalizations for acute exacerbations of COPD in UK electronic health care records. Clin Epidemiol . 2016;8:771–782. doi: 10.2147/CLEP.S117867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. 2022. https://www.cdc.gov/flu/about/season/index.html
- 34. VanderWeele TJ. Principles of confounder selection. Eur J Epidemiol . 2019;34:211–219. doi: 10.1007/s10654-019-00494-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang W, Mei A, Qian H, Li D, Xu H, Chen J, et al. The role of glucagon-like peptide-1 receptor agonists in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis . 2023;18:129–137. doi: 10.2147/COPD.S393323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Han MK, Quibrera PM, Carretta EE, Barr RG, Bleecker ER, Bowler RP, et al. SPIROMICS Investigators Frequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med . 2017;5:619–626. doi: 10.1016/S2213-2600(17)30207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peters MC, Schiebler ML, Cardet JC, Johansson MW, Sorkness R, DeBoer MD, et al. National Heart, Lung, and Blood Institute Severe Asthma Research Program-3 The impact of insulin resistance on loss of lung function and response to treatment in asthma. Am J Respir Crit Care Med . 2022;206:1096–1106. doi: 10.1164/rccm.202112-2745OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gasparini A. Comorbidity: an R package for computing comorbidity scores. J Open Source Softw . 2018;3:648. [Google Scholar]
- 39. Cazzola M, Rogliani P, Barnes PJ, Blasi F, Celli B, Hanania NA, et al. An update on outcomes for COPD pharmacological trials: a COPD investigators report—reassessment of the 2008 American Thoracic Society/European Respiratory Society statement on outcomes for COPD pharmacological trials. Am J Respir Crit Care Med . 2023;208:374–394. doi: 10.1164/rccm.202303-0400SO. [DOI] [PubMed] [Google Scholar]
- 40.Feingold KR. In: Endotext. Feingold KR, Anawalt B, Blackman MR, editors. South Dartmouth, MA: MDText.com; 2022. Oral and injectable (non-insulin) pharmacological agents for the treatment of type 2 diabetes.https://www.ncbi.nlm.nih.gov/books/NBK279141/ [Google Scholar]
- 41. Lambert AA, Putcha N, Drummond MB, Boriek AM, Hanania NA, Kim V, et al. COPDGene Investigators Obesity is associated with increased morbidity in moderate to aevere COPD. Chest . 2017;151:68–77. doi: 10.1016/j.chest.2016.08.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Viby NE, Isidor MS, Buggeskov KB, Poulsen SS, Hansen JB, Kissow H. Glucagon-like peptide-1 (GLP-1) reduces mortality and improves lung function in a model of experimental obstructive lung disease in female mice. Endocrinology . 2013;154:4503–4511. doi: 10.1210/en.2013-1666. [DOI] [PubMed] [Google Scholar]
- 43. Foer D, Beeler PE, Cui J, Snyder WE, Mashayekhi M, Nian H, et al. Glucagon-like peptide-1 receptor agonist use is associated with lower serum periostin. Clin Exp Allergy . 2023;53:469–473. doi: 10.1111/cea.14284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jiang M, Tao S, Zhang S, Wang J, Zhang F, Li F, et al. Type 2 innate lymphoid cells participate in IL-33-stimulated Th2-associated immune response in chronic obstructive pulmonary disease. Exp Ther Med . 2019;18:3109–3116. doi: 10.3892/etm.2019.7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gorska K, Nejman-Gryz P, Paplinska-Goryca M, Korczynski P, Prochorec-Sobieszek M, Krenke R. Comparative study of IL-33 and IL-6 levels in different respiratory samples in mild-to-moderate asthma and COPD. COPD . 2018;15:36–45. doi: 10.1080/15412555.2017.1416074. [DOI] [PubMed] [Google Scholar]
- 46. Joo H, Park SJ, Min KH, Rhee CK. Association between plasma interleukin-33 level and acute exacerbation of chronic obstructive pulmonary disease. BMC Pulm Med . 2021;21:86. doi: 10.1186/s12890-021-01423-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rabe KF, Celli BR, Wechsler ME, Abdulai RM, Luo X, Boomsma MM, et al. Safety and efficacy of itepekimab in patients with moderate-to-severe COPD: a genetic association study and randomised, double-blind, phase 2a trial. Lancet Respir Med . 2021;9:1288–1298. doi: 10.1016/S2213-2600(21)00167-3. [DOI] [PubMed] [Google Scholar]
- 48. Rabe KF, Rennard S, Martinez FJ, Celli BR, Singh D, Papi A, et al. Targeting type 2 inflammation and epithelial alarmins in chronic obstructive pulmonary disease: a biologics outlook. Am J Respir Crit Care Med . 2023;208:395–405. doi: 10.1164/rccm.202303-0455CI. [DOI] [PubMed] [Google Scholar]
- 49. Jin Y, Schneeweiss S, Merola D, Lin KJ. Impact of longitudinal data-completeness of electronic health record data on risk score misclassification. J Am Med Inform Assoc . 2022;29:1225–1232. doi: 10.1093/jamia/ocac043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Young AL, Bragman FJS, Rangelov B, Han MK, Galbán CJ, Lynch DA, et al. COPDGene Investigators Disease progression modeling in chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2020;201:294–302. doi: 10.1164/rccm.201908-1600OC. [DOI] [PMC free article] [PubMed] [Google Scholar]