Abstract
Rationale
Spirometry is essential for diagnosis and assessment of prognosis in patients with chronic obstructive pulmonary disease (COPD).
Objectives
To identify FEV1 trajectories and their determinants on the basis of annual spirometry measurements among individuals with and without airway obstruction (AO) and to assess mortality in relation to trajectories.
Methods
From 2002 through 2004, individuals with AO (FEV1/VC < 0.70, n = 993) and age- and sex-matched nonobstructive (NO) referents were recruited from population-based cohorts. Annual spirometry until 2014 was used in joint-survival latent-class mixed models to identify lung function trajectories. Mortality data were collected during 15 years of follow-up.
Measurements and Main Results
Three trajectories were identified among the subjects with AO and two among the NO referents. Trajectory membership was driven by baseline FEV1% predicted (FEV1%pred) in both groups and also by pack-years in subjects with AO and current smoking in NO referents. Longitudinal FEV1%pred depended on baseline FEV1%pred, pack-years, and obesity. The trajectories were distributed as follows: among individuals with AO, 79.6% in AO trajectory 1 (FEV1 high with normal decline), 12.8% in AO trajectory 2 (FEV1 high with rapid decline), and 7.7% in AO trajectory 3 (FEV1 low with normal decline) (mean, 27, 72, and 26 ml/yr, respectively) and, among NO referents, 96.7% in NO trajectory 1 (FEV1 high with normal decline) and 3.3% in NO trajectory 2 (FEV1 high with rapid decline) (mean, 34 and 173 ml/yr, respectively). Hazard for death was increased for AO trajectories 2 (hazard ratio [HR], 1.56) and 3 (HR, 3.45) versus AO trajectory 1 and for NO trajectory 2 (HR, 2.99) versus NO trajectory 1.
Conclusions
Three different FEV1 trajectories were identified among subjects with AO and two among NO referents, with different outcomes in terms of FEV1 decline and mortality. The FEV1 trajectories among subjects with AO and the relationship between low FVC and trajectory outcome are of particular clinical interest.
Keywords: prognosis, chronic obstructive pulmonary disease, FEV1, natural history
At a Glance Commentary
Scientific Knowledge on the Subject
Lung function trajectories have received increased attention during the past decade. However, the few publications on long-term follow-up of lung function in chronic obstructive pulmonary disease usually include a limited number of lung function tests during the observation period or include selected patient populations. Some studies provide mean or median decline estimates without illustrating trajectories. In addition, hardly any studies evaluating long-term lung function development and survival in chronic obstructive pulmonary disease are population based.
What This Study Adds to the Field
Our prospective population-based study, including repeated measurements of FEV1% predicted over 10 years, identified three lung function trajectories with different outcomes in terms of initial FEV1, subsequent FEV1 decline, and mortality among adults with airway obstruction. A majority belonged to the trajectory “FEV1 low with normal decline,” and compared with them, those in the trajectories “FEV1 high with rapid decline” and “FEV1 low with normal decline” had increased mortality. Among nonobstructive referents, two trajectories were identified: a small group with FEV1 high with rapid decline had increased mortality compared with FEV1 high with normal decline. The different trajectories among those with airway obstruction are clinically relevant and suggestive of diverse underlying disease mechanisms for which future studies may reveal new treatable traits.
The global burden of chronic obstructive pulmonary disease (COPD) is high, with an expected overall prevalence of about 10% (1). A cornerstone in the diagnosis of COPD is the confirmation of chronic airway obstruction (AO) by spirometry and assessment of severity on the basis of FEV1% predicted (FEV1%pred) (2). Decline in FEV1 is an important prognostic marker, and a rapid decline is associated with both a high burden of disease and mortality (3). Although there is no gold standard for defining a rapid decline in FEV1, a limit of at least 60 ml/yr was discussed in a classic paper by Fletcher and Peto published in 1977 (4).
Decline in FEV1 among individuals with COPD should be evaluated across several years to identify a reliable measure, as lung function values may naturally fluctuate between examinations performed at shorter time intervals (5). On the basis of large samples of patients with COPD, such estimates of mean or median rate of decline have been presented from both primary care and pharmacological trials (6, 7). Still, the well-known underdiagnosis of COPD (8) entails the need for population-based studies in which individuals across all severity stages of COPD can be identified to further unravel lung function changes over time. By using two time points of lung function measurements, it has been shown that not only a rapid but also a normal rate of decline in FEV1 among individuals with submaximally attained lung function may contribute to the development of COPD (9). In addition, prognosis differed between groups, and both all-cause and respiratory mortality were higher in COPD associated with a maximally attained FEV1 followed by a more rapid FEV1 decline than in COPD associated with submaximally attained lung function but a normal rate of FEV1 decline (10). Besides presenting mean rates of decline in COPD, the importance of identifying different lung function trajectories in the population has been increasingly highlighted in recent decades, revealing diverse clinically meaningful endpoints from childhood to middle age (11) and also among adults (12). However, there is a lack of longitudinal data from population-based COPD cohorts, especially studies including repeated lung function measurements, which are of importance to provide further insights into the natural history of COPD.
Thus, the overall aim of this study was to identify lung function trajectories and their determinants, on the basis of annual spirometry measurements, in a long-term study including adult individuals with and without AO sampled from the general population. A further aim was to evaluate prognosis, assessed as mortality, in relation to lung function trajectories.
Some of the results of this study have been previously reported in the form of an abstract (13).
Methods
Study Population
The first four OLIN (Obstructive Lung Disease in Northern Sweden) adult population-based cohorts were recruited in 1985, 1992 (age-stratified samples), 1993, and 1996 (random samples) using postal questionnaire surveys, in total comprising about 30,000 individuals. Random and stratified samples of responders were invited to clinical examinations initiated the year after the postal questionnaire surveys (14). Previously examined individuals from the four cohorts were invited to reexaminations from 2002 through 2004, after which we identified the present study population (14), all individuals with AO (FEV1/VC < 0.70, n = 993 cases) together with 993 age- and sex-matched nonobstructive (NO) referents. This study population (n = 1,986) constitutes the longitudinal OLIN COPD study and has since 2005 been invited to annual examinations including spirometry and a structured interview following a validated questionnaire (14). The 2002–2004 clinical examinations constitute the baseline (recruitment), and the last clinical follow-up included in the present paper was conducted in 2014. The Swedish National Board of Health and Welfare provided data on all-cause mortality during 15 years of follow-up from baseline. Ethical approval was given by the Regional Ethics Committee at Umeå University, and the study was performed according to the Declaration of Helsinki.
Spirometry at Baseline and at Each Clinical Examination
Spirometry was performed in accordance with the American Thoracic Society guidelines (15) using the same set of dry volume spirometers, the Mijnhardt Vicatest 5 (Mijnhardt), throughout. VC was defined as the highest of FVC and slow VC, and VC was used as the denominator when defining AO as FEV1/VC < 0.70 before bronchodilation (i.e., modified Global Initiative for Chronic Obstructive Lung Disease criteria) (2). Subjects with FEV1/VC < 0.70 were also invited to bronchodilation testing, using 4 × 0.2 mg salbutamol. The Swedish OLIN reference values for spirometry (16) were used.
Definition of Variables at Baseline
Pack-years of cigarette smoking was defined as (number of cigarettes smoked per day/20) × number of years.
Respiratory symptoms during the past 12 months included productive cough (cough with phlegm most days for at least three months), recurrent wheeze (usually having wheezing and whistling in the chest), and dyspnea (modified Medical Research Council Dyspnea Scale score ⩾ 2).
Any respiratory symptom was defined as any of the aforementioned respiratory symptoms during the past 12 months.
Any exacerbations were defined as healthcare contact because of respiratory symptoms during the past 12 months.
Definitions of Time-Varying Variables Collected at Baseline and at Each Examination
Height and weight were measured before spirometry in indoor clothing without shoes.
Body mass index (BMI) was calculated (weight [kilograms] divided by height [meters] [2]) and categorized as underweight (<18.5 kg/m2), normal weight (⩾18.5–24.9 kg/m2), overweight (⩾25–29.9 kg/m2), and obesity (⩾30.0 kg/m2).
Smoking status was divided into never-smokers, ex-smokers (for at least one year), and current smokers.
Statistics
The combined longitudinal and survival data were analyzed using joint latent-class mixed models (J-LCMM) (17), enabling a data-driven approach to identify subgroups with different lung function trajectories (see the online supplement for further details) while accounting for different survival patterns across subgroups. The J-LCMM analyses were performed using the lcmm package in R (R Core Team). FEV1%pred was used as the longitudinal outcome variable in the models, and covariates were included to analyze associations with trajectory membership (baseline age, sex, height, pack-years, and smoking habit) and longitudinal FEV1%pred (baseline age, sex, height, pack-years and the time-varying covariates, smoking habit, and BMI category) on the basis of previous evidence and clinical experience. For subjects with AO who participated in bronchodilation testing, the highest FEV1%pred values from pre- or postbronchodilation testing were used as the outcome. For the NO referents, prebronchodilatory FEV1%pred values were used as the outcome. For a description of the survival part of the model, see the online supplement. Models identifying two, three, or four trajectories were constructed, and the models with the lowest values of the Bayesian information criterion were chosen among subjects with AO and NO referents, respectively, and presented as main results, while the discarded models are described in Figures E1–E4 in the online supplement.
Differences in characteristics at baseline among the identified trajectories were analyzed using chi-square testing and ANOVA, as appropriate, using SPSS (version 26; IBM). A significance threshold of P < 0.05 was chosen. Individual annual decline estimates in terms of prebronchodilatory FEV1 (milliliters) are estimated by subjectwise linear regression with time as a covariate. Differences in 15-year all-cause mortality among the identified trajectories were analyzed using Cox proportional-hazards models including sex, age, pack-years of smoking, and overweight and obesity at baseline as covariates. Hazard ratios (HR) with 95% confidence intervals (CI) and survival functions for each trajectory were estimated.
Sensitivity Analysis
J-LCMM were also applied to the subsample of individuals with postbronchodilatory (post-BD) AO (post-BD FEV1/VC < 0.70; n = 736 of 993 cases) at baseline. Differences in 15-year all-cause mortality among trajectories were analyzed using similar Cox proportional-hazards models as in the main analyses. These results are presented in the online supplement.
Results
The lung function trajectories are based on a total of >11,000 data points for FEV1%pred, and the number of observations at each time point is presented in Table E1. Trajectories among the subjects with AO and NO referents are illustrated in Figure 1 and the corresponding raw data plots in Figures E5 and E6.
Figure 1.
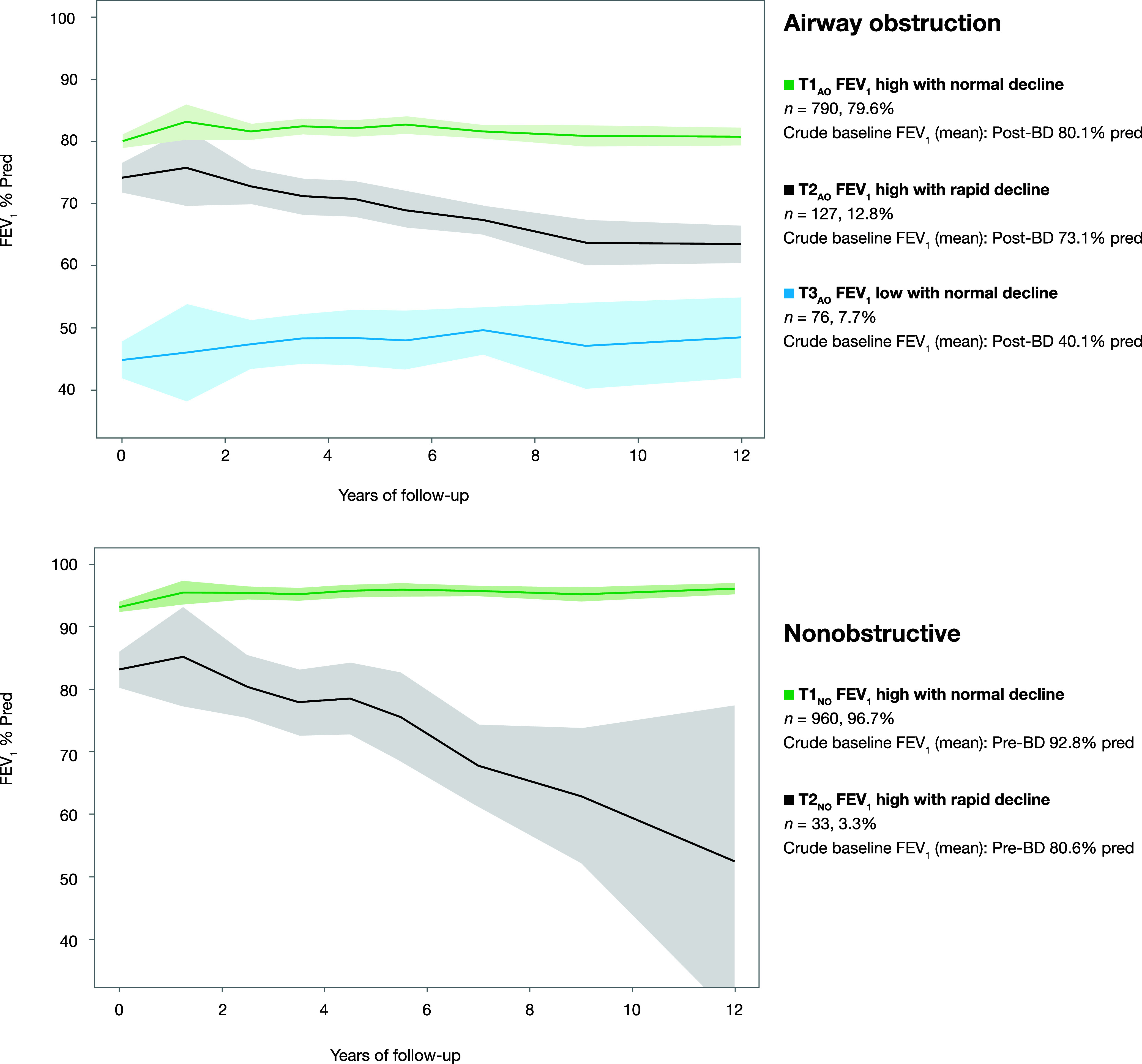
FEV1% predicted (FEV1%pred) trajectories estimated using joint-survival latent class mixed models separately among 993 individuals with airway obstruction (AO) (FEV1/VC < 0.70) and 993 nonobstructive (NO) referents without AO (FEV1/VC ⩾ 0.70) at baseline. The solid lines represent the observed means of FEV1%pred, and the shaded areas represent 95% confidence intervals of the means. For subjects with AO who participated in bronchodilation testing, the highest FEV1%pred values from pre- or postbronchodilation testing were used as outcomes. For the NO referents, pre-BD FEV1%pred values were used as outcomes. BD = bronchodilatory; T1 = trajectory 1 (FEV1 high with normal decline); T2 = trajectory 2 (FEV1 high with rapid decline); T3 = trajectory 3 (FEV1 low with normal decline).
FEV1 Trajectories among Individuals with AO
The three identified FEV1%pred trajectories among individuals with AO are illustrated in Figure 1, and additional characteristics at baseline are presented in Table 1. AO trajectory 1 (T1AO), FEV1 high with normal decline, consisted of 790 individuals with a mean age of 65.4 years, 51.4% of whom were men and 23.2% of whom were current smokers. The mean FEV1 at baseline was higher and the prevalence of any respiratory symptom lower than in the other trajectories. AO trajectory 2 (T2AO), FEV1 high with rapid decline, consisted of 127 individuals, 100% of whom were current smokers, with a higher proportion of parental smoking before school age (65.4%), a lower mean age (57.5 yr), and a higher proportion of men (81.1%) than in the other trajectories. The mean FEV1%pred at baseline was 73.1, and 79.5% of subjects reported any respiratory symptoms, whereof productive cough was most common. AO trajectory 3 (T3AO), FEV1 low with normal decline, consisted of 76 individuals with the highest mean age (70.8 yr), 43.4% of whom were men and 27.6% of whom were current smokers. The proportions with dyspnea (75.0%), any respiratory symptoms (93.4%), and any exacerbations (52.6%) were higher than in the other trajectories. Both mean FEV1%pred, VC% predicted and FEV1/VC at baseline were lower than in other trajectories. Ever having had an asthma diagnosis was most commonly reported in T3AO, by 44.7%, while the proportion with significant bronchodilation response was the lowest at 3.2%. The prevalence of obesity was slightly higher in T3AO compared with T1AO and T2AO (17.1% vs. 15.8% in T1AO and 11.8% in T2AO).
Table 1.
Characteristics at Baseline by Trajectories of FEV1% Predicted Separately among Individuals with and without AO
| AO |
NO |
||||||
|---|---|---|---|---|---|---|---|
| Characteristic at Baseline | T1AO (FEV1 High with Normal Decline) | T2AO (FEV1 High with Rapid Decline) | T3AO (FEV1 Low with Normal Decline) | P Value* | T1NO (FEV1 High with Normal Decline) | T2NO (FEV1 High with Rapid Decline) | P Value* |
| (n = 790) | (n = 127) | (n = 76) | |||||
| (n = 960) | (n = 33) | ||||||
| Clinical characteristics | |||||||
| Age, yr, mean (SD) | 65.4 (11.5) | 57.7 (9.3) | 70.8 (8.0) | <0.001 | 64.1 (11.3) | 72.8 (9.4) | <0.001 |
| Age, yr, range | 25–85 | 32–83 | 52–83 | — | 27–84 | 53–83 | — |
| Male sex | 51.4 | 81.1 | 43.4 | <0.001 | 54.2 | 66.7 | 0.156 |
| Pack-years, mean (SD) | 16.9 (14.9) | 31.9 (14.6) | 25.6 (15.5) | <0.001 | 13.3 (12.5) | 8.8 (9.6) | 0.130 |
| Nonsmokers | 28.0 | 0.0 | 22.4 | — | 47.9 | 45.5 | — |
| Former smokers | 48.8 | 0.0 | 50.0 | — | 39.4 | 42.4 | — |
| Current smokers | 23.2 | 100.0 | 27.6 | <0.001 | 12.7 | 12.1 | 0.940 |
| BMI, kg/m2, mean (SD) | 26.2 (4.1) | 25.7 (3.9) | 25.6 (5.2) | 0.276 | 26.6 (3.9) | 25.6 (3.1) | 0.150 |
| BMI categories | |||||||
| Underweight | 0.8 | 1.6 | 5.3 | — | 0.5 | 0.0 | — |
| Normal weight | 43.2 | 46.5 | 43.4 | — | 36.3 | 45.5 | — |
| Overweight | 40.3 | 40.2 | 34.2 | — | 46.6 | 48.5 | — |
| Obesity | 15.8 | 11.8 | 17.1 | 0.030 | 16.6 | 6.1 | 0.376 |
| Parental smoking before school age | 53.4 | 65.4 | 56.6 | 0.041 | 49.8 | 51.5 | 0.846 |
| Maternal smoking during pregnancy | 4.0 | 7.9 | 2.6 | 0.095 | 4.4 | 0.0 | 0.219 |
| Productive cough | 38.2 | 63.0 | 56.6 | <0.001 | 22.8 | 36.4 | 0.070 |
| Recurrent wheeze | 45.8 | 58.3 | 72.4 | <0.001 | 18.3 | 33.3 | 0.030 |
| Dyspnea (mMRC score ⩾ 2) | 22.5 | 22.0 | 75.0 | <0.001 | 9.2 | 15.2 | 0.252 |
| Any respiratory symptoms† | 69.0 | 79.5 | 93.4 | <0.001 | 41.0 | 51.5 | 0.230 |
| Any exacerbation last 12 mo | 18.0 | 18.9 | 52.6 | <0.001 | 9.1 | 6.1 | 0.553 |
| Any airway medication use last 12 mo | 32.5 | 25.2 | 82.9 | <0.001 | 10.0 | 12.1 | 0.691 |
| Self-reported asthma diagnosis, ever | 31.9 | 20.5 | 44.7 | 0.001 | 11.1 | 18.2 | 0.211 |
| Postbronchodilator‡ FEV1 increase of ⩾200 ml and ⩾12% | 16.0 | 10.8 | 3.2 | 0.012 | N/A | N/A | — |
| Lung function at baseline, mean (SD) | |||||||
| FEV1 pre-BD | |||||||
| Liters | 2.28 (0.73) | 2.49 (0.73) | 0.98 (0.32) | <0.001 | 2.81 (0.80) | 2.31 (0.72) | <0.001 |
| % predicted | 75.7 (13.9) | 69.6 (15.6) | 37.7 (10.9) | <0.001 | 92.8 (12.9) | 80.6 (17.3) | <0.001 |
| FEV1 post-BD | |||||||
| Liters | 2.41 (0.74) | 2.61 (0.73) | 1.04 (0.32) | <0.001 | N/A | N/A | — |
| % predicted | 80.1 (13.6) | 73.1 (15.3) | 40.1 (11.2) | <0.001 | N/A | N/A | — |
| VC pre-BD | |||||||
| Liters | 3.55 (1.06) | 4.06 (1.00) | 2.15 (0.68) | <0.001 | 3.61 (1.01) | 3.01 (0.93) | 0.001 |
| % predicted | 86.7 (14.1) | 85.7 (14.9) | 58.8 (13.4) | <0.001 | 87.9 (12.5) | 75.9 (16.2) | <0.001 |
| VC post-BD | |||||||
| Liters | 3.59 (1.05) | 4.11 (0.99) | 2.19 (0.68) | <0.001 | N/A | N/A | — |
| % predicted | 87.7 (13.7) | 86.8 (14.6) | 59.8 (13.4) | <0.001 | N/A | N/A | — |
| FEV1/VC pre-BD, z-score | −1.6 (1.0) | −2.6 (1.3) | −4.2 (2.3) | <0.001 | 0.63 (0.82) | 0.71 (0.93) | 0.587 |
| FEV1/VC post-BD, z-score | −1.1 (1.1) | −2.2 (1.3) | −3.8 (2.4) | <0.001 | N/A | N/A | — |
Definition of abbreviations: AO = airway obstruction; BMI = body mass index; mMRC = modified Medical Research Council Dyspnea Scale; N/A = not applicable; NO = nonobstructive; pre-BD = prebronchodilatory value; post-BD = highest of pre- and postbronchodilatory values; T1 = trajectory 1; T2 = trajectory 2; T3 = trajectory 3.
Data are presented as column percentage unless otherwise stated. Mean pack-years of smoking is calculated among ex-smokers and current smokers.
Chi-square test for proportions, ANOVA for means.
Any of productive cough, recurrent wheeze, attacks of shortness of breath, or dyspnea (mMRC score ⩾ 2).
Results from bronchodilatation testing available for 846 subjects with AO.
Regarding determinants of FEV1%pred, age and pack-years of smoking at baseline and the time-varying variable obesity were significantly associated with lower FEV1%pred (Tables 2 and 3).
Table 2.
Determinants of FEV1%Pred Trajectory Membership Separately among Individuals with and without AO Using Joint Latent-Class Mixed Models
| LC Part of the Model: Baseline Determinants of Trajectory Membership | AO |
NO |
||||
|---|---|---|---|---|---|---|
| T2AO (FEV1 High with Rapid Decline) |
T3AO (FEV1 Low with Normal Decline) |
T2NO (FEV1 High with Rapid Decline) |
||||
| Coef (SE) | P Value | Coef (SE) | P Value | Coef (SE) | P Value | |
| Age | −0.08 (0.04) | 0.055 | 0.10 (0.02) | <0.001 | 0.21 (0.04) | <0.001 |
| Male sex | 1.60 (0.64) | 0.013 | −0.11 (0.34) | 0.744 | 2.16 (1.29) | 0.095 |
| Pack-years | 0.07 (0.03) | 0.001 | 0.03 (0.01) | <0.001 | 0.06 (0.07) | 0.385 |
| Current smoking | 12.77 (37.02) | 0.730 | 0.50 (0.39) | 0.205 | 2.84 (1.41) | 0.045 |
Definition of abbreviations: AO = airway obstruction; Coef = coefficient from joint latent-class mixed models; FEV1%pred = FEV1% predicted; LC = latent class; NO = nonobstructive; T1 = trajectory 1; T2 = trajectory 2; T3 = trajectory 3.
All variables listed in the table were included in the models, and all models are also adjusted for squared height. Trajectory 1 (FEV1 high with normal decline) is used as the reference category in the model of determinants of trajectory membership among those with airway obstruction (T1AO) and among the NO referents (T1NO).
Table 3.
Determinants of FEV1%Pred Trajectory Shape Separately among Individuals with and without AO Using Joint Latent-Class Mixed Models
| MM Part of the Model: Longitudinal Modeling of Determinants of FEV1%pred | AO |
NO |
||
|---|---|---|---|---|
| Coef (SE) | P Value | Coef (SE) | P Value | |
| Baseline age | −0.10 (0.05) | 0.026 | −0.04 (0.04) | 0.293 |
| Baseline male sex | −2.01 (1.05) | 0.056 | −0.80 (0.84) | 0.339 |
| Baseline pack-years | −0.20 (0.04) | <0.001 | −0.10 (0.04) | 0.008 |
| Current smoking (time varying) | −0.55 (0.47) | 0.232 | 0.26 (0.50) | 0.606 |
| Overweight (BMI 25–30 kg/m2) (time varying) | −0.29 (0.30) | 0.345 | −1.18 (0.26) | <0.001 |
| Obesity (BMI > 30 kg/m2) (time varying) | −1.92 (0.47) | <0.001 | −2.73 (0.38) | <0.001 |
Definition of abbreviations: AO = airway obstruction; BMI = body mass index; Coef = coefficient from joint latent-class mixed models; FEV1%pred = FEV1% predicted; MM = mixed models; NO = nonobstructive.
All variables listed in the table were included in the models, and all models are also adjusted for squared height. Smoking, overweight, and obesity were included as time-varying covariates in the longitudinal analysis of determinants of FEV1%pred.
FEV1 Trajectories among Referents without AO
The two identified FEV1%pred trajectories among the NO referents are illustrated in Figure 1, and characteristics at baseline are presented in Table 1. NO trajectory 1 (T1NO), FEV1 high with normal decline, consisted of 960 individuals characterized by a mean age of 64.1 years, 12.7% of whom were current smokers, 46.6% overweight, and 16.6% obese; their mean FEV1%pred was 92.8% at baseline. NO trajectory 2 (T2NO), FEV1 high with rapid decline, consisted of 33 individuals with a higher mean age (72.8 yr), 12.1% of whom were current smokers, 48.5% overweight, and 6.1% obese; their mean FEV1%pred was 80.6% at baseline. Any respiratory symptoms were similarly common in T1NO and T2NO, at 41.0% and 51.5%, respectively.
Pack-years of smoking at baseline and the time-varying variables overweight and obesity were significantly associated with lower FEV1%pred (Tables 2 and 3).
Annual Rate of FEV1 Decline for Each Trajectory
In Table 4, subject-specific rates of decline in prebronchodilatory FEV1 are presented as loss of milliliters per year and also using different cutoffs to define accelerated decline. Among those with AO, the mean estimated annual FEV1 decline was 27 ml in T1AO, 72 ml in T2AO, and 26 ml in T3AO (P < 0.001), and the distribution of individual decline estimates by trajectory is illustrated in Figure 2. The proportions with annual FEV1 decline of at least 30 ml were 49.3% in T1AO, 90.7% in T2AO, and 47.1% in T3AO (P < 0.001).
Table 4.
Annual Decline in Prebronchodilatory FEV1 by Trajectory, Separately among Individuals with and without AO and at Least Two Measurements of FEV1
| AO |
NO |
||||||
|---|---|---|---|---|---|---|---|
| T1AO (High FEV1 with Normal Decline) | T2AO (High FEV1 with Rapid Decline) | T3AO (Low FEV1 with Normal Decline) | P Value | T1NO (High FEV1 with Normal Decline) | T2NO (High FEV1 with Rapid Decline) | P Value | |
| (n = 690) | (n = 108) | (n = 51) | (n = 861) | (n = 25) | |||
| Annual decline in FEV1, ml | |||||||
| Mean (SD) | −27 (55) | −72 (49) | −26 (68) | <0.001 | −34 (34) | −173 (172) | <0.001 |
| Median (IQR) | −29 (−48 to −11) | −69 (−96 to −49) | −30 (−50 to −3) | — | −32 (−50 to −18) | −123 (−183 to −100) | — |
| Categories of annual decline in FEV1, ml | |||||||
| ⩾30-ml decline, % | 49.3 | 90.7 | 47.1 | <0.001 | 54.6 | 96.0 | <0.001 |
| ⩾40-ml decline, % | 33.0 | 82.4 | 41.2 | <0.001 | 38.1 | 92.0 | <0.001 |
| ⩾50-ml decline, % | 22.8 | 73.1 | 23.5 | <0.001 | 24.6 | 92.0 | <0.001 |
| ⩾60-ml decline, % | 15.4 | 58.3 | 13.7 | <0.001 | 15.6 | 88.0 | <0.001 |
| ⩾70-ml decline, % | 11.4 | 49.1 | 13.7 | <0.001 | 9.9 | 88.0 | <0.001 |
| ⩾80-ml decline, % | 8.7 | 39.8 | 13.7 | <0.001 | 6.2 | 88.0 | <0.001 |
| ⩾90-ml decline, % | 6.2 | 29.6 | 9.8 | <0.001 | 4.3 | 84.0 | <0.001 |
Definition of abbreviations: AO = airway obstruction; IQR = interquartile range; NO = nonobstructive; T1 = trajectory 1; T2 = trajectory 2; T3 = trajectory 3.
Individual decline estimates in terms of FEV1% predicted and milliliters per year are estimated by subjectwise linear regression with time as a covariate.
Figure 2.
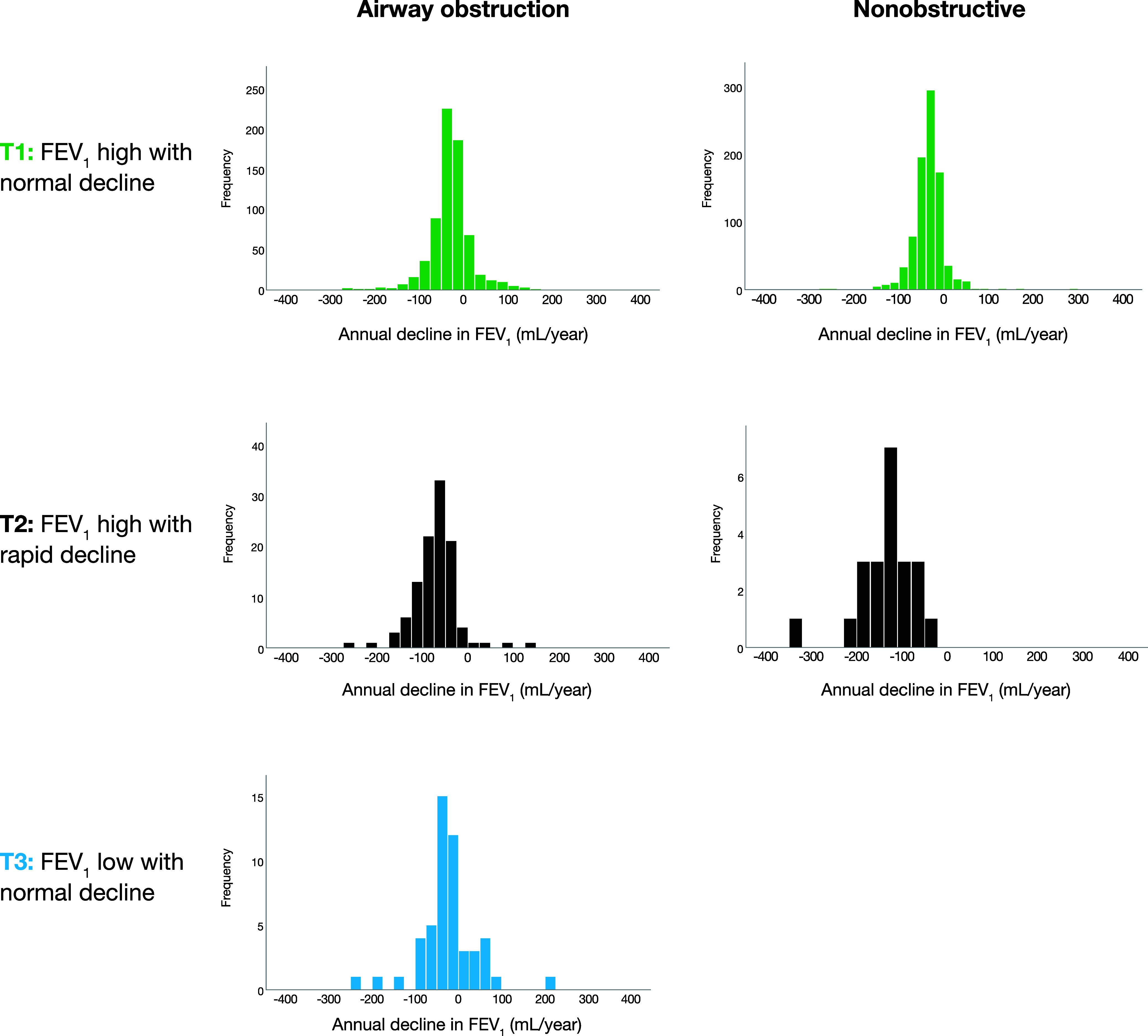
Histograms of individual prebronchodilatory FEV1 decline (ml/yr) estimates within each of the trajectories, separately among individuals with airway obstruction (FEV1/VC < 0.70) and referents without airway obstruction (FEV1/VC ⩾ 0.70) at baseline. T1 = trajectory 1; T2 = trajectory 2; T3 = trajectory 3.
Among the NO referents, the mean estimated annual FEV1 decline was 34 ml in T1NO and 173 ml in T2NO (P < 0.001), and the proportion with annual FEV1 decline of at least 30 ml was 54.6% in T1NO and 96.0% in T2NO (P < 0.001) (Table 4 and Figure 2).
Fifteen-Year All-Cause Mortality for Each Trajectory
Among individuals with AO, the crude mean survival was 12.3 years (95% CI, 12.0–12.6 yr) in T1AO, 11.9 years (95% CI, 11.1–12.7 yr) in T2AO, and 7.0 years (95% CI, 5.9–8.0 yr) in T3AO. The corresponding figures in the NO group were 12.9 years (95% CI, 12.6–13.1 yr) in T1NO and 6.8 years (95% CI, 5.3–8.3 yr) in T2NO. Crude mortality rates per 1,000 person-years were 34.3 in T1AO, 38.6 in T2AO, and 126.7 in T3AO, with corresponding figures of 25.8 in T1NO and 138.1 in T2NO. Among subjects with AO, survival curves illustrate a higher hazard for 15-year all-cause mortality in T2AO and T3AO than in T1AO (adjusted HRs, 1.56 [95% CI, 1.12–2.15] and 3.45 [95% CI, 2.63–4.52], respectively), when adjusted for age, sex, pack-years and BMI categories. Also among the NO referents, the hazard for 15-year mortality was higher in the trajectory with rapid decline in FEV1 (T2NO) than in that with normal decline in FEV1 (T1NO) (adjusted HR, 2.99 [95% CI, 2.03–4.40]) (Figure 3).
Figure 3.
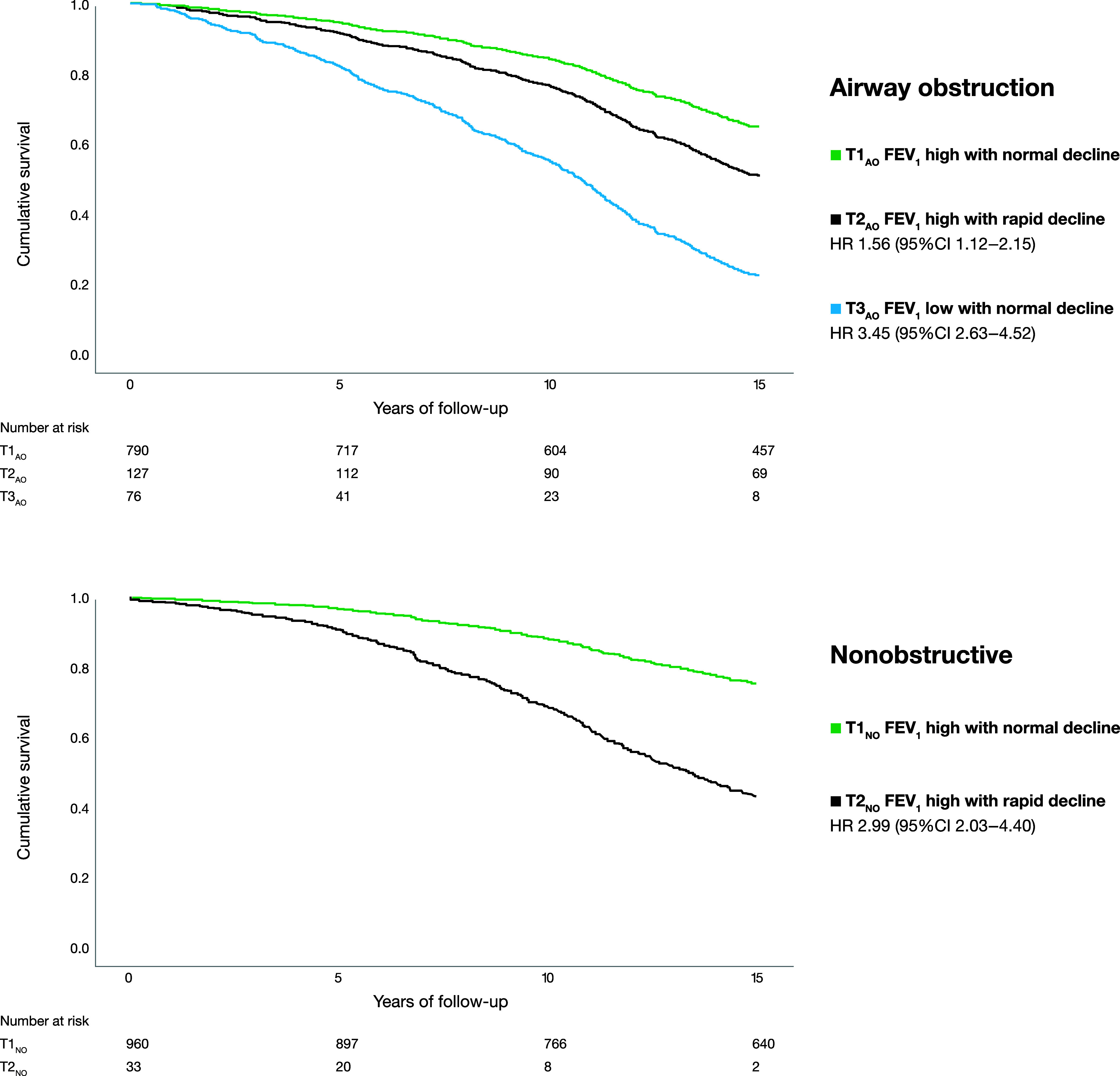
Survival functions for each trajectory and HRs with 95% CIs (T1 as reference), estimated using Cox regression models including sex, age, pack-years of smoking, and overweight and obesity as covariates, separately among 993 subjects with AO and 993 age- and sex-matched NO referents. AO = airway obstruction; CI = confidence interval; HR = hazard ratio; NO = nonobstructive; T1 = trajectory 1 (FEV1 high with normal decline); T2 = trajectory 2 (FEV1 high with rapid decline); T3 = trajectory 3 (FEV1 low with normal decline).
Sensitivity Analysis
The separate analysis among 736 individuals with post-BD obstruction confirmed the main findings in terms of both trajectory shapes and survival (see Figure E7).
Discussion
In this prospective, population-based case-referent study including repeated lung function testing across more than 10 years of follow-up, data-driven analyses identified three lung function trajectories with clinically different outcomes in terms of both FEV1 decline and mortality among those with AO, while two trajectories were identified among the NO referents. Among individuals with AO, T2AO (FEV1 high with rapid decline) (mean, 72 ml/yr) had 56% increased mortality, and T3AO (FEV1 low with normal decline) (mean, 26 ml/yr) had 245% increased mortality compared with T1AO (FEV1 high with normal decline). Among the NO referents, the small group of individuals in T2NO (FEV1 high with rapid decline) (mean, 173 ml/yr) had 199% increased mortality compared with T1NO (FEV1 high with normal decline).
Among subjects with AO in the present study, a majority (79%) belonged to T1AO (FEV1 high with normal decline), with an estimated mean FEV1 decline of 27 ml/yr. Our results thus indicate that on a population level, a majority of those with AO aged 25–85 years at baseline maintain fairly normal lung function. For comparison, about 80% remained in the same Global Initiative for Chronic Obstructive Lung Disease stage during 4–8 years of follow-up of a COPD cohort (18), indicating a good prognosis with respect to lung function decline in the majority of patients. Besides preserved lung function, T1AO also had the best survival among those with AO in the present study, although the mortality rate was slightly higher than that of T1NO (i.e., referents with high FEV1 with normal decline).
In contrast, T2AO (FEV1 high with rapid decline) was a smaller group (12.8% of subjects with AO) than T1AO, but the majority in this trajectory fulfilled the criterion for rapid decline suggested by Fletcher and Peto (4). All individuals in this trajectory were current smokers at baseline, predominantly men, but with the lowest mean age of all trajectories and a higher hazard of death than subjects in T1AO. Studies focusing on lung function trajectories in COPD have been increasingly highlighted during the past decade (19), and there are, for comparison, a few studies including longitudinal lung function data in patients with COPD. In the Normative Aging Study, four distinct FEV1 trajectories were identified using data-driven modeling. These trajectories were then applied to a subsample of smoking men from the COPDGene study, resulting in 17% of subjects being assigned to the trajectory with the most rapid decline in FEV1 (20). In two other studies including patients with COPD with at least three lung function measurements over 10–12 years, the groups with the most rapid decline in lung function constituted 18–30% of the patients, with a mean FEV1 decline of 78–86 ml/yr (21, 22). The proportion with rapid FEV1 declines in these studies of patients with COPD (20–22) was slightly higher than in the present study. However, the AO group in our population-based study included mainly individuals with mild to moderate airflow limitation, known to be largely underdiagnosed (8) but still representative of individuals with AO on a societal level. Thus, our results highlight that case finding with repeated lung function measurements among smokers, regardless of age, may identify individuals with rapid lung function declines and a worse prognosis at earlier stages of disease and may also be useful for the detection of early COPD (23) with an unfavorable prognosis.
T3AO (FEV1 low with normal decline) included 76 individuals with a mean FEV1 decline of 26 ml/yr. Among the AO trajectories, mean age and proportions of respiratory symptoms and exacerbations were highest and survival was worst in T3AO. Besides the lowest FEV1%pred, in this trajectory VC% predicted was also lower than in T1AO and T2AO. In a recently published study, different trajectories were observed in a cohort followed from 7 years to middle age (24). The mixed lifetime spirometry pattern (having both low FEV1:FVC and low FVC) was associated with childhood illnesses, and when reaching middle age, these subjects had the highest prevalence of COPD and biomarkers indicating increased inflammation. Taken together across the lifespan, the previous study (24) and T3AO in the present study indicate that obstruction with accompanying low VC may indicate the most severe form of COPD. Our assumption is that T3AO includes individuals with low maximally attained lung function or previous periods with rapid decline to which earlier life events or childhood disadvantages, including asthma, may have contributed (25–27). Future studies on causes of death could potentially reveal underlying reasons besides respiratory causes for the increased mortality of this trajectory.
We hypothesize that the observed three well-separated trajectories among subjects with AO represent clinically relevant underlying biological mechanisms. Several biomarkers have been associated with COPD pathogenesis, such as reduced concentrations of CC16 (club cell protein 16) and sRAGE (soluble receptor for advanced glycation end‐products), different interleukins, and other inflammatory markers (28). Also, genetics and epigenetics may play a role in accelerated lung aging and early COPD (29, 30). In addition, early life events may affect lung function development into adulthood (31). However, the biomarker pattern in COPD is heterogeneous, and different biological processes can be involved in patients with similar degrees of airflow limitation (32), but there are few studies evaluating biomarkers in relation to long-term outcomes. The present study offers clinical aspects to the understanding of lung pathogenesis in COPD, and we propose future studies specifically on biological mechanisms in relation to these lung function trajectories.
Smoking, assessed by pack-years at baseline and the time-varying variable current smoking, was not unexpectedly associated with both trajectory membership and FEV1%pred. Furthermore, parental smoking before school age was most common (65%) in the rapid-decline trajectory (T2AO), which is in line with previous studies showing associations between parental COPD and more severe COPD in the offspring (33). However, the relationship between parental smoking and smokers with COPD is difficult to disentangle, as it may mirror familial smoking behaviors but also lower socioeconomic status (34) or a genetic predisposition to the harmful effects of smoking (33).
In COPD, underweight and malnutrition have been observed as risk factors associated with increased mortality (35, 36). However, it has been increasingly recognized that obesity is also common among subjects with COPD (37–39), and in the present study, obesity was associated with more rapid FEV1 declines in both individuals with AO and NO referents. Even though obesity is a recognized treatable trait in COPD (39, 40), it has rarely been evaluated in relation to changes in lung function. In selected populations of pharmacological COPD trials, higher BMI was, on the contrary, associated with reduced lung function loss among men (41). Further studies are needed to disentangle the relationship between BMI or BMI changes and lung function decline in COPD.
Among NO individuals, we identified two well-defined and delimited lung function trajectories, and the great majority (96.7%) belonged to T1NO (FEV1 high with normal decline), thus with declines in FEV1 comparable with that of a population sample (42, 43). A strikingly deviant pattern was observed in the small group belonging to T2NO (FEV1 high with rapid decline). This trajectory membership was driven by older age and was associated with a substantially increased hazard for death compared with T1NO. The underlying biological mechanisms may be heterogeneous in this small group.
There are previous population-based studies on lung function among adults with long-term follow-up. However, because of a prevalence of COPD of about 10% (1), the total number of individuals with COPD in these studies is limited (11, 12). Furthermore, in most studies on lung function decline, the annualized estimates are based on two or a few lung function measurements divided by the time of follow-up to yield an average (5, 9, 23, 44). Some pharmacological COPD trials have provided repeated measurements of lung function (6, 45, 46), but they generally include highly selected COPD populations, most often with moderate to severe disease, providing results not generalizable to the COPD spectra in society. Both baseline lung function and rapid decline matter for prognosis (44), and there is evidence showing that low peak lung function in early adulthood is associated with mortality (47). However, lung function groups are most often predefined, and thereafter prognosis is assessed, and an important contribution of our study is the data-driven modeling using FEV1%pred to illustrate lung function trajectories, in contrast to studies of predefined groups.
We further want to highlight the following strengths of our study. The population-based design includes a large sample of individuals with AO who, together with age- and sex-matched NO referents, have been followed with annual clinical examinations over several years. The age distribution in the study sample is reasonable for clinically relevant COPD. The standardized methods were ensured by specifically trained personnel, the use of the same spirometers, adherence to guidelines for lung function testing, and, besides lung function, the collection of time-dependent variables such as smoking habit and BMI at each examination. Mortality during the observation period was taken into account by the data-driven modeling of the trajectories. Among the individuals with AO, three trajectories were clearly delimited from one another, all with distinctively different mortality rates, and the findings were confirmed in sensitivity analyses performed in cases with post-BD FEV1/VC < 0.70. The results provide another important piece of the puzzle to understand the complex syndrome of COPD, still to be evaluated in relation to clinical phenotypes and underlying disease mechanisms.
The study also has weaknesses. First, spirometry findings are limited to adult life, and we can only make assumptions, on the basis of data from birth cohorts (11, 26, 27), regarding an association between T3AO and earlier life events. Second, medication was not accounted for and may have affected rates of decline in lung function (48). Last, the AO population in this study is likely to be representative of AO in the source population, whereas the original study design with age and sex matching does not imply a corresponding representativeness for the NO referent group. Thus, the lung function trajectories in the NO group are not considered generalizable to the general population but rather aid in highlighting differences between individuals with AO and NO referents that are unrelated to age and sex.
Conclusions
In this prospective, population-based study including repeated clinical examination across more than 10 years, we identified three different FEV1 trajectories among subjects with AO and two among the NO referents. The trajectories were clinically distinguishable between as well as within the groups in terms of initial FEV1, subsequent FEV1 decline, and mortality. The well-separated different FEV1 trajectories among subjects with AO and the relationship between low FVC and trajectory outcome are of particular clinical interest. Future studies may reveal tools for both preventive measures and new treatable traits.
Acknowledgments
Acknowledgment
The authors acknowledge the research staff and the late Bo Lundbäck, founder of the OLIN studies, as well as all study participants.
Footnotes
Supported by grants from the Swedish Heart and Lung Foundation, a regional agreement between Umeå University and Region Västerbotten (ALF), the Swedish Respiratory Society, VISARE NORR Fund Northern County Councils Regional Federation, and the Norrbotten County Council. The funders had no role in data collection, data analysis, data interpretation, writing of the manuscript, or the decision to submit for publication.
Author Contributions: H.B., A.B., E.R., and A. Lindberg contributed to the protocol and design of the study. H.B., A. Lundquist, and A. Lindberg did the statistical analysis and verified the underlying data. H.B. and A. Lindberg drafted the report. All authors contributed to data interpretation, provided important content, and reviewed and approved the final report.
Data Sharing: Data are available from the authors upon reasonable request and with approval from the Swedish Ethical Review Authority.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202211-2166OC on July 17, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Adeloye D, Song P, Zhu Y, Campbell H, Sheikh A, Rudan I, NIHR RESPIRE Global Respiratory Health Unit Global, regional, and national prevalence of, and risk factors for, chronic obstructive pulmonary disease (COPD) in 2019: a systematic review and modelling analysis. Lancet Respir Med . 2022;10:447–458. doi: 10.1016/S2213-2600(21)00511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Agustí A, Celli BR, Criner GJ, Halpin D, Anzueto A, Barnes P, et al. Global Initiative for Chronic Obstructive Lung Disease 2023 report: GOLD executive summary. Am J Respir Crit Care Med . 2023;207:819–837. doi: 10.1164/rccm.202301-0106PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wise RA. The value of forced expiratory volume in 1 second decline in the assessment of chronic obstructive pulmonary disease progression. Am J Med . 2006;119:4–11. doi: 10.1016/j.amjmed.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 4. Fletcher C, Peto R. The natural history of chronic airflow obstruction. BMJ . 1977;1:1645–1648. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vestbo J, Lange P. Natural history of COPD: focusing on change in FEV1. Respirology . 2016;21:34–43. doi: 10.1111/resp.12589. [DOI] [PubMed] [Google Scholar]
- 6. Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, et al. ECLIPSE Investigators Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med . 2011;365:1184–1192. doi: 10.1056/NEJMoa1105482. [DOI] [PubMed] [Google Scholar]
- 7. Whittaker HR, Pimenta JM, Jarvis D, Kiddle SJ, Quint JK. Characteristics associated with accelerated lung function decline in a primary care population with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis . 2020;15:3079–3091. doi: 10.2147/COPD.S278981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lamprecht B, Soriano JB, Studnicka M, Kaiser B, Vanfleteren LE, Gnatiuc L, et al. BOLD Collaborative Research Group, the EPI-SCAN Team, the PLATINO Team, and the PREPOCOL Study Group Determinants of underdiagnosis of COPD in national and international surveys. Chest . 2015;148:971–985. doi: 10.1378/chest.14-2535. [DOI] [PubMed] [Google Scholar]
- 9. Lange P, Celli B, Agustí A, Boje Jensen G, Divo M, Faner R, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med . 2015;373:111–122. doi: 10.1056/NEJMoa1411532. [DOI] [PubMed] [Google Scholar]
- 10. Marott JL, Ingebrigtsen TS, Çolak Y, Vestbo J, Lange P. Lung function trajectories leading to chronic obstructive pulmonary disease as predictors of exacerbations and mortality. Am J Respir Crit Care Med . 2020;202:210–218. doi: 10.1164/rccm.201911-2115OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bui DS, Lodge CJ, Burgess JA, Lowe AJ, Perret J, Bui MQ, et al. Childhood predictors of lung function trajectories and future COPD risk: a prospective cohort study from the first to the sixth decade of life. Lancet Respir Med . 2018;6:535–544. doi: 10.1016/S2213-2600(18)30100-0. [DOI] [PubMed] [Google Scholar]
- 12. Washko GR, Colangelo LA, Estépar RSJ, Ash SY, Bhatt SP, Okajima Y, et al. Adult life-course trajectories of lung function and the development of emphysema: the CARDIA Lung Study. Am J Med . 2020;133:222–230.e11. doi: 10.1016/j.amjmed.2019.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Backman H, Blomberg A, Lundquist A, Strandkvist V, Sawalha S, Nilsson U, et al. Lung function trajectories based on annual measurements for 10 years in adults with airway obstruction. Eur Respir J . 2022;60:481. doi: 10.1164/rccm.202211-2166OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindberg A, Lundbäck B. The Obstructive Lung Disease in Northern Sweden Chronic Obstructive Pulmonary Disease Study: design, the first year participation and mortality. Clin Respir J. 2008;2:64–71. doi: 10.1111/j.1752-699X.2008.00086.x. [DOI] [PubMed] [Google Scholar]
- 15. American Thoracic Society. Standardization of spirometry, 1994 update. Am J Respir Crit Care Med . 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 16. Backman H, Lindberg A, Odén A, Ekerljung L, Hedman L, Kainu A, et al. Reference values for spirometry—report from the Obstructive Lung Disease in Northern Sweden studies. Eur Clin Respir J . 2015;2 doi: 10.3402/ecrj.v2.26375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Proust-Lima C, Séne M, Taylor JMG, Jacqmin-Gadda H. Joint latent class models for longitudinal and time-to-event data: a review. Stat Methods Med Res . 2014;23:74–90. doi: 10.1177/0962280212445839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de-Torres JP, Marín JM, Pinto-Plata V, Divo M, Sanchez-Salcedo P, Zagaceta J, et al. Is COPD a progressive disease? A long term bode cohort observation. PLoS ONE . 2016;11:e0151856. doi: 10.1371/journal.pone.0151856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Agusti A, Faner R. Lung function trajectories in health and disease. Lancet Respir Med . 2019;7:358–364. doi: 10.1016/S2213-2600(18)30529-0. [DOI] [PubMed] [Google Scholar]
- 20. Ross JC, Castaldi PJ, Cho MH, Hersh CP, Rahaghi FN, Sánchez-Ferrero GV, et al. Longitudinal modeling of lung function trajectories in smokers with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 2018;198:1033–1042. doi: 10.1164/rccm.201707-1405OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koskela J, Katajisto M, Kallio A, Kilpeläinen M, Lindqvist A, Laitinen T. Individual FEV1 trajectories can be identified from a COPD cohort. COPD . 2016;13:425–430. doi: 10.3109/15412555.2015.1043423. [DOI] [PubMed] [Google Scholar]
- 22. Casanova C, de Torres JP, Aguirre-Jaíme A, Pinto-Plata V, Marin JM, Cordoba E, et al. The progression of chronic obstructive pulmonary disease is heterogeneous: the experience of the BODE cohort. Am J Respir Crit Care Med . 2011;184:1015–1021. doi: 10.1164/rccm.201105-0831OC. [DOI] [PubMed] [Google Scholar]
- 23. Çolak Y, Afzal S, Nordestgaard BG, Lange P, Vestbo J. Importance of early COPD in young adults for development of clinical COPD: findings from the Copenhagen General Population Study. Am J Respir Crit Care Med . 2021;203:1245–1256. doi: 10.1164/rccm.202003-0532OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dharmage SC, Bui DS, Walters EH, Lowe AJ, Thompson B, Bowatte G, et al. Lifetime spirometry patterns of obstruction and restriction, and their risk factors and outcomes: a prospective cohort study. Lancet Respir Med . 2023;11:273–282. doi: 10.1016/S2213-2600(22)00364-2. [DOI] [PubMed] [Google Scholar]
- 25. Svanes C, Sunyer J, Plana E, Dharmage S, Heinrich J, Jarvis D, et al. Early life origins of chronic obstructive pulmonary disease. Thorax . 2010;65:14–20. doi: 10.1136/thx.2008.112136. [DOI] [PubMed] [Google Scholar]
- 26. Stocks J, Hislop A, Sonnappa S. Early lung development: lifelong effect on respiratory health and disease. Lancet Respir Med . 2013;1:728–742. doi: 10.1016/S2213-2600(13)70118-8. [DOI] [PubMed] [Google Scholar]
- 27. Bui DS, Perret JL, Walters EH, Lodge CJ, Bowatte G, Hamilton GS, et al. Association between very to moderate preterm births, lung function deficits, and COPD at age 53 years: analysis of a prospective cohort study. Lancet Respir Med . 2022;10:478–484. doi: 10.1016/S2213-2600(21)00508-7. [DOI] [PubMed] [Google Scholar]
- 28. Faner R, Tal-Singer R, Riley JH, Celli B, Vestbo J, MacNee W, et al. ECLIPSE Study Investigators Lessons from ECLIPSE: a review of COPD biomarkers. Thorax . 2014;69:666–672. doi: 10.1136/thoraxjnl-2013-204778. [DOI] [PubMed] [Google Scholar]
- 29. Eriksson Ström J, Kebede Merid S, Pourazar J, Blomberg A, Lindberg A, Ringh MV, et al. Chronic obstructive pulmonary disease is associated with epigenome-wide differential methylation in BAL lung cells. Am J Respir Cell Mol Biol . 2022;66:638–647. doi: 10.1165/rcmb.2021-0403OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang J, Xu H, Qiao D, DeMeo DL, Silverman EK, O’Connor GT, et al. A polygenic risk score and age of diagnosis of COPD. Eur Respir J . 2022;60:2101954. doi: 10.1183/13993003.01954-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang G, Hallberg J, Faner R, Koefoed HJ, Kebede Merid S, Klevebro S, et al. Plasticity of individual lung function states from childhood to adulthood. Am J Respir Crit Care Med . 2023;207:406–415. doi: 10.1164/rccm.202203-0444OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vanfleteren LEGW, Weidner J, Franssen FME, Gaffron S, Reynaert NL, Wouters EFM, et al. Biomarker-based clustering of patients with chronic obstructive pulmonary disease. ERJ Open Res . 2023;9:00301-02022. doi: 10.1183/23120541.00301-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hersh CP, Hokanson JE, Lynch DA, Washko GR, Make BJ, Crapo JD, et al. COPDGene Investigators Family history is a risk factor for COPD. Chest . 2011;140:343–350. doi: 10.1378/chest.10-2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tjora T, Hetland J, Aarø LE, Øverland S. Distal and proximal family predictors of adolescents’ smoking initiation and development: a longitudinal latent curve model analysis. BMC Public Health . 2011;11:911. doi: 10.1186/1471-2458-11-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Prescott E, Almdal T, Mikkelsen KL, Tofteng CL, Vestbo J, Lange P. Prognostic value of weight change in chronic obstructive pulmonary disease: results from the Copenhagen City Heart Study. Eur Respir J . 2002;20:539–544. doi: 10.1183/09031936.02.00532002. [DOI] [PubMed] [Google Scholar]
- 36. Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med . 1999;160:1856–1861. doi: 10.1164/ajrccm.160.6.9902115. [DOI] [PubMed] [Google Scholar]
- 37. Franssen FM, O’Donnell DE, Goossens GH, Blaak EE, Schols AM. Obesity and the lung: 5. Obesity and COPD. Thorax . 2008;63:1110–1117. doi: 10.1136/thx.2007.086827. [DOI] [PubMed] [Google Scholar]
- 38. Lambert AA, Putcha N, Drummond MB, Boriek AM, Hanania NA, Kim V, et al. COPDGene Investigators Obesity is associated with increased morbidity in moderate to severe COPD. Chest . 2017;151:68–77. doi: 10.1016/j.chest.2016.08.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Agustí A, Rapsomaniki E, Beasley R, Hughes R, Müllerová H, Papi A, et al. NOVELTY Study Investigators Treatable traits in the NOVELTY study. Respirology . 2022;27:929–940. doi: 10.1111/resp.14325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Agusti A, Bel E, Thomas M, Vogelmeier C, Brusselle G, Holgate S, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J . 2016;47:410–419. doi: 10.1183/13993003.01359-2015. [DOI] [PubMed] [Google Scholar]
- 41. Chen W, Sadatsafavi M, FitzGerald JM, Lynd LD, Sin DD. Gender modifies the effect of body mass index on lung function decline in mild-to-moderate COPD patients: a pooled analysis. Respir Res . 2021;22:59. doi: 10.1186/s12931-021-01656-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lindberg A, Larsson LG, Rönmark E, Jonsson AC, Larsson K, Lundbäck B. Decline in FEV1 in relation to incident chronic obstructive pulmonary disease in a cohort with respiratory symptoms. COPD . 2007;4:5–13. doi: 10.1080/15412550601168358. [DOI] [PubMed] [Google Scholar]
- 43. Aanerud M, Carsin AE, Sunyer J, Dratva J, Gislason T, Jarvis D, et al. Interaction between asthma and smoking increases the risk of adult airway obstruction. Eur Respir J . 2015;45:635–643. doi: 10.1183/09031936.00055514. [DOI] [PubMed] [Google Scholar]
- 44. Mannino DM, Reichert MM, Davis KJ. Lung function decline and outcomes in an adult population. Am J Respir Crit Care Med . 2006;173:985–990. doi: 10.1164/rccm.200508-1344OC. [DOI] [PubMed] [Google Scholar]
- 45. Celli BR, Thomas NE, Anderson JA, Ferguson GT, Jenkins CR, Jones PW, et al. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med . 2008;178:332–338. doi: 10.1164/rccm.200712-1869OC. [DOI] [PubMed] [Google Scholar]
- 46. Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, et al. UPLIFT Study Investigators A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med . 2008;359:1543–1554. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- 47. Agustí A, Noell G, Brugada J, Faner R. Lung function in early adulthood and health in later life: a transgenerational cohort analysis. Lancet Respir Med . 2017;5:935–945. doi: 10.1016/S2213-2600(17)30434-4. [DOI] [PubMed] [Google Scholar]
- 48. Celli BR, Anderson JA, Cowans NJ, Crim C, Hartley BF, Martinez FJ, et al. Pharmacotherapy and lung function decline in patients with chronic obstructive pulmonary disease: a systematic review. Am J Respir Crit Care Med . 2021;203:689–698. doi: 10.1164/rccm.202005-1854OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


