Abstract
We have analyzed antibody reactivity to a fibronectin-binding microbial surface component that recognizes adhesive matrix molecules (MSCRAMM) in blood plasma collected from patients with staphylococcal infections. All patients had elevated levels of anti-MSCRAMM antibodies compared to those of young children who, presumably, had not been exposed to staphylococcal infections. The anti-MSCRAMM antibodies preferentially reacted with the ligand-binding repeat domain of the adhesin. However, these antibodies did not inhibit fibronectin binding. Essentially, all patients had antibodies which specifically recognized the fibronectin-MSCRAMM complex but not the isolated components. Epitopes recognized by these anti-ligand-induced binding sites antibodies were found in each repeat unit of the MSCRAMM. These results demonstrate that staphylococci have bound fibronectin some time during infection and that each repeat unit in the MSCRAMM can engage in ligand binding. Furthermore, our previously proposed model, suggesting that an unordered structure in the MSCRAMM undergoes a conformational change upon ligand binding (K. House-Pompeo, Y. Xu, D. Joh, P. Speziale, and M. Höök, J. Biol. Chem. 271:1379–1384, 1996), is presumably operational in patients during infections.
Adherence to host tissues is the initial critical step in the pathogenic process of most bacterial infections. Tissue adherence is mediated by bacterial surface components called adhesins, which recognize target ligands on host cells or in the extracellular matrix. Because of the importance of host tissue adherence in the pathogenic process, bacteria have adopted strategies to secure adhesion steps and to protect adhesins against attacks by the host defense system. Adhesins on bacteria which are extracellular pathogens are particularly vulnerable, since throughout their lives in an animal these organisms are exposed to the host’s defense systems. We have used the abbreviation MSCRAMM (microbial surface component recognizing adhesive matrix molecules) for the family of surface proteins binding to extracellular matrix molecules (11, 12). Molecular studies of MSCRAMM have revealed unexpectedly sophisticated mechanisms of ligand interactions in which host systems are often mimicked and great efforts are made to avoid detection by the host’s immune system.
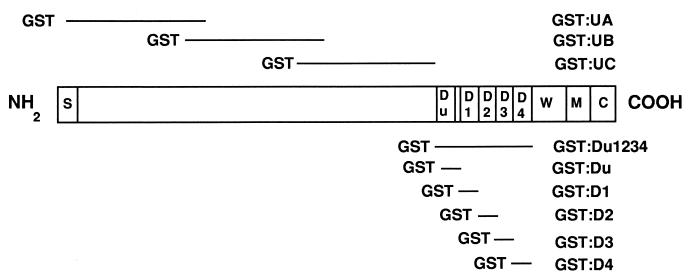
Fibronectin (Fn)-binding MSCRAMM are present on many pathogenic gram-positive bacteria. To date, the sequences of almost a dozen of these MSCRAMM have been determined (1, 4–5, 8–10, 13–17). Most have very similar structural organizations and molecular sizes of around 100 kDa. The N terminus contains a long signal sequence characteristic of many exported proteins in gram-positive bacteria. Following is a long stretch of unique sequence, which may be interrupted by a 30- to 35-amino-acid-(aa) repeated motif of unknown function. The primary ligand-binding domain consists of three to six repeats of a 40- to 50-aa motif. Synthetic peptides mimicking individual repeat units often bind Fn and effectively inhibit the binding of Fn to bacteria. The ligand-binding domain is found just outside a cell wall attachment region present in many surface proteins on gram-positive bacteria. At the C terminus is a putative transmembrane segment rich in hydrophobic residues, followed by a short cytoplasmic domain dominated by positively charged residues. A model of an Fn-binding MSCRAMM is presented in Fig. 1.
FIG. 1.
Schematic representation of recombinant proteins containing fragments of MSCRAMM FnbpA. All the segments were expressed in fusion with GST carrier. Fn-binding repeat units are indicated by Du, D1, D2, D3, and D4; S, signal sequence; W, cell wall spanning region; M, membrane-spanning region.
We recently demonstrated that the ligand-binding domains of the Fn-binding MSCRAMM do not have an organized structure but that a conformation is induced in the repeat units on ligand binding (6). This induced fit conformation could be detected by a specific monoclonal antibody which does not react with unoccupied MSCRAMM (16). We speculated that this induced-fit mechanism of ligand binding might affect the production of inhibiting antibodies, interfering with the Fn-MSCRAMM interaction.
In the present study, we have examined the antibody response and specificity to the staphylococcal Fn-binding MSCRAMM FnbpA in patients with staphylococcal infections.
MATERIALS AND METHODS
Sera.
Serum specimens from 33 individuals with staphylococcal infections (Staphylococcus aureus) were obtained from the Ospedale di Circolo, Varese, Italy. All sera were from patients ranging from 21 to 86 years of age, with the majority of patients over 65 years old. Antibodies from the sera were purified by chromatography on protein A-Sepharose (Pharmacia), and the concentration of the purified immunoglobulin G (IgG) was quantitated by absorbance at 280 nm, with human IgG as the standard. Control IgG was obtained from pooled sera of healthy 2-year-old children. Analysis of IgG from individual children suggests that children have a uniformly low reactivity to the Fn-binding protein FnbpA from S. aureus. The patients were diagnosed with a variety of staphylococcal infections, including sepsis, pneumonia, and peritonitis. S. aureus was the only pathogen isolated in most cases, although some multimicrobial infections were recorded. In general, blood samples were obtained 2 days to 3 weeks after the original diagnoses. The histories of the patients were not available.
Isolation and labeling of ligands.
Human Fn was prepared as previously reported (18). The N-terminal Fn fragment (N29) was isolated as described previously (6). The N29 fragment was 125I labeled with IODO-BEADS iodination reagent as recommended by the manufacturer (Pierce, Rockford, Ill.).
Recombinant proteins.
Recombinant proteins were expressed from plasmids derived from pGEX-2T (Pharmacia) or pGEX-2H (see below). The pGEX vectors drive the production of fusion proteins in which gluthatione S-transferase (GST) precedes the unique polypeptide segment encoded by the inserted DNA. The N-terminal GST carrier allows the fusion proteins to be purified by affinity chromatography with gluthatione-coupled matrix.
DNA fragments encoding the polypeptide segments indicated in Fig. 1 were produced by PCR and cloned into the vector as previously reported (7). Table 1 describes the oligonucleotide primers used in PCR and the vector used for cloning each PCR fragment. To construct the plasmid expressing GST-UA, GST-UB, and GST-UC, DNA amplified with the indicated primers was cloned into the BamHI/HindIII site of the vector pGEX-2H. This vector is a modified version of pGEX-2T in which the SmaI restriction sequence was replaced by a HindIII restriction sequence as follows. A double-stranded oligonucleotide fragment, 5′GGAAGCTTCC3′, was blunt-end ligated to pGEX-2T digested with SmaI. The resulting vector, named pGEX-2H, lacks the SmaI restriction sequence but possesses a HindIII restriction sequence. Table 1 describes the oligonucleotide primers used in PCR and the vector used for the amplified fragment.
TABLE 1.
Oligonucleotide primers and vectors used in the construction of plasmids expressing recombinant Fn-binding protein fragments
| Expression product (vector) | Direction | Primera |
|---|---|---|
| GST-UA (pGEX-2H) | Forward | 5′ACGTGGATCCAATCTTAGGTACGGCATTAG3′ |
| Reverse | 5′ACGTAAGCTTTCTAATCTTTCCACCTTC3′ | |
| GST-UB (pGEX-2H) | Forward | 5′ACGTGGATCCACGCATGGCGTATCAACTGC3′ |
| Reverse | 5′ACGTAAGCTTACCATTATCCCAAGTTAAGG3′ | |
| GST-UC (pGEX-2H) | Forward | 5′ACGTGGATCCGGACATCCAGAGCAAC3′ |
| Reverse | 5′ACGTAAGCTTCGAATGACTGGTTACCGC3′ | |
| GST-Du (pGEX-2T) | Forward | 5′ACACATGGATCCTCAAAACATCACGCTGATG3′ |
| Reverse | 5′TTCAACGAATTCATGATCGCTGCTCACTGCGCC3′ | |
| GST-D1 (pGEX-2T) | Forward | 5′ATCCGGATCCGAAGGTGGCCAAAATAGCG3′ |
| Reverse | 5′TGCTTTGAATTCCCATGAATTTGAGGT3′ | |
| GST-D2 (pGEX-2T) | Forward | 5′TTTGATGGATCCCCTCAAATTCATGGT3′ |
| Reverse | 5′TGCTTAGAATTCCCGTGAATATGTGGC3′ | |
| GST-D3 (pGEX-2T) | Forward | 5′GACAGTGGATCCCATATTCACGGATTC3′ |
| Reverse | 5′GACCTTGAATTCGGCCGCTTACTTTTGG3′ | |
| GST-D4 (pGEX-2T) | Forward | 5′GAAGATGGATCCCCAAAAGTAAGCGGCC3′ |
| Reverse | 5′TCTGGTGAATTCGGCGTTGGTGGCACG3′ | |
| GST-Du1234 (pGEX-2T) | Forward | 5′ACACATGGATCCTCAAAACATCACGCTGATG3′ |
| Reverse | 5′TCTGGTGAATTCGGCGTTGGTGGCACG3′ |
Restriction endonuclease sites are underlined.
ELISA.
Igs isolated from human sera were tested for antibodies against FnbpA recombinant proteins by enzyme-linked immunosorbent assay (ELISA). Microtiter wells (Costar, Cambridge, Mass.) were incubated overnight at 4°C with 100 μl of 50 mM sodium carbonate, pH 9.5, containing 10 μg of protein per ml. Additional protein binding sites in the wells were blocked by incubation for 1 h with 200 μl of 2% (wt/vol) bovine serum albumin (BSA) in 10 mM sodium phosphate, pH 7.4, containing 0.13 M NaCl (phosphate-buffered saline; PBS). The wells were then washed five times with PBST (0.1% Tween 20 in PBS-NaCl) and incubated with 2 μg of antibody dissolved in 100 μl of 2% BSA in PBS at 22°C. Unbound antibody was removed by washing the wells five times with PBST. Bound antibody was detected by incubation (1 h at 37°C) with peroxidase-conjugated rabbit anti-human IgG (Dako, Gostrup, Denmark) diluted 1:2,000. After being washed the conjugated enzyme was reacted with o-phenylenediamine dihydrochloride (Sigma), and the absorbance at 492 nm was monitored with a microplate reader (Bio-Rad).
Fn-binding assay.
Binding of 125I-labeled N29 to surface-immobilized MSCRAMM was performed on microtiter plates. Wells were coated with 100 μl of GST-Du1234 (10 μg/ml) in 50 mM sodium carbonate, incubated overnight at 4°C, and then subjected to blocking with 200 μl of 2% (wt/vol) BSA in PBS. The wells were subsequently incubated for 2 h at 37°C with 125I-labeled N29 (8 × 104 cpm), and after extensive washing (five times) with PBST, radioactivity associated with the wells was quantitated with a gamma counter. The binding of 125I-labeled N29 to staphylococci was quantitated as described previously (16).
Isolation of anti-ligand-induced binding site (LIBS) antibodies by affinity chromatography on GST-Du1234–Sepharose.
Eight milligrams of IgG isolated from patient 5 was passed through a gelatin-Sepharose column to remove possible contaminating Fn. This material was subsequently passed through a column (1 by 4 cm) of Sepharose 4B coupled with GST-Du1234 recombinant protein and equilibrated with PBS-azide. The column was washed with equilibration buffer (PBS) until a stable baseline level of absorbance at 280 nm of the column effluent was observed (flowthrough) and then with 0.4 M NaCl in 10 mM phosphate buffer, pH 7.4. The material which specifically bound to the column was eluted with 0.1 M glycine, pH 2.8, and the fractions were neutralized with 1 M Tris. The unadsorbed material and the material bound and eluted from the column were analyzed by ELISA and by Western blot analysis and dot blot analysis.
Western blotting.
FnbpA recombinant GST-Du1234 protein was run in sodium dodecyl sulfate (SDS)–10% polyacrylamide gel under reducing conditions and then electroblotted onto nitrocellulose membranes (Sartorius, Gottingen, Germany) for 2 h at 200 mA in transfer buffer (20 mM Tris-HCl, 150 mM glycine, 20% [vol/vol] methanol, pH 8.3). The membranes were then treated for 1 h with a solution containing 5% (wt/vol) dried skim milk in TBS (20 mM Tris-HCl containing 0.5 M NaCl, pH 7.5), washed three times for 10 min each time with TBS, and followed by 1 h of incubation with 0.7 μg of N29 per ml in 1% milk in TTBS (TBS containing 0.05% Tween 20). Membranes were washed and incubated for 2 h with IgG (0.7 μg/ml), washed three times for 10 min each time in TTBS, and subsequently incubated for 1 h in TTBS containing 1% milk and 5,000-fold-diluted horseradish peroxidase-conjugated rabbit anti-human IgG (Dako). After being washed, the membrane was treated with enhanced chemiluminescence detection reagents (NEN, Boston, Mass.) according to the procedure recommended by the manufacturer and exposed to X-ray film for 30 to 60 s.
Dot blot analysis.
Affinity membranes of Immobilon AV (Millipore) were activated in PBS for 1 min and then dried with filter paper. Recombinant GST-Du1234 protein (100 ng) dissolved in the coupling buffer (0.5 M KH2PO4, pH 7.4) was spotted and covalently bound to the membrane. To detect the immunoreactive spots, the membrane was probed with antibodies in the presence and absence of N29 and treated following the procedure described for the Western blot assay.
RESULTS
IgG reacts with the repeat domain of the Fn-binding MSCRAMM.
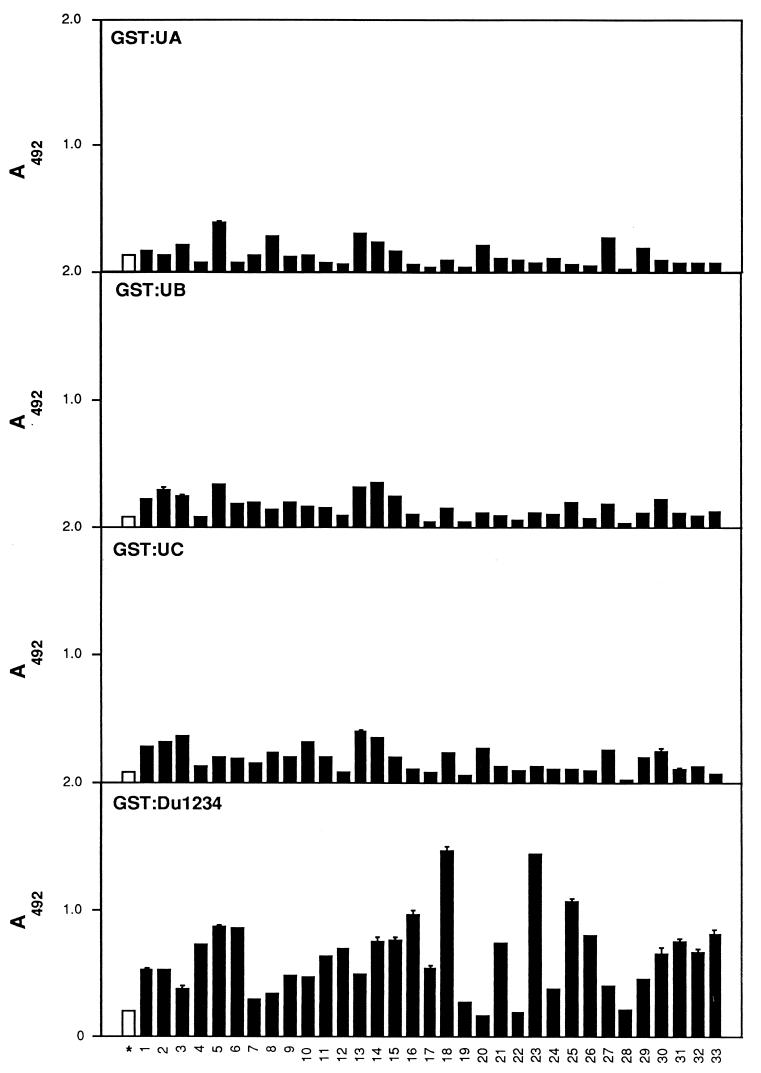
Different segments of the S. aureus Fn-binding MSCRAMM were expressed as recombinant proteins and purified. The reactivity of IgG to these segments coated onto microtiter plates was subsequently examined (Fig. 2). In general, more antibodies bound to wells coated with the ligand-binding repeat domain GST-Du1234 than to wells coated with any other recombinant MSCRAMM domain.
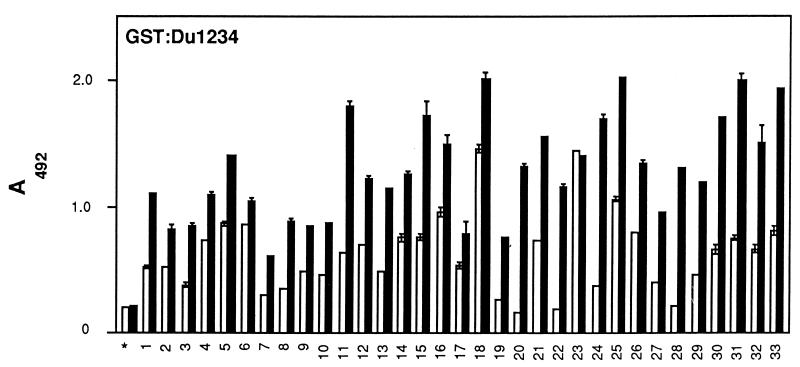
FIG. 2.
Mapping of epitopes in GST-FnbpA fusion protein derivatives. GST-FnbpA polypeptides GST-UA, GST-UB, GST-UC and GST-Du1234 (1 μg in 100 μl) were immobilized in microtiter wells and probed with IgG preparations (2 μg in 100 μl). Bound antibody was detected as described in Materials and Methods. Pooled sera from 2-year-old children were used as the control (∗). Results are expressed as means ± standard deviations (error bars). Each sample point was done in duplicate.
Patients varied substantially in terms of IgG reactivity to the GST-Du1234 protein. IgG from patients 20, 22, and 28 seemed to bind to the MSCRAMM at levels that were the same or lower than those of the control IgG isolated from young children with no history of staphylococcal infection. On the other hand, most of the IgG from the adults bound to the MSCRAMM at levels significantly higher than those of the control IgG, and patients 18 and 23 gave a 10-fold-higher response signal. Taken together, these results suggest that patients exposed to staphylococcal infections respond by producing antibodies to the Fn-binding MSCRAMM that preferentially target the ligand-binding domain of the MSCRAMM. A sample of adult IgG preparations was analyzed for concentration-dependent binding to the immobilized GST-Du1234. In general, a linear relationship was observed when the amount of IgG added varied from 0 to 2 μg (data not shown). The difference in reactivities among IgG preparations, seen in Fig. 2, is therefore a reflection of relative titers.
Adult IgG does not inhibit Fn-MSCRAMM interaction.
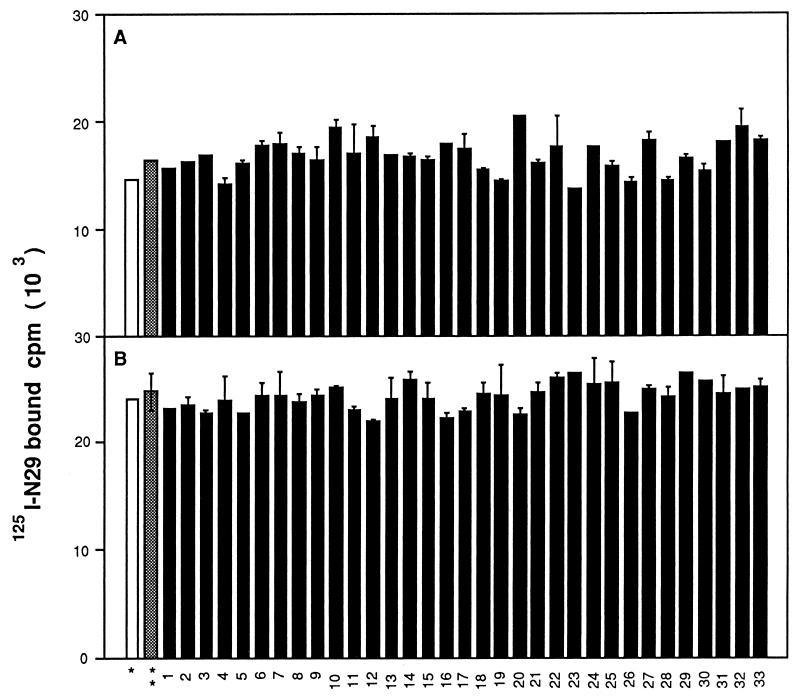
The ability of the isolated adult IgG to interfere with the binding of the 125I-labeled N-terminal fragment of Fn to staphylococcal cells or to GST-Du1234 immobilized on microtiter wells was analyzed. At 50 μg, none of the IgG preparations significantly interfered with Fn binding to staphylococcal cells (Fig. 3A). The antibody preparations at 2 μg did not significantly affect the binding of Fn to the immobilized GST-Du1234 fusion protein either (Fig. 3B). Selected patient IgG preparations were further examined for the presence of a low concentration of antibodies capable of interfering with the ligand-binding activity of the MSCRAMM. Thus, increasing the added IgG from patients 1, 2, 32, and 33 up to 10 μg had no effect on Fn binding to the immobilized GST-Du1234 (data not shown).
FIG. 3.
Antibody preparations from patients do not inhibit Fn binding to FnbpA. (A) Cells of S. aureus Cowan 1 (108) were incubated with 125I-labeled N29 (5 × 104) in the presence of 50 μg of each IgG preparation, and the radioactivity bound to the cells was quantitated. (B) GST-Du1234 was immobilized onto microtiter wells (1 μg/well) and probed with 8 × 104 cpm of 125I-labeled N29 in the presence of 2 μg of each IgG preparation. After being washed extensively with PBS containing 0.1% Tween 20, the plates were incubated with 200 μl of 2% SDS at 37°C for 30 min, and the radioactivity released from the wells was quantitated with a gamma counter. Controls are reported when bacteria or GST-Du1234-coated plates were incubated without antibodies (∗) or with antibodies isolated from pooled sera from young children (∗∗). Data are reported as means ± standard deviations (error bars).
Patients’ IgG contains anti-LIBS antibodies.
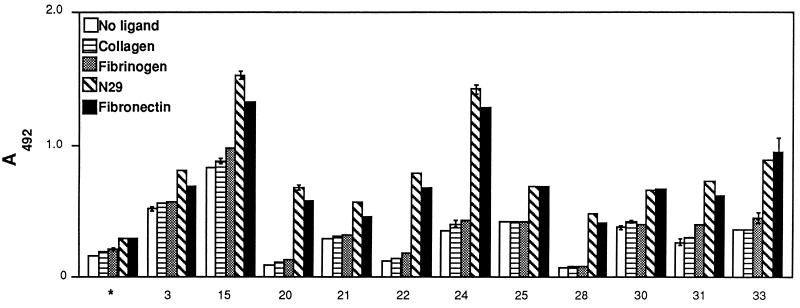
A sample of adult IgG preparation was examined for the presence of anti-LIBS antibodies, which recognize epitopes formed by the binding of the ligand to the MSCRAMM (2, 3). Antibody binding to microtiter wells coated with GST-Du1234 was enhanced by the presence of Fn for most of the IgG preparations tested (Fig. 4). This enhanced reactivity was specifically induced by Fn and its N-terminal fragment N29 but not by fibrinogen and collagen, which are known to interact with different MSCRAMM of staphylococci. When the whole panel of IgG preparations was examined for its reactivity toward GST-Du1234 in the presence or absence of N29, essentially all preparations showed strong anti-LIBS activity (Fig. 5). The extent of anti-LIBS reactivity varied from one IgG preparation to another. For patient 23, the presence of Fn did not increase the amount of antibody bound to the immobilized GST-Du1234 recombinant protein. Other IgG preparations gave a signal up to fivefold higher in the presence of the ligand. It is noteworthy that IgG from patients 20, 22, and 28, which gave a low signal in the absence of Fn, seemed to have a very high proportion of anti-LIBS antibodies. The control IgG preparation contained very low anti-LIBS activity. Taken together, these data suggest that most individuals with staphylococcal infections develop anti-LIBS antibodies to the Fn-binding MSCRAMM. On the other hand, fractionation of IgG from patient 23 on a GST-Du1234–Sepharose column did not reveal any detectable anti-LIBS antibodies (data not shown). This result is consistent with the observation that the reactivity of this patient’s IgG to GST-Du1234 was not enhanced by the presence of Fn (Fig. 5).
FIG. 4.
Specific formation of LIBS epitopes induced by Fn. GST-Du1234 recombinant protein was immobilized onto microtiter wells (1 μg in 100 μl) and probed with each IgG preparation (2 μg in 100 μl) in the presence of Fn, the N-terminal Fn fragment (N29), collagen, or fibrinogen. Binding of antibodies to the recombinant protein in absence of ligand is also shown. Bound antibody was detected as described in Materials and Methods. Bars represent means ± standard deviations with duplicate testing. Controls (∗) represent immobilized GST-Du1234 incubated with antibodies from young children.
FIG. 5.
Screening of the whole panel of IgG preparations for anti-LIBS activity. Microtiter wells were coated with GST-Du1234 (1 μg in 100 μl) and incubated with each IgG preparation (2 μg in 100 μl) either in the absence (□) or presence (■) of 0.5 μg of N29. Bound antibody was detected as described in Materials and Methods. Data are expressed as means ± standard deviations (error bars) of duplicate testing.
Anti-LIBS antibodies can be isolated.
To separate the anti-LIBS antibodies from antibodies binding to the MSCRAMM in the absence of Fn, a selected IgG preparation from patient 5 was passed through an affinity column composed of GST-Du1234 coupled with Sepharose. The antibodies that did not bind to the column (flowthrough) were collected separately. The column was washed extensively, and bound antibodies were subsequently eluted with 0.1 M glycine, pH 2.8, and neutralized. The unfractionated and fractionated antibodies were then analyzed. By an ELISA carried out in the presence or absence of Fn, the unfractionated antibodies gave a stronger signal in the presence of the ligand at the different concentrations of antibody tested (Fig. 6A). Antibodies bound and eluted from the affinity matrix gave the same response regardless of whether Fn was present in the ELISA or not (Fig. 6C). The flowthrough IgG preparation showed weak binding to the immobilized MSCRAMM in the absence of Fn. When Fn was included, a markedly stronger, concentration-dependent binding of the antibody to the MSCRAMM was noted (Fig. 6B). Fractionation of IgG from patients 15 and 26 gave similar results. In both cases, IgG that did not bind to the GST-Du1234–Sepharose column were highly enriched with anti-LIBS antibodies (data not shown).
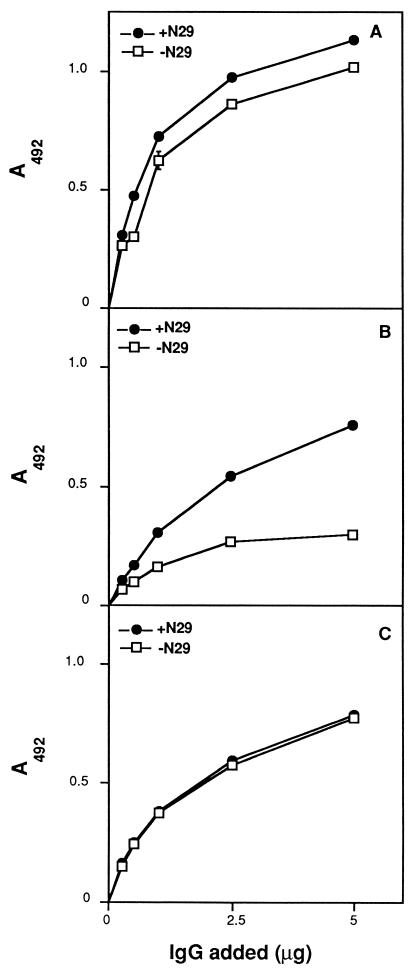
FIG. 6.
ELISA of anti-LIBS antibodies isolated by affinity chromatography on GST-Du1234. A preparation of IgG obtained from patient 5 was fractionated onto a GST-Du1234–Sepharose column as described in Materials and Methods. GST-Du1234 protein was immobilized onto microtiter wells (1 μg in 100 μl) and assayed by ELISA with increasing concentrations of IgG from the flowthrough of the column (B) or with antibodies bound and eluted from the affinity matrix (C). Reactivity of the unfractionated IgG preparation is shown in panel A. Assays were carried out in the absence or presence of N29. Bound antibody was detected as described in Materials and Methods.
These results were subsequently confirmed in dot blot and Western blot assays of IgG from patient 5 (data not shown). Unfractionated antibody reacted with MSCRAMM in the presence and absence of Fn, although the presence of the ligand seemed to induce a somewhat stronger signal. The affinity-purified antibody recognized the MSCRAMM in the process, which was seemingly unaffected by the presence or absence of Fn. The flowthrough IgG, on the other hand, detected the MSCRAMM only when Fn was present in the incubation mixture.
Localization of the LIBS epitopes.
The Fn-binding MSCRAMM FnbpA of S. aureus contains four repeats (Du, D1, D2, and D3) of a ligand-binding sequence and a fifth partial repeat, D4. All these units have been shown to bind Fn (data not shown) and could potentially form epitopes recognized by anti-LIBS antibodies. To determine to which repeat unit(s) the LIBS antibodies were directed, we expressed the individual units as GST fusion proteins and purified the recombinant proteins as described previously. The whole panel of patient IgG preparations was screened with the individual recombinant repeat units in the presence or absence of Fn. ELISA (Fig. 7) demonstrated that the repeat units, Du, D1, D2, and D3, as well as the incomplete repeat unit, D4, formed epitopes in the presence of Fn which could be recognized by anti-LIBS antibodies. These results confirm that all the individual repeat units are capable of binding Fn and that this interaction can occur in patients. Presumably, the binding of repeat units of the MSCRAMM to Fn involves a conformational change in the MSCRAMM repeats which can be manifested as an epitope recognized by an anti-LIBS antibody.
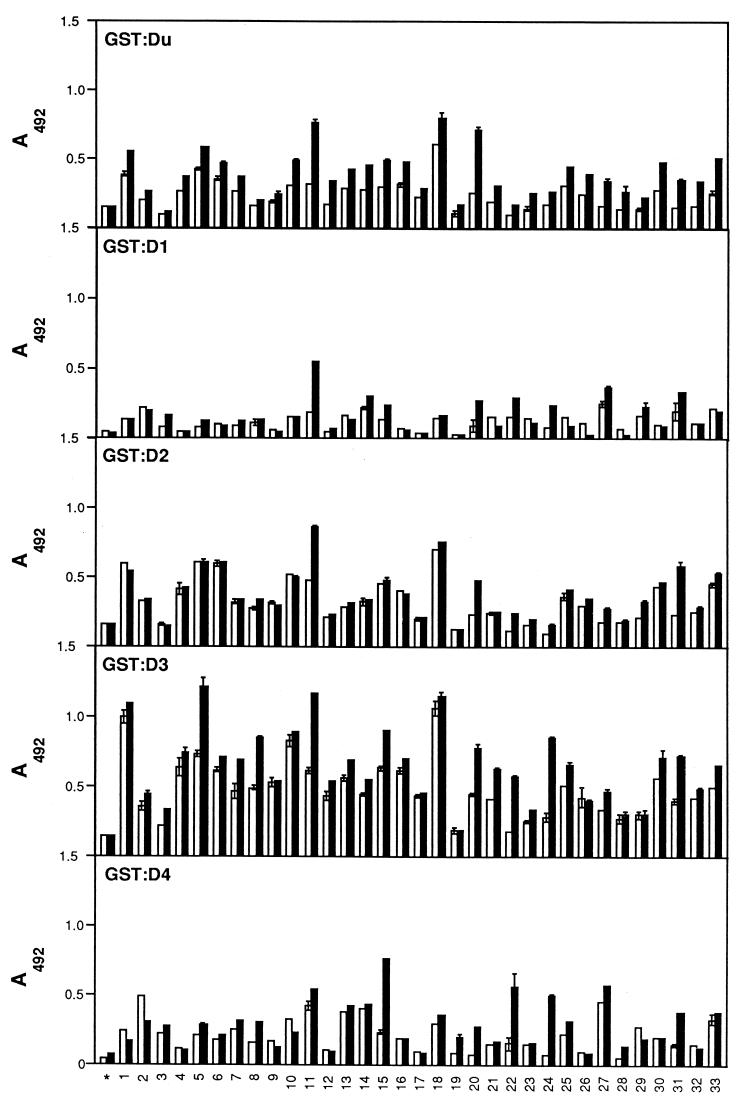
FIG. 7.
Localization of LIBS epitopes in the individual Fn-binding motifs. The Fn-binding motifs Du, D1, D2, D3, and D4 fused to the GST carrier were immobilized onto microtiter wells (1 μg in 100 μl) and probed with the panel of IgG preparations either in the absence (□) or presence (■) of the N-terminal fragment (N29) of Fn. Bound antibody was detected as described in Materials and Methods.
The variation in anti-LIBS antibody signals to the individual recombinant MSCRAMM repeat units differed greatly among different IgG preparations. In general, most IgG preparations contained anti-LIBS antibodies which recognized epitopes formed by the Du and D3 repeat units, whereas only occasional IgG preparations contained anti-LIBS antibodies recognizing the complex formed by the binding of D1, D2, or D4 to Fn.
It is noteworthy that IgG from essentially all patients contained anti-LIBS antibodies by analysis with the GST-Du1234 fragments, whereas IgG reactivity to individual recombinant MSCRAMM repeat units revealed a lower frequency of anti-LIBS antibodies. This result is not surprising, since anti-LIBS antibodies detected with the GST-Du1234 fragment should include the combined reactivities of the anti-LIBS antibodies and those of the different individual MSCRAMM repeat units.
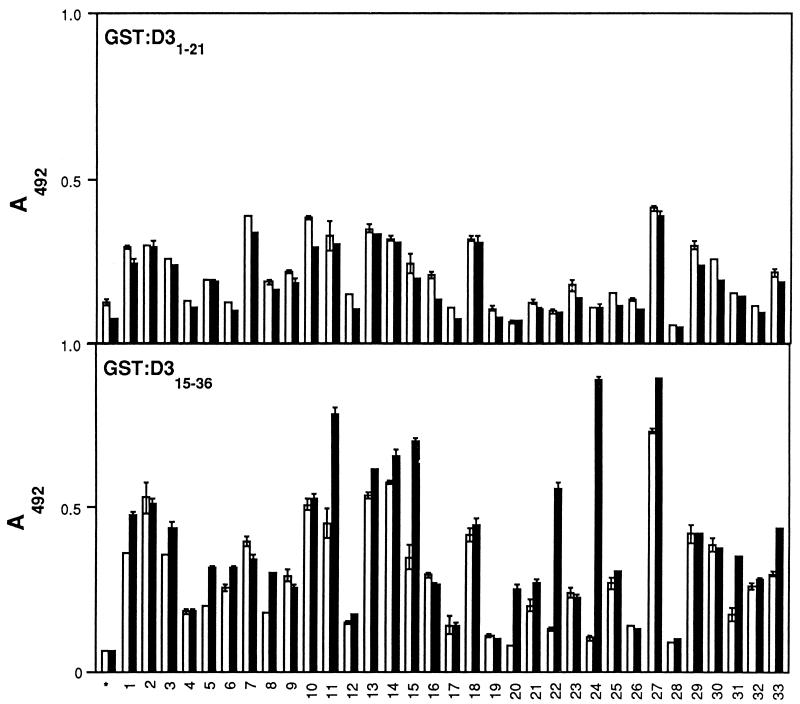
The D3 repeat unit contains a strong Fn-binding motif located in the C terminus, corresponding to residues 15 to 36 of the repeat, whereas residues 1 to 21 contain one (or two) weaker Fn-binding motifs (data not shown). To determine if both motifs could contain and support the formation of epitopes recognized by anti-LIBS antibodies, the different D3 segments were expressed as recombinants and targeted by ELISA. The results of this experiment showed that the C-terminal, but not the N-terminal, motif could form epitopes recognized by anti-LIBS antibodies (Fig. 8).
FIG. 8.
Localization of LIBS epitopes in the subfragments of the D3 motif. Microtiter wells were coated with recombinant subfragments GST-D31–21 and GST-D315–36 (1 μg in 100 μl) and incubated with IgG preparations in either the absence (□) or presence (■) of N29 (see Results). Bound antibody was detected as described in Materials and Methods.
DISCUSSION
We have analyzed the immune response to the Fn-binding MSCRAMM in patients with S. aureus infections. The patient population was quite varied, and in this study, we did not attempt to correlate the immune response to onset, type of infection, or patient group. Rather, we were interested in determining the reactivity of antibodies produced by humans in response to staphylococcal infection. We found that antibodies purified by affinity chromatography on a protein A-Sepharose column bound preferentially to the Fn-binding repeat domain of the MSCRAMM. This observation suggests that the repeat domain is immunodominant over other MSCRAMM domains, a conclusion which is supported by a recent study in which ∼80% of monoclonal antibodies generated from mice immunized with the full-length MSCRAMM were found to be directed to the repeat domain (data not shown). Alternatively, these observations could reflect variations in serum type. Analyses of strain collections suggest that most S. aureus strains contain the gene encoding the Fn-binding MSCRAMM FnbpA. However, the sequence variability of the gene in different isolates has not been determined. The patients from whom the IgG was derived most likely were infected by strains whose Fn-binding MSCRAMM sequences differ. The observed low IgG binding to MSCRAMM regions outside the repeat region may simply reflect a great sequence variability in this region among different strains compared to a presumably conserved ligand-binding domain.
None of the IgG preparations significantly inhibited the binding of Fn to isolated recombinant MSCRAMM or to intact bacteria. This surprising result does not have an obvious molecular explanation. Is it possible that the MSCRAMM binds Fn with a much higher affinity than a competing potentially inhibiting antibody and that consequently inhibiting antibodies are not detected? Is the ligand-binding surface of the MSCRAMM always occupied by Fn and therefore never detected as foreign and a target for an immune response? The observed conformational changes induced in the MSCRAMM on ligand binding could help protect the binding surface of the MSCRAMM, since it is not formed in the absence of Fn. The presence of anti-LIBS antibodies demonstrates that the Fn-MSCRAMM complex is exposed to the immune system. Epitopes recognized by the anti-LIBS antibodies were detected in each of the repeat units in the ligand-binding domain when they were presented as recombinant individual units. These observations demonstrate that each repeat unit is capable of binding Fn and suggest that these interactions involve conformational changes in the MSCRAMM repeat units. Furthermore, when recombinant fragments of the D3 unit were examined, we found that the C-terminal 20-amino-acid residues which contain a high-affinity binding site also harbor a LIBS, suggesting that LIBS can be located close to the ligand-binding surface. It is not clear if the LIBS is formed only by residues in the MSCRAMM or if residues in Fn can contribute to the epitope. So far, we have not been able to demonstrate the direct binding of these IgG preparations to Fn by ELISA. The value of LIBS antibodies to the patient is not known. LIBS antibodies could be opsonic and stimulate phagocytic clearance of the bacteria. On the other hand, previous studies suggest that these antibodies may be advantageous to bacteria by stabilizing Fn binding and thus enhancing bacterial adherence to the host tissue (16).
Since MSCRAMM are currently being targeted in different bacterial vaccination strategies, further studies to determine the significance of anti-LIBS antibodies are clearly warranted.
ACKNOWLEDGMENTS
This work was supported by the Italian Ministry of University and Scientific Research and Techology, by National Institutes of Health grant AI20624, and by grant CRG931412 from the North Atlantic Treaty Organization.
REFERENCES
- 1.Courtney H S, Li Y, Dale J B, Hasty D L. Cloning, sequencing, and expression of a fibronectin/fibrinogen-binding protein from group A streptococci. Infect Immun. 1994;62:3937–3946. doi: 10.1128/iai.62.9.3937-3946.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frelinger A L, Cohen I, Plow E F, Smith M A, Roberts J, Lam S C, Ginsberg M H. Selective inhibition of integrin function by antibodies specific for ligand-occupied receptor conformers. J Biol Chem. 1990;265:6346–6352. [PubMed] [Google Scholar]
- 3.Frelinger A L, Du X, Plow E F, Ginsberg M H. Monoclonal antibodies to ligand-occupied conformers of integrin αIIbβ3 (glycoprotein IIb-IIIa) alter receptor affinity, specificity, and function. J Biol Chem. 1991;266:17105–17111. [PubMed] [Google Scholar]
- 4.Frick I-M, Crossin K L, Edelman G M, Biörck L. Protein H—a bacterial surface protein with affinity for both immunoglobulin and fibronectin type III domains. EMBO J. 1995;14:1674–1679. doi: 10.1002/j.1460-2075.1995.tb07156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanski E, Caparon M. Protein F, a fibronectin-binding protein, is an adhesin of the group A streptococcus Streptococcus pyogenes. Proc Natl Acad Sci USA. 1992;89:6172–6176. doi: 10.1073/pnas.89.13.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.House-Pompeo K, Xu Y, Joh D, Speziale P, Höök M. Conformational changes in the fibronectin binding MSCRAMM are induced by ligand binding. J Biol Chem. 1996;271:1379–1384. doi: 10.1074/jbc.271.3.1379. [DOI] [PubMed] [Google Scholar]
- 7.Joh H J, House-Pompeo K, Patti J M, Gurusiddappa S, Höök M. Fibronectin receptors from Gram-positive bacteria: comparison of active sites. Biochemistry. 1994;33:6086–6092. doi: 10.1021/bi00186a007. [DOI] [PubMed] [Google Scholar]
- 8.Jönsson C, Signäs C, Müller H P, Lindberg M. Two different genes encode fibronectin-binding proteins in Staphylococcus aureus. The complete nucleotide sequence and characterization of the second gene. Eur J Biochem. 1991;202:1041–1048. doi: 10.1111/j.1432-1033.1991.tb16468.x. [DOI] [PubMed] [Google Scholar]
- 9.Lindgren P-E, McGavin M J, Signäs C, Guss B, Gurusidappa S, Höök M, Lindberg M. Two different genes coding for fibronectin-binding proteins from Streptococcus dysgalactiae. Eur J Biochem. 1993;214:819–827. doi: 10.1111/j.1432-1033.1993.tb17985.x. [DOI] [PubMed] [Google Scholar]
- 10.Panchioli V, Fischetti V A. A major surface protein on group A streptococci is a glyceraldehyde-3-phosphate dehydrogenase with multiple binding activity. J Exp Med. 1992;176:415–426. doi: 10.1084/jem.176.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patti J M, Allen B L, McGavin M J, Höök M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol. 1994;48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- 12.Patti J M, Höök M. Microbial adhesins recognizing extracellular matrix macromolecules. Curr Opin Cell Biol. 1994;6:752–758. doi: 10.1016/0955-0674(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 13.Rakonjac J V, Robbins J C, Fischetti V A. DNA sequence of the serum opacity factor of group A streptococci: identification of a fibronectin binding repeat domain. Infect Immun. 1995;63:622–631. doi: 10.1128/iai.63.2.622-631.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt K H, Mann K, Cooney J, Köhler W. Multiple binding of type 3 streptococcal M protein to human fibrinogen, albumin and fibronectin. FEMS Immun Med Microbiol. 1993;7:135–144. doi: 10.1111/j.1574-695X.1993.tb00392.x. [DOI] [PubMed] [Google Scholar]
- 15.Signäs C, Raucci G, Jönsson K, Lindgren P-E, Anantharamaiah G M, Höök M, Lindberg M. Nucleotide sequence of the gene for a fibronectin-binding protein from Staphylococcus aureus and its use in the synthesis of biologically active peptides. Proc Natl Acad Sci USA. 1989;86:699–703. doi: 10.1073/pnas.86.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Speziale P, Joh D, Visai L, Bozzini S, House-Pompeo K, Lindberg M, Höök M. A monoclonal antibody enhances ligand binding of fibronectin MSCRAMM adhesin from Streptococcus dysgalactiae. J Biol Chem. 1996;271:1371–1378. doi: 10.1074/jbc.271.3.1371. [DOI] [PubMed] [Google Scholar]
- 17.Talay S R, Valentin-Weigand P, Timmis K N, Chhatwal G S. Domain structure and conserved epitopes of the Sfb protein, the fibronectin-binding adhesin of Streptococcus pyogenes. Mol Microbiol. 1994;13:531–539. doi: 10.1111/j.1365-2958.1994.tb00448.x. [DOI] [PubMed] [Google Scholar]
- 18.Vuento M, Vaheri A. Purification of fibronectin from human plasma by affinity chromatography under non-denaturing conditions. Biochem J. 1979;183:331–337. doi: 10.1042/bj1830331. [DOI] [PMC free article] [PubMed] [Google Scholar]