Abstract
Background
Several scores aimed at predicting COVID-19 progression have been proposed. As the variables vaccination and early SARS-CoV-2 treatment were systematically excluded from the prognostic scores, the present study's objective was to develop a new model adapted to the current epidemiological scenario.
Methods
We included all patients evaluated by the Infectious Disease Unit in Sassari, with SARS-CoV-2 infection and without signs of respiratory failure at the first evaluation (P/F > 300). Disease progression was defined by the prescription of supplemental oxygen. In addition, variables related to demographics, vaccines, comorbidities, symptoms, CT scans, blood tests, and therapies were collected. Multivariate logistic regression modelling was performed to determine factors associated with progression; any variable with significant univariate test or clinical relevance was selected as a candidate for multivariate analysis. Hosmer–Lemeshow (HL) goodness of fit statistic was calculated. Odds ratio values were used to derive an integer score for developing an easy-to-use progression risk score. The discrimination performance of the risk index was determined using the AUC, and the best cut-off point, according to the Youden index, sensitivity, specificity, predictive value, and likelihood ratio, was chosen.
Results
1145 patients [median (IQR) age 74 (62–83) years; 53.5% males] were enrolled; 336 (29.3%) had disease progression. Patients with a clinical progression were older and showed more comorbidities; furthermore, they were less vaccinated and exposed to preventive therapy. In the multivariate logistic regression analysis, age ≥ 60 years, COPD, dementia, haematological tumours, heart failure, exposure to no or one vaccine dose, fever, dyspnoea, GGO, consolidation, ferritin, De Ritis ≥ 1.2, LDH, and no exposure to early anti-SARS-CoV-2 treatment were associated with disease progression. The final risk score ranged from 0 to 45. The ROC curve analysis showed an AUC of 0.92 (95% CI 0.90–0.93) with a 93.7% specificity and 72.9% sensitivity. Low risk was defined when the cut-off value was less than 23. Three risk levels were identified: low (0–23 points), medium (24–35), and high (≥ 36).
Conclusions
The proportion of patients with progression increases with high scores: the assessment of the risk could be helpful for clinicians to plan appropriate therapeutic strategies.
Keywords: Progression risk score, COVID-19, Score, SARS-CoV-2, Antiviral treatment, Vaccination
Introduction
More than six million deaths have occurred since the emergence and spread of SARS-CoV-2 worldwide; however, asymptomatic or mild forms of disease are recorded in the majority of the cases [1]. The most prevalent symptoms of Coronavirus Disease 19 (COVID-19) are fever, cough, and dyspnoea; a low proportion complains of gastrointestinal symptoms, anosmia, dysgeusia, headache, and skin lesions [2–4] life-threatening systemic inflammation, respiratory failure, and multiorgan dysfunction [5, 6].
Several factors are associated with COVID-19 severity and death: older age, being male, being a smoker, Chronic Obstructive Pulmonary Disease (COPD), cardiovascular disease (CVD), diabetes, hypertension, obesity, cancer, and acute kidney injury are associated with increased mortality [7].
Several scores were created to identify individuals with a higher risk of severe disease and death [6, 8–12]. The 4C-score, developed at the beginning of the pandemic after the recruitment of a cohort in the ISARIC Coronavirus Clinical Characterization Consortium (ICARIC-4C), has been frequently adopted [13]. However, in these years, substantial changes occurred. First, the virus mutated with new viral variants [14, 15]. In addition, several vaccines were commercially distributed, reducing the risk of severe illness and death [16, 17]. Finally, more effective drugs and adequate management of people with severe diseases [18–23]. For all these reasons, it is not easy for clinicians to predict the patients' evolution, given the numerous factors that come into play and decide when hospital admission could be necessary. In this regard, Drake et al. found that only half of the hospitalized people developed complications needing support [24].
Therefore, aim of the present study was to create a new score to predict the risk of disease severity.
Methods
Study design
A retrospective cohort study was conducted by recruiting individuals with a SARS-CoV-2 infection diagnosed by Polymerase Chain Reaction (PCR) between January and September 2022 in an Italian university hospital. Those aged < 18 years, those with incomplete clinical data, and with severe COVID-19 needing oxygen supplementation at the first evaluation were excluded.
The primary study objective was to create a score to predict disease progression (i.e., administration or increase of oxygen supplementation).
Data on demographics (age, gender, and weight), medical history (chronic renal disease, dialysis, immunodeficiency, transplantation, rheumatologic disease, diabetes, COPD, hemoglobinopathy, neurological disease, cancer, and cardiovascular disease), Charlson Comorbidity Index (CCI) [25], vaccination status (number of doses, time from the last dose), ward and symptoms at the admission (fever, cough, tachypnea, ageusia, pharyngodynia, chills, asthenia, headache, myalgia, gastrointestinal symptoms, dyspnoea, nasal congestion, and anosmia), computed tomography (CT) signs, biochemical indicators at admission (white blood cells -WBC-, neutrophils lymphocyte, neutrophil–lymphocyte-ratio-NLR-, ferritin, procalcitonin -PCT-, urea, creatinine, Estimated Glomerular Filtration Rate –eGFR—calculated with Cockcroft-Gault formula [26], aspartate aminotransferase -AST-, alanine aminotransferase -ALT-, De Ritis ratio -AST/ALT-, lactate dehydrogenase -LDH-, C-reactive protein -CRP-, and D-Dimer), early treatment with antivirals [monlupiravir, nirmatrelvir/ritonavir(r), and remdesivir] and monoclonal antibodies (casirivimab/imdevimab, and sotrovimab), and data of negativization were collected.
Ethics
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee with the protocol code PG/2022/20481.
Statistical analysis
Sample characteristics were described using absolute and relative (percentage) frequencies or median and Interquartile Range (IQR); Shapiro–Wilk test was used to assess the normality distribution of quantitative variables. Differences between quantitative and qualitative variables were evaluated by the Mann–Whitney U test and by Pearson Chi-Square or Fisher exact test, respectively. A multivariate stepwise logistic regression was performed to assess disease severity-related factors. The variables included in the multivariate model were evaluated based on their clinical or statistical significance at the obtained in the univariate analysis. Hosmer–Lemeshow (HL), goodness of fit statistic was calculated. Odds ratio values were used to derive an integer score from developing an easy-to-use progression risk score. The discrimination performance of the risk index was determined using the AUC, and the best cut-off, according to the Youden index, sensitivity, specificity, predictive value, and likelihood ratio, was chosen. Furthermore, performance of the score (AUC) was tested to a half cohort randomly selected from the total cohort. A two-tailed p-value less than 0.05 was considered statistically significant. Statistical analysis was carried out using STATA 17 (StataCorp, TX, USA).
Results
A total of 1652 individuals with a SARS-CoV-2 infection were evaluated: 245 were excluded because of missing data, as well as 262 owing to severe COVID-19 at the admission.
Overall, 1145 patients with a median (IQR) age of 74 (62–83) years were included in the study. During the follow-up, 336 (29.3%) developed a severe disease needing oxygen supplementation or an increased administration. When compared with patients who did not experience disease progression, they were older, with a higher comorbidity index, less frequently vaccinated and exposed to early antiviral therapies and monoclonal antibodies, with fever, cough, and dyspnea at admission, and more frequently showed CT and blood biochemical indexes abnormalities (Table 1).
Table 1.
Demographic characteristics, comorbidities, symptoms, radiological findings, biochemical features, and treatments of 1145 patients with SARS-CoV-2 infection with or without disease progression
| Variables | Total cohort (n = 1145) | Non-severe disease (n = 809) | Severe disease (n = 336) | p-value |
|---|---|---|---|---|
| Males, n (%) | 612 (53.5) | 426 (52.7) | 186 (55.4) | 0.404 |
| Age, years, median (IQR) | 74 (62–83) | 72 (59–82) | 77.5 (66.5–86.0) | < 0.001 |
| Age groups, n (%) | ||||
| < 50 years | 134 (11.7) | 118 (14.6) | 16 (4.8) | < 0.001 |
| 50–59 years | 115 (10.0) | 90 (11.1) | 25 (7.4) | 0.058 |
| 60–69 years | 209 (18.3) | 147 (18.2) | 62 (18.5) | 0.905 |
| 70–79 years | 270 (23.6) | 182 (22.5) | 88 (26.2) | 0.179 |
| ≥ 80 years | 417 (36.4) | 272 (33.6) | 145 (43.2) | 0.002 |
| Age ≥ 60 years, n (%) | 896 (78.3) | 601 (74.3) | 295 (87.8) | < 0.001 |
| Patient provenience, n (%) | ||||
| ED | 762 (66.7) | 508 (62.9) | 254 (75.8) | < 0.001 |
| Ward | 334 (29.2) | 255 (31.6) | 79 (23.6) | 0.007 |
| Domicile | 47 (4.1) | 45 (5.6) | 2 (0.6) | 0.001 |
| Comorbidities | ||||
| Weight, kg, median (IQR) | 70 (60–80) | 70 (60–80) | 70 (62–80) | 0.111 |
| BMI > 30 kg/m2, n (%) | 260 (22.7) | 173 (21.4) | 87 (25.9) | 0.097 |
| Chronic renal failure, n (%) | 186 (16.2) | 127 (15.7) | 59 (17.6) | 0.437 |
| Dialysis, n (%) | 24 (2.1) | 15 (1.9) | 9 (2.7) | 0.375 |
| Immunodeficit, n (%) | 156 (13.6) | 104 (12.9) | 52 (15.5) | 0.239 |
| Transplant recipients, n (%) | 17 (1.5) | 10 (1.2) | 7 (2.8) | 0.280 |
| Rheumatological disease, n (%) | 62 (5.4) | 42 (5.2) | 20 (6.0) | 0.604 |
| Decompensated diabetes, n (%) | 183 (16.0) | 109 (13.5) | 74 (22.0) | < 0.001 |
| Diabetes, n (%) | 252 (22.0) | 172 (21.3) | 80 (23.8) | 0.343 |
| Chronic liver disease, n (%) | 66 (5.8) | 42 (5.2) | 24 (7.1) | 0.197 |
| COPD, n (%) | 222 (19.4) | 137 (16.9) | 85 (25.3) | 0.001 |
| Hemoglobinopathies, n (%) | 5 (0.4) | 4 (0.5) | 1 (0.3) | 0.646 |
| Neurodevelopmental/neurodegenerative diseases, n (%) | 321 (28.0) | 203 (25.1) | 118 (35.1) | 0.001 |
| Dementia, n (%) | 176 (15.4) | 100 (12.4) | 76 (22.6) | < 0.001 |
| Chromosopathies/hypoxia, n (%) | 8 (0.7) | 4 (0.5) | 4 (1.2) | 0.198 |
| Neuromuscular disease, n (%) | 33 (2.9) | 25 (3.1) | 8 (2.4) | 0.514 |
| Cerebrovascular events, n (%) | 134 (11.7) | 88 (10.9) | 46 (13.7) | 0.178 |
| Oncological disease, n (%) | 170 (14.9) | 133 (16.4) | 37 (11.0) | 0.019 |
| Metastasis, n (%) | 58 (5.1) | 41 (5.1) | 17 (5.1) | 0.995 |
| Terminal cancer, n (%) | 20 (1.8) | 6 (0.7) | 14 (4.2) | < 0.001 |
| Haematological tumours, n (%) | 71 (6.2) | 44 (5.4) | 27 (8.0) | 0.097 |
| Solid tumours in chemotherapy, n (%) | 33 (2.9) | 26 (3.2) | 7 (2.1) | 0.298 |
| Haematological tumours in chemotherapy, n (%) | 48 (4.2) | 30 (3.7) | 18 (5.4) | 0.205 |
| Cardiovascular diseases, n (%) | 417 (36.4) | 276 (34.1) | 141 (42.0) | 0.012 |
| Heart failure, n (%) | 370 (32.3) | 241 (29.8) | 129 (38.4) | 0.005 |
| Previous acute myocardial infarction, n (%) | 147 (12.8) | 101 (12.5) | 46 (13.7) | 0.579 |
| Hypertension, n (%) | 547 (47.8) | 361 (44.6) | 186 (55.4) | 0.001 |
| Median (IQR) number of comorbidities | 2 (1–3) | 2 (1–3) | 2 (1–3) | < 0.001 |
| CCI, median (IQR) | 5 (3–7) | 5 (3–7) | 5 (4–7) | < 0.001 |
| Vaccine, n (%) | 937 (81.8) | 721 (89.1) | 216 (64.3) | < 0.001 |
| N. of doses, n (%) | ||||
| 0 | 208 (18.2) | 88 (10.9) | 120 (35.7) | < 0.001 |
| 1 | 26 (2.3) | 16 (2.0) | 10 (3.0) | 0.303 |
| 2 | 187 (16.3) | 140 (17.3) | 47 (14.0) | 0.169 |
| 3 | 698 (61.0) | 543 (67.1) | 155 (46.1) | < 0.001 |
| 4 | 26 (2.3) | 22 (2.7) | 4 (1.2) | 0.120 |
| Time between vaccination and SARS-CoV-2 infection, median (IQR) | 147 (84–204) | 136 (82–191) | 171.5 (98–227) | 0.001 |
| Symptomsa | 954 (83.3) | 639 (79.0) | 315 (93.8) | < 0.001 |
| Fever, n (%) | 538 (47.0) | 335 (41.4) | 203 (60.4) | < 0.001 |
| Cough, n (%) | 410 (35.8) | 257 (31.8) | 153 (45.5) | < 0.001 |
| Tachypnoea, n (%) | 35 (3.1) | 12 (1.5) | 23 (6.9) | < 0.001 |
| Ageusia, n (%) | 17 (1.5) | 13 (1.6) | 4 (1.2) | 0.596 |
| Pharyngodynia, n (%) | 162 (14.2) | 132 (16.3) | 30 (8.9) | 0.001 |
| Chills, n (%) | 40 (3.5) | 33 (4.1) | 7 (2.1) | 0.094 |
| Asthenia, n (%) | 410 (35.8) | 290 (35.9) | 120 (35.7) | 0.996 |
| Headache, n (%) | 127 (11.1) | 94 (11.6) | 33 (9.8) | 0.378 |
| Myalgias, n (%) | 184 (16.1) | 134 (16.6) | 50 (14.9) | 0.480 |
| Gastrointestinal symptoms, n (%) | 171 (14.9) | 120 (14.8) | 51 (15.2) | 0.881 |
| Dyspnoea, n (%) | 281 (24.5) | 102 (12.6) | 179 (53.3) | < 0.001 |
| Nasal congestion, n (%) | 53 (4.6) | 49 (6.1) | 4 (1.2) | < 0.001 |
| Anosmia, n (%) | 21 (1.8) | 14 (1.7) | 7 (2.1) | 0.685 |
| Radiological findings | ||||
| CT pneumonia, n (%) | 449 (39.2) | 193 (23.9) | 256 (79.2) | < 0.0001 |
| GGO, n (%) | 375 (32.8) | 157 (19.4) | 218 (64.9) | < 0.0001 |
| Consolidation, n (%) | 227 (19.8) | 79 (9.8) | 148 (44.1) | < 0.0001 |
| Pulmonary embolism, n (%) | 21 (1.8) | 12 (1.5) | 9 (2.7) | 0.170 |
| Biochemical indexes | ||||
| WBC (× 103), median (IQR) | 6.9 (5.3–9.3) | 6.7 (5.1–9.0) | 7.6 (5.7–10.4) | 0.001 |
| Neutrophils, median (IQR) | 4.9 (3.4–7.1) | 4.8 (3.3–6.6) | 5.7 (3.9–8.3) | < 0.001 |
| Lymphocytes, median (IQR) | 1.1 (0.7–1.6) | 1.1 (0.8–1.6) | 0.9 (0.6–1.4) | 0.001 |
| NLR, median (IQR) | 4.5 (2.6–8.0) | 4 (2.5–7.2) | 5.8 (3.1–10.2) | < 0.001 |
| Ferritin, median (IQR) | 222 (121–454) | 187 (107–349) | 411.5 (199.5–874.0) | < 0.001 |
| Ferritin/10, median (IQR) | 22.2 (12.1–45.4) | 18.7 (10.7–34.9) | 41.2 (20.0–87.4) | < 0.001 |
| Ferritin/50, median (IQR) | 4.4 (2.4–9.1) | 3.7 (2.1–7.0) | 8.2 (4.0–17.5) | < 0.001 |
| PCT, median (IQR) | 0.07 (0.02–0.22) | 0.05 (0.02–0.15) | 0.15 (0.06–0.46) | < 0.001 |
| PCT > 0.5, n (%) | 174 (15.2) | 93 (11.5) | 81 (24.1) | |
| Urea, median (IQR) | 35 (27–55) | 33 (25–50) | 44 (32–65) | < 0.001 |
| Creatinine, median (IQR) | 0.9 (0.7–1.3) | 0.8 (0.7–1.2) | 1.0 (0.8–1.3) | 0.002 |
| eGFR, median (IQR) | 67.4 (43.6–90.7) | 71.4 (45.8–92.7) | 57.4 (37.9–82.2) | < 0.001 |
| AST, median (IQR) | 23 (17–33) | 22 (17–30) | 26.5 (19.0–40.5) | < 0.001 |
| ALT, median (IQR) | 19 (13–30) | 18 (13–29) | 20 (13–32) | 0.212 |
| De Ritis, median (IQR) | 1.2 (0.9–1.7) | 1.2 (0.9–1.5) | 1.3 (1.1–1.8) | < 0.001 |
| LDH, median (IQR) | 222 (177–280) | 206 (168–250) | 262 (212.5–333.5) | < 0.001 |
| LDH/10, median (IQR) | 22.2 (17.7–28.0) | 20.6 (16.8–25.0) | 26.2 (21.3–33.4) | < 0.001 |
| LDH/50, median (IQR) | 4.4 (3.5–5.6) | 4.2 (3.4–5.0) | 5.2 (4.3–6.7) | < 0.001 |
| CRP, median (IQR) | 2.6 (1.1–6.6) | 2.0 (1.0–5.2) | 5.6 (2.3–11.1) | < 0.001 |
| D-Dimer, median (IQR) | 1 (0.5–2.1) | 0.8 (0.4–1.8) | 1.5 (0.8–3.3) | < 0.001 |
| Therapy | ||||
| Days between symptoms onset and start of treatment, median (IQR) | 2 (1–3) | 2 (1–3) | 2 (1–4) | 0.154 |
| Early treatment, n (%) | 214 (65.6) | 190 (66.7) | 24 (58.5) | 0.305 |
| Antiviral, n (%) | 389 (34.0) | 352 (43.5) | 37 (11.0) | < 0.001 |
| Monlupiravir, n (%) | 242 (21.1) | 224 (27.7) | 18 (5.4) | < 0.001 |
| Nirmatrelvir/ritonavir, n (%) | 39 (3.4) | 36 (4.5) | 3 (0.9) | 0.002 |
| Remdesivir, n (%) | 108 (9.4) | 92 (11.4) | 16 (4.8) | < 0.001 |
| Monoclonal antibodies, n (%) | 237 (20.7) | 207 (25.6) | 30 (8.9) | < 0.001 |
| Casirivimab/Imdevimab, n (%) | 110 (9.6) | 91(11.3) | 19 (5.7) | 0.003 |
| Sotrovimab, n (%) | 130 (11.4) | 118 (14.6) | 12 (3.6) | < 0.001 |
| Hospital-acquired infection, n (%) | 304 (26.6) | 233 (28.8) | 71 (21.1) | 0.007 |
| Bacterial co-infection, n (%) | 129 (11.3) | 71 (8.8) | 58 (17.3) | < 0.001 |
aPeople with at least one symptom
Having more than 60 years, COPD, dementia, haematological tumor, heart failure, having not received at least two doses of vaccine, fever and dyspnea, CT ground glass opacities (GGO) and consolidation, higher ferritin and LDH level, De Ritis ratio, and not having received antivirals or monoclonal antibodies were associated with an increased risk of disease severity (Table 2).
Table 2.
Logistic regression analysis to assess the relationship between demographic, clinical characteristics and the need to start oxygen therapy (n = 1145)
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Males | 1.12 (0.86–1.44) | 0.404 | – | – |
| Age groups ≥ 60 yearsa | 2.49 (1.73–3.58) | < 0.0001 | 2.86 (1.52–5.37) | 0.001 |
| Comorbidity | ||||
| Weight, kg | 1.00 (0.99–1.01) | 0.298 | – | – |
| BMI > 30 kg/m2 | 1.28 (0.96–1.73) | 0.098 | – | – |
| Chronic renal failure, yes | 1.14 (0.82–1.61) | 0.437 | – | – |
| Dialysis, yes | 1.46 (0.63–3.36) | 0.378 | – | – |
| Immunodeficit, yes | 1.24 (0.87–1.78) | 0.240 | – | – |
| Transplant recipients, yes | 1.70 (0.64–4.50) | 0.286 | – | – |
| Rheumatological disease, yes | 1.16 (0.67–2.00) | 0.605 | – | – |
| Decompensated diabetes, yesa | 1.81 (1.31–2.52) | < 0.001 | 1.40 (0.84–2.32) | 0.198 |
| Diabetes, yes | 1.16 (0.86–1.57) | 0.343 | – | – |
| Chronic liver disease, yes | 1.41 (0.84–23.36) | 0.199 | – | – |
| COPD, yesa | 1.66 (1.22–2.26) | 0.001 | 1.69 (1.03–2.78) | 0.038 |
| Hemoglobinopathies, yes | 0.60 (0.07–5.40) | 0.649 | – | – |
| Neurodevelopmental/neurodegenerative diseases, yes | 1.62 (1.23–2.13) | 0.001 | – | – |
| Dementia, yesa | 2.07 (1.49–2.88) | < 0.0001 | 2.17 (1.32–3.56) | 0.002 |
| Chromosopathies/hypoxia, yes | 2.43 (0.60–9.75) | 0.212 | – | – |
| Neuromuscular disease, yes | 0.77 (0.34–1.71) | 0.515 | – | – |
| Cerebrovascular events, yes | 1.30 (0.89–1.90) | 0.178 | – | – |
| Oncological disease, yesa | 0.63 (0.43–0.93) | 0.019 | 0.93 (0.51–1.72) | 0.822 |
| Metastasis, yes | 1.00 (0.56–1.78) | 0.995 | – | – |
| Terminal cancer, yes | 5.82 (2.22–15.27) | < 0.0001 | – | – |
| Hematological tumors, yesa | 1.52 (0.92–2.50) | 0.099 | 3.01 (1.41–6.543) | 0.004 |
| Solid tumors in chemotherapy, yes | 0.64 (0.28–1.49) | 0.302 | – | – |
| Hematological tumors in chemotherapy, yes | 1.47 (0.81–2.68) | 0.207 | – | – |
| Cardiovascular diseases, yes | 1.40 (1.08–1.81) | 0.012 | – | – |
| Heart failure, yesa | 1.47 (1.13–1.92) | 0.005 | 1.55 (1.01–2.38) | 0.046 |
| Previous acute myocardial infarction, yes | 1.11 (0.76–1.62) | 0.579 | – | – |
| Hypertension, yes | 1.54 (1.19–1.99) | 0.001 | – | – |
| Number of comorbidities, yes | 1.22 (1.12–1.34) | < 0.001 | – | – |
| CCI, yes | 1.11 (1.06–1.16) | < 0.001 | – | – |
| Vaccine 0–1 dosesa | 4.28 (3.17–5.78) | < 0.001 | 3.46 (2.19–5.49) | < 0.001 |
| Symptoms | 3.99 (2.49–6.40) | < 0.001 | – | – |
| Fever, yesa | 2.16 (1.67–2.80) | < 0.001 | 2.60 (1.75–3.88) | < 0.001 |
| Cough, yes | 1.80 (1.39–2.33) | < 0.001 | – | – |
| Tachypnea, yes | 4.88 (2.40–9.93) | < 0.001 | – | – |
| Ageusia, yes | 0.74 (0.24–2.28) | 0.597 | – | – |
| Pharyngodynia, yes | 0.50 (0.33–0.76) | 0.001 | – | – |
| Chills, yes | 0.50 (0.22–1.14) | 0.100 | – | – |
| Asthenia, yes | 0.99 (0.76–1.30) | 0.966 | – | – |
| Headache, yes | 0.83 (0.55–1.26) | 0.378 | – | – |
| Myalgias, yes | 0.88 (0.62–1.25) | 0.480 | – | – |
| Gastrointestinal symptoms, yes | 1.03 (0.72–1.47) | 0.881 | – | – |
| Dyspnoea, yesa | 7.90 (5.86–10.65) | < 0.001 | 5.04 (3.24–7.83) | < 0.001 |
| Nasal congestion, yes | 0.19 (0.07–0.52) | 0.001 | – | – |
| Anosmia, yes | 1.21 (0.48–3.02) | 0.686 | – | – |
| Radiological findings | – | – | ||
| CT pneumonia, yes | 10.21 (7.58–13.77) | < 0.0001 | – | – |
| GGO, yesa | 7.67 (5.78–10.19) | < 0.0001 | 3.52 (2.33–5.32) | < 0.001 |
| Consolidation, yesa | 7.27 (5.30–9.98) | < 0.0001 | 2.67 (1.66–4.29) | < 0.001 |
| Pulmonary Embolism, yes | 1.83 (0.76–4.38) | 0.176 | – | – |
| Biochemical indexes | ||||
| WBC (× 103) ≥ 11a | 2.10 (1.50–2.95) | < 0.0001 | 1.35 (0.79–2.31) | 0.271 |
| Neutrophils | 1.01 (0.99–1.03) | 0.161 | – | – |
| Lymphocytes | 1.04 (0.99–1.09) | 0.115 | – | – |
| NLR | 1.01 (0.99–1.03) | 0.095 | – | – |
| Ferritin | 1.00 (1.00–1.00) | < 0.001 | – | – |
| Ferritin > 336 ng/mL in male and > 307 ng/mL in femalea | 3.75 (2.87–4.89) | < 0.001 | 3.24 (2.16–4.85) | < 0.001 |
| PCT | 1.01 (0.99–1.03) | 0.317 | – | – |
| PCT > 0.5 ng/mLa | 2.45 (1.76–3.40) | < 0.001 | 1.03 (0.61–1.73) | 0.914 |
| Urea | 1.01 (1.01–1.01) | < 0.001 | – | – |
| Creatinine | 1.01 (0.97–1.05) | 0.713 | – | – |
| eGFR ≥ 67.4 mL/min/1.73m2a | 0.50 (0.38–0.65) | < 0.001 | 0.75 (0.49–1.15) | 0.186 |
| AST | 1.00 (1.00–1.01) | 0.004 | – | – |
| ALT | 1.00 (1.00–1.00) | 0.093 | – | – |
| De Ritis ≥ 1.2a | 1.92 (1.48–2.50) | < 0.0001 | 1.60 (1.07–2.39) | 0.022 |
| LDH | 1.00 (1.00–1.01) | < 0.001 | – | – |
| LDH > 333 UI/La | 3.37 (2.39–4.74) | < 0.001 | 1.79 (1.01–3.18) | 0.048 |
| CRP | 1.00 (0.99–1.00) | 0.785 | – | – |
| D-Dimer ≥ 1a | 2.21 (1.70–2.88) | < 0.001 | 1.27 (0.86–1.90) | 0.234 |
| Therapy | ||||
| Early treatment, yes | 0.71 (0.36–1.38) | 0.307 | – | – |
| Exposure to antiviral | 0.16 (0.11–0.23) | < 0.001 | – | – |
| Exposure to Monlupiravir, yes | 0.15 (0.09–0.24) | < 0.001 | – | – |
| Exposure to Nirmatrelvir/ritonavir | 0.19 (0.06–0.63) | 0.007 | – | – |
| Exposure to Remdesivir | 0.39 (0.23–0.67) | 0.001 | – | – |
| Not therapy with antiviral or monoclonal antibodies treatmenta | 7.13 (5.12–9.93) | < 0.001 | 11.07 (6.99–17.54) | < 0.001 |
| Exposure to Casirivimab/Imdevimab | 0.47 (0.28–0.79) | 0.004 | – | – |
| Exposure to Sotrovimab | 0.22 (0.12–0.40) | < 0.001 | – | – |
| Hospital infection, yes | 0.66 (0.49–0.90) | 0.008 | – | – |
| Bacterial co-infection, yesa | 2.17 (1.49–3.15) | < 0.001 | 1.55 (0.88–2.74) | 0.131 |
Hosmer–Lemeshow p-value = 0.36
aVariables included in the multivariate analysis
A score was assigned according to the odd ratio (Table 3).
Table 3.
Variables included for the score estimate
| Variables | OR (95% CI) | Score |
|---|---|---|
| Age groups ≥ 60 years | 2.86 (1.52–5.37) | 3 |
| Decompensated diabetes, yes | 1.40 (0.84–2.32) | 1 |
| COPD/Emphysema, yes | 1.69 (1.03–2.78) | 2 |
| Dementia, yes | 2.17 (1.32–3.56) | 2 |
| Oncological disease, yes | 0.93 (0.51–1.72) | 1 |
| Hematological tumors, yes | 3.01 (1.41–6.543) | 3 |
| Heart failure, yes | 1.55 (1.01–2.38) | 2 |
| Vaccine 0–1 doses, yes | 3.46 (2.19–5.49) | 3 |
| Fever, yes | 2.60 (1.75–3.88) | 3 |
| Dyspnoea, yes | 5.04 (3.24–7.83) | 5 |
| GGO, yes | 3.52 (2.33–5.32) | 4 |
| Consolidation | 2.67 (1.66–4.29) | 3 |
| WBC (× 10^3) ≥ 11 | 1.35 (0.79–2.31) | 1 |
| Ferritin > 336 ng/mL in male and > 336 ng/mL in female | 3.24 (2.16–4.85) | 3 |
| PCT > 0.5 | 1.03 (0.61–1.73) | 1 |
| eGFR ≥ 67.4 | 0.75 (0.49–1.15) | 1 |
| De Ritis ≥ 1.2 | 1.60 (1.07–2.39) | 2 |
| LDH > 333 Ui/L | 1.79 (1.01–3.18) | 2 |
| D-Dimer ≥ 1 | 1.27 (0.86–1.90) | 1 |
| Not therapy with antiviral or monoclonal antibodies | 11.07 (6.99–17.54) | 11 |
| Bacterial infection, yes | 1.55 (0.88–2.74) | 2 |
The risk score ranged from 0 to 45 points. According to the Youden Index and clinical considerations, a low risk of clinical progression was considered for values less than 23 (Table 4). Patients were divided into three different risk groups: Low (0–23 points), Medium (24–35 points), and High (≥ 36 points).
Table 4.
Progression risk score description
| Total cohort (n = 1145) | Non-severe disease (n = 809) | Severe disease (n = 336) | p-value | |
|---|---|---|---|---|
| Progression risk score, median (IQR) | 19 (13–26) | 15 (11–20) | 30 (25–35) | < 0.001 |
| Risk score cut-off > 23, n (%) | 357 (31.2) | 92 (11.4) | 265 (78.9) | < 0.001 |
| Risk score levels, n (%) | ||||
| Low | 788 (88.8) | 717 (88.6) | 71 (21.1) | < 0.001 |
| Medium | 288 (25.2) | 90 (11.1) | 198 (58.9) | |
| High | 69 (6.0) | 2 (0.3) | 67 (19.9) |
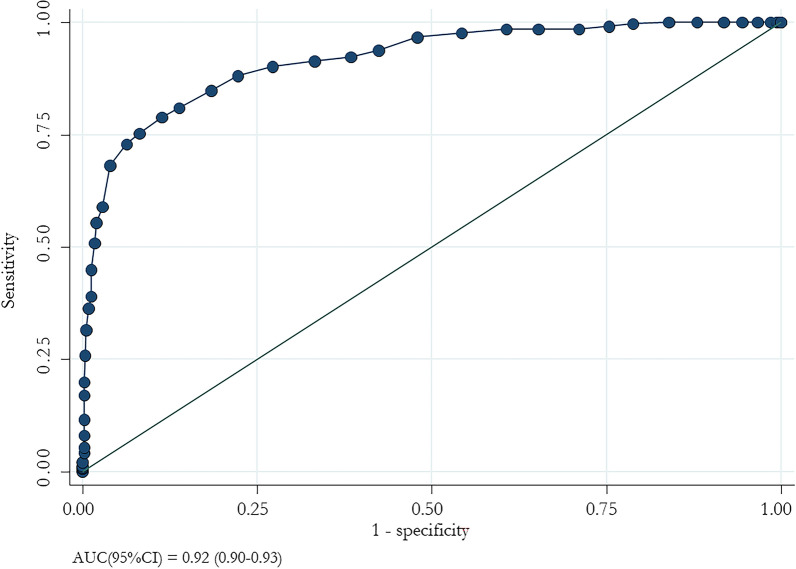
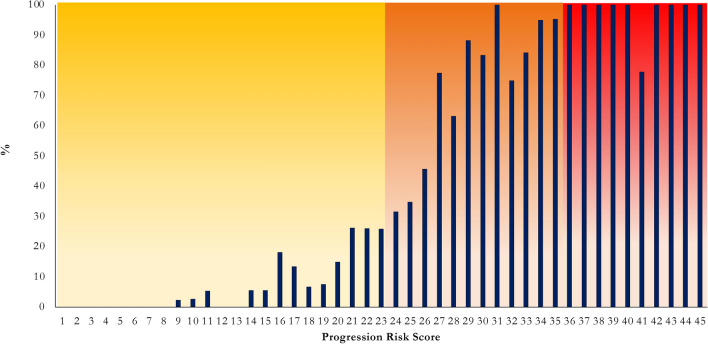
The ROC curve showed an AUC (95% CI) value of 0.92 (0.90–0.93; Fig. 1). 87.6% were correctly classified with a sensitivity of 72.9% and a specificity 93.7% (Fig. 2).
Fig. 1.
ROC curve analysis of progression risk score
Fig. 2.
Frequency distribution of progression among different risk score cut points
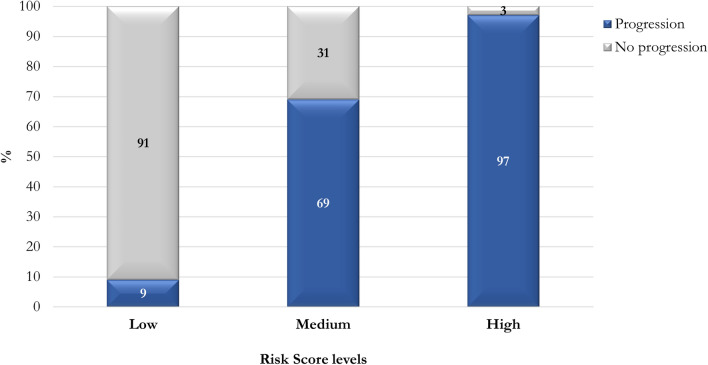
In low-risk group only 9% experienced disease progression, whereas in the medium-risk group the percentage increased to 69%, and to 97% in the high-risk group (Fig. 3).
Fig. 3.
Frequency distribution of progression among different risk score levels
Discussion
Our score effectively predicts the progression risk in people with SARS-CoV-2 infection in a pandemic setting with an overworked healthcare system. We identified different variables associated with increased risk.
Authors found that older age is associated with an increased risk of disease progression and death [27–29]. In Italy, only 3.7% of deaths occurred in people under 60 years; the higher risk of death could be explained by the inflammaging, a chronic upregulation of pro-inflammatory status associated with immunosenescence [29].
Our study found that COPD, dementia, hematological tumours, and heart failure were associated with an increased risk of disease severity.
It is well-established that individuals with COPD are at increased risk of developing severe COVID-19 due to chronic lung damage [30, 31]. Numerous studies have investigated this specific population, consistently demonstrating a worse prognosis in terms of severity, complications, and mortality when compared with the general population, confirmed by a meta-analysis which found that the risk of developing COVID-19 was 3.8 times higher among individuals with COPD than those without [30].
However, studies on the role of dementia in COVID-19 are poor. Bianchetti et al. found that among 627 people admitted to a COVID-19 ward, 13.1% had dementia, and 62.2% of them died versus 26.2% without dementia [32]. Additionally, a retrospective study by Harrison et al. found that dementia was a risk factor for increased mortality, potentially explained by alterations of the neuroendocrine and immune systems, as well as by their high frailty index [32].
In our study, hematological malignancy did not reach statistical significance in the univariate analysis, but it was included in the multivariate analysis due to solid clinical evidence [33, 34]. Passamonti et al. reported that among 536 patients with COVID-19 and hematological malignancy, 36.9% died, which is 4 times higher than the general Italian population [33]. Moreover, individuals with hematological malignancy exhibited a lower response to SARS-CoV-2 vaccination. In our study, hematological malignancy was found to increase the risk of disease progression (OR 3.01).
Moreover, chronic heart failure was found to increase disease progression in our study. In another study, individuals with chronic heart failure had a higher percentage of developing severe disease (62% vs. 36.9%) and a 4.6-fold risk of death [35].
Numerous studies on vaccine efficacy found that vaccinated individuals had a significantly lower risk of disease progression (risk ratio 0.38) and COVID-19-related death (risk ratio 0.16) [36].
Ferritin was associated with severity and death due to COVID [37, 38]. It increases in several inflammatory conditions and has an important immunomodulatory effect on mortality and inflammatory processes. The De Ritis ratio (AST/ALT) was also associated with an increased risk of death, although cut-off values were not established [39, 40]. LDH, an enzyme present in the cytosol of all nucleated cells that catalyzes the final step of glycolysis, is a highly sensitive but nonspecific marker of tissue damage due to its distribution [41, 42]. Regarding the WBC, an increase number could be an expression of elevated WBC counts may be indicative of a heightened inflammatory response, which is often associated with severe disease manifestations in COVID-19. This hyperactive immune response can lead to complications such as cytokine storm, contributing to the deterioration of clinical conditions and increasing the risk of severe outcomes [7–10]. Elevated procalcitonin levels in COVID-19 patients are a significant indicator of bacterial co-infection, as procalcitonin is a biomarker that rises in response to bacterial infections. This concomitant bacterial infection can exacerbate the severity of COVID-19 by amplifying the body's inflammatory response and potentially leading to more severe respiratory and systemic complications.
About D-dimer, elevated levels are often associated with an increased risk of thrombotic events, which are common complications in severe COVID-19 cases. High D-Dimer levels can indicate a hypercoagulable state, potentially leading to complications such as deep vein thrombosis or pulmonary embolism, thereby increasing the risk of severe disease progression.
Administration of antivirals and monoclonal antibodies can significantly predict the progression to severe disease. When the study was carried out, the available drugs were monlupiravir, nirmatrelvir/ritonavir, remdesivir (3-day course), casirivimab/imdevimab, and sotrovimab.
Our scoring system was developed when vaccines and early antiviral therapies were available. In the context of predicting COVID-19 progression, Ji et al. described the CALL score model, which includes age, comorbidities, lymphopenia, and LDH and requires only basic laboratory tests. However, it was based on a poor sample size and defined progression based on chest radiological findings rather than the need for oxygen therapy [43]. Gong et al. developed a prognostic nomogram for patients at risk of severe COVID-19 based on age, LDH, CRP, direct bilirubin, red blood cell distribution width, urea, and albumin. However, this complex model does not account for radiological findings [44]. Lee et al. published the KDDH score, which considers age, CRP, LDH, and hemoglobin, and defines progression as the need for oxygen therapy. However, this model does not include radiological signs [45].
The main limitations of those scores are that they did not consider symptoms, such as fever and dyspnea, and were developed using data collected in 2020 when all patients were infected with the wild-type SARS-CoV-2, and no vaccines or preventive therapies were available. Although they show a good sensitivity and specificity, it is unlikely that they could be currently adopted with different variants and multiple treatment options available.
The strength of the present study relies on its high specificity and inclusion of previous vaccination and the use of early antiviral treatment, whereas the limitations are its retrospective and monocenter design, incomplete data on the type of vaccine, unavailability of tixagevimab/cilgavimab, and the lack of data on SARS-CoV-2 previous infections.
Conclusion
The score showed high specificity, and the risk of underestimating patients' clinical status is low. The main risk factor for disease progression is not having received antiviral or monoclonal antibodies, underscoring that early treatment is fundamental to prevent the disease progression.
In conclusion, this score could significantly affect clinical practice and support clinical decisions at hospital admission.
Author contributions
ADV, GS, and GiMa have made substantial contributions to the conception; ADV, GS, and GiMa designed the work; LS, MVP, GS performed the statistical analysis and prepared the figures; ADV, and AC interpreted the data; ADV, LS, MVP, GS, and GiMa wrote the main manuscript text; AC, BZ, VF, MF, MCM, AB, GiMo, IM, SB were responsible for the data acquisition. All authors reviewed the manuscript.
Funding
No funding.
Availability of data and materials
All data will be available upon specific request to the authors.
Declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee with the protocol code PG/2022/20481.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Andrea De Vito and Laura Saderi have contributed equally to the manuscript.
References
- 1.He J, Guo Y, Mao R, Zhang J. Proportion of asymptomatic coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. J Med Virol. 2020;2020:26326. doi: 10.1002/jmv.26326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Vito A, Fiore V, Princic E, Geremia N, Panu Napodano CM, Muredda AA, Maida I, Madeddu G, Babudieri S. Predictors of infection, symptoms development, and mortality in people with SARS-CoV-2 living in retirement nursing homes. PLoS ONE. 2021;16(3):e0248009. doi: 10.1371/journal.pone.0248009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geremia N, De Vito A, Gunnella S, Fiore V, Princic E, Panu Napodano C, Madeddu G, Babudieri S. A case of vasculitis-like skin eruption associated with COVID-19. Infect Dis Clin Pract. 2020;28(6):e30–e31. doi: 10.1097/IPC.0000000000000952. [DOI] [Google Scholar]
- 4.Vaira LA, De Vito A, Lechien JR, Chiesa-Estomba CM, Mayo-Yàñez M, Calvo-Henrìquez C, Saussez S, Madeddu G, Babudieri S, Boscolo-Rizzo P, Hopkins C, De Riu G. New onset of smell and taste loss are common findings also in patients with symptomatic COVID-19 after complete vaccination. Laryngoscope. 2022;132(2):419–421. doi: 10.1002/lary.29964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iba T, Connors JM, Levy JH. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflamm Res. 2020;69(12):1181–1189. doi: 10.1007/s00011-020-01401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zinellu A, De Vito A, Scano V, Paliogiannis P, Fiore V, Madeddu G, Maida I, Zinellu E, Mangoni AA, Arru LB, Carru C, Babudieri S, Pirina P, Fois AG. The PaO2/FiO2 ratio on admission is independently associated with prolonged hospitalization in COVID-19 patients. J Infect Dev Ctries. 2021;15(3):353–359. doi: 10.3855/jidc.13288. [DOI] [PubMed] [Google Scholar]
- 7.Dessie ZG, Zewotir T. Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect Dis. 2021;21(1):1. doi: 10.1186/s12879-021-06536-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King JT, Yoon JS, Rentsch CT, Tate JP, Park LS, Kidwai-Khan F, Skanderson M, Hauser RG, Jacobson DA, Erdos J, Cho K, Ramoni R, Gagnon DR, Justice AC. Development and validation of a 30-day mortality index based on pre-existing medical administrative data from 13,323 COVID-19 patients: The Veterans Health Administration COVID-19 (VACO) Index. PLoS ONE. 2020;15(11):1. doi: 10.1371/journal.pone.0241825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garibaldi BT, Fiksel J, Muschelli J, Robinson ML, Rouhizadeh M, Perin J, Schumock G, Nagy P, Gray JH, Malapati H, Ghobadi-Krueger M, Niessen TM, Kim BS, Hill PM, Ahmed MS, Dobkin ED, Blanding R, Abele J, Woods B, Harkness K, Thiemann DR, Bowring MG, Shah AB, Wang MC, Bandeen-Roche K, Rosen A, Zeger SL, Gupta A. Patient trajectories among persons hospitalized for COVID-19: a cohort study. Ann Intern Med. 2021;174(1):33–41. doi: 10.7326/M20-3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodacre S, Thomas B, Sutton L, Burnsall M, Lee E, Bradburn M, Loban A, Waterhouse S, Simmonds R, Biggs K, Marincowitz C, Schutter J, Connelly S, Sheldon E, Hall J, Young E, Bentley A, Challen K, Fitzsimmons C, Harris T, Lecky F, Lee A, MacOnochie I, Walter D. Derivation and validation of a clinical severity score for acutely ill adults with suspected COVID-19: the PRIEST observational cohort study. PLoS ONE. 2021;16(1):e0245840. doi: 10.1371/journal.pone.0245840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wynants L, Van Calster B, Collins GS, Riley RD, Heinze G, Schuit E, Bonten MMJ, Damen JAA, Debray TPA, De Vos M, Dhiman P, Haller MC, Harhay MO, Henckaerts L, Kreuzberger N, Lohmann A, Luijken K, Ma J, Andaur Navarro CL, Reitsma JB, Sergeant JC, Shi C, Skoetz N, Smits LJM, Snell KIE, Sperrin M, Spijker R, Steyerberg EW, Takada T, Van Kuijk SMJ, Van Royen FS, Wallisch C, Hooft L, Moons KGM, Van Smeden M. Prediction models for diagnosis and prognosis of COVID-19: systematic review and critical appraisal. BMJ. 2020;369:29. doi: 10.1136/bmj.m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrari F, Puci MV, Ferraro OE, Romero-González G, Husain-Syed F, Rizo-Topete L, Senzolo M, Lorenzin A, Muraro E, Baracca A, Serrano-Soto M, Triviño AM, Castro AC, De Cal M, Corradi V, Brendolan A, Scarpa M, Carta MR, Giavarina D, Bonato R, Iotti GA, Ronco C. Development and validation of quick Acute Kidney Injury-score (q-AKI) to predict acute kidney injury at admission to a multidisciplinary intensive care unit. PLoS ONE. 2019;14(6):1. doi: 10.1371/journal.pone.0217424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knight SR, Ho A, Pius R, Buchan I, Carson G, Drake TM, Dunning J, Fairfield CJ, Gamble C, Green CA, Gupta R, Halpin S, Hardwick HE, Holden KA, Horby PW, Jackson C, McLean KA, Merson L, Nguyen-Van-Tam JS, Norman L, Noursadeghi M, Olliaro PL, Pritchard MG, Russell CD, Shaw CA, Sheikh A, Solomon T, Sudlow C, Swann OV, Turtle LCW, Openshaw PJM, Baillie JK, Semple MG, Docherty AB, Harrison EM. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: development and validation of the 4C Mortality Score. BMJ. 2020;370:22. doi: 10.1136/bmj.m3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karimizadeh Z, Dowran R, Mokhtari-azad T, Shafiei-Jandaghi NZ. The reproduction rate of severe acute respiratory syndrome coronavirus 2 different variants recently circulated in human: a narrative review. Eur J Med Res. 2023;28(1):1–10. doi: 10.1186/s40001-023-01047-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carabelli AM, Peacock TP, Thorne LG, Harvey WT, Hughes J, de Silva TI, Peacock SJ, Barclay WS, de Silva TI, Towers GJ, Robertson DL. SARS-CoV-2 variant biology: immune escape, transmission and fitness. Nat Rev Microbiol. 2023;21(3):162–177. doi: 10.1038/s41579-022-00841-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chi W-Y, Li Y-D, Huang H-C, Chan TEH, Chow S-Y, Su J-H, Ferrall L, Hung C-F, Wu T-C. COVID-19 vaccine update: vaccine effectiveness, SARS-CoV-2 variants, boosters, adverse effects, and immune correlates of protection. J Biomed Sci. 2022;29(1):82. doi: 10.1186/s12929-022-00853-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Vito A, Colpani A, Saderi L, Puci M, Zauli B, Fiore V, Fois M, Meloni MC, Bitti A, Di Castri C, Maida I, Babudieri S, Sotgiu G, Madeddu G. Impact of early SARS-CoV-2 antiviral therapy on disease progression. Viruses. 2023;15(1):71. doi: 10.3390/v15010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Vito A, Colpani A, Bitti A, Zauli B, Meloni MC, Fois M, Denti L, Bacciu S, Marcia C, Maida I, Babudieri S, Madeddu G. Safety and efficacy of molnupiravir in SARS-CoV-2 infected patients: a real-life experience. J Med Virol. 2022;2022:1. doi: 10.1002/jmv.28011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gottlieb RL, Vaca CE, Paredes R, Mera J, Webb BJ, Perez G, Oguchi G, Ryan P, Nielsen BU, Brown M, Hidalgo A, Sachdeva Y, Mittal S, Osiyemi O, Skarbinski J, Juneja K, Hyland RH, Osinusi A, Chen S, Camus G, Abdelghany M, Davies S, Behenna-Renton N, Duff F, Marty FM, Katz MJ, Ginde AA, Brown SM, Schiffer JT, Hill JA. Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med. 2022;386(4):305–315. doi: 10.1056/NEJMoa2116846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Najjar-Debbiny R, Gronich N, Weber G, Khoury J, Amar M, Stein N, Goldstein LH, Saliba W. Effectiveness of paxlovid in reducing severe coronavirus disease 2019 and mortality in high-risk patients. Clin Infect Dis. 2022;2022:1. doi: 10.1093/cid/ciac443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arbel R, Wolff Sagy Y, Hoshen M, Battat E, Lavie G, Sergienko R, Friger M, Waxman JG, Dagan N, Balicer R, Ben-Shlomo Y, Peretz A, Yaron S, Serby D, Hammerman A, Netzer D. Nirmatrelvir use and severe covid-19 outcomes during the omicron surge. N Engl J Med. 2022;387(9):790–798. doi: 10.1056/NEJMoa2204919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jayk Bernal A, Gomes da Silva MM, Musungaie DB, Kovalchuk E, Gonzalez A, Delos Reyes V, Martín-Quirós A, Caraco Y, Williams-Diaz A, Brown ML, Du J, Pedley A, Assaid C, Strizki J, Grobler JA, Shamsuddin HH, Tipping R, Wan H, Paschke A, Butterton JR, Johnson MG, De Anda C. Molnupiravir for oral treatment of covid-19 in nonhospitalized patients. N Engl J Med. 2022;386(6):509–520. doi: 10.1056/NEJMoa2116044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drake TM, Riad AM, Fairfield CJ, Egan C, Knight SR, Pius R, Hardwick HE, Norman L, Shaw CA, Mclean KA, Thompson AAR, Ho A, Swann OV, Sullivan M, Soares F, Holden KA, Merson L, Plotkin D, Sigfrid L, de Silva TI, Girvan M, Jackson C, Russell CD, Dunning J, Solomon T, Carson G, Olliaro P, Nguyen-Van-Tam JS, Turtle L, Docherty AB, Openshaw PJ, Baillie JK, Harrison EM, Semple MG, Alex B, Bach B, Barclay WS, Bogaert D, Chand M, Cooke GS, da Silva Filipe A, Fletcher T, Green CA, Hiscox JA, Ho AY, Horby PW, Ijaz S, Khoo S, Klenerman P, Law A, Lim WS, Mentzer AJ, Meynert AM, Noursadeghi M, Moore SC, Palmarini M, Paxton WA, Pollakis G, Price N, Rambaut A, Robertson DL, Sancho-Shimizu V, Scott JT, de Silva T, Sriskandan S, Stuart D, Summers C, Tedder RS, Thomson EC, Thwaites RS, Gupta RK, Palmieri C, Zambon M, Dumas ME, Griffin J, Takats Z, Chechi K, Andrikopoulos P, Osagie A, Olanipekun M, Liggi S, Lewis M, dos Santos Correia G, Sands C, Takis P, Maslen L, Hardwick H, Donohue C, Griffiths F, Oosthuyzen W, Murphy D, Dalton J, Saviciute E, Roberts S, Harrison J, Marsh L, Connor M, Halpin S, Gamble C, Lee J, Leeming G, Wham M, Clohisey S, Hendry R, Scott-Brown J, Greenhalf W, Shaw V, McDonald SE, Keating S, Ahmed KA, Armstrong JA, Ashworth M, Asiimwe IG, Bakshi S, Barlow SL, Booth L, Brennan B, Bullock K, Catterall BW, Clark JJ, Clarke EA, Cole S, Cooper L, Cox H, Davis C, Dincarslan O, Dunn C, Dyer P, Elliott A, Evans A, Finch L, Fisher LW, Foster T, Garcia-Dorival I, Gunning P, Hartley C, Jensen RL, Jones CB, Jones TR, Khandaker S, King K, Kiy RT, Koukorava C, Lake A, Lant S, Latawiec D, Lavelle-Langham L, Lefteri D, Lett L, Livoti LA, Mancini M, McDonald S, McEvoy L, McLauchlan J, Metelmann S, Miah NS, Middleton J, Mitchell J, Murphy EG, Penrice-Randal R, Pilgrim J, Prince T, Reynolds W, Ridley PM, Sales D, Shaw VE, Shears RK, Small B, Subramaniam KS, Szemiel A, Taggart A, Tanianis-Hughes J, Thomas J, Trochu E, van Tonder L, Wilcock E, Zhang JE, Flaherty L, Maziere N, Cass E, Doce Carracedo A, Carlucci N, Holmes A, Massey H, Murphy L, Wrobel N, McCafferty S, Morrice K, MacLean A, Adeniji K, Agranoff D, Agwuh K, Ail D, Aldera EL, Alegria A, Angus B, Ashish A, Atkinson D, Bari S, Barlow G, Barnass S, Barrett N, Bassford C, Basude S, Baxter D, Beadsworth M, Bernatoniene J, Berridge J, Best N, Bothma P, Chadwick D, Brittain-Long R, Bulteel N, Burden T, Burtenshaw A, Caruth V, Chambler D, Chee N, Child J, Chukkambotla S, Clark T, Collini P, Cosgrove C, Cupitt J, Cutino-Moguel MT, Dark P, Dawson C, Dervisevic S, Donnison P, Douthwaite S, Drummond A, DuRand I, Dushianthan A, Dyer T, Evans C, Eziefula C, Fegan C, Finn A, Fullerton D, Garg S, Garg A, Gkrania-Klotsas E, Godden J, Goldsmith A, Graham C, Hardy E, Hartshorn S, Harvey D, Havalda P, Hawcutt DB, Hobrok M, Hodgson L, Hormis A, Jacobs M, Jain S, Jennings P, Kaliappan A, Kasipandian V, Kegg S, Kelsey M, Kendall J, Kerrison C, Kerslake I, Koch O, Koduri G, Koshy G, Laha S, Laird S, Larkin S, Leiner T, Lillie P, Limb J, Linnett V, Little J, Lyttle M, MacMahon M, MacNaughton E, Mankregod R, Masson H, Matovu E, McCullough K, McEwen R, Meda M, Mills G, Minton J, Mirfenderesky M, Mohandas K, Mok Q, Moon J, Moore E, Morgan P, Morris C, Mortimore K, Moses S, Mpenge M, Mulla R, Murphy M, Nagel M, Nagarajan T, Nelson M, Norris L, O'Shea MK, Otahal I, Ostermann M, Pais M, Panchatsharam S, Papakonstantinou D, Paraiso H, Patel B, Pattison N, Pepperell J, Peters M, Phull M, Pintus S, Singh Pooni J, Post F, Price D, Prout R, Rae N, Reschreiter H, Reynolds T, Richardson N, Roberts M, Roberts D, Rose A, Rousseau G, Ryan B, Saluja T, Shah A, Shanmuga P, Sharma A, Shawcross A, Sizer J, Shankar-Hari M, Smith R, Snelson C, Spittle N, Staines N, Stambach T, Stewart R, Subudhi P, Szakmany T, Tatham K, Thomas J, Thompson C, Thompson R, Tridente A, Tupper-Carey D, Twagira M, Vallotton N, Vancheeswaran R, Vincent-Smith L, Visuvanathan S, Vuylsteke A, Waddy S, Wake R, Walden A, Welters I, Whitehouse T, Whittaker P, Whittington A, Papineni P, Wijesinghe M, Williams M, Wilson L, Sarah S, Winchester S, Wiselka M, Wolverson A, Wootton DG, Workman A, Yates B, Young P. Characterisation of in-hospital complications associated with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol UK: a prospective, multicentre cohort study. Lancet. 2021;398(10296):223–37. [DOI] [PMC free article] [PubMed]
- 25.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 26.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 27.Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging: an evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908(1):244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 28.Lithander FE, Neumann S, Tenison E, Lloyd K, Welsh TJ, Rodrigues JCL, Higgins JPT, Scourfield L, Christensen H, Haunton VJ, Henderson EJ. COVID-19 in older people: a rapid clinical review. Age Ageing. 2020;49(4):501–515. doi: 10.1093/ageing/afaa093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonafè M, Prattichizzo F, Giuliani A, Storci G, Sabbatinelli J, Olivieri F. Inflamm-aging: why older men are the most susceptible to SARS-CoV-2 complicated outcomes. Cytokine Growth Factor Rev. 2020;53:33–37. doi: 10.1016/j.cytogfr.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uruma Y, Manabe T, Fujikura Y, Iikura M, Hojo M, Kudo K. Effect of asthma, COPD, and ACO on COVID-19: a systematic review and meta-analysis. PLoS ONE. 2022;17(11):e0276774. doi: 10.1371/journal.pone.0276774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moreno-Martos D, Verhamme K, Ostropolets A, Kostka K, Duarte-Sales T, Prieto-Alhambra D, Alshammari TM, Alghoul H, Ahmed W-U-R, Blacketer C, DuVall S, Lai L, Matheny M, Nyberg F, Posada J, Rijnbeek P, Spotnitz M, Sena A, Shah N, Suchard M, Chan You S, Hripcsak G, Ryan P, Morales D. Characteristics and outcomes of COVID-19 patients with COPD from the United States, South Korea, and Europe. Wellcome Open Res. 2022;7:22. doi: 10.12688/wellcomeopenres.17403.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bianchetti A, Rozzini R, Guerini F, Boffelli S, Ranieri P, Minelli G, Bianchetti L, Trabucchi M. Clinical presentation of COVID19 in dementia patients. J Nutr Health Aging. 2020;24(6):560–562. doi: 10.1007/s12603-020-1389-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Passamonti F, Cattaneo C, Arcaini L, Bruna R, Cavo M, Merli F, Angelucci E, Krampera M, Cairoli R, Della Porta MG, Fracchiolla N, Ladetto M, Gambacorti Passerini C, Salvini M, Marchetti M, Lemoli R, Molteni A, Busca A, Cuneo A, Romano A, Giuliani N, Galimberti S, Corso A, Morotti A, Falini B, Billio A, Gherlinzoni F, Visani G, Tisi MC, Tafuri A, Tosi P, Lanza F, Massaia M, Turrini M, Ferrara F, Gurrieri C, Vallisa D, Martelli M, Derenzini E, Guarini A, Conconi A, Cuccaro A, Cudillo L, Russo D, Ciambelli F, Scattolin AM, Luppi M, Selleri C, Ortu La Barbera E, Ferrandina C, Di Renzo N, Olivieri A, Bocchia M, Gentile M, Marchesi F, Musto P, Federici AB, Candoni A, Venditti A, Fava C, Pinto A, Galieni P, Rigacci L, Armiento D, Pane F, Oberti M, Zappasodi P, Visco C, Franchi M, Grossi PA, Bertù L, Corrao G, Pagano L, Corradini P. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020;7(10):e737–e745. doi: 10.1016/S2352-3026(20)30251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langerbeins P, Hallek M. COVID-19 in patients with hematologic malignancy. Blood. 2022;140(3):236. doi: 10.1182/blood.2021012251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arutyunov GP, Tarlovskaya EI, Arutyunov AG, Lopatin YM. Impact of heart failure on all-cause mortality in COVID-19: findings from the Eurasian International Registry. ESC Heart Fail. 2023;10(2):1013. doi: 10.1002/ehf2.14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu K, Wang Z, Qin M, Gao Y, Luo N, Xie W, Zou Y, Wang J, Ma X. A systematic review and meta-analysis of the effectiveness and safety of COVID-19 vaccination in older adults. Front Immunol. 2023;14:1. doi: 10.3389/fimmu.2023.1113156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feld J, Tremblay D, Thibaud S, Kessler A, Naymagon L. Ferritin levels in patients with COVID-19: a poor predictor of mortality and hemophagocytic lymphohistiocytosis. Int J Lab Hematol. 2020;42(6):773–779. doi: 10.1111/ijlh.13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huyut MT, Huyut Z. Effect of ferritin, INR, and D-dimer immunological parameters levels as predictors of COVID-19 mortality: a strong prediction with the decision trees. Heliyon. 2023;9(3):e14015. doi: 10.1016/j.heliyon.2023.e14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zinellu A, Arru F, De Vito A, Sassu A, Valdes G, Scano V, Zinellu E, Perra R, Madeddu G, Carru C, Pirina P, Mangoni AA, Babudieri S, Fois AG. The De Ritis ratio as prognostic biomarker of in-hospital mortality in COVID-19 patients. Eur J Clin Invest. 2020;2020:e13427. doi: 10.1111/eci.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pranata R, Huang I, Lim MA, Yonas E, Vania R, Lukito AA, Nasution SA, Siswanto BB, Kuswardhani RAT. Elevated De Ritis ratio is associated with poor prognosis in COVID-19: a systematic review and meta-analysis. Front Med (Lausanne) 2021;8:676581. doi: 10.3389/fmed.2021.676581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fialek B, Pruc M, Smereka J, Jas R, Rahnama-Hezavah M, Denegri A, Szarpak A, Jaguszewski MJ, Peacock FW, Szarpak L. Diagnostic value of lactate dehydrogenase in COVID-19: a systematic review and meta-analysis. Cardiol J. 2022;29(5):751. doi: 10.5603/CJ.a2022.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kojima K, Yoon H, Okishio K, Tsuyuguchi K. Increased lactate dehydrogenase reflects the progression of COVID-19 pneumonia on chest computed tomography and predicts subsequent severe disease. Sci Rep. 2023;13(1):1–10. doi: 10.1038/s41598-023-28201-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ji D, Zhang D, Xu J, Chen Z, Yang T, Zhao P, Chen G, Cheng G, Wang Y, Bi J, Tan L, Lau G, Qin E. Prediction for progression risk in patients with COVID-19 pneumonia: the CALL score. Clin Infect Dis. 2020;71(6):1393–1399. doi: 10.1093/cid/ciaa414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gong J, Ou J, Qiu X, Jie Y, Chen Y, Yuan L, Cao J, Tan M, Xu W, Zheng F, Shi Y, Hu B. A tool for early prediction of severe coronavirus disease 2019 (COVID-19): a multicenter study using the risk nomogram in Wuhan and Guangdong. China Clin Infect Dis. 2020;71(15):833–840. doi: 10.1093/cid/ciaa443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee JY, Nam BH, Kim M, Hwang J, Kim JY, Hyun M, Kim HA, Cho CH. A risk scoring system to predict progression to severe pneumonia in patients with Covid-19. Sci Rep. 2022;12(1):5390. doi: 10.1038/s41598-022-07610-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data will be available upon specific request to the authors.