Abstract
People infected with Blastomyces dermatitidis develop strong immunity to the yeast surface adhesin WI-1, including antibody responses to the adhesive domain, a 25-amino-acid repeat, and cellular responses to the N terminus. We studied the immunogenicity of WI-1 and the ability of anti-WI-1 immune responses to protect against lethal pulmonary infection in mice. WI-1 immunization, given in Freund’s adjuvant subcutaneously in two doses 2 weeks apart, evoked delayed hypersensitivity responses in a concentration-dependent manner. Immunized mice also had anti-WI-1 antibody responses, with titers reaching an endpoint dilution of approximately 1:800,000. Anti-WI-1 immunoglobulin G (IgG) antibody subclasses were IgG1 > IgG2b > IgG2a > IgG3, indicating a mixed T helper 1 and T helper 2 immune response. In protection experiments, WI-1 immunization significantly prolonged the survival of C57BL/6 and BALB/c mice compared to controls following intranasal administration of a lethal dose of B. dermatitidis yeasts (Kaplan-Meier survival curve P values of 0.027 to 0.0002) and also protected a proportion of the animals from death due to progressive pulmonary blastomycosis. Taken together, our results suggest that administration of WI-1 raises antibody and cell-mediated immune responses, which enhance resistance against pulmonary infection with B. dermatitidis. Mechanisms of vaccine-induced resistance require further investigation.
Blastomyces dermatitidis is a thermal dimorphic fungus and the causal agent of blastomycosis, which is one of the principal endemic systemic mycoses of humans and other mammals. Inhaled conidia of B. dermatitidis initiate the infection, and at body temperature they convert to invasive yeast forms that produce a chronic, progressive pneumonia, which often disseminates to extrapulmonary organs (25). Infections that go undiagnosed or untreated may progress and become fatal even in immunocompetent hosts. Patients with AIDS or other immunosuppressive conditions are prone to disseminated, often lethal infections (22, 23). Dogs that reside in the zone where the disease is endemic are a common victim of blastomycosis; incidence rates approach 1 to 2% of susceptible animals (4).
Innate and adaptive mechanisms that limit infection and promote clearance of the fungus that have been characterized include polymorphonuclear leukocytes, mononuclear phagocytes, and antigen-specific T lymphocytes (14). However, the antigens of B. dermatitidis that stimulate clearance of the fungus have not been identified. We previously identified a 120-kDa protein on the surface of B. dermatitidis yeasts, designated WI-1 (17). WI-1 is an adhesin that binds the fungus to complement and CD14 receptors on host cells (21) and an immunodominant antigen (17). Most infected patients develop strong humoral and cell-mediated immune responses to WI-1 during the course of illness (17, 18). Despite the fact that WI-1 is consistently recognized as an antigen by infected patients, the value of these immune responses in resistance to B. dermatitidis infection has not been studied.
In this study we investigated the immunogenicity of WI-1 and its protective efficacy in an experimental infection of mice. The goals of our study were to (i) raise immune responses to WI-1 in inbred strains of mice, (ii) characterize humoral and cellular anti-WI-1 responses elicited by the immunization, and (iii) assess the protective efficacy of these immune responses in a murine model of lethal pulmonary blastomycosis. Our findings demonstrate that administration of WI-1 elicits immune responses that significantly enhance resistance against a lethal pulmonary infection.
MATERIALS AND METHODS
Fungal strains and growth.
Strains of B. dermatitidis used here include ATCC (American Type Culture Collection) 60636, originally isolated from soil and patients during an outbreak of blastomycosis in Wisconsin (19), and ATCC 26199, originally isolated from a human patient in South Carolina (5). Isolates were maintained in the yeast form on Middlebrook 7H10 agar slants with oleic acid-albumin complex (Sigma Chemical Co., St. Louis, Mo.) at 37°C. Histoplasma capsulatum 184 AS ura 5-11 is a uracil auxotroph of a smooth variant of the parental isolate 184 AR (28, 29). The variant is highly attenuated in virulence for mice due to two independent alterations, including loss of surface α-(1,3)-glucan and uracil auxotrophy. This isolate was grown in Histoplasma macrophage medium supplemented with uracil (50 μg/ml) as described previously (28, 29).
Mouse strains.
Male C57BL/6 and BALB/c strains of mice were 5 to 6 weeks old at the time of purchase from The Jackson Laboratory. They were housed and cared for throughout these experiments according to guidelines of the University of Wisconsin Animal Care Committee, which approved all aspects of this work.
Antigens.
Secreted WI-1 was purified from the ATCC 60636 yeasts as previously described (3). Briefly, yeasts were grown in liquid Histoplasma macrophage medium in a gyratory shaker at 37°C for 2 weeks. Supernatants enriched for WI-1 were collected and purified in a two-step process using anion-exchange chromatography followed by hydrophobic interaction chromatography. The homogeneity of purified WI-1 was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and staining with silver nitrate.
Candida albicans yeast cell extract was purchased from Bayer Corporation Pharmaceutical Division, Elkhart, Ind. (formerly Hollister-Stier, Spokane, Wash.) and used at the optimal dilution of 1:100 (wt/vol) for in vitro stimulation of lymphocytes (18).
Immunizations.
Purified WI-1 or bovine serum albumin (BSA) as a control was administered to mice in Freund’s adjuvant subcutaneously at the base of the tail. The immunogens were diluted to the desired concentration in phosphate-buffered saline (PBS) and emulsified in an equal volume of either complete Freund’s adjuvant (for the first immunization) or incomplete Freund’s adjuvant (for the booster). Each immunogen was administered in a volume of 0.2 ml of emulsion.
Some mice were immunized with live or dead yeasts of strain ATCC 60636. Based on previously published work by Brummer et al. (7), mice were given a sublethal infection with 4 × 104 live B. dermatitidis yeasts administered once subcutaneously into two sites at the base of the tail. Other mice received 106 heat-killed B. dermatitidis yeasts subcutaneously in 0.2 ml of saline, twice, according to the same schedule used for WI-1 protein immunization.
An additional group of mice were immunized with live yeast cells of H. capsulatum 184 AS ura 5-11/WI-1 as an alternative vehicle for delivering WI-1. The recombinant strain was isolated by electrotransformation (28, 29). Briefly, genomic WI-1 and its own promoter (isolated from strain ATCC 26199) was cloned on a 6.3-kb BsrG1 fragment into the vector pMAD93, kindly provided by Jon Woods (University of Wisconsin—Madison). Yeast-form cells were electrotransformed with HpaI-linearized pMAD93/WI-1, designed to expose Histoplasma telomeres. Transformed yeast cells contained the WI-1 transgene expressed off a multicopy, extrachromosomal plasmid and displayed abundant amounts of the WI-1 protein on their surfaces as determined by fluorescence-activated cell sorting analysis and SDS-PAGE (data not shown). As a control, the same strain of H. capsulatum also was transformed with pMAD93 not containing the WI-1 coding sequence to repair the defect in uracil metabolism. For immunization with these recombinant strains, mice were infected intranasally with a sublethal dose of 107 yeasts, given twice, 1 month apart.
Measurement of immune responses.
Mice were tested 2 weeks after the second immunization to assess humoral and cellular immune responses to WI-1. For measurement of antibody responses, mice were bled from the tail vein to obtain serum samples. Anti-WI-1 antibody was detected and titered in serum by using a previously described solution-phase radioimmunoassay (17). A positive test in this assay is defined as at least 20% specific binding of the radiolabeled WI-1 antigen target at a serum dilution of 1:40 or greater. The endpoint titer of a serum sample is defined as that dilution of serum yielding 20% specific binding of the radiolabeled WI-1 antigen target.
The isotype and subclass of anti-WI-1 antibody raised by immunization was determined by enzyme-linked immunosorbent assay (ELISA). The wells of C8 Maxisorp plates (Nunc [Roskilde, Denmark] catalog no. 445101) were coated with 100 μl of WI-1 per well at a concentration of 4 μg per ml of PBS and incubated overnight. Coated plates were washed four times with PBS-Tween buffer. Washed plates were blocked for 1 h at 37°C with PBS containing 2% BSA. Test serum diluted in PBS–2% BSA was added to the blocked plates and incubated for 2 h at 37°C. After incubation, serum-treated wells were washed four times with PBS-Tween buffer. A 100-μl volume of horseradish peroxidase-conjugated detector antibody (Zymed Laboratories, South San Francisco, Calif., or Pharmingen, San Diego, Calif.), specific for the various immunoglobulin isotypes or immunoglobulin G (IgG) subclasses and diluted 1:1,000 to 1:2,000 in PBS–2% BSA, was added to the well. A substrate solution of ortho-phenylenediamine dihydrochloride (Sigma catalog no. P6912), 5 mg in 12.5 ml of 25 mM sodium citrate–50 mM NaPO4 (pH 5.0) containing 5 μl of fresh 30% H2O2, was added to the detector antibody-treated wells in a volume of 100 μl per well and incubated for 5 to 10 min at room temperature, protected from the light. A stop solution of 1 M HCl was added, and color development was quantified at an optical density of 492 nm on an ELISA plate reader.
Antigen-induced splenocyte proliferation was determined by standard microtiter methods (18). In brief, 2 × 105 splenocytes were cultured in triplicate in 0.25-ml flat-bottomed microtiter plates (Costar, Cambridge, Mass.) containing 0.1 ml of test antigen and 0.1 ml of RPMI 1640 medium supplemented with 25 mM HEPES buffer, l-glutamine, penicillin, streptomycin (Flow Laboratories, McLean, Va.), and 10% (by volume) heat-inactivated fetal bovine serum (HyClone Laboratories, Logan, Utah). Following incubation for 5 days with the antigen at 37°C in a 5% CO2 humidified incubator, the cultures were pulsed with 1 μCi of [methyl-3H]thymidine ([3H]TdR); New England Nuclear, Boston, Mass.). Radiolabeled cultures were incubated for 18 h and harvested with a cell harvester (Packard Filter Mate 196; Packard Instrument Company Inc., Downers Grove, Ill.). The amount of [3H]TdR incorporation was quantified by a beta counter (Matrix 9600; Packard Instrument Company). Data are expressed as mean ± standard error counts per minute for antigen-stimulated cells divided by that for unstimulated cells.
Delayed-type hypersensitivity responses were assessed by measuring footpad swelling of immunized and control mice 2 weeks after they were boosted. For each mouse, 10 μg of WI-1 in 50 μl of normal saline was injected into the footpad of one hind leg and 50 μl of saline alone as a control was injected into the footpad of the other hind leg. Initial experiments demonstrated that footpad swelling in response to antigen peaked at 24 h, was reduced at 48 h, and was undetectable at 72 h after the antigen was administered. Consequently, the swelling of each footpad was measured 24 h after antigen was administered, and the delayed-type hypersensitivity response of the mouse was defined as the swelling due to WI-1 antigen minus that due to saline control, expressed in millimeters.
Experimental infection.
Two weeks after mice received a second immunization, they were infected with B. dermatitidis yeasts intranasally. After mice were anesthetized with inhaled Metafane (Mallinckrodt Veterinary Inc., Mundelein, Ill.), a suspension of yeast cells was administered in a volume of 25 μl dropwise into their nares. The number of yeasts needed to achieve a lethal pulmonary infection was established in preliminary studies as 104 yeasts for both ATCC 60636 and ATCC 26199. Unimmunized mice that received a lethal dose died from a progressive pulmonary infection 14 to 21 days after they were challenged. To evaluate the protective efficacy of immunization, WI-1-immunized mice and control mice were given a lethal dose of yeast cells, and their survival was assessed daily over 30 days after challenge.
Statistical analysis.
Kaplan-Meier curves were generated for mice that survived a lethal challenge with B. dermatitidis. The survival times of mice that were alive at the end of the study were regarded as censored. Time data were analyzed by the log rank statistic, which summarizes the extent to which the observed survival times in the two groups of data (immunized versus control) deviate from those expected under the null hypothesis of no group differences. Since the number of mice per group is considered small, the exact P values were computed by using the statistical package StatXact-3 (CYTEL Software Corporation). The survival rates of two groups are considered to be significantly different if the two-sided P value is less than 0.05. When multiple comparisons were made simultaneously, the P values were adjusted according to Bonferroni’s correction in order to protect the overall significance level of 0.05.
RESULTS
Delayed-type hypersensitivity responses in immunized mice.
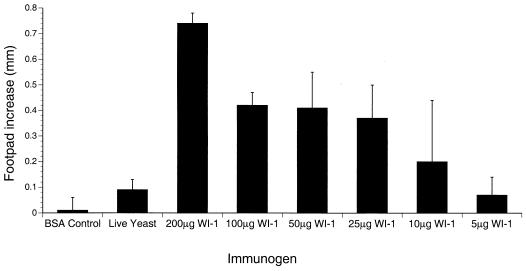
Previous work in a murine model of blastomycosis has demonstrated that the development of delayed-type hypersensitivity correlates temporally with the ability to resist a lethal experimental infection (10, 27). To investigate whether WI-1 immunization evoked delayed-type hypersensitivity responses, we measured the swelling of footpads injected with WI-1. C57BL/6 mice immunized with WI-1 showed substantial amounts of footpad swelling upon recall with WI-1, whereas mice immunized with BSA showed no footpad swelling in response to WI-1 (Fig. 1). The size of the delayed-type hypersensitivity response in WI-1-immunized mice increased in a concentration-dependent manner with the immunizing dose of antigen. Mice that received a subcutaneous infection with 4 × 104 live B. dermatitidis yeasts had weaker delayed-type hypersensitivity responses to WI-1.
FIG. 1.
Immunization with WI-1 induces delayed-type hypersensitivity responses in a concentration-dependent manner. Groups of three C57BL/6 mice were immunized with various doses of WI-1 or live yeast according to the protocol described in Materials and Methods. Mice were tested 2 weeks after the second immunization for delayed-type hypersensitivity by administration of 10 μg of WI-1 in saline into one footpad and saline alone into the other footpad. Measurements were taken 24 h later. Error bars represent standard errors of the mean.
C57BL/6 mice immunized with 100 μg of WI-1 were studied for the in vitro response of their splenocytes to WI-1 and a control antigen from C. albicans. Splenocytes from these mice responded to WI-1 in a concentration-dependent manner, yielding a maximal stimulation index of threefold over background in response to 20 μg of WI-1, whereas they did not respond to Candida antigen (data not shown).
Antibody responses in immunized mice.
Natural infection with B. dermatitidis is accompanied by a strong antibody response to WI-1. To determine whether immunization evokes an immune response that resembles the natural one, we investigated the antibody response. C57BL/6 mice immunized with 100 μg of WI-1 and boosted 2 weeks later with the same dose demonstrated strong anti-WI-1 antibody responses. Sera from the immunized mice showed an average of 80% specific binding of 125I-WI-1 by radioimmunoassay and an endpoint titer of 1:814,013. Mice that received either heat-killed yeasts or viable yeasts also had detectable anti-WI-1 antibodies; however, the average endpoint titers were 16-fold lower (1:49,799) and 358-fold lower (1:2,274) in these two groups of mice, respectively.
Anti-WI-1 IgG subclass profile in immunized mice.
Because cell-mediated immunity is important in developing acquired resistance to B. dermatitidis infection, we characterized aspects of the cellular immune response in WI-1-immunized mice. To investigate the phenotype of T helper cells that arise in immunized mice, we investigated the subclass of serum IgG antibodies specific for WI-1. This parameter has been used as a surrogate marker to indicate T helper 1 responses (IgG2a and IgG3 antibodies) and T helper 2 responses (IgG1 and IgG2b antibodies) (12, 26).
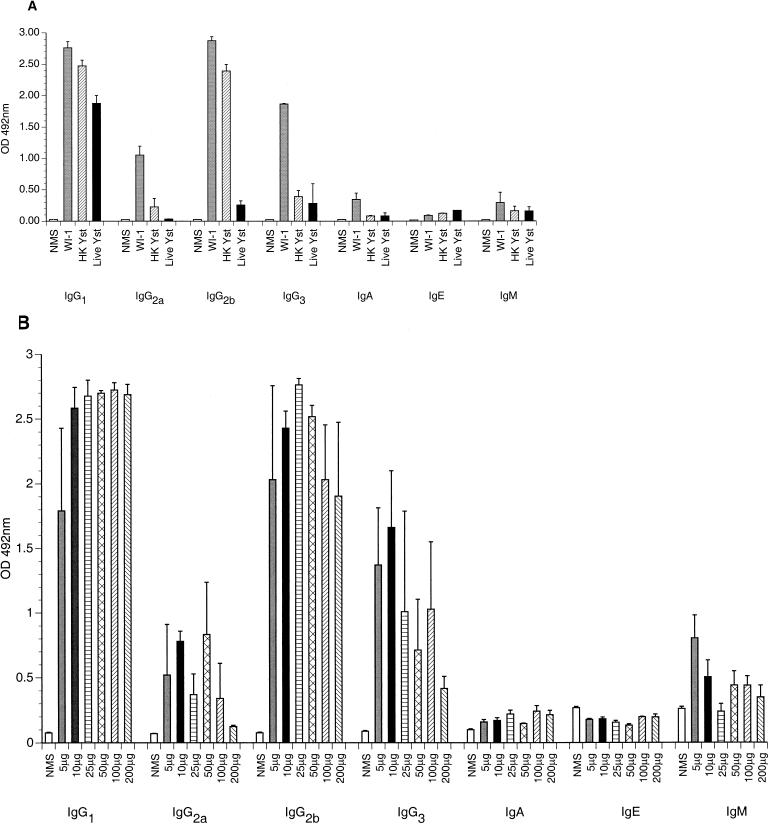
Anti-WI-1 IgG antibody subclasses were IgG1 > IgG2b > IgG2a > IgG3 in C57BL/6 mice immunized with WI-1 (Fig. 2A), indicating a mixed T helper phenotype. The IgG subclasses were distributed similarly in the other groups of immunized mice that had received either heat-killed yeasts or live B. dermatitidis yeasts, even though the antibody titer was lower in these mice than in the WI-1-immunized mice. In addition, BALB/c mice immunized with WI-1 showed an IgG subclass distribution similar to that of the C57BL/6 mice (data not shown). Because some studies have demonstrated that the dose of soluble antigen used for immunization may influence the phenotype of T helper cells (low doses leading to T helper 1 and high doses leading to T helper 2) (24), we assessed the distribution of anti-WI-1 IgG subclasses according to the dose of WI-1 used for immunization, ranging from 10 to 200 μg of antigen. However, the distribution of IgG subclasses did not change substantially according to the immunizing dose of WI-1 (Fig. 2B).
FIG. 2.
Anti-WI-1 IgG subclasses in four C57BL/6 mice per group immunized with either 100 μg of WI-1, 106 heat-killed yeasts (HK Yst), or 4 × 104 live yeast (Live Yst) subcutaneously (A) and three C57BL/6 mice per group immunized with various doses of WI-1 as shown (B). In each experiment, sera were obtained 2 weeks after the second immunization and analyzed for anti-WI-1 IgG subclass by ELISA. Error bars indicate standard error of the mean. OD, optical density. NMS, normal mouse serum.
Protective efficacy of WI-1.
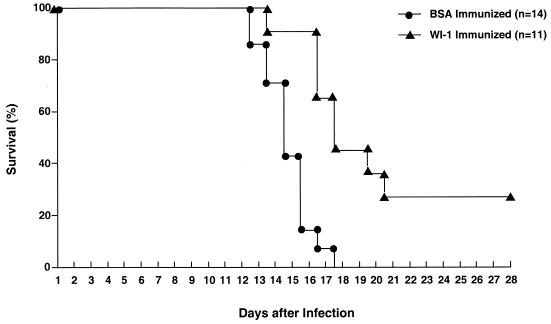
The above results indicated that WI-1 is immunogenic and raises both humoral and cell-mediated immune responses. We next sought to determine whether these anti-WI-1 immune responses confer any protective benefit on the host following a lethal pulmonary challenge with yeasts. WI-1 immunization of C57BL/6 mice and BALB/c mice significantly protected these animals against death after infectious challenge (Fig. 3 and Table 1). The immunizing dose of WI-1 was found to be important since mice immunized with 200 μg of antigen fared significantly better than those immunized with only 10 μg (Table 1, experiment 3). In other experiments (Table 1, experiments 1, 2, and 4), mice were immunized with 100 μg of WI-1 protein according to the protocol described in Materials and Methods. This vaccination significantly prolonged the lives of the mice in each of the experiments and protected a proportion of them from dying during the observation interval. Figure 3 shows a representative survival analysis of C57BL/6 mice immunized with either WI-1 or BSA as a control (P = 0.0002).
FIG. 3.
Protective efficacy of WI-1 immunization against lethal pulmonary blastomycosis. Groups of C57BL/6 mice were immunized subcutaneously either with 100 μg of WI-1 or with BSA according to the protocol described in Materials and Methods. Two weeks after a second immunization, the mice were challenged with a lethal dose of 104 B. dermatitidis ATCC 60636 yeasts and observed for survival. Kaplan-Meier survival curves were generated for WI-1-immunized mice and control mice during an observation interval of 30 days after experimental infection.
TABLE 1.
WI-1 prolongs the survival of mice challenged with a lethal dose of B. dermatitidisa
| Expt | Mouse strains | Group (n) | Mean survival (days) | % Survival at 30 days postinfection (no. of mice that survived/total no. of mice) | Significanceb (P) |
|---|---|---|---|---|---|
| 1 | C57BL/6 | WI-1 (8) | 28 | 75 (6/8) | 0.0002 |
| BSA (8) | 18 | 0 | |||
| 2 | C57BL/6 | WI-1 (11) | 21 | 27 (3/11) | 0.0002 |
| BSA (14) | 15 | 0 | |||
| 3 | C57BL/6 | WI-1, 200 μg (12) | 17 | 0 | 0.009 |
| WI-1, 10 μg (12) | 13 | 0 | |||
| 4 | BALB/c | WI-1 (8) | 21 | 25 (2/8) | 0.027 |
| BSA (8) | 16 | 0 | |||
| 5 | C57BL/6 | Histo/WI-1 (7) | 25 | 29 (2/7) | 0.026† |
| Histo alone (15) | 20 | 0 | 0.045‡ | ||
| Unimmunized (10) | 18 | 10 (1/10) |
Summary of five separate protection experiments performed in C57BL/6 mice and BALB/c mice. C57BL/6 or BALB/c mice were immunized with WI-1 or BSA according to the protocol described in Materials and Methods. Mice were observed for survival after intranasal infection with a lethal dose of 104 ATCC 60636 yeasts. In experiment 5, mice were intranasally infected twice (1 month apart) with a sublethal dose of either H. capsulatum 184 AS ura 5-11 expressing WI-1 off the telomeric, extrachromosomal plasmid pMAD93/WI-1 (Histo/WI-1) or the control strain transformed with plasmid pMAD93 lacking WI-1 (Histo alone). These immunized and control mice were challenged intranasally with a lethal dose of 104 ATCC 26199 yeasts 2 weeks after the second immunization and monitored for survival.
Two-sided P value. †, comparison of Histo/WI-1 group versus Histo alone group; ‡, comparison of Histo/WI-1 group versus unimmunized group.
Experiments shown in Table 1 analyzed survival over a 30-day observation period after infection, explaining why the mean survival periods were never greater than 30 days in mice immunized with WI-1, even though the mice may have survived beyond that interval. The proportion of WI-1-immunized mice surviving in the individual experiments varied, being up to 75% survival in experiment 1. The median proportion of mice surviving for the five total experiments was 27%. Despite this variation between individual experiments, mice immunized with WI-1 survived significantly longer than control mice in each of the experiments.
Some of the surviving mice were analyzed for burden of infection at 30 days postinfection. Half of the mice in experiment 1 showed detectable CFU in the lungs ranging from 2 × 103 to 5 × 105. In one mouse, the pathogen had disseminated to the kidneys and spleen. Thus, the fungi had not been eliminated in the surviving mice.
In experiment 5, WI-1 was delivered to the mice in a different vehicle, H. capsulatum 184 AS ura 5-11 yeasts, in an effort to alter the phenotype of T helper cells responding to WI-1. We reasoned that the predominantly intracellular location of this fungus and the strong T helper 1 response generally evoked by H. capsulatum (2, 30) would offer an immunomodulating effect. Surprisingly, these mice demonstrated barely detectable anti-WI-1 antibody responses in the radioimmunoassay and undetectable IgG antibody subclasses by ELISA. However, anti-WI-1 IgM antibody responses were detected in the ELISA (data not shown).
Mice immunized with WI-1 borne by H. capsulatum yeasts were again significantly protected from lethal infection compared to control mice who received H. capsulatum without WI-1 (P = 0.026) or no immunization at all (P = 0.045).
DISCUSSION
In prior work, WI-1, an abundant surface protein on B. dermatitidis yeasts, has been shown to be a chief antigen of both humoral and cellular immune responses in people and dogs infected with the fungus. Antibody to WI-1 was detected in 85% of infected patients (17) and almost 90% of infected dogs (15). Peripheral blood mononuclear cells from human blastomycosis patients also react strongly in an in vitro proliferation assay after stimulation with WI-1 (18).
In this study, we sought to investigate the immunogenicity of purified WI-1, characterize features of the humoral and cellular response to the immunogen, and analyze whether these anti-WI-1 immune responses are able to enhance resistance against experimental infection. Our results demonstrate that WI-1 administration prompts antibody and cell-mediated immune responses in two inbred strains of mice. The immune responses after administration of WI-1 were qualitatively similar to those after administration of live or dead yeasts but were quantitatively much stronger. Thus, WI-1 is immunogenic and evokes immune responses that resemble the ones observed following exposure to the intact yeast, whether it is dead or alive.
The immune responses engendered by WI-1 administration enhanced the resistance of mice against a lethal experimental infection. Mice immunized with WI-1 lived significantly longer than control mice that were unimmunized or received BSA. Ultimately, only a small proportion of the immunized mice survived the infection. Thus, it would be desirable to investigate methods of enhancing the protective efficacy of WI-1. Alternatively, our model of experimental infection may be overly rigorous. A lethal dose of yeast from cells, as delivered in this study, might overwhelm even a robust, effective immune response. Under circumstances of natural infection, the host is exposed to conidia rather than yeasts, and the infection evolves slowly as a subacute or chronic process. In contrast, animals infected in this study developed a progressive pulmonary infection and died of overwhelming pneumonia 14 to 21 days after the infection. In the related mycosis, histoplasmosis, hsp-80 immunization protects mice against a sublethal infection but not a lethal one. Thus, WI-1 immunization might be more protective in a model of blastomycosis producing a sublethal infection or one that is less rapidly lethal.
The profile of the immune response to WI-1 administration may help explain its modest protective benefit and offer a clue on how to improve the protective efficacy of WI-1. WI-1 evokes a robust immune response, with a humoral component illustrating a mixed T helper phenotype that is biased toward a T helper 2 phenotype. Antibodies offer no established benefit in conferring resistance to B. dermatitidis infection (7) and could harm the host more than help it, as reported for Coccidioides immitis infection (9). Conceivably, some antibodies might benefit the host, as in experimental infections with Cryptococcus neoformans (20) and C. albicans (13). Nonetheless, a T helper 2 response, as suggested by the distribution of anti-WI-1 IgG subclasses, is probably not optimally protective.
Our inferences about T helper phenotype responses to WI-1 immunization were based on analyzing the subclass of WI-1-specific antibodies rather than on directly measuring the cytokines themselves. This approach is indirect, and our data should be interpreted cautiously. However, the approach is supported by a substantial body of literature (12, 26). Moreover, Brummer et al. (6) has shown that mice infected with B. dermatitidis yeasts develop features of a T helper 2 immune response during chronic progression of the infection and features of a T helper 1 immune response during a healing phase of the infection after antifungal therapy. Those findings indicate that both elements of T helper immunity can be observed during infection and suggest that T helper 2 responses to WI-1 may not help in clearing B. dermatitidis infection and may even retard its clearance.
The protective efficacy of WI-1 might be enhanced by modifying the composition of the immunogen. For example, the immunogen might be delivered with adjuvant that preferentially drives T helper 1 immune responses, a possibility that had been a consideration in immunizing mice with H. capsulatum yeasts transfected with WI-1. This approach showed some benefit. However, the protective efficacy was not that different from what we observed with WI-1 protein alone, and we were unable to assess whether the T helper phenotype had been altered using IgG subclass analyses since only IgM antibody was present.
Interleukin-12 (IL-12) as an adjuvant together with WI-1 may offer the most direct way to alter the phenotype of T helper cells that arise after vaccination. IL-12 was first described as a vaccine adjuvant in experimental leishmaniasis (1). Soluble leishmania antigen administered to BALB/c mice led to antigen-specific immune responses with a T helper 2 phenotype and progressive infection after challenge. The addition of IL-12 to soluble leishmania antigen converted it from a nonprotective antigen to a protective antigen by enhancing the differentiation of CD4+ T cells toward a T helper 1 subset and cytokine profile needed to promote delayed-type hypersensitivity responses. Administration of IL-12 together with WI-1 is presently under investigation.
We chose not to investigate various schedules of WI-1 administration in this study. Immunized mice evinced strong antibody and delayed hypersensitivity responses with the protocol described, and their immune responses and survival appeared to plateau with doses of 100 to 200 μg per immunization. Consequently, we judged that improved protection is more likely to be achieved by changing the profile, rather than the intensity, of the response.
It will be informative to dissect the correlates of resistance after immunization with WI-1, in view of the current reexamination of the contributions of humoral and cellular immunity in resistance to medical fungi (8). This reevaluation has been prompted by work involving the passive transfer of protective monoclonal antibodies against experimental cryptococcosus, candidiasis, and Pneumocystis infection (8). The prevailing view in the literature is that resistance to B. dermatitidis is mediated by cellular immunity, but the roles of humoral immunity and anti-WI-1 antibodies have not been studied systematically. Although antigen-specific T cells are likely to be important in resistance to this fungus, and WI-1 reactive T cells are likely to help in this regard, the host mounts a strong antibody response to WI-1. We speculate that WI-1-reactive antibodies may benefit the host, possibly by promoting opsonic clearance of fungus from tissue or by enhancing the formation of granulomas, as reported for experimental Cryptococcus infection (11). Alternatively, the antibodies might interfere with adhesive properties of WI-1, as many of them are directed at the tandem repeat (16), which mediates binding to complement and CD14 receptors (21). The exact role and action of anti-WI-1 antibodies and T cells require further studies.
ACKNOWLEDGMENTS
This work was supported by grants from the USPHS (B. S. Klein) and from the Swiss National Science Foundation (M. Wüthrich). B. S. Klein is the recipient of a Research Career Development Award from the National Institutes of Health and is a Burroughs Wellcome Fund Scholar in Molecular Pathogenic Mycology.
We thank George Deepe and Ruth Allendorfer, Division of Infectious Diseases, University of Cincinnati, for advice and instruction in developing the murine model of infection for this work; Charles Czuprynski for advice and instruction in measurement of delayed hypersensitivity responses; Lan Zeng, Department of Biostatistics and Medical Informatics at the University of Wisconsin—Madison, for assistance with statistical analyses; and Robert Audet and George Cook for help in purifying secreted WI-1 used in this study.
REFERENCES
- 1.Afonso L C, Scharton T M, Vieira L Q, Wysocka M, Trinchieri G, Scott P. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science. 1994;263:235–237. doi: 10.1126/science.7904381. [DOI] [PubMed] [Google Scholar]
- 2.Allendoerfer R, Biovin G P, Deepe G S., Jr Modulation of immune responses in murine pulmonary histoplasmosis. J Infect Dis. 1997;175:905–914. doi: 10.1086/513989. [DOI] [PubMed] [Google Scholar]
- 3.Audet R, Brandhorst T T, Klein B. Purification in quantity of the secreted form of WI-1: a major adhesin on Blastomyces dermatitidis yeasts. Protein Expression Purif. 1997;11:219–226. doi: 10.1006/prep.1997.0783. [DOI] [PubMed] [Google Scholar]
- 4.Baumgardner D J, Paretsky D P, Yopp A C. The epidemiology of blastomycosis in dogs: north central Wisconsin, USA. J Med Vet Mycol. 1995;33:171–176. doi: 10.1080/02681219580000361. [DOI] [PubMed] [Google Scholar]
- 5.Brass C, Volkmann C M, Philpott D E, Klein H P, Halde C J, Stevens D A. Spontaneous mutant of Blastomyces dermatitidis attenuated in virulence for mice. Sabouraudia. 1982;20:145–158. [PubMed] [Google Scholar]
- 6.Brummer E, Hanson L H, Stevens D A. IL-4, IgE, and interferon-gamma production in pulmonary blastomycosis: comparison in mice untreated, immunized, or treated with an antifungal (SCH 39304) Cell Immunol. 1993;149:258–267. doi: 10.1006/cimm.1993.1153. [DOI] [PubMed] [Google Scholar]
- 7.Brummer E, Morozumi P A, Vo P T, Stevens D A. Protection against pulmonary blastomycosis: adoptive transfer with T lymphocytes, but not serum, from resistant mice. Cell Immunol. 1982;73:349–359. doi: 10.1016/0008-8749(82)90461-0. [DOI] [PubMed] [Google Scholar]
- 8.Casadevall A. Antibody immunity and invasive fungal infections. Infect Immun. 1995;63:4211–4218. doi: 10.1128/iai.63.11.4211-4218.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox R A. Coccidioidomycosis. In: Cox R A, editor. Immunology of the fungal diseases. Boca Raton, Fla: CRC Press, Inc.; 1989. pp. 139–164. [Google Scholar]
- 10.Cozad G C, Chang C T. Cell-mediated immunoprotection in blastomycosis. Infect Immun. 1980;28:398–403. doi: 10.1128/iai.28.2.398-403.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldmesser M, Casadevall A. Effect of serum IgG1 to Cryptococcus neoformans glucuronoxylomannan on murine pulmonary infection. J Immunol. 1997;158:790–799. [PubMed] [Google Scholar]
- 12.Finkelman F D, Holmes J, Katona I M, Urban J F, Jr, Beckmann M P, Park L S, Schooley K A, Coffman R L, Mosmann T R, Paul W E. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 13.Han Y, Cutler J E. Antibody response that protects against disseminated candidiasis. Infect Immun. 1995;63:2714–2719. doi: 10.1128/iai.63.7.2714-2719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein B S. Immunology of blastomycosis. In: Al-Doory Y, DiSalvo A F, editors. Blastomycosis. New York, N.Y: Plenum Publishing Corporation; 1992. pp. 133–158. [Google Scholar]
- 15.Klein, B. S. Unpublished data.
- 16.Klein B S, Hogan L H, Jones J M. Immunologic recognition of a 25-amino acid repeat arrayed in tandem on a major antigen of Blastomyces dermatitidis. J Clin Investig. 1993;92:330–337. doi: 10.1172/JCI116571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein B S, Jones J M. Isolation, purification, and radiolabeling of a novel 120-kD surface protein on Blastomyces dermatitidis yeasts to detect antibody in infected patients. J Clin Investig. 1990;85:152–161. doi: 10.1172/JCI114406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein B S, Sondel P M, Jones J M. WI-1, a novel 120-kilodalton surface protein on Blastomyces dermatitidis yeast cells, is a target antigen of cell-mediated immunity in human blastomycosis. Infect Immun. 1992;60:4291–4300. doi: 10.1128/iai.60.10.4291-4300.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein B S, Vergeront J M, Weeks R J, Kumar U N, Mathai G, Varkey B, Kaufman L, Bradsher R W, Stoebig J F, Davis J P. Isolation of Blastomyces dermatitidis in soil associated with a large outbreak of blastomycosis in Wisconsin. N Engl J Med. 1986;314:529–534. doi: 10.1056/NEJM198602273140901. [DOI] [PubMed] [Google Scholar]
- 20.Mukherjee J, Pirofski L A, Scharff M D, Casadevall A. Antibody-mediated protection in mice with lethal intracerebral Cryptococcus neoformans infection. Proc Natl Acad Sci USA. 1993;90:3636–3640. doi: 10.1073/pnas.90.8.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newman S L, Chaturvedi S, Klein B S. The WI-1 antigen of Blastomyces dermatitidis yeasts mediates binding to human macrophage CD11b/CD18 (CR3) and CD14. J Immunol. 1995;154:753–761. [PubMed] [Google Scholar]
- 22.Pappas P G, Pottage J C, Powderly W G, Fraser V J, Stratton C W, McKenzie S, Tapper M L, Chmel H, Bonebrake F C, Blum R, et al. Blastomycosis in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1992;116:847–853. doi: 10.7326/0003-4819-116-10-847. [DOI] [PubMed] [Google Scholar]
- 23.Pappas P G, Threlkeld M G, Bedsole G D, Cleveland K O, Gelfand M S, Dismukes W E. Blastomycosis in immunocompromised patients. Medicine. 1993;72:311–325. doi: 10.1097/00005792-199309000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Pearce E J, Reiner S L. Induction of Th2 responses in infectious diseases. Curr Opin Immunol. 1995;7:497–504. doi: 10.1016/0952-7915(95)80094-8. [DOI] [PubMed] [Google Scholar]
- 25.Sarosi G A, Davies S F. Blastomycosis. Am Rev Respir Dis. 1979;120:911–938. doi: 10.1164/arrd.1979.120.4.911. [DOI] [PubMed] [Google Scholar]
- 26.Snapper C M, Finkelman F D. Immunoglobulin class switching. In: Paul W E, editor. Fundamental immunology. 3rd ed. New York, N.Y: Raven Press; 1993. pp. 837–864. [Google Scholar]
- 27.Spencer H D, Cozad G C. Role of delayed hypersensitivity in blastomycosis of mice. Infect Immun. 1973;7:329–334. doi: 10.1128/iai.7.3.329-334.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woods J P, Goldman W E. Autonomous replication of foreign DNA in Histoplasma capsulatum: role of native telomeric sequences. J Bacteriol. 1993;175:636–641. doi: 10.1128/jb.175.3.636-641.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woods J P, Goldman W E. In vivo generation of linear plasmids with addition of telomeric sequences by Histoplasma capsulatum. Mol Microbiol. 1992;6:3603–3610. doi: 10.1111/j.1365-2958.1992.tb01796.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhou P, Sieve M C, Bennett J, Kwon-Chung K J, Tewari R P, Gazzinelli R T, Sher A, Seder R A. IL-12 prevents mortality in mice infected with Histoplasma capsulatum through induction of IFN-gamma. J Immunol. 1995;155:785–795. [PubMed] [Google Scholar]