Abstract
Extensive research in countries with high sociodemographic indices (SDIs) to date has shown that coronavirus disease 2019 (COVID-19) may be directly associated with more severe outcomes among patients living with haematological disorders and malignancies (HDMs). Because individuals with moderate to severe immunodeficiency are likely to undergo persistent infections, shed virus particles for prolonged periods, and lack an inflammatory or abortive phase, this represents an overall risk of morbidity and mortality from COVID-19. In cases suffering from HDMs, further investigation is needed to achieve a better understanding of triviruses and a group of related variants in patients with anemia and HDMs, as well as their treatment through vaccines, drugs, and other methods. Against this background, the present study aimed to delineate the relationship between HDMs and the novel COVID-19, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Besides, effective treatment options for HDM cases were further explored to address this epidemic and its variants. Therefore, learning about how COVID-19 manifests in these patients, along with exploiting the most appropriate treatments, may lead to the development of treatment and care strategies by clinicians and researchers to help patients recover faster.
Video Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12964-023-01316-9.
Keywords: COVID-19, SARS-CoV-2, Anemia, Haematological disorders and malignancies, Immune response
Introduction
As of December 2019, the first confirmed cases of new COVID-19, SARS-CoV-2, were reported in Wuhan, China, and the situation has affected more than 200 countries [1]. The infection may cause severe illness with shortness of breath and some symptoms of chest pain, which can potentially develop into pneumonia [2]. On the other hand, at the same time as the spread of this virus worldwide, its detection of this virus through laboratory tests such as real-time reverse transcription polymerase chain reaction (rRT-PCR), chest CT scans, Immunoglobulin Rapid Diagnostic Tests (Ig-RDTs), Elisa-linked Immunosorbent Assays (ELISAs), and detecting seroconverted IgA, IgM and IgG antibodies were performed in serum or blood [3, 4].
Therefore, the respiratory system involved during SARS-CoV-2 could be associated with the dysregulated expression of some biomarkers [5]. In addition, lymphopenia is nothing special in this condition and high patients, which indicates anemia in cases of COVID-19. The ferritin test shows higher than normal levels, indicating an acute inflammatory response in patients or the entry of the virus and its effects on iron metabolism, which can reduce the bioavailability of iron, thereby depriving the virus of this element and leading to anemia [6, 7]. As of October 2021, there have been abundant reports of high mortality rates in HDM patients co-infected with COVID-19 [8]. Notably, HDMs caused by overproduction of blood cells are assumed as an abnormal phenomenon that leads to improper control of these cells in fighting infections or preventing serious bleeding [9, 10]. Such malignancies and their treatment may affect the human immune system and put them at high risk of contracting COVID-19 and suffering its consequences [11]. As evidenced in related studies, patients with HDM, above all those with acute lymphoblastic leukemia (ALL), essential thrombocytopenia (ET), multiple myeloma (MM), and chronic myeloid leukemia (CML), compared to people without such conditions, have experienced a higher chance of infection with COVID-19 [12]. Like other ribonucleic acid (RNA) viruses, CoV is constantly changing during the mutation process, producing new variants with a high risk of transmission and pathogenicity [13]. New variants can mainly escape the immune response caused by infection and even vaccination [14]. Considering this issue, dealing with this virus and its types in patients with anemia and HDM creates different scenarios and hypotheses or offers suggestions for preventive or therapeutic purposes that should be considered in the treatment and care of affected patients. Because this virus and its variants significantly affect all aspects of human life worldwide, measuring these risk factors among patients with COVID-19 is crucial to help treat the disease. On the other hand, the possibility of false results in diagnostic tests, especially rRT-PCR, has been abundantly observed. According to the research conducted on patients with blood malignancies, there is a higher probability of false negatives, which can depend on endogenous factors such as hematocrit, triglycerides, cholesterol, and other blood substances [15, 16]. Hence, it is necessary to identify the virus in these patients by more sensitive tests such as CRISPR [17]. Therefore, this review article can help young infectious disease specialists, hematologists, and physicians gain a deeper understanding of this condition and quickly look at recent events caused by HDMs.
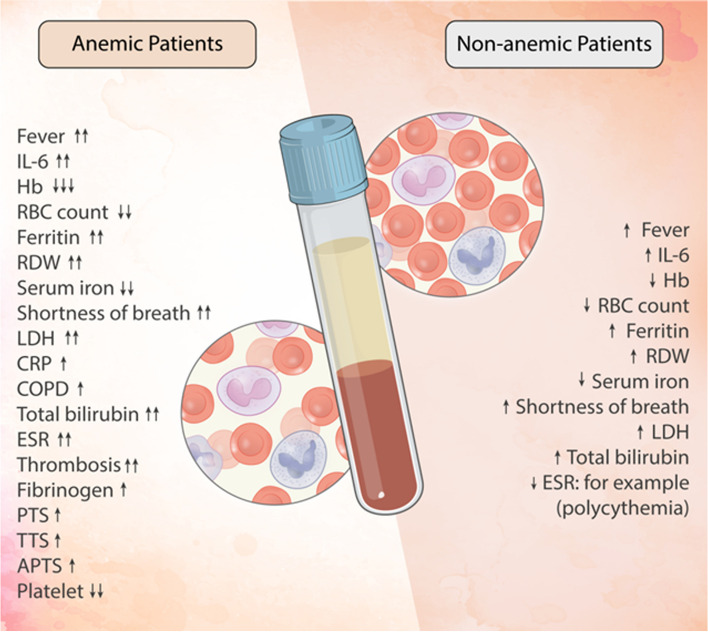
COVID-19 in patients with anemia and haematological malignancies
A diverse range of the most common haematological manifestations, including lymphopenia, anemia, thrombocytopenia, hyperferritinemia, coagulopathy, and high D-dimer levels, can be closely related to COVID-19 [18, 19]. The pathogenesis of this condition is mostly attributed to the severe increase of pro-inflammatory markers such as interleukin-1 beta (IL-1β), IL-2, IL-4, IL-6, IL-10, tumor necrosis factor-alpha (TNF-α), and interferon-gamma (IFN-γ) which cause an exaggerated immune response [20]. The inflammatory response also causes disturbances in iron metabolism, contributing to high hepcidin levels, decreased iron utilization, hyperferritinemia, and anemia [21] (Fig. 1). Such manifestations can be observed during the infection of COVID-19, which often disappear after the resolution of the infection [22]. Due to the rapid spread of the virus, it is necessary to take necessary precautions regarding the risk factors that make these patients more vulnerable to this disease.
Fig. 1.
Graphic overview of factors affecting some types of anemia and haematological disorders in the exacerbation of COVID-19
Beta-thalassemia
Beta (β)-thalassemias represent a group of inherited autosomal recessive anemias that occur due to a reduction or absence of beta globulin tetramers (β4), also called Hb-H [23]. In cases with β-thalassemia, some polymorphisms of the heme oxygenase-1 (HO-1) gene, especially repeat mutations in the dinucleotide (GT) promoter region, induce the HO-1 gene to generate reactive oxygen species (ROS), which protects the cell [24] ( Fig. 2). In patients with COVID-19, longer GT sequences are likely to be present, resulting in modulation of blood flow [25]. During β-thalassemia (mainly partial form), porphyrin deficiency is not observed, but ferritin and excess serum ferritin [26, 27] and iron have been confirmed as risk factors for the exacerbation of COVID-19 [24].
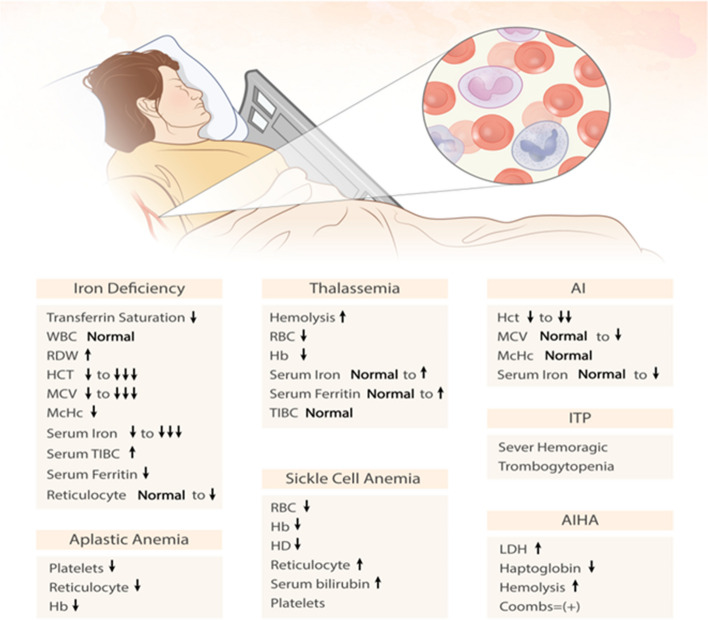
Fig. 2.
Abnormalities in haematological parameters of patients with anemia and RBC disorders
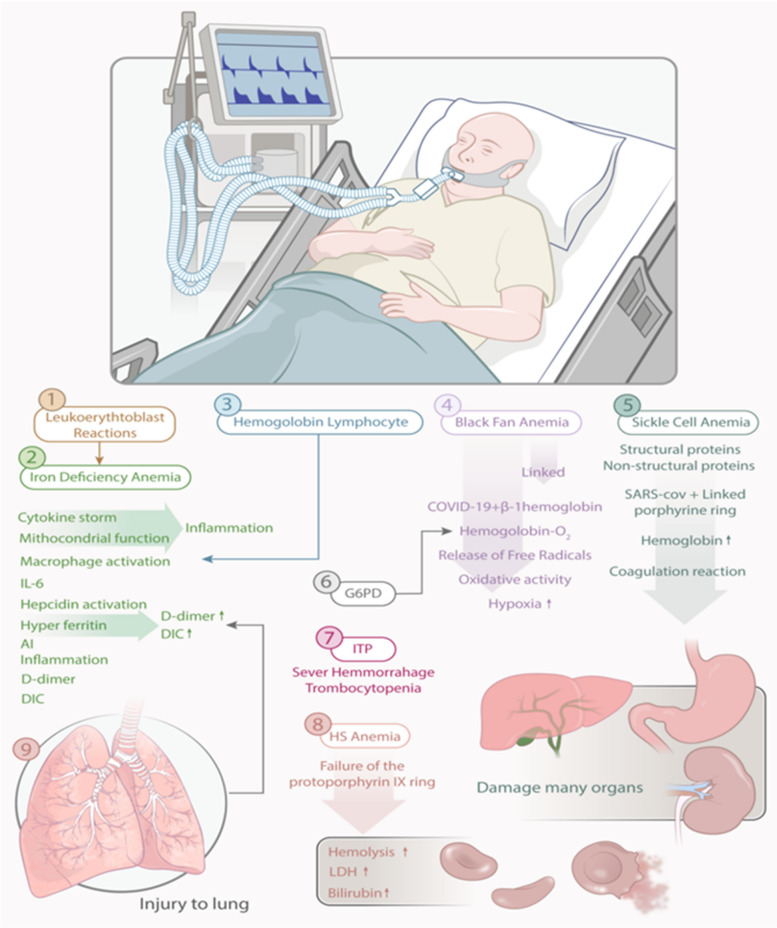
Recent studies have also shown that β-thalassemia patients may contribute to increased susceptibility to SARS-CoV-2 infection due to the nature of their chronic disease [28]. β thalassemias can be defined as an inherited disorder caused by a defect in hemoglobin synthesis, thus accelerating the continuous hemolysis and premature destruction of red blood cells (RBCs) in the bone marrow [29]. These patients are exposed to infectious diseases, especially bacterial infections, acute respiratory infections, and SARS-CoV-2. Therefore, during COVID-19, people with beta-thalassemia are threatened by SARS-CoV-2 infection, as most of them have underlying diseases such as diabetes, common heart diseases, liver problems, and various endocrine disorders [28] (Fig. 3).
Fig. 3.
Graphic illustration of the potential role of different types of anemia and blood disorders in the clinical manifestations of people with COVID-19, possibly influencing the pathophysiology of COVID-19
Sickle cell disease
Sickle cell disease (SCD) refers to a type of HDM that has recently been associated with SARS-CoV-2. As confirmed in previous research, people with swine flu (H1N1) commonly develop respiratory complications, including acute chest syndrome (ACS) [30]. Compared to other children with influenza, children with SCD are also referred to health centers much more often [31]. Consequently, such complications are likely to occur more rapidly in patients with comorbid SCD and COVID-19 [32]. Also, SCD leads to an increased chance of several diseases, such as pulmonary hypertension, chronic lung disease, and kidney failure [33]. In this vein, chronic pulmonary injury from thrombo-inflammation caused by COVID-19 exacerbates SCD complications and increases mortality rates [34]. Likewise, acute vaso-occlusive crisis (VOC) attributed to SCD in COVID-19 patients increases the likelihood of pulmonary embolism (PE) and ACS [35]. Thus, individuals with COVID-19 who are co-infected with SCD have a 3.5-fold increased risk of developing PE compared to patients without the disease. Furthermore, the preclinical and procoagulant status of patients with comorbid SCD and COVID-19 may contribute to milder clinical symptoms [36]. According to some clinical studies, patients with SCD and subsequently infected with COVID-19 do not face the risk of complications or mortality from the epidemic. Still, the hospitalization rate is higher in these people, which raises two different hypotheses [37]. First, patients with SCD have severe hemolysis with continuous release of heme, consisting of toll-like receptor 4 (TLR4) with proinflammatory and procoagulant states [38]. In addition, elevated levels of plasma cytokines, such as IL-1, IL-6, and TNF-α, have been reported in individuals with persistent SCD, and S protein up-regulated them for SARS-CoV-2 [39]. As reported in a study, COVID-19 patients with SCD (age > 50 years), presenting a growth in the serum D-dimer, creatinine (Cr), and lactate dehydrogenase (LDH) levels are at greater risk of mortality regardless of their genotype or gender [30]. Accordingly, the pathophysiology of SCD accounts for chronic anemia, endothelial dysfunction, chronic inflammation, immunodeficiency disease, and hypercoagulability, all as risk factors for the worst outcomes of COVID-19 in individuals with a hypercoagulable state and minor pathophysiology during Hypoxia is considered. As with renal modules, such patients could potentially be at increased risk for contracting COVID-19 [38]. Furthermore, people with SCD are likely to develop some neurological complications during their lifetime. Since SARS-CoV-2 adversely affects the central nervous system (CNS) and patients with SCD are completely immunocompromised, many concerns arise that require further investigation [38] (Figs. 2 and 3).
Iron-deficiency anemia
As iron is required for the growth and reproduction of various cells in the immune system, iron deficiency (ID) can impair the host immune response [40]. As an essential trace element for the host, iron is essential for many enzymatic and non-enzymatic reactions as well as various physiological processes [41]. For example, iron significantly contributes to mitochondrial function in adenosine triphosphate (ATP) production or synthesis, RNA and deoxyribonucleic acid (DNA) repair, cell survival, and ferroptosis [42, 43]. In addition, this valuable element is vital for the multiplication of viruses. On the other hand, IL-6 is a key mediator in post-inflammatory iron management, as hepcidin produces iron [44, 45]. As a key regulator of iron homeostasis, hepcidin further destroys the duodenum by damaging the cellular iron exporter, ferroportin (FPN1), which helps promote cell retention in macrophages and regulates cellular iron metabolism. Therefore, inflammation causes some changes in iron homeostasis due to its dysfunction [46, 47]. This deficiency is often compensated by high levels of iron in the reticuloendothelial cells and ultimately by hyperferritinemia, while low levels of iron are present in the bloodstream [48]. Subsequently, inflammation limits iron in RBCs, leading to anemia known as anemia of inflammation (AI), which is commonly seen in pregnant women with decreased red blood cell quantity and quality along with increased erythrocyte sedimentation rate (ESR). It is related to the gas exchange that occurs during the reduction of RBCs [49, 50] (Fig. 2). This can be caused by a deficiency of folate and other B vitamins. Therefore, pregnancy when infected with COVID-19, especially in IDA, makes this viral infection more visible in the third trimester because inflammatory processes occur much more often. As reported in some studies, many viruses, including SARS-CoV-2, disrupt iron homeostasis in cells, caused by hemolysis, and then enhance intercellular iron load, which steps it accelerates the multiplication of the virus and ultimately increases the severity of the disease [51, 52]. In any case, this iron overload increases serum ferritin and is further associated with rheumatoid arthritis (RA), multiple sclerosis (MS), antiphospholipid syndrome (APS), macrophage activation syndrome (MAS), adult-onset steel disease (AOSD), catastrophic APS (cAPS), and then septic shock [53, 54] (Fig. 3).
Aplastic anemia
Aplastic anemia (AA), also known as rare HDM, is characterized by central pancytopenia due to bone marrow failure [55]. Although the pathogenesis of this type of anemia is still unclear, it is hypothesized to result from the destruction of hematopoietic stem cells (HPSCs) secondary to an aberrant immune response [56]. More than 50% of AA cases are also idiopathic in nature [55]. Despite this, chemotherapy (chemo), ionizing radiation, and viral infections also contribute to this disease [57]. Accordingly, the most common infectious agents include viral hepatitis, human immunodeficiency virus (HIV) [58], cytomegalovirus (CMV) [59], parvovirus B19 (PVB19) [60], and Epstein-Barr virus (EBV) [59]. In this regard, SARS-CoV-2 mainly affects the pulmonary system, which in rarer cases leads to central neutropenia, lymphopenia, and pancytopenia and disrupts the hematopoietic system [61]. Moreover, the overproduction of inflammatory cytokines in infectious viruses, such as IL-1ß, IL-6, TNF-α, and IFN-γ [62], disrupts the bone marrow microenvironment and subsequently causes bone marrow failure [63, 64] (Fig. 3).
Diamond-blackfan anemia
Diamond-blackfan anemia (DBA) mainly affects the bone marrow and causes some physical abnormalities in many parts of the body. During DBA, the bone marrow is normally disrupted, resulting in reduced red blood cells to supply oxygen to the tissues [65, 66]. Actually, changes in Hb levels are predictive of worsening clinical progression in patients with COVID-19, as the bone marrow is unable to make RBCs. SARS-CoV-2 can attack the β-1 chain of Hb and detach it from iron to form perforin. Therefore, often less Hb is available to carry oxygen and carbon dioxide (CO2). Here, binding of the virus to Hb and subsequent release of ions produces free radicals that increase oxidative stress (OS) in organs and lead to hypoxia. Each Hb molecule also contains four hemes during chemical interactions, each of which binds precisely to oxygen in the lungs [67]. In addition, iron (II) and (III) ions (Fe2+ or Fe3+), as part of the toxic structure of oxyhemoglobin in a free state, augment OS in the blood. If SARS-CoV-2 binds to Hb Fe2+ and Fe3+, it may be released into blood and tissues, thus determining the main effects of the virus. In this case, the function of Hb is disturbed, the oxygen supply decreases and finally, hypoxia increases. As a result, shortness of breath and fatigue may persist even after recovery in some patients with COVID-19 [68, 69] (Fig. 3).
Hereditary spherocytosis
In patients with hereditary spherocytosis (HS), cellular stress combined with splenic clearance multiplies the chance of hemolysis [70]. Such individuals may live with chronic baseline hemolysis, sometimes requiring splenectomy to treat severe chronic anemia, or there may be intermittent major hemolysis and splenomegaly [71, 72]. Since the spleen is the site of RBC clearance in HS patients, splenectomy is often advocated as a treatment option [73]. Nonetheless, this surgical procedure does not resolve the defects in the function of the erythrocyte membrane and exposes patients to severe cellular stress and a higher chance of hemolysis [74]. Accordingly, many hemolytic markers can be considered during this emergency. For example, bilirubin (BLR) levels are a significant indicator, the increase of which can be attributed to the breakdown of the protoporphyrin IX (PPIX) ring [70]. Likewise, ferritin is another hemolytic marker, as an acute phase reactant (APR), which is increased in patients with severe COVID-19 with cytokine storm (CS) [75]. Furthermore, LDH levels in severely infected individuals with COVID-19 are compounded due to increased cytokine activity, and decreased monitoring of Hb and hemolytic markers in cases with hemolytic disorders and COVID-19 [76]. Notably, HS can have varying degrees of hemolysis and may be the first hemolytic event at the onset of COVID-19 infection [70].
Leukoerythroblastic reaction
During the leukoerythroblastic reaction (LER), immature RBCs and myeloid cells often circulate in the peripheral blood [77, 78]. This reaction is commonly reported in some disorders related to bone marrow fibrosis, including myeloproliferative disorders (MPDs) and cancer types associated with bone marrow metastatic problems [79]. LER has been identified mostly in viral infections, such as polycythemia vera (PV) and COVID-19. In severe cases, SARS-CoV-2 infection is associated with overproduction of proinflammatory cytokines such as IL-2, IL-6, IL-7, IL-8, IFN-γ, TNF-α, transforming growth factor-beta (TGF-β), C-X-C motif chemokine ligand 8 (CXCL8), CXCL10, chemokine ligand 3 (CCL3), macrophage inflammatory protein-1 alpha (MIP-1α), and -1β, known as CS. In patients infected with SARS-CoV-2, this condition results in LER, with increased production and the presence of immature myeloid cells in the circulatory system [80]. Leukoerythroblastosis can occur in children with Kawasaki disease. The exact etiology of Kawasaki disease is unknown, although an infectious agent appears to be the source of its initiation [81]. Hypersensitivity reactions or inappropriate immune responses, possibly caused by viruses or bacteria, can trigger an inflammatory process that damages blood vessels in people who are genetically predisposed to the disease. Notably, KD and COVID-19 are very similar in this respect [82–84] (Fig. 3).
Hemophagocytic lymphocytosis
Hemophagocytic lymphocytosis (HLH), introduced as a less common symptom in viral proinflammatory conditions, has a high consequence in most patients with COVID-19 [85]. It is an ambiguous clinical condition, followed by immune-mediated tissue damage, which occurs irregularly due to viral infections or HDM. This phenomenon may not be observed in patients with COVID-19, where phagocytosis is also observed in bone marrow aspirates, cytopenias are present, and serum ferritin elevations below ≥ 2000 ng/mL occur [86]. Also, MAS is a life-threatening proinflammatory syndrome that is likely to appear in patients with severe viral infections, such as those with EBV. There is also an interesting pathophysiological similarity between EBV infection and COVID-19 in the case of MAS. During both infections, uncontrolled and hyperactive macrophages cause hypercytokinemia, organ damage, cytopenias, and coagulopathy [85]. Besides, there are several associations between descriptions of severe forms of COVID-19 infection and secondary HLH (sHLH). For example, elevated serum ferritin and C-reactive protein (CRP) levels are commonly observed in patients with severe COVID-19 and sHLH [87]. Moreover, people with severe COVID-19 develop many complications that resemble multi-organ failure (MOF) in HLH [88]. Since COVID-19 has the same pathogenesis compared to sHLH, early diagnosis, and rapid immunosuppression before MOF are often of particular importance [89]. Therefore, all patients with severe COVID-19 should be screened with standard laboratory tests, such as HScore to detect severe inflammation [90].
Sideroblastic anemia
Sideroblastic anemia (SA) encompasses a group of inherited and acquired anemias characterized by ineffective erythropoiesis. In this type of anemia, there is an accumulation of ring sideroblasts (RS) in the bone marrow and a decrease in the production of fully developed red blood cells. These ring sideroblasts are nucleated erythroblasts that show abnormal accumulation of iron granules in the mitochondrial matrix [91]. Such mechanisms contribute to the formation of iron-rich mitochondrial complexes around erythroblast nuclei instead of the standard incorporation of iron into PPIX in the mitochondria [92]. It has then again been hypothesized that COVID-19 may induce an immune-mediated genetic defect in a hematopoietic clone, resulting in ineffective erythropoiesis and the development of RS cells [93]. Accordingly, COVID-19 likely induces a genetic change in new genes that cause SA [93]. In addition, SARS-CoV-2 interacts with Hb molecules through a cluster of differentiation 147 (CD147), CD2b, and other receptors commonly found on erythrocytes and other Hb cells, leading to Hb denaturation [94]. Considering that, hemoglobin concentration decreases and toxic heme is released, which usually causes hypoxia [94]. Furthermore, a gradual decrease in the Hb concentration may promote SA and increase erythrocyte distribution width (RDW), indicating overproduction of immature erythrocytes and an increased risk of mortality [95] (Fig. 2).
Megaloblastic anemia
In some cases, megaloblastic anemia (MA), impaired nerve myelin sheath integrity, impaired immune response, neurological complications, and degenerative conditions of the spine can be caused by some effects of low cobalamin levels [96–98]. Following these conditions, symptoms of vitamin B12 deficiency, including elevated OS and LDH, intravascular coagulation and thrombosis, hyperhomocysteinemia, coagulation cascade, subnormal reticulocyte count, vasoconstriction, and renal failure may often accompany COVID-19 [99, 100]. As suggested, high doses of methylcobalamin could potentiate the RNA-dependent RNA polymerase (RdRp) activity of SARS-CoV-2 nonstructural protein 12 (NSP12) enzymes, which then reduces the viral infection and severity of COVID-19. Overall, methylcobalamin helps reduce the severity of COVID-19 [101]. Vitamin B12 deficiency mostly causes two conditions. Sometimes, parietal cells can't make enough vitamin B12 because people don't have a diet rich in this vitamin. In this case, the megaloblast normally forms in the cells and becomes asynchronous when the nucleus and cytoplasm are mixed, also called MA [102]. Accordingly, those suffering from MA do not have enough red blood cells to carry oxygen properly. A second scenario is that vitamin B12 is produced by the parietal cells because a person receives an adequate vitamin B12-rich diet but has difficulty absorbing it [103]. Bacteria in the large intestine also mutate so they cannot absorb vitamin B12 or have challenges in the ion channels that absorb vitamin B12 [104]. To absorb vitamin B12, folate is needed in a trivalent form, and this dihydrofolate is converted from tetrahydrofolate by these colon bacteria with the help of the dihydrofolate reductase (DHFR) enzyme. Therefore, mutations frequently occur that produce this enzyme and lead to pernicious anemia [105]. In an interesting study, vitamin B12 was identified as one of the viral proteins of SARS-CoV-2, so it can easily bind to it and reduce its effects [106]. Therefore, it is necessary to maintain its level. Furthermore, SARS-CoV-2 may interact with the metabolic activities of vitamin B12 and possibly shape the microbiological distribution in the intestine. It occurs if symptoms such as vasoconstriction, increased OS, coagulation cascade, high LDH levels, pulmonary-renal syndrome (PRS), and hyperhomocysteinemia are present. In addition, B12 deficiency can lead to some abnormalities in the CNS, gastrointestinal (GI), and respiratory systems [107]. Accordingly, a recent study has shown that extra doses of methylcobalamin may help minimize organ damage and even some symptoms associated with COVID-19. For example, a study in Singapore showed a significant reduction in existing symptoms of severe COVID-19 in patients taking magnesium, vitamin D (1000 IU), and vitamin B12 (500 μg) supplements [108]. Prenatal pancytopenia is also a rare manifestation, causing anemia, leukopenia, and thrombocytopenia with a simultaneous decline in all blood cell lineages. Vitamin B12 and folate deficiency generally present as MA, but rare manifestations of pancytopenia have been reported so far. The prevalence of vitamin B12 and folate deficiency during pregnancy is currently significantly high in developing countries due to their poor socioeconomic status and nutrition. Hemodilution with interplacental transfer of vitamin B12 further contributes to the physiological reduction of vitamin B12 levels. In addition, pancytopenia is a rare manifestation of some viral infections, including the novel COVID-19 [109].
Autoimmune and inflammatory haematological complications and COVID-19
As confirmed in related studies, COVID-19 is associated with some autoimmune diseases, including autoimmune cytopenias, cutaneous vasculitis, encephalitis, and Guillain–Barre syndrome (GBS). Among them, autoimmune hemolytic anemia (AIHA) and immune thrombocytopenic purpura (ITP) are the most common [110] (Fig. 2).
Autoimmune hemolytic anemia
In confirmed cases of COVID-19, AIHA or its reactivation has been reported so far, which can be attributed to severe anemia or rituximab treatment. During SARS-CoV-2 infection, anemia associated with elevated LDH and other hemolytic markers may be more frequently observed, and even if the anemia appears unexplained and discontinuous, AIHA is also suspected [111]. Also, molecular mimicry could be the highest factor in the development of AIHA caused by SARS-CoV-2. Immunological cross-reactivity between ankyrin 1 (ANK-1), an RBC membrane protein, and spike proteins in a virus has been implicated in the pathogenesis of AIHA among patients with COVID-19 [112]. Some researchers also believe that the induction of AIHA in children with HLH is due to OS stimulation by SARS-CoV-2. In addition, the acute phase response of COVID-19 induces the formation of aberrant complement immune complexes and complement products on the RBC surface, leading to intravascular thrombosis [113]. This could be consistent with disseminated intravascular coagulation (DIC) with MOF induced by AIHA in COVID-19 patients. Concomitantly, hypercoagulability and inflammatory responses are exacerbated and may affect red blood cells, rupture their membranes, and in such cases lead to PE and vascular coagulation. In this regard, iron and ferritin caused by hemolysis lead to OS. Accordingly, hyperferritinemia and impaired iron homeostasis contribute to endothelial damage and structural changes in red blood cells in cases of COVID-19 [113]. Besides, there are reports of AIHA in patients receiving the vaccination against this disease, particularly with influenza and diphtheria-tetanus-pertussis (DTP) vaccines, due to the induction of warm and cold anti-RBC antibodies [114]. Therefore, vaccines as infectious agents can cause HDMs by molecular mimicry, lymphocyte polyclonal activation, epitope release, and presentation of cryptic antigens [115]. On the other hand, the use of some vaccines cannot protect people with anemia and HDM against SARS-CoV-2 and lead to haematological complications (Table 1).
Table 1.
Summary of studies showing haematological complications of some post-injection COVID-19 vaccines
| Patient | Vaccine or drugs | Results | Ref |
|---|---|---|---|
| A 60-year-old man | Moderna mRNA vaccination |
No personal or family history of haematological conditions Bone marrow biopsy confirmed very severe aplastic anemia with severely hypocellular bone marrow |
[116] |
| 84-year-old man | Pfizer and BioNTech | One case of severe autoimmune hemolytic anemia was identified in the third week after administration of the Pfizer-BioNTech COVID-19 vaccine. However, the condition improved after corticosteroid treatment | [117] |
| 25-year-old man | Spikevax (mRNA-1273, Moderna Biotech, USA) | Diagnosis of thrombotic thrombocytopenic purpura after SARS-CoV-2 vaccine | [118] |
| 75-year-old woman | BNT162b2 | Development of autoimmune hemolytic anemia after vaccination | [119] |
| 69-year-old man | ChAdOx1 and BNT162b2 | TTP | [120] |
| 56-year-old male | Pfizer-BioNTech mRNA vaccine | AA | [121] |
Abbreviations: AA Aplastic anemia, TTP, Thrombotic thrombocytopenic purpura
Idiopathic thrombocytopenic purpura
Depending on viruses and immune and environmental factors, idiopathic thrombocytopenic purpura (ITP) refers to a disease with isolated thrombocytopenia and platelets less than 100 × 109/L, the causes of which are still unknown. Accordingly, autoantibodies reduce platelet synthesis, antibodies against platelet membrane antigens, increase platelet secretion, and prolong life, while platelet production in the bone marrow is reduced due to thrombocytopenia [122]. Acute ITP is usually initiated by a viral infection, and platelet levels usually improve independently after a few weeks or months. Of note, acute ITP lasts more than a year if thrombocytopenia persists (Fig. 2). The most potential viruses as triggers are cytomegalovirus, hepatitis C virus (HCV), herpes simplex virus (HSV), varicella-zoster virus (VZV), rubella virus, EBV, influenza virus, HIV, and SARS-CoV. Furthermore, molecular mimicry between virus-specific antibodies and host proteins may cause virus-mediated ITP [123–125]. The main cause of increased mortality in SARS-CoV-2 cases is thrombocytopenia, which can be caused by DIC, thrombosis, septicemia, or drugs. ITP most often occurs during and up to ten days after a COVID-19 infection. In this regard, antibodies directed against viral glycoproteins can interact with platelet surface integrins, such as glycoprotein IIb/IIIa (GP IIb/IIIa) or GPIb-IX-V, which accounts for approximately 5–10% of cases of ITP caused by SARS-CoV-2 [126]. Therefore, patients with COVID-19 and ITP can present with increased thrombocytopenia and excessive bleeding, mainly in the second stage of the disease [127]. According to this, ITP thrombocytopenia is one of the mechanisms that is at the top of the decrease in the number of platelets in patients with COVID-19 [115]. Until now, this phenomenon has been explained for some reasons, especially virus-induced autoimmunity. Therefore, molecular mimicry along with the expression of cryptic antigens or the release of epitopes can clarify this immune disorder. In most cases, ITP can appear two to three weeks after COVID-19 infection and even before vaccination [128] (Fig. 3). On the other hand, it is noteworthy that patients with previous SARS-CoV-2 infection may have excessive procoagulation factors that can lead to thrombosis and thrombocytopenia. However, currently its pathophysiology is unknown. Experimental studies currently show that a type of soluble adenoviral spike protein leads to the formation of thrombosis, which ultimately results from graft events creates significant endothelial inflammatory events, and leads to binding with endothelial cells expressing ACE2 [129].
Haematological malignancies and COVID-19
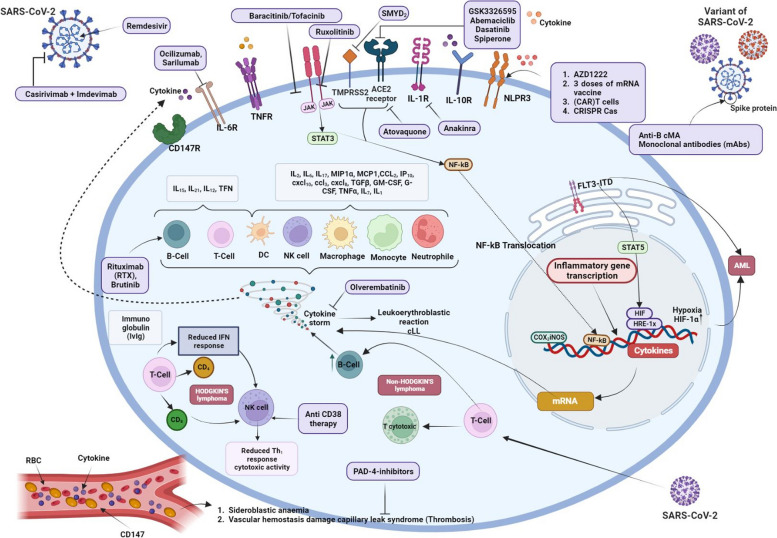
Patients living with HDM are at increased risk of contracting COVID-19 compared to patients without such symptoms [130]. Such malignancies can affect the production and function of blood cells to fight viral infections [131]. For example, HDM cases often have multiple abnormalities in the innate and adaptive immune system, including low levels of immunoglobulin G (IgG) in patients with chronic lymphocytic leukemia (CLL) or other B-cell neoplasms, as well as immature or neoplastic dysfunctions [132, 133]. Therefore, such immune disorders could make people with HDM susceptible to COVID-19 [134] (Figs. 4 (some are hypotheses and are listed in Tables 4 and 5) and 5 (hypothesis)).
Fig. 4.
Graphical overview of the effectiveness of different treatments on the mechanisms of patients with haematological malignancies and severe COVID-19. Created with BioRender.com
Table 4.
Potential therapeutic candidates for immunomodulation in COVID-19 infection
| Therapeutic candidates | Types of vaccines or drugs | Haematological malignancies | Description (advantage, disadvantage, function, or target) | Ref |
|---|---|---|---|---|
| BNT162b2 | mRNA vaccine | Lymphoma patients | BNT162b2 vaccine induces a significant humoral response in a significant proportion of patients with B-cell non-Hodgkin's lymphoma (B-NHL) regardless of gender or age. However, patients receiving active treatment with anti-CD20 antibodies experience impaired humoral responses. In fact, it is impossible to achieve a humoral response in the first 9 months after anti-CD20 therapy. However, response rates gradually improved after this period and continued to increase over time. | [221] |
| mRNA-1273 (Moderna) | Nanoparticle–encapsulated mRNA vaccine | Lymphoma patients | Vaccination with mRNA-1273 or Ad26.CoV2.S prime vaccination, as well as the mRNA-1273 booster compared to BNT162b2, resulted in higher levels of antiviral antibodies in patients with HM, (haematological malignancies), unlike the similar efficacy observed in healthy individuals. These differences in immunogenicity could be attributed to variations in spike mRNA quantity, coding sequence, lipid composition of vaccines, and dosing schedules. | [222, 223] |
| Recombinant subunit zoster vaccine* | Recombinant subunit | B-NHL |
• Prevention of HZ in adults over 50 years of age • Provide immunity in a significant proportion of immunocompromised adult patients over 18 years of age, while maintaining an acceptable safety profile |
[224–226] |
| Johnson & Johnson* | Adenovirus based vaccine | N.A |
• A single vaccination was up to 66% successful in preventing moderate to severe COVID-19, while completely preventing hospitalization and death from COVID-19 • There were no reports of severe hypersensitivity or adverse reactions to vaccination |
[227, 228] |
| Dexamethasone* | Small-molecule inhibitor | CLL | • A glucocorticoid drug | [229] |
| Baracitinib/tofacinib* | Small-molecule inhibitor | Pyoderma gangrenosum | • JAK–inhibitor | [230] |
| TKI* | Small-molecule inhibitor | CLL | • Tyrosine kinase | [231] |
| Hydroxyurea therapy* | Small-molecule inhibitor | SCA and CML |
• Increases fetal hemoglobin and reduces the number of attacks • Inhibiting the enzyme ribonucleotide reductase by scavenging tyrosyl free radicals decreases the production of deoxyribonucleotides as these radicals play a role in reducing NDPs |
[232] |
| Remdesivir* | Small-molecule inhibitor | CLL, Lymphoma |
• It is a nucleoside-like compound that inhibits the RdRp of coronaviruses • Dynamics of temperature, C-reactive protein, and lymphocyte count indicate SARS-CoV-2 reinfection |
[233] |
| Ibrutinib* | Small-molecule inhibitor | N.A |
• XLA • MCL • CLL |
[234] |
| Ruxolitinib* (Jakafi, Incyte) | Small-molecule inhibitor | N.A | • Myelofibrosis | [235] |
| Venetoclax* | Small-molecule inhibitor | SLL, CLL and AML | • Bcl-2 inhibitor | [236] |
| RTX* | Anti-CD20 Monoclonal antibody | Non-Hodgkin's B-cell lymphoma and rheumatoid arthritis |
• This results in the removal of CD20 from the cells, allowing them to persist and resist clearance • Used to treat haematological and autoimmune diseases by depleting CD20-expressing B-cells |
[237] |
| Casirivimab/Imdevimab* | Monoclonal antibody | Leukemias, myelodysplastic syndromes, myeloproliferative neoplasms, lymphomas, and MM | • A mixture of two MABs neutralizing human IgG1 against SARS-CoV-2 spike protein | [238, 239] |
| Tocilizumab, sarilumab* | Human monoclonal antibody | HLH | • Anti-IL-6 | [240] |
| Temelimab* | IgG4 monoclonal antibody | N.A |
• Increased intensity of HERV-W envelope protein expression in leukocytes of COVID-19 patients • HERV-W is a potential biomarker in severe cases of COVID-19 • Temelimab targets the HERV-W** protein and can be investigated as an option to reduce the severity of COVID-19 in patients with blood malignancies |
[241, 242] |
| Isatuximab/daratumumab* | Anti-CD38 antibody | MM, CLL, AML, etc | • Allosteric kinetic inhibitors of CD38 | [243] |
| hATG* | An infusion of horse or rabbit-derived antibodies against human T cells and their precursors | Severe acquired aplastic anemia and AML | • The use of hATG in AML patients receiving intensive ventilation had no significant adverse effect on clinical outcomes | [244] |
| HCT and cell therapy* | N.A | Many inherited or acquired disorders of the hematopoietic system such as ALL | • High safety and low toxicity data from patients treated with allogeneic HCT after receiving CAR-T therapy | [245] |
| Convalescent plasma therapy* | N.A | ALL | • Shorter hospital stay and lower mortality | [246] |
| IG* | Mediated by the Fe portion of IgG and by the spectrum of variable (V) regions contained in the immune globulin preparations | Malignant lymphoma and MM idiopathic thrombocytopenic purpura, Kawasaki disease, Guillain-Barré syndrome, dermatomyositis |
• Immunomodulatory properties • Involved in association with T cell surface molecules critical for immune regulation, such as αβ TCR, CD5, CD4, invariant components of MHC class I molecules, and T- and B-cell adhesion molecules • Increased platelet count |
[247] |
| AZD1222* | Replication-deficient simian adenovirus expressing SARS-CoV-2 spike protein | WM, CLL, and NHL |
• Neutralization of antibodies and antigen-specific T cells against SARS-CoV-2 spike protein • After the first vaccine dose, WM/CLL/NHL patients had lower neutralizing Ab titers than controls |
[248, 249] |
| Anakinra* | Recombinant human IL-1 receptor antagonis | HLH | • Anti-IL1 | [250] |
Abbreviations: ALL Acute lymphoid leukemia, AML Acute myeloid leukemia, B-NHL B-cell non-Hodgkin lymphoma, CAR Chimeric antigen receptor, CLL Chronic lymphocytic leukemia, CML Chronic myeloid leukemia, DVM Donor versus malignancy, DVR Donor versus recipient, HCT Hematopoietic cell transplantation, HSCT Hematopoietic stem cell transplantation, HLH Hemophagocytic lymphohistiocytosis, HZ herpes zoster, Hatg Horse anti-thymocyte globulin, IG Immune globulin, IgG Immunoglobulin gamma, IL Interleukin, MCL Mantle cell lymphoma, NHL Non-Hodgkin lymphoma, NDPs Nucleoside diphosphates, RVD Recipient versus donor, RTX Rituximab, RdRp RNA-dependent RNA polymerase, SCA Sickle cell anemia, SLL Small lymphocytic lymphoma, WM Waldenstrom Macroglobulinemia, XLA X-linked agammaglobulinemia
*Hypotheses about the use of vaccines and other treatments, as well as the possibility of finding new treatment methods in people with blood diseases exposed to this virus, have been proposed with further studies
**It is important to mention that with the progress in treatments, HERVs can be part of side effects related to treatment and drug resistance mechanisms. For example, aberrant transcription of MDR-1 by ERV1 LTR MER57 found in lymphoma cells is a clear example. As a result, to reduce the transcription of harmful genes, several inhibitors of DNA methyltransferase (DNMT) and histone deacetylase (HDAC) are used in the treatment of malignancies
Table 5.
SARS-CoV-2 variants in patients with haematological malignancies: summary of received treatments and hypothesis for haematological malignancies
| Types of vaccines or drugs | SARS-CoV-2 variants | Haematological malignancies | Description (advantage, disadvantage, function, or target) | Ref |
|---|---|---|---|---|
| Drugs | ||||
| AZD7442 (tixagevimab–cilgavimab) | Omicron |
NHL B-cell malignancies |
• AZD7442 did not successfully neutralize Omicron-RBD in patients with haematological malignancies who received a single dose of 150 mg • Neutralization increased above the positive threshold after a 300 mg dose, although it was still variable • AZD7442 counteracts weak performance against contemporary Omicron SARS-CoV-2 strains |
[251–254] |
| Dexamethasone | Omicron | Myeloma | • Administration of dexamethasone resulted in a reduction in 28-day mortality in subjects randomized to receive oxygen therapy or invasive mechanical ventilation. No significant reduction in mortality was observed in patients who received no respiratory support | [8, 255, 256] |
| Plitidepsin | SARS-CoV-2 B.1.1.7 | Haematological malignancies |
• It inhibits the translation of ORFs, ORF1A and ORF1B, leading to reduced synthesis of pp, pp1a and pp1ab, and through eEF1A drive, reduces the amount of repetitive nonstructural proteins such as RNA-dependent RNA polymerase • Inhibits the translation of various sub-genomic mRNAs, resulting in insufficient production of structural and auxiliary viral proteins • The absence of essential viral proteins such as RdRp and structural proteins simultaneously prevents the production of virus copies |
[257–259] |
| Remdesivir and plasma therapy and free remdesivir | SARS-CoV-2 variants and Omicron |
Acute B Lymphoblastic leukemia, AML, ALL, NHL, myeloma/plasmacytoma, myelodysplastic syndrome, CLL, active neoplasia |
• Accelerate resolution of infection and safe initiation of immunosuppressive therapy • It is a nucleoside-like compound that inhibits the RdRp of coronaviruses |
[260, 261] |
| Bamlanivimab D etesevimab | Alpha variant | B-cell malignancies | • Causes SARS-CoV-2 immune escape mutations and secondary clinical deterioration in COVID-19 patients with B-cell malignancies | [262] |
| Obatoclax* | Alpha (B.1.1.7), Beta (B.1.351), and Delta (B.1.617) | N.A | • Block endocytosis and membrane fusion | [263] |
| Olverembatinib | Omicron | CML | • Inhibits the release of cytokines | [264] |
| Abemaciclib, Dasatinib and Spiperone* | Omicron | N.A | • Block the interaction between the omicron spike protein and the host cell receptor ACE2 | [265] |
| Azacytidine* | Delta | N.A | • As a potent inhibitor of DNA methylation, both in preclinical models and in cancer patients | [266] |
| Atovaquone* | SARS-CoV-2 and other variants of concern including the alpha, beta, and delta variants | N.A |
• Inhibits replication • Its ability is partly related to the expression of TMPRSS2, and the drug can prevent the spike protein from binding to the viral receptor, ACE2 • Spike-mediated membrane fusion was also reduced in the presence of atovaquone |
[267] |
| Venetoclax (BTK inhibitors) | Delta | AML, CLL, SLL, B-cell malignancies, and MCL |
• Reduction of mortality • Antitumor agents |
[268, 269] |
| Vaccines | ||||
| BNT162b2* | Alpha and Delta | MM | BNT162b2 induced a specific T cell response in healthy individuals. Conversely, in MM patents, T cell responses are weaker and more heterogeneous than healthy controls. | [270, 271] |
| 3 doses of mRNA vaccine | SARS-CoV-2 variants expect Omicron | CLL | • Robust hybrid immunity in serum, saliva, and T-cell compartments in patients | [272] |
| mRNA-1273 | Omicron | MM |
• MM patients who received mRNA-1273 vaccination developed severe infections 10 weeks after vaccination, despite the production of protective antibodies • After administration of two mRNA vaccines to people with NSCLC and healthy participants, it was observed that NSCLC patients showed less neutralizing activity against live viruses compared to their healthy counterparts |
[273] |
|
Moderna (second vaccine) Pfizer (second vaccine) Janssen (1 vaccine) |
Omicron | AML | • Boosted immunity | [274] |
| Monoclonal antibodies | ||||
| Anti-CD38 therapy | Alpha and delta | MM |
• It is approved in the first line in combination with other agents (immunomodulatory drugs—IMID or PI) and high-dose steroids • Anti-CD38 therapy was even less associated with NAbs response among MM patients • Anti-CD38 antibodies also cause a relative depletion of NK cells, which could contribute to immunodeficiency in MM receiving these immunosuppressive regimens |
[275] |
| Anti-CD20 | SARS-CoV-2 (delta) | B-cell malignancies |
• Disrupts humoral responses after two or three vaccinations • Patients who received anti-CD20 antibodies showed limited efficacy of a BNT162b2 booster dose |
[276–279] |
| Anti-BCMA MAbs | Omicron, WA1, and delta | MM | • After the third time, no significant increase in anti-spike Ab levels was observed in patients treated with anti-BCMA MAbs | [280] |
| ACE2-blocking antibody | Omicron BA.1 and BA.2 and other SARS-CoV-2 variants | Haematological malignancies | • Maintain potent neutralization and protection against Omicron and other SARS-CoV-2 variants | [281, 282] |
| Antibody-containing plasma | Omicron variant | Haematological malignancies | • Control virus replication | [283] |
| CAR-T cells | SARS-CoV-2 variants (Omicron variant) |
CLL B-cell-depleted lymphoma |
• Vaccine-induced T-cell response against SARS-CoV-2 and its Omicron variant in patients • CD19-CAR-T therapy selectively depleted all CD191/CD201 B-cells from the blood of our patients, thus eradicating the immune cell compartment secreting anti-SARS-CoV-2 antibodies |
[280, 284] |
| Cellular therapies | SARS-CoV-2 variants | Haematological malignancies |
• Cell therapy used by adoption to prevent or treat viral infections in cases of natural or transplant immune errors is safe and effective against herpes viruses, polyomaviruses, and certain respiratory pathogens such as adenovirus • Adoptive T-cell therapy has been investigated as a prophylactic or curative adjuvant therapy against SARS-CoV-2 |
[285–287] |
| GSK3326595* | Omicron, delta, and beta variants | N.A |
• By inhibiting ACE2-R671 dimethylation • Able to significantly reduce ACE2 binding to RBD • Strongly reduces the interaction of ACE2 with Spike1 |
[288] |
| T-cell or B-cell immunotherapy | Omicron | Haematological malignancy (MM, lymphoma, CLL, and etc.) |
• Low levels of nAb after two or three doses of vaccination • SARS-CoV-2 variants showed partial vaccine escape, especially the Omicron variant (BA.1) compared to the Delta variant (B.1.617.2) • Resistance to anti-SARS-CoV-2 mAb |
[289] |
| ALVR109* | Alpha, Beta, Gamma, Delta, Epsilon, and Kappa | N.A |
• On day 10 after the second injection of ALVR109, the patient`s nasopharyngeal SARS-CoV-2 viral load became persistently untraceable • After the second infusion, the patient was discharged on minimal oxygen support and received a second negative result of quantitative nasopharyngeal PCR |
[290] |
| CAR-NK cell therapy* | SARS-CoV-2 variant | Leukemia and MM |
• CAR-NK cells target SARS-CoV-2 spike protein with CR3022 scFv domain, a potent neutralizing antibody targeting SARS-CoV-2 S protein • The results showed that CR3022-CAR-NK cells can kill SARS-CoV-2 infected cells in vitro |
[291] |
| Inhibitors | ||||
| PAD-4 inhibitors | SARS-CoV-2 variants | MM |
• Offers broad therapeutic potential in a wide range of inflammatory diseases such as COVID-19 through the formation of NETs • In reducing thrombotic complications in various inflammatory disorders such as COVID-19 and suggests that these inhibitors may be valuable in the treatment of immunothrombotic origin of SARS-CoV-2 |
[292] |
| FLT3 inhibitors | SARS-CoV-2 variant | AML | • Decreased autophagy can reduce the risk of chemotherapy resistance and mortality | [293] |
| SMYD2* | Delta variant | N.A |
• Inhibition downregulates TMPRSS2 • Reduces SARS-CoV-2 infection |
[294] |
| Other treatment | ||||
| Convalescent plasma | Alpha |
B-cell lymphoid malignancy AML |
• Reduction of mortality | [295, 296] |
| Nanobodies* | Emerging mutant variants like Alpha (B.1.1.7), Beta (B.1.351), and Gamma (P.1) | N.A | • Exclusive neutralization efficiency | [297] |
| CRISPR-Cas* | SARS-CoV-2 variant | T cell ALL |
• Development of global T cells against COVID-19 • Enormous potential for the advancement of CAR-NK cell therapy through the use of CAR-NK cells derived from induced pluripotent stem cells. This is due to their ability to manipulate genes and cell surface receptors that have already been studied in the development of CAR-T cells or genetic mutations that cause disease |
[291] |
Abbreviations: ALL Acute lymphoid leukemia, AML Acute myeloid leukemia, BTK Bruton’s tyrosine kinase, CAR Chimeric antigen receptor, CLL Chronic lymphocytic leukemia, CRISPR Clustered regularly interspaced short palindromic repeats, CML Chronic myeloid leukemia, MCL Mantle cell lymphoma, MM Multiple myeloma, NK Natural killer, NETs Neutrophil extracellular traps, nAb Neutralizing antibodies, NHL Non-hodgkin’s lymphoma, NSCLC non-small cell lung cancer, ORFs Open reading frames, PCR Polymerase chain reaction, pp Polyproteins, PI Proteasome inhibitors, RBD Receptor‐binding domain, RdRp RNA-dependent RNA polymerase, SLL Small lymphocytic lymphoma
*Hypotheses about the use of vaccines and other treatments, as well as the possibility of finding new treatment methods in people with blood diseases exposed to this virus, have been proposed with further studies
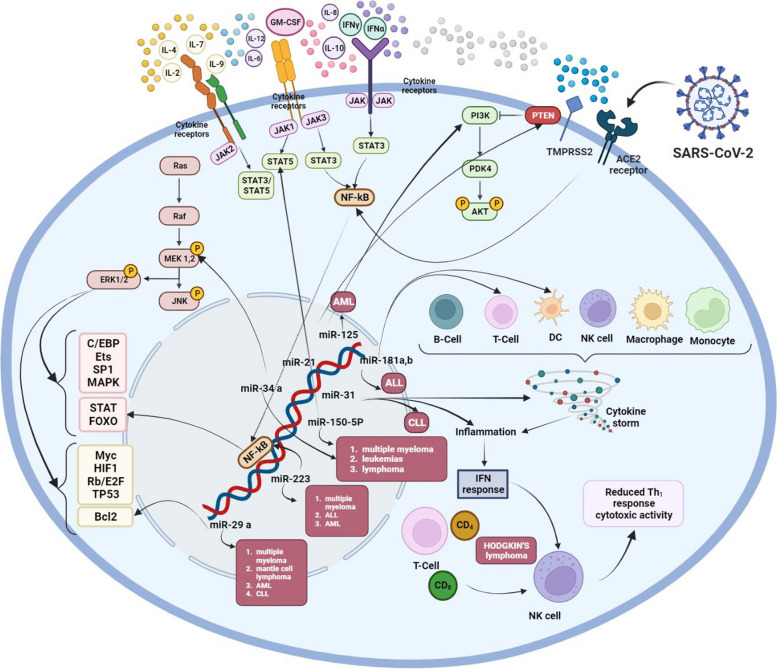
Fig. 5.
Plausible host miRNA action modes in SARS-CoV-2 infection. Host miRNAs may regulate COVID-19 infection in patients with haematological malignancies. Created with BioRender.com
Chronic lymphocytic leukemia
As a malignancy, chronic lymphocytic leukemia (CLL) is characterized by an increase in monoclonal CD5+ B lymphocytes, leading to intrinsic and extrinsic triggering events [135]. For example, the function of various elements in the immune system during viral infections can determine the onset of CLL [136]. Certain factors such as high levels of markers of immune activation such as IL-4, IL-10, and TNF-α, or cytokine release syndrome (CRS) in patients with COVID-19 and high levels of granulocyte colony-stimulating factor (G-CSF), IL-6, IL-7, IL-8, IL-10, IL-1Rα, IFN-γ, TNF-α, granulocyte–macrophage (GM)-CSF, and monocyte chemoattractant protein-1 (MCP-1) are of great importance in this malignancy. Such cytokines can lead to a rapid increase in the clonal expansion of lymphocytes in COVID-19 patients, potentially increasing the chance of malignancy (Fig. 4) [137]. After cancer patients were infected with SARS-CoV-2, CS could effectively induce a severe form of the disease. In this respect, if the patient is healthy enough, the CS will end and the cancer treatment process will not be interrupted [138]. Therefore, activated signaling pathways may negatively affect the therapeutic response and survival rate in cancer patients just at the beginning and before the end of CS. Accordingly, early detection of CS in such patients (such as patients with CLL) with COVID-19 is critical to multiply the effectiveness of targeted therapy [139, 140]. During acute inflammation, this condition may be caused by high endogenous hormone levels, but additional processes may be beneficial that require further investigation [140]. Moreover, it is not known whether the growth of lymphocyte count is a prognostic marker in patients with severe type of COVID-19 and untreated CLL. Furthermore, similar results are unavailable for CLL subjects who have never been treated. For this reason, treatment for people with COVID-19 and CLL poses great challenges [141]. It also gives the impression that the immune system is ineffective in CLL patients and that lymphocytes do not respond strongly to viral infection. Accordingly, such an agent is likely to help protect these patients against CRS and its subsequent damage and MOF [142]. However, chemotherapy in CLL and COVID-19 cases remains controversial, as it may increase the risk of cardiotoxicity, SARS-CoV-2-induced immunodeficiency, and prognosis [143]. To minimize treatment-induced immunodeficiency and drug interactions, it is therefore best to avoid chemotherapy in patients with comorbid CLL and COVID-19 [144, 145].
Acute lymphocytic leukemia
Among the most common types of cancer recognized as the leading cause of death in young adults is acute lymphocytic leukemia (ALL) [146]. Thus, disruption of transcription factors that contribute to direct lymphocyte growth [147, 148], abnormal activation of key signaling pathways, and loss of tumor suppressor genes required for cell cycle regulation [149] are commonly associated with ALL pathogenesis. Furthermore, this condition is often implicated in gene mutations that provide epigenetic regulatory codes [149]. Notably, most cases of ALL and COVID-19 infection have so far not been reported during the pandemic, and the disease progresses slowly in ALL patients with or without clinical symptoms. Therefore, systemic therapy should be delayed in SARS-CoV-2-positive patients (e.g., following the absence of primary hyperleukocytosis). Then, some symptoms such as dry cough, high temperature, anosmia and gastrointestinal problems should be carefully evaluated. If a diagnostic test for SARS-CoV-2 is not possible, a CT scan of the chest should be performed. Furthermore, serological tests should be performed on all patients as soon as they are available [150]. In this line, some studies have further emphasized the abnormally expressed micro (mi)RNAs in ALL patients, as they seem to play a central role in controlling carcinogenesis and drug resistance [151]. Therefore, the etiopathogenesis of HDMs is related to many members of this family, namely miRNA-181a and -181b. Since the expression of miRNA-181a and -181b is much higher in ALL patients than in healthy patients, these findings raise the possibility of using miRNA-181a and miRNA-181b as biomarkers [152]. Researchers have similarly found that all patients living with COVID-19 showed significantly higher levels of miRNA-181a expression, indicating the pathogenic function and prognostic significance of miRNA-181 in patients with comorbid ALL and COVID-19 [153] (Fig. 5 and Table 2).
Table 2.
COVID-19-associated miRNAs involved in immune response
| MicroRNAs | Functions | Types of malignancy | Ref |
|---|---|---|---|
| miRNA-181a and -b | • Regulation of differentiation and development of immune cells and involvement in pathogenesis | ALL | [153, 154] |
| miRNA-129-2 |
• It is associated with lung adenocarcinoma and hepatocellular carcinoma through the control of cell growth • In head and neck squamous cell carcinoma with HPV positive and keratinocyte cells transfected with HPV, miRNA-129–2-3p shows increased expression • Given the growing understanding of the relationship between SARS-CoV-2 and comorbidities, changes in miRNA-129–2-3p expression could be of significant importance |
Haematological malignancies, including lymphoma | [155] |
| Hypothesis* | |||
| miRNA-223-3p |
• Capable of directly targeting NF-κB inhibitor alpha • Notch and NF-κB signaling pathways increase miRNA-223 transcription and decrease FBXW7 tumor suppressor transcription in T-cell ALL cases |
ALL | [156] |
| miRNA-16 and miRNA-511 | • miRNA-16 and miRNA-511 were significantly overexpressed in adult B-ALL | ALL | [157] |
| MSTRG.119845.30/hsa-miRNA-20a-5p/TNFRSF1B, MSTRG.119845.30/hsa-miRNA-29b-2-5p/FCGR2A, and MST RG.106112.2/hsa-miRNA-6501-5p/STAT3 | • Investigating DEGs related to the immune and/or inflammatory response of the host and interaction networks of regulatory genes | Peripheral blood samples of COVID-19 patients | [158] |
| miRNA-155 |
• An essential role in the pathogenesis and severity of COVID-19 • Good diagnostic clinical biomarker for diagnosis of COVID-19 disease and severity of infection • A key target in cancer and an ideal target for therapeutic inhibition |
DLBCL, AML, ALL and CLL | [159, 160] |
| miRNA-34a and miRNA-34a-5p |
• Up-regulated mRNAs associated with cell proliferation interacted • Decreased expression of more than 30 distinct oncogenes in different cancer pathways (such as MET, MEK1, MYC, PDGFR-α, CDK4/6, BCL2, WNT 1/3, NOTCH1, CD44), along with genes that help tumors evade the immune system response (PD-L1, DGK) |
Lymphoma, MM, and leukemias | [161, 162] |
| miRNA-125 |
• Related to the formation and growth of tumors, including proliferation of cancer cells, programmed cell death, invasion and dissemination to other parts of the body, metabolic activity, and immune reactions • Acts as an oncogenic factor or tumor suppressor gene and is also associated with drug resistance in various types of leukemia • Manages cervical cancer progression by controlling VEGF and PI3K/AKT signaling pathways |
AML | [163, 164] |
| miRNA-223 |
• Reduce inflammation to prevent secondary damage during infection and prevent cancerous changes in myeloid cells • Targets of miRNA-223 involved in inflammation and infection include granzyme B, IKKa, Roquin, and STAT3 • In cancer, confirmed targets are C/EBPb, E2F1, FOXO1, and NFI-A • Viral nucleocapsid proteins can suppress BASCs and regulate the release of pro-inflammatory cytokines |
AML, MM, and ALL |
[156, 165] |
| miRNA- 150-5p |
• It directly inhibits the translation of STAT5b mRNA, which leads to a decrease in total STAT5 protein phosphorylation and can be targeted therapeutically • Overexpression of cellular miRNA-150-5p, which is reduced in COVID-19 patients, can inhibit infection of SARS-CoV-2 target cells • Interaction between cellular miRNA-150-5p and a unique MRE in the NSP10 gene encoded by SARS-CoV-2 |
MM, AML, malignant lymphoma, Burkitt lymphomas | [166–168] |
| miRNA-21 |
• Alterations in miRNA-21 concentrations have the potential to modulate LZTFL1 gene expression, which may ultimately lead to organ fibrosis and inflammatory processes • This may provide valuable insights into the progression of severe symptoms associated with COVID-19 • miRNA-21 is effective in inducing apoptosis in cancer therapies targeting p53-deficient tumors • As miRNA-21 exhibits oncogenic properties, it presents an appealing target for the treatment of MM • Confirmed targets of miRNA-21 (PTEN, Rho-B, and BTG2) are associated with AKT dysfunction and extracellular signal-regulated kinase signaling |
Haematological cancers (MM) |
[169–171] |
| miRNA-29a |
• Controls ACE2 and may play an important role in the initiation of COVID-19 infection • miRNA-29 participates in a signaling cascade that includes the phosphatase Ppm1d/Wip1, an important modulator of the DNA damage response, and the tumor suppressor p53 • Genes targeted for the regulation of programmed cell death such as the Bcl-2 family of proteins (cellular leukemia/lymphoma) • Dysregulation of the crucial anti-apoptotic protein Mcl-1 is frequently observed in cancerous cells |
AML, CLL, MCL, and MM | [172–174] |
| miRNA-31 |
• Suppresses the expression of multiple pro-metastatic target genes, thus inhibiting various components of the invasion-metastasis process, including motility, invasion, and anoikis resistance • Significantly up-regulated microRNA in COVID-19 patients was miRNA-31-5p, which could be related to its function in regulating inflammation |
CML | [175, 176] |
Abbreviations: ALL Acute lymphoid leukemia, AML Acute myeloid leukemia, BASCs bronchoalveolar stem cells, CLL Chronic lymphocytic leukemia, CML Chronic myeloid leukemia, DEGs Differentially expressed genes, HPV Human papillomavirus, MCL Mantle cell lymphoma, MRE miRNA recognition element, MM Multiple myeloma, NSP10 nonstructural protein 10, NF-κB Nuclear factor kappa B
*Explanations about the function of miRNAs in various haematological malignancies or in COVID-19 raise hypotheses to find a new relationship between COVID-19 and miRNAs involved in the immune response in individuals with haematological malignancies exposed to this virus. It needs more studies and research
Chronic myeloid leukemia
Chronic myeloid leukemia (CML) is usually initiated by BCR-ABL1 as a hybrid gene in cells with innate or acquired biological potential [177]. This type of cancer can initiate complications in patients with COVID-19 [178]. Furthermore, drug-drug interactions between tyrosine kinase inhibitors (TKIs) for the treatment of this malignancy and those targeting COVID-19 infection may be very hazardous [179]. Also, the side effects of TKI are unbearable for patients with SARS-CoV-2. TKI is often used as an initial treatment for patients with CML and has resulted in a good prognosis and significant improvement [180]. Therefore, treatment with TKIs in cases with CML, which shows a slight increase in the risk of infection, may be due to off-target inhibition of immune-related kinases [181]. Therefore, the decision to withhold or continue TKI-based treatment during the period of COVID-19 seems challenging and needs further investigation. Some studies have also concluded that TKIs help control the immune response to infection [181]. Anti-immune genes such as CD28, CCL55, and IFN-γ and low expression of some such as arginase 1 (ARG1) and fucosyltransferase 4 (FUT4) have been observed so far [182]. Overall, the mortality rate of COVID-19 in Latin American patients with CML has been higher than in the general population. Accordingly, this type of leukemia can cause problems during SARS-CoV-2. Drug interactions between TKIs and COVID-19 treatments can be more dangerous and require careful monitoring [183].
Acute myeloid leukemia
Acute myeloid leukemia (AML), as one of the most common HDMs, can have adverse effects on blood, bone marrow, and other tissues [184]. This condition is characterized by abnormal proliferation or differentiation of clonal cells and a weakened immune system [185]. Currently, several effective treatments are available for AML, especially for young adults [185]. Therefore, infections, including viral infections, can be a major complication of AML treatment, as in other HDMs. Treatment regimens used for patients with AML can also lead to severe granulocytopenia and an increased risk of serious infections. Therefore, a person with AML and SARS-CoV-2 is at high risk of respiratory failure, which requires a reduction in drug dosage and the fact that antiviral drugs [186].
Besides, it is hypothesized that AML patients with COVID-19 undergo a more severe form of the disease. Some even expire due to these conditions, experience significant progression to recurrence of the malignancy, or become resistant to therapeutic drugs, especially if they harbor FMS-like intronic tyrosine kinase-3 tandem mutations (FLT3-ITD). Based on the theory developed by Zalpoor et al. the pharmacological targeting of autophagy and hypoxia-inducible factor 1 alpha (HIF-1α) may be a potential treatment for FLT3-ITD mutations with COVID-19 and risk of mortality, development of HDMs, and drug resistance [187]. In a remarkable study, Deeb et al. established that cytoplasmic expression of HIF-1α was associated with poor prognosis following conventional therapy in older AML patients with normal karyotype [188]. Therefore, they suggested that stimulation of autophagy and HIF-1α by COVID-19 may be a marker for AML patients, especially those with FLT3-ITD mutations. They also hypothesized that autophagy associated with COVID-19, FLT3-ITD, and overexpression of HIF-1α may cause leukemia and drug resistance in these patients. It probably increases the severity of COVID-19 [188]. However, more studies are still needed to support it. Furthermore, autophagy-related drugs have recently been proposed as potential SARS-CoV-2 treatments based on some in vitro and in vivo studies [189]. Accordingly, it has been hypothesized that autophagy induced by COVID-19 may contribute to cancer growth, chemotherapy resistance, and tumor recurrence in patients. These data also suggest that COVID-19 can induce autophagy due to various factors [190]. In addition to being an antiviral therapeutic strategy, targeting autophagy may be a viable option for treating cancer patients with COVID-19 to reduce the risk of mortality, progression, chemotherapy resistance, and tumor recurrence in a variety of cancers [191] (Fig. 4).
Multiple myeloma
Another type of HDM is multiple myeloma (MM), which affects plasma cells in the bone marrow [192]. In cases with MM, the immune system is often compromised by various factors making people with this malignancy susceptible to infection [193]. People with a mean age of 65 years have more underlying diseases, so they are at risk of infection [194]. CD4 depletion, lymphopenia, and loss of functional immunoglobulins can increase the chance of viral, bacterial, and fungal infections [195]. Thus, immunosuppressive drugs advocated in this regard can lead to neutropenia, thereby increasing the risk of contracting COVID-19, as the virus exacerbates the cause of abnormally low concentrations of neutrophils in the blood [196]. Notably, new apheresis testing using autologous stem cell transplantation (ASCT) and polymerase chain reaction (PCR) is required before hospitalization in epidemic-affected countries [197]. While living with this anemia, these patients receive treatments that cause some changes in immune system function, such as humoral immunodeficiency, hypogammaglobulinemia, and impaired B-lymphocyte response to SARS-COV-2. Management of MM in the era of COVID-19 accordingly calls for a thorough assessment of patient- and disease-related variables in order to reduce the risk of developing MM through effective treatment [198].
Myeloproliferative neoplasm
In myeloproliferative neoplasm (MPN), platelets, RBCs, and leukocytes are continuously activated from clonal progenitor cells to hematopoietic cells [199]. PV, ET, and myelofibrosis are also among the leading active neoplasms that can affect mortality [200]. In ET, it is often associated with a persistent increase in the number of platelets that have a propensity for thrombosis, bleeding, and activation of inflammatory mechanisms [201]. In patients with COVID-19 and this malignancy, the lungs may be involved first and then adverse effects on different organs may be observed. In various reports, thrombosis has been presented with some complications of COVID-19 [202]. Therefore, virus-induced thrombosis is a very important genetic thrombosis mechanism in this disease. Accordingly, patients with MPN and COVID-19 are more prone to thrombotic complications and higher mortality [203].
Hodgkin’s lymphoma
Known as a curable malignancy, Hodgkin’s lymphoma (HL) is probably associated with EBV [204]. First, COVID-19 infection may play a significant role in the transient improvement of HL [205]. Decreased peripheral blood lymphocytes (PBLs) and natural killer (NK) cells can be observed in COVID-19 patients [206, 207]. As well, the total number of lymphocytes (here, the CD4+ and CD8+ cells) decreases in severe forms of the disease, more than in mild cases [208]. Second, inflammatory microenvironments may minimize the effective function of NK cells. The high levels of IL-6 and IL-10 in these patients therefore add to the capacity to reduce the cytotoxic process and increase the expression of NKG2A in killing virus-infected cells [205]. In these patients, it also binds to angiotensin-converting enzyme 2 (ACE2) in NK cells and suppresses their function [205]. SARS-CoV-2 and subsequent immune cell inflammatory responses inhibit NK cell cytotoxicity, induce CRS, and amplify inadequate immune responses [209]. Third, individuals with EBV-positive HL is that the NSP10/NSP7/3CLpro/major protease (Mpro) and SARS-CoV-2 S proteins bind to the tumor necrosis factor receptor type 1-associated death domain protein (TRADD) at the binding site of latent membrane protein 1 (LMP1), blocking LMP1 binds to TRADD. This interaction may therefore inhibit LMP1-mediated nuclear factor kappa B (NF-κB) signaling to induce remission [206] (Figs. 4 and 5).
Non- Hodgkin's lymphoma
The most common type of cancer in HIV-infected individuals is non-Hodgkin's lymphoma (NHL), with an increased incidence of B-cell aggressive NHL [210]. Factors affecting the emergence and development of NHL include HIV infection with high viremia, the presence of EBV, and possibly SARS-CoV-2 infection [210]. Recent data also suggest that patients with HDM, including patients with B-cell NHL, are at high risk of severe COVID-19 and may act as a persistent viral reservoir that gives rise to new and potentially more aggressive mutations. Therefore, prevention of COVID-19 or at least modulating its severity in these patients is of great importance [211]. Moreover, patients with B-cell NHL have lower rates of seroconversion and antibody levels compared to other subjects with HDM. Patients with B-cell NHL are also at increased risk of complications and mortality from SARS-CoV-2 [212]. Vaccination against SARS-CoV-2 reduces COVID-19-related deaths and hospitalizations. However, NHL cases experience suboptimal antibody responses to COVID-19 vaccines before and after B-cell-targeted therapies, such as the rituximab anti-CD20 antibody therapy [213] (Fig. 4).
Overview of therapeutic candidates for COVID- 19 infection and related variants
The current pandemic of SARS-CoV-2 and COVID-19 has so far resulted in high rates of mortality and morbidity worldwide. Hematology societies are therefore suggested to conduct prospective and multicenter studies to clarify the effects of this virus and even measure disease severity in patients with anemia and HDMs [214]. In this regard, various blood markers that act as prognostic markers in the severity of the disease have been investigated in previous researches. In this case, patients with HDM are at a higher risk of contracting various infections, including SARS-CoV-2 [215] (Table 3).
Table 3.
Summary of studies evaluating the impacts of COVID-19 in patients with anemia and haematological malignancies
| Country | Time of study | Patient | Results | Type of malignancy | Ref |
|---|---|---|---|---|---|
| Italy | Feb 25 and May 18, 2020 |
• 198 (37%) of 536 patients died • Compared to the general Italian population with covid-19, the standardized mortality ratio was 2.04 in our entire study group and 72.3 in people younger than 70 years • Compared to the non-COVID-19 group with haematological malignancies, the standardized mortality ratio was 41·3 |
People with haematological malignancies experience worse outcomes compared to the general population with COVID-19 and patients with haematological malignancies who are not infected with COVID-19 | Leukaemias, myelodysplastic syndromes, myeloproliferative neoplasms, lymphomas, and MM | [214] |
| Turkey | 11 March 2020 and 22 June 2020 | • COVID-19 patients with haematological malignancy (n = 740) | Patients with haematological malignancies are at increased risk of experiencing severe COVID-19 events, such as ICU admission, MV support, or death, compared with non-malignant COVID-19 patients | NHL (30.1%), myelodysplastic syndrome (19.7%), and myeloproliferative neoplasm (15.7%) were the most common haematological malignancies | [215] |
| China | Jan 13 and Mar 18, 2020 | • 205 patients with cancer and laboratory-confirmed SARS-CoV-2 infection and 22 patients (11%) had haematological malignancies | Patients with cancer and COVID-19 who were hospitalized had a high mortality rate | Haematological malignancies | [216] |
| Spain | March 7, 2020, and April 7, 2020 | • Mortality of patients with haematological malignancies compared to non-cancer patients (35.9% vs. 13.2%) | Mortality from COVID-19 is significantly higher in patients with haematological malignancies compared to non-cancer patients | Lymphoma (30%) and MM (30%) | [217] |
| Italy | March 1, 2020, and April 11, 2020 | • 206 patients with COVID-19 and the prevalence of anemia in COVID-19 was 61% | COVID-19 often leads to anemia as a widespread symptom. While anemia may not have a direct impact on mortality, it can significantly affect the well-being of the elderly and vulnerable, thereby reducing their quality of life | Non-malignant | [218] |
| China | 1 December 2019 to 20 March 2020 | • 222 confirmed patients including 79 patients with anaemia and 143 patients without anaemia | Anemic COVID-19 patients showed higher rates of comorbidities, more severe inflammatory responses, and organ damage compared to non-anemic controls | Non-malignant | [219] |
| Italy | May 01 and June 15, 2020 | • 860 patients with malignancy were tested, of which 474/860 (55%) had haematological malignancies and 386/860 (45%) had solid tumors | Increased risk of contracting COVID‐19 in patients with haematological malignancy | Neoplasms were lymphomas in 198/860 (23%) cases, breast cancer in 103/860 (12%) cases, MM in 103/860 (12%) cases, acute leukemia in 83/860 (9.5%) cases, and lung cancer in 81/860 (9%) cases | [220] |
| Japan | Feb-22 | • The average (± SD) age of 9 individuals was 74 ± 7 years (ranging from 61 to 85 years), and 6 people (66.7%) were male. All participants had a case of ongoing HM: 3 (33.3%) with myeloproliferative disorder. 6 (66.7%) of the patients had received two vaccines more than half a year ago | Although the Omicron strain can be more serious than previous cases, the mortality rate for hospitalized people with underlying health conditions who are infected with the Omicron strain is still significant | 4 (44.4%) with malignant lymphoma, and 2 (22.2%) with MM | [8] |
Abbreviations: MM Multiple myeloma, NHL Non-hodgkin’s lymphoma
On the other hand, this study is very important in the management of patients with blood cancers in the face of SARS-COV-2 and its variants. It also emphasizes the priority of these patients in receiving vaccines and many other treatments. Also, various vaccines and treatment methods SARS-COV-2 and its variants have been considered in different patients (Tables 4 and 5).
Conclusion and future directions
In conclusion, COVID-19 has presented unique and significant challenges for patients with anemia and hematological malignancies, who face a higher risk of severe illness and mortality. This review article has examined the risk factors, clinical guidelines, and emerging therapeutic approaches for managing COVID-19 in this patient population. While much progress has been made in understanding COVID-19 in this context, there are still many areas that require further research.
Prospective type comparative studies using different vaccines, drugs, or combinations against SARS-CoV-2 and its multiple variants in HDM cases are necessary to discover the best options for this specific scenario. Considering the effects of anemia and HDMs on the quality of human life, this issue cannot be ignored, especially during the COVID-19 pandemic, and even because of the high costs, side effects, and shortage of blood. It is hoped that knowing the types of anemia and HDM in the face of this virus, as well as the vaccines and drugs used for the virus itself and related types, will help reduce the clinical burden of COVID-19 and its variants in terms of treatment and care.
For future directions, researchers must focus on uncovering the long-term effects of COVID-19 on patients with anemia and hematological malignancies. Specifically, understanding the cellular and molecular mechanisms of SARS-CoV-2 in potential long-term effects such as chemotherapy resistance, metastasis, and recurrence can open new avenues for developing therapeutic and preventive strategies. In addition, future studies should evaluate the efficacy and potential side effects of vaccination and emerging therapeutic approaches, with a focus on developing new vaccines and drugs that are more suited to the clinical, cellular, and molecular conditions of these diseases to improve efficacy and reduce side effects.
This study provides a potent foundation for preparing for future outbreaks of newly emerged coronaviruses. By examining the risk factors, clinical guidelines, and emerging therapeutic approaches for managing COVID-19 in this patient population, we can gain valuable insights into the challenges of managing patients with underlying health conditions during a possible upcoming new pandemic. This information can be used to guide the development of clinical guidelines and protocols for managing patients with newly emerged coronaviruses in the future, as well as to inform the development of therapeutic approaches and vaccination strategies. Overall, this review article on COVID-19 in patients with anemia and hematological malignancies is an important tool in preparing for and managing future outbreaks of newly emerged coronaviruses.
Acknowledgements
We are deeply grateful to BioChem Studio for designing some of the very attractive schematic illustrations in this article. We also are thankful to BioRender for providing such attractive templates.
Abbreviations
- ACS
Acute chest syndrome
- ALL
Acute lymphoid leukemia
- AML
Acute myeloid leukemia
- AI
Anemia of inflammation
- APS
Antiphospholipid syndrome
- AA
Aplastic anemia
- AIHA
Autoimmune hemolytic anemia
- CNS
Central nervous system
- CAR
Chimeric antigen receptor
- CLL
Chronic lymphocytic leukemia
- CML
Chronic myeloid leukemia
- CSF
Colony-stimulating factor
- COVID-19
Coronavirus disease 2019
- CRS
Cytokine release syndrome
- CS
Cytokine storm
- DBA
Diamond-blackfan anemia
- DIC
Disseminated intravascular coagulation
- EBV
Epstein-Barr virus
- ET
Essential thrombocytopenia
- FLT3-ITD
FMS-like tyrosine kinase-3 internal tandem duplication
- GI
Gastrointestinal
- HDMs
Haematological disorders and malignancies
- Hb
Hemoglobin
- HLH
Hemophagocytic lymphocytosis
- HS
Hereditary spherocytosis
- HL
Hodgkin’s lymphoma
- hATG
Horse antithymocyte globulin
- HIV
Human immunodeficiency virus
- HIF-1α
Hypoxia-inducible factor 1 alpha
- IFN-γ
Interferon gamma
- IL-1β
Interleukin-1 beta
- IgG
Immunoglobulin G
- ITP
Immune thrombocytopenic purpura
- IL
Interleukin
- KD
Kawasaki disease
- LER
Leukoerythroblastic reaction
- MAS
Macrophage activation syndrome
- MCL
Mantle cell lymphoma
- MAbs
Monoclonal antibodies
- MM
Multiple myeloma
- MOF
Multiple-organ failure
- MPN
Myeloproliferative neoplasm
- NK
Natural killer
- NHL
Non-hodgkin’s lymphoma
- ORF
Open reading frames
- OS
Oxidative stress
- PV
Polycythemia vera
- PPIX
Protoporphyrin IX
- ROS
Reactive oxygen species
- RBCs
Red blood cells
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- SCD
Sickle cell disease
- SLL
Small lymphocytic lymphoma
- SDIs
Sociodemographic indices
- TNF-α
Tumor necrosis factor alpha
- TKIs
Tyrosine kinase inhibitors
Authors’ contributions
S.K., B.H., P.A., F.Z., M.N.A., M.R.F., H.Z., M.A., M.I.S., B.G.F., E.T., and I.Z.: Conceptualization, Data curation, Formal analysis, and Investigation; S.K., F.Z., and I.Z.: Visualization; S.K., B.H., P.A., M.N.A.: Writing—original draft; S.K. and I.Z.: Project administration; Supervision: I.Z. and M.M.; S.K., M.R.F., H.Z., M.A., M.I.S., B.G.F., E.T., I.Z., and MM: Editing; S.K., I.Z., and M.M.: Editing Validation.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Iman Zare, Email: izare@sinabioco.com.
Mohammad Motamedifar, Email: motamedm@sums.ac.ir.
References
- 1.Costa BA, da Luz KV, Campos SEV, Lopes GS, Leitão JP, Duarte FB. Can SARS-CoV-2 induce hematologic malignancies in predisposed individuals? A case series and review of the literature. Hematol Transfus Cell Ther. 2022;44:26–31. doi: 10.1016/j.htct.2021.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aggarwal A, Shrivastava A, Kumar A, Ali A. Clinical and epidemiological features of SARS-CoV-2 patients in SARI ward of a tertiary care centre in New Delhi. J Assoc Physicians India. 2020;68:19–26. [PubMed] [Google Scholar]
- 3.GenScript Rare Codon Analysis Tool, http://www.genscript.com/cgi-bin/tools/rare_codon_analysis. 2014.
- 4.Zhai P, Ding Y, Wu X, Long J, Zhong Y, Li Y. The epidemiology, diagnosis and treatment of COVID-19. Int J Antimicrob Agents. 2020;55:105955. doi: 10.1016/j.ijantimicag.2020.105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L, Guo H. Biomarkers of COVID-19 and technologies to combat SARS-CoV-2. Adv Biomark Sci Technol. 2020;2:1–23. doi: 10.1016/j.abst.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Algassim AA, Elghazaly AA, Alnahdi AS, Mohammed-Rahim OM, Alanazi AG, Aldhuwayhi NA, Alanazi MM, Almutairi MF, Aldeailej IM, Kamli NA. Prognostic significance of hemoglobin level and autoimmune hemolytic anemia in SARS-CoV-2 infection. Ann Hematol. 2021;100:37–43. doi: 10.1007/s00277-020-04256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taneri PE, Gómez-Ochoa SA, Llanaj E, Raguindin PF, Rojas LZ, Roa-Díaz ZM, Salvador D, Groothof D, Minder B, Kopp-Heim D. Anemia and iron metabolism in COVID-19: a systematic review and meta-analysis. Eur J Epidemiol. 2020;35:763–773. doi: 10.1007/s10654-020-00678-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taenaka R, Obara T, Kohno K, Aoki K, Ogawa R. Infections with the SARS-CoV-2 Omicron variant show a similar outcome as infections with the previous variants in patients with hematologic malignancies. Ann Hematol. 2022;101:1877–1878. doi: 10.1007/s00277-022-04833-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller MA, Zachary JF. Mechanisms and morphology of cellular injury, adaptation, and death. Pathol Basis Vet Dis. 2017;2–43.e19.
- 10.Habib HM, Ibrahim S, Zaim A, Ibrahim WH. The role of iron in the pathogenesis of COVID-19 and possible treatment with lactoferrin and other iron chelators. Biomed Pharmacother. 2021;136:111228. doi: 10.1016/j.biopha.2021.111228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vivarelli S, Falzone L, Grillo CM, Scandurra G, Torino F, Libra M. Cancer management during COVID-19 pandemic: is immune checkpoint inhibitors-based immunotherapy harmful or beneficial? Cancers. 2020;12:2237. doi: 10.3390/cancers12082237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Q, Berger NA, Xu R. When hematologic malignancies meet COVID-19 in the United States: infections, death and disparities. Blood Rev. 2021;47:100775. doi: 10.1016/j.blre.2020.100775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen KV. Containing the spread of COVID-19 virus facing to its high mutation rate: approach to intervention using a nonspecific way of blocking its entry into the cells. Nucleosides Nucleotides Nucleic Acids. 2022;41:778–814. doi: 10.1080/15257770.2022.2071937. [DOI] [PubMed] [Google Scholar]
- 14.Castro Dopico X, Ols S, Loré K, Karlsson Hedestam GB. Immunity to SARS-CoV-2 induced by infection or vaccination. J Intern Med. 2022;291:32–50. doi: 10.1111/joim.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mouliou DS. Managing viral emerging infectious diseases via current molecular diagnostics in the emergency department: the tricky cases. Expert Rev Anti Infect Ther. 2022;20:1163–1169. doi: 10.1080/14787210.2022.2089653. [DOI] [PubMed] [Google Scholar]
- 16.Mouliou DS, Gourgoulianis KI. False-positive and false-negative COVID-19 cases: respiratory prevention and management strategies, vaccination, and further perspectives. Expert Revi Respir Med. 2021;15:993–1002. doi: 10.1080/17476348.2021.1917389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ibrahim M, Vegel A, Niu A, Panse K, Chen R, Safah H, Socola F, Luk A, Saba NS. Reinfection versus failure of viral clearance in a COVID-19 patient with hematologic malignancy. Leukemia Res. 2021;101:106514. doi: 10.1016/j.leukres.2021.106514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agbuduwe C, Basu S. Haematological manifestations of COVID-19: from cytopenia to coagulopathy. Eur J Haematol. 2020;105:540–546. doi: 10.1111/ejh.13491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korompoki E, Gavriatopoulou M, Fotiou D, Ntanasis-Stathopoulos I, Dimopoulos MA, Terpos E. Late-onset hematological complications post COVID-19: an emerging medical problem for the hematologist. Am J Hematol. 2022;97:119–128. doi: 10.1002/ajh.26384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomes SM, Brito AC, Manfro WF, Ribeiro-Alves M, Ribeiro RS, da Cal MS, Lisboa VD, Abreu DP, Castilho LD, Porto LC. High levels of pro-inflammatory SARS-CoV-2-specific biomarkers revealed by in vitro whole blood cytokine release assay (CRA) in recovered and long-COVID-19 patients. Plos one. 2023;18:e0283983. doi: 10.1371/journal.pone.0283983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Camaschella C, Nai A, Silvestri L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica. 2020;105:260. doi: 10.3324/haematol.2019.232124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mancilha EMB, Oliveira JS. SARS-CoV-2 association with hemoglobin and iron metabolism. Revista da Associação Médica Brasileira. 2021;67:1349–1352. doi: 10.1590/1806-9282.20210555. [DOI] [PubMed] [Google Scholar]
- 23.Urbinati F, Madigan C, Malik P. Pathophysiology and therapy for haemoglobinopathies; part II: thalassaemias. Expert Rev Mol Med. 2006;8:1–26. doi: 10.1017/S1462399406010805. [DOI] [PubMed] [Google Scholar]
- 24.Lansiaux E, Drouin E, Bolm C. Beta-thalassemia minor and SARS-CoV-2: physiopathology, prevalence, severity, morbidity, and mortality. Thalassemia Rep. 2023;13:21–32. doi: 10.3390/thalassrep13010003. [DOI] [Google Scholar]
- 25.Fakhouri EW, Peterson SJ, Kothari J, Alex R, Shapiro JI, Abraham NG. Genetic polymorphisms complicate COVID-19 therapy: pivotal role of HO-1 in cytokine storm. Antioxidants. 2020;9:636. doi: 10.3390/antiox9070636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kong Y, Zhou S, Kihm AJ, Katein AM, Yu X, Gell DA, Mackay JP, Adachi K, Foster-Brown L, Louden CS. Loss of α-hemoglobin–stabilizing protein impairs erythropoiesis and exacerbates β-thalassemia. J Clin Investig. 2004;114:1457–1466. doi: 10.1172/JCI21982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stockman JA, Weiner LS, Simon GE, Stuart MJ, Oski FA. The measurement of free erythrocyte porphyrin (FEP) as a simple means of distinguishing iron deficiency from beta-thalassemia trait in subjects with microcytosis. J Lab Clin Med. 1975;85:113–119. [PubMed] [Google Scholar]
- 28.Sotiriou S, Samara AA, Lachanas KE, Vamvakopoulou D, Vamvakopoulos KO, Vamvakopoulos N, Janho MB, Perivoliotis K, Donoudis C, Daponte A, Gourgoulianis KI (2022) Vulnerability of β-thalassemia heterozygotes to COVID-19: results from a cohort study. J Pers Med 12:352 [DOI] [PMC free article] [PubMed]
- 29.Marengo-Rowe AJ. The thalassemias and related disorders. Proc (Bayl Univ Med Cent) 2007;20:27–31. doi: 10.1080/08998280.2007.11928230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teulier M, Elabbadi A, Gerotziafas G, Lionnet F, Voiriot G, Fartoukh M (2021) Severe COVID-19 with acute respiratory distress syndrome (ARDS) in a sickle cell disease adult patient: case report. BMC Pulm Med 21:1–5 [DOI] [PMC free article] [PubMed]
- 31.Alsayegh F, Mousa SA. Challenges in the management of sickle cell disease during SARS-CoV-2 pandemic. Clin Appl Thromb Hemost. 2020;26:1–8. doi: 10.1177/1076029620955240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Umar Z, Ilyas U, Nso N. Sickle cell disease and COVID-19 infection: importance of COVID-19 testing and approach to management. Cureus. 2022;14:e23604. doi: 10.7759/cureus.23604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Machado RF, Gladwin MT. Chronic sickle cell lung disease: new insights into the diagnosis, pathogenesis and treatment of pulmonary hypertension. Br J Haematol. 2005;129:449–464. doi: 10.1111/j.1365-2141.2005.05432.x. [DOI] [PubMed] [Google Scholar]
- 34.Chiang KC, Gupta A, Sundd P, Krishnamurti L. Thrombo-inflammation in COVID-19 and sickle cell disease: two faces of the same coin. Biomedicines. 2023;11:338. doi: 10.3390/biomedicines11020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sivalingam T, Inusa B, Doyle P, Oteng-Ntim E. COVID-19 and the pulmonary complications of sickle cell disease. EJHaem. 2020;1:545–547. doi: 10.1002/jha2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alhazmi A, Moafa WA, Madkhali J, Saifain O, Alyahyawi F, Adhabi O, Alharbi AA. Coronavirus disease 2019 in patients with sickle cell disease: a cross-sectional study from Jazan Province, Saudi Arabia. J Nat Sci Med. 2022;5:199. doi: 10.4103/jnsm.jnsm_49_21. [DOI] [Google Scholar]
- 37.Alyammahi SK, Abdin SM, Alhamad DW, Elgendy SM, Altell AT, Omar HA. The dynamic association between COVID-19 and chronic disorders: an updated insight into prevalence, mechanisms and therapeutic modalities. Infect Genet Evol. 2021;87:104647. doi: 10.1016/j.meegid.2020.104647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoogenboom WS, Alamuri TT, McMahon DM, Balanchivadze N, Dabak V, Mitchell WB, Morrone KB, Manwani D, Duong TQ. Clinical outcomes of COVID-19 in patients with sickle cell disease and sickle cell trait: a critical appraisal of the literature. Blood Rev. 2022;53:100911. doi: 10.1016/j.blre.2021.100911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kakavandi S, Zare I, VaezJalali M, Dadashi M, Azarian M, Akbari A, Ramezani Farani M, Zalpoor H, Hajikhani B. Structural and non-structural proteins in SARS-CoV-2: potential aspects to COVID-19 treatment or prevention of progression of related diseases. Cell Commun Signal. 2023;21:110. doi: 10.1186/s12964-023-01104-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ward RJ, Crichton RR, Taylor DL, Corte LD, Srai SK, Dexter DT. Iron and the immune system. J Neural Transm. 2011;118:315–328. doi: 10.1007/s00702-010-0479-3. [DOI] [PubMed] [Google Scholar]
- 41.Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22:266–282. doi: 10.1038/s41580-020-00324-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodriguez R, Schreiber SL, Conrad M. Persister cancer cells: Iron addiction and vulnerability to ferroptosis. Mol Cell. 2022;82:728–740. doi: 10.1016/j.molcel.2021.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Panda SP, Kesharwani A. Micronutrients/miRs/ATP networking in mitochondria: clinical intervention with ferroptosis, cuproptosis, and calcium burden. Mitochondrion. 2023;71:1–16. doi: 10.1016/j.mito.2023.05.003. [DOI] [PubMed] [Google Scholar]
- 44.McGonagle D, Sharif K, O'Regan A, Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19:102537. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nemeth E, Ganz T. Regulation of iron metabolism by hepcidin. Annu Rev Nutr. 2006;26:323–342. doi: 10.1146/annurev.nutr.26.061505.111303. [DOI] [PubMed] [Google Scholar]
- 46.Wlazlo E, Mehrad B, Morel L, Scindia Y. Iron metabolism: an under investigated driver of renal pathology in lupus nephritis. Frontiers in Medicine. 2021;8:643686. doi: 10.3389/fmed.2021.643686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vogt A-CS, Arsiwala T, Mohsen M, Vogel M, Manolova V, Bachmann MF. On iron metabolism and its regulation. Int J Mol Sci. 2021;22:4591. doi: 10.3390/ijms22094591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bellmann-Weiler R, Lanser L, Barket R, Rangger L, Schapfl A, Schaber M, Fritsche G, Wöll E, Weiss G. Prevalence and predictive value of anemia and dysregulated iron homeostasis in patients with COVID-19 infection. J Clin Med. 2020;9:2429. doi: 10.3390/jcm9082429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lv Y, Chen L, Liang X, Liu X, Gao M, Wang Q, Wei Q, Liu L. Association between iron status and the risk of adverse outcomes in COVID-19. Clin Nutr. 2021;40:3462–3469. doi: 10.1016/j.clnu.2020.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi L, Wang Y, Yang H, Duan G, Wang Y. Laboratory abnormalities in pregnant women with novel coronavirus disease 2019. Am J Perinatol. 2020;37:1070–1073. doi: 10.1055/s-0040-1712181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ward JL, Torres-Gonzalez M, Ammons MCB. The influence of viral infections on iron homeostasis and the potential for lactoferrin as a therapeutic in the age of the SARS-CoV-2 pandemic. Nutrients. 2022;14:3090. doi: 10.3390/nu14153090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kronstein-Wiedemann R, Stadtmüller M, Traikov S, Georgi M, Teichert M, Yosef H, Wallenborn J, Karl A, Schütze K, Wagner M. SARS-CoV-2 Infects red blood cell progenitors and dysregulates hemoglobin and iron metabolism. Stem Cell Rev Rep. 2022;18:1809–1821. doi: 10.1007/s12015-021-10322-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosário C, Zandman-Goddard G, Meyron-Holtz EG, D’Cruz DP, Shoenfeld Y. The hyperferritinemic syndrome: macrophage activation syndrome, Still’s disease, septic shock and catastrophic antiphospholipid syndrome. BMC Med. 2013;11:1–11. doi: 10.1186/1741-7015-11-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giemza-Stokłosa J, Islam MA, Kotyla PJ. Hyperferritinaemia: an iron sword of autoimmunity. Curr Pharm Des. 2019;25:2909–2918. doi: 10.2174/1381612825666190709202804. [DOI] [PubMed] [Google Scholar]
- 55.Sumbly V, Siddiqui R, Alshamam M, Kurbanova T, Rizzo V. New onset aplastic anemia after a COVID-19 infection: a case report. Am J Med Case Rep. 2021;9:451–455. [Google Scholar]
- 56.Shallis RM, Ahmad R, Zeidan AM. Aplastic anemia: etiology, molecular pathogenesis, and emerging concepts. Eur J Haematol. 2018;101:711–720. doi: 10.1111/ejh.13153. [DOI] [PubMed] [Google Scholar]
- 57.Khalid I, Abbas F, Mustafa Z, Kamran M. Association of various etiological factors with idiopathic acquired aplastic anemia. J Haematol Stem Cell Res. 2022;2:19–25. [Google Scholar]
- 58.Shehi E, Ghazanfar H, Fortuzi K, Gonzalez E, Zeana C. A rare case of parvovirus B19 infection manifesting as chronic aplastic anemia and neutropenia in a human immunodeficiency virus-infected patient. Cureus. 2020;12:e12174. doi: 10.7759/cureus.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scheinberg P, Fischer SH, Li L, Nunez O, Wu CO, Sloand EM, Cohen JI, Young NS, John Barrett A. Distinct EBV and CMV reactivation patterns following antibody-based immunosuppressive regimens in patients with severe aplastic anemia. Blood. 2007;109:3219–3224. doi: 10.1182/blood-2006-09-045625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kerr JR. A review of blood diseases and cytopenias associated with human parvovirus B19 infection. Rev Med Virol. 2015;25:224–240. doi: 10.1002/rmv.1839. [DOI] [PubMed] [Google Scholar]
- 61.Nasiri K, Mohammadzadehsaliani S, Kheradjoo H, Shabestari AM, Eshaghizadeh P, Pakmehr A, Alsaffar MF, Al-Naqeeb BZT, Yasamineh S, Gholizadeh O. Spotlight on the impact of viral infections on Hematopoietic Stem Cells (HSCs) with a focus on COVID-19 effects. Cell Commun Signal. 2023;21:1–15. doi: 10.1186/s12964-023-01122-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Karki R, Sharma BR, Tuladhar S, Williams EP, Zalduondo L, Samir P, Zheng M, Sundaram B, Banoth B, Malireddi RS. Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell. 2021;184:149–168. doi: 10.1016/j.cell.2020.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kitagawa M, Saito I, Kuwata T, Yoshida S, Yamaguchi S, Takahashi M, Tanizawa T, Kamiyama R, Hirokawa K. Overexpression of tumor necrosis factor (TNF)-α and interferon (IFN)-γ by bone marrow cells from patients with myelodysplastic syndromes. Leukemia. 1997;11:2049–2054. doi: 10.1038/sj.leu.2400844. [DOI] [PubMed] [Google Scholar]
- 64.Zheng S, Vrindts Y, Lopez M, De Groote D, Zangerlé P-F, Collette J, Franchimont N, Geenen V, Albert A, Reginster J-Y. Increase in cytokine production (IL-1β, IL-6, TNF-α but not IFN-γ, GM-CSF or LIF) by stimulated whole blood cells in postmenopausal osteoporosis. Maturitas. 1997;26:63–71. doi: 10.1016/S0378-5122(96)01080-8. [DOI] [PubMed] [Google Scholar]
- 65.Iskander D, Wang G, Heuston EF, Christodoulidou C, Psaila B, Ponnusamy K, Ren H, Mokhtari Z, Robinson M, Chaidos A. Single-cell profiling of human bone marrow progenitors reveals mechanisms of failing erythropoiesis in Diamond-Blackfan anemia. Sci Transl Med. 2021;13:eabf0113. doi: 10.1126/scitranslmed.abf0113. [DOI] [PubMed] [Google Scholar]
- 66.Barber C. Rare health conditions 44: ataxia, Diamond Blackfan anaemia, sexual identities. Br J Healthcare Assist. 2021;15:78–83. doi: 10.12968/bjha.2021.15.2.78. [DOI] [Google Scholar]
- 67.Wenzhong, Liu, and Li Hualan (2020) COVID-19: attacks the 1-beta chain of hemoglobin and captures the porphyrin to inhibit human heme metabolism. ChemRxiv Preprint
- 68.Dutt S, Hamza I, Bartnikas TB (2022) Molecular mechanisms of iron and heme metabolism. Annu Rev Nutr 42:311–35 [DOI] [PMC free article] [PubMed]
- 69.Lee JX, Chieng WK, Lau SC, Tan CE (2021) COVID-19 and hemoglobinopathies: a systematic review of clinical presentations, investigations, and outcomes. Front Med 8:757510 [DOI] [PMC free article] [PubMed]
- 70.Severance T, Rahim M, French J, Baker R, Shriner A, Khaitan A, Overholt K. COVID-19 and hereditary spherocytosis: a recipe for hemolysis. Pediatr Blood Cancer. 2021;68:e28548. doi: 10.1002/pbc.28548. [DOI] [PubMed] [Google Scholar]
- 71.Rothman JA, Stevens JL, Gray FL, Kalfa TA. How I approach hereditary hemolytic anemia and splenectomy. Pediatr Blood Cancer. 2020;67:e28337. doi: 10.1002/pbc.28337. [DOI] [PubMed] [Google Scholar]
- 72.Schwartz SI, Bernard RP, Adams JT, Bauman AW. Splenectomy for hematologic disorders. Arch Surg. 1970;101:338–347. doi: 10.1001/archsurg.1970.01340260242036. [DOI] [PubMed] [Google Scholar]
- 73.Buesing KL, Tracy ET, Kiernan C, Pastor AC, Cassidy LD, Scott JP, Ware RE, Davidoff AM, Rescorla FJ, Langer JC. Partial splenectomy for hereditary spherocytosis: a multi-institutional review. J Pediatr Surg. 2011;46:178–183. doi: 10.1016/j.jpedsurg.2010.09.090. [DOI] [PubMed] [Google Scholar]
- 74.Manciu S, Matei E, Trandafir B. Hereditary spherocytosis-diagnosis, surgical treatment and outcomes. A literature review. Chirurgia (Bucur) 2017;112:110–116. doi: 10.21614/chirurgia.112.2.110. [DOI] [PubMed] [Google Scholar]
- 75.Severance T, Rahim M, French J, Baker R, Shriner A, Khaitan A, Overholt K (2020) COVID-19 and hereditary spherocytosis: a recipe for hemolysis. Authorea Preprints [DOI] [PubMed]
- 76.Cavezzi A, Menicagli R, Troiani E, Corrao S. COVID-19, cation dysmetabolism, sialic acid, CD147, ACE2, viroporins, hepcidin and ferroptosis: a possible unifying hypothesis. F1000Research. 2022;11:102. doi: 10.12688/f1000research.108667.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mitra A, Dwyre DM, Schivo M, Thompson GR, III, Cohen SH, Ku N, Graff JP. Leukoerythroblastic reaction in a patient with COVID-19 infection. Am J Hematol. 2020;95:999–1000. doi: 10.1002/ajh.25793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tabares Calvache E, Tabares Calvache AD, Faulhaber GAM. Systematic review about etiologic association to the leukoerythroblastic reaction. Int J Lab Hematol. 2020;42:495–500. doi: 10.1111/ijlh.13238. [DOI] [PubMed] [Google Scholar]
- 79.Wolf BC, Neiman RS. Myelofibrosis with myeloid metaplasia: pathophysiologic implications of the correlation between bone marrow changes and progression of splenomegaly. Blood. 1985;65:803–809. doi: 10.1182/blood.V65.4.803.803. [DOI] [PubMed] [Google Scholar]
- 80.Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodríguez L. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee WS, Margolskee E (2020) Leukoerythroblastosis and plasmacytoid lymphocytes in a child with SARS-CoV-2–associated multisystem inflammatory syndrome. Blood 136(7):914 [DOI] [PMC free article] [PubMed]
- 82.Rowley AH, Shulman ST. Pathogenesis and management of Kawasaki disease. Expert Rev Anti Infect Ther. 2010;8:197–203. doi: 10.1586/eri.09.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dajani AS, Taubert KA, Gerber MA, Shulman ST, Ferrieri P, Freed M, Takahashi M, Bierman FZ, Karchmer AW, Wilson W. Diagnosis and therapy of Kawasaki disease in children. Circulation. 1993;87:1776–1780. doi: 10.1161/01.CIR.87.5.1776. [DOI] [PubMed] [Google Scholar]
- 84.Esper F, Shapiro ED, Weibel C, Ferguson D, Landry ML, Kahn JS. Association between a novel human coronavirus and Kawasaki disease. J Infect Dis. 2005;191:499–502. doi: 10.1086/428291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Soy M, Atagündüz P, Atagündüz I, Sucak GT. Hemophagocytic lymphohistiocytosis: a review inspired by the COVID-19 pandemic. Rheumatol Int. 2021;41:7–18. doi: 10.1007/s00296-020-04636-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Loscocco GG. Secondary hemophagocytic lymphohistiocytosis, HScore and COVID-19. Int J Hematol. 2020;112:125–126. doi: 10.1007/s12185-020-02895-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kiran KS, Yasitha K, Panakala S, Pitcairn N, Tiwari RV, Sharma M, Tiwari H. Role of C-reactive protein, serum ferritin and D-Dimer in Covid cases: systematic review & meta analysis. Ann Romanian Soc Cell Biol. 2021;25:10807–10816. [Google Scholar]
- 88.Hsu R-J, Yu W-C, Peng G-R, Ye C-H, Hu S, Chong PCT, Yap KY, Lee JYC, Lin W-C, Yu S-H. The role of cytokines and chemokines in severe acute respiratory syndrome coronavirus 2 infections. Front Immunol. 2022;13:832394. doi: 10.3389/fimmu.2022.832394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim JS, Lee JY, Yang JW, Lee KH, Effenberger M, Szpirt W, Kronbichler A, Shin JI. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics. 2021;11:316. doi: 10.7150/thno.49713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bordbar M, Sanaei Dashti A, Amanati A, Shorafa E, Mansoori Y, Dehghani SJ, Molavi Vardanjani H (2021) Assessment of the HScore as a predictor of disease outcome in patients with COVID-19. BMC Pulm Med 21:1-9 [DOI] [PMC free article] [PubMed]
- 91.Abu-Zeinah G, DeSancho MT. Understanding sideroblastic anemia: an overview of genetics, epidemiology, pathophysiology and current therapeutic options. J Blood Med. 2021;25:305–318. doi: 10.2147/JBM.S232644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brissot P, Bernard DG, Brissot E, Loréal O, Troadec M-B. Rare anemias due to genetic iron metabolism defects. Mutat Res Rev Mutat Res. 2018;777:52–63. doi: 10.1016/j.mrrev.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 93.Mukhi N, Soto LR, Vuppala A. Transient sideroblastic anemia post-COVID-19 infection. Cureus. 2022;14:e30275. doi: 10.7759/cureus.30275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.El-Sayed EM, Ibrahim KS. Ameliorating effects of probiotics on alterations in iron homeostasis and inflammation in COVID-19. Mol Biol R. 2022;49:5153–5163. doi: 10.1007/s11033-022-07226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ayyashi M, Darbashi H, Hakami A, Sharahili F, Sharahili F., Sr Evaluation of remdesivir utilization pattern in critically Ill patients with COVID-19 in Jazan Province. Cureus. 2023;15:e36247. doi: 10.7759/cureus.36247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wolffenbuttel BH, Wouters HJ, Heiner-Fokkema MR, van der Klauw MM. The many faces of cobalamin (vitamin B12) deficiency. Mayo Clin Proc Innov Qual Outcomes. 2019;3:200–214. doi: 10.1016/j.mayocpiqo.2019.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Allen LH. Efficacy and safety of vitamin B12 fortification. In: Food Fortification in a Globalized World. Elsevier. 2018:255-261.
- 98.Fath MK, Naderi M, Hamzavi H, Ganji M, Shabani S, Khalesi B, Pourzardosht N, Hashemi ZS, Khalili S. Molecular Mechanisms and therapeutic effects of different vitamins and minerals in COVID-19 patients. J Trace Elem Med Biol. 2022;73:127044. doi: 10.1016/j.jtemb.2022.127044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sabry W, Elemary M, Burnouf T, Seghatchian J, Goubran H. Vitamin B12 deficiency and metabolism-mediated thrombotic microangiopathy (MM-TMA) Transfus Apher Sci. 2020;59:102717. doi: 10.1016/j.transci.2019.102717. [DOI] [PubMed] [Google Scholar]
- 100.Stokes MB, Zviti R, Lin F, D’Agati VD. An unusual cause of hypertension with hematuria and proteinuria: answers. Pediatr Nephrol. 2016;31:2265–2270. doi: 10.1007/s00467-016-3348-y. [DOI] [PubMed] [Google Scholar]
- 101.Tan CW, Ho LP, Kalimuddin S, Cherng BPZ, Teh YE, Thien SY, Wong HM, Tern PJW, Chandran M, Chay JWM. Cohort study to evaluate the effect of vitamin D, magnesium, and vitamin B12 in combination on progression to severe outcomes in older patients with coronavirus (COVID-19) Nutrition. 2020;79:111017. doi: 10.1016/j.nut.2020.111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stabler SP. Vitamin B12 deficiency. N Engl J Med. 2013;368:149–160. doi: 10.1056/NEJMcp1113996. [DOI] [PubMed] [Google Scholar]
- 103.Green R, Allen LH, Bjørke-Monsen A-L, Brito A, Guéant J-L, Miller JW, Molloy AM, Nexo E, Stabler S, Toh B-H. Vitamin B12 deficiency. Nat Rev Dis Primers. 2017;3:1–20. doi: 10.1038/nrdp.2017.40. [DOI] [PubMed] [Google Scholar]
- 104.Guéant J-L, Guéant-Rodriguez R-M, Alpers DH. Vitamin B12 absorption and malabsorption. Vitam Horm. 2022;119:241–274. doi: 10.1016/bs.vh.2022.01.016. [DOI] [PubMed] [Google Scholar]
- 105.Kuijpers TW, de Vries AC, van Leeuwen EM, Ermens AT, de Pont S, Smith DE, Wamelink MM, Mensenkamp AR, Nelen MR, Lango Allen H, Pals ST (2022) Megalobastic anemia, infantile leukemia, and immunodeficiency caused by a novel homozygous mutation in the DHFR gene. Blood Adv 6:5829-34 [DOI] [PMC free article] [PubMed]
- 106.Batista KS, Cintra VM, Lucena PA, Manhães-de-Castro R, Toscano AE, Costa LP, Queiroz ME, de Andrade SM, Guzman-Quevedo O. Aquino JdS: The role of vitamin B12 in viral infections: a comprehensive review of its relationship with the muscle–gut–brain axis and implications for SARS-CoV-2 infection. Nutr Rev. 2022;80:561–578. doi: 10.1093/nutrit/nuab092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.dos Santos LMJ. Can vitamin B12 be an adjuvant to COVID-19 treatment? GSC Biol Pharm Sci. 2020;11:001–005. doi: 10.30574/gscbps.2020.11.3.0155. [DOI] [Google Scholar]
- 108.Babar Q, Ali A, Saeed A, Tahir MF: Novel treatment strategy against COVID-19 through anti-inflammatory, antioxidant and immunostimulatory properties of the b vitamin complex. In: B-Complex Vitamins-Sources, Intakes and Novel Applications. Intechopen. 2021.
- 109.Agarwal N, Khatri N, Singh P (2021) Pancytopenia in pregnant patients with COVID-19 infection and vitamin B12 deficiency: a case report study. JIDHealth 4:415-8
- 110.Tang K-T, Hsu B-C, Chen D-Y. Autoimmune and rheumatic manifestations associated with COVID-19 in adults: an updated systematic review. Front Immunol. 2021;12:645013. doi: 10.3389/fimmu.2021.645013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fattizzo B, Pasquale R, Bellani V, Barcellini W, Kulasekararaj AG (2021) Complement mediated hemolytic anemias in the COVID-19 era: case series and review of the literature. Front Immunol 12:791429. [DOI] [PMC free article] [PubMed]
- 112.Dotan A, Muller S, Kanduc D, David P, Halpert G, Shoenfeld Y (2021) The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun Rev 20:102792. [DOI] [PMC free article] [PubMed]
- 113.Al-Kuraishy HM, Al-Gareeb AI, Kaushik A, Kujawska M. Batiha GE-S: Hemolytic anemia in COVID-19. Ann Hematol. 2022;101:1887–1895. doi: 10.1007/s00277-022-04907-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jafarzadeh A, Jafarzadeh S, Pardehshenas M, Nemati M, Mortazavi SMJ. Development and exacerbation of autoimmune hemolytic anemia following COVID-19 vaccination: a systematic review. Int J Lab Hematol. 2023;45:145–155. doi: 10.1111/ijlh.13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.González-López TJ, Bárez A, Bernardo-Gutiérrez A, Bernat S, Canaro-Hirnyk M, Entrena-Ureña L, Fernández-Fuertes F, de GuineaCastro JM, Jiménez-Bárcenas R, Pascual-Izquierdo C. Recommendations on the management of patients with immune thrombocytopenia (ITP) in the context of SARS-CoV-2 infection and vaccination: consensus guidelines from a Spanish ITP expert group. Infect Dis Ther. 2023;12:303–315. doi: 10.1007/s40121-022-00745-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sridhara S, Nair R, Stanek M. Severe aplastic anemia after receiving SARS-CoV-2 Moderna mRNA vaccination. J Hematol. 2022;11:34. doi: 10.14740/jh954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Murdych TM. A case of severe autoimmune hemolytic anemia after a receipt of a first dose of SARS-CoV-2 vaccine. Int J Lab Hematol. 2022;44:e10. doi: 10.1111/ijlh.13653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Osmanodja B, Schreiber A, Schrezenmeier E, Seelow E. First diagnosis of thrombotic thrombocytopenic purpura after SARS-CoV-2 vaccine–case report. BMC Nephrol. 2021;22:1–5. doi: 10.1186/s12882-021-02616-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Okuno S, Hashimoto K, Shimizu R, Takagi E, Kajiguchi T. Development of autoimmune hemolytic anemia after BNT162b2 mRNA COVID-19 vaccination. Jpn J Clin Hematol. 2021;62:1510–1514. doi: 10.11406/rinketsu.62.1510. [DOI] [PubMed] [Google Scholar]
- 120.Waqar SHB, Khan AA, Memon S. Thrombotic thrombocytopenic purpura: a new menace after COVID bnt162b2 vaccine. Int J Hematol. 2021;114:626–629. doi: 10.1007/s12185-021-03190-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tabata S, Hosoi H, Murata S, Takeda S, Mushino T, Sonoki T. Severe aplastic anemia after COVID-19 mRNA vaccination: causality or coincidence? J Autoimmun. 2022;126:102782. doi: 10.1016/j.jaut.2021.102782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Julian JA, Mathern DR, Fernando D. Idiopathic thrombocytopenic purpura and the Moderna COVID-19 vaccine. Ann Emerg Med. 2021;77:654–656. doi: 10.1016/j.annemergmed.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liebman HA, Weitz IC. Autoimmune hemolytic anemia. Med Clin. 2017;101:351–359. doi: 10.1016/j.mcna.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 124.Hill A, Hill QA. Autoimmune hemolytic anemia. Hematology 2014, the American Society of Hematology Education Program Book. 2018:382-389. [DOI] [PMC free article] [PubMed]
- 125.Barcellini W, Zaninoni A, Giannotta JA, Fattizzo B. New insights in autoimmune hemolytic anemia: from pathogenesis to therapy. J Clin Med. 2020;9:3859. doi: 10.3390/jcm9123859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mahévas M, Moulis G, Andres E, Riviere E, Garzaro M, Crickx E, Guillotin V, Malphettes M, Galicier L, Noel N, Darnige L (2020) Clinical characteristics, management and outcome of COVID‐19‐associated immune thrombocytopenia: a French multicentre series. Br J Haematol 190:e224-9 [DOI] [PMC free article] [PubMed]
- 127.Mahévas M, Moulis G, Andres E, Riviere E, Garzaro M, Crickx E, Guillotin V, Malphettes M, Galicier L, Noel N. Clinical characteristics, management and outcome of COVID-19-associated immune thrombocytopenia: a French multicentre series. Br J Haematol. 2020;190:e224–e229. doi: 10.1111/bjh.17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chou S-C, Chang Y-C, Liao C-K, Chen T-C, Sun K-J, Huang W-H, Wu Y-F. New presentations and exacerbations of immune thrombocytopenia after coronavirus disease 2019 vaccinations: the Taiwan experience. Platelets. 2022;33:531–535. doi: 10.1080/09537104.2022.2042237. [DOI] [PubMed] [Google Scholar]
- 129.Mouliou DS, Dardiotis E. Current evidence in SARS-CoV-2 mRNA vaccines and post-vaccination adverse reports: knowns and unknowns. Diagnostics. 2022;12:1555. doi: 10.3390/diagnostics12071555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tian J, Yuan X, Xiao J, Zhong Q, Yang C, Liu B, Cai Y, Lu Z, Wang J, Wang Y. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21:893–903. doi: 10.1016/S1470-2045(20)30309-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pascutti MF, Erkelens MN, Nolte MA. Impact of viral infections on hematopoiesis: from beneficial to detrimental effects on bone marrow output. Front Immunol. 2016;7:364. doi: 10.3389/fimmu.2016.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Griggio V, Perutelli F, Salvetti C, Boccellato E, Boccadoro M, Vitale C, Coscia M. Immune dysfunctions and immune-based therapeutic interventions in chronic lymphocytic leukemia. Front Immunol. 2020;11:594556. doi: 10.3389/fimmu.2020.594556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Allegra A, Tonacci A, Musolino C, Pioggia G, Gangemi S. Secondary immunodeficiency in hematological malignancies: focus on multiple myeloma and chronic lymphocytic leukemia. Front Immunol. 2021;12:738915. doi: 10.3389/fimmu.2021.738915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Martínez JC, Sica RA, Stockerl-Goldstein K, Rubinstein SM. COVID-19 in patients with hematologic malignancies: outcomes and options for treatments. Acta Haematologica. 2022;145:244–256. doi: 10.1159/000522436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cutrona G, Tripodo C, Matis S, Recchia AG, Massucco C, Fabbi M, Colombo M, Emionite L, Sangaletti S, Gulino A, Reverberi D (2018) Microenvironmental regulation of the IL-23R/IL-23 axis overrides chronic lymphocytic leukemia indolence. Sci Transl Med 10:eaal1571 [DOI] [PubMed]
- 136.Ye X, Xiao X, Li B, Zhu W, Li Y, Wu J, Huang X, Jin J, Chen D, Jin J, Huang J (2020) Low humoral immune response and ineffective clearance of SARS-Cov-2 in a COVID-19 patient with CLL during a 69-day follow-up. Front Oncol 10:1272 [DOI] [PMC free article] [PubMed]
- 137.Largeaud L, Ribes A, Dubois-Galopin F, Memier V, Rolland Y, Gaudin C, Rousset D, Geeraerts T, Noel-Savina E, Rieu JB, Vergez F (2021) Major rise of a chronic lymphoid leukemia clone during the course of COVID-19. Int J Lab Hematol 43:e82–3 [DOI] [PubMed]
- 138.Allegra A, Pioggia G, Tonacci A, Musolino C, Gangemi S (2020) Cancer and SARS-CoV-2 infection: diagnostic and therapeutic challenges. Cancers 12:1581 [DOI] [PMC free article] [PubMed]
- 139.Li X, Shao M, Zeng X, Qian P, Huang H (2021) Signaling pathways in the regulation of cytokine release syndrome in human diseases and intervention therapy. Signal Transduct Target Ther 6:367 [DOI] [PMC free article] [PubMed]
- 140.Dettorre GM, Patel M, Gennari A, Pentheroudakis G, Romano E, Cortellini A, Pinato DJ (2021) The systemic pro-inflammatory response: targeting the dangerous liaison between COVID-19 and cancer. ESMO open 6:100123 [DOI] [PMC free article] [PubMed]
- 141.Ujjani CS, Gooley TA, Spurgeon SE, Stephens DM, Lai C, Broome CM, O'Brien SM, Zhu H, Laing KJ, Winter AM. Diminished humoral and cellular responses to SARS-CoV-2 Vaccines in patients with chronic lymphocytic leukemia. Blood Adv. 2022:bloodadvances. 2022009164. [DOI] [PMC free article] [PubMed]
- 142.Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- 143.Roeker LE, Knorr DA, Pessin MS, Ramanathan LV, Thompson MC, Leslie LA, Zelenetz AD, Mato AR. Anti-SARS-CoV-2 antibody response in patients with chronic lymphocytic leukemia. Leukemia. 2020;34:3047–3049. doi: 10.1038/s41375-020-01030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Heydarian M, Mohammadtaghizadeh M, Shojaei M, Babazadeh M, Abbasian S, Amrovani M. The effect of COVID-19 derived cytokine storm on cancer cells progression: double-edged sword. Mol Biol Rep. 2022;49:605–615. doi: 10.1007/s11033-021-06800-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gandomi-Mohammadabadi A, Divsalar F, Taram S, Kateb Z, Montazer F, Farbod Y. COVID-19 in a patient with newly diagnosed chronic lymphocytic leukemia (CLL): a case report. J Res Appl Basic Med Sci. 2021;7:26–30. doi: 10.52547/rabms.7.1.26. [DOI] [Google Scholar]
- 146.Bernar B, Kropshofer G, Crazzolara R, Kapelari K, Griesmacher A, Müller T, Scholl-Bürgi S. SARS-CoV-2 infection in a 7-year-old girl with pancytopenia during acute lymphocytic leukemia maintenance therapy. Pediatr Blood Cancer. 2020;67:e28391. doi: 10.1002/pbc.28391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Somasundaram R, Prasad MA, Ungerbäck J, Sigvardsson M. Transcription factor networks in B-cell differentiation link development to acute lymphoid leukemia. Blood J Am Soc Hematol. 2015;126:144–152. doi: 10.1182/blood-2014-12-575688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sanda T, Leong WZ. TAL1 as a master oncogenic transcription factor in T-cell acute lymphoblastic leukemia. Exp Hematol. 2017;53:7–15. doi: 10.1016/j.exphem.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 149.Montaño A, Forero-Castro M, Marchena-Mendoza D, Benito R, Hernández-Rivas JM. New challenges in targeting signaling pathways in acute lymphoblastic leukemia by NGS approaches: an update. Cancers. 2018;10:110. doi: 10.3390/cancers10040110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Baruchel A, Bertrand Y, Boissel N, Brethon B, Ducassou S, Gandemer V, Halfon-Domenech C, Leblanc T, Leverger G, Michel G. COVID-19 and acute lymphoblastic leukemias of children and adolescents: First recommendations of the Leukemia committee of the French Society for the fight against cancers and leukemias in children and adolescents (SFCE) Bulletin Du Cancer. 2020;107:629–632. doi: 10.1016/j.bulcan.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Huang FL, Liao EC, Li CL, Yen CY, Yu SJ. Pathogenesis of pediatric B-cell acute lymphoblastic leukemia: molecular pathways and disease treatments. Oncol Lett. 2020;20:448–454. doi: 10.3892/ol.2020.11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yan W, Song L, Wang H, Yang W, Hu L, Yang Y. Extracellular vesicles carrying miRNA-181b-5p affects the malignant progression of acute lymphoblastic leukemia. J Transl Med. 2021;19:1–9. doi: 10.1186/s12967-021-03174-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Iravani Saadi M, Ramzi M, Hesami Z, Kheradmand N, Owjfard M, Nabi Abdolyousefi E, Karimi Z. MiR-181a and-b expression in acute lymphoblastic leukemia and its correlation with acute graft-versus-host disease after hematopoietic stem cell transplantation, COVID-19 and torque teno viruses. Virusdisease. 2021;32:727–736. doi: 10.1007/s13337-021-00743-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Baghbani E, Khaze V, Sadreddini S, Mokhtarzadeh A, Mansoori B, Mohammadi A, Vatankhahan V, Toosi P, Baradaran B. PTPN22 silencing in human acute T-cell leukemia cell line (Jurkat cell) and its effect on the expression of miR-181a and miR-181b. Adv Pharm Bull. 2018;8:277. doi: 10.15171/apb.2018.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Arisan ED, Dart A, Grant GH, Arisan S, Cuhadaroglu S, Lange S, Uysal-Onganer P. The prediction of miRNAs in SARS-CoV-2 genomes: hsa-miR databases identify 7 key miRs linked to host responses and virus pathogenicity-related KEGG pathways significant for comorbidities. Viruses. 2020;12:614. doi: 10.3390/v12060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Amini-Farsani Z, Yadollahi-Farsani M, Arab S, Forouzanfar F, Yadollahi M, Asgharzade S. Prediction and analysis of microRNAs involved in COVID-19 inflammatory processes associated with the NF-kB and JAK/STAT signaling pathways. Int immunopharmacol. 2021;100:108071. doi: 10.1016/j.intimp.2021.108071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Badreldein M, Elsorady M, Alhadidi A, Gallab O, Eldabah N. ALL-443: diagnostic and prognostic significance of miRNA-511 and miRNA-16 expression in adult B-Acute lymphoblastic leukemia patients. Clin Lymphoma Myeloma Leuk. 2021;21:S277. doi: 10.1016/S2152-2650(21)01667-0. [DOI] [Google Scholar]
- 158.Li C-X, Chen J, Lv S-K, Li J-H, Li L-L, Hu X. Whole-transcriptome RNA sequencing reveals significant differentially expressed mRNAs, miRNAs, and lncRNAs and related regulating biological pathways in the peripheral blood of COVID-19 patients. Mediators Inflamm. 2021;6635925:22. doi: 10.1155/2021/6635925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Witten L, Slack FJ. miR-155 as a novel clinical target for hematological malignancies. Carcinogenesis. 2020;41:2–7. doi: 10.1093/carcin/bgz183. [DOI] [PubMed] [Google Scholar]
- 160.Haroun RA-H, Osman WH, Amin RE, Hassan AK, Abo-Shanab WS, Eessa AM. Circulating plasma miR-155 is a potential biomarker for the detection of SARS-CoV-2 infection. Pathology. 2022;54:104–110. doi: 10.1016/j.pathol.2021.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Di Martino MT, Leone E, Amodio N, Foresta U, Lionetti M, Pitari MR, Cantafio MEG, Gullà A, Conforti F, Morelli E. Synthetic miR-34a Mimics as a Novel Therapeutic Agent for Multiple Myeloma: In Vitro and In Vivo EvidenceAntitumor Activity of miR-34a in Multiple Myeloma. Clin Cancer Res. 2012;18:6260–6270. doi: 10.1158/1078-0432.CCR-12-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sagulkoo P, Chuntakaruk H, Rungrotmongkol T, Suratanee A, Plaimas K. Multi-level biological network analysis and drug repurposing based on leukocyte transcriptomics in severe COVID-19: in silico systems biology to precision medicine. J Personal Med. 2022;12:1030. doi: 10.3390/jpm12071030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liu A-F, Wang J-X, Li F-L, Chen Y-J. Research progress on miR-125 family in malignant hematologic diseases-review. J Exp Hematol. 2017;25:1842–1846. doi: 10.7534/j.issn.1009-2137.2017.06.049. [DOI] [PubMed] [Google Scholar]
- 164.Zhang S, Amahong K, Sun X, Lian X, Liu J, Sun H, Lou Y, Zhu F, Qiu Y. The miRNA: a small but powerful RNA for COVID-19. Brief Bioinform. 2021;22:1137–1149. doi: 10.1093/bib/bbab062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Haneklaus M, Gerlic M, O'Neill LA, Masters S. miR-223: infection, inflammation and cancer. J Intern Med. 2013;274:215–226. doi: 10.1111/joim.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Leoncini PP, Bertaina A, Papaioannou D, Flotho C, Masetti R, Bresolin S, Menna G, Santoro N, Zecca M, Basso G. MicroRNA-150 regulates STAT5b levels in juvenile myelomonocytic leukemia (JMML) Blood. 2015;126:2851. doi: 10.1182/blood.V126.23.2851.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Akula SM, Bolin P, Cook PP. Cellular miR-150-5p may have a crucial role to play in the biology of SARS-CoV-2 infection by regulating nsp10 gene. RNA Biol. 2022;19:1–11. doi: 10.1080/15476286.2021.2010959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lee JH, Choi YS, Park JH, Kim H, Lee I, Won YB, Yun BH, Park JH, Seo SK, Lee BS. miR-150-5p may contribute to pathogenesis of human leiomyoma via regulation of the Akt/p27Kip1 pathway in vitro. Int J Mol Sci. 2019;20:2684. doi: 10.3390/ijms20112684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Derda AA, Garg A, Bär C, Thum T. Reply to ‘COVID-19 severity, miR-21 targets, and common human genetic variation’. Eur J Heart Fail. 2021;23:1987. doi: 10.1002/ejhf.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ma X, Choudhury SN, Hua X, Dai Z, Li Y. Interaction of the oncogenic miR-21 microRNA and the p53 tumor suppressor pathway. Carcinogenesis. 2013;34:1216–1223. doi: 10.1093/carcin/bgt044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Leone E, Morelli E, Di Martino MT, Amodio N, Foresta U, Gullà A, Rossi M, Neri A, Giordano A, Munshi NC. Targeting miR-21 inhibits in vitro and in vivo multiple myeloma cell growthantitumor activity of mir-21 inhibitors in multiple myeloma. Clin Cancer Res. 2013;19:2096–2106. doi: 10.1158/1078-0432.CCR-12-3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Askari N, Hadizadeh M, Rashidifar M. A new insight into sex-specific non-coding RNAs and networks in response to SARS-CoV-2. Infect Genet Evol. 2022;97:105195. doi: 10.1016/j.meegid.2021.105195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sevcikova S, Kubiczkova L, Sedlarikova L, Slaby O, Hajek R. Serum miR-29a as a marker of multiple myeloma. Leuk Lymphoma. 2013;54:189–191. doi: 10.3109/10428194.2012.704030. [DOI] [PubMed] [Google Scholar]
- 174.Fiserovaa B, Kubiczkova L, Sedlarikova L, Hajek R, Sevcikova S. The miR-29 family in hematological malignancies. Biomed Pap Med Fac Palacky Univ Olomouc. 2015;159:184–191. doi: 10.5507/bp.2014.037. [DOI] [PubMed] [Google Scholar]
- 175.Hershkovitz Rokah O, Granot G, Ovcharenko A, Modai S, Pasmanik-Chor M, Toren A, Shomron N, Shpilberg O. Downregulation of miR-31, miR-155, and miR-564 in chronic myeloid leukemia cells. PloS one. 2012;7:e35501. doi: 10.1371/journal.pone.0035501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Farr RJ, Rootes CL, Rowntree LC, Nguyen TH, Hensen L, Kedzierski L, Cheng AC, Kedzierska K, Au GG, Marsh GA. Altered microRNA expression in COVID-19 patients enables identification of SARS-CoV-2 infection. PLoS Pathogens. 2021;17:e1009759. doi: 10.1371/journal.ppat.1009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhou T, Medeiros LJ, Hu S. Chronic myeloid leukemia: beyond BCR-ABL1. Curr Hematol Malig Rep. 2018;13:435–445. doi: 10.1007/s11899-018-0474-6. [DOI] [PubMed] [Google Scholar]
- 178.Delgado N, Torres A. What do we currently know about chronic myeloid leukemia (CML) and COVID-19? Curr Oncol Rep. 2022;24:645–650. doi: 10.1007/s11912-021-01169-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Eşkazan AE. Chronic myeloid leukaemia and the use of tyrosine kinase inhibitors in the days of COVID-19 pandemic. Br J Clin Pharmacol. 2020;86:1790. doi: 10.1111/bcp.14353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.García-Gutiérrez V, Hernández-Boluda JC. Tyrosine kinase inhibitors available for chronic myeloid leukemia: efficacy and safety. Front Oncol. 2019;9:603. doi: 10.3389/fonc.2019.00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Alves R, McArdle SE, Vadakekolathu J, Gonçalves AC, Freitas-Tavares P, Pereira A, Almeida AM, Sarmento-Ribeiro AB, Rutella S. Flow cytometry and targeted immune transcriptomics identify distinct profiles in patients with chronic myeloid leukemia receiving tyrosine kinase inhibitors with or without interferon-α. J Transl Med. 2020;18:1–15. doi: 10.1186/s12967-019-02194-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Galimberti S, Petrini M, Baratè C, Ricci F, Balducci S, Grassi S, Guerrini F, Ciabatti E, Mechelli S, Di Paolo A. Tyrosine kinase inhibitors play an antiviral action in patients affected by chronic myeloid leukemia: a possible model supporting their use in the fight against SARS-CoV-2. Front Oncol. 2020;10:1428. doi: 10.3389/fonc.2020.01428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pagnano KB, Peralta EH, Navarro JR, David Salas LDR, Delgado N, Moiraghi B, Toreli ACM, Perobelli LM, Fechio L, Quixada AT. COVID-19 in chronic myeloid leukemia patients in Latin America. Leuk Lymphoma. 2021;62:3212–3218. doi: 10.1080/10428194.2021.1950709. [DOI] [PubMed] [Google Scholar]
- 184.Chiarini F, Lonetti A, Evangelisti C, Buontempo F, Orsini E, Evangelisti C, Cappellini A, Neri LM, McCubrey JA, Martelli AM. Advances in understanding the acute lymphoblastic leukemia bone marrow microenvironment: from biology to therapeutic targeting. Biochim Biophys Acta. 2016;1863:449–463. doi: 10.1016/j.bbamcr.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 185.Grimwade D, Ivey A, Huntly BJ (2016) Molecular landscape of acute myeloid leukemia in younger adults and its clinical relevance. Blood 127:29-41 [DOI] [PMC free article] [PubMed]
- 186.Ferrara F, Zappasodi P, Roncoroni E, Borlenghi E, Rossi G (2020) Impact of COVID-19 on the treatment of acute myeloid leukemia. Leukemia 34:2254-6 [DOI] [PMC free article] [PubMed]
- 187.Zalpoor H, Bakhtiyari M, Akbari A, Aziziyan F, Shapourian H, Liaghat M, Zare-Badie Z, Yahyazadeh S, Tarhriz V, Ganjalikhani-Hakemi M (2022) Potential role of autophagy induced by FLT3-ITD and acid ceramidase in acute myeloid leukemia chemo-resistance: new insights. Cell Commun Signal 20:172 [DOI] [PMC free article] [PubMed]
- 188.Deeb G, Vaughan MM, McInnis I, Ford LA, Sait SN, Starostik P, Wetzler M, Mashtare T, Wang ES. Hypoxia-inducible factor-1α protein expression is associated with poor survival in normal karyotype adult acute myeloid leukemia. Leuk Res. 2011;35:579–584. doi: 10.1016/j.leukres.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 189.Sargazi S, Sheervalilou R, Rokni M, Shirvaliloo M, Shahraki O, Rezaei N. The role of autophagy in controlling SARS-CoV-2 infection: An overview on virophagy-mediated molecular drug targets. Cell Biol Int. 2021;45:1599–1612. doi: 10.1002/cbin.11609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bartlett DL, Howe JR, Chang G, Crago A, Hogg M, Karakousis G, Levine E, Maker A, Mamounas E, McGuire K. Management of cancer surgery cases during the COVID-19 pandemic: considerations. Ann Surg Oncol. 2020;27:1717–1720. doi: 10.1245/s10434-020-08461-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zalpoor H, Akbari A, Nayerain Jazi N, Liaghat M, Bakhtiyari M. Possible role of autophagy induced by COVID-19 in cancer progression, chemo-resistance, and tumor recurrence. Infect Agents Cancer. 2022;17:38. doi: 10.1186/s13027-022-00450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Klimienė I, Radzevičius M, Matuzevičienė R, Sinkevič-Belliot K, Kučinskienė ZA, Pečeliūnas V. Adhesion molecule immunophenotype of bone marrow multiple myeloma plasma cells impacts the presence of malignant circulating plasma cells in peripheral blood. Int J Lab Hematol. 2021;43:403–408. doi: 10.1111/ijlh.13387. [DOI] [PubMed] [Google Scholar]
- 193.Dhodapkar MV. The immune system in multiple myeloma and precursor states: lessons and implications for immunotherapy and interception. Am J Hematol. 2023;98:S4–S12. doi: 10.1002/ajh.26752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Akhlaghi T, Maclachlan K, Korde N, Mailankody S, Lesokhin AM, Hassoun H, Lu SX, Patel D, Shah UA, Tan C. African American patients with smoldering multiple myeloma may have a lower risk of progression compared to White patients. Am Soc Clin Oncol. 2022;40:8045–8045. doi: 10.1200/JCO.2022.40.16_suppl.8045. [DOI] [Google Scholar]
- 195.Yarmohammadi H, Cunningham-Rundles C (2017) Idiopathic CD4 lymphocytopenia: Pathogenesis, etiologies, clinical presentations and treatment strategies. Ann Allergy Asthma Immunol 119:374-8 [DOI] [PMC free article] [PubMed]
- 196.Sereno M, Gutiérrez-Gutiérrez G, Sandoval C, Falagan S, Jimenez-Gordo AM, Merino M, López-Menchaca R, Martínez-Martin P, Roa S, Casado E. A favorable outcome of pneumonia COVID 19 in an advanced lung cancer patient with severe neutropenia: is immunosuppression a risk factor for SARS-COV2 infection? Lung Cancer. 2020;145:213–215. doi: 10.1016/j.lungcan.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wang B, Van Oekelen O, Mouhieddine TH, Del Valle DM, Richter J, Cho HJ, Richard S, Chari A, Gnjatic S, Merad M. A tertiary center experience of multiple myeloma patients with COVID-19: lessons learned and the path forward. J Hematol Oncol. 2020;13:94. doi: 10.1186/s13045-020-00934-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ribas A, Dhodapkar MV, Campbell KM, Davies FE, Gore SD, Levy R, Greenberger LM. How to provide the needed protection from COVID-19 to patients with hematologic malignancies. Blood Cancer Discov. 2021;2:562–567. doi: 10.1158/2643-3230.BCD-21-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hasselbalch HC, Elvers M, Schafer AI. The pathobiology of thrombosis, microvascular disease, and hemorrhage in the myeloproliferative neoplasms. Blood. 2021;137:2152–2160. doi: 10.1182/blood.2020008109. [DOI] [PubMed] [Google Scholar]
- 200.Landgren O, Goldin LR, Kristinsson SY, Helgadottir EA, Samuelsson J, Björkholm M. Increased risks of polycythemia vera, essential thrombocythemia, and myelofibrosis among 24 577 first-degree relatives of 11 039 patients with myeloproliferative neoplasms in Sweden. Blood. 2008;112:2199–2204. doi: 10.1182/blood-2008-03-143602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Veninga A, De Simone I, Heemskerk JW, Ten Cate H, van der Meijden PE. Clonal hematopoietic mutations linked to platelet traits and the risk of thrombosis or bleeding. Haematologica. 2020;105:2020–2031. doi: 10.3324/haematol.2019.235994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ejaz H, Alsrhani A, Zafar A, Javed H, Junaid K, Abdalla AE, Abosalif KO, Ahmed Z, Younas S. COVID-19 and comorbidities: deleterious impact on infected patients. J Infect Public Health. 2020;13:1833–1839. doi: 10.1016/j.jiph.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Wolach O, Shacham Abulafia A (2021) Can Novel Insights into the Pathogenesis of Myeloproliferative Neoplasm-Related Thrombosis Inform Novel Treatment Approaches?. Hemato 2:305-28.
- 204.Murray PG, Young LS. An etiological role for the Epstein-Barr virus in the pathogenesis of classical Hodgkin lymphoma. Blood. 2019;134:591–596. doi: 10.1182/blood.2019000568. [DOI] [PubMed] [Google Scholar]
- 205.Pasin F, Calveri MM, Pizzarelli G, Calabrese A, Andreoli M, Bongiovanni I, Cattaneo C, Rignanese G (2020) Oncolytic effect of SARS-CoV2 in a patient with NK lymphoma. Acta Biomed 91:e2020047 [DOI] [PMC free article] [PubMed]
- 206.Yousif NG, Oton A, Chetterje K, Gupta M, Shankar M (2022) Complete remission of Hodgkin Lymphoma after a concurrent infection with COVID-19: systematic review and meta-analysis. Muthanna Med J 6:57-67
- 207.Osman M, Faridi RM, Sligl W, Shabani-Rad MT, Dharmani-Khan P, Parker A, Kalra A, Tripathi MB, Storek J, Cohen Tervaert JW, Khan FM (2020) Impaired natural killer cell counts and cytolytic activity in patients with severe COVID-19. Blood Adv 4:5035-9 [DOI] [PMC free article] [PubMed]
- 208.Huang I, Pranata R (2020) Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intensive Care 8:1-0 [DOI] [PMC free article] [PubMed]
- 209.van Eeden C, Khan L, Osman MS, Cohen Tervaert JW. Natural killer cell dysfunction and its role in COVID-19. Int J Mol Sci. 2020;21:6351. doi: 10.3390/ijms21176351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sparano JA, Lee JY, Kaplan LD, Levine AM, Ramos JC, Ambinder RF, Wachsman W, Aboulafia D, Noy A, Henry DH. Rituximab plus concurrent infusional EPOCH chemotherapy is highly effective in HIV-associated B-cell non-Hodgkin lymphoma. Blood. 2010;115:3008–3016. doi: 10.1182/blood-2009-08-231613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Perry C, Luttwak E, Balaban R, Shefer G, Morales MM, Aharon A, Tabib Y, Cohen YC, Benyamini N, Beyar-Katz O, Neaman M (2021) Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with B-cell non-Hodgkin lymphoma. Blood advances 5:3053–61 [DOI] [PMC free article] [PubMed]
- 212.Terpos E, Gavriatopoulou M, Fotiou D, Giatra C, Asimakopoulos I, Dimou M, Sklirou AD, Ntanasis-Stathopoulos I, Darmani I, Briasoulis A, Kastritis E (2021) Poor neutralizing antibody responses in 132 patients with CLL, NHL and HL after vaccination against SARS-CoV-2: a prospective study. Cancers 13:4480 [DOI] [PMC free article] [PubMed]
- 213.Riise J, Meyer S, Blaas I, Chopra A, Tran TT, Delic-Sarac M, Hestdalen ML, Brodin E, Rustad EH, Dai KZ. Rituximab-treated patients with lymphoma develop strong CD8 T-cell responses following COVID-19 vaccination. Br J Haematol. 2022;197:697–708. doi: 10.1111/bjh.18149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Passamonti F, Cattaneo C, Arcaini L, Bruna R, Cavo M, Merli F, Angelucci E, Krampera M, Cairoli R, Della Porta MG. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020;7:e737–e745. doi: 10.1016/S2352-3026(20)30251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Yigenoglu TN, Ata N, Altuntas F, Bascı S, Dal MS, Korkmaz S, Namdaroglu S, Basturk A, Hacıbekiroglu T, Dogu MH. The outcome of COVID-19 in patients with hematological malignancy. J Med Virol. 2021;93:1099–1104. doi: 10.1002/jmv.26404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Yang K, Sheng Y, Huang C, Jin Y, Xiong N, Jiang K, Lu H, Liu J, Yang J, Dong Y. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol. 2020;21:904–913. doi: 10.1016/S1470-2045(20)30310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Sanchez-Pina JM, Rodríguez Rodriguez M, Castro Quismondo N, Gil Manso R, Colmenares R, Gil Alos D, Paciello ML, Zafra D, Garcia-Sanchez C, Villegas C. Clinical course and risk factors for mortality from COVID-19 in patients with haematological malignancies. Eur J Haematol. 2020;105:597–607. doi: 10.1111/ejh.13493. [DOI] [PubMed] [Google Scholar]
- 218.Bergamaschi G, de BorrelliAndreis F, Aronico N, Lenti MV, Barteselli C, Merli S, Pellegrino I, Coppola L, Cremonte EM, Croce G. Anemia in patients with Covid-19: pathogenesis and clinical significance. Clin Exp Med. 2021;21:239–246. doi: 10.1007/s10238-020-00679-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Tao Z, Xu J, Chen W, Yang Z, Xu X, Liu L, Chen R, Xie J, Liu M, Wu J. Anemia is associated with severe illness in COVID-19: a retrospective cohort study. J Med Virol. 2021;93:1478–1488. doi: 10.1002/jmv.26444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Candoni A, Petruzzellis G, Sperotto A, Andreotti V, Giavarra M, Corvaja C, Minisini A, Comuzzi C, Tascini C, Fanin R. Detection of SARS-CoV-2 infection prevalence in 860 cancer patients with a combined screening procedure including triage, molecular nasopharyngeal swabs and rapid serological test. A report from the first epidemic wave. PloS one. 2022;17:e0262784. doi: 10.1371/journal.pone.0262784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Perry C, Luttwak E, Balaban R, Shefer G, Morales MM, Aharon A, Tabib Y, Cohen YC, Benyamini N, Beyar-Katz O, Neaman M (2021) Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with B-cell non-Hodgkin lymphoma. Blood Adv 5:3053-61 [DOI] [PMC free article] [PubMed]
- 222.Langerbeins P, Hallek M (2022) COVID-19 in patients with hematologic malignancy. Blood 140:236-52 [DOI] [PMC free article] [PubMed]
- 223.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Racine É, Gilca V, Amini R, Tunis M, Ismail S, Sauvageau C (2020) A systematic literature review of the recombinant subunit herpes zoster vaccine use in immunocompromised 18–49 year old patients. Vaccine 38:6205-14 [DOI] [PubMed]
- 225.Dooling KL, Guo A, Patel M, Lee GM, Moore K, Belongia EA, Harpaz R. Recommendations of the advisory committee on immunization practices for use of herpes zoster vaccines. Am J Transpl. 2018;18:756–762. doi: 10.1111/ajt.14683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Dagnew AF, Ilhan O, Lee WS, Woszczyk D, Kwak JY, Bowcock S, Sohn SK, Macías GR, Chiou TJ, Quiel D, Aoun M (2019) Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis. Lancet Infect Dis 19:988-1000 [DOI] [PubMed]
- 227.Natarajan K, Prasad N, Dascomb K, Irving SA, Yang DH, Gaglani M, Klein NP, DeSilva MB, Ong TC, Grannis SJ, Stenehjem E (2022) Effectiveness of homologous and heterologous COVID-19 booster doses following 1 Ad. 26. COV2. S (Janssen [Johnson & Johnson]) vaccine dose against COVID-19–associated emergency department and urgent care encounters and hospitalizations among adults—VISION Network, 10 states, December 2021–March 2022. Morbidity and Mortality Weekly Report. 71:495 [DOI] [PMC free article] [PubMed]
- 228.Livingston EH, Malani PN, Creech CB. The Johnson & Johnson vaccine for COVID-19. Jama. 2021;325:1575–1575. doi: 10.1001/jama.2021.2927. [DOI] [PubMed] [Google Scholar]
- 229.Rossignol J, Michallet A, Oberic L, Picard M, Garon A, Willekens C, Dulery R, Leleu X, Cazin B, Ysebaert L. Rituximab–cyclophosphamide–dexamethasone combination in the management of autoimmune cytopenias associated with chronic lymphocytic leukemia. Leukemia. 2011;25:473–478. doi: 10.1038/leu.2010.278. [DOI] [PubMed] [Google Scholar]
- 230.Scheinberg M, Machado LA, Castro LGM, Ferreira SB, Michalany N. Successful treatment of ulcerated pyoderma gangrenosum with baricitinib, a novel JAK inhibitor. J Transl Autoimmun. 2021;4:100099. doi: 10.1016/j.jtauto.2021.100099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Campbell R, Chong G, Hawkes EA. Novel indications for Bruton’s tyrosine kinase inhibitors, beyond hematological malignancies. J Clin Med. 2018;7(4):62. doi: 10.3390/jcm7040062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.McGann PT, Ware RE. Hydroxyurea therapy for sickle cell anemia. Exp Opin Drug Saf. 2015;14:1749–1758. doi: 10.1517/14740338.2015.1088827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zhang R, Mylonakis E. In inpatients with COVID-19, none of remdesivir, hydroxychloroquine, lopinavir, or interferon β-1a differed from standard care for in-hospital mortality. Ann Intern Med. 2021;174:JC17. doi: 10.7326/ACPJ202102160-017. [DOI] [PubMed] [Google Scholar]
- 234.Hendriks RW, Yuvaraj S, Kil LP. Targeting Bruton's tyrosine kinase in B cell malignancies. Nat Rev Cancer. 2014;14:219–232. doi: 10.1038/nrc3702. [DOI] [PubMed] [Google Scholar]
- 235.Pardanani A, Tefferi A. Definition and management of ruxolitinib treatment failure in myelofibrosis. Blood Cancer J. 2014;4:e268–e268. doi: 10.1038/bcj.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Juárez-Salcedo LM, Desai V, Dalia S. Venetoclax: evidence to date and clinical potential. Drugs Context. 2019;8:212574. doi: 10.7573/dic.212574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ng CM, Bruno R, Combs D, Davies B. Population pharmacokinetics of rituximab (anti-CD20 monoclonal antibody) in rheumatoid arthritis patients during a phase II clinical trial. J Clin Pharmacol. 2005;45:792–801. doi: 10.1177/0091270005277075. [DOI] [PubMed] [Google Scholar]
- 238.Owen C, Robinson S, Christofides A, Sehn LH. A Canadian perspective: Monoclonal antibodies for pre-and post-exposure protection from COVID-19 in vulnerable patients with hematological malignancies. Curr Oncol. 2022;29:3940–3949. doi: 10.3390/curroncol29060315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Cicchitto G, Cardillo L, de Martinis C, Sabatini P, Marchitiello R, Abate G, Rovetti A, Cavallera A, Apuzzo C, Ferrigno F. Effects of casirivimab/imdevimab monoclonal antibody treatment among vaccinated patients infected by SARS-CoV-2 delta variant. Viruses. 2022;14:650. doi: 10.3390/v14030650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Xu C, Rafique A, Potocky T, Paccaly A, Nolain P, Lu Q, Iglesias-Rodriguez M, St John G, Nivens MC, Kanamaluru V. Differential binding of Sarilumab and Tocilizumab to IL-6Rα and effects of receptor occupancy on clinical parameters. J Clin Pharmacol. 2021;61:714–724. doi: 10.1002/jcph.1795. [DOI] [PubMed] [Google Scholar]
- 241.Mouliou DS, Dardiotis E. Temelimab for MS and SARS-CoV-2: could it be a double-edged blessing? Mult Scler Relat Disord. 2022;64:103938. doi: 10.1016/j.msard.2022.103938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Stricker E, Peckham-Gregory EC, Scheurer ME. HERVs and cancer—A comprehensive review of the relationship of human endogenous retroviruses and human cancers. Biomedicines. 2023;11:936. doi: 10.3390/biomedicines11030936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Mikhael J, Belhadj-Merzoug K, Hulin C, Vincent L, Moreau P, Gasparetto C, Pour L, Spicka I, Vij R, Zonder J. A phase 2 study of isatuximab monotherapy in patients with multiple myeloma who are refractory to daratumumab. Blood cancer journal. 2021;11:89. doi: 10.1038/s41408-021-00478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Scheinberg P, Nunez O, Weinstein B, Scheinberg P, Biancotto A, Wu CO, Young NS. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Engl J Med. 2011;365:430–438. doi: 10.1056/NEJMoa1103975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Passweg JR, Baldomero H, Basak GW, Chabannon C, Corbacioglu S, Duarte R, Kuball J, Lankester A, Montoto S, de Latour RP. The EBMT activity survey report 2017: a focus on allogeneic HCT for nonmalignant indications and on the use of non-HCT cell therapies. Bone Marrow Transpl. 2019;54:1575–1585. doi: 10.1038/s41409-019-0465-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Shankar R, Radhakrishnan N, Dua S, Arora S, Rana M, Sahu DK, Rai S, Gupta DK. Convalescent plasma to aid in recovery of COVID-19 pneumonia in a child with acute lymphoblastic leukemia. Transfus Apher Sci. 2021;60:102956. doi: 10.1016/j.transci.2020.102956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Hashino K, Ishii M, Iemura M, Akagi T, Kato H. Re-treatment for immune globulin-resistant Kawasaki disease: a comparative study of additional immune globulin and steroid pulse therapy. Pediatr Int. 2001;43:211–217. doi: 10.1046/j.1442-200x.2001.01373.x. [DOI] [PubMed] [Google Scholar]
- 248.Swanson PA, Padilla M, Hoyland W, McGlinchey K, Fields PA, Bibi S, Faust SN, McDermott AB, Lambe T, Pollard AJ. AZD1222/ChAdOx1 nCoV-19 vaccination induces a polyfunctional spike protein–specific TH1 response with a diverse TCR repertoire. Sci Transl Med. 2021;13:eabj7211. doi: 10.1126/scitranslmed.abj7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Ewer KJ, Barrett JR, Belij-Rammerstorfer S, Sharpe H, Makinson R, Morter R, Flaxman A, Wright D, Bellamy D, Bittaye M. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial. Nat Med. 2021;27:270–278. doi: 10.1038/s41591-020-01194-5. [DOI] [PubMed] [Google Scholar]
- 250.Welniak LA, Blazar BR, Murphy WJ (2007) Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol 25:139-70 [DOI] [PubMed]
- 251.Stuver R, Shah GL, Korde NS, Roeker LE, Mato AR, Batlevi CL, Chung DJ, Doddi S, Falchi L, Gyurkocza B. Activity of AZD7442 (tixagevimab-cilgavimab) against Omicron SARS-CoV-2 in patients with hematologic malignancies. Cancer Cell. 2022;40:590–591. doi: 10.1016/j.ccell.2022.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Chang A, Koff JL, Lai L, Orellana-Noia VM, Surati M, Leal AM, Ellis ML, Wali B, Moreno A, Linderman SL. Low neutralizing activity of AZD7442 against current SARS-CoV-2 Omicron variants in patients with B cell malignancies. Blood Adv. 2023;7:2459–2462. doi: 10.1182/bloodadvances.2022009475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Otiniano A, van de Wyngaert Z, Brissot E, Dulery R, Gozlan J, Daguenel A, Abi Aad Y, Ricard L, Stocker N, Banet A. Tixagevimab/cilgavimab for Omicron SARS-CoV-2 infection in patients with haematologic diseases. Bone Marrow Transpl. 2023;58:340–342. doi: 10.1038/s41409-022-01894-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Scarfò L, Herishanu Y. CLL and COVID-19: light at the end of the tunnel? Blood. 2022;140:407–409. doi: 10.1182/blood.2022017071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Djebbari F, Rampotas A, Vallance G, Panitsas F, Basker N, Sangha G, Salhan B, Karim F, Firas A-K, Gudger A. Infections in relapsed myeloma patients treated with isatuximab plus pomalidomide and dexamethasone during the COVID-19 pandemic: Initial results of a UK-wide real-world study. Hematology. 2022;27:691–699. doi: 10.1080/16078454.2022.2082725. [DOI] [PubMed] [Google Scholar]
- 256.Group RC Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Martinez MA. Plitidepsin: a repurposed drug for the treatment of COVID-19. Antimicrob Agents Chemother. 2021;65:e00200–00221. doi: 10.1128/AAC.00200-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.He W, Gao Y, Zhou J, Shi Y, Xia D, Shen H-M. Friend or Foe? Implication of the autophagy-lysosome pathway in SARS-CoV-2 infection and COVID-19. Int J Biol Sci. 2022;18:4690. doi: 10.7150/ijbs.72544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Papapanou M, Papoutsi E, Giannakas T, Katsaounou P. Plitidepsin: Mechanisms and clinical profile of a promising antiviral agent against COVID-19. J Personal Med. 2021;11:668. doi: 10.3390/jpm11070668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Dell'Isola GB, Felicioni M, Ferraro L, Capolsini I, Cerri C, Gurdo G, Mastrodicasa E, Massei MS, Perruccio K, Brogna M. Case report: remdesivir and convalescent plasma in a newly acute B lymphoblastic leukemia diagnosis with concomitant sars-CoV-2 infection. Front Pediatr. 2021;9:712603. doi: 10.3389/fped.2021.712603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Guo W, Zheng Y, Feng S (2023) Omicron related COVID-19 prevention and treatment measures for patients with hematological malignancy and strategies for modifying hematologic treatment regimes. Frontiers in Cellular and Infection Microbiology 13: 1207225 [DOI] [PMC free article] [PubMed]
- 262.Pommeret F, Colomba J, Bigenwald C, Laparra A, Bockel S, Bayle A, Michot J-M, Hueso T, Albiges L, Tiberghien P. Bamlanivimab+ etesevimab therapy induces SARS-CoV-2 immune escape mutations and secondary clinical deterioration in COVID-19 patients with B-cell malignancies. Ann Oncol. 2021;32:1445–1447. doi: 10.1016/j.annonc.2021.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Mao B, Le-Trilling VTK, Wang K, Mennerich D, Hu J, Zhao Z, Zheng J, Deng Y, Katschinski B, Xu S. Obatoclax inhibits SARS-CoV-2 entry by altered endosomal acidification and impaired cathepsin and furin activity in vitro. Emerg Microbes Infect. 2022;11:483–497. doi: 10.1080/22221751.2022.2026739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Chan M, Holland EC, Gujral TS. Olverembatinib inhibits SARS-CoV-2-Omicron variant-mediated cytokine release in human peripheral blood mononuclear cells. EMBO Mol Med. 2022;14:e15919. doi: 10.15252/emmm.202215919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Gurung AB, Ali MA, Elshikh MS, Aref I, Amina M, Lee J. An in silico approach unveils the potential of antiviral compounds in preclinical and clinical trials as SARS-CoV-2 omicron inhibitors. Saudi J Biol Sci. 2022;29:103297. doi: 10.1016/j.sjbs.2022.103297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Lin X, Ke X, Jian X, Xia L, Yang Y, Zhang T, Xiong H, Zhao B, Liu W, Chen Q. Azacytidine targeting SARS-CoV-2 viral RNA as a potential treatment for COVID-19. Sci Bullet. 2022;67:1022. doi: 10.1016/j.scib.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Carter-Timofte ME, Arulanandam R, Kurmasheva N, Fu K, Laroche G, Taha Z, van Der Horst D, Cassin L, van der Sluis RM, Palermo E. Antiviral potential of the antimicrobial drug atovaquone against SARS-CoV-2 and emerging variants of concern. ACS Infect Dis. 2021;7:3034–3051. doi: 10.1021/acsinfecdis.1c00278. [DOI] [PubMed] [Google Scholar]
- 268.Parry H, McIlroy G, Bruton R, Damery S, Tyson G, Logan N, Davis C, Willett B, Zuo J, Ali M. Impaired neutralisation of SARS-CoV-2 delta variant in vaccinated patients with B cell chronic lymphocytic leukaemia. J Hematol Oncol. 2022;15:1–12. doi: 10.1186/s13045-021-01219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Brullo C, Villa C, Tasso B, Russo E, Spallarossa A. Btk inhibitors: a medicinal chemistry and drug delivery perspective. Int J Mol Sci. 2021;22:7641. doi: 10.3390/ijms22147641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Henriquez S, Zerbit J, Bruel T, Ouedrani A, Planas D, Deschamps P, Staropoli I, Hadjadj J, Varet B, Suarez F, Ermark N (2021) Anti-CD38 therapy impairs SARS-CoV-2 vaccine response in multiple myeloma patients. MedRxiv [DOI] [PMC free article] [PubMed]
- 271.Nooka AK, Shanmugasundaram U, Cheedarla N, Verkerke H, Edara VV, Valanparambil R, Kaufman JL, Hofmeister CC, Joseph NS, Lonial S, Azeem M (2022) Determinants of neutralizing antibody response after SARS CoV-2 vaccination in patients with myeloma. J Clin Oncol 40:3057 [DOI] [PMC free article] [PubMed]
- 272.Blixt L, Gao Y, Wullimann D, Murén Ingelman-Sundberg H, Muschiol S, Healy K, Bogdanovic G, Pin E, Nilsson P, Kjellander C. Hybrid immunity in immunocompromised patients with CLL after SARS-CoV-2 infection followed by booster mRNA vaccination. Blood. 2022;140:2403–2407. doi: 10.1182/blood.2022016815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Xu A, Hong B, Lou F, Wang S, Li W, Shafqat A, An X, Zhao Y, Song L, Tong Y. Sub-lineages of the SARS-CoV-2 Omicron variants: characteristics and prevention. MedComm. 2022;3:e172. doi: 10.1002/mco2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Bellusci L, Grubbs G, Srivastava P, Nemeth MJ, Griffiths EA, Golding H, Khurana S. Neutralization of SARS-CoV-2 Omicron after vaccination of patients with myelodysplastic syndromes or acute myeloid leukemia. Blood. 2022;139:2842–2846. doi: 10.1182/blood.2022016087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Henriquez S, Zerbit J, Bruel T, Ouedrani A, Planas D, Deschamps P, Staropoli I, Hadjadj J, Varet B, Ermak N. Anti-CD38 therapy impairs SARS-CoV-2 vaccine response against alpha and delta variants in patients with multiple myeloma. Blood. 2022;139:942–946. doi: 10.1182/blood.2021013714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Greenberger LM, Saltzman LA, Senefeld JW, Johnson PW, DeGennaro LJ, Nichols GL. Anti-spike antibody response to SARS-CoV-2 booster vaccination in patients with B cell-derived hematologic malignancies. Cancer Cell. 2021;39:1297–1299. doi: 10.1016/j.ccell.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Lim SH, Stuart B, Joseph-Pietras D, Johnson M, Campbell N, Kelly A, Jeffrey D, Turaj AH, Rolfvondenbaumen K, Galloway C. Immune responses against SARS-CoV-2 variants after two and three doses of vaccine in B-cell malignancies: UK PROSECO study. Nat Cancer. 2022;3:552–564. doi: 10.1038/s43018-022-00364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Kornek B, Leutmezer F, Rommer PS, Koblischke M, Schneider L, Haslacher H, Thalhammer R, Zimprich F, Zulehner G, Bsteh G. B cell depletion and SARS-CoV-2 vaccine responses in neuroimmunologic patients. Ann Neurol. 2022;91:342–352. doi: 10.1002/ana.26309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Gressens SB, Wiedemann A, Déchenaud M, Dupuis J, Gallien S, Melica G, Haioun C, Lemonnier F, Levy Y. Anti-SARS-CoV-2 cellular response after 2 and 3 doses of BNT162b2 mRNA vaccine in lymphoma patients receiving anti-CD20 antibodies. Vaccine. 2023;41:1550–1553. doi: 10.1016/j.vaccine.2023.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Terpos E, Rosati M, Bear J, Burns R, Ntanasis-Stathopoulos I, Devasundaram S, Gavriatopoulou M, Kastritis E, Dimopoulos M-A, Pavlakis GN. Antibody Response to COVID-19 mRNA vaccine in patients with multiple myeloma and waldenstrom's macroglobulinemia after primary immunization and booster: reactivity to the Sars-Cov-2 WT virus, delta and omicron variants. Blood. 2022;140:4334–4335. doi: 10.1182/blood-2022-163521. [DOI] [Google Scholar]
- 281.Fredericks AM, East KW, Shi Y, Liu J, Maschietto F, Ayala A, Cioffi WG, Cohen M, Fairbrother WG, Lefort CT. Identification and mechanistic basis of non-ACE2 blocking neutralizing antibodies from COVID-19 patients with deep RNA sequencing and molecular dynamics simulations. Front Mol Biosci. 2022;9:1362. doi: 10.3389/fmolb.2022.1080964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Du W, Hurdiss DL, Drabek D, Mykytyn AZ, Kaiser FK, González-Hernández M, Muñoz-Santos D, Lamers MM, van Haperen R, Li W. An ACE2-blocking antibody confers broad neutralization and protection against Omicron and other SARS-CoV-2 variants of concern. Sci Immunol. 2022;7:eabp9312. doi: 10.1126/sciimmunol.abp9312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Denkinger CM, Janssen M, Schaekel U, Gall J, Leo A, Stelmach P, Weber SF, Krisam J, Baumann L, Stermann J. Anti-SARS-CoV-2 antibody-containing plasma improves outcome in patients with hematologic or solid cancer and severe COVID-19: a randomized clinical trial. Nat Cancer. 2023;4:96–107. doi: 10.1038/s43018-022-00503-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Atanackovic D, Luetkens T, Omili D, Iraguha T, Lutfi F, Hardy NM, Fan X, Avila SV, Saharia KK, Husson JS. Vaccine-induced T-cell responses against SARS-CoV-2 and its Omicron variant in patients with B cell–depleted lymphoma after CART therapy. Blood. 2022;140:152–156. doi: 10.1182/blood.2022016175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Zerbit J, Detroit M, Meyer A, Decroocq J, Deau-Fischer B, Deschamps P, Birsen R, Mondesir J, Franchi P, Miekoutima E. Patients with hematological malignancies treated with T-cell or B-cell immunotherapy remain at high risk of severe forms of COVID-19 in the omicron era. Viruses. 2022;14:2377. doi: 10.3390/v14112377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Haggenburg S, Hofsink Q, Rutten CE, Nijhof IS, Hazenberg MD, Goorhuis A. SARS-CoV-2 vaccine-induced humoral and cellular immunity in patients with hematologic malignancies. Semin Hematol. 2022;59:192–197. doi: 10.1053/j.seminhematol.2022.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Conway SR, Keller MD, Bollard CM. Cellular therapies for the treatment and prevention of SARS-CoV-2 infection. Blood. 2022;140:208–221. doi: 10.1182/blood.2021012249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Li Z, Yong H, Wang W, Gao Y, Wang P, Chen X, Lu J, Zheng J, Bai J. GSK3326595 is a promising drug to prevent SARS-CoV-2 Omicron and other variants infection by inhibiting ACE2-R671 di-methylation. J Med Virol. 2023;95:e28158. doi: 10.1002/jmv.28158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Keppler-Hafkemeyer A, Greil C, Wratil PR, Shoumariyeh K, Stern M, Hafkemeyer A, Ashok D, Hollaus A, Lupoli G, Priller A. Potent high-avidity neutralizing antibodies and T cell responses after COVID-19 vaccination in individuals with B cell lymphoma and multiple myeloma. Nat Cancer. 2023;4:81–95. doi: 10.1038/s43018-022-00502-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Martits-Chalangari K, Spak CW, Askar M, Killian A, Fisher TL, Atillasoy E, Marshall WL, McNeel D, Miller MD, Mathai SK. ALVR109, an off-the-shelf partially HLA matched SARS-CoV-2–specific T cell therapy, to treat refractory severe COVID-19 pneumonia in a heart transplant patient: case report. Am Jo Transpl. 2022;22:1261–1265. doi: 10.1111/ajt.16927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.He X, Zeng XX. Immunotherapy and CRISPR cas systems: potential cure of COVID-19? Drug Des Devel Ther. 2022;16:951–972. doi: 10.2147/DDDT.S347297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Elliott W, Jr, Guda MR, Asuthkar S, Teluguakula N, Prasad DV, Tsung AJ, Velpula KK. PAD inhibitors as a potential treatment for SARS-CoV-2 immunothrombosis. Biomedicines. 2021;9:1867. doi: 10.3390/biomedicines9121867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Buske C, Dreyling M, Alvarez-Larrán A, Apperley J, Arcaini L, Besson C, Bullinger L, Corradini P, Della Porta MG, Dimopoulos M. Managing hematological cancer patients during the COVID-19 pandemic: an ESMO-EHA Interdisciplinary Expert Consensus. ESMO open. 2022;7:100403. doi: 10.1016/j.esmoop.2022.100403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Yu Y-Q, Herrmann A, Thonn V, Cordsmeier A, Neurath MF, Ensser A, Becker C. SMYD2 inhibition downregulates TMPRSS2 and decreases SARS-CoV-2 infection in human intestinal and airway epithelial cells. Cells. 2022;11:1262. doi: 10.3390/cells11081262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Hueso T, Godron A-S, Lanoy E, Pacanowski J, Levi LI, Gras E, Surgers L, Guemriche A, Meynard J-L, Pirenne F. Convalescent plasma improves overall survival in patients with B-cell lymphoid malignancy and COVID-19: a longitudinal cohort and propensity score analysis. Leukemia. 2022;36:1025–1034. doi: 10.1038/s41375-022-01511-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Palanques-Pastor T, Megías-Vericat JE, Martínez P, López Lorenzo JL, Cornago Navascués J, Rodriguez Macias G, Cano I, Arnan Sangerman M, Vidriales Vicente MB, Algarra Algarra JL. Characteristics, clinical outcomes, and risk factors of SARS-COV-2 infection in adult acute myeloid leukemia patients: experience of the PETHEMA group. Leukemia & Lymphoma. 2021;62:2928–2938. doi: 10.1080/10428194.2021.1948031. [DOI] [PubMed] [Google Scholar]
- 297.Weinstein JB, Bates TA, Leier HC, McBride SK, Barklis E, Tafesse FG. A potent alpaca-derived nanobody that neutralizes SARS-CoV-2 variants. Iscience. 2022;25:103960. doi: 10.1016/j.isci.2022.103960. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.