Abstract
The polysaccharide (PS) capsules of many pathogenic bacteria are poor immunogens in infants and young children as a result of the delayed response to PS antigens during ontogeny. The development of polysaccharide-protein conjugate vaccines for Haemophilus influenzae type b, which have proven to be efficacious in this age group, has led to active development by a number of investigators of conjugate vaccines for other diseases. We describe here the response of several mouse strains to the capsular PS of Neisseria meningitidis group C (MCPS) conjugated to tetanus toxoid (MCPS-TT) and the same response in BALB/c mice as a model of the immune consequences of conjugate vaccine immunization. The use of a conjugate vaccine results in a shift in the isotype elicited in response to the MCPS, from immunoglobulin M (IgM) and IgG3 to primarily IgG1. A response to MCPS-TT is seen even among mouse strains which respond poorly to MCPS itself, emphasizing the importance of a strain survey when choosing a mouse model for a vaccine. The marked increase in IgG1 antibody titer was accompanied by a large increase in bactericidal activity of sera from these animals. Animals primed with the conjugate vaccine demonstrated a booster response after secondary immunization with either the MCPS or the conjugate. The ability to produce a boosted IgG1 anti-MCPS response to the MCPS can be transferred to adoptive recipients by B cells alone from mice primed with MCPS-TT but not mice primed with MCPS alone. These data indicate that in BALB/c mice a single immunization with MCPS-TT is sufficient to induce a shift to IgG1 and generate a memory B-cell population that does not require T cells for boosting.
The capsular polysaccharides (PS) constitute the major virulence factor of many pathogenic bacteria that cause invasive diseases and, therefore, have been employed in a number of vaccines against diseases caused by encapsulated organisms. These PS are classified as thymus-independent (TI) antigens, because they do not require mature T cells to elicit a humoral response in vivo, although they do require a late developing subset of B cells (37). These PS antigens are immunogenic in adults but only poorly immunogenic or nonimmunogenic in infants and young children, who are highly susceptible to infection caused by encapsulated bacteria (22, 43, 64).
The unresponsiveness of some animals to these PS has led to investigations into the nature of this lack of response. Avery and Goebel (5) showed that when rabbits which were unresponsive to type III pneumococcal PS were immunized with type III PS coupled to horse serum albumin, they produced an anti-type III response. Thus, the unresponsiveness to the pure, TI PS could be overcome by the use of a thymus-dependent (TD) conjugate vaccine (62).
A number of studies have demonstrated the utility of conjugate vaccines. Stein et al. (59) compared the immune response to a dextran-derived oligosaccharide-protein conjugate to the response to the dextran polysaccharide itself during ontogeny and found that the conjugate shifted the peak antidextran response from 12 weeks of age to 3 to 4 weeks of age but that it was still age related. Our mouse models have proven to be useful in understanding human responses to PS and were completely predictive of the age-related ability of human infants to respond to Haemophilus influenzae type b (Hib) conjugate vaccines (2, 35, 60). Despite an age-related increase in response, both vaccines (2, 10) have been demonstrated to be efficacious in preventing invasive diseases caused by Hib in infants.
The use of conjugate vaccines against human disease was pioneered by the studies of Robbins, Schneerson, Smith, and their colleagues on Hib conjugate vaccines (26, 35, 57). Early vaccines against Hib were composed of Hib PS capsules and were shown to be effective in the prevention of invasive disease in older children but not in infants (42, 44). Conjugating the Hib PS to different proteins has produced several vaccines with better immunogenicity and safety in the elderly and with efficacy in infants as well; both groups are at an increased risk for disease caused by encapsulated bacteria (11, 29, 38, 55). These vaccines have been particularly useful for prevention of Hib infection in high-risk infant populations (25, 53–55). The almost complete disappearance of Hib disease and the reduction in pharyngeal carriage of Hib (6) testify to the usefulness of these conjugated vaccines (6, 63). Despite the significant public health advances seen with the introduction of Hib conjugate vaccines, the mechanisms by which these conjugate vaccines induce a response in infants is not well understood. The mechanism(s) of action of conjugate vaccines and the reasons why the response is still age related are the subjects of ongoing studies in our laboratory. A thorough understanding of the underlying mechanism of the immune response to PS determinants is essential in order to develop improved, second-generation vaccines to Hib and other PS (12, 30, 48, 58), to mix several or many conjugate vaccines on their own or with other types of vaccines successfully, and to be able to immunize infants at birth.
The capsular PS of Neisseria meningitidis group C (MCPS) is a linear homopolymer of α(2→9)-linked sialic acid residues (9) that are O acetylated at carbons 7 and/or 8 (15). N. meningitidis PS vaccines have been available for quite some time (23); however, the PS is a TI type 2 (TI-2) antigen which is poorly immunogenic in infants and has a short duration of protection in young children (13, 19, 21, 33, 64). Combination of a noncovalent complex of MCPS with outer membrane proteins of group B meningococci did not transform the MCPS into a TD antigen (36). Furthermore, although high levels of antibody were demonstrated, bactericidal activity was not observed in serum from children younger than 24 months (36). Early murine studies on meningococcal conjugate vaccines showed mainly immunoglobulin G1 (IgG1) antibodies to PS and carrier after one dose (8) and increased IgG titers after a second dose (14). However, neither study investigated all isotypes in both primary or secondary responses or the effects of conjugate priming and PS boosting. Several oligosaccharide-protein conjugate vaccines have been developed to elicit a TD response to protect young children against invasive meningococcal disease (14, 27) and are currently being evaluated in clinical trials (4, 14, 17, 32, 34, 49, 65).
Newer vaccines currently in development, with conjugate proteins similar to the Hib vaccines, promise to be more immunogenic against N. meningitidis groups A and C in young children (17, 32, 34, 39). We have compared the BALB/c mouse response to MCPS with that to an MCPS-tetanus toxoid (MCPS-TT) conjugate, a TD form of the antigen. We report here that the meningococcal group C conjugate stimulates significant antibody responses in mouse strains that are unresponsive to PS and that the conjugate primes for an IgG1 memory response that can be transferred to an adoptive host by B cells alone. The response to the conjugate is accompanied by a significant increase in serum bactericidal capacity, compared to the response to PS alone.
MATERIALS AND METHODS
Animals.
Four-week-old female BALB/cAnN (H-2d Igha) (BALB/c), A/HeN (H-2a Ighe) (A/He), C57BL/6N (H-2b Ighb) (B6), DBA/2N (H-2d Ighc) (DBA/2), and pregnant female BALB/cAnN mice were purchased from the Charles River Laboratories through the National Institutes of Health Small Animal Section and maintained in our animal rooms. All animal protocols were approved by the Center for Biologics Evaluation and Research (CBER) Animal Care and Use Committee.
PS.
The MCPS prepared from N. meningitidis C11 was obtained from Merck Sharp and Dohme, West Point, Pa. (lot 1815T). The MCPS used in this study is a homopolymer of α(2→9)-linked sialic acid with O-acetyl groups on C-7 and/or C-8 (9, 15).
Conjugate vaccines.
Two conjugate vaccines were used in this study. The first, MCPS-TT, was prepared as previously described (7) and kindly provided by E. C. Beuvery, Bilthoven, The Netherlands. This conjugate was used only for the strain distribution experiments. The second, a group C meningococcal oligosaccharide coupled to TT (also referred to as MCPS-TT), was prepared as previously described (27) and was used for all other experiments. The periodate-oxidized group C PS was size fractionated on a Bio-Gel A5 column. The selection of a 10-kDa fragment with a narrow size range (9 to 11 kDa) was accomplished by screening the resultant fractions by size exclusion high-performance liquid chromatography (Superose-12; Pharmacia Biotech, Uppsala, Sweden) and comparing them with a calibration curve constructed with a series of sialic acid oligosaccharides.
Immunization protocol.
A/He, BALB/c, B6, and DBA/2 mice were immunized intraperitoneally at 10 to 12 weeks of age with either 10 μg of MCPS as free PS or in the form of MCPS-TT in 5% Maalox used as an adjuvant (modified from reference 46). These mice were bled by retro-orbital plexus puncture at 2 and 4 weeks after primary immunization, and they rested for 4 weeks after the last bleeding. The mice were then given a secondary immunization identical to the primary one and bled 1 and 3 weeks later.
B-cell purification.
Spleen cells from 12- to 16-week-old BALB/c mice were treated with a cocktail of anti-T-cell antibodies (anti-Thy 1.2 [clone 13.4-2.2; NEN Research Products, Boston, Mass.] and anti-Lyt2.2 [anti-CD8; NEN Research Products]) and then with Low Tox M rabbit complement (Accurate Chemical & Scientific Corp., Westbury, N.Y.). The resulting T-cell-depleted cells were evaluated for purity by examining the population for concanavalin A (ConA)- and phytohemagglutinin (PHA)-reactive T cells, and the cells were more than 96% T cell depleted. Only background [3H]thymidine incorporation was detected (1,599 ± 192 [standard error of the mean] cpm for ConA, 570 ± 71 cpm for PHA, 962 ± 112 cpm for medium control) in the T-cell-depleted population. Unseparated spleen cells responded to ConA with 57,593 ± 4,118 cpm, to PHA with 13,751 ± 1,944 cpm, and to a medium control with 979 ± 61 cpm. Purity was evaluated in this manner in order to exclude functional T cells.
Adoptive transfer.
A cell-free control or 5 × 107 B cells (purified as indicated above) from normal, MCPS- or MCPS-TT-primed BALB/c mice were mixed with a challenge antigen (antigen-free control, 10 μg of MCPS, 10 μg of MCPS-TT, or 10 μg of MCPS and 20 μg of TT) and were injected intravenously into 600-R-irradiated (CBA/N × BALB/c)F1 male mice. The mice were bled on day 7 after injection, and serial threefold dilutions of serum were assayed for IgG1 antibodies by fluorescence enzyme-linked immunosorbent assay (FELISA) on MCPS-coated plates.
FELISA.
The anti-MCPS FELISA has been described in detail elsewhere (51). Antibody titers in equal aliquots of pooled sera from 10 individual mice per strain were determined with antigen for eight serial twofold dilutions, starting at 1:20. Earlier experiments had shown that the endpoint titer of an equal aliquot of serum from 10 mice was the same as the mean of pooled sera from 10 individuals, validating the experimental approach (52a). Anti-TT-antibody titers were measured by using tetanus toxoid provided by the Laboratory of Bacterial Toxins, CBER, Food and Drug Administration, which was used to coat plates diluted in 0.015 M sodium bicarbonate, pH 9, at a concentration of 1 μg/ml. Antibodies to TT were developed with an alkaline phosphatase-coupled anti-total Ig, rather than with class- and subclass-specific reagents. Titers are expressed as reciprocal dilutions, determined by extrapolation to 0 from the linear part of the titration curve.
Bactericidal assay.
The bactericidal assay has been described in detail previously (52). Briefly, threefold serial dilutions of sera were incubated with bacteria and complement at 37°C for 30 min, and 50-μl volumes of the mixture were plated on agar. The number of colonies was counted, the percent bacterial killing was calculated for each titration, and the mean of three to seven replicates was calculated. Bacteria were incubated with complement alone in eight replicates in each assay, and the mean of this value was used as 0% killing.
RESULTS
Anti-MCPS responses of mice with different genetic backgrounds.
Four strains of mice which differed in their H-2 and Igh haplotypes were immunized with either one or two injections of MCPS or MCPS-TT. The isotype distribution of the anti-MCPS response was evaluated.
There were no detectable levels of IgM antibodies at a 1:10 dilution of the preimmune sera from these animals (data not shown). After a primary immunization with MCPS, both A/He and BALB/c mice responded, but B6 and DBA/2 mice did not respond significantly. A second immunization with MCPS also did not stimulate a response in B6 or DBA/2 mice and resulted in clearance of antibodies from A/He and BALB/c mouse sera. After a primary immunization with MCPS-TT the IgM titers were equivalent to those in response to MCPS alone; however, after a second immunization there was clear evidence of a booster effect in BALB/c and DBA/2 mice (data not shown).
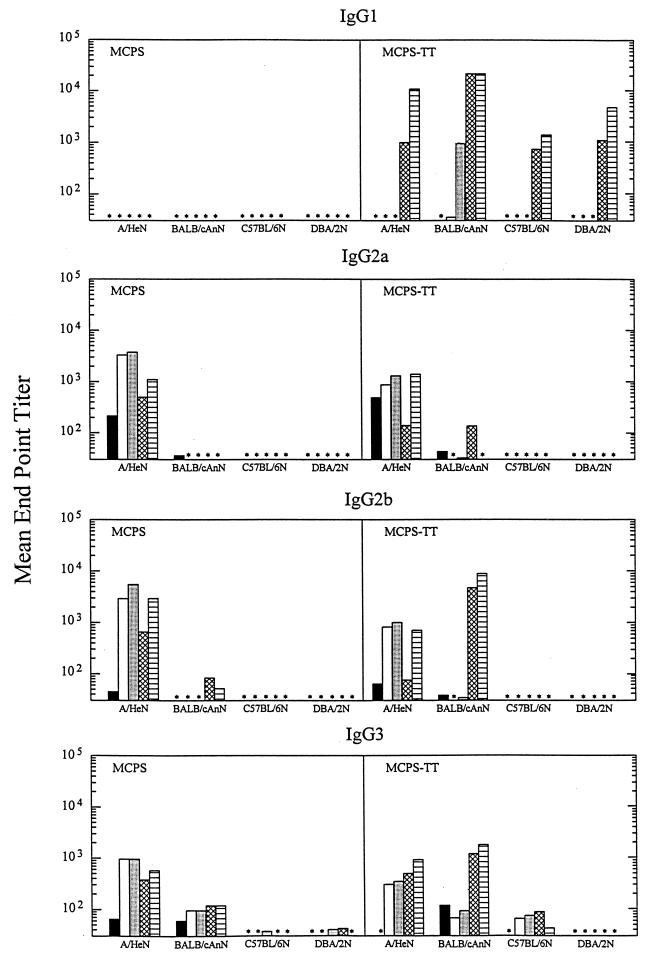
There were no detectable levels of IgG1 in any sera following immunization with MCPS (Fig. 1); however, there was a dramatic difference seen after immunization with MCPS-TT. Only BALB/c mice showed evidence of a primary IgG1 response to MCPS-TT, but all strains of mice exhibited very high levels of IgG1 anti-MCPS antibodies after the secondary immunization with MCPS-TT.
FIG. 1.
Titers of antibodies specific for MCPS, by IgG subclass, in sera of four strains of mice with different H-2 and Igh haplotypes immunized with MCPS or MCPS-TT and then given boosters of the same antigen. Mice were bled prior to immunization (solid bars), 2 weeks (open bars) and 4 weeks (stippled bars) after primary immunization, and 1 week (crosshatched bars) and 3 weeks (striped bars) after secondary immunization. Some titers were below detectable levels (∗).
Only the A/He mice produced IgG2a antibodies (Fig. 1) following immunization with either MCPS or MCPS-TT; however, the preimmune levels in these animals were also substantial. None of the other strains exhibited significant IgG2a responses to either MCPS or MCPS-TT. BALB/c mice had detectable anti-MCPS IgG2a levels after two immunizations with MCPS-TT.
The IgG2b anti-MCPS antibody titers are shown in Fig. 1. The A/He mice showed a response similar to the IgG2a response. BALB/c mice made very little IgG2b antibody following immunization with MCPS but exhibited a substantial response after a secondary immunization with MCPS-TT. As with IgG2a, B6 and DBA/2 mice showed no IgG2b response to either antigen.
IgG3 anti-MCPS antibody titers (Fig. 1) of A/He mice in response to both MCPS and MCPS-TT were substantial and similar for both antigens after a primary immunization; however, in neither case was this response boosted after a secondary immunization. BALB/c mice showed measurable preimmune titers of IgG3 anti-MCPS antibodies, which increased somewhat after MCPS immunization and markedly after a secondary immunization with MCPS-TT. B6 and DBA/2 mice had no IgG3 response to MCPS, but B6 mice had a small response to MCPS-TT.
Booster response to MCPS and MCPS-TT in BALB/c mice.
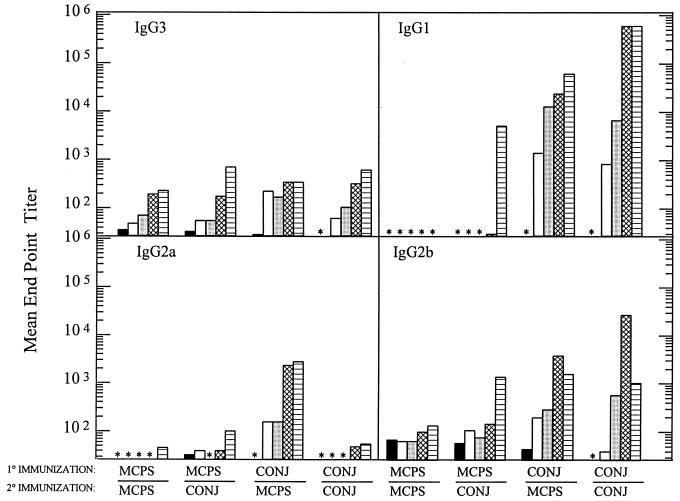
BALB/c mice, because of their consistent response to MCPS and MCPS-TT, were chosen to further investigate the immune response to MCPS and its conjugate vaccine counterpart. In these experiments mice were immunized with an oligosaccharide conjugate. In order to answer the question of whether conjugate vaccine could prime for a boosted response to PS, four different immunization schemes were employed. Figure 2 shows the mean endpoint titers (reciprocal serum dilution) of sera from mice, taken before immunization, at 2 and 4 weeks following a primary immunization, or at 1 and 3 weeks following a secondary immunization with MCPS or MCPS-TT. Primarily IgM (data not shown), IgG3, and IgG2b were present in similar concentrations after both primary and secondary immunizations with MCPS. The studies show that IgG1 antibodies are not produced in response to MCPS alone and that this subclass is the dominant isotype produced in response to MCPS-TT. Moreover, the data show that either MCPS-TT or MCPS alone can boost the IgG1 response. Following a secondary immunization with MCPS-TT, not only was the IgG1 response enhanced, but also increases of IgM (data not shown) and other IgG isotypes were observed (Fig. 2). This indicates that the use of a TD antigen resulted in cells being switched to produce isotypes not seen in response to a TI-2 antigen.
FIG. 2.
Titers of antibodies specific for MCPS, by isotype, in sera of BALB/c mice immunized with MCPS or MCPS-TT and given boosters as indicated. Mice were bled prior to immunization (solid bars), 2 weeks (open bars) and 4 weeks (stippled bars) after primary immunization, and 1 week (crosshatched bars) and 3 weeks (striped bars) after secondary immunization. Some titers were below detectable levels (∗).
Another way to monitor if the mice responded to the conjugate was to examine the response to the carrier protein. The anti-TT antibody titers in all groups immunized with conjugate were greater than 105 at the bleeding 4 weeks after the primary immunization and greater than 106 1 week after boosting. Mice immunized with MCPS alone did not respond to TT.
Memory response.
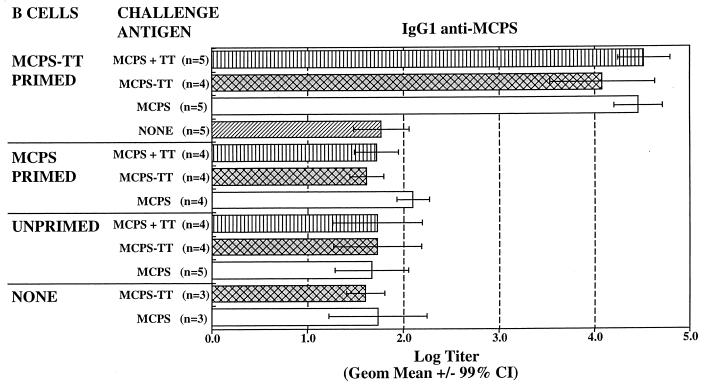
To prove that MCPS-TT stimulated a memory response, adoptive transfer experiments were conducted with purified B cells from mice immunized with MCPS or MCPS-TT transferred into an irradiated host [(CBA/N × BALB/c)F1 male (xid) mice, which have no anti-PS antibodies, were used as the host]. The mice were then boosted with MCPS or MCPS-TT. Because an IgG1 serum response is seen in response to MCPS-TT but not MCPS alone, an IgG1 anti-MCPS response in adoptive recipients is indicative of a memory response. As seen in Fig. 3, MCPS-TT induced a memory response that could be boosted by either MCPS or MCPS-TT in the absence of T cells with a 2-log increase in IgG1 antibody. In contrast, background levels were seen with B cells from mice primed with unconjugated PS, and antibody titers were equivalent to those in mice given unprimed B cells or primed cells but no antigen booster. These data demonstrate that the effect of the TD antigen during the primary immunization is to induce class switching and a memory B-cell population that can be boosted by either a TI-2 or a TD antigen.
FIG. 3.
IgG1 antibodies specific for MCPS in sera from irradiated (CBA/N × BALB/c)F1 male mice that were recipients of B cells from BALB/c mice primed with MCPS or MCPS-TT or from unprimed BALB/c mice. The irradiated hosts were given MCPS or MCPS-TT boosters and bled on day 7 after injection. n, number of mice per group. Data are the geometric means ± 99% confidence intervals (CI). Nonoverlapping confidence intervals indicate a significant difference (P ≤ 0.01).
Bactericidal activity in BALB/c mice.
Because of the large increases in levels of antibody to MCPS in serum following conjugate immunization, the bactericidal activity of these sera was also examined. The response to MCPS-TT also was accompanied by an increase in serum bactericidal activity, as seen in Table 1. The response to the primary immunization with MCPS-TT showed a titer of approximately 1:100, in contrast to the response to MCPS, which was <1:10. The response to MCPS-TT was boosted by MCPS (3-fold increase) and by MCPS-TT (19-fold increase).
TABLE 1.
Serum bactericidal capacity
| Primary and secondary immunization | Serum dilution resulting in 50% killing
|
||
|---|---|---|---|
| PIa | Primaryb | Secondaryc | |
| MCPS, MCPS | <1:10 | <1:10 | <1:10 |
| MCPS-TT, MCPS | <1:10 | 1:117 | 1:311 |
| MCPS-TT, MCPS-TT | <1:10 | 1:100 | 1:1,885 |
a Preimmunization sera.
b Sera obtained from bleedings 4 weeks after the primary immunization.
c Sera obtained from bleedings 3 weeks after the secondary immunization.
DISCUSSION
One of the body’s defense mechanisms against the invasion of encapsulated pathogenic bacteria is to mount an immune response to the capsular PS. The response to capsular PS (TI antigens) is markedly different from the response to most proteins (TD antigens) and has been classified as a TI-2 response. The antibody response to TI-2 antigens develops late in ontogeny (20, 43) and utilizes a particular subset of B cells (37). TI antigens generally fail to elicit a memory response and usually do not show affinity maturation (1, 62). In contrast, the ability to respond to a TD antigen is present at birth (60) and results in the formation of memory cells, and the response undergoes subsequent affinity maturation after reimmunization. For TI antigens, IgG3 and IgM are the major isotypes expressed, even after secondary immunization (45). In contrast, for TD antigens, the ratio of IgG to IgM increases, with IgG1 being the major subclass after secondary immunization (59–61).
We have explored strain differences with regard to the immune response to both MCPS and its conjugate counterpart. A wide range of responses was seen among mice with different genetic backgrounds. Importantly, all strains, even those which were low-level responders or nonresponders to MCPS, showed high levels of IgG1 antibodies after a secondary immunization with MCPS-TT (Fig. 1). The importance of choosing the right strain for model studies has been emphasized by other investigators for a variety of immunogens (28, 50, 56).
The levels of antibodies elicited in mice immunized with the MCPS conjugate vaccine were long lasting. The IgG1 titers that we observed remained at a significant level for as long as 1 year after the primary injection (data not shown). The maintenance of high IgG1 anti-MCPS antibody titers may be due to a boosting effect by an environmental antigen which is cross-reactive with the MCPS (18) and which may be responsible for the appearance of natural antibodies in BALB/c mice (51) and in humans (22).
Other investigators have reported rises in levels of total antibody, isotype-specific antibody, and bactericidal antibody following both meningococcal disease and meningococcal PS vaccination (4, 22). We observed a significant rise in bactericidal-antibody titers after immunization with the conjugate. The response to MCPS-TT showed bactericidal activity at least 10-fold higher than the response to MCPS alone and was boosted by MCPS and by MCPS-TT. The sera from mice immunized with MCPS alone did not have significant bactericidal activity; however, there was a good correlation between IgG1 and IgG2b antibody levels and the bactericidal activity in mice primed with MCPS-TT and boosted with either MCPS or MCPS-TT. It is not clear which isotype(s) contributes to the increased bactericidal activity. Although IgG1 titers were approximately 10-fold higher than those for IgG2b, mouse IgG1 generally does not fix complement (16, 41). Thus, if IgG1 is the protective isotype, its in vivo action is unlikely to be via complement-dependent killing. It is also unlikely that IgG3 is the protective isotype, as all four immunization groups showed similar levels of IgG3 antibody. The mechanism involved might be opsonophagocytosis via specific receptors for mouse IgG2b and IgG1 (66). Consistent with this hypothesis are the findings of Pon et al. (47), who have shown that IgG1 antibodies to N-propionylated group B meningococcal PS that are not bactericidal in vitro can protect in vivo by adoptive transfer. Furthermore, models that include IgG1 and opsonic activity can be used to predict mouse protection against pneumococci as suggested by Alonso DeVelasco et al. (3).
MCPS-specific IgG1 memory B cells were observed in the MCPS-TT-primed mice as shown in adoptive-transfer experiments (Fig. 3). This response could be boosted by either MCPS or MCPS-TT in the absence of T cells. In contrast, B cells from mice primed with unconjugated PS did not show an IgG1 increase, and antibody titers were equivalent to those in control mice given unprimed B cells or primed cells but no antigen boost. These data demonstrate that the effect of the TD antigen during the primary immunization is to induce class switching and a memory B-cell population that can be boosted by either a TI-2 or a TD antigen. An analysis of monoclonal antibodies derived from mice primed with MCPS-TT and boosted with MCPS or MCPS-TT showed that an increase in antibody diversity and avidity is also a feature of the response to TD priming (17a).
An important requirement for conjugate vaccines is that they be able to prime the immune system for a booster response upon encounter with encapsulated bacteria. Indeed, our studies show that MCPS as well as conjugate can clearly elicit a secondary immune response in animals primed with conjugate vaccine. Data for human infants immunized with Hib (24, 31, 67), pneumococcal PS type 6A and 23F (40), or meningococcal group A and C (32) conjugates also suggest that conjugates induce a memory B-cell response and that, for meningococcal group C conjugates, this response was boosted by either MCPS or the conjugate (32).
In conclusion, our data show that the use of a TD form of MCPS, compared to the TI form, resulted in an isotype shift and the generation of memory B cells and bactericidal antibodies. Moreover, these data suggest that MCPS-TT will elicit a protective response to a booster not only with the conjugate but, more importantly, upon exposure to meningococcal group C encapsulated bacteria.
ACKNOWLEDGMENTS
We thank Karen L. Elkins and Carl E. Frasch (CBER, FDA) for critical review of the manuscript and Ezio Bonvini for the statistical analysis of the data on adoptive transfer.
REFERENCES
- 1.Aaberge I S, Løvik M. The antibody response to secondary immunization with pneumococcal polysaccharides in mice. Scand J Immunol. 1995;42:617–625. doi: 10.1111/j.1365-3083.1995.tb03704.x. [DOI] [PubMed] [Google Scholar]
- 2.Ahonkhai V I, Lukacs L J, Jonas L C, Matthews H, Vella P P, Ellis R W, Staub J M, Dolan K T, Rusk C M, Calandra G B, Gerety R J. Haemophilus influenzae type b conjugate vaccine (meningococcal protein conjugate) (PedvaxHIB™): clinical evaluation. Pediatrics. 1990;85:676–681. [PubMed] [Google Scholar]
- 3.Alonso DeVelasco E, Dekker B A T, Verheul A F M, Feldman R G, Verhoef J, Snippe H. Anti-polysaccharide immunoglobulin isotype levels and opsonic activity of antisera: relationships with protection against Streptococcus pneumoniae infection in mice. J Infect Dis. 1995;172:562–565. doi: 10.1093/infdis/172.2.562. [DOI] [PubMed] [Google Scholar]
- 4.Anderson E L, Bowers T, Mink C M, Kennedy D J, Belshe R B, Harakeh H, Pais L, Holder P, Carlone G M. Safety and immunogenicity of meningococcal A and C polysaccharide conjugate vaccine in adults. Infect Immun. 1994;62:3391–3395. doi: 10.1128/iai.62.8.3391-3395.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avery O T, Goebel W F. Chemo-immunological studies on conjugated carbohydrate-proteins. V. The immunological specificity of an antigen prepared by combining the capsular polysaccharide of type III pneumococcus with foreign protein. J Exp Med. 1931;54:437–447. doi: 10.1084/jem.54.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbour M L. Conjugate vaccines and the carriage of Haemophilus influenzae type B. Emerg Infect Dis. 1996;2:176–182. doi: 10.3201/eid0203.960303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beuvery E C, Miedema F, van Delft R, Haverkamp J. Preparation and immunochemical characterization of meningococcal group C polysaccharide-tetanus toxoid conjugates as a new generation of vaccines. Infect Immun. 1983;40:39–45. doi: 10.1128/iai.40.1.39-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beuvery E C, van Delft R W, Miedema F, Kanhai V, Nagel J. Immunological evaluation of meningococcal group C polysaccharide-tetanus toxoid conjugate in mice. Infect Immun. 1983;41:609–617. doi: 10.1128/iai.41.2.609-617.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhattacharjee A K, Jennings H J, Kenny C P, Martin A, Smith I C P. Structural determination of the sialic acid polysaccharide antigens of Neisseria meningitidis serogroup B and C with carbon 13 nuclear magnetic resonance. J Biol Chem. 1975;250:1926–1932. [PubMed] [Google Scholar]
- 10.Black S B, Shinefield H R, Fireman B, et al. Efficacy in infancy of oligosaccharide conjugate Haemophilus influenzae type b (HbOC) vaccine in a United States population of 61,080 children. Pediatr Infect Dis J. 1991;10:97–104. doi: 10.1097/00006454-199102000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Booy R, Heath P T, Slack M P E, Begg N, Moxon E R. Vaccine failures after primary immunization with Haemophilus influenzae type-b conjugate vaccine without booster. Lancet. 1997;349:1197–1202. doi: 10.1016/s0140-6736(96)06392-1. [DOI] [PubMed] [Google Scholar]
- 12.Campbell W N, Hendrix E, Cryz S, Jr, Cross A S. Immunogenicity of a 24-valent Klebsiella capsular polysaccharide vaccine and an eight-valent Pseudomonas O-polysaccharide conjugate vaccine administered to victims of acute trauma. Clin Infect Dis. 1996;23:179–181. doi: 10.1093/clinids/23.1.179. [DOI] [PubMed] [Google Scholar]
- 13.Ceesay S J, Allen S J, Menon A, Todd J E, Cham K, Carlone G M, Turner S H, Gheesling L L, DeWitt W, Plikaytis B D, Greenwood B. Decline in meningococcal antibody levels in African children 5 years after vaccination and the lack of an effect of booster immunization. J Infect Dis. 1993;167:1212–1216. doi: 10.1093/infdis/167.5.1212. [DOI] [PubMed] [Google Scholar]
- 14.Costantino P, Viti S, Podda A, Velmonte M A, Nencioni L, Rappuoli R. Development and phase 1 clinical testing of a conjugate vaccine against meningococcus A and C. Vaccine. 1992;10:691–698. doi: 10.1016/0264-410x(92)90091-w. [DOI] [PubMed] [Google Scholar]
- 15.Egan W. Structure of the capsular polysaccharide antigens from Haemophilus influenzae and Neisseria meningitidis by 13C NMR spectroscopy. In: Cohen J S, editor. Magnetic resonance in biology. Vol. 1. New York, N.Y: John Wiley & Sons; 1980. p. 197. [Google Scholar]
- 16.Ey P L, Russel-Jones G J, Jenkin C R. Isotypes of mouse IgG—I. Evidence for ‘non-complement-fixing’ IgG1 antibodies and characterization of their capacity to interfere with IgG2 sensitization of target red blood cells for lysis by complement. Mol Immunol. 1980;17:699–710. doi: 10.1016/0161-5890(80)90139-x. [DOI] [PubMed] [Google Scholar]
- 17.Fairley C K, Begg N, Borrow R, Fox A J, Jones D M, Cartwright K. Conjugate meningococcal serogroup A and C vaccine: reactogenicity and immunogenicity in United Kingdom infants. J Infect Dis. 1996;174:1360–1363. doi: 10.1093/infdis/174.6.1360. [DOI] [PubMed] [Google Scholar]
- 17a.García-Ojeda, P. A., et al. Unpublished data.
- 18.Glode M P, Robbins J B, Liu T-Y, Gotschlich E C, Ørskov I, Ørskov F. Cross-antigenicity and immunogenicity between capsular polysaccharides of group C Neisseria meningitidis and of Escherichia coli K92. J Infect Dis. 1977;135:94–102. doi: 10.1093/infdis/135.1.94. [DOI] [PubMed] [Google Scholar]
- 19.Gold R, Lepow M L, Goldschneider I, Draper T L, Gotschlich E C. Clinical evaluation of group A and group C meningococcal polysaccharide vaccines in infants. J Clin Investig. 1975;56:1536–1547. doi: 10.1172/JCI108235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gold, R., M. L. Lepow, I. Goldschneider, and E. C. Gotschlich. 1977. Immune response of human infants to polysaccharide vaccines of group A and C Neisseria meningitidis. J. Infect. Dis. 136(Suppl.):S31–S35. [DOI] [PubMed]
- 21.Gold R, Lepow M L, Goldschneider I, Draper T L, Gotschlich E C. Kinetics of antibody production to group A and group C meningococcal polysaccharide vaccines administered during the first six years of life: prospects for routine immunization of infants and children. J Infect Dis. 1979;140:690–697. doi: 10.1093/infdis/140.5.690. [DOI] [PubMed] [Google Scholar]
- 22.Goldschneider I, Gotschlich E C, Artenstein M S. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969;129:1327–1348. doi: 10.1084/jem.129.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gotschlich E C, Goldschneider I, Artenstein M S. Human immunity to the meningococcus. IV. Immunogenicity of group A and group C meningococcal polysaccharides in human volunteers. J Exp Med. 1969;129:1367–1384. doi: 10.1084/jem.129.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Granoff D M, Holmes S J, Osterholm M T, McHugh J E, Lucas A H, Anderson E A, Belshe R B, Jacobs J L, Medley F, Murphy T V. Induction of immunologic memory in infants primed with Haemophilus influenzae type b conjugate vaccines. J Infect Dis. 1993;168:663–671. doi: 10.1093/infdis/168.3.663. [DOI] [PubMed] [Google Scholar]
- 25.Harrison L H, Tajkowski C, Croll J, Reid R, Hu D, Brenneman G, Weatherholtz R C, Santosham M. Postlicensure effectiveness of Haemophilus influenzae type b polysaccharide—Neisseria meningitidis outer-membrane protein complex conjugate vaccine among Navajo children. J Pediatr. 1994;125:571–576. doi: 10.1016/s0022-3476(94)70009-5. [DOI] [PubMed] [Google Scholar]
- 26.Insel R A, Anderson P W. Oligosaccharide-protein conjugate vaccines induce and prime for oligoclonal IgG antibody responses to the Haemophilus influenzae b capsular polysaccharide in human infants. J Exp Med. 1986;163:262–269. doi: 10.1084/jem.163.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jennings H J, Lugowski C. Immunochemistry of groups A, B, and C meningococcal polysaccharide-tetanus toxoid conjugates. J Immunol. 1981;127:1011–1018. [PubMed] [Google Scholar]
- 28.Jiménez de Bagüés M P, Elzer P H, Blasco J M, Marín C M, Gamazo C, Winter A J. Protective immunity to Brucella ovis in BALB/c mice following recovery from primary infection or immunization with subcellular vaccines. Infect Immun. 1994;62:632–638. doi: 10.1128/iai.62.2.632-638.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kantor E, Luxenberg J S, Lucas A H, Granoff D M. Phase I study of the immunogenicity and safety of conjugated Haemophilus influenzae type b vaccines in the elderly. Vaccine. 1997;15:129–132. doi: 10.1016/s0264-410x(96)00164-8. [DOI] [PubMed] [Google Scholar]
- 30.Kasper D L, Paoletti L C, Wessels M R, Guttormsen H-K, Carey V J, Jennings H J, Baker C J. Immune response to type III group B streptococcal polysaccharide-tetanus toxoid conjugate vaccine. J Clin Investig. 1996;98:2308–2314. doi: 10.1172/JCI119042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurikka S. Priming with diphtheria-tetanus-pertussis vaccine enhances the response to the Haemophilus influenzae type b tetanus conjugate vaccine in infancy. Vaccine. 1996;14:1239–1242. doi: 10.1016/s0264-410x(96)00025-4. [DOI] [PubMed] [Google Scholar]
- 32.Leach A, Twumasi P A, Kumah S, Banya W S, Jaffar S, Forrest B D, Granoff D M, LiButti D E, Carlone G M, Pais L B, Broome C V, Greenwood B M. Induction of immunologic memory in Gambian children by vaccination in infancy with a group A plus group C meningococcal polysaccharide-protein conjugate vaccine. J Infect Dis. 1997;175:200–204. doi: 10.1093/infdis/175.1.200. [DOI] [PubMed] [Google Scholar]
- 33.Lepow M L, Goldschneider I, Gold R, Randolph M, Gotschlich E C. Persistence of antibody following immunization of children with groups A and C meningococcal polysaccharide vaccines. Pediatrics. 1977;60:673–680. [PubMed] [Google Scholar]
- 34.Lieberman J M, Chiu S S, Wong V K, Partridge S, Chang S-J, Chiu C-Y, Gheesling L L, Carlone G M, Ward J I. Safety and immunogenicity of serogroup A/C Neisseria meningitidis oligosaccharide-protein conjugate vaccine in young children. A randomized controlled trial. JAMA. 1996;275:1499–1503. [PubMed] [Google Scholar]
- 35.Madore D V, Johnson C L, Phipps D C, Popejoy L A, Eby R, Smith D H. Safety and immunologic response to Haemophilus influenzae type b oligosaccharide-CRM197 conjugate vaccine in 1-to 6-month old infants. Pediatrics. 1990;85:331–337. [PubMed] [Google Scholar]
- 36.Milagres L G, Lemos A P S, Meles C E A, Silva E L, Ferreira L H M L, Souza J A M, Carlone G M. Antibody response after immunization of Brazilian children with serogroup C meningococcal polysaccharide noncovalently complexed with outer membrane proteins. Braz J Med Biol Res. 1995;28:981–989. [PubMed] [Google Scholar]
- 37.Mosier D E, Zitron I M, Mond J J, Ahmed A, Scher I, Paul W E. Surface immunoglobulin D as a functional receptor for a subclass of B lymphocytes. Immunol Rev. 1977;37:89–104. doi: 10.1111/j.1600-065x.1977.tb00246.x. [DOI] [PubMed] [Google Scholar]
- 38.Mulholland K, Hilton S, Adegbola R, Usen S, Oparaugo A, Omosigho C, Weber M, Palmer A, Schneider G, Jobe K, Lahai G, Jaffar S, Secka O, Lin K, Ethevenaux C, Greenwood B. Randomised trial of Haemophilus influenzae type-b tetanus protein conjugate for the prevention of pneumonia and meningitis in Gambian infants. Lancet. 1997;349:1191–1197. doi: 10.1016/s0140-6736(96)09267-7. [DOI] [PubMed] [Google Scholar]
- 39.Nieminen T, Käyhty H, Kantele A. Circulating antibody secreting cells and humoral antibody response after parenteral immunization with a meningococcal polysaccharide vaccine. Scand J Infect Dis. 1996;28:53–58. doi: 10.3109/00365549609027150. [DOI] [PubMed] [Google Scholar]
- 40.O’Brien K L, Steinhoff M C, Edwards K, Keyserling H, Thoms M L, Madore D. Immunologic priming of young children by pneumococcal glycoprotein conjugate, but not polysaccharide, vaccines. Pediatr Infect Dis J. 1996;15:425–430. doi: 10.1097/00006454-199605000-00009. [DOI] [PubMed] [Google Scholar]
- 41.Oi V T, Vuong T M, Hardy R, Reidler J, Dangl J, Herzenberg L A, Stryer L. Correlation between segmental flexibility and effector function of antibodies. Nature. 1984;307:136–140. doi: 10.1038/307136a0. [DOI] [PubMed] [Google Scholar]
- 42.Parke, J. C., R. Schneerson, J. B. Robbins, and J. J. Schlesselman. 1977. Interim report of a controlled field trial of immunization with capsular polysaccharides of Haemophilus influenzae type b and group C Neisseria meningitidis in Mecklenburg County, North Carolina (March 1974–March 1976). J. Infect. Dis. 136(Suppl.):S51–S56. [DOI] [PubMed]
- 43.Peltola H, Kayhty H, Sivonen A, Makela P H. Haemophilus influenzae type b capsular polysaccharide vaccine in children: a double-blind field of study of 100,000 vaccinees 3 months to 5 years of age in Finland. Pediatrics. 1977;60:730–737. [PubMed] [Google Scholar]
- 44.Peltola H, Makela P H, Kayhty H, Jousimies H, Herva E, Hallstrom K, Sivonen A, Renkonen O V, Pettay O, Karanko V, Ahvonen P, Sarna S. Clinical efficacy of meningococcus group A capsular polysaccharide vaccine in children three months to five years of age. N Engl J Med. 1977;297:686–691. doi: 10.1056/NEJM197709292971302. [DOI] [PubMed] [Google Scholar]
- 45.Perlmutter R M, Hansburg D, Briles D E, Nicolotti R A, Davie J M. Subclass restriction of murine anti-carbohydrate antibodies. J Immunol. 1978;121:566–572. [PubMed] [Google Scholar]
- 46.Pierres M, Germain R N, Dorf M E, Benacerraf B. In vivo effects of anti-Ia alloantisera. I. Elimination of specific suppression by in vivo administration of antisera specific for I-J controlled determinants. J Exp Med. 1978;147:656–666. doi: 10.1084/jem.147.3.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pon R A, Lussier M, Yang Q-L, Jennings H J. N-Propionylated group B meningococcal polysaccharide mimics a unique bactericidal capsular epitope in group B Neisseria meningitidis. J Exp Med. 1997;185:1929–1938. doi: 10.1084/jem.185.11.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Powers D C, Anderson E L, Lottenbach K, Mink C M. Reactogenicity and immunogenicity of a protein-conjugated pneumococcal oligosaccharide vaccine in older adults. J Infect Dis. 1996;173:1014–1018. doi: 10.1093/infdis/173.4.1014. [DOI] [PubMed] [Google Scholar]
- 49.Richmond P, Goldblatt D, Fusco P, Fusco J, Heron I, Michon F. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Phase I evaluation of a meningococcal C-tetanus toxoid conjugate vaccine, abstr. G-111; p. 212. [Google Scholar]
- 50.Rose M E, Hesketh P, Wakelin D. Immunization against experimental coccidiosis produces contrasting results in inbred mice of differing susceptibility to infection. Infect Immun. 1994;62:733–737. doi: 10.1128/iai.62.2.733-737.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rubinstein L J, Stein K E. Murine immune response to the Neisseria meningitidis group C capsular polysaccharide. I. Ontogeny. J Immunol. 1988;141:4352–4356. [PubMed] [Google Scholar]
- 52.Rubinstein L J, Stein K E. Murine immune response to the Neisseria meningitidis group C capsular polysaccharide. II. Specificity. J Immunol. 1988;141:4357–4362. [PubMed] [Google Scholar]
- 52a.Rubinstein, L. J., and K. E. Stein. Unpublished data.
- 53.Santosham M, Hill J, Wolff M, Reid R, Lukacs L, Ahonkhai V. Safety and immunogenicity of a Haemophilus influenzae type b conjugate vaccine in a high risk American Indian population. Pediatr Infect Dis J. 1991;10:113–117. doi: 10.1097/00006454-199102000-00007. [DOI] [PubMed] [Google Scholar]
- 54.Santosham M, Wolff M, Reid R, Hohenboken M, et al. The efficacy in Navajo infants of a conjugate vaccine consisting of Haemophilus influenzae type b polysaccharide and Neisseria meningitidis outer-membrane protein complex. N Engl J Med. 1991;324:1767–1772. doi: 10.1056/NEJM199106203242503. [DOI] [PubMed] [Google Scholar]
- 55.Santosham, M., B. Rivin, M. Wolff, R. Reid, W. Newcomer, G. W. Letson, J. Almeido-Hill, C. Thompson, and G. R. Siber. 1992. Prevention of Haemophilus influenzae type b infection in Apache and Navajo children. J. Infect. Dis. 165(Suppl. 1):S144–S151. [DOI] [PubMed]
- 56.Scheller L F, Wirtz R A, Azad A F. Susceptibility of different strains of mice to hepatic infection with Plasmodium berghei. Infect Immun. 1994;62:4844–4847. doi: 10.1128/iai.62.11.4844-4847.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schneerson R, Barrera O, Sutton A, Robbins J B. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J Exp Med. 1980;152:361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shelly M A, Jacoby H, Riley G J, Graves B T, Pichichero M, Treanor J J. Comparison of pneumococcal polysaccharide and CRM197-conjugated pneumococcal oligosaccharide vaccines in young and elderly adults. Infect Immun. 1997;65:242–247. doi: 10.1128/iai.65.1.242-247.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stein K E, Zopf D A, Johnson B M, Miller C B, Paul W E. The immune response to an isomaltohexosyl-protein conjugate, a thymus-dependent analogue of α(1→6) dextran. J Immunol. 1982;128:1350–1354. [PubMed] [Google Scholar]
- 60.Stein K E, Zopf D A, Miller C B, Johnson B M, Mongini P K A, Ahmed A, Paul W E. The immune response to a thymus-dependent form of B512 dextran requires the presence of Lyb5+ lymphocytes. J Exp Med. 1983;157:657–666. doi: 10.1084/jem.157.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stein K E, Rubinstein L J. Regulation of the immune response to Neisseria meningitidis group C polysaccharide. In: Osterhaus A, UytdeHaag F, editors. Proceedings of the congress on idiotype networks in biology and medicine. New York, N.Y: Elsevier; 1990. pp. 189–193. [Google Scholar]
- 62.Stein, K. E. 1992. Thymus-independent and thymus-dependent responses to polysaccharide antigens. J. Infect. Dis. 165(Suppl. 1):S49–S52. [DOI] [PubMed]
- 63.Steinhoff M C. Haemophilus influenzae type b infections are preventable everywhere. Lancet. 1997;349:1186–1187. doi: 10.1016/S0140-6736(97)22017-9. [DOI] [PubMed] [Google Scholar]
- 64.Taunay A E, Feldman R A, Bastos C O, Galvão P A A, Morais J S, Castro I O. Avaliação do efeito protetor de vacina polissacarídica antimeningocócica do grupo C, em crianças de 6 a 36 meses. Rev Inst Adolfo Lutz. 1978;38:77–82. [Google Scholar]
- 65.Twumasi P A, Jr, Kumah S, Leach A, O’Dempsey T J D, Ceesay S J, Todd J, Broome C V, Carlone G M, Pais L B, Holder P K, Plikaytis B D, Greenwood B M. A trial of a group A plus group C meningococcal polysaccharide-protein conjugate vaccine in African infants. J Infect Dis. 1995;171:632–638. doi: 10.1093/infdis/171.3.632. [DOI] [PubMed] [Google Scholar]
- 66.Unkeless J C, Scigliano E, Freedman V H. Structure and function of human and murine receptors for IgG. Annu Rev Immunol. 1988;6:251–281. doi: 10.1146/annurev.iy.06.040188.001343. [DOI] [PubMed] [Google Scholar]
- 67.Zepp F, Schmitt H J, Kaufhold A, Schuind A, Knuf M, Habermehl P, Meyer C, Bogaerts H, Slaoui M, Clemens R. Evidence for induction of polysaccharide specific B-cell-memory in the 1st year of life: plain Haemophilus influenzae type b-PRP (Hib) boosters children primed with a tetanus-conjugate Hib-DTPa-HBV combined vaccine. Eur J Pediatr. 1997;156:18–24. doi: 10.1007/s004310050544. [DOI] [PubMed] [Google Scholar]