ABSTRACT
Here we report the complete genome sequence of two moderately thermophilic methanotrophs isolated from a landfill methane biofilter, Methylococcus capsulatus (Norfolk) and Methylocaldum szegediense (Norfolk).
KEYWORDS: methanotrophs, genomes, landfill, biofilter
ANNOUNCEMENT
The Strumpshaw closed landfill features a biofilter for the mitigation of the climate active gas methane, generated by the anaerobic breakdown of organic waste. This biofilter harnesses methanotrophic bacteria in a soil matrix for methane bio-oxidation. Two methanotrophs, Methylococcus capsulatus (Norfolk) and Methylocaldum szegediense (Norfolk), were isolated from this system. Biofilter soil was used to inoculate vials containing nitrate mineral salt (NMS) medium (1) and supplied with 20% (vol/vol) methane. Isolates were obtained from enrichment cultures by serial dilution and plating onto NMS agar plates, incubated in gas-tight containers supplied with 50% (vol/vol) methane. Optimal growth temperatures of the Methylococcus and Methylocaldum isolates were 45°C and 50°C, respectively. M. capsulatus (Norfolk) also grew on methanol (1%–5% vol/vol) as did Methylococcus strain MIR (2).
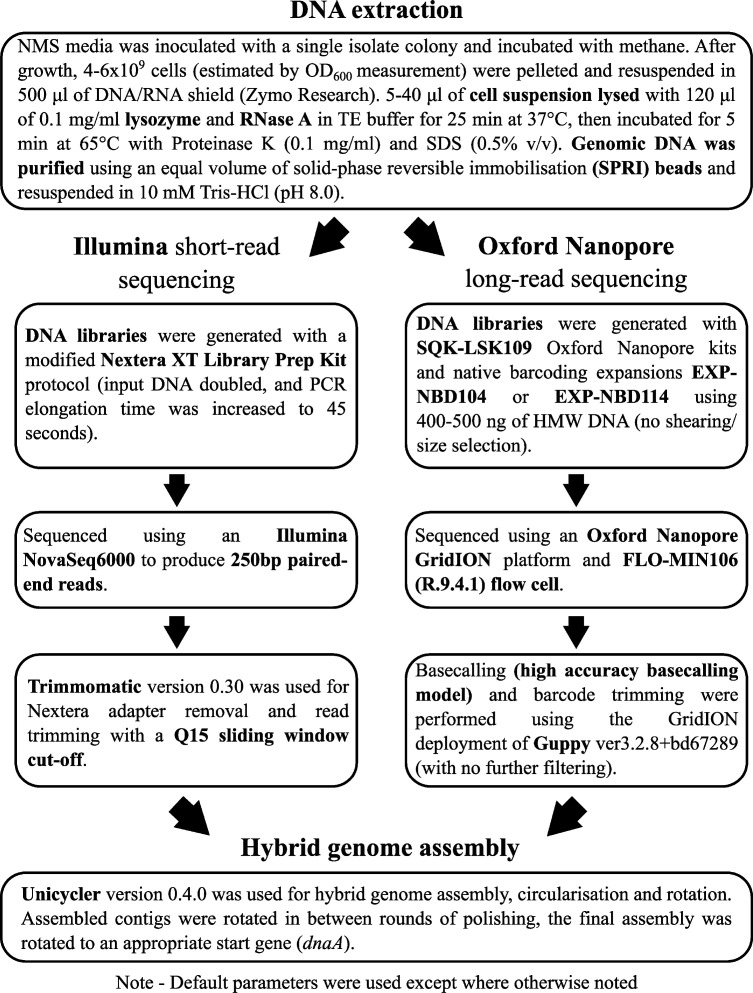
DNA extraction, sequencing, and genome assembly were done using a combined long- and short-read sequencing service at MicrobesNG (Birmingham, UK) as described in Fig. 1. This pipeline was used to construct genomes for M. capsulatus (Norfolk) and M. szegediense (Norfolk), producing a closed genome in both cases.
Fig 1.
Sequencing and assembly pipeline.
MicroScope v.3.16.0 (3) was used for automated annotation and taxonomic assignment of assembled genomes before further manual curation. Genome assembly and sequencing read summaries are shown in Table 1.
TABLE 1.
Methylocaldum szegediense (Norfolk) and Methylococcus capsulatus (Norfolk) genome summaries
| DNA sequencing reads | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Isolate | Illumina total reads | Illumina read length (bp) | Nanopore total reads | Nanopore N50 (bp) | Illumina reads ENA accession no. | Nanopore reads ENA accession no. | |||
| Methylocaldum | 936,436 | 250 | 184,537 | 4,370 | ERR11151912 | ERR11151913 | |||
| Methylococcus | 891,006 | 250 | 15,738 | 13,497 | ERR11151914 | ERR11151915 | |||
The Norfolk isolates were assigned to the Methylococcus capsulatus and Methylocaldum szegediense spp. first described by Foster and Davis (4) and Bodrossy et al. (5). Based on average nucleotide identity (ANI) scores generated using CJ Bioscience’s online ANI calculator (6), the sequenced genomes with the highest similarity to Methylococcus capsulatus (Norfolk) and Methylocaldum szegediense (Norfolk) are Methylococcus capsulatus (Texas) (99.56%) and Methylocaldum szegediense (O-12) (99.64%), respectively (GenBank accession numbers GCA_000297615.1 and GCA_000427385.1).
Both genomes contain genes encoding a full methane oxidation pathway. Two pmoCAB clusters encoding particulate methane monooxygenase were found in each genome (7), and the Methylococcus capsulatus (Norfolk) genome also possesses a single soluble methane monooxygenase mmoXYBZDCGQSR cluster (8) and a putative copper chaperone (mopE) gene (9). Calcium-dependent (mxaFJGIRSACKLD) and lanthanide-dependent (xoxFJ) methanol dehydrogenase gene clusters (10, 11) were found in these genomes, with a clade 5 xoxF gene present in each and an additional clade 3 xoxF in Methylocaldum szegediense (Norfolk) (12). Both genomes feature complete gene inventories for tetrahydromethanopterin and tetrahydrofolate-linked formaldehyde oxidation, in addition to formate dehydrogenase genes (13). Carbon is presumed to be assimilated primarily via the ribulose monophosphate pathway as in Methylococcus capsulatus (Bath), although genes for a partial serine cycle and complete Calvin-Benson-Bassham pathway were detected (14). Alanine dehydrogenase and GS/GOGAT cycle genes for ammonia assimilation were present (15).
In addition to the 4.87 Mbp chromosome, Methylocaldum szegediense (Norfolk) also contained a ~25-kbp plasmid, encoding a plasmid replication initiator protein (TrfA), replication protein (RepA) and a toxin anti-toxin plasmid retention mechanism. A gene encoding a putative siphovirus Gp157 protein was also found, which may confer increased bacteriophage resistance (16, 17).
ACKNOWLEDGMENTS
Genome sequencing was provided by MicrobesNG (http://www.microbesng.com). This work was funded by the EnvEast DTP (NERC NE/L002582/1) and Norfolk County Council. The LABGeM (CEA/Genoscope and CNRS UMR8030), the France Génomique, and French Bioinformatics Institute National Infrastructures (funded as part of Investissement d'Avenir program managed by Agence Nationale pour la Recherche, contracts ANR-10-INBS-09 and ANR-11-INBS-0013) are acknowledged for support within the MicroScope annotation platform.
Contributor Information
David Pearce, Email: David.Pearce@uea.ac.uk.
Elinne Becket, California State University San Marcos, USA.
DATA AVAILABILITY
Genome assembly and raw read accession numbers are listed in Table 1.
REFERENCES
- 1. Whittenbury R, Phillips KC, Wilkinson JF. 1970. Enrichment, isolation and some properties of methane-utilizing bacteria. J Gen Microbiol 61:205–218. doi: 10.1099/00221287-61-2-205 [DOI] [PubMed] [Google Scholar]
- 2. Oshkin IY, Suleimanov RZ, Khmelenina VN, Mardanov AV, Pimenov NV, Dedysh SN. 2022. Complete genome sequence of Methylococcus capsulatus MIR, a methanotroph capable of growth on methanol. Microbiol Resour Announc 11:e0054222. doi: 10.1128/mra.00542-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vallenet D, Calteau A, Dubois M, Amours P, Bazin A, Beuvin M, Burlot L, Bussell X, Fouteau S, Gautreau G, Lajus A, Langlois J, Planel R, Roche D, Rollin J, Rouy Z, Sabatet V, Médigue C. 2020. Microscope: an integrated platform for the annotation and exploration of microbial gene functions through genomic, pangenomic and metabolic comparative analysis. Nucleic Acids Res 48:D579–D589. doi: 10.1093/nar/gkz926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Foster JW, Davis RH. 1966. A methane-dependent coccus, with notes on classification and nomenclature of obligate, methane-utilizing bacteria. J Bacteriol 91:1924–1931. doi: 10.1128/jb.91.5.1924-1931.1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bodrossy L, Holmes EM, Holmes AJ, Kovács KL, Murrell JC. 1997. Analysis of 16S rRNA and methane Monooxygenase gene sequences reveals a novel group of Thermotolerant and Thermophilic Methanotrophs, Methylocaldum gen. nov.. Arch Microbiol 168:493–503. doi: 10.1007/s002030050527 [DOI] [PubMed] [Google Scholar]
- 6. Yoon SH, Ha SM, Lim J, Kwon S, Chun J. 2017. A large-scale evaluation of algorithms to calculate average nucleotide identity. Antonie Van Leeuwenhoek 110:1281–1286. doi: 10.1007/s10482-017-0844-4 [DOI] [PubMed] [Google Scholar]
- 7. Lieberman RL, Rosenzweig AC. 2004. Biological methane oxidation: regulation, biochemistry, and active site structure of particulate methane monooxygenase. Crit Rev Biochem Mol Biol 39:147–164. doi: 10.1080/10409230490475507 [DOI] [PubMed] [Google Scholar]
- 8. Lee SJ. 2016. Hydroxylation of methane through component interactions in soluble methane monooxygenases. J Microbiol 54:277–282. doi: 10.1007/s12275-016-5642-6 [DOI] [PubMed] [Google Scholar]
- 9. Ve T, Mathisen K, Helland R, Karlsen OA, Fjellbirkeland A, Røhr ÅK, Andersson KK, Pedersen R-B, Lillehaug JR, Jensen HB. 2012. The Methylococcus capsulatus (Bath) secreted protein, MopE*, binds both reduced and oxidized copper. PLoS One 7:e43146. doi: 10.1371/journal.pone.0043146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Amaratunga K, Goodwin PM, O’Connor CD, Anthony C. 1997. The methanol oxidation genes mxaFJGIR(S)ACKLD in Methylobacterium extorquens. FEMS Microbiol Lett 146:31–38. doi: 10.1111/j.1574-6968.1997.tb10167.x [DOI] [PubMed] [Google Scholar]
- 11. Oshkin IY, Danilova OV, But SY, Miroshnikov KK, Suleimanov RZ, Belova SE, Tikhonova EN, Kuznetsov NN, Khmelenina VN, Pimenov NV, Dedysh SN. 2021. Expanding characterized diversity and the pool of complete genome sequences of Methylococcus species, the bacteria of high environmental and biotechnological relevance. Front Microbiol 12:756830. doi: 10.3389/fmicb.2021.756830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Keltjens JT, Pol A, Reimann J, Op den Camp HJM. 2014. PQQ-dependent methanol dehydrogenases: rare-earth elements make a difference. Appl Microbiol Biotechnol 98:6163–6183. doi: 10.1007/s00253-014-5766-8 [DOI] [PubMed] [Google Scholar]
- 13. Trotsenko YA, Murrell JC. 2008. Metabolic aspects of aerobic obligate methanotrophy. Adv Appl Microbiol 63:183–229. doi: 10.1016/S0065-2164(07)00005-6 [DOI] [PubMed] [Google Scholar]
- 14. Ward N, Larsen O, Sakwa J, Bruseth L, Khouri H, Durkin AS, Dimitrov G, Jiang L, Scanlan D, Kang KH, et al. 2004. Genomic insights into methanotrophy: the complete genome sequence of Methylococcus capsulatus (Bath). PLoS Biol 2:e303. doi: 10.1371/journal.pbio.0020303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Murrell JC, Dalton H. 1983. Ammonia assimilation in Methylococcus capsulatus (Bath) and other obligate methanotrophs. Microbiology 129:1197–1206. doi: 10.1099/00221287-129-4-1197 [DOI] [Google Scholar]
- 16. Zhong KX, Suttle CA, Baudoux A-C, Derelle E, Colombet J, Cho A, Caleta J, Six C, Jacquet S. 2018. A new freshwater cyanosiphovirus harboring integrase. Front Microbiol 9:2204. doi: 10.3389/fmicb.2018.02204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Foley S, Lucchini S, Zwahlen M-C, Brüssow H. 1998. A short noncoding viral DNA element showing characteristics of a replication origin confers bacteriophage resistance to Streptococcus thermophilus. Virology 250:377–387. doi: 10.1006/viro.1998.9387 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Genome assembly and raw read accession numbers are listed in Table 1.