Abstract
In this study, we used mice in which the gene for gamma interferon (IFN-γ) has been disrupted (IFN-γ−/− mice) to study the role of this cytokine in the resolution of Chlamydia trachomatis infection. We show that IFN-γ−/− mice are impaired in the ability to clear infection with C. trachomatis compared to IFN-γ+/+ control mice. Activated CD8+ cytotoxic T lymphocytes (CTL) secrete IFN-γ in response to intracellular infection, and we have shown previously that a Chlamydia-specific CTL line can reduce C. trachomatis infection when adoptively transferred into infected mice. In the present study, we found that when these IFN-γ+/+ CTL lines are transferred into Chlamydia-infected IFN-γ−/− mice, the transferred CTL cannot overcome the immune defect seen in the IFN-γ−/− mice. We also show that Chlamydia-specific CTL can be cultured from IFN-γ-deficient mice infected with C. trachomatis; however, the adoptive transfer of IFN-γ−/− CTL into infected IFN-γ+/+ mice does not reduce the level of infection. These results suggest that IFN-γ production by CTL is not sufficient to overcome the defect that IFN-γ−/− mice have in the resolution of Chlamydia infection, yet IFN-γ production by CTL is required for the protective effect seen upon adoptive transfer of CTL into IFN-γ+/+ mice.
Cytotoxic T lymphocytes (CTL) play a role in the resolution of many intracellular infections. When CTL recognize an infected cell, the activated CTL clones lyse the infected cell and secrete cytokines. Each of these effector functions may be involved in limiting further spread of the pathogen. Lysis of infected cells deprives organisms of their replicative niche; release of cytokines, such as gamma interferon (IFN-γ), stimulates and activates other elements of the immune system.
In a previous report, we demonstrated that CTL were primed during murine infection with the obligate intracellular parasite Chlamydia trachomatis (16). When cultured Chlamydia-specific CTL were adoptively transferred into C. trachomatis-infected animals, the CTL effected a reduction in the number of organisms recovered from the spleen. Additional experiments showed that in animals treated with neutralizing anti-IFN-γ antibody, the protective effect seen upon adoptive transfer was abrogated, suggesting that the protection by adoptive transfer required the activity of IFN-γ. Because these data were obtained by systemic elimination of this cytokine by using antibody, it was not possible to define whether neutralization of IFN-γ produced by the transferred cells or IFN-γ produced by another resident cell type was responsible for the loss of the protective effect. In this report, we expand on these previous findings, separating the effect of IFN-γ production by resident host cells from IFN-γ production by the transferred CTL lines.
Here we use mice in which the gene for IFN-γ is disrupted (5) (IFN-γ−/− mice) to explore the effect of IFN-γ on C. trachomatis infection and the CTL response to this organism. We show that animals deficient in the ability to produce IFN-γ are impaired in the ability to clear C. trachomatis infection and that transfer of IFN-γ-producing C. trachomatis-specific CTL is not sufficient to mediate clearance. We further demonstrate that Chlamydia-specific CTL can be cultured from infected IFN-γ−/− mice and that these CTL lines, unlike wild-type (IFN-γ+/+) lines, are not able to protect mice against Chlamydia challenge.
MATERIALS AND METHODS
Mice.
Female BALB/c mice (H-2d) were purchased from The Jackson Laboratory and used at 10 to 14 weeks of age. IFN-γ−/− and heterozygous (IFN-γ−/+) mice in the C57/BL6 strain background were generously provided by Dyana Dalton and her colleagues at Genentech (South San Francisco, Calif.). These mice were mated through seven backcrosses to the BALB/c strain. Mice used in these experiments resulted from matings of IFN-γ−/− with IFN-γ+/− mice. IFN-γ−/− animals were differentiated from IFN-γ+/− animals by a PCR screen of DNA from tail biopsy specimens for the IFN-γ gene. Additional IFN-γ−/− mice (BALB/c-Ifngtm1Ts mice) were purchased from The Jackson Laboratory.
Tissue culture.
Medium used for all tissue culture was RPMI 1640 supplemented with l-glutamine, 50 μM 2-mercaptoethanol, antibiotics (except where noted), and 10% fetal calf serum (RP-10). All cells were cultured at 37°C in 7.0% CO2. The cell lines used in this study were J774A.1 (H-2d), derived from a BALB/c monocyte-macrophage tumor, and BALB/3T3 (H-2d), derived from a BALB/c embryo.
Growth, isolation, and detection of C. trachomatis.
C. trachomatis LGV serovar L2 (434) has been described previously (18). Elementary bodies were propagated on human epithelial (HeLa) monolayers and purified on Renografin density gradients (7). The isolated elementary bodies were titered on McCoy cells (15) and stored at −70°C in a medium containing sucrose (219 mM), phosphate (3.8 mM KH2PO4, 8.6 mM Na2HPO4), and glutamate (4.9 mM glutamic acid) (SPG) (9). Aliquots of C. trachomatis elementary bodies were thawed at 37°C immediately prior to use, diluted in sterile SPG, and injected into mice or used to infect monolayers. To quantitate C. trachomatis, spleens from infected mice were homogenized, sonicated, diluted, and applied to McCoy cell monolayers. Inclusions were counted by immunofluorescent microscopy 42 h after infection and reported as inclusion-forming units (IFU) (15).
Infection of tissue culture monolayers with C. trachomatis.
J774 cells used for stimulation of C. trachomatis-specific CTL were seeded at a density of 2 × 105 cells per well in a 24-well tissue culture plate in RP-10 without antibiotics. The following day the medium was removed from each well and an aliquot of thawed C. trachomatis containing 107 IFU was added to each well. To facilitate infection, the plates were centrifuged at 1,200 × g for 1 h at 37°C. The inoculum was then removed by aspiration and replaced with 2 ml of RP-10 without antibiotics. After incubation for 24 h at 37°C, the cells were rinsed twice with RP-10 containing antibiotics and used in CTL cultures. All subsequent additions to the CTL cultures contained antibiotics.
BALB/3T3 cells to be used as targets in CTL assays were seeded into six-well tissue culture plates at a density of 106/well in RP-10 without antibiotics and incubated overnight. The medium was then removed and replaced with 5 × 107 IFU of C. trachomatis. The plates were centrifuged at 1,200 × g for 1 h at 37°C, the inoculum was removed, and 5 ml of RP-10 without antibiotics was added to each well. These Chlamydia-infected cells were incubated for 18 h and then removed from the tissue culture plate with phosphate-buffered saline (PBS) containing 0.1 M EDTA. The suspended cells were washed twice by centrifugation with RP-10 containing antibiotics and used as target cells in CTL assays. Uninfected cells were prepared exactly as were infected cells, using SPG without C. trachomatis.
Stimulation and maintenance of C. trachomatis-specific CTL.
Spleen cells from infected mice were washed in RP-10, and cultures containing 4 × 106 splenocytes and 2 × 105 irradiated (2 × 104 rads) C. trachomatis-infected J774 cells were established in 2 ml of RP-10 in 12 wells of a 24-well tissue culture plate. Infected J774 cells used for stimulation were prepared as described above. Primary cytotoxic effector populations were harvested from the 12 wells after 7 days, and half of the recovered cells were restimulated again in 12 wells of a new 24-well tissue culture plate. In addition to the effector cells, each culture well contained 4 × 106 irradiated (2,000 rads) syngeneic spleen cells and 2 × 105 irradiated (2 × 104 rads) C. trachomatis-infected J774 cells. Subsequent weekly stimulations were carried out with 2-ml cultures in multiple wells of a 24-well tissue culture plate. Each culture consisted of 105 responder cells, 4 × 106 irradiated (2,000 rads) syngeneic spleen cells, and 2 × 105 irradiated (2 × 104 rads) C. trachomatis-infected J774 cells in a medium containing RP-10 supplemented with 5% supernatant from concanavalin A-stimulated rat spleen cells and 50 mM α-methylmannoside.
CTL assays.
Activity of CTL was determined by chromium release assay. Infected and uninfected BALB/3T3 target cells were resuspended in 100 μl to which 100 μCi of sodium 51chromate was added. The cells were incubated at 37°C for 1 h, washed three times with RP-10, and diluted for use at 104 cells per assay well in 96-well plates. Serial dilutions of CTL were added to the assay wells such that the final assay volume was 200 μl in RP-10. Spontaneous release was determined in wells with target cells but without CTL. Maximum release was determined by addition of detergent to wells containing target cells. Following a 4-h incubation at 37°C, 100 μl of supernatant was evaluated on a Wallac 1470 gamma counter. Percent specific lysis was determined as 100 × [(release by CTL − spontaneous release)/(maximal release − spontaneous release)]. In all experiments, spontaneous release was less than 30% of maximal release by detergent.
Assay for IFN-γ production.
The amount of IFN-γ produced by the CTL lines stimulated from the IFN-γ+/+, IFN-γ+/−, and IFN-γ−/− mice was determined by incubating 105 CTL with 104 irradiated (2 × 104 rads) C. trachomatis-infected J774 cells and 2.5 × 105 irradiated (2 × 103 rads) syngeneic spleen cells. These cultures were established in triplicate in 200 μl of RP-10 in 96-well assay dishes. Control wells contained uninfected rather than infected J774 cells. After 12 h of incubation, the culture medium was removed from each well, diluted, and assayed for mouse IFN-γ by using a commercially available enzyme-linked immunosorbent assay kit (Endogen, Woburn, Mass.). Antigen-specific IFN-γ production was determined by subtracting the concentration of IFN-γ in the wells with infected J774 cells from the concentration of IFN-γ in the wells with uninfected J774 cells. The average amounts of IFN-γ produced in the assay wells with uninfected J774 cells were 20 ng/ml for the IFN-γ+/+ CTL line, 8 ng/ml for the IFN-γ+/− line, and 0 ng/ml for the IFN-γ−/− line.
RESULTS
IFN-γ−/− mice are impaired in the ability to clear infection with C. trachomatis.
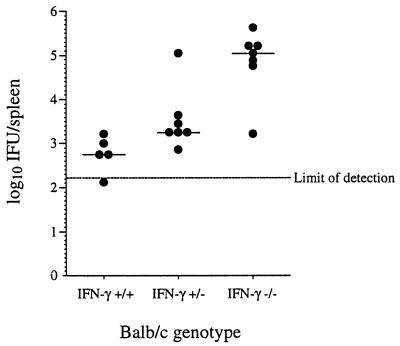
To confirm that IFN-γ plays a role in clearance of C. trachomatis, whether produced by CTL or by other cells of the immune system, we infected three groups (IFN-γ−/−, IFN-γ+/−, and IFN-γ+/+) of BALB/c mice intravenously (i.v.) with 106 C. trachomatis IFU per mouse. The mice were sacrificed 14 days after infection, and the number of IFU per spleen was determined. While wild-type mice were able to reduce the number of C. trachomatis IFU almost to the level of detectability, mice in which the gene for IFN-γ has been disrupted were impaired in the ability to resolve the infection (Fig. 1). Interestingly, heterozygous mice, which have cells producing, on average, half as much IFN-γ also show a deficit in the resolution of infection, although not to the extent of the IFN-γ−/− mice.
FIG. 1.
Effect of the IFN-γ gene mutation on the ability of mice to resolve infection with C. trachomatis. Mice were sacrificed 14 days after infection with 106 IFU of C. trachomatis. Bars indicate median values within the group. A P value of 0.000058 for these data is based on a one-way analysis of variance and a trend test on ranked data.
IFN-γ−/− mice fail to completely resolve C. trachomatis infection within 4 weeks of infection.
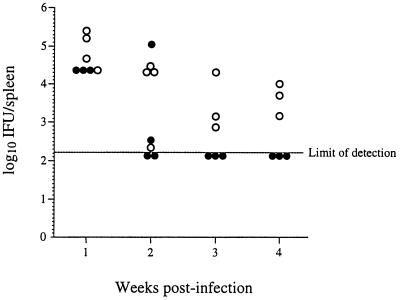
To determine the extent to which infection is prolonged in IFN-γ−/− knockout mice, we expanded the experiment described above to compare the level of infection in IFN-γ+/+ mice over 4 weeks to the level of infection in IFN-γ−/− mice. Groups of IFN-γ+/+ mice and IFN-γ−/− mice were infected with 106 C. trachomatis IFU, and members of the groups were sacrificed 1, 2, 3, and 4 weeks postinfection. Except for one animal sacrificed at week 2, all of the IFN-γ−/− mice failed to completely resolve the infection before sacrifice (Fig. 2). All IFN-γ+/+ mice sacrificed after week 2 had resolved the infection. These results demonstrate that the IFN-γ−/− mice have a deficit in the resolution of C. trachomatis infection which extends at least 1 month postinfection.
FIG. 2.
Ability of IFN-γ+/+ and IFN-γ−/− mice to resolve infection with C. trachomatis. Groups of IFN-γ+/+ mice (•) and IFN-γ−/− mice (○) were infected with 106 IFU of C. trachomatis. Members of each group were sacrificed at 1, 2, 3, and 4 weeks as shown. For the IFN-γ+/+ versus IFN-γ−/− values at each time point, P < 0.01.
The immune defect in IFN-γ−/− mice cannot be overcome by adoptive transfer of IFN-γ+/+ CTL.
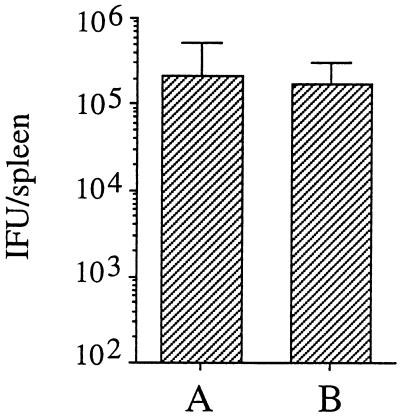
We previously have shown that adoptive transfer of Chlamydia-specific CTL into infected mice reduces the number of C. trachomatis IFU per spleen 20- to 30-fold (16). In the present experiments, we sought to determine whether adoptive transfer of IFN-γ+/+ CTL into infected IFN-γ−/− mice would reduce or resolve the immune deficit seen in these mice in the experiments described above. Groups of mice were infected i.v. with 106 C. trachomatis IFU and 30 min later were given 107 C. trachomatis-specific CTL from culture (line 69, described in reference 16). This cell line has been shown previously (16) and in this study (see Fig. 5) to confer protection against Chlamydia. Control groups were infected and given injections of PBS in place of CTL. Three days later the animals were sacrificed and the number of IFU per spleen was determined. As shown in Fig. 3, adoptive transfer of CTL able to secrete IFN-γ did not reduce the level of infection as compared to control groups not receiving CTL.
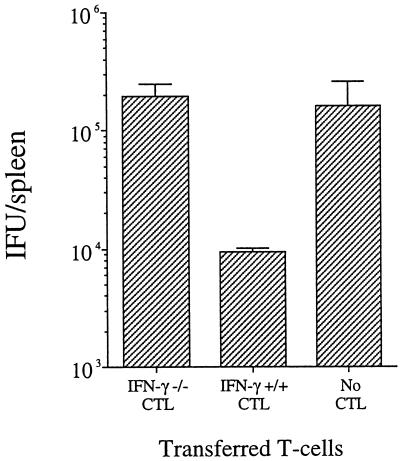
FIG. 5.
Effect of adoptive transfer of IFN-γ−/− CTL on infection with C. trachomatis. Mice were infected i.v. with 106 C. trachomatis IFU. Thirty minutes after infection, animals were injected i.v. with IFN-γ−/− CTL, IFN-γ+/+ CTL, or PBS without CTL. Groups consisted of five animals each. Bars show the number of IFU per spleen determined 3 days after infection.
FIG. 3.
Effect of adoptive transfer of T cells on infection levels in IFN-γ−/− mice. Mice were infected i.v. with 106 IFU of C. trachomatis and 30 min later with Chlamydia-specific CTL from IFN-γ+/+ mice (A) or PBS without T cells (B). Groups consisted of five animals each. Bars show the number of IFU per spleen determined 3 days after infection (P > 0.05).
Chlamydia-specific CTL can be primed in IFN-γ−/− mice.
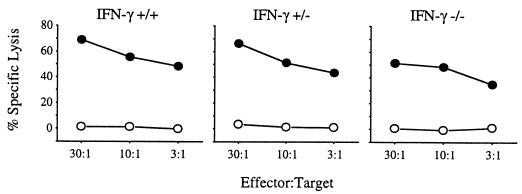
To determine if the inability of IFN-γ to resolve infection results from an inability to mount a specific CTL response, we attempted to culture C. trachomatis-specific CTL lines from IFN-γ+/+, IFN-γ+/−, and IFN-γ−/− mice. BALB/c mice with IFN-γ+/+, IFN-γ+/−, and IFN-γ−/− genotypes were infected intraperitoneally with 107 IFU of C. trachomatis, allowed to recover for 14 days, and then sacrificed. The spleen cells were then stimulated in vitro on C. trachomatis-infected J774 cells for 3 weeks, and the resulting CTL lines were tested for the ability to lyse BALB/3T3 cells infected with C. trachomatis. As shown in Fig. 4, Chlamydia-specific CTL were primed in animals of all three IFN-γ genotypes. No lysis of the uninfected controls was apparent with any of the resulting CTL lines. Each of the CTL cell lines was >90% CD8+ T cells, as measured by flow cytometry (data not shown). These results suggest that although the mice in which the gene for IFN-γ has been disrupted are impaired in the ability to resolve C. trachomatis infection, they are able to mount a Chlamydia-specific CTL response.
FIG. 4.
Lysis of cells infected with C. trachomatis by CTL in a 51Cr release assay. The CTL lines were stimulated from spleen cells of IFN-γ+/+, IFN-γ+/−, and IFN-γ−/− mice following intraperitoneal infection with C. trachomatis. Target cells were C. trachomatis serovar L2-infected (•) or uninfected (○) BALB/3T3 cells.
The amount of IFN-γ produced by these IFN-γ+/+, IFN-γ+/−, and IFN-γ−/− CTL lines was measured 12 h after stimulation on C. trachomatis-infected J774 cells as described above. The concentrations of IFN-γ in the assay wells following stimulation were 158 ng/ml (±23 ng/ml) for the IFN-γ+/+ line, 81 ng/ml (±22 ng/ml) for the IFN-γ+/− line, and 0 ng/ml for the IFN-γ−/− line.
Adoptive transfer of IFN-γ−/− CTL does not protect against C. trachomatis infection.
We have shown previously that the protective effect seen following adoptive transfer of CTL into infected mice is abrogated when the animals have been treated previously with a neutralizing antibody specific for IFN-γ. This finding suggested that IFN-γ production by CTL is required for the observed protective effect. To more directly test this hypothesis, we conducted adoptive transfer studies using a CTL line cultured from an IFN-γ−/− mouse (the line represented in Fig. 4). To determine whether these IFN-γ−/− CTL (which are unable to produce IFN-γ) could adoptively transfer protection, we infected groups of BALB/c mice i.v. with 106 IFU of C. trachomatis, followed 30 min later with 107 IFN-γ−/− CTL. Control groups were infected and then injected with 107 IFN-γ+/+ CTL (line 69 [16]) (as a positive control) or with PBS in place of CTL (as a negative control). As shown in Fig. 5, mice injected with IFN-γ−/− CTL showed no protective effect—the level of infection was indistinguishable from that in animals given no CTL (P > 0.05). Animals given IFN-γ+/+ CTL showed the same level of protection as reported previously (P < 0.01) (16). These data suggest that IFN-γ production by CTL is required for their ability to confer protection upon adoptive transfer.
DISCUSSION
The results presented here (Fig. 1 and 2) demonstrate that mice deficient in IFN-γ are impaired in the ability to resolve C. trachomatis infection. There are at least two mechanisms by which IFN-γ might act to resolve these infections. IFN-γ is a potent activator of macrophages, and organisms within activated macrophages are readily destroyed. It could be envisioned that IFN-γ-activated macrophages destroy elementary bodies phagocytosed at the site of infection. Alternatively, the IFN-γ may act to directly limit C. trachomatis replication. Many in vitro studies have shown that treatment of infected cells with IFN-γ limits the replication of Chlamydia (1–3, 6, 11, 13, 14). Previous experiments examining the course of C. trachomatis infections in mice treated with neutralizing anti-IFN-γ antibody showed a pronounced increase in organisms recovered from multiple organs (19, 20). The results shown in Fig. 1 and 2 support the notion that IFN-γ is an important mediator of resistance to C. trachomatis. Studies are now being conducted to define the cell type and site where IFN-γ acts to provide protection against C. trachomatis. The mechanism of protection at this site also remains undetermined.
Others have recently used IFN-γ−/− mice to study the course of Chlamydia genital tract infection in the absence of this cytokine (4, 12). Consistent with the data shown here, these reports describe a delay in the ability of IFN-γ−/− mice to resolve these infections (4, 12). Although in these studies the IFN-γ−/− mice were deficient in clearing the infections, they could resolve subsequent reinfection to the same extent as control IFN-γ+/+ mice. Experiments using IFN-γ receptor-deficient (IFN-γR−/−) mice also revealed an inability to control genital infection with C. trachomatis (10). However, in contrast to the studies using IFN-γ−/− mice, infected IFN-γR−/− mice which had resolved infection were not protected upon reinfection (10).
In this study, we find that although IFN-γ+/+ mice show protection upon adoptive transfer of CD8+ T cells, infection levels in IFN-γ−/− mice are not reduced by adoptive transfer of known protective IFN-γ+/+ CTL lines. This result suggests that adoptive transfer of CD8+ T cells cannot overcome the immune deficit seen in IFN-γ−/− mice. It is likely that the transfer of other cell types which produce IFN-γ, including Chlamydia-specific CD4+ T cells, would be required to resolve infection in IFN-γ−/− mice. We also show that despite the limited ability of IFN-γ−/− mice to control infection with C. trachomatis, there is a CD8+ T-cell response primed in these mice during infection. We were able to culture Chlamydia-specific CTL lines from these mice and use these IFN-γ−/− CTL lines to pursue the role of IFN-γ in the adoptive transfer of protection.
Two mechanisms by which transferred CTL might mediate protection were considered. First, CTL lysis of cells during intracellular replication of C. trachomatis might release the metabolically active reticulate bodies, depriving the organism of its intracellular niche and releasing a form of the organism unable to infect neighboring cells. Alternatively, the protective effect might be primarily driven by the release of IFN-γ from CTL activated through recognition of a Chlamydia-infected cell. The local increase in IFN-γ concentration might act either by stimulating the antimicrobial activity of nonspecific effector cells such as macrophages, clearing the area of organisms and limiting spread, or by exerting a direct inhibitory effect on C. trachomatis replication. In experiments designed to differentiate these two possibilities, we infected groups of IFN-γ+/+ mice with C. trachomatis and then transferred into these animals either an IFN-γ+/+ or an IFN-γ−/− CTL line. Only the IFN-γ+/+ CTL line was able to reduce the level of C. trachomatis infection in these animals (Fig. 5). This finding suggests that production of IFN-γ by the transferred CTL is required for the protective effect and supports the hypothesis that a protective mechanism dependent on IFN-γ is responsible for protection rather than cytotoxicity. As perforin-, granzyme B-, and Fas ligand (gld)-deficient mice become available in the H-2d haplotype, we will attempt to culture Chlamydia-specific CD8+ T cells from these mice and assess their ability to confer protection. These CD8+ T cells would have deficits in the ability to induce cell death by cytotoxic mechanisms and apoptosis.
Both CD8+ CTL and CD4+ T cells produce IFN-γ in response to activation. The ability of both types of T cells to reduce C. trachomatis infection has been described elsewhere (8, 16, 17) and may allow for redundant or supplementary systems to ensure the production of this cytokine in response to infection.
ACKNOWLEDGMENTS
We thank Lamar M. Ballweber and Andrey O. Kiselev for excellent technical assistance, Jim Hughes for help with statistical analysis, and Sarah D’Orazio for critical reading of the manuscript.
This work was supported by Public Health Service grants AI39558, AI31448, and HD18184. M.N.S. is the recipient of a Junior Faculty Research Award from the American Cancer Society.
REFERENCES
- 1.Beatty W L, Byrne G I, Morrison R P. Morphologic and antigenic characterization of interferon gamma-mediated persistent Chlamydia trachomatis infection in vitro. Proc Natl Acad Sci USA. 1993;90:3998–4002. doi: 10.1073/pnas.90.9.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beatty W L, Morrison R P, Byrne G I. Persistent chlamydiae: from cell culture to a paradigm for chlamydial pathogenesis. Microbiol Rev. 1994;58:686–699. doi: 10.1128/mr.58.4.686-699.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byrne G I, Lehmann L K, Landry G J. Induction of tryptophan catabolism is the mechanism for gamma-interferon-mediated inhibition of intracellular Chlamydia psittaci replication in T24 cells. Infect Immun. 1986;53:347–351. doi: 10.1128/iai.53.2.347-351.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cotter T W, Ramsey K H, Miranpuri G S, Poulsen C E, Byrne G I. Dissemination of Chlamydia trachomatis chronic genital tract infection in gamma interferon gene knockout mice. Infect Immun. 1997;65:2145–2152. doi: 10.1128/iai.65.6.2145-2152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalton D K, Pitts-Meek S, Keshav S, Figari I S, Bradley A, Stewart T A. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 6.de la Maza L M, Plunkett M J, Carlson E J, Peterson E M, Czarniecki C W. Ultrastructural analysis of the anti-chlamydial activity of recombinant murine interferon-gamma. Exp Mol Pathol. 1987;47:13–25. doi: 10.1016/0014-4800(87)90003-7. [DOI] [PubMed] [Google Scholar]
- 7.Howard L, Orenstein N S, King N W. Purification on Renografin density gradients of Chlamydia trachomatis grown in the yolk sac of eggs. Appl Microbiol. 1974;27:102–106. doi: 10.1128/am.27.1.102-106.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Igietseme J U, Rank R G. Susceptibility to reinfection after a primary chlamydial genital infection is associated with a decrease of antigen-specific T cells in the genital tract. Infect Immun. 1991;59:1346–1351. doi: 10.1128/iai.59.4.1346-1351.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson E B, Smadel J E. Immunization against scrub typhus. II. Preparation of lyophilized living vaccine. Am J Hyg. 1951;53:326–331. doi: 10.1093/oxfordjournals.aje.a119457. [DOI] [PubMed] [Google Scholar]
- 10.Johansson M, Schon K, Ward M, Lycke N. Genital tract infection with Chlamydia trachomatis fails to induce protective immunity in gamma interferon receptor-deficient mice despite a strong local immunoglobulin A response. Infect Immun. 1997;65:1032–1044. doi: 10.1128/iai.65.3.1032-1044.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayer J, Woods M L, Vavrin Z, Hibbs J B J. Gamma interferon-induced nitric oxide production reduces Chlamydia trachomatis infectivity in McCoy cells. Infect Immun. 1993;61:491–497. doi: 10.1128/iai.61.2.491-497.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perry L L, Feilzer K, Caldwell H D. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. J Immunol. 1997;158:3344–3352. [PubMed] [Google Scholar]
- 13.Rothermel C D, Byrne G I, Havell E A. Effect of interferon on the growth of Chlamydia trachomatis in mouse fibroblasts (L cells) Infect Immun. 1983;39:362–370. doi: 10.1128/iai.39.1.362-370.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shemer Y, Sarov I. Inhibition of growth of Chlamydia trachomatis by human gamma interferon. Infect Immun. 1985;48:592–596. doi: 10.1128/iai.48.2.592-596.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stamm W E, Tam M, Koester M, Cles L. Detection of Chlamydia trachomatis inclusions in McCoy cell cultures with fluorescein-conjugated monoclonal antibodies. J Clin Microbiol. 1983;17:666–668. doi: 10.1128/jcm.17.4.666-668.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Starnbach M N, Bevan M J, Lampe M F. Protective cytotoxic T lymphocytes are induced during murine infection with Chlamydia trachomatis. J Immunol. 1994;153:5183–5189. [PubMed] [Google Scholar]
- 17.Su H, Caldwell H D. CD4+ T cells play a significant role in adoptive immunity to Chlamydia trachomatis infection of the mouse genital tract. Infect Immun. 1995;63:3302–3308. doi: 10.1128/iai.63.9.3302-3308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang S-P, Kuo C-C, Grayston J T. A simplified method for immunological typing of trachoma-inclusion conjunctivitis-lymphogranuloma venereum organisms. Infect Immun. 1973;7:356–360. doi: 10.1128/iai.7.3.356-360.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams D M, Byrne G I, Grubbs B, Marshal T J, Schachter J. Role in vivo for gamma interferon in control of pneumonia caused by Chlamydia trachomatis in mice. Infect Immun. 1988;56:3004–3006. doi: 10.1128/iai.56.11.3004-3006.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhong G, Peterson E M, Czarniecki C W, Schreiber R D, de la Maza L M. Role of endogenous gamma interferon in host defense against Chlamydia trachomatis infections. Infect Immun. 1989;57:152–157. doi: 10.1128/iai.57.1.152-157.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]