Abstract
Introduction
Human albumin is used in the treatment of complications of cirrhosis. However, the use of long-term human albumin administration is costly and resource demanding for both patients and healthcare systems. A precision medicine approach with biomarkers to predict human albumin treatment response, so-called predictive biomarkers, could make this a viable treatment option in patients with cirrhosis and ascites.
Methods and analysis
ALB-TRIAL is a multinational, double-blind, placebo-controlled randomised controlled trial. We aim to validate a predictive biomarker, consisting of a panel of circulating metabolites, to predict the treatment response to human albumin in patients with cirrhosis and ascites. All enrolled patients are stratified into a high-expected or low-expected effect stratum of human albumin based on the biomarker outcome. After stratification, patients in each group are randomised into either active treatment (20% human albumin) or corresponding placebo (0.9% NaCl) every 10th day for 6 months. The primary outcome is the cumulative number of liver-related events (composite of decompensation episodes, transjugular intrahepatic shunt insertion, liver transplantation and death). Key secondary outcomes include time-to-event analysis of primary outcome components, an analysis of the total healthcare burden and a health economic analysis.
Ethics and dissemination
The trial obtained ethical and regulatory approval in Denmark, Germany, the Netherlands, Belgium, Hungary and Spain through the Clinical Trials Information System (CTIS) from 13 February 2023, while UK approvals from the Health Regulatory Authority, Medicines and Healthcare products Regulatory Agency and Research Ethics Committee are pending. Findings will be published in peer-reviewed journals, presented at conferences, communicated to relevant stakeholders and in the public registry of CTIS, following trial completion.
Trial registration number
NCT05056220 EU CT: 2022-501006-34-01
Keywords: hepatology, protocols & guidelines, randomized controlled trial, neglected diseases, health economics, hepatobiliary disease
STRENGTHS AND LIMITATIONS OF THIS STUDY.
The predictive biomarker of human albumin treatment response will be validated in a randomised clinical trial.
The inclusion of patients with both a high-expected and low-expected effect of human albumin treatment allows for more granular insight into biomarker cut-off values and cost-effectiveness of the intervention.
Patients, investigators and outcome assessors are all masked to the outcome of the predictive biomarker response during the trial.
Since serum albumin is routinely measured in patients with liver cirrhosis, it is not possible to blind investigators to treatment groups (human albumin or saline) during the trial.
However, masking of patients and outcome assessors will ensure treatment masking at the highest methodological attainable level.
Introduction
Background and rationale
Patients with liver cirrhosis and ascites have a reduced quality of life, frequent hospitalisations and a high mortality.1 Part of the pathophysiology of ascites is a reduced vascular oncotic pressure due to hypoalbuminaemia. For decades, plasma-derived human albumin has been used to treat patients with complications of cirrhosis, despite its limited availability, as no synthetic agent with similar properties exists.2 Current evidence-based guidelines support the use of human albumin in patients with cirrhosis during large volume paracentesis, hepatorenal syndrome and spontaneous bacterial peritonitis (SBP).3 Several other indications with short-term treatment have been investigated, such as the prevention of non-SBP infections, kidney dysfunction and minimal hepatic encephalopathy.4–6 With either negative primary outcomes or a low number of participants, the results for these novel indications have been insufficient to become standard-of-care. In addition to this, the efficacy of long-term human albumin administration in patients with cirrhosis and ascites has been investigated in several trials, but with conflicting results. The midodrine and albumin for cirrhotic patients in the waiting list for liver transplantation (MACHT) trial was a double-blind trial of 196 patients where an effect of long-term human albumin on the development of complications to cirrhosis was not shown.7 On the contrary, the Italian open-label human albumin for the treatment of ascites in patients with hepatic cirrhosis (ANSWER) trial with 440 patients observed an 18-month survival benefit of long-term human albumin administration.8 However, in the general population with cirrhosis and ascites, the effect size of the treatment was modest and corresponded to a number-needed-to-treat (NNT) of 24, making wide uptake difficult. Therefore, a more targeted approach is necessary to make long-term administration of human albumin an available treatment option in this patient population.
Precision medicine in patients with cirrhosis and ascites
Biomarkers that can predict the response to long-term human albumin treatment could serve to identify patients eligible for albumin treatment and reduce the NNT substantially. As part of the MICROB-PREDICT Horizon 2020 EU Consortium (www.microb-predict.eu), we have developed a panel of predictive blood metabolite biomarkers capable of distinguishing between human albumin treatment responders and non-responders in an observational setting.9 However, the observational nature of the findings limits causal inference, necessitating a randomised clinical trial for the validation of the biomarker panel.
Trial objective
The primary objective of ALB-TRIAL is to validate the predictive blood metabolite biomarkers of treatment response to human albumin treatment in patients with cirrhosis and ascites.
Methods and analysis
Setting
ALB-TRIAL is an investigator-initiated, randomised, double-blind, placebo-controlled, multicentre clinical biomarker validation trial. Participants will be stratified to high-expected or low-expected effect of albumin according to the biomarker and treated for 6 months with human albumin or placebo (saline) in a 1:1 ratio.
The trial setting is outpatient clinics of hospitals in Belgium, Denmark, Germany, Hungary, Spain, the Netherlands and the UK. A full list of recruiting sites is available at ClinicalTrials.gov (NCT05056220). We used the Standard Protocol Items: Recommendations for Interventional Trials checklist when writing our report.10
Eligibility criteria
The target population consists of adult patients with cirrhosis and ascites that are clinically stable (defined as >5 days since the resolution of a decompensating event or any other condition requiring hospitalisation) at the time of enrolment. There is no upper age limit for this trial. Exclusion criteria include severe extrahepatic comorbidities and known hypersensitivity to human albumin. The full list of inclusion and exclusion criteria is available in box 1.
Box 1. Inclusion and exclusion criteria.
Inclusion criteria
Decompensated liver cirrhosis defined as Child-Pugh score 7-12
Clinical and/or ultrasound evidenced ascites
Age >18 years
>5 days since resolution of a decompensation event or any condition requiring hospitalisation
Exclusion criteria
Patients with acute or subacute liver failure without underlying cirrhosis
Patients with cirrhosis who develop decompensation in the postoperative period following partial hepatectomy
Refractory ascites as defined by the International Ascites Club
Existing Transjugular intrahepatic portosystemic shunt
Portal vein thrombosis
Severe alcoholic hepatitis (Glasgow Alcoholic Hepatitis Score > 11)
Hepatic encephalopathy grade III-IV according to West-Haven criteria
Current, planned or previous treatment with direct antiviral agents for HCV in the last six months
Contraindications for human albumin infusion (pulmonary oedema, hypersensitivity etc.)
Evidence of current malignancy except for non-melanocytic skin cancer and hepatocellular carcinoma within BCLC-0 or BCLC-A
Presence or history of severe extra-hepatic diseases (e.g., chronic renal failure requiring hemodialysis, severe heart disease (NYHA > II); severe chronic pulmonary disease (GOLD Score > C), severe neurological and psychiatric disorders, pulmonary arterial hypertension)
HIV positive or other condition associated with and/or requiring immunosuppression
Previous liver or other transplantation
Pregnancy
Breastfeeding
Patients who decline to participate, patients who cannot provide prior written informed consent due to other causes than hepatic encephalopathy or patients with hepatic encephalopathy who cannot provide prior written informed consent and when there is documented evidence that the patient has no legal surrogate decision maker or sufficient ability to provide delayed informed consent
Physician’s denial (investigator considers that the patient will not adhere to the study protocol scheduled, e.g. in case of heavy drinking)
Participation in another study within 3 months prior to screening
Interventions
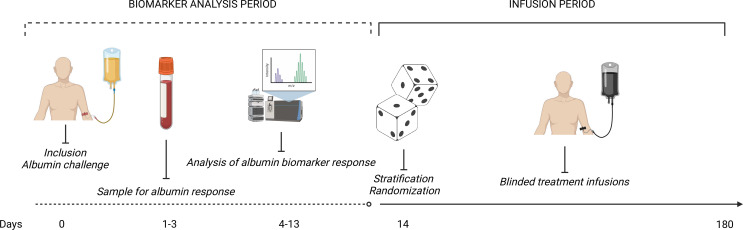
After enrolment, all participants will undergo an open-label human albumin challenge. The albumin challenge will be a weight-adjusted dose of a 20% human albumin solution of 1.5 g/kg of bodyweight with a maximum of 100 g (500 mL). The predictive biomarker of response to human albumin is sampled 1–3 days after the albumin challenge (figure 1). The biomarker sample is thereafter shipped to a central laboratory in Münster, Germany, where the panel of metabolites are measured. The specific metabolites in the biomarker panel will become available with the study protocol in the Clinical Trials Information Systems (CTIS) public registry. The outcome of the blood metabolite panel will stratify patients into high-expected or low-expected effect of human albumin. Following stratification, randomisation occurs into either active treatment with 20% human albumin (1.5 g/kg of bodyweight with a maximum of 100 g) or placebo (0.9% NaCl in the corresponding volume). The initial infusion rate will be 100 mL every 30 min, with the possibility of adjusting the rate according to the state of the individual participant. Infusion visits are scheduled at 10 days intervals during the follow-up period. In case a participant is hospitalised during a scheduled infusion visit, the infusion can take place in an inpatient setting with consideration given to the participant’s status. Since human albumin is recommended following large-volume paracentesis, in the treatment of spontaneous bacterial peritonitis and hepatorenal syndrome, some participants may receive human albumin infusions as part of the standard medical treatment. We allow concomitant human albumin during the whole trial period, and dose adjustment of the protocoled infusions will only occur in cases where site investigators foresee an elevated and clinically relevant risk of fluid overloading the participant.
Figure 1.
Trial overview. Overview of the trial from the perspective of the participant. All enrolled patients will undergo an open-label, weight-adjusted, human albumin challenge. Treatment response biomarker will be collected 1–3 days after this challenge. Within 14 days shall the biomarker response be available, so that stratification (high-expected or low-expected effect) and randomisation (20% human albumin or 0.9% NaCl) can occur. Patients will receive blinded treatment infusion of their assigned intervention up until day 180 after inclusion.
Outcomes
The primary outcome is the cumulative number of liver-related events (box 2). The primary analysis will be based on the difference in the incidence of events between the two strata, that is, high-expected versus low-expected effect (figure 2). Death, insertion of transjugular intrahepatic shunt (TIPS) and liver transplantation (LTx) all serve as counting and censoring events. We chose the cumulative composite primary endpoint of decompensation episodes, death, insertion of TIPS and LTx to evaluate the predictive biomarker efficacy, since it reflects the total healthcare burden for patients with cirrhosis and ascites. Importantly, TIPS insertion is a counting event since it often reflects a deteriorated state of health for patients with ascites and furthermore a censoring event due to the clinically relevant increased risk of hepatic encephalopathy, which without censoring may skew the primary outcome model.11 Key secondary outcomes include time-to-event and incidence rates of the primary outcome, hospitalisation rates, health economic and treatment safety analyses. Full list of secondary outcomes is available in online supplemental table 1.
Box 2. Primary outcome: Liver-related events.
-
Decompensation episodes
Variceal bleeding (gastro-oesophageal variceal haemorrhage endoscopical confirmed)
Ascites (large-volume paracentesis defined as removal of > 5 litres)
Spontaneous bacterial peritonitis (ascitic fluid neutrophil count > 250/mm3 on flow cytometry or microscopy without an evident intraabdominal surgically treatable source)
-
Infections requiring hospitalisation (urinary tract infection, pneumonia or bloodstream infection)
AKI >1B or HRS-AKI defined as:
Diagnosis of AKI
No response after two consecutive days of diuretic withdrawal and plasma volume expansion with albumin (1 g per kg of bodyweight)
Absence of shock
No current or recent use of nephrotoxic drugs (NSAIDs, aminoglycosides, iodinated contrast medias etc.)
-
No macroscopic signs of structural kidney injury, defined as:
Absence of proteinuria (>500 mg/day)
Absence of microhaematuria (>50 RBCs per high power field)
Normal findings on renal ultrasonography
Overt hepatic encephalopathy defined as grade II-IV according to West-Haven criteria
Insertion of transjugular intrahepatic shunt
Liver transplantation
Death
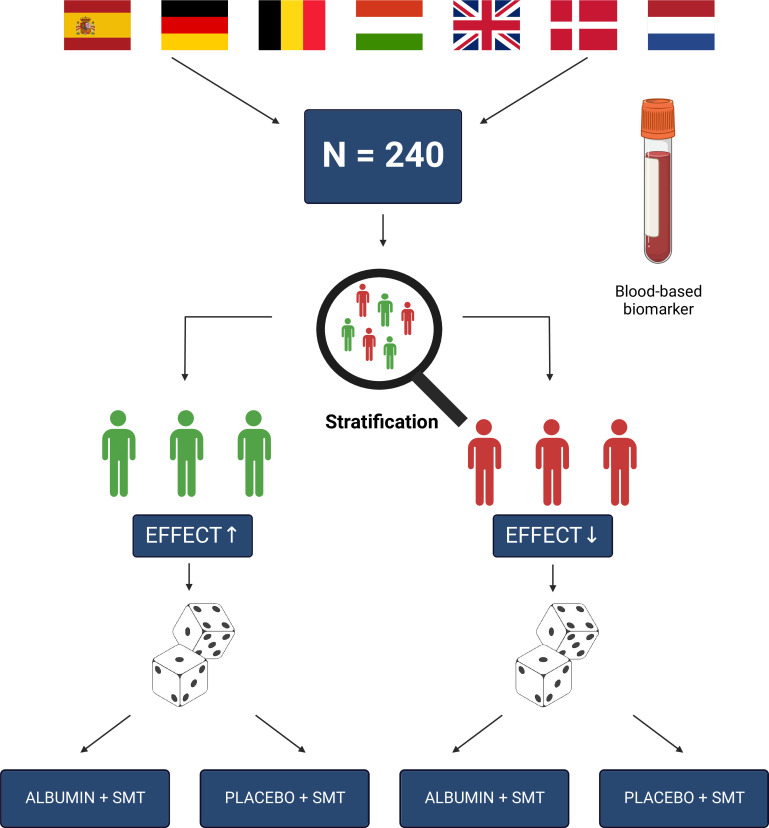
Figure 2.
Trial design. Overview of the trial design. ALB-TRIAL is a multinational trial with recruiting sites from Spain, Germany, Belgium, Hungary, the UK, Denmark and the Netherlands. In total, 240 participants will be enrolled and undergo a biomarker-based stratification according to their expected response to human albumin infusions, before they are randomisation into an active treatment group (albumin) or corresponding placebo (saline). SMT, standard medical treatment.
bmjopen-2023-079309supp001.pdf (127.9KB, pdf)
Participant timeline
We plan to recruit the first participant in March 2024, while last patient last visit is expected in March 2025 with the ending of the MICROB-PREDICT Consortium funding period. On inclusion and prior to randomisation, all participants undergo an open-label weight-adjusted human albumin challenge for stratification purposes (figure 1). We sample blood for the biomarker to predict treatment response on days 1–3 after the human albumin challenge. Stratification, randomisation and first blinded treatment visit are expected to occur within 14 days from inclusion. Every 10th day, we plan an infusion visit with an acceptable deviation of ±4 days. Two extended visits (after 2 and 4 months) are planned, where biobank sampling, in addition to a regular infusion, is scheduled. The last visit is scheduled at 6 months after inclusion. A full schedule of assessment is available in online supplemental table 2.
Sample size
We expect to estimate the incidence of events using an exponential function. We assume a mean observation time of 5.5 months while accounting for censoring events. Based on the arithmetic mean of complication rates from the largest RCT testing albumin in outpatients with decompensated cirrhosis, we expect an incidence rate ratio of 0.65.8 We based our sample size calculation on an equal size of each treatment arm (θ=1) in each stratum, a type I error of 5% and a power of 80%. We used a dispersion parameter (κ) of 0.12 to correct for expected overdispersion from the Poisson distribution to the gamma distribution (negative binomial model) in the low-expected effect stratum. This yielded a total of 55 patients. In the high-expected effect stratum, with the same dispersion parameter, but with an incidence rate ratio of 0.55, we would need 31 patients (n1). Since we expect an equal distribution of patients in the two strata according to the biomarker, we used the most conservative calculation with 55 patients for each treatment arm in each stratum. Expecting a drop-out rate of 8%, we will include a total of 240 patients. This sample size will enable us to show a statistically significant difference of treatment effect in both the high-expected and low-expected effect stratum. The sample size formula below shows that zα/2 is the two-sided type I error, zα is the power. V0 and V1 are the variances of the parameter β1 under the null and alternative hypothesis, respectively; λ1 and λ0 are the ratios of the true event rates between two groups in the low-expected effect stratum, while λ2 and λ3 are the ratios in the high-expected effect stratum.
Recruitment
A participant enrolment rate of between two and three participants per trial site per month was originally estimated to reach the sample size. To account for the delay of trial start, we will activate new trial sites in countries, where ethical and regulatory approval is already granted.
Allocation
We stratify the allocation according to: (1) biomarker response (ie, high-expected or low-expected effect of human albumin), (2) Child-Pugh class (B or C), (3) aetiology of liver cirrhosis (alcohol-related vs others) and (4) trial site. For each stratum, we use a computer-generated random numbers system (www.sealedenvelope.com) to create a 1:1 allocation sequence for active treatment (20% human albumin) or corresponding placebo (0.9% NaCl). The allocation will happen in permuted blocks of two and four. The allocation sequence is generated by a data manager from Odense University Hospital, who is otherwise not involved in the trial. The electronic case report format (eCRF) contains the complete randomisation list, which is inaccessible to participants, investigators and outcome assessors during the trial. The assigned treatment will be allocated directly through a designated instrument in the eCRF.
Blinding
This trial will implement two levels of blinding: (1) blinding to biomarker stratification and (2) blinding to treatment group. At the first level, participants, investigators and outcome assessors will be blinded to the result of the biomarker response stratification. This is ensured by having a separate instrument in the eCRF for biomarker response, which is only visible for the delegated personnel at the central laboratory in Münster, Germany.
At the second level of blinding, the participants and outcome assessors are masked to treatment group. Participants will be masked using infusion vials covered by hanger boxes, while infusion lines will be yellow coloured to ensure an identical visual appearance of the human albumin and saline. Blinded outcome assessment will be performed at the end of the trial, where outcome assessors will review printouts of trial participants’ medical history, where any mention of treatment and serum albumin levels will be cross-marked and left out. Personnel involved during the trial will not be blinded to treatment group, as serum albumin is a routinely measured laboratory value that is difficult to conceal. Unblinding of participants during the trial is only expected to be relevant in cases, where there is suspicion of a hypersensitive reaction and urgent medical care is needed.
Data collection methods
Unblinded outcome assessment will be done consecutively by delegated investigators according to trial-specific standard operating procedures, after 2, 4 and 6 months. For the final efficacy evaluation, blinded outcome assessors will perform the outcome assessment when enrolment and all scheduled visits are completed for the trial site. In case of discrepancy between the blinded and unblinded outcome assessment, an independent representative from the sponsor organisation will adjudicate the outcomes in question.
For the purpose of quality of life outcomes, we will use the Chronic Liver Disease Questionnaire, Short Form-36 and EuroQol-5D which are all validated.12–14 A small and large selection of routine laboratory tests are scheduled at designated visits during the trial (online supplemental table 2). All measurements of the predictive biomarker for the stratification will be performed with validated assays on a LCMS-8050 CL mass spectrometer platform (Shimadzu, Japan).
In cases where trial participants discontinue the trial or reach the prespecified non-compliance threshold, any data that can be captured through the patients (electronic) medical record will be recorded, at the designated time points for the visits. If a participant discontinues due to an adverse reaction to the trial treatment, the remaining visits will be done as planned, but without any infusions.
Data management
Trial data will be collected and managed using Research Electronic Data Capture (REDCap) electronic data capture tools hosted at Open Patient data Explorative Network, Odense University Hospital, Region of Southern Denmark.15 16 A risk-based approach is applied to the monitoring of data entries. An initial Good Clinical Practice (GCP) on-site monitoring visit with full data review is scheduled after the inclusion of two participants at each trial site. The on-site monitoring is accompanied by central monitoring, where the sponsor review data entries according to the central monitoring plan. Range checks are defined for routine laboratory tests according to local units of trial sites.
Statistical methods
The primary outcome of liver-related clinical outcomes will be analysed as a binomial recurrent event model with the objective of testing the difference in events between the high-expected and low-expected effect strata (figure 3). All time-to-event secondary outcomes are analysed as incidences and compared by Kaplan-Meier curves with log-rank tests. For numerical continuous secondary outcomes, analyses will be based on Student’s t-test or Wilcoxon Mann-Whitney U test depending on the distribution of the data. Subgroup analyses will be performed according to:
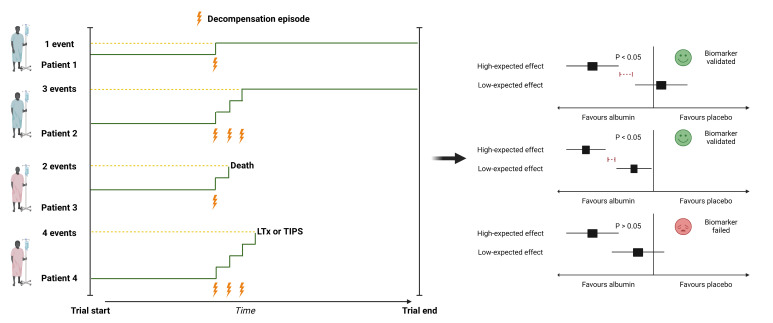
Figure 3.
Primary outcome: recurrent event model. Left part of the figure depicts the conceptual model of the primary outcome (composite end point of liver-related events). Participants are followed and treated for up to 6 months. Decompensation episodes, insertion of a transjugular intrahepatic portosystemic shunt (TIPS), liver transplantation (LTx) and death are counting events. TIPS and LTx are in addition to death censoring events for the purpose of the primary analysis. Right part of the figure shows the potential scenarios and outcome of the primary analysis. The primary analysis is based on the comparison between the number of events in the high-expected and low-expected effect strata. The predictive biomarker of treatment response to human albumin is considered validated if the rate of events between treatment arms (albumin compared with placebo) is lower in the high-expected effect stratum compared with the low-expected effect stratum.
Trial site
Compliance (≥80% of scheduled treatments)
Child-Pugh score (≤9)
Initial serum albumin concentration (<35 g/L)
Aetiology (alcohol-related vs others)
High biomarker response (defined as highest quartile) versus low biomarker response (defined as lowest quartile)
All primary analyses will be adjusted for stratifying variables.
The intention-to-treat (ITT) population is defined as all randomised patients.
The per-protocol population is defined as the ITT population, who did not present with serious protocol violations, received >80% of the scheduled trial treatments and who were not withdrawn from the trial due to non-adherence (interruption of treatment for >4 weeks).
In cases where time-to-event data are missing, participants are censored from the time of their latest trial visit. For dichotomous missing data, we will assume that participants did not experience the outcome of interest. For continuous missing data the last observation will be carried forward. If no observation can be carried forward, we will perform multiple imputations based on age, gender, aetiology of liver cirrhosis and severity of liver disease (Child-Pugh score).
Data monitoring
A Data and Safety Monitoring Committee (DSMC) consisting of three independent clinical and statistical experts within the field of hepatology will oversee the trial. The DSMC will monitor and oversee the safety of trial participants, review participant recruitment, accrual, retention and withdrawal. Finally, the DSMC will review biomarker data with the possibility of recalibration of the biomarker cut-off in cases of significant imbalances between the stratification groups.
The first full review meeting of the DSMC will occur after 10% (n=24) of participants has been included. An interim analysis of the biomarker cut-off is planned after the inclusion of 40 participants, while the subsequent meeting frequency will be once yearly. The DSMC charter is available as part of the application dossier for trial in the Clinical Trials Information System (EU CT: 2022-501006-34-01).
Harms
Adverse events (AEs) and serious adverse events (SAEs) are defined according to the International Conference for Harmonisation (ICH-GCP). Since SAEs that concern the primary outcome are expected in patients with liver cirrhosis and ascites, they are exempted from immediate reporting within 24 hours. Instead, local investigators are required to report within 7 days, unless the AE in question is considered attributed to the intervention. Such cases follow the regular reporting timelines within 24 hours.
Patient and public involvement
As a partner in the MICROB-PREDICT Horizon 2020 consortium, the European Liver Patient Association (ELPA) was actively involved in the design of the trial. Here, ELPA has served as the voice of the potential participant regarding the burden of the intervention and potential patient population based on the eligibility criteria.
Protocol amendments
Any amendments to the approved protocol will undergo ethical and regulatory review prior to implementation. Investigators will be informed about changes at virtual investigator meetings every second week and through other electronic means of communication. Trial registries will be updated as appropriate. If changes to the trial protocol affect the treatment or follow-up of participants, trial site staff will inform participants at the first upcoming scheduled trial visit.
Consent procedures
Informed consent to participate in the trial will primarily be sought from patients themselves with appropriate reflection time as per national legislation. Hepatic encephalopathy is a cerebral manifestation of liver cirrhosis which affects neurological function in varying degree with a dynamic course. Even though all potential participants are outpatients, it is believed that some eligible may be affected by a mild degree hepatic encephalopathy. In such cases, a surrogate consent will be sought as per national regulation in the participating countries. For participants who during the trial may regain sufficient neurological function to understand the implications of the trial, a renewed informed consent from the patient will be sought. Model consent forms are available in online supplemental file 1.
Confidentially
All data captured in the eCRF will be processed and stored in a pseudonymised manner. Personal sensitive information linking the patient to their participant ID will only be kept on-file at trial sites in a secure place. Trial-specific documents and data will be kept on-file at trial sites as part of the investigator site files for 25 years after the termination of the trial as per the Clinical Trial Regulation (536/2014).
Access to data
Access to the final trial dataset is governed by the Joint Data Controllership Agreement of the MICROB-PREDICT participating parties together with the Data Management Plan of ALB-TRIAL.
Dissemination policy
Both positive and negative results will be published in peer-reviewed academic journals and at scientific congresses within the field of hepatology. Participants will be asked if they would like to be contacted about the final results together with their treatment and stratification group after the end of the trial. Primary and secondary outcomes will be published in CTIS, maximum 12 months after trial completion where the full trial protocol will also be available. There is no planned use of professional academic writers. Inquiries for participant-level datasets will be evaluated on a case-by-case basis to ensure that any sharing is in line with applicable data protection regulation and the consent of the participants.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the contributions the following: Maria Fogt, Peter Andersen, Emil Deleuran Hansen, Ida Spedtsberg, Stine Johansen and Charlotte Calov for their supportive work at Odense University Hospital sponsor site. Anna Bosch, Cristina Sánchez Garrido, Patricia Sierra and Yolanda Godoy for their work with the biobank and co-sponsor responsibilities at European Foundation for the Study of Chronic Liver Failure. Ameli Schwalber (concentris research management GmbH) for the project management of MICROB-PREDICT. Christophe Junot (Commissariat à l'énergie atomique et aux énergies alternatives) for his work with the biomarker. Victoria Kronsten for her help with the ethical and regulatory application submission process in the United Kingdom. Robert Schierwagen (Universitätsklinikum Münster), Wenyi Gu (Universitätsklinikum Münster), Christel Langenstroer (Universitätsklinikum Münster), Balogh Boglárka (Debreceni Egyetem), Annelotte Broekhoven (Leiden University Medical Centre) and Susan Fischer (Leiden University Medical Centre) all for their help and support with the application dossier for the Clinical Trials Information Systems at European Union trial sites. Figures created with Biorender.
Footnotes
Twitter: @NikolajTorp_, @IsraelsenMads, @MinnekeC, @DebbieShawcros1, @Marko_Korenjak, @ProfPaoloAngeli, @JonelTrebicka, @AleksanderKrag
Collaborators: MICROB-PREDICT Consortium: Vicente Arroyo, Cristina Sanchez-Garrido, Ferran Aguilar-Parera, Patricia Sierra-Casas, Jonel Trebicka, Anna Bosch-Comas, Maria Papp, David Tornai, Boglarka Balogh, Aleksander Krag, Nikolaj Torp, Maja Thiele, Katrine Holtz Thorhauge, Mads Israelsen, Casper Sahl Poulsen, Helene Baek Juel, Torben Hansen, Camila Alvarez-Silva, Manimozhiyan Arumugam, Peer Bork, Stefanie Kandels, Michael Kuhn, Thea Van Rossum, Suguru Nishijima, Marisa I Keller, Diënty H M Hazenbrink, Christophe Junot, François Fenaille, Sylvain Dechaumet, Sebastian D Burz, Florence A Castelli, Emeline Chu-Van, Etienne Thévenot, Alain Pruvost, Florian A Rosenberger, Peter V Treit, Philipp E Geyer, Matthias Mann, Benjamin Lelouvier, Florence Servant, Sebastian Van Blerk, Amirouche Ouzerdine, Alain Roulet, Jean-Louis-Marie Insonere, Céline Serres, Manolo Laiola, Mathieu Almeida, Florence Thirion, Camille Champion, Hervé M Blottière, Chaima Ezzine, Célia Chamignon, Nicolas Lapaque, Mamadou Gabou Thiam, Nathalie Galleron, Benoit Quinquis, Lore Van Espen, Wim Laleman, Jelle Matthijnssens, Minneke J Coenraad, Ed J Kuijper, Quinten R Ducarmon, Annelotte GC Broekhoven, Susan Fischer, Romy Zwittink, Itziar De Lecuona, Ivica Letunic, Giulio Rosati, Celia Fuentes-Chust, Arben Merkoçi, Massimo Urban, José Alfonso Marrugo-Ramirez, Hans Olav Melberg, Lars Asphaug, Marko Korenjak, Ameli Schwalber, Debbie Lindsay Shawcross, Vishal C Patel, Mark J W McPhail, Lindsey Ann Edwards, Victoria Tatiana Kronsten, David Moyes, Saeed Shoaie, Azadeh Harzandi, Adrià Juanola, Elisa Pose, Jordi Gratacós-Ginès, Martina Pérez-Guasch, Pere Ginès, Miriam Pellón, Joan Clària, Cristina López-Vicario, Bryan Contreras, Sabine Klein, Robert Schierwagen, Wenyi Gu, Frank Erhard Uschner, Max Brol, Michael Praktiknjo, Manfred Fobker, Renata Antonina Feuerborn.
Contributors: NT, MI, JT and AK drafted this manuscript and first versions of the trial protocol. MC, MP, DS, MK, PA, WL, AJ and PG all reviewed this manuscript, approved the final version and came with contributions to the development of the trial protocol. MK is a patient representative and provided expertise from his perspective. JT received the funding for this trial as the project coordinator of MICROB-PREDICT Horizon 2020 consortium, which all coauthors are part of.
Funding: The MICROB-PREDICT project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 825694.This reflects only the author's view, and the European Commission is not responsible for any use that may be made of the information it contains.
Disclaimer: The funders had no influence in the study design, data collection and analysis, decision to publish or preparation of the current manuscript.
Competing interests: PG received grants form Gilead and Grifols, served on advisory boards for Gilead, RallyBio, SeaBeLife, Merck Sharp and Dohme, Ocelot Bio, Behring and received speaking fees from Pfizer. JT has received speaking and/or consulting fees from Versantis, Gore, Boehringer-Ingelheim, Alexion, Falk, Mallinckrodt, Grifols and CSL Behring and was supported by the German Research Foundation (DFG) project ID 403224013-SFB 1382 (A09), by the German Federal Ministry of Education and Research (BMBF) for the DEEP-HCC project and by the Hessian Ministry of Higher Education, Research and the Arts (HMWK) for the ENABLE and ACLF-I cluster projects. The MICROB-PREDICT (project ID 825694), DECISION (project ID 847949), GALAXY (project ID 668031), LIVERHOPE (project ID 731875) and IHMCSA (project ID 964590) projects have received funding from the European Union’s Horizon 2020 research and innovation programme. AK has served as speaker for Novo Nordisk, Norgine, Siemens and Nordic Bioscience and participated in advisory boards for Norgine, Siemens, Resalis Therapeutics and Novo Nordisk, all outside the submitted work. Research support; Norgine, Siemens, Nordic Bioscience, Astra, Echosense. Consulting Takeda, Resalis Therapeutics, Zealand Pharma, Novo Nordisk, B&I. Board member and co-founder Evido. All other authors have no competing interests to declare.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the 'Methods' section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
Collaborators: MICROB-PREDICT Consortium, Vicente Arroyo, Cristina Sanchez-Garrido, Ferran Aguilar-Parera, Patricia Sierra-Casas, Jonel Trebicka, Anna Bosch-Comas, Maria Papp, David Tornai, Boglarka Balogh, Aleksander Krag, Nikolaj Torp, Maja Thiele, Katrine Holtz Thorhauge, Mads Israelsen, Casper Sahl Poulsen, Helene Baek Juel, Torben Hansen, Camila Alvarez-Silva, Manimozhiyan Arumugam, Peer Bork, Stefanie Kandels, Michael Kuhn, Thea Van Rossum, Suguru Nishijima, Marisa I Keller, Diënty H M Hazenbrink, Christophe Junot, François Fenaille, Sylvain Dechaumet, Sebastian D Burz, Florence A Castelli, Emeline Chu-Van, Etienne Thévenot, Alain Pruvost, Florian A Rosenberger, Peter V Treit, Philipp E Geyer, Matthias Mann, Benjamin Lelouvier, Florence Servant, Sebastian Van Blerk, Amirouche Ouzerdine, Alain Roulet, Jean-Louis-Marie Insonere, Céline Serres, Manolo Laiola, Mathieu Almeida, Florence Thirion, Camille Champion, Hervé M Blottière, Chaima Ezzine, Célia Chamignon, Nicolas Lapaque, Mamadou Gabou Thiam, Nathalie Galleron, Benoit Quinquis, Lore Van Espen, Wim Laleman, Jelle Matthijnssens, Minneke J Coenraad, Ed J Kuijper, Quinten R Ducarmon, Annelotte GC Broekhoven, Susan Fischer, Romy Zwittink, Itziar De Lecuona, Ivica Letunic, Giulio Rosati, Celia Fuentes-Chust, Arben Merkoçi, Massimo Urban, José Alfonso Marrugo-Ramirez, Hans Olav Melberg, Lars Asphaug, Marko Korenjak, Ameli Schwalber, Debbie Lindsay Shawcross, Vishal C Patel, Mark J W McPhail, Lindsey Ann Edwards, Victoria Tatiana Kronsten, David Moyes, Saeed Shoaie, Azadeh Harzandi, Adrià Juanola, Elisa Pose, Jordi Gratacós-Ginès, Martina Pérez-Guasch, Pere Ginès, Miriam Pellón, Joan Clària, Cristina López-Vicario, Bryan Contreras, Sabine Klein, Robert Schierwagen, Wenyi Gu, Frank Erhard Uschner, Max Brol, Michael Praktiknjo, Manfred Fobker, and Renata Antonina Feuerborn
Data availability statement
Data may be obtained from a third party and are not publicly available. Access to the final trial dataset is governed by the Joint Data Controllership Agreement of the MICROB-PREDICT participating parties together with the Data Management Plan of ALB-TRIAL.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Ginès P, Krag A, Abraldes JG, et al. Liver cirrhosis. Lancet 2021;398:1359–76. 10.1016/S0140-6736(21)01374-X [DOI] [PubMed] [Google Scholar]
- 2.Janeway CA, Gibson ST, Woodruff LM, et al. CHEMICAL, clinical, and immunological studies on the products of human plasma fractionation. VII. concentrated human serum albumin. J Clin Invest 1944;23:465–90. 10.1172/JCI101514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol 2018;69:406–60. 10.1016/j.jhep.2018.03.024 [DOI] [PubMed] [Google Scholar]
- 4.Fernández J, Angeli P, Trebicka J, et al. Efficacy of albumin treatment for patients with cirrhosis and infections unrelated to spontaneous bacterial Peritonitis. Clin Gastroenterol Hepatol 2020;18:963–73. 10.1016/j.cgh.2019.07.055 [DOI] [PubMed] [Google Scholar]
- 5.China L, Freemantle N, Forrest E, et al. A randomized trial of albumin infusions in hospitalized patients with cirrhosis. N Engl J Med 2021;384:808–17. 10.1056/NEJMoa2022166 [DOI] [PubMed] [Google Scholar]
- 6.Fagan A, Gavis EA, Gallagher ML, et al. A double-blind randomized placebo-controlled trial of albumin in outpatients with hepatic encephalopathy: HEAL study. J Hepatol 2023;78:312–21. 10.1016/j.jhep.2022.09.009 [DOI] [PubMed] [Google Scholar]
- 7.Solà E, Solé C, Simón-Talero M, et al. Midodrine and albumin for prevention of complications in patients with cirrhosis awaiting liver transplantation. A randomized placebo-controlled trial. J Hepatol 2018;69:1250–9. 10.1016/j.jhep.2018.08.006 [DOI] [PubMed] [Google Scholar]
- 8.Caraceni P, Riggio O, Angeli P, et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): an open-label randomised trial. Lancet 2018;391:2417–29. 10.1016/S0140-6736(18)30840-7 [DOI] [PubMed] [Google Scholar]
- 9.Trebicka J, Fernandez J, Papp M, et al. The PREDICT study Uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J Hepatol 2020;73:842–54. 10.1016/j.jhep.2020.06.013 [DOI] [PubMed] [Google Scholar]
- 10.Chan A-W, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schindler P, Heinzow H, Trebicka J, et al. Shunt-induced hepatic encephalopathy in TIPS: Current approaches and clinical challenges. J Clin Med 2020;9:11. 10.3390/jcm9113784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Younossi ZM, Guyatt G, Kiwi M, et al. Development of a disease specific questionnaire to measure health related quality of life in patients with chronic liver disease. Gut 1999;45:295–300. 10.1136/gut.45.2.295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brazier JE, Harper R, Jones NM, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ 1992;305:160–4. 10.1136/bmj.305.6846.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727–36. 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (Redcap)--A Metadata-driven methodology and Workflow process for providing Translational research Informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris PA, Taylor R, Minor BL, et al. The Redcap consortium: building an international community of software platform partners. J Biomed Inform 2019;95. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-079309supp001.pdf (127.9KB, pdf)
Data Availability Statement
Data may be obtained from a third party and are not publicly available. Access to the final trial dataset is governed by the Joint Data Controllership Agreement of the MICROB-PREDICT participating parties together with the Data Management Plan of ALB-TRIAL.