Abstract
We have previously shown that Clostridium difficile toxin A induces detachment of human colonic epithelial cells from the basement membrane and subsequent cell death by apoptosis. Because these cells require adhesion-dependent signalling from the extracellular matrix for survival, their detachment from the basement membrane by other means also induces apoptosis. The role of toxin A in the induction of apoptosis therefore remains to be determined. In addition, sensitivities to C. difficile toxin A of lamina propria lymphocytes, macrophages, and eosinophils, which lie below the surface epithelium, are not known. In contrast to epithelial cells, these lamina propria cells do not require adhesion-dependent signalling from the extracellular matrix for survival, and this may allow the mechanisms of toxin A-induced cell death to be further investigated. The aim of this study was to investigate the effect of purified C. difficile toxin A on human colonic lamina propria T cells, macrophages, and eosinophils. We show that C. difficile toxin A induces loss of viability in isolated colonic lamina propria cell preparations containing the three different cell types in a dose- and time-dependent fashion. Exposure to high concentrations of the toxin led to loss of macrophages within 72 h. T-lymphocyte and eosinophil cell death was prominent at later time points and occurred by apoptosis. Exposure to toxin A also induced the production of tumor necrosis factor alpha by the isolated colonic lamina propria cells. However, the presence of neutralizing antibodies to this cytokine did not influence C. difficile toxin A-induced T-cell apoptosis. Moreover, purified T cells also underwent apoptosis following exposure to toxin A, implying that apoptosis occurred as a consequence of a direct interaction between T cells and the toxin. Our studies suggest that C. difficile toxin A is capable of suppressing human colonic mucosal immune responses by inducing early loss of macrophages followed by T-cell apoptosis.
Clostridium difficile, a gram-positive anaerobic bacillus, is the most common identifiable bacterial cause of diarrhea in hospitalized patients. It induces colonic disease that is characterized by the infiltration of inflammatory cells into the mucosa. Pseudomembranous colitis is a severe form of the colonic disease in which the pseudomembrane covering the mucosa comprises cells derived from the lamina propria, sloughed epithelial cells, mucin, and fibrin. Toxigenic C. difficile secretes high-molecular-weight toxins A and B, and animal studies have shown that they are responsible for inducing the intestinal disease (14, 20, 27).
The first host cells that C. difficile toxins would interact with in the colon are epithelial cells. In vitro studies have shown that purified toxin A has potent effects on human colonic epithelial cells (5, 8, 17). We have recently shown that in primary human colonocytes and in epithelial cell lines toxin A induces cell rounding and detachment from the basement membrane, followed by programmed cell death (apoptosis) (17). Epithelial cell rounding and detachment preceded apoptosis. Loss of adherence to the extracellular matrix component of the basement membrane per se has also been shown to trigger apoptosis in epithelial and endothelial cells (designated anoikis) (7, 17, 19, 26). It is possible, therefore, that C. difficile toxin A-induced apoptosis in intestinal epithelial cells occurs as a consequence of cell detachment rather than as a direct effect of the toxin. However, the toxin has also been shown to have potent effects within cells, characterized by disruption of the actin cytoskeleton following interaction with the Rho family of GTP binding proteins (1). It is possible that such intracellular action of the toxin in itself subsequently leads to apoptosis. Lamina propria T lymphocytes and macrophages lie below the surface epithelium and are phenotypically and functionally distinct from peripheral blood cells (10, 16). These two cell types are important components of the mucosal immune system, mediating functions such as antigen presentation (15) and cytokine production (2, 10). Following toxin A-induced loss of the colonic surface epithelium in vivo, lamina propria T cells, macrophages, and eosinophils would be exposed to C. difficile toxins via discrete pores in the basement membrane (18). However, responses by these lamina propria cells to C. difficile toxin A are not known. Since they do not require adherence-dependent signalling from components of the extracellular matrix for survival, the induction of apoptosis by C. difficile toxin A in them may allow further studies to investigate the mechanisms of toxin A-induced cell death.
The aim of this study was therefore to investigate responses by isolated normal human colonic lamina propria T cells, macrophages, and eosinophils to purified C. difficile toxin A. We show that the toxin induces loss of viability of the isolated lamina propria cells and demonstrate that there is an early loss of macrophages, followed by T-lymphocyte and eosinophil cell death by apoptosis. C. difficile toxin A also induced the release of tumor necrosis factor alpha (TNF-α) by lamina propria cells, but neutralization of this cytokine did not influence T-cell apoptosis. In addition, exposure of purified T cells to toxin A also induced apoptosis.
MATERIALS AND METHODS
Purification of toxin A.
C. difficile toxin A was purified as described previously (13). Briefly, strain VPI 10463 of toxigenic C. difficile was cultured in dialysis tubing and culture filtrates were applied to a thyroglobulin affinity column. Fractions demonstrating cytotoxicity (as assessed by observing the rounding of Vero cells) and hemagglutinating activity (determined by using a 1% rabbit erythrocyte suspension) were subsequently subjected to two sequential anion exchange chromatographic steps with Q Sepharose FF and Mono Q (both obtained from Pharmacia Biotech, Brussels, Belgium) columns incorporated into a fast protein liquid chromatography apparatus (from Pharmacia Biotech). Aliquots of the purified toxin A (at concentrations of 50 to 100 μg/ml) were frozen at −70°C until used.
Isolation of colonic lamina propria cells.
Human colonic lamina propria cells were obtained by using our recently described model (18), in which there is no requirement for enzymatic digestion. In brief, fresh normal colonic mucosal samples were obtained from specimens of colon resected because of a carcinoma. The samples used were obtained >5 cm from the tumor and were confirmed to be histologically normal. Following incubation (for 15 min at room temperature) with 1 mmol of dithiothreitol (from Sigma Chemical Co., St. Louis, Mo.) per liter, mucosal strips were denuded of epithelial cells by three 30-min incubations (at 37°C) in 1 mmol of EDTA (Sigma Chemical Co.) per liter. The mucosal samples were extensively washed with calcium- and magnesium-free Hanks balanced salt solution (from Gibco BRL, Gaithersburg, Md.) after each EDTA treatment and were subsequently cultured (at 37°C in 5% CO2) in RPMI 1640 containing 10% fetal calf serum (FCS; Gibco BRL). During culture, lymphocytes, macrophages, and eosinophils migrated out of the lamina propria via basement membrane pores (18) and appeared in suspension (approximately 2 × 106 cells/g of tissue per 24-h period; viability, 90 to 95%). After 24 h of culture, the mucosal pieces were removed and the culture dish (containing cells in suspension and those adhering to the culture dish) was incubated at 4°C for 1 h. Following vigorous pipetting to detach adherent cells, the cells in suspension were collected, centrifuged (at 400 × g), and resuspended in fresh medium (10% FCS, RPMI 1640). The proportions of T cells, macrophages, and eosinophils in these cell preparations were determined by immunohistochemical studies (using anti-CD68 and anti-CD3 mouse monoclonal antibodies; from Dako Ltd., High Wycombe, United Kingdom) and toluidine blue staining of cells in cytospin preparations, as previously described (18).
The isolated lamina propria cells (at a final concentration of 106 or 2 × 106/ml) were exposed to C. difficile toxin A at concentrations ranging from 0 to 1,000 ng/ml. In some studies, 10 μl of neutralizing rabbit anti-human TNF-α antibody (capable of neutralizing approximately 1,000 U [25 ng] of TNF-α per ml; information from Genzyme, Cambridge, Mass.) was added to cells cultured in control medium or with toxin A. Following culture for 24 to 120 h, viability of the isolated lamina propria cells was assessed by measurement of mitochondrial dehydrogenase activity (22). The cells were also studied by electron microscopy, by immunohistochemistry (in cytospin preparations), and by fluorescence-activated cell sorter (FACS) analysis (see below).
Peripheral blood cells.
Studies were also performed with peripheral blood mononuclear cells and purified T cells isolated from healthy subjects. Mononuclear cells were obtained by centrifugation with lymphocyte separation medium (from ICN Biomedicals Inc., Irvine, Calif.). T cells were purified as described previously (15). In brief, the mononuclear cells were depleted of monocytes because monocytes adhered to tissue culture plates; B cells and remaining monocytes were subsequently removed by passage through a nylon wool column.
Assay for mitochondrial dehydrogenase activity.
This assay was used to assess the viability of toxin A-exposed lamina propria cells. Metabolism by mitochondrial dehydrogenase of the yellow tetrazolium salt 3-(4,5-dimethylthiozol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to the purple formazan reaction product can be quantified spectrophotometrically (22).
Lamina propria cells (105 in 100 μl per well) were cultured in 96-well tissue culture plates (Nunc A/S, Roskilde, Denmark; Gibco BRL) in the absence or presence of toxin A. An additional control included the culture of cells in the presence of the mitogen phytohemagglutinin (PHA; from Sigma Chemical Co.) at a final concentration of 10 μg/ml. After culturing the cells for various periods (24 to 96 h) of time, MTT (Sigma Chemical Co.) was added (to a final concentration of 0.5 mg/ml) to each well, and incubation continued for 4 h before the addition of 100 μl of solubilization solution (5% sodium dodecyl sulfate in 0.1 mM HCl). Following overnight incubation, a spectrophotometric absorbance assay of the samples was performed with an enzyme-linked immunosorbent assay (ELISA) plate reader (Labsystems iEMS; Reader MF) equipped with a 570-nm filter.
[3H]thymidine incorporation.
Colonic lamina propria cells (105 per well) were cultured in 10% human AB serum–RPMI 1640 in 96-well microtiter plates (Nunc A/S) in the presence of PHA (final concentration, 10 μg/ml) or purified C. difficile toxin A (final concentration, 1,000 ng/ml). The cells were cultured for a total of 72 h, the last 18 h of which was in the presence of [3H]thymidine (0.8 μCi/well; Amersham International PLC, Aylesbury, United Kingdom). The cells were harvested with a Filtermate Packard cell harvester (Packard, Pangbourne, United Kingdom), and [3H]thymidine uptake was determined with a Top Count microplate scintillation counter (Packard).
Electron microscopy.
Control and toxin A-exposed lamina propria cells were fixed for 2 h in 2.5% gluteraldehyde (in 0.1 M cacodylate buffer, pH 7.4) and subsequently washed with phosphate-buffered saline (PBS) (by centrifugation at 600 × g for 10 min). Cell pellets were recentrifuged in plasma and fixed for a further 4 h before being cut into 1-mm cubes. Following fixation in 1% sodium tetroxide for 1 h, the samples were dehydrated in ethanol and embedded in Epon resin as previously described (25). Sections were stained with uranyl acetate and lead citrate, before observation with a Jeol 1200 EX transmission electron microscope.
Immunohistochemistry.
Cytospin preparations of control and toxin A-exposed lamina propria cells were made by using approximately 50,000 cells per slide. They were air dried, fixed in acetone for 10 min, and stored at −70°C until used for immunohistochemistry, which was performed with the Vectastain ABC peroxidase kit (Vecta Lab, Burlingame, Calif.) and the mouse monoclonal antibodies specific for macrophages (KP1 [CD68]; from Dako Ltd.) and T cells (UCHT1 [CD3]; from Dako Ltd.). Endogenous peroxidase activity within the cells had previously been blocked (with H2O2 and methanol). For each batch, two control slides were used, with PBS (pH 7) instead of the mouse monoclonal antibody. In one of these controls, endogenous peroxidase activity was not blocked and was used to determine the proportion of eosinophils (which strongly express endogenous peroxidase activity) present (the proportions of eosinophils determined in this way were similar to those in toluidine blue-stained cytospin preparations). The proportion of positively stained (with the monoclonal antibody) cells or eosinophils in each cytospin preparation was determined by analyzing a total of at least 200 cells.
FACS analysis.
Control and toxin A-exposed lamina propria and peripheral blood cells were analyzed by FACS for phenotypic studies and to determine the type of cell death occurring.
For the analysis of apoptotic nuclei, a previously described technique was used (23). The cells were centrifuged (at 400 × g for 10 min), and cell pellets were fixed in 70% ethanol (on ice) for 60 min. Following a wash in PBS, the cells were incubated with propidium iodide (PI; 100 μg/ml in PBS; Sigma Chemical Co.) at room temperature in the dark for 15 min. The PI fluorescence of nuclei was measured with a FACScan flow cytometer (Becton Dickinson Immunocytometry Systems, Mountain View, Calif.) as previously described (23).
Apoptotic cells were also analyzed with fluorescein isothiocyanate (FITC)-conjugated annexin V (from Bioproducts, Boehringer Ingelheim, Heidelberg, Germany) (29) and PI (100 μg/ml in PBS; Sigma Chemical Co.). Cells (unfixed; 2 × 105 to 5 × 105/ml) were incubated with FITC-conjugated annexin V (in binding buffer: 10 mM HEPES, 140 mM NaCl, 2.5 mM CaCl2) at room temperature, in the dark, for 10 min. After being washed in PBS, the cells were incubated with PI for 15 min (in the dark), before analysis on a FACScan flow cytometer.
For phenotypic studies, lamina propria cells (at a concentration of 106/ml) were labelled with FITC- or phycoerythrin-conjugated monoclonal antibodies and analyzed by FACS as previously described (24). Following preincubation with mouse serum (final dilution, 1:100; at 4°C for 30 min), cell suspensions (100 μl; containing 105 cells) were incubated (on ice, in the dark) with the labelled monoclonal antibody solutions for 30 min. After being washed with PBS (pH 7.0; containing 0.1% sodium azide), the cells were fixed with FACS fix (0.5% formaldehyde in sheath fluid [6.38 mmol of NaCl, 0.5 mmol of sodium tetraborate, 16.2 mmol of boric acid, and 0.5 mmol of EDTA per liter; from Sysmex, Hamburg, Germany]). Subsequent analysis was performed by two-color flow cytometry with a FACScan flow cytometer.
The following antibody pairs to CD45/CD14 and CD4/CD8 were used: Hle-1/Leu-M3 and Leu-3a/Leu-2a, respectively (Becton Dickinson Immunocytometry Systems). Individual FITC- or phycoerythrin-labelled monoclonal antibodies to CD3, CD45RO, CD45RA, and CD25 (from Dako Ltd.) and Apo 2.7 (from Immunotech, Coulter, Marseille, France) were used. In double-labelling studies, surface-binding monoclonal antibodies were used before permeabilization of cells and application of the anti-Apo 2.7 antibody, which binds to an epitope on mitochondria (30).
TNF-α assay.
TNF-α levels in cell culture supernatants were measured by ELISA (Pelikine Compact human TNF-α ELISA kit; from Central Laboratory of the Netherlands Red Cross Blood Transfusion Service, Amsterdam, The Netherlands).
Statistical analyses.
Data were analyzed by paired or unpaired t test or Wilcoxon test as appropriate.
RESULTS
Human colonic lamina propria cells were obtained by our recently described technique in which mucosal samples are completely denuded of epithelial cells and cultured in 10% FCS–RPMI 1640 (18). During 24 h of culture, large numbers (approximately 2 × 106/g) of cells migrate out of the lamina propria via discrete pores in the basement membrane, and these cells were collected for the studies described below. Compatible with our previous studies (18), the isolated lamina propria cells consisted predominantly of T cells (CD3+; mean ± standard error of the mean [SEM], 75.7% ± 5.9%) but also included macrophages (CD68+; 9.0% ± 0.8%) and eosinophils (8.3% ± 1.4%). The viability of the cells obtained after a 24-h culture of denuded mucosal samples was 90 to 95% (as assessed by trypan blue exclusion).
C. difficile toxin A induces loss of viability of human colonic lamina propria cells.
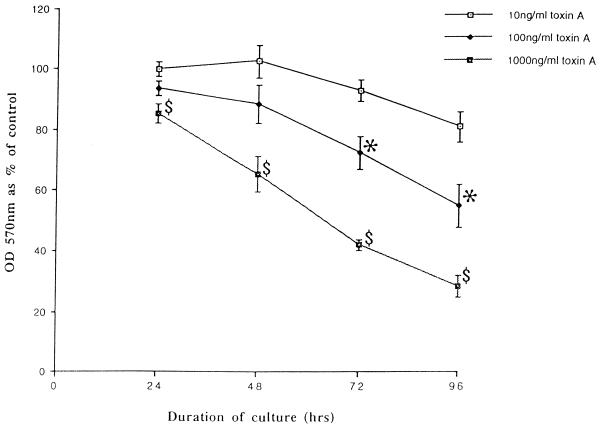
MTT assays showed that exposure to purified C. difficile toxin A induced loss of viability of isolated colonic lamina propria cells in a dose- and time-dependent fashion (Fig. 1). By contrast, PHA induced proliferation of the lamina propria cells (optical density at 570 nm, 174.0% ± 5.2% of that of control cells at day 3). C. difficile toxin A-induced loss of cell viability was confirmed by cell counts (data not shown). For all the subsequent studies described below, colonic lamina propria cells were exposed to purified toxin A at a final concentration of 1,000 ng/ml.
FIG. 1.
C. difficile toxin A induces the loss of viability of human colonic lamina propria cells. Cells were obtained from normal colonic mucosal samples and cultured with various concentrations of purified toxin A. After culture for 24 to 96 h, mitochondrial dehydrogenase activity was assessed by a MTT assay and expressed as the percentage of activity in cells cultured in control medium. The figure shows mean (± standard deviation) data from experiments performed, at different time points, with cells obtained from five separate resection specimens. OD 570 nm, optical density at 570 nm;  , P < 0.01 versus control; ∗, P < 0.05 versus control.
, P < 0.01 versus control; ∗, P < 0.05 versus control.
Studies of [3H]thymidine incorporation supported the results of MTT assays. Thus, unlike those cultured with PHA (for which the level of [3H]thymidine incorporation was 17,937.1 ± 255.7 dpm), cells cultured with toxin A (final concentration, 1,000 ng/ml) incorporated significantly less [3H]thymidine (653.1 ± 95.2 dpm) than control cells (2,365.9 ± 105.3 dpm; P < 0.001).
Immunohistochemical studies on cytospin preparations.
The isolated colonic lamina propria cells consist of T cells, macrophages, and eosinophils. In order to investigate the sensitivities of the different cell populations to the effects of toxin A, immunohistochemical studies were performed on cytospin preparations of control and toxin-exposed cells.
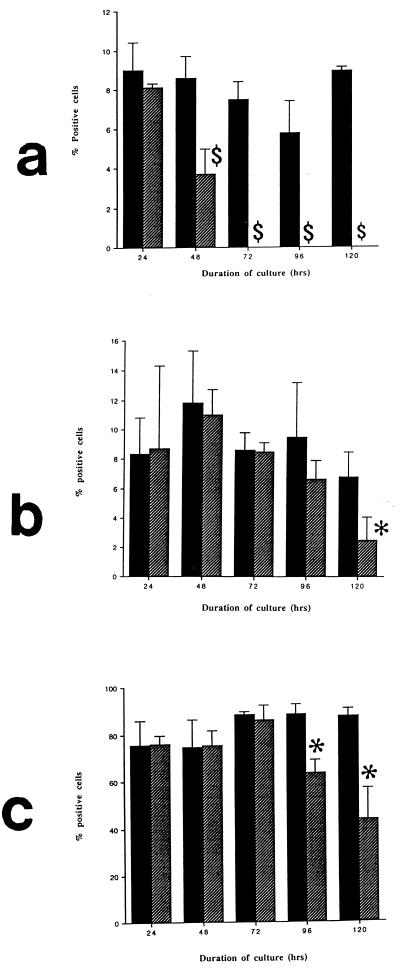
There was an early loss of macrophages from toxin A-exposed colonic lamina propria cell preparations as shown by a significant reduction in the proportion of CD68+ cells present at 48 h. From 72 h onwards, no CD68+ cells were seen in lamina propria cell preparations cultured with toxin A (Fig. 2a). By contrast, immunoreactive T cells (CD3+) and eosinophils were seen in colonic cell preparations exposed to toxin A for up to 120 h (Fig. 2b and c). From 72 h onwards many cellular fragments and debris were seen in all the toxin A-exposed cell preparations. In addition, many CD3-immunoreactive cells and eosinophils were much smaller than those in control cultures (data not shown).
FIG. 2.
Proportions of macrophages (a), eosinophils (b), and T cells (c) present following the culture of isolated colonic lamina propria cells with C. difficile toxin A (1,000 ng/ml; hatched bars) or control medium (solid bars) for 24 to 120 h. The proportions of each cell type in cytospin preparations were determined. $, P < 0.01 versus control; ∗, P < 0.03 versus control.
Electron microscopy.
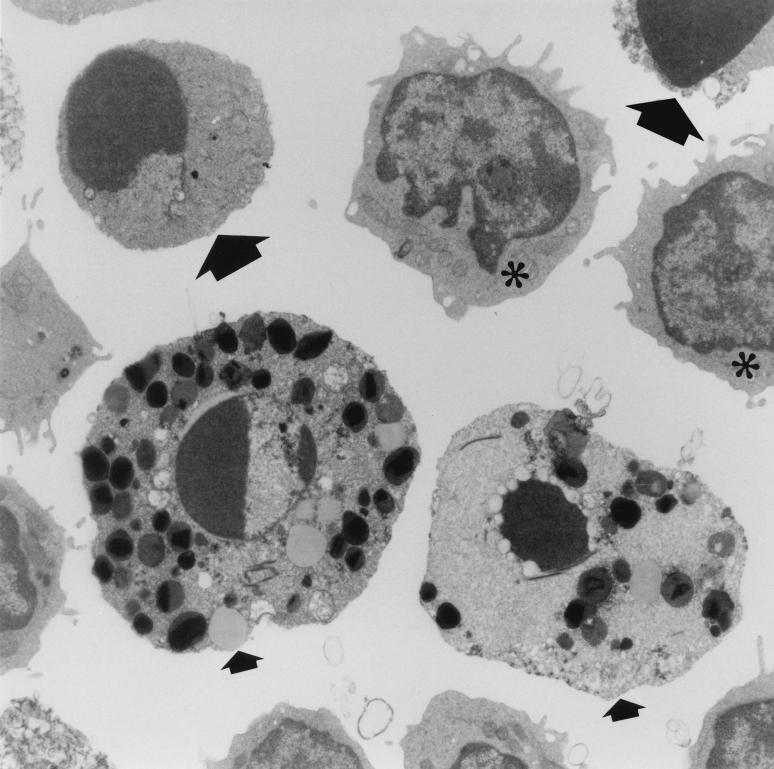
Transmission electron microscopy (TEM) confirmed the absence of macrophages in lamina propria cell preparations exposed to toxin A for 72 h or more. TEM studies also confirmed the presence of viable-looking lymphocytes and eosinophils in cell preparations exposed to toxin for 72 to 120 h. In addition, lymphocytes and eosinophils with characteristic morphological features of apoptosis were also seen in cell preparations exposed to toxin A for 72 h or more (Fig. 3).
FIG. 3.
Transmission electron micrograph of isolated colonic lamina propria cells exposed to C. difficile toxin A (1,000 ng/ml) for 96 h. Two eosinophils (small arrows) and lymphocytes (large arrows) are undergoing apoptosis. Viable lymphocytes (∗) are also present.
FACS analysis.
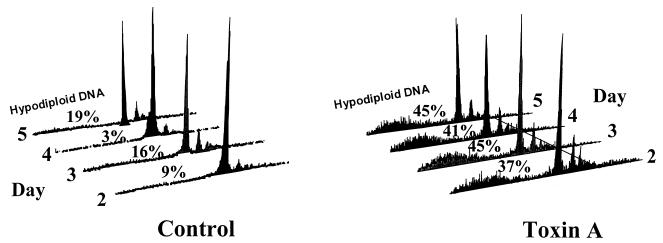
To confirm and quantify apoptotic cell death in isolated lamina propria cells exposed to C. difficile toxin A, studies were performed by FACS analysis. Nuclei of cells made permeable by alcohol fixation were labelled with PI and analyzed by flow cytometry. Apoptotic nuclei produce a broad hypodiploid DNA peak, which is easily distinguished from the narrow peak of cells with normal DNA content (23). Studies of control and toxin A-exposed cells were performed every 24 h (over a total of 120 h). In contrast to what was seen for cells cultured in control medium, large hypodiploid DNA peaks were seen for lamina propria cells exposed to toxin A (Fig. 4).
FIG. 4.
DNA fluorescence flow cytometric profiles of alcohol-fixed, PI-stained human colonic lamina propria cells cultured in control medium or in the presence of C. difficile toxin A. Apoptotic nuclei produce broad hypodiploid peaks, which can be easily distinguished from the tall narrow peaks produced by cells with normal DNA content. For control versus toxin A-exposed cells, P = 0.001. The figure shown presents results representative of three experiments.
Studies using FITC-conjugated annexin V were also performed. Soon after the initiation of apoptosis, cells translocate phosphatidylserine (PS) from the inner face of the plasma membrane to the cell surface (29). Annexin V is a protein that binds PS with high affinity, and therefore FITC-conjugated annexin V can be used to detect cells during the early stages of apoptosis, when they are also impermeable to PI (29).
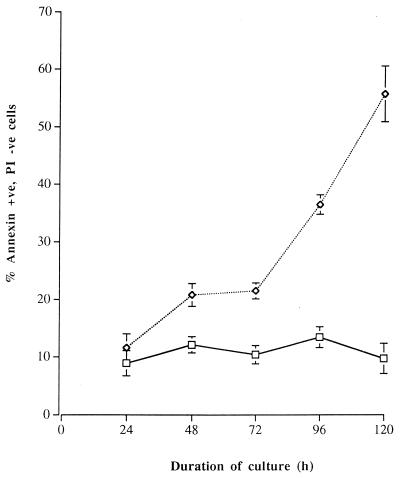
FACS analysis was performed on unfixed cells, after gating for lymphocytes. In studies every 24 h (over a total of 120 h), 8.9 to 13.4% (mean percentage) of lamina propria cells cultured in control medium demonstrated features of early apoptosis (annexin V positive, PI negative). Upon exposure to C. difficile toxin A, there was a significant time-dependent increase in the proportion of annexin V-positive (PI-negative) cells (mean, 11.6 to 55.6%; Fig. 5).
FIG. 5.
Percentage of annexin V-positive, PI-negative normal colonic lamina propria cells cultured in control medium or with 1,000 ng of C. difficile toxin A per ml for 24 to 120 h. Experiments were performed with lamina propria cells isolated from normal mucosal samples of four resected colons. For the control cell value (square) versus the toxin A-exposed cell value (diamond) at 24 h, P = 0.07; for corresponding values at other time points, P < 0.01.
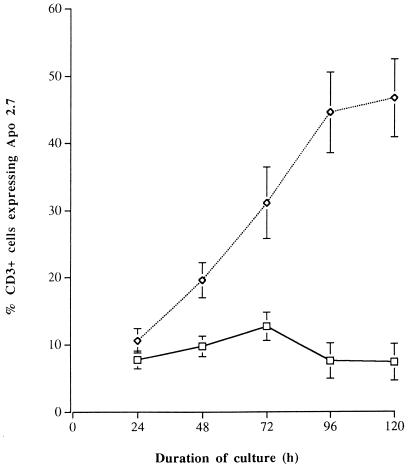
To confirm that the cells undergoing apoptosis were T cells, double-labelling studies were performed with monoclonal antibodies to Apo 2.7 and CD3. Monoclonal antibody to Apo 2.7 (or anti-7A6) reacts strongly with an epitope on a mitochondrial membrane protein that is exposed on cells undergoing apoptosis (30). FACS analysis confirmed that the toxin A-exposed colonic lamina propria cells undergoing apoptosis were T cells (CD3+; Fig. 6). In contrast to what was seen for control cells, exposure to toxin A led to a progressive increase in the proportion of CD3+ cells that expressed Apo 2.7 (after 120 h of culture; for control and toxin A-exposed cells, mean percentages of CD3+ cells ± SEM were 7.4% ± 2.7% and 46.6% ± 5.8%, respectively; P < 0.01). The Apo 2.7-positive T cells were predominantly CD45RO+ and included both CD4+ and CD8+ subpopulations (data not shown).
FIG. 6.
Percentage of CD3+ (T) cells that expressed Apo 2.7 in normal colonic lamina propria cell preparations cultured in control medium or with C. difficile toxin A (1,000 ng/ml) for 24 to 120 h. FACS analysis was performed on lamina propria cells isolated from normal mucosal samples of four resected colons. For the control cell values (squares) versus the toxin A-exposed cell values (diamonds) at 24 and 72 h, P = 0.02; for corresponding values at other time points, P < 0.005.
Following exposure to C. difficile toxin A, there was also a significant increase in the proportion of T cells that expressed the interleukin 2 receptor (CD25; Table 1).
TABLE 1.
Percentages of CD25+ normal colonic lamina propria T lymphocytes following culture in control medium or with C. difficile toxin Aa
| Culture medium contents | % CD25+ T lymphocytesb ± SEM following culture for:
|
||||
|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 96 h | 120 h | |
| Control | 7.2 ± 1.0 | 7.0 ± 1.0 | 7.3 ± 0.7 | 6.4 ± 0.4 | 14.3 ± 0.8 |
| Toxin A | 10.7 ± 1.1 | 19.1 ± 0.7 | 11.0 ± 0.9 | 9.8 ± 0.6 | 21.2 ± 3.6 |
a FACS analysis was performed on lamina propria lymphocytes isolated from normal mucosal samples of four resected colons. C. difficile toxin A was used at 1,000 ng/ml.
b For control medium percentages versus toxin A percentages, P < 0.03 at all time points except 120 h, for which P = 0.18.
TNF-α production and its neutralization.
Supernatants of lamina propria cells cultured in the presence of C. difficile toxin A for 24 h contained significantly higher levels of TNF-α than controls (medians [ranges], control cells, 112.6 pg/ml [21.0 to 310.0 pg/ml]; toxin A-exposed cells, 419.2 pg/ml [35.1 to 2,112 pg/ml]; P = 0.02). However, there was no significant difference in TNF-α levels in supernatants of cells cultured for 48 h in control medium (61.2 pg/ml [18.4 to 1,225.0 pg/ml]) or with C. difficile toxin A (55.4 pg/ml [18.2 to 598.3 pg/ml]). In order to investigate the possible role of TNF-α in the induction of T-cell apoptosis, studies using neutralizing antibody to the cytokine were performed. However, the presence of neutralizing antibody to TNF-α did not make any significant difference to the proportion of C. difficile toxin A-exposed T cells undergoing apoptosis (Table 2).
TABLE 2.
C. difficile toxin A-induced apoptosis in human colonic lamina propria T cells is not influenced by the presence of neutralizing antibodies to TNF-α
| Speci-men | % Apo 2.7+ cellsa following culture in medium with indicated contents for:
|
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 h
|
48 h
|
72 h
|
96 h
|
120 h
|
||||||||||||||||
| Control medium | Control medium + anti-TNF Ab | Toxin A | Toxin A + anti-TNF Ab | Control medium | Control medium + anti-TNF Ab | Toxin A | Toxin A + anti-TNF Ab | Control medium | Control medium + anti-TNF Ab | Toxin A | Toxin A + anti-TNF Ab | Control medium | Control medium + anti-TNF Ab | Toxin A | Toxin A + anti-TNF Ab | Control medium | Control medium + anti-TNF Ab | Toxin A | Toxin A + anti-TNF Ab | |
| I | 4.7 | 4.4 | 7.6 | 8.1 | 10.4 | 7.1 | 24.6 | 28.1 | 14.0 | 15.6 | 39.2 | 45.0 | 19.9 | 14.7 | 61.2 | 61.3 | 18.5 | 17.3 | 61.5 | 67.5 |
| II | 7.7 | 11.7 | 39.4 | 40.1 | 6.3 | 3.8 | 35.3 | 32.5 | ||||||||||||
| III | 11.9 | 9.9 | 13.8 | 12.6 | 10.5 | 9.9 | 19.7 | 18.4 | ||||||||||||
a The proportion of CD3+ cells that expressed Apo 2.7 was determined by FACS analysis of lamina propria cells isolated from normal mucosal samples of three colonic resection specimens cultured in control medium or with toxin A (1,000 ng/ml), in the absence or presence of neutralizing antibody (Ab) to TNF-α.
Studies with peripheral blood T cells.
When cultured with peripheral blood mononuclear cells, C. difficile toxin A induced programmed cell death as demonstrated by the expression of annexin V (but the absence of PI staining; Table 3). Purified populations of T cells (<2% contaminating B cells and monocytes) also underwent apoptosis upon exposure to toxin A (Table 3). Similar data were obtained upon FACS analysis of cells (from a smaller number of subjects) labelled with monoclonal antibodies to Apo 2.7 and to CD3 (data not shown).
TABLE 3.
Percentages of annexin V-positive, PI-negative (apoptotic) cells in unfractionated peripheral blood mononuclear cells and following purification of T cellsa
| Cell type | Mean % of annexin V+, PI− cellsb ± SD following culture in medium with the indicated contents for:
|
|||
|---|---|---|---|---|
| 72 h
|
96 h
|
|||
| Control | Toxin A | Control | Toxin A | |
| Unfractionated PBMCc | 8.6 ± 2.7 | 46.7 ± 3.5 | 6.2 ± 2.9 | 53.6 ± 2.6 |
| Purified T cells | 16.2 ± 4.6 | 39.3 ± 3.0 | 15.4 ± 2.7 | 49.2 ± 6.1 |
a Cells were isolated from five healthy volunteers and were cultured in control medium or with C. difficile toxin A (1,000 ng/ml) for 72 or 96 h before FACS analysis of gated lymphocytes.
b For control medium percentages versus toxin A percentages, for both unfractionated peripheral blood mononuclear cells and purified T cells, P < 0.001 at both 72 and 96 h.
c PBMC, peripheral blood mononuclear cells.
DISCUSSION
Our previous studies have shown that purified C. difficile toxin A induces cell rounding, detachment from the basement membrane, and apoptosis in primary human colonic epithelial cells (17). In vivo, loss of the surface epithelial cells would expose cells in the underlying lamina propria to C. difficile toxins. A large number of T cells, which are phenotypically and functionally distinct from peripheral blood cells (10), are resident in the normal intestinal lamina propria. In addition to eosinophils, there is also a significant heterogenous population of macrophages that is especially prominent below the surface epithelium and the basement membrane (16). Following the loss of the injured epithelium, lamina propria T cells, macrophages, and eosinophils would be exposed to C. difficile toxin A via pores in the basement membrane (18). In this study, we have investigated the responses of these resident colonic lamina propria cells to purified C. difficile toxin A.
Culture with toxin A led to a loss of viability of isolated colonic lamina propria cells in a dose- and time-dependent fashion. Subsequent phenotypic studies (on cytospin preparations) using high concentrations of toxin A showed that macrophages were more sensitive than T cells and eosinophils. In studies confirmed by electron microscopy, no macrophages were seen following exposure to the toxin for 72 h. Since they are a small proportion of the isolated lamina propria cell population, it has not been possible to determine the type of cell death occurring in the toxin-exposed macrophages. By contrast, our studies convincingly demonstrate that toxin A-induced T-cell and eosinophil cell death occurs by apoptosis. In ultrastructural studies, the retention of characteristic granules in the cytoplasm allowed the identification of apoptotic eosinophils. C. difficile toxin A-induced apoptosis of colonic T cells was confirmed by FACS analysis using dual staining with monoclonal antibodies to Apo 2.7 and CD3. Studies by electron microscopy and FACS analysis of cells expressing annexin V and Apo 2.7 showed that the number of T cells undergoing apoptosis progressively increased with the duration of exposure to toxin A. In studies performed after each 24-h period of exposure to toxin A, there was a modest but significant increase in the proportion of T cells expressing the interleukin 2 receptor (CD25). It is possible that toxin A-induced activation of T cells preceded cell death by apoptosis.
Previous studies have shown that antigen-induced apoptosis of mature T cells occurs in distinct phases (4). Following activation and expression of growth factors and their receptors, there is a proliferation of T cells before programmed cell death. Staphylococcal enterotoxin B binds to major histocompatibility complex class II molecules and the Vβ region of a T-cell receptor to induce activation and proliferation of T lymphocytes (9) and subsequent cell death by apoptosis (11, 28). However, in our studies there was a significant reduction in the incorporation of [3H]thymidine by colonic lamina propria cells exposed to C. difficile toxin A, consistent with cell death in the absence of proliferation.
Following exposure to toxin A for 24 h, there was induction of TNF-α production by the isolated lamina propria cells. However, after culture with toxin A for 48 h, the amount TNF-α produced was not significantly different from the amount produced by control cells. The likely explanation for this is the loss of TNF-α-producing macrophages after culture with the toxin for 48 h. Since TNF-α may induce apoptosis in T cells (6), studies were performed to investigate its role in toxin A-induced cell death. However, exposure to toxin A induced apoptosis in colonic T cells despite the presence of the specific neutralizing anti-TNF-α antibody. Another mechanism by which apoptosis may occur in T cells is by the interaction of Fas with the Fas ligand (12). In our studies, a majority (>90%) of the control and toxin A-exposed colonic lamina propria T cells expressed Fas but only 0.7 to 2.3% of the cells expressed the Fas ligand (unpublished observations).
We have shown that C. difficile toxin A also induced apoptosis in purified T cells. This implies that C. difficile toxin A induces cell death following a direct interaction with T lymphocytes. After this toxin binds to specific receptors, its biological effects would be expected to occur following internalization. Recent studies with other cells have shown that subtypes of the Rho family of GTP binding proteins, which regulate the cytoskeleton, are substrates for C. difficile toxin A (1). The role of the Rho family of GTP binding proteins in T-cell apoptosis remains to be characterized. One recent study has shown that transient expression (using a Sindbis virus-based transient gene expression system) of Clostridium botulinum exoenzyme C3, which ADP ribosylates Rho, induced apoptosis in EL4 murine lymphoma cells (21). The authors of this study postulated that apoptosis may have resulted from the combined effects of inactivation of Rho and the stress induced by viral replication (21). It can be postulated from our studies that the inactivation of Rho is sufficient to induce T-cell apoptosis.
We have previously shown that C. difficile toxin A induces apoptosis in colonic epithelial cells (17). The epithelial cell apoptosis was preceded by cell rounding and detachment from the basement membrane. However, detachment of epithelial cells from the basement membrane, in the absence of the toxin, also induced apoptosis (17). We therefore postulated in that study that toxin A induced programmed cell death by causing a loss of epithelial cell adherence to extracellular matrix components of the basement membrane. However, our current studies of T cells (which are not dependent upon adhesion to the extracellular matrix for survival) suggest that C. difficile toxin A can itself directly induce apoptosis.
There is increasing interest in apoptosis in host-microbial interactions (31), but the significance to the host or the bacterium of C. difficile toxin A-induced programmed cell death in the colonic mucosa remains to be determined. Macrophages and T cells are important components of the colonic mucosal immune system (10, 15). C. difficile toxin A-induced early loss of macrophages and T cells would be expected to lead to suppression of the host mucosal immune system. Our studies suggest that such impairment of the mucosal immune system would be dependent upon the concentration of toxin A to which the colonic mucosa is exposed. Such a dose-dependent effect of C. difficile toxin A has also been seen with intestinal epithelial cells (17) and may determine the severity of clinical disease.
In the present study, we also show that a small population of isolated colonic lamina propria T cells spontaneously undergo apoptosis in culture. An increased level of apoptosis in unstimulated human intestinal T cells (isolated by collagenase digestion), compared to the level in peripheral blood T cells, has also been reported recently (3). One explanation for this spontaneous apoptosis of intestinal T cells could be that they represent lymphocytes that were destined to die in the lamina propria.
In conclusion, our study has shown for the first time that C. difficile toxin A induces a rapid loss of resident colonic lamina propria macrophages, followed by T cells and eosinophils. For the last two cell types, cell death occurred by apoptosis. Exposure of normal colonic lamina propria cells to toxin A for 24 h induces the release of TNF-α, but its neutralization did not influence toxin-induced apoptosis in T cells. In addition, our studies with purified cells suggest that toxin A induces apoptosis after interacting directly with the T cells. C. difficile toxin A-induced loss of lamina propria macrophages and T cells in vivo may suppress the colonic mucosal immune system and lead to severe disease.
ACKNOWLEDGMENTS
This work was supported by the Medical Research Council (United Kingdom). The electron microscopy studies used equipment funded by The Wellcome Trust.
We thank Trevor Gray for assistance in the studies by electron microscopy and Adrian Robins for assistance in FACS analysis.
REFERENCES
- 1.Aktories K. Rho proteins: targets for bacterial toxins. Trends Microbiol. 1997;5:282–288. doi: 10.1016/S0966-842X(97)01067-6. [DOI] [PubMed] [Google Scholar]
- 2.Anaya-Velazquez, F., G. D. F. Jackson, P. B. Ernt, B. J. Underdown, and J. Gauldie. Cytokines in the liver and gastrointestinal tract, p. 315–323. In P. L. Ogra, M. E. Lamm, J. R. McGhee, J. Mestecky, W. Strober, and J. Bienenstock (ed.), Handbook of mucosal immunology. Academic Press, San Diego, Calif.
- 3.Boirivant M, Pica R, De Maria R, Testi R, Pallone F, Strober W. Stimulated human lamina propria T cells manifest enhanced Fas-mediated apoptosis. J Clin Investig. 1996;98:2616–2622. doi: 10.1172/JCI119082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Critchfield J M, Lanardo M J. Antigen-induced programmed cell death as a new approach to immune therapy. Clin Immunol Immunopathol. 1995;75:13–19. doi: 10.1006/clin.1995.1046. [DOI] [PubMed] [Google Scholar]
- 5.Fiorentini C, Thelestam M. Clostridium difficile toxin A and its effects on cells. Toxicon. 1991;29:543–567. doi: 10.1016/0041-0101(91)90050-2. [DOI] [PubMed] [Google Scholar]
- 6.Fraser A, Evans G. A license to kill. Cell. 1996;85:781–784. doi: 10.1016/s0092-8674(00)81005-3. [DOI] [PubMed] [Google Scholar]
- 7.Frisch S M, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hecht G, Pothoulakis C, LaMont J T, Carlson S, Madara J L. C. difficile toxin A perturbs cytoskeletal structure and tight junction permeability of cultured human intestinal epithelial monolayers. J Clin Investig. 1988;82:1516–1524. doi: 10.1172/JCI113760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herman A, Kapper J W, Marrack P, Pullen A M. Superantigens: mechanism of T-cell stimulation and role in immune responses. Annu Rev Immunol. 1991;9:745–772. doi: 10.1146/annurev.iy.09.040191.003525. [DOI] [PubMed] [Google Scholar]
- 10.James S P, Zeitz M. Human gastrointestinal mucosal T cells. In: Ogra P L, Lamm M E, McGhee J R, Mestecky J, Strober W, Bienenstock J, editors. Handbook of mucosal immunology. San Diego, Calif: Academic Press; 1994. pp. 275–285. [Google Scholar]
- 11.Jenkinson E J, Kingston R, Smith C A, Williams G T, Owen J J T. Antigen-induced apoptosis in developing T cells: a mechanism for negative selection of the T cell receptor repertoire. Eur J Immunol. 1989;19:2175–2177. doi: 10.1002/eji.1830191132. [DOI] [PubMed] [Google Scholar]
- 12.Ju S, Panka D J, Cui H, Ettinger R, El-Khatib M, Sherr D H, Stanger B Z, Marshak-Rothstein A. Fas (CD95)/Fas interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 13.Kamiya S, Reed P J, Borriello S P. Purification and characterization of Clostridium difficile toxin A by bovine thyroglobulin affinity chromatography and dissociation in denaturing conditions with or without reduction. J Med Microbiol. 1989;30:69–77. doi: 10.1099/00222615-30-1-69. [DOI] [PubMed] [Google Scholar]
- 14.Lyerly D M, Saum K E, MacDonald D K, Wilkins T D. Effects of Clostridium difficile toxins given intragastrically to animals. Infect Immun. 1985;47:349–352. doi: 10.1128/iai.47.2.349-352.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahida Y R, Wu K, Jewell D P. Characterization of antigen presenting activity of mononuclear cells isolated from normal and inflammatory bowel disease colon and ileum. Immunology. 1988;65:543–549. [PMC free article] [PubMed] [Google Scholar]
- 16.Mahida Y R, Patel S, Gionchetti P, Vaux D, Jewell D P. Macrophage sub-populations in lamina propria of normal and inflamed colon and terminal ileum. Gut. 1989;30:826–834. doi: 10.1136/gut.30.6.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahida Y R, Makh S, Hyde S, Gray T, Borriello S P. Effect of Clostridium difficile toxin A on human intestinal epithelial cells: induction of interleukin 8 production and apoptosis after cell detachment. Gut. 1996;38:337–347. doi: 10.1136/gut.38.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahida Y R, Galvin A M, Gray T, Makh S, McAlindon M E, Sewell H F, Podolsky D K. Migration of human intestinal lamina propria lymphocytes, macrophages and eosinophils following the loss of surface epithelial cells. Clin Exp Immunol. 1997;109:377–386. doi: 10.1046/j.1365-2249.1997.4481346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meredith J E, Fazeli B, Schwartz M A. The extracellular matrix as a cell survival factor. Mol Biol Cell. 1993;4:953–961. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell T J, Ketley J M, Haslam S C, Stephen J, Burdon D W, Candy D C A, Daniel R. Effect of toxin A and B of Clostridium difficile on rabbit ileum and colon. Gut. 1986;27:78–85. doi: 10.1136/gut.27.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moorman J P, Bobak D A, Hahn C S. Inactivation of the small GTP binding protein Rho induces multinucleate cell formation and apoptosis in murine T lymphoma EL4. J Immunol. 1996;156:4146–4153. [PubMed] [Google Scholar]
- 22.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxic assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 23.Nicoletti I, Migliorati G, Pagliacci M C, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 24.Pallis M, Robins A, Powell R. Quantitative analysis of lymphocyte CD11a using standardised flow cytometry. Scand J Immunol. 1993;38:558–564. doi: 10.1111/j.1365-3083.1993.tb03241.x. [DOI] [PubMed] [Google Scholar]
- 25.Robinson G, Gray T. Electron microscopy 2. Tissue preparation, sectioning and staining. In: Bancroft J D, Stevens A, editors. Theory and practice of histological techniques. 3rd ed. London, United Kingdom: Churchill Livingstone; 1990. pp. 525–562. [Google Scholar]
- 26.Ruoslahti E, Reed J C. Anchorage dependence, integrins and apoptosis. Cell. 1994;77:477–478. doi: 10.1016/0092-8674(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 27.Triadafilopoulos G, Pothoulakis C, O’Brien M J, LaMont T J. Differential effects of Clostridium difficile toxins A and B on rabbit ileum. Gastroenterology. 1987;93:273–279. doi: 10.1016/0016-5085(87)91014-6. [DOI] [PubMed] [Google Scholar]
- 28.Vabulas R, Bittlingmaier R, Heeg K, Wagner H, Miethke T. Rapid clearance of bacterial superantigen staphylococcal enterotoxin B in vivo. Infect Immun. 1996;64:4567–4573. doi: 10.1128/iai.64.11.4567-4573.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 30.Zhang C, Ao Z, Seth A, Schlossman S F. A mitochondrial membrane protein defined by a novel monoclonal antibody is preferentially detected in apoptotic cells. J Immunol. 1996;157:3980–3987. [PubMed] [Google Scholar]
- 31.Zychlinsky A, Sansonetti P. Apoptosis in bacterial pathogenesis. J Clin Investig. 1997;100:493–496. doi: 10.1172/JCI119557. [DOI] [PMC free article] [PubMed] [Google Scholar]