Abstract
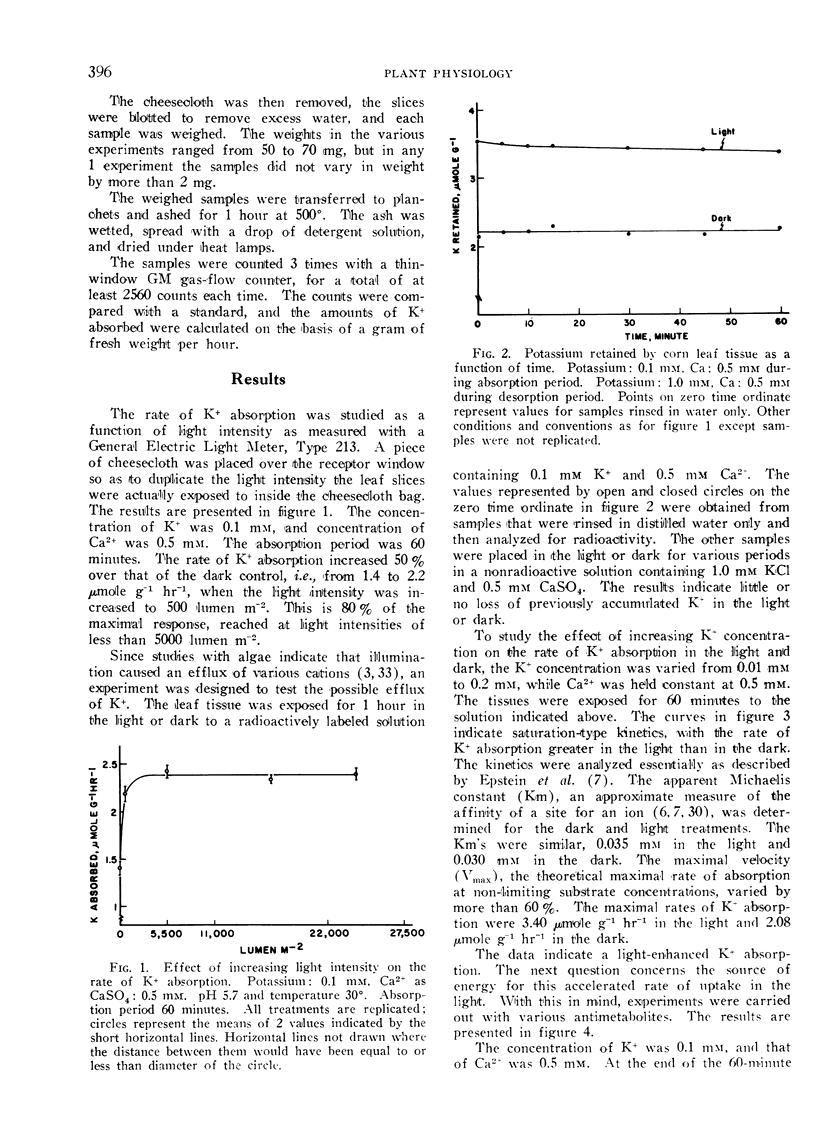
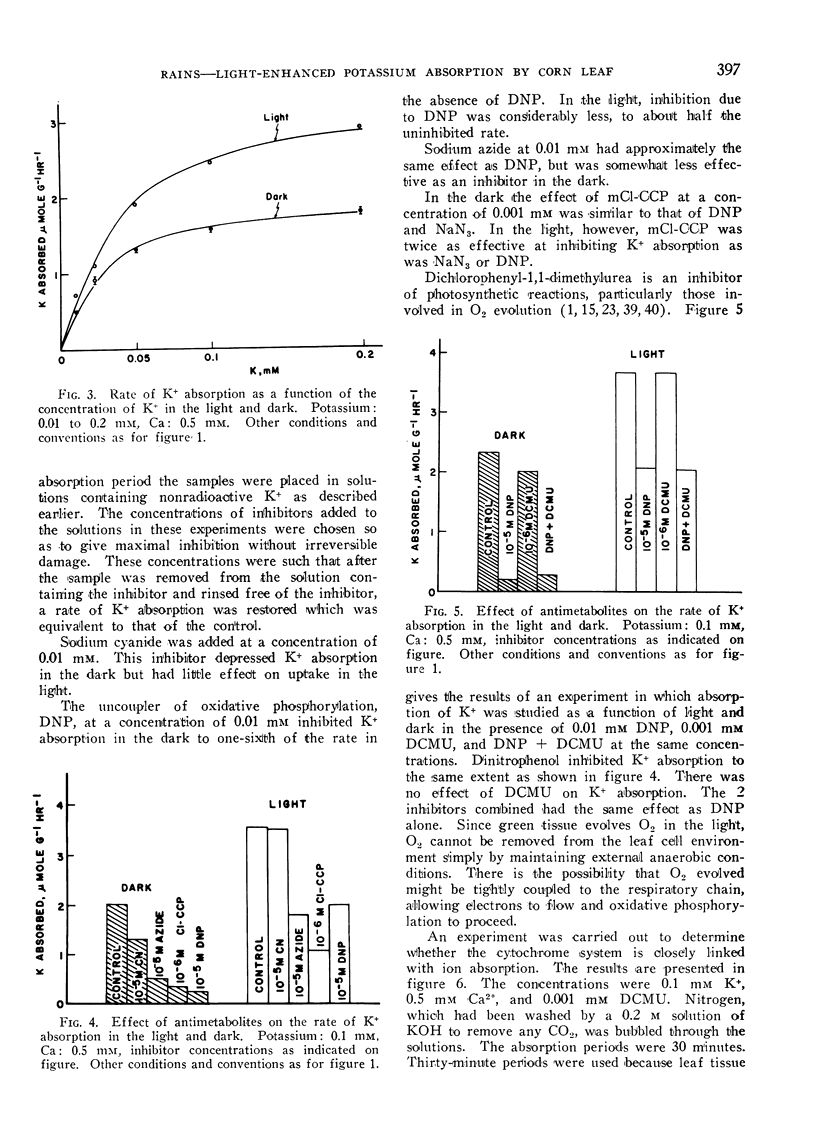
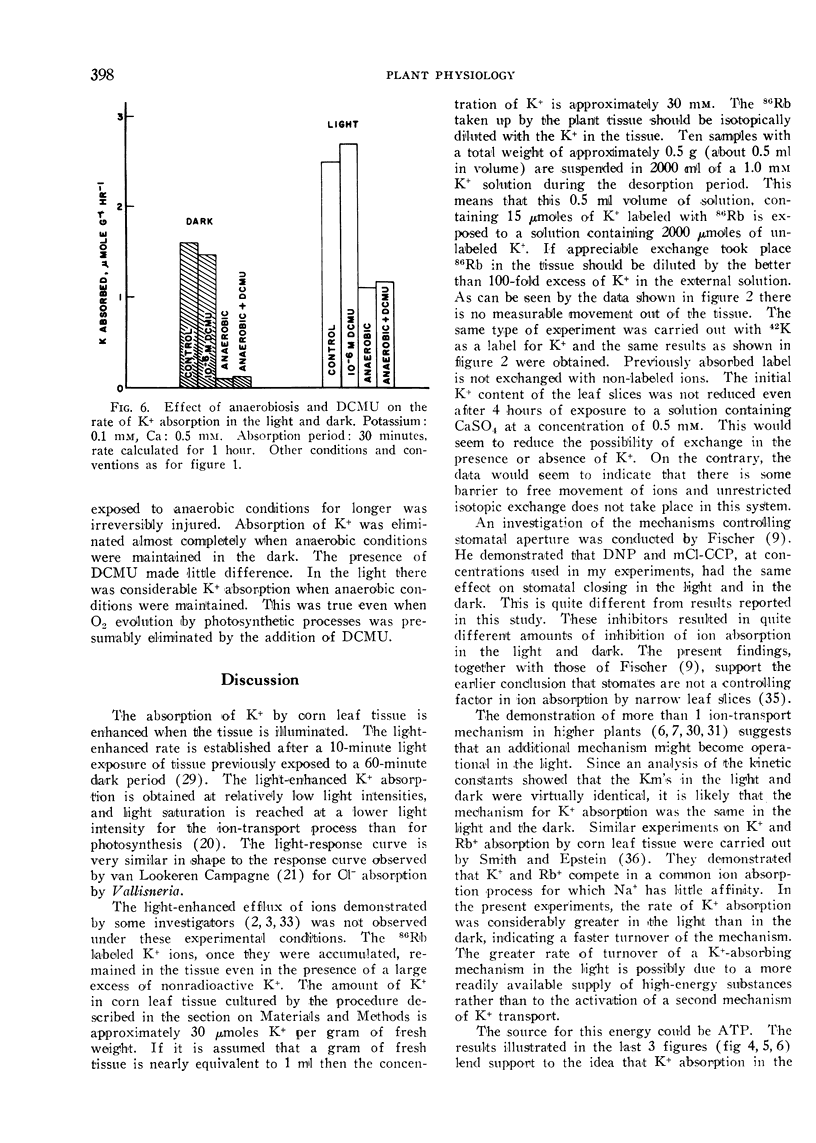
The effect of illumination on the absorption of K+ by leaf tissue of Zea mays was investigated. The rate of K+ absorption was enhanced by exposure of slices of corn leaf tissue to light, even of relatively low intensities. Potassium was transported inward, with virtually no efflux of previously accumulated K+. The evidence indicates that the transport mechanism for absorption of K+ is the same in the light as in the dark, but that the source of energy for absorption of K+ is different in the light from that in the dark. Various anti-metabolites were used to establish that the energy utilized for active ion transport in the light came partly from ATP supplied by cyclic photophosphorylation. Expenditure of ATP was required in the dark too, but this ATP was formed by oxidative phosphorylation. Establishing the ultimate source of energy for active ion uptake by higher plants might be facilitated by demonstration of an ion-transport process that is not linked directly with the transfer of electrons in the mitochondrial cytochrome chain.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I., Tsujimoto H. Y., McSwain B. D. Ferredoxin and photosynthetic phosphorylation. Nature. 1967 May 6;214(5088):562–566. doi: 10.1038/214562a0. [DOI] [PubMed] [Google Scholar]
- EPPLEY R. W. Sodium exclusion and potassium retention by the red marine alga, Porphyra perforata. J Gen Physiol. 1958 May 20;41(5):901–911. doi: 10.1085/jgp.41.5.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E., Rains D. W., Elzam O. E. RESOLUTION OF DUAL MECHANISMS OF POTASSIUM ABSORPTION BY BARLEY ROOTS. Proc Natl Acad Sci U S A. 1963 May;49(5):684–692. doi: 10.1073/pnas.49.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E. The essential role of calcium in selective cation transport by plant cells. Plant Physiol. 1961 Jul;36(4):437–444. doi: 10.1104/pp.36.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester M. L., Krotkov G., Nelson C. D. Effect of Oxygen on Photosynthesis, Photorespiration and Respiration in Detached Leaves. II. Corn and other Monocotyledons. Plant Physiol. 1966 Mar;41(3):428–431. doi: 10.1104/pp.41.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEYTLER P. G. uncoupling of oxidative phosphorylation by carbonyl cyanide phenylhydrazones. I. Some characteristics of m-Cl-CCP action on mitochondria and chloroplasts. Biochemistry. 1963 Mar-Apr;2:357–361. doi: 10.1021/bi00902a031. [DOI] [PubMed] [Google Scholar]
- Hodges T. K., Elzam O. E. Effect of azide and oligomycin on the transport of calcium ions in corn mitochondria. Nature. 1967 Aug 26;215(5104):970–972. doi: 10.1038/215970a0. [DOI] [PubMed] [Google Scholar]
- Klepper B., Kaufmann M. R. Removal of salt from xylem sap by leaves and stems of guttating plants. Plant Physiol. 1966 Dec;41(10):1743–1747. doi: 10.1104/pp.41.10.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACROBBIE E. A. THE NATURE OF THE COUPLING BETWEEN LIGHT ENERGY AND ACTIVE ION TRANSPORT IN NITELLA TRANSLUCENS. Biochim Biophys Acta. 1965 Jan 25;94:64–73. doi: 10.1016/0926-6585(65)90008-7. [DOI] [PubMed] [Google Scholar]
- NOBEL P. S., PACKER L. ENERGY-DEPENDENT ION UPTAKE IN SPINACH CHLOROPLASTS. Biochim Biophys Acta. 1964 Sep 25;88:453–455. doi: 10.1016/0926-6577(64)90206-2. [DOI] [PubMed] [Google Scholar]
- Neumann J., Jagendorf A. T. Dinitrophenol as an uncoupler of photosynthetic phosphorylation. Biochem Biophys Res Commun. 1964 Aug 11;16(6):562–567. doi: 10.1016/0006-291x(64)90193-7. [DOI] [PubMed] [Google Scholar]
- Rains D. W., Epstein E. Sodium absorption by barley roots: role of the dual mechanisms of alkali cation transport. Plant Physiol. 1967 Mar;42(3):314–318. doi: 10.1104/pp.42.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rains D. W. Light-enhanced potassium absorption by corn leaf tissue. Science. 1967 Jun 9;156(3780):1382–1383. doi: 10.1126/science.156.3780.1382. [DOI] [PubMed] [Google Scholar]
- SALTMAN P., FORTE J. G., FORTE G. M. Permeability studies on chloroplasts from Nitella. Exp Cell Res. 1963 Feb;29:504–514. doi: 10.1016/s0014-4827(63)80013-0. [DOI] [PubMed] [Google Scholar]
- Smith F. A. Active phosphate uptake by Nitella translucens. Biochim Biophys Acta. 1966 Sep 5;126(1):94–99. doi: 10.1016/0926-6585(66)90040-9. [DOI] [PubMed] [Google Scholar]
- Smith R. C., Epstein E. Ion Absorption by Shoot Tissue: Kinetics of Potassium and Rubidium Absorption by Corn Leaf Tissue. Plant Physiol. 1964 Nov;39(6):992–996. doi: 10.1104/pp.39.6.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. C., Epstein E. Ion Absorption by Shoot Tissue: Technique and First Findings with Excised Leaf Tissue of Corn. Plant Physiol. 1964 May;39(3):338–341. doi: 10.1104/pp.39.3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichler-Zallen D., Hoch G. Cyclic electron transport in algae. Arch Biochem Biophys. 1967 Apr;120(1):227–230. doi: 10.1016/0003-9861(67)90620-0. [DOI] [PubMed] [Google Scholar]
- VERNON L. P., AVRON M. PHOTOSYNTHESIS. Annu Rev Biochem. 1965;34:269–296. doi: 10.1146/annurev.bi.34.070165.001413. [DOI] [PubMed] [Google Scholar]
- WEIGL J. UBER DEN ZUSAMMENHANG VON PHOTOPHOSPHORYLIERUNG UND AKTIVER LONENAUFNAHME. Z Naturforsch B. 1964 Sep;19:845–851. [PubMed] [Google Scholar]


