Abstract
Large variability in the individual response to even the most-efficacious pain treatments is observed clinically, which has led to calls for a more personalized, tailored approach to treating patients with pain (ie, “precision pain medicine”). Precision pain medicine, currently an aspirational goal, would consist of empirically based algorithms that determine the optimal treatments, or treatment combinations, for specific patients (ie, targeting the right treatment, in the right dose, to the right patient, at the right time). Answering this question of “what works for whom” will certainly improve the clinical care of patients with pain. It may also support the success of novel drug development in pain, making it easier to identify novel treatments that work for certain patients and more accurately identify the magnitude of the treatment effect for those subgroups. Significant preliminary work has been done in this area, and analgesic trials are beginning to utilize precision pain medicine approaches such as stratified allocation on the basis of prespecified patient phenotypes using assessment methodologies such as quantitative sensory testing. Current major challenges within the field include: 1) identifying optimal measurement approaches to assessing patient characteristics that are most robustly and consistently predictive of inter-patient variation in specific analgesic treatment outcomes, 2) designing clinical trials that can identify treatment-by-phenotype interactions, and 3) selecting the most promising therapeutics to be tested in this way. This review surveys the current state of precision pain medicine, with a focus on drug treatments (which have been most-studied in a precision pain medicine context). It further presents a set of evidence-based recommendations for accelerating the application of precision pain methods in chronic pain research.
Keywords: Pain, precision, personalized, biomarker, phenotype, neuropathic, quantitative sensory testing
Chronic pain, which persists or recurs for at least 3 months,189 is a public health epidemic. For decades, spinal pain, headache disorders, and knee pain have ranked among the top global causes of years lived with disability.46,163 A 2018 analysis of Medical Expenditure Panel Survey data found that the proportion of U.S. adults reporting painful health conditions increased from just over 30% in 1997 to 1998 to 41% several decades later.138 Chronic pain is notoriously difficult to “cure,” is a leading global cause of reduced quality of life and carries direct and indirect costs approaching 1 trillion dollars annually in the U.S. alone.183,204
People with chronic pain often receive numerous treatments, with analgesic medications among the most common. However, long-term administration of analgesics such as nonsteroidal anti-inflammatory drugs (NSAIDs), antidepressant medications, and opioids involves substantial risk, exemplified by the contribution of prescribed analgesics to the ongoing opioid crisis in some countries.93 These findings, together with frustration stemming from the failure of most treatments to produce substantial benefits in the majority of patients,45 have stimulated intensive efforts to match patients with the best treatment for them.134,192,214 Progress in developing and implementing such precision medicine approaches has been significant in fields such as oncology, cardiology, neurology, and psychiatry,127 though it has been somewhat slower to reach maturity for pain.45
Decades ago, Mitchell Max proposed that “more effective, less toxic treatments” could be developed by targeting specific pathophysiologic mechanisms in specific persons being treated for pain.133 Precision medicine is an approach that accounts for individual variation in patient characteristics and disease mechanisms, with the primary goal of optimizing treatment outcomes.33,127 It is a strategy that seeks to provide the right treatment to the right patient, at the right dose, at the right time, with the expectation of better health outcomes at a lower cost. We use the term precision medicine instead of “personalized medicine,” which is sometimes misinterpreted as implying that unique treatments can be designed for each person.48,49 Chronic pain is an area in desperate need of precision medicine advances, as inter-patient variability in treatment outcomes (even for efficacious treatments) is impressively broad.57,80,81 While some variability is likely random, there is optimism that patient-by-treatment interactions can be identified.80 In short, just as cancer is conceptualized as hundreds of (genetically) distinct diseases,128,184 chronic pain is becoming an umbrella term encompassing overlapping conditions to which many pain-generating mechanisms contribute.
As noted in recent reviews, numerous high-quality, randomized, controlled trials (RCTs) have not produced significant overall treatment effects, despite encouraging results from early-phase drug studies.14,45,57,79 In addition to reflecting bias in preclinical research,35,202 such results derive from patient heterogeneity, which obscures positive treatment outcomes in certain subgroups. Within any given painful diagnosis, multiple pain mechanisms may be active to varying degrees in different patients at different time points over the course of the disease and the lifespan, leading to marked intersubject variation in treatment effects; this variability within a given pain condition is greater than that between different conditions.66,67 Conceptually, the field generally recognizes that certain patient phenotypes are associated with differential likelihood of response to other treatments such as surgery. For example, people with radicular leg pain and a large disc herniation are most likely to benefit from discectomy relative to those with persistent back pain who do not have those features,114 and patients with histories of substance use disorders are least likely to be helped and most likely to be harmed by long-term opioid treatment.47,205 However, progress toward a comprehensive precision medicine approach to managing chronic pain has been gradual.
Added to issues of mechanistic heterogeneity is the additional concern that specific mechanisms may contribute to multiple conditions, suggesting that: 1) substantial comorbidity may exist across distinct pain conditions, and 2) pain treatments may be most effective when tailored to patient phenotypes rather than pain diagnoses. This is reflected in the substantial overlap in pain diagnoses,131 especially in older adults, who often report function-limiting pain in multiple body locations.116,146 The high prevalence of chronic overlapping pain conditions (COPCs) highlights the presence of common mechanisms and shared phenotypes across chronic pain syndromes, which may reflect a major contribution of central factors to these conditions.77,131 For example, someone outside the field would have no reason to suspect that irritable bowel syndrome (IBS) and temporomandibular joint disorder (TMD) would be highly comorbid. After all, the pain symptoms differ phenomenologically, they affect distinct anatomic regions, one condition is visceral in nature while the other affects primarily muscles and joints. However, IBS and TMD are in fact highly comorbid COPCs.31 Collectively, clinical studies have revealed that comorbid pain conditions may exacerbate one another, and treatment of one may result in improvement of others.34,82 Consequently, it is important to investigate COPCs collectively when evaluating the efficacy of potential treatments.
The aims of this comprehensive review include: 1) elucidating the challenges of taxonomy and framework that have previously been utilized for clinical trials of pain-relieving treatments; 2) highlighting specific examples of seminal precision pain medicine studies in the last several decades; 3) identifying key components of pain phenotyping that will help advance precision pain medicine; 4) summarizing the current state of knowledge in precision pain medicine; 5) developing recommendations for the design of clinical trials of pain treatments in order to continue to build an evidence base for precision pain medicine.
Methods
IMMPACT Meeting
An Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) consensus meeting in 2016 included attendees from academia, government, pharmaceutical companies, and patient advocacy organizations. The aim of this 2-day meeting on “Accelerating the Development of Precision Pain Medicine” was to summarize the field and develop recommendations for clinical trials. Meeting organizers conducted a narrative background review of publications, and articles were circulated prior to the meeting. Titles of the meeting talks are listed in Appendix 1; meeting materials are available on the IMMPACT website: http://www.immpact.org/meetings/Immpact19/participants19.html. After the meeting, additional literature searches were incorporated into the summary of the discussions and recommendations. In light of past IMMPACT reviews on related topics,57 emphasis was placed on recent studies. Electronic versions of the manuscript were circulated to all authors; final agreement was achieved through discussion and iterative review of the draft manuscript. This manuscript, which focuses on foundational precision medicine approaches such as applying predictive enrichment strategies, was approved by all authors.
Challenges to creating a mechanistic pain taxonomy
Historically, pain conditions have been defined anatomically rather than on the basis of mechanisms, though this approach is steadily shifting.189 Moreover, most chronic pain conditions may not be easily classified by predominant category (eg, nociceptive, inflammatory, neuropathic, nociplastic), and may contain multiple overlapping pain mechanisms with varying loci involving the peripheral and central nervous systems (eg, peripheral sensitization, ectopic activity, neuroinflammation, central sensitization).197,203 An ongoing point of debate concerns what measurable phenotypic characteristics are most predictive of variability in analgesic outcomes, and what measurement approaches are best suited to evaluate these characteristics. Although we know a great deal about the general predictors of persistent pain and disability, less is known about the phenotypes that predict individual responses to specific pain treatments, and indeed, we cannot assume that these factors, or factor combinations, are the same.56,81 Recent work has identified core domains (eg, psychosocial status, sleep, pain-modulatory capacity) that have proven to be robustly important in shaping outcomes in clinical trials of pain treatments (see57). As we discuss prediction studies in this review, wherever possible we focus on treatment effect modification, in which a phenotype is differentially associated with outcomes in different treatment arms. Such findings are also sometimes referred to as Heterogeneity of Treatment Effect (HTE) or moderation effects80; these studies are essential in facilitating precision pain medicine, which relies on identifying and harnessing differential effects across treatments in specific patient subgroups.
Caveats
Rigorous moderation studies, in which a phenotype is differentially associated with outcomes in different treatment arms (ie, most often an active and a placebo arm) are more common in certain areas (eg, pharmacologic treatment of neuropathic pain). In contrast, perhaps because controls are more challenging (or absent), there are relatively fewer trials of medical devices that examine treatment-by-patient interactions. General prediction studies abound, and some use quite sophisticated statistical approaches: For example, artificial neural networks have identified predictive factors for successful treatment with extracorporeal shock wave therapy for chronic plantar fasciitis.221 Factors such as shorter-duration pain, higher-intensity pain, and the presence of spurs are important positive prognostic factors. However, with no control group, we cannot determine whether these are general predictive factors, or whether they are specifically important in this treatment’s outcomes. Finally, we note that while most of the reviewed studies utilize pain intensity as the primary outcome, a broader range of outcomes (each of which may have unique predictors) are important to patients and should be considered in the future.193
Results
Biomarkers
A full examination of pain biomarker research is beyond our scope (recent reviews offer excellent summaries:37), but the search for pain biomarkers is an instructive example of integrating information across multiple domains towards a personalized approach to pain. Precision pain medicine overlaps with the categories of pharmacodynamic/response biomarkers (ie, biomarkers which reflect target engagement), predictive biomarkers (ie, biomarkers which can predict response to a therapy), and safety biomarkers (ie, biomarkers which reflect the potential or presence of toxicity related to a therapeutic agent). Rigorously-validated biomarkers, once they have cleared regulatory hurdles associated with the status of in vitro diagnostic (IVD) devices, have great potential to provide objective measures of pain as complements to the “gold-standard” of pain self-reports, confirm that a therapeutic intervention has reached its intended molecular target, and predict treatment responses.37,178,188 Many pain biomarker studies have identified multimodal predictors of analgesic response to a specific treatment. For example, a recent fMRI study of people with neuropathic pain treated with ketamine revealed that high pre-treatment levels of temporal summation of pain, as well as high pretreatment dynamic functional connectivity between regions of the default mode network and descending antinociceptive brain circuits, were both associated with better analgesic response to ketamine.20 This study was limited by the lack of a control treatment group, but the findings offer an intriguing glimpse into the potential future of precision pain medicine, involving comprehensive multimodal assessment and subsequent clustering of patients into subtypes.
Using Biomarkers/Phenotyping in Precision Pain Medicine
Peripheral Nerve Assessment
Skin punch biopsy involves taking representative sections of skin, immunohistochemically staining them to reveal intra- and subepidermal nerve fibers and quantifying the number/density of those fibers.43 These biopsies are a critical tool in the diagnosis of small fiber neuropathy (SFN), a common source of chronic neuropathic pain. Compared to clinical examination, skin biopsy more accurately identified people with and without SFN.42,44 Interestingly, it is not only neuropathic pain conditions that show loss of peripheral nerve fibers on skin punch biopsy. Compared with controls, people with fibromyalgia also exhibited decreased intra-epidermal nerve fiber density (IENFD).141 People with HIV-associated peripheral neuropathy who exhibited lower IENFD at the distal leg reported more intense pain than those who had higher IENFD.155,224 Others have reported similar findings in groups of people with SFN and diabetes,157,179 though a direct relationship between IENFD and pain severity has not been conclusively established.104–106
There is some evidence that inter-patient variability in IENFD may predict likelihood of treatment benefit. In an early PHN study, participants with normal nerve fiber density and preserved sensation respond well to topical treatments.166,167 In a more recent enrichment-design, placebo-controlled crossover trial of pregabalin for the treatment of prediabetic neuropathic pain, pretreatment IENFD was associated with treatment response.90 Pregabalin responders (after 1 month of treatment) had a higher IENFD compared to those who were classified as pregabalin nonresponders (compared to placebo). Collectively, IENFD has emerged as a sensitive and efficient diagnostic tool to identify individuals with SFN, and it may be useful in the early diagnosis of other neuropathic conditions.42,43 However, further research is needed to determine whether skin biopsy can predict treatment benefit or to distinguish between people with neuropathy who will or will not experience neuropathic pain.
Brain Imaging
Magnetic resonance imaging (MRI) has opened a window into the evaluation of the human brain by allowing noninvasive study of brain structure and function.129,154 Applying neuroimaging-based biomarkers for pain is proving be useful in numerous ways, including: diagnosis, prognosis, identifying treatment responders, identifying therapeutic targets, and defining surrogate endpoints.126 Many of these studies use machine learning systems to work with the enormous data sets generated by imaging methods125 to identify neural signatures associated with pain: for example, the Neurological Pain Signature (NPS) and Pain-Analgesic Network.188,206 Neuroimaging has also been productively combined with other biomarker-based approaches such as genetics. For example, recent studies have identified brain axonogenesis as a major contributing pathway to chronic pain through a functional genomics approach combined with structural neuroimaging.110
A challenge for neuroimaging is to deliver actionable information at an individual level. To date, multiple studies have met this challenge,20,120,187,191 with recent work applying whole-brain functional connectivity to develop a brain connectivity biomarker for sustained experimental pain as well as clinical pain.126 The neural signature predicted clinical pain severity and classified patients versus controls in two independent studies of low back pain. Similar work in fibromyalgia (FM) has shown that fMRI responses to an aversive visual stimulus could distinguish not only between people with FM and healthy controls, but also between those taking pregabalin versus placebo at greater than 80% accuracy.95 Importantly these neuroimaging predictors overlapped within the same insula region, suggesting that a specific maladaptive pattern of chronic pain-related functional brain organization could serve as the target of a successful analgesic.
Recent investigations have also sought to identify the mechanism of ketamine’s analgesic effect using resting-state functional magnetic resonance imaging (rs-fMRI).20,36,164 Persons with chronic pain were divided into ketamine responders (≥50% improvement in pain intensity) and nonresponders. Responders exhibited significantly lower functional connectivity within the default mode network (DMN), and higher connectivity between the overall DMN network and descending pain-modulatory regions (eg, the periaqueductal grey and the rostroventral medulla). This connectivity may represent an important biomarker, providing evidence that reducing an overactive ascending nociceptive system that is suppressing a strong descending modulation system is associated with ketamine benefits.20 Some acupuncture studies have also evaluated functional connectivity in DMN regions as a predictor of outcomes. For example, connectivity between the medial prefrontal cortex (mPFC, a part of the DMN) and insula, putamen, and caudate was significantly correlated with treatment responses after 4 weeks of acupuncture treatment.191 The insula is a key region integrating sensory processing and cognitive modulation, and it is activated during acupuncture.27,89 In particular, mPFC-insula connectivity was previously reported to be altered and correlated with changes in knee pain after acupuncture treatment.29,58 It is possible that mPFC-insula connectivity may reflect patients’ unique internal sensory and cognitive states (eg, reward) for acupuncture treatment, that consequently influence treatment response. In contrast, connectivity in different circuits (ie, mPFC to anterior cingulate cortex connectivity) was predictive of treatment response to sham acupuncture, suggesting that sham acupuncture may reduce symptoms in cLBP via an alternate pathway29,89; such work highlights the possibility that neuroimaging may allow for trial enrichment or stratification approaches that could improve assay sensitivity in clinical trials and advance the development of precision pain medicine.
Psychosocial Factors
The biopsychosocial model describes pain as a multidimensional, dynamic interaction among physiological, psychological, and social factors that reciprocally influence one another, resulting in chronic and complex pain syndromes. Psychosocial variables such as depression, anxiety, and distress are among the most robust predictors of the transition from acute to chronic pain, especially musculoskeletal pain.56,156 Some evidence also suggests that high levels of negative affect and pain-specific distress are associated with reduced benefit from a variety of potentially pain-reducing treatments.53,207,208 Importantly, trials of opioid analgesics have noted that elevated pre-treatment scores on measures of depression and anxiety are associated with reduced opioid analgesic benefit101,161,207,210 within the active treatment group. Similarly, pain catastrophizing is a psychosocial construct comprised of cognitive and emotional processes such as helplessness, pessimism, rumination, and magnification of pain reports.53,173,182 Uncontrolled studies suggest that risk factors such as catastrophizing, along with positive resilience factors, can independently predict inter-patient variation in treatment outcomes. For example, higher baseline pain resilience was associated with better quality-of-life outcomes, whereas higher baseline catastrophizing was associated with poorer outcomes following multidisciplinary treatment,69 and similar findings have emerged from other studies.56,60,173 There are also some moderational findings from controlled studies; for example, an RCT of transcutaneous electrical nerve stimulation (TENS) for postoperative pain reported strong effect-modification results.158 Surgical patients were randomized to receive TENS, placebo TENS, or standard care for 6 weeks. Those in the TENS group with high baseline catastrophizing scores showed less pain reduction and reduced range of motion at 6 weeks. In contrast, there was no predictive effect of catastrophizing in the other 2 groups. Other effect modification findings have suggested that different treatments may be most effective in people reporting relatively elevated catastrophizing; for example, among women undergoing mastectomy, regional anesthesia (compared to surgery as usual with no regional anesthesia) reduced postoperative acute226 and chronic225 pain and opioid use to a greater degree in high-catastrophizing relative to low-catastrophizing women. Similarly, higher baseline pain catastrophizing was associated with a greater benefit of a conditioned open-label placebo intervention following spine surgery.65
Sleep
Pain can be both a cause and a consequence of disruption in sleep patterns. Persistent pain and sleep deficiency share a variety of mechanisms, including perturbations of opioid, monoaminergic, immune, and endocannabinoid systems.92 Experimental, clinical, and epidemiologic studies have suggested that sleep disruption or deprivation has a variety of negative effects such as: enhanced pain sensitivity, reduced pain inhibition, elevated chronic pain severity and disability, and increases in the frequency and impact of daily musculoskeletal pains.62 Persistent sleep disturbance is a robust and independent predictor of chronic postsurgical pain development.172 It is also clear that insomnia and its associated symptoms are a major contributor to poor pain-related quality of life; an IMMPACT survey found that trouble falling asleep, trouble staying asleep, and feeling tired, are 3 of the top 10 importance-rated domains for people with persistent pain.193
To date, several studies have shown that variation in sleep can predict pain-related outcomes. In preclinical studies, sleep-deprived animals derive reduced analgesic benefit from opioids and at least one controlled human study has shown similar effects.180 The SPACE trial,119 which randomized patients to opioid or nonopioid treatment, suggested that baseline sleep quality consistently predicted treatment outcomes across groups, with higher sleep disturbance scores at baseline predicting less improvement in Brief Pain Inventory (BPI) interference (P < .001) and BPI severity at 1-year follow-up.115 Interestingly, a post-hoc analysis of data pooled from 16 placebo-controlled trials of pregabalin in patients with neuropathic pain conditions (ie, DPN or PHN) revealed that, among thousands of patients, one of the best predictors of pregabalin-associated pain reduction was a high degree of sleep disruption at baseline.198,199 This small set of apparently disparate findings suggests that phenotypic measures of sleep disturbance are likely to have treatment-specific effects (eg, people with severe insomnia may benefit most from pregabalin and least from opioids), which could be identified using predictive algorithms in RCTs.
Quantitative sensory testing (QST)
QST refers to psychophysical methods used to quantify somatosensory functioning. It has been used to diagnose and monitor conditions such as sensory neuropathies,42 probe the function of specific nerve fiber populations, investigate pain mechanisms, characterize somatosensory profiles, and measure individual variability in pain sensitivity and modulation.2 QST can quantify the severity of positive (eg, hyperalgesia) and negative sensory phenomena (eg, hypoesthesia and hypoalgesia).103 It has perhaps been most frequently applied to study maladaptive sensory responses in chronic pain; indeed, recent reviews highlight the extent to which pain conditions with disparate etiologies demonstrate widespread hyperalgesia.7
Numerous large studies have applied QST to patients with a variety of pain conditions (often neuropathic pain) in order to examine sensory profiles or subgroups.70,84,130 Many of these studies use the German Research Network on Neuropathic Pain (DFNS) testing protocol, which is highly standardized and which assesses numerous parameters: for example, thermal and mechanical pain thresholds, temporal summation, dynamic mechanical allodynia.165 In general, these sensory profiling studies have determined that66,67: 1) Most participants exhibit at least 1 sensory abnormality, which is expected, given that many diagnostic criteria for pain require positive or negative sensory symptoms/signs, 2) every measured somatosensory abnormality occurs at least occasionally across every pain condition, 3) no particular QST profile is unique to a given pain diagnosis, and 4) painful and painless neuropathies express similar clusters of QST abnormalities.68 This last observation, that quite different neuropathies are not distinguishable on the basis of QST, but that similar subgroups can be defined in each diagnostic group, has been especially surprising. These observed “transetiological” patterns of sensory symptoms and deficits may reflect unique pain mechanisms, which may be a fruitful target for specific therapeutic approaches.
QST has also been applied in predictive contexts. Pre-surgical individual differences in sensory profiles have shown prospective associations with acute and chronic post-operative pain across a number of procedures.169,196 In musculoskeletal pain, QST-assessed hypersensitivity due to central pain mechanisms can impair recovery and lead to worse clinical outcomes. A recent systematic review of nearly 40 prospective studies concluded that baseline QST predicted musculoskeletal pain and disability measures, and that sensory profiling could help develop targeted interventions across a range of musculoskeletal conditions.78 QST is sometimes combined with other phenotypic information to enhance prognosis of pain outcomes: for example, low pressure pain thresholds together with features of neuropathic pain, more widespread pain, higher patient-reported distress, and poor sleep were all predictive of persistent and worsening joint pain over 1 year in a community sample.1 Promising findings are emerging from diverse neuropathic pain trials examining pretreatment QST responses as predictors of response to therapy.14,15,67,153,160
Using multinational DFNS data collected by 3 research consortia, Baron et al. conducted cluster analyses to identify and cross-validate 3 subgroups of patients with peripheral neuropathic pain16 (see Figure 1). The sensory profiles—termed “sensory loss,” “thermal hyperalgesia,” and “mechanical hyperalgesia”—bear a striking resemblance to the 3 subgroups identified in some of the initial mechanism-focused research on post-herpetic neuralgia.144 Moreover, similar profiles emerged in a large sample of healthy participants undergoing surrogate experimental models of nerve block, primary hyperalgesia, and secondary hyperalgesia.200 Such QST-identified sensory phenotypes show robust temporal stability in the absence of intervention, but some abnormal sensory findings in neuropathic pain have been shown to resolve with effective disease-modifying treatment (ie, in the case of successful surgery for carpal tunnel syndrome:109). Recent work has also validated brief “bedside” QST, which can generally be conveniently performed in a half hour or less.117 Importantly, the sensory phenotypes derived from QST are not likely to be amenable to assessment via patient self-report. While some questionnaire measures assessing sensory features of neuropathic pain have proven useful both as phenotyping and outcome measures (see the later “Pain Qualities” section), patient-reported neuropathic symptoms on measures such as the Neuropathic Pain Symptom Inventory (NPSI) show minimal associations with QST-assessed measures of allodynia, hyperalgesia, etc.70,85,117
Figure 1.
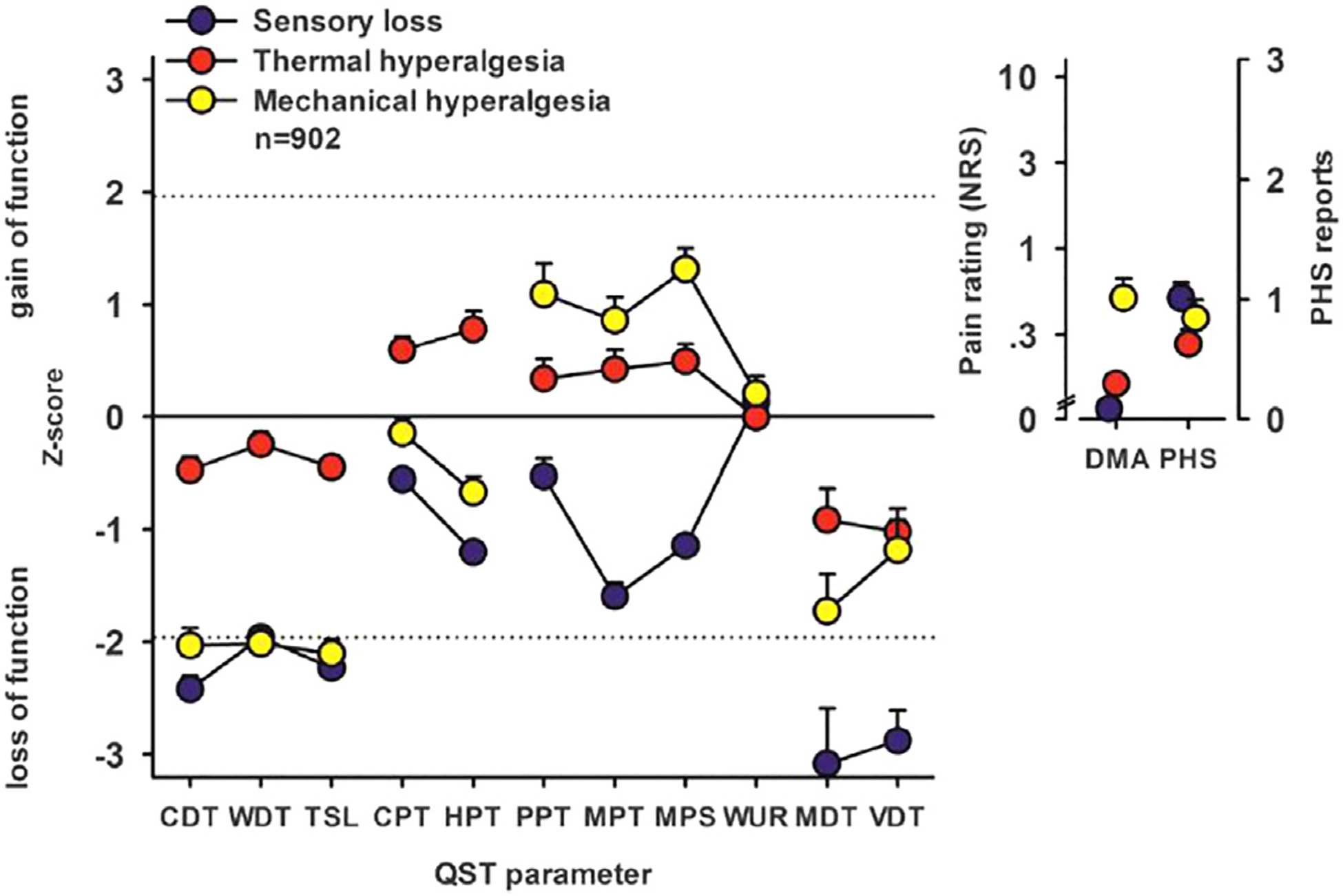
DFNS-assessed 3-cluster sensory profiles (Adapted with permission from Baron et al., 2017). Cluster analysis results: Sensory profiles of the 3 clusters presented as mean z scores ± 95% confidence interval for the test data set (n = 902). Positive z scores indicate positive sensory signs (hyperalgesia), whereas negative z values indicate negative sensory signs (hypoesthesia and hypoalgesia). Dashed lines: 95% confidence interval for healthy subjects. Insets show numeric pain ratings for dynamic mechanical allodynia (DMA) on a logarithmic scale (0–100) and frequency of paradoxical heat sensation (PHS; 0–3). Blue symbols: cluster 1 “sensory loss” (42% of sample). Red symbols: cluster 2 “thermal hyperalgesia” (33% of sample). Yellow symbols: cluster 3 “mechanical hyperalgesia” (24% of sample). CDT, cold detection threshold; CPT, cold pain threshold; HPT, heat pain threshold; MDT, mechanical detection threshold; MPS, mechanical pain sensitivity; MPT, mechanical pain threshold; NRS, Numerical Rating Scale; PPT, pressure pain threshold; QST, quantitative sensory testing; TSL, thermal sensory limen; VDT, vibration detection threshold; WDT, warm detection threshold; WUR, wind-up ratio.
Several trials have reported that QST-assessed indices of hyperalgesia are associated with better analgesic responses to neuropathic pain medications compared to placebo: among patients with PHN, those with mechanical allodynia had a better outcome with intravenous lidocaine than with placebo,10 a finding that was also observed among patients with spinal cord injury pain treated with lamotrigine,64 patients with peripheral neuropathic pain treated with botulinum toxin A,9 and patients with HIV neuropathy or chronic visceral pain treated with pregabalin.143,175 In painful diabetic neuropathy, an oral transient receptor potential ankyrin 1 (TRPA1) antagonist produced statistically significant improvement in pain specifically in a sub population of patients with preserved small fiber function defined by QST.97 To date, the majority of the positive findings involving QST-assessed phenotypes have been identified in post-hoc analyses. However, some trials have incorporated pre-specified phenotypic hypotheses into their study designs. For example, a 2014 RCT of oxcarbazepine showed effect modification using elements of the DFNS QST paradigm.40 At baseline, patients were phenotyped into “irritable nociceptor” (ie, those with sensory gain) and “nonirritable nociceptor” groups. The irritable nociceptor group derived substantially greater benefit from oxcarbazepine compared to the nonirritable nociceptor group, with no differences in placebo effects. The number needed to treat (NNT) for 50% pain relief was 3.9 in the irritable nociceptor group, compared with an NNT of 13 in the remainder of the sample.40 Collectively, the hypotheses presented by Baron and colleagues regarding the classes of pharmacologic treatments expected to be efficacious for each of the QST-identified subgroups of patients they identified will be a valuable guide for the design of future clinical trials16,201 (see Table 1).
Table 1.
(Adapted from Vollert et al., 2017). Predicted Benefits of Different Analgesic Classes in 3 DFNS-defined Subgroups
| Cluster Characteristics, Hypotheses About Underlying Pathophysiology, and Expected Treatment Efficacy | |||
|---|---|---|---|
| Sensory Loss | Thermal Hyperalgesia | Mechanical Hyperalgesia | |
| Sensory profile | |||
| Sensory Loss | Touch, thermal, pain | None | Mostly thermal |
| Hyperalgesia | None | Mostly cold & heat | Mostly pressure & pain |
| DMA | Little | Little | Much |
| PHS | Much | Little | Little |
| Pathophysiology | |||
| Sensory Loss | Small & large fibers | — | Mostly small fibers |
| Hyperalgesia | — | Peripheral sensitization | Central sensitization |
| Ongoing Pain | Ectopic activity | Spontaneous activity | Ectopic activity |
| Predicted Findings | |||
| IENFD | Loss | None | Mild loss |
| CCM | Loss | None | Mild loss |
| Peripheral MRI | Damage | None | Mild damage |
| LEP | Reduction | Normal or gain | Mild reduction |
| RIII | Reduction | Normal or gain | Gain |
| μENG | Denervation | Sensitization | Little denervation |
| Predicted Efficacy | |||
| NSAIDS | — | (+) | — |
| Botox | + | ||
| Topical capsaicin | + | ||
| NMDA antagonist | + | ||
| SNRI | ++ | + | + |
| Gabapentinoid | + | + | ++ |
| Sodium channel blocker | + | ++ | ++ |
| Opioid | ++ | + | + |
CCM, confocal corneal microscopy; DMA, dynamic mechanical allodynia; IENFD, intraepidermal nerve fiber density; LEP, laser-evoked potentials; μENG, microneurography; PHS, paradoxical heat sensation; RIII, nociceptive flexion reflex; SNRI, serotonin-norepinephrine reuptake inhibitor.
Some QST prediction work is also being done on non-pharmacologic therapies. A recent secondary analysis examined whether pressure pain tolerance predicted response to CBT or emotional awareness and expression therapy (EAET) compared to an education-based control condition.19 The analysis revealed an interaction between treatment assignment and QST phenotype; among patients with low pain tolerance, both EAET and CBT led to small but significant improvements in pain severity compared to the education control group. Conversely, in the subset of patients with normal pain tolerance, the patients receiving EAET reported a much larger reduction in pain than the other groups. The authors suggested that QST may provide insights about individual responses to psychologically based therapies for chronic pain.19 Interestingly, recent studies of high-frequency TENS treatment for musculoskeletal pain report that patients who are most sensitive to mechanical noxious stimuli are more likely to benefit from active TENS treatment relative to sham/placebo.98,100,102
Endogenous Pain Modulation
Nociceptive signals are modulated by pain-inhibitory and facilitatory processes which operate across the central nervous system and shape inter-individual variability in the trajectory of many persistent pain conditions. For instance, conditioned pain modulation (CPM) and temporal summation (TS) paradigms have been used as indices of pain-inhibitory and pain-facilitatory processesm,2,7 respectively. Psychophysical assessment of pain facilitation is most often assessed using TS, which involves applying a series of identical noxious stimuli and measuring the increase in the percept of pain.8 People differ broadly in their degree of temporal summation, and many persistent pain groups demonstrate increased TS relative to controls. Its neural correlates are increasingly being identified,30,111 and TS can be reduced by a variety of centrally acting analgesic treatments, from ketamine6 to spinal cord stimulation59 to acupuncture222 to exercise.194 Recent studies of postoperative pain have highlighted the potential prognostic value of TS for predicting the development of persistent postoperative pain.153
In addition, CPM has emerged as a predictor of post-operative pain.121 CPM was originally studied in animals as diffuse noxious inhibitory controls (DNIC), a physiological counter-irritation phenomenon described decades ago.122–124 CPM reflects CNS endogenous pain-inhibitory mechanisms; a noxious stimulus applied to one body region can reduce spinal neuronal responses (and the perception of pain) in response to a second noxious stimulus applied elsewhere on the body.217,218 Investigations of the temporal stability of CPM have suggested that it is generally reliable and can be effectively utilized as a phenotyping measure.108 Currently, the CPM concept is best viewed as the net effect of various facilitating and inhibiting systems exerting their activity at spinal or supraspinal levels.136,150
Impaired CPM and facilitated TS appear in patients with chronic musculoskeletal, visceral, and neuropathic pain conditions (for reviews, see.7,41,78,113,153 There has been growing interest in characterizing people based on their pain modulation profiles (PMPs),178 as inter-patient variability in pain modulation has been shown to predict clinical outcomes such as development or worsening of pain after surgery.153 Several studies have also found that TS is a predictor of responses to COX-2 inhibitors4 and that CPM is a predictor of responses to topical NSAID54 as well as pregabalin24 and duloxetine219 treatment. Such PMP subgrouping might contribute to individualized treatment selection. For example, Yarnitsky and colleagues postulated that patients showing decrements in CPM should benefit most from serotonin-noradrenaline re-uptake inhibitors (SNRIs), which augment descending inhibition.219 In patients with diabetic neuropathic pain who were treated with duloxetine, those with low pretreatment CPM derived substantial pain relief, while those with efficient baseline CPM did not benefit. Further, for the low CPM group, duloxetine-related changes in pain intensity paralleled changes in CPM. A placebo-controlled follow-up study also reported that CPM improved with duloxetine administration, this time in a group of migraine patients. However, it was TS rather than CPM that showed significant effect modification; higher TS predicted more pain improvement in the migraine patients receiving duloxetine, but not in those receiving placebo.112 Since poor CPM was correlated with elevated temporal summation, as has been observed in other chronic pain studies,132 it may be the case that clusters of patients with low CPM and high TS are most likely to respond to duloxetine versus placebo, with individual variables not necessarily emerging as significant predictors in multivariate models run in relatively small samples. Interestingly, CPM may be somewhat specific in its treatment-predictive capacity; in contrast to the SNRI findings, an RCT in patients with chronic pancreatitis suggested that pretreatment CPM was not associated with the analgesic effectiveness of pregabalin143 and was in turn unaffected by subsequent pregabalin treatment.23 Such specificity is expected, given the overlap between CPM mechanisms and SNRI mechanisms.219
Patient-Reported Pain Qualities & Characteristics
There is great interest in using electronic tools to perform real-time, frequent, “ecological” assessment of pain that has traditionally been accomplished by asking respondents for retrospective reports.168,170 Ecological Momentary Assessment (EMA) indices of daily pain show good reliability135 and may offer valuable supplemental information about treatment effects in RCTs,51 though there is no firm evidence for enhanced assay sensitivity with these methods.171 EMA also offers potential value in studies of precision pain medicine, as patients differ widely in the degree of temporal variability in their ratings of pain intensity. Several RCTs have assessed within-subject pain variability as a phenotypic predictor of trial outcomes in patients with musculoskeletal pain94 as well as neuropathic pain61; in each case, people with greater daily variability in pain intensity were more likely to be classified as placebo responders but were not more likely to respond to active medications. Such effect-modification results might suggest that people with high pretreatment variability in pain intensity (generally measured as the standard deviation of daily pain intensity ratings collected over 1 week) could be excluded from RCTs in order to minimize placebo responses and maximize assay sensitivity. Overall, it has proven challenging to identify robust predictors of placebo responses in clinical trials of neuropathic pain treatments,86 other than variability in pain ratings.50,57,61,190
In addition, questionnaires measuring pain qualities (eg, “burning,” “shooting,” “aching”) may be useful in precision pain medicine. For example, patients with neuropathic pain who reported their pain as paroxysmal, deep, electrical, and radiating reported greater analgesic benefit from pregabalin (but there was no association with placebo benefits), highlighting the potential benefits of phenotyping pain qualities.72 Similar findings emerged in a pooled post-hoc analysis of Phase 3 trials of pregabalin70; several subgroups of patients with specific patterns of neuropathic pain symptoms had greater pain improvement after taking pregabalin than did those who took placebo. Exploratory analyses of data from a trial of a morphine-gabapentin combination for neuropathic pain also suggested that baseline pain descriptors may be predictive of analgesic treatment response.87,88 A trial of the sodium channel blocker oxcarbazepine noted that the subgroup of patients reporting “paroxysmal” and “burning” pain symptoms showed significantly better pain reduction with oxcarbazepine than placebo.40 Paroxysmal and deep pain phenotypes were also associated with benefit from lidocaine patches39 and from subcutaneous injections of botulinum toxin A.21 Interestingly, a comparison of pregabalin and duloxetine in patients with diabetic neuropathic pain suggested that the cluster of patients with the least neuropathic pain symptoms responded better to duloxetine than to pregabalin.22 Finally, pain duration may also play a role in shaping the relative benefits of antidepressants and anticonvulsants in neuropathic pain.148 In a review of crossover trials, patients with shorter pain durations reported more pain improvement from antidepressant treatment, while those with longer duration responded better to anticonvulsants.176
Intersections of Phenotypes with Specific Treatments
Sodium Channel Antagonists
Multiple RCTs have reported that QST-assessed indices of hyperalgesia are associated with better analgesic responses to sodium channel antagonists compared to placebo: IV lidocaine in PHN,10 lamotrigine in central neuropathic pain,64 and oxcarbazepine in patients with peripheral neuropathic pain conditions.40 Moreover, recent efforts at back-translation of these studies have produced exciting results.45 For example, in a rat model of neuropathy, spontaneous activity in the thalamus was substantially attenuated by spinal lidocaine, as well as intraplantar lidocaine and systemic oxcarbazepine.147 Intraplantar injection of oxcarbazepine’s active metabolite licarbazepine replicated the effects of systemic oxcarbazepine, supporting a peripheral locus of action.147 These findings suggest that ongoing activity in primary afferent fibers drives spontaneous thalamic firing after spinal nerve injury; the inhibitory effects of both lidocaine and oxcarbazepine suggest that this rat model of neuropathy, involving a partial ligation of spinal nerves, resembles the irritable nociceptor patient subgroup identified in human studies.
Like oxcarbazepine, lacosamide is a nonselective sodium channel blocker and also reduced evoked spinal neuronal responses in an experimental rat model.18 Prior negative trials in neuropathic pain may stem from a lack of patient stratification rather than lack of efficacy as such. A recently registered trial will attempt to address this by investigating whether a similar drug-by-sensory phenotype interaction exists.28 A multimodal genetic, electrophysiological, and sensory profiling approach has already showed promise for treatment selection; several recent studies support that patients with Nav1.7 variant-driven small fiber neuropathies can benefit from lacosamide treatment.38,139
Calcium Channel Antagonists
The α2δ−1/2 ligands pregabalin and gabapentin have seen steady increases in use across the globe (eg,223). Their effects have been comprehensively characterized in rodent injury models, and both gabapentin and pregabalin attenuate ongoing pain and evoked hypersensitivity through central mechanisms, particularly where central sensitization is present.13,14,213 However, many patients do not derive substantially greater benefit over placebo, with NNTs in the range of 5 to 6.145 This may be at least partly attributable to the fact that, in animal models, the gabapentinoids are particularly effective at inhibiting high-intensity mechanically-evoked neuronal responses.13 There is some evidence from human studies that pregabalin may have similarly selective effects. Post hoc analysis of clinical trial data revealed that pregabalin did not separate from placebo in patients with HIV neuropathy, but provided pain relief in a subgroup characterized by severe mechanical hyperalgesia.175 This is consistent with Baron and colleagues’ proposal that central sensitization may be the predominant pathophysiological mechanism for this mechanically sensitive patient phenotype.16 Consistent with the features of this QST-derived sensory profile, analysis of a separate pregabalin trial concluded that analgesia corresponded with preserved large fiber function and poorer outcomes were observed with loss of fibers.96 In addition, post-hoc analysis of questionnaire and bedside QST data in a series of 5 pregabalin trials in neuropathic pain revealed that patient-reported hyperalgesia on the Neuropathic Pain Symptom Inventory was associated with a significantly better response to pregabalin than to placebo in both primary and confirmatory analysis, and that the presence of severe punctate hyperalgesia, moderate-to-severe cold hyperalgesia, and moderate-to-severe temporal summation to tactile stimuli were all associated with a better response to pregabalin over placebo.21,70 Collectively, these disparate studies suggest that pregabalin is likely to be differentially effective in reducing neuropathic pain in patients who demonstrate a mechanically sensitized sensory profile, characterized by at least moderate hyperalgesia.
Opioids
Psychosocial factors are known to be strong predictors of opioid-related outcomes, with high levels of distress, negative effect, and catastrophizing predicting less opioid analgesia, more side effects, and a greater propensity to misuse opioids.12,55,76,99,107,210 Most of this work involves general prediction studies, though several trials have identified differential response to opioid vs. placebo as a function of psychosocial status.101,207, 209 Previous QST findings have suggested that the magnitude of CPM is lower for opioid users than nonusers, suggesting that long-term opioid use might dampen the functioning of endogenous pain-inhibitory systems.55,132,159 Interestingly, several recent experimental studies have found that acute opioid administration may enhance endogenous pain inhibition,3,140 though other reports of short-term administration have suggested minimal effects.185 Further work has indicated that higher levels of pre-treatment CPM are associated with enhanced morphine analgesia (measured as a reduction in experimental pain sensitivity) in patients with chronic low back pain as well as healthy adults.25 Collectively, these findings may suggest that the impact of opioid use on indices of pain inhibition shows a biphasic time course, with acute potentiation of CPM followed by long-term decrements of CPM in persistent opioid users. To the extent that endogenous pain-inhibitory systems exert a modulatory influence upon sensitization processes,5,52,91,215 opioid-induced disruption of CPM might compromise the expected association between pain inhibition and pain facilitation, as a recent study has observed.132 Collectively more precision medicine data from opioid trials is necessary in order to determine which phenotypic patient characteristics are associated with relatively better or worse pain-related outcomes associated with opioid treatment.
NSAIDs
Most reviews and meta-analyses of NSAID effects have focused on features of the specific medications themselves (eg, comparing drugs, or dosages, or durations of treatment) rather than patient-level characteristics as predictors of analgesic responses to NSAIDS, though one recent individual patient meta-analysis of topical NSAIDS did report superior benefit over placebo in women relative to men.149 Collectively, we know relatively little about QST’s role in the prediction of NSAID-associated pain relief, but there is some evidence that, contrary to some of the neuropathic pain treatments, a less favorable, more sensitized pain modulation profile is associated with reduced responsiveness to NSAID treatment. For example, after 3 weeks of treatment with NSAIDs and paracetamol in patients with knee osteoarthritis, high TSP was associated with a lower likelihood of response.151 Furthermore, in contrast to the duloxetine findings, better CPM was associated with better analgesic effects of topical NSAIDs for painful knee osteoarthritis and with better response to NSAIDs and paracetamol in patients with knee osteoarthritis.54,152 Both of these were uncontrolled “general prediction” studies, highlighting the preliminary stage of the NSAID data.
SNRIs
An RCT in diabetic neuropathic pain revealed that patients with the lowest burden of neuropathic pain symptoms responded better to duloxetine than to pregabalin,22 and QST studies have also suggested that patients with poor CPM and elevated temporal summation (ie, those with relatively maladaptive pain modulation profiles132 are most likely to respond to duloxetine.112,219 Given the prior findings of a review in painful polyneuropathy (ie, the benefits of antidepressant treatment over placebo are significantly greater in those who have experienced pain for less than 3 years176), it appears important to consider the duration of patient-reported pain symptoms as a potentially important factor as well. Finally, recent neuroimaging studies have suggested that predictive brain biomarkers of placebo responses differ from the predictive brain biomarkers of duloxetine responses, generating hope that personalized treatment algorithms will eventually be possible.186,187
Recommendations (see Table 2)
Table 2.
Recommendations for Precision Pain Medicine Studies
| Recommendation | Benefit | |
|---|---|---|
| 1. | Test for heterogeneity of treatment effect | Confirms an adequate degree of inter-patient variation in treatment responsiveness to test phenotype-by-treatment interactions. |
| 2. | Select validated phenotyping measures | Maximizes precision in quantifying phenotypes of interest. Facilitates comparison of findings across studies (that use validated measures). |
| 3. | Carefully consider sample size requirements | Testing for phenotype-by-treatment interactions often requires large samples. Adequately powering a trial is essential in minimizing Type II error. |
| 4. | Consider crossover, or N-of-1 trials | Offers much greater power (i.e., greatly reduced sample size requirements) when examining subgroup/phenotype differences in treatment response. |
| 5. | Consider stratified allocation based on phenotypes | Maximizes power to detect phenotype-by-treatment interactions. When possible, implement 50:50 (i.e., equal group sizes) stratified allocation. |
| 6. | When possible, implement back-translation approaches | Facilitates confirmation of hypothesized treatment targets and localization of drug/treatment effects in the nervous system. |
| 7. | Plan for phenotypic clustering | Reduces concerns related to testing multiple, correlated, individual variables. Enhances power by minimizing the need for multiple comparison corrections. |
| 8. | Implement dynamic measurement in trials | Accounts for naturally-occurring phenotypic variability over time, increases reliability of phenotyping measurements. |
Test for heterogeneity of treatment effect
Before examining (differential) prediction of outcomes in different groups in RCTs, it would ideally be important to demonstrate statistically that patients vary significantly (over and above the “natural” fluctuations that occur in outcome variables such as pain intensity) in their response to intervention. It seems obvious that such variability would be significant, given that a sizable percentage of patients respond even to placebo treatments,86,149 but not all treatments produce definitive statistical evidence of heterogeneity. For example, a recent analysis of 4 multiperiod crossover trials of fentanyl treatment for cancer pain revealed firm evidence of heterogeneity (ie, significant treatment-by- patient interactions) for at least 3 of the trials.80 That is, patients differed from one another in their differential response to fentanyl compared to placebo, and those individual differences persisted across treatment episodes. In contrast, a meta-analysis of an education-focused behavioral treatment (Pain Neuroscience Education) revealed insufficient evidence for inter-patient variation (over and above random variation over time) in treatment response.211 These disparate findings highlight the potential importance of evaluating response heterogeneity before undertaking resource-intensive precision pain medicine approaches to evaluate predictors of inter-patient variation in treatment responsiveness.
Select validated phenotyping measures
In a 2016 review, we offered recommendations for including phenotypic factors (and validated phenotyping measures) for Phase 2 and 3 trials of chronic pain treatments.57 Additional advances have been made in some of the fields: for example, we recommended considering the DFNS QST battery for QST phenotyping, and that recommendation remains solidly evidence-based. Since then, though, further work has been done on brief, less resource-intensive “bedside” QST protocols that can generally be conveniently performed in a half hour or less.117 Such assessments may be less burdensome and more feasible to apply in multisite trials and could be considered in place of the full DFNS battery. It is also recommended that investigators strongly consider including assessment of commonly-assessed phenotypes that are not necessarily the primary phenotypic factors that are being studied: for example, pain variability, sleep, mood. Over time, this will help to promote additional precision pain medicine investigations, allow for pooling of data, etc.
Carefully consider sample size requirements
In order to rigorously show the ability of a phenotype to predict response to active treatment, an RCT with a prespecified primary analysis that tests the significance of the difference between the effect sizes in the 2 subgroups must be conducted. This is accomplished by testing for treatment-by-phenotype interactions. Powering a trial to test such interactions generally requires quite large sample sizes.81,201 For example, if the subgroups are of equal size (50:50 allocation) and the Standardized Effect Sizes (SESs) in the subgroups are 0.2 and 0.5, respectively (ie, an SES of 0.35 for the overall treatment effect), the total sample size required to demonstrate a significant treatment-by-subgroup interaction (for this subgroup difference in SES of 0.3) is over 1,300 participants.
Consider crossover, or N-of-1 trials
The sample sizes required to detect treatment-by-phenotype interactions are far more reasonable in crossover designs.80,81 For example, in the hypothetical investigation above (phenotypic subgroups of equal size (50:50 allocation), with SESs of .2 and .5), if we assume a moderate (r = .4–.6) within-patient correlation in treatment effects, only around 300 to 400 patients are required to achieve 80% power (in contrast to over 1,300 patients in the parallel-design study). Multiperiod crossover N-of-1 trials require even smaller samples, have been facilitated by the broad adoption of mobile data collection platforms (e.g., smartphones), and have been used in both recent and older studies to assess individual treatment responses.17,118,142,174,220 Additional design for consideration include enriched enrollment randomized withdrawal designs; these have been routinely used, especially for opioid trials,137 though it is not clear whether such trials provide greater power for subgroup analyses than standard non-withdrawal designs.71
Consider stratified allocation
While past trials have performed post-hoc analyses, we recommend 50:50 stratified allocation based on a defined phenotype. This approach will maximize power to detect phenotype-by-treatment interactions (eg, see:66 and81). Some ongoing trials in neuropathic pain appear to be taking this type of approach. For example, a recently described phase 2, proof-of-concept, phenotype-stratified study is enrolling patients with peripheral neuropathic pain who will be randomized to a 12-week treatment with lacosamide or placebo.28 The primary objective is to compare change in daily ratings of average pain intensity in patients with and without the irritable nociceptor phenotype.
Back-translation
When possible, consider back-translation (also termed reverse translation) approaches which can help to confirm effects and targets and localize where in the nervous system drug effects are unfolding.14,45,162 Reverse translation, also called bedside–to–benchtop research, begins with clinical experiences or clinical research findings, and works backward to uncover the mechanistic basis for these observations. For example, Rice and colleagues have proposed a back-translational approach involving classifying animal models of neuropathic pain by their sensory response profiles, which would be defined on the basis of human QST studies (eg, sensory loss, thermal hyperalgesia, mechanical hyperalgesia).162 Collectively, progress in the area of precision approaches to treatment of chronic neuropathic pain with sodium channel antagonists appears to have been substantively facilitated by back-translational work.14,28,147,216
Consider phenotypic clusters
It may eventually be prudent to utilize cluster-based approaches to define phenotypes (since seemingly disparate characteristics are often inter-correlated:11,77,177). This is an area where modern multivariable and machine-learning approaches that overcome the limitations of single-variable prediction studies may be invaluable, as has been demonstrated in the Precision Psychiatry literature.32,195,212 Advanced machine-learning algorithms should be developed along with digital phenotyping and other data-rich measurement techniques; these are at present being most frequently applied in the area of neuroimaging.37,129
Implement dynamic measurement
To advance precision pain medicine more rapidly, we may need frequent, dynamic measurement of predictors.127 Though investigators often assume that a disorder’s pathophysiology is well-known, such that a static baseline marker can reliably predict treatment effects, a treatment course in neuro-behavioral conditions is a complex, evolving interplay between a patient and their treatment. Dynamically assessed phenotypic changes, which may occur as patient behavior and neurobiology evolve during the course of treatment, may provide more robust individual prediction of pain treatment outcomes.26,73–75,83
Summary & Conclusion
In the field of pain, many treatments are available but most are only partially beneficial for a subset of patients, and the consequences of poor pain control are frequently dire, including severe suffering, disability, and elevated mortality. Numerous stakeholders would benefit tremendously from our ability to identify, for a given patient, the available intervention(s) most likely to yield the best response. While challenges abound,63 and the slow pace of findings to date suggests that success in the goal of matching patients to treatments has been elusive, the accelerating success of precision medicine in other disciplines offers reason for optimism.32,127,181 The tremendous heterogeneity among patients with persistent pain, and the disappointing, negative results of many analgesic trials may be harbingers of a future in which patients are comprehensively phenotyped (in addition to being diagnosed), then are managed according to an empirically-supported algorithm that matches those patient profiles to the optimal combination of treatments.57,66 We hope that this summary of the current state of precision pain medicine, as well as evidence-based recommendations for implementing these methods in research, can facilitate the further development and indeed the acceleration of precision medicine in chronic pain management.
Perspective:
Given the considerable variability in treatment outcomes for chronic pain, progress in precision pain treatment is critical for the field. An array of phenotypes and mechanisms contribute to chronic pain; this review summarizes current knowledge regarding which treatments are most effective for patients with specific biopsychosocial characteristics.
Acknowledgments
We thank Andrea Speckin and Valorie Thompson for their assistance in organizing the meeting.
Conflicts of interest statement:
all funding sources supporting the work and all institutional and corporate affiliations of mine are acknowledged. Except as disclosed on a separate attachment, I certify that I have no commercial associations (eg, consultancies, stock ownership, equity interests, and patent licensing arrangements) that might pose a conflict of interest in connection with the submitted article.
Disclosures:
The views expressed in this article are those of the authors, none of whom have financial conflicts of interest specifically related to the issues discussed in this article. At the time of the meeting on which this article is based, several authors were employed by pharmaceutical companies and others had received consulting fees or honoraria from one or more pharmaceutical or device companies. Authors of this article who were not employed by industry or government at the time of the meeting received travel stipends, hotel accommodations, and meals during the meeting provided by the Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks (ACTTION) public-private partnership with the US Food and Drug Administration (FDA), which has received research contracts, grants, or other revenue from the FDA, multiple pharmaceutical and device companies, and other sources. Preparation of this article was supported by ACTTION. No official endorsement by the FDA, US National Institutes of Health, or the pharmaceutical and device companies that have provided unrestricted grants to support the activities of ACTTION should be inferred.
RRE has received in the past 3 years research grants and contracts from NIH, ACTTION, and the NIH-DoD-VA Pain Management Collaboratory, and compensation for consulting for GW Pharmaceuticals.
RD has received in the past 5 years research grants and contracts from the FDA and the NIH, and compensation for serving on advisory boards or consulting on clinical trial methods from Abide, Acadia, Adynxx, Analgesic Solutions, Aptinyx, Aquinox, Asahi Kasei, Astellas, Biogen, Biohaven, Biosplice, Boston Scientific, Braeburn, Cardialen, Celgene, Centrexion, Chiesi, Chromocell, Clexio, Collegium, Concert, Confo, Decibel, Editas, Eli Lilly, Endo, Ethismos (equity), Eupraxia, Exicure, Glenmark, Gloriana, Grace, Hope, Lotus, Mainstay, Merck, Mind Medicine (also equity), Neumentum, Neurana, NeuroBo, Novaremed, Novartis, OliPass, Pfizer, Q-State, Reckitt Benckiser, Regenacy (also equity), Sangamo, Sanifit, Scilex, Semnur, SIMR Biotech, Sinfonia, SK Biopharmaceuticals, Sollis, SPRIM, Teva, Theranexus, Toray, Vertex, Vizuri, and WCG
RB provides consulting services for: Pfizer Pharma GmbH, Genzyme GmbH, Grünenthal GmbH, Mundipharma Research GmbH und Co. KG, Allergan, Sanofi Pasteur, Medtronic, Eisai, Lilly GmbH, Boehrin-ger Ingelheim Pharma GmbH&Co.KG, Astellas Pharma GmbH, Novartis Pharma GmbH, Bristol-Myers Squibb, Biogenidec, AstraZeneca GmbH, Merck, Abbvie, Daiichi Sankyo, Glenmark Pharmaceuticals S.A., Seqirus Australia Pty. Ltd, Teva Pharmaceuticals Europe Niederlande, Teva GmbH, Genentech, Mundipharma International Ltd. UK, Astellas Pharma Ltd. UK, Galapagos NV, Kyowa Kirin GmbH, Vertex Pharmaceuticals Inc., Biotest AG, Celgene GmbH, Desitin Arzneimittel GmbH, Regeneron Pharmaceuticals Inc. USA, Theranexus DSV CEA Frankreich, Abbott Pro-ducts Operations AG Schweiz, Bayer AG, Grünenthal Pharma AG Schweiz, Mundipharma Research Ltd. UK, Akcea Therapeutics Germany GmbH, Asahi Kasei Pharma Corporation, AbbVie Deutschland GmbH & Co. KG, Air Liquide Sante Inter-national Frankreich, Alnylam Germany GmbH, Lateral Pharma Pty Ltd, Hexal AG, An-gelini, Janssen, SIMR Biotech Pty Ltd Australien, Confo Therapeutics N. V. Belgium, Merz Pharmaceuticals GmbH, Neumentum Inc., F. Hoffmann-La Roche Ltd. Switzer-land, AlgoTherapeutix SAS France.
RDK reports research grants from the NIH, the Patient Centered Outcomes Research Initiative, and the Department of Veterans Affairs. He also receives an honorarium as a member of the Chronic Pain Centre of Excellence for Canadian Veterans Scientific Advisory Board and a stipend as Senior Executive Editor of the journal, Pain Medicine.
LC received in the past 5 years research grants from the US National Institutes of Health, and honoraria for lecturing, serving as an expert witness and/or panelist or consulting for methodological aspects from Chiesi, Averitas, Shionogi and Patient-Centered Outcomes Research Institute.
ASCR undertakes consultancy and advisory board work for Imperial College Consultants- in the last 36 months this has included remunerated work for: Abide, Confo, Vertex, Pharmanovo, Lateral, Novartis, Mundipharma, Orion, Shanghai SIMR BiotechAsahi Kasei, Toray & Theranexis. ASCR holds the following positions: IASP Councillor, NIHR- Chair of the Trial Steering Committee for the OPTION-DM trial, Advisor to the British National Formulary, Member Joint Committee on Vaccine and Immunisation- varicella sub-committee, ACTTION steering committee member, Member of the Non Freezing Cold Injury Independent Senior Advisory Committee (NISAC), Member of the Medicines and Healthcare products Regulatory Agency (MHRA) Commission on Human Medicines - Neurology, Pain & Psychiatry Expert Advisory Group. ASCR is named as an inventor on patents: Rice A.S.C., Vandevoorde S. and Lambert D.M Methods using N-(2-propenyl) hexadecanamide and related amides to relieve pain (WO 2005/079771). And: Okuse K. et al Methods of treating pain by inhibition of vgf activity EP13702262.0 (WO2013 110945).
UW reports research grants from the US National Institutes of Health. In her capacity as a special government employee of the US Food and Drug Administration (FDA), she has served as a voting member of the FDA Anesthetic and Analgesic Drug Products Advisory Committee. She serves as a consultant for Aphrodite Health Inc., Wilmington, DE, Bayer Aktiengesellschaft, Leverkusen, Germany, and Biohaven Pharmaceuticals, New Haven, CT.
Appendix 1. IMMPACT Precision Pain Medicine Meeting Talks
Precision Pain Medicine: Accomplishments of the Past 25 Years, and Prospects for the Next 10 (Clifford Woolf).
Preclinical Research Obstacles and Opportunities in Developing Precision Pain Medicine: An Overview (Andrew Rice).
Clinical Research Obstacles and Opportunities in Developing Precision Pain Medicine: An Overview (Michael Rowbotham).
Precision Medicine at the NIH (William Riley).
Rare vs Common Gene Variants as Guides to Pain Mechanisms and Drug Development (Alban Latremoliere).
Sodium Channels as Targets for Precision Pain Medicine: Preclinical Perspectives (Simon Tate).
Sodium Channels as Targets for Precision Pain Medicine: “Irritable Nociceptors” and Other Phenotypes in the Design of Clinical Trials (Troels Jensen).
COX Inhibitors and NGF Antibodies as Targets for Precision Pain Medicine (Nathaniel Katz).
Descending Inhibition as a Target for Precision Pain Medicine (Roland Staud).
Signs, Symptoms, and Comprehensive QST: A Perspective from the German Research Network on Neuropathic Pain (Ralf Baron).
Signs, Symptoms, and Bedside QST: α2-δ and Other Targets (Roy Freeman).
Non-pharmacologic Treatments in Precision Pain Medicine: Rationale for Splitting (Stratifying) vs. Lumping (Dennis Turk).
What Else Needs to be Included When Phenotyping is Considered? (Robert Edwards).
References
- 1.Akin-Akinyosoye K, Sarmanova A, Fernandes GS, Frowd N, Swaithes L, Stocks J, Valdes A, McWilliams DF, Zhang W, Doherty M, Ferguson E, Walsh DA: Baseline self-report ‘central mechanisms’ trait predicts persistent knee pain in the Knee Pain in the Community (KPIC) cohort. Osteoarthritis Cartilage 28:173–181, 2020 [DOI] [PubMed] [Google Scholar]
- 2.Arendt-Nielsen L: Central sensitization in humans: Assessment and pharmacology. Handb Exp Pharmacol 227:79–102, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Arendt-Nielsen L, Andresen T, Malver LP, Oksche A, Mansikka H, Drewes AM: A double-blind, placebo-controlled study on the effect of buprenorphine and fentanyl on descending pain modulation: A human experimental study. Clin J Pain 28:623–627, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Arendt-Nielsen L, Egsgaard LL, Petersen KK: Evidence for a central mode of action for etoricoxib (COX-2 inhibitor) in patients with painful knee osteoarthritis. Pain 157:1634–1644, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Arendt-Nielsen L, Graven-Nielsen T: Translational musculoskeletal pain research. Best Pract Res Clin Rheumatol 25:209–226, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Arendt-Nielsen L, Mansikka H, Staahl C, Rees H, Tan K, Smart TS, Monhemius R, Suzuki R, Drewes AM: A translational study of the effects of ketamine and pregabalin on temporal summation of experimental pain. Reg Anesth Pain Med 36:585–591, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Arendt-Nielsen L, Morlion B, Perrot S, Dahan A, Dickenson A, Kress HG, Wells C, Bouhassira D, Mohr Drewes A: Assessment and manifestation of central sensitisation across different chronic pain conditions. Eur J Pain 22:216–241, 2018 [DOI] [PubMed] [Google Scholar]
- 8.Arendt-Nielsen L, Yarnitsky D: Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. J Pain 10:556–572, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Attal N, de Andrade DC, Adam F, Ranoux D, Teixeira MJ, Galhardoni R, Raicher I, Uceyler N, Sommer C, Bouhassira D: Safety and efficacy of repeated injections of botulinum toxin A in peripheral neuropathic pain (BOTNEP): A randomised, double-blind, placebo-controlled trial. Lancet Neurol 15:555–565, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Attal N, Rouaud J, Brasseur L, Chauvin M, Bouhassira D: Systemic lidocaine in pain due to peripheral nerve injury and predictors of response. Neurology 62:218–225, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Bair E, Gaynor S, Slade GD, Ohrbach R, Fillingim RB, Greenspan JD, Dubner R, Smith SB, Diatchenko L, Maixner W: Identification of clusters of individuals relevant to temporomandibular disorders and other chronic pain conditions: The OPPERA study. Pain 157:1266–1278, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ballantyne JC: Opioids for the treatment of chronic pain: Mistakes made, lessons learned, and future directions. Anesth Analg 125:1769–1778, 2017 [DOI] [PubMed] [Google Scholar]
- 13.Bannister K, Qu C, Navratilova E, Oyarzo J, Xie JY, King T, Dickenson AH, Porreca F: Multiple sites and actions of gabapentin-induced relief of ongoing experimental neuropathic pain. Pain 158:2386–2395, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bannister K, Sachau J, Baron R, Dickenson AH: Neuropathic pain: Mechanism-based therapeutics. Annu Rev Pharmacol Toxicol 60:257–274, 2020 [DOI] [PubMed] [Google Scholar]
- 15.Baron R, Dickenson AH: Neuropathic pain: Precise sensory profiling improves treatment and calls for back-translation. Pain 155:2215–2217, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Baron R, Maier C, Attal N, Binder A, Bouhassira D, Cruccu G, Finnerup NB, Haanpaa M, Hansson P, Hullemann P, Jensen TS, Freynhagen R, Kennedy JD, Magerl W, Mainka T, Reimer M, Rice AS, Segerdahl M, Serra J, Sindrup S, Sommer C, Tolle T, Vollert J, Treede RD: Peripheral neuropathic pain: A mechanism-related organizing principle based on sensory profiles. Pain 158:261–272, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barr C, Marois M, Sim I, Schmid CH, Wilsey B, Ward D, Duan N, Hays RD, Selsky J, Servadio J, Schwartz M, Dsouza C, Dhammi N, Holt Z, Baquero V, MacDonald S, Jerant A, Sprinkle R, Kravitz RL: The PREEMPT study - evaluating smartphone-assisted n-of-1 trials in patients with chronic pain: Study protocol for a randomized controlled trial. Trials 16:67, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bee LA, Dickenson AH: Effects of lacosamide, a novel sodium channel modulator, on dorsal horn neuronal responses in a rat model of neuropathy. Neuropharmacology 57:472–479, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Bellomo TR, Schrepf A, Kruger GH, Lumley MA, Schubiner H, Clauw DJ, Williams DA, Harte SE: Pressure pain tolerance predicts the success of emotional awareness and expression therapy in patients with fibromyalgia. Clin J Pain 36:562–566, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bosma RL, Cheng JC, Rogachov A, Kim JA, Hemington KS, Osborne NR, Venkat Raghavan L, Bhatia A, Davis KD: Brain dynamics and temporal summation of pain predicts neuropathic pain relief from ketamine infusion. Anesthesiology 129:1015–1024, 2018 [DOI] [PubMed] [Google Scholar]
- 21.Bouhassira D, Branders S, Attal N, Fernandes AM, Demolle D, Barbour J, Ciampi de Andrade D, Pereira A: Stratification of patients based on the Neuropathic Pain Symptom Inventory: Development and validation of a new algorithm. Pain 162:1038–1046, 2021 [DOI] [PubMed] [Google Scholar]
- 22.Bouhassira D, Wilhelm S, Schacht A, Perrot S, Kosek E, Cruccu G, Freynhagen R, Tesfaye S, Lledo A, Choy E, Marchettini P, Mico JA, Spaeth M, Skljarevski V, Tolle T: Neuropathic pain phenotyping as a predictor of treatment response in painful diabetic neuropathy: Data from the randomized, double-blind, COMBO-DN study. Pain 155:2171–2179, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Bouwense SA, Olesen SS, Drewes AM, Poley JW, van Goor H, Wilder-Smith OH: Effects of pregabalin on central sensitization in patients with chronic pancreatitis in a randomized, controlled trial. PLoS. One 7:e42096, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouwense SA, Olesen SS, Drewes AM, van Goor H, Wilder-Smith OH: Pregabalin and placebo responders show different effects on central pain processing in chronic pancreatitis patients. J Pain Res 8:375–386, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruehl S, France CR, Stone AL, Gupta R, Buvanendran A, Chont M, Burns JW: Greater conditioned pain modulation is associated with enhanced morphine analgesia in healthy individuals and patients with chronic low back pain. Clin J Pain 37:20–27, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell CM, McCauley L, Bounds SC, Mathur VA, Conn L, Simango M, Edwards RR, Fontaine KR: Changes in pain catastrophizing predict later changes in fibromyalgia clinical and experimental pain report: Cross-lagged panel analyses of dispositional and situational catastrophizing. Arthritis Res Ther 14:R231, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao J, Tu Y, Orr SP, Lang C, Park J, Vangel M, Chen L, Gollub R, Kong J: Analgesic effects evoked by real and imagined acupuncture: A neuroimaging study. Cereb Cortex 29:3220–3231, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carmland ME, Kreutzfeldt M, Holbech JV, Andersen NT, Jensen TS, Bach FW, Sindrup SH, Finnerup NB: Effect of lacosamide in peripheral neuropathic pain: Study protocol for a randomized, placebo-controlled, phenotype-stratified trial. Trials 20:588, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X, Spaeth RB, Freeman SG, Scarborough DM, Hashmi JA, Wey HY, Egorova N, Vangel M, Mao J, Wasan AD, Edwards RR, Gollub RL, Kong J: The modulation effect of longitudinal acupuncture on resting state functional connectivity in knee osteoarthritis patients. Mol Pain 11:67, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng JC, Anzolin A, Berry M, Honari H, Paschali M, Lazaridou A, Lee J, Ellingsen DM, Loggia ML, Grahl A, Lindquist MA, Edwards RR, Napadow V: Dynamic functional brain connectivity underlying temporal summation of pain in fibromyalgia. Arthritis Rheumatol 74:700–710, 2022 [DOI] [PubMed] [Google Scholar]
- 31.Clemens JQ, Mullins C, Ackerman AL, Bavendam T, van Bokhoven A, Ellingson BM, Harte SE, Kutch JJ, Lai HH, Martucci KT, Moldwin R, Naliboff BD, Pontari MA, Sutcliffe S, Landis JR, Group MRNS: Urologic chronic pelvic pain syndrome: Insights from the MAPP research network. Nat Rev Urol 16:187–200, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen ZD, DeRubeis RJ: Treatment selection in depression. Annu Rev Clin Psychol 14:209–236, 2018 [DOI] [PubMed] [Google Scholar]
- 33.Collins FS, Varmus H: A new initiative on precision medicine. N Engl J Med 372:793–795, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Costantini R, Affaitati G, Wesselmann U, Czakanski P, Giamberardino MA: Visceral pain as a triggering factor for fibromyalgia symptoms in comorbid patients. Pain 158:1925–1937, 2017 [DOI] [PubMed] [Google Scholar]
- 35.Currie GL, Angel-Scott HN, Colvin L, Cramond F, Hair K, Khandoker L, Liao J, Macleod M, McCann SK, Morland R, Sherratt N, Stewart R, Tanriver-Ayder E, Thomas J, Wang Q, Wodarski R, Xiong R, Rice ASC, Sena ES: Animal models of chemotherapy-induced peripheral neuropathy: A machine-assisted systematic review and meta-analysis. PLoS Biol 17: e3000243, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis KD: Imaging vs quantitative sensory testing to predict chronic pain treatment outcomes. Pain 160(Suppl 1):S59–S65, 2019 [DOI] [PubMed] [Google Scholar]
- 37.Davis KD, Aghaeepour N, Ahn AH, Angst MS, Borsook D, Brenton A, Burczynski ME, Crean C, Edwards R, Gaudilliere B, Hergenroeder GW, Iadarola MJ, Iyengar S, Jiang Y, Kong JT, Mackey S, Saab CY, Sang CN, Scholz J, Segerdahl M, Tracey I, Veasley C, Wang J, Wager TD, Wasan AD, Pelleymounter MA: Discovery and validation of biomarkers to aid the development of safe and effective pain therapeutics: Challenges and opportunities. Nat Rev Neurol 16:381–400, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Greef BTA, Hoeijmakers JGJ, Geerts M, Oakes M, Church TJE, Waxman SG, Dib-Hajj SD, Faber CG, Merkies ISJ: Lacosamide in patients with Nav1.7 mutations-related small fibre neuropathy: A randomized controlled trial. Brain 142:263–275, 2019 [DOI] [PubMed] [Google Scholar]
- 39.Demant DT, Lund K, Finnerup NB, Vollert J, Maier C, Segerdahl MS, Jensen TS, Sindrup SH: Pain relief with lidocaine 5% patch in localized peripheral neuropathic pain in relation to pain phenotype: A randomised, double-blind, and placebo-controlled, phenotype panel study. Pain 156:2234–2244, 2015 [DOI] [PubMed] [Google Scholar]
- 40.Demant DT, Lund K, Vollert J, Maier C, Segerdahl M, Finnerup NB, Jensen TS, Sindrup SH: The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: A randomised, double-blind, placebo-controlled phenotype-stratified study. Pain 155:2263–2273, 2014 [DOI] [PubMed] [Google Scholar]
- 41.den Boer C, Dries L, Terluin B, van der Wouden JC, Blankenstein AH, van Wilgen CP, Lucassen P, van der Horst HE: Central sensitization in chronic pain and medically unexplained symptom research: A systematic review of definitions, operationalizations and measurement instruments. J Psychosom Res 117:32–40, 2019 [DOI] [PubMed] [Google Scholar]
- 42.Devigili G, Cazzato D, Lauria G: Clinical diagnosis and management of small fiber neuropathy: An update on best practice. Expert Rev Neurother 20:967–980, 2020 [DOI] [PubMed] [Google Scholar]
- 43.Devigili G, Rinaldo S, Lombardi R, Cazzato D, Marchi M, Salvi E, Eleopra R, Lauria G: Diagnostic criteria for small fibre neuropathy in clinical practice and research. Brain 142:3728–3736, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Devigili G, Tugnoli V, Penza P, Camozzi F, Lombardi R, Melli G, Broglio L, Granieri E, Lauria G: The diagnostic criteria for small fibre neuropathy: From symptoms to neuropathology. Brain 131:1912–1925, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dickenson AH, Patel R: Translational issues in precision medicine in neuropathic pain. Can J Pain 4:30–38, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Disease GBD, Injury I, Prevalence C: Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 392:1789–1858, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dowell D, Haegerich TM, Chou R: CDC guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep 65:1–49, 2016 [DOI] [PubMed] [Google Scholar]
- 48.Dworkin RH, Kerns RD, McDermott MP, Turk DC, Veasley C: The ACTTION guide to clinical trials of pain treatments: Standing on the shoulders of giants. Pain Rep 4: e757, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dworkin RH, Kerns RD, McDermott MP, Turk DC, Veasley C: The ACTTION guide to clinical trials of pain treatments, part II: Mitigating bias, maximizing value. Pain Rep 6:e886, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dworkin RH, Turk DC, Peirce-Sandner S, Baron R, Bellamy N, Burke LB, Chappell A, Chartier K, Cleeland CS, Costello A, Cowan P, Dimitrova R, Ellenberg S, Farrar JT, French JA, Gilron I, Hertz S, Jadad AR, Jay GW, Kalliomaki J, Katz NP, Kerns RD, Manning DC, McDermott MP, McGrath PJ, Narayana A, Porter L, Quessy S, Rappaport BA, Rauschkolb C, Reeve BB, Rhodes T, Sampaio C, Simpson DM, Stauffer JW, Stucki G, Tobias J, White RE, Witter J: Research design considerations for confirmatory chronic pain clinical trials: IMMPACT recommendations. Pain 149:177–193, 2010 [DOI] [PubMed] [Google Scholar]
- 51.Dworkin RH, Turk DC, Peirce-Sandner S, Burke LB, Farrar JT, Gilron I, Jensen MP, Katz NP, Raja SN, Rappaport BA, Rowbotham MC, Backonja MM, Baron R, Bellamy N, Bhagwagar Z, Costello A, Cowan P, Fang WC, Hertz S, Jay GW, Junor R, Kerns RD, Kerwin R, Kopecky EA, Lissin D, Malamut R, Markman JD, McDermott MP, Munera C, Porter L, Rauschkolb C, Rice AS, Sampaio C, Skljarevski V, Sommerville K, Stacey BR, Steigerwald I, Tobias J, Trentacosti AM, Wasan AD, Wells GA, Williams J, Witter J, Ziegler D: Considerations for improving assay sensitivity in chronic pain clinical trials: IMMPACT recommendations. Pain 153:1148–1158, 2012 [DOI] [PubMed] [Google Scholar]
- 52.Edwards RR: Individual differences in endogenous pain modulation as a risk factor for chronic pain. Neurology 65:437–443, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Edwards RR, Cahalan C, Mensing G, Smith M, Haythornthwaite JA: Pain, catastrophizing, and depression in the rheumatic diseases. Nat. Rev Rheumatol 7:216–224, 2011 [DOI] [PubMed] [Google Scholar]
- 54.Edwards RR, Dolman AJ, Martel MO, Finan PH, Lazaridou A, Cornelius M, Wasan AD: Variability in conditioned pain modulation predicts response to NSAID treatment in patients with knee osteoarthritis. BMC Musculoskelet Disord 17:284, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Edwards RR, Dolman AJ, Michna E, Katz JN, Nedeljkovic SS, Janfaza D, Isaac Z, Martel MO, Jamison RN, Wasan AD: Changes in pain sensitivity and pain modulation during oral opioid treatment: The impact of negative affect. Pain Med 17:1882–1891, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Edwards RR, Dworkin RH, Sullivan MD, Turk DC, Wasan AD: The role of psychosocial processes in the development and maintenance of chronic pain. J Pain 17:T70–T92, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Edwards RR, Dworkin RH, Turk DC, Angst MS, Dionne R, Freeman R, Hansson P, Haroutounian S, Arendt-Nielsen L, Attal N, Baron R, Brell J, Bujanover S, Burke LB, Carr D, Chappell AS, Cowan P, Etropolski M, Fillingim RB, Gewandter JS, Katz NP, Kopecky EA, Markman JD, Nomikos G, Porter L, Rappaport BA, Rice AS, Scavone JM, Scholz J, Simon LS, Smith SM, Tobias J, Tockarshewsky T, Veasley C, Versavel M, Wasan AD, Wen W, Yarnitsky D: Patient phenotyping in clinical trials of chronic pain treatments: IMMPACT recommendations. Pain 157:1851–1871, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Egorova N, Gollub RL, Kong J: Repeated verum but not placebo acupuncture normalizes connectivity in brain regions dysregulated in chronic pain. Neuroimage Clin 9:430–435, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eisenberg E, Burstein Y, Suzan E, Treister R, Aviram J: Spinal cord stimulation attenuates temporal summation in patients with neuropathic pain. Pain 156:381–385, 2015 [DOI] [PubMed] [Google Scholar]
- 60.Elman I, Borsook D: Common brain mechanisms of chronic pain and addiction. Neuron 89:11–36, 2016 [DOI] [PubMed] [Google Scholar]
- 61.Farrar JT, Troxel AB, Haynes K, Gilron I, Kerns RD, Katz NP, Rappaport BA, Rowbotham MC, Tierney AM, Turk DC, Dworkin RH: Effect of variability in the 7-day baseline pain diary on the assay sensitivity of neuropathic pain randomized clinical trials: An ACTTION study. Pain 155:1622–1631, 2014 [DOI] [PubMed] [Google Scholar]
- 62.Finan PH, Goodin BR, Smith MT: The association of sleep and pain: An update and a path forward. J Pain 14:1539–1552, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Finnerup NB, Kuner R, Jensen TS: Neuropathic pain: From mechanisms to treatment. Physiol Rev 101:259–301, 2021 [DOI] [PubMed] [Google Scholar]
- 64.Finnerup NB, Sindrup SH, Bach FW, Johannesen IL, Jensen TS: Lamotrigine in spinal cord injury pain: A randomized controlled trial. Pain 96:375–383, 2002 [DOI] [PubMed] [Google Scholar]
- 65.Flowers KM, Patton ME, Hruschak VJ, Fields KG, Schwartz E, Zeballos J, Kang JD, Edwards RR, Kaptchuk TJ, Schreiber KL: Conditioned open-label placebo for opioid reduction after spine surgery: A randomized controlled trial. Pain 162:1828–1839, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Forstenpointner J, Otto J, Baron R: Individualized neuropathic pain therapy based on phenotyping: Are we there yet? Pain 159:569–575, 2018 [DOI] [PubMed] [Google Scholar]
- 67.Forstenpointner J, Rehm S, Gierthmuhlen J, Baron R: Stratification of neuropathic pain patients: The road to mechanism-based therapy? Curr Opin Anaesthesiol 31:562–568, 2018 [DOI] [PubMed] [Google Scholar]
- 68.Forstenpointner J, Ruscheweyh R, Attal N, Baron R, Bouhassira D, Enax-Krumova EK, Finnerup NB, Freynhagen R, Gierthmuhlen J, Hansson P, Jensen TS, Maier C, Rice ASC, Segerdahl M, Tolle T, Treede RD, Vollert J: No pain, still gain (of function): The relation between sensory profiles and the presence or absence of self-reported pain in a large multicenter cohort of patients with neuropathy. Pain 162:718–727, 2021 [DOI] [PubMed] [Google Scholar]
- 69.France CR, Ysidron DW, Slepian PM, French DJ, Evans RT: Pain resilience and catastrophizing combine to predict functional restoration program outcomes. Health Psychol 39:573–579, 2020 [DOI] [PubMed] [Google Scholar]
- 70.Freeman R, Baron R, Bouhassira D, Cabrera J, Emir B: Sensory profiles of patients with neuropathic pain based on the neuropathic pain symptoms and signs. Pain 155:367–376, 2014 [DOI] [PubMed] [Google Scholar]
- 71.Furlan A, Chaparro LE, Irvin E, Mailis-Gagnon A: A comparison between enriched and nonenriched enrollment randomized withdrawal trials of opioids for chronic non-cancer pain. Pain Res Manag 16:337–351, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gammaitoni AR, Smugar SS, Jensen MP, Galer BS, Bolognese JA, Alon A, Hewitt DJ: Predicting response to pregabalin from pretreatment pain quality: Clinical applications of the pain quality assessment scale. Pain Med 14:526–532, 2013 [DOI] [PubMed] [Google Scholar]
- 73.Garland EL, Froeliger B, Howard MO: Effects of mindfulness-oriented recovery enhancement on reward responsiveness and opioid cue-reactivity. Psychopharmacology (Berl) 231:3229–3238, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Garland EL, Hanley AW, Riquino MR, Reese SE, Baker AK, Salas K, Yack BP, Bedford CE, Bryan MA, Atchley R, Nakamura Y, Froeliger B, Howard MO: Mindfulness-oriented recovery enhancement reduces opioid misuse risk via analgesic and positive psychological mechanisms: A randomized controlled trial. J Consult Clin Psychol 87:927–940, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garland EL, Howard MO: Enhancing natural reward responsiveness among opioid users predicts chronic pain relief: EEG analyses from a trial of mindfulness-oriented recovery enhancement. J Soc Social Work Res 9:285–303, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Garland EL, Trostheim M, Eikemo M, Ernst G, Leknes S: Anhedonia in chronic pain and prescription opioid misuse. Psychol Med 50:1977–1988, 2020. [DOI] [PubMed] [Google Scholar]
- 77.Gaynor SM, Bortsov A, Bair E, Fillingim RB, Greenspan JD, Ohrbach R, Diatchenko L, Nackley A, Tchivileva IE, Whitehead W, Alonso AA, Buchheit TE, Boortz-Marx RL, Liedtke W, Park JJ, Maixner W, Smith SB: Phenotypic profile clustering pragmatically identifies diagnostically and mechanistically informative subgroups of chronic pain patients. Pain 162:1528–1538, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Georgopoulos V, Akin-Akinyosoye K, Zhang W, McWilliams DF, Hendrick P, Walsh DA: Quantitative sensory testing and predicting outcomes for musculoskeletal pain, disability, and negative affect: A systematic review and meta-analysis. Pain 160:1920–1932, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gewandter JS, Dworkin RH, Turk DC, Devine EG, Hewitt D, Jensen MP, Katz NP, Kirkwood AA, Malamut R, Markman JD, Vrijens B, Burke L, Campbell JN, Carr DB, Conaghan PG, Cowan P, Doyle MK, Edwards RR, Evans SR, Farrar JT, Freeman R, Gilron I, Juge D, Kerns RD, Kopecky EA, McDermott MP, Niebler G, Patel KV, Rauck R, Rice ASC, Rowbotham M, Sessler NE, Simon LS, Singla N, Skljarevski V, Tockarshewsky T, Vanhove GF, Wasan AD, Witter J: Improving study conduct and data quality in clinical trials of chronic pain treatments: IMMPACT recommendations. J Pain 21:931–942, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gewandter JS, McDermott MP, He H, Gao S, Cai X, Farrar JT, Katz NP, Markman JD, Senn S, Turk DC, Dworkin RH: Demonstrating heterogeneity of treatment effects among patients: An overlooked but important step toward precision medicine. Clin Pharmacol Ther 106:204–210, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gewandter JS, McDermott MP, Mbowe O, Edwards RR, Katz NP, Turk DC, Dworkin RH: Navigating trials of personalized pain treatments: We’re going to need a bigger boat. Pain 160:1235–1239, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Giamberardino MA, Affaitati G, Martelletti P, Tana C, Negro A, Lapenna D, Curto M, Schiavone C, Stellin L, Cipollone F, Costantini R: Impact of migraine on fibromyalgia symptoms. J Headache Pain 17:28, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Giannoni-Luza S, Pacheco-Barrios K, Cardenas-Rojas A, Mejia-Pando PF, Luna-Cuadros MA, Barouh JL, Gnoatto-Medeiros M, Candido-Santos L, Barra A, Caumo W, Fregni F: Noninvasive motor cortex stimulation effects on quantitative sensory testing in healthy and chronic pain subjects: A systematic review and meta-analysis. Pain 161:1955–1975, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gierthmuhlen J, Maier C, Baron R, Tolle T, Treede RD, Birbaumer N, Huge V, Koroschetz J, Krumova EK, Lauchart M, Maihofner C, Richter H, Westermann A: Sensory signs in complex regional pain syndrome and peripheral nerve injury. Pain 153:765–774, 2012 [DOI] [PubMed] [Google Scholar]
- 85.Gierthmuhlen J, Schneider U, Seemann M, Freitag-Wolf S, Maihofner C, Enax-Krumova EK, Azad SC, Uceyler N, Birklein F, Maier C, Tolle T, Treede RD, Baron R: Can self-reported pain characteristics and bedside test be used for the assessment of pain mechanisms? An analysis of results of neuropathic pain questionnaires and quantitative sensory testing. Pain 160:2093–2104, 2019 [DOI] [PubMed] [Google Scholar]
- 86.Gillving M, Demant D, Lund K, Holbech JV, Otto M, Vase L, Jensen TS, Bach FW, Finnerup NB, Sindrup SH: Factors with impact on magnitude of the placebo response in randomized, controlled, cross-over trials in peripheral neuropathic pain. Pain 161:2731–2736, 2020 [DOI] [PubMed] [Google Scholar]
- 87.Gilron I, Bailey JM, Tu D, Holden RR, Weaver DF, Houlden RL: Morphine, gabapentin, or their combination for neuropathic pain. N Engl J Med 352:1324–1334, 2005 [DOI] [PubMed] [Google Scholar]
- 88.Gilron I, Tu D, Holden RR: Sensory and affective pain descriptors respond differentially to pharmacological interventions in neuropathic conditions. Clin J Pain 29:124–131, 2013 [DOI] [PubMed] [Google Scholar]
- 89.Gollub RL, Kirsch I, Maleki N, Wasan AD, Edwards RR, Tu Y, Kaptchuk TJ, Kong J: A functional neuroimaging study of expectancy effects on pain response in patients with knee osteoarthritis. J Pain 19:515–527, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gonzalez-Duarte A, Lem M, Diaz-Diaz E, Castillo C, Cardenas-Soto K: The efficacy of pregabalin in the treatment of prediabetic neuropathic pain. Clin J Pain 32:927–932, 2016 [DOI] [PubMed] [Google Scholar]
- 91.Graven-Nielsen T, Arendt-Nielsen L: Assessment of mechanisms in localized and widespread musculoskeletal pain. Nat Rev Rheumatol. 6:599–606, 2010 [DOI] [PubMed] [Google Scholar]
- 92.Haack M, Simpson N, Sethna N, Kaur S, Mullington J: Sleep deficiency and chronic pain: Potential underlying mechanisms and clinical implications. Neuropsychopharmacology 45:205–216, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Han B, Compton WM, Blanco C, Crane E, Lee J, Jones CM: Prescription opioid use, misuse, and use disorders in U. S. adults: 2015 National Survey on Drug Use and Health. Ann Intern Med 167:293–301, 2017 [DOI] [PubMed] [Google Scholar]
- 94.Harris RE, Williams DA, McLean SA, Sen A, Hufford M, Gendreau RM, Gracely RH, Clauw DJ: Characterization and consequences of pain variability in individuals with fibromyalgia. Arthritis Rheum 52:3670–3674, 2005 [DOI] [PubMed] [Google Scholar]
- 95.Harte SE, Ichesco E, Hampson JP, Peltier SJ, Schmidt-Wilcke T, Clauw DJ, Harris RE: Pharmacologic attenuation of cross-modal sensory augmentation within the chronic pain insula. Pain 157:1933–1945, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Holbech JV, Bach FW, Finnerup NB, Jensen TS, Sindrup SH: Pain phenotype as a predictor for drug response in painful polyneuropathy-a retrospective analysis of data from controlled clinical trials. Pain 157:1305–1313, 2016 [DOI] [PubMed] [Google Scholar]
- 97.Jain SM, Balamurugan R, Tandon M, Mozaffarian N, Gudi G, Salhi Y, Holland R, Freeman R, Baron R: Randomized, double-blind, placebo-controlled trial of ISC 17536, an oral inhibitor of transient receptor potential ankyrin 1, in patients with painful diabetic peripheral neuropathy: Impact of preserved small nerve fiber function. Pain 163:e738–e747, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jamison RN, Curran S, Wan L, Ross EL, Gilligan CJ, Edwards RR: Higher pain sensitivity predicts efficacy of a wearable transcutaneous electrical nerve stimulation device for persons with fibromyalgia: A randomized double-blind sham-controlled trial. Neuromodulation 25:1410–1420, 2021. [DOI] [PubMed] [Google Scholar]
- 99.Jamison RN, Dorado K, Mei A, Edwards RR, Martel MO: Influence of opioid-related side effects on disability, mood, and opioid misuse risk among patients with chronic pain in primary care. Pain Rep. 2:e589, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jamison RN, Edwards RR, Curran S, Wan L, Ross EL, Gilligan CJ, Gozani SN: Effects of wearable transcutaneous electrical nerve stimulation on fibromyalgia: A randomized controlled trial. J Pain Res 14:2265–2282, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jamison RN, Edwards RR, Liu X, Ross EL, Michna E, Warnick M, Wasan AD: Relationship of negative affect and outcome of an opioid therapy trial among low back pain patients. Pain Pract 13:173–181, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jamison RN, Wan L, Edwards RR, Mei A, Ross EL: Outcome of a high-frequency transcutaneous electrical nerve stimulator (hfTENS) device for low back pain: A randomized controlled trial. Pain Pract 19:466–475, 2019 [DOI] [PubMed] [Google Scholar]
- 103.Jensen TS, Finnerup NB: Allodynia and hyperalgesia in neuropathic pain: Clinical manifestations and mechanisms. Lancet Neurol 13:924–935, 2014 [DOI] [PubMed] [Google Scholar]
- 104.Jensen TS, Karlsson P, Gylfadottir SS, Andersen ST, Bennett DL, Tankisi H, Finnerup NB, Terkelsen AJ, Khan K, Themistocleous AC, Kristensen AG, Itani M, Sindrup SH, Andersen H, Charles M, Feldman EL, Callaghan BC: Painful and non-painful diabetic neuropathy, diagnostic challenges and implications for future management. Brain 144:1632–1645, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Karlsson P, Hincker AM, Jensen TS, Freeman R, Haroutounian S: Structural, functional, and symptom relations in painful distal symmetric polyneuropathies: A systematic review. Pain 160:286–297, 2019 [DOI] [PubMed] [Google Scholar]
- 106.Karlsson P, Provitera V, Caporaso G, Stancanelli A, Saltalamacchia AM, Borreca I, Manganelli F, Santoro L, Jensen TS, Nolano M: Increased peptidergic fibers as a potential cutaneous marker of pain in diabetic small fiber neuropathy. Pain 162:778–786, 2021 [DOI] [PubMed] [Google Scholar]
- 107.Kaye AD, Jones MR, Kaye AM, Ripoll JG, Jones DE, Galan V, Beakley BD, Calixto F, Bolden JL, Urman RD, Manchikanti L: Prescription opioid Abuse in chronic pain: An updated review of opioid abuse predictors and strategies to curb opioid abuse (Part 2). Pain Physician 20:S111–S133, 2017 [PubMed] [Google Scholar]
- 108.Kennedy DL, Kemp HI, Ridout D, Yarnitsky D, Rice ASC: Reliability of conditioned pain modulation: A systematic review. Pain 157:2410–2419, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kennedy DL, Vollert J, Ridout D, Alexander CM, Rice AS: The responsiveness of quantitative sensory testing-derived sensory phenotype to disease-modifying intervention in patients with entrapment neuropathy: A longitudinal study. Pain 162:2881–2893, 2021. [DOI] [PubMed] [Google Scholar]
- 110.Khoury S, Parisien M, Thompson SJ, Vachon-Presseau E, Roy M, Martinsen AE, Winsvold BS, Pain HA-I, Mundal IP, Zwart JA, Kania A, Mogil JS, Diatchenko L: Genome-wide analysis identifies impaired axonogenesis in chronic overlapping pain conditions. Brain 145:1111–1123, 2022. [DOI] [PubMed] [Google Scholar]
- 111.Kim J, Loggia ML, Cahalan CM, Harris RE, Beissner F, Garcia RG, Kim H, Barbieri R, Wasan AD, Edwards RR, Napadow V: The somatosensory link in fibromyalgia: Functional connectivity of the primary somatosensory cortex is altered by sustained pain and is associated with clinical/autonomic dysfunction. Arthritis Rheumatol 67:1395–1405, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kisler LB, Weissman-Fogel I, Coghill RC, Sprecher E, Yarnitsky D, Granovsky Y: Individualization of migraine prevention: A randomized controlled trial of psychophysical-based prediction of duloxetine efficacy. Clin J Pain 35:753–765, 2019 [DOI] [PubMed] [Google Scholar]
- 113.Knudsen L, Petersen GL, Norskov KN, Vase L, Finnerup N, Jensen TS, Svensson P: Review of neuroimaging studies related to pain modulation. Scand J Pain 2:108–120, 2018 [DOI] [PubMed] [Google Scholar]
- 114.Koerner JD, Glaser J, Radcliff K: Which variables are associated with patient-reported outcomes after discectomy? Review of SPORT disc herniation studies. Clin Orthop Relat Res 473:2000–2006, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Koffel E, Kats AM, Kroenke K, Bair MJ, Gravely A, DeRonne B, Donaldson MT, Goldsmith ES, Noorbaloochi S, Krebs EE: Sleep disturbance predicts less improvement in pain outcomes: Secondary analysis of the SPACE randomized clinical trial. Pain Med 21:1162–1167, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Koroukian SM, Schiltz N, Warner DF, Sun J, Bakaki PM, Smyth KA, Stange KC, Given CW: Combinations of chronic conditions, functional limitations, and geriatric syndromes that predict health outcomes. J Gen Intern Med 31:630–637, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Koulouris AE, Edwards RR, Dorado K, Schreiber KL, Lazaridou A, Rajan S, White J, Garcia J, Gibbons C, Freeman R: Reliability and validity of the boston bedside quantitative sensory testing battery for neuropathic pain. Pain Med 21:2336–2347, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kravitz RL, Schmid CH, Marois M, Wilsey B, Ward D, Hays RD, Duan N, Wang Y, MacDonald S, Jerant A, Servadio JL, Haddad D, Sim I: Effect of mobile device-supported single-patient multi-crossover trials on treatment of chronic musculoskeletal pain: A randomized clinical trial. JAMA Intern Med 178:1368–1377, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Krebs EE, Gravely A, Nugent S, Jensen AC, DeRonne B, Goldsmith ES, Kroenke K, Bair MJ, Noorbaloochi S: Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: The SPACE randomized clinical trial. JAMA 319:872–882, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kutch JJ, Labus JS, Harris RE, Martucci KT, Farmer MA, Fenske S, Fling C, Ichesco E, Peltier S, Petre B, Guo W, Hou X, Stephens AJ, Mullins C, Clauw DJ, Mackey SC, Apkarian AV, Landis JR, Mayer EA, Network MR: Resting-state functional connectivity predicts longitudinal pain symptom change in urologic chronic pelvic pain syndrome: A MAPP network study. Pain 158:1069–1082, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Larsen DB, Laursen M, Edwards RR, Simonsen O, Arendt-Nielsen L, Petersen KK: The combination of preoperative pain, conditioned pain modulation, and pain catastrophizing predicts postoperative pain 12 months after total knee arthroplasty. Pain Med 22:1583–1590, 2021 [DOI] [PubMed] [Google Scholar]
- 122.Le Bars D, Dickenson AH, Besson JM: Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurones in the rat. Pain 6:283–304, 1979 [DOI] [PubMed] [Google Scholar]
- 123.Le Bars D, Dickenson AH, Besson JM: Diffuse noxious inhibitory controls (DNIC). II. Lack of effect on non-convergent neurones, supraspinal involvement and theoretical implications. Pain 6:305–327, 1979 [DOI] [PubMed] [Google Scholar]
- 124.Le Bars D, Villanueva L, Bouhassira D, Willer JC: Diffuse noxious inhibitory controls (DNIC) in animals and in man. Patol Fiziol Eksp Ter 65:55–65, 1992 [PubMed] [Google Scholar]
- 125.Lee J, Mawla I, Kim J, Loggia ML, Ortiz A, Jung C, Chan ST, Gerber J, Schmithorst VJ, Edwards RR, Wasan AD, Berna C, Kong J, Kaptchuk TJ, Gollub RL, Rosen BR, Napadow V: Machine learning-based prediction of clinical pain using multimodal neuroimaging and autonomic metrics. Pain 160:550–560, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lee JJ, Kim HJ, Ceko M, Park BY, Lee SA, Park H, Roy M, Kim SG, Wager TD, Woo CW: A neuroimaging biomarker for sustained experimental and clinical pain. Nat Med 27:174–182, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lenze EJ, Nicol GE, Barbour DL, Kannampallil T, Wong AWK, Piccirillo J, Drysdale AT, Sylvester CM, Haddad R, Miller JP, Low CA, Lenze SN, Freedland KE, Rodebaugh TL: Precision clinical trials: A framework for getting to precision medicine for neurobehavioural disorders. J Psychiatry Neurosci 46:E97–E110, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Leroux C, Konstantinidou G: Targeted therapies for pancreatic cancer: Overview of current treatments and new opportunities for personalized oncology. Cancers (Basel) 13, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mackey S, Greely HT, Martucci KT: Neuroimaging-based pain biomarkers: Definitions, clinical and research applications, and evaluation frameworks to achieve personalized pain medicine. Pain Rep 4:e762, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Maier C, Baron R, Tolle TR, Binder A, Birbaumer N, Birklein F, Gierthmuhlen J, Flor H, Geber C, Huge V, Krumova EK, Landwehrmeyer GB, Magerl W, Maihofner C, Richter H, Rolke R, Scherens A, Schwarz A, Sommer C, Tronnier V, Uceyler N, Valet M, Wasner G, Treede RD: Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain 150:439–450, 2010 [DOI] [PubMed] [Google Scholar]
- 131.Maixner W, Fillingim RB, Williams DA, Smith SB, Slade GD: Overlapping chronic pain conditions: Implications for diagnosis and classification. J Pain 17:T93–T107, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Martel MO, Petersen K, Cornelius M, Arendt-Nielsen L, Edwards R: Endogenous pain modulation profiles among individuals with chronic pain: relation to opioid use. J Pain 20:462–471, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Max MB: Towards physiologically based treatment of patients with neuropathic pain. Pain 42:131–137, 1990 [DOI] [PubMed] [Google Scholar]
- 134.Max MB: Is mechanism-based pain treatment attainable? Clinical trial issues. J Pain 1:2–9, 2000 [DOI] [PubMed] [Google Scholar]
- 135.May M, Junghaenel DU, Ono M, Stone AA, Schneider S: Ecological momentary assessment methodology in chronic pain research: A systematic review. J Pain 19:699–716, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.McPhee ME, Vaegter HB, Graven-Nielsen T: Alterations in pronociceptive and antinociceptive mechanisms in patients with low back pain: A systematic review with meta-analysis. Pain 161:464–475, 2020 [DOI] [PubMed] [Google Scholar]
- 137.Meske DS, Lawal OD, Elder H, Langberg V, Paillard F, Katz N: Efficacy of opioids versus placebo in chronic pain: A systematic review and meta-analysis of enriched enrollment randomized withdrawal trials. J Pain Res 11:923–934, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nahin RL, Sayer B, Stussman BJ, Feinberg TM: Eighteen-year trends in the prevalence of, and health care use for, noncancer pain in the United States: Data from the Medical Expenditure Panel Survey. J Pain 20:796–809, 2019 [DOI] [PubMed] [Google Scholar]
- 139.Namer B, Schmidt D, Eberhardt E, Maroni M, Dorf-meister E, Kleggetveit IP, Kaluza L, Meents J, Gerlach A, Lin Z, Winterpacht A, Dragicevic E, Kohl Z, Schuttler J, Kurth I, Warncke T, Jorum E, Winner B, Lampert A: Pain relief in a neuropathy patient by lacosamide: Proof of principle of clinical translation from patient-specific iPS cell-derived nociceptors. EBioMedicine 39:401–408, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Niesters M, Proto PL, Aarts L, Sarton EY, Drewes AM, Dahan A: Tapentadol potentiates descending pain inhibition in chronic pain patients with diabetic polyneuropathy. Br J Anaesth 113:148–156, 2014 [DOI] [PubMed] [Google Scholar]
- 141.Oaklander AL, Herzog ZD, Downs HM, Klein MM: Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. Pain 154:2310–2316, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Odineal DD, Marois MT, Ward D, Schmid CH, Cabrera R, Sim I, Wang Y, Wilsey B, Duan N, Henry SG, Kravitz RL: Effect of mobile device-assisted N-of-1 trial participation on analgesic prescribing for chronic pain: Randomized controlled trial. J Gen Intern Med 35:102–111, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Olesen SS, Graversen C, Bouwense SA, van Goor H, Wilder-Smith OH, Drewes AM: Quantitative sensory testing predicts pregabalin efficacy in painful chronic pancreatitis. PLoS One 8:e57963, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pappagallo M, Oaklander AL, Quatrano-Piacentini AL, Clark MR, Raja SN: Heterogenous patterns of sensory dysfunction in postherpetic neuralgia suggest multiple pathophysiologic mechanisms. Anesthesiology 92:691–698, 2000 [DOI] [PubMed] [Google Scholar]
- 145.Patel KV, Allen R, Burke L, Farrar JT, Gewandter JS, Gilron I, Katz NP, Markman JD, Marshall SF, Resnick M, Rice ASC, Rowbotham MC, Smith SM, Vanhove GF, Wasan AD, Zhang S, Dworkin RH, Turk DC: Evaluation of composite responder outcomes of pain intensity and physical function in neuropathic pain clinical trials: An ACTTION individual patient data analysis. Pain 159:2245–2254, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Patel KV, Guralnik JM, Phelan EA, Gell NM, Wallace RB, Sullivan MD, Turk DC: Symptom burden among community-dwelling older adults in the United States. J Am Geriatr Soc 67:223–231, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Patel R, Kucharczyk M, Montagut-Bordas C, Lockwood S, Dickenson AH: Neuropathy following spinal nerve injury shares features with the irritable nociceptor phenotype: A back-translational study of oxcarbazepine. Eur J Pain 23:183–197, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Perez C, Latymer M, Almas M, Ortiz M, Clair A, Parsons B, Varvara R: Does duration of neuropathic pain impact the effectiveness of pregabalin? Pain Pract 17:470–479, 2017 [DOI] [PubMed] [Google Scholar]
- 149.Persson MSM, Stocks J, Varadi G, Hashempur MH, van Middelkoop M, Bierma-Zeinstra S, Walsh DA, Doherty M, Zhang W: Predicting response to topical non-steroidal anti-inflammatory drugs in osteoarthritis: An individual patient data meta-analysis of randomized controlled trials. Rheumatology (Oxford) 59:2207–2216, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Petersen KK, McPhee ME, Hoegh MS, Graven-Nielsen T: Assessment of conditioned pain modulation in healthy participants and patients with chronic pain: Manifestations and implications for pain progression. Curr Opin Support Palliat Care 13:99–106, 2019 [DOI] [PubMed] [Google Scholar]
- 151.Petersen KK, Olesen AE, Simonsen O, Arendt-Nielsen L: Mechanistic pain profiling as a tool to predict the efficacy of 3-week nonsteroidal anti-inflammatory drugs plus paracetamol in patients with painful knee osteoarthritis. Pain 160:486–492, 2019 [DOI] [PubMed] [Google Scholar]
- 152.Petersen KK, Simonsen O, Olesen AE, Morch CD: Arendt-Nielsen L. Pain inhibitory mechanisms and response to weak analgesics in patients with knee osteoarthritis. Eur J Pain 23:1904–1912, 2019 [DOI] [PubMed] [Google Scholar]
- 153.Petersen KK, Vaegter HB, Stubhaug A, Wolff A, Scammell BE, Arendt-Nielsen L, Larsen DB: The predictive value of quantitative sensory testing: A systematic review on chronic postoperative pain and the analgesic effect of pharmacological therapies in patients with chronic pain. Pain 162:31–44, 2021 [DOI] [PubMed] [Google Scholar]
- 154.Peyron R, Fauchon C: Functional imaging of pain. Rev Neurol (Paris) 175:38–45, 2019 [DOI] [PubMed] [Google Scholar]
- 155.Polydefkis M, Yiannoutsos CT, Cohen BA, Hollander H, Schifitto G, Clifford DB, Simpson DM, Katzenstein D, Shriver S, Hauer P, Brown A, Haidich AB, Moo L, McArthur JC: Reduced intraepidermal nerve fiber density in HIV-associated sensory neuropathy. Neurology 58:115–119, 2002 [DOI] [PubMed] [Google Scholar]
- 156.Price TJ, Basbaum AI, Bresnahan J, Chambers JF, De Koninck Y, Edwards RR, Ji RR, Katz J, Kavelaars A, Levine JD, Porter L, Schechter N, Sluka KA, Terman GW, Wager TD, Yaksh TL, Dworkin RH: Transition to chronic pain: Opportunities for novel therapeutics. Nat Rev Neurosci 19:383–384, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Quattrini C, Tavakoli M, Jeziorska M, Kallinikos P, Tesfaye S, Finnigan J, Marshall A, Boulton AJ, Efron N, Malik RA: Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes 56:2148–2154, 2007 [DOI] [PubMed] [Google Scholar]
- 158.Rakel BA, Zimmerman MB, Geasland K, Embree J, Clark CR, Noiseux NO, Callaghan JJ, Herr K, Walsh D, Sluka KA: Transcutaneous electrical nerve stimulation for the control of pain during rehabilitation after total knee arthroplasty: A randomized, blinded, placebo-controlled trial. Pain 155:2599–2611, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ram KC, Eisenberg E, Haddad M, Pud D: Oral opioid use alters DNIC but not cold pain perception in patients with chronic pain - new perspective of opioid-induced hyperalgesia. Pain 139:431–438, 2008 [DOI] [PubMed] [Google Scholar]
- 160.Reimer M, Helfert SM, Baron R: Phenotyping neuropathic pain patients: Implications for individual therapy and clinical trials. Curr Opin Support Palliat Care 8:124–129, 2014 [DOI] [PubMed] [Google Scholar]
- 161.Reimer M, Hullemann P, Hukauf M, Keller T, Binder A, Gierthmuhlen J, Baron R: Prediction of response to tapentadol in chronic low back pain. Eur J Pain 21:322–333, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rice ASC, Finnerup NB, Kemp HI, Currie GL, Baron R: Sensory profiling in animal models of neuropathic pain: A call for back-translation. Pain 159:819–824, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rice ASC, Smith BH, Blyth FM: Pain and the global burden of disease. Pain 157:791–796, 2016 [DOI] [PubMed] [Google Scholar]
- 164.Rogachov A, Bhatia A, Cheng JC, Bosma RL, Kim JA, Osborne NR, Hemington KS, Venkatraghavan L, Davis KD: Plasticity in the dynamic pain connectome associated with ketamine-induced neuropathic pain relief. Pain 160:1670–1679, 2019 [DOI] [PubMed] [Google Scholar]
- 165.Rolke R, Baron R, Maier C, Tolle TR, Treede RD, Beyer A, Binder A, Birbaumer N, Birklein F, Botefur IC, Braune S, Flor H, Huge V, Klug R, Landwehrmeyer GB, Magerl W, Maihofner C, Rolko C, Schaub C, Scherens A, Sprenger T, Valet M, Wasserka B: Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): Standardized protocol and reference values. Pain 123:231–243, 2006 [DOI] [PubMed] [Google Scholar]
- 166.Rowbotham MC, Fields HL: Post-herpetic neuralgia: The relation of pain complaint, sensory disturbance, and skin temperature. Pain 39:129–144, 1989 [DOI] [PubMed] [Google Scholar]
- 167.Rowbotham MC, Yosipovitch G, Connolly MK, Finlay D, Forde G, Fields HL: Cutaneous innervation density in the allodynic form of postherpetic neuralgia. Neurobiol Dis 3:205–214, 1996 [DOI] [PubMed] [Google Scholar]
- 168.Salaffi F, Sarzi-Puttini P, Atzeni F: How to measure chronic pain: New concepts. Best Pract Res Clin Rheumatol 29:164–186, 2015 [DOI] [PubMed] [Google Scholar]
- 169.Sangesland A, Storen C, Vaegter HB: Are preoperative experimental pain assessments correlated with clinical pain outcomes after surgery? A systematic review. Scand J Pain 15:44–52, 2017 [DOI] [PubMed] [Google Scholar]
- 170.Scher C, Petti E, Meador L, Van Cleave JH, Liang E, Reid MC: Multidimensional pain assessment tools for ambulatory and inpatient nursing practice. Pain Manag Nurs 21:416–422, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Schneider S, Junghaenel DU, Ono M, Broderick JE, Stone AA. III: Detecting treatment effects in clinical trials with different indices of pain intensity derived from ecological momentary assessment. J Pain 22:386–399, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Schreiber KL, Zinboonyahgoon N, Flowers KM, Hruschak V, Fields KG, Patton ME, Schwartz E, Azizoddin D, Soens M, King T, Partridge A, Pusic A, Golshan M, Edwards RR: Prediction of persistent pain severity and impact 12 months after breast surgery using comprehensive preoperative assessment of biopsychosocial pain modulators. Ann Surg Oncol 28:5015–5038, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Schutze R, Rees C, Smith A, Slater H, Campbell JM, O’Sullivan P: How can we best reduce pain catastrophizing in adults with chronic noncancer pain? A systematic review and meta-analysis. J Pain 19:233–256, 2018 [DOI] [PubMed] [Google Scholar]
- 174.Sheather-Reid RB, Cohen M: Efficacy of analgesics in chronic pain: A series of N-of-1 studies. J Pain Symptom Manage 15:244–252, 1998 [DOI] [PubMed] [Google Scholar]
- 175.Simpson DM, Schifitto G, Clifford DB, Murphy TK, Durso-De Cruz E, Glue P, Whalen E, Emir B, Scott GN, Freeman R: Pregabalin for painful HIV neuropathy: A randomized, double-blind, placebo-controlled trial. Neurology 74:413–420, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sindrup SH, Holbech J, Demant D, Finnerup NB, Bach FW, Jensen TS: Impact of etiology and duration of pain on pharmacological treatment effects in painful polyneuropathy. Eur J Pain 21:1443–1450, 2017 [DOI] [PubMed] [Google Scholar]
- 177.Slade GD, Ohrbach R, Greenspan JD, Fillingim RB, Bair E, Sanders AE, Dubner R, Diatchenko L, Meloto CB, Smith S, Maixner W: Painful temporomandibular disorder: Decade of discovery from OPPERA studies. J Dent Res 95:1084–1092, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Smith SM, Dworkin RH, Turk DC, Baron R, Polydefkis M, Tracey I, Borsook D, Edwards RR, Harris RE, Wager TD, Arendt-Nielsen L, Burke LB, Carr DB, Chappell A, Farrar JT, Freeman R, Gilron I, Goli V, Haeussler J, Jensen T, Katz NP, Kent J, Kopecky EA, Lee DA, Maixner W, Markman JD, McArthur JC, McDermott MP, Parvathenani L, Raja SN, Rappaport BA, Rice ASC, Rowbotham MC, Tobias JK, Wasan AD, Witter J: The potential role of sensory testing, skin biopsy, and functional brain imaging as biomarkers in chronic pain clinical trials: IMMPACT considerations. J Pain 18:757–777, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sorensen L, Molyneaux L, Yue DK: The relationship among pain, sensory loss, and small nerve fibers in diabetes. Diabetes Care 29:883–887, 2006 [DOI] [PubMed] [Google Scholar]
- 180.Steinmiller CL, Roehrs TA, Harris E, Hyde M, Green-wald MK, Roth T: Differential effect of codeine on thermal nociceptive sensitivity in sleepy versus nonsleepy healthy subjects. Exp Clin Psychopharmacol 18:277–283, 2010 [DOI] [PubMed] [Google Scholar]
- 181.Strianese O, Rizzo F, Ciccarelli M, Galasso G, D’Agostino Y, Salvati A, Del Giudice C, Tesorio P, Rusciano MR: Precision and personalized medicine: How genomic approach improves the management of cardiovascular and neurodegenerative disease. Genes (Basel) 11, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sullivan MJ, Thorn B, Haythornthwaite JA, Keefe F, Martin M, Bradley LA, Lefebvre JC: Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain 17:52–64, 2001 [DOI] [PubMed] [Google Scholar]
- 183.Sullivan W, Hirst M, Beard S, Gladwell D, Fagnani F, Lopez Bastida J, Phillips C, Dunlop WC: Economic evaluation in chronic pain: A systematic review and de novo flexible economic model. Eur J Health Econ 17:755–770, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Supplitt S, Karpinski P, Sasiadek M, Laczmanska I: Current achievements and applications of transcriptomics in personalized cancer medicine. Int J Mol Sci 22:1422, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Suzan E, Midbari A, Treister R, Haddad M, Pud D, Eisenberg E: Oxycodone alters temporal summation but not conditioned pain modulation: Preclinical findings and possible relations to mechanisms of opioid analgesia. Pain 154:1413–1418, 2013 [DOI] [PubMed] [Google Scholar]
- 186.Tetreault P, Baliki MN, Baria AT, Bauer WR, Schnitzer TJ, Apkarian AV: Inferring distinct mechanisms in the absence of subjective differences: Placebo and centrally acting analgesic underlie unique brain adaptations. Hum Brain Mapp 39:2210–2223, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tetreault P, Mansour A, Vachon-Presseau E, Schnitzer TJ, Apkarian AV, Baliki MN: Brain connectivity predicts placebo response across chronic pain clinical trials. PLoS Biol 14:e1002570, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tracey I, Woolf CJ, Andrews NA: Composite pain biomarker signatures for objective assessment and effective treatment. Neuron 101:783–800, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB, Giamberardino MA, Kaasa S, Korwisi B, Kosek E, Lavand’homme P, Nicholas M, Perrot S, Scholz J, Schug S, Smith BH, Svensson P, Vlaeyen JWS, Wang SJ: Chronic pain as a symptom or a disease: The IASP classification of chronic pain for the international classification of diseases (ICD-11). Pain 160:19–27, 2019 [DOI] [PubMed] [Google Scholar]
- 190.Treister R, Honigman L, Lawal OD, Lanier RK, Katz NP: A deeper look at pain variability and its relationship with the placebo response: Results from a randomized, double-blind, placebo-controlled clinical trial of naproxen in osteoarthritis of the knee. Pain 160:1522–1528, 2019 [DOI] [PubMed] [Google Scholar]
- 191.Tu Y, Ortiz A, Gollub RL, Cao J, Gerber J, Lang C, Park J, Wilson G, Shen W, Chan ST, Wasan AD, Edwards RR, Napadow V, Kaptchuk TJ, Rosen B, Kong J: Multivariate resting-state functional connectivity predicts responses to real and sham acupuncture treatment in chronic low back pain. Neuroimage Clin 23:101885, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Turk DC: Customizing treatment for chronic pain patients: Who, what, and why. Clin J Pain 6:255–270, 1990 [DOI] [PubMed] [Google Scholar]
- 193.Turk DC, Dworkin RH, Revicki D, Harding G, Burke LB, Cella D, Cleeland CS, Cowan P, Farrar JT, Hertz S, Max MB, Rappaport BA: Identifying important outcome domains for chronic pain clinical trials: An IMMPACT survey of people with pain. Pain 137:276–285, 2008 [DOI] [PubMed] [Google Scholar]
- 194.Vaegter HB, Handberg G, Graven-Nielsen T: Isometric exercises reduce temporal summation of pressure pain in humans. Eur J Pain 19:973–983, 2015. [DOI] [PubMed] [Google Scholar]
- 195.Van Bronswijk SC, Bruijniks SJE, Lorenzo-Luaces L, Derubeis RJ, Lemmens L, Peeters F, Huibers MJH: Cross-trial prediction in psychotherapy: External validation of the personalized advantage index using machine learning in two Dutch randomized trials comparing CBT versus IPT for depression. Psychother Res 31:78–91, 2021. [DOI] [PubMed] [Google Scholar]
- 196.van Helmond N, Aarts HM, Timmerman H, Olesen SS, Drewes AM, Wilder-Smith OH, Steegers MA, Vissers KC: Is preoperative quantitative sensory testing related to persistent postsurgical pain? A systematic literature review. Anesth Analg 131:1146–1155, 2020 [DOI] [PubMed] [Google Scholar]
- 197.Vardeh D, Mannion RJ, Woolf CJ: Toward a mechanism-based approach to pain diagnosis. J Pain 17:T50–T69, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Vinik A, Emir B, Cheung R, Whalen E: Relationship between pain relief and improvements in patient function/quality of life in patients with painful diabetic peripheral neuropathy or postherpetic neuralgia treated with pregabalin. Clin Ther 35:612–623, 2013 [DOI] [PubMed] [Google Scholar]
- 199.Vinik A, Emir B, Parsons B, Cheung R: Prediction of pregabalin-mediated pain response by severity of sleep disturbance in patients with painful diabetic neuropathy and post-herpetic neuralgia. Pain Med 15:661–670, 2014 [DOI] [PubMed] [Google Scholar]
- 200.Vollert J, Magerl W, Baron R, Binder A, Enax-Krumova EK, Geisslinger G, Gierthmuhlen J, Henrich F, Hullemann P, Klein T, Lotsch J, Maier C, Oertel B, Schuh-Hofer S, Tolle TR, Treede RD: Pathophysiological mechanisms of neuropathic pain: Comparison of sensory phenotypes in patients and human surrogate pain models. Pain 159:1090–1102, 2018 [DOI] [PubMed] [Google Scholar]
- 201.Vollert J, Maier C, Attal N, Bennett DLH, Bouhassira D, Enax-Krumova EK, Finnerup NB, Freynhagen R, Gierthmuhlen J, Haanpaa M, Hansson P, Hullemann P, Jensen TS, Magerl W, Ramirez JD, Rice ASC, Schuh-Hofer S, Segerdahl M, Serra J, Shillo PR, Sindrup S, Tesfaye S, Themistocleous AC, Tolle TR, Treede RD, Baron R: Stratifying patients with peripheral neuropathic pain based on sensory profiles: Algorithm and sample size recommendations. Pain 158:1446–1455, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Vollert J, Schenker E, Macleod M, Bespalov A, Wuerbel H, Michel M, Dirnagl U, Potschka H, Waldron AM, Wever K, Steckler T, van de Casteele T, Altevogt B, Sil A, Rice ASC, members EWsg: Systematic review of guidelines for internal validity in the design, conduct and analysis of preclinical biomedical experiments involving laboratory animals. BMJ Open Sci 4:e100046, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.von Hehn CA, Baron R, Woolf CJ: Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron 73:638–652, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Von Korff M, Scher AI, Helmick C, Carter-Pokras O, Dodick DW, Goulet J, Hamill-Ruth R, LeResche L, Porter L, Tait R, Terman G, Veasley C, Mackey S: United States National Pain Strategy for population research: Concepts, definitions, and pilot data. J Pain 17:1068–1080, 2016 [DOI] [PubMed] [Google Scholar]
- 205.Voon P, Karamouzian M, Kerr T: Chronic pain and opioid misuse: A review of reviews. Subst Abuse Treat Prev Policy 12:36, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E: An fMRI-based neurologic signature of physical pain. N Engl J Med 368:1388–1397, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wasan AD, Davar G, Jamison R: The association between negative affect and opioid analgesia in patients with discogenic low back pain. Pain 117:450–461, 2005 [DOI] [PubMed] [Google Scholar]
- 208.Wasan AD, Jamison RN, Pham L, Tipirneni N, Nedeljkovic SS, Katz JN: Psychopathology predicts the outcome of medial branch blocks with corticosteroid for chronic axial low back or cervical pain: A prospective cohort study. BMC Musculoskelet Disord 10:22, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wasan AD, Kaptchuk TJ, Davar G, Jamison RN: The association between psychopathology and placebo analgesia in patients with discogenic low back pain. Pain Med 7:217–228, 2006 [DOI] [PubMed] [Google Scholar]
- 210.Wasan AD, Michna E, Edwards RR, Katz JN, Nedeljkovic SS, Dolman AJ, Janfaza D, Isaac Z, Jamison RN: Psychiatric comorbidity is associated prospectively with diminished opioid analgesia and increased opioid misuse in patients with chronic low back pain. Anesthesiology 123:861–872, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Watson JA, Ryan CG, Atkinson G, Williamson P, Ellington D, Whittle R, Dixon J, Martin DJ: Inter-individual differences in the responses to pain neuroscience education in adults with chronic musculoskeletal pain: A systematic review and meta-analysis of randomized controlled trials. J Pain 22:9–20, 2021. [DOI] [PubMed] [Google Scholar]
- 212.Webb CA, Trivedi MH, Cohen ZD, Dillon DG, Fournier JC, Goer F, Fava M, McGrath PJ, Weissman M, Parsey R, Adams P, Trombello JM, Cooper C, Deldin P, Oquendo MA, McInnis MG, Huys Q, Bruder G, Kurian BT, Jha M, DeRubeis RJ, Pizzagalli DA: Personalized prediction of antidepressant v. placebo response: Evidence from the EMBARC study. Psychol Med 49:1118–1127, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Westermann A, Krumova EK, Pennekamp W, Horch C, Baron R, Maier C: Different underlying pain mechanisms despite identical pain characteristics: A case report of a patient with spinal cord injury. Pain 153:1537–1540, 2012 [DOI] [PubMed] [Google Scholar]
- 214.Woolf CJ: Pain: Moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med 140:441–451, 2004 [DOI] [PubMed] [Google Scholar]
- 215.Woolf CJ: Central sensitization: Uncovering the relation between pain and plasticity. Anesthesiology 106:864–867, 2007 [DOI] [PubMed] [Google Scholar]
- 216.Yang Y, Mis MA, Estacion M, Dib-Hajj SD, Waxman SG: NaV1.7 as a pharmacogenomic target for pain: Moving toward precision medicine. Trends Pharmacol Sci 39:258–275, 2018 [DOI] [PubMed] [Google Scholar]
- 217.Yarnitsky D: Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): Its relevance for acute and chronic pain states. Curr Opin Anaesthesiol 23:611–615, 2010 [DOI] [PubMed] [Google Scholar]
- 218.Yarnitsky D, Arendt-Nielsen L, Bouhassira D, Edwards RR, Fillingim RB, Granot M, Hansson P, Lautenbacher S, Marchand S, Wilder-Smith O: Recommendations on terminology and practice of psychophysical DNIC testing. Eur J Pain 14:339, 2010 [DOI] [PubMed] [Google Scholar]
- 219.Yarnitsky D, Granot M, Nahman-Averbuch H, Khamaisi M, Granovsky Y: Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. Pain 153:1193–1198, 2012 [DOI] [PubMed] [Google Scholar]
- 220.Yelland MJ, Poulos CJ, Pillans PI, Bashford GM, Nikles CJ, Sturtevant JM, Vine N, Del Mar CB, Schluter PJ, Tan M, Chan J, Mackenzie F, Brown R: N-of-1 randomized trials to assess the efficacy of gabapentin for chronic neuropathic pain. Pain Med 10:754–761, 2009 [DOI] [PubMed] [Google Scholar]
- 221.Yin M, Ma J, Xu J, Li L, Chen G, Sun Z, Liu Y, He S, Ye J, Mo W: Use of artificial neural networks to identify the predictive factors of extracorporeal shock wave therapy treating patients with chronic plantar fasciitis. Sci Rep 9:4207, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zheng Z, Feng SJ, Costa C, Li CG, Lu D, Xue CC: Acupuncture analgesia for temporal summation of experimental pain: A randomised controlled study. Eur J Pain 14:725–731, 2010 [DOI] [PubMed] [Google Scholar]
- 223.Zhou L, Bhattacharjee S, Kwoh CK, Tighe PJ, Malone DC, Slack M, Wilson DL, Brown JD, Trends Lo-Ciganic WH: Patient and prescriber characteristics in gabapentinoid use in a sample of United States Ambulatory Care Visits from 2003 to 2016. J Clin Med 9, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zhou L, Kitch DW, Evans SR, Hauer P, Raman S, Ebenezer GJ, Gerschenson M, Marra CM, Valcour V, Diaz-Arrastia R, Goodkin K, Millar L, Shriver S, Asmuth DM, Clifford DB, Simpson DM, McArthur JC, Narc Group AAS: Correlates of epidermal nerve fiber densities in HIV-associated distal sensory polyneuropathy. Neurology 68:2113–2119, 2007 [DOI] [PubMed] [Google Scholar]
- 225.Zinboonyahgoon N, Patton ME, Chen YK, Edwards RR, Schreiber KL: Persistent post-mastectomy pain: The impact of regional anesthesia among patients with high vs low baseline catastrophizing. Pain Med 22:1767–1775, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zinboonyahgoon N, Vlassakov K, Lirk P, Spivey T, King T, Dominici L, Golshan M, Strichartz G, Edwards R, Schreiber K: Benefit of regional anaesthesia on postoperative pain following mastectomy: The influence of catastrophising. Br J Anaesth 123:e293–e302, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]


