Abstract
Concurrent chemoradiation therapy (CRT) is the standard of care for none—small cell lung cancer. We studied the effects of minor differences in the CRT start dates in a cohort of 11,119 patients and found that minimal differences, as few as 3 days, were associated with worse survival rates. Efforts to mitigate the factors that interfere with the synchronous delivery of CRT are needed.
Background:
We evaluated trends in administration of concurrent chemoradiation therapy (CRT) and how variations in start dates of chemotherapy and radiotherapy affected overall survival (OS) in patients with non—small cell lung cancer (NSCLC) undergoing a course of definitive CRT.
Materials and Methods:
Cases of NSCLC treated with definitive CRT were obtained from the National Cancer Database. A survival analysis was performed with Kaplan-Meier curves and Cox proportional hazards models. Propensity score matching was conducted.
Results:
On a national level, only 48.6% of patients began concurrent CRT on the same day. In a propensity-matched population, starting CRT within 6 days was associated with improved OS (17.9 months) compared with starting 7 to 13 days apart (16.5 months; P = .04). Starting dual therapy within 6 days of each other was associated with a 7% reduction in the risk of death (hazard ratio, 0.93; P = .05). Furthermore, in a propensity-matched cohort, starting CRT within 3 days was associated with longer survival (18.7 months) compared with 4 to 6 days apart (17.5 months; P = .02). Starting treatment 4 to 6 days apart was associated with an 8% increased risk of death (hazard ratio, 1.08; P = .04).
Conclusion:
A large proportion (48.6%) of patients with unresectable NSCLC do not initiate CRT on the same day as is considered standard by national guidelines. In this population, nonsimultaneous initiation of CRT was associated with differences in OS. Further efforts to understand the mitigating factors and barriers that interfere with timely delivery of concurrent CRT are needed.
Keywords: Chemoradiation therapy, NSCLC, OS, Prognostic factors, Treatment delivery
Introduction
Non—small cell lung cancer (NSCLC) remains a leading cause of cancer mortality in Americans each year. Approximately 25% to 35% of cases of NSCLC initially present as stage III, locally advanced, inoperable disease.1,2 For locally advanced NSCLC, initial trials of dual modality therapy compared sequential chemotherapy followed by radiotherapy (RT) to RT alone and found improved overall survival (OS) with dual therapy.3,4 Building on these results, further trials compared sequential chemotherapy and RT to concurrent chemo-RT (CRT) and found a 6% to 7% OS benefit at 5 years with concurrent CRT.5,6 Consequently, the current standard of care for unresectable stage II and III NSCLC is concurrent CRT.
In many larger trials investigating concurrent CRT, the national oncology cooperative group protocols historically mandated that concurrent CRT begin on the same day, as do the current National Comprehensive Cancer Network guidelines. Although it might be intuitive chemotherapy and radiotherapy start on the same day with concurrent treatment, no studies are available to support this practice nor have any examined the possible effect of minor delays on patient outcomes. However, data on this topic is needed owing to the numerous external factors that can drive differences in CRT start times. For example, RT planning is a time-intensive process; therefore, for some patients (ie, large volume lung cancer or bulky lymph nodes), it might seem appropriate to quickly start chemotherapy before RT. Additionally, other non—cancer-related factors can also result in barriers that prohibit patients from initiating dual modality treatment simultaneously. For example, an increased distance from the treatment facility and regional residency differences can decrease the proportion of rectal cancer patients who receive RT.7 Furthermore, multimodality therapy requires coordination among multiple physicians, often creating logistical difficulties. However, when well implemented, physician interaction and a shared cohort of patients among oncologic subspecialists improves survival outcomes for cancer patients.8,9
Ideally, we strive to begin dual modality therapy as simultaneously as possible; however, in practical terms, patients might not start both chemotherapy and RT on the same day. Therefore, we evaluated, on a national level, trends in the administration of concurrent CRT, factors associated with nonsynchronous delivery of dual therapy, and how variations in start dates of chemotherapy and RT affected OS in NSCLC patients undergoing a course of definitive CRT. We hypothesized that nonsynchronous administration of chemotherapy and RT would adversely affect OS in patients with inoperable NSCLC.
Materials and Methods
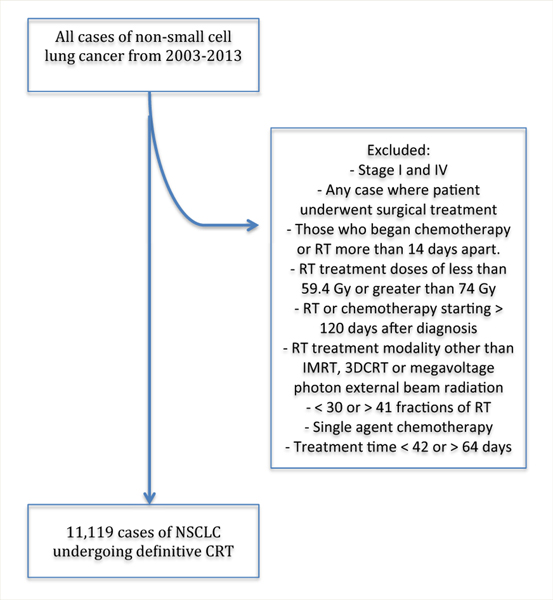
We included cases of stage II and III NSCLC from the anonymous National Cancer Database from 2003 to 2011.10 Patient data were coded using the AJCC Cancer Staging Manual according to the edition in use during the year of each patient’s diagnosis. Cases of interest included those in which the patients had undergone concurrent CRT. A flowchart of the patient inclusion criteria is shown in Figure 1. We excluded any case in which the patient underwent surgical treatment. To identify individuals who had undergone concurrent CRT, we excluded those who began chemotherapy or RT > 14 days apart. Other exclusion criteria included RT doses of < 59.4 Gy or > 74 Gy, RT or chemotherapy starting ≥ 120 days after diagnosis, any RT modality other than intensity-modulated RT, 3-dimensional conformal RT, or megavoltage photon external beam RT, use of < 30 or > 41 fractions of RT, single-agent chemotherapy, and a treatment time of < 42 or > 64 days. We used these elapsed-day exclusions to remove patients who likely received palliative treatment or those whose treatment became so protracted as to no longer represent concurrent therapy. We based these upper and lower bounds of elapsed days to reflect the shortest (60 Gy in 30 fractions) and longest (73.8 Gy in 41 fractions) treatment regimens in the inclusion criteria. We then calculated the treatment time according to the expected length of treatment, including weekends, for these regimens, plus an added 7 days to also include reasonable treatment breaks.
Figure 1. Flow Chart Documenting How the Final Study Population Was Attained.
Abbreviations: CRT = concurrent chemoradiotherapy; 3DCRT = 3-dimensional conformal radiotherapy; IMRT = intensity-modulated radiotherapy; NSCLC = non—small cell lung cancer; RT = radiotherapy.
We obtained patient, tumor, and treatment variables from the database. We dichotomized some variables, including treatment facility type (academic vs. nonacademic), race (caucasian vs. non-caucasian), treatment location (urban/metropolitan vs. rural), and median income according to patient origin (> $35,000 vs. ≤ $35,000). Other variables of interest included the Charlson/Deyo comorbidity score, gender, clinical stage, age, distance from treatment facility, T stage, insurance status, regional treatment location, and histologic subtype.
We compared baseline characteristics of cases using χ2 tests of significance for categorical variables and reported their corresponding P values. Continuous variables were assessed for normality. We compared normally distributed variables using the 2-sample t test. Non-normally distributed variables were compared using the Wilcoxon rank sum test. To identify factors associated with starting CRT within 6 days, we used the generalized estimating equations approach in univariate and multivariable settings. For each model, a logit link function was specified to model the probability of receiving CRT within 6 days, and a set of factors and exchangeable correlation structures within facility was assumed. Unadjusted Kaplan-Meier curves for product limit survival estimates were computed for the subjects. We defined OS time from the start of treatment to death or the last known follow-up visit. We stratified these curves on whether dual therapy started within 6 days versus 7 to 13 days of each other, as well as treatment initiation within 3 days versus 4 to 6 days. Patients who started chemotherapy and RT on the same day were categorized as starting on day 0; those who started on back to back days (ie, chemotherapy on Monday and RT on Tuesday) were categorized as starting 1 day apart. Survival differences were compared using the log-rank test. Survival analyses were performed with Cox proportional hazards models to calculate the unadjusted or adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) relating to the variables of interest. Proportional hazards assumptions for the variables were checked graphically using log-log survival plots. Variables with a P value in the univariate setting of < 0.5 were selected for multivariate Cox regression analyses of the entire unmatched cohort. This threshold was chosen to consider possible changes in the significance of variables in the presence of other variables in the multivariate setting. In an attempt to reduce the bias commonly observed in a large observational study such as ours, we used propensity score matching in the cohorts. Matching between the 2 groups (initiation of CRT within 6 days vs. 7–13 days) was performed in a 3:1 nearest neighbor matching without replacement and with a caliper of 0.048. Distributions of covariates were re-evaluated after matching. Once we completed matching, we evaluated the effect of variations in start dates in Cox proportional hazard regression models adjusted for propensity scores. Proportional hazard assumptions were tested and verified. We repeated a similar propensity matching approach in the subgroup analysis (0–3 days vs. 4–6 days apart). In this matching (2:1), we used a caliper of 0.051. The variables included in propensity matching were age, RT dose, RT treatment time, race, gender, tumor stage, Charlson/Deyo score, insurance provider, tumor histologic type, median household income (according to patient location of origin), patient origin (urban, rural, or suburban), distance to treatment facility, facility type (ie, academic vs. nonacademic center), and treatment facility location within the United States. All analyses were conducted using SAS, version 9.4 (SAS Institute, Cary, NC), with the statistical level of significance set as P < .05.
Results
Baseline Characteristics
A total of 11,119 patients from 2003 to 2011 treated with definitive CRT were available for analysis. For the group as a whole, the mean and median ages were 65.6 and 66 years (range, 19–90 years), respectively. Of the 11,119 patients, 1,065 (9.6%) had stage II NSCLC and 10,054 (90.4%) had stage III NSCLC. The median RT dose was 64.8 Gy (range, 59.4–74 Gy). RT was delivered over a median of 52 days (range, 42–64 days). Of the 11,119 patients, 5,399 (48.6%) began RT and chemotherapy on the same day, 8,296 (74.6%) began RT and chemotherapy within 3 days, 9,688 (87.1%) started chemotherapy and RT within 6 days, and 1,431 (12.9%) started CRT within 7 to 13 days of each other.
Two separate analyses were conducted according to the interval between chemotherapy and RT. The first compared those who started CRT within 6 days of each other with those who started CRT within 7 to 13 days of each other. The second compared those who started CRT within 3 days with those who started CRT within 4 to 6 days of each other. The distribution of variables in the unmatched cohort are listed in Supplemental Table 1 (available in the online version). The distribution of variables in the matched cohort are listed in Table 1, with no differences found between the 2 groups.
Table 1.
Baseline Characteristics of the Matched Population
| Characteristic | Treatment Start 0–13 d | Treatment Start 0–6 d | ||||
|---|---|---|---|---|---|---|
| 0–6 d | 7–13 d | P Value | 0–3 d | 4–6 d | P Value | |
| Patients, n | 4278 | 1426 | NS | 2774 | 1387 | NS |
| Age, y | ||||||
| Mean | 65.7 ± 9.6 | 65.7 ± 9.7 | .78 | 65.3 ± 9.8 | 65.4 ±9.9 | .74 |
| Median (range) | 66 (40–90) | 66 (40–90) | .66 | 66 (40–90) | 67 (40–89) | .57 |
| RT, Gy | ||||||
| Mean | 52.3 ± 5.2 | 52.5 ± 5.1 | .27 | 65.0 ± 3.6 | 65.0 ± 3.7 | .64 |
| Median (range) | 52 (42–64) | 52 (42–64) | .26 | 65.0 (59.4–74) | 65.0 (59.4–74) | .70 |
| RT duration, d | ||||||
| Mean | 64.8 ±3.8 | 64.9 (3.6) | .53 | 52.7 ± 5.1 | 52.6 ± 5.2 | .74 |
| Median (range) | 64.8 (59.4–74.0) | 64.8 (59.4–74.0) | .40 | 52 (42–64) | 52 (42–64) | .55 |
| Race | .78 | .90 | ||||
| White | 3638 (85) | 1217 (85.3) | 2388 (86.1) | 1196 (86.2) | ||
| Nonwhite | 640 (15) | 209 (14.7) | 386 (13.9) | 191 (13.8) | ||
| Gender | .60 | .96 | ||||
| Female | 1768 (41.3) | 578 (40.5) | 1038 (37.4) | 518 (37.4) | ||
| Male | 2510 (58.7) | 848 (59.5) | 1736 (62.6) | 869 (62.6) | ||
| Stage | .98 | .84 | ||||
| II | 6 (0.1) | 1 (0.1) | 4 (0.1) | 3 (0.2) | ||
| IIA | 67 (1.6) | 22 (1.5) | 50 (1.8) | 26 (1.9) | ||
| IIB | 279 (6.5) | 97 (6.8) | 190 (6.9) | 93 (6.7) | ||
| III | 17 (0.4) | 7 (0.5) | 24 (0.9) | 14 (1) | ||
| IIIA | 2113 (49.4) | 701 (49.2) | 1245 (44.9) | 648 (46.7) | ||
| IIIB | 1796 (42) | 598 (41.9) | 1261 (45.5) | 603 (43.5) | ||
| CD score | .98 | .99 | ||||
| 0 | 2662 (62.2) | 884 (62) | 1766 (63.7) | 886 (63.9) | ||
| 1 | 1211 (28.3) | 405 (28.4) | 699 (25.2) | 347 (25) | ||
| ≥2 | 405 (9.5) | 137 (9.6) | 309 (11.1) | 154 (11.1) | ||
| Insurance | .90 | .97 | ||||
| Private | 1339 (31.3) | 461 (32.3) | 898 (32.4) | 439 (31.7) | ||
| None | 157 (3.7) | 50 (3.5) | 124 (4.5) | 63 (4.5) | ||
| Government | 2739 (64) | 900 (63.1) | 1730 (62.4) | 874 (63) | ||
| Unknown | 43 (1.1) | 15 (1.1) | 22 (0.8) | 11 (0.8) | ||
| Histologic type | .99 | .99 | ||||
| Adenocarcinoma | 1146 (26.8) | 384 (26.9) | 693 (25) | 349 (25.2) | ||
| SCC | 1097 (25.6) | 366 (25.7) | 1292 (46.6) | 642 (46.3) | ||
| Bronchoalveolar | 14 (0.3) | 5 (0.4) | 123 (0.5) | 7 (0.5) | ||
| Large cell | 158 (3.7) | 54 (3.8) | 75 (2.7) | 37 (2.8) | ||
| Other | 1863 (43.6) | 617 (43.3) | 701 (25.3) | 352 (25.4) | ||
| Income | .78 | .33 | ||||
| >$35,000 | 2385 (55.8) | 789 (55.3) | 1650 (59.5) | 803 (57.9) | ||
| <$35,000 | 1893 (44.2) | 637 (44.7) | 1124 (40.5) | 584 (42.1) | ||
| Residential area | .24 | .90 | ||||
| Urban | 4134 (96.6) | 1387 (97.3) | 2692 (97) | 1341 (94.7) | ||
| Rural | 144 (3.4) | 39 (2.7) | 82 (3) | 46 (5.3) | ||
| Distance, miles | .05 | .28 | ||||
| 0–49.9 | 3993 (93.3) | 1352 (94.8) | 2605 (93.9) | 1341 (96.7) | ||
| >50 | 285 (6.7) | 74 (5.2) | 169 (6.1) | 73 (3.3) | ||
| Facility type | .77 | .49 | ||||
| Academic | 3495 (81.7) | 1170 (82.1) | 555 (20) | 265 (19.1) | ||
| Other | 783 (18.3) | 256 (17.9) | 2219 (80) | 1122 (80.9) | ||
| Location | .99 | .63 | ||||
| Northeast | 778 (18.2) | 256 (18) | 507 (18.3) | 259 (18.7) | ||
| Central | 1326 (31) | 447 (31.3) | 956 (34.5) | 457 (32.9) | ||
| South | 1775 (41.5) | 591 (41.4) | 1086 (39.2) | 545 (39.3) | ||
| West | 399 (9.3) | 132 (9.3) | 225 (8) | 126 (9.1) | ||
Data presented as n (%), unless noted otherwise.
Abbreviations: CD = Charlson/Deyo (comorbidity); RT = radiation therapy; SCC = squamous cell carcinoma.
Survival Outcomes With Asynchronous CRT
On univariate regression analysis, factors associated with an increased risk of mortality included starting dual therapy 7 to 13 days apart, increasing age, caucasian ethnicity, male gender, higher stage NSCLC, government-based insurance, Charlson/Deyo score of 1 or 2, and increasing duration to complete RT. Adenocarcinoma histologic type, increasing RT dose, lower T stage, origin in an area with a median income > $35,000, treatment on the West coast (vs. the Northeast), and treatment in an academic center were associated with improved survival rates.
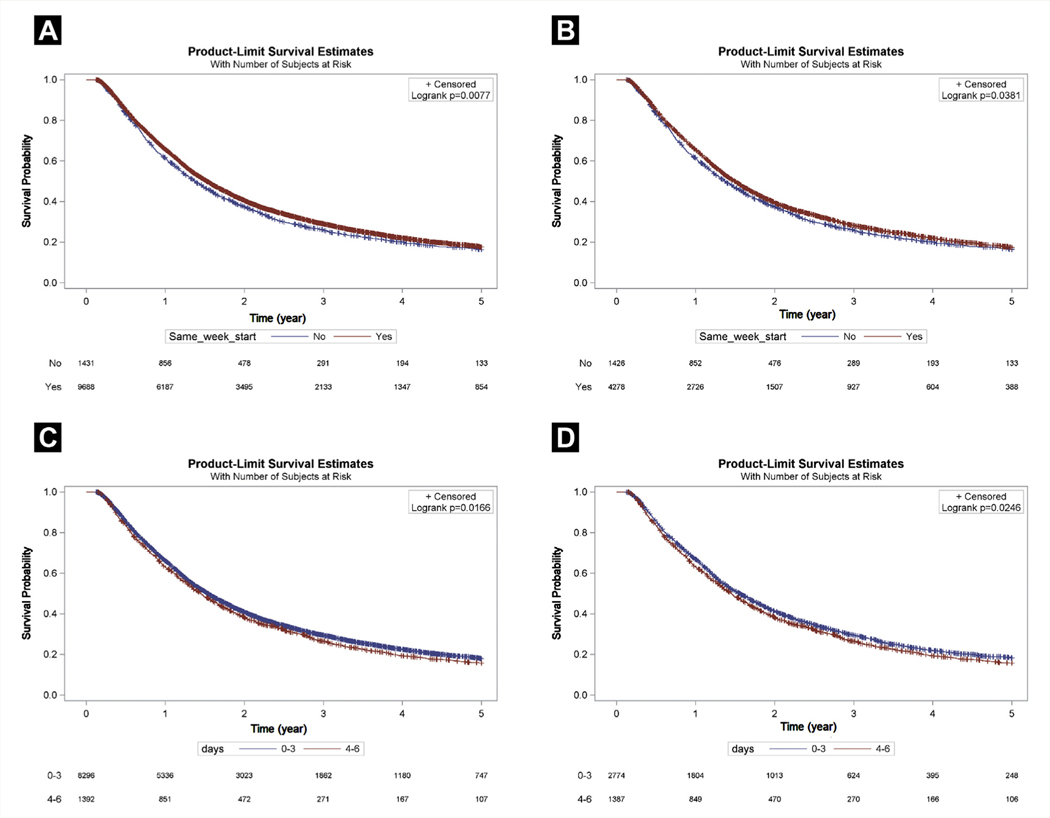
The median OS for patients who started dual therapy within 6 days of each other was 18.3 months (95% CI, 17.8–18.8 months) compared with 16.5 months (95% CI, 15.4–17.6 months) for those who started dual therapy 7 to 13 days apart (P = .008; Figure 2A). On multivariate analysis (Table 2), starting dual therapy within 6 days was associated with improved mortality (HR, 0.94; 95% CI, 0.88–0.99; P = .03).
Figure 2.
Comparison of Median Overall Survival (OS) Stratified by Start Date. (A) The Median OS for an Unmatched Population Who Started Dual Therapy Within 6 Days of Each Other Was 18.3 Months (95% Confidence Interval [CI], 17.8–18.8 Months) Compared With 16.5 Months (95% CI, 15.4–17.6 Months) for Those Who Started Dual Therapy 7 to 13 Days Apart (P = .008). (B) In a Matched Population, the Median OS Differences Between Those Who Started Treatment Within 6 Days (17.9 Months; 95% CI, 17.2–18.7 Months) Versus Those Who Started Treatment 7 to 13 Days Apart (16.5 Months; 95% CI, 15.4–17.6 Months) Remained Significantly Different (P = .04). (C) In an Unmatched Population, the Subgroup That Started Dual Therapy Within 3 Days Showed Superior Median Survival (18.5 Months; 95% CI, 18.0–19.0 Months) Compared With That for the Subgroup That Started Therapy Within 4 to 6 Days (17.4 Months; 95% CI, 16.1–18.7 Months; P = .02). (D) In a Matched Cohort, the Median OS Between the Subgroup That Started Treatment Within 3 Days (18.7 Months; 95% CI, 17.6–19.9 Months) Was Significantly Longer Compared With Starting Treatment 4 to 6 Days Apart (17.5 Months; 95% CI, 16.1–18.7 Months; P = .02)
Table 2.
Multivariate Analysis for Overall Survival of Patients Who Started RT and Chemotherapy Within 6 Days Versus 7 to 13 Days Apart
| Characteristic | HR (95% CI) | P Value |
|---|---|---|
| RT/chemotherapy within 6 d | 0.94 (0.88–0.99) | .03 |
| White race | 1.13 (1.06–1.21) | .0003 |
| Female gender | 0.84 (0.81–0.88) | <.0001 |
| Stage (vs. IIIB) | ||
| II | 0.70 (0.42–1.16) | .16 |
| IIA | 0.64 (0.52–0.78) | <.0001 |
| IIB | 0.72 (0.66–0.79) | <.0001 |
| III | 0.93 (0.72–1.21) | .60 |
| IIIA | 0.87 (0.82–0.92) | <.0001 |
| CD score (vs. 0) | ||
| 1 | 1.09 (1.04–1.15) | .0004 |
| 2 | 1.19 (1.10–1.28) | <.0001 |
| RT dose (vs. <66 Gy) | ||
| 66–72 Gy | 0.88 (0.84–0.92) | <.0001 |
| >72 Gy | 0.75 (0.61–0.92) | <.0001 |
| Agea | 1.11 (1.08–1.13) | <.0001 |
| RT treatment timeb | 1.06 (1.03–1.08) | <.0001 |
| T stage (vs. 4) | ||
| 1 | 0.84 (0.77–0.91) | .08 |
| 1A | 0.84 (0.68–1.02) | .25 |
| 1B | 0.90 (0.75–1.08) | .76 |
| 2 | 0.99 (0.93–1.06) | .29 |
| 2A | 0.93 (0.81–1.06) | .95 |
| 2B | 1.01 (0.87–1.16) | .19 |
| 3 | 1.05 (0.98–1.06) | .81 |
| Histologic type (vs. SCC) | ||
| Adenocarcinoma | 1.01 (0.96–1.06) | .81 |
| Bronchoalveolar | 1.23 (0.89–1.64) | .94 |
| Large cell | 1.01 (0.89–1.12) | .94 |
| Other | 1.07 (1.01–1.12) | .02 |
| Income >$ 35,000 | 0.91 (0.88–0.98) | <.0001 |
| Academic facility | 0.93 (0.88–0.98) | .009 |
| Facility location (vs. NE) | ||
| Central | 0.97 (0.91–1.03) | .36 |
| South | 0.95 (0.89–1.01) | .11 |
| West | 0.86 (0.79–0.94) | .0007 |
Abbreviations: CD = Charlson/Deyo (comorbidity); CI = confidence interval; HR = hazard ratio; NE = northeast; RT = radiation therapy.
Per 10-year increase.
Per 5-day increase.
In our matched population, 4,278 patients started CRT within 6 days of each other and 1,426 patients started therapy within 7 to 13 days of each other. The median OS (Figure 2B) differences between those who started treatment within 6 days (17.9 months; 95% CI, 17.2–18.7 months) and those who started treatment 7 to 13 days apart (16.5 months; 95% CI, 15.4–17.6 months) remained statistically significant (P = .04). Starting dual therapy within 6 days of each other was associated with a 7% reduction in the risk of death (HR, 0.93; 95% CI, 0.87–0.99; P = .05).
Smaller differences in CRT start dates were also associated with survival differences. On univariate analysis, starting treatment 4 to 6 days apart was associated with worse survival compared with starting treatment within 3 days of each other (HR, 1.07; 95% CI, 1.01–1.14; P = .03). Similar to the previous analysis, factors associated with a greater risk of mortality included increasing age, caucasian ethnicity, male gender, Charlson/Deyo comorbidity score of 1 or 2, and increasing RT elapsed treatment days. Adenocarcinoma histologic subtype, private insurance, increasing RT dose, lower clinical stage, lower T stage, origin from an area with a median income > $35,000, treatment on the West coast, and treatment at an academic center were associated with improved survival.
The subgroup that started dual therapy within 3 days showed a superior median OS (18.5 months; 95% CI, 18.0–19.0 months) compared with the subgroup that started therapy within 4 to 6 days (17.4 months; 95% CI, 16.1–18.7 months; P = .02; Figure 2C). On multivariate analysis (Table 3), starting dual therapy within 4 to 6 days of each other showed a trend toward worse mortality (HR, 1.06; 95% CI, 0.99–1.13; P = .09) compared with starting treatment within 3 days. In the matched cohort (Figure 2D), differences in median OS in the subgroup that started treatment within 3 days (18.7 months; 95% CI, 17.6–19.9 months) was significantly greater than that for the subgroup that started treatment 4 to 6 days apart (17.5 months; 95% CI, 16.1–18.7 months; P = .02). In addition, the 5-year OS rate was significantly greater (20% vs. 18%; P = .03). Starting treatment 4 to 6 days apart was associated with an 8% increased risk of death compared with starting treatment within 3 days (HR, 1.08; 95% CI, 1.003–1.161; P = .04).
Table 3.
Multivariate Analysis for Overall Survival of Patients Who Started RT and Chemotherapy Within 3 Days Versus 4 to 6 Days Apart
| Characteristic | HR (95% CI) | P Value |
|---|---|---|
| RT/chemotherapy within 4–6 d | 1.06 (0.99–1.13) | .09 |
| White race | 1.13 (1.05–1.21) | .001 |
| Female gender | 0.84 (0.80–0.88) | <.0001 |
| Stage (vs. IIIB) | ||
| II | 0.69 (0.41–1.16) | .16 |
| IIA | 0.64 (0.52–0.79) | <.0001 |
| IIB | 0.71 (0.64–0.78) | <.0001 |
| III | 0.93 (0.70–1.22) | .59 |
| IIIA | 0.87 (0.82–0.93) | <.0001 |
| CD score (vs. 0) | ||
| 1 | 1.08 (1.02–1.14) | .007 |
| 2 | 1.20 (1.11–1.30) | <.0001 |
| RT dose (vs. <66 Gy) | ||
| 66–72 Gy | 0.88 (0.83–0.92) | <.0001 |
| >72 Gy | 0.75 (0.59–0.95) | .02 |
| Agea | 1.11 (1.09–1.14) | <.0001 |
| T stage (vs. 4) | ||
| 1 | 0.82 (0.75–0.90) | <.0001 |
| 1A | 0.80 (0.65–0.99) | .04 |
| 1B | 0.90 (0.74–1.08) | .26 |
| 2 | 0.99 (0.93–1.07) | .89 |
| 2A | 0.93 (0.81–1.07) | .30 |
| 2B | 0.99 (0.86–1.16) | .94 |
| 3 | 1.06 (0.85–0.94) | <.0001 |
| Histologic type (vs. SCC) | ||
| Adenocarcinoma | 1.02 (0.96–1.08) | .58 |
| Bronchoalveolar | 1.23 (0.91–1.66) | .17 |
| Large cell | 1.04 (0.91–1.18) | .59 |
| Other | 1.07 (1.01–1.13) | .02 |
| Treatment durationb | 1.06 (1.03–1.09) | <.0001 |
| Income >$ 35,000 | 0.89 (0.85–0.94) | <.0001 |
| Academic facility | 0.93 (0.87–0.98) | .01 |
| Facility location (vs. NE) | ||
| Central | 0.97 (0.91–1.04) | .41 |
| South | 0.95 (0.89–1.02) | .14 |
| West | 0.85 (0.78–0.93) | .0005 |
Abbreviations: CD = Charlson/Deyo (comorbidity); CI = confidence interval; HR = hazard ratio; NE = northeast; RT = radiation therapy.
Per 10-year increase.
Per 5-day increase.
Additionally, we investigated whether starting chemotherapy or RT first affected OS. However, no difference was found when comparing patients who started RT first versus those who started chemotherapy first (P = .19).
Factors Associated With Asynchronous CRT
Using the generalized estimating equations in the univariate setting, we found that caucasian patients (P = .04) and those originating from an area with a median income > $35,000 (P = .0004) were more likely to receive CRT within 6 days. In contrast, patients with a Charlson/Deyo score of 1 compared with 0 were less likely to receive dual therapy within 6 days. On multivariable analysis, patients originating from an area with a median income > $35,000 (P = .003) were more likely to start CRT within 6 days and patients with a Charlson/Deyo comorbidity score of 1 were less likely to start the dual therapy within 6 days (P = .008).
Discussion
Our results demonstrated that a significant proportion of patients, nearly 50%, do not start RT and chemotherapy on the same day, which was historically dictated in many trials the standard of care for unresectable NSCLC is based on. Factors associated with more synchronous starts in RT and chemotherapy were caucasian ethnicity, origin from an area with a median income > $35,000, and lower Charlson/Deyo morbidity score. Furthermore, the results of our analysis suggest that these relatively minor variations in starting chemotherapy and RT as a part of concurrent CRT could be detrimental to patient outcomes. Initiating both RT and chemotherapy within 6 days of each other was associated with a better median OS compared with starting the 2 modalities 7 to 13 days apart. In addition, we found apparent differences in the median OS for a subgroup that started dual therapy within the same week; namely, starting both elements of dual therapy within 3 days of each other associated with better median OS compared to starting treatment within 4 to 6 days of each other. The trend of these results suggest that starting chemotherapy and RT on the same day optimizes the effects of concurrent treatment, an observation that builds upon the prior data derived from randomized trials comparing concurrent and sequential CRT.
The Radiation Therapy Oncology Group (RTOG) 9410 trial demonstrated a 2.5-month median survival benefit (17 months vs. 14.6 months) and a 6% 5-year OS benefit (16% vs. 10%) for concurrent CRT compared with sequential CRT.5 With improvements in technology, staging, and treatment, OS improved, and RTOG 0617 recently demonstrated a median survival of 28.7 months for the standard 60-Gy treatment arm.11 The current estimated 5-year OS rates for patients with stage II and III NSCLC range from 5% to 30%,12 which are similar to the rates presented in this report.13,14 The results of our study indicate that starting dual therapy within 6 days associated with a better median survival than starting both modalities 7 to 13 days apart. Furthermore, our results suggest that the subgroup that started the dual modality therapy within 3 days of each other benefited from a measurable survival improvement compared with the subgroup starting dual therapy within 4 to 6 days of each other. When considered in isolation, these differences might seem relatively modest; however, in the context of a disease that measures treatment benefits on the order of months, these results suggest an incremental and meaningful improvement.
One weaknesses of the present study stems from the nature of the National Cancer Database, which summarizes gross elements of stage and outcomes, but contains none of the individual variables that often affect treatment decisions and potentially influence outcomes. We attempted to mitigate these factors by performing propensity matching, such that our comparator groups had similar distributions of variables likely to affect survival outcomes. However, some gaps remain; for example, the database carries no information about comorbidities such as heart or lung disease, which could have been imbalanced between the 2 groups and affected survival outcomes. Additionally, extenuating circumstances could have been present, such as respiratory failure from mass compression, which might have necessitated an urgent start of RT over chemotherapy, and could possibly have portended a worse prognosis for these patients owing to larger volume of disease.
Also, the database does not document which chemotherapeutic agents were used; thus, nonstandard chemotherapy regimens could have been prescribed because of patient-specific factors (ie, kidney disease). The 2 most commonly used regimens during CRT are cisplatin/etoposide or carboplatin/paclitaxel, which have not shown apparent survival differences.13 However, cisplatin/etoposide is thought to deliver systemic doses of therapy unlike carboplatin/paclitaxel; therefore, 2 full doses of carboplatin/paclitaxel are needed after completion of CRT. Nonetheless regardless of the chemotherapy regimen chosen, the RTOG still mandates RT and chemotherapy beginning on day 1. Finally, although we found a correlation between asynchronous CRT start dates and worse OS, this correlation might not represent causation, and other variables could account for these differences.
In addition, we found social variables, such as ethnicity and income, potentially contribute to differences in CRT start times. Similar trends have been documented in other malignancies. For example, Corso et al15 showed that African Americans are less likely to receive stereotactic body RT for early-stage NSCLC, and Martinez et al16 reported a similar effect for RT in the treatment of breast cancer. In addition to socioeconomic factors, physician-related factors also play a role in treatment outcomes. Physician interactions, in the form of collaboration and shared treatment of patients between oncologic subspecialists, a surrogate for inter-physician communication, is correlated with improved outcomes in rectal cancer patients.9 Communication can be facilitated through collaborative sessions such as tumor boards at which multidisciplinary coordinated discussions of care occur. Previous studies have demonstrated that patients at academic centers might fare better with regard to their cancer outcomes,17,18 which could be a surrogate for a setting in which all providers are under a single roof, facilitating communication across specialties. Therefore, these external factors also remain ripe for study to improve and optimize delivery of multimodality cancer therapy.
Conclusion
Our study results highlight the importance of initiating chemotherapy and RT as synchronously as possible for the treatment of patients with inoperable NSCLC for whom definitive CRT is planned. Further efforts to understand the factors that interfere with the synchronous delivery of CRT are needed.
Supplementary Material
Clinical Practice Points.
CRT is the standard of care for locally advanced inoperable NSCLC.
Many of the large clinical trials of CRT on which we have based this standard of care dictated that chemotherapy and RT should begin concurrently on day 1.
Although this might be intuitive, no studies are available to support this practice nor have any examined the effect, if any, of minor delays on patient outcomes.
Our results have demonstrated that a significant proportion of patients, nearly 50%, will not start RT and chemotherapy on the same day.
In a propensity-matched cohort, patients who started dual therapy within 3 days had a significantly longer median OS (18.7 months; 95% CI, 17.6–19.9 months) compared with those starting treatment 4 to 6 days apart (17.5 months; 95% CI, 16.1–18.7 months; P = .02).
We found that these differences held true as the delays in the initiation of dual treatment increased.
In a propensity-matched cohort, we found that starting dual therapy within 6 days of each other corresponded to a 7% reduction in the risk of death (HR, 0.93; 95% CI, 0.87–0.99; P = .05), and the median OS for those who started treatment within 6 days (17.9 months; 95% CI, 17.2–18.7 months) was longer than that for those who started treatment 7 to 13 days apart (16.5 months; 95% CI, 15.4–17.6 months; P = .04).
Further efforts to understand factors that interfere with the synchronous delivery of CRT are needed.
Footnotes
Supplemental Data
Supplemental table accompanying this article can be found in the online version at https://doi.org/10.1016/j.cllc.2018.03.007.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Provencio M, Isla D, Sanchez A, Cantos B. Inoperable stage III non-small cell lung cancer: current treatment and role of vinorelbine. J Thorac Dis 2011; 3: 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen VW, Ruiz BA, Hsieh MC, Wu XC, Ries LA, Lewis DR. Analysis of stage and clinical/prognostic factors for lung cancer from SEER registries: AJCC staging and collaborative stage data collection system. Cancer 2014; 120(suppl 23):3781–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dillman RO, Seagren SL, Propert KJ, et al. A randomized trial of induction chemotherapy plus high-dose radiation versus radiation alone in stage III non-small-cell lung cancer. N Engl J Med 1990; 323:940–5. [DOI] [PubMed] [Google Scholar]
- 4.Sause WT, Scott C, Taylor S, et al. Radiation Therapy Oncology Group (RTOG) 88–08 and Eastern Cooperative Oncology Group (ECOG) 4588: preliminary results of a phase III trial in regionally advanced, unresectable non-small-cell lung cancer. J Natl Cancer Inst 1995; 87:198–205. [DOI] [PubMed] [Google Scholar]
- 5.Curran WJ Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011; 103:1452–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fournel P, Robinet G, Thomas P, et al. Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d’Oncologie Thoracique-Groupe Francais de Pneumo-Cancerologie NPC 95–01 Study. J Clin Oncol 2005; 23:5910–7. [DOI] [PubMed] [Google Scholar]
- 7.Lin CC, Bruinooge SS, Kirkwood MK, et al. Association between geographic access to cancer care and receipt of radiation therapy for rectal cancer. Int J Radiat Oncol Biol Phys 2016; 94:719–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnett ML, Landon BE, O’Malley AJ, Keating NL, Christakis NA. Mapping physician networks with self-reported and administrative data. Health Serv Res 2011; 46:1592–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hussain T, Chang HY, Veenstra CM, Pollack CE. Collaboration between surgeons and medical oncologists and outcomes for patients with stage III colon cancer. J Oncol Pract 2015; 11:e388–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Partridge EE. The National Cancer Data Base: ten years of growth and commitment. CA Cancer J Clin 1998; 48:131–3. [DOI] [PubMed] [Google Scholar]
- 11.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015; 16:187–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 2014; 64:252–71. [DOI] [PubMed] [Google Scholar]
- 13.Santana-Davila R, Devisetty K, Szabo A, et al. Cisplatin and etoposide versus carboplatin and paclitaxel with concurrent radiotherapy for stage III non-small-cell lung cancer: an analysis of Veterans Health Administration data. J Clin Oncol 2015; 33:567–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang EH, Rutter CE, Corso CD, et al. Patients selected for definitive concurrent chemoradiation at high-volume facilities achieve improved survival in stage III non-small-cell lung cancer. J Thorac Oncol 2015; 10:937–43. [DOI] [PubMed] [Google Scholar]
- 15.Corso CD, Park HS, Kim AW, Yu JB, Husain Z, Decker RH. Racial disparities in the use of SBRT for treating early-stage lung cancer. Lung Cancer 2015; 89:133–8. [DOI] [PubMed] [Google Scholar]
- 16.Martinez SR, Beal SH, Chen SL, et al. Disparities in the use of radiation therapy in patients with local-regionally advanced breast cancer. Int J Radiat Oncol Biol Phys 2010; 78:787–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lassig AA, Joseph AM, Lindgren BR, et al. The effect of treating institution on outcomes in head and neck cancer. Otolaryngol Head Neck Surg 2012; 147:1083–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samson P, Patel A, Crabtree TD, et al. Multidisciplinary treatment for stage IIIA non-small cell lung cancer: does institution type matter? Ann Thorac Surg 2015; 100:1773–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.