Abstract
Radiation dose escalation has been shown to improve local control and survival in patients with non–small cell lung cancer in some studies, but randomized data have not supported this premise, possibly owing to adverse effects. Because of the physical characteristics of the Bragg peak, proton therapy (PT) delivers minimal exit dose distal to the target volume, resulting in better sparing of normal tissues in comparison to photon-based radiation therapy. This is particularly important for lung cancer given the proximity of the lung, heart, esophagus, major airways, large blood vessels, and spinal cord. However, PT is associated with more uncertainty because of the finite range of the proton beam and motion for thoracic cancers. PT is more costly than traditional photon therapy but may reduce side effects and toxicity-related hospitalization, which has its own associated cost. The cost of PT is decreasing over time because of reduced prices for the building, machine, maintenance, and overhead, as well as newer, shorter treatment programs. PT is improving rapidly as more research is performed particularly with the implementation of 4-dimensional computed tomography–based motion management and intensity modulated PT. Given these controversies, there is much debate in the oncology community about which patients with lung cancer benefit significantly from PT. The Particle Therapy Co-operative Group (PTCOG) Thoracic Subcommittee task group intends to address the issues of PT indications, advantages and limitations, cost-effectiveness, technology improvement, clinical trials, and future research directions. This consensus report can be used to guide clinical practice and indications for PT, insurance approval, and clinical or translational research directions.
Introduction
Non–small cell lung cancer (NSCLC) remains the number one cause of cancer death in the world. It is anticipated that the incidence and mortality will continue to increase worldwide because of smoking, environmental pollution, and an aging population. The anticipated increase in curable lung cancer incidence resulting from lung screening throughout the world demands more effective and less morbid treatments for lung cancer.
Clinical studies in early-stage NSCLC have shown that an ablative dose can improve local disease control and potentially affect survival. For example, stereotactic ablative radiation therapy, which delivers a biological effective dose >100 Gy, achieves local control rates of >95%, improves overall survival compared with conventional radiation therapy, and achieves similar or better survival compared with surgical resection in stage I NSCLC (1–3). This success is due to improvements in imaging and precision delivery of radiation therapy for small tumors. However, dose escalation with photon-based radiation therapy in patients with locally advanced NSCLC is limited because of potential severe toxicities, with rates of grade 3 or higher toxicities of 76% to 79% as seen in the Radiation Therapy Oncology Group (RTOG) 0617 trial, which can result from incidental irradiation of intrathoracic structures (4). The heart dose was found to be inversely proportional to overall survival (5). The survival was worse with 74 Gy than with 60 Gy when conventionally fractionated.
The unique characteristic of proton therapy is the Bragg peak, which deposits the bulk of its cancer killing at a particular depth. The Bragg peak is dependent on the protons’ initial energy and the density and depth of the tissue in the beam path. Proton therapy decreases radiation exposure to organs at risk. Unlike photons that cause ionizing damage to DNA throughout the beam penetration, including the significant exit dose, protons have the potential to reduce radiation-induced toxicities by their lack of exit dose. These features of proton therapy could be particularly beneficial in patients who have poor pulmonary function, patients with cardiovascular disease or recurrent disease, and other individuals at high risk of the development of severe side effects in general (such as elderly persons).
Despite proton therapy’s theoretical advantages, there is debate in the oncology community regarding its use in lung cancer. First, the technical challenges of proton therapy limit its broad adoption. Second, given the higher cost of proton therapy, a definitive side-by-side comparison of proton therapy with modern photon radiation therapy such as intensity modulated radiation therapy (IMRT) or volumetric intensity modulated arc therapy (VMAT) is needed. The Particle Therapy Co-operative Group (PTCOG) Thoracic Subcommittee task group intended to review the potentials, limitations, and future optimization of proton therapy in early-stage and advanced NSCLC.
What Are the Potentials and Limitations of Proton Therapy?
There are 2 basic modes of delivering the proton beam, either through a passively scattered approach (passive scattering proton therapy [PSPT]) or through a pencil beam scanning (PBS) approach. The PBS approach can either be planned with single-field optimization or multi-field optimization to create intensity modulated proton therapy (IMPT). In PSPT, a 3-dimensional (3D) treatment planning technique is used to design a conformal radiation dose distribution. An individualized compensator is used to shape the distal edge of the beam, and an aperture is used to limit the perimeter of the radiation field (6, 7). With the PBS approach, PBS with different energies is used to deliver treatment using individually weighted “spots” (Bragg peaks) to achieve a desired proton beam dose distribution. In contrast to photon-based IMRT or VMAT that typically needs 5 to 12 beam fields (IMRT) or a constantly changing field with arc rotation (VMAT), IMPT typically only needs 2 to 4 fields. Treatment planning for single-field or multi-field optimization, an inverse treatment planning procedure, uses an objective function to simultaneously optimize the intensity and energy of each pencil beam required to deliver the desired radiation dose distribution to maximize dose to tumor and minimize dose to surrounding critical normal structures (8). This intensity modulation can be conducted within 1 field or multiple fields. Uniform scanning, another form of scanning beam technology used at some institutions, delivers radiation dose to planes or layers of tissue with uniform dose without dose painting of individual voxels. The uniform scanning technique is a hybrid of passive scattering and scanning. It is similar to PSPT in terms of using range modulation wheels, apertures, compensators, and dose distributions.
PSPT relies on 3D treatment planning, cannot perform intensity modulation, and lacks proximate conformation to the target volume (6). In general, PSPT reduces the radiation dose to the lungs, esophagus, or heart compared with photon-based radiation therapy. However, the benefit varies depending on the location and size or shape of the tumor and adenopathy (Table 1) (9). In some PSPT cases, a dose reduction may not be evident when tumors are located in complex anatomic positions or curve around critical structures. In such cases, dose reductions to normal organs may not be as significant or compromised target coverage must be considered to avoid injury to critical normal tissue structures (8). On the other hand, PSPT provides a more robust method of delivering proton therapy in lung cancer that is less sensitive to motion than IMPT and similar in robustness to IMRT (10).
Table 1.
Comparisons of and indications for VMAT-IMRT, PSPT, and IMPT
| Technique | Pros | Cons | Clinical scenarios beneficial to proton therapy |
|---|---|---|---|
| IMRT-VMAT | High conformity between prescription isodose line and target | Higher low to medium dose to normal tissues limiting the ability for dose escalation | |
| Robust with respect to changes in motion or anatomy | |||
| Lower cost and higher availability | |||
| PSPT | Limited low or medium dose to normal tissues enabling target dose escalation | Possibly higher lung mean dose and volume receiving 20 Gy and higher for complicated anatomy, lack of proximate conformation to target | Centrally located stage I disease |
| Can be made robust with respect to changes in motion or anatomy | Poor conformality of prescription isodose line to target due to 3D planning, lack of conformity in the proximal end of the target volume and range uncertainty | Stage II to III disease without contralateral hilar lymph node involvement | |
| IMPT | High conformity between prescription isodose line and target | Because of range uncertainty, less robust with respect to motion and/or changes in anatomy, making the treatment of mobile targets difficult | Centrally located stage I disease |
| Spares more normal tissues than IMRT or PSPT including the heart, cord, lung, esophagus, and so on | Complexity of motion management, plan optimization, and quality assurance | Stage II to III disease with adequate motion management, robustness optimization, and strict quality assurance |
Abbreviations: IMPT = intensity modulated proton therapy; IMRT = intensity modulated radiation therapy; PSPT = passive scattering proton therapy; 3D = 3-dimensional; VMAT = volumetric intensity modulated arc therapy.
In contrast to PSPT, IMPT simultaneously optimizes the intensities and the energies of all pencil beams by using an objective function that accounts for both target coverage and normal tissue sparing. IMPT is an analogous improvement in proton delivery technology as IMRT was to photon therapy (Table 1). Dosimetric studies indicate that IMPT reduces the dose to the critical normal tissues relative to IMRT or PSPT and allows individualized radical radiation therapy for stage I and stage III NSCLC in clinically challenging cases (8, 11). This is because of the superior dose distribution from each proton field compared with photon fields. However, uncertainties regarding proton range, tumor motion, and other aspects of treatment planning, treatment optimization, and quality assurance for IMPT can be more challenging and complex than those for PSPT or IMRT (12, 13). Implementation of this technology had been delayed in thoracic cancers until recently, when 4-dimensional computed tomography (CT)–based motion analysis and management and new robust planning algorithms were successfully applied (14). Comparisons of and indications for IMRT-VMAT, PSPT, and IMPT are listed in Table 1. Preliminary data indicated that IMPT could be safely delivered to patients with stage III or recurrent NSCLC when strict motion management and quality assurance were applied (14). Guidelines for clinical implementation of IMPT in thoracic cancers are being discussed by the PTCOG Thoracic Subcommittee and will be published in the near future.
As compared with photon therapy, the theoretical advantage of proton radiation therapy is its superiority in sparing normal tissues while administering an equivalent or higher dose to the tumor volume. Two approaches can be considered to improve clinical outcomes: The first is to maintain set dose constraints and deliver an escalated or accelerated radiation dose to the target. This is most applicable to tumor situations associated with poor local control with current photon-based technology, as is the case with stage III NSCLC. The second approach is to keep the target dose the same but minimize radiation exposure to normal critical tissues as much as possible. This would be applicable to use for specific situations such as stage I lung cancers in difficult stereotactic body radiation therapy (SBRT) situations such as tumors near the sensitive central structures. Illustrating the first strategy, virtual clinical studies indicated that, given a constant set of normal tissue dose-volume constraints, higher radiation doses can be delivered more safely with proton therapy than with IMRT in patients with locally advanced NSCLC, particularly when IMPT is used (8). IMPT spared more of the lung, heart, spinal cord, and esophagus than IMRT or PSPT did, thereby allowing dose escalation from 63 Gy (relative biological effectiveness [RBE]) to 74 Gy.
Using a constant prescribed target dose and minimizing radiation exposure to normal critical tissues, a study compared PSPT, IMPT, and photon-based radiation therapy to deliver stereotactic ablative radiation therapy, 50 Gy in 4 fractions in centrally located stage I NSCLC, and reported significant reduction of radiation doses to critical structures (11). Other studies reported that the proton-based SBRT plans delivered a lower radiation dose to normal tissues including the lungs, esophagus, bronchial tree, and spinal cord than photon-based radiation therapy did (15, 16). Proton radiation therapy was noted to deliver a slightly higher radiation dose to the skin and chest wall when fewer than 3 beams were used (16). However, the dose to the skin and chest wall could be decreased with the use of a greater number of proton beam fields (about 4 beams) (17) or arc proton therapy (18), when concern arises regarding the chest wall dose.
Because of the characteristics of the Bragg peak, with the same dosimetric features used to focus the radiation dose to the target and reduce the radiation dose to normal tissue, proton therapy is made less forgiving in moving lung cancer given the Bragg peak’s sensitivity to motion, anatomy, and density changes (12, 13, 19). Therefore, proton therapy is required to be optimized for respiratory motion and anatomy or density changes for NSCLC. Using 4-dimensional CT-based planning, we are now able to evaluate tumor motion across an entire respiratory cycle and deliver radiation therapy accordingly (20). For stereotactic ablative radiation therapy, on-board volumetric imaging should be performed before each fraction to verify tumor location. In selected patients, adaptive re-planning is needed to compensate for tumor shrinkage and anatomy or motion changes that could significantly alter the radiation dose distribution (21). Indeed, it was reported that 20% of cases using PSPT (22) and 30% of cases using IMPT (23) need adaptive re-planning during the course of proton therapy.
Because PSPT uses 3D chemoradiation planning techniques, complicated dose distributions are challenging to achieve when the dose needs be conformed to eccentric or curved shapes. As IMRT-VMAT has become more mature (24), PSPT is not always better in sparing all critical structures, particularly for the esophagus or lung receiving 20 Gy or higher, as compared with IMRT-VMAT (Fig. 1, Table 1). For this reason, personalized treatment planning and comparison plans are required to decide which critical structure sparing is the priority for the individual patient. Compared with optimized IMRT-VMAT, optimized PSPT typically better spares the heart, lung volume receiving 5 Gy and higher, contralateral lung, spinal cord, and integral dose compared with photon plans (Fig. 1). However, PSPT may not spare the esophagus more if it abuts the tumor volume (Fig. 1). PSPT may not improve ipsilateral lung volume receiving 20 Gy and higher or mean lung dose because of limited beam numbers and a larger uncertainty margin (Fig. 1). In addition, in contrast to IMRT-VMAT, PSPT may not always have improved tumor coverage and conformality (Fig. 1). Therefore, PSPT may be better suited for tumors with simple anatomy and without involvement of the contralateral lung, contralateral hilum, or contralateral mediastinal lymph node, whereas IMPT may be more suitable for tumors with limited motion (14). On the other hand, motion mitigation techniques for PBS proton therapy are being developed (eg, re-scanning and/or gating), and the sensitivity of this technique to motion will certainly be reduced in the future with the introduction of such techniques to the clinic. The tradeoff in conformality for PSPT is balanced against the significant need for accurate motion control that is necessary with IMPT.
Fig. 1.
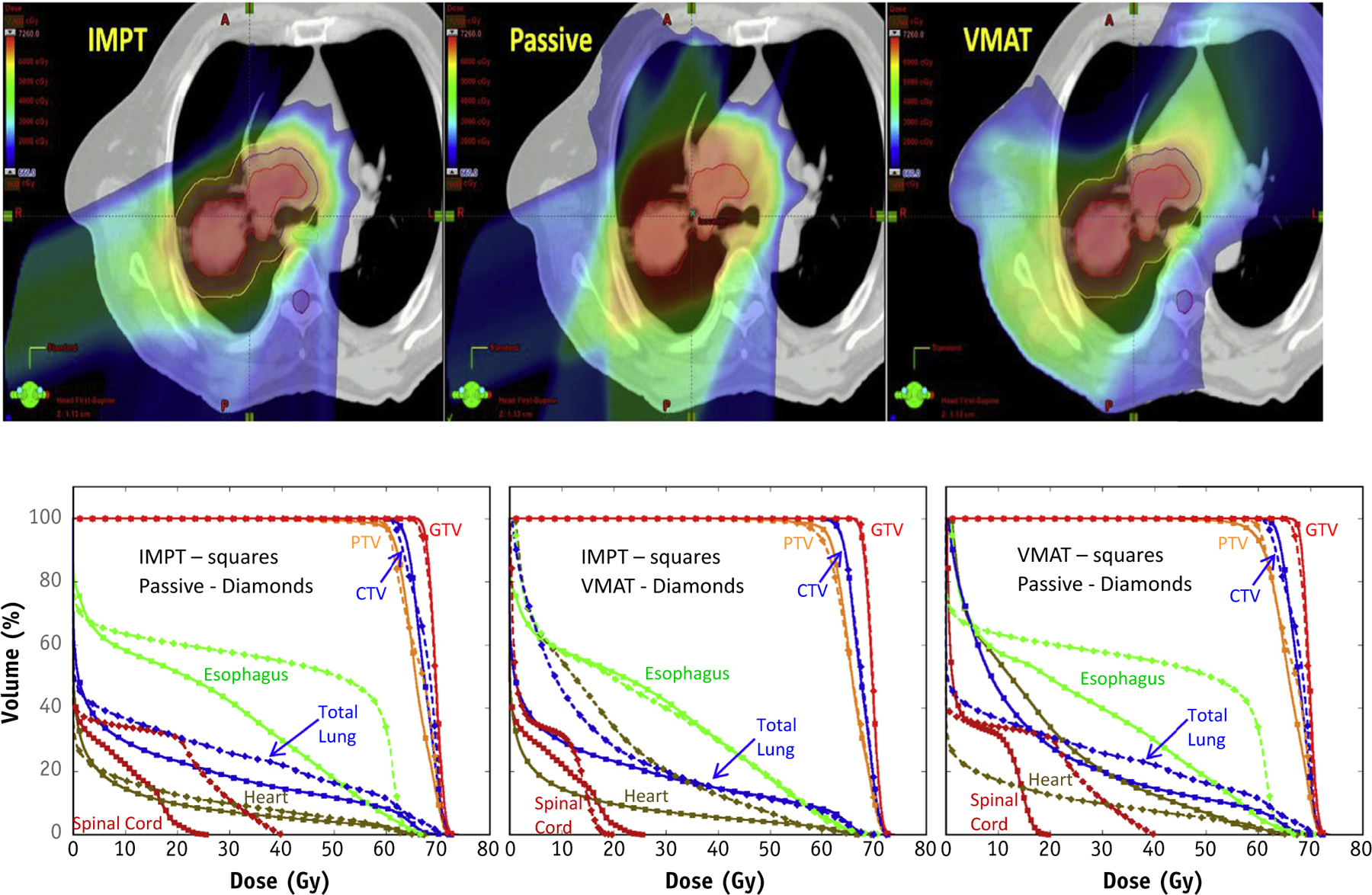
Comparison of intensity modulated proton therapy (IMPT) versus passive scattering proton therapy (PSPT) and volumetric intensity modulated arc therapy (VMAT) in stage III non–small cell lung cancer. The IMPT plan achieves the best sparing of all critical structures. PSPT spares more of the heart and contralateral lung but not the esophagus ipsilateral lung, lung mean dose, or volume receiving a dose of 20 Gy or higher as compared with VMAT. Abbreviations: CTV = clinical target volume; GTV = gross tumor volume; PTV = planning target volume.
IMPT generally provides better conformality than PSPT. Optimized IMPT can almost always spare all critical structures even with complicated anatomy. However, the greater precision of IMPT makes proper motion management, plan optimization, and meticulous quality assurance more imperative (14). Robust optimization of the IMPT plan is crucial and required to improve conformality and minimize the uncertainty of radiation dose delivery resulting from motion or anatomy changes. Therefore, IMPT is well suited for complicated anatomy with minimal motion. Preliminary clinical outcome data indicate that IMPT may potentially reduce the side effects in NSCLC particularly for cases of reirradiation (23). Because IMPT was recently implemented in the clinic, more research is necessary. Currently, the most advanced IMPT robustness optimization methods are still in the research phases and have not been commercialized.
What Are the Indications for Proton Therapy?
Early-stage NSCLC
Contrary to new technologies in the treatment of most cancers, the use of proton therapy in the management of stage I NSCLC preceded dosimetric comparison studies. In the mid-1990s, after having successfully developed a niche for treating prostate cancer with proton therapy, the Loma Linda University (California) lung cancer program developed techniques for managing early-stage NSCLC using proton therapy. Initially, the patients were treated under a phase 1 and 2 dose-intensification study that used hypofractionated accelerated treatment with a dose of 50 Gy (RBE) over 10 fractions (25). Over the next 20 years, with increased experience, the dose was intensified to 70 Gy (RBE) over 10 fractions (26). Loma Linda University’s early work led numerous proton centers in Japan and in the United States to consider hypofractionated and accelerated proton treatments to manage stage I NSCLC. The 2-year local control rates from these studies ranged from 80% to 100% (27–34).
As reported by Grutters et al (35), the use of proton therapy and particle beam therapy in early-stage NSCLC was associated with improved outcomes over conventionally fractionated radiation therapy. In fact, the 5-year overall survival rate doubled among patients treated with proton or carbon therapy. However, shortly after the excellent results on the use of proton therapy for early-stage NSCLC began to emerge, the studies on photon-based SBRT (also called stereotactic ablative radiation therapy) from Europe, Japan, and the United States (1–3, 35) reported similar, improved rates of disease control with low rates of grade 3 or higher toxicity.
Following published clinical experiences with hypofractionated proton therapy for early-stage NSCLC, several groups began dosimetric studies to understand if proton therapy added benefits to photon-based SBRT (36, 37). A major limitation to proton therapy in early-stage NSCLC was the lack of volumetric image guidance, which would ultimately require slightly larger planning target volume (PTV) margins compared with photon-based SBRT using cone beam CT scans. This issue was rarely if ever addressed in the dosimetric comparison studies, with the same PTV margins given for the comparison plans. In addition, the dosimetric studies that were published lacked consensus results, with some showing benefits for proton therapy that others did not find. Furthermore, even when a statistical benefit may have been seen with dose-volume histogram measures, these theoretical benefits did not always translate into meaningful clinical benefits. A more detailed look at the studies shows that the benefit was likely due in part to the target volumes and locations, as described later. Importantly, the cost differential between proton therapy and photon radiation becomes smaller as fewer fractions are delivered, such that when SBRT fractions are considered, the cost becomes very similar to proton radiation (38).
Small peripheral lesions
A vast majority of early-stage NSCLCs are small peripheral tumors. These tumors are located far from critical normal tissue, and the risk of side effects is mostly attributed to rib fracture and chest wall pain. These patients generally benefit most from volumetric imaging, especially to avoid placement of CT-guided markers that carry the risk of pneumothorax. Proton therapy, in these cases, rarely improves dosimetry in a clinically meaningful way, partially because protons have a hard time stopping sharply in the middle of the lung. Consequently, there is more exit dose within the lung parenchyma compared with soft tissue (like the mediastinum) when using proton therapy. This dilemma likely explains why Georg et al (36) failed to see a dosimetric benefit for proton therapy over SBRT. Tumors in this study were quite small and peripheral.
Larger tumors
Virtual clinical studies showed improved dose distributions to the organs at risk with the use of proton therapy, which was attributed to the larger PTVs used in these studies (12, 37). Consequently, the larger the tumor, the more benefit derived from proton therapy. Similar data have been reported in the management of brain metastases with proton-based stereotactic radiosurgery (39). Moreover, another study showed reduced dose to the chest wall and ribs among patients with larger peripheral lesions, suggesting some clinical benefit in this scenario (17).
Central tumors
One of the few major studies published on toxicity after SBRT showed an increased risk of grade 3 or higher toxicities among patients with centrally located tumors (40). RTOG 0813 investigated the use of SBRT for centrally located tumors, and the preliminary data showed significant toxicity, even death (6% mortality, 4 of 81 evaluable patients received 52.5 Gy or higher delivered in 5 fractions), could occur when an ablative dose was delivered to the bronchus (41). In fact, for the highest dose tested (60 Gy in 5 fractions), among 30 patients evaluable for dose-limiting toxicity, 7 had grade 3 to 5 toxicities develop. Longer follow-up is still needed to better understand the role of SBRT for central tumors because many of the toxicities in the report of Timmerman et al (40) occurred >1 year after treatment. Nevertheless, Bush et al (26) showed no increased risk of toxicities for patients with centrally located stage I NSCLC treated with proton therapy. The rationale for these findings can be explained by some work demonstrating that tumors located centrally may benefit more from proton therapy (11). This concept makes sense because the mediastinum is adjacent to the tumor and allows a location for the protons to stop and reduce the dose to the mediastinum. Thus radiation dose to the esophagus, heart, major vessels, and spinal cord can be substantially reduced, without having to redistribute an increase in dose to the lungs. On the other hand, proton therapy may not have as much of a benefit in reducing the radiation dose to the major bronchi when disease is directly adjacent to them, because the major bronchi are generally surrounded by lung parenchyma in which the protons travel farther. Figure 2 shows comparison plans for a patient with a small central tumor wherein proton therapy could deliver the dose while meeting dose constraints; however, the SBRT photon plan drove up the lung and heart dose in a clinically meaningful way to meet the dose constraints to the esophagus. In fact, on the basis of the dosimetric benefit, a randomized trial has been developed between SBRT and stereotactic body proton therapy.
Fig. 2.

Comparison of proton- versus proton-based stereotactic body radiation therapy in a centrally located lesion in stage I non–small cell lung cancer. As compared with photon therapy, proton therapy spares more of the bronchial tree, lung, major vessels, heart, and spinal cord.
Tumors near the brachial plexus
Tumors located in the apex of the lung may be adjacent to the brachial plexus. In addition to demonstrating lower doses to the organs at risk in centrally located tumors, a study showed improved dosimetry in apical tumors with proton therapy (11). The rationale for considering proton therapy in these patients is the possibility of brachial plexopathy. A study reported a risk of brachial plexopathy among patients treated with SBRT for tumors in the apical region (42). Thus caution is needed as is consideration of proton therapy to reduce the dose to the brachial plexus.
Multiple tumors
Another phenomenon is the use of proton therapy in patients with multiple tumors (37). Shi et al (43) reported on a patient with bilateral early-stage NSCLC for whom proton therapy could better deliver the necessary dose of radiation than other modalities.
In summary, proton therapy delivers excellent dose distributions in patients with early-stage NSCLC with associated high rates of local control and survival. However, similar outcomes have been achieved with the use of SBRT, which is more widely available, diminishing the relative benefits to some degree. Nevertheless, patients with larger early-stage tumors and tumors located more centrally or close to the brachial plexus may benefit more from the use of proton therapy. In particular, in patients who do not meet dosimetric constraints with SBRT, proton therapy may allow patients to receive a more effective treatment than standard fractionated radiation therapy. The use of volumetric image guidance will help to minimize the PTV margin such that target volume comparisons between proton therapy and SBRT are identical.
Locally advanced NSCLC
Locally advanced (stage III) lung cancer typically presents with large primary tumors with mediastinal node involvement that are directly adjacent to critical central structures such as the heart, esophagus, spinal cord, and major vessels. Both high local failure and distant metastasis pose challenges for a cure in this group of patients. Prospective randomized studies have shown that improved local control with concurrent chemoradiation may be associated with better overall survival (44–46).
The proximity of advanced tumors to critical normal structures limits dose escalation or acceleration in patients with NSCLC. In RTOG 0617, patients with stage III NSCLC were randomized to receive 2 radiation doses (60 Gy and 74 Gy) using photon with concurrent chemotherapy with or without cetuximab (4). Unexpectedly, the 74-Gy dose of radiation was associated with poorer median survival as compared with the 60-Gy arm (20.3 months vs 28.7 months, P=.004). Criticisms of RTOG 0617 include lack of mandated dose-volume constraints for most of the major organs such as the lung and heart. In fact, the median mean lung dose in the 74-Gy arm was 19.9 Gy, meaning that half of the patients received an excessive lung dose. On a secondary analysis of this trial, the dose delivered to the heart was found to be an independent predictor of survival. In addition, IMRT was found to spare more of the heart and lung, reduce radiation pneumonitis, and improve quality of life as compared with 3D conformal radiation therapy (5). Virtual clinical studies showed that proton plans better reduce radiation exposure to normal tissues, particularly the heart, than do photon plans (6, 47). Several clinical studies have also confirmed that proton therapy can deliver adequate radiation dose to target while avoiding normal tissue in locally advanced NSCLC (48–52).
A phase 2 study of 44 patients with stage III NSCLC who received 74 Gy (RBE) via conventional fractionation (2 Gy (RBE) per fraction) with weekly concurrent carboplatin and paclitaxel, the same chemoradiation regimen as RTOG 0617, reported no grade 4 or 5 toxicities, and grade 3 toxicities were minimal, including dermatitis (n=5), esophagitis (n=5), and pneumonitis (n=1) (48). Because of the tolerability of this high-dose regimen, patients were more likely to complete chemotherapy and radiation treatment without a treatment break. The median survival was 29.4 months, as compared with 20.3 months for the 74-Gy arm in RTOG 0617. Two similarly designed studies that included fewer patients showed equally excellent outcomes supporting its use (49, 50).
Another phase 2 randomized study, supported by a National Institutes of Health program grant, to compare PSPT with IMRT in stage III NSCLC treated with radiation therapy to 74 Gy with concurrent carboplatin and paclitaxel has finished enrollment and is pending clinical outcome analysis. The primary endpoint of this trial was time to grade 3 radiation pneumonitis or any recurrence. An ongoing phase 3 study, RTOG 1308, comparing IMRT with proton therapy in stage III NSCLC, seeks to evaluate overall survival as its primary objective because both arms use the same radiation dose and same chemotherapy regimen. Table 2 lists available information about proton therapy clinical trials in lung cancer.
Table 2.
Clinical trials using proton therapy in non–small cell lung cancer
| Status | Stage | Title | ClinicalTrials.gov identifier | Center | Phase |
|---|---|---|---|---|---|
| Early stage | |||||
| Recruiting | I | Hypofractionated, Image-Guided Radiation Therapy With Proton Therapy for Stage I Non-Small Cell Lung Cancer | NCT00875901 | UF | |
| Recruiting | I | Proton Stereotactic Body Radiation Therapy (SBRT) for Medically Inoperable, Peripheral Early-Stage Non-Small Cell Lung Cancer (NSCLC): A Pilot Study | NCT01525446 | MGH | - |
| Final result pending | I | Phase II Escalated/Accelerated Proton Radiotherapy for Inoperable Stage I (T1-T2N0M0) and Selected Stage II (T3N0M0) Non-Small Cell Lung Cancer (NSCLC) | NCT00495040 | MDA | 2 |
| On hold for CBCT | I or II | Randomized Phase II Study Comparing Stereotactic Body Radiotherapy (SBRT) With Stereotactic Body Proton Therapy (SBPT) for Centrally Located Stage I, Selected Stage II and Recurrent Non-Small Cell | NCT01511081 | MDA | 2 |
| Locally advanced stage | |||||
| Terminated early | III | A Phase II Trial of 3 Dimensional Proton Radiotherapy With Concomitant Chemotherapy for Patients With Initially Unresectable Stage III Non-Small Cell Lung Cancer | NCT00881712 | UF | 2 |
| Recruiting | II or III | Phase III Randomized Trial Comparing Overall Survival After Photon versus Proton Chemoradiotherapy for Inoperable Stage II-IIIB NSCLC | NCT01993810 | NRG | 3 |
| Recruiting | II or III | A Phase I/II Study of Hypofractionated Proton Therapy for Stage II-III Non-Small Cell Lung Cancer | NCT01770418 | PCG | 1, 2 |
| Recruiting | II or III | A Phase I Study of Radiation Dose Intensification With Accelerated Hypofractionated Proton Therapy and Chemotherapy for Non-Small Cell Lung Cancer | NCT02172846 | WU | 1 |
| Recruiting | III | Phase I/II Trial of Image-Guided, Intensity-Modulated Photon (IMRT) or Scanning Beam Proton Therapy (IMPT) Both With Simultaneous Integrated Boost (SIB) Dose Escalation to the Gross Tumor Volume (GTV) With Concurrent Chemotherapy for Stage II/III Non-Small Cell Lung Cancer (NSCLC) | NCT01629498 | MDA | 1, 2 |
| Recruiting | III | Feasibility and Phase I/II Trial of Preoperative Proton Beam Radiotherapy With Concurrent Chemotherapy for Resectable Stage IIIA or Superior Sulcus NSCLC | NCT01076231 | UP | 1, 2 |
| Final result pending | II or III | A Bayesian Randomized Trial of Image-Guided Adaptive Conformal Photon versus Proton Therapy, With Concurrent Chemotherapy, for Locally Advanced Non-Small Cell Lung Carcinoma: Treatment Related Pneumonitis and Locoregional Recurrence | NCT00915005 | MDA | 2 |
| Final result pending | III | Phase II Concurrent Proton and Chemotherapy in Locally Advanced Stage IIIA/B Non-Small Cell Lung Cancer (NSCLC) | NCT00495170 | MDA | 2 |
| Completed | III | Phase I Dose Escalation Trial of Proton Beam Radiotherapy With Concurrent Chemotherapy and Nelfinavir for Inoperable Stage III NSCLC | NCT01108666 | UP | 1 |
| Terminated early | III | Phase I/II Study of Combined Chemotherapy and High Dose, Accelerated Proton Radiation for the Treatment of Locally Advanced Non-Small Cell Lung Carcinoma | NCT00614484 | LL | 1, 2 |
| Terminated early | III | A Phase I Trial of Hypofractionated Proton Radiation Therapy With Cisplatin and Etoposide Followed by Surgery in Stage III Non-Small Cell Lung Cancer | NCT01565772 | MGH | 1 |
| Relapse and other | |||||
| Recruiting | Relapse | Definitive Re-irradiation With Proton Beam Radiotherapy for Patients With Recurrent Thoracic Cancers | NCT02204761 | UW | - |
| Recruiting | Relapse | Proton Radiotherapy for Recurrent Tumors | NCT01126476 | UP | - |
| Completed | I-IV, relapse | Phase I Study of Hypofractionated Proton Radiation Therapy in Thoracic Malignancies | NCT01165658 | MDA | 1 |
Abbreviations: CBCT = cone beam computed tomography; LL = Loma Linda University; MDA = MD Anderson Cancer Center; MGH = Massachusetts General Hospital; NRG = NRG Oncology; PCG = Proton Collaborative Group; UF = University of Florida; UP = University of Pennsylvania; UW = University of Washington; WU = Washington University.
Although randomized studies are the optimal approach to evaluate the efficacy and toxicity of photon versus proton modalities, there are some limitations to this approach. It may be prudent to design trials stratifying for different modalities of proton therapy given the pros and cons, as mentioned in the previous sections, for planning of PSPT and IMPT (Table 1), and such trials may need to be based on varying anatomy and personalized planning approaches. It may not be wise to compare matured IMRT-VMAT with maturing proton technology, particularly regarding inferior image guidance. IMRT ideally should be compared with IMPT using similar volumetric image guidance and motion management strategies. A randomized study to compare IMRT with IMPT using an integrated boost approach to boost gross tumor volume to 72 Gy while keeping PTV dose to 60 Gy with concurrent chemotherapy is ongoing (Table 2).
Another potential advantage to using proton therapy in locally advanced NSCLC is to explore the use of modest hypofractionation with or without concurrent chemotherapy as a way to accelerate treatment and deliver a more biologically effective dose, similar to that being done in stage I NSCLC. Not only may this improve local control, but it also makes the treatment shorter and more cost-effective (53). Two studies are currently exploring hypofractionation with concurrent chemotherapy—a single-institution study and a multicenter study being performed through the Proton Collaborative Group (Table 2). The Proton Collaborative Group study is a combined phase 1 and 2 study, where the maximum tolerated dose is achieved in the phase 1 component starting at a dose level of 60 Gy (RBE) at 2.5 Gy (RBE) per fraction and with each subsequent dose arm increasing the dose per fraction (3 Gy [RBE], 3.53 Gy [RBE], and 4 Gy [RBE]) while keeping the final dose at 60 Gy (RBE).
Recurrent NSCLC
As more patients with lung cancer live longer, recurrence in the previously irradiated area can occur more often and imposes significant clinical challenges because of limitation of normal tissue tolerance. Clinical data indicated that a higher reirradiation dose and/or concurrent chemotherapy are associated with improved overall survival, but the results of photon reirradiation remain unsatisfactory (54, 55). Proton therapy can benefit this group dramatically, particularly for mediastinal lymph nodes, and allow more patients to receive a definitive dose of radiation therapy with concurrent chemotherapy (55, 56) (Table 2).
Cost Analysis of Proton and Photon Radiation Therapy
A significant source of concern regarding proton beam therapy is the added cost of this therapy compared with photon-based conformal radiation therapy or IMRT (57). It is hoped that the efficacy of proton beam therapy will lead to wider acceptance of this therapy by improving the outcomes for many lung cancers despite the increased treatment cost. Proton therapy may also allow for safer hypofractionation, which can reduce the number of fractions of therapy, thereby reducing the costs of treatment. Altered fractionation has already been shown to be cost-effective in photon therapy, and this will be even more pronounced for proton therapy (58).
Overall, lung cancer therapy is least costly when patients require only initial therapy. Conversely, the cost of lung cancer care increases at the time of recurrence because of added hospitalizations and outpatient visits, with hospitalizations being the main cost driver (59, 60). Novel strategies to prevent or delay treatment failure of lung cancer may offset some of the economic burden (59). For the initial management of lung cancer, the cost of radiation therapy is known to be inexpensive and provides notable tumor control and symptom palliation at a population level (60). The cost-effectiveness of chemoradiation for stage III NSCLC is superior to the best supportive care (58). These economic improvements of radiation therapy have been measured by life-years gained, cost-utility analyses, and quality-adjusted life-years (61–64). In addition, more complex radiation planning for stage I and II NSCLC has been shown to improve survival in photon radiation therapy (65). There is further opportunity to build on the gains made by standard radiation therapy techniques by possible toxicity reduction with proton therapy, particularly long-term cardiac, pulmonary, and esophageal toxicity (66). The relative cost of proton therapy has been estimated to be 1.6 to 2.4 times as expensive as photon therapy (67). Overall, the cost of these treatments should not serve as the primary driver of decision making, but ideally each case would be evaluated to determine whether proton therapy provides sufficient dosimetric advantages (68) and may enable patients to receive therapies with lower toxicity, avoid hospitalizations, and maximize functional status. Economic models have been published to take into account the effect of uncertainties in the decision to reimburse proton therapy (69), as well as how to deal with the economic effects of patient heterogeneity (70).
Conclusions
Because of its Bragg peak, proton radiation therapy is a promising modality for delivering adequate dose to the target to potentially improve local control and survival and decrease side effects in patients with NSCLC. Virtual clinical studies have shown the dosimetric advantages of proton-based radiation therapy over photon-based radiation therapy in sparing normal critical structures, particularly the heart and spinal cord, with low-dose exposure of the lung. Sparing the heart could be crucial to improving survival based on recent data from RTOG 0617. Promising preliminary clinical outcomes have been reported for patients with early-stage or locally advanced NSCLC who received proton therapy. However, the expense and technical challenges of proton therapy demand further technique optimization and more clinical studies. A one-size-fits-all approach does not apply for any treatment including proton therapy. Therefore, we cannot provide generalized statements about proton therapies being of universal benefit to all patients for both early-stage and locally advanced lung cancers. Compared with IMRT-VMAT, PSPT is better suited to spare critical structures for tumors without extensive involvement of the contralateral lung, hilum, and mediastinal lymph node (Table 1). As compared with both IMRT-VMAT and PSPT, IMPT is better suited for both simple and complicated tumor anatomy with adequate motion management. For other clinical situations, an individualized approach using virtual clinical plan comparison should be considered. Personalized proton therapy optimization with adequate quality assurance to consider each patient’s anatomy and motion, other uncertainties, and priority of individual critical structure sparing is crucial and required. Given this emerging and novel technology, we should proceed methodically while we optimize the technology and provide an opportunity for maturing data on this technology to develop. It is anticipated that there is a significant learning curve, particularly for small centers without strong academic and physics support. On the basis of its unique physical characteristics, proton therapy should be better than photon therapy to spare critical normal structures if the similar technique and maturity are applied and adequate motion management is used. Proton therapy may potentially result in reduced long-term medical costs because of reduced toxicities. It behooves us to continue to optimize proton therapy, enroll patients in clinical studies, and establish evidence-based clinical indications and technology guidelines to provide maximal benefit to the patient while reducing the side effects of therapy.
Acknowledgments—
The authors thank all members of the Particle Therapy Co-operative Group (PTCOG) Thoracic Subcommittee and PTCOG Executive Committee for their help and support.
Footnotes
Conflict of interest: none.
References
- 1.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303: 1070–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shirvani SM, Jiang J, Chang JY, et al. Lobectomy, sublobar resection, and stereotactic ablative radiotherapy for early-stage non-small cell lung cancers in the elderly. JAMA Surg 2014;149:1244–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: A pooled analysis of two randomised trials. Lancet Oncol 2015;16:630–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015; 16:187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chun SG, Hu C, Choy H, et al. Outcomes of intensity modulated and 3D-conformal radiotherapy for stage III non-small cell lung cancer in NRG oncology/RTOG 0617 Presented at: 16th World Conference on Lung Cancer. September 6–10, 2015; Denver, CO. [Google Scholar]
- 6.Chang JY, Zhang X, Wang X, et al. Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in stage I or stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2006;65: 1087–1096. [DOI] [PubMed] [Google Scholar]
- 7.Auberger T, Seydl K, Futschek T, et al. Photons or protons: Precision radiotherapy of lung cancer. Strahlenther Onkol 2007;183:3–6. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Li Y, Pan X, et al. Intensity-modulated proton therapy reduces the dose to normal tissue compared with intensity-modulated radiation therapy or passive scattering proton therapy and enables individualized radical radiotherapy for extensive stage IIIB non-small-cell lung cancer: A virtual clinical study. Int J Radiat Oncol Biol Phys 2009;77:357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohan R, Matney J, Bluett J, et al. IMRT versus passively scattered proton therapy (PSPT) for locally advanced NSCLC: Impact of changing techniques and technologies over the course of a randomized trial [abstract]. Int J Radiat Oncol Biol Phys 2012;84: S565–S566. [Google Scholar]
- 10.Matney J, Park PC, Bluett J, et al. Effects of respiratory motion on passively scattered proton therapy versus IMRT for stage III lung cancer: Are proton plans more sensitive to breathing motion? Int J Radiat Oncol Biol Phys 2013;87:576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Register SP, Zhang X, Mohan R, et al. Proton stereotactic body radiation therapy for clinically challenging cases of centrally and superiorly located stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2011;80:1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albertini F, Bolsi A, Lomax AJ, et al. Sensitivity of intensity modulated proton therapy plans to changes in patient weight. Radiother Oncol 2008;86:187–194. [DOI] [PubMed] [Google Scholar]
- 13.Engelsman M, Rietzel E, Kooy HM. Four-dimensional proton treatment planning for lung tumors. Int J Radiat Oncol Biol Phys 2006;64:1589–1595. [DOI] [PubMed] [Google Scholar]
- 14.Chang JY, Li H, Zhu XR, et al. Clinical implementation of intensity modulated proton therapy for thoracic malignancies. Int J Radiat Oncol Biol Phys 2014;90:809–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoppe BS, Huh S, Flampouri S, et al. Double-scattered proton-based stereotactic body radiotherapy for stage I lung cancer: A dosimetric comparison with photon-based stereotactic body radiotherapy. Radiother Oncol 2010;97:425–430. [DOI] [PubMed] [Google Scholar]
- 16.Macdonald OK, Kruse JJ, Miller JM, et al. Proton beam radiotherapy versus three-dimensional conformal stereotactic body radiotherapy in primary peripheral, early-stage non-small-cell lung carcinoma: A comparative dosimetric analysis. Int J Radiat Oncol Biol Phys 2009; 75:950–958. [DOI] [PubMed] [Google Scholar]
- 17.Welsh J, Amini A, Ciura K, et al. Evaluating proton stereotactic body radiotherapy to reduce chest wall dose in the treatment of lung cancer. Med Dosim 2013;38:442–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seco J, Gu G, Marcelos T, et al. Proton arc reduces range uncertainty effects and improves conformality compared with photon volumetric modulated arc therapy in stereotactic body radiation therapy for non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2013;87:188–194. [DOI] [PubMed] [Google Scholar]
- 19.Zhao L, Sandison GA, Farr JB, et al. Dosimetric impact of intra-fraction motion for compensator-based proton therapy of lung cancer. Phys Med Biol 2008;53:3343–3364. [DOI] [PubMed] [Google Scholar]
- 20.Chang JY, Dong L, Liu H, et al. Image-guided radiation therapy for non-small cell lung cancer. J Thorac Oncol 2008;3:177–186. [DOI] [PubMed] [Google Scholar]
- 21.Hui Z, Zhang X, Starkschall G, et al. Effects of interfractional motion and anatomic changes on proton therapy dose distribution in lung cancer. Int J Radiat Oncol Biol Phys 2008;72:1385–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koay EJ, Lege D, Mohan R, et al. Adaptive/nonadaptive proton radiation planning and outcomes in a phase II trial for locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2012;84: 1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho J, Li H, Zhang X, et al. Clinical outcome of intensity modulated proton therapy for NSCLC Presented at: 57th Annual Meeting of the American Society for Radiation Oncology. October 18–21, 2015; San Antonio, TX. [Google Scholar]
- 24.Chang JY. Intensity-modulated radiotherapy, not 3D conformal, is the preferred technique for treating locally advanced lung cancer. Semin Radiat Oncol 2015;25:110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bush DA, Slater JD, Bonnet R, et al. Proton-beam radiotherapy for early-stage lung cancer. Chest 1999;116:1313–1319. [DOI] [PubMed] [Google Scholar]
- 26.Bush DA, Cheek G, Zaheer S, et al. High-dose hypofractionated proton beam radiation therapy is safe and effective for central and peripheral early-stage non-small cell lung cancer: Results of a 12-year experience at Loma Linda University Medical Center. Int J Radiat Oncol Biol Phys 2013;86:964–968. [DOI] [PubMed] [Google Scholar]
- 27.Shioyama Y, Tokuuye K, Okumura T, et al. Clinical evaluation of proton radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2003;56:7–13. [DOI] [PubMed] [Google Scholar]
- 28.Nihei K, Ogino T, Ishikura S, et al. High-dose proton beam therapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2006;65:107–111. [DOI] [PubMed] [Google Scholar]
- 29.Hata M, Tokuuye K, Kagei K, et al. Hypofractionated high-dose proton beam therapy for stage I non-small-cell lung cancer: Preliminary results of a phase I/II clinical study. Int J Radiat Oncol Biol Phys 2007;68:786–793. [DOI] [PubMed] [Google Scholar]
- 30.Nakayama H, Sugahara S, Tokita M, et al. Proton beam therapy for patients with medically inoperable stage I non-small-cell lung cancer at the University of Tsukuba. Int J Radiat Oncol Biol Phys 2010;78: 467–471. [DOI] [PubMed] [Google Scholar]
- 31.Chang JY, Komaki R, Wen HY, et al. Toxicity and patterns of failure of adaptive/ablative proton therapy for early-stage, medically inoperable non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2011; 80:1350–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Westover KD, Seco J, Adams JA, et al. Proton SBRT for medically inoperable stage I NSCLC. J Thorac Oncol 2012;7:1021–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanemoto A, Okumura T, Ishikawa H, et al. Outcomes and prognostic factors for recurrence after high-dose proton beam therapy for centrally and peripherally located stage I non–small-cell lung cancer. Clin Lung Cancer 2014;15:e7–e12. [DOI] [PubMed] [Google Scholar]
- 34.Iwata H, Murakami M, Demizu Y, et al. High-dose proton therapy and carbon-ion therapy for stage I nonsmall cell lung cancer. Cancer 2010;116:2476–2485. [DOI] [PubMed] [Google Scholar]
- 35.Grutters JP, Kessels AG, Pijls-Johannesma M, et al. Comparison of the effectiveness of radiotherapy with photons, protons and carbonions for non-small cell lung cancer: A meta-analysis. Radiother Oncol 2010;95:32–40. [DOI] [PubMed] [Google Scholar]
- 36.Georg D, Hillbrand M, Stock M, et al. Can protons improve SBRT for lung lesions? Dosimetric considerations. Radiother Oncol 2008; 88:368–375. [DOI] [PubMed] [Google Scholar]
- 37.Kadoya N, Obata Y, Kato T, et al. Dose-volume comparison of proton radiotherapy and stereotactic body radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2011;79:1225–1231. [DOI] [PubMed] [Google Scholar]
- 38.Peeters A, Grutters J, Pijls-Johannesma M, et al. How costly is particle therapy? Cost analysis of external beam radiotherapy with carbon-ions, protons, and photons. Radiother Oncol 2010;95:45–53. [DOI] [PubMed] [Google Scholar]
- 39.Serago CF, Thornton AF, Urie MM, et al. Comparison of proton and x-ray conformal dose distributions for radiosurgery applications. Med Phys 1995;22:2111–2116. [DOI] [PubMed] [Google Scholar]
- 40.Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of SBRT for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24: 4833–4839. [DOI] [PubMed] [Google Scholar]
- 41.Bezjak A, Paulus R, Gaspar LE, et al. Primary study endpoint analysis for NRG Oncology/RTOG 0813 trial of stereotactic body radiotherapy (SBRT) for centrally located non-small cell lung cancer (NSCLC) Presented at: 57th Annual Meeting of the American Society for Radiation Oncology. October 18–21, 2015; San Antonio, TX. [Google Scholar]
- 42.Chang JY, Balter PA, Dong L, et al. Stereotactic body radiation therapy in centrally and superiorly located stage I or isolated recurrent non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2008; 72:967–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi W, Nichols JRC, Flampouri S, et al. Proton-based chemoradiation for synchronous bilateral non-small-cell lung cancers: A case report. Thorac Cancer 2013;4:198–202. [DOI] [PubMed] [Google Scholar]
- 44.Schaake-Koning C, van den Bogaert W, Dalesio O, et al. Effects of concomitant cisplatin and radiotherapy on inoperable non-small-cell lung cancer. N Engl J Med 1992;326:524–530. [DOI] [PubMed] [Google Scholar]
- 45.Furuse K, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol 1999;17:2692–2699. [DOI] [PubMed] [Google Scholar]
- 46.Curran WJ Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: Randomized phase III trial RTOG 9410. J Natl Cancer Inst 2011;103:1452–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nichols RC, Huh SN, Henderson RH, et al. Proton radiation therapy offers reduced normal lung and bone marrow exposure for patients receiving dose-escalated radiation therapy for unresectable stage III non-small-cell lung cancer: A dosimetric study. Clin Lung Cancer 2011;12:252–257. [DOI] [PubMed] [Google Scholar]
- 48.Chang JY, Komaki R, Lu C, et al. Phase 2 study of high-dose proton therapy with concurrent chemotherapy for unresectable stage III nonsmall cell lung cancer. Cancer 2011;117:4707–4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoppe BS. Phase II trial of concurrent chemotherapy and proton therapy for stage 3 NSCLC. Int J Particle Ther 2014;2:58. [Google Scholar]
- 50.Oshiro Y, Okumura T, Kurishima K, et al. High-dose concurrent chemo-proton therapy for stage III NSCLC: Preliminary results of a phase II study. J Radiat Res 2014;55:959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nguyen QN, Ly NB, Komaki R, et al. Long-term outcomes after proton therapy, with concurrent chemotherapy, for stage II-III inoperable non-small cell lung cancer. Radiother Oncol 2015; 115:367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sejpal S, Komaki R, Tsao A, et al. Early findings on toxicity of proton beam therapy with concurrent chemotherapy for nonsmall cell lung cancer. Cancer 2011;117:3004–3013. [DOI] [PubMed] [Google Scholar]
- 53.Gomez DR, Gillin M, Liao Z, et al. Phase 1 study of dose escalation in hypofractionated proton beam therapy for non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2013;86:665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McAvoy S, Ciura K, Wei C, et al. Definitive reirradiation for locoregionally recurrent non-small cell lung cancer with proton beam therapy or intensity modulated radiation therapy: Predictors of high-grade toxicity and survival outcomes. Int J Radiat Oncol Biol Phys 2014;90:819–827. [DOI] [PubMed] [Google Scholar]
- 55.McAvoy SA, Ciura KT, Rineer JM, et al. Feasibility of proton beam therapy for reirradiation of locoregionally recurrent non-small cell lung cancer. Radiother Oncol 2013;109:38–44. [DOI] [PubMed] [Google Scholar]
- 56.Rutenberg MS, Nichols RC, Flampouri S, et al. Proton radiotherapy in reirradiation for recurrent NSCLC. Int J Particle Ther 2014;1:774–775. [Google Scholar]
- 57.Foote RL, Stafford SL, Petersen IA, et al. The clinical case for proton beam therapy. Radiat Oncol 2012;7:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramaekers BL, Joore MA, Lueza B, et al. Cost effectiveness of modified fractionation radiotherapy versus conventional radiotherapy for unresected non-small-cell lung cancer patients. J Thorac Oncol 2013;8:1295–1307. [DOI] [PubMed] [Google Scholar]
- 59.Kutikova L, Bowman L, Chang S, et al. The economic burden of lung cancer and the associated costs of treatment failure in the United States. Lung Cancer 2005;50:143–154. [DOI] [PubMed] [Google Scholar]
- 60.Pompen M, Gok M, Novak A, et al. Direct costs associated with the disease management of patients with unresectable advanced non-small-cell lung cancer in the Netherlands. Lung Cancer 2009;64: 110–116. [DOI] [PubMed] [Google Scholar]
- 61.Barbera L, Walker H, Foroudi F, et al. Estimating the benefit and cost of radiotherapy for lung cancer. Int J Technol Assess Health Care 2004;20:545–551. [DOI] [PubMed] [Google Scholar]
- 62.Chouaid C, Atsou K, Hejblum G, et al. Economics of treatments for non-small cell lung cancer. Pharmacoeconomics 2009;27:113–125. [DOI] [PubMed] [Google Scholar]
- 63.Lievens Y, Kesteloot K, Van den Bogaert W. Chart in lung cancer: Economic evaluation and incentives for implementation. Radiother Oncol 2005;75:171–178. [DOI] [PubMed] [Google Scholar]
- 64.Vergnenegre A, Combescure C, Fournel P, et al. Cost-minimization analysis of a phase III trial comparing concurrent versus sequential radiochemotherapy for locally advanced non-small-cell lung cancer (GFPC-GLOT 95–01). Ann Oncol 2006;17:1269–1274. [DOI] [PubMed] [Google Scholar]
- 65.Park CH, Bonomi M, Cesaretti J, et al. Effect of radiotherapy planning complexity on survival of elderly patients with unresected localized lung cancer. Int J Radiat Oncol Biol Phys 2011; 81:706–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin SH, Wang L, Myles B, et al. Propensity score-based comparison of long-term outcomes with 3-dimensional conformal radiotherapy vs intensity-modulated radiotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys 2012;84:1078–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goitein M, Jermann M. The relative costs of proton and x-ray radiation therapy. Clin Oncol 2003;15:S37–S50. [DOI] [PubMed] [Google Scholar]
- 68.Lievens Y, Van den Bogaert W. Proton beam therapy: Too expensive to become true? Radiother Oncol 2005;75:131–133. [DOI] [PubMed] [Google Scholar]
- 69.Grutters JP, Abrams KR, de Ruysscher D, et al. When to wait for more evidence? Real options analysis in proton therapy. Oncologist 2011;16:1752–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grutters JP, Sculpher M, Briggs AH, et al. Acknowledging patient heterogeneity in economic evaluation: A systematic literature review. Pharmacoeconomics 2013;31:111–123. [DOI] [PubMed] [Google Scholar]


