Abstract
This study aimed to determine the association between severity of autism spectrum disorder (ASD) and cognitive, behavioral, and molecular measures in individuals with Fragile X Syndrome (FXS). Study inclusion criteria included individuals with FXS and 1) age 6 – 40 years, 2) full scale IQ < 84 and 3) language ≥ 3-word phrases. ASD symptom severity was determined by Autism Diagnostic Observation Schedule-2 (ADOS-2). Other measures identified non-verbal IQ, adaptive skills and aberrant behaviors. Molecular measures included blood FMR1 and CYFIP1 mRNA levels, FMRP and MMP9 levels. Analysis of Variance (ANOVA) and Spearman’s correlations were used to compare between ASD severity groups. Data from 54 individuals was included with no/mild(N=7), moderate(N=18) and severe(N=29) ASD. Individuals with high ASD severity had lower adaptive behavior scores (47.48 ± 17.49) than the no/mild group(69.00 ± 20.45, p=0.0366); they also had more challenging behaviors, lethargy and stereotypic behaviors. CYFIP1 mRNA expression levels positively correlated with the ADOS-2 comparison score(r2= 0.33, p=0.0349), with no significant correlations with other molecular markers. In conclusion, autism symptom severity is associated with more adverse cognitive, adaptive skills and specific behaviors in FXS while CYFIP1 mRNA expression levels may be a potential biomarker for severity of ASD in FXS.
Keywords: fragile X syndrome, Autism, CYFIP1 mRNA, FMRP, MMP9
1. Introduction
Fragile X syndrome (FXS) is the most common inherited form of intellectual disability (ID) with an estimated prevalence of 1 in 7000 males and 1 in11,000 females [1]. FXS is caused by a non-coding CGG trinucleotide expansion (>200 repeats) within the Fragile X messenger ribonucleprotein 1 (FMR1) gene, which typically results in methylation and gene silencing. Consequent deficiency/absence of the FMR1 protein (FMRP) leads to the clinical features of FXS including ID, language impairment, repetitive behaviors and social anxiety [2,3]. FXS is the leading single-gene cause of autism spectrum disorder (ASD), accounting for 2–5% of all cases of ASD [4].
FMRP plays a key role in regulating mRNA in pre- and post- synaptic neurons [3]. With the loss of FMRP, there is altered protein synthesis of several signaling effectors such as excitatory metabotropic glutamate receptor (mGluR), matrix metalloproteinase 9 (MMP-9), and mammalian target of rapamycin (mTOR) [5–7]. The inhibitory effects of FMRP on translation are also manifested through its interactions with the cytoplasmic FMRP interacting protein (CYFIP1) and the eukaryotic translation initiation factor (eIF4E) to form an inhibitory complex that regulates long-term synaptic plasticity [5]. Lack of FMRP, with loss of its role in gene modulation and synaptic plasticity, largely determine the FXS phenotype.
A co-occurring diagnosis of ASD is common in FXS and more common in males than females [8,9]. Previous studies suggest that individuals with a dual diagnosis of FXS and ASD have more significant developmental delays than those with FXS alone [10,11]. These delays likely contribute to the lower level of adaptive functioning seen in these individuals [12]. There is significant phenotypic overlap between the FXS and idiopathic ASD phenotypes [13]. Social avoidance, poor eye contact, tactile defensiveness, stereotypies including hand flapping or hand biting are common in both disorders. Studies have suggested the presence of autistic features in up to 90% of males with FXS, with up to 60% presenting with symptoms that meet diagnostic criteria for ASD [14]. In addition, perseverative speech and repetitive movements are often observed in both ASD and FXS. [15,16]. However, distinct features of idiopathic ASD that characterize it as a different condition from FXS alone include more severe difficulties with social communication, social reciprocity, strong fixated interests, rigidity and adherence to routines. All of these are the hallmark features of ASD and not necessarily seen in individuals with FXS alone. Individuals with ASD can also have a wide range of cognition, including normal and above-average cognition while this is rare in FXS. There is also a possible overlap between FXS and idiopathic ASD at the molecular level, with proteins associated with FXS and FMRP deficits being implicated in pathways of ASD. FMRP regulates the translation of approximately 30% of genes that when mutated can cause ASD [17]. For example, the CYFIP family of proteins has been associated with ASD as an autism-risk gene involved in axonogenesis and synaptogenesis, and FMRP is known to control the translation of CYFIP1 [18,19]. However, there has not been a clear identification of the relationship between FXS molecular markers and ASD severity, as well as other phenotypic features of FXS.
The aim of this study was to elucidate the phenotypic profile of individuals with FXS across varying degrees of ASD severity, in terms of cognitive, adaptive and behavioral functioning and molecular measures (specifically FMR1 and CYFIP1 mRNAs, FMRP and MMP9). We hypothesized that greater severity of ASD will be correlated with more severe behavioral impairments, including hyperactivity, and lower cognitive and adaptive functioning. We also postulated that levels of FMR1 mRNA and FMRP will be positively associated with adaptive functioning, cognition and ASD severity.
2. Materials and Methods
2.1. Study Sample and Procedure
Data for this study was obtained as part of an Institutional Human Subjects-approved clinical trial on individuals with FXS that commenced in 2019. Pertinent inclusion criteria included: 1. Diagnosis of FXS, confirmed by genetic testing showing the FMR1 full mutation (≥ 200 CGG repeats) 2. Age between 6 to 40 years inclusive, 3. Overall IQ as assessed by the Leiter-3 non-verbal scale of < 84, and 4. Oral production of at least 3-word phrases based on care-giver report and clinical assessment. Presence of co-occurring conditions common to FXS, including anxiety, and/or depression were not exclusion criteria; however, individuals with any serious chronic systemic medical illness were excluded (N=0).
Data from 54 participants was included in this analysis (age 13.9 ± 5.3, range = 6–27; 49 males (94.2%). ASD severity was divided into three groups: no/mild ASD (N=7), moderate (N=18) and high (N=29) based on ADOS-2 comparison scores. Descriptive statistics of study measures among participants are listed in Table 1. The sample consisted of predominantly males. More than half (29/50) of the participants had the full FXS mutation, while 10 participants were mosaic for methylation status and 8 were mosaic for CGG expansion size. The average non-verbal IQ of participants was 48.13 (SD 14.82) with a median of 46.00 and interquartile range of 35.00 to 58.50. Participants who received the BOSA did not differ from those who received the ADOS-2 in terms of age, mean non-verbal IQ score or any other measure.
Table 1.
Descriptive data of study participants.
| Variable | Mean (SD) or N (%) | ||
|---|---|---|---|
| Age (years) | 13.85 (5.28) | ||
| Male Gender | 49 (94.2%) | ||
| ADOS: Severity | No/Mild | 7 (13.0%) | |
| Moderate | 18 (33.3%) | ||
| High | 29 (53.7%) | ||
| Mean (SD) | Median (Q1, Q3) | ||
| MMP9 | 0.52 (0.35) | 0.41 (0.29, 0.64) | |
| CYFIP1 mRNA | 0.35 (0.18) | 0.32 (0.23, 0.39) | |
| FMR1 mRNA | 0.30 (0.48) | 0.07 (0, 0.53) | |
| FMRPrel* | 0.21 (0.24) | 0.12 (0.08, 0.24) | |
| ABC: Composite Score | 51.15 (29.88) | 49.00 (31, 65) | |
| ABC: Irritability | 16.25 (14.15) | 12.00 (4, 21) | |
| ABC: Lethargy | 7.66 (6.11) | 5.00 (4, 11) | |
| ABC: Stereotypy | 6.40 (5.12) | 5.00 (3, 9) | |
| ABC: Hyperactivity | 12.08 (7.62) | 11.00 (5, 17) | |
| ABC: Inappropriate Speech | 5.81 (3.23) | 6 (3, 9) | |
| ABC: Social Avoidance | 2.96 (2.89) | 3 (0, 4) | |
| ADAMS: Manic/Hyperactive Behavior | 7.44 (3.72) | 7 (4.25,10) | |
| ADAMS: Depressed Mood | 2.06 (2.46) | 1 (0, 3) | |
| ADAMS: Social Avoidance | 7.74 (4.15) | 7.5 (5,11) | |
| ADAMS: General Anxiety | 6.96 (4.05) | 7.5 (4, 10) | |
| ADAMS: Obsessive Compulsive Behavior | 2.80 (2.53) | 3 (0, 4) | |
| ADAMS: Total Score | 25 (14.15) | 24.50 (16, 35.75) | |
| VABS: Adaptive Behavior Composite | 51.22 (17.44) | 54 (34.5, 63.5) | |
| VABS: Communication | 44.20 (19.24) | 46 (24, 60) | |
| VABS: Daily Living Skills | 55.73 (24.03) | 59 (34.50, 72) | |
| VABS: Socialization | 52.43 (18.42) | 50 (38, 67.5) | |
| Leiter: Nonverbal IQ | 48.13 (14.82) | 46 (35, 58.5) | |
| SNAP - IV: ADHD Combined Total | 27.91 (12.31) | 30 (18,36) | |
| ELS: Narration | 56.71 (29.97) | 50.5 (37.25, 70.25) | |
| ELS: Conversation | 73.18 (36.55) | 69.5 (45, 94.75) | |
| ELS: Composite | 64.94 (30.74) | 61.25 (41.25, 80.62) | |
| PEDSQL: Total Score | 66.37 (13.25) | 67.81 (55.94, 75) | |
| ADOS-2: Comparison Score | 7.24 (2.14) | 8 (6, 9) | |
| CSHQ: Total Sleep Disturbance score | 46.42 (6.24) | 47 (44, 50) | |
Abbreviations: ABC: Aberrant Behavior Checklist (ABCFX), ADAMS: The Anxiety Depression and Mood Screen, VABS: Vineland Adaptive Behavioral Score, SNAP-IV: Swanson, Nolan and Pelham Questionnaire, ELS: Expressive Language Sampling, PEDSQL: Pediatric Quality of Life, ADOS-2: Autism Diagnostic Observation Scale 2, CSHQ: Child Sleep Habits Questionnaire
FMRP values are normalized to the mean of samples with normal alleles to represent a relative ratio. For example, 0.21 = 21% FMRP compared to patients with control alleles.
Study participants and/or their caregivers were administered various study measures over two days as part of assessments to determine eligibility and baseline for the clinical trial. Data from these baseline assessments were used in this manuscript.
2.2. Study Measures
Participants completed several standardized assessments and parent/caregiver completed questionnaires as part of study measures.
The Leiter-3 –
The Leiter-3 [20] is a standardized measure of cognition that is unique in that it is a nonverbally administered assessment of nonverbal cognition. The examiner administers the test using nonverbal cues (e.g.; affect, gestures, pantomime). The non-verbal IQ score obtained at the end of the assessment was utilized in the study.
Autism Diagnostic Observation Scale (ADOS-2) –
The ADOS-2 [21] is a semi-structured standardized assessment that uses developmentally appropriate social and object-based interactions to elicit symptoms of ASD in social communication, and repetitive and restrictive behaviors. Modules of the ADOS-2 were selected based on participant age and language levels and was administered by trained study personnel. Overall total scores were used to generate the calibrated comparison score (CCS); this score can also be used to generally describe the level of ASD severity (i.e., CCS score 1–2: minimal-to-no evidence, 3–4: low, 5–7: moderate, 8–10: high) as per protocol. The oldest age group was used to assign CCSs for those participants who were out of the norming age-range. Using this CCS, ASD severity groups were obtained; those in the ‘minimal-to-no evidence’ (N=5) and ‘low’ (N=2) group were combined to create a no/mild severity group with those in the ‘moderate’ and ‘high’ groups being classified as moderate and high severity ASD respectively. In the no/mild severity ASD group, 5 individuals did not meet criteria for ASD while 1 had low level of ASD symptoms. Those in the moderate and high severity groups both met DSM-5 criteria for ASD diagnosis as well.
Brief Observation of Symptoms of Autism (BOSA) –
The ongoing COVID-19 pandemic impacted the administration of the ADOS-2, necessitating the use of the BOSA [22] for 5 study participants. The BOSA is a standardized measure adapted from the ADOS-2, designed to evaluate for the presence of ASD based on an observed patient-caregiver interaction. The BOSA was administered by trained study personnel familiar with the ADOS-2. Following the BOSA administration, the ADOS-2 scoring sheet was used to derive total ADOS-2 scores and subsequently the standardized comparison score to generate ASD severity classification [23].
Vineland Adaptive Behavior Scales–Third Edition (VABS-3) –
The VABS-3 [24] is a well-validated and widely used assessment that measures everyday functioning in the domains of communication, social, and daily living skills. The comprehensive VABS-3 was administered in an interview format by research personnel and the informant was a parent/caregiver. Standard scores for each of the domains as well as an overall Adaptive Behavior Composite (ABC) standard score are obtained.
Expressive Language Sampling (ELS) –
The ELS is a validated measure to assess expressive language in individuals with FXS and ID [25]. ELS procedures are administered by a trained examiner who interacts with the participant to elicit samples of conversational language (topics including school and games) and narrative language (using a wordless picture book depicting a story). Using a script for prompts and his/her responses, the examiner minimizes their participation, maximizes the participant’s contribution, and avoids the use of examiner language that would constrain the participant’s talk. Recorded language samples are transcribed into text files that are then analyzed using specialized software to generate ELS scores representing the number of different words used in the first 50 complete and fully intelligible C-units (or the full sample if <50), with a C-unit defined as any verbal unit from a single word up to an independent clause and its associated modifiers for Narration, Conversation and for both sampling contexts combined. ELS has been used previously in several studies of individuals with FXS and shown to have high test-retest reliability and construct validity including in those with low cognitive abilities [26].
The Aberrant Behavior Checklist – Community Edition (ABC-C) –
The ABC-C is a caregiver-completed questionnaire measure of problem and interfering behaviors. It has been studied in individuals with FXS, with a FXS-specific factoring system of scoring validated [27], this was used in this study. This uses the 54-item responses to generate a composite score and 6 subscales; namely irritability, lethargy, social avoidance, stereotypic behavior, hyperactivity, and inappropriate speech. Higher scores represent greater challenging behaviors.
The Anxiety Depression and Mood Screen (ADAMS) –
The ADAMS [28] is a caregiver-completed questionnaire designed to assess for symptoms of anxiety, mood, and depression among individuals with ID. The ADAMS yields a total score and 5 subscale scores: general anxiety, social avoidance, depression, manic/hyperactive and obsessive-compulsive behavior. Higher scores represent presence of more symptoms of each sub-scale.
Swanson, Nolan and Pelham Questionnaire (SNAP-IV) –
The SNAP-IV [29] is a caregiver-completed standardized questionnaire based that measures symptom of attention-deficit hyperactivity disorder and gives sub-scale scores for inattention, hyperactivity/impulsivity and combined symptoms.
Pediatric Quality of Life Questionnaire (PedsQL) Parent Proxy –
The PedsQL [30] is a validated measure of quality of life of children and adolescents that is caregiver-reported. It provides an overall quality of life score and sub-scale scores in physical functioning, emotional functioning, social functioning and school functioning with higher scores indicating better quality.
Child Sleep Habits Questionnaire (CSHQ) –
The CHSQ [31] is a caregiver-completed standardized measure of sleep problems. The CHSQ provides a total sleep score and domain scores for sleep duration, routine, resistance, night awakenings, parasomnias, sleep-disordered breathing, morning behavior, daytime behavior and parental perception. Higher scores represent greater sleep problems.
2.3. Molecular measures
CGG repeat sizing:
CGG repeat sizing was carried out on genomic DNA isolated from 5ml of whole blood using standard procedures (Qiagen, Valencia, CA). A combination of PCR and Southern Blot analysis was used as previously reported [32,33]. Methylation status was used to determine the presence of mosaicism and was measured by densitometric analysis as described in Tassone et al. 1999. Methylation status included the percentage of methylation (% of methylated alleles) and in females, the activation ratio (AR), which expresses the percentage of cells carrying the normal allele on the active X chromosome [34].
FMR1 and CYFIP1 mRNA expression levels:
Total RNA was isolated from 2.5 ml of peripheral blood collected in PAXgene Blood RNA tubes using the PAXgene Blood RNA Kit (Qiagen, Valencia, CA, United States). RNA concentration was calculated using the Agilent 2,100 Bioanalyzer system. cDNA synthesis and mRNA expression levels were determined using real-time PCRs (qRT-PCR) with gene specific primers and probes; β-Glucuronidase (GUS) was used as reference gene [35]. qRT-PCR were run in duplicate and in three different concentrations as detailed in Tassone et al. 2000. Probe and primer sequences, PCR conditions and details of the method are as reported in Tassone et al, 2000 [35].
MMP9 levels:
MMP-9 activity was measured using the Human MMP-Magnetic Bead Panel 2 (Merck Millipore, Billerica, MA). Preparation of plasma samples and reagents was performed according to the manufacturer’s protocol. Plates were run on Luminex® (50 μL, 50 beads per bead set), quality controls, and negative and positive controls, were included; target samples were run in duplicate. The plates were run on Luminex® with xPONENT software; the Median Fluorescent Intensities (MFIs) were analyzed using the spline curve-fitting method for calculating the concentrations of MMP9 in each sample.
FMRP quantification:
The time-resolved fluorescence resonance energy transfer (TR-FRET) method was used to quantify FMRP using the Cisbio Human FMRP assay (Cisbio US, Bedford, MA) following the manufacturer’s protocol, with the following modifications: frozen peripheral blood mononuclear cells (PBMCs) were thawed in the presence of protease inhibitors; the Cisbio lysis buffer was supplemented with Benzonase (MilliporeSigma, Burlington, MA) and MgCl2; samples were incubated with fluorescent antibody conjugates by rocking overnight at room temperature. Relative FMRP levels were quantified by interpolating the percent change in fluorescence (ΔF%) on a standard curve generated by a control fibroblast fiducial line, as performed in Kim et al. (2019) [36], using either a four-factor fit (ΔF% > 65; 0 to 3 μg of the fiducial) or a linear fit (ΔF% ≤ 65; 0 to 0.4 μg of the fiducial); negative values were replaced by zero. Finally, after correcting for total protein loaded, as determined by BCA Protein Assay (Thermo Fisher Scientific, Rockford, IL), FMRP was normalized to the mean of samples with control alleles, FMRPre, (data not shown).
2.4. Statistical analysis
Statistical analyses of data were performed with an open-source R software (version 4.2). Descriptive statistics were expressed as mean ± standard deviation (SD) of mean or median ± interquartile range (Q1 = 1st Quartile, Q3 = 3rd Quartile) for continuous variables and proportion (%) for categorical variables. Prior to statistical inferential tests, the Shapiro-Wilk test was used to check if a continuous variable follows a normal distribution. For quantitative variables, group comparisons in means or medians were determined by analysis of variance (ANOVA) or Kruskal-Wallis test as appropriate, followed by Tukey’s honest significant difference (HSD) or Conover-Iman post-hoc tests [37]. Spearman correlations were used to measure the strength and direction of correlation between variables. Two-tailed p-values less than 0.05 were considered statistically significant as appropriate.
3. Results
Figure 1 shows a heatmap of correlations of ADOS-2 calibrated comparison scores (CCS) and various clinical and molecular measures. ASD severity as measured by the ADOS-2 CCS correlated negatively with the Leiter non-verbal IQ score (r = − 0.36, p = 0.0067) and the total sleep disturbance score on the CSHQ (r = −0.36, p = 0.0092). In addition, there was a positive correlation with CYFIP1 levels with higher ADOS-2 CCSs being correlated with higher levels of CYFIP1 (r = 0.33, p=0.0349). The relationship between CYFIP1 and ASD CCS is also depicted visually in Supplemental Figure 1. There were no other significant correlations with ASD severity noted with molecular measures (Figure 1).
Figure 1:
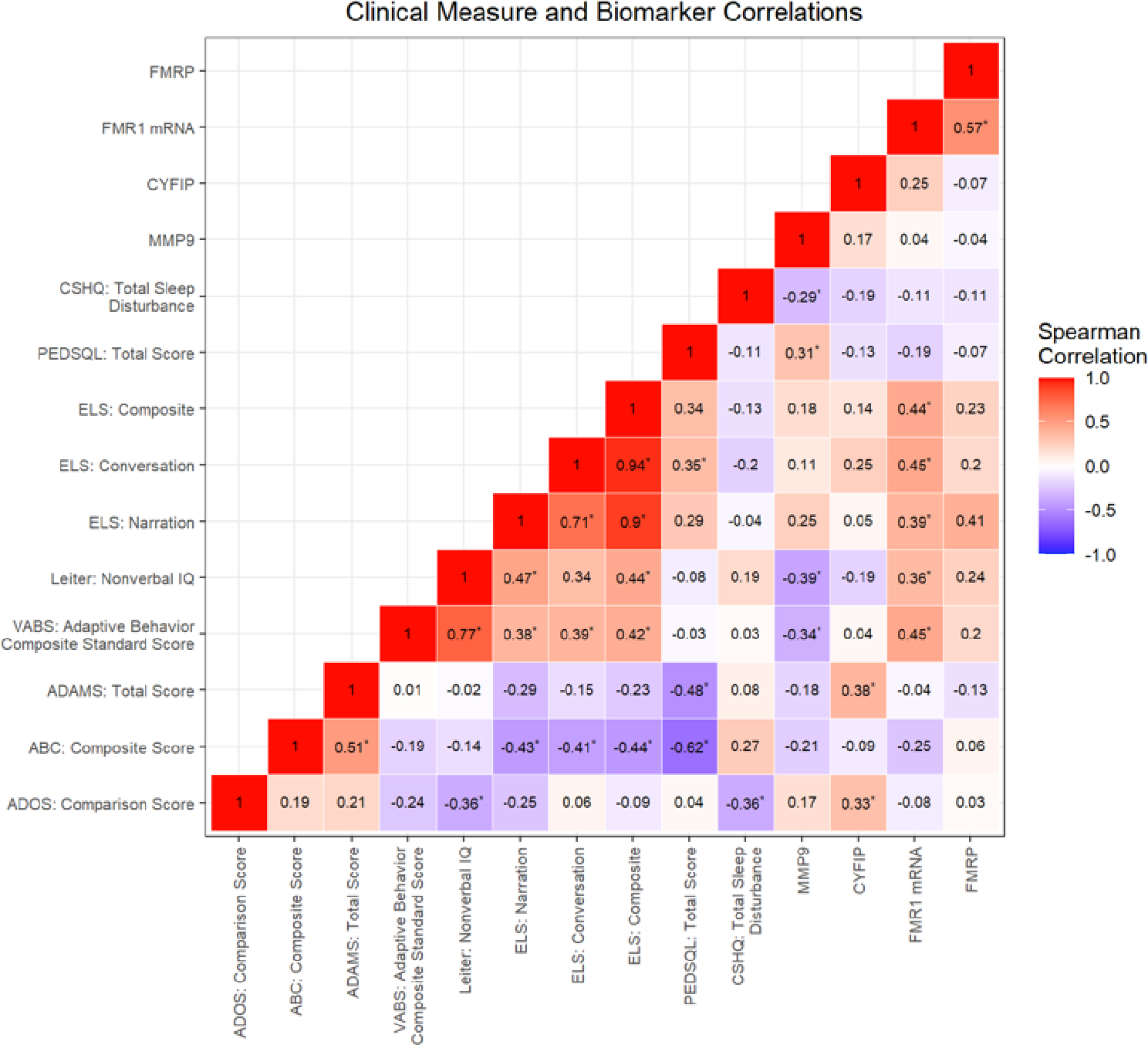
Correlation heat map of ADOS-2 calibrated comparison score (CCS) with clinical measures and molecular markers. Note: ADOS-2: Autism Diagnostic Observation Scale, *: p < 0.05
Additional positive correlations include the following: The FMR1 mRNA levels correlated positively with the ELS scores, the Leiter Nonverbal score and the VABS composite score which is expected since higher levels of mRNA typically means more FMRP and higher functioning levels and less involvement from FXS. The MMP9 levels which are elevated in those with FXS correlated negatively with the Leiter IQ, the VABS composite score and the CSHQ sleep disturbance score which is expected since the higher MMP9 level elevation means more involvement with FXS. However, the positive correlation with quality-of-life score is difficult to explain, but perhaps the lower functioning participants caused fewer problems for the family’s quality of life rather than the higher functioning participants who perhaps may have more behavioral problems or verbal requests. These correlations are shown in Figure 1.
Comparisons amongst participants across varying ASD severity (Table 2) demonstrate that individuals in the no/mild ASD group had significantly higher IQ scores (68.14 ± 12.16) compared to those from the moderate (44.78 ± 8.91, p = 0.00639) and high severity (45.38 ± 14.86, p=0.00134) groups. Similarly, individuals in the no/mild ASD group had significantly higher VABS-3 adaptive behavior composite scores (69.00 ± 20.45) than the high severity group (47.48 ± 17.49, p = 0.0366). Sub-scale scores on the VABS-3 socialization sub-scale were also higher in those individuals with no/mild severity ASD compared to those from the other two groups. ELS Narration scores were higher among individuals with no/mild severity ASD (95.25 ± 25.12) as compared to those with high severity (46.44 ± 20.54, p=0.00612). Supplemental Table 1 presents median values by ASD severity group for measures tested with the non-parametric Kruskal-Wallis test for group comparison. Figure 2 depicts these comparisons for selected measures.
Table 2.
Comparisons of ASD severity groups across clinical and molecular measures
| Variable [mean (SD)] | Autism severity | |||
|---|---|---|---|---|
| None/Mild (N=7) | Moderate (N=18) | High (N=29) | p-value | |
| MMP9 | 0.42 (0.37) | 0.53 (0.38) | 0.54 (0.33) | 0.5921 |
| CYFIP1 mRNA | 0.29 (0.08) | 0.33 (0.19) | 0.37 (0.19) | 0.4041 |
| FMR1 mRNA | 0.42 (0.23) | 0.16 (0.22) | 0.35 (0.58) | 0.2701 |
| FMRPrel | 0.12 (0.04) | 0.12 (0.12) | 0.20 (0.28) | 0.6391 |
| Age (years) | 14.13 (5.68) | 12.34 (5.23) | 14.68 (5.23) | 0.355 |
| ABC: Composite Score | 27.29 (19.91) | 57.44 (30.49) | 53.07 (29.46) | 0.0461 |
| ABC: Irritability | 8.86 (10.33) | 19.06 (13.44) | 16.29 (15.12) | 0.1391 |
| ABC: Lethargy | 2.86 (1.77) | 8.11 (6.65) | 8.57 (6.03) | 0.0141 |
| ABC: Stereotypy | 1.86 (2.12) | 6.28 (4.44) | 7.61 (5.50) | 0.026 |
| ABC: Hyperactivity | 8.43 (8.06) | 14.11 (7.23) | 11.68 (7.62) | 0.230 |
| ABC: Inappropriate Speech | 4.14 (2.73) | 6.22 (3.23) | 5.96 (3.32) | 0.335 |
| ABC: Social Avoidance | 1.14 (1.46) | 3.67 (3.41) | 2.96 (2.66) | 0.1251 |
| ADAMS: Manic/Hyperactive Behavior | 5.86 (3.34) | 7.62 (4.05) | 7.74 (3.63) | 0.485 |
| ADAMS: Depressed Mood | 2.00 (2.89) | 1.88 (2.22) | 2.19 (2.57) | 0.9381 |
| ADAMS: Social Avoidance | 5.43 (3.51) | 7.69 (4.35) | 8.37 (4.12) | 0.252 |
| ADAMS: General Anxiety | 5.86 (3.85) | 7.56 (4.52) | 6.89 (3.90) | 0.653 |
| ADAMS: Obsessive Compulsive Behavior | 1.00 (1.53) | 2.50 (2.50) | 3.44 (2.56) | 0.060 |
| ADAMS: Total Score | 20.14 (11.35) | 24.22 (15.55) | 26.66 (13.99) | 0.537 |
| VABS: Adaptive Behavior Composite Score | 69.00 (20.45) | 52.35 (13.59) | 47.48 (17.49) | 0.0491 |
| VABS: Communication | 58.80 (24.67) | 44.71 (16.14) | 41.38 (19.47) | 0.1661 |
| VABS: Daily Living Skills | 77.20 (28.75) | 58.53 (16.99) | 50.38 (25.14) | 0.0911 |
| VABS: Socialization | 75.60 (21.13) | 51.59 (18.29) | 48.93 (15.56) | 0.009 |
| Leiter: Nonverbal IQ | 68.14 (12.16) | 44.78 (8.91) | 45.38 (14.86) | 0.0031 |
| SNAP - IV: Combined Total | 21.43 (13.16) | 30.24 (10.62) | 28.10 (12.88) | 0.284 |
| ELS: Narration | 95.25 (25.12) | 59.25 (33.93) | 46.44 (20.54) | 0.008 |
| ELS: Conversation | 92.75 (25.20) | 67.83 (36.85) | 72.39 (38.62) | 0.3851 |
| ELS: Composite | 94.00 (24.90) | 63.54 (34.65) | 59.42 (26.74) | 0.122 |
| PEDSQL: Total Score | 69.80 (9.67) | 63.46 (14.41) | 67.26 (13.35) | 0.500 |
| CSHQ: Total Sleep Disturbance score | 47.33 (3.27) | 48.06 (7.97) | 45.28 (5.43) | 0.1511 |
Abbreviations: ABC: Aberrant Behavior Checklist, ADAMS: The Anxiety Depression and Mood Screen, VABS: Vineland Adaptive Behavioral Score, SNAP-IV: Swanson, Nolan and Pelham Questionnaire, ELS: Expressive Language Sampling, PEDSQL: Pediatric Quality of Life, CSHQ: Child Sleep Habits Questionnaire
Group comparisons were performed by Kruskal-Wallis test followed by Conover-Iman post-hoc tests for not normally distributed variables (see Supplemental Table 1 for medians (Q1, Q3)); otherwise, regular ANOVA followed by Tukey’s HSD post-hoc tests.
Figure 2:
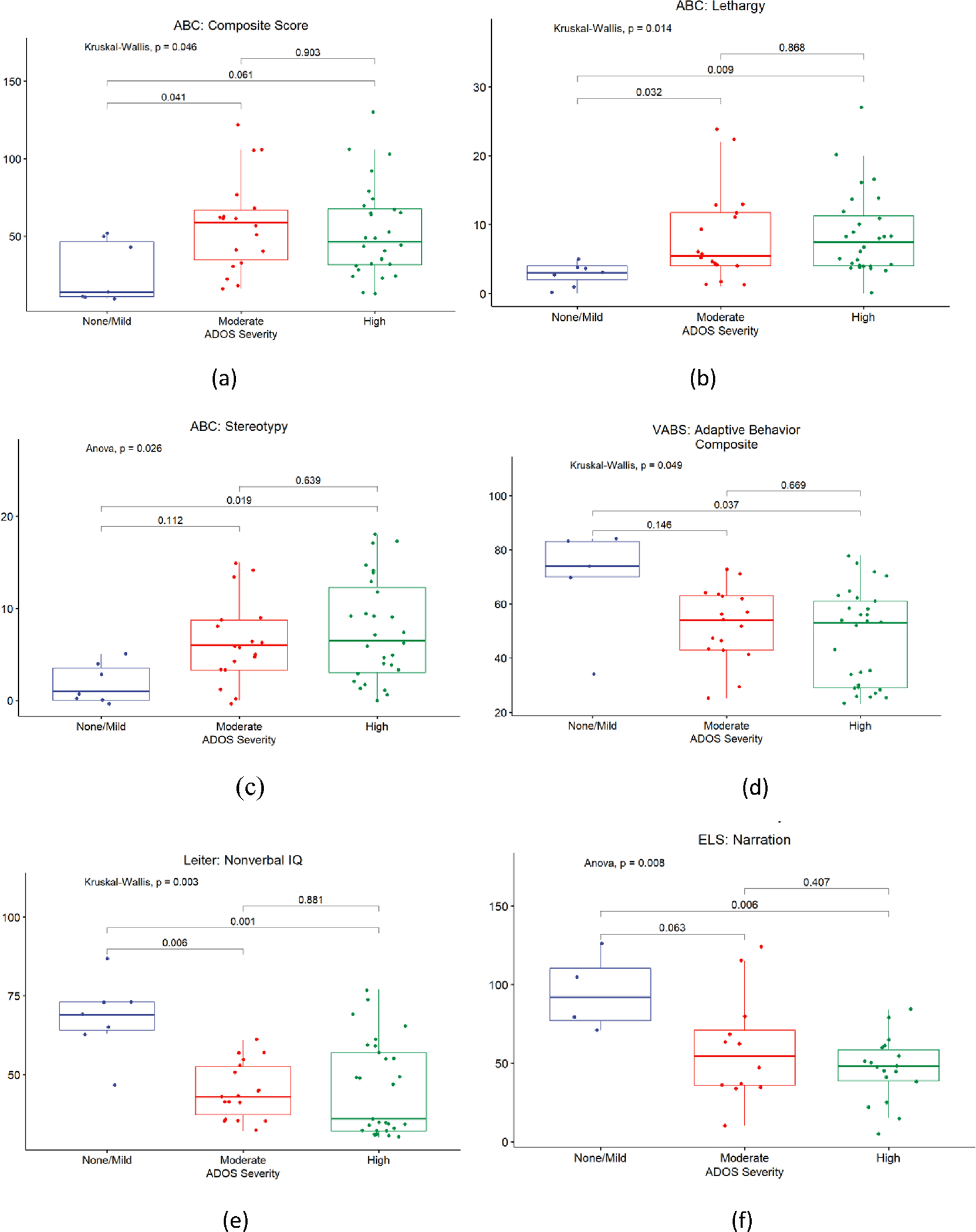
ASD severity versus selected clinical measures: (a) ASD severity correlated with ABC Composite Score (b) ASD severity correlated with ABC Lethargy Score (c) ASD severity correlated with ABC Stereotypy score (d) ASD severity correlated with VABS Adaptive Behavior Composite score (e) ASD severity correlated with Leiter: Nonverbal IQ (f) ASD severity correlated with ELS: Narration score
In terms of behavior, those with moderate severity ASD had more challenging behaviors with a higher composite score (57.44 ± 30.49) on the ABC-C as compared to individuals in the no/mild group (27.29 ± 19.91, p = 0.0408). Further, individuals in the no/mild severity group (1.86 ± 2.12) had fewer stereotypic behaviors compared to those in the high severity groups (7.61 ± 5.50, p=0.0194); this former group (2.86 ± 1.77) also had less lethargic behavior compared to those in the moderate (8.11 ± 6.65, p=0.032) and high (8.57 ± 6.03, p=0.0086) severity groups (Figure 2 and Table 2). Severity of ASD did not significantly associate with any other behavioral features including hyperactivity and inattention, nor with quality-of-life measures.
4. Discussion
This study sought to investigate the behavioral, cognitive, and molecular profiles of individuals with FXS with respect to the presence and severity of ASD. Our main results show that greater severity of ASD is associated with lower IQ scores, adaptive skills and expressive vocabulary. Those with more severe ASD also display more pronounced problem behaviors such as lethargy, social avoidance and stereotypic behavior. Interestingly, autism severity is also positively correlated with CYFIP1 mRNA expression levels.
Our findings in regards to limited cognitive (non-verbal IQ and language) and adaptive functioning in those with FXS and ASD as opposed to FXS alone are consistent with previous studies [38, 39]. In addition, we observed a decrease in cognitive and behavioral functioning with increasing severity of ASD. It is possible that ASD contributes additively to the language impairments seen in FXS, although the reverse is also possible i.e FXS individuals with low cognitive abilities being more likely to have ASD-like symptoms. Numerous behavior stereotypies (i.e., repetitive or restricted interest behaviors) have been observed in patients with FXS [40]. The higher levels of stereotypy scores seen in children with more severe ASD in our study possibly reflects an additive effect of ASD as stereotypic behaviors are also a hallmark of ASD. Lethargy is a behavior finding that is not as commonly associated with FXS and interestingly, lethargy scores were higher in individuals with more severe ASD. Alternatively, this could be related to the lower functioning levels of these individuals due to their lower intellectual and adaptive skills; but lethargy needs to be further studied in FXS, regardless of ASD severity. Social avoidance worsened with greater ASD severity in our study. Although problems related to eye contact and social interaction are present in FXS, the presence of ASD takes an additional toll on social impairment in individuals with FXS.
We did not observe any significant correlation between the severity of ASD and either FMRP or FMR1 mRNA levels, which was not a surprise, since such levels are very low in those with FXS. However, increased CYFIP1 mRNA levels were potentially associated with the severity of ASD based on correlational analysis, although categorical analysis based on groups of varying ASD severity did not illustrate significant differences. The CYFIP1 gene, located in the chromosomal region 15q11.2 plays a role in actin dynamics and protein synthesis through binding with specific mRNAs which in turn affect synaptic development implicated in the pathogenesis of ASD [41]. The CYFIP family of genes has been associated with ASD [18]; it is thus possible that excess levels of CYFIP1 may interfere with optimal synaptic functioning. A paper by Noorozi et al also showed increased levels of both CYFIP1 and CYFIP2 in individuals with ASD as compared to controls without ASD [42]. These results lend weight to the possible role of this family of genes in the pathogenesis of ASD. In our study, we observed this similar relationship in individuals who have FXS as well; although it is hard to delineate if this relationship is due to ASD or FXS or a combination of both. For example, the higher levels of CYFIP1 in our sample could be related to lower FMRP levels due to the presence of FXS. Since FMRP is a known regulator of CYFIP1, lower levels of FMRP as seen in FXS can lead to higher levels of CYFIP1. While the literature on CYFIP1 expression in FXS is limited, decreased CYFIP1 mRNA levels in FXS individuals with the Prader-Willi like phenotype (PWP) has been previously reported; however, this phenotype likely has unique molecular underpinnings that does not apply to the typical individual with FXS [43]. None of the individuals in the current study had the PWP. Another study of Fragile X premutation alleles (55 – 200 CGG repeats) in human cells showed elevated levels of CYFIP1 expression [44]. Given emerging literature suggesting a higher incidence of ASD in premutation carriers [45], the role of CYFIP1 expression in ASD and in Fragile X mutation states needs further investigation. Nonetheless, our preliminary results suggest a potential role for the use of CYFIP1 mRNA levels as a potential biomarker for the presence of ASD. This area needs further research with larger samples of individuals with FXS across varying ASD severity. This could be especially useful for infants and young children with FXS who are ineligible for or awaiting a diagnostic evaluation for ASD. Identifying these children with FXS who are at risk for ASD at a younger age could facilitate more intensive early intervention, especially focusing on language and cognitive development.
Clinical implications of our study include reinforcing the importance of an evaluation for and diagnosis of ASD in individuals with FXS with difficulties with social communication, language delays and stereotypic behaviors. Given that presence of severe ASD is associated with lower cognitive and adapting functioning and expressive language, selected individuals with FXS may warrant targeted intervention focusing on communication and adaptive skills, starting from childhood. Use of CYFIP levels as a marker for the presence and severity of ASD and potentially identify children for intensive intervention is also an area for future research. Future studies can further investigate correlations between cognitive and behavioral measures and molecular markers.
Strengths of our study include the use of comprehensive phenotypic measures of behavior and adaptive skills combined with biomarker analysis. We sought to capture the spectrum of ASD severity and how it associates with the phenotype as opposed to a dichotomous classification of presence/absence of ASD alone so as to capture the real-life variation of the phenotype better. However, our study has several limitations. First, the current study sample is not representative of the general FXS population with the small proportion of females sampled. This is likely due to the study’s inclusion criteria specifying an IQ < 84, with females with FXS more likely to have average cognitive functioning. Inclusion of a greater number of females in future studies could help address this. Second, the behavior measures used in the study were primarily caregiver completed and there may be responder bias introduced due to their knowledge of their child’s ASD severity status. Despite this, the results related to molecular measures and standardized assessments add reliability to the results. The use of the BOSA in lieu of the ADOS-2 for a small number of subjects was also unavoidable due to pandemic-related measures. Third, the study comprised an overall small total number of participants, of which the proportion of subjects with mild severity autism was relatively small and so was the number with FMRP values. This could have limited some of our correlations that probably would have been positive with greater numbers. Such examples include the relationship between FMRP and IQ or FMRP and the severity of ASD. Future studies should have larger sample sizes with intentional recruitment of subjects with mild severity autism to address this. Lastly, the current sample does not include a control group of individuals without FXS or ASD and this may limit interpretation of the implications of the molecular correlations in our results. Addition of such a group in future studies should be done to facilitate this.
5. Conclusion
In conclusion, this study adds to the literature describing the varying cognitive and behavior profiles of individuals with FXS depending on the presence and severity of ASD. The role of CYFIP1 mRNA in individuals with FXS is also an area for further research. Assessing for the presence of ASD throughout childhood and evaluating further where necessary – especially in terms of adaptive/aberrant behavior, social anxiety, non-verbal IQ, and language ability—will be important in the management of children with FXS. This can be useful for prognostication of their developmental trajectory as well as identifying individuals who need greater intervention and support throughout their childhood and adolescent period.
Supplementary Material
Table S1: Comparisons of ASD severity groups across measures for non normally distributed measures
Figure S1: Scatterplot of CYFIP1 levels against ADOS-2 comparison scores
Funding:
This study was supported by the Azrieli Foundation and the MIND Institute IDDRC from NICHD P50 HD103526.
Footnotes
Conflicts of Interest: RH has received funding from the Azrieli Foundation to carry out metformin studies in those with FXS. The other authors have indicated that they have no conflicts of interest to declare that are relevant to the content of this article.
Institutional Review Board Statement: The study was approved by all the Institutional Review Board of the MIND Institute (protocol code 1068417, approved 11/01/2017).
Informed Consent Statement: Written informed consent was obtained from all individual participants included in the study.
Data Availability Statement:
Data can be shared on request and also available on www.clinicaltrials.org
References:
- 1.Hunter J, Rivero-Arias O, Angelov A, Kim E, Fotheringham I, Leal J. Epidemiology of fragile X syndrome: A systematic review and meta-analysis. Am J Med Genet Part A. 2014. 164A:1648–1658 [DOI] [PubMed] [Google Scholar]
- 2.Saldarriaga W, Tassone F, Gonzalez-Teshima LY, et al. Fragile X syndrome. Colomb Med (Cali). 2014;45(4):190–198. [PMC free article] [PubMed] [Google Scholar]
- 3.Bagni C, Tassone F, Neri G, et al. Fragile X syndrome: causes, diagnosis, mechanisms, and therapeutics. J Clin Invest. 2012;122(12):4314–4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaufmann WE, Kidd SA, Andrews HF, et al. Autism Spectrum Disorder in Fragile X Syndrome: Cooccurring Conditions and Current Treatment. Pediatrics. 2017;139(Suppl 3):S194–s206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Napoli I, Mercaldo V, Boyl PP, et al. The fragile X syndrome protein represses activity-dependent translation through CYFIP1, a new 4E-BP. Cell. 2008;134(6):1042–1054. [DOI] [PubMed] [Google Scholar]
- 6.Bagni C, Zukin RS. A Synaptic Perspective of Fragile X Syndrome and Autism Spectrum Disorders. Neuron. 2019;101(6):1070–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romagnoli A, Di Marino D. The Use of Peptides in the Treatment of Fragile X Syndrome: Challenges and Opportunities. Front Psychiatry. 2021;12:754485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Budimirovic D, Haas-Givler B, Blitz R, et al. Autism Spectrum Disorder in Fragile X Syndrome. https://fragilex.org/wp-content/uploads/2012/08/Autism-Spectrum-Disorder-in-Fragile-X-Syndrome-2014-Nov.pdf. Published 2014 (updated 2020). Accessed May 18., 2021. [Google Scholar]
- 9.Klusek J, Martin GE, Losh M. Consistency between research and clinical diagnoses of autism among boys and girls with fragile X syndrome. J Intellect Disabil Res. 2014;58(10):940–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thurman AJ, McDuffie A, Hagerman RJ, et al. Language Skills of Males with Fragile X Syndrome or Nonsyndromic Autism Spectrum Disorder. J Autism Dev Disord. 2017;47(3):728–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thurman AJ, McDuffie A, Kover ST, et al. Autism Symptomatology in Boys with Fragile X Syndrome: A Cross Sectional Developmental Trajectories Comparison with Nonsyndromic Autism Spectrum Disorder. J Autism Dev Disord. 2015;45(9):2816–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raspa M, Franco V, Bishop E, et al. A comparison of functional academic and daily living skills in males with fragile X syndrome with and without autism. Res Dev Disabil. 2018;78:1–14. [DOI] [PubMed] [Google Scholar]
- 13.Hagerman RJH PJ Fragile X Syndrome and Premutation Disorders. London: Mac Keith Press; 2020. [Google Scholar]
- 14.Niu M, Han Y, Dy ABC, Du J, et al. Autism Symptoms in Fragile X Syndrome. Journal of child neurology. 2017;32(10):903–909. [DOI] [PubMed] [Google Scholar]
- 15.Martin GE, Roberts JE, Helm-Estabrooks N, et al. Perseveration in the connected speech of boys with Fragile X syndrome with and without autism spectrum disorder. Am J Intellect Dev Disabil. 2012;117(5):384–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolff JJ, Bodfish JW, Hazlett HC, et al. Evidence of a distinct behavioral phenotype in young boys with fragile X syndrome and autism. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51(12):1324–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iossifov I, Ronemus M, Levy D, et al. De novo gene disruptions in children on the autistic spectrum. Neuron. 2012. Apr 26;74(2):285–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abekhoukh S, Bardoni B. CYFIP family proteins between autism and intellectual disability: links with Fragile X syndrome. Front Cell Neurosci. 2014;8:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ingason A, Kirov G, Giegling I, et al. Maternally derived microduplications at 15q11-q13: implication of imprinted genes in psychotic illness. Am J Psychiatry. 2011;168(4):408–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roid GH, Koch C. Leiter-3: Nonverbal Cognitive and Neuropsychological Assessment. [Google Scholar]
- 21.Lord C, Rutter M, DiLavore P, et al. Autism Diagnostic Observation Schedule Second Edition (ADOS-2) Manual (Part 1): Modules 1–4. Torrance, CA: Western Psychological Services. 2012. [Google Scholar]
- 22.Dow D, Holbrook A, Toolan C, et al. The Brief Observation of Symptoms of Autism (BOSA): Development of a New Adapted Assessment Measure for Remote Telehealth Administration Through COVID-19 and Beyond. J Autism Dev Disord. 2021:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dow D, Holbrook A, Toolan C et al. The Brief Observation of Symptoms of Autism (BOSA): Development of a New Adapted Assessment Measure for Remote Telehealth Administration Through COVID-19 and Beyond. J Autism Dev Disord 52, 5383–5394 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sparrow S, Cicchetti D, Balla D. Vineland adaptive behavior scales: (Vineland II), survey interview form/caregiver rating form. Livonia, MN: Pearson Assessments. 2005. [Google Scholar]
- 25.Abbeduto L, Berry-Kravis E, Sterling A, et al. Expressive language sampling as a source of outcome measures for treatment studies in fragile X syndrome: feasibility, practice effects, test-retest reliability, and construct validity. J Neurodev Disord. 2020;12(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berry-Kravis E, Doll E, Sterling A, et al. Development of an expressive language sampling procedure in fragile X syndrome: a pilot study. J Dev Behav Pediatr. 2013;34(4):245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sansone SM, Widaman KF, Hall SS, et al. Psychometric study of the Aberrant Behavior Checklist in Fragile X Syndrome and implications for targeted treatment. J Autism Dev Disord. 2012;42(7):1377–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esbensen AJ, Rojahn J, Aman MG, et al. Reliability and validity of an assessment instrument for anxiety, depression, and mood among individuals with mental retardation. J Autism Dev Disord. 2003;33(6):617–629. [DOI] [PubMed] [Google Scholar]
- 29.Hall CL, Guo B, Valentine AZ, et al. The Validity of the SNAP-IV in Children Displaying ADHD Symptoms. Assessment. 2020;27(6):1258–1271. [DOI] [PubMed] [Google Scholar]
- 30.Varni JW, Seid M, Rode CA. The PedsQL™: measurement model for the pediatric quality of life inventory. Medical care. 1999:126–139. [DOI] [PubMed] [Google Scholar]
- 31.Owens JA, Spirito A, McGuinn M. The Children’s Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23(8):1043–1051. [PubMed] [Google Scholar]
- 32.Tassone F, Pan R, Amiri K, et al. A rapid polymerase chain reaction-based screening method for identification of all expanded alleles of the fragile X (FMR1) gene in newborn and high-risk populations. J Mol Diagn. 2008;10(1):43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Filipovic-Sadic S, Sah S, Chen L, et al. A novel FMR1 PCR method for the routine detection of low abundance expanded alleles and full mutations in fragile X syndrome. Clinical chemistry. 2010;56(3):399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tassone F, Hagerman RJ, Iklé DN, et al. FMRP expression as a potential prognostic indicator in fragile X syndrome. Am J Med Genet. 1999;84(3):250–261. [PubMed] [Google Scholar]
- 35.Tassone F, Hagerman RJ, Taylor AK, et al. Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in the fragile-X syndrome. Am J Hum Genet. 2000;66(1):6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim K, Hessl D, Randol JL, et al. Association between IQ and FMR1 protein (FMRP) across the spectrum of CGG repeat expansions. PLoS One. 2019;14(12):e0226811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conover WJ. Practical nonparametric statistics: john wiley & sons; 1999. [Google Scholar]
- 38.Skinner M, Hooper S, Hatton DD, et al. Mapping nonverbal IQ in young boys with fragile X syndrome. Am J Med Genet A. 2005;132a(1):25–32. [DOI] [PubMed] [Google Scholar]
- 39.Thurman AJ, Hoyos Alvarez C. Language Performance in Preschool-Aged Boys with Nonsyndromic Autism Spectrum Disorder or Fragile X Syndrome. J Autism Dev Disord. 2020;50(5):1621–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oakes A, Thurman AJ, McDuffie A, et al. Characterising repetitive behaviours in young boys with fragile X syndrome. J Intellect Disabil Res. 2016;60(1):54–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Domínguez-Iturza N, Lo AC, Shah D, et al. The autism-and schizophrenia-associated protein CYFIP1 regulates bilateral brain connectivity and behaviour. Nature communications. 2019;10(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noroozi R, Omrani MD, Sayad A, et al. Cytoplasmic FMRP interacting protein 1/2 (CYFIP1/2) expression analysis in autism. Metabolic Brain Disease. 2018. Aug;33:1353–8. [DOI] [PubMed] [Google Scholar]
- 43.Nowicki ST, Tassone F, Ono MY, et al. The Prader-Willi phenotype of fragile X syndrome. J Dev Behav Pediatr. 2007;28(2):133–138. [DOI] [PubMed] [Google Scholar]
- 44.Handa V, Goldwater D, Stiles D, et al. Long CGG-repeat tracts are toxic to human cells: implications for carriers of Fragile X premutation alleles. FEBS Lett. 2005;579(12):2702–2708. [DOI] [PubMed] [Google Scholar]
- 45.Aishworiya R, Protic D, Hagerman R. Autism spectrum disorder in the fragile X premutation state: possible mechanisms and implications. J Neurol. 2022;269(9):4676–4683. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Comparisons of ASD severity groups across measures for non normally distributed measures
Figure S1: Scatterplot of CYFIP1 levels against ADOS-2 comparison scores
Data Availability Statement
Data can be shared on request and also available on www.clinicaltrials.org


