Abstract
There are limited data on influenza vaccination coverage among pregnant people in the United States during the coronavirus disease 2019 (COVID-19) pandemic. Within the Vaccine Safety Datalink, we conducted a retrospective cohort study to examine influenza vaccination coverage during the 2016–2017 through the 2021–2022 influenza seasons among pregnant people aged 18–49 years. Using influenza vaccines administered through March each season, we assessed crude coverage by demographic and clinical characteristics. Annual influenza vaccination coverage increased from the 2016–2017 season (63.0%) to a high of 71.0% in the 2019–2020 season. After the start of the COVID-19 pandemic, it decreased to a low of 56.4% (2021–2022). In each of the six seasons, coverage was lowest among pregnant people aged 18–24 years and among non-Hispanic Black pregnant people. The 2021–2022 season had the lowest coverage across all age and race and ethnicity groups. The recent decreases highlight the need for continued efforts to improve coverage among pregnant people.
There are limited data on influenza vaccination coverage among pregnant people in the United States during the coronavirus disease 2019 (COVID-19) pandemic.1,2 We report influenza vaccine coverage among pregnant people within the Vaccine Safety Datalink3 during the 2016–2017 through the 2021–2022 influenza seasons by demographic and clinical characteristics.
METHODS
Comprehensive methodology is provided in Appendix 1, available online at http://links.lww.com/AOG/D258. Data for this analysis were obtained from eight Vaccine Safety Datalink sites in six U.S. states (California, Colorado, Minnesota, Oregon, Washington, and Wisconsin). This research was approved by the Centers for Disease Control and Prevention and the IRBs of the participating Vaccine Safety Datalink sites. Influenza vaccination coverage was evaluated retrospectively for the August–March periods of the 2016–2017 through the 2021–2022 influenza seasons.
Each influenza season’s eligible population consisted of pregnant people aged 18–49 years. Pregnancies were identified using a validated Vaccine Safety Datalink algorithm,4 and patient age was determined as of August 1 each year. Pregnancies that ended in spontaneous or induced abortion, pregnancies that ended before 14 weeks of gestation regardless of the outcome, and pregnancies for which the Vaccine Safety Datalink algorithm could not identify a pregnancy outcome were excluded from analyses. Additional inclusion and exclusion criteria, as well as handling of pregnancies that spanned two seasons and people with multiple pregnancies per season, are described in Appendix 1 (http://links.lww.com/AOG/D258).
All influenza vaccines administered in the eligible study population were identified from standardized Vaccine Safety Datalink files (Appendix 2, available online at http://links.lww.com/AOG/D258). Influenza vaccines administered between July and March were included in season-specific analyses, irrespective of the administration relative to pregnancy (ie, before, during, or after pregnancy).
For each season, crude influenza vaccination coverage was calculated by dividing the number of pregnant people vaccinated by the total number of pregnant people. Coverage was calculated by age group, self-reported race and ethnicity, health care utilization, pregnancy comorbidities or complications, and conditions associated with increased risk of severe illness and complications from influenza. Coverage strata are defined in Appendix 1 (http://links.lww.com/AOG/D258).
In each season, we calculated influenza vaccination coverage according to the calendar month of pregnancy start. In these analyses, coverage was calculated by dividing the number of pregnant people vaccinated among those with a pregnancy start in a given calendar month. Additional details are available in Appendix 1 (http://links.lww.com/AOG/D258). Among people vaccinated, we examined the timing relative to pregnancy: before pregnancy, during pregnancy by trimester (before 14 weeks of gestation, 14–27 weeks, after 27 weeks), at delivery (63 days of pregnancy end date), and after pregnancy.
RESULTS
Across the 2016–2017 through the 2021–2022 influenza seasons, the number of pregnant people identified in the Vaccine Safety Datalink ranged from 128,267 to 139,927. Overall, crude influenza vaccination coverage ranged from 56.4% to 71.0% in the influenza seasons examined, with coverage peaking in 2019–2020 and declining over the subsequent two seasons (Table 1). In all six seasons, coverage was highest among pregnant people aged 35–49 years, ranging from 64.2% to 75.3%; coverage was lowest among pregnant people aged 18–24 years, ranging from 43.7% to 62.5% (Appendix 3, available online at http://links.lww.com/AOG/D258). Coverage was lowest for all age groups in the 2021–2022 influenza season. Influenza vaccination coverage was highest among non-Hispanic Asian pregnant people in all seasons, ranging from 71.3% to 81.1% (Fig. 1). Among those with known race and ethnicity, coverage was lowest in all seasons among non-Hispanic Black pregnant people, ranging from 35.5% to 51.3%. Coverage was lowest for all known race and ethnicity categories in the 2021–2022 influenza season. In all seasons, influenza vaccination coverage increased with increasing clinical encounters (Appendix 4, available online at http://links.lww.com/AOG/D258). Influenza vaccination coverage among pregnant people diagnosed with prespecified pregnancy comorbidities or complications (Appendix 5, available online at http://links.lww.com/AOG/D258) is presented in Appendix 6, available online at http://links.lww.com/AOG/D258.
Table 1.
Demographic and Clinical Characteristics and Influenza Vaccination Status of Pregnant People in the 2016–2017 Through the 2021–2022 Influenza Seasons
| Influenza Season | ||||||
|---|---|---|---|---|---|---|
| 2016–2017 | 2017–2018 | 2018–2019 | 2019–2020 | 2020–2021 | 2021–2022 | |
| Pregnant people* | 128,267 | 133,056 | 136,441 | 135,554 | 139,927 | 139,478 |
| Age group (y) | ||||||
| 18–24 | 20,580 (16.0) | 19,831 (14.9) | 19,550 (14.3) | 18,428 (13.6) | 18,432 (13.2) | 17,152 (12.3) |
| 25–34 | 80,042 (62.4) | 83,274 (62.6) | 85,443 (62.6) | 85,005 (62.7) | 88,360 (63.1) | 87,219 (62.5) |
| 35–49 | 27,645 (21.6) | 29,951 (22.5) | 31,448 (23.0) | 32,121 (23.7) | 33,135 (23.7) | 35,107 (25.2) |
| Race and ethnicity | ||||||
| Asian, non-Hispanic | 20,162 (15.7) | 21,119 (15.9) | 22,231 (16.3) | 22,156 (16.3) | 22,406 (16.0) | 22,695 (16.3) |
| Black, non-Hispanic | 8,067 (6.3) | 8,444 (6.3) | 8,662 (6.3) | 8,901 (6.6) | 9,456 (6.8) | 9,336 (6.7) |
| Hispanic | 43,252 (33.7) | 44,967 (33.8) | 46,354 (34.0) | 46,456 (34.3) | 48,383 (34.6) | 50,011 (35.9) |
| White, non-Hispanic | 48,182 (37.6) | 48,988 (36.8) | 49,227 (36.1) | 48,065 (35.5) | 49,000 (35.0) | 46,467 (33.3) |
| None of the above | 5,789 (4.5) | 6,149 (4.6) | 6,541 (4.8) | 6,174 (4.6) | 6,403 (4.6) | 6,564 (4.7) |
| Unknown or missing | 2,815 (2.2) | 3,389 (2.5) | 3,426 (2.5) | 3,802 (2.8) | 4,279 (3.1) | 4,405 (3.2) |
| Pregnancy comorbidities(s)† | 61,716 (48.1) | 67,955 (51.1) | 71,721 (52.6) | 74,095 (54.7) | 78,649 (56.2) | 64,874 (46.5) |
| High-risk condition(s)‡ | 44,543 (34.7) | 47,409 (35.6) | 48,565 (35.6) | 48,660 (35.9) | 51,120 (36.5) | 44,619 (32.0) |
| Health care utilization (no. of encounters)§ | ||||||
| Fewer than 6 | 6,761 (5.3) | 7,026 (5.3) | 7,294 (5.3) | 11,896 (8.8) | 13,166 (9.4) | 10,293 (7.4) |
| 6–10 | 22,597 (17.6) | 23,302 (17.5) | 23,361 (17.1) | 25,581 (18.9) | 30,832 (22.0) | 27,703 (19.9) |
| 11–20 | 57,786 (45.1) | 58,915 (44.3) | 60,258 (44.2) | 59,529 (43.9) | 58,587 (41.9) | 60,171 (43.1) |
| More than 20 | 41,123 (32.1) | 43,813 (32.9) | 45,528 (33.4) | 38,548 (28.4) | 37,342 (26.7) | 41,311 (29.6) |
| Influenza vaccine receipt‖ | 80,787 (63.0) | 85,762 (64.5) | 92,354 (67.7) | 96,202 (71.0) | 92,436 (66.1) | 78,683 (56.4) |
Data are n or n (column %).
Eligible pregnancies spanned at least 1 day during the August 1 through March 31 period of an influenza season and met enrollment criteria.
Individuals with at least one complication or comorbidity. Predefined pregnancy comorbidities or complications included the following, identified in the electronic health record during the pregnancy: diabetes or hypertension; liver disorders; renal disease; nervous system, respiratory, or digestive system diseases complicating pregnancy; obesity complicating pregnancy; alcohol use, drug use, smoking complicating pregnancy (Appendix 5, available online at http://links.lww.com/AOG/D258).
Individuals with at least one condition. Predefined conditions associated with increased risk of severe illness and complications from influenza (high-risk conditions) included the following, identified in the electronic health record during the pregnancy: cardiac, cerebrovascular, chronic pulmonary, liver, peptic ulcer, peripheral vascular, or renal diseases; diabetes; nondiabetic metabolic, endocrine, immunosuppressive, or neurologic or musculoskeletal disorders; hemoglobinopathies; long-term medication use; malignancy; morbid obesity (Appendix 7, available online at http://links.lww.com/AOG/D258).
Number of medical encounters in the July 1 through June 30 period overlapping the influenza season of interest.
Receipt of same-season influenza vaccine between August 1 and March 31, including before, during, or after pregnancy.
Fig. 1.
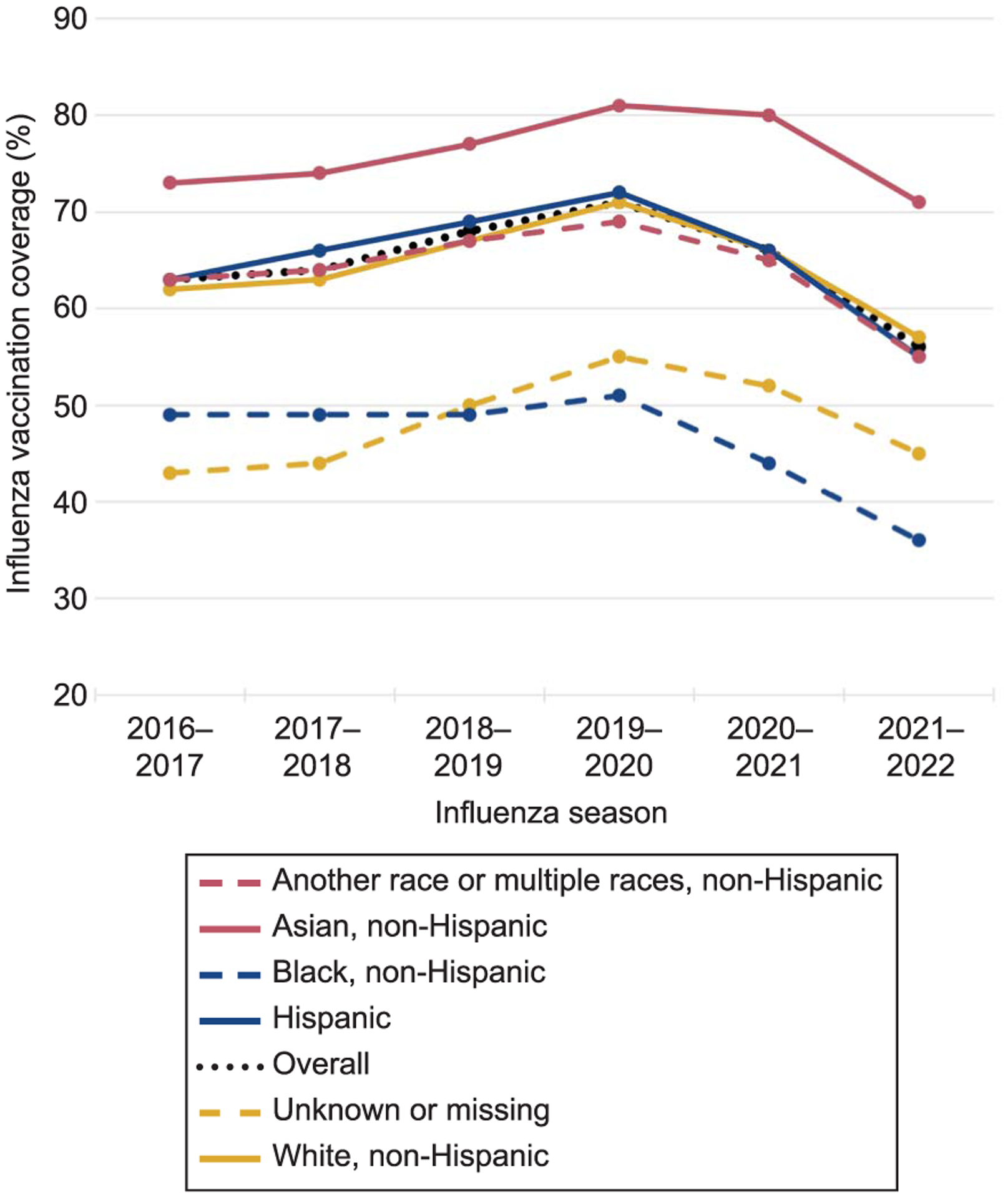
Influenza vaccination coverage among pregnant people aged 18–49 years, overall and by race and ethnicity, 2016–2017 through 2021–2022 influenza seasons. Eligible pregnancies spanned at least 1 day during the August 1 through March 31 period of an influenza season and met enrollment criteria. All same-season influenza vaccines administered between July 1 and March 31, including before, during, or after pregnancy, were included in coverage estimates.
Influenza vaccination coverage among pregnant people diagnosed with prespecified conditions (Appendix 7, available online at http://links.lww.com/AOG/D258) known to be risk factors for severe influenza, by calendar month of start of pregnancy and timing of vaccination relative to pregnancy, are presented in Appendices 8–10 (available online at http://links.lww.com/AOG/D258).
DISCUSSION
This large retrospective analysis found that, from the 2016–2017 through the 2019–2020 influenza seasons, influenza vaccination coverage increased among pregnant people in the Vaccine Safety Datalink, peaking at an overall crude coverage of 71.0%. However, coverage fell in the 2020–2021 season and declined even more in the 2021–2022 season, to a low of 56.4% overall. We identified differences in coverage across demographic and clinical characteristics, with the lowest coverage among younger (age 18–24 years) and non-Hispanic Black pregnant people, and those with fewer medical encounters. We include a more comprehensive discussion of the findings, including the limitations of the source data, in Appendix 11 (available online at http://links.lww.com/AOG/D258).
The decreases in coverage in recent seasons shown here have important public health ramifications. Continued efforts to improve vaccination coverage in the pregnant population, especially in specific subpopulations with lower vaccination coverage such as younger and non-Hispanic Black pregnant people, are essential.
Supplementary Material
Financial Disclosure
Edward A. Belongia’s institution received payment from Seqirus. Darios Getahun’s institution received payment from Hologic, Inc., Johnson & Johnson, and the NIH/NICHD. Lisa A. Jackson reports research support in the form of grants to her institution from Pfizer in the last 3 years. Nicola P. Klein reports research support in the form of grants to her institution from GlaxoSmithKline, Sanofi Pasteur, Merck, and Pfizer in the last 3 years. Allison L. Naleway reports research support in the form of grants to her institution from Pfizer and Vir Biotechnology in the last 3 years. The other authors did not report any potential conflicts of interest.
This work was supported by the U.S. Centers for Disease Control and Prevention (contract #200-2012-53584). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Khan KE, Razzaghi H, Jatlaoui TC, Skoff TH, Ellington SR, Black CL. Flu, Tdap, and COVID-19 vaccination coverage among pregnant women – United States, April 2022. Accessed January 10, 2023. https://www.cdc.gov/flu/fluvaxview/pregnant-women-apr2022.htm#:~:text=The%20Advisory%20Committee%20on%20Immunization,time%20during%20pregnancy%20%5B1%5D [DOI] [PMC free article] [PubMed]
- 2.Centers for Disease Control and Prevention. Influenza vaccination coverage, pregnant persons, United States. Accessed January 31, 2023. https://www.cdc.gov/flu/fluvaxview/dashboard/vaccination-coverage-pregnant.html
- 3.Baggs J, Gee J, Lewis E, Fowler G, Benson P, Lieu T, et al. The Vaccine Safety Datalink: a model for monitoring immunization safety. Pediatrics 2011;127:S45–53. doi: 10.1542/peds.2010-1722H [DOI] [PubMed] [Google Scholar]
- 4.Naleway AL, Crane B, Irving SA, Bachman D, Vesco KK, Daley MF, et al. Vaccine Safety Datalink infrastructure enhancements for evaluating the safety of maternal vaccination. Ther Adv Drug Saf 2021;12:204209862110212. doi: 10.1177/20420986211021233 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


