Abstract
Introduction
The term “atypical melanocytic nevus” (AMN) is used as a synonym for dysplastic nevus (DN) in clinical practice. Although the criteria for diagnosis of AMN/DN by the Agency for Research on Cancer helps to differentiate AMN/DN from common acquired nevi, they do not have high degrees of specificity, as they are similar to those used for the diagnosis of melanoma.
Objectives
In this retrospective study we evaluated the correlation and diagnostic concordance of dermoscopy, confocal microscopy, and histological examination in 50 AMN.
Methods
A graded scale was used to compare histological examination with dermoscopy and confocal microscopy. Low magnification histological images of only the central part of lesions were examined. This allowed histological diagnoses based almost exclusively on architectural criteria instead of simultaneously architectural and cytological, as in the global histological examination.
Results
Our data demonstrate that the diagnostic accuracy of dermoscopy and confocal microscopy diagnosis of the clinical aspects of AMN/DN as nevi or melanomas tends to be equivalent, being fair for nevi and excellent for melanomas. The total percentage of AMN suggested that the accuracy of confocal microscopy in the diagnosis of melanoma (86.7%) is greater than that of dermoscopy (73.3%).
Conclusions
This study demonstrated that diagnostic assessments of AMN/DN by dermoscopy and confocal microscopy are accurate and often coincide with those of histological examination and that their combined use helps to better manage and monitor these patients by facilitating early detection of melanomas and reducing unnecessary excisions of benign melanocytic lesions.
Keywords: atypical nevi, confocal microscopy, dermoscopy, dysplastic nevi, histopathology, melanoma
Introduction
Dysplastic nevi (DN) are acquired melanocytic nevi that, compared to common nevi, appear larger and less symmetrical in their configuration. For this reason, the clinical term “atypical melanocytic nevus” (AMN) is used as a synonym for dysplastic nevus (ND) in clinical practice. In 1990 the International Agency for Research on Cancer established the following criteria for the clinical diagnosis of AMN/DN: all AMN/DN have a macular component, at least in one area of the lesion. To this criterion should be added at least three more of the following: size > 5 mm, fuzzy lesion, border not well defined, color variability, irregularity of lesion contour, erythema [1,2]. Often the nevus has a central raised area surrounded by a macular component giving a ‘fried egg’ or ‘target’ appearance. Although these criteria help in differentiating AMN/DN from common acquired nevi, they do not have a high degree of specificity as they are very similar to those used for the diagnosis of melanoma. Generally, with many exceptions, the clinical difference between an AMN/DN and a melanoma depends on the extent of these criteria: the more the lesion has them, the more likely the diagnosis of melanoma.
Histologically, a nevus is dysplastic when it simultaneously presents an architectural disorder and cytological atypia [3]. The typical common junctional melanocytic nevus is formed by vertically oriented thecae of melanocytes located at the apex of the epidermal ridges. Architectural disorder means a deviation from this histological pattern in a lesion that is generally larger than the stereotypical common acquired melanocytic nevus. In particular, architectural disorder is characterized by the presence of a junctional “shoulder” extending laterally to the dermal component, thecae of melanocytes varying in shape and size, thecae of melanocytes oriented horizontally at the dermo-epidermal junction bridging adjacent epidermal ridges, poorly cohesive intraepidermal melanocytic thecae, presence of a greater density of single melanocytes at the dermo-epidermal junction (more melanocytes than keratinocytes in an area > 1 mm2), presence of a few single melanocytes in the suprabasal layer of the epidermis (typically fewer in number than in melanoma), lamellar and concentric fibroplasia and lymphocyte infiltrate in the papillary dermis [4,5]. Cytological atypia is characterized by the focal presence of melanocytes with increased nuclear size, pleomorphic nuclei, hyperchromatic nuclei with chromatin thickening and prominent nucleoli. Although there is some variability by even experienced dermatopathologists in applying the criteria of histological dysplasia, numerous studies have demonstrated significant intra-observer and inter-observer reproducibility in the histological diagnosis of dysplastic nevus and thus good reliability in the use of these histological criteria [6,7]. In order to identify classes of patients with different risk of melanoma, attempts have also been made to classify dysplastic nevi into two or more histological grades (mild, moderate and severe dysplasia). Although many authors have proposed various grading schemes, few studies have addressed the issue of diagnostic reproducibility and melanoma risk associated with histological dysplasia grade [8,9]. These studies have shown both poor inter-observer reproducibility of histological criteria for mild, moderate and severe dysplasia grades and a lack of significant correlation between atypia grades and melanoma risk.
Currently, most authors agree that the diagnosis of AMN/DN is clinic-histological, ie it requires the simultaneous presence of the criteria for clinical and histological dysplasia [10–12]. Other research, instead, have highlighted a significant presence of histological dysplasia in nevi lacking clinical dysplasia and therefore a limited specificity and sensitivity of the clinical criteria for dysplastic nevus when compared with the histological ones [13]. The possible lack of good clinical-histological correlation of the criteria for identifying dysplastic nevus casts some doubt on the real existence of AMN/DN as a clinicopathological entity. Added to this is the fact that no study to date has demonstrated the value of histological dysplasia as an independent risk factor for melanoma. It follows that, at present, AMN/DN is only of relevance on the clinical side. In practice, only the clinical presence of nevi with ND/AMN features (independent of their histological aspects) is to be considered a risk factor for melanoma independent of other factors such as total number of nevi, family history of melanoma, phototype and exposure to ultraviolet radiation.
As mentioned, AMN/DN often have clinical features overlapping with melanoma in situ and thin melanoma (SSM). From a prevention and patient care point of view, it is therefore essential to use all the tools at our disposal to be able to make the best differential diagnosis of these lesions.
Among the most easily used non-invasive diagnostic methods is dermoscopy, which allows the visualization of structural features of pigmented lesions that cannot be appreciated by simple clinical observation [14–16]. However, most authors agree that currently no qualitative parameters have been discovered or semi-quantitative systems devised that can accurately and reproducibly differentiate AMN/DN from melanoma in situ or melanoma in its early growth phase.
In vivo reflectance confocal microscopy (RCM) is a non-invasive technology that allows real-time visualization of skin structures at a resolution similar to histology. Overall, the diagnostic accuracy of RCM for melanoma has been shown to be superior to that of dermoscopy [17]. The RCM has also been shown to identify characteristic features of AMN/DN [18]. The basic histopathological criteria for histological dysplasia have precise correlates in confocal microscopy. In this regard, a simple algorithm has been proposed to differentiate AMN/DN from common acquired melanocytic nevi and melanoma. The possibility of recognizing histological dysplasia in vivo can lead to the correct removal of lesions most at risk for melanoma. RCM can complement the histopathological report by improving the distinction between melanoma and AMN/DN.
Objectives
The present study is a retrospective clinical case-control study based on microscopic and instrumental images and aims to evaluate the accuracy, correlation and diagnostic concordance of dermoscopic examination, confocal microscopy and histological examination in a population of AMN/DN in order to improve the clinical behavior and diagnosis of atypical melanocytic lesions.
Methods
Study Population
Atypical melanocytic lesions excised from 50 patients between 2018 and 2020 were studied to rule out melanoma. All lesions were selected as having the clinical features of AMN/DN, ie appearing as barely palpable macules or plaques, larger than 0.5 cm in diameter and having at least two of the following criteria: shaded border, color variability, irregularity of lesion margins and erythema. Both dermoscopic and confocal microscopy images acquired at the Department of Dermatology of the University of Modena and Reggio Emilia were available of all lesions.
Dermoscopic Study
Dermoscopic images were obtained through the use of a polarized light dermoscope (DermLite Photo 3Gen LLC). All dermoscopic images were analyzed by an expert in dermoscopy unaware of both the histological diagnosis and any clinical information, and confocal microscopy referable to the lesions under examination. Using the pattern analysis method each lesion was classified according to a graded scale as: −3 nevus certain; −2 nevus probable; −1 nevus possible; +1 melanoma possible; +2 melanoma probable; +3 melanoma certain.
Confocal Microscopy Study
Images were acquired through the use of a laser scanning confocal microscope (Vivascope 1500; MAVIG GmBH). The mosaics were acquired at three levels corresponding to the superficial layer of the epidermis (stratum spinosum and stratum granulosum), at the level of the dermo-epidermal junction and at the level of the papillary dermis.
For our study, mosaic images showing the dermo-epidermal junction of individual lesions were examined. In particular, only the central portion of each image corresponding to a lesion area of approximately 3 mm × 3 mm was cut out and evaluated.
The images of each individual lesion were examined by a confocal microscopy expert who was kept unaware of both the global histological diagnosis and any other clinical and dermoscopic information referable to the lesions under study. Using the parameters introduced by Pellacani and collaborators, each lesion was classified according to a graded scale as: −3 nevus certain; −2 nevus probable; −1 nevus possible; +1 melanoma possible; +2 melanoma probable; +3 melanoma certain [22].
Histopathological Study
For each lesion, a histological image of 10x magnification was scanned using a digital histological slide scanner (D-sight, Menarini SpA). Subsequently, in order to analyze approximately the same area of the lesion as the one examined by confocal microscopy, a portion of the scanned histological image representing approximately the central 3 mm of the lesion was cut out from each scanned histological image. At this point, the images were submitted to an expert dermatopathologist for examination. Obviously, the viewing of partial and low-magnification histological images of the lesions allowed a histological evaluation based mainly on architectural parameters rather than cytological ones. This was intended to make the diagnostic comparison between histological images and those of dermoscopy and confocal microscopy, which are known to base their diagnostic capacity mainly on architectural criteria, more homogeneous. Therefore, the dermatopathologist, on the basis of the histological criteria described in the literature [19,20] and in the absence of the histological diagnosis and of any clinical, dermoscopic and confocal microscopy information on the lesions under examination, was called to classify each lesion using the following gradation: −3 nevus certain; −2 nevus probable; −1 nevus possible; +1 melanoma possible; +2 melanoma probable; +3 melanoma certain [19,20].
Statistical Study
The statistical study was conducted by deriving the relative and absolute frequencies of the various dermoscopic, confocal microscopy and histological diagnoses obtained by applying the grading system described above to our population of AMN/DN.
The correlation coefficient (Spearman Rho) was also used to assess the presence of any correlations between the dermoscopic, confocal microscopy and histological diagnostic grading systems used in this study.
Finally, the concordance between the dermoscopic diagnostic grading, confocal microscopy and histological grading systems was calculated through Cohen κ value. A κ value of 0 denotes agreement that can only be explained by chance, a value of +1 means absolute agreement while a negative value implies complete disagreement. Intermediate κ values of denote non-random agreement as follows: less than 0.20 negligible; 0.20–0.40 poor; 0.40–0.60 moderate; 0.60–0.80 good; greater than 0.80 excellent.
Results
Of the 50 patients enrolled, 42% were male and 58% female; the mean age was 47 years; 46% of the lesions were located on the trunk, 27% on the lower limbs; 21% on the upper limbs and 6% in the head and neck region.
Of the 50 lesions, 20 were histologically diagnosed as melanocytic nevi, and 30 as melanomas (4 melanomas in situ, 24 superficial spreading melanomas of Breslow thickness less than one millimeter and 2 melanomas arising on melanocytic nevus). In this study, the global histological evaluation ie based on architectural and cytological criteria was considered as the diagnostic gold standard.
Tables 1–3 show the absolute and relative frequencies of the individual diagnostic grades found by dermoscopic, confocal microscopic and histological examination compared with the global histological diagnoses. On dermoscopic examination, of 20 lesions with a histological diagnosis of nevus, 11 were graded −3, −2 and −1 and therefore classified as nevi and 9 (grades +1, +2 and +3) as possible or probable melanoma. In contrast, all lesions with a histological diagnosis of melanoma were also dermoscopically interpreted as melanoma (grade +1, 8 cases; grade +2, 14 cases; grade +3, 8 cases). Similarly, confocal microscopy correctly classified all cases with a histological diagnosis of melanoma as melanoma (grade +1, 4 cases; grade +2, 11 cases; grade +3, 15 cases). Furthermore, confocal microscopy evaluated 50% of the AMN/DNs in our study with histological diagnosis of nevus as benign lesions and the other 50% as possible or probable melanoma. Finally, the predominantly architectural histological examination also correctly interpreted all lesions with histological diagnosis of melanoma, whereas 7 nevi were considered as possible melanoma (grade +1) and 2 nevi probable melanoma (grade +2), respectively.
Table 1.
Absolute and relative frequencies of individual diagnostic grade values on dermoscopic examination.
| Global Histological Diagnosis | Dermoscopic diagnostic grading | Total | |||||
|---|---|---|---|---|---|---|---|
| −3 | −2 | −1 | 1 | 2 | 3 | ||
| Nevus n°(%) | 1(5%) | 5(25%) | 5(25%) | 4(20%) | 4(20%) | 1(5%) | 20(100%) |
| Melanoma n°(%) | 0(%) | 0(%) | 0(%) | 8(26,7%) | 14(46,7%) | 8(26,7%) | 30(100%) |
| Total n°(%) | 1(2%) | 5(10%) | 5(10%) | 12(24%) | 18(36%) | 9(18%) | 50(100%) |
Table 2.
Absolute and relative frequencies of individual diagnostic grade values on confocal microscopy examination.
| Global Histological Diagnosis | Confocal diagnostic grading | Total | |||||
|---|---|---|---|---|---|---|---|
| −3 | −2 | −1 | 1 | 2 | 3 | ||
| Nevus n°(%) | 4(20%) | 3(15%) | 3(15%) | 5(25%) | 3(15%) | 2(10%) | 20(100%) |
| Melanoma n°(%) | 0(%) | 0(%) | 0(%) | 4(13,3%) | 11(36,7%) | 15(50%) | 30(100% |
| Total n°(%) | 4(8%) | 3(6%) | 3(6%) | 9(18%) | 14(28%) | 17(34%) | 50(100%) |
Table 3.
Absolute and relative frequencies of individual diagnostic grade values on histological examination.
| Global Histological Diagnosis | Histological diagnostic grading | Total | |||||
|---|---|---|---|---|---|---|---|
| −3 | −2 | −1 | 1 | 2 | 3 | ||
| Nevus n°(%) | 3(15%) | 6(30%) | 2(10%) | 7(35%) | 2(10%) | 0(0%) | 20(100%) |
| Melanoma n°(%) | 0(%) | 0(%) | 0(%) | 0(%) | 11(36,7%) | 19(63,3%) | 30(100%) |
| Total n°(%) | 3(6%) | 6(12%) | 2(4%) | 7(14%) | 13(26%) | 19(38%) | 50(100%) |
In order to facilitate the interpretation of the data, we then converted the results into a three-grade system called −1, 0, +1, which was created on the basis of the degree of diagnostic certainty. In grade −1 (very high probability of nevus diagnosis) we grouped the cases previously classified as −3 and −2; in grade +1 (very high probability of melanoma diagnosis) we included all the cases classified as +2 and +3; finally, in grade 0 (cases with uncertain diagnosis between nevus/melanoma) we included the cases diagnosed as −1 and +1. This subdivision shows how the percentage of cases graded as uncertain (grade 0) progressively decreases from 34% with dermoscopic examination, to 24% with confocal microscopy, to 18% with histological examination (Figures 1–4). Spearman analysis showed a statistically significant correlation between both the dermoscopic and confocal microscopy diagnostic grading system (ρ= 0.584, P < 0.001), between dermoscopy and histology ((ρ= 0.600, p < 0.001) and also between confocal microscopy and histology (ρ= 0.707, P < 0.001).
Figure 1.
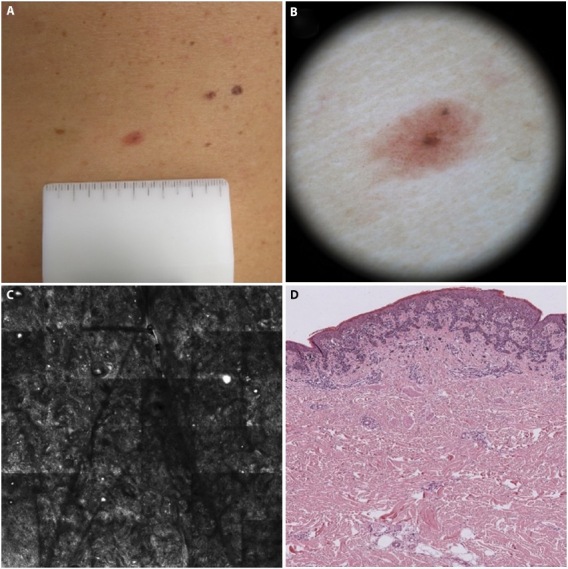
Good correlation between diagnostic grading with dermoscopy, confocal microscopy and histology. (A) AMN/DN with nevus histologic diagnosis. (B) Dermoscopic diagnosis: −3 (nevus certain). (C) Confocal microscopy diagnosis: −2 (nevus probable). (D)) histologic diagnosis: −3 (nevus certain).
Figure 2.
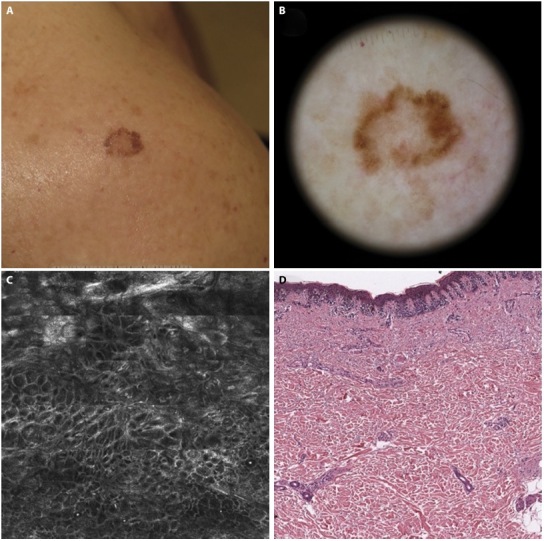
Disagreement between confocal diagnosis with dermoscopic and histologic ones. (A) AMN/DN with nevus histologic diagnosis. (B) Dermoscopic diagnosis: −1 (nevus possible). (C) Confocal microscopy diagnosis: +2 (melanoma probable). (D) histologic diagnosis: −1 (nevus possible).
Figure 3.
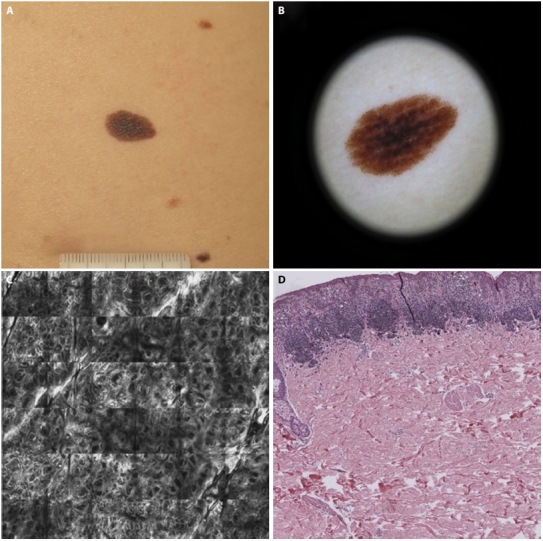
Disagreement between dermoscopic diagnosis with confocal and histologic ones. (A) AMN/DN with nevus histologic diagnosis. (B) Dermoscopic diagnosis: −1 (nevus possible). (C) Confocal microscopy diagnosis: +2 (melanoma probable). (D) Histologic diagnosis: +1 (melanoma possible).
Figure 4.
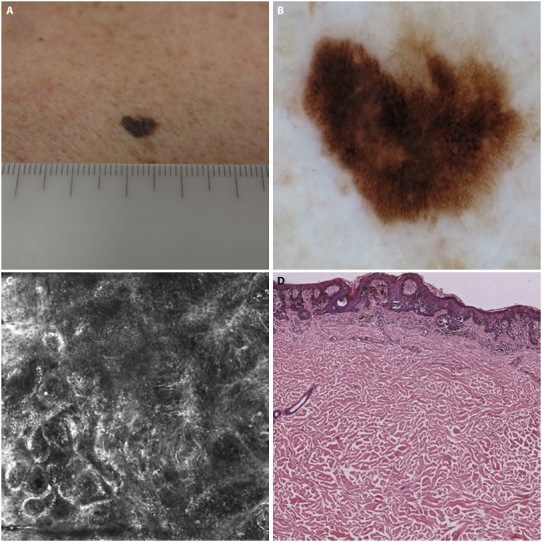
Good correlation between dermoscopic, confocal and histologic diagnosis. (A) AMN/DN with melanoma histologic diagnosis. (B) Dermoscopic diagnosis: +3 (melanoma certain). (C) confocal microscopy diagnosis: +3 (melanoma certain). (D) histologic diagnosis: +3 (melanoma certain).
Calculation of the agreement between the diagnoses made using our grading system gave a statistical value of 0.104 (agreement between dermoscopy and confocal microscopy), 0.162 (agreement between dermoscopy and histological examination EIa) and 0.314 (agreement between confocal microscopy and histological examination).
Conclusions
In this study we evaluated the accuracy of the main methods available for the diagnosis of melanocytic lesions in a population of clinically doubtful lesions. We also looked for possible correlation and diagnostic concordance between the different methods. The dermoscopic, confocal microscopic and histological diagnoses were graded according to the degree of diagnostic certainty as follows: nevus certain (−3); nevus probable (−2); nevus possible (−1); melanoma possible (+1); melanoma probable (+2); melanoma certain (+3). This made it possible to evaluate the correlations between the diagnostic methods in a very detailed way. In addition, it was decided to use histological images taken at low magnification and representing only the central part of the lesion examined. This allowed histological diagnoses to be based almost exclusively on architectural criteria, rather than on both architectural and cytological criteria as in the global histological examination. In practice, by removing the cytological details from the histological image analysis, the histological classification of lesions based on architectural criteria alone was brought into line with that of dermoscopy and confocal microscopy, which are also predominantly based on architectural criteria, thus allowing a more reliable assessment of the correlation between the different methods. Examination of the absolute and relative frequencies of the different diagnostic grades (Tables 1–3) shows that the dermoscopic and histological examination were able to correctly diagnose as nevi 55% (N = 11) of the lesions with clinical features of AMN/DN, compared to 50% (No = 10) of the confocal microscopic examination. On the other hand, only 5% (N =1), 10% (N = 2) and 0% (N = 0) of lesions with histological diagnosis of nevus were considered as definite melanoma (+3) by dermoscopy, confocal microscopy and histological examination respectively. It should also be noted that no lesion with a histological diagnosis of melanoma was classified as a nevus by any of the three methods. Taken together, these data suggest, firstly, that even a histological examination based almost exclusively on architectural aspects can correctly diagnose lesions presenting clinically as AMN/DN as melanoma. Secondly, the diagnostic accuracy of both dermoscopy and confocal microscopy in correctly diagnosing lesions with clinical aspects of AMN/DN as nevi or melanoma tends to be equivalent, being fair for nevi and excellent for melanoma Finally, architectural histology leaves doubt in 45% of the nevi, whereas it leaves none for melanoma, as all melanomas were correctly diagnosed as such. Taken together, these data indicate that all three methods for AMN/DN examination detect cases in which the diagnosis is uncertain. Histology also classified some lesions as suspicious, but at a lower rate than dermoscopy and confocal microscopy, and all suspicious lesions were found to be nevi on total histology.
The results of the Spearman test showed a statistically significant correlation between the dermoscopic and confocal microscopy diagnostic grading system, between the dermoscopic and histological grading system and between the confocal microscopy and histological grading system. This suggests that the combination of dermoscopic and confocal examination can provide a good indication of the architectural substrate of the lesion being examined.
Finally, Cohen test showed poor agreement between the dermoscopic and histological diagnostic grading system. Better agreement, although not high, was observed between confocal microscopy and histological examination It is noteworthy that all discordant cases were found to be melanocytic nevi on global histology. It follows that there appears to be good diagnostic agreement between the different methods when limited to the diagnosis of melanoma.
Overall, this study shows if a lesion is classified as a definite/probable nevus or definite/probable melanoma by dermoscopy or confocal microscopy, there is a high probability that this lesion will also be diagnosed as a nevus or melanoma by histology. However, the correlation between dermoscopic and histological diagnosis, although good, is lower than that between confocal microscopy and histology. From a prognostic point of view, we have seen how crucial it is to detect melanoma in its early growth phase and how this can be very difficult in patients with a high number of melanocytic nevi and, in particular, AMN/DN with clinical features very similar to those of melanoma. In the past, when the clinical diagnosis was in doubt, many lesions were surgically removed that later proved to be simple melanocytic nevi on histological examination, which in retrospect was unnecessary. This study, together with others in the literature, by showing a remarkable correlation between dermoscopic, confocal microscopic and histological diagnoses, demonstrates that non-invasive dermoscopic and confocal microscopic examinations provide in vivo accurate and reliable diagnoses of AMN/DN almost as much as histological ones. In particular, our study based on a diagnostic grading system shows that when faced with a patient with AMN/DN, dermoscopic examination should be performed first. If the dermoscopic diagnosis is definite/probable melanoma, possibly confirmed by confocal microscopy examination, the lesion should be removed immediately as there is a high probability that it will turn out to be melanoma on histological examination. Similarly, if the dermoscopic and/or confocal microscopic diagnosis is a definite/probable nevus, there is a very high probability that the histological examination will also be a nevus. In this case, it is sufficient to recommend follow-up of the lesion for 6–12 months, possibly with RCM evaluation to increase diagnostic confidence. Finally, if the dermoscopic examination of an AMN/NMD gives an uncertain result of a possible nevus/melanoma, it is advisable to proceed to confocal microscopy examination. If the confocal examination is inconclusive, the lesion should be excised or monitored for a short period of time, depending on the clinical features and the patient overall perception. At present, we know that a patient with multiple AMN/DN is at risk of developing melanoma, but we have no means of predicting which lesion is more likely to develop into melanoma, assuming that this occurs as a result of subsequent transformations. Therefore, at present, we can only act through secondary prevention processes, ie trying to detect melanoma in its early stages. In fact, in addition to the time and effort required to perform and periodically repeat total body mapping and digital dermoscopic monitoring, one must rely on the patient compliance, which must be maintained throughout life (estimated at less than 30% for 12-month follow-ups), especially given the long time period [24]. Therefore, the ultimate benefit of being able to predict and intervene in possible precursors could be effective in making the surveillance and prevention system simpler and less burdensome, both in terms of clinical and economic burden. Of course, the scientific approach obliges us to consider the null hypothesis, ie that melanoma does not arise through a process of evolutionary stages, but from a single clonal expanding cell, generating a random effect of appearance from healthy skin or on a melanocytic lesion. In such a case, it would be useful to orient the screening system towards whole-body imaging systems, possibly combined with automatic systems for identifying variations/new lesions. In conclusion, the combined use of dermoscopic and confocal microscopic examination in subjects with AMN/DN allows better management and monitoring of these patients, favoring early detection of possible melanoma and a significant reduction in unnecessary excision of benign melanocytic lesions.
Footnotes
Funding: None.
Competing Interests: None.
Authorship: All authors have contributed significantly to this publication.
References
- 1.Elder DE, Massi D, Scolyer RA, Willemze R. WHO classification of skin tumor. 4th ed. 2018. [Google Scholar]
- 2.Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: I. Common and atypical naevi. Eur J Cancer. 2005;41(1):28–44. doi: 10.1016/j.ejca.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 3.Elder DE. Dysplastic naevi: an update. Histopathology. 2010;56(1):112–120. doi: 10.1111/j.1365-2559.2009.03450.x. [DOI] [PubMed] [Google Scholar]
- 4.Shors AR, Kim S, White E, et al. Dysplastic naevi with moderate to severe histological dysplasia: a risk factor for melanoma. Br J Dermatol. 2006;155(5):988–993. doi: 10.1111/j.1365-2133.2006.07466.x. [DOI] [PubMed] [Google Scholar]
- 5.Xiong MY, Rabkin MS, Piepkorn MW, et al. Diameter of dysplastic nevi is a more robust biomarker of increased melanoma risk than degree of histologic dysplasia: a case-control study. J Am Acad Dermatol. 2014;71(6):1257–1258.e4. doi: 10.1016/j.jaad.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 6.Clemente C, Chocrane AJ, Eldwer DE, et al. Histopathologic diagnosis of dysplastic nevi: concordance among pathologists convened by the World Health Organization Melanoma Programme. Hum Pathol. 1991;22(4):313–319. doi: 10.1016/0046-8177(91)90078-4. [DOI] [PubMed] [Google Scholar]
- 7.Weinstock MA, Barnhill RL, Rhodes AR, Brodsky GL. Reliability of the histopathologic diagnosis of melanocytic dysplasia. The Dysplastic Nevus Panel. Arch Dermatol. 1997;133(8):953–958. [PubMed] [Google Scholar]
- 8.Ahmed I, Piepkorn MW, Rabkins MS, et al. Histopathologic characteristics of dysplastic nevi. Limited association of conventional histologic criteria with melanoma risk group. J Am Acad Dermatol. 1990;22(5 Pt 1):727–733. [PubMed] [Google Scholar]
- 9.Rhodes AR, Mihm MC, Jr, Weinstock MA. Dysplastic Melanocytic Nevi: a Reproducible Histologic Definition Emphasizing Cellular Morphology. Mod Pathol. 1989;2:306. [PubMed] [Google Scholar]; Mod Pathol. 1990;3(1):99. [PubMed] [Google Scholar]
- 10.Kelly JW, Crutcher WA, Sagebiel RW. Clinical diagnosis of dysplastic melanocytic nevi. A clinicopathologic correlation. J Am Acad Dermatol. 1986;14(6):1044–1052. doi: 10.1016/s0190-9622(86)70131-x. [DOI] [PubMed] [Google Scholar]
- 11.Black WC, Hunt WC. Histologic correlations with the clinical diagnosis of dysplastic nevus. Am J Surg Pathol. 1990;14(1):44–52. doi: 10.1097/00000478-199001000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Barnhill RL, Roush GC. Correlation of clinical and histopathologic features in clinically atypical melanocytic nevi. Cancer. 1991;67(12):3157–3164. doi: 10.1002/1097-0142(19910615)67:12<3157::aid-cncr2820671237>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 13.Annessi G, Cattaruzza MF, Abeni D, et al. Correlation between clinical atypia and histologic dysplasia in acquired melanocytic nevi. J Am Acad Dermatol. 2001;45(1):77–85. doi: 10.1067/mjd.2001.114580. [DOI] [PubMed] [Google Scholar]
- 14.Suh KS, Park JB, Kim JH, et al. Dysplastic nevus: Clinical features and usefulness of dermoscopy. J Dermatol. 2019;46(2):e76–e77. doi: 10.1111/1346-8138.14583. [DOI] [PubMed] [Google Scholar]
- 15.Grichner JM. Dermoscopy of melanocytic neoplasms: subpatterns of dysplastic/atypical nevi. Arch Dermatol. 2003;139(9):1238. doi: 10.1001/archderm.139.9.1238. [DOI] [PubMed] [Google Scholar]
- 16.Grichner JM. Dermoscopy of melanocytic neoplasms: subpatterns of dysplastic/atypical nevi. Arch Dermatol. 2003;139(7):970. doi: 10.1001/archderm.139.7.970. [DOI] [PubMed] [Google Scholar]
- 17.Guitera P, Pellacani G, Longo C, Seidenari S, Avramidis M, Menzies SW. In vivo reflectance confocal microscopy enhances secondary evaluation of melanocytic lesions. J Invest Dermatol. 2009;129(1):131–138. doi: 10.1038/jid.2008.193. [DOI] [PubMed] [Google Scholar]
- 18.Pellacani G, Farnetani F, Gonzalez S, et al. In vivo confocal microscopy for detection and grading of dysplastic nevi: a pilot study. J Am Acad Dermatol. 2012;66(3):e109–e121. doi: 10.1016/j.jaad.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 19.Maize JC, Ackerman AB. Pigmented lesions of the skin. Philadelphia: Lea & Fabiger; 1987. [Google Scholar]
- 20.Elder DE. Lever’s Histopathology of the skin. 9th Ed. Philadelphia: Lippincott Willams & Wilkins; 2005. [Google Scholar]
- 21.Yelamos O, Manubens E, Jain M, et al. Improvement of diagnostic confidence and management of equivocal skin lesions by integration of reflectance confocal microscopy in daily practice: Prospective study in 2 referral skin cancer centers. J Am Acad Dermatol. 2020;83(4):1057–1063. doi: 10.1016/j.jaad.2019.05.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Condorelli AG, Farnetani F, Ciardo S, et al. Dynamic dermoscopic and reflectance confocal microscopic changes of melanocytic lesions excised during follow up. J Am Acad Dermatol. 2022;86(5):1049–1057. doi: 10.1016/j.jaad.2021.03.081. [DOI] [PubMed] [Google Scholar]
- 23.Muruzabal UF, Villegas RG, Franco AP, et al. Combined in vivo reflectance confocal microscopy and digital dermoscopy for follow up of patients at high risk of malignant melanoma: A prospective case series study. J Dermatol. 2017;44(6):681–689. doi: 10.1111/1346-8138.13743. [DOI] [PubMed] [Google Scholar]
- 24.Argenziano G, Mordente I, Ferrara G, Sgambato A, Annese P, Zalaudek I. Dermoscopic monitoring of melanocytic skin lesions: clinical outcome and patient compliance vary according to follow-up protocols. Br J Dermatol. 2008;159(2):331–336. doi: 10.1111/j.1365-2133.2008.08649.x. [DOI] [PubMed] [Google Scholar]


