Abstract
The EspB protein of enteropathogenic Escherichia coli (EPEC) is exported via a type III secretion apparatus. EspB is critical for signaling the host cell and for the development of the attaching and effacing lesion characteristic of EPEC infection. We used cellular fractionation and confocal laser scanning microscopy to determine the cellular location of EspB during infection of HeLa cells. Both methods indicated that EspB is targeted to the cytoplasm of infected cells. Using mutants, we found that EspB targeting to the host cell cytoplasm requires the type III secretion apparatus and the secreted proteins EspA and EspD, but not intimin. These results provide insights into the function of the type III secretion apparatus of EPEC and the functions of the Esp proteins.
Enteropathogenic Escherichia coli (EPEC) is a leading cause of infantile diarrhea in developing countries throughout the world. EPEC forms clusters of bacteria on the surface of infected epithelial cells in a pattern referred to as localized adherence. Subsequently, signals are transduced to the host cell via the secretion of several EPEC effector molecules. This signaling cascade culminates in the formation of the attaching and effacing lesion, which is characterized by intimate attachment of the bacteria and localized degeneration of the epithelial microvilli (reviewed in reference 9). Highly organized cytoskeletal structures, referred to as pedestals, form directly beneath adherent bacteria. These pedestals are composed of actin filaments and several other cytoskeletal proteins (12, 23).
The bacterial genes that encode all of the factors necessary for pedestal formation are found within a large pathogenicity island in the E. coli chromosome known as the LEE (28) (for locus of enterocyte effacement), which is roughly organized into three regions (11). Many of the genes located in the left-hand region of the island encode a type III secretion apparatus, which exports several effector molecules encoded by genes located in the right-hand region (16). Between these regions are the gene eae, which encodes the bacterial adhesin intimin (17), and a recently identified gene that encodes Tir. Upon translocation into the host cell membrane, Tir becomes tyrosine phosphorylated and serves as the receptor for intimin (19).
Intimin is a 94-kDa outer membrane protein that is required for intimate adherence of EPEC to the epithelial cell membrane (8, 18). Mutants defective in eae are unable to sharply focus cytoskeletal components under adherent bacteria (6), although the signal transduction process remains intact (33). At least three proteins secreted by EPEC, EspB, EspA, and EspD, are involved in activating signals in infected epithelial cells (13, 22, 26). These signals include calcium and inositol phosphate fluxes (1, 10, 14); activation of phospholipase C-γ (21), protein kinase C (5), and NF-κB (35); and changes in membrane potential and short-circuit current (4, 38). The Esp proteins are also required for tyrosine phosphorylation of the Tir protein (13, 22, 26). Signaling through these proteins must occur prior to intimate attachment of the bacteria. Mutations in espB, espA, or espD or those in the type III secretion apparatus (esc, formerly cfm, and sep) render EPEC unable to signal or to induce the formation of attaching and effacing lesions. Genetic analysis has also shown that the translocation of Tir into the host cell membrane requires EspB, EspA, and the type III secretion apparatus (19). In contrast to EspA, EspB adopts a protease-resistant form upon contact between EPEC and epithelial cells (20). This finding suggests that EspA and EspB have separate functions in the signal transduction process. In this study, we present the results of experiments designed to determine whether EspB is delivered to the host cytoplasm during infection.
MATERIALS AND METHODS
Bacterial strains and tissue culture.
EPEC E2348/69 and isogenic mutants CVD206 (eae), UMD864 (espB), UMD872 (espA), UMD870 (espD), and CVD452 (escN) have been described previously (8, 13, 16, 22, 26). These bacteria were routinely cultured in Luria-Bertani (LB) broth or on LB plates. To induce EPEC virulence factor expression, the bacteria were grown in DMEM/F12 (Gibco-BRL, Gaithersburg, Md.). Plasmid pIL14 (25) was introduced into electrocompetent cells of CVD452, UMD864, UMD870, and UMD872 as previously described (26). HeLa cells (ATCC CCL2) were seeded (106 per well) in six-well tissue culture plates in Dulbecco’s modified Eagle’s medium supplemented with 10% (vol/vol) fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 μg/ml) and incubated overnight in an atmosphere of 95% air–5% CO2 at 37°C.
Cellular fractionation.
One-half hour prior to infection, HeLa cells were washed with phosphate-buffered saline (PBS) and incubated with DMEM/F12 lacking additives. Bacteria grown overnight in LB broth were diluted 1:100 into DMEM/F12 and grown at 37°C in an atmosphere of 95% air–5% CO2 for 4 h. HeLa cell monolayers were infected with 3-ml volumes of each EPEC culture for 1 h. Four fractions were obtained from these infections as follows. First, the culture supernatant was removed, combined with PBS washes of the HeLa cell monolayer, and subjected to centrifugation (14,000 × g; 10 min; room temperature) to yield a pellet (fraction 1) that contained nonadherent bacteria. The supernatant was then passed through a 0.2-μm filter to remove any residual bacteria, yielding a filtrate (fraction 2) that contained secreted proteins not associated with the host cell. This fraction was concentrated by precipitation with trichloroacetic acid and resuspended in sample buffer containing 10% (vol/vol) saturated Tris base. Infected HeLa cell monolayers were lysed in a buffer consisting of 0.25 M Tris-HCl, pH 7.5, phenylmethylsulfonyl fluoride (50 μg/ml), aprotinin (0.5 μg/ml), and EDTA (0.5 μM) by three freeze-thaw cycles consisting of alternating 5-min incubations in a dry ice-ethanol bath and a water bath set at 37°C. Lysates were subjected to ultracentrifugation (100,000 × g; 1 h; 4°C). This procedure yielded two fractions: a supernatant fraction containing soluble cytoplasmic contents (fraction 3) and a pellet consisting of adherent bacteria, HeLa cell membranes, nuclei, and cytoskeletal proteins (fraction 4). Equivalent volumes of these fractions were analyzed on sodium dodecyl sulfate (SDS)–12% polyacrylamide gels and electrotransferred in Towbin’s buffer (39) to polyvinylidene difluoride membranes (Immobilon-P; Millipore, Bedford, Mass.). In some experiments, HeLa cell cultures were treated with either cytochalasin D (2 μM) or genistein (250 μM) prior to and during the infection process.
Antibodies.
Primary antibodies were diluted in PBS–0.1% Tween 20 for immunoblotting. A monoclonal antibody to phospholipase C-γ was provided by M. Chedid, National Institutes of Health, and was used in the form of undiluted ascites fluid. A monoclonal antibody to the human fibronectin receptor was provided by R. Isberg, Tufts University, and was used at a dilution of 1:4,000. A polyclonal antibody against the EPEC adhesin intimin has been previously described (17) and was used at a dilution of 1:1,000. A previously described polyclonal antiserum against EspB (31) was affinity purified with the peptide to which the antiserum was raised immobilized on a column (Aminolink; Pierce, Rockford, Ill.) according to the manufacturer’s instructions and was used at a dilution of 1:100. Membranes were blocked in PBS containing 0.5% (vol/vol) Tween 20 and 5% (wt/vol) nonfat dry milk and were developed with secondary antibodies conjugated to alkaline phosphatase and 5-bromo-4-chloro-3-indolylphosphate and nitroblue tetrazolium (Sigma, St. Louis, Mo.) or with secondary antibody conjugated to horseradish peroxidase and enhanced chemiluminescence reagents (Amersham, Naperville, Ill.).
Confocal microscopy.
HeLa cells were incubated on coverslips that were placed in 24-well plates in DMEM/F12 supplemented with 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 μg/ml) and incubated in an atmosphere of 95% air–5% CO2 until 85% confluence was reached. Overnight static cultures of EPEC strains grown in LB broth at 37°C were diluted 1:100 in DMEM/F12 without additives and incubated with aeration for 3 h at 37°C. HeLa cell cultures were washed three times with PBS, and the medium was replaced with DMEM/F12 without additives. One-milliliter volumes of EPEC cultures were added to 1 ml of base medium overlaying the HeLa cell monolayers and centrifuged at 800 × g for 10 min. Infected HeLa monolayers were incubated in an atmosphere of 95% air–5% CO2 at 37°C for 3 h. Following infection, the cell monolayers were washed extensively in PBS, fixed with 2% formaldehyde, and permeabilized with 0.1% Triton X-100. The fixed monolayers were initially stained with 4′,6′-diamidino-2-phenylindole (DAPI; Sigma) (5 μg/ml in H2O) for 1 h. This compound, which binds to DNA and fluoresces bright blue after excitation with a UV laser, was used to label adherent bacteria and host cell nuclei. The monolayers were then blocked overnight at 4°C in 3% bovine serum albumin (BSA)–0.2% sodium azide. All subsequent antibody treatments were performed at room temperature for 3 h. The affinity-purified anti-EspB antibody was used at a dilution of 1:10 in 0.3% BSA in PBS and detected in stained cells with an anti-rabbit immunoglobulin G antibody conjugated to lissamine rhodamine B (Molecular Probes, Eugene, Oreg.) at a dilution of 1:200 in 0.3% BSA-PBS. Filamentous actin was detected with fluorescein isothiocyanate-phalloidin (5 μg/ml) in PBS. The samples were examined with a Zeiss LSM410 confocal laser scanning microscope with a 63×, numerical aperture 1.4 objective. Fluorescein and lissamine rhodamine signals were excited with the 488- and 568-nm lines of a 50-mW KrAr laser and detected through 515- to 540-nm band-pass and 590-nm long-pass filters, respectively. DAPI fluorescence was excited with the 351- and 364-nm lines of a 100-mW Ar UV laser and detected through a 472.5- to 492.5-nm band-pass filter. The diameter of the detector pinhole corresponded to one Airy unit at 590 nm, which corresponds to an optical thickness of 1 μm along the z axis. Conditions for laser attenuation and detector black level and gain were established by using cultures infected with wild-type EPEC, and these settings were maintained for the other samples. Image analysis was performed with LSM410 software.
RESULTS
Cellular fractionation of HeLa cells infected with EPEC.
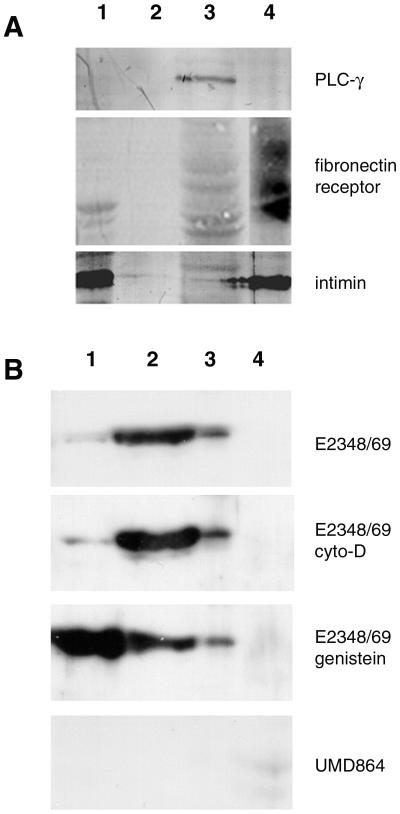
To determine whether EspB is targeted to the host cell cytoplasm, we performed cellular fractionation experiments on HeLa cells that had been infected with the prototypic wild-type EPEC strain, E2348/69, or the isogenic espB mutant UMD864. Several primary antibodies were employed to evaluate the purity of the fractions. We were particularly concerned with the separation of the cytoplasmic and membrane fractions of the infected HeLa cells, to allow us to distinguish whether EspB gains access to the cytoplasm or interacts with the host at the interface between the cell membrane and the adherent bacteria. As seen in Fig. 1A, phospholipase C-γ, a cytoplasmic enzyme, was detected only in the HeLa soluble lysate sample (fraction 3). In contrast, a diffuse signal representing glycosylated forms of the human fibronectin receptor, a membrane integrin, was detected only in the HeLa cell pellet sample (fraction 4). The bacterial outer membrane protein intimin was found in the pellet of the culture medium containing nonadherent bacteria (fraction 1) and in the pellet from the HeLa cell lysate (fraction 4), which also contains adherent bacteria. These results indicate that, within the detection limits of our assay, our fractionation procedure successfully isolated HeLa cell membranes from cytoplasm. These results also indicate that neither the bacterial secreted protein fraction nor the soluble cytoplasmic fraction is contaminated with whole bacteria.
FIG. 1.
(A) Cellular fractionation of HeLa cells infected with wild-type EPEC. Samples were derived from the pellet (lane 1) or supernatant (lane 2) of the medium obtained from HeLa cells infected with EPEC, from the lysate of the infected HeLa cells (lane 3), or from the pellet of lysed infected cells (lane 4). Equivalent volumes of these samples were separated by SDS-polyacrylamide gel electrophoresis, transferred to membranes, and probed with a monoclonal antibody to phospholipase C-γ (PLC-γ), a monoclonal antibody to the human fibronectin receptor, or a polyclonal antibody to the EPEC adhesin intimin. (B) Detection of EspB in fractions from infected HeLa cells. HeLa cell fractions, as defined for panel A, were separated by SDS-polyacrylamide gel electrophoresis, transferred to membranes, and probed with an affinity-purified anti-EspB antibody. Samples were prepared from HeLa cells infected with wild-type EPEC E2348/69, with the wild-type strain in the presence of cytochalasin D (cyto-D) or genistein, and with espB mutant strain UMD864, as indicated.
Similar fractions were analyzed with an affinity-purified antibody against EspB. As expected, EspB was found in both the bacterial pellet and the culture supernatant, corresponding to protein associated with nonadherent bacteria and protein secreted into the medium (Fig. 1B). EspB was also found in the HeLa cell lysate, but not in the pellet of the high-speed centrifugation, indicating that the protein also fractionates with the HeLa cell cytoplasm but not with the host cell membrane. Furthermore, the absence of EspB in fraction 4, which contains intimin, indicates that the bacteria adherent to the host cell have undetectable levels of EspB. In contrast, both intimin and EspB are detected in the fraction containing the nonadherent bacteria (fraction 1). Neither cytochalasin D nor genistein inhibited the association of EspB with the cytoplasmic fraction. Since these compounds are potent inhibitors of EPEC invasion (7, 33), these results indicate that fractionation of EspB with the host cell cytoplasm does not require intracellular bacteria. When an espB deletion mutant, UMD864, was tested in this assay, no bands with the mobility of EspB were seen. Thus, cell fractionation studies indicate that EspB is directed to the cytoplasm of host cells during infection by EPEC.
Localization of EspB in infected HeLa cells by laser scanning confocal microscopy.
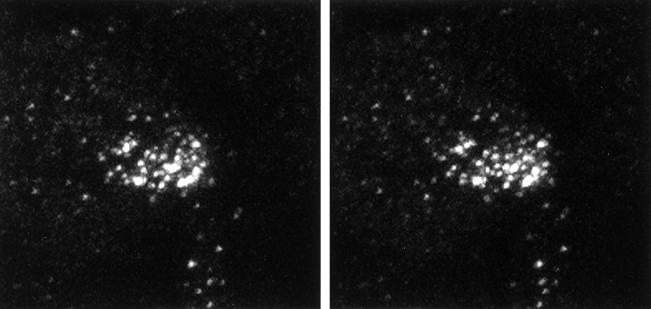
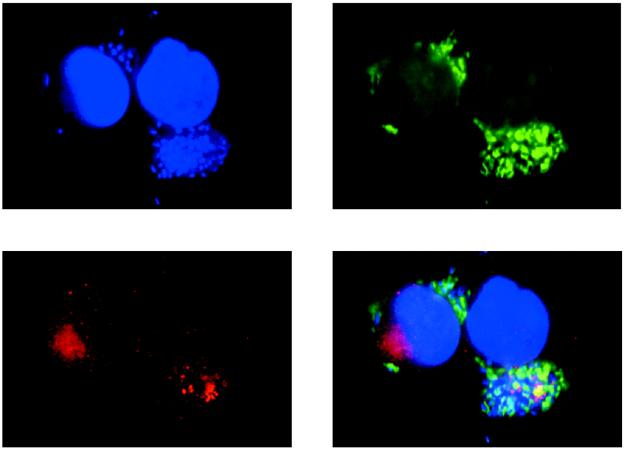
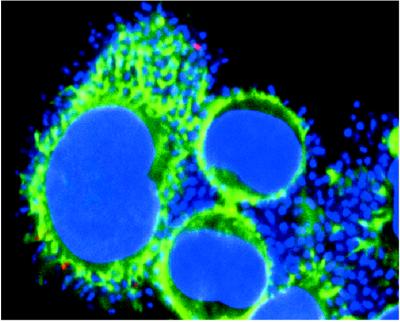
We used confocal microscopy with the affinity-purified EspB antibody to confirm the results obtained by cell fractionation and to provide information on the subcellular location of the protein. Figure 2 shows a stereo image reconstructed from a series of optical sections taken at 0.5-μm spacing from the surface through the basal border of a HeLa cell that had been infected with wild-type EPEC bacteria. These images confirm that the EspB signal can be found throughout the depth of an infected cell, clearly indicating the intracellular location of the protein. Figure 3 demonstrates the relationship between EspB and other structures in HeLa cells infected with wild-type EPEC bacteria. The EspB protein appears as an accumulation of bright dots that coincide spatially with the plane in which condensed actin appears directly under adherent bacteria. This result provides additional evidence that EspB is targeted to the cytoplasm of infected cells. Although at times colocalization of actin and EspB was observed, as indicated by the fusion of the green and red signals to give a yellow color, colocalization was not common. Instead, EspB was found most often in the same vicinity as the actin, yet emitted a distinct signal. We also observed that the EspB staining often radiated outward and downward from the perimeter of a defined bacterial colony, suggesting that the protein diffused somewhat from the point of entry yet did not spread throughout the cytoplasm. An example of this effect is seen in Fig. 3, where the bacterial colony associated with the EspB in the cell on the left is out of the plane of the section. These observations indicate that EspB is translocated into infected cells and is localized in the vicinity of adherent bacteria.
FIG. 2.
Stereo image of a HeLa cell infected with wild-type EPEC. A series of 18 images, representing 0.5-μm optical sections in the z axis, were taken of a representative HeLa cell stained with an affinity-purified anti-EspB antiserum. The stereo image was reconstructed from these sections.
FIG. 3.
Confocal laser scanning microscopy of HeLa cells infected with wild-type EPEC. Bacterial and cellular nucleic acid was labeled with DAPI (blue). Actin was labeled with fluorescein isothiocyanate-phalloidin (green). EspB was labeled with affinity-purified EspB antiserum and detected with a secondary antibody against immunoglobulin G conjugated to lissamine rhodamine (red). Areas of colocalization of EspB and actin appear yellow.
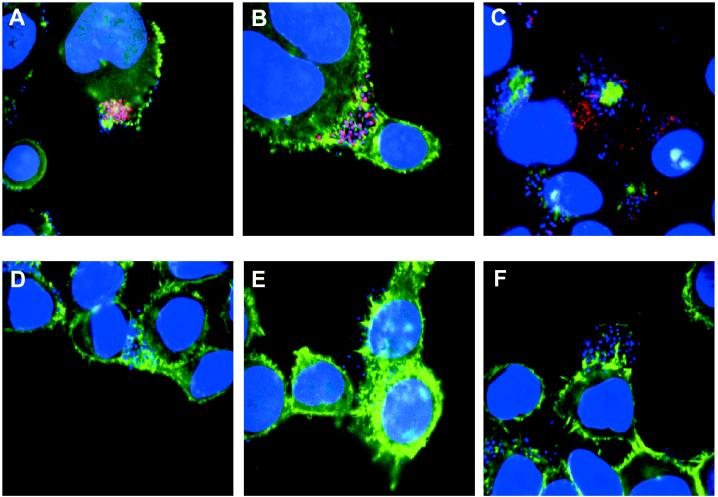
HeLa cells were also infected with the escN (formerly sepB) mutant, CVD452. Since this strain is defective in a component of the type III secretion apparatus, it is unable to secrete any of the Esp proteins or to induce epithelial signaling or actin condensation (16). As shown in Fig. 4B, the pattern of EspB staining observed was quite different from that seen in wild-type infection. In this case, the EspB antiserum stained the bacteria on the HeLa cell surface. In contrast to the wild type, punctate cytoplasmic staining was not observed in cells infected with the escN mutant. The EspB signal (red) colocalized with the DAPI signal (blue), rendering most bacteria purple. These results provide further evidence that the pattern of EspB staining seen in wild-type infection represents intracellular protein. This result also indicates, as expected, that the type III secretion apparatus is required to deliver EspB to the host cytoplasm.
FIG. 4.
Confocal laser scanning microscopy of HeLa cells infected with wild-type and mutant EPEC. HeLa cell were infected with wild-type bacteria (A), escN mutant CVD452 deficient in a component of the type III secretion apparatus (B), eae mutant CVD206 (C), espB mutant UMD864 (D), espA mutant UMD872 (E), and espD mutant UMD870 (F). Labeling was done as described in the legend to Fig. 3. Areas of colocalization of EspB and actin appear yellow, and areas of colocalization of EspB and nucleic acid appear purple.
Figure 4C shows HeLa cells infected with the eae mutant, CVD206 (8). This mutant is defective in the gene encoding intimin and is unable to induce the highly organized cytoskeletal pedestal structures, leading to an immature attaching and effacing lesion referred to as an actin shadow when it is observed by fluorescence microscopy (6). Despite this defect, this mutant is able to deliver Tir to host cells (19, 33). As shown here, the pattern of EspB staining in CVD206 is similar to that of wild-type EPEC. These observations indicate that intimin is not necessary for targeting EspB to the cytoplasm of host cells.
We also infected HeLa cells with a series of mutants defective in each of the esp genes. Previous studies have determined that these mutants are unable to signal epithelial cells nor are they able to induce the reorganization of actin in infected cells (13, 22, 26). Fig. 4D, E, and F show infection with the espB mutant, the espA mutant, and the espD mutant, respectively. Each mutant in these experiments was negative for EspB staining, although colonies of bacteria could clearly be observed adhering to the epithelial cell surface. Although the espA and espD mutants secrete EspB, this result suggests that EspB requires the other Esp proteins for efficient translocation into the host cytoplasm. These results contrast with those obtained with the escN mutant, which stained purple due to colocalization of EspB and the bacteria. In the esp mutants there was no colocalization of EspB with the bacteria. Therefore, in the espA and espD mutants, either all of the EspB made by these bacteria was secreted into the medium or the protein was not accessible to the antiserum as it was in the escN mutant. These results also provide an explanation for an earlier observation. In previous experiments it was demonstrated that coinfection of host cells with an eae mutant and either an escV mutant (formerly cfm or sepA), an espB mutant, or an espA mutant results in attaching and effacing lesions and invasion levels similar to those seen in wild-type infections (13, 22, 33). The fact that the eae mutant is able to deliver EspB to the host cell cytoplasm while escN, espA, and espD mutants are not suggests that intracellular EspB is at least one factor missing from host cells infected with the signaling mutants, which is supplied in trans by the eae mutant to allow normal infection. Similarly, since neither the espA mutant nor the espB mutant is able to deliver EspB to the host cytoplasm, this also explains the earlier observation that the espA and espB mutants are unable to complement each other in coinfection experiments (22).
Since the esp and escN mutants bind to HeLa cells at a lower density than does wild-type EPEC, these experiments do not exclude the possibility that our failure to observe EspB translocation with these mutants was due to insufficient numbers of adherent bacteria. To address this question, we introduced a plasmid that encodes an afimbrial adhesin (AFA/I) of uropathogenic E. coli into each of these mutants. Figure 5 shows HeLa cells infected with the espA mutant expressing AFA/I. Despite the greater density of bacteria binding to the cell monolayers in comparison to the wild-type strain, little or no intracellular EspB staining was detected. Similar results were obtained with the espD and espB mutants transformed with pIL14 and with the escN mutant transformed with pIL14, except that in the last case many of the bacteria appeared purple due to colocalization of the DAPI and EspB signals (data not shown). This result demonstrates that the lack of intracellular EspB labeling in host cells infected with these mutants is not due to insufficient numbers of adherent bacteria. We conclude that the EspA and EspD proteins and an intact type III secretion apparatus are required for targeting EspB to the epithelial cell cytoplasm.
FIG. 5.
Confocal laser scanning microscopy of HeLa cells infected with a hyperadherent mutant EPEC. HeLa cell samples were infected with the espA mutant UMD872, which had been transformed with a plasmid encoding an afimbrial adhesin. Labeling was done as described in the legend to Fig. 3.
DISCUSSION
We have shown that EPEC targets the EspB protein to the host cytoplasm of infected epithelial cells. Translocation of EspB requires EspA and EspD as well as products of the type III secretion machinery. This process does not, however, require the expression of the bacterial adhesin intimin. Although EspA and EspD are required for the delivery of EspB into the cytoplasm, we have little insight into how these proteins function to achieve this purpose. A recent study suggests that EspA is a component of a pilus on the surface of EPEC that may function in adherence (24). Whether EspD is also a component of such a structure is not known. One hypothesis is that these two proteins form a translocation apparatus which functions to deliver EspB, as well as the Tir protein, to the host cell. Similar functions have been proposed for a pilus associated with the type III secretion system of Pseudomonas syringae (32). Alternatively, it may be necessary for EspB and EspD to form a complex that allows both proteins to enter the cytoplasm. Formation of a translocation apparatus may be the only function of EspA and EspD, or these proteins may share a dual function as translocators and effectors by inducing signals at the level of the host membrane or cytoplasm in the process of translocating EspB. Similarly, while EspB enters the cytoplasm, this protein is also required for the membrane localization of Tir (19).
It is becoming increasingly clear that EPEC shares many features with other pathogens, such as Yersinia, Salmonella, Shigella, and Pseudomonas species, that use a type III secretion apparatus to target effector molecules to host cells (27, 29). Each of these pathogens uses this export system to affect host cell signaling, yet each species appears to have different and defined end points in this process. In recent studies it has been shown that Salmonella typhimurium exports several type III-dependent proteins known as Sips, which share many features with EPEC Esp proteins. SipB and -C are both targeted to the host cell cytoplasm, whereas SipA remains associated with the bacterial cell surface (3). A fourth protein, SipD, is required for translocation of the other Sips, but its function is not yet defined. Two other proteins, SopE and SopB, have recently been reported to be translocated into host cells by a Sip-dependent mechanism in Salmonella dublin (15, 41). It is tempting to draw analogies between the Sips and Sops of Salmonella and the Esps of EPEC. Although sequence analysis shows no obvious amino acid homologies, the functional similarities between these two groups of effector molecules are compelling.
Yersinia species target several Yops to the host cytoplasm (2, 30, 34, 36, 37). Undirected secretion of the Yop proteins occurs in low-calcium medium, but bacteria cultured in the presence of epithelial cells target YopE and YopH to the host cell cytoplasm without secreting the proteins in the medium (30, 34). In contrast, our findings with cell fractionation suggest that in EPEC, secretion of EspB into the medium occurs in addition to translocation into the host cell. However, cell fractionation indicates that, in contrast to nonadherent bacteria, bacteria adherent to the host cell contain undetectable levels of EspB, suggesting that secretion of EspB has been triggered by contact with the host cell. Furthermore, no EspB colocalizing with adherent wild-type or esp mutant bacteria is detected by confocal microscopy, whereas EspB colocalizes with adherent type III secretion-deficient bacteria. These data indicate that secretion of EspB is contact dependent. In previous studies we reported that, although the espA and espD mutants secrete EspB, they are incapable of signaling host cells (22, 26). In this study we have determined that these mutants are not capable of delivering EspB to the host cytoplasm, despite their ability to secrete the protein.
The fact that most EspB does not directly colocalize with condensed actin under adherent bacteria and the fact that this protein is not capable of focusing actin in the absence of intimin suggest that the direct function of EspB is not actin binding. It seems more likely that this protein interacts with cellular signaling pathways that are important in EPEC-induced cytoskeletal reorganization. It remains a possibility that EspB functions merely to facilitate the entry of the Tir protein, and apart from becoming the receptor for intimin, the Tir protein itself may direct all of the signaling cascades observed. It is equally possible that the Esps and Tir act in concert, with each protein having distinct functions in cellular signaling.
EPEC is now firmly established as a member of a rapidly expanding group of gram-negative bacteria that use a type III secretion apparatus to insert bacterial effector molecules inside host cells. The interactions between EspB, Tir, and the host cell may in fact be unique among this group of pathogens. In any case, this system serves to emphasize the complexity of pathogen-host interactions and will certainly provide important insight for understanding similar interactions in other pathogens.
ACKNOWLEDGMENTS
We thank Valentina Shustova for technical assistance and Marcio Chedid and Ralph Isberg for supplying antibodies.
This work was supported by Public Health Service awards AI32074 (M.S.D.) and AI09651 (K.A.T.) from the National Institutes of Health and IBN 9309510 from the National Science Foundation (P.W.L.). The University of Maryland Facility for Confocal Microscopy was created with National Science Foundation instrumentation grants BRI 9318061 and DBI 9512985.
ADDENDUM
Since submission of the manuscript, another group has reported that EspB is targeted to the host cell cytoplasm (40). These authors also find that delivery of EspB to the cytoplasm requires both EspA and EspB (24, 40).
REFERENCES
- 1.Baldwin T J, Ward W, Aitken A, Knutton S, Williams P H. Elevation of intracellular free calcium levels in HEp-2 cells infected with enteropathogenic Escherichia coli. Infect Immun. 1991;59:1599–1604. doi: 10.1128/iai.59.5.1599-1604.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boland A, Sory M P, Iriarte M, Kerbourch C, Wattiau P, Cornelis G R. Status of YopM and YopN in the Yersinia Yop virulon: YopM of Y. enterocolitica is internalized inside the cytosol of PU5-1.8 macrophages by the YopB, D, N delivery apparatus. EMBO J. 1996;15:5191–5201. [PMC free article] [PubMed] [Google Scholar]
- 3.Collazo C M, Galán J E. The invasion-associated type III system of Salmonella typhimurium directs the translocation of Sip proteins into the host cell. Mol Microbiol. 1997;24:747–756. doi: 10.1046/j.1365-2958.1997.3781740.x. [DOI] [PubMed] [Google Scholar]
- 4.Collington G K, Booth I W, Knutton S. Rapid modulation of electrolyte transport in Caco-2 cell monolayers by enteropathogenic Escherichia coli (EPEC) infection. Gut. 1998;42:200–207. doi: 10.1136/gut.42.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crane J K, Oh J S. Activation of host cell protein kinase C by enteropathogenic Escherichia coli. Infect Immun. 1997;65:3277–3285. doi: 10.1128/iai.65.8.3277-3285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donnenberg M S, Calderwood S B, Donohue-Rolfe A, Keusch G T, Kaper J B. Construction and analysis of TnphoA mutants of enteropathogenic Escherichia coli unable to invade HEp-2 cells. Infect Immun. 1990;58:1565–1571. doi: 10.1128/iai.58.6.1565-1571.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donnenberg M S, Donohue-Rolfe A, Keusch G T. A comparison of HEp-2 cell invasion by enteropathogenic and enteroinvasive Escherichia coli. FEMS Microbiol Lett. 1990;57:83–86. doi: 10.1016/0378-1097(90)90417-o. [DOI] [PubMed] [Google Scholar]
- 8.Donnenberg M S, Kaper J B. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donnenberg M S, Kaper J B, Finlay B B. Interactions between enteropathogenic Escherichia coli and host epithelial cells. Trends Microbiol. 1997;5:109–114. doi: 10.1016/S0966-842X(97)01000-7. [DOI] [PubMed] [Google Scholar]
- 10.Dytoc M, Fedorko L, Sherman P M. Signal transduction in human epithelial cells infected with attaching and effacing Escherichia coli in vitro. Gastroenterology. 1994;106:1150–1161. doi: 10.1016/0016-5085(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 11.Elliott S J, Wainwright L A, McDaniel T K, Jarvis K G, Deng Y, Lai L-C, McNamara B P, Donnenberg M S, Kaper J B. The complete sequence of the locus of enterocyte effacement (LEE) of enteropathogenic E. coli E2348/69. Mol Microbiol. 1998;28:1–4. doi: 10.1046/j.1365-2958.1998.00783.x. [DOI] [PubMed] [Google Scholar]
- 12.Finlay B B, Rosenshine I, Donnenberg M S, Kaper J B. Cytoskeletal composition of attaching and effacing lesions associated with enteropathogenic Escherichia coli adherence to HeLa cells. Infect Immun. 1992;60:2541–2543. doi: 10.1128/iai.60.6.2541-2543.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foubister V, Rosenshine I, Donnenberg M S, Finlay B B. The eaeB gene of enteropathogenic Escherichia coli is necessary for signal transduction in epithelial cells. Infect Immun. 1994;62:3038–3040. doi: 10.1128/iai.62.7.3038-3040.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foubister V, Rosenshine I, Finlay B B. A diarrheal pathogen, enteropathogenic Escherichia coli (EPEC), triggers a flux of inositol phosphates in infected epithelial cells. J Exp Med. 1994;179:993–998. doi: 10.1084/jem.179.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galyov E E, Wood M W, Rosqvist R, Mullan P B, Watson P R, Hedges S, Wallis T S. A secreted effector protein of Salmonella dublin is translocated into eukaryotic cells and mediates inflammation and fluid secretion in infected ileal mucosa. Mol Microbiol. 1997;25:903–912. doi: 10.1111/j.1365-2958.1997.mmi525.x. [DOI] [PubMed] [Google Scholar]
- 16.Jarvis K G, Girón J A, Jerse A E, McDaniel T K, Donnenberg M S, Kaper J B. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc Natl Acad Sci USA. 1995;92:7996–8000. doi: 10.1073/pnas.92.17.7996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jerse A E, Kaper J B. The eae gene of enteropathogenic Escherichia coli encodes a 94-kilodalton membrane protein, the expression of which is influenced by the EAF plasmid. Infect Immun. 1991;59:4302–4309. doi: 10.1128/iai.59.12.4302-4309.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jerse A E, Yu J, Tall B D, Kaper J B. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci USA. 1990;87:7839–7843. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kenny B, DeVinney R, Stein M, Reinscheid D J, Frey E A, Finlay B B. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 20.Kenny B, Finlay B B. Protein secretion by enteropathogenic Escherichia coli is essential for transducing signals to epithelial cells. Proc Natl Acad Sci USA. 1995;92:7991–7995. doi: 10.1073/pnas.92.17.7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kenny B, Finlay B B. Intimin-dependent binding of enteropathogenic Escherichia coli to host cells triggers novel signaling events, including tyrosine phosphorylation of phospholipase C-γ1. Infect Immun. 1997;65:2528–2536. doi: 10.1128/iai.65.7.2528-2536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenny B, Lai L-C, Finlay B B, Donnenberg M S. EspA, a protein secreted by enteropathogenic Escherichia coli (EPEC), is required to induce signals in epithelial cells. Mol Microbiol. 1996;20:313–323. doi: 10.1111/j.1365-2958.1996.tb02619.x. [DOI] [PubMed] [Google Scholar]
- 23.Knutton S, Baldwin T, Williams P H, McNeish A S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989;57:1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knutton S, Rosenshine I, Pallen M J, Nisan I, Neves B C, Bain C, Wolff C, Dougan G, Frankel G. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 1998;17:2166–2176. doi: 10.1093/emboj/17.8.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Labigne-Roussel A F, Lark D, Schoolnik G, Falkow S. Cloning and expression of an afimbrial adhesin (AFA-I) responsible for P blood group-independent, mannose-resistant hemagglutination from a pyelonephritic Escherichia coli strain. Infect Immun. 1984;46:251–259. doi: 10.1128/iai.46.1.251-259.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai L C, Wainwright L A, Stone K D, Donnenberg M S. A third secreted protein that is encoded by the enteropathogenic Escherichia coli pathogenicity island is required for transduction of signals and for attaching and effacing activities in host cells. Infect Immun. 1997;65:2211–2217. doi: 10.1128/iai.65.6.2211-2217.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee C A. Type III secretion systems: machines to deliver bacterial proteins into eukaryotic cells? Trends Microbiol. 1997;5:148–156. doi: 10.1016/S0966-842X(97)01029-9. [DOI] [PubMed] [Google Scholar]
- 28.McDaniel T K, Jarvis K G, Donnenberg M S, Kaper J B. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci USA. 1995;92:1664–1668. doi: 10.1073/pnas.92.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mecsas J J, Strauss E J. Molecular mechanisms of bacterial virulence: type III secretion and pathogenicity islands. Emerg Infect Dis. 1996;2:270–288. doi: 10.3201/eid0204.960403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Persson C, Nordfelth R, Holmström A, Håkansson S, Rosqvist R, Wolf-Watz H. Cell-surface-bound Yersinia translocate the protein tyrosine phosphatase YopH by a polarized mechanism into the target cell. Mol Microbiol. 1995;18:135–150. doi: 10.1111/j.1365-2958.1995.mmi_18010135.x. [DOI] [PubMed] [Google Scholar]
- 31.Rabinowitz R P, Lai L-C, Jarvis K, McDaniel T K, Kaper J B, Stone K D, Donnenberg M S. Attaching and effacing of host cells by enteropathogenic Escherichia coli in the absence of detectable tyrosine kinase mediated signal transduction. Microb Pathog. 1996;21:157–171. doi: 10.1006/mpat.1996.0051. [DOI] [PubMed] [Google Scholar]
- 32.Roine E, Wei W S, Yuan J, Nurmiaho-Lassila E L, Kalkkinen N, Romantschuk M, He S Y. Hrp pilus: an hrp-dependent bacterial surface appendage produced by Pseudomonas syringae pv tomato DC3000. Proc Natl Acad Sci USA. 1997;94:3459–3464. doi: 10.1073/pnas.94.7.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenshine I, Donnenberg M S, Kaper J B, Finlay B B. Signal exchange between enteropathogenic Escherichia coli (EPEC) and epithelial cells: EPEC induce tyrosine phosphorylation of host cell protein to initiate cytoskeletal rearrangement and bacterial uptake. EMBO J. 1992;11:3551–3560. doi: 10.1002/j.1460-2075.1992.tb05438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosqvist R, Magnusson K-E, Wolf-Watz H. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 1994;13:964–972. doi: 10.1002/j.1460-2075.1994.tb06341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Savkovic S D, Koutsouris A, Hecht G. Activation of NF-kappaB in intestinal epithelial cells by enteropathogenic Escherichia coli. Am J Physiol. 1997;273:C1160–C1167. doi: 10.1152/ajpcell.1997.273.4.C1160. [DOI] [PubMed] [Google Scholar]
- 36.Sory M-P, Cornelis G R. Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol Microbiol. 1994;14:583–594. doi: 10.1111/j.1365-2958.1994.tb02191.x. [DOI] [PubMed] [Google Scholar]
- 37.Sory M P, Boland A, Lambermont I, Cornelis G R. Identification of the YopE and YopH domains required for secretion and internalization into the cytosol of macrophages, using the cyaA gene fusion approach. Proc Natl Acad Sci USA. 1995;92:11998–12002. doi: 10.1073/pnas.92.26.11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stein M A, Mathers D A, Yan H, Baimbridge K G, Finlay B B. Enteropathogenic Escherichia coli (EPEC) markedly decreases the resting membrane potential of Caco-2 and HeLa human epithelial cells. Infect Immun. 1996;64:4820–4825. doi: 10.1128/iai.64.11.4820-4825.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolff C, Nisan I, Hanski E, Frankel G, Rosenshine I. Protein translocation into host epithelial cells by infecting enteropathogenic Escherichia coli. Mol Microbiol. 1998;28:143–155. doi: 10.1046/j.1365-2958.1998.00782.x. [DOI] [PubMed] [Google Scholar]
- 41.Wood M W, Rosqvist R, Mullan P B, Edwards M H, Galyov E E. SopE, a secreted protein of Salmonella dublin, is translocated into the target eukaryotic cell via a sip-dependent mechanism and promotes bacterial entry. Mol Microbiol. 1996;22:327–338. doi: 10.1046/j.1365-2958.1996.00116.x. [DOI] [PubMed] [Google Scholar]