Abstract
Sentinel lymph node biopsy (SLNB) is a surgical procedure aimed to detect nodal metastases in patients with clinically occult disease. Since the advent of new systemic therapies, its role in melanoma has been extensively debated over the last years. In this article, three possible scenarios are discussed, considering the SLNB impact on the management of melanoma patients. First, pT1b and pT2a patients with negative SLNB (stages IA and IB) and those with positive SLNB (stage IIIA) would all not benefit from adjuvant treatment. Therefore, SLNB might be avoided in these categories of patients. Second, in IIB and IIC, melanoma patients are already candidates for adjuvant treatment; therefore, SLNB in patients with T3b, T4a, or T4b melanoma would not change treatment decisions. On the other end of the spectrum, patients with pT2b and pT3a melanomas (clinical stage IIA) represent the only two groups whose management would be significantly affected by the SLNB status, being adjuvant therapy only indicated for SLN-positive patients. Further studies are needed to investigate which melanoma patient deserves SLNB.
Keywords: melanoma, adjuvant therapy, sentinel node biopsy
Introduction
Sentinel lymph node biopsy (SLNB) is a surgical procedure aimed to detect nodal metastases in patients with clinically occult disease. The role of SLNB in melanoma has been extensively debated over the last years. SLNB intended as a therapeutic technique evolved from the observation that most primary cutaneous melanomas spread initially through the intradermal lymphatics to the regional nodes and then move to distant sites [1]. This sequential model of melanoma metastasis has been lately disproved [2,3] and sentinel node is now considered an “indicator” of disease as its status illustrates metastatic potential, but its removal cannot prevent further spread. Although SLNB does not impact survival, it is associated with a benefit in terms of rate of recurrence within the primary tumor region [4].
Currently SLNB is recommended as a staging procedure that can help identify those patients with at least pT1b melanoma (Tables 1 and 2) who may benefit from adjuvant therapy [3,5,6]. SLNB positivity rates vary depending on histopathological procedures, with a reported false negative rate up to 10% [7]. Adjuvant treatment in stage III melanoma patients is the standard of care from 2018, when both immunotherapy (PD-1 inhibitors) and molecular targeted therapy were worldwide approved thanks to the excellent results obtained from Checkmate 238, Keynote-054 and COMBI-AD trials [8–10]. These clinical trials recruited only high-risk stage III patients, meaning that patients with stage IIIA (Table 2) melanoma could only be included if the metastasis in the SLN were larger than 1 mm. However, adjuvant therapies are approved in all stage III subgroups [11].
Table 1.
Melanoma stage I and II (SLNB-) according to the AJCC classification system 8th Edition.
| Breslow thickness | Ulceration | T Category | N category | Stage |
|---|---|---|---|---|
| <0.8 mm | no | T1a | N0 | IA |
| <0.8 mm | yes | T1b | N0 | IB |
| 0.8–1.0 mm | Yes/no | T1b | N0 | IB |
| >1.0–2.0 mm | no | T2a | N0 | IB |
| >1.0–2.0 mm | yes | T2b | N0 | IIA |
| >2.0–4.0 mm | no | T3a | N0 | IIA |
| >2.0–4.0 mm | yes | T3b | N0 | IIB |
| >4.0 mm | no | T4a | N0 | IIB |
| >4.0 mm | yes | T4b | N0 | IIC |
Adapted from: Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017; doi:10.3322/caac.21409.
Table 2.
Melanoma stage III subgroups according to the AJCC classification system 8th Edition.
| N Category | T Category | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T0 Occult primary tumor | T1a < 0.8 | T1b < 0.8 U or 0.8–1.0 | T2a >1.0–2.0 | T2b >1.0–2.0 U | T3a >2.0–4.0 | T3b >2.0–4.0 U | T4a >4.0 | T4b >4.0 U | ||
| N1a | 1 node c.o. | N/A | A | A | A | B | B | C | C | C |
| N1b | 1 node c.d | B | B | B | B | B | B | C | C | C |
| N1c | only S/T mets | B | B | B | B | B | B | C | C | C |
| N2a | 2/3 node c.o. | N/A | A | A | A | B | B | C | C | C |
| N2b | 2/3 node, at least 1 c.d. | C | B | B | B | B | B | C | C | C |
| N2c | S/T mets + 1 nodea | C | C | C | C | C | C | C | C | C |
| N3a | ≥4 node c.o. | N/A | C | C | C | C | C | C | C | D |
| N3b | ≥4 node, at least 1 c.d. or matted nodes | C | C | C | C | C | C | C | C | D |
| N3c | S/T mets + ≥2 nodea or matted nodes | C | C | C | C | C | C | C | C | D |
c.o. = clinically occult (diagnosed by sentinel node biopsy); c.d. = clinically detected (by palpation or imaging); N/A = not applicable. S/T mets = satellite and/or in-transit metastases. T category is expressed in mm.U = ulceration;
in N2c and N3c subcategories involved nodes may be either clinically occult or clinically detected.
Adapted from: Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017; doi:10.3322/caac.21409.
Adjuvant treatment in stage II melanoma patients is the current focus of oncology research [12]. Despite the absence of identified lymph node involvement, patients with stage IIB and IIC (Table 1) melanoma have a greater risk of recurrence/death than those with stage IIIA disease and similar to those with stage IIIB disease. Pembrolizumab 200 mg for one year was compared against placebo in more than 900 patients with stage IIB and IIC melanoma in KEYNOTE 716 trial resulting in a 18-months recurrence-free-survival rate of 86% (95% CI 82–89) in the treatment group versus 77% (95% CI 73–81) in the placebo group, which led to approval by the FDA and EMA [13]. Final results from the CheckMate −76K trial (expected soon) seem to confirm the efficacy of Nivolumab in stage IIB and IIC melanoma patients [14]. With regards to target therapy, a phase III trial comparing Encorafenib plus Binimetinib versus placebo in these subsets of patients is currently enrolling (Columbus-Ad study, NCT05270044).
Objectives
With the access of negative-SLN patients to adjuvant treatment a question rises accordingly: is SLNB still needed [15]? Which melanoma patient does deserve it?
Results
Impact of SLNB on Staging Workup: Potential Scenarios After the Indication of Adjuvant Therapy in Stage IIB and IIC Patients
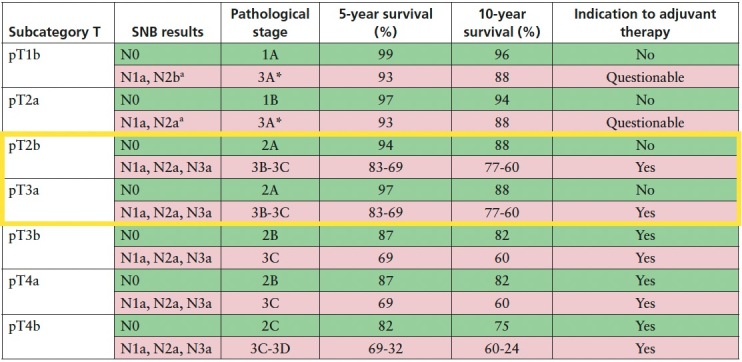
In Table 3 the staging workups with indication to SLNB are reported. According to NCCN guidelines, SLNB should be discussed in patients with microsatellites, as SLN status does have prognostic significance, with a positive SLN upstaging a patient from at least N1c and at least stage IIIB disease, to at least N2c, which corresponds to stage IIIC disease (Table 2) [5]. However, although this may have a prognostic impact (from 83% to 69% and from 77% to 60% 5-year and 10-year melanoma specific survival [MSS] respectively), the upgrade from stage IIIB to stage IIIC does not change the therapeutic management of this subgroup of patients, which would all be candidates for adjuvant therapy [16,17].
Table 3.
Staging workup and consequent indication to adjuvant therapy.
pT1b and pT2a Melanomas
Patients with pT1b melanoma and a negative SLNB (N0) are included in pathological stage IA, which exhibit an excellent 5- and 10-year MSS (99% and 96% respectively). Similarly, patients with pT2a N0 melanoma are characterized by an excellent survival (97% and 94% 5- and 10-year MSS) [17] although are belonging to stage IB. Consequently, they do not require adjuvant therapy.
In the literature the probability of finding a metastatic node in pT1b and pT2a melanomas, meaning N1a subcategory, ranges from 2.3% and 8.4% to 7.5% and 14.6% [18,19], respectively. Among those, the overall incidence of a maximum deposit size greater than 1.0 mm is likewise slightly above 3% [18,19]. As shown in Table 1, pT1b-N1a, pT2a-N1a, pT1b-N2a and pT2b-N2a melanomas are included in stage IIIA. The probability to find four or more metastatic nodes (N3a) through SLNB in pT1b and pT2a melanomas, which would be upgraded to stage IIIC, can be considered not relevant (0.1%) [17,19].
Stage IIIA carries a better prognosis of stage IIB and IIC (5-year MSS: 93% versus 87% and 82%; 10-year MSS: 88% versus 82% and 75%), thus it remains highly questionable whether adjuvant treatment is needed in this stage [20,21]. Recent updates on adjuvant therapy survival rates highlighted that the benefit in stage IIIA patients was lower than other stage III subgroups [22]. Consequently, the ESMO consensus on melanoma management underlined that there is currently insufficient evidence to support the routine use of adjuvant therapy in stage IIIA melanoma [23]. NCCN guidelines states that in patients with a low risk of recurrence (for example, stage IIIA with <1 mm of nodal tumor burden), the toxicity of adjuvant therapy may outweigh the benefit and observation is preferred. Although adjuvant systemic therapy is approved for these patients, they were excluded from the prospective adjuvant therapy trials [5].
In this light, weighing the very low frequency of a positive SLN (especially greater than 1 mm), the morbidity and costs of the surgical procedure and the benefit–risk ratio of adjuvant therapy, the indication to performing SLNB in pT1b and pT2a melanomas can be questioned.
pT2b and pT3a Melanomas
Patients with pT2b or pT3a N0 melanoma are included in stage IIA, characterized by 94% and 88% 5- and 10- year MSS. Stage IIA melanoma patients were not included in adjuvant therapy registration trial due to their rather favorable prognosis. In contrast, the presence of a metastatic node detected through SLNB upgrades pT2b and pT3a melanoma patients to stage IIIB or IIIC according to the number of nodes involved (Table 3). The prognosis of stages IIIB and IIIC justifies adjuvant treatment as 5-year MSS ranges from 83% to 69% and 10-year MSS ranges from 77% to 60%, respectively. Thus, performing SLNB in this group of patients does actually change the management by selecting those patients who may benefit from adjuvant therapy.
pT3b, pT4a and pT4b Melanomas
Patients with pT3b or pT4a N0 melanoma are included in stage IIB, while those with pT4b N0 melanoma in stage IIC. 5- and 10-year MSS resulted to be 87% and 82% for stage IIB and 82% and 75% for stage IIC. In case of a metastatic node these subsets of patients are upgraded to stages IIIC–IIID according to the number of nodes involved. As mentioned before, IIB and IIC melanoma patients are already candidates for adjuvant treatment, therefore, SLNB in patients with T3b, T4a, or T4b melanoma would not change treatment decisions and the procedure may be omitted.
Conclusions
SLNB is a surgical staging procedure aimed at identifying patient who may benefit from adjuvant treatment. Since the approval of adjuvant therapy in negative SLN patients, namely stages IIB and IIC, its role may be reappraised. Patients with pT3b, pT4a and pT4b melanomas and negative SLNB (stages IIB–IIC) and those with positive SLNB (stages IIIC–IIID) would all be candidates for adjuvant therapy for a duration of 1 year. In these patients SLNB might be avoided since it does not alter the therapeutic management. On the other side of the spectrum, pT1b and pT2a patients with negative SLNB (stages IA and IB) and those with positive SLNB (stage IIIA) would all not benefit from adjuvant treatment. Therefore, SLNB might be avoided also in these categories of patients. Patients with pT2b and pT3a melanomas (clinical stage IIA) represent the only two groups whose management would be significantly affected by the SLNB status, being adjuvant therapy only indicated for SLN positive patients.
More studies are needed to improve evidence around SLNB and its potential role in only a limited number of patients.
Footnotes
Funding: None.
Competing Interests: None.
Authorship: All authors have contributed significantly to this publication.
References
- 1.Morton D. Overview and update of the phase III Multicenter Selective Lymphadenectomy Trials (MSLT-I and MSLT-II) in melanoma. Clin Exp Metastasis. 2012;29(7):699–706. doi: 10.1007/s10585-012-9503-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faries MB, Thompson JF, Cochran AJ, et al. Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma. N Engl J Med. 2017;376(23):2211–2222. doi: 10.1056/NEJMoa1613210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong SL, Faries MB, Kennedy EB, et al. Sentinel lymph node biopsy and management of regional lymph nodes in Melanoma: American society of clinical oncology and society of surgical oncology clinical practice guideline update. J Clin Oncol. 2018;36(4):399–413. doi: 10.1200/JCO.2017.75.7724. [DOI] [PubMed] [Google Scholar]
- 4.Morton DL, Cochran AJ, Thompson JF, et al. Sentinel node biopsy for early-stage melanoma: Accuracy and morbidity in MSLT-I, an international multicenter trial. Ann Surg. 2005;242(3):302–311. doi: 10.1097/01.sla.0000181092.50141.fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. Melanoma: Cutaneous (Version 1.2023) [Internet] 2023. Available from: https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf PLEASE PROVIDE DATE OF ACCESS.
- 6.Garbe C, Amaral T, Peris K, et al. European consensus-based interdisciplinary guideline for melanoma. Part 2: Treatment - Update 2022. Eur J Cancer. 2022;170:256–284. doi: 10.1016/j.ejca.2022.04.018. Epub 2022 May 24. [DOI] [PubMed] [Google Scholar]
- 7.Nieweg OE. False-negative sentinel node biopsy: Editorial. Ann Surg Oncol. 2009;16(8):2089–2091. doi: 10.1245/s10434-009-0540-3. Epub 2009 Jun 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weber J, Mandala M, Del Vecchio M, et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N Engl J Med. 2017;377(19):1824–1835. doi: 10.1056/NEJMoa1709030. [DOI] [PubMed] [Google Scholar]
- 9.Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N Engl J Med. 2018;378(19):1789–1801. doi: 10.1056/NEJMoa1802357. Epub 2018 Apr 15. [DOI] [PubMed] [Google Scholar]
- 10.Long GV, Hauschild A, Santinami M, et al. Adjuvant Dabrafenib plus Trametinib in Stage III BRAF-Mutated Melanoma. N Engl J Med. 2017;377(19):1813–1823. doi: 10.1056/NEJMoa1708539. [DOI] [PubMed] [Google Scholar]
- 11.Napolitano S, Brancaccio G, Argenziano G, et al. It is finally time for adjuvant therapy in melanoma. Cancer Treat Rev. 2018;69:101–111. doi: 10.1016/j.ctrv.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Brancaccio G, Napolitano S, Troiani T, et al. Eighth American Joint Committee on Cancer (AJCC) melanoma classification: what about stage IIC? Br J Dermatol. 2018;179(6):1422–1423. doi: 10.1111/bjd.17145. [DOI] [PubMed] [Google Scholar]
- 13.Long GV, Luke JJ, Khattak MA, et al. Pembrolizumab versus placebo as adjuvant therapy in resected stage IIB or IIC melanoma (KEYNOTE-716): distant metastasis-free survival results of a multicentre, double-blind, randomised, phase 3 trial. Lancet Oncol. 2022;23(11):1378–1388. doi: 10.1016/S1470-2045(22)00559-9. [DOI] [PubMed] [Google Scholar]
- 14.Wilson M, Geskin LJ, Carvajal RD, et al. Adjuvant nivolumab in high-risk stage IIb/IIc melanoma patients: Results from investigator initiated clinical trial. J Clin Oncol. 2021;(suppl):9583. doi: 10.1200/JCO.2021.39.15_. [DOI] [Google Scholar]
- 15.Hindié E. Adjuvant therapy in stage IIB and IIC melanoma: is sentinel biopsy needed? Lancet. 2022;400(10352):559. doi: 10.1016/S0140-6736(22)01388-5. [DOI] [PubMed] [Google Scholar]
- 16.Amin MB, Edge SB, Greene FL, et al. American Joint Committee on Cancer (AJCC) AJCC Cancer Staging Manual. 2017 [Google Scholar]
- 17.Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(6):472–492. doi: 10.3322/caac.21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brancaccio G, Pellerone S, Scharf C, et al. Sentinel node biopsy in thin melanoma: a retrospective descriptive cohort study. J Eur Acad Dermatol Venereol. 2022;36(10):e795–e796. doi: 10.1111/jdv.18274. [DOI] [PubMed] [Google Scholar]
- 19.Moncrieff MD, Lo SN, Scolyer RA, et al. Evaluation of the Indications for Sentinel Node Biopsy in Early-Stage Melanoma with the Advent of Adjuvant Systemic Therapy: An International, Multicenter Study. Ann Surg Oncol. 2022;29(9):5937–5945. doi: 10.1245/s10434-022-11761-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moncrieff MD, Lo SN, Scolyer RA, et al. Clinical Outcomes and Risk Stratification of Early-Stage Melanoma Micrometastases From an International Multicenter Study: Implications for the Management of American Joint Committee on Cancer IIIA Disease. J Clin Oncol. 2022;40(34):3940–3951. doi: 10.1200/JCO.21.02488. [DOI] [PubMed] [Google Scholar]
- 21.Hindié E. Adjuvant therapy in stage IIIA melanoma. Lancet Oncol. 2021;22(7):e299. doi: 10.1016/S1470-2045(21)00346-6. [DOI] [PubMed] [Google Scholar]
- 22.Eggermont AMM, Suciu S, Robert C. Adjuvant therapy in stage IIIA melanoma - Authors’ reply. Lancet Oncol. 2021;22(7):e300. doi: 10.1016/S1470-2045(21)00354-5. [DOI] [PubMed] [Google Scholar]
- 23.Michielin O, van Akkooi A, Lorigan P, et al. ESMO consensus conference recommendations on the management of locoregional melanoma: under the auspices of the ESMO Guidelines Committee. Ann Oncol. 2020;31(11):1449–1461. doi: 10.1016/j.annonc.2020.07.005. [DOI] [PubMed] [Google Scholar]


