Abstract
Objectives:
Cognitive decline and gait speed slowing are independent predictors of disability and mortality. While both factors increase in prevalence with advancing age, little is known about their combined patterns of change. The study goal was to identify joint trajectories of cognition and gait speed within an aging bi-ethnic cohort of Mexican Americans and European Americans.
Methods/Design:
Participants included 182 Mexican Americans and 188 European Americans, ages 65 to 74, who were followed over a mean of 9.5 years. Cognition was assessed with the mini-mental state examination and gait speed was examined with a timed 10-ft walk. Joint trajectory classes of cognition and gait speed were identified with latent growth mixture modeling. Odd-ratios assessed predictors for trajectory classes.
Results:
Three latent trajectory classes were identified: (a) relatively stable cognition and gait (termed stable cognition and gait class, 65.4%); (b) deteriorating cognition and gait (termed cognitive and physical vulnerability class, 22.2%); (c) stable cognition and deteriorating gait (termed physical vulnerability class, 12.4%). The odds of classification in the cognitive and physical vulnerability class vs stable cognition and gait class was associated with Mexican American ethnicity (OR = 3.771, P = .016), age (OR = 1.186, P = .017), income (OR = 0.828, P = .029), education (OR = 0.703, P < .001), and diabetes (OR = 4.547, P = .010). The odds of classification in the physical vulnerability class was associated with female sex (OR = 6.481, P = .004) and body mass index (OR = 1.118, P = .025).
Conclusions:
The trajectories of cognition and gait speed were generally parallel, suggesting the two domains may act synergistically to shape important health outcomes. Socioeconomic disparities and Mexican American ethnicity independently conferred risk for accelerated decline.
Keywords: cognition, gait speed, Mexican Americans, older adults, trajectories
1 |. INTRODUCTION
The proportion of older adults in the population is accelerating with ethnic minorities, particularly Hispanics, growing at the fastest rates.1 With the emerging population shift, prevention of functional decline and disability across diverse ethnic subgroups has become a global health priority. Biological aging is associated with gradual decline across multiple physiological systems, conferring risk for disability.2 In particular, deterioration in physical and cognitive functioning are salient risk factors for disablement as they hinder navigation in both personal and social environments. Across indices of physical functioning, slowed gait speed is a highly sensitive predictor of adverse health outcomes including functional decline, institutionalization, and mortality.3–5 Even in the absence of disease, gait speed gradually decreases by 1% each year after age 65 with more rapid deceleration of approximately 4% after age 80.5,6 In addition to slowed gait, cognitive impairment is an independent risk factor for poor quality of life, functional decline, and nursing home placement.7–9 Cognitive impairment increases in prevalence across the lifespan from approximately 4% in those ages 65 to 69 years to more than 30% after age 85.10,11
While both cognition and gait speed decline with advancing age, the interdependent relationship between the two domains remains poorly defined.12 Numerous cross-sectional studies have reported an association between cognitive decline and gait speed slowing,12–16 alluding to an overlapping etiological cause. Motoric cognitive risk syndrome, a classification defined by cognitive complaints and slowed gait speed, has been strongly associated with cognitive decline and incident dementia risk across numerous cohorts around the globe.17,18
Additional longitudinal studies have attempted to clarify the onset of gait speed slowing relative to the development of cognitive decline. To date, mixed findings have emerged with some studies identifying gait speed slowing as a predictor of incident cognitive decline and others identifying cognitive impairment as a risk factor for subsequent gait slowing.12,16,19–21 The varied findings have been interpreted as support for bidirectional associations between gait slowing and cognitive decline.19,21 In contrast to baseline predictions, far less is known regarding how change in one domain may affect the rate of change in the other domain. Health transitions in aging occur as a result of dynamic interactions between multiple health domains.22 Thus, declines in gait speed and cognition may iteratively affect one another, shaping important health-related outcomes.
Examination of combined cognition and gait speed trajectories further enables characterization of heterogeneity within the population. While biological aging consists of gradual declines across multiple systems, there is substantial variation in the relative onset and slope of these changes across individuals.22–24 Some individuals remain remarkably resilient to age-related declines and enjoy a high degree of functional independence. In contrast, others contend with numerous chronic medical conditions and become increasingly disabled. White et al. previously identified three distinct gait speed trajectories in older adults as characterized by mild, moderate, and fast decline.5 The groups represented 27%, 51%, and 22% of the sample, respectively. Similarly, in a sample of older Mexican Americans, Downer et al. reported three trajectories of global cognitive change with 31% of the sample maintaining high cognition, 52% displaying mild decline, and 15% evidencing pronounced decline.25 As declines in gait speed and cognition may interact synergistically, concomitant examinations of both domains may yield meaningful information regarding the complexity of health-related changes related to these two domains.
The identification of distinct trajectories can also be leveraged to determine risk factors associated with accelerated decline. Interventions specifically designed to target chronic health conditions may attenuate the rate of decline in high risk sub-groups. Moreover, examination of joint cognition and gait speed trajectories may identify factors associated with well-established ethnic disparities in health outcomes.10,26 Hispanics of Mexican descent represent the fastest growing sector of the elderly population27 and will exert significant influence on the emerging healthcare landscape. As compared to non-Hispanic whites, Hispanics have higher rates of cognitive impairment and slower gait speed,10,28,29 which have largely been attributed to disparities in socioeconomic status and medical comorbidities. Despite the ample literature documenting poorer health outcomes in Mexican Americans, research is lacking on the determinants of temporal trajectories of decline. With improved understanding of ethnic differences in multidomain health trajectories, a more comprehensive and tailored approach to alleviate health disparities may be developed.
The aim of the current study was to evaluate joint trajectories of cognition and gait speed in an aging, bi-ethnic cohort of older Mexican Americans and European Americans from the San Antonio Longitudinal Study of Aging (SALSA). We applied latent growth mixture modeling to explore heterogeneity in trajectories over the course of the average 9.5-year follow-up period. In line with previous research, we hypothesized identification of distinct trajectory patterns within the sample.22–24 We further aimed to define how ethnicity, socioeconomic factors, and medical comorbidities influenced classification within trajectory classes. As compared to European Americans, Mexican Americans were anticipated to display increased vulnerability for accelerated cognitive and gait speed decline, attributable at least in part to ethnic disparities in socioeconomic factors and the prevalence of medical comorbidities.29
2 |. MATERIALS AND METHODS
2.1 |. Participants
Participants included 182 Mexican Americans and 188 European Americans, who participated in both the baseline examination and the first follow-up examination of SALSA. The SALSA sample is comprised of the oldest members (≥65 years) of the San Antonio Heart Study (SAHS) cohort,30 which was conducted in two waves: October 1979 to November 1982 and October 1984 to October 1988. In SAHS, Mexican Americans and European Americans were randomly sampled from low- (Mexican American only), middle-, and high-income neighborhoods to maximize sociocultural diversity among the Mexican Americans. The two ethnic groups were classified using a standardized, validated algorithm.31 The SALSA baseline examination was completed between 1992 and 1996. Three follow-up examinations were conducted at eighteen-month intervals between 2000 and 2005. Of the 749 participants who completed the baseline examination, 246 did not return to the first follow-up examination. Thirty-eight participants had missing MMSE and/or gait speed at the baseline visit and an additional 82 participants had missing MMSE and/or gait speed at the first follow-up examination. Finally, 13 participants were excluded due to missing covariates (Figure 1). Relative to the individuals from the entire SALSA cohort who were not included in the study, the study sample was younger, had higher incomes, education, and baseline MMSE and gait speed scores, and lower rates of diabetes and coronary artery disease (Table 1). The study was approved by the Institutional Review Board at The University of Texas Health Science Center at San Antonio, and all participants provided written informed consent prior to enrollment.
FIGURE 1.
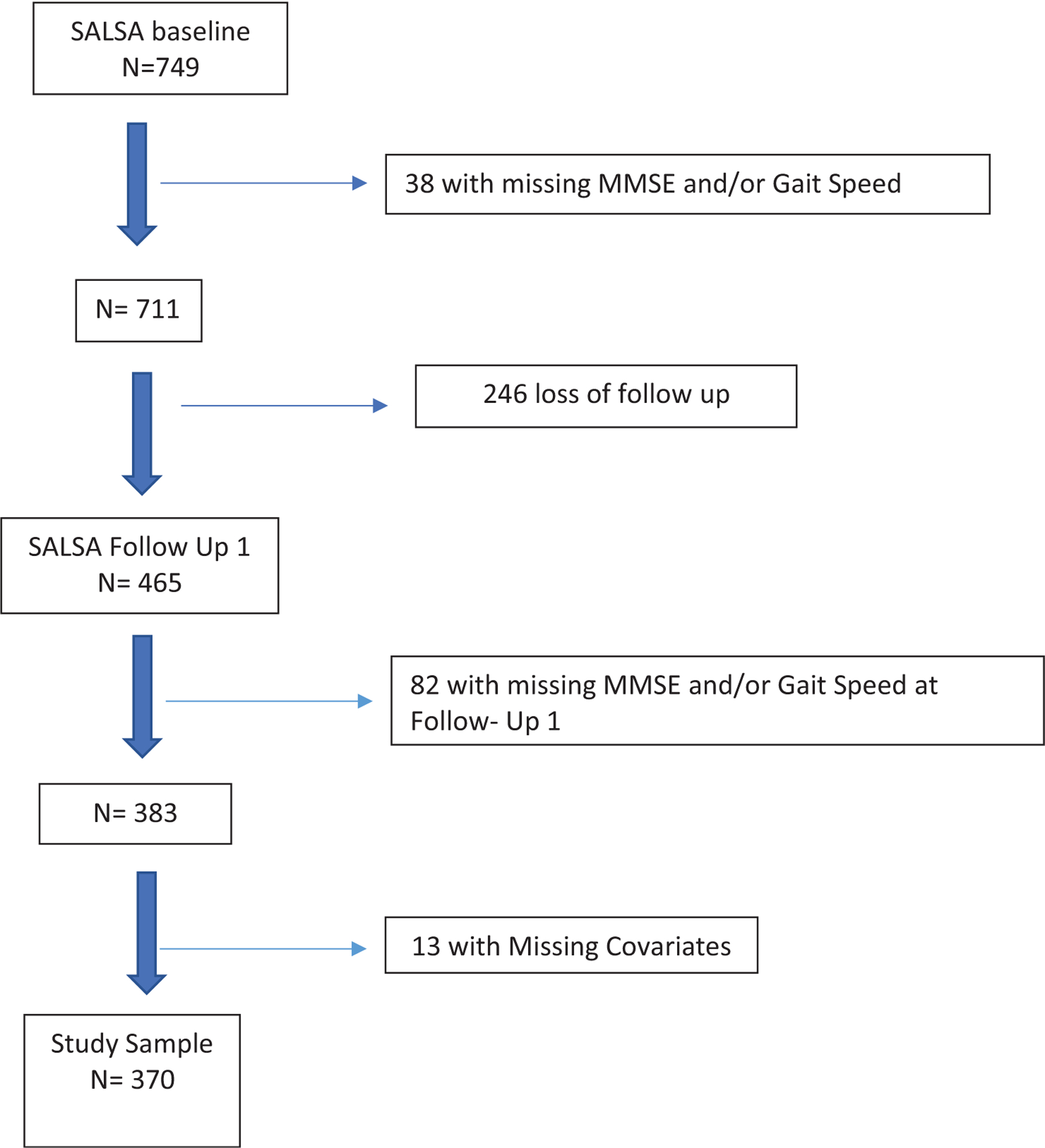
Chart of the included participants relative to the total San Antonio longitudinal study of aging (SALSA) cohort
TABLE 1.
Demographic and clinical characteristics of the included participants relative to the entire San Antonio longitudinal study of aging sample
| Included sample (n = 370) | Excluded sample (n = 379) | P-value | |
|---|---|---|---|
| Age, years | 69.28 ± 3.25 | 70.23 ± 3.48 | <.001* |
| Sex (M:F) | 220:150 | 212:167 | .329 |
| Income category | 12.21 ± 2.83 | 10.96 ± 3.13 | <.001* |
| Education, years | 11.87 ± 3.91 | 10.19 ± 4.72 | <.001* |
| Ethnicity (Mexican American:European American) | 182:188 | 175:204 | .065 |
| Body mass index, kg/m2 | 28.28 ± 4.90 | 28.47 ± 5.61 | .620 |
| Diabetes, (%) | 16.80 | 31.00 | <.001* |
| Hypertension, (%) | 47.84 | 53.89 | .099 |
| Coronary artery disease (%) | 17.84 | 24.01 | .037* |
| MMSE total score | 27.03 ± 2.76 | 25.03 ± 4.13 | <.001* |
| Gait speed, m/sec | 0.92 ± 0.20 | 0.79 ± 400.28 | <.001* |
Note: Group differences were analyzed using independent t-tests for continuous measures and the chi-squared statistic for categorical measures. All values represent mean ± SD unless otherwise noted. Income Category: 11 = monthly household income of $1250–1499 and 13 = monthly household income of $2000 to $2499.
Abbreviation: MMSE, mini-mental status examination.
2.2 |. Procedures
At the baseline and follow-up examinations, participants completed a comprehensive home-based assessment within their residences and a performance-based assessment at a clinical research center. The assessments were administered in English or Spanish by trained bilingual research staff in alignment with participants’ language preferences. Approximately 25% of the Mexican American participants completed the evaluations in Spanish.32
2.3 |. Measures
2.3.1 |. Sociodemographic factors
Sociodemographic factors including age, sex, years of formal education, and monthly household income were obtained from self-report. To alleviate participant discomfort in reporting specific incomes, monthly household income was categorized in fifteen classes as follows: (a). $0–49; (b). $50–99; (c). $100–149; (d). $150–199; (e). $200–299; (f). $300–399; (g). $400–499; (h). $500–749; (i). $750–999; (j). $1000–1249; (k). $1250–1499; (l). $1500–1999; (m). $2000–2499; (n). $2500–2999; (o). ≥$3000.
2.3.2 |. Cognitive function
Global cognition was assessed using English or Spanish versions of the Folstein Mini-Mental State Examination (MMSE).33 The MMSE is comprised 30 items, which assess orientation to time and place (10-points), attention (5-points), registration (3-points), recall (3-points), language (8-points), and visuoconstructional abilities (1-point). The measure has been previously validated in Mexican Americans and European Americans.32,34,35
2.3.3 |. Gait speed
Participants were instructed to walk at their usual pace over a 10-ft distance beginning from a standing position. Gait speed was calculated as distance in meters divided by time in seconds. The faster of two trials was used for analysis.
2.3.4 |. Health and chronic medical conditions
Weight in kilograms and height in centimeters were obtained for calculation of body mass index (BMI). Diabetes mellitus was categorized based on the American Diabetes Association criteria (fasting blood glucose ≥126 mg/dL and/or currently taking glucose lowering medication).36 Hypertension was defined as systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg and/or current antihypertensive medication based on the sixth report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure.37 Coronary artery disease (CAD) was classified based on the presence of ischemic electrocardiogram abnormalities and/or angina pectoris as measured by the Rose questionnaire.38,39
2.3.5 |. Statistical analyses
Ethnic group differences in demographic and clinical factors were assessed with independent samples t-tests for continuous variables and the chi-squared statistic for categorical variables. Distinct classes associated with the joint trajectories of cognition and gait speed changes were identified using latent growth mixture modeling (LGMM).40 LGMM is an exploratory multivariable statistical technique, similar to cluster analysis, which enables identification of distinct latent classes associated with repeated measures of continuous outcomes while adjusting for covariates.41 As a part of the LGMM specification, odds ratios of the class membership associated with covariates are assessed by a multinomial logistic regression model. For the current study, the unobserved latent classes were determined based on heterogeneity of both means and within-subject variability for cognition and gait speed over repeated measures from baseline to Follow-up 3. Age, sex, ethnicity, education, income, BMI, diabetes, hypertension, and CAD at baseline were included as predictors of class membership in a single multivariable model in the LGMM analysis. For exploratory analyses, interactions of ethnicity with income, education, and diabetes for class membership were conducted. The growth parameters estimated from LGMM were used to obtain the mean trajectory for each class, where the time covariates reflect time intervals between adjacent repeated measures. The optimal number of classes was determined based on the Bayesian Information Criterion (BIC).42 Each participant’s joint trajectory class membership identified by LGMM analyses was characterized in terms of their propensity score for membership in each trajectory class (or the poster probabilities of class membership conditioned on the participant’s observed data and the LGMM estimates). The pseudoclass technique was used to obtain class estimates for each individual by drawing a random sample from a multinomial distribution with probabilities being the propensity scores of class membership. For example, in a model with two latent classes, propensity scores of 0.75 and 0.25 for a given individual suggest that he or she is likely to belong to the first class with a probability of 0.75 (75%) and to the second class with a probability of 0.25 (25%). These pseudoclasses can then be used to derive unbiased estimates for each trajectory class including class-specific trajectory means. Specifically, the class-specific empirical means of MMSE and gait speed were obtained by first estimating the pseudoclass membership for each individual by drawing a random sample from a multinomial distribution. Finally, the means of MMSE and gait speed were calculated for each pseudoclass Additionally, logistic regression was employed to examine potential differences in mortality across the derived classes with adjustment for age, sex, ethnicity, education, income, BMI, diabetes, hypertension, and CAD at baseline.
3 |. RESULTS
3.1 |. Participant characteristics
Table 2 displays participant characteristics at baseline by ethnic group. As compared to European Americans, Mexican Americans were younger and had lower incomes and educational attainment, higher BMI, and increased prevalence of diabetes. There were no ethnic group differences in sex distribution or prevalence of CAD. The Mexican American group also had lower average MMSE scores and slower gait speed at the baseline examination.
TABLE 2.
Demographic and clinical characteristics of Mexican American and European American groups at baseline (N = 370)
| Mexican American (n = 182) | European American (n = 188) | P-value | |
|---|---|---|---|
| Age, years | 68.67 ± 2.92 | 69.86 ± 3.44 | <.001* |
| Sex (M:F) | 73:109 | 77:111 | .868 |
| Income category | 11.15 ± 3.17 | 13.24 ± 1.99 | <.001* |
| Education, years | 10.16 ± 4.36 | 13.53 ± 2.49 | <.001* |
| Body mass index, kg/m2 | 29.19 ± 4.79 | 27.40 ± 4.85 | <.001* |
| Diabetes, (%) | 23.08 | 7.45 | <.001* |
| Hypertension, (%) | 44.5 0 | 51.1 0 | .20 |
| Coronary artery disease (%) | 15.38 | 20.21 | .225 |
| MMSE total score | 25.91 ± 3.15 | 28.11 ± 1.75 | <.001* |
| Gait speed, m/sec | 0.88 ± 0.20 | 0.95 ± 40.19 | <.001* |
Note: Group differences were analyzed using independent t-tests for continuous measures and the chi-squared statistic for categorical measures. All values represent mean ± SD unless otherwise noted. Income Category: 11 = monthly household income of $1250–1499 and 13 = monthly household income of $2000 to $2499.
Abbreviation: MMSE, mini-mental status examination.
3.2 |. Joint trajectory classes for cognition and gait speed
A three-class solution was identified as the optimal model among models that converged. Constraints on growth parameters were imposed to achieve model convergence (reliable estimates of standard errors) of 3-class solutions. The best-fit three-class solution obtained the lowest BIC score, and the Lo-Mendal-Rubin likelihood ratio test was significant when compared to models of two-class solutions (P < .001). The first trajectory class, comprising 65.4% of the sample, displayed relatively stable cognition and gait speed over time and was labeled as the stable cognition and gait class. The second trajectory class, comprising 22.2% of the sample, demonstrated deteriorating cognition and gait speed over time and was labeled as the cognitive and physical vulnerability class. The third trajectory class included 12.4% of the sample and was characterized by stable cognition and deteriorating gait speed over time. This trajectory was labeled as the physical vulnerability class.
The empirical mean trajectories of global cognition and gait speed for each class are displayed in Table 3 and Figure 2. Participants in the cognitive and physical vulnerability class compared with those in the stable cognition and gait class had a lower mean MMSE score (−5 points) and slower gait speed (−0.19 m/sec) at baseline and demonstrated accelerated decline in cognition and gait speed over an average of the 9.5-year study interval. Participants in the physical vulnerability class had similar mean MMSE scores (28.11 vs 28.12), but slower mean baseline gait speed (−0.16 m/sec) at baseline with more rapid gait slowing over the study follow-up period.
TABLE 3.
Average mini-mental state examination (MMSE) and gait speed performance across the three joint trajectory classes at baseline and follow-up 3
| Stable cognition and gait class (64.5%) | Physical and cognitive vulnerability class (22.2%) | Physical vulnerability class (12.4%) | |
|---|---|---|---|
| MMSE, baseline | 28.13 ± 1.51 | 23.23 ± 2.72 | 28.11 ± 1.61 |
| MMSE, follow-up 3 | 27.27 ± 2.14 | 21.24 ± 3.97 | 27.21 ± 2.65 |
| Gait speed (m/sec), baseline | 0.98 ± 0.18 | 0.79 ± 0.17 | 0.82 ± 0.19 |
| Gait speed (m/sec), follow-up 3 | 0.96 ± 0.18 | 0.57 ± 0.36 | 0.44 ± 0.32 |
FIGURE 2.
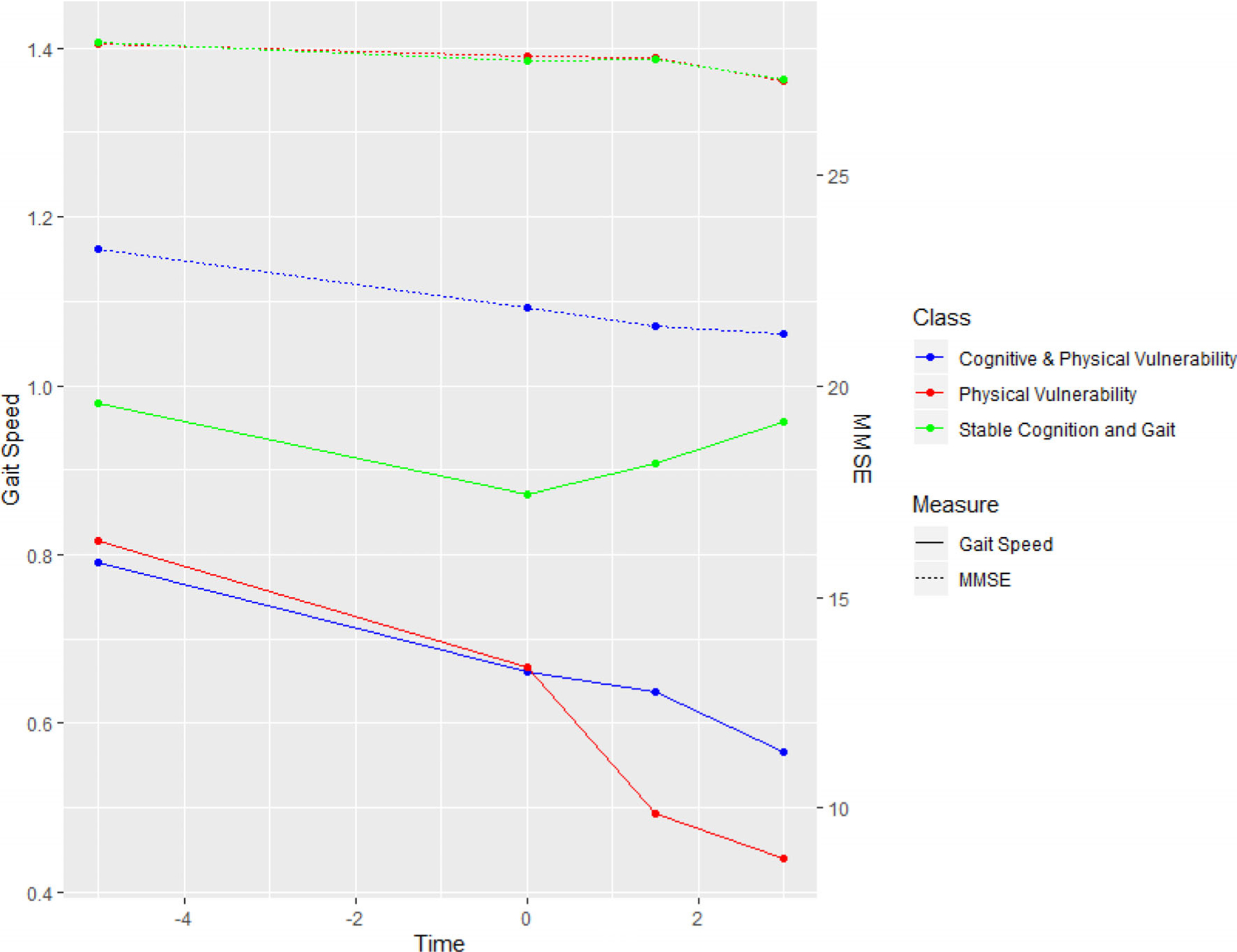
Latent growth mixture modeling results for identification of joint trajectory cognition and gait speed classes. The pseudoclass technique was used to obtain class estimates for each individual with adjustment for age, sex, ethnicity, income category, education, body mass index, diabetes, hypertension, and coronary artery disease
3.3 |. Participant characteristics of joint cognition and gait speed trajectory classes
As seen in Table 4, the odds of classification within the cognitive and physical vulnerability class as opposed to the stable cognition, and gait class were significantly associated with Mexican American ethnicity, older age, lower educational attainment, reduced income, and diabetes. In contrast, the odds of classification within the physical vulnerability class vs the stable cognition, and gait class were significantly associated with female sex and higher BMI.
TABLE 4.
Odds ratios of participant characteristics associated with the cognitive and physical vulnerability class and physical vulnerability class relative to the stable cognition and gait class
| Cognitive and physical vulnerability odds ratio (P-value) | Physical vulnerability odds ratio (P-value) | |
|---|---|---|
| Age, years | 1.186* (.017) | 1.028 (.680) |
| Female sex | 2.237 (.128) | 6.481 (.004) |
| Mexican American ethnicity | 3.771 (.016) | 0.338(.068) |
| Income category | 0.828 (.029) | 0.913 (.373) |
| Education, years | 0.703 (<.001) | 1.035 (.772) |
| Body mass index, kg/m2 | 0.999 (.993) | 1.118 (.025) |
| Diabetes | 4.547 (.010*) | 3.248 (.098) |
| Hypertension | 0.727 (.478) | 1.309 (.577) |
| Coronary artery disease | 1.727 (.396) | 2.749 (.061) |
Note: P < .05;
odds ratios of the class membership were assessed by a single multivariable logistic regression with inclusion of all listed variables as covariates.
In exploratory analyses, there were no statistically significant interactions between ethnicity with income, education, and diabetes for class membership (all P > .05).
3.4 |. Mortality outcomes of joint cognition and gait speed trajectory classes
Logistic regression adjusted for covariates indicated significant differences in mortality across the three classes throughout the study period (chi-square = 13.283, P = .0013). Relative to the stable cognition and gait class, the physical vulnerability class had a 7-fold increased risk of mortality (odds ratio = 7.249 [95% CI: 2.23–23.58]). The cognitive and physical vulnerability class had an approximate 5-fold increased risk of mortality relative to the successful aging class (odds ratio = 5.08 [95% CI: 1.45–17.75]).
4 |. DISCUSSION
In a community-based sample of Mexican American and European American older adults, three distinct joint cognitive and gait speed trajectory classes were identified. The majority of the sample (64.5%) maintained relatively stable cognition and gait speed across the mean 9.5-year follow-up interval and were labeled the stable cognition and gait class. In contrast, 22.2% of the sample demonstrated pronounced deterioration in both health domains and were labeled the cognitive and physical vulnerability class. Finally, a sizable minority of the sample (12.4%) evidenced decline in gait speed while maintaining relatively stable cognition and were labeled the physical vulnerability class. While there was significant heterogeneity in the rate of change across the sample, distinct factors predicted more rapid deterioration. Specifically, socioeconomic factors including lower education and income were associated with a progressive declining course. Moreover, even after adjustment for socioeconomic factors and chronic medical conditions, Mexican Americans were almost four times more likely than European Americans to be classified in the joint cognitive and physical vulnerability class. Categorization in the the joint cognitive and gait speed trajectory classes was highly clinically meaningful. Across the 9.5-year study follow-up period, individuals classified in the cognitive and physical vulnerability class or physical vulnerability class had a five-to seven-fold increased risk of mortality relative to the stable cognition and gait class.
For most of the sample, temporal changes in cognition and gait speed were parallel — characterized by joint stability or decline over time. These results are consistent with cross-sectional investigations demonstrating strong associations between gait speed and cognition.12–16 Additionally, longitudinal studies have provided support for bidirectional associations between the two health domains.19,21 Several shared mechanisms may account for the observed interdependent relationship. Chronic small vessel ischemic disease is a risk factor for both cognitive decline and gait slowing.43–45 White matter pathology may contribute to declines in both domains through disconnection of cortical regions integral to both cognition and gait coordination.46–48 Abnormal amyloid beta and tau deposition, the hallmarks of Alzheimer’s disease, predict progressive cognitive decline and gait slowing.49–52 Abnormal protein deposition propagates neurodegeneration and synaptic loss, which may induce dysfunction in brain regions governing cognition and gait.53 Beyond cerebral pathology, systemic changes in hormonal regulation and cardiovascular functioning have been associated with deterioration in cognition and gait speed.54 It is important to note that while the majority of the sample demonstrated similar slopes across health domains, there was a subgroup that evidenced declines in gait speed, while maintaining relatively stable global cognition. The results highlight the importance of evaluating interindividual variations in temporal health changes that may not be captured by cross-sectional studies or average population trends.24
Another important finding that emerged from the study was the pervasive impact of baseline cognition and gait speed on interval change over time. Specifically, the cognitive and physical vulnerability class displayed poorer global cognition and slower gait speed at the baseline examination followed by an accelerated deteriorating course across the average 9.5-year study interval. Modeling of longitudinal outcomes captured the baseline performance within the trajectory, indicating steeper rates of decline over and above the low starting point. Similar to our findings, White et al. reported strong associations between baseline gait speed and trajectories of gait speed decline in the Health, Aging, and Body Composition Study.5 The results are clinically relevant as they suggest that participants presenting with poorer performance at baseline may have already begun on a progressive downward course. Alternatively, underlying and unmeasured factors may be contributing to both lower baseline scores and rate of decline.55 Nonetheless, the results suggest preventive efforts should ideally target young and middle-aged adults as declining trajectories may initiate or be caused by influences experienced prior to late life.
Within our sample, lower educational attainment and income were independently related to increased risk of classification within the cognitive and physical vulnerability class. The results highlight the enduring impact of disparity on cognitive and physical health outcomes across the lifespan. Consistent with our findings, socioeconomic inequalities including unstable housing, poor nutrition, and reduced access to health care have been found to contribute to health disparities in old age.56,57 Importantly, Mexican Americans were almost four times more likely than European Americans to be classified in the joint cognitive and physical vulnerability class even after adjustment for educational attainment, income, and chronic medical conditions. Akin to our results, Black et al. reported that Hispanic Americans were three times more likely to experience cognitive decline than non-Hispanic whites after adjusting for socioeconomic factors.10 Many Mexican Americans contend with higher levels of perceived discrimination, which has been associated with elevated stress levels and adverse health outcomes.58 Over the lifespan, repeated exposure to discrimination and related stressors may cumulatively contribute to progressive cognitive and physical decline, increasing vulnerability for disability.26 Health disparities also contribute to differential prevalence of chronic diseases. In our sample, type 2 diabetes was significantly elevated in the Mexican American (23%) group as compared to the European American (7%) group. As displayed in Table 3, diabetes was associated with a 4.5-fold increase of classification within the cognitive and physical vulnerability class after adjustment for other covariates. Similar to our findings, diabetes has been associated with accelerated cognitive and physical decline in other studies,59,60 which may be attributable to ischemic white matter damage.46,61
A distinguishing feature of the physical vulnerability class relative to the other trajectory classes was the reduced concordance between changes in cognition and gait speed over time. While the central nervous system plays an important role in gait coordination, several other physiological parameters have been linked to slower gait speed including loss of bone density, lower muscle mass, degenerative joint disease, and orthopedic injury.62 In our sample, female gender and higher BMI were risk factors for categorization within the physical vulnerability class. Prior investigations have reported sex differences in several gait parameters.63,64 Declining estrogen levels contribute to bone loss,65 which may be one mechanism placing women at higher risk for physical decline. Elevated BMI has also been associated with more rapid gait slowing in longitudinal studies.66,67 Elevated BMI may be related to a sedentary lifestyle, contributing to physical deconditioning, loss of muscle mass, and lower aerobic capacity.66,68 Excess body weight also places strain on muscles and joints, which may further hinder movement and slow gait speed.66
The results of the present study must be interpreted in the context of the limitations. First, cognitive outcomes were only assessed with the MMSE. While the MMSE is one of the most frequently used cognitive measures in both clinical and research settings, it is susceptible to ceiling effects that limit sensitivity.69 To help mitigate this factor, the MMSE was scored only using serial sevens rather than WORLD backwards as our previous research has demonstrated that this approach increases within group variability and maximizes sensitivity.35 Further research is needed examining joint cognitive and gait speed trajectories with specific cognitive domains as prior studies have demonstrated specific associations with executive function, processing speed, and memory.14–16,19–21,70 It is also important to note that participants were lost to follow-up due to mortality and other causes of attrition across the follow-up period. Due to survival biases, our study may have underestimated the magnitude of cognitive and physical decline in the sample. Finally, our sample was comprised of Mexican Americans and European Americans residing in South Texas studied between the years of 1992 and 2005. Thus, our results may not fully generalize to other geographic regions and time periods. Within San Antonio, the proportion of individuals of Hispanic ethnicity increased by 24.9% from 2000 to 2010, the proportion of individuals with less than a high school degree only marginally changed from 2000 to 2017 (25% vs 18%), and median income growth was amongst the slowest in the state of Texas with 18.6% of the population living in poverty from 2014 to 2018 (US Census Bureau). Thus, studies on ethnic and socioeconomic disparities and their impact on health remain highly relevant to the region.
In summary, Mexican American and European American older adults displayed substantial heterogeneity in joint cognitive and gait speed trajectories across the 9.5-year study interval. Individuals with lower scores at the baseline examination evidenced increased likelihood of a progressive declining course, underscoring the importance of initiating interventions in early and mid-life. Exploration of distinct multidomain health trajectories further enabled identification of high-risk subgroups. Within our sample, lower educational attainment, reduced income, and Mexican American ethnicity independently conferred vulnerability for accelerated cognitive and gait speed decline. The pervasive impact of socioeconomic status and ethnicity for shaping multidomain health trajectories highlights the need for tailored approaches to alleviate health disparities and preserve functional independence across the lifespan.
Key Points.
In a community-based sample of Mexican American and European American older adults, three distinct joint cognitive and gait speed trajectory classes were identified.
Mexican American ethnicity, older age, lower educational attainment, reduced income, and diabetes were associated with accelerated cognition and gait speed decline.
Female sex and higher body mass index were associated with more rapid gait speed slowing in the context of relatively stable global cognition.
ACKNOWLEDGEMENTS
The SALSA study was supported by National Institute on Aging (NIA) Grant 1-R01-AG10444, NIA Grant 1-R01-AG16518, National Institute of Diabetes and Digestive and Kidney Diseases Grant 1-K25 DK075092, and National Center for Research Resources Grant M01-RR01346.
Funding information
National Center for Research Resources, Grant/Award Number: M01-RR01346; National Institute of Diabetes and Digestive and Kidney Diseases, Grant/Award Number: 1-K25 DK075092; National Institute on Aging, Grant/Award Numbers: 1-R01-AG10444, 1-R01-AG16518
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Prevention CDC. Trends in aging—United States and worldwide. MMWR Morb Mortal Wkly Rep. 2003;52:101–104. [PubMed] [Google Scholar]
- 2.Mitnitski A, Song X, Rockwood K. Assessing biological aging: the origin of deficit accumulation. Biogerontology. 2013;14:709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abellan Van Kan G, Rolland Y, Andrieu S, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13:881–889. [DOI] [PubMed] [Google Scholar]
- 4.Rothman MD, Leo-Summers L, Gill TM. Prognostic significance of potential frailty criteria. J Am Geriatr Soc. 2008;56:2211–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White DK, Neogi T, Nevitt MC, et al. Trajectories of gait speed predict mortality in well-functioning older ddults: The Health, Aging and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2012;68: 456–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Himann JE, Cunningham DA, Rechnitzer PA, Paterson DH. Age-related changes in speed of walking. Med Sci Sports Exerc. 1988;20:161–166. [DOI] [PubMed] [Google Scholar]
- 7.Andel R, Hyer K, Slack A. Risk factors for nursing home placement in older adults with and without dementia. J Aging Health. 2007;19: 213–228. [DOI] [PubMed] [Google Scholar]
- 8.McGuire LC, Ford ES, Ajani UA. Cognitive functioning as a predictor of functional disability in later life. Am J Geriatr Psychiatry. 2006;14: 36–42. [DOI] [PubMed] [Google Scholar]
- 9.Di Carlo A, Baldereschi M, Amaducci L, et al. Cognitive impairment without dementia in older people: Prevalence, vascular risk factors, impact on disability. The Italian Longitudinal Study on Aging. J Am Geriatr Soc. 2000;48:775–782. [DOI] [PubMed] [Google Scholar]
- 10.Black SA, Rush RD. Cognitive and functional decline in adults aged 75 and older. J Am Geriatr Soc. 2002;50:1978–1986. [DOI] [PubMed] [Google Scholar]
- 11.Alzheimer’s Association. 2018 Alzheimer’s disease facts and figures. Alzheimers Dement. 2018;14:367–429. [Google Scholar]
- 12.Clouston SAP, Brewster P, Kuh D, et al. The dynamic relationship between physical function and cognition in Longitudinal Aging Cohorts. Epidemiol Rev. 2013;35:33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitzpatrick AL, Buchanan CK, Nahin RL. Associations of gait speed and other measures of physical function with cognition in a healthy cohort of elderly persons. J Gerontol A Biol Sci Med Sci. 2007;62(11):1244–1251. [DOI] [PubMed] [Google Scholar]
- 14.Ble A, Volpato S, Zuliani G, et al. Executive function correlates with walking speed in older persons: the InCHIANTI study. J Am Geriatr Soc. 2005;53:410–415. [DOI] [PubMed] [Google Scholar]
- 15.Holtzer R, Verghese J, Xue X, Lipton RB. Cognitive processes related to gait velocity: results from the Einstein aging study. Neuropsychology. 2006;20:215–223. [DOI] [PubMed] [Google Scholar]
- 16.Watson NL, Rosano C, Boudreau RM, et al. Executive function, memory, and gait speed decline in well-functioning older adults. J Gerontol A Biol Sci Med Sci. 2010;65:1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verghese J, Annweiler C, Ayers E, et al. Motoric cognitive risk syndrome: multicountry prevalence and dementia risk. Neurology. 2014; 83:718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verghese J, Wang C, Lipton RB, Holtzer R. Motoric cognitive risk syndrome and the risk of dementia. J Gerontol A Biol Sci Med Sci. 2013; 68:412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gale CR, Allerhand M, Sayer AA, Cooper C, Deary IJ. The dynamic relationship between cognitive function and walking speed: the English longitudinal study of ageing. Age. 2014;36:9682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camargo EC, Weinstein G, Beiser AS, et al. Association of physical function with clinical and subclinical brain disease: the Framingham offspring study. J Alzheimers Dis. 2016;53:1597–1608. [DOI] [PubMed] [Google Scholar]
- 21.Krall JR, Carlson MC, Fried LP, Xue QL. Examining the dynamic, bidirectional associations between cognitive and physical functioning in older adults. Am J Epidemiol. 2014;180:838–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang WC, Lu FP, Lan TY, Wu SC. Multidimensional health-transition patterns among a middle-aged and older population. Geriatr Gerontol Int. 2013;13:571–579. [DOI] [PubMed] [Google Scholar]
- 23.Hsu HC, Jones BL. Multiple trajectories of successful aging of older and younger cohorts. Gerontologist. 2012;52:843–856. [DOI] [PubMed] [Google Scholar]
- 24.Wickrama K, Mancini JA, Kwag K, Kwon J. Heterogeneity in multidimensional health trajectories of late old years and socioeconomic stratification: a latent trajectory class analysis. J Gerontol B Psychol Sci Soc Sci. 2012;68:290–297. [DOI] [PubMed] [Google Scholar]
- 25.Downer B, Chen NW, Raji M, Markides KS. A longitudinal study of cognitive trajectories in Mexican Americans age 75 and older. Int J Geriatr Psychiatry. 2017;32:1122–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown TH, O’Rand AM, Adkins DE. Race-ethnicity and health trajectories: Tests of three hypotheses across multiple groups and health outcomes. J Health Soc Behav. 2012;53:359–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guzmán B, McConnell ED. The Hispanic population: 1990–2000 growth and change. Popul Res Policy Rev. 2002;21:109–128. [Google Scholar]
- 28.Quiben MU, Hazuda HP. Factors contributing to 50-ft walking speed and observed ethnic differences in older community-dwelling Mexican Americans and European Americans. Phys Ther. 2015;95: 871–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haas SA, Krueger PM, Rohlfsen L. Race/ethnic and nativity disparities in later life physical performance: The role of health and socioeconomic status over the life course. J Gerontol B Psychol Sci Soc Sci. 2012;67:238–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stern MP, Pugh JA, Gaskill SP, Hazuda HP. Knowledge, attitudes, and behavior related to obesity and dieting in Mexican Americans and Anglos: the San Antonio heart study. Am J Epidemiol. 1982;115: 917–928. [DOI] [PubMed] [Google Scholar]
- 31.Hazuda HP, Comeaux PJ, Stern MP, Haffner SM, Eifler CW, Rosenthal M. A Comparison of three indicators for identifying Mexican Americans in epidemiological research: methodological findings from the San Antonio heart study. Am J Epidemiol. 1986; 123:96–112. [DOI] [PubMed] [Google Scholar]
- 32.Dergance JM, Mouton CP, Lichtenstein MJ, Hazuda HP. Potential mediators of ethnic differences in physical activity in older Mexican Americans and European Americans: results from the San Antonio longitudinal study of aging. J Am Geriatr Soc. 2005;53:1240–1247. [DOI] [PubMed] [Google Scholar]
- 33.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 34.Espino DV, Lichtenstein MJ, Palmer RF, Hazuda HP. Ethnic differences in mini-mental state examination (MMSE) scores: where you live makes a difference. J Am Geriatr Soc. 2001;49:538–548. [DOI] [PubMed] [Google Scholar]
- 35.Espino DV, Lichtenstein MJ, Palmer RF, Hazuda HP. Evaluation of the mini-mental state examination’s internal consistency in a community-based sample of Mexican-American and European-American elders: results from the San Antonio longitudinal study of aging. J Am Geriatr Soc. 2004;52:822–827. [DOI] [PubMed] [Google Scholar]
- 36.American Diabetes Association. 2. classification and diagnosis of diabetes. diabetes care. 2016;39:S13–S22. [DOI] [PubMed] [Google Scholar]
- 37.Joint National Committee on Prevention Detection, and Treatment of High Blood Pressure. The 6th Report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Arch Intern Med. 1997;157:2413–2446. [DOI] [PubMed] [Google Scholar]
- 38.Rose GA. The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bull World Health Organ. 1962;27:645–658. [PMC free article] [PubMed] [Google Scholar]
- 39.Rose GA, Blackburn H, Gillum RF, Prineas RJ. Cardiovascular survey methods. Vol 56. Geneva, Switzerland: WHO; 1982. [Google Scholar]
- 40.Muthén BO. Beyond SEM: General latent variable modeling. Behav Ther. 2002;29:81–117. [Google Scholar]
- 41.Wang CP, Hazuda HP. Better glycemic control is associated with maintenance of lower-extremity function over time in Mexican American and European American older adults with diabetes. Diabetes Care. 2011;34:268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang CP, Hendricks Brown C, Bandeen-Roche K. Residual diagnostics for growth mixture models: examining the impact of a preventive intervention on multiple trajectories of aggressive behavior. J Am Stat Assoc. 2005;100:1054–1076. [Google Scholar]
- 43.Prins ND, van Dijk EJ, den Heijer T, et al. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain. 2005;128:2034–2041. [DOI] [PubMed] [Google Scholar]
- 44.de Laat KF, van Norden AGW, Gons RAR, et al. Gait in elderly with cerebral small vessel disease. Stroke. 2010;41:1652–1658. [DOI] [PubMed] [Google Scholar]
- 45.Smith EE, O’Donnell M, Dagenais G, et al. Early cerebral small vessel disease and brain volume, cognition, and gait. Ann Neurol. 2015;77:251–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Laat KF, Tuladhar AM, van Norden AGW, Norris DG, Zwiers MP, de Leeuw F-E. Loss of white matter integrity is associated with gait disorders in cerebral small vessel disease. Brain. 2010;134:73–83. [DOI] [PubMed] [Google Scholar]
- 47.Tuladhar AM, van Norden AGW, de Laat KF, et al. White matter integrity in small vessel disease is related to cognition. NeuroImage: Clinical. 2015;7:518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valkanova V, Ebmeier KP. What can gait tell us about dementia? Review of epidemiological and neuropsychological evidence. Gait Posture. 2017;53:215–223. [DOI] [PubMed] [Google Scholar]
- 49.Ossenkoppele R, Schonhaut DR, Schöll M, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain. 2016;139:1551–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pontecorvo MJ, Devous MD Sr, Navitsky M, et al. Relationships between flortaucipir PET tau binding and amyloid burden, clinical diagnosis, age and cognition. Brain. 2017;140:748–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schneider JA, Li JL, Li Y, Wilson RS, Kordower JH, Bennett DA. Substantia nigra tangles are related to gait impairment in older persons. Ann Neurol. 2006;59:166–173. [DOI] [PubMed] [Google Scholar]
- 52.Del Campo N, Payoux P, Djilali A, et al. Relationship of regional brain β-amyloid to gait speed. Neurology. 2016;86:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wennberg AMV, Lesnick TG, Schwarz CG, et al. Longitudinal association between brain amyloid-beta and gait in the mayo clinic study of aging. J Gerontol A Biol Sci Med Sci. 2017;73:1244–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robertson DA, Savva GM, Kenny RA. Frailty and cognitive impairment—A review of the evidence and causal mechanisms. Ageing Res Rev. 2013;12:840–851. [DOI] [PubMed] [Google Scholar]
- 55.Glymour MM, Weuve J, Berkman LF, Kawachi I, Robins JM. When Is baseline adjustment useful in analyses of change? An example with education and cognitive change. Am J Epidemiol. 2005;162:267–278. [DOI] [PubMed] [Google Scholar]
- 56.Haas S, Rohlfsen L. Life course determinants of racial and ethnic disparities in functional health trajectories. Soc Sci Med. 2010;70:240–250. [DOI] [PubMed] [Google Scholar]
- 57.Inzitari M, Doets E, Bartali B, et al. Nutrition in the age-related disablement process. J Nutr Health Aging. 2011;15:599–604. [DOI] [PubMed] [Google Scholar]
- 58.Flores E, Tschann JM, Dimas JM, Bachen EA, Pasch LA, de Groat CL. Perceived discrimination, perceived stress, and mental and physical health among Mexican-origin adults. His J Behav Sci. 2008;30:401–424. [Google Scholar]
- 59.Kuo HK, Jones RN, Milberg WP, et al. Effect of blood pressure and diabetes mellitus on cognitive and physical functions in older adults: a longitudinal analysis of the advanced cognitive training for independent and vital elderly cohort. J Am Geriatr Soc. 2005;53:1154–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mayeda ER, Haan MN, Kanaya AM, Yaffe K, Neuhaus J. Type 2 diabetes and 10-year risk of dementia and cognitive impairment among older Mexican Americans. Diabetes Care. 2013;36:2600–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Verdelho A, Madureira S, Moleiro C, et al. White matter changes and diabetes predict cognitive decline in the elderly: the LADIS study. Neurology. 2010;75:160–167. [DOI] [PubMed] [Google Scholar]
- 62.Alexander NB, Goldberg A. Gait disorders: search for multiple causes. Clev Clin J Med. 2005;72:586. [DOI] [PubMed] [Google Scholar]
- 63.Callisaya ML, Blizzard L, Schmidt MD, McGinley JL, Srikanth VK. Sex modifies the relationship between age and gait: a population-based study of older adults. J Gerontol A Biol Sci Med Sci. 2008;63:165–170. [DOI] [PubMed] [Google Scholar]
- 64.S-u K, Tolea MI, Hausdorff JM, Ferrucci L. Sex-specific differences in gait patterns of healthy older adults: results from the Baltimore longitudinal study of aging. J Biomech. 2011;44:1974–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Joseph C, Kenny AM, Taxel P, Lorenzo JA, Duque G, Kuchel GA. Role of endocrine-immune dysregulation in osteoporosis, sarcopenia, frailty and fracture risk. Mol Aspects Med. 2005;26: 181–201. [DOI] [PubMed] [Google Scholar]
- 66.Xu B, Houston DK, Gropper SS, Zizza CA. Race/ethnicity differences in the relationship between obesity and gait speed among older Americans. J Nutri Elderly. 2009;28:372–385. [DOI] [PubMed] [Google Scholar]
- 67.Woo J, Leung J, Kwok T. BMI, Body composition, and physical functioning in older adults. Obesity. 2007;15:1886–1894. [DOI] [PubMed] [Google Scholar]
- 68.Jensen GL. Obesity and functional decline: epidemiology and geriatric consequences. Clin Geriatr Med. 2005;21:677–687. [DOI] [PubMed] [Google Scholar]
- 69.de Jager CA, Milwain E, Budge M. Early detection of isolated memory deficits in the elderly: the need for more sensitive neuropsychological tests. Psychol Med. 2002;32:483–491. [DOI] [PubMed] [Google Scholar]
- 70.Van Lersel MB, Kessels RPC, Bloem BR, Verbeek ALM, Olde Rikkert MGM. Executive functions are associated with gait and balance in community-living elderly people. J Gerontol A Biol Sci Med Sci. 2008;63:1344–1349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


