Abstract
For over a decade Enterocytozoon bieneusi infections in people with AIDS have been linked with chronic diarrhea and wasting. The slow scientific progress in treating these infections is attributed to the inability of investigators to cultivate the parasite, which has also precluded evaluation of effective therapies. We report here successful serial transmissions of E. bieneusi from patients with AIDS and from macaques with AIDS to immunosuppressed gnotobiotic piglets. One infected piglet was still excreting spores at necropsy 50 days after an oral challenge. Spores in feces were detected microscopically by trichrome stain and by PCR and within enterocytes by in situ hybridization and immunohistochemistry. E. bieneusi infection induced no symptoms. The development of an animal model for E. bieneusi will open up new opportunities for investigating this parasite.
The AIDS epidemic has led to the emergence of many infectious agents previously unrecognized or poorly defined. Among these agents are species of the phylum Microspora, including members of the genera Encephalitozoon, Enterocytozoon, Vittaforma, Nosema, and Trachipleistophora (1, 3, 5–7, 15, 20). These obligate intracellular protozoan parasites lack mitochondria, eukaryotic ribosomal characteristics, and Golgi membranes (26). Microsporidia form spores that are highly resistant to environmental influences and possess a unique extrusion apparatus composed of an anchoring disk, polar tubule, and polaroplast that allows them to penetrate and infect eukaryotic cells.
Enterocytozoon bieneusi, the most commonly diagnosed microsporidian in humans (16, 19), was first recognized in biopsy specimens from persons with AIDS in 1985 (6). It has been identified in some 30 to 50% of human immunodeficiency virus (HIV)-infected patients with chronic diarrhea and wasting (9–11, 18, 27) and at higher frequencies in developing countries (2). E. bieneusi infects mostly the gastrointestinal tract, where it becomes localized in the upper small intestine, and in the hepatobiliary tract (26), and it is thought to contribute to chronic diarrhea and wasting in humans with AIDS (17). Infections in HIV-negative immunocompetent individuals have also been reported (24). Little progress has been made in studying the parasite and the disease it induces. The available information is largely circumstantial and is based on limited direct studies in humans. The slow progress in studying these infections is due to the inability to cultivate E. bieneusi in vitro or in vivo, which has markedly curtailed laboratory investigations of E. bieneusi and many aspects of the host-pathogen interaction. The contribution of the immune status of the host to pathogenesis and the clinical manifestations in AIDS remains unclear. The lack of a suitable animal model has also limited the evaluation of chemotherapeutic formulations against E. bieneusi to direct testing in human patients. Consistently effective drugs against E. bieneusi are currently unavailable.
The development of animal models for E. bieneusi infection has been identified as a research priority by the ad hoc subpanel on opportunistic infections of the National Institutes of Health AIDS Research Evaluation Program and would greatly aid the investigation of E. bieneusi pathogenesis and drug therapy. Our effort to develop an animal model, which began more than 4 years ago, led to the first successful transmission and establishment of a persistent E. bieneusi infection in simian immunodeficiency virus (SIV)-infected rhesus macaques (21). We subsequently reported the spontaneous occurrence of E. bieneusi infections in three species of macaques with AIDS (13). We describe here infections and the serial propagation of E. bieneusi obtained from chronically infected human patients and macaques in chemically immunosuppressed gnotobiotic piglets.
MATERIALS AND METHODS
Animals and procedures.
Twenty-nine large white piglets from five consecutive litters were derived by cesarean section and were maintained within microbiological isolators for the duration of the experiment (22). The cesarean-derived piglets were fed a sterile milk replacement diet (Similac Infant Formula; Ross Abbot Laboratories, Columbus, Ohio) given twice daily. They were colostrum deprived and, since piglets receive all their maternal immunoglobulin after birth (28), they were agammaglobulinemic. Twenty of them were immediately started on a course of immunosuppressive therapy for the duration of the experiment; this therapy included a daily oral dose of 15 mg of cyclosporine solution per kg of body weight (Sandoz Pharma Ltd., Basel, Switzerland) and daily intramuscular administration of 25 mg of methylprednisolone sodium per kg (Upjohn, Kalamazoo, Mich.). A total of 23 piglets (20 immunosuppressed and 3 normal) were inoculated within 24 h of delivery with spores of E. bieneusi derived from humans (two isolates), macaques (three isolates), or piglets originally infected with either a human or a macaque isolate (two isolates). The oral inoculum varied from 5 × 103 to 5 × 105 spores per animal and was suspended in 2 ml of phosphate-buffered saline. Infected (23 animals) and control (6 animals) piglets were monitored daily for symptoms, changes in body weight, and shedding of spores in the feces. Piglets were euthanatized at different intervals after infection as detailed in Table 1, and multiple sections from the gastrointestinal tract and viscera were taken for histology, immunohistochemistry, and for in situ hybridization analyses. Mesenteric lymph nodes, distributed throughout the mesentery of the small intestine, were removed aseptically from representative immunosuppressed and normal piglets to perform proliferative assays on B and T lymphocytes.
TABLE 1.
Summary of experimental inoculation of newborn gnotobiotic piglets with spores of E. bieneusi isolates of human (H) or macaque (M) origin or after propagation in piglets (P)
| No. of animals | Inoculum (5 × 103 to 5 × 105) | Immuno-suppressed | Onset of spore/excretion (score)a | Euthanatization point (days postchallenge) |
|---|---|---|---|---|
| 4 | 320/97M | + | 6–8 (3+) | 9, 10, 20, 31 |
| 2 | 320/97P | + | 5–6 (3+) | 28 |
| 2 | 114/97M | + | 8 (3+) | 20 |
| 1 | FP/95Hb | + | 6 (3+) | 12 |
| 4 | FP/95P | + | 5 (3+) | 8, 14, 19 |
| 3 | JC/96H | + | 6 (2+) | 16 |
| 4 | A57/97M | + | 4–6 (2+) | 12, 17 |
| 3 | A57/97M | − | 9–12 (1+) | 14, 17 |
| 2 | Control | + | NA | 14, 50 |
| 4 | Control | − | NA | 21 |
Maximum recorded shedding score over the observation period. 1+, <10 spores identified in the entire smear; 2+, spores readily found in most HP fields; 3+, >2 per HP field. NA, not applicable.
“Isolate” FP/95H was actually a mixture of duodenal aspirates from two persons with AIDS designated BF/95H and WP/95H; these were mixed together because only small quantities of the material, stored over 2 years, were available.
Sources and preparation of E. bieneusi.
Two human isolates were concentrated by centrifugation from duodenal aspirates of individuals with AIDS that had been confirmed to have E. bieneusi by electron microscopy. Isolates FP/95H (a mixture of BF/95H and WP/95H [see Table 1 and Fig. 3]) and ER/95H were obtained from Donald Kotler (St. Luke’s-Roosevelt Hospital Center, New York, N.Y.), and JC/96H was obtained from Louis Weiss (Albert Einstein College of Medicine, New York, N.Y.). Three macaque isolates were obtained from the New England Regional Primate Research Center in Southborough, Mass. The spores from three macaques were aspirated from the gallbladder of euthanatized, terminally ill animals with AIDS and confirmed as E. bieneusi by PCR and in situ hybridization (see below). These included isolates 320/97M, 114/97M, and A57/97M. The isolate designations reflect the year of isolation and whether they were of human (H) or macaque (M) origin. After propagation in piglets, the spores purified from the feces of piglets originally infected with a human or a macaque isolate were designated 320/97P and FP/95P, respectively (Table 1). Regardless of the origin of E. bieneusi, whether from aspirates or fecal specimens, the spores were washed twice with phosphate-buffered saline by suspension and centrifugation at 5,000 × g for 30-min cycles. The total number of spores in a 5-μl volume, smeared and stained with modified trichrome, was counted under high-power (HP) (×1,200 magnification) microscopy. The suspension was then divided among the available number of piglets (Table 1). The shedding of spores in feces was semiquantified as follows: 1+, <10 spores identified under an HP field in approximately 5 μl of fecal smear; 2+, spores readily found in most HP fields; and 3+, >2 spores observed per HP field. Despite rigorous processing of the inocula obtained from humans, macaques, or piglets, some still contained defined yeast and bacterial contaminants. These were monitored at regular intervals (the spectrum of the microorganisms is not shown).
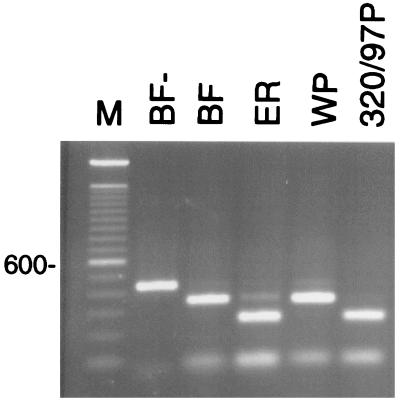
FIG. 3.
Restriction polymorphism in the E. bieneusi ITS region. PCR products amplified from the ribosomal ITS of three human and one pig-propagated isolate were restricted with MspA1I. Notice the shared genotype between the human sample ER and the pig sample 320/97P (see Table 1). The remaining human samples BF and WP share a different genotype at this locus. BF−, undigested PCR product; M, 100-bp ladder. The position of the 600-bp band is shown on the left.
DNA extraction and PCR amplification.
A procedure described previously (2) was used with some modifications. Approximately 200 μl of feces was transferred to a 2-ml screw cap conical tube containing 200 μl of 0.5-mm glass beads (Biospec Products, Inc.) and 400 μl of digestion buffer (100 mM NaCl, 25 mM EDTA, 10 mM Tris-Cl [pH 8.0], 1% sodium dodecyl sulfate, and 100 μg of proteinase K per ml). The sample was then placed in a minibead beater (Biospec) at 5,000 rpm for 2 min and incubated for 1 h to overnight at 50°C. Samples were spun in a microcentrifuge for 2 min at top speed, and the supernatant was then transferred to a new tube and extracted with an equal volume of phenol-chloroform. Next, 300 μl of supernatant was adjusted to 0.7 M. The sample was incubated for 10 min at 65°C. After incubation, the solution was extracted with an equal volume of chloroform, and the DNA was recovered from the resulting supernatant by using the Geneclean system (Bio 101) according to the manufacturer’s protocol for liquid samples except that the DNA was resuspended in 20 μl of TE. PCR amplification was performed on 1 μl of the preparation described above with the EBIER1 and EBIEF1 primers as described by da Silva et al. (4) and cycling parameters consisting of 45 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 40 s in a Mini-cycler (MJ Research, Inc., Watertown, Mass.). Products were visualized in ethidium-stained 1.5% agarose gels. The presence of spores in the feces was also confirmed by the modified trichrome stain method (8, 25).
Detection of E. bieneusi in tissue by in situ hybridization and immunohistochemistry.
To identify E. bieneusi infection in piglet tissue, the in situ hybridization technique we developed and reported previously (2) was used. E. bieneusi small subunit (SSU) rRNA was detected in routinely processed, formalin-fixed, paraffin-embedded tissue sections. Immunohistochemistry analysis was performed as described previously (23) with specific antibodies directed against the spores.
RFLP analysis of the ribosomal internal transcribed spacer.
A restriction fragment length polymorphism (RFLP) assay was developed based on two ribosomal internal transcribed spacer (ITS) sequences deposited in GenBank. The alignment of these sequences (accession numbers L29290 and U61180) revealed several polymorphic restriction sites. PCR products spanning the entire ITS sequence were generated from three human isolates and the pig-passaged isolate 320/97P by using primers located at the 3′ end of the SSU rRNA (5′-ggtcatagggatgaagagc-3′) and the 5′ end of the large subunit (LSU) rRNA gene (5′-ttcgagttctttcgcgctcg-3′). Restriction endonuclease MspA1I, expected to cut once or twice depending on the ITS genotype, was used to digest the ITS amplification products.
RESULTS
The regimen developed in this study induced immunosuppression and was well tolerated by gnotobiotic piglets. The battery of laboratory and molecular tools required to monitor infections with E. bieneusi which we had described earlier (2) were further refined. All 23 challenged animals became infected with E. bieneusi regardless of the origin of the spores or the immune status of piglets. Immunosuppressive treatment, however, led to an earlier onset and to considerably higher quantities of spore shedding in feces, compared to the nonimmunosuppressed animals. Thus, as illustrated in Table 1, four piglets infected with isolate A57/97M shed earlier (4 to 6 days after challenge), with more spores in the feces, than did the other three animals from the same litter (9 to 12 days after challenge), which had received the same isolate but which were not treated with immunosuppressive drugs. The human and macaque isolates equally and readily infected gnotobiotic piglets, and spores concentrated from the feces of infected piglets were equally infectious to other piglets as shown in Table 1. This indicated that E. bieneusi of either origin could potentially be continuously propagated in piglets. This is significant since E. bieneusi from human patients is not readily available and obtaining samples requires endoscopy, a highly invasive technique. This is why we had to use spores that were 2 years old, and the number of spores of the human isolate JC/96H (5 × 103) was only sufficient to infect a single piglet. Spores concentrated from the feces and gut contents of this piglet were then used to infect four animals. The excretion of spore in feces continued in all animals until euthanasia, the longest duration being 50 days (Table 1).
Animals showed little or no symptoms of diarrhea or wasting, as judged by body weight gains that were equal to those of littermate uninfected animals (data not shown) over the observation period. It is conceivable that the lack of symptoms was due to the relatively short observation period, and symptoms may have developed after extended immunosuppressive treatment, as with individuals with AIDS who appear to develop symptoms when their CD4 T-cell counts drop to below 100/mm3 (16, 18).
Animals were euthanatized at different time points (Table 1) in an attempt to detect mucosal lesions and to identify the optimal time for parasite recovery from the tissue (see Fig. 2). No specific mucosal or cellular lesions were identified at any of the time points examined, and in situ hybridization showed a sparse distribution of parasites within enterocytes in the duodenum, in the jejunum, and in the associated lamina propria. The predominant site, however, was the lymphoid tissue-rich terminal ileum, in which multiple lymphoid cells contained parasite antigen beneath the dome-shaped villi. It is not clear whether cells in gut-associated lymphoid tissue were infected or contained parasite antigen. Enterocytes and M cells were also heavily infected in this region of the gut. No evidence of infection in the gallbladder or the hepatobiliary tree was found.
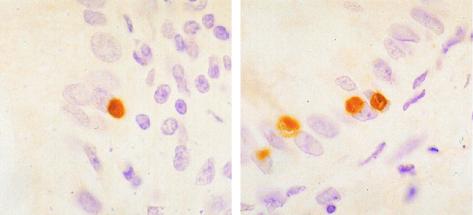
FIG. 2.
In situ hybridization in the small intestine of a piglet infected with isolate 320/97M (Table 1) showing several E. bieneusi plasmodia within the cytoplasm of the enterocytes. Note the characteristic nonstanding clefts in two of them. Magnification, ×200.
Spores were identified by staining fecal smears with modified trichrome and were confirmed by PCR (Fig. 1). Although the number of spores detected varied over time, all animals continued to shed spores daily until they were euthanatized. PCR analysis of fecal DNA normally identified the E. bieneusi spores in the feces a day or so earlier than did microscopy.
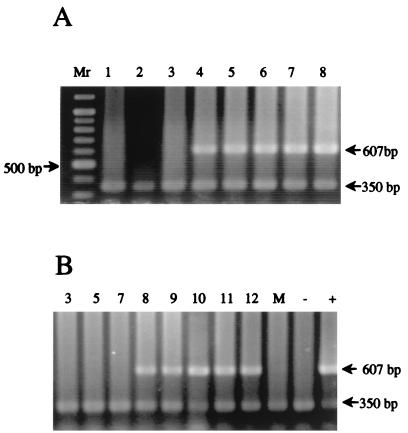
FIG. 1.
PCR amplification of DNA extracted from feces of four piglets infected with E. bieneusi isolate A57/97M (Table 1). Extraction and amplification were performed as described in the text. The numbers indicate days since challenge. Panels A and B show the results from an immunosuppressed and a nonimmunosuppressed piglet, respectively. Mr, 100-bp ladder with a 500-bp fragment indicated. The mock extraction (M), negative (−), and positive (+) controls for this experiment are shown in panel B. The 607-bp band indicates a positive sample, and the 350-bp band is an internal control to prevent false negatives due to PCR inhibition. Lane numbers indicate days postinfection.
The most consistent indication of effective immunosuppression in these animals was the much-atrophied mesenteric lymph nodes at euthanasia compared to those of the control animals. This often resulted in the recovery of insufficient quantities of lymphocytes for proliferative assays. A comparison of the proliferative responses of mesenteric lymph node lymphocytes to T-cell mitogen concanavalin A (ConA) and to B-cell mitogen lipopolysaccharide (LPS) is shown in Table 2 for one such experiment.
TABLE 2.
Analysis of proliferative T- and B-cell responses of the mesenteric lymph node of two immunosuppressed piglets compared with an untreated immunocompetent animala
| Piglet and mutagen | T- and B-cell response (mean cpm ± SD) at:
|
||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| Untreated animal | |||
| Control | 2,452 ± 472 | 2,539 ± 392 | 2,877 ± 1,102 |
| ConA | 78,102 ± 7,399 | 73,069 ± 3,738 | 62,338 ± 6,036 |
| LPS | 5,352 ± 1,575 | 6,225 ± 1,525 | 6,745 ± 2,520 |
| Immunosuppressed animal 1 | |||
| Control | 136 ± 61 | 111 ± 27 | 174 ± 31 |
| ConA | 193 ± 60 | 195 ± 23 | 193 ± 65 |
| LPS | 155 ± 44 | 176 ± 41 | 141 ± 11 |
| Immunosuppressed animal 2 | |||
| Control | 157 ± 17 | 184 ± 24 | 201 ± 38 |
| ConA | 221 ± 16 | 198 ± 40 | 226 ± 102 |
| LPS | 118 ± 13 | 148 ± 33 | 143 ± 28 |
Mutagens used included ConA for T cells and LPS for B cells.
Genotyping of piglet-propagated E. bieneusi by RFLP analysis of the ribosomal internal transcribed spacer.
To investigate potential genetic differences between E. bieneusi recovered from patients and that from experimentally infected piglets, an RFLP analysis of the ribosomal ITS was carried out. As shown in Fig. 3, two restriction profiles were obtained: one with the pig-propagated macaque isolate 320/97P (identical to 320/97M [data not shown]) and an isolate from one of the three patients (i.e., isolate ER/95H) and one with isolates from the remaining two patients (BF/95H and WP/95H). Because of the similar profiles, BF/95H and WP/95H were mixed to obtain sufficient material to form inoculum FP/95H, which was then given to one piglet (Table 1). The origin of a second, low-mobility band in some piglets is unknown at this point. These data demonstrate not only that genetically diverse E. bieneusi isolates occur in AIDS patients but also that some ITS profiles are shared with those obtained from macaques and that macaque and human isolates infect piglets equally well.
DISCUSSION
Based on the circumstantial evidence, E. bieneusi has been linked with chronic diarrhea and wasting in persons with AIDS (9, 10, 11, 16, 18, 19, 26, 27). Although E. bieneusi was discovered in 1985 (6), little progress has been made in studying the nature of this infection in humans due to the lack of essential tools for laboratory investigations. It was only recently that our group successfully transmitted E. bieneusi of human origin to SIV-infected macaques in which persistent infections, similar to those observed in humans with AIDS, were established (21). The ability to transmit and serially propagate E. bieneusi in piglets should facilitate future investigations.
All challenged piglets became infected with E. bieneusi, regardless of whether the isolates were derived from humans or macaques or were pig propagated. In addition, these experiments demonstrated that cesarean-derived newborn piglets can consistently be immunosuppressed by the procedure used here. Although immunosuppression was not a prerequisite, as we had originally thought, it nevertheless shortened considerably the incubation period and increased the quantity of spores excreted in the feces. The extent of infection, as judged by in situ hybridization and immunohistochemistry, was sparse and confined to the gut, resulting in few or no mucosal lesions. Infected enterocytes and cells with the appearance of macrophages in the lamina propria were scattered throughout the mucosa of the upper small intestine. There was no evidence of infection of the hepatobiliary tree or in the other visceral organs in piglets over the course of these experiments. Based on our findings with piglets and on our observations in naturally infected SIV-negative macaques in which long periods of asymptomatic carriage were evident (13), we believe that prolonged survival of the parasite in the immunocompetent host is due to an intrinsically low-grade infection that appears to stimulate little immune or inflammatory response. In immunocompetent people, asymptomatic low-grade E. bieneusi infections presumably also follow the same natural course. Depending on the nature and extent of the host immune defect, E. bieneusi infection may become somewhat more extensive, as seen in moderately immunosuppressed piglets, or profoundly more extensive, as seen in severely immunodeficient individuals with AIDS (18, 27) or in macaques with AIDS (14). It is possible that in piglets with a more rigorous immunosuppressive regimen sustained over months rather than weeks, extensive mucosal lesions involving the hepatobiliary tract and clinically manifested with diarrhea and wasting can also be induced.
The experiments described here showed both that the spores can remain infectious for at least 2 years when stored at 4°C, although the extent, if any, of the loss of viability over this period remains unknown, and that E. bieneusi can be passed from macaques to piglets, from humans to piglets, and from piglets to other piglets. The piglet model will be useful for the evaluation of therapeutic agents against E. bieneusi. Infected piglets can also be used to generate parasite material for laboratory investigations, including genomic libraries and parasite antigen for specific antibody production.
Since naturally born conventional piglets receive all of their maternal immunoglobulin after birth, gnotobiotic piglets, which are colostrum deprived, are agammaglobulinemic (28). This means they are free of passively acquired and potentially interfering specific antibodies. They are also free of all other competing microorganisms that normally colonize the gut of conventional animals. The absence of maternally acquired protection, on the other hand, makes these piglets extremely susceptible to infections with other microorganisms, including some that under normal conditions are nonpathogenic. Hence, keeping these animals in isolation is essential. On balance, however, this provides a model that can readily be standardized for laboratory investigations with minimum variability.
It was important to ascertain that E. bieneusi strains infecting macaques and humans are genetically related. Our RFLP analysis provided results consistent with this assumption, although the scope of the analysis was limited by the fact that it was based on a single gene. Significantly, the restriction profiles revealed a heterogeneity among the three human samples, one of which was similar to the piglet-propagated macaque isolate, and that piglets appear to be susceptible to both ITS genotypes. Future studies will assess the extent of heterogeneity by using additional genetic markers.
ACKNOWLEDGMENTS
This work was supported in part by NIH grants RR0700 and RR00168.
REFERENCES
- 1.Cali A, Kotler D P, Orenstein J M. Septata intestinalis, n.g. n.sp., an intestinal microsporidian associated with chronic diarrhea and dissemination in AIDS patients. J Protozool. 1993;40:101–112. doi: 10.1111/j.1550-7408.1993.tb04889.x. [DOI] [PubMed] [Google Scholar]
- 2.Carville A, Mansfield K, Widmer G, Lackner A, Kotler D, Wiest P, Gumbo T, Sarbah S, Tzipori S. Development and application of genetic probes for detection of Enterocytozoon bieneusi in formalin-fixed stools and in intestinal biopsy specimens from infected patients. Clin Diagn Lab Immunol. 1997;4:405–408. doi: 10.1128/cdli.4.4.405-408.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chupp G L, Alroy J, Adelman L S, Breen J C, Skolnick P R. Myositis due to Pleistophora (microsporidia) in a patient with AIDS. Clin Infect Dis. 1993;16:15–21. doi: 10.1093/clinids/16.1.15. [DOI] [PubMed] [Google Scholar]
- 4.da Silva A J, Schwartz D A, Visvesvara G S, de Moura H, Slemenda S B, Pieniazek N J. Sensitive PCR diagnosis of infections by Enterocytozoon bieneusi (microsporidia) using primers based on the region coding for small-subunit rRNA. J Clin Microbiol. 1996;34:986–987. doi: 10.1128/jcm.34.4.986-987.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Groote M A, Visvesvara G, Wilson M L, Pieniazek N J. Polymerase chain reaction and culture confirmation of disseminated Encephalitozoon cuniculi in a patient with AIDS: successful therapy with albendazole. J Infect Dis. 1995;171:1375–1378. doi: 10.1093/infdis/171.5.1375. [DOI] [PubMed] [Google Scholar]
- 6.Desportes I, Le Charpentier Y, Galian A, Cochand-Priollet B, Lavergne A, Ravisse P, Modigliani R. Occurrence of a new microsporidian: Enterocytozoon bieneusi n.g., n.sp., in the enterocytes of a human patient with AIDS. J Protozool. 1985;32:250–254. doi: 10.1111/j.1550-7408.1985.tb03046.x. [DOI] [PubMed] [Google Scholar]
- 7.Didier E S, Didier P J, Friedberg D N, Stenson S M, Millichamp J M, Shadduck J A. Isolation and characterization of a new human microsporidian, Encephalitozoon hellem (n.sp.), from three AIDS patients with keratoconjunctivitis. J Infect Dis. 1991;163:617–621. doi: 10.1093/infdis/163.3.617. [DOI] [PubMed] [Google Scholar]
- 8.Didier E S, Orenstein J M, Aldras A, Bertucci D, Rogers L B, Janney F A. Comparison of three staining methods for detecting microsporidia in fluids. J Clin Microbiol. 1995;33:3138–3145. doi: 10.1128/jcm.33.12.3138-3145.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eeftinck-Schattenkerk J K M, Van Gool T, van Ketel R J, Bartelsman J F W M, Kuiken C L, Terpstra W J, Reiss P. Clinical significance of small-intestinal microsporidiosis in HIV-1-infected individuals. Lancet. 1991;337:895–898. doi: 10.1016/0140-6736(91)90215-b. [DOI] [PubMed] [Google Scholar]
- 10.Field A M, Hing M, Milliken S, Marriott D J. Microsporidia in the small intestine of HIV-infected patients: a new diagnostic technique and a new species. Med J Aust. 1993;158:390–394. [PubMed] [Google Scholar]
- 11.Greensom W K, Belitsos P C, Yardley J H, Bartlett J G. AIDS enteropathy: occult enteric infections and duodenal mucosal alterations in chronic diarrhea. Ann Intern Med. 1991;114:366–372. doi: 10.7326/0003-4819-114-5-366. [DOI] [PubMed] [Google Scholar]
- 12.Hollister W S, Canning E U, Weidner E U, Field A S, Kench J, Marriott D J. Development and ultrastructure of Trachipleistophora hominis n.g., n.sp. after in vitro isolation from an AIDS patient and inoculation into athymic mice. Parasitology. 1996;112:143–154. doi: 10.1017/s0031182000065185. [DOI] [PubMed] [Google Scholar]
- 13.Mansfield, K. G., A. Carville, D. Hebert, L. Chalifoux, D. Shvetz, K. C. Lin, S. Tzipori, A. A. Lackner. Persistent infection of normal rhesus macaques (Macaca mulatta) with Enterocytozoon bieneusi localizes to the hepatobiliary tree. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 14.Mansfield K G, Carville A, Shvetz D, MacKey J, Tzipori S, Lackner A A. Identification of an Enterocytozoon bieneusi-like microsporidian parasite in simian immunodeficiency virus-inoculated macaques with hepatobiliary disease. Am J Pathol. 1997;150:1395–1405. [PMC free article] [PubMed] [Google Scholar]
- 15.Margileth A M, Strano A J, Chandra R, Neafie R, Blum M, McCully R M. Disseminated nosematosis in an immunologically compromised infant. Arch Pathol. 1973;95:145–150. [PubMed] [Google Scholar]
- 16.Molina J M, Sarfati B, Beauvais M, Lemann A, Lesourd A, Ferchal F, Casin I, Lagrange P, Modigliani R, Derouin F, et al. Intestinal microsporidiosis in human immunodeficiency virus-infected patients with chronic unexplained diarrhea. J Infect Dis. 1993;167:217–221. doi: 10.1093/infdis/167.1.217. [DOI] [PubMed] [Google Scholar]
- 17.Orenstein J M. Intestinal microsporidiosis. Adv Anat Pathol. 1996;3:46–58. [Google Scholar]
- 18.Pol S, Romana C A, Richard S, Amouyal P, Desportes-Livage I, Carnot F, Pays J, Berthelot P. Microsporidia infection in patients with the human immunodeficiency virus and unexplained cholangitis. N Engl J Med. 1993;328:95–99. doi: 10.1056/NEJM199301143280204. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz D A, Sobottka I, Leitch G L, Cali A, Visvesvara G S. Pathology of microsporidiosis: emerging parasitic infections in patients with acquired immunodeficiency syndrome. Arch Pathol Lab Med. 1996;120:173–188. [PubMed] [Google Scholar]
- 20.Silveira J A, Canning E U. Vittaforma corneae n. comb. for the human microsporidium Nosema corneum Shadduck, Mecoli, Davis & Font, 1990, based on its ultrastructure in the liver of experimentally infected athymic mice. J Eukaryot Microbiol. 1995;42:158–165. doi: 10.1111/j.1550-7408.1995.tb01557.x. [DOI] [PubMed] [Google Scholar]
- 21.Tzipori S, Carville A, Widmer G, Kotler D, Mansfield K G, Lackner A. Transmission and establishment of a persistent infection of E. bieneusi derived from a human with AIDS in SIV-infected rhesus monkeys. J Infect Dis. 1997;175:1016–1020. doi: 10.1086/513962. [DOI] [PubMed] [Google Scholar]
- 22.Tzipori S, Rand W, Griffiths J, Widmer G, Crabb J. Evaluation of an animal model for cryptosporidiosis: therapeutic efficacy of paromomycin and hyperimmune bovine colostrum-immunoglobulin. Clin Diagn Lab Immunol. 1994;1:450–463. doi: 10.1128/cdli.1.4.450-463.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veazey R S, Rosenzweig M, Johnson P, Chalifoux L, Lackner A A. Acute infection of the intestine by pathogenic molecular clones of SIV results in rapid changes in the immunophenotypic composition of gut-associated lymphoid tissues. J Med Primatol. 1995;24:201. [Google Scholar]
- 24.Wanke C A, Degirolami P, Federman M. E. bieneusi infection and diarrheal disease in patients who were not infected with HIV: case report and review. Clin Infect Dis. 1996;23:816–818. doi: 10.1093/clinids/23.4.816. [DOI] [PubMed] [Google Scholar]
- 25.Weber R, Bryan R T, Owen R L, Wilcox C M, Gorelkin L, Visvesvara G S. Improved light microscopic detection of microsporidian spores in stools and duodenal aspirates. N Engl J Med. 1992;326:161–166. doi: 10.1056/NEJM199201163260304. [DOI] [PubMed] [Google Scholar]
- 26.Weber R, Bryan R T, Schwartz D A, Owen R L. Human microsporidial infections. Clin Microbiol Rev. 1994;7:426–461. doi: 10.1128/cmr.7.4.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weber R, Kuster H, Keller R, Bachi T, Spycher M A, Briner J, Russi E, Luthy R. Pulmonary and intestinal microsporidiosis in a patient with the acquired immunodeficiency syndrome. Am Rev Respir Dis. 1992;146:1603–1605. doi: 10.1164/ajrccm/146.6.1603. [DOI] [PubMed] [Google Scholar]
- 28.Wilson M R. Immunologic development of the neonatal pig. J Anim Sci. 1974;38:1018–1021. doi: 10.2527/jas1974.3851018x. [DOI] [PubMed] [Google Scholar]