Abstract
Electrospun nanofibers have been found in many applications such as air/water filtration, performance apparel, drug delivery, and scaffold for tissue engineering and started to be integrated in commercial products, which leads to their exposure to environment. Electrospun nanofibrous material is a relatively new material to microorganism in nature and little is known about the biological implication of interactions between electrospun nanofibrous mats and cellular fungal cells. Herein the interaction between electrospun polyacrylonitrile (ESPAN) nanofibrous mat and representative non-pathogenic/pathogenic cellular yeasts (Saccharomyces cerevisiae and Candida albicans) was investigated. It is demonstrated for the first time that when these cellular yeasts, species of the kingdom fungi, were exposed to ESPAN nanofibrous mat, they exhibited lower growth rate, radical change to morphology, and reduced viability without presence of any chemical antifungal agent. These responses were distinct from the cellular interactions with other forms of PAN materials (e.g. solid film or microfibrous mat). Exploration of mechanism indicated that the interaction between yeast cell and electrospun nanofibrous mat is a complex phenomenon in which both nanofibrous morphology and fiber surface composition/property play significant roles. The inherent anti-yeast and potential anti-fungal functionality of ESPAN nanofibrous mat may make an immediate impact on environmental microorganism and could also benefit the next-generation material design to control microbial growth through solely physical contact.
Keywords: Polyacrylonitrile, Electrospinning, Nanofiber, Anti-fungal
1. INTRODUCTION
Electrospinning is a rapidly developing technique that utilizes electrical force to drive the spinning process and produces fibers with diameters ranging from tens to hundreds of nanometers, which have seen a wide range of applications [1,2]. Although they are not yet as popular as textile fibers in our daily life, electrospun nanofibers are already present in the form of nanofibrous mat in commercial products these days and inevitably encounter microorganism in our environment. The impact of electrospun nanofibers on environmental microorganism such as cellular fungi, however, has not been thoroughly addressed. Little is known about biological interaction between electrospun nanofibrous mat and fungal cell. In this study, the interactions between electrospun nanofibrous mats and representative cellular yeast species were investigated and alterations to morphology, viability and proliferation, and mitochondrial function of the yeast cells when they were cultured with electrospun nanofibrous mats were assessed using standard microbiological assays and confocal microscopy. Two representative yeast species, i.e. Saccharomyces cerevisiae (S. cerevisiae, also known as the baker’s yeast and one of the most intensively studied eukaryotic model organisms) and Candida albicans (C. albicans, an opportunistic pathogenic yeast and a popular model organism to study fungal pathogens) [3], were selected as industrially and biomedically relevant fungal species and the interactions between these fungal species and electrospun polyacrylonitrile (ESPAN) nanofibrous mats were examined.
2. Results and discussion
In addition to the ESPAN nanofibrous mat, two control PAN materials including PAN film and PAN microfibrous mat were also prepared. The ESPAN nanofibrous mat was a non-woven material comprised of fibers with average diameter of ~500 nm. The prepared PAN film exhibited a solid but somewhat crimpled surface. The PAN microfibrous mat was a non-woven material consisted of fibers with average diameter of ~10 μm, i.e. roughly 20 times larger than that of the nanofibrous counterpart. On the control PAN materials, mid-log phase S. cerevisiae strain SK1 exhibited ellipsoidal shape with varied sizes in the range of 3–4 μm, characteristics of normal and healthy cells. There was no appreciable change to the morphology of SK1 cells on either PAN film or PAN microfibrous mat after 30-min contact (Fig. 1a and b). However, radicle change to the SK1 cell morphology was observed after just 30-min exposure to the ESPAN nanofibrous mat. In this case, SK1 cells exhibited a distinctly flattened abnormal morphology (Fig. 1c), a common indication of cell rupture and/or death [4]. The abnormal cell morphology of SK1 yeast cells upon exposure to the ESPAN nanofibrous mat suggested that the ESPAN nanofibrous mat might inhibit S. cerevisiae growth without addition of a fungicide.
Fig. 1.
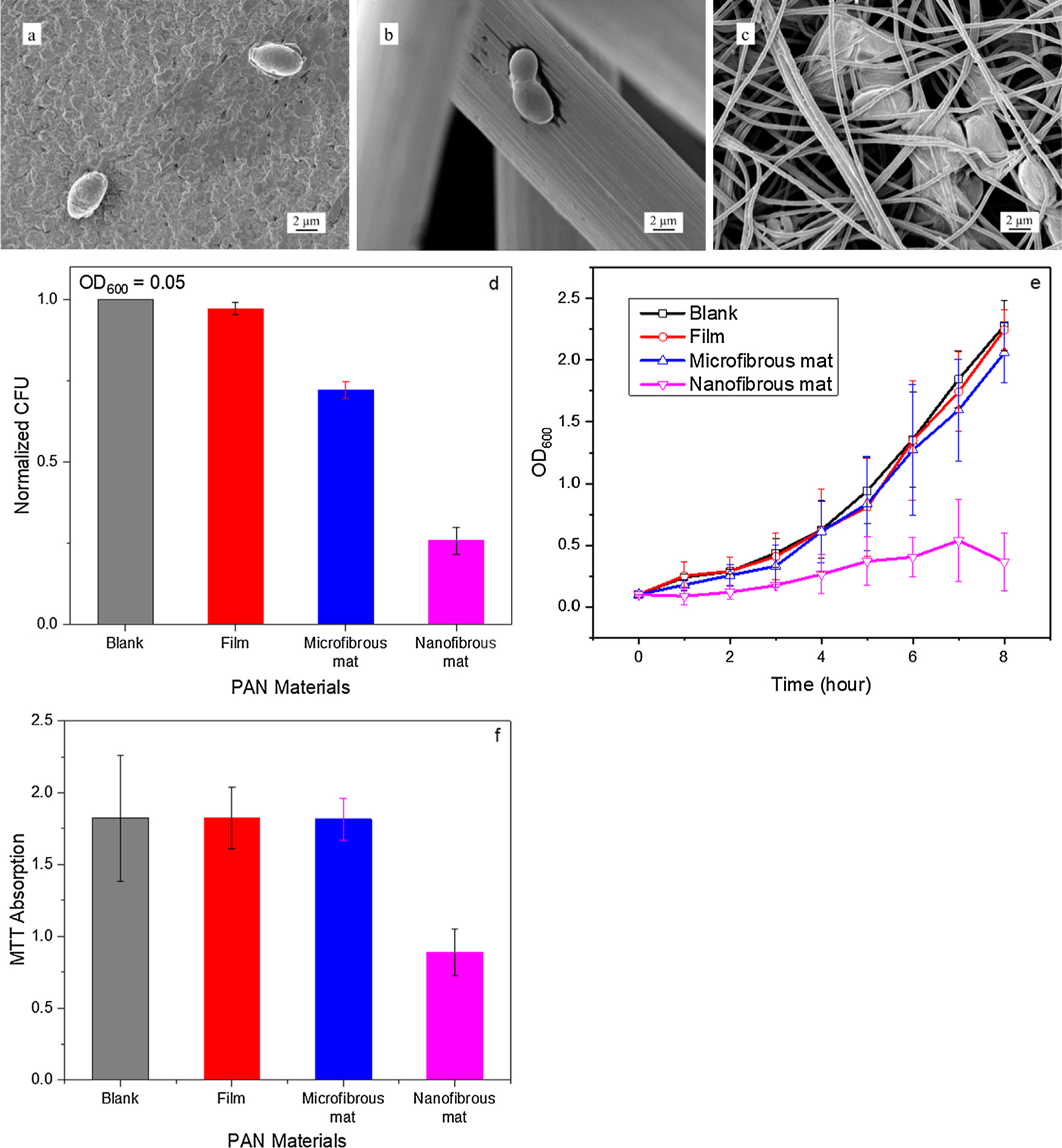
Morphology and viability of S. cerevisiae SK1 cultured on PAN materials. (a–c) Representative SEM images of SK1 cells cultured on PAN film, microfibrous mat, and nanofibrous mat, respectively, after 30-min contact. (d) Normalized CFU of SK1 cultures with different PAN materials starting at after 1-h contact based on the CFU of blank SK1 culture. (e) OD600 variation of SK1 cultures with different PAN materials within 8-h growth period. (f) MTT assay of SK1 cultures with different PAN materials after 8-h contact.
To assess viability of S. cerevisiae strain SK1 cells on the ESPAN nanofibrous mat, a CFU assay was performed. The CFU assay was carried out in SK1 culture grown to an [5] and the CFU was measured after 1-h contact and incubation with corresponding PAN materials. No alteration of CFU was observed when SK1 cells were placed on PAN film and PAN microfibrous mat, but a significant 66% reduction of cell number in the yeast culture was observed with the ESPAN nanofibrous mat (Fig. 1d). This is an indication that the ESPAN nanofibrous mat was capable of significantly reducing number of living cells of S. cerevisiae in culture.
The growth of S. cerevisiae strain SK1 yeast in contact with the ESPAN nanofibrous mat was next monitored using OD600 measurement over the course of 8 h. While SK1 cells demonstrated a standard log growth pattern in blank control culture, SK1 yeast cultured with the ESPAN nanofibrous mat exhibited a significantly slower growth rate (Fig. 1e). This result demonstrated an immediate growth inhibition of S. cerevisiae upon contact with the ESPAN nanofibrous mat. SK1 cultures were further extended to 18 h and a similar trend was observed. In the experiment, the cell growth was initiated with two SK1 yeast cultures: a mid-log phase starting point , and a lag phase starting point. In the blank control culture (without the ESPAN nanofibrous mat), SK1 cells grew robustly in both cases, reaching final OD600 of 2.2 and 0.5 for mid-log phase and lag phase starts, respectively. Again, in presence of the ESPAN nanofibrous mat, significantly reduced OD600 was observed after 18-h culture: OD600 of 0.5 for the mid-log phase start and a negligible OD600 for the lag phase start.
To characterize the inhibition of S. cerevisiae in presence of the ESPAN nanofibrous mat at metabolic level, metabolic activity of SK1 cells was assessed using an MTT assay. SK1 cells that were incubated with the ESPAN nanofibrous mat for 8 h demonstrated a significant reduction in MTT absorbance (Fig. 1f) as compared to those with the control PAN materials.
The lack of proliferation as well as reduced metabolic activity of S. cerevisiae was consistent with the alteration of cell morphology and indicated that the ESPAN nanofibrous mat inhibits the viability of S. cerevisiae upon contact. To test if the number reduction of S. cerevisiae in the cell culture with the ESPAN nanofibrous mat was caused by a trapping/sequestering effect as a result of high inter-fiber porosity of the ESPAN nanofibrous mat, a vital dye (MitoTracker Red CMX/Ros) and confocal microscopy were used to examine whether the contact with the ESPAN nanofibrous mat altered the viability/metabolic activity of SK1 cells. MitoTracker Red accumulates in active mitochondria in an inner mitochondrial membrane dependent fashion [6]. Fluorescence emission intensity of the dye correlates directly with mitochondrial inner membrane potential and low mitochondrial membrane potential correspondingly indicates apoptosis [7,8]. Live cell imaging was performed over a course of 10 min using a Zeiss Axiovision Spinning Confocal Microscope. In control sample, i.e. S. cerevisiae strain SK1 cells on a flat glass substrate, mitochondrial morphology of the cells showed an intricate reticular structure and remained intact with increase of contact time over the 10-min observation period (Fig. 2a). However, SK1 cells that were in contact with the ESPAN nanofibrous mat exhibited a remarkable transformation in mitochondrial morphology, i.e. a rapid reduction of their inner mitochondrial membrane potential as indicated by the reduction of fluorescence intensity of the MitoTracker Red CMX/Ros dye (Fig. 2b). Initially all the SK1 cells that were attached to ESPAN nanofibers in the mat exhibited a normal mitochondria morphology and a similar intensity as those mitochondria found in the cells that were attached to the flat control surface (Fig. 2c). During the course of exposure to the ESPAN nanofibrous mat, the spindle shaped mitochondrion that was found in the attached SK1 cells swelled until becoming diffuse with an almost complete loss of fluorescent signature. These results confirmed that the S. cerevisiae suffered a rapid mitochondrial collapse when come into contact with the ESPAN nanofibrous mat. The alteration in mitochondrial activity appeared to be basis for the changes to the proliferation and overall viability of the S. cerevisiae that we observed.
Fig. 2.
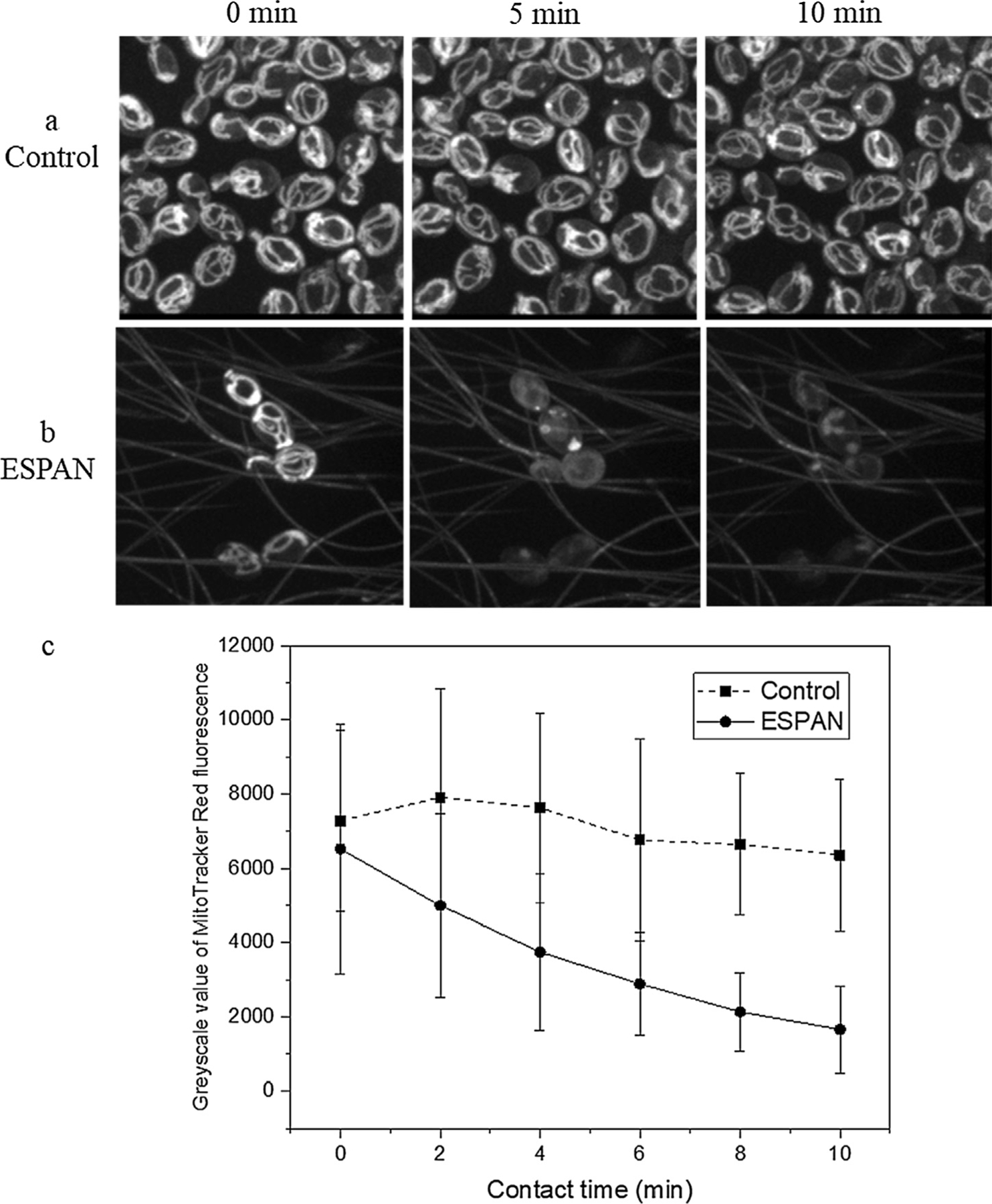
Fluorescent measurement of MitoTracker Red stained S. cerevisiae SK1 cells within 10 min on the ESPAN nanofibrous mat. (a) Control; (b) ESPAN nanofibrous mat; (c) Greyscale value of MitoTracker Red fluorescence.
All above results indicated that the ESPAN nanofibrous mat may have natural anti-yeast/fungal functionality. To explore if this functionality applies to other yeast/fungal species, we also investigated growth behavior of C. albicans upon contact with the ESPAN nanofibrous mat. Strikingly the ESPAN nanofibrous mat demonstrated even more inhibition effect on the growth of C. albicans compared to S. cerevisiae (Fig. 3a). Experimental work has demonstrated that there is a relationship between S. cerevisiae and some nanostructured surface [4], in which spherically capped cylindrical protrusions with aspect ratio ~5 and spacing ~120 nm on a flat surface could generate yeast cell rupturing/death. As in the case of electrospun nanofibrous mat, yeast cells sit in micrometer-scale inter-fiber pores and contact with a few surrounding fibers with diameter of a few hundred nanometers. This is a different environment compared to the reported nanostructured surface. The yeast cells may experience an asymmetrical contact with surrounding ultra-thin fibers (approximately one tenth of the size of the yeast cells) in culture, which might have caused internal stress in the yeast cells during growth and eventually lead to dead/ruptured cells. If this is the case, other electrospun nanofibrous mat with similar fiber size should have similar effect on yeast cells’ growth. To verify this hypothesis, two other electrospun nanofibrous mats including electrospun carbon nanofibrous mat and electrospun cellulose nanofibrous mat, which represented hydrophobic and hydrophilic surface, were prepared and growth behavior of S. cerevisiae SK1 upon contact with these two nanofibrous mats was evaluated (Fig. 3b). Based on similar fiber size, it was observed that the carbon nanofibrous mat showed the least cell inhibition effect (93% OD600 normalized to blank control) and the PAN nanofibrous mat showed the most cell inhibition effect (22% OD600 normalized to blank control) while the cellulose nanofibrous mat showed its cell inhibition effect in the middle (65% OD600 normalized to blank control). These results indicated that the interaction between yeast cell and electrospun PAN nanofibrous mat is too complex to be explained with a single factor. The nanofibrous morphology plays an important role but there are other factors in control. Surface composition and property as well as the resultant cell-surface adhesion should also take effect, which may counteract the nanofibrous morphology effect.
Fig. 3.
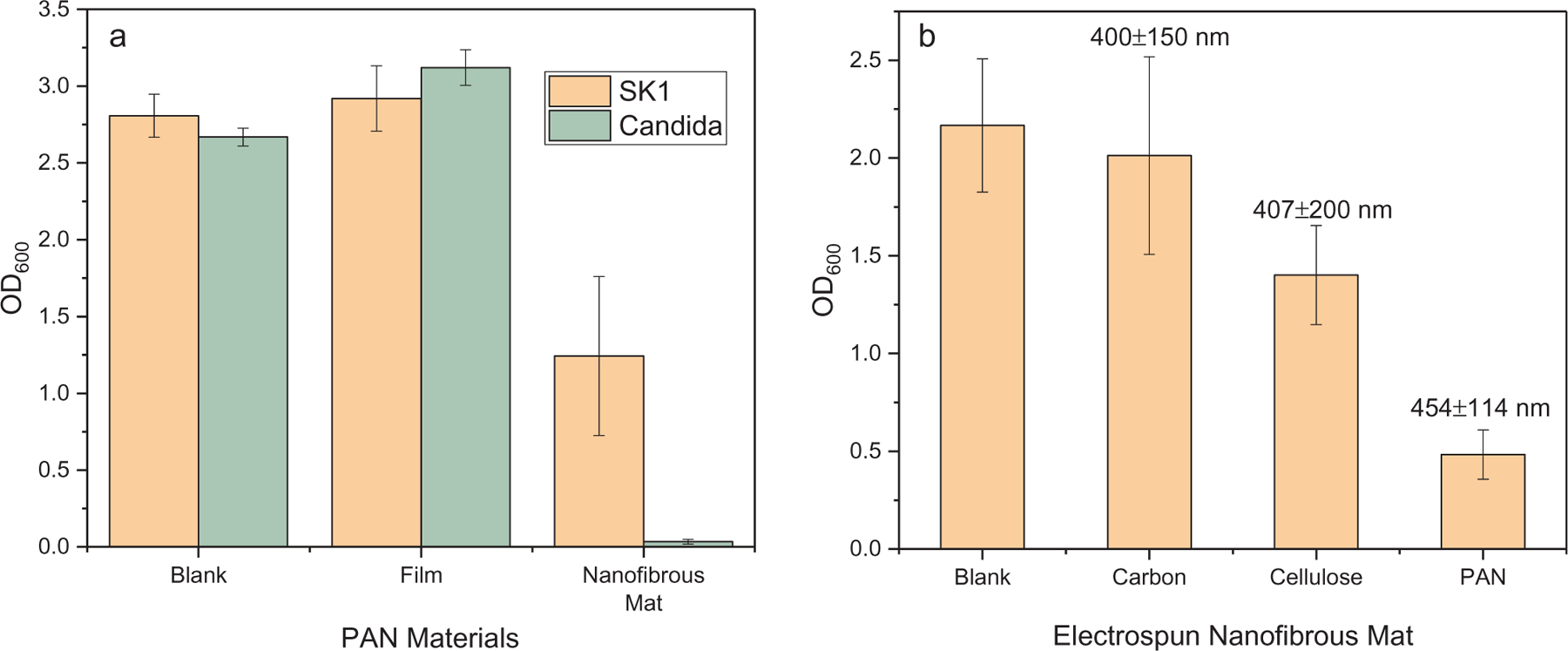
Viability of yeast cells on electrospun nanofibrous mat after 18 h contact. (a) S. cerevisiae SK1 and C. albicans cultured on PAN materials; (b) S. cerevisiae SK1 cultured on different electrospun nanofibrous mats with average fiber sizes marked.
3. Conclusions
Overall our results indicated that electrospun PAN nanofibrous mat may have natural and universal anti-yeast or even anti-fungal functionality. This is an unforeseen biological impact of electrospun nanofibers since electrospun nanofibrous mats have been recognized as scaffold material for tissue engineering for more than a decade [9]. The discovery from this research provided new information to address environmental concerns upon application of electrospun nanofibers, revealed exciting promises to control fungal growth through only physical contact, and could serve as a cautionary tale for unforeseen and unpredicted biological impact of nanomaterials. Further investigation is still going on.
Acknowledgements
This work was supported by the National Science Foundation (CBET-1743607), the National Institutes of Health (1R15EB024921-01), and the Joint School of Nanoscience and Nanoengineering of North Carolina A&T State University and the University of North Carolina at Greensboro, a member of Southeastern Nanotechnology Infrastructure Corridor (SENIC) and National Nanotechnology Coordinated Infrastructure (NNCI) by the National Science Foundation (ECCS-1542174).
Footnotes
Declaration of Competing Interest
There are no conflicts of interest to declare.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.eurpolymj.2019.07.035.
Data availability
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
References
- 1.Dzenis Y, Science 304 (2004) 1917. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal S, Greimer A, Wendorff JH, Adv. Func. Mater 19 (2009) 2863. [DOI] [PubMed] [Google Scholar]
- 3.Kabir MA, Hussain MA, Ahmad Z, ISRN Microbiol 2012 (2012) 538694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nowlin K, Boseman A, Covell A, LaJeunesse D, Soc JR. Interface 12 (2015) 20140999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kregiel D, Berlowska J, Ambroziak W, World J Microbiol. Biotechnol 8 (2012) 3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehrenreich IM, Torabi N, Jia Y, Kent J, Martis S, Shapiro JA, Gresham D, Caudy AA, Kruglyak L, Nature 464 (2010) 1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perry SW, Norman JP, Barbieri J, Brown EB, Gelbard HA, Biotechniques 50 (2011) 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agnello M, Morici G, Rinaldi AM, Cytotechnology 56 (2008) 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li W-J, Laurencin CT, Caterson EJ, Tuan RS, Ko FK, J. Biomed. Mater. Res 60 (2002) 613. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.


