Abstract
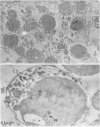
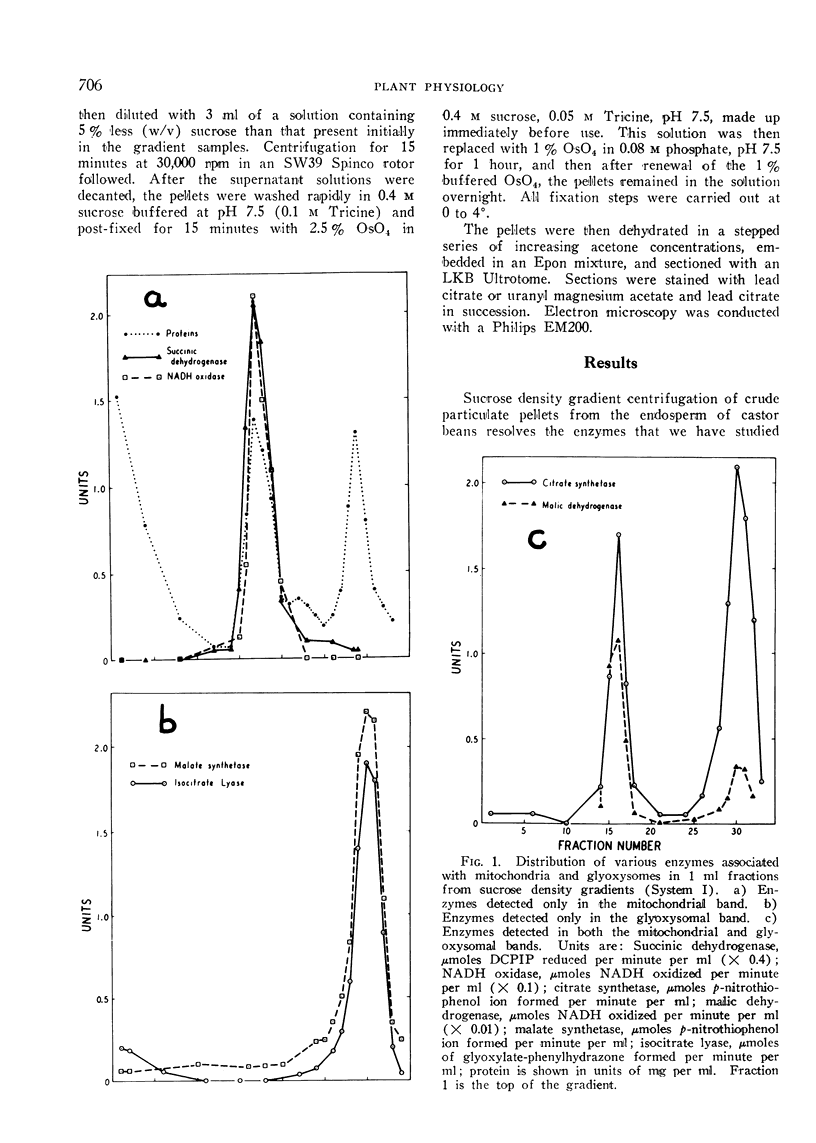
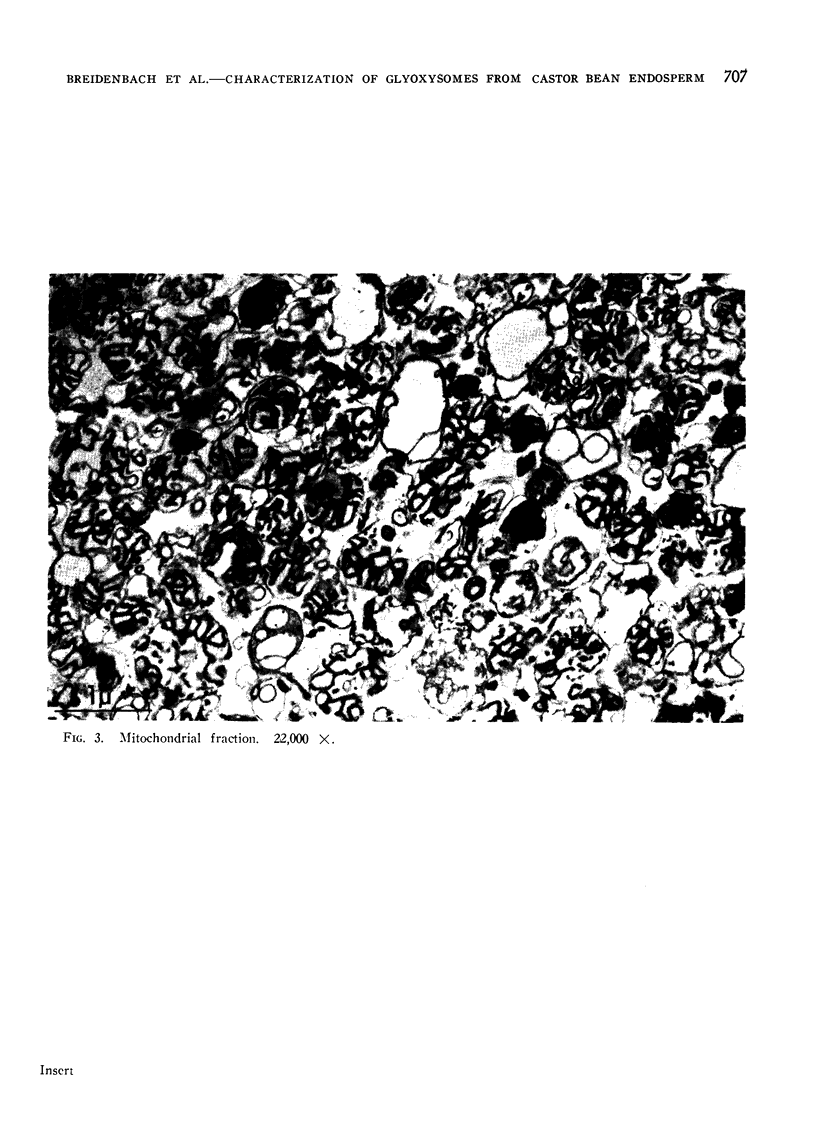
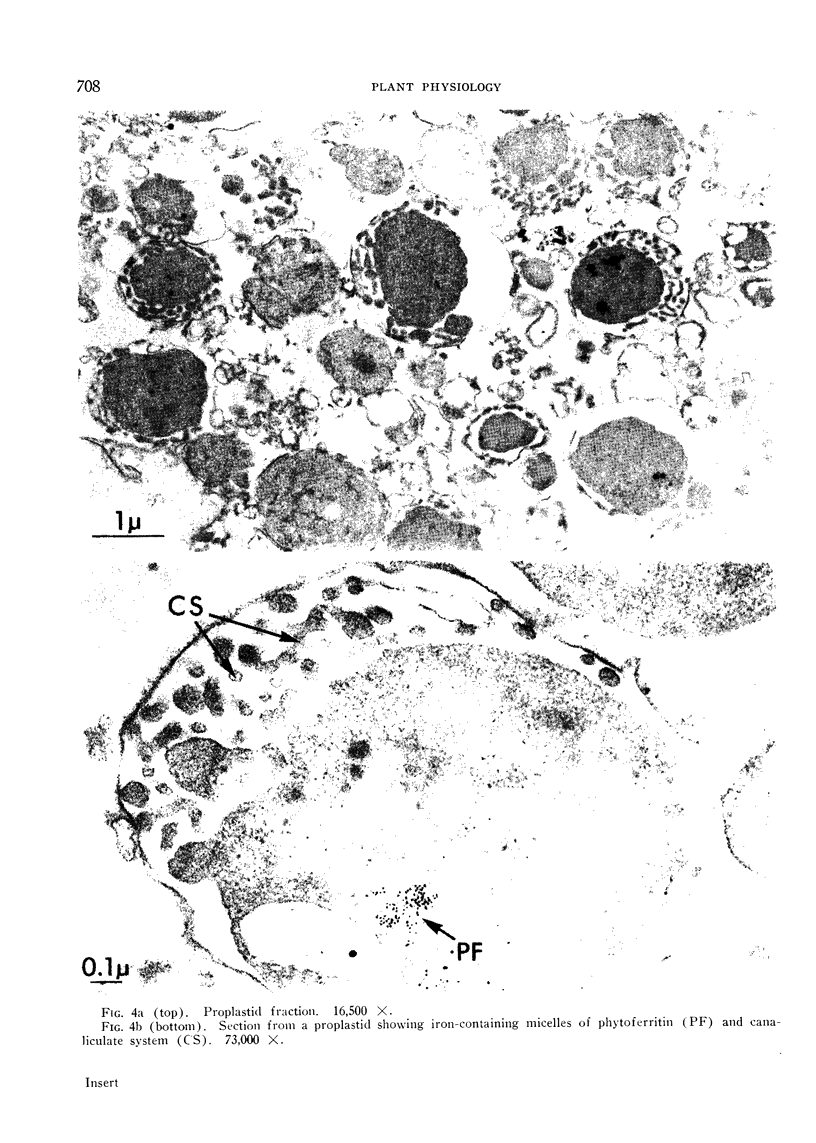
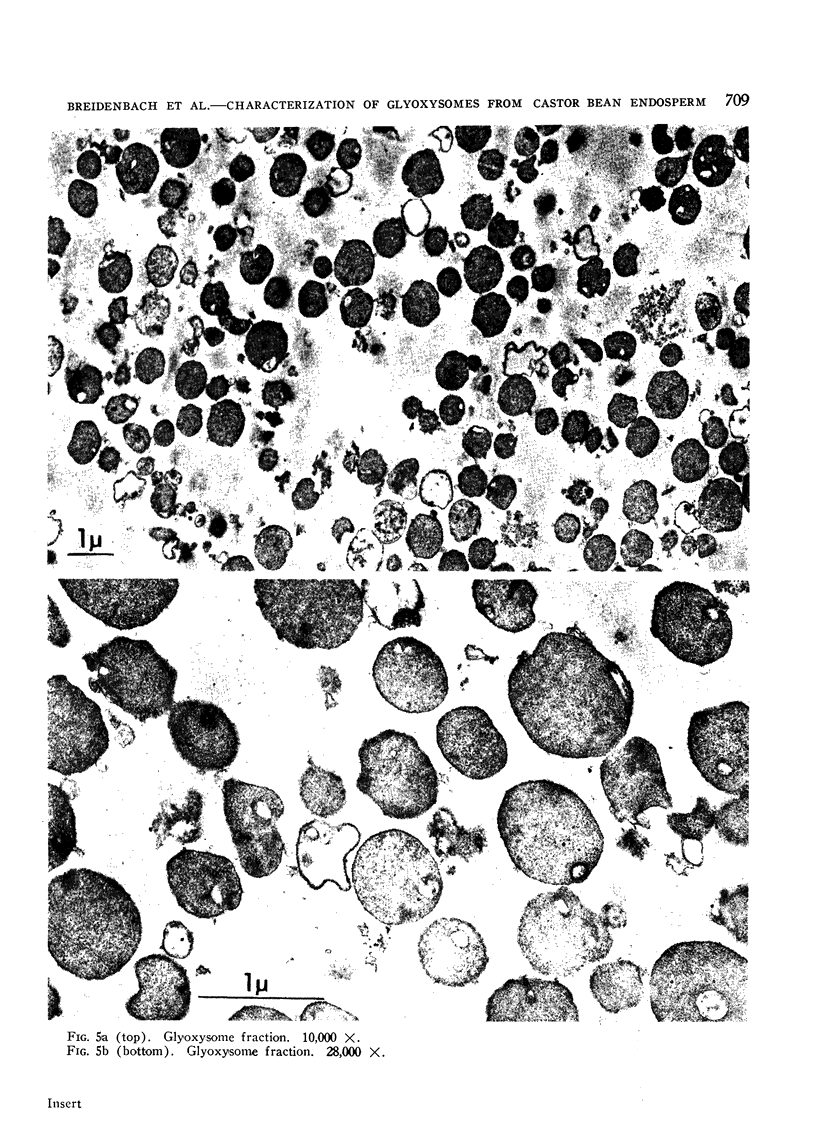
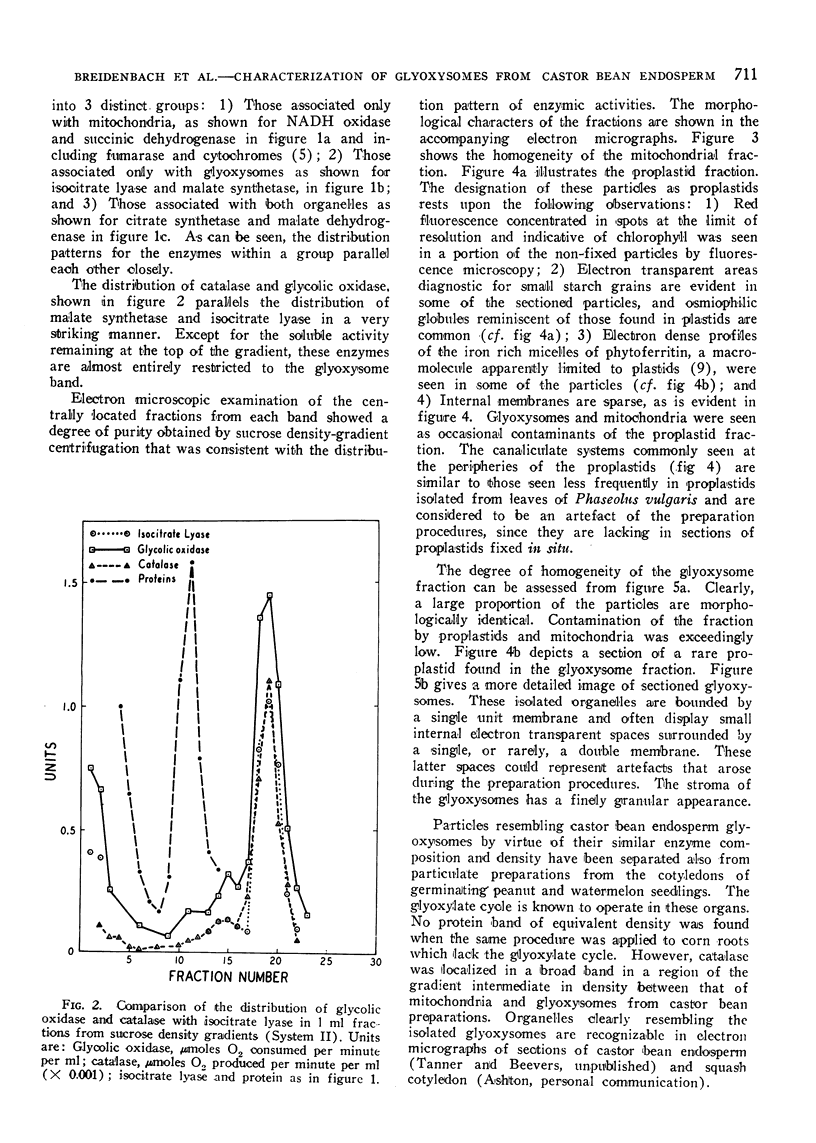
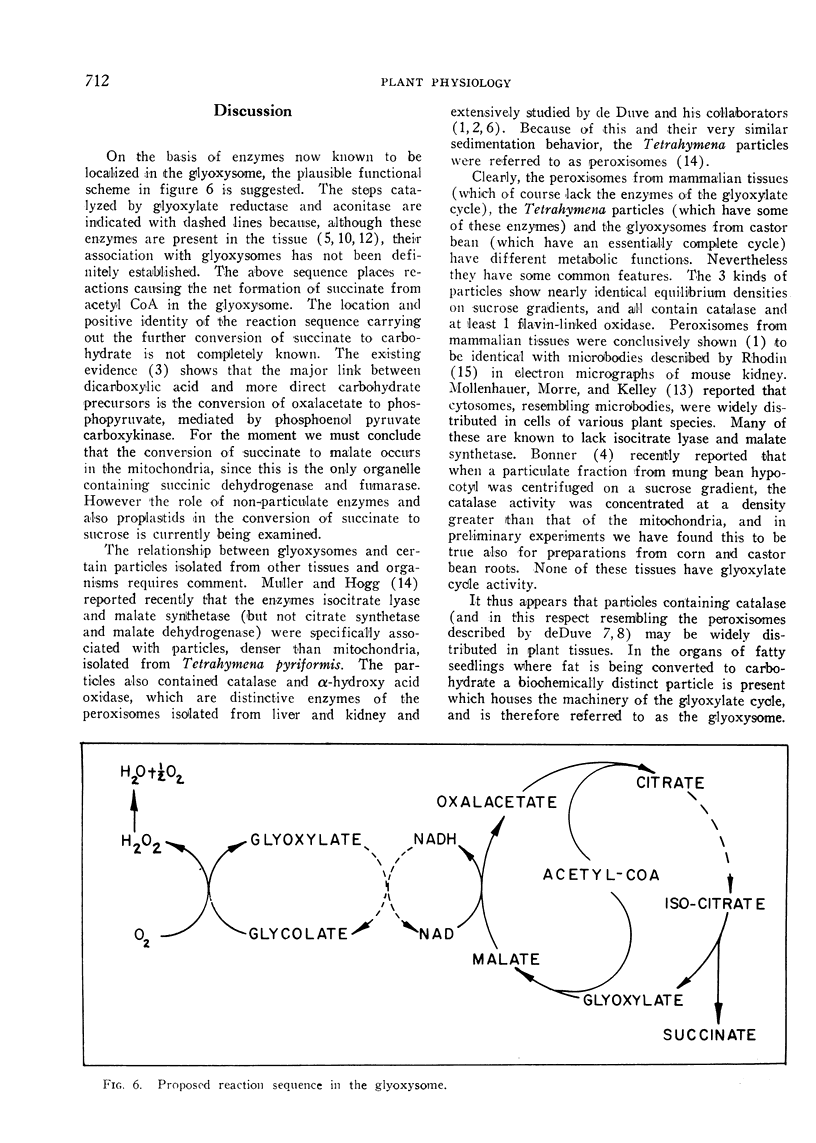
Electron micrographs are presented which establish the identity of the components of the 3 major bands observed after sucrose density centrifugation of the crude particulate fraction from the endosperm of germinating castor bean seedlings. These are: mitochondria (density 1.19 g/cc), proplastids (density 1.23 g/cc) and glyoxysomes (density 1.25 g/cc). Further evidence is provided on the enzymatic composition of the glyoxysomes. Essentially all of the particulate malate synthetase, isocitrate lyase, catalase, and glycolic oxidase is present in these organelles. The distribution of glyoxysomal enzymes on sucrose density gradients is contrasted with that of the strictly mitochondrial enzymes fumarase, NADH oxidase, and succinoxidase. Malate dehydrogenase and citrate synthetase are present in both organelles. The functional role of glyoxysomes and their relationship to cytosomes from other tissues is discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEEVERS H. Metabolic production of sucrose from fat. Nature. 1961 Jul 29;191:433–436. doi: 10.1038/191433a0. [DOI] [PubMed] [Google Scholar]
- Baudhuin P., Beaufay H., De Duve C. Combined biochemical and morphological study of particulate fractions from rat liver. Analysis of preparations enriched in lysosomes or in particles containing urate oxidase, D-amino acid oxidase, and catalase. J Cell Biol. 1965 Jul;26(1):219–243. doi: 10.1083/jcb.26.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaufay H., Jacques P., Baudhuin P., Sellinger O. Z., Berthet J., De Duve C. Tissue fractionation studies. 18. Resolution of mitochondrial fractions from rat liver into three distinct populations of cytoplasmic particles by means of density equilibration in various gradients. Biochem J. 1964 Jul;92(1):184–205. doi: 10.1042/bj0920184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breidenbach R. W., Beevers H. Association of the glyoxylate cycle enzymes in a novel subcellular particle from castor bean endosperm. Biochem Biophys Res Commun. 1967 May 25;27(4):462–469. doi: 10.1016/s0006-291x(67)80007-x. [DOI] [PubMed] [Google Scholar]
- DE DUVE C., BEAUFAY H., JACQUES P., RAHMAN-LI Y., SELLINGER O. Z., WATTIAUX R., DE CONINCK S. Intracellular localization of catalase and of some oxidases in rat liver. Biochim Biophys Acta. 1960 May 6;40:186–187. doi: 10.1016/0006-3002(60)91338-x. [DOI] [PubMed] [Google Scholar]
- De Duve C., Baudhuin P. Peroxisomes (microbodies and related particles). Physiol Rev. 1966 Apr;46(2):323–357. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
- HYDE B. B., HODGE A. J., KAHN A., BIRNSTIEL M. L. STUDIES ON PHYTOFERRITIN. I. IDENTIFICATION AND LOCALIZATION. J Ultrastruct Res. 1963 Oct;59:248–258. doi: 10.1016/s0022-5320(63)80005-2. [DOI] [PubMed] [Google Scholar]
- KORNBERG H. L., BEEVERS H. The glyoxylate cycle as a stage in the conversion of fat to carbohydrate in castor beans. Biochim Biophys Acta. 1957 Dec;26(3):531–537. doi: 10.1016/0006-3002(57)90101-4. [DOI] [PubMed] [Google Scholar]
- MAEHLY A. C., CHANCE B. The assay of catalases and peroxidases. Methods Biochem Anal. 1954;1:357–424. doi: 10.1002/9780470110171.ch14. [DOI] [PubMed] [Google Scholar]
- MARCUS A., VELASCO J. Enzymes of the glyoxylate cycle in germinating peanuts and castor beans. J Biol Chem. 1960 Mar;235:563–567. [PubMed] [Google Scholar]
- Plesnicar M., Bonner W. D., Jr, Storey B. T. Peroxidase associated with higher plant mitochondria. Plant Physiol. 1967 Mar;42(3):366–370. doi: 10.1104/pp.42.3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]