Abstract
Splenic macrophages from Histoplasma capsulatum-infected mice express inducible nitric oxide synthase (iNOS), and the iNOS expression correlates with severity of the infection. We examined whether production of NO is responsible for apoptosis and the anti-lymphoproliferative response of splenocytes from mice infected with H. capsulatum. In situ terminal deoxynucleotidyl transferase nick end labeling revealed apoptotic nuclei in cryosections of spleen from infected but not normal mice. Splenocytes of infected mice were unresponsive to stimulation by either concanavalin A or heat-killed H. capsulatum yeast cells. Splenocyte responsiveness was restored by addition to the medium of NG-monomethyl-l-arginine, a known inhibitor of NO production. The proliferative response of splenocytes from infected mice was also restored by depletion of macrophages or by replacement with macrophages from normal mice. In addition, expression of iNOS returned to its basal level when the animals had recovered from infection. These results suggest that suppressor cell activity of macrophages is associated with production of NO, which also appears to be an effector molecule for apoptosis of cultured splenocytes from infected mice.
Nitric oxide (NO) has been reported to induce apoptosis in many cells including smooth muscle cells (20), oligodendrocytes (27), pancreatic β cells (11), melanoma cells (35), thymocytes (7), B lymphocytes (4), and macrophages (2). Fehsel et al. recently demonstrated apoptosis in freshly isolated thymocytes after exposure to NO (7). In the same report, they also showed apoptotic foci in close proximity to blood vessels after lipopolysaccharide treatment. Capillary endothelial and dendritic cells adjacent to apoptotic foci stained strongly for inducible nitric oxide synthase (iNOS), suggesting that NO may be the mediator for thymic apoptosis (7). Data from another laboratory also showed that cloned thymic stromal cell monolayers eliminate thymocytes in vitro through production of NO (26). Furthermore, apoptosis has been suggested as a mechanism by which the immune system replenishes itself and maintains homeostasis (30).
The dimorphic fungus Histoplasma capsulatum is a facultative intracellular pathogen of the macrophage (32). Although it is not an obligate intracellular pathogen, the organism is found almost exclusively inside host cells during histoplasmosis (5). In our in vitro studies, H. capsulatum exhibits uninhibited growth in normal unstimulated murine macrophages (32). In activated macrophages, either peritoneal macrophages and cells from the Raw 264.7 line stimulated by gamma interferon (IFN-γ) or splenic macrophages stimulated by IFN-γ and lipopolysaccharide, growth of the fungus is inhibited (13, 18, 32). Furthermore, the anti-histoplasma activity of macrophages is dependent on the expression of iNOS and the production of NO (14, 18). However, the significance of NO production in immunoregulation of histoplasmosis is not clearly defined.
In this study, we examined whether NO can act as a regulator of apoptosis in lymphoproliferative responses of splenocytes from H. capsulatum-infected mice. We showed that iNOS was induced in splenic macrophages during active infection and the expression of iNOS coincided with active infection. We also observed by in situ terminal deoxynucleotidyl transferase (TdT) nick end labeling (TUNEL) of spleen sections that apoptosis occurred in immune cells in the spleens of infected mice but was minimal in control mice. The link between apoptosis and NO production was established by inclusion of NG-monomethyl-l-arginine (NMMA) in the culture medium. Inhibition of NO production reduced the amount of apoptosis in splenocyte culture. Thereby, we also confirmed the findings of Zhou et al. (36) that production of NO by splenocytes of H. capsulatum-infected mice suppressed the splenic lymphocyte proliferative response. In addition, we showed that macrophages were mediators of splenocyte unresponsiveness through the NO that they produced and that NO production was associated with apoptotic changes in cultured splenocytes from infected mice.
MATERIALS AND METHODS
Animals.
C57BL/6 mice at 6 to 8 weeks of age were obtained from Jackson Laboratory (Bar Harbor, Maine) and were housed in sterilized plastic cages fitted with filter cage tops. The animals were fed with sterilized food and water.
Animal model.
H. capsulatum 505 was grown at 37°C for 72 h on brain heart infusion agar supplemented with cysteine and glucose. A suspension of washed yeast cells was prepared. Mice were injected intravenously with 2 × 105 H. capsulatum yeast cells (sublethal dose) as described previously (33).
Reagents.
RPMI 1640 culture medium (GIBCO-BRL, Grand Island, N.Y.) was supplemented with 10% heat-inactivated fetal bovine serum (HyClone, Logan, Utah), 1 mM sodium pyruvate, 2 mM l-glutamine, 0.1 mM nonessential amino acids, 5 × 10−5 M 2-mercaptoethanol, and 50 μM HEPES buffer. All the supplements were purchased from GIBCO-BRL. Tritiated thymidine (1 mCi/ml) was obtained from Du Pont-NEN (Boston, Mass.); propidium iodide and NMMA were obtained from Calbiochem (San Diego, Calif.); and RNase A, Triton X-100, sodium citrate, and concanavalin A (ConA) were obtained from Sigma (St. Louis, Mo.). The TUNEL reaction mixture was purchased from Boehringer GmbH, Mannheim, Germany.
Nitrite assay.
Splenocytes from normal and infected mice were cultured (107 per ml) for 24 h in modified Eagle’s medium (GIBCO) containing 5% heat-inactivated fetal bovine serum. Culture supernatant fluids were collected and analyzed for nitrite levels. The nitrite concentration in the supernatant fluids was determined by a colorimetric assay with Griess reagents as described previously (14).
Semiquantitative reverse transcription-PCR.
Under RNase-free conditions, total RNA was extracted from freshly harvested mouse spleen. The spleen was homogenized in TRIzol reagent (GIBCO-BRL), containing phenol and guanidine thiocyanate, as specified by the manufacturer. The homogenate was then centrifuged for 10 min at 12,000 × g at 4°C. The supernatant fluid was collected and mixed with chloroform. After settling at room temperature for a few minutes, the mixture was centrifuged at 12,000 × g for 15 min and total RNA was recovered from the water-soluble layer. RNA was precipitated by addition of an equal volume of isopropanol. After centrifugation at 12,000 × g for 10 min, the precipitate was recovered and washed repeatedly with 75% alcohol, and the RNA was dissolved in 20 to 40 μl of diethypyrocarbonate-H2O. The quality and quantity of extracted RNA were assessed by examining the ratio of spectrophotometric readings at optical densities of 260 and 280 as well as by 28S and 18S RNA banding in a 1% agarose gel. Extracted RNA was stored at −80°C before being subjected to reverse transcription.
A 2-μg quantity of RNA (1 μg/μl) was reverse transcribed with 200 U of Superscript TM II RNase H− reverse transcriptase (GIBCO-BRL) and 1 μl of NotI-(dT)18 primer (0.48 μg/μl) in a total volume of 12 μl. After addition of primer, the mixture was incubated at 70°C for 10 min and then at 0°C for 1 to 2 min. Deoxynucleoside triphosphate, dithiothreitol, and first-strand buffer were added before the mixture was heated to 37°C for 5 min. Reverse transcriptase was then added. The reaction mixture was incubated at 37°C for 50 min, and the reaction was stopped by incubation at 70°C for 20 min. cDNA was stored at −20°C.
iNOS-specific cDNA was amplified by the use of paired primers: 5′-TGGGAATGGAGACTGTCCCAG and 3′-GGGATCTGAATGTGATGTTTG (22). Hypoxanthine phosphoribosyltransferase (HPRT)-specific primers were 5′-GTTGGATACAGGCCAGACTTTGTTG and 3′-GAGGGTAGGCTGGCCTATAGGCT (22). A semiquantitative PCR was carried out with the following reaction mixture: 1 μl of sample RNA; 2.5 μl each of HPRT 3′ primer, HPRT 5′ primer, iNOS 3′ primer, and iNOS 5′ primer (each at 2 μM); 0.5 μl of 10 mM deoxynucleoside triphosphate; 0.25 μl of Taq polymerase (5 U/μl) and 10.25 μl of diethylpyrocarbonate-H2O. The thermal cycles were 1 cycle of 90°C for 3 min followed by 35 cycles of 94°C for 40 s, 60°C for 60 s, and 72°C for 60 s. Our pilot runs confirmed that under these conditions, PCR products of each primer set fell within the linear range. The reaction was terminated by incubation at 72°C for 8 min and then stored at 4°C.
PCR products were separated by electrophoresis on a 2% agarose gel (NuSieve 3:1 agarose; FMC BioProducts, Rockland, Maine). The bands were stained in ethidium bromide for 10 min. The differential quantity of each band was analyzed with a densitometer (UltraScanXL laser densitometer; Pharmacia). The ratio of iNOS and HPRT PCR products was derived from densitometer readings.
In situ iNOS staining.
Spleens removed from mice were immediately fixed overnight in 4% paraformaldehyde before being serially immersed in 10, 20, and 30% sucrose solutions. The spleens were then embedded in O.C.T. (Optimal Control Temperature Compound; Miles Incorp., Elkhart, Ind.) in liquid nitrogen and stored at −80°C before being subjected to cryosection. The frozen spleens were warmed to −20°C and sectioned at a thickness of <5 μm. The sections were dried, fixed in 4% paraformaldehyde for 10 min, and rinsed in phosphate-buffered saline (PBS). Fluorescein isothiocyanate (FITC)-conjugated rabbit anti-mouse iNOS polyclonal antibody (Transduction Laboratories, Lexington, Ky.) at a 1:100 dilution was applied to the section. After a 2-h incubation at 37°C, the slides were washed in PBS and mounted in 1:1 glycerol-PBS mounting fluid. Immunofluorescence staining was viewed under a Olympus fluorescence microscope, and photographs were taken with Fuji color film (ASA 400).
Nuclear staining with propidium iodide.
Splenocytes in single-cell suspension were isolated from normal and infected mice. At 2, 22, and 44 h of incubation in RPMI medium containing supplements, the cells were centrifuged and the supernatant fluids were discarded. The cell pellets were resuspended in 1 ml of hypotonic DNA staining buffer that contained sodium citrate (3.4 mM), Triton X-100 (0.3%), propidium iodide (0.15 mM), and RNase A (1.5 U/ml). Samples were kept at 4°C and protected from light until analyzed by flow cytometry at an excitation wavelength of 488 nm. The fluorescence intensity of degraded DNA debris fell below 101. Intact DNA in cells fluoresced at an intensity above 103 (12). Nuclei with fragmented DNA fluoresced at an intensity between 101 and 103. The percentage of nuclei with fragmented DNA was recorded as percent apoptotic cells.
Splenocyte proliferation assay.
Splenocytes (5 × 105/100 μl) from normal and infected mice were added to separate wells in a 96-well flat-bottom plate. A 100-μl volume of supplemented medium or medium containing ConA (2 μg/ml) or heat-killed (60°C for 1 h) H. capsulatum yeast cells at a 1:40 splenocyte-to-yeast ratio was added to triplicate wells in the presence or absence of NMMA (1.2 mM). The cells were pulsed with 1 μCi of [3H]thymidine from 0 to 24 h. A Filtermate 196 harvester (Packard Instrument Co., Meridien, Conn.) was used to wash and to lyse the cells. Cell lysate was collected onto a 24-well microplate, and the radioisotope uptake was determined in a Topcount Microplate scintillation and luminescence counter (Packard Instrument).
Macrophage depletion by plastic adherence and nylon wool.
Splenocytes were isolated from normal or infected mice. Single-cell suspensions were adjusted to 5 × 107 to 10 × 107 cells per 10 ml of complete RPMI 1640 medium and plated in heat-inactivated fetal bovine serum-precoated tissue culture dishes. After incubation for 1 h at 37°C, nonadherent cells in the suspension were collected and the plate was washed with warm medium. The cells were centrifuged and resuspended in 10 ml of medium for another cycle of adherence. Nonadherent cells were collected as above. After centrifugation, the cell pellet was resuspended in 2 ml of medium and loaded onto a prewashed nylon wool column. After a 45-min incubation at 37°C, the cells were eluted with 15 ml of warm medium.
Nylon wool-nonadherent cells (5 × 105) alone or cocultured with 105 peritoneal macrophages from either normal or infected animals were stimulated with ConA (2 μg/ml) for 48 h. At 16 h before harvest, the cells were pulsed with 1 μCi of [3H]thymidine per well. The cells were harvested and the radioisotope uptake was determined as described above.
TUNEL staining for detection of apoptosis.
We used the TUNEL method to detect DNA fragmentation in cells of spleen sections (8, 9). Spleen sections were prepared and cryosectioned as described above for in situ iNOS staining. The cryosections were then dried, fixed again in paraformaldehyde, and washed before treatment with 3% H2O2. After thorough washing with PBS, TdT-mediated, FITC-conjugated dUTP was applied to the sections. The reaction was allowed to take place at 37°C for 90 min. The sections were then washed in PBS for 15 min at room temperature before avidin-biotin-peroxidase-conjugated anti-FITC antibody was added. After a 30-min incubation at room temperature and washing, diaminobenzidine tetrahydrochloride (DAB) was added for color development. The sections were then counterstained with hematoxylin, and microphotographs were taken with a Fuji film (ASA 400).
To determine the phenotype of apoptotic splenocytes, freshly harvested spleen cells were prepared as single-cell suspensions and delivered to V-bottom 96-well plates at 106 per 100 μl. In separate wells, monoclonal phycoerythrin (PE)-conjugated hamster anti-mouse CD3, PE-conjugated rat anti-mouse B220, or PE-conjugated rat anti-mouse Mac-1 antibody at 0.1 μg in 100 μl was added. After a 30-min staining on ice with constant shaking, the cells were centrifuged, washed, and resuspended in 2% paraformaldehyde solution. After 30 min of fixation, the cells were washed and permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate for 2 min on ice. After they were washed, TUNEL reaction mixture was added to each well. The culture plates were then wrapped in aluminum foil and placed in a 37°C water bath for 1 h. The cells were then washed again and analyzed by flow cytometry.
Trypan blue exclusion.
Splenocytes from normal or infected animals were added (5 × 105 per 100 μl) in triplicate to flat-bottom 96-well culture plates and cultured in supplemented RPMI 1640 medium with or without NMMA. Immediately after plating (0 h) and at 20 and 46 h of incubation, the cell suspension in each well was thoroughly mixed and the numbers of viable and dead cells were determined by the use of 0.1% trypan blue. To minimize errors introduced by plating, the percentages of viable cells at 20 or 46 h were calculated by dividing the number of viable cells at 20 or 46 h by that at 0 h.
RESULTS
Splenic macrophages are activated to express iNOS in response to H. capsulatum infection.
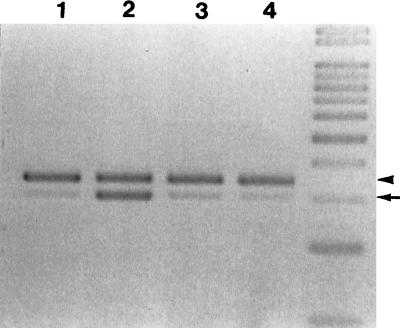
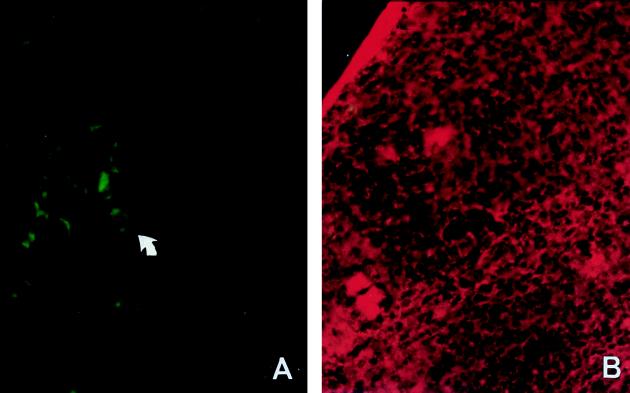
Intravenous inoculation of mice with yeast cells of H. capsulatum results in disseminated infection (3, 33). By semiquantitative reverse transcription-PCR methods, we found that iNOS was expressed in spleens of infected mice during the course of active infection (Fig. 1). Between 1 and 2 weeks of infection, when mouse spleen was enlarged with the highest fungal burden (33), iNOS mRNA was induced. At this time, iNOS mRNA expression was about 0.77 times that of a housekeeping gene. In contrast, iNOS mRNA in normal spleen was only minimally detectable (0.12 iNOS/HPRT ratio). At 6 to 8 weeks of infection, when the fungus was cleared and the spleen had returned to its normal size, iNOS expression was at a basal level (0.21 iNOS/HPRT ratio). It is apparent that iNOS expression coincided with active infection. Furthermore, we observed by immunohistochemical staining (Fig. 2) that the most prominent iNOS-producing cells found in the spleen of an infected mouse were cells with extensive cytoplasm, morphologically similar to macrophages and giant cells. Taken together, we conclude that splenic macrophages and giant cells were activated to produce iNOS during the active inflammatory response to H. capsulatum. However, we are unable to rule out the additional involvement of granulocytes and endothelial cells, which are also known to produce iNOS.
FIG. 1.
iNOS mRNA expression in spleens of H. capsulatum-infected mice coincides with active infection. Total RNA extracted from normal (lane 1) and infected mice on days 10 (lane 2), 35 (lane 3), and 55 (lane 4) after infection. RNA was reverse transcribed and cDNA was amplified by PCR with paired iNOS primers and HPRT primers simultaneously. The arrowhead points to HPRT PCR products (352 bp), and the arrow points to iNOS PCR products (306 bp). The ratios of iNOS to HPRT are 0.12, 0.77, 0.39, and 0.21 for lanes 1, 2, 3, and 4, respectively.
FIG. 2.
Splenic macrophages express iNOS protein in H. capsulatum-infected mice. Spleen cryosections from H. capsulatum-infected (2 to 3 weeks postinfection) (A) and normal (B) mice were stained with FITC-conjugated polyclonal rabbit anti-mouse iNOS antibody. (A) The arrow points to a cell, most probably a macrophage or giant cell by morphology, which stains positive for iNOS. (B) The spleen section was stained with ethidium bromide as a counterstain. Magnification, ×400.
Apoptosis of splenocytes in response to H. capsulatum infection.
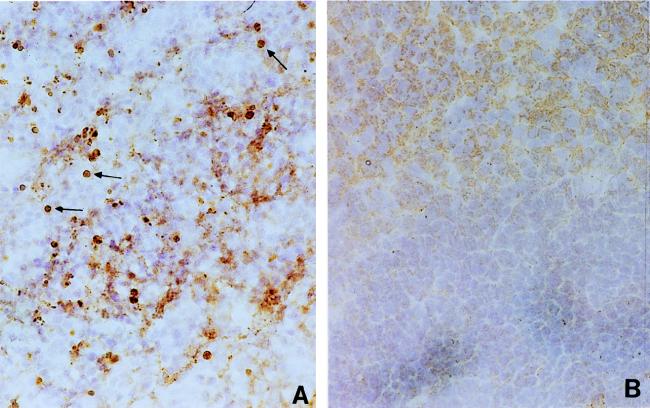
In view of the known toxicity of NO to mammalian cells, we examined whether iNOS expression in the spleen is associated with the death of splenocytes. By use of TUNEL reagents, we examined spleen sections of infected and normal mice for the presence of apoptotic nuclei. Apoptotic nuclei were detected in the spleen of an infected mouse (Fig. 3A), whereas they were minimally detectable in the spleen of a normal mouse (Fig. 3B). Furthermore, double staining with anti-CD3, anti-B220, or anti-Mac-1 antibodies and TUNEL reagents and analysis by flow cytometry demonstrated that apoptotic cells in the spleens of infected mice included T and B lymphocytes and macrophages. Apoptotic cells in the freshly harvested splenocyte population of infected mice consisted of 4.0 × 106 T cells, 3.9 × 106 B cells, and 0.6 × 106 macrophages. In contrast, only 0.84 × 106 T cells, 0.84 × 106 B cells, and 0.04 × 106 macrophages were found to be apoptotic in freshly harvested normal splenocytes.
FIG. 3.
Apoptosis in spleen cells in H. capsulatum-infected mice. Spleen cryosections of H. capsulatum-infected (2 to 3 weeks postinfection) (A) and normal (B) mice were stained with TUNEL reagents, and DAB substrate was used for color development. The arrows point to brown apoptotic nuclei. Magnification, ×200.
Splenocyte apoptosis is reduced by inhibition of NO production.
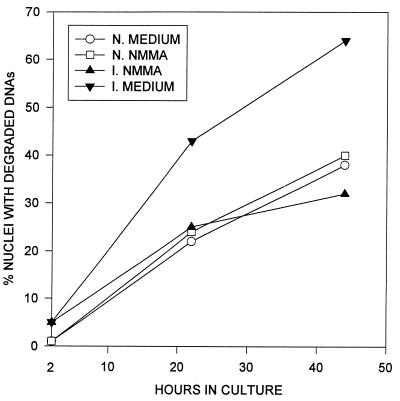
To determine if NO production was the cause of splenocyte apoptosis, spleen cells were cultured in medium with or without NMMA. The percentage of apoptotic cells in the splenocyte population was assessed by propidium iodide staining of the nuclei (Fig. 4). While the total number of nuclei remained constant over the observed period, at 2 h of incubation 5% of the nuclei in spleen cells from infected mice were apoptotic, compared with <2% for normal mice. Apoptotic cells in cultures of spleen cells from infected mice increased from 5 to 43% by 22 h and to 65% by 44 h, compared with 24 and 38% apoptosis at 22 and 44 h, respectively, in normal splenocyte cultures. Addition of NMMA reduced the number of apoptotic cells in cultures of infected splenocytes from infected mice to a level comparable to that for normal splenocytes. Since NMMA competitively inhibits NO production, we conclude that while spontaneous apoptosis occurred in normal splenocytes in culture, NO induced significantly higher apoptosis in splenocytes of infected mice (P < 0.05).
FIG. 4.
Inhibition of NO production reduces apoptosis in splenocytes of infected mice. Splenocytes isolated from normal (open symbols) and infected (2 to 3 weeks postinfection [solid symbols]) mice were cultured in the presence or absence of 1.2 mM NMMA. The graph was plotted according to data from three experiments.
Analysis of cell viability by the trypan blue exclusion assay showed that 77% ± 14% and 70% ± 10% of normal splenocytes were viable at 20 and 46 h, respectively. In contrast, only 55% ± 13% (P < 0.01) and 22% ± 10% (P < 0.01) of splenocytes from infected mice survived after similar incubation periods. Moreover, addition of NMMA increased the percentage of viable cells in cultured splenocytes of infected mice to 75% ± 14% (P < 0.05) and 59% ± 8% (P < 0.01) at 20 and 46 h, respectively. However, the addition of NMMA did not change the viability of normal splenocytes in culture. The viability was 82% ± 10% (P > 0.05) and 68% ± 1% (P > 0.05) at the two respective time points. These results, together with those in Fig. 4, indicate that splenocytes from infected mice died in culture and that NO was the cause of cell death.
Effect of NO on the proliferative response of splenocytes.
During the height of disseminated disease, unfractionated splenocytes from Histoplasma-infected mice are unresponsive to stimulation by ConA or heat-killed yeast cells of H. capsulatum, as shown by their failure to incorporate [3H]thymidine (3). In the present study, we found that unfractionated splenocytes from Histoplasma-infected mice secreted nitrite into the culture supernatant fluids. Stimulation with ConA or heat-killed yeast cells increased the level of nitrite (Table 1). These data indicate that unfractionated splenocytes produced NO. Furthermore, inhibition of NO production restored the responsiveness of splenocytes from Histoplasma-infected mice to normal levels (Table 2). Thus, we conclude that NO was responsible for the unresponsiveness of splenocytes from infected animals and is also associated with apoptosis of splenocytes.
TABLE 1.
Presence of nitrite in splenocyte culture supernatant fluids
| Source of splenocytesa | Stimulant | Level of nitrite (μM)d |
|---|---|---|
| Normal mice | None | 2.3 |
| ConAb | 0.5 | |
| H. capsulatumc | 1.1 | |
| Infected mice | None | 13.6 |
| ConA | 21.1 | |
| H. capsulatum | 18.4 |
Splenocytes from normal and H. capsulatum-infected mice at 2 to 3 weeks after infection were cultured at 107 cells per ml. Supernatant fluids were collected 24 h after incubation.
ConA was added at 2 μg/ml.
Heat-killed H. capsulatum yeasts were added at a 1:40 splenocyte-to-yeast ratio.
These data are representative of three separate experiments.
TABLE 2.
Effect of NO on the proliferative response of splenocytes from H. capsulatum-infected mice
| Source of splenocytea | NMMAb | ConAb | Heat-killed H. capsulatumc | SId |
|---|---|---|---|---|
| Normal mice | − | − | − | 1.0 |
| − | + | − | 7.3 | |
| − | − | + | 1.2 | |
| + | − | − | <1.0 | |
| + | + | − | 7.3 | |
| + | − | + | 1.3 | |
| Infected mice | − | − | − | 1.0 |
| − | + | − | <1.0 | |
| − | − | + | 1.7 | |
| + | − | − | 7.3 | |
| + | + | − | 21.5 | |
| + | − | + | 9.0 |
Splenocytes from normal and H. capsulatum-infected mice at 2 to 3 weeks after infection were cultured at 5 × 105 cells in a 96-well plate.
NMMA was added at 1.2 mM. Con A was added at 2 μg/ml.
Heat-killed H. capsulatum yeasts were added at a 1:40 splenocyte-to-yeast ratio.
The stimulation index (SI) is the counts per minute (cpm) of splenocytes from either normal or infected mice stimulated with ConA or heat-killed yeast cells divided by the cpm of splenocytes from either normal or infected mice without stimulation. Background thymidine uptakes were 2,934 ± 580 for normal splenocytes and 2,250 ± 982 for splenocytes from infected mice.
Macrophage depletion restores lymphocyte responsiveness.
To determine the cells responsible for the production of NO that is associated with lymphocyte unresponsiveness, we depleted macrophages from the splenocytes of infected and normal mice and studied their proliferative responses with and without macrophages. The results in Table 3 show that depletion of macrophages from infected splenocytes restored their responsiveness to ConA. Furthermore, replacing macrophages in a normal splenocyte population with peritoneal macrophages from infected mice suppressed the normal splenocyte proliferative response to ConA. Conversely, replacing macrophages in splenocytes of infected mice with normal macrophages restored splenocyte responsiveness. Since we have observed that peritoneal as well as splenic macrophages from H. capsulatum-infected mice can express iNOS, we conclude that NO produced by activated macrophages of infected mice is involved in splenocyte unresponsiveness.
TABLE 3.
Effect of macrophages from H. capsulatum-infected mice on the lymphocyte response to ConAa
| Source of whole splenocytes | Macrophage depletionb | Macrophage additionc | SId |
|---|---|---|---|
| Normal mice | − | − | 8.5 |
| Infected mice | − | − | 1.2 |
| Normal mice | + | − | 4.7 |
| Infected mice | + | − | 5.5 |
| Normal mice | + | + (normal mice) | 6.9 |
| Normal mice | + | + (infected mice) | <1.0 |
| Infected mice | + | + (normal mice) | 4.1 |
| Infected mice | + | + (infected mice) | 1.9 |
The effect of macrophages on the splenocyte response to ConA (2 μg/ml) was assessed by the depletion of macrophages from splenocytes with or without the addition of peritoneal macrophages from normal or H. capsulatum-infected mice. Data represent the results of three separate experiments.
Splenocytes from normal mice and mice at 2 to 3 weeks after infection were depleted of macrophages by adherence to plastic and nylon wool.
Peritoneal macrophages (3 × 105) from either normal or infected mice were added to macrophage-depleted splenocyte culture.
Stimulation index (SI) is defined as the cpm of splenocyte culture in the presence of ConA divided by the cpm of splenocyte culture without ConA. Background thymidine uptakes were 201.9 ± 25.0 for normal splenocytes and 140.7 ± 16.3 for splenocytes from infected mice. Addition of macrophages without ConA did not change the background counts.
Splenocytes recover responsiveness to ConA after fungal clearance.
We have previously observed that C57BL/6 mice clear a sublethal dose of H. capsulatum from most organs by 5 weeks postinoculation (33). At this stage, infected mice in the recovery phase of the infection exhibited a spleen size and splenic architecture comparable to those in healthy mice, and only basal levels of iNOS expression could be detected by reverse transcription-PCR (Fig. 1). Interestingly, splenocytes from such recovering mice were responsive to ConA, and the addition of NMMA did not change their responsiveness (Table 4), showing a direct correlation between splenic macrophage iNOS expression and splenocyte unresponsiveness.
TABLE 4.
Splenocytes recover from unresponsiveness to ConA after fungal clearance
| Source of splenocytesa | Culture medium containing:
|
SIb | |
|---|---|---|---|
| NMMA | ConA | ||
| Normal mice | − | + | 10.7 |
| + | + | 10.5 | |
| Infected mice | − | + | 6.0 |
| + | + | 7.2 | |
Splenocytes were isolated from normal mice and mice inoculated 6 weeks previously with H. capsulatum. No fungus was recovered from the spleen at the time of the experiment. The size of the spleen had returned to a size comparable to that of a normal spleen.
The stimulation index (SI) is defined as the cpm of splenocytes from normal or infected mice stimulated with ConA divided by the cpm of splenocytes from normal or infected mice without ConA.
DISCUSSION
NO production by macrophages via iNOS (NOS 2) is described as a high-output NO pathway, in contrast to the low-output pathway via nNOS (neuronal NOS, NOS 1) and eNOS (endothelial NOS, NOS 3) (16). The high-output NO pathway has been shown to be crucial in antimicrobial functions of macrophages (16). NO is also known to be anti-proliferative. With its anti-proliferative property, NO has been described as an immunosuppressant (16). However, the question of how NO functions as an immunsuppressant has not been resolved.
In murine models of experimental histoplasmosis, the anti-histoplasma activity of macrophages is dependent on the expression of iNOS and the production of NO (14, 18). It has been shown that spleen cells from H. capsulatum-infected mice do not respond to antigenic or mitogenic stimulation during the active phase of the infection (3). Macrophages and/or some factors produced by macrophages were described as the mediator for immune suppression in murine histoplasmosis (19).
In this study, we have demonstrated that splenic macrophages in H. capsulatum-infected animals expressed iNOS mRNA and protein and that the expression of iNOS coincided with the acute inflammatory response. Spleen cells isolated from animals during the course of active infection died in culture more rapidly than did normal splenocytes, and the increased death rate was directly related to the production of NO. We also observed that splenocytes from infected mice were unresponsive to specific antigenic or mitogenic stimulation and that responsiveness was restored by inhibition of NO. These results are consistent with results reported previously in experimental histoplasmosis (36). Furthermore, we showed that the unresponsiveness could be corrected by removal of macrophages from infected splenocytes. It is apparent that the production of NO is associated with splenocyte unresponsiveness in disseminated histoplasmosis, which is similar to the immunosuppression described for other disseminated infections by intracellular pathogens (6, 10, 23–25). One common feature of these infections is macrophage activation, and the suppression is due at least in part to “suppressor macrophages,” which down regulate the T-cell proliferative response to specific antigens or mitogens. In some cases, macrophage production of NO is the cause of the observed suppression (15, 36). It was recently shown that after infection with Leishmania major, spleen cells from homozygous mice lacking the iNOS gene had significantly higher levels of T-cell proliferation when stimulated by leishmanial antigen or ConA than did spleen cells from heterozygous or wild-type controls (29). The results of these experiments support the notion that NO is anti-proliferative (1, 17). However, the conclusion that NO suppresses lymphocyte proliferation was drawn from experiments based on the thymidine uptake assay, which did not differentiate active suppression and cell death. Based on our findings, we propose that through production of NO, there is an association between macrophage-mediated splenocyte unresponsiveness and apoptotic cell death. However, the direct causal link between unresponsiveness and apoptosis still awaits clarification. It will be possible when the mechanism of NO-induced apoptosis is better understood.
In situ staining with TUNEL reagents showed that the spleens of infected mice exhibited apoptotic nuclei (Fig. 3), indicating that apoptosis occurred in the spleens of infected mice. The apoptotic population included T and B lymphocytes as well as macrophages. Interestingly, the appearance and disappearance of apoptotic nuclei in infected mice coincided with the kinetics of iNOS expression (31). Although direct evidence showing apoptosis in the vicinity of iNOS expression is lacking, our data point to the possibility that during an acute inflammatory response, high-output NO causes the death of spleen cells in vivo (7).
Splenomegaly is a hallmark of disseminated histoplasmosis (34). Analysis of single-cell suspensions of splenocytes by flow cytometry revealed an increase in the number of T and B lymphocytes and macrophages in infected mice (28). Due to the adherent nature of macrophages, they are not readily isolated from the spleen, and hence their number is often underrepresented in single-cell suspensions of splenocytes (21). In an earlier study of H. capsulatum-infected mice, we found that in cryosectioned spleen, infiltrating macrophages account for the majority of cells in the enlarged spleen (34). While infiltrating macrophages did not remain in the marginal zones but invaded the follicles, T lymphocytes migrated from the follicles into the marginal zones and intermingled with macrophages. Furthermore, the number of T lymphocytes was comparatively small and both the CD4 and CD8 cells are sparsely distributed in the spleen. Taken together, these findings indicate that during the inflammatory response to Histoplasma infection, macrophages are activated to produce NO, which in turn negatively regulates T-lymphocyte expansion by inducing their apoptosis.
Based on these and our previous findings, we propose a working model for the role of NO in the clearance of H. capsulatum and the maintenance of homeostasis of cells in the immune system in the course of acute infection. During primary H. capsulatum infection, the spleen is enlarged due to massive macrophage infiltration and lymphocyte expansion. Upon activation, T lymphocytes produce IFN-γ and other cytokines, which in turn activate macrophages. The high-output pathway of NOS is induced in activated macrophages, which are armed to control the growth of the fungus (14, 18). Due to a bystander effect, cells in the vicinity of NO producers and the producers themselves are also killed. Through this mechanism, splenocytes are reduced in number and the spleen returns to its normal size after recovery from infection. After all, upon clearance of the pathogen, recruited inflammatory cells are no longer needed in the microenvironment. It is both economical and efficient for the immune system to use the high-output NO pathway as a double-edged sword not only to kill the pathogen but also to maintain homeostasis.
Recently, NO was shown not to be important in secondary histoplasmosis and its role in primary infection was confirmed (37). It will be of interest to investigate splenocyte responsiveness in relation to NO and macrophages in animals with secondary histoplasmosis.
ACKNOWLEDGMENTS
This study was supported in part by R.O.C. National Science Council grants NSC 86-2314-B-002-125 and 87-2314-B-002-257 and in part by U.S. Public Health Service grant R01 AI-32630 from the National Institutes of Health.
We gratefully acknowledge John Kung and Ping-Ning Hsu for their critical reading of the manuscript. We thank Sylvia Odesa for her excellent technical assistance.
REFERENCES
- 1.Albina J E, Abate J A, Henry W L., Jr Nitric oxide production is required for murine resident peritoneal macrophages to suppress mitogen-stimulated T-cell proliferation. Role of IFNγ in the induction of the nitric oxide-synthesizing pathway. J Immunol. 1991;147:144–148. [PubMed] [Google Scholar]
- 2.Albina J E, Cui S, Mateo R B, Reichner J S. Nitric oxide-mediated apoptosis in murine peritoneal macrophages. J Immunol. 1993;150:5080–5085. [PubMed] [Google Scholar]
- 3.Artz R P, Bullock W E. Immunoregulatory responses in experimental disseminated histoplasmosis: depression of T-cell-dependent and T-effector responses by activation of splenic suppressor cells. Infect Immun. 1979;23:884–892. doi: 10.1128/iai.23.3.893-902.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosca L, Stauber C, Hortelano S, Baixeras E, Martinez C. Characterization of signals leading to clonal expansion or cell death during lymphocyte B activation. Curr Top Microbiol Immunol. 1995;200:39–50. doi: 10.1007/978-3-642-79437-7_3. [DOI] [PubMed] [Google Scholar]
- 5.Deepe G S, Bullock W E. Histoplasmosis: a granulomatous inflammatory response. In: Gallin J J, Goldstein I M, Snyder R, editors. Inflammation: basic principles and clinical correlates. New York, N.Y: Raven Press; 1988. p. 733. [Google Scholar]
- 6.Eisenstein T K, Huang D, Meissler J, Al-Ramadi B. Macrophage nitric oxide mediates immunosuppression in infectious inflammation. Immunobiology. 1994;191:493–502. doi: 10.1016/S0171-2985(11)80455-9. [DOI] [PubMed] [Google Scholar]
- 7.Fehsel K, Kroncke K-D, Meyer K L, Huber H, Wahn V, Kolb-Bachofen V. Nitric oxide induces apoptosis in mouse thymocytes. J Immunol. 1995;155:2858–2865. [PubMed] [Google Scholar]
- 8.Gavrieli Y, Sherman Y, Ben-Sasson S A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gold R, Shmied M, Rothe G, Zischler H, Breitschope H, Wekerle H, Lassmann H. Detection of DNA fragmentation in apoptosis: application of in situ nick translation to cell culture system and tissue sections. J Histochem Cytochem. 1993;41:1023–1030. doi: 10.1177/41.7.8515045. [DOI] [PubMed] [Google Scholar]
- 10.Gregory S H, Wing E J, Hoffman R A, Simons R L. Reactive nitrogen intermediates suppress the primary immune response to listeria. J Immunol. 1993;150:2901–2909. [PubMed] [Google Scholar]
- 11.Kaneto H, Fuji I, Seo H J, Suzuki K, Matsuoka T, Nakamura M, Tatsumi H, Yamasaki Y, Kamada T, Taniguchi N. Apoptotic cell death triggered by NO in pancreatic β cells. Diabetes. 1995;44:733–738. doi: 10.2337/diab.44.7.733. [DOI] [PubMed] [Google Scholar]
- 12.Krishan A. Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. J Cell Biol. 1975;66:188–193. doi: 10.1083/jcb.66.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lane T E, Wu-Hsieh B A, Howard D H. Gamma interferon cooperates with lipopolysaccharide to activate mouse splenic macrophages to an antihistoplasma state. Infect Immun. 1993;61:1468–1473. doi: 10.1128/iai.61.4.1468-1473.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lane T E, Otero G C, Wu-Hsieh B, Howard D H. Expression of inducible nitric oxide synthase by activated macrophages correlates with their antihistoplasma activity. Infect Immun. 1994;62:1940–1945. doi: 10.1128/iai.62.4.1478-1479.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mabbott N A, Sutherland I A, Sternberg J M. Suppressor macrophages in Trypanosoma brucei infection: nitric oxide is related to both suppressive activity and life span in vivo. Parasite Immunol. 1995;17:143–150. doi: 10.1111/j.1365-3024.1995.tb01016.x. [DOI] [PubMed] [Google Scholar]
- 16.Macmicking J, Xie Q-W, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 17.Mills C D. Molecular basis of ‘suppressor’ macrophages: arginine metabolism via the nitric oxide synthetase pathway. J Immunol. 1991;146:2719–2723. [PubMed] [Google Scholar]
- 18.Nakamura T, Wu-Hsieh B, Howard D H. Recombinant murine gamma interferon stimulates macrophages of the RAW cell line to inhibit the intracellular growth of Histoplasma capsulatum. Infect Immun. 1994;62:680–684. doi: 10.1128/iai.62.2.680-684.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nickerson D A, Havens R A, Bullock W E. Immunoregulation in disseminated histoplasmosis: characterization of splenic suppressor cell populations. Cell Immunol. 1981;60:287–297. doi: 10.1016/0008-8749(81)90270-7. [DOI] [PubMed] [Google Scholar]
- 20.Nishio E, Fukushima K, Shiozaki M, Watanabe Y. Nitric oxide donor SNAP induces apoptosis in smooth muscle cells through cGMP-independent mechanism. Biochem Biophys Res Commun. 1996;221:163–168. doi: 10.1006/bbrc.1996.0563. [DOI] [PubMed] [Google Scholar]
- 21.Nusrat A R, Wright S D, Aderam A A, Steinman R M, Cohn Z A. Properties of isolated red pulp macrophages from mouse spleen. J Exp Med. 1988;168:1505–1510. doi: 10.1084/jem.168.4.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reiner S L, Zheng S, Corry D B, Locksley R M. Constructing polycompetitor cDNAs for quantitative PCR. J Immunol Methods. 1994;165:37–46. doi: 10.1016/0022-1759(93)90104-f. [DOI] [PubMed] [Google Scholar]
- 23.Rockett K A, Awburn M M, Rockett E J, Cowden W B, Clark I A. Possible role of nitric oxide in malarial immunosuppression. Parasite Immunol. 1994;16:243–249. doi: 10.1111/j.1365-3024.1994.tb00346.x. [DOI] [PubMed] [Google Scholar]
- 24.Schleifer K W, Mansfield J M. Suppressor macrophages in African trypanosomiasis inhibit T cell proliferative responses by nitric oxide and prostaglandins. J Immunol. 1993;151:5492–5503. [PubMed] [Google Scholar]
- 25.Sternberg J, McGuigan F. Nitric oxide mediates suppression of T cell responses in murine Trypanosoma brucei infection. Eur J Immunol. 1992;22:2741–2744. doi: 10.1002/eji.1830221041. [DOI] [PubMed] [Google Scholar]
- 26.Tai X G, Saitoh Y, Satoh T, Yamamoto N, Kita Y, Takenaka G, Yu W G, Zou J P, Hamaoka T, Fujiwara H. –1995. Thymic stromal cells eliminate T cells stimulated with antigen plus stromal Ia molecules through their cross-talk involving the production of interferon-gamma and nitric oxide. Thymus. 1994;24:41–56. [PubMed] [Google Scholar]
- 27.Vartanian T, Li Y, Zhao M, Stefansson K. Interferon-gamma-induced oligodendrocyte cell death; implications for the pathogenesis of multiple sclerosis. Mol Med. 1995;1:732–743. [PMC free article] [PubMed] [Google Scholar]
- 28.Watson S, Miller T B, Redington T J, Bullock W E. Immunoregulation in experimental disseminated histoplasmosis: flow microfluorometry (FMF) studies of the Thy and Lyt phenotypes of T lymphocytes from infected mice. J Immunol. 1983;131:984–990. [PubMed] [Google Scholar]
- 29.Wei X-Q, Charles I G, Smith A, Ure J, Feng G-J, Huang F-P, Xu D, Muller W, Monocada S, Liew F Y. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- 30.Williams G T. Apoptosis in the immune system. J Pathol. 1994;173:1–4. doi: 10.1002/path.1711730102. [DOI] [PubMed] [Google Scholar]
- 31.Wu-Hsieh, B. Unpublished data.
- 32.Wu-Hsieh B, Howard D H. Inhibition of intracellular growth of Histoplasma capsulatum by recombinant murine gamma interferon. Infect Immun. 1987;55:1014–1016. doi: 10.1128/iai.55.4.1014-1016.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu-Hsieh B. Relative susceptibility of inbred mouse strains C57BL/6 and A/J to infection with Histoplasma capsulatum. Infect Immun. 1989;57:3788–3792. doi: 10.1128/iai.57.12.3788-3792.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu-Hsieh B, Lee G-S, Franco M, Hofman F. Early activation of splenic macrophages by tumor necrosis factor alpha is important in determining the outcome of experimental histoplasmosis in mice. Infect Immun. 1992;60:4230–4238. doi: 10.1128/iai.60.10.4230-4238.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie K, Huang S, Dong Z, Juang S H, Gutman M, Xie Q W, Nathan C, Fidler I J. Transfection with the iNOS gene suppresses tumorigenicity and abrogates metastasis by K-1735 melanoma cells. J Exp Med. 1995;181:1333–1343. doi: 10.1084/jem.181.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou P, Sieve M C, Bennett J, Kwon-Chung K J, Tewari R P, Gazzinelli R T, Sher A, Seder R A. IL-12 prevents mortality of mice infected with Histoplasma capsulatum through induction of IFN-gamma. J Immunol. 1995;155:785–795. [PubMed] [Google Scholar]
- 37.Zhou P, Miller G, Seder R A. Factors involved in regulating primary and secondary immunity to infection with Histoplasma capsulatum: TNF-α plays a critical role in maintaining secondary immunity in the absence of IFN-γ. J Immunol. 1998;160:1359–1368. [PubMed] [Google Scholar]