Abstract
Background and study aims The treatment of anorectal strictures is particularly challenging and historically focused on surgical resection and/or diversion. There are a number of endoscopic options, but repeat interventions are common. The use of the needle knife stricturotomy technique as an alternative to surgery in the treatment of a variety of strictures has been described, but its use for the treatment of severe anorectal and anopouch strictures has not been studied.
Patients and methods Our Inflammatory Bowel Disease department’s records were queried to identify patients with endoscopic non-traversable anorectal/anopouch strictures. Consecutive patients that underwent insulated tip/needle-knife endoscopic stricturotomy treatment were included. Primary outcome was immediate traversability of the treated stricture by the endoscope. Other outcomes included need for reintervention, 30-day post-procedure events, and follow-up period events.
Results All strictures were immediately successfully traversed following endoscopic stricturotomy treatment. The mean time to endoscopic reintervention was 5.3 months, with the majority of these patients undergoing repeat stricturotomy. Over a mean follow-up period of 12.8 months, two patients (8%) required surgical intervention (resection with coloanal anastomosis with a colostomy and complete proctectomy) for refractory stricture disease following initial endoscopic stricturotomy. Seven patients (29%) in our study have not required any further reintervention throughout the study period. There were no 30-day post-procedure adverse events and no adverse post-procedure events.
Conclusions Endoscopic stricturotomy is safe and effective in treating severe anorectal/anopouch strictures.
Keywords: Endoscopy Lower GI Tract, Inflammatory bowel disease, Endoscopy Small Bowel
Introduction
Stricture disease is a common manifestation of inflammatory bowel disease (IBD), particularly in Crohn’s disease (CD) patients 1 . Ileal pouch-anal anastomosis (IPAA) is a common surgery performed for treatment of refractory ulcerative colitis (UC), familial adenomatous polyposis (FAP), and select cases of Crohn’s colitis. Strictures of the ileal pouch and pouch anastomosis may be primary or secondary to the surgery. The treatment of anorectal and anopouch strictures are particularly challenging, and historically focused on surgical resection and/or diversion 2 . Other treatment options include digital 3 , balloon 4 , or bougie 5 dilatation, stenting 6 , and corticosteroid injection 7 . Many patients require repeated interventions.
Endoscopic balloon dilation (EBD) has been shown to be safe and effective in treating ileal pouch strictures 8 , primary and anastomotic CD strictures 9 10 . However, EBD can be ineffective for particularly tight and long strictures. Approximately 25% of patients will require surgery within 3 to 4 years following EBD treatment 11 .
Endoscopic electroincision therapy involves the disruption of fibrosed and/or strictured tissue with controlled electrocautery. Endoscopic electroincision therapy has been described as a treatment option for esophageal and upper gastrointestinal disease, but its use in colorectal disease remains uncharted. A case series by Truong et al. explored the use of combined endoscopic electroincision therapy and balloon dilatation for treatment of benign colorectal anastomotic strictures in 35 patients 12 . Clinical data on the use of endoscopic electroincision to treat strictures related to CD is also scarce. A single case report from Korea described the successful use of an endoscopic insulated-tip knife to treat a CD-related anorectal stricture 13 .
Shen et al. first described the novel needle-knife stricturotomy technique to treat ileal pouch strictures 14 and subsequently proved that it is a safe and effective alternative to surgery for a variety of refractory CD-related strictures (including ileal pouch anastomosis, pouch inlet/afferent limb, and anal strictures) 15 . Endoscopic stricturotomy has been favorably compared with EBD for the treatment of pouch inlet/afferent limb strictures 16 . Endoscopic stricturotomy results in comparable surgery-free survival time as compared with surgical resection for treatment of ileocolonic anastomotic strictures, with the additional benefit of reduced morbidity 17 .
Ongoing research on the effectiveness of this technique in treating anorectal/anopouch strictures, and specifically its most severe type (endoscopically non-traversable), are important and necessary as these strictures often preclude patients from definitive surgical intervention for more proximal disease. This study aimed to evaluate the technical success and post-procedure outcomes of endoscopic insulated-tip (IT)/needle-knife (NK) stricturotomy in treating severe, endoscopically non-traversable anorectal and anopouch strictures at our tertiary-care center.
Patients and methods
Data sources
This study was approved by the Columbia University Irving Medical Center Institutional Review Board (IRB). At the Interventional IBD Center at Columbia University Irving Medical Center, endoscopic electroincision with stricturotomy has become the first and standard therapy for patients with anorectal/anopouch strictures since December 2019. In clinical practice, we have noticed a better efficacy of endoscopic stricturotomy than EBD. More importantly, stricturotomy with electroincision in a circumferential fashion may avoid iatrogenic trauma to anal sphincters from radial tears of EBD.
All consecutive patients with anorectal or anopouch strictures treated with endoscopic stricturotomy at our IBD Center between January 2020 and March 2022 were identified from our institution’s IBD registry. Demographic and clinical data, endoscopic procedural data, and post-procedure outcomes data were reviewed from the medical records.
Inclusion and exclusion criteria
Inclusion criteria included any patient with an endoscopically non-traversable anorectal or anopouch stricture that was treated by IT/NK endoscopic stricturotomy with or without prior endoscopic or other intervention (i.e., EBD). Patients who did not meet the above criteria or lacked complete clinical documentation or follow-up information were excluded from the study.
Endoscopic stricturotomy procedure
Every patient had a visit with the gastroenterologist prior to endoscopy, during which a complete medical and surgical history was obtained and physical exam performed. Endoscopy and/or imaging was used to diagnosis a stricture prior to planned stricturotomy intervention. The decision to treat a stricture with IT/NK stricturotomy was based solely on the clinical judgment of the patient’s gastroenterologist. All IT/NK stricturotomy procedures were performed by the senior author, an experienced interventional endoscopist. All patients without diverting ostomies received oral polyethylene glycol-based bowel preparation the day prior to their planned procedure. The procedure was performed under conscious sedation or monitored anesthesia care in an outpatient setting.
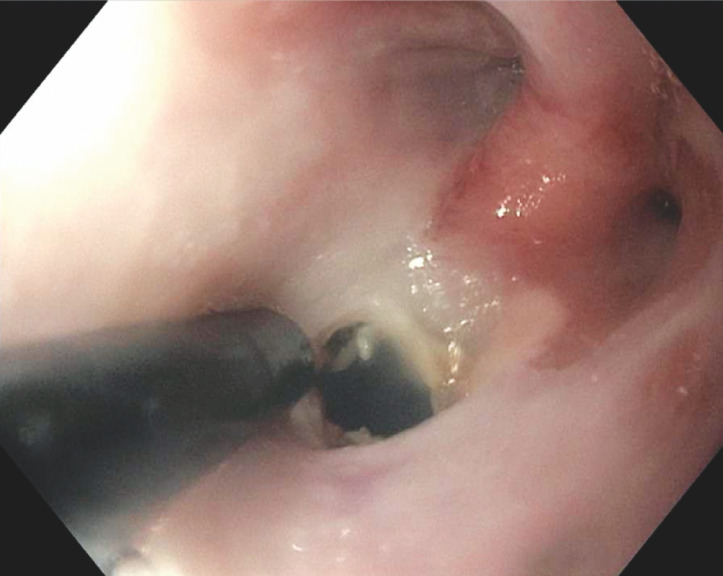
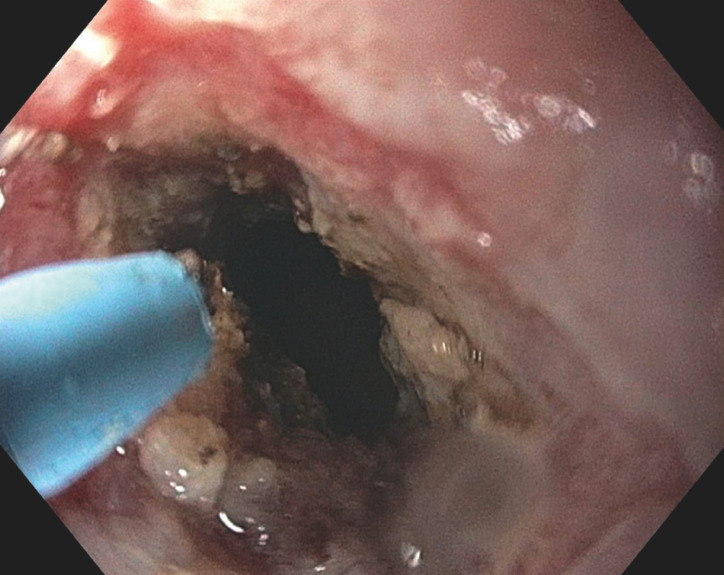
The procedure begins with a thorough examination and any perianal/anal abnormalities are documented. An upper endoscope (GIF series, Olympus, Tokyo) is then advanced to the level of the stricture. This study included only strictures noted to classified as either anorectal (anorectal ring) or anopouch. The degree of stricture is then noted (classification 0–4) 15 . The estimated length of the stricture is noted. Soft-tip guidewire was used in selected patients with pinhole strictures or adjacent fistulae. The IT ( Fig. 1 ), IT2 (Olympus, Tokyo), NK ( Fig. 2 ) (Boston Scientific, 300 Boston Scientific Way Marlborough, Massachusetts, United States) and Erbe VIO 300 D electrosurgical generator (ERBE USA, Marietta, Georgia, United States) with a setting of Endocut mode were used to perform electroincision and/or electrocautery treatment. The Endocut mode on the Erbe electrosurgical generator combines both cutting and coagulating, in short bursts. The ability to traverse the prior stricture site was tested immediately following treatment. Post-procedure patients were observed 30–45 minutes in the recovery room before discharge home.
Fig. 1.
Endoscope reintervention-free survival.
Fig. 2.
Surgery reintervention-free survival.
Endoscopic reintervention
All patients who underwent endoscopic stricturotomy intervention were seen in the office for follow-up visits. Success of intervention was marked by documentation of symptom improvement or relief – namely reduced or resolved pain and improved bowel function. Return or worsening of symptoms prompted reevaluation. Endoscopy was performed to evaluate and define the character of the stricture. The decision to perform repeat IT/NK stricturotomy was made by the senior author.
Data collection
Patient demographic information was collected, including age, sex, race/ethnicity, body mass index (BMI), smoking status, primary diagnosis, and medical comorbidity history. Smoking status was designated as either active smoker or non-active smoker. Primary diagnosis represented the patient’s IBD diagnosis of either CD or UC. Two patients included in the study had a primary diagnosis of colonic neoplasm or FAP. Active IBD-related medications, including biologics and corticosteroids, were documented.
Procedural details and stricture data was obtained from the medical record, as well patient follow-up data which was recorded as per the usual clinical practice. The need for reintervention, whether endoscopic or surgical, was noted through procedural notes documented in the medical record, which was reviewed over the course of the study period.
Outcomes
The primary outcome was immediate technical success, as measured by the ability to traverse the strictured site with the endoscope 18 . Secondary outcomes were procedure-associated adverse events (AEs) (both 30-day post-procedure and the individual complete post-procedure follow-up times), as well as endoscopy reintervention-free survival and surgery-free survival 18 . Post-procedure follow-up time was defined as the time from the index endoscopic IT/NK stricturotomy to the end of the study period.
Statistical analysis
Descriptive statistics were performed for all appropriate variables. Categorical variables were reported as percentages, while quantitative variables were reported as mean ±standard deviations. Repeat endoscopy-free and surgery-free survival were evaluated with Kaplan-Meier curves.
Results
A total of 24 patients met the criteria for inclusion in the study. All patient and stricture characteristics are listed in Table 1 .
Table 1 Patient and stricture characteristics (N=24).
| Patient c haracteristics, n (%) | |
| SD, standard deviation; ASA, American Society of Anesthesiologists. | |
| Age, years, mean ±SD | 39.4 ± 14.5 |
| Female gender, n (%) | 16 (66.7) |
| Race/ethnicity, n (%) | |
|
14 (58.3) |
|
10 (41.7) |
| ASA class, n (%) | |
|
18 (75) |
|
6 (25) |
| Body mass index, kg/mm 2 , mean (SD) | 23.9 (5.4) |
| Non-active smoker, n (%) | 24 (100) |
| Primary diagnosis, n (%) | |
|
18 (75) |
|
4 (17) |
|
1 (4) |
|
1 (4) |
| Active medications, n (%) | |
| |
|
2 (8) |
|
4 (16) |
|
7 (29) |
|
3 (12.5) |
| |
|
2 (8) |
|
2 (8) |
| |
|
3 (12.5) |
|
1 (4) |
| Characterization of strictures | |
| |
|
17 (71) |
|
7 (29) |
|
24 (100%) |
|
2.4 ± 1.2 |
Demographic and clinical data
The mean age was 39.4±12.4 years, and the majority of patients were female (66.7%) and White (58.3%). The mean BMI was 23.9 ± 5.4, and nearly all patients were American Society of Anesthesiologists Class 1 and 2 (75%). All patients aside from two had a primary diagnosis of CD (75%) or UC (17%). Most patients (71%) were actively receiving biological agents, glucocorticoids, or an anti-metabolite as a part of their treatment plans.
Characteristics of strictures
The majority of strictures were anorectal in type (71%). The mean stricture length was 2.4 (± 1.2 cm), which is considered short (< 4 cm). 3 patients (12.5%) had documented history of EBD for the treatment of the stricture prior to the initial IT/NK stricturotomy. The decision to perform EBD prior to stricturotomy therapy was based on stricture type and clinical judgment of the primary gastroenterologist.
Outcomes
The results of endoscopic IT/NK stricturotomy intervention are listed in Table 2 . All patients achieved immediate technical success with documented traversability by the endoscope. There were no 30-day post-procedure AEs that required readmission or intervention. We highlighted common complications including perforation, ileus, and bleeding. There were no significant AEs for any patients over the entirety of the post-procedure follow-up period.
Table 2 Procedure outcomes (N = 24).
| Immediate technical success, n (100%) | 24 (100) |
| Reintervention, n (%) | |
|
16 (66.6) |
|
2 (8.3) |
|
1 |
|
1 |
|
1 (4.2) |
|
7 (29) |
| Time to reintervention, months ± mean SD | 5.3 ± 4.0 |
| Follow-up time, months, mean ± SD | 12.8 ± 6.4 |
| 30-day post-procedure complications, n (%) | |
|
0 (0) |
|
0 (0) |
|
0 (0) |
| Post-procedure adverse events (total follow-up period), n (%) | 0 (0) |
| IT, insulated tip; NK, needle knife; SD, standard deviation | |
Repeat endoscopic IT/NK stricturotomy intervention was required for 67% of patients, but only two patients (11%) required surgical intervention following initial IT/NK stricturotomy. The mean time to reintervention was 5.3 ± 4.0 months. One patient required a completion proctectomy and end ileostomy creation for a rectovaginal fistula with associated abscesses. The other patient ultimately required takedown of the coloanal anastomosis and end colostomy creation due to the severity of disease. Seven patients (29%) did not require any reintervention after initial IT/NK stricturotomy therapy.
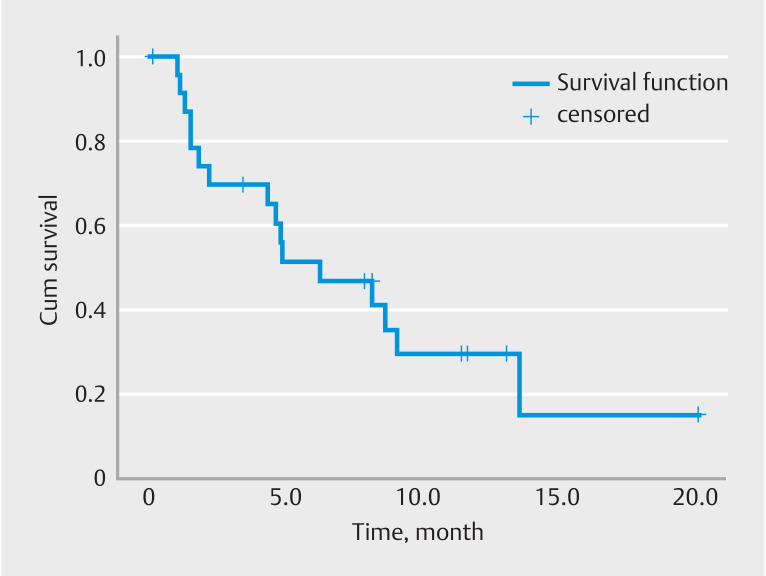
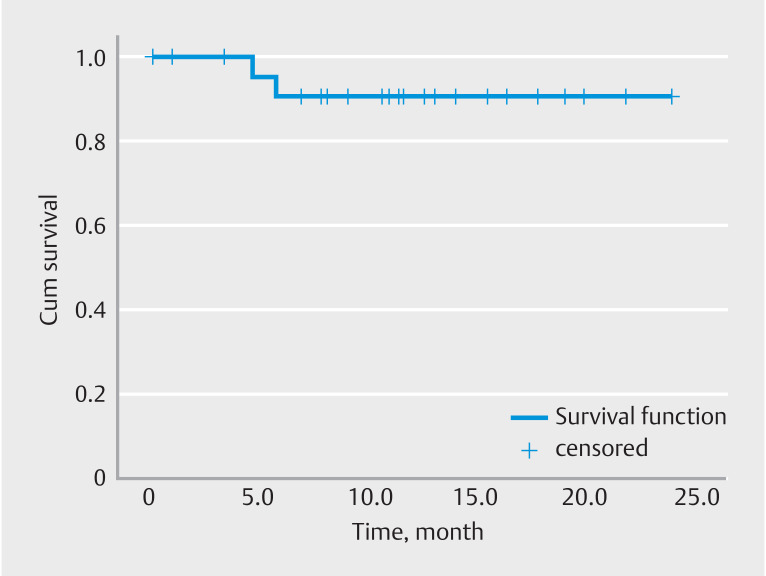
The mean follow-up time was 12.8 ± 6.4 months. The interval between the index endoscopic intervention and the first endoscopic reintervention and/or first surgical interventions were calculated for survival curves. Kaplan-Meier analysis was performed to evaluate repeat endoscopic intervention-free survival ( Fig. 3 ) Cumulative endoscopic reintervention-free survival was 33%. Kaplan-Meier analysis was performed to evaluate surgery-free survival. ( Fig. 4 ) Cumulative surgery-free survival was 92% during the follow-up period.
Fig. 3.
Insulated-tip endoscopic stricturotomy.
Fig. 4.
Needle-knife endoscopic stricturotomy.
Discussion
Our study evaluated 24 patients with non-traversable anorectal/anopouch strictures of various etiologies treated with endoscopic IT/NK stricturotomy. All patients achieved immediate technical success without any post-procedure AEs. A substantial number of patients required repeat endoscopic intervention, but the majority of patients were able to avoid surgical intervention.
Anorectal strictures represent some of the most challenging manifestations of IBD, and anopouch strictures represent a serious complication for patients who undergo IPAA surgery. A case series of CD patients reported a prevalence of 8.9% for anorectal stricture disease 19 and so this represents a common problem for IBD patients and their clinicians. Strictures are defined by etiology type (primary vs anastomotic), length (long vs short), number, degree of fibrosis and inflammation, and presence of concurrent fistulae/abscess. These characteristics are meant to assist in treatment planning, however, optimal treatment for such strictures is not clearly defined in either the medical or surgical literature. There are no prospective studies examining the management of anorectal strictures 20 and the role of medical therapy in the treatment of anorectal or anopouch strictures has not been well defined.
Interventional endoscopy has expanded to position itself as a bridge between medical therapy and surgical intervention. Endoscopic therapies for strictures include EBD, stent placement, intralesional injection, and stricturotomy. Among the endoscopic options, EBD is the most studied. The Global Interventional IBD Group, consisting of IBD experts and specialists, published formal position statements on the role of these endoscopic interventions 21 . They outlined the safety of EBD for primary and secondary strictures, although acknowledged that achieving clinical success with this method was unlikely and that repeat EBD is often necessary. Endoscopic stenting with covered removable metal stents were shown to be less effective and is more complication-prone than EBD in a recent randomized controlled trial (RCT) 22 .
Endoscopic stricturotomy was first described to be safe and effective for the treatment of ileal pouch strictures and various other IBD-related fibrotic strictures 14 15 . Endoscopic stricturotomy is more technically demanding than EBD but is associated with a higher rate of technical success and reduced rate of subsequent surgery than EBD for treatment of CD-related anastomotic strictures (included in this cohort were six ileo-rectal anastomotic strictures) 9 . However, endoscopic stricturotomy does carry a higher risk of bleeding than EBD. Bleeding associated with endoscopic stricturotomy in the anorectal area can usually be readily managed with topical tamponade.
The Global Interventional IBD Group noted that stricturotomy may be the ideal treatment modality for Crohn’s-related distal bowel or anal strictures. Endoscopic stricturotomy offers control over the location, depth, and orientation of electroincision, avoiding inadvertent injury to the anal sphincter or anorectal fistula formation. Our study supports this notion by demonstrating the safety of the IT/NK endoscopic stricturotomy technique in treating severe anorectal/anopouch strictures. It also affirms the efficacy of this treatment modality in achieving technical success, as well clinical success by preventing or delaying the need for surgical intervention. Our study reported a surgical intervention rate of 11% over a mean follow-up period of 11 months, as compared with a rate of 27% over a median follow-up period of 15 to 70 months in a meta-analysis of EBD treatment of CD-related strictures 23 . This is also comparable to a previous study of various IBD-related strictures treated with endoscopic stricturotomy that reported a 15.3% rate of subsequent surgery over a follow-up of 0.9 years 9 . Only three of our patients underwent EBD prior to IT/NK stricturotomy, so these results should not be attributed to a combined effect of EBD+IT/NK Stricturotomy.
This study has limitations in its generalizability, as the IT/NK stricturotomy was performed by an experienced endoscopist with the skilled support staff at a tertiary-care center. This may also contribute to a selection bias. In addition, the sample size is small and follow-up time was relatively short. We aim to gather more data with a larger sample size in follow-up studies, and those will likely include treatment comparison outcomes. Anorectal ring strictures in patients with IBD are newly recognized disease entities and there is scant literature on endoscopic therapy. There have been concerns about the iatrogenic injury to anal sphincters with previously published size of balloons (18–20 mm). However, the authors are exploring drug-coated balloons for the treatment of refractory anorectal and lower GI strictures. In fact, our Columbia University team is leading the Patent B trial (NCT03885310). It will be interesting to compare the safety and efficacy of endoscopic stricturotomy and drug-coated balloons in the treatment anorectal and anopouch strictures.
The majority of the patients in this series required endoscopic reintervention although almost all of them avoided surgery during the follow-up. Clinically, the management of anorectal strictures has been challenging. Various non-surgical options have been explored, including EBD (with radial force) and bougie (with shear force) dilation. The recurrence of strictures after non-surgical treatment is common and most patients required frequent treatment. On the other hand, surgical therapy including completion proctocolectomy, completion proctectomy, pouch excision with permanent fecal diversion is not a valid option for most patients. Morbidities following “more definitive” surgical therapy include stoma complication, persistent perineal sinus (especially in those with current perianal fistula), and iatrogenic injuries to pelvic organs. Therefore, we believe that endoscopic stricturotomy remains a valid treatment option for those with refractory anorectal strictures before more effective and safer treatment modalities are available.
Conclusions
In conclusion, IT/NK endoscopic stricturotomy is safe and effective in the treatment of primary and secondary severe (non-traversable) and short (< 4 cm) anorectal or anopouch strictures, when performed by an experienced interventional endoscopist. We recommend its consideration in the treatment plan for patients with such strictures. The emphasis on advanced endoscopic training during gastroenterology/IBD fellowship is essential so that this technique may become more widely available for further use and study. Ongoing clinical research with larger sample sizes and longer follow-up time is necessary, as well as prospective and RCTs evaluating endoscopic stricturotomy vs EBD or surgical treatment are crucial.
Footnotes
Conflict of Interest The authors declare that they have no conflict of interest.
Correction.
Insulated tip/needle-knife endoscopic stricturotomy is safe and effective for treatment of non-traversable anorectal stricture Koby Herman, Ravi P. Kiran, Bo Shen Endoscopy International Open 2024; 12: E231–E236. DOI: 10.1055/a-2230-7372 In the above-mentioned article an authorʼs correspondence address was corrected. This was corrected in the online version on 26.02.2024.
References
- 1.Linares L, Moreira LF, Andrews H et al. Natural history and treatment of anorectal strictures complicating Crohnʼs disease. Br J Surg. 1988;75:653–655. doi: 10.1002/bjs.1800750711. [DOI] [PubMed] [Google Scholar]
- 2.Rieder F, Zimmermann EM, Remzi FH et al. Crohnʼs disease complicated by strictures: a systematic review. Gut. 2013;62:1072–1084. doi: 10.1136/gutjnl-2012-304353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jumbi T, Kuria K, Osawa F et al. The effectiveness of digital anal dilatation in preventing anal strictures after anorectal malformation repair. J Pediatr Surg. 2019;54:2178–2181. doi: 10.1016/j.jpedsurg.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Singh VV, Draganov P, Valentine J. Efficacy and safety of endoscopic balloon dilation of symptomatic upper and lower gastrointestinal Crohnʼs disease strictures. J Clin Gastroenterol. 2005;39:284–290. doi: 10.1097/01.mcg.0000155128.31208.44. [DOI] [PubMed] [Google Scholar]
- 5.Werre A, Mulder C, van Heteren C et al. Dilation of benign strictures following low anterior resection using Savary-Gilliard bougies. Endoscopy. 2000;32:385–388. doi: 10.1055/s-2000-8999. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki N, Saunders BP, Thomas-Gibson S et al. Colorectal stenting for malignant and benign disease: outcomes in colorectal stenting. Dis Colon Rectum. 2004;47:1201–1207. doi: 10.1007/s10350-004-0556-5. [DOI] [PubMed] [Google Scholar]
- 7.Lavy A. Triamcinolone improves outcome in Crohnʼs disease strictures. Dis Colon Rectum. 1997;40:184–186. doi: 10.1007/BF02054985. [DOI] [PubMed] [Google Scholar]
- 8.Shen B, Fazio VW, Remzi FH et al. Endoscopic balloon dilation of ileal pouch strictures. Am J Gastroenterol. 2004;99:2340–2347. doi: 10.1111/j.1572-0241.2004.40604.x. [DOI] [PubMed] [Google Scholar]
- 9.Lan N, Shen B. Endoscopic stricturotomy versus balloon dilation in the treatment of anastomotic strictures in Crohnʼs disease. Inflamm Bowel Dis. 2018;24:897–907. doi: 10.1093/ibd/izx085. [DOI] [PubMed] [Google Scholar]
- 10.Lian L, Stocchi L, Remzi FH et al. Comparison of endoscopic dilation vs surgery for anastomotic stricture in patients with Crohnʼs disease following ileocolonic resection. Clin Gastroenterol Hepatol. 2017;15:1226–1231. doi: 10.1016/j.cgh.2016.10.030. [DOI] [PubMed] [Google Scholar]
- 11.Mueller T, Rieder B, Bechtner G et al. The response of Crohnʼs strictures to endoscopic balloon dilation. Aliment Pharmacol Ther. 2010;31:634–639. doi: 10.1111/j.1365-2036.2009.04225.x. [DOI] [PubMed] [Google Scholar]
- 12.Truong S, Willis S, Schumpelick V. Endoscopic therapy of benign anastomotic strictures of the colorectum by electroincision and balloon dilatation. Endoscopy. 1997;29:845–849. doi: 10.1055/s-2007-1004319. [DOI] [PubMed] [Google Scholar]
- 13.Chon HK, Shin IS, Kim SW et al. High grade anorectal stricture complicating Crohnʼs disease: endoscopic treatment using insulated-tip knife. Intest Res. 2016;14:285–288. doi: 10.5217/ir.2016.14.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen B, Lian L, Kiran RP et al. Efficacy and safety of endoscopic treatment of ileal pouch strictures. Inflamm Bowel Dis. 2011;17:2527–2535. doi: 10.1002/ibd.21644. [DOI] [PubMed] [Google Scholar]
- 15.Lan N, Shen B. Endoscopic stricturotomy with needle knife in the treatment of strictures from inflammatory bowel disease. Inflamm Bowel Dis. 2017;23:502–513. doi: 10.1097/MIB.0000000000001044. [DOI] [PubMed] [Google Scholar]
- 16.Lan N, Wu JJ, Wu XR et al. Endoscopic treatment of pouch inlet and afferent limb strictures: stricturotomy vs. balloon dilation. Surg Endoscopy. 2021;35:1722–1733. doi: 10.1007/s00464-020-07562-z. [DOI] [PubMed] [Google Scholar]
- 17.Lan N, Stocchi L, Delaney CP et al. Endoscopic stricturotomy versus ileocolonic resection in the treatment of ileocolonic anastomotic strictures in Crohnʼs disease. Gastrointest Endoscopy. 2019;90:259–268. doi: 10.1016/j.gie.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 18.Shen B, Kochhar G, Navaneethan U et al. Practical guidelines on endoscopic treatment for Crohnʼs disease strictures: a consensus statement from the Global Interventional Inflammatory Bowel Disease Group. Lancet Gastroenterol Hepatol. 2020;5:393–405. doi: 10.1016/S2468-1253(19)30366-8. [DOI] [PubMed] [Google Scholar]
- 19.Keighley MR, Allan RN. Current status and influence of operation on perianal Crohnʼs disease. Int J Colorectal Dis. 1986;1:104–107. doi: 10.1007/BF01648416. [DOI] [PubMed] [Google Scholar]
- 20.Lightner AL, Click B, Yamamoto T et al. Management of isolated anal strictures in Crohnʼs disease. Dis Colon Rectum. 2020;63:1639–1647. doi: 10.1097/DCR.0000000000001834. [DOI] [PubMed] [Google Scholar]
- 21.Shen B, Kochhar G, Navaneethan U et al. Role of interventional inflammatory bowel disease in the era of biologic therapy: a position statement from the Global Interventional IBD Group. Gastrointest Endosc. 2019;89:215–237. doi: 10.1016/j.gie.2018.09.045. [DOI] [PubMed] [Google Scholar]
- 22.Loras C, Andújar X, Gornals JB et al. Self-expandable metal stents versus endoscopic balloon dilation for the treatment of strictures in Crohnʼs disease (ProtDilat study): an open-label, multicentre, randomised trial. Lancet Gastroenterol Hepatol. 2022;7:332–341. doi: 10.1016/S2468-1253(21)00386-1. [DOI] [PubMed] [Google Scholar]
- 23.Navaneethan U, Lourdusamy V, Njei B et al. Endoscopic balloon dilation in the management of strictures in Crohnʼs disease: a systematic review and meta-analysis of non-randomized trials. Surg Endosc. 2016;30:5434–5443. doi: 10.1007/s00464-016-4902-1. [DOI] [PubMed] [Google Scholar]