Abstract
Esophageal cancer (EC) is a leading cause of cancer-related death in the west 1 . Esophageal squamous cell carcinoma (SCC) is the most common type of EC worldwide. However, in Western countries, including the United States, esophageal adenocarcinoma (EAC) is the most common 2 . EAC is most common in the lower esophagus whereas SCC is most common in the middle and upper esophagus 3 . The incidence of EAC has increased dramatically in western countries over the past few decades. 2 3 The exact reason for this rise in EAC has not been clearly understood. However, an increase in the prevalence of EAC risk factors is postulated as a potential explanation 4 . Although there are many identifiable EAC risk factors, including gastroesophageal reflux disease (GERD), obesity, male sex, White race, and smoking 5 6 7 , Barrett’s esophagus (BE) remains the major precursor lesion of esophageal adenocarcinoma. BE develops when there is a change in the normal squamous lining of the esophageal mucosa into intestinal metaplasia 8 9 . The incidence has also increased in the population over the past few decades 10 11 . There is a well-described progression within BE from non-dysplastic BE (NDBE), low-grade dysplasia (LGD), high-grade dysplasia (HGD), intramucosal carcinoma (IMC), to invasive EAC 12 13 . Recent data suggest that the increased incidence of EAC may have plateaued 1 . However, we questioned whether the prevalence of EAC is still increasing, especially at younger ages in lieu of recent trends showing an increase in the prevalence of colorectal cancer in younger patients. These findings resulted in a lowering of the colorectal cancer screening age cutoff to 45 years from 50 years 14 15 16 . Therefore, we aimed to assess the time trends in the prevalence and incidence of EAC and some of its risk factors in a large population of patients in Florida and to assess these trends based on age categories. We hypothesized that the prevalence of EAC and BE has increased over time at younger age groups.
Keywords: Reflux disease, Barrett's and adenocarcinoma, Epidemiology
Patients and methods
Database
We conducted a retrospective, longitudinal analysis using the OneFlorida Clinical Data Research Network, a statewide network of healthcare systems that provides medical care to more than 40% of the population in Florida (the third most populous state in the United States) 17 . OneFlorida contains electronic health records and health care claims from over 11 healthcare systems and affiliated practices covering most of Florida and is representative of the whole population in Florida 17 . It includes data on diagnoses, vital signs, laboratory tests, and procedures, among others. All the data were de-identified.
Inclusion criteria
We included all adult patients (aged >18 years) who had at least one entry in the database between 2012 and 2019. All patients who reached the age threshold during the study period were included in the study. We used the International Classification of Diseases 9 th and 10 th Revision (ICD-9/10) codes to identify patients with EAC (Appendix 1) who had at least one clinical encounter diagnosis during the research period. No pathological diagnoses were available given the study design. Therefore, all diagnoses were based on medical coding. EAC was defined if a patient was diagnosed with lower EC: ICD-9 codes 150.2 (malignant neoplasm of lower esophagus) or 150.5 (malignant neoplasm of lower esophagus), or ICD-10 code C15.5 (malignant neoplasm of lower third of esophagus). Other ECs were identified by the following codes: upper EC (ICD 9 code 150.4 or ICD 10 codes C15.4 and C15.8), and unspecific EC (ICD 9 codes 150.9, 150, 150.0,150.1,150.3,150.8 or ICD 10 codes C15 and C15.9). BE and GERD were identified using ICD 9/10 codes and at least one diagnosis during the research period. The primary outcome of interest was the prevalence (per 100,000 patients) of EAC in the population. We assumed that the patient was observable in one consecutive year if they had at least one entry. We assumed that a patient was censored in one consecutive year if they had no entry into the data in that year.
Outcomes and definitions
The primary outcome of this study was disease prevalence. The prevalence of disease was defined as follows:
Patients were divided into the following risk categories based on their age: young (age 18–44 years), middle-aged (45–64 years), and elderly (> 65 years). In the subgroup analysis, we further divided the age groups into 41 to 50 years, 51 to 60 years, and 61 to 70 years. We reported the prevalence of EAC and BE over the study period, stratified by age category.
We reported time trends in the risk factors for EC, including BE, GERD, male sex, White race ethnicity, and obesity. Once a patient had an outcome (.ie. EAC), they did not count again for that outcome in future during the study. Patients who had BE with HGD were considered as BE and not as EAC. Chronic GERD was defined as at least three diagnoses, with any two diagnoses occurring at least 30 days apart during the research period. We reported the prevalence of BE and GERD according to age group. Upper Endoscopy (EGD) was identified using Current Procedural Terminology (CPT) codes (Appendix 1). Obesity was estimated using the average body mass index (BMI) over the study period and an average BMI > 30 as obesity.
We reported the yearly prevalence of obesity and endoscopy (for any indication) over the study period. Race-ethnicity was categorized as non-Hispanic White, non-Hispanic Black, non-Hispanic Other, and Hispanic. For the outcome of incidence rate, this was calculated as follows:
Statistical analysis
All statistical analyses were performed by an expert statistician (SY) using SAS (v.9.4; SAS Institute). Results are reported as means and standard deviations (SD) for normally distributed data or medians with ranges (for skewed data). Proportions were reported for discrete data. Chi-squared tests were used to assess differences between proportions. Statistical significance was set at P < 0.05. Linear regression analysis was used to visualize disease progression over time. We report the beta coefficient for the regression analysis and R 2 . We assessed the time to the diagnosis of EAC and BE as a measure of cumulative incidence. Multivariable hazard cox regression analysis was used to assess the association between new cases and various predictors, including age, sex, race, GERD, and proton pump inhibitors (PPIs). We assessed the effect modification using interaction terms. Hazard ratios (HRs) with 95% confidence intervals and P values were reported. Martingale residuals were used to test the proportional hazard assumption. The study was approved by the Institutional Review Board of the University of Florida.
Results
During our study period from 2012 to 2019, the OneFlorida database included 6,872,194 adult patients who formed the study group. None of the patients were excluded from the study. The mean age at the last encounter of the study group was 49.7 (± 26.2) years, 51.5% were White race, 34.4% were obese, 39.3% were current smokers, and the majority (86.9%) lived in urban areas. The prevalence of GERD in the overall cohort was 11.7% (n=802,014). Other patient characteristics are summarized in Table 1 .
Table 1 Baseline patient characteristics of over 6 million patients included in the study.
| Characteristic (%) | Overall N = 6,872,194 (5) |
| SD, standard deviation; GERD, gastroesophageal reflux disease; PPI, proton pump inhibitor; BMI, body mass index. | |
| Age | |
|
49.7 (26.2) |
|
2,770,417 (40.3%) |
|
2,581,748 (37.6%) |
|
1,520,028 (22.1%) |
| Race | |
|
3,104,625 (51.5%) |
|
2,919,926 (48.5%) |
| EGD | |
|
209,120 (3%) |
|
6,663,073 (97%) |
| GERD | |
|
802, 014 (11.7%) |
|
6,070,179 (88.3%) |
| Chronic GERD | |
|
237,437 (3.5%) |
|
6,634,756(96.5%) |
| Chronic PPI use | |
|
160,185 (2.3%) |
|
6,712,008 (97.7%) |
| Obese | |
|
1,267,526 (34.4%) |
|
2,413,405 (65.6%) |
|
3191262 |
| Residence | |
|
694,821 (13.1%) |
|
4,622,985 (86.9%) |
|
155,4387 |
| Current smoker | |
|
250,959 (39.3%) |
|
388,186 (60.7%) |
|
623,3048 |
| Payer | |
|
1114,268 (19.5%) |
|
688,854 (12.1%) |
|
2,303,760 (40.3%) |
|
543,491 (9.5%) |
|
500,840 (8.8%) |
|
565,505 (9.9%) |
|
1,155,475 |
Overall prevalence
The overall prevalence of EC in the population was 100 per 100,000 patients (n = 7,067). The prevalence of EC was higher in men than in women (180/100,000 vs.40/100,000, P < 0.0001) and in the elderly group than in the middle-aged group (260/100,000 vs. 120/100,000, P < 0.0001). The distribution of esophageal cancer location is summarized in Table 2 . In both ICD-9 and ICD-10 codes, the most common site of EC was unspecified, and the prevalence of EC was on the rise at all sites; however, the highest rate of increase was noted in the lower esophagus.
Table 2 Prevalence of Barrett’s esophagus and esophageal cancer in the study group of 6,872,194 patients.
| Condition | Prevalence |
| BE, Barrett’s esophagus; EC, esophageal cancer. | |
| BE | |
|
311,88 (0.5%) |
|
6,841,005 (99.5%) |
| EC | |
|
7, 067 (0.1%) |
|
6,865,126 (99.9%) |
| BE by codes | |
|
17,315 (55.5%) |
|
1 (0) |
|
331 (1.1%) |
|
328 (1.1%) |
|
515 (1.7%) |
|
12,698 (40.7%) |
| EC by codes | |
|
20 (0.3%) |
|
326 (4.7%) |
|
1301 (18.8%) |
|
115 (1.7%) |
|
2,869 (40.6.7%) |
|
75 (1.1%) |
|
37 (0.5%) |
|
26 (0.4%) |
|
44 (0.6%) |
|
93 (1.3%) |
|
376 (5.3%) |
|
376 (5.3%) |
|
1,626 (23.0%) |
| EC by sites | |
|
1,702 (24.1%) |
|
540 (7.8%) |
|
4825 (68.1%) |
The overall prevalence of EAC in the population was 25 per 100,000 patients (n =1702). The prevalence of EAC was higher in males compared than in females (50/100,000 vs.10/100,000, P < 0.0001) and in the elderly group than in the middle-aged group (60/100,000 vs. 30/100,000, P < 0.0001). The overall prevalence of BE in the study population was 450 per 100,000 patients (n = 31,188). Distribution of BE cases in summarized in Table 2 .
Time trends
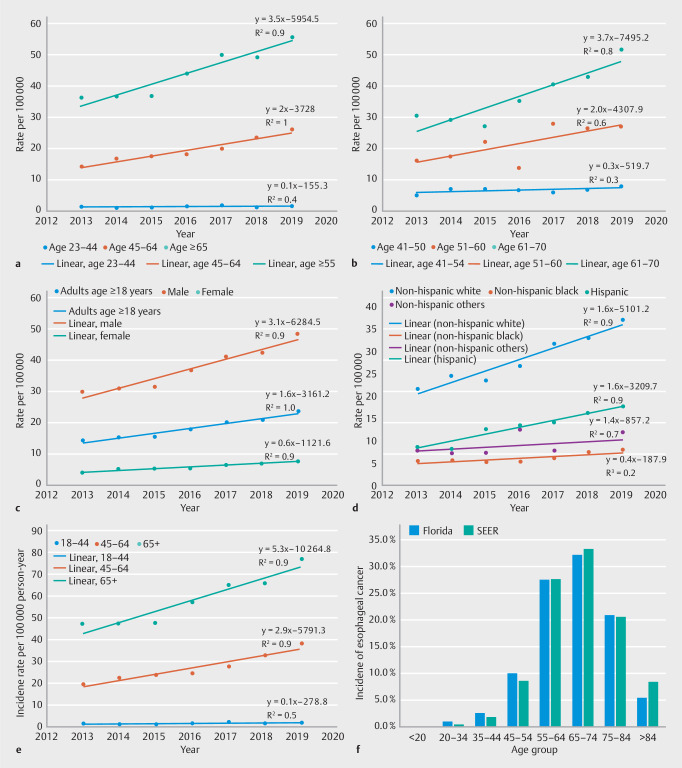
In the middle-aged group, the prevalence of EAC increased from 14.6 to 24.1 cases per 100,000 individuals during the study period. Regression analysis showed a linear increase in the prevalence of EAC in this age group over the study period (y = 2 × – 3718, Fig. 3 a ). In subgroup analyses, the rate of increase in EAC prevalence was highest in the 61 to 70-years age group (y = 4 × – 7496), followed by 51 to 60 years (y = 2 × – 4108), then 41 to 50 years (y = 0.3 × – 520), Fig. 3 b . The prevalence of EAC in the elderly group also increased from 361to 55.7 per 100,000 individuals (y = 3.5 × – 6954).
Fig. 3.
Prevalence of esophageal cancer over time stratified by: a age groups; b age groups within the age 41 to 70 years patient population; c sex; and d race; e middle-aged and elderly grou; f incidence rate of esophageal cancer over time; and h age-adjusted incidence of esophageal cancer in the study population compared to the Surveillance, Epidemiology, and End Results (SEER) database.
The prevalence of lower EAC increased in both males (30.4 to 48.8/100,000; y = 3.1 × – 6284) and females (4.4 to 8.1 per 100,000; y = 0.6 × – 1122, Fig. 3 c ). The prevalence of EAC also increased in all ethnic groups of races. The highest rate increase was noted in the non-Hispanic white group (21.7 to 37.1 per 100,000; y = 2.6 × – × 5121). This was followed by Hispanic (8.9 to 17.9 per 100,000; y = 1.6 × – 3208), Fig. 3 d .
The incidence rate of EAC over time increased in the middle-aged group and in the elderly group ( Fig. 3 e ). In Cox proportional hazards models, controlling for sex, race, GERD, and PPI use, middle-age was independently associated with time to incident diagnosis of EAC (HR:0.04 [0.03–0.06], P < 0.001, Appendix 2). The age-adjusted incidence of EAC in our cohort was similar to that reported by Surveillance, Epidemiology, and End Results (SEER) 18 ( Fig. 3 f ).
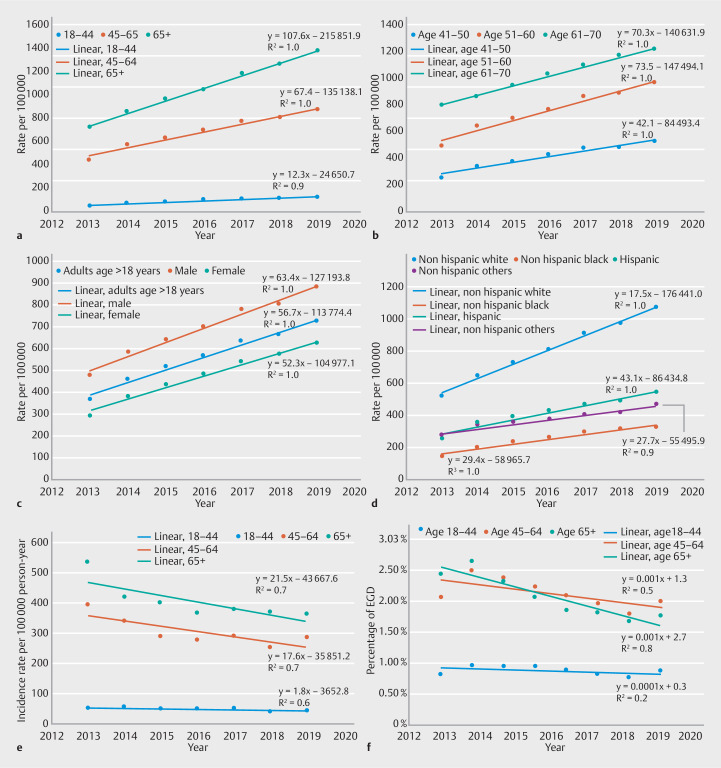
Mirroring the trends in EAC, the prevalence of BE in the middle-aged group nearly doubled (96% increase) in a linear trend (y = 67.37 × – 135138) from 447/100,000 in 2012 to 877/100,000 in 2019 ( Fig. 4 a ). In subgroup analysis, the rate of increase in BE prevalence was highest in the 51–60 years age group (y = 73.5 × –147494), followed by the 61–70 years (y =70.3 × –140682), the 41–50 group (y = 42.1 × –84493), Fig. 4 b .
Fig. 4.
Prevalence of Barrett’s esophagus stratified by: a age group; b age groups from 41–70; c sex; d race-ethnicity; e Incidence rate of Barrett’s esophagus overtime; and f rates of upper endoscopy over time stratified by age group.
The prevalence of BE increased in both males (475/100,000 to 876/100,000; y = 63.4 × 127194) and females (294–621 per 100,000; y = 52.3 × – 104977, Fig. 4 c ). The prevalence of BE also increased in all race-ethnicity groups. The highest rate of increase was noted in the non-Hispanic White group (529 to 1,071/100,000; y = 87.9 × – 176440, Fig. 4 d ). This was followed by non-Hispanic Black (152 to 331 per 100,000; y = 76.6 × –153650), Hispanic (266 to 548 per 100,000; y = 43.1 × –86435), and non-Hispanic other (279–471 per 100,000; y = 27.7 × –55486).
The incidence of BE decreased in both age groups ( Fig. 4 e ). In Cox proportional hazards models, controlling for sex, race, GERD, and BMI, the middle-age group was independently associated with time to incident diagnosis of BE (HR:0.21 [0.20–0.22], P < 0.001) (Appendix 3).
Trends in endoscopy, GERD, and obesity
Over the study period, the use of upper endoscopy decreased from 2.4% in 2013 to 1.9% in 2019 (y = –0.0006 × + 1.3) and from 2.6% in 2013 to 1.7% in 2019 (y = –0.001 × + 2.7, Fig. 4 e ) in the elderly group.
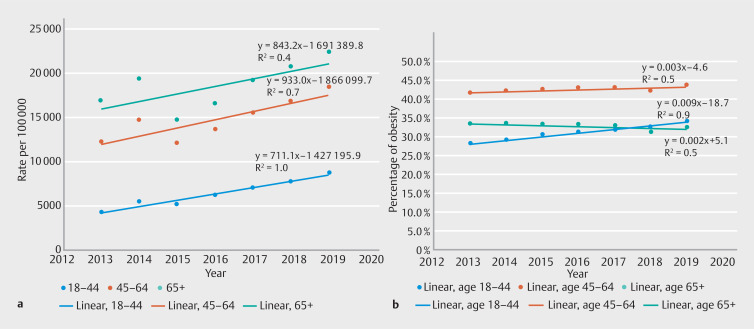
The prevalence of GERD increased by 33% (from 16,831 to 22,351 per 100,000 patients) in the elderly group (y = 848.17 × – 2 × 10 –6 ) and by 50.2% (from 12,278 to 18,443 per 100,000 patients) in the middle-aged group (y = 932.98x – 2 × 10 –6 , Fig. 5 a ). The highest rate increase was noted in the age group 51 to 60 years (y = 958.1x – 2 × 10 –6 ) from 12,950 to 19,272 per 100,000 patients.
Fig. 5.
a Prevalence of GERD by age group and b percentage of adults > 18 years who were obesity over the study period stratified by age group.
For obesity, the highest rate of increase was noted in the young patient population (from 28.1–34.2%). The rate of increase was less profound in the middle-aged group (from 41.6% in 2013 to 43.8% in 2019; Fig. 5 b ). However, obesity was more prevalent in the middle-aged group than in the other groups ( P < 0.0001). Obesity rates declined in the elderly group (from 33.4% in 2013 to 32.7% in 2019).
Discussion
Using a large database of over 6 million patients in Florida, we report that the prevalence of EAC, BE, GERD, and obesity is increasing in middle-aged patients. This increase in EAC and BE prevalence was noted in both sexes and across race-ethnic groups. Incidence rates of EAC were also increasing in middle-aged and elderly groups. Middle age was an independent risk factor for incident EAC diagnosis when controlling for confounders. These trends were noted despite the absence of an increase in the endoscopy rate over the study period.
Clinical implications
Cancer is a leading cause of death worldwide and ranks second only to heart disease 19 . The incidence of many cancers has been declining, including gastric and colorectal cancers 20 21 . On the other hand, the incidence of EAC, has increased dramatically over the past five decades 22 23 , with more recent data from SEER suggesting a possible plateau in this trend over the past few years 24 .
A disturbing trend has recently emerged for some gastrointestinal malignancies. Wang et al. 25 reported that the incidence rates of gastric cancer decreased overall but increased in people < 50 years of age. Similarly, several studies 15 16 26 have reported an increased incidence of colorectal cancer in young adults. In this study, the incidence rate of EAC appears to be on the rise in the middle-aged patients. In addition, we reported an increasing prevalence of EAC and its precursor, BE, in middle-aged patients. In the subgroup analysis, the prevalence of EAC and BE increased even among patients aged 41 to 50 years.
In general, an increased prevalence of a disease is most likely related to two factors: increased incidence and/or longer survival. We noted that the incidence rate of EAC in this age group is increasing. This implies that there is an increase in the rate of new (incident) cases. Coupled with the longer life expectancy in this age group, this may explain the observed trends in prevalence.
The observed increase in BE and EAC prevalence in younger patients has several important clinical implications. In our study, the prevalence of BE in the middle-aged group doubled over the study period. This was mirrored by an increase in the prevalence of EAC, which became more pronounced by the 50s, but is also on the rise in patients aged 41 to 50 years. An important implication of these results is the age cut-off, which is considered a risk factor for BE and EAC. BE is the major precursor lesion for EAC 13 27 28 . Surveillance in patients with BE is associated with a lower risk of cancer progression and higher survival 29 30 . Recent guidelines from major gastroenterology societies in the United States consider age > 50 years to be a risk factor for BE and EAC 30 31 32 . However, the rising prevalence of BE and EAC in the young population may shift this age cut-off to 45 years. More than 10% of all EC were detected in the age group of 45 to 54 years. Changing the screening age to 45 years (if the patient had other risk factors) would match the age at which colorectal screening was initiated. We hope that our findings will encourage future research on this important topic.
EAC and its primary precursor, BE, are thought to be diseases of elderly patients. The reasons why the prevalence of EAC and BE is increasing at younger ages are not easily explained but should be of concern to patients and their physicians. Changing dietary habits, sedentary lifestyles, and changes in the gut microbiome have all been implicated as potential causes 33 . These factors may have played a role in the observed trends. In a database study, we could not establish causality for this observed trend. However, one of the most important risk factors for both EAC and BE, GERD, was noted to have increased in prevalence at a younger age. We believe that the increase in the prevalence of GERD is likely a major contributor to the observed trends. Obesity is another important risk factor for EAC and BE 34 35 . We observed that the prevalence of obesity increased in young patients, and was very high in middle-aged patients. The higher rates of GERD and obesity, along with an aging population, could be major contributors to the increasing prevalence of EC and BE observed in this study.
The rate of increase in obesity prevalence was highest in the youngest population (18–44 years) and peaked in the middle-aged group at > 40%. The rate increase in BE prevalence was highest in the 51- to 60-year age group. The rate increase in EAC prevalence was highest in the 61- to 70-year age group. While the exact mechanisms for these observations are unclear, it is plausible to hypothesize that some of the predisposing factors for EAC, namely obesity and GERD, seem to be accelerating in the younger population (< 45 years old). Approximately 10 years later, the prevalence of BE seems to be accelerating in the 50- to 60-year age group. This later translates to the peaking in the prevalence of EAC in the 60s and 70s. If this hypothesis is accurate, then we may need to intervene in younger age groups to treat obesity and GERD to lower the risk of BE later in life and ultimately lower the rates of EAC at the peak of incidence. These findings have important implications for public health policies.
It is plausible that the observed trends in BE and EAC could be related to improved screening and the wider availability of endoscopy in the population. However, in our cohort, endoscopic utilization, defined as the number of endoscopies performed adjusted for the number of patients in the population, was unlikely to be a contributing factor.
Strengths and limitations
This study is novel in several ways. We focused on the prevalence of EAC in younger patients and found that the time trends indicate a concerning increase in their prevalence. In addition, the study highlights the link between rise in prevalence of EAC precursor (BE, GERD, and obesity) and how this could be leading to the increased EAC prevalence. To our knowledge, such data have not been previously reported. Our data provide evidence from millions of patients that the prevalence of BE is increasing among middle-aged patients. Our sample size was large, which allowed us to compute the desired statistical analyses which we presented in this study. We believe that these results have high validity and are likely to impact in the field of EAC and BE.
Despite its strengths, our study had several limitations. First, the ICD codes did not allow for easy differentiation between esophageal adenocarcinoma and esophageal squamous cell cancer. Neither ICD-9 nor ICD-10 codes differentiated between the two disease entities. However, we were able to separate the patients based on the location of the cancer within the esophagus. Adenocarcinoma is predominantly seen in the lower esophagus, while SCC is more common in the middle or upper part of the esophagus. In the United States, most ECs are EAC. Therefore, after limiting the results to lower esophagus, where EAC is more common, we assumed that lower ECs in this study represent EAC. We believe that our assumption is valid, and that the results presented here are reflective of trends in EAC.
Second, the true prevalence of BE may have been underestimated by the study because patients may have asymptomatic disease. This is less of an issue for EAC, which is progressive and symptomatic, especially in later stages. Furthermore, this limitation is inherent to all population-based study designs. In addition, selection bias cannot be ruled out as access to healthcare in the United States is limited due to lack of universal healthcare coverage. However, previous studies have shown that this OneFlorida database is reflective of the general population in Florida at is covers most localities in the State including urban and rural areas.
Finally, despite the very large sample size of over 6 million patients, the study population came from one state, Florida. Therefore, the generalizability of the results to the general US population may be limited. However, our age-adjusted incidence data closely follow those reported by SEER, which indicates that our results are likely generalizable to a larger US population. In addition, our results are well aligned with the published EC rates in the population, suggesting the external validity of our data.
Conclusions
In this large study of healthcare databases in Florida, we found that the prevalence and incidence rate of EAC and BE to be increasing in middle-aged patients (45 to 64 years old) with a stable incidence rate. The observed trends are not due to increased utilization of endoscopy. These findings are concerning and require further investigation and quantification.
Footnotes
Conflict of Interest B Qumseya: Consultant for: Medtronics; Assertio Management; Endogastric Solutions. Speaker for: Castle Biosciences The remaining authors have no conflict of interest to declare.
Supplementary Material
References
- 1.Islami F, Ward EM, Sung H et al. Annual Report to the Nation on the Status of Cancer, Part 1: National Cancer Statistics. J Natl Cancer Inst. 2021;113:1648–1669. doi: 10.1093/jnci/djab131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Njei B, McCarty TR, Birk JW. Trends in esophageal cancer survival in United States adults from 1973 to 2009: A SEER database analysis. J Gastroenterol Hepatol. 2016;31:1141–1146. doi: 10.1111/jgh.13289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Then EO, Lopez M, Saleem S et al. Esophageal cancer: an updated surveillance epidemiology and end results database analysis. World J Oncol. 2020;11:55–64. doi: 10.14740/wjon1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chai J, Jamal MM. Esophageal malignancy: a growing concern. World J Gastroenterol. 2012;18:6521–6526. doi: 10.3748/wjg.v18.i45.6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qumseya BJ, Bukannan A, Gendy S et al. Systematic review and meta-analysis of prevalence and risk factors for Barrett's esophagus. Gastrointest Endosc. 2019;90:707–717 e701. doi: 10.1016/j.gie.2019.05.030. [DOI] [PubMed] [Google Scholar]
- 6.Qumseya BJ, Wolfsen C, Wang Y et al. Factors associated with increased bleeding post-endoscopic mucosal resection. J Dig Dis. 2013;14:140–146. doi: 10.1111/1751-2980.12002. [DOI] [PubMed] [Google Scholar]
- 7.van Blankenstein M, Looman CW, Johnston BJ et al. Age and sex distribution of the prevalence of Barrett's esophagus found in a primary referral endoscopy center. Am J Gastroenterol. 2005;100:568–576. doi: 10.1111/j.1572-0241.2005.40187.x. [DOI] [PubMed] [Google Scholar]
- 8.Lenglinger J, Riegler M, Cosentini E et al. Review on the annual cancer risk of Barrett's esophagus in persons with symptoms of gastroesophageal reflux disease. Anticancer Res. 2012;32:5465–5473. [PubMed] [Google Scholar]
- 9.Kestens C, Offerhaus GJ, van Baal JW et al. Patients With barrett's esophagus and persistent low-grade dysplasia have an increased risk for high-grade dysplasia and cancer. Clin Gastroenterol Hepatol. 2016;14:956–962 e951. doi: 10.1016/j.cgh.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 10.Conio M, Cameron AJ, Romero Y et al. Secular trends in the epidemiology and outcome of Barrett's oesophagus in Olmsted County, Minnesota. Gut. 2001;48:304–309. doi: 10.1136/gut.48.3.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Soest EM, Dieleman JP, Siersema PD et al. Increasing incidence of Barrett's oesophagus in the general population. Gut. 2005;54:1062–1066. doi: 10.1136/gut.2004.063685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.den Hoed CM, van Blankenstein M, Dees J et al. The minimal incubation period from the onset of Barrett's oesophagus to symptomatic adenocarcinoma. Br J Cancer. 2011;105:200–205. doi: 10.1038/bjc.2011.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qumseya BJ, Wani S, Gendy S et al. Disease progression in Barrett's low-grade dysplasia with radiofrequency ablation compared with surveillance: systematic review and meta-analysis. Am J Gastroenterol. 2017;112:849–865. doi: 10.1038/ajg.2017.70. [DOI] [PubMed] [Google Scholar]
- 14.Shaukat A, Kahi CJ, Burke CA et al. ACG Clinical Guidelines: Colorectal Cancer Screening 2021. Am J Gastroenterol. 2021;116:458–479. doi: 10.14309/ajg.0000000000001122. [DOI] [PubMed] [Google Scholar]
- 15.Hussan H, Patel A, Le Roux M et al. Rising incidence of colorectal cancer in young adults corresponds with increasing surgical resections in obese patients. Clin Transl Gastroenterol. 2020;11:e00160. doi: 10.14309/ctg.0000000000000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loomans-Kropp HA, Umar A. Increasing incidence of colorectal cancer in young adults. J Cancer Epidemiol. 2019;2019:9.841295E6. doi: 10.1155/2019/9841295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hogan WR, Shenkman EA, Robinson T et al. The OneFlorida Data Trust: a centralized, translational research data infrastructure of statewide scope. J Am Med Inform Assoc. 2022;29:686–693. doi: 10.1093/jamia/ocab221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Cancer Institue Surveillance, Epidemiology, and End Results Program . Cancer Stat Facts: Esophageal cancer. https://seer.cancer.gov/statfacts/html/esoph.html https://seer.cancer.gov/statfacts/html/esoph.html
- 19.Torre LA, Bray F, Siegel RL et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 20.Dharwadkar P, Lai A, Brown G et al. Decreasing Rates of Gastrointestinal Cancer at a Veterans Affairs Hospital Between 1994 to 2013: 1000. American Journal of Gastroenterology. 2016;111:S436. [Google Scholar]
- 21.Xie Y, Shi L, He X et al. Gastrointestinal cancers in China, the USA, and Europe. Gastroenterol Rep (Oxf) 2021;9:91–104. doi: 10.1093/gastro/goab010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pera M, Manterola C, Vidal O et al. Epidemiology of esophageal adenocarcinoma. J Surg Oncol. 2005;92:151–159. doi: 10.1002/jso.20357. [DOI] [PubMed] [Google Scholar]
- 23.Patel N, Benipal B. Incidence of esophageal cancer in the United States from 2001–2015: A United States cancer statistics analysis of 50 states. Cureus. 2018;10:e3709. doi: 10.7759/cureus.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Cancer Society . Key Statistics for Esophageal Cancer. https://www.cancer.org/cancer/esophagus-cancer/about/key-statistics.html#references https://www.cancer.org/cancer/esophagus-cancer/about/key-statistics.html#references
- 25.Wang Z, Graham DY, Khan A et al. Incidence of gastric cancer in the USA during 1999 to 2013: a 50-state analysis. Int J Epidemiol. 2018;47:966–975. doi: 10.1093/ije/dyy055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Troeung L, Sodhi-Berry N, Martini A et al. Increasing Incidence of colorectal cancer in adolescents and young adults aged 15–39 years in Western Australia 1982–2007: examination of colonoscopy history. Front Public Health. 2017;5:179. doi: 10.3389/fpubh.2017.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klaver E, Bureo Gonzalez A, Mostafavi N et al. Barrett's esophagus surveillance in a prospective Dutch multi-center community-based cohort of 985 patients demonstrates low risk of neoplastic progression. United Europ Gastroenterol J. 2021;9:929–937. doi: 10.1002/ueg2.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rugge M, Fassan M, Cavallin F et al. Risk of malignant progression in Barrett's esophagus patients: results from a large population-based study. J Natl Cancer Inst. 2012;104:1771–1772. doi: 10.1093/jnci/djs426. [DOI] [PubMed] [Google Scholar]
- 29.Codipilly DC, Chandar AK, Singh S et al. The Effect of endoscopic surveillance in patients with Barrett's esophagus: a systematic review and meta-analysis. Gastroenterology. 2018;154:2068–2086 e2065. doi: 10.1053/j.gastro.2018.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Society of Gastrointestinal Endoscopy Standards Of Practice Committee . Qumseya B, Sultan S et al. ASGE guideline on screening and surveillance of Barrett's esophagus. Gastrointest Endosc. 2019;90:335–359 e332. doi: 10.1016/j.gie.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 31.Shaheen NJ, Falk GW, Iyer PG et al. Diagnosis and management of Barrett's Esophagus: An updated ACG guideline. Am J Gastroenterol. 2022;117:559–587. doi: 10.14309/ajg.0000000000001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muthusamy VR, Wani S, Gyawali CP et al. AGA clinical practice update on new technology and innovation for surveillance and screening in Barrett's esophagus: expert review. Clin Gastroenterol Hepatol. 2022 doi: 10.1016/j.cgh.2022.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stoffel EM, Murphy CC. Epidemiology and mechanisms of the increasing incidence of colon and rectal cancers in young adults. Gastroenterology. 2020;158:341–353. doi: 10.1053/j.gastro.2019.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlottmann F, Dreifuss NH, Patti MG. Obesity and esophageal cancer: GERD, Barrett s esophagus, and molecular carcinogenic pathways. Expert Rev Gastroenterol Hepatol. 2020;14:425–433. doi: 10.1080/17474124.2020.1764348. [DOI] [PubMed] [Google Scholar]
- 35.Cho JH, Shin CM, Han KD et al. Abdominal obesity increases risk for esophageal cancer: a nationwide population-based cohort study of South Korea. J Gastroenterol. 2020;55:307–316. doi: 10.1007/s00535-019-01648-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.