Abstract
This experiment aimed to investigate the effect of Lonicerae flos and Turmeric extracts (LTE) added to diets on growth performance and intestinal health of broilers. A total of 720 healthy 21-day-old yellow-feathered broilers were randomly divided into 3 treatment groups, with 6 replicates and 40 broilers per replicate. These 3 dietary treatments included a basal diet + 0 g/t LTE (CON), a basal diet + 300 g/t LTE (LTE300), and a basal diet + 500 g/t LTE (LTE500). The results showed that dietary supplementation of LTE linearly increased (P < 0.05) average daily gain (d 21–38) and average daily feed intake (d 21–60). At d 60, LTE300 had the highest serum total antioxidant capacity and total superoxide dismutase (P < 0.05), and LTE500 had the lowest malondialdehyde level (P < 0.05) among the three groups. Moreover, compared to CON, LTE300 significantly (P < 0.05) reduced endotoxin (d 38 and d 60) and diamine oxidase activity (d 38); LTE500 significantly (P < 0.05) reduced endotoxin (d 38 and d 60) and diamine oxidase levels (d 60) in the serum. LTE groups significantly (P < 0.05) increased ileal the ratio of villus height to crypt depth and serum immunoglobulin G. Furthermore, dietary supplementation of LTE also improved the intestinal epithelial barrier by the up-regulated mRNA expression of Claudin-1, Occludin and zonula occludens-1, and decreased the mRNA expression of interleukin-2, interleukin-8, tumor necrosis factor-α, nuclear factor κB, myeloid differentiation factor 88 and toll-like receptor 4. Compared to CON, 16S rRNA sequencing analysis showed that LTE300 had a better effect on the microbial diversity and composition in the ileum, and Bacillus and Lactobacillus_agilis were significantly enriched in LTE300. PICRUSt results showed that LTE300 was significantly (P < 0.05) enriched in four pathway pathways at KEGG level 2. In conclusion, dietary supplementation with LTE improved growth performance and intestinal health by enhancing antioxidant capacity, intestinal barrier and immune function, and regulating intestinal flora of yellow-feathered broilers.
Key words: yellow-feathered broiler, chlorogenic acid, curcumin, growth performance, intestinal health
INTRODUCTION
Feed antibiotics can effectively solve many disease problems in poultry production, but it also brings problems such as bacterial resistance, drug residues and food safety (Sreejith et al., 2020). Therefore, the research and development of alternative antibiotic products and technologies that are safe, reliable, stable and environmentally friendly have become a hot research topic in the animal production. Natural antibiotic alternatives include probiotics, prebiotics, organic acids, essential oils, enzymes, and plant extracts have positive impact on poultry production due to their unique properties and positive effects (Salem et al., 2023). Studies have shown that plant extracts act as a natural feed additive with growth promoting, antimicrobial, antioxidant and anti-inflammatory properties (Ding et al., 2017; Abou-Elkhair et al., 2018; Abd et al., 2020).
Lonicerae flos and Turmeric extracts (LTE) is a novel complex plant extract with chlorogenic acid and curcumin as its main components. Lonicerae flos is a Chinese herbal medicine (called Shanyinhua in Chinese) with similar pharmacological effects as Lonicerae japonicae flos (called Jinyinhua in Chinese), such as antimicrobial, antioxidant, and growth-promoting effects (Li et al., 2020; Li et al., 2023), and its main active ingredient is chlorogenic acid. Turmeric is a polyphenolic compound that has been shown to improve growth performance, anti-inflammatory and immunomodulatory effects in poultry and its main active ingredient is curcumin (Pan et al., 2022; Aderemi et al., 2023). Studies have shown that chlorogenic acid is widely used as a feed additive to improve growth performance and intestinal health of animals (Li et al., 2010; Naveed et al., 2018; Bagdas et al., 2020). Also, dietary addition of curcumin improves growth performance, antioxidant capacity and relieves intestinal damage in broilers (Zhang et al., 2019a Yadav et al., 2020).
A growing body of research supports the important role of polyphenolic plant-derived products in animal growth and gut health (Xie et al., 2019; Zhao et al., 2019; Guo et al., 2023). However, there is a paucity of literature on the effects of LTE on the health of yellow-feathered broilers. It is unclear whether the addition of LTE enhances growth performance and improves gut health in healthy yellow-feathered broilers. Therefore, the aim of this study was to evaluate the effects of LTE addition to the diet on growth performance, antioxidant function, and intestinal barrier function of broilers, and to investigate the possible modulatory effects of LTE on broiler intestinal health.
MATERIALS AND METHODS
All experimental procedures were carried out in accordance with the Chinese Guidelines for Animal Welfare Act and were approved by the Institutional Animal Care and Use Committee of China Agricultural University (permission number: AW31903202-1-2).
Lonicerae Flos and Turmeric Extracts
The Lonicerae flos and Turmeric extracts used in this study was supplied by Beijing Centre Biology Co., Ltd. (Beijing, China) and the active ingredients of this product are chlorogenic acid 100 g/t and curcumin 200 g/t.
Experimental Design and Diets
A total of 720 healthy 21-day-old yellow-feathered broilers were selected and randomly divided into 3 treatment groups with 6 replicates and 40 broilers per replicate. These 3 dietary treatments included a basal diet + 0 g/t LTE (CON), a basal diet + 300 g/t LTE (LTE300), and a basal diet + 500 g/t LTE (LTE500). The experiment lasted for 40 d, including 21 to 38 d in the first period and 39 to 60 d in the second period. The basal diet was in pellet form and formulated to meet the recommendation (NY/T33-2004) in Table 1 and all chickens were fed with feed and water freely. Dry matter (DM), crude protein (CP), calcium and total phosphorus in the diet were determined. Dry matter and CP were determined according to GB/T 6435-2014 and GB/T 6432-2018, respectively. Calcium and total phosphorus were determined according to GB/T 6436-2018 and GB/T 6437-2018. In the first 3 d, the room temperature was maintained at 34°C. And then, the room temperature decreased by 3°C per week until 24°C.
Table 1.
Composition and nutrients levels of the basal diet (as-fed basis).
| Ingredient (%) | Days 21–38 | Days 39–60 |
|---|---|---|
| Corn | 61.90 | 62.90 |
| 46% soybean meal | 27.50 | 25.00 |
| Soybean oil | 2.80 | 3.50 |
| Low-gluten flour | 4.00 | 5.00 |
| Dicalcium phosphate | 0.80 | 0.80 |
| Limestone | 1.50 | 1.35 |
| DL-methionine | 0.25 | 0.20 |
| Sodium chloride | 0.25 | 0.25 |
| Premix1 | 1.00 | 1.00 |
| Total | 100 | 100 |
| Analyzed nutrients levels | ||
| Dry matter, % | 89.34 | 88.73 |
| Crude protein, % | 19.14 | 18.24 |
| Calcium, % | 0.86 | 0.79 |
| Total phosphorus, % | 0.64 | 0.59 |
| Calculated nutrients levels | ||
| Metabolizable energy, kcal/kg | 3010.00 | 3070.00 |
| Available phosphorus, % | 0.48 | 0.47 |
| Lysine, % | 1.12 | 1.05 |
| Methionine, % | 0.55 | 0.50 |
The premix provided the following per kilogram of the diet: VA 6,000 IU; VD3 2,000 IU; VE 30 mg; VK3 2 mg; VB1 3 mg; VB2 5 mg; VB12 1 mg; pantothenic acid 800 mg; choline chloride 1,500 mg; nicotinic acid 30 mg; pyridoxine 3 mg; folic acid 500 mg; biotin 0.2 mg; Fe 100 mg; Cu 8 mg; Mn 100 mg; Zn 100 mg; I 0.42 mg; Se 0.3 mg.
Sample Collection
At 38 and 60 d of age, one broiler was randomly selected from each replicate and blood was drawn from the wing vein. The blood was centrifuged at 3,000 r/min for 10 min, and the serum was separated and stored at −20°C. Subsequently, the selected broilers were euthanized by severing the jugular vein, while ileum tissues, and content were collected, and then stored at −80°C for further analysis.
Growth Performance
The body weight (BW) of the broilers was measured at 21, 38, and 60 d. The growth performance of broilers at 21 to 38 and 38 to 60 d of age was observed and recorded. The average daily gain (ADG), average daily feed intake (ADFI), feed conversion ratio (FCR), and mortality rate were calculated for each group.
Immune Organ Index
The liver, spleen, and bursa of the selected birds were removed and weighed, while the immune organ indices were calculated as follows: relative organ weight = organ weight (g) / live weight (kg).
Intestinal Morphology
About 1 cm of the middle of the broiler ileum was taken, rinsed with saline, placed in 4% paraformaldehyde solution, then embedded in paraffin, cut into thin slices and stained with hematoxylin and eosin (H&E) for morphometric analyses. The villus was observed under a light microscope (Leica DM750, Shanghai, China), and villus height (VH), crypt depth (CD), and ratio of villus height to crypt depth (VH/CD) were measured and calculated using the microscope image processing software (LIOO 3.7).
Determination of Immunoglobulins, Antioxidant Capacity, and Intestinal Permeability
Immunoglobulin G (IgG) and immunoglobulin M (IgM) levels were measured in serum using the ELISA kit for chicken (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China). Secretory immunoglobulin A (sIgA) level was determined using ELISA kits (Cloud-Clone Corp., Wuhan, China). The serum and ileum were analyzed for superoxide dismutase (SOD), total antioxidant capacity (T-AOC) and malondialdehyde (MDA) with the aid of a spectrophotometer according to the instruction manual of the kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The determination of diamine oxidase (DAO), endotoxin and D-lactic acid (D-LA) in the serum using commercial kits (Beijing Jinhai Kecum Biotechnology Development Co., Ltd., Beijing, China) in accordance with the manufacturer's instructions.
Tissue RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction Analysis
The mRNA expression of immune and tight junction genes, including zonula occludens-1 (ZO-1), Claudin-1, Occludin, tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), transforming growth factor-β2 (TGF-β2), interleukin-1β (IL-1β), interleukin-2 (IL-2), interleukin-8 (IL-8), interleukin-4 (IL-4), nuclear factor κB (NF-κB), myeloid differentiation factor 88 (MyD88), and Toll-Like receptor 4 (TLR4) was determined using real-time quantitative polymerase chain reaction (RT-qPCR) (ABI 7500, Thermo Fisher Scientific, Waltham, MA). Total mRNA was extracted from broiler ileal tissues at 60 d of age using an RNA extraction kit (TaKaRa, Shiga, Japan) according to the operating instructions, and the purity and quantification were detected using a NanoDrop 1000 Ultra-Micro Spectrophotometer. One microliter of total mRNA was taken, and cDNA was synthesized using a reverse transcription kit (TaKaRa, Shiga, Japan). cDNA was synthesized using a real-time fluorescence quantitative PCR amplification system of 20.0 μL, which included: SYBR Premix Ex TaqTM 10.0 μL, upstream and downstream primers of 0.5 μL each, 1.0 μL of cDNA, and ddH2O of 8.0 μL. The PCR program was 95°C, pre-denaturation 30 s, and 95°C. The PCR reaction program was 95°C, pre-denaturation for 30 s; 95°C denaturation for 5 s, 60°C annealing for 30 s, a total of 40 cycles. The relative expression of mRNA of target gene was calculated by 2−ΔΔCt method using β-actin as the internal reference gene. The primer information is shown in Table 2.
Table 2.
Primers for RT-qPCR analysis of tissue RNA.
| Gene names | Primers sequence 5′→3′ | Accession no. |
|---|---|---|
| Claudin-1 | F: AAGTGCATGGAGGATGACCA R: GCCACTCTGTTGCCATACCA |
NM_001013611.2 |
| Occludin | F: AGTTCGACACCGACCTGAAG R: TCCTGGTATTGAGGGCTGTC |
NM_205128.1 |
| ZO-1 | F: ACAGCTCATCACAGCCTCCT R: TGAAGGGCTTACAGGAATGG |
XM_015278981.1 |
| IL-1β | F: TCATCTTCTACCGCCTGGAC R: GTAGGTGGCGATGTTGACCT |
NM_204524.1 |
| IL-2 | F: GAGTGCACCCAGCAAACTCT R: CCGGTGTGATTTAGACCCGT |
NM_204153.1 |
| IL-4 | F: GTGCCCACGCTGTGCTTAC R: AGGAAACCTCTCCCTGGATGTC |
NM_001007079.1 |
| IL-8 | F: GGCTTGCTAGGGGAAATGA R:AGCTGACTCTGACTAGGAAACTGT |
NM_205498.1 |
| TNF-α | F: CCCCTACCCTGTCCCACAA R: TGAGTACTGCGGAGGGTTCAT |
NM_204267.1 |
| TGF-β2 | F: TCATCACCAGGACAGCGTTA R: TGTGATGGAGCCATTCATGT |
NM_001031045.3 |
| NF-κB | F: TGGAGAAGGCTATGCAGCTT R: CATCCTGGACAGCAGTGAGA |
NM_205134.1 |
| MyD88 | F: TGCAAGACCATGAAGAACGA R: TCACGGCAGCAAGAGAGATT |
NM_001030962.3 |
| TLR4 | F:GATGCATCCCCAGTCCGTG R:CCAGGGTGGTGTTTGGGATT |
NM_001030693 |
| β-actin | F: GAGAAATTGTGCGTGACATCA R: CCTGAACCTCTCATTGCCA |
NM_205518.1 |
Abbreviations: IFN-γ, interferon-γ; IL-1β, interleukin-1β; IL-2, interleukin-2; IL-4, interleukin-4; IL-8, interleukin-8; MyD88, myeloid differentiation factor 88; NF-κB, nuclear factor κB; TGF-β2, transforming growth factor-β2; TLR4, Toll-like receptor 4; TNF-α, tumor necrosis factor-α; ZO-1, zonula occludens-1.
Ileal Microbiota Analysis
On d 60, one chicken per replicate was randomly selected, weighed, and the contents were collected from the ileum and stored at −80°C for subsequent microbiological analysis. Microbiota genomic DNA was extracted from the ileal contents of broilers from the CON and LTE300 groups using the FastDNA Spin Kit for Soil (MP Biomedicals, Southern California, CA) according to the instructions. After completion of genomic DNA extraction, the extracted genomic DNA was detected using 1% agarose gel electrophoresis. The V3 to V4 region of the bacterial 16S rRNA gene was amplified by PCR. The PCR amplification products were extracted from a 2% agarose gel and purified using the AxyPrep DNA Gel Extraction Kit (AXYGEN, New York, NY) according to the manufacturer's instructions. After quantitative purification, sequencing was performed using the Nextseq 2000 PE300 platform from Majorbio Bio-Pharm Technology Co Ltd (Shanghai, China). The original sequences were screened and clustered into operational taxonomic units (OTU) with 97% similarity by QIIME software (version 1.9.1). Finally, data were analyzed by the online Majorbio cloud platform (www.majorbio.com), including α, β diversity, Venn plots, principal coordinate analysis (PCoA), partial least squares discriminant analysis (PLS-DA), Linear discriminant analysis effect size (LEfSe) and PICRUSt analyses.
Statistical Analysis
Data were analyzed preliminarily by Excel and one-way analysis of variance (ANOVA) using SPSS 20.0 statistical software. The linear and quadratic effects of adding LTE were determined using polynomial contrasts. Duncan's multiple comparison test was used to analyze the differences between groups. P < 0.05 was used as the criterion for determining significant differences. Graphics were then visualized using GraphPad prism 9.0 (GraphPad Software Inc., San Diego, CA).
RESULTS
Growth Performance
The effects of LTE on the growth performance of broilers are presented in Table 3. The ADG of broilers during the overall period (21–60 d) linearly increased with increasing LTE levels (P < 0.05). Also, there is a potential linear trend of increase in BW at LTE levels on d 38 and d 60 (0.05 < P < 0.1).
Table 3.
Effect of Lonicerae flos and Turmeric extracts on the growth performance in broilers.
| Items | CON | LTE300 | LTE500 | SEM |
P value |
||
|---|---|---|---|---|---|---|---|
| ANOVA | Linear | Quadratic | |||||
| Body weight, g | |||||||
| Day 21 | 293.33 | 293.75 | 290.42 | 0.857 | 0.234 | 0.212 | 0.240 |
| Day 38 | 744.58 | 753.46 | 776.90 | 6.332 | 0.085 | 0.035 | 0.535 |
| Day 60 | 1790.70 | 1826.03 | 1855.68 | 11.793 | 0.062 | 0.022 | 0.892 |
| 21–38 d | |||||||
| ADFI (g/d) | 60.34 | 60.34 | 62.39 | 0.549 | 0.228 | 0.140 | 0.373 |
| ADG (g/d) | 24.61 | 25.02 | 25.63 | 0.335 | 0.495 | 0.252 | 0.892 |
| FCR | 2.45 | 2.41 | 2.44 | 0.018 | 0.702 | 0.717 | 0.459 |
| Mortality rate (%) | 0.63 | 0.00 | 0.00 | 0.208 | 0.405 | 0.252 | 0.497 |
| 39–60 d | |||||||
| ADFI (g/d) | 115.68 | 115.81 | 118.15 | 0.803 | 0.404 | 0.240 | 0.533 |
| ADG (g/d) | 47.71 | 48.98 | 49.32 | 0.352 | 0.139 | 0.064 | 0.498 |
| FCR | 2.43 | 2.37 | 2.39 | 0.018 | 0.418 | 0.458 | 0.279 |
| Mortality rate (%) | 1.28 | 1.28 | 1.28 | 0.386 | 1.000 | 1.000 | 1.000 |
| 21–60 d | |||||||
| ADFI (g/d) | 90.78 | 90.85 | 93.06 | 0.604 | 0.228 | 0.134 | 0.397 |
| ADG (g/d) | 37.45b | 38.31ab | 39.11a | 0.291 | 0.048 | 0.016 | 0.958 |
| FCR | 2.43 | 2.37 | 2.38 | 0.013 | 0.242 | 0.181 | 0.294 |
| Mortality rate (%) | 1.88 | 1.25 | 1.25 | 0.372 | 0.767 | 0.538 | 0.720 |
Abbreviations: ADFI, average daily feed intake; ADG, average daily gain; BW, body weight; CON, control group, basal diet; FCR, feed conversion ratio; LTE300, basal diet supplemented with 300 g/t LTE; LTE500, basal diet supplemented with 500 g/t LTE.
Means within a row with different superscripts differ significantly (P < 0.05).
Organ Index
The effects of LTE on the relative organ weight in broilers are shown in Table 4. On 60 d, the liver index was quadratically increased by LTE levels (P < 0.05); moreover, the spleen index was linearly affected by LTE levels (P < 0.05).
Table 4.
Effect of Lonicerae flos and Turmeric extracts on the relative organ weight in broilers.
| Items | CON | LTE300 | LTE500 | SEM |
P value |
||
|---|---|---|---|---|---|---|---|
| ANOVA | Linear | Quadratic | |||||
| 38 d | |||||||
| Liver index (g/kg) | 35.97 | 37.26 | 38.01 | 0.474 | 0.224 | 0.091 | 0.949 |
| Spleen index (g/kg) | 1.54 | 1.68 | 1.49 | 0.094 | 0.724 | 1.000 | 0.431 |
| Bursal index (g/kg) | 3.11 | 2.98 | 3.71 | 0.187 | 0.223 | 0.221 | 0.212 |
| 60 d | |||||||
| Liver index (g/kg) | 26.06b | 29.71a | 25.18b | 0.822 | 0.044 | 0.848 | 0.014 |
| Spleen index (g/kg) | 1.10b | 1.19ab | 1.42a | 0.055 | 0.036 | 0.016 | 0.338 |
| Bursal index (g/kg) | 2.07 | 2.07 | 1.98 | 0.093 | 0.923 | 0.749 | 0.820 |
Abbreviations: CON, control group, basal diet; LTE300, basal diet supplemented with 300 g/t LTE; LTE500, basal diet supplemented with 500 g/t LTE.
Means within a row with different superscripts differ significantly (P < 0.05).
Antioxidant Capacity in Serum and Ileum
The effects of LTE on the antioxidant capacity of 60-day-old broilers are shown in Table 5. Dietary supplementation improved the antioxidant capacity of 60-day-old broilers to a certain extent. LTE levels linearly affected T-AOC and SOD levels in serum at d 60, 300 g/t LTE had the highest T-AOC and SOD activity (P < 0.05). In addition, SOD quadratically increased and MDA level linearly decreased with increasing LTE levels (P < 0.05).
Table 5.
Effect of Lonicerae flos and Turmeric extracts on antioxidant capacity of 60-day-old broilers.
| Items | CON | LTE300 | LTE500 | SEM |
P value |
||
|---|---|---|---|---|---|---|---|
| ANOVA | Linear | Quadratic | |||||
| Serum | |||||||
| T-AOC (U/mL) | 9.30b | 10.33a | 10.21ab | 0.199 | 0.050 | 0.024 | 0.319 |
| SOD (U/mL) | 158.12b | 169.69a | 163.28ab | 1.644 | 0.022 | 0.043 | 0.038 |
| MDA (nmol/mL) | 4.58 | 4.04 | 4.18 | 0.109 | 0.087 | 0.056 | 0.245 |
| Ileum | |||||||
| T-AOC (mmol/g prot) | 0.18 | 0.21 | 0.18 | 0.006 | 0.069 | 0.994 | 0.024 |
| SOD (U/mg prot) | 19.99b | 22.65a | 21.58ab | 0.442 | 0.032 | 0.060 | 0.046 |
| MDA (nmol/mg prot) | 2.38a | 2.01ab | 1.45b | 0.154 | 0.038 | 0.014 | 0.514 |
Abbreviations: CON, control group, basal diet; LTE300, basal diet supplemented with 300 g/t LTE; LTE500, basal diet supplemented with 500 g/t LTE; MDA, malondialdehyde; SOD, superoxide dismutase; T-AOC, total antioxidant capacity.
Means within a row with different superscripts differ significantly (P < 0.05).
Intestinal Permeability
The effects of LTE on the intestinal permeability of broilers are summarized in Table 6. Compared with the CON group, the addition of 300 g/t LTE to the diet significantly reduced endotoxin level (P < 0.05). And LTE levels quadratically reduced DAO activity at d 38 (P < 0.05). At d 60, LTE levels linearly reduced endotoxin and DAO levels (P < 0.05).
Table 6.
Effect of Lonicerae flos and Turmeric extracts on intestinal permeability in broilers.
| Items | CON | LTE300 | LTE500 | SEM |
P value |
||
|---|---|---|---|---|---|---|---|
| ANOVA | Linear | Quadratic | |||||
| 38 d | |||||||
| Endotoxin (EU/L) | 11.15a | 10.16b | 10.45b | 0.156 | 0.014 | 0.018 | 0.044 |
| DAO (ng/mL) | 4.00a | 3.42b | 3.87ab | 0.106 | 0.048 | 0.495 | 0.019 |
| D-LA (nmol/L) | 7.20 | 6.60 | 7.04 | 0.121 | 0.094 | 0.408 | 0.044 |
| 60 d | |||||||
| Endotoxin (EU/L) | 10.11a | 9.45b | 8.90b | 0.172 | 0.002 | 0.001 | 0.773 |
| DAO (ng/mL) | 3.53a | 3.26ab | 2.87b | 0.102 | 0.016 | 0.006 | 0.448 |
| D-LA (nmol/L) | 6.52 | 6.06 | 6.03 | 0.099 | 0.066 | 0.030 | 0.379 |
Abbreviations: CON, control group, basal diet; DAO, diamine oxidase; D-LA, D-lactic acid; LTE300, basal diet supplemented with 300 g/t LTE; LTE500, basal diet supplemented with 500 g/t LTE.
Means within a row with different superscripts differ significantly (P < 0.05).
Immune Function
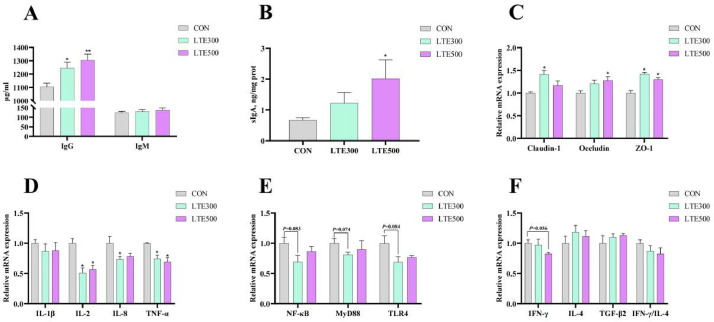
The effects of LTE on immune function of broilers are shown in Figure 1. Compared with the CON group, the serum IgG level and ileal mucosa sIgA level in LTE500 group were significantly increased (P < 0.05, Figures 1A and 1B); the serum IgG level in LTE300 group was also significantly increased (P < 0.05, Figure 1A).
Figure 1.
Effect of Lonicerae flos and Turmeric extracts on ileum barrier and immune function in broilers (n = 6). (A) immunoglobulin G (IgG); immunoglobulin M (IgM); (B) secretory immunoglobulin A (sIgA); (C) Claudin-1, Occludin, ZO-1; (D) tumor necrosis factor-α (TNF-α); interleukin-1β (IL-1β); interleukin-2 (IL-2); interleukin-8 (IL-8); (E) nuclear factor κB (NF-κB); myeloid differentiation factor 88 (MyD88); Toll-Like receptor 4 (TLR4); (F) interferon-γ (IFN-γ); transforming growth factor-β2 (TGF-β2); interleukin-4 (IL-4); the ratio of IFN-γ to IL-4 (IFN-γ/IL-4); CON, control group, basal diet; LTE300, basal diet supplemented with 300 g/t LTE; LTE500, basal diet supplemented with 500 g/t LTE. Asterisk indicates a significant difference from the control group, *P < 0.05, ** P < 0.01 (n = 6).
Ileum Morphology
The effects of LTE on intestinal morphology in broilers are shown in Table 7. At 38 d of age, both dietary additive levels of LTE significantly decreased the CD (P < 0.001) and significantly increased the VH/CD of ileum (P < 0.05). At 60 d of age, LTE levels linearly increased the VH of ileum and quadratically increased the VH/CD of ileum (P < 0.05).
Table 7.
Effect of Lonicerae flos and Turmeric extracts on intestinal morphology in broilers.
| Items | CON | LTE300 | LTE500 | SEM |
P value |
||
|---|---|---|---|---|---|---|---|
| ANOVA | Linear | Quadratic | |||||
| 38 d | |||||||
| VH (μm) | 800.00 | 846.13 | 879.63 | 15.408 | 0.060 | 0.020 | 0.959 |
| CD (μm) | 127.00a | 101.75b | 107.14b | 3.268 | <0.001 | <0.001 | 0.005 |
| VH/CD | 6.34b | 8.18a | 8.21a | 0.269 | <0.001 | <0.001 | 0.024 |
| 60 d | |||||||
| VH (μm) | 918.78b | 1126.74a | 1073.31ab | 36.777 | 0.036 | 0.035 | 0.087 |
| CD (μm) | 107.83 | 108.98 | 119.94 | 3.783 | 0.390 | 0.249 | 0.466 |
| VH/CD | 8.58b | 10.58a | 8.59b | 0.345 | 0.020 | 0.337 | 0.008 |
Abbreviations: CD, crypt depth; CON, control group, basal diet; LTE300, basal diet supplemented with 300 g/t LTE; LTE500, basal diet supplemented with 500 g/t LTE; VH, villus height; VH/CD, the ratio of villus height to crypt depth.
Means within a row with different superscripts differ significantly (P < 0.05).
Ileum Tight-Junction and Immune-Related Factors
The effects of LTE on ileal barrier-related and immunity genes in broilers are shown in Figure 1. The addition of 300 g/t LTE to the diet significantly increased the expression levels of Claudin-1, ZO-1 genes (P < 0.05), and the addition of 500 g/t LTE to the diet significantly increased the expression levels of Occludin, ZO-1 genes (P < 0.05, Figure 1C). Therefore, our data showed that LTE up-regulated the expression level of genes such as tight junction and improved the intestinal barrier function. Dietary addition of LTE significantly decreased the expression levels of IL-2, IL-8, TNF-α pro-inflammatory genes (P < 0.05, Figure 1D), and there was a trend of decreasing IFN-γ in LTE500 group (P = 0.056, Figure 1F). And the addition of 300 g/t of LTE to the diet decreased the expression levels of TLR4, NF-κB, and MyD88 gene expression levels (0.05 < P < 0.1, Figure 1E).
Ileal Microbiota Diversity
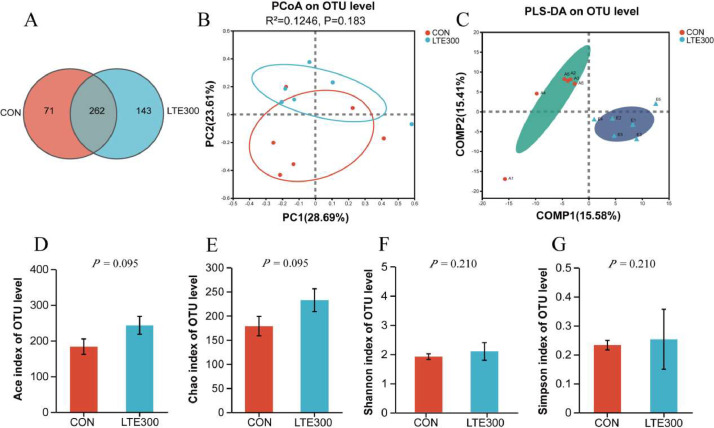
Broilers fed 300 g/t diet of LTE showed better growth performance, antioxidant capacity, immune function, ileal morphology and barrier function. Therefore, the effect of LTE300 group on intestinal flora was further investigated in this experiment. The results of this study showed that LTE300 had a tendency to increase the Ace (P = 0.095) and Chao (P = 0.095) indices compared with the CON group, and the bacterial richness was improved by the treatment (Figures 2D and 2G). The Venn diagrams showed that the CON and LTE300 groups contained 262 common OTUs on d 60, in addition, LTE300 group contained 143 unique OTUs, 72 more than the CON group (Figure 2A). PCoA and PLS-DA calculations showed that the samples from the treatment and control groups showed potential trend of discrimination (Figures 2B and 2C).
Figure 2.
Effect of dietary addition of Lonicerae flos and Turmeric extracts on microbiota diversity and composition of broiler ileum. (A) Venn diagram between treatments on OTUs level. (B and C) Principal coordinate analysis (PCoA) and partial least squares discriminant analysis (PLS-DA) of ileal microbiota on OTU level. (D–G) Alpha diversity at the OTU level (n = 6). CON, control group, basal diet; LTE300, basal diet supplemented with 300 g/t LTE; LTE500, basal diet supplemented with 500 g/t LTE (n = 6).
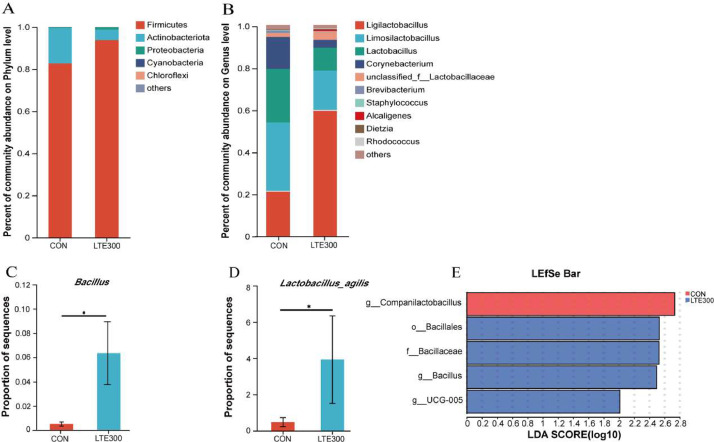
At the phylum level, ileal microbiota were mainly composed of Firmicutes and Actinobacterioa; at the genus level, Lactobacillus, Ligilactobacillus, Limosilactobacillus, Corynebacterium were the major ileal genera (Figures 3A and 3B). In addition, dietary addition of 300 g/t of LTE significantly increased the relative abundance of Bacillus (P < 0.05, Figure 3C); further analysis revealed that the relative abundance of Lactobacillus_agilis was significantly higher in LTE300 group than in the CON group (P < 0.05, Figure 3D).
Figure 3.
Effect of dietary addition of Lonicerae flos and Turmeric extracts on the ileal microbiota at the phylum and genus levels. (A and B) Percentage of community abundance of ileal microbiota at phylum and genus levels. (C and D) significantly different bacteria at the genus and species levels in the LTE300 compared to the CON group. (E) LDA effect size (LEfSe) analysis from phylum level to genus level (LDA score 2.0). CON, control group, basal diet; LTE300, basal diet supplemented with 300 g/t LTE. Asterisk indicates a significant difference from the control group, *P < 0.05 (n = 6).
Biomarkers that were statistically different between the CON and LTE300 groups were identified by linear discriminant analysis (LEfSe analysis). Five biomarkers from the gate level to the genus level were identified by LEfSe analysis (LDA threshold of 2, Figure 3E), and Bacillus and UCG_005 were enriched as biomarkers in LTE300 compared with the CON group at the genus level.
Predicting the Function of Intestinal Bacteria
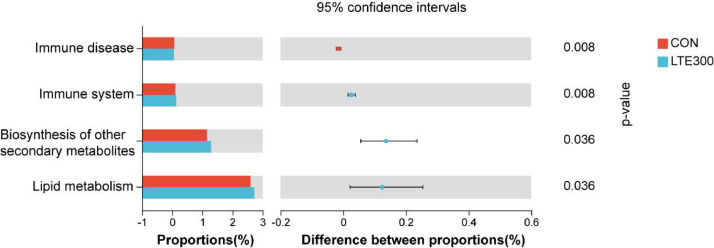
To predict functional alterations of microbes in the ileum, PICRUSt showed significant functional gene differences between LTE300 and the CON group (Figure 4). In this experiment, we obtained a total of 46 KEGG pathways at the level 2. Among them, four pathways with significant differences (P < 0.05) were found between LTE300 and CON groups, namely immune system, immune disease, lipid metabolism and biosynthesis of other secondary metabolites.
Figure 4.
Differences in the KEGG pathway at level 2 of the ileal microbiota. CON, control group, basal diet; LTE300, basal diet supplemented with 300 g/t LTE (n = 6).
DISCUSSION
As novel feed additives, chlorogenic acid and curcumin possess anti-inflammatory, antibacterial, antiviral, antioxidant, and immunomodulatory effects (Naveed et al., 2018; Bagdas et al., 2020; Yadav et al., 2020). Several previous studies have shown that chlorogenic acid contributes to the ability to prevent disease, minimize environmental stress, control glucose and lipid metabolism, and the growth performance of animals (Lu et al., 2020; Miao and Xiang, 2020; Sranujit et al., 2021). Hafez et al., 2022 showed that broilers supplemented with 200 g/t curcumin exhibited better ADG and FCR. In addition, Zhao et al. (2019) demonstrated that the addition of 1,000 g/t chlorogenic acid to the diet could alleviate the adverse effects of heat stress on the growth performance in broilers. In this study, our results are partially consistent with previous findings in broilers, which showed broilers fed LTE linearly increased ADG during the full growth period, and there is a potential linear trend of increase in BW at LTE levels on d 38 and d 60. The dose, method and environmental conditions of the extracts in this trial influenced the differences between these studies. Therefore, the composition and dosage of additives should be considered in practical poultry production.
Liver, spleen, and bursa are important immune organs of broilers. At the later stage of broilers, compared to the CON group, the liver index in LTE300 was higher as well as the spleen index in LTE500. We hypothesized that this may be due to the fact that broilers adapted to the LTE in the diets as they matured in terms of growth and development.
There is a certain dynamic balance of free radicals in animals, and an excess or deficiency of free radicals can disrupt the dynamic balance. The addition of chlorogenic acid can eliminate the activity of hydroxyl radicals or stimulate the antioxidant capacity by affecting the signaling pathway, thus exerting antioxidant effects (Tošović et al., 2017). Studies have shown that dietary chlorogenic acid supplementation improves the free radical scavenging ability, protects the integrity of the intestinal mucosa, increases the antioxidant capacity and the growth performance (Chen et al., 2022). Injecting 4 mg chlorogenic acid into eggs also enhanced the antioxidant capacity of the intestinal tract of broilers (Pan et al., 2023). In this study, we examined oxidative products and important antioxidant enzymes in serum and intestine. SOD, a metalloenzyme, is one of the most potent antioxidant enzymes, effectively balancing the oxidative and antioxidant levels of the organism, and is the first line of defense for living cells against reactive oxygen species. LTE300 group significantly increased the serum levels of T-AOC, SOD, and the ileal SOD content; LTE500 group significantly reduced the ileal MDA content on 60 d. This is consistent with previous findings that addition of 500 or 1,000 g/t chlorogenic acid to the diet had an ameliorating effect on serum SOD activity and MDA concentration in broilers (Zha et al., 2023). Based on the above results, we speculated that feeding LTE can modulate the antioxidant capacity in broilers by enhancing the activity of the antioxidant enzymes.
As is well known, the gut is an important place to digest and absorb nutrients. In the maturation of the digestive tract, an increase in VH, a shallower CD, and an increase in VH/CD indicate a crucial role in the digestive and absorptive function and in the intestinal mucosal structure (Yamauchi et al., 2010). Studies have shown that treatment with chlorogenic acid increased intestinal VH and CD and increased intestinal area expansion in mice compared to control group (Qin et al., 2023). Dietary chlorogenic acid and curcumin supplementation both increased VH/CD in broilers after lipopolysaccharide infection (Zhang et al., 2021a; Tan et al., 2023). The results we obtained were LTE groups reduced the ileal CD and increased VH/CD, the additive leads to a well-developed gut and enhances the digestive and absorptive capacity of broilers at maturity.
Blood and intestinal parameters are important indicators that respond to the physiological state of the animal. Among them, the change of intestinal mucosal permeability can accurately reflect the degree of intestinal mucosal damage, which is an important indicator to monitor the intestinal barrier function (Duangnumsawang et al., 2021). This can be reflected by DAO, D-LA and endotoxin indicators (Dieryck et al., 2022; Zhang et al., 2022). In the present study, LTE supplementation improved the intestinal mucosal permeability of broilers by decreasing endotoxin and DAO levels. Tight junctions play a crucial role in maintaining normal intestinal function, and their damage can disrupt the barrier structure, thereby increasing intestinal permeability (Zhao et al., 2021). In this experiment, the effect of LTE on ileal barrier function and immune-related genes in broilers was further investigated. Dietary LTE at 300 g/t up-regulated the expression levels of Claudin-1 and ZO-1; at 500 g/t up-regulated the expression levels of Occludin and ZO-1. Hence, these results suggest that LTE up-regulates the expression level of genes such as tight junction protein, which improves the intestinal barrier function in broilers and is consistent with growth performance.
Cytokines are one of the key indicators of immunity, which are divided into two categories: Th1-type and Th2-type. Moderate expression of Th1-type factors (e.g., IFN-γ, IL-2, and TNF-α) stimulates the maturation of the immune system, and once over-expressed, they cause inflammatory reactions (Smith and Humphries, 2009); Th2-type factors (e.g., IL-4 and IL-10) are generally anti-inflammatory factors, and their moderate expression suppresses inflammatory reactions, but their excessive expression tends to cause immunosuppression (O'Garra and Vieira, 2007). Synergistic expression among cytokines maintains the immune homeostasis. IFN-γ/IL-4 has been used as one of the indicators of the immune homeostasis (Koarada et al., 2002). Research evidence has shown that chlorogenic acid increases the expression levels of immune factors such as IL-2 and IFN-γ in mice and promotes the activation and proliferation of T cells, macrophages and natural killer cells (Wu et al., 2004). In this study, we found that dietary LTE reduced the expression levels of IL-2, IL-8, TNF-α, and there was a trend to reduce IFN-γ in LTE500 group, but LTE had no effect on IFN-γ/IL-4. This suggests that broilers fed diets containing LTE can inhibit the expression of pro-inflammatory factors and improve the intestinal immune function. While chlorogenic acid itself has an immunostimulatory effect can promote the development of immune system by stimulating the secretion of IFN-γ from Th1-type cytokines; urcumin and chlorogenic acid both can regulate immune homeostasis by modulating the NF-κB signaling pathway, which in turn regulates the secretion of pro-inflammatory factors (Wang et al., 2019; Jin et al., 2021; Li et al., 2021). The classical inflammatory signaling pathway of TLR4/MyD88/NF-κB was analyzed in this study. The results showed that dietary 300 g/t LTE had a tendency to reduce the expression of TLR4, NF-κB, and MyD88, suggesting that extracts may reduce the expression level of pro-inflammatory cytokines through the TLR4/MyD88/NF-κB signaling pathway.
In addition, chlorogenic acid may also enhance the immune system of animals and protect them from disease infections, which require immunoglobulins to regulate immune function (Chen et al., 2018). In this experiment, serum IgG level and ileal mucosal sIgA level were significantly increased in broilers of LTE500 group compared to the CON group; serum IgG levels were also increased in broilers of LTE300 group. This increased immunoglobulins could stimulate complementary components to enhance specific immune mechanisms in poultry, thus protecting them from infections. Among them, the secretion of sIgA is regulated by the amount and type of microbiota in the intestinal, which prevents pathogens from colonizing the intestinal mucosa, and is the main immune barrier to maintain the homeostasis of the commensal microbiota (Papp et al., 2013).
Gut microbiota is not only involved in regulating the absorption and transport of nutrients, but also in modulating the immune function of the gut (Abd et al., 2022). The abundance and diversity of the gut microbiota are a reliable indicator of host health (Chen et al., 2017). Alpha diversity analysis refers to the diversity of a particular ecosystem, and the community diversity and richness indices are commonly used to respond to the species and structural diversity of the microbiota (Wagner et al., 2018; Magurran, 2021). Previous studies have shown that the addition of chlorogenic acid to broiler diets not only significantly altered the intestinal structure, but also increased the alpha diversity of cecum microorganisms and decreased the relative abundance of Firmicutes and Proteobacteria (Chen et al., 2019); moreover, dietary addition of 300 g/t curcumin increased Enterococcus and decreased unclassified_f_Ruminococcaceae and Alistipes abundances (Ruan et al., 2022). Feed supplementation with curcumin increased the abundance of Faecalibacterium prausnitzii associated with anti-inflammatory properties in the cecum of chickens (Zhang et al., 2021a). In the present study, dietary addition of 300 g/t LTE improved the abundance of gut microbiota. Changes in microbiota composition were also observed in broilers treated with this extracts. At the phylum level, the dominant ileal phylum in this test consisted of Firmicutes and Actinobacteria. Some studies showed that Firmicutes, Tenericutes, Bacteroidetes, Proteobacteria, and Actinobacteria were the major bacterial in broilers; and both Firmicutes and Tenericutes in the intestine were are strongly associated with performance in poultry (Wei et al., 2013; Singh et al., 2014), and play an important role in polysaccharide catabolism and subsequent production of short-chain fatty acids (Postler and Ghosh, 2017). Our findings showed that at the genus level, LTE300 group contained a high level of Bacillus. Probiotics of the Bacillus (e.g., Bacillus subtilis, Bacillus licheniformis, Bacillus coagulans) are widely used in poultry production as antibiotic alternatives to enhance growth performance, immunity, and improve intestinal health (Grant et al., 2018; Abd et al., 2020; Buiatte et al., 2023).
Further analysis revealed that Lactobacillus had high abundance in LTE300 group, and the relative abundance of Lactobacillus_agilis was significantly higher than that of the control. Lactobacillus is the major bacterial taxon found in crop, stomach, duodenum, and ileum (Bindari and Gerber, 2022). Studies have shown that chlorogenic acid can modulate the intestinal microbiota to increase the diversity. Compared to the CON, the intestinal tract of broilers after dietary addition of chlorogenic acid was enriched with Lactobacillaceae, Lactobacillus, and Lactobacillales, which play an important role in maintaining the health (Teixeira et al., 2021; Zhang et al., 2021b; Liu et al., 2023a). Chen and Yu et al. (2020) showed that the proportion of Lactobacillus was positively correlated with ADFI results in broilers. In addition, Liu et al., 2023b, Liu et al., 2023a showed that increased abundance of Lactobacillus was also associated with improved growth performance, immunity and antioxidant capacity in yellow-feathered broilers. In mouse fecal microorganisms, chlorogenic acid mitigated the decrease in microbiota diversity, up-regulated the relative abundance of Lactobacillus and attenuated dextran sulfate-induced colon damage (Zhang et al., 2019b).
PICRUSt can predict unobserved characteristics from phylogenetic information of organisms in the community (Langille et al., 2013; Douglas et al., 2018). The results showed that the addition of 300 g/t of LTE to the diet showed significant differences in four pathways, namely immune system, immune disease, lipid metabolism and biosynthesis of other secondary metabolites in the ileal microbiota of broilers. The four pathways indirectly reflect the involvement of ileal microbiota in intestinal immunity and nutrient digestion and absorption in broilers. Previous studies have shown that chlorogenic acid can affect lipid metabolism in diabetic mice by regulating fatty acid oxidation and transport as well as triglyceride catabolism and synthesis; also chlorogenic acid restored the abundance of Lactobacillus in the cecum of mice and improved bacterial diversity (Yan et al., 2022). In the present study, we found that treatment with extracts significantly increased the biosynthetic pathways of lipid metabolism and other secondary metabolites as well as the relative abundance of Lactobacillus and was associated with improved growth performance and intestinal health in broilers. Gut environmental factors, including nutrition and microbiota, play an important role in controlling immune responses and maintaining homeostasis, and that gut microbiota metabolize dietary lipids that can modulate immune system (Saika et al., 2019). The gut microbiota can both transform and synthesize lipids and catabolize dietary lipids to produce secondary metabolites with host-regulatory properties (Brown et al., 2023). It is evident that the addition of LTE may improve growth performance and immune function of broilers by regulating the ileal microbiota, which may be associated with improved intestinal health.
CONCLUSIONS
In conclusion, dietary supplementation with Lonicerae flos and Turmeric extracts improved the growth performance and intestinal health of yellow-feathered broilers by enhancing antioxidant capacity and immune function, improving intestinal morphology and barrier function. Also LTE regulated intestinal flora may be associated with increasing the abundance of Bacillus and Lactobacillus. Considering the cost and the efficacy of LTE, an inclusion level of 300 g/t LTE in broilers diets is recommended.
ACKNOWLEDGMENTS
The authors thank Beijing Centre Biology Co., Ltd. for the gift of Lonicerae flos and Turmeric extracts in this study. And thanks for support of the State Key Laboratory of Animal Nutrition of China Agricultural University for this experiment.
DISCLOSURES
The authors declare that there is no conflict of interest in the present study.
REFERENCES
- Abd E.M., El-Saadony M.T., Alqhtani A.H., Swelum A.A., Salem H.M., Elbestawy A.R., Noreldin A.E., Babalghith A.O., Khafaga A.F., Hassan M.I., El-Tarabily K.A. The relationship among avian influenza, gut microbiota and chicken immunity: an updated overview. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.102021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd E.M., El-Saadony M.T., Shafi M.E., Qattan S., Batiha G.E., Khafaga A.F., Abdel-Moneim A.E., Alagawany M. Probiotics in poultry feed: a comprehensive review. J. Anim. Physiol. Anim. Nutr. (Berl). 2020;104:1835–1850. doi: 10.1111/jpn.13454. [DOI] [PubMed] [Google Scholar]
- Abou-Elkhair R., Selim S., Hussein E. Effect of supplementing layer hen diet with phytogenic feed additives on laying performance, egg quality, egg lipid peroxidation and blood biochemical constituents. Anim. Nutr. 2018;4:394–400. doi: 10.1016/j.aninu.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aderemi F.A., Alabi O.M. Turmeric (Curcuma longa): an alternative to antibiotics in poultry nutrition. Transl. Anim. Sci. 2023;7:d133. doi: 10.1093/tas/txad133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdas D., Gul Z., Meade J.A., Cam B., Cinkilic N., Gurun M.S. Pharmacologic overview of chlorogenic acid and its metabolites in chronic pain and inflammation. Curr. Neuropharmacol. 2020;18:216–228. doi: 10.2174/1570159X17666191021111809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindari Y.R., Gerber P.F. Centennial review: factors affecting the chicken gastrointestinal microbial composition and their association with gut health and productive performance. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E.M., Clardy J., Xavier R.J. Gut microbiome lipid metabolism and its impact on host physiology. Cell Host Microbe. 2023;31:173–186. doi: 10.1016/j.chom.2023.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buiatte V., Schultheis M., Lorenzoni A.G. Deconstruction of a multi-strain Bacillus-based probiotic used for poultry: an in vitro assessment of its individual components against C. perfringens. BMC Res. Notes. 2023;16:117. doi: 10.1186/s13104-023-06384-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Chen D., Yu B., Luo Y., Zheng P., Mao X., Yu J., Luo J., Huang Z., Yan H., He J. Chlorogenic acid attenuates oxidative stress-induced intestinal mucosa disruption in weaned pigs. Front. Vet. Sci. 2022;9 doi: 10.3389/fvets.2022.806253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Xie H., Chen D., Yu B., Mao X., Zheng P., Yu J., Luo Y., Luo J., He J. Chlorogenic acid improves intestinal development via suppressing mucosa inflammation and cell apoptosis in weaned pigs. ACS Omega. 2018;3:2211–2219. doi: 10.1021/acsomega.7b01971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Yu B., Chen D., Zheng P., Luo Y., Huang Z., Luo J., Mao X., Yu J., He J. Changes of porcine gut microbiota in response to dietary chlorogenic acid supplementation. Appl. Microbiol. Biotechnol. 2019;103:8157–8168. doi: 10.1007/s00253-019-10025-8. [DOI] [PubMed] [Google Scholar]
- Chen L., Xu Y., Chen X., Fang C., Zhao L., Chen F. The maturing development of gut microbiota in commercial piglets during the weaning transition. Front. Microbiol. 2017;8:1688. doi: 10.3389/fmicb.2017.01688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.C., Yu Y.H. Bacillus licheniformis-fermented products improve growth performance and the fecal microbiota community in broilers. Poult. Sci. 2020;99:1432–1443. doi: 10.1016/j.psj.2019.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieryck I., De Backere J., Paeshuyse J. Effect of hatching system and prophylactic antibiotic use on serum levels of intestinal health biomarker diamine oxidase in broilers at an early age. Animal. 2022;16 doi: 10.1016/j.animal.2022.100493. [DOI] [PubMed] [Google Scholar]
- Ding X., Yu Y., Su Z., Zhang K. Effects of essential oils on performance, egg quality, nutrient digestibility and yolk fatty acid profile in laying hens. Anim. Nutr. 2017;3:127–131. doi: 10.1016/j.aninu.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas G.M., Beiko R.G., Langille M. Predicting the functional potential of the microbiome from marker genes using PICRUSt. Methods Mol. Biol. 2018;1849:169–177. doi: 10.1007/978-1-4939-8728-3_11. [DOI] [PubMed] [Google Scholar]
- Duangnumsawang Y., Zentek J., Goodarzi B.F. Development and functional properties of intestinal mucus layer in poultry. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.745849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant A., Gay C.G., Lillehoj H.S. Bacillus spp. as direct-fed microbial antibiotic alternatives to enhance growth, immunity, and gut health in poultry. Avian Pathol. 2018;47:339–351. doi: 10.1080/03079457.2018.1464117. [DOI] [PubMed] [Google Scholar]
- Guo S., Hu J., Ai S., Li L., Ding B., Zhao D., Wang L., Hou Y. Effects of Pueraria extract and curcumin on growth performance, antioxidant status and intestinal integrity of broiler chickens. Animals (Basel) 2023;13:1276. doi: 10.3390/ani13081276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafez M.H., El-Kazaz S.E., Alharthi B., Ghamry H.I., Alshehri M.A., Sayed S., Shukry M., El-Sayed Y.S. The impact of curcumin on growth performance, growth-related gene expression, oxidative stress, and immunological biomarkers in broiler chickens at different stocking densities. Animals (Basel) 2022;12:958. doi: 10.3390/ani12080958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S., Yang H., Jiao Y., Pang Q., Wang Y., Wang M., Shan A., Feng X. Dietary curcumin alleviated acute ileum damage of ducks (Anas platyrhynchos) induced by AFB1 through regulating Nrf2-ARE and NF-κB signaling pathways. Foods. 2021;1370:303. doi: 10.3390/foods10061370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koarada S., Wu Y., Olshansky G., Ridgway W.M. Increased nonobese diabetic Th1:Th2 (IFN-gamma:IL-4) ratio is CD4+ T cell intrinsic and independent of APC genetic background. J. Immunol. 2002;169:6580–6587. doi: 10.4049/jimmunol.169.11.6580. [DOI] [PubMed] [Google Scholar]
- Langille M.G., Zaneveld J., Caporaso J.G., McDonald D., Knights D., Reyes J.A., Clemente J.C., Burkepile D.E., Vega T.R., Knight R., Beiko R.G., Huttenhower C. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013;31:814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Zhang L., He P., Li H., Pan X., Zhang W., Xiao M., He F. Traditional uses, botany, phytochemistry, and pharmacology of Lonicerae japonicae flos and Lonicerae flos: a systematic comparative review. J. Ethnopharmacol. 2023;322 doi: 10.1016/j.jep.2023.117278. [DOI] [PubMed] [Google Scholar]
- Li Y., Li W., Fu C., Song Y., Fu Q. Lonicerae japonicae flos and Lonicerae flos: a systematic review of ethnopharmacology, phytochemistry and pharmacology. Phytochem. Rev. 2020;19:1–61. doi: 10.1007/s11101-019-09655-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Wang Q., Yao X., Li Y. Induction of CYP3A4 and MDR1 gene expression by baicalin, baicalein, chlorogenic acid, and ginsenoside Rf through constitutive androstane receptor- and pregnane X receptor-mediated pathways. Eur. J. Pharmacol. 2010;640:46–54. doi: 10.1016/j.ejphar.2010.05.017. [DOI] [PubMed] [Google Scholar]
- Li Y., Yang D., Jia Y., He L., Li J., Yu C., Liao C., Yu Z., Zhang C. Research note: anti-inflammatory effects and antiviral activities of baicalein and chlorogenic acid against infectious bursal disease virus in embryonic eggs. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Zhou J., Li Y., Ding Y., Lian J., Dong Q., Qu Q., Lv W., Guo S. Effects of dietary polyherbal mixtures on growth performance, antioxidant capacity, immune function and jejunal health of yellow-feathered broilers. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Zhang Y., Bai D., Li Y., He X., Ito K., Tan H., Zhen W., Zhang B., Ma Y. Dietary supplementation with chlorogenic acid enhances antioxidant capacity, which promotes growth, jejunum barrier function, and cecum microbiota in broilers under high stocking density stress. Animals (Basel) 2023;13:303. doi: 10.3390/ani13020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H., Tian Z., Cui Y., Liu Z., Ma X. Chlorogenic acid: a comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr. Rev. Food Sci. Food Saf. 2020;19:3130–3158. doi: 10.1111/1541-4337.12620. [DOI] [PubMed] [Google Scholar]
- Magurran A.E. Measuring biological diversity. Curr. Biol. 2021;31:R1174–R1177. doi: 10.1016/j.cub.2021.07.049. [DOI] [PubMed] [Google Scholar]
- Miao M., Xiang L. Pharmacological action and potential targets of chlorogenic acid. Adv. Pharmacol. 2020;87:71–88. doi: 10.1016/bs.apha.2019.12.002. [DOI] [PubMed] [Google Scholar]
- Naveed M., Hejazi V., Abbas M., Kamboh A.A., Khan G.J., Shumzaid M., Ahmad F., Babazadeh D., FangFang X., Modarresi-Ghazani F., WenHua L., XiaoHui Z. Chlorogenic acid (CGA): a pharmacological review and call for further research. Biomed. Pharmacother. 2018;97:67–74. doi: 10.1016/j.biopha.2017.10.064. [DOI] [PubMed] [Google Scholar]
- O'Garra A., Vieira P. T(H)1 cells control themselves by producing interleukin-10. Nat. Rev. Immunol. 2007;7:425–428. doi: 10.1038/nri2097. [DOI] [PubMed] [Google Scholar]
- Pan S., Yan J., Xu X., Chen Y., Chen X., Li F., Xing H. Current development and future application prospects of plants-derived polyphenol bioactive substance curcumin as a novel feed additive in livestock and poultry. Int. J. Mol. Sci. 2022;23:11905. doi: 10.3390/ijms231911905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Lin H., Jiao H., Zhao J., Wang X. Effects of in ovo feeding of chlorogenic acid on antioxidant capacity of postnatal broilers. Front. Physiol. 2023;14 doi: 10.3389/fphys.2023.1091520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp M., Sipeki N., Vitalis Z., Tornai T., Altorjay I., Tornai I., Udvardy M., Fechner K., Jacobsen S., Teegen B., Sumegi A., Veres G., Lakatos P.L., Kappelmayer J., Antal-Szalmas P. High prevalence of IgA class anti-neutrophil cytoplasmic antibodies (ANCA) is associated with increased risk of bacterial infection in patients with cirrhosis. J. Hepatol. 2013;59:457–466. doi: 10.1016/j.jhep.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Postler T.S., Ghosh S. Understanding the holobiont: how microbial metabolites affect human health and shape the immune system. Cell. Metab. 2017;26:110–130. doi: 10.1016/j.cmet.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y., Wang S., Huang W., Li K., Wu M., Liu W., Han J. Chlorogenic acid improves intestinal morphology by enhancing intestinal stem-cell activity. J. Sci. Food. Agric. 2023;103:3287–3294. doi: 10.1002/jsfa.12469. [DOI] [PubMed] [Google Scholar]
- Ruan D., Wu S., Fouad A.M., Zhu Y., Huang W., Chen Z., Gou Z., Wang Y., Han Y., Yan S., Zheng C., Jiang S. Curcumin alleviates LPS-induced intestinal homeostatic imbalance through reshaping gut microbiota structure and regulating group 3 innate lymphoid cells in chickens. Food Funct. 2022;13:11811–11824. doi: 10.1039/d2fo02598a. [DOI] [PubMed] [Google Scholar]
- Saika A., Nagatake T., Kunisawa J. Host- and microbe-dependent dietary lipid metabolism in the control of allergy, inflammation, and immunity. Front. Nutr. 2019;6:36. doi: 10.3389/fnut.2019.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem H.M., Saad A.M., Soliman S.M., Selim S., Mosa W., Ahmed A.E., Al J.S., Almuhayawi M.S., Abd E.M., El-Tarabily K.A., El-Saadony M.T. Ameliorative avian gut environment and bird productivity through the application of safe antibiotics alternatives: a comprehensive review. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K.M., Shah T.M., Reddy B., Deshpande S., Rank D.N., Joshi C.G. Taxonomic and gene-centric metagenomics of the fecal microbiome of low and high feed conversion ratio (FCR) broilers. J. Appl. Genet. 2014;55:145–154. doi: 10.1007/s13353-013-0179-4. [DOI] [PubMed] [Google Scholar]
- Smith A.J., Humphries S.E. Cytokine and cytokine receptor gene polymorphisms and their functionality. Cytokine Growth Factor Rev. 2009;20:43–59. doi: 10.1016/j.cytogfr.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Sranujit R.P., Noysang C., Tippayawat P., Kooltheat N., Luetragoon T., Usuwanthim K. Phytochemicals and immunomodulatory effect of Nelumbo nucifera flower extracts on human macrophages. Plants (Basel) 2021;10:2007. doi: 10.3390/plants10102007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreejith S., Shajahan S., Prathiush P.R., Anjana V.M., Viswanathan A., Chandran V., Ajith K.G., Jayachandran R., Mathew J., Radhakrishnan E.K. Healthy broilers disseminate antibiotic resistance in response to tetracycline input in feed concentrates. Microb. Pathog. 2020;149 doi: 10.1016/j.micpath.2020.104562. [DOI] [PubMed] [Google Scholar]
- Tan H., Zhen W., Bai D., Liu K., He X., Ito K., Liu Y., Li Y., Zhang Y., Zhang B., Ma Y. Effects of dietary chlorogenic acid on intestinal barrier function and the inflammatory response in broilers during lipopolysaccharide-induced immune stress. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira C.G., Fusieger A., Milião G.L., Martins E., Drider D., Nero L.A., de Carvalho A.F. Weissella: an emerging bacterium with promising health benefits. Probiotics Antimicrob. Proteins. 2021;13:915–925. doi: 10.1007/s12602-021-09751-1. [DOI] [PubMed] [Google Scholar]
- Tošović J., Marković S., Dimitrić M.J., Mojović M., Milenković D. Antioxidative mechanisms in chlorogenic acid. Food. Chem. 2017;237:390–398. doi: 10.1016/j.foodchem.2017.05.080. [DOI] [PubMed] [Google Scholar]
- Wagner B.D., Grunwald G.K., Zerbe G.O., Mikulich-Gilbertson S.K., Robertson C.E., Zemanick E.T., Harris J.K. On the use of diversity measures in longitudinal sequencing studies of microbial communities. Front. Microbiol. 2018;9:1037. doi: 10.3389/fmicb.2018.01037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.W., Jia H.J., Zhang H.J., Wang J., Lv H.Y., Wu S.G., Qi G.H. Supplemental plant extracts from Flos lonicerae in combination with baikal skullcap attenuate intestinal disruption and modulate gut microbiota in laying hens challenged by Salmonella pullorum. Front. Microbiol. 2019;10:1681. doi: 10.3389/fmicb.2019.01681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S., Morrison M., Yu Z. Bacterial census of poultry intestinal microbiome. Poult. Sci. 2013;92:671–683. doi: 10.3382/ps.2012-02822. [DOI] [PubMed] [Google Scholar]
- Wu H.Z., Luo J., Yin Y.X., Wei Q. Effects of chlorogenic acid, an active compound activating calcineurin, purified from Flos Lonicerae on macrophage. Acta Pharmacol. Sin. 2004;25:1685–1689. [PubMed] [Google Scholar]
- Xie Z., Shen G., Wang Y., Wu C. Curcumin supplementation regulates lipid metabolism in broiler chickens. Poult. Sci. 2019;98:422–429. doi: 10.3382/ps/pey315. [DOI] [PubMed] [Google Scholar]
- Yadav S., Teng P.Y., Souza D.S.T., Gould R.L., Craig S.W., Lorraine F.A., Pazdro R., Kim W.K. The effects of different doses of curcumin compound on growth performance, antioxidant status, and gut health of broiler chickens challenged with Eimeria species. Poult. Sci. 2020;99:5936–5945. doi: 10.1016/j.psj.2020.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi K.E., Incharoen T., Yamauchi K. The relationship between intestinal histology and function as shown by compensatory enlargement of remnant villi after midgut resection in chickens. Anat. Rec (Hoboken). 2010;293:2071–2079. doi: 10.1002/ar.21268. [DOI] [PubMed] [Google Scholar]
- Yan Y., Li Q., Shen L., Guo K., Zhou X. Chlorogenic acid improves glucose tolerance, lipid metabolism, inflammation and microbiota composition in diabetic db/db mice. Front. Endocrinol. (Lausanne) 2022;13 doi: 10.3389/fendo.2022.1042044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha P., Wei L., Liu W., Chen Y., Zhou Y. Effects of dietary supplementation with chlorogenic acid on growth performance, antioxidant capacity, and hepatic inflammation in broiler chickens subjected to diquat-induced oxidative stress. Poult. Sci. 2023;102 doi: 10.1016/j.psj.2023.102479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Gao X., Wu J., Chen M. The correlation between endotoxin, D-lactate, and diamine oxidase with endoscopic activity in inflammatory bowel disease. Dis. Markers. 2022;2022 doi: 10.1155/2022/9171436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Jiao H., Wang C., Lin Y., You S. Chlorogenic acid ameliorates colitis and alters colonic microbiota in a mouse model of dextran sulfate sodium-induced colitis. Front. Physiol. 2019;10:325. doi: 10.3389/fphys.2019.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Han H., Shen M., Zhang L., Wang T. Comparative studies on the antioxidant profiles of curcumin and bisdemethoxycurcumin in erythrocytes and broiler chickens. Animals (Basel) 2019;9:953. doi: 10.3390/ani9110953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Yang Y., Han H., Zhang L., Wang T. Bisdemethoxycurcumin attenuates lipopolysaccharide-induced intestinal damage through improving barrier integrity, suppressing inflammation, and modulating gut microbiota in broilers. J. Anim. Sci. 2021;99 doi: 10.1093/jas/skab296. skab296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Shi L., Chen R., Zhao Y., Ren D., Yang X. Chlorogenic acid inhibits trimethylamine-N-oxide formation and remodels intestinal microbiota to alleviate liver dysfunction in high L-carnitine feeding mice. Food. Funct. 2021;12:10500–10511. doi: 10.1039/d1fo01778k. [DOI] [PubMed] [Google Scholar]
- Zhao J.S., Deng W., Liu H.W. Effects of chlorogenic acid-enriched extract from Eucommia ulmoides leaf on performance, meat quality, oxidative stability, and fatty acid profile of meat in heat-stressed broilers. Poult. Sci. 2019;98:3040–3049. doi: 10.3382/ps/pez081. [DOI] [PubMed] [Google Scholar]
- Zhao L., Xie Q., Etareri E.S., Liu D., Dong J., Ping L., Liu F., Li B., Huo G. Bifidobacterium dentium N8 with potential probiotic characteristics prevents LPS-induced intestinal barrier injury by alleviating the inflammatory response and regulating the tight junction in Caco-2 cell monolayers. Food Funct. 2021;12:7171–7184. doi: 10.1039/d1fo01164b. [DOI] [PubMed] [Google Scholar]