Abstract
Coxiella burnetii, an obligate intracellular bacterium which survives in myeloid cells, causes Q fever in humans. We previously demonstrated that virulent C. burnetii organisms are poorly internalized by monocytes compared to avirulent variants. We hypothesized that a differential mobilization of the actin cytoskeleton may account for this distinct phagocytic behavior. Scanning electron microscopy demonstrated that virulent C. burnetii stimulated profound and polymorphic changes in the morphology of THP-1 monocytes, consisting of membrane protrusions and polarized projections. These changes were transient, requiring 5 min to reach their maximum extent and vanishing after 60 min of incubation. In contrast, avirulent variants of C. burnetii did not induce any significant changes in cell morphology. The distribution of filamentous actin (F-actin) was then studied with a specific probe, bodipy phallacidin. Virulent C. burnetii induced a profound and transient reorganization of F-actin, accompanied by an increase in the F-actin content of THP-1 cells. F-actin was colocalized with myosin in cell protrusions, suggesting that actin polymerization and the tension of actin-myosin filaments play a role in C. burnetii-induced morphological changes. In addition, contact between the cell and the bacterium seems to be necessary to induce cytoskeleton reorganization. Bacterial supernatants did not stimulate actin remodeling, and virulent C. burnetii organisms were found in close apposition with F-actin protrusions. The manipulation of the actin cytoskeleton by C. burnetii may therefore play a critical role in the internalization strategy of this bacterium.
Coxiella burnetii is a strictly intracellular bacterium classified in the gamma subdivision of Proteobacteria. It multiplies in acidic phagosomes of myeloid cells (27). C. burnetii causes Q fever, a disease which manifests as an acute form involving febrile illness, pneumonia, or hepatitis, or as a chronic form, usually involving endocarditis with a poor prognosis in the context of cell-mediated immune deficiency (24, 26). The survival of C. burnetii in monocytes and macrophages is essential for the development of Q fever (29). Monocytes from patients with chronic Q fever produce tumor necrosis factor and interleukin-1β, which probably accounts for the inflammatory syndrome of Q fever (11), and also interleukin-10, which is associated with Q fever relapses (10).
The mechanism of entry of C. burnetii into monocytes may determine the intracellular fate of the bacteria and consequently the successful development of Q fever. Bacterial uptake by macrophages is initiated by the interaction of plasma membrane receptors and bacterial ligands. Receptors for the Fc portion of immunoglobulins (FcγR) and receptors for complement (CR) recognize bacteria opsonized with immunoglobulin G (IgG) and complement components, respectively (38). Receptors such as integrins are involved in the recognition of nonopsonized pathogens (22). Hence, phase I C. burnetii organisms, isolated from natural infections (20), were ingested by human monocytes through αvβ3 integrin. Avirulent (phase II) variants were internalized through αvβ3 integrin and αMβ2 integrin (CR3 or CD11b/CD18) (28). The actin cytoskeleton is differentially modulated to support bacterial internalization (34). In zipper phagocytosis, the uptake of opsonized bacteria by macrophages requires the sequential recruitment of membrane receptors, resulting in the formation of a pseudopod apposed to the bacterium surface (6). Actin polymerization, which leads to a dense network of actin filaments, occurs in an area of the plasma membrane in contact with the bacterium (phagocytic cups) (18). Actin disassembles from the phagosome once particle internalization is completed (31). In triggered phagocytosis, Salmonella typhimurium stimulates generalized surface ruffling of macrophages, i.e., unguided pseudopodia which trap bacteria by the formation of macropinosomes. The macrophage response requires an intense cytoskeletal reorganization (8).
The precise role of the actin cytoskeleton in phagocytosis remains unclear. Actin polymerization may provide the mechanical force for particle engulfment (33). Myosin also accumulates in the cytoplasm underneath phagocytic cups (5), suggesting that the resulting tension generates the force necessary for phagocytosis. The organization of the actin cytoskeleton in macrophages, as well as in other eukaryotic cells, is under the control of the Rho family of GTP-binding proteins, including Rho, Rac, and Cdc42 (7, 21). The Rho protein is likely to play a role in the FcγR-mediated phagocytosis of zymosan particles by macrophages (19), but Rac and Cdc42 might also be required for FcγR-dependent phagocytosis (15).
We recently demonstrated that virulent C. burnetii organisms are poorly phagocytosed by human monocytes whereas avirulent variants are efficiently phagocytosed (28). We hypothesize that a differential mobilization of actin cytoskeleton may account for this distinct phagocytic behavior. In this study, we have investigated the effect of C. burnetii on the morphology of THP-1 monocytes and actin organization. Virulent C. burnetii organisms induced intense cell protrusions, while avirulent variants did not induce any cell projections. These morphological changes were associated to a profound reorganization of actin cytoskeleton dependent on the GTP-binding protein Rho. We therefore suggest that C. burnetii organisms exploit the cytoskeleton to modulate their internalization by myeloid cells.
MATERIALS AND METHODS
Reagents.
Monoclonal antibodies (MAb) directed against human myosin (clone 2F12-A9, mouse IgM), control IgM, fluorescein-conjugated goat F(ab′)2 anti-mouse IgM, and fluorescein-conjugated goat F(ab′)2 anti-rabbit IgG were supplied by Immunotech, Marseilles, France. Bodipy phallacidin and rhodamine phalloidin were purchased from Molecular Probes (Eugene, Oreg.). C3 exotransferase from Clostridium botulinum was purified as previously described (12). RPMI 1640, Eagle minimal essential medium, Hanks’ balanced salt solution (HBSS), fetal calf serum (FCS), l-glutamine, penicillin, and streptomycin were from Gibco-BRL Life Technologies (Eragny, France). All media were checked for the absence of endotoxins with Limulus amebocyte lysate (Boehringer Ingelheim, Gagny, France). Other reagents were from Sigma Chemicals (St. Louis, Mo.).
Preparation of bacteria.
Phase I C. burnetii (Nine Mile strain, ATCC VR-615) organisms were injected into mice and were recovered from spleens 10 days later. They were then cultured for two passages in mouse L929 fibroblasts maintained in antibiotic-free Eagle minimal essential medium supplemented with 4% FCS and 2 mM l-glutamine. Phase II C. burnetii (Nine Mile strain, avirulent variants) organisms were cultured in L929 cells by repeated passages. Virulent C. burnetii organisms were also isolated from two patients with acute Q fever and two patients with Q fever endocarditis by establishing the strains in HEL cells and culturing them in L929 cells for two passages (29). After 1 week of infection, L929 cells were sonicated and the homogenates were centrifuged at 5,000 × g for 10 min. Bacteria were layered onto 25 to 45% linear Renografin gradient (37). Next, the gradients were centrifuged and the bacteria were collected, washed, and suspended in HBSS before being stored at −80°C.
Monocyte-C. burnetii interaction.
The human myelomonocytic cell line THP-1 was provided by the European Collection of Animal Cell Cultures (Cerdic, Sophia-Antipolis, France). The cells were propagated by means of biweekly passages at an initial density of 4 × 105 cells per ml in RPMI 1640 containing 20 mM HEPES, 10% heat-inactivated FCS, 2 mM l-glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml. Monocytes (106 cells per assay) were incubated at 37°C with C. burnetii at different bacterium-to-cell ratios in HBSS. The cells were washed after different periods and fixed with 1% formaldehyde. In some experiments, the monocytes were incubated at 37°C with C3 exotransferase for 24 h before the addition of bacteria. In another series of experiments, bacteria (2 × 108 per assay) were incubated in HBSS for 2 h at 37°C before centrifugation and the bacterial supernatants were filtered to remove bacteria and then added to the monocytes.
Morphological changes of monocytes.
Morphological changes of THP-1 cells were assessed by scanning electron microscopy (25). The cells were incubated with C. burnetii and fixed for 30 min in 0.1 M cacodylate buffer (pH 7.2) containing 2.5% glutaraldehyde. After extensive washings, the cells were dehydrated through graded ethanol concentrations and critical point dried under CO2. The monocytes were examined with a scanning electron microscope (JEOL 35CF).
Determination of filamentous actin.
THP-1 cells were fixed with 1% formaldehyde and incubated for 20 min with phosphate-buffered saline (PBS) containing 10 U of bodipy phallacidin per ml and 100 μg of lysophosphatidylcholine (LPC) per ml. After the cells were washed, their content in filamentous actin (F-actin) was determined (25). The fluorescence was measured with an EPICS Profile (Coulter, Hialeah, Fla.) equipped with an argon laser (488-nm excitation and 525-nm fluorescence emission). Linear fluorescence intensities of 10,000 cells were expressed as the mean of arbitrary units ± standard deviation (SD), as provided by the data-processing software. The intracellular distribution of F-actin was examined with a laser scanning confocal fluorescence microscope (Leica, Lyon, France) equipped with a 60× (NA 1.4) oil immersion lens. Serial optical sections of images were collected at 0.5-μm intervals along the z axis, analyzed with Adobe Photoshop 3.0, and printed with a Mavigraph color video printer (Sony).
The colocalization of F-actin with myosin was studied by incubating monocytes with anti-myosin MAb (or control IgM) and 10 U of rhodamine phalloidin per ml for 30 min in PBS containing 1% bovine serum albumin and 100 μg of LPC per ml. The cells were rinsed with PBS and then incubated with fluorescein-conjugated F(ab′)2 anti-mouse IgM for 30 min. The specimens were mounted in Slowfade solution (Molecular Probes) and examined with the confocal fluorescence microscope equipped with separate filters for each fluorochrome. THP-1 monocytes were single labeled with each fluorochrome to ensure that no cross talk was occurring for the given confocal conditions. Fluorescein and rhodamine images were adjusted with roughly equal intensities, converted into green and red images, respectively, and merged to synthesize the yellow color.
Determination of C. burnetii localization.
Bacterial localization was determined as follows. Monocytes incubated with C. burnetii were fixed with 1% formaldehyde. Bacterial labeling was performed by incubating cell preparations with rabbit Ab directed to C. burnetii or control serum at 1:250 for 30 min in PBS containing 1% bovine serum albumin, 10 U of rhodamine phalloidin per ml, and 100 μg of LPC per ml. After the cells were washed, a 1:200 dilution of fluorescein-conjugated F(ab′)2 anti-rabbit IgG was added to the cell preparations for 30 min. The monocytes were then examined with the confocal fluorescence microscope.
RESULTS
C. burnetii-induced morphological changes.
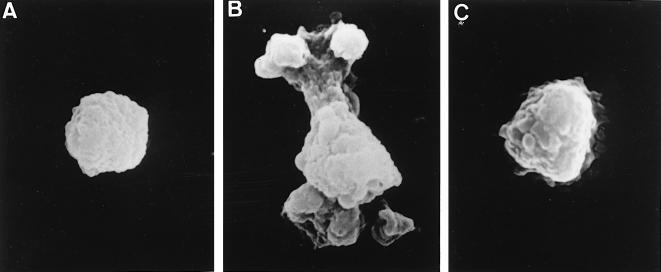
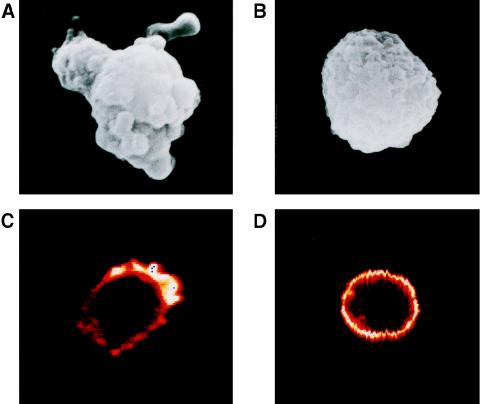
THP-1 cells were incubated with C. burnetii (Nine Mile strain) at a 200:1 bacterium-to-cell ratio for different periods and studied by scanning electron microscopy. Control monocytes were perfectly spherical (Fig. 1A). After 5 min of incubation with virulent C. burnetii, monocytes exhibited increased size and morphological changes consisting of membrane extensions and polarized protrusions (Fig. 1B). These morphological changes were observed in about 70% of monocytes. We also investigated the role of C. burnetii strains isolated from patients with acute Q fever or Q fever endocarditis in cell morphology. After a 5-min incubation period, membrane extensions and polarized protrusions on monocytes were observed in response to the different strains of C. burnetii (data not shown). The percentage of monocytes exhibiting these morphological changes (about 70%) was similar whatever the origin of the C. burnetii strain. After 1 h of incubation, the monocytes recovered a rounded morphology (data not shown). In contrast, avirulent variants of C. burnetii (Nine Mile strain) did not modify the morphology of monocytes, whatever the duration of the incubation, although a few membrane folds were observed after 5 min of incubation (Fig. 1C).
FIG. 1.
Morphological changes of monocytes incubated with C. burnetii. THP-1 cells were incubated with C. burnetii for 5 min. After fixation and dehydration, about 100 cells were examined with a scanning electron microscope and representative cells were photographed. (A) Control monocyte. (B) Monocyte incubated with virulent bacteria. (C) Monocyte incubated with avirulent variants of C. burnetii.
C. burnetii-induced reorganization of the actin cytoskeleton.
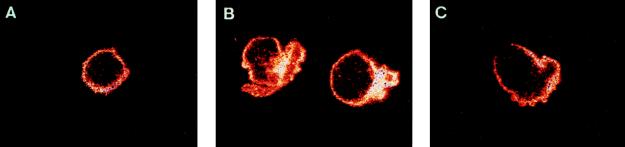
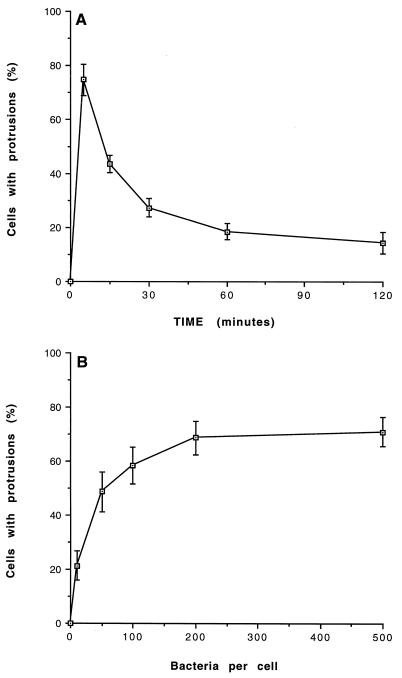
The cell protrusions induced by virulent C. burnetii prompted us to study the actin cytoskeleton of monocytes. The intracellular localization of F-actin was examined with a specific fluorescent probe, bodipy phallacidin. Control monocytes exhibited a homogeneous and peripheral ring of F-actin (Fig. 2A). Incubation of monocytes with virulent C. burnetii at a bacterium-to-cell ratio of 200:1 intensively reorganized F-actin distribution. After 5 min, F-actin was found on the inside of the protrusions. In areas away from cell deformation, F-actin was found as a continuous submembranous ring. After 15 min, the F-actin concentration and number of cell protrusions decreased (Fig. 2C), and after 60 min, the distribution of F-actin was similar to that in control monocytes (data not shown). C. burnetii-induced F-actin reorganization was observed in 75% ± 6% of monocytes after 5 min (Fig. 3A). Most of these cells returned to a homogeneous distribution after 1 h. We also studied the effect of bacterial concentration on remodeling of the actin cytoskeleton. For a bacterium-to-cell ratio as low as 10:1, cytoskeletal reorganization induced by C. burnetii was detected in 21% ± 5% of monocytes. Reorganization was detected in 58% ± 7% of monocytes with a ratio of 100:1 and reached a plateau when a ratio of 200:1 was used (Fig. 3B). In contrast, avirulent variants of C. burnetii did not stimulate any F-actin remodeling, even for a bacterium-to-cell ratio of 500:1 and regardless of their incubation time with monocytes (Table 1 and data not shown).
FIG. 2.
Kinetics of the F-actin reorganization induced by C. burnetii. Monocytes were incubated with virulent C. burnetii for different periods, fixed, and labeled with bodipy phallacidin. The cells were examined by laser scanning confocal microscopy, and representative cells were photographed. (A) Control monocyte. (B and C) Monocytes incubated with bacteria for 5 and 15 min, respectively.
FIG. 3.
Cytoskeletal remodeling induced by C. burnetii. (A) Monocytes were incubated with virulent C. burnetii (at a bacterium-to-cell ratio of 200:1) for different periods. (B) Monocytes were incubated with bacteria at different bacterium-to-cell ratios for 5 min. The cells were then fixed, labeled with bodipy phallacidin, and examined by laser scanning confocal microscopy. The results are expressed as the percentage of monocytes showing a cytoskeletal reorganization and represent the mean ± SD of three different experiments.
TABLE 1.
Successive C. burnetii stimulations of F-actin reorganizationa
| C. burnetii strain | % of monocytes reorganized afterb:
|
||
|---|---|---|---|
| First stimulation
|
Second stimulation (5 min) | ||
| 5 min | 60 min | ||
| Virulent | 72 ± 5 | 15 ± 4 | 22 ± 6 |
| Avirulent | 8 ± 4 | 6 ± 3 | 68 ± 8 |
Monocytes were incubated with virulent C. burnetii or an avirulent variant (at a bacterium-to-cell ratio of 200:1) for 5 or 60 min (first stimulation). After recovery of the normal actin organization (i.e., after 60 min), the monocytes were washed to remove unbound bacteria and incubated with virulent bacteria (at a bacterium-to-cell ratio of 200:1) for 5 min (second stimulation). The monocytes were fixed, labeled with bodipy phallacidin, and examined by laser scanning confocal microscopy.
The results are expressed in the percentage of monocytes showing a cytoskeletal reorganization and represent the mean ± SD of three different experiments.
We assessed the ability of different clinical strains of C. burnetii to induce F-actin rearrangement in monocytes. Strains derived from both acute and chronic forms of Q fever were tested, and strains from both sources were found to elicit F-actin reorganization in the majority of monocytes observed (Table 2). After 15 min of stimulation, the percentage of monocytes showing cytoskeletal remodeling progressively decreased, and after 1 h, the F-actin distribution of most monocytes returned to normal (data not shown).
TABLE 2.
Cytoskeletal remodeling induced by different strains of C. burnetiia
| Source of C. burnetii | % of monocytes with protrusionsb |
|---|---|
| Patient 1 | 65 ± 8 |
| Patient 2 | 69 ± 7 |
| Patient 3 | 74 ± 7 |
| Patient 4 | 77 ± 5 |
Strains of C. burnetii were isolated from two patients with acute Q fever (patients 1 and 2) and two patients with Q fever endocarditis (patients 3 and 4). Monocytes were incubated with C. burnetii (at a bacterium-to-cell ratio of 200:1) for 5 min. They were then fixed, labeled with bodipy phallacidin, and examined by laser scanning confocal microscopy.
The results are expressed as the percentage of monocytes showing cytoskeletal reorganization and represent the mean ± SD of three different experiments.
Since the monocyte cytoskeletal organization returned to near normal after 60 min of incubation with virulent bacteria, we investigated whether F-actin rearrangement in these monocytes could be stimulated for a second time by a subsequent bacterial challenge (Table 1). When monocytes were pretreated with avirulent bacteria, they retained the ability to reorganize F-actin, since 68% ± 8% of monocytes demonstrated a cytoskeletal reorganization in response to virulent C. burnetii. In contrast, when monocytes were pretreated with virulent bacteria, they lost the ability to reorganize F-actin in response to a second stimulation with virulent C. burnetii, since only 22% ± 6% of cells showed protrusions rich in F-actin.
Localization of bacteria with F-actin protrusions.
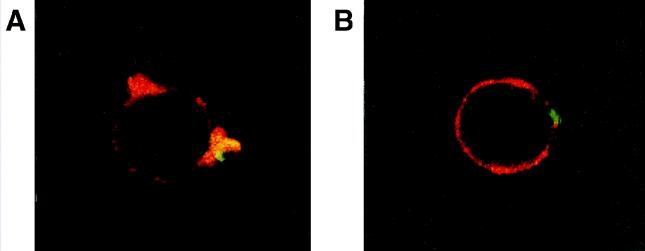
We also considered whether contact of virulent C. burnetii with monocytes was necessary to stimulate cytoskeletal reorganization. First, bacterial supernatants were incubated with monocytes for 5 min. These did not induce F-actin rearrangements (data not shown). Second, C. burnetii localization was studied by double fluorescence. After 5 min of incubation with virulent C. burnetii, monocytes reorganized the F-actin distribution (Fig. 4A, red); virulent bacteria (green) were closely apposed to the protrusions. All the bacteria detected were apposed to F-actin protrusions, and none of the bacteria were outside these protrusions (data not shown). Avirulent variants of C. burnetii did not modify the distribution of F-actin, and the bacteria were localized along the F-actin peripheral ring (Fig. 4B).
FIG. 4.
Localization of C. burnetii with F-actin protrusions. Monocytes were incubated with C. burnetii for 5 min, fixed, and labeled with rhodamine phalloidin and fluorescein-conjugated Ab directed against C. burnetii. They were then examined by laser scanning confocal microscopy (F-actin is shown in red, and bacteria are shown in green), and representative cells were photographed. (A) Monocyte incubated with virulent C. burnetii. (B) Monocyte incubated with an avirulent variant of C. burnetii.
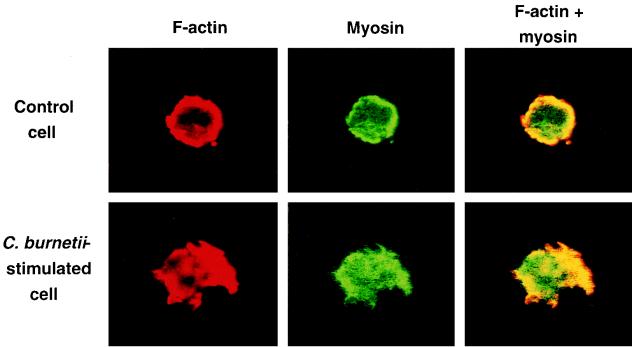
Study of actin-myosin colocalization.
The colocalization of F-actin and myosin was investigated by labeling F-actin with rhodamine phalloidin and labeling myosin with fluorescein-conjugated Ab (Fig. 5). In control monocytes, F-actin (red) was homogeneously distributed and essentially submembraneous; myosin (green) was also distributed mainly at the periphery of cells, with a minor portion being dispersed throughout the cytoplasm. F-actin and myosin were colocalized at the cell periphery (yellow). After 5 min of incubation with virulent C. burnetii, F-actin and myosin were colocalized in cell protrusions (yellow). F-actin and myosin were homogeneously distributed, as in control cells, 1 h after incubation (data not shown).
FIG. 5.
Colocalization of F-actin and myosin. Monocytes were incubated with virulent C. burnetii for 5 min. F-actin was labeled with rhodamine phalloidin, and myosin was labeled with fluorescein-conjugated Ab. The monocytes were then examined by laser scanning confocal microscopy, and representative cells were photographed. (Top) Control monocyte. (Bottom) Monocyte incubated with bacteria. Note that the colocalization of F-actin and myosin appears in yellow.
Study of the F-actin increase stimulated by C. burnetii.
The remodeling of intracellular F-actin may result from the shift of preformed microfilaments without actin assembly or from the local assembly of actin with an associated increase in the cellular F-actin content. Virulent C. burnetii induced a transient polymerization of actin in monocytes. The increase in the level of F-actin was maximal (a twofold increase) after 5 min, and the F-actin content returned progressively to its baseline level (Fig. 6). The durations of the F-actin increase, actin reorganization, and cell protrusions were superimposable, suggesting that these events are related.
FIG. 6.
Kinetics of F-actin increase induced by C. burnetii. Monocytes were incubated with virulent C. burnetii for different periods, fixed, and labeled with bodipy phallacidin. The cell fluorescence (expressed on a linear scale) was determined by flow cytometry. A total of 10,000 monocytes were scored each time. Results are expressed as mean fluorescence intensity ± standard error of the mean relative to controls and represent the average of three experiments.
Effects of Rho on C. burnetii-induced cytoskeletal changes.
The C3 exotransferase of Clostridium botulinum, which selectively inhibits the activity of the GTPase Rho, was used to investigate the possible role of Rho on the morphological changes and the reorganization of F-actin induced by C. burnetii. First, a 24-h pretreatment of monocytes with 2 nM C3 exotransferase inhibited the morphological changes stimulated by C. burnetii (compare Fig. 7A and B). Complete inhibition of cell deformation was observed in all monocytes incubated with C3 exotransferase (data not shown). Pretreatment with C3 exotransferase did not affect cell viability, as assessed by trypan blue exclusion (data not shown). Second, the distribution of F-actin remained homogeneous without any local reorganization (compare Fig. 7C and D). In a series of three experiments, 77% ± 3% of monocytes showed F-actin protrusions when incubated with C. burnetii for 5 min. When the monocytes were pretreated with C3 exotransferase and then stimulated with bacteria for 5 min, only 21% ± 3% of cells showed protrusions with F-actin concentration. Third, preincubation of monocytes with C3 exotransferase inhibited the increase in the F-actin content induced by C. burnetii (data not shown). These results indicate that the Rho protein may regulate the morphological changes and the reorganization of F-actin stimulated by C. burnetii.
FIG. 7.
Effects of C3 exotransferase. Monocytes were preincubated with (B and D) or without (A and C) 2 nM C3 exotransferase from Clostridium botulinum for 24 h. They were then stimulated with virulent C. burnetii for 5 min. After fixation and dehydration, about 100 cells were examined with a scanning electron microscope (A and B). Monocytes were also examined by laser scanning confocal microscopy after F-actin labeling (C and D). Representative cells were photographed.
DISCUSSION
In this report, we show that virulent C. burnetii stimulates morphological changes in THP-1 monocytes, consisting of membrane extensions and polarized protrusions. This finding deserves several comments. Virulent bacteria isolated from ticks and bacteria isolated from patients suffering from acute Q fever or chronic Q fever induce morphological changes in THP-1 monocytes, emphasizing the relationship of morphological changes in monocytes to the virulence of C. burnetii. In addition, while virulent C. burnetii induced transient cell projections, avirulent variants of C. burnetii did not induce any morphological changes. Thus, the reorganization of cell morphology is related to bacterial virulence. Cell protrusions were induced by virulent C. burnetii in suspended THP-1 monocytes and in adherent macrophages, such as human monocytes and murine bone marrow-derived macrophages (data not shown). Hence, the ability to project cell extensions is not dependent on the state (suspended or adherent) of the myeloid cells. The morphological changes covered an extensive region of the surface of the THP-1 monocytes, as reported for murine macrophages stimulated by Salmonella typhimurium (8). Membrane ruffling or folding was limited to the region adjacent to S. typhimurium or Shigella flexneri in epithelial cells (1, 17), suggesting that intense cell protrusions characterize myeloid cells, which possess the large reservoir of membrane necessary for locomotion and phagocytosis (30).
The morphological changes induced by C. burnetii result from the mobilization of the actin cytoskeleton. First, F-actin was concentrated in cell protrusions; the times required for F-actin redistribution and shape changes were similar. Second, the transient increase in the F-actin content and the occurrence of cell deformations were concomitant. Third, an increase in the F-actin content of monocytes was required for C. burnetii-stimulated morphological changes, since cytochalasin D blocked F-actin redistribution and cell protrusions (data not shown). How actin filaments are related to the force generating cell movements and morphological changes remains unclear. Actin polymerization and pseudopodal extensions are coupled, but ATPase motor activity delivered by myosin may be involved in the leading-edge structures in motile cells (36). Colocalization studies suggest that myosin acts as a mechanical motor in phagocytosing macrophages. Indeed, myosin accumulates on the phagocytic cups (32) and colocalizes with F-actin on forming phagosomes (5). In this paper we demonstrate that myosin and F-actin are selectively colocalized in the cell protrusions, indicating that the tension of actin-myosin filaments may drive the morphological changes stimulated by C. burnetii.
The regulation of actin organization in several adherent cell types, including fibroblasts and epithelial cells, involves the GTP-binding proteins of the Rho family (21). The role of Rho proteins in actin-dependent phenomena such as phagocytosis may be specific (3, 7). We show here that C3 exotransferase from C. botulinum, which specifically inhibits Rho via ADP-ribosylation (4, 12), inhibits morphological changes and F-actin reorganization generated by C. burnetii. Other pathogens than C. burnetii exploit the actin cytoskeleton by acting on Rho proteins (14). The Rho protein has been involved in Shigella-induced folding essential for the invasion of epithelial cells (2, 35) but not in Salmonella-stimulated ruffles (13, 23). Rho was recently demonstrated to be required for FcγR-dependent phagocytosis in macrophages (19), but Rac and Cdc42 were also shown to be involved in membrane ruffling and FcγR-mediated phagocytosis (15). Our results suggest that the Rho protein is involved in the generation of cell protrusions stimulated by C. burnetii. We cannot exclude the possibility that Rac or Cdc42 was involved in the polymorphic changes stimulated by C. burnetii in monocyte morphology. Indeed, the GTPases of the Rho family may function in a coordinated, and possibly hierarchical manner, as described for fibroblasts (21, 39).
Although we did not aim to study the functional consequences of the reorganization of the actin cytoskeleton in great detail, we made several pertinent observations. First, we observed a prolonged impairment of cytoskeleton mobilization. Monocytes which were pretreated with virulent C. burnetii but had recovered a normal morphology were unable to mobilize F-actin in response to a second stimulation. Second, the cytoskeletal rearrangements may be stimulated by contact between monocytes and bacteria. Indeed, bacterial supernatants were unable to stimulate F-actin rearrangements in monocytes. Moreover, virulent C. burnetii organisms were closely apposed to F-actin protrusions. Finally, while S. typhimurium-stimulated microprojections allow the uptake of bacteria by macrophages (8), C. burnetii-induced morphological changes may have different consequences. Virulent C. burnetii organisms were poorly phagocytosed by monocytes compared to avirulent variants and did not engage CR3 (28). It is thus likely that membrane projections restrict the engagement of CR3. Indeed, the functional activation of CR3 is dependent on the organization of actin filaments (16). We recently demonstrated that the preincubation of monocytes with virulent C. burnetii prevented CR3-dependent phagocytosis but did not affect FcγR-dependent phagocytosis (9). The mechanism of CR3 inactivation induced by C. burnetii may involve the association of CR3 with the actin cytoskeleton and/or the clustering of CR3.
In conclusion, virulent C. burnetii stimulated morphological changes in human monocytes via the reorganization of actin cytoskeleton. The actin cytoskeleton may be exploited by C. burnetii organisms to modulate their internalization by host cells.
REFERENCES
- 1.Adam T, Arpin M, Prévost M C, Gounon P, Sansonetti P J. Cytoskeletal rearrangements and the functional role of T-plastin during entry of Shigella flexneri into HeLa cells. J Cell Biol. 1995;129:367–381. doi: 10.1083/jcb.129.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adam T, Giry M, Boquet P, Sansonetti P. Rho-dependent membrane folding causes Shigella entry into epithelial cells. EMBO J. 1996;15:3315–3321. [PMC free article] [PubMed] [Google Scholar]
- 3.Aepfelbacher M, Essler M, Huber E, Czech A, Weber P C. Rho is a negative regulator of human monocyte spreading. J Immunol. 1996;157:5070–5075. [PubMed] [Google Scholar]
- 4.Aktories K. Bacterial toxins that target Rho proteins. J Clin Invest. 1997;5:827–829. doi: 10.1172/JCI119245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen L A, Aderem A. A role for MARCKS, the α isozyme of protein kinase C and myosin I in zymosan phagocytosis by macrophages. J Exp Med. 1995;182:829–840. doi: 10.1084/jem.182.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen L A, Aderem A. Molecular definition of distinct cytoskeletal structures involved in complement- and Fc receptor-mediated phagocytosis in macrophages. J Exp Med. 1996;184:627–637. doi: 10.1084/jem.184.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen W E, Jones G E, Pollard J W, Ridley A J. Rho, Rac and Cdc42 regulate actin organization and cell adhesion in macrophages. J Cell Sci. 1997;110:707–720. doi: 10.1242/jcs.110.6.707. [DOI] [PubMed] [Google Scholar]
- 8.Alpuche-Aranda C M, Racoosin E L, Swanson J A, Miller S I. Salmonella stimulate macrophage macropinocytosis and persist within spacious phagosomes. J Exp Med. 1994;179:601–608. doi: 10.1084/jem.179.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capo, C., F. P. Lindberg, Y. Zaffran, G. Tardei, D. Raoult, E. J. Brown, and J. L. Mege. Unpublished data. [PubMed]
- 10.Capo, C., Y. Zaffran, F. Zugun, P. Houpikian, D. Raoult, and J. L. Mege. Production of interleukin-10 and transforming growth factor β by peripheral blood mononuclear cells in Q fever endocarditis. Infect. Immun. 64:4143–4147. [DOI] [PMC free article] [PubMed]
- 11.Capo C, Zugun F, Stein A, Tardei G, Lepidi H, Raoult D, Mege J L. Upregulation of tumor necrosis factor alpha and interleukin-1β in Q fever endocarditis. Infect Immun. 1996;64:1638–1642. doi: 10.1128/iai.64.5.1638-1642.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chardin P, Boquet P, Madaule P, Popoff M R, Rubin E J, Gill D M. The mammalian G protein Rho C is ADP-ribosylated by Clostridium botulinum exoenzyme C3 and affects actin microfilaments in Vero cells. EMBO J. 1989;8:1087–1092. doi: 10.1002/j.1460-2075.1989.tb03477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L M, Hobbie S, Galan J E. Requirement of Cdc42 for Salmonella-induced cytoskeletal and nuclear responses. Science. 1996;274:2115–2118. doi: 10.1126/science.274.5295.2115. [DOI] [PubMed] [Google Scholar]
- 14.Cossart P. Subversion of the mammalian cell cytoskeleton by invasive bacteria. J Clin Invest. 1997;99:2307–2311. doi: 10.1172/JCI119409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox D, Chang P, Zhang Q, Reddy P G, Bokoch G M, Greenberg S. Requirements for both Rac1 and Cdc42 in membrane ruffling and phagocytosis in leukocytes. J Exp Med. 1997;186:1487–1494. doi: 10.1084/jem.186.9.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elemer G S, Edgington T S. Microfilament reorganization is associated with functional activation of αMβ2 on monocytic cells. J Biol Chem. 1994;269:3159–3166. [PubMed] [Google Scholar]
- 17.Francis C L, Ryan T A, Jones B D, Smith S J, Falkow S. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature. 1993;364:639–642. doi: 10.1038/364639a0. [DOI] [PubMed] [Google Scholar]
- 18.Greenberg S, Burridge K, Silverstein S C. Colocalization of F-actin and talin during Fc receptor-mediated phagocytosis in mouse macrophages. J Exp Med. 1990;172:1853–1856. doi: 10.1084/jem.172.6.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hackam D J, Rotstein O D, Schreiber A, Zhang W J, Grinstein S. Rho is required for the initiation of calcium signaling and phagocytosis by Fcγ receptors in macrophages. J Exp Med. 1997;186:955–966. doi: 10.1084/jem.186.6.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hackstadt T. The role of lipopolysaccharides in the virulence of Coxiella burnetii. Ann N Y Acad Sci. 1990;590:27–32. doi: 10.1111/j.1749-6632.1990.tb42203.x. [DOI] [PubMed] [Google Scholar]
- 21.Hall A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annu Rev Cell Biol. 1994;10:31–54. doi: 10.1146/annurev.cb.10.110194.000335. [DOI] [PubMed] [Google Scholar]
- 22.Isberg R R, Tran Van Nhieu G. Binding and internalization of microorganisms by integrin receptors. Trends Microbiol. 1994;2:10–14. doi: 10.1016/0966-842x(94)90338-7. [DOI] [PubMed] [Google Scholar]
- 23.Jones B D, Paterson H F, Hall A, Falkow S. Salmonella typhimurium induces membrane ruffling by a growth factor-receptor-independent mechanism. Proc Natl Acad Sci USA. 1993;90:10390–10394. doi: 10.1073/pnas.90.21.10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koster F T, Williams J C, Goodwin J S. Cellular immunity in Q fever: modulation of responsiveness by a suppressor T cell-monocyte circuit. J Immunol. 1985;135:1067–1072. [PubMed] [Google Scholar]
- 25.Lepidi H, Zaffran Y, Ansaldi J L, Mege J L, Capo C. Morphological polarization of human polymorphonuclear leucocytes in response to three different chemoattractants: an effector response independent of calcium rise and tyrosine kinases. J Cell Sci. 1995;108:1771–1778. doi: 10.1242/jcs.108.4.1771. [DOI] [PubMed] [Google Scholar]
- 26.Marrie T J, Raoult D. Q fever: a review and issues for the next century. Int J Antimicrob Agents. 1997;8:145–161. doi: 10.1016/s0924-8579(96)00369-x. [DOI] [PubMed] [Google Scholar]
- 27.Maurin M, Benoliel A M, Bongrand P, Raoult D. Phagolysosomal alkalinisation and the bactericidal effect of antibiotics: the Coxiella burnetii paradigm. J Infect Dis. 1992;166:1097–1102. doi: 10.1093/infdis/166.5.1097. [DOI] [PubMed] [Google Scholar]
- 28.Mege J L, Maurin M, Capo C, Raoult D. Coxiella burnetii: the ‘query’ fever bacterium. A model of immune subversion by a strictly intracellular microorganism. FEMS Microbiol Rev. 1997;19:209–217. doi: 10.1111/j.1574-6976.1997.tb00298.x. [DOI] [PubMed] [Google Scholar]
- 29.Raoult D, Vestris G, Enea M. Isolation of 16 strains of Coxiella burnetii from patients by using a sensitive centrifugation cell culture system and establishment of the strains in HEL cells. J Clin Microbiol. 1990;28:2482–2484. doi: 10.1128/jcm.28.11.2482-2484.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmid-Schönbein G W. Leukocyte biophysics. Cell Biophys. 1990;17:107–135. doi: 10.1007/BF02990492. [DOI] [PubMed] [Google Scholar]
- 31.Silverstein S C, Greenberg S, Di Virgilio F, Steinberg T H. Phagocytosis. In: Paul W E, editor. Fundamental immunology. New York, N.Y: Raven Press; 1989. pp. 703–720. [Google Scholar]
- 32.Stendahl O I, Hartwig J H, Brotschi E A, Stossel T P. Distribution of actin-binding protein and myosin in macrophages during spreading and phagocytosis. J Cell Biol. 1980;84:215–224. doi: 10.1083/jcb.84.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stossel T P. The mechanical responses of white blood cells. In: Gallin J, Goldstein I, Snyderman R, editors. Inflammation. Basic principles and clinical correlates. New York, N.Y: Raven Press; 1992. pp. 459–475. [Google Scholar]
- 34.Swanson J A, Baer S C. Phagocytosis by zippers and triggers. Trends Cell Biol. 1995;5:89–93. doi: 10.1016/s0962-8924(00)88956-4. [DOI] [PubMed] [Google Scholar]
- 35.Watarai M, Kamata Y, Kozaki S, Sasakawa C. Rho, a small GTP-binding protein, is essential for Shigella invasion of epithelial cells. J Exp Med. 1997;185:281–292. doi: 10.1084/jem.185.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Welch M D, Mallavarapu A, Rosenblatt J, Mitchison T J. Actin dynamics in vivo. Curr Opin Cell Biol. 1997;9:54–61. doi: 10.1016/s0955-0674(97)80152-4. [DOI] [PubMed] [Google Scholar]
- 37.Williams J C, Peacock M G, McCaul T F. Immunological and biological characterization of Coxiella burnetii, phases I and II, separated from host components. Infect Immun. 1981;32:840–851. doi: 10.1128/iai.32.2.840-851.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wright S D. Receptors for complement and the biology of phagocytosis. In: Gallin J, Goldstein I, Snyderman R, editors. Inflammation. Basic principles and clinical correlates. New York, N.Y: Raven Press; 1992. pp. 477–495. [Google Scholar]
- 39.Zigmond S H. Signal transduction and actin filament organization. Curr Opin Cell Biol. 1996;8:66–73. doi: 10.1016/s0955-0674(96)80050-0. [DOI] [PubMed] [Google Scholar]