To the Editor: Early identification and management of children at high-risk of diabetes is instrumental in preventing complications in adulthood.1 Atopic dermatitis (AD) is one of the most common chronic childhood conditions and may be associated with diabetes because of (1) inflammatory processes involving insulin-driven increases in adipokines that worsen AD and (2) increased insulin resistance due to systemic inflammation from AD.2,3 Therefore, it is important to understand whether children with AD would benefit from diabetes risk assessment and treatment. While some prior studies found a positive association between childhood AD and diabetes, others found a negative association or no association at all, and most have not assessed AD severity or biomarkers.4,5
Our study aims to determine the extent to which AD activity and severity are associated with fasting blood glucose and insulin in late childhood and adolescence. We performed a secondary analysis of the Avon Longitudinal Study of Parents and Children cohort, an ongoing longitudinal birth cohort from Avon, England. The study website contains additional details: http://www.bristol.ac.uk/alspac/researchers/our-data/. We included children with AD data at ages 9, 15, and/or 17 (n = 9067). Using AD activity and severity as the exposure and fasting glucose and insulin levels as the outcomes, we performed cross-sectional analyses at ages 9, 15, and 17 using logistic regression models. AD activity was based on a maternal- or self-reported questionnaire that asked about disease activity and severity over the past 12 months. AD severity was categorized as “no problem/mild” and “moderate/severe.” Glucose measurements at age 9 were limited to a random sample of participants as part of a substudy (n = 851), while all participants were invited to participate in the glucose measurements at ages 15 and 17. High fasting glucose was defined as ≥5.6 mmol/L based on the International Diabetes Federation guidelines. Since insulin is not included as a diagnostic measure for diabetes in the International Diabetes Federation consensus, we used a cut-off of those who have values equal to or over the 90th percentile in the cohort to indicate being at risk.
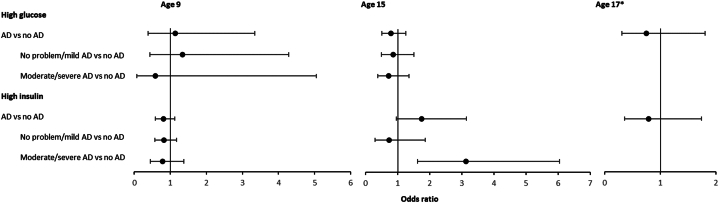
The study population were half female (50.85%) and mostly White (95.98%). The annual period prevalence of active AD ranged from 14.45% at age 15 to 18.82% at age 9 (Table I). We did not find significant associations between AD and blood glucose at any time point, and there did not appear to be consistent trends by severity. However, we did find a significant positive association between moderate/severe AD and insulin levels at age 15 (Fig 1). Linear models using log-transformed continuous blood values and complete case analysis designed to address missing data concerns showed similar results (Supplementary Tables, available via Mendeley at https://data.mendeley.com/datasets/bxhvcftyns/1). Our study was limited by a single blood value per participant at each time point, which may not be an accurate reflection of the participant’s average blood level. Moreover, as our cohort was predominantly White, these results should be tested in more diverse populations. The lack of consistent trends in our results, combined with mixed findings from prior studies, do not support a strong association between AD and fasting blood glucose.
Table I.
Cohort characteristics
| 9 y old | 15 y old | 17 y old | ||||
|---|---|---|---|---|---|---|
| Characteristic | N = 4457 | % | N = 2394 | % | N = 1644 | % |
| BMI | 4380 | Mean (SD): 17.57 (2.76) | 2367 | Mean (SD): 21.43 (3.42) | 1610 | Mean (SD): 22.38 (3.51) |
| Cholesterol (mmol/L) | 4135 | Mean (SD): 4.28 (0.68) | 2394 | Mean (SD): 3.77 2(0.65) | 1638 | Mean (SD): 3.79 (0.68) |
| Sex | 4457 | 2390 | 1643 | |||
| Female | 2180 | 48.91 | 1340 | 56.07 | 983 | 59.83 |
| Race | 4161 | 2205 | 1527 | |||
| Non-White | 155 | 3.73 | 82 | 3.72 | 52 | 3.41 |
| Highest education of parent∗ | 4210 | 2231 | 1545 | |||
| Vocational | 197 | 4.68 | 74 | 3.32 | 46 | 2.98 |
| O level/CSE | 1337 | 31.46 | 598 | 26.80 | 382 | 24.72 |
| A level | 1473 | 34.99 | 805 | 36.08 | 536 | 34.69 |
| Degree | 1203 | 28.57 | 754 | 33.80 | 581 | 37.61 |
| Mother had diabetes | 4190 | 2219 | 1534 | |||
| Yes | 39 | 0.93 | 19 | 0.86 | 17 | 1.11 |
| Mother had AD | 4457 | 2394 | 1644 | |||
| Yes | 1009 | 22.64 | 598 | 24.98 | 382 | 23.24 |
| Social class† | 4140 | 2198 | 1516 | |||
| Professional | 714 | 17.25 | 459 | 20.88 | 351 | 23.15 |
| Managerial and technical | 2016 | 48.70 | 1084 | 49.32 | 761 | 50.2 |
| Skilled nonmanual | 983 | 23.74 | 468 | 21.29 | 285 | 18.8 |
| Skilled manual | 290 | 7.00 | 127 | 5.78 | 80 | 5.28 |
| Partly skilled | 120 | 2.90 | 51 | 2.32 | 35 | 2.31 |
| Unskilled | 17 | 0.41 | 9 | 0.41 | <5 | <0.5 |
| Exposures | ||||||
| Current active AD | 839 | 18.82 | 346 | 14.45 | 242 | 14.72 |
| Definite, no prob/mild | 632 | 14.18 | 198 | 8.27 | N/A | N/A |
| Definite, mod/severe | 206 | 4.62 | 148 | 6.18 | N/A | N/A |
| Outcomes | ||||||
| Glucose (mmol/L) | 810 | Mean (SD): 4.95 (0.40) | 2394 | Mean (SD): 5.20 (0.37) | 1638 | Mean (SD): 5.01 (0.49) |
| High glucose (fasting: ≥5.6 mmol/L) | 53 | 6.54 | 242 | 10.11 | 88 | 5.37 |
| Insulin (IU/L) | 4196 | Mean (SD): 11.52 (13.91) | 2390 | Mean (SD): 10.11 (5.69) | 1610 | Mean (SD): 7.95 (6.94) |
| High insulin‡ | 422 | 10.06 | 220 | 9.21 | 131 | 8.14 |
To reduce skewness, the glucose and insulin measurements were log-transformed for the purposes of analyses.
A, Advanced; AD, atopic dermatitis; BMI, body mass index; CSE, certificate of secondary education; O, ordinary.
Based on British-based education levels. Vocational training is occupation-based training. O level represents 11 y of academic study and marks the end of the secondary education cycle. O level and CSE are equivalent. A level represents an additional 2 y of study (13 total) after the O level is obtained and is an admission requirement for university to pursue a degree.
Based on highest parental occupation level at time of initial study enrollment.
High insulin (≥90th percentile insulin for each age group within cohort).
Fig 1.
Adjusted odds of high glucose and insulin according to atopic dermatitis disease activity and severity. Logistic regression model was adjusted for sex, race, maternal delivery age, highest parental education level, social class assessed through parental occupation, body mass index, cholesterol, parental history of atopic dermatitis, and family history of diabetes. Sample sizes by age group are as follows: glucose analysis: age 9 (n = 490), age 15 (n = 1992), age 17 (n = 1357); insulin analysis: age 9 (n = 3681), age 15 (n = 1995), age 17 (n = 1302). 95% CIs are denoted by the error bars. ∗AD severity data were not captured at age 17. AD, Atopic dermatitis.
Conflicts of interest
Dr Abuabara is a consultant to Target RWE and receives research grants to her institution from Pfizer. Dr Langan is an investigator on the European Union Horizon 2020-funded BIOMAP Consortium (http://www.biomap-imi.eu/) but is not in receipt of industry funding. Author Shan, Author Ye, Dr Ku, and Dr McCulloch have no conflicts of interest to declare.
Acknowledgments
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses.
Footnotes
Funding sources: The UK Medical Research Council and Wellcome (Grant ref: 217065/Z/19/Z) and the University of Bristol provide core support for ALSPAC. This publication is the work of the authors, who will serve as guarantors for the contents of this paper. A comprehensive list of grants funding is available on the ALSPAC website. This research was specifically funded by MRC (Grant ref: MC_UU_12013/1). This study was also supported by UCSF Summer Explore Inquiry Funding and NIAMS5R03AR078962. Dr Langan was also supported by Health Data Research UK (Grant number: LOND1), which is funded by the UK Medical Research Council, Engineering and Physical Sciences Research Council, Economic and Social Research Council, Department of Health and Social Care (England), Chief Scientist Office of the Scottish Government Health and Social Care Directorates, Health and Social Care Research and Development Division (Welsh Government), Public Health Agency (Northern Ireland), British Heart Foundation and Wellcome Trust. Funders had no role in the study design, collection, analysis, and interpretation of data; in the writing of the report; and in the decision, submit the article for publication. Dr Langan was funded by the Wellcome Trust [205039/Z/16/Z]. For the purpose of Open Access, the author has applied a CC BY public copyright license to any Author Accepted Manuscript (AAM) version arising from this submission.
Patient consent: Participants in ALSPAC provided written informed consent, and ethical approval was obtained from the ALSPAC Ethics and Law Committee and the Local Research Ethics Committees.
IRB approval status: The present study was considered exempt from review by the University of California San Francisco Institutional Review Board because all of the data obtained by investigators were fully deidentified.
References
- 1.Virani S.S., Alonso A., Benjamin E.J., et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 2.Machura E., Szczepanska M., Ziora K., et al. Evaluation of adipokines: apelin, visfatin, and resistin in children with atopic dermatitis. Mediators Inflamm. 2013;2013 doi: 10.1155/2013/760691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunner P.M., Silverberg J.I., Guttman-Yassky E., et al. Councilors of the International Eczema Council. Increasing comorbidities suggest that atopic dermatitis is a systemic Disorder. J Invest Dermatol. 2017;137(1):18–25. doi: 10.1016/j.jid.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 4.Drucker A.M., Qureshi A.A., Dummer T.J.B., Parker L., Li W.Q. Atopic dermatitis and risk of hypertension, type 2 diabetes, myocardial infarction and stroke in a cross-sectional analysis from the Canadian Partnership for Tomorrow Project. Br J Dermatol. 2017;177(4):1043–1051. doi: 10.1111/bjd.15727. [DOI] [PubMed] [Google Scholar]
- 5.Huang A.H., Roh Y.S., Sutaria N., et al. Real-world comorbidities of atopic dermatitis in the pediatric ambulatory population in the United States. J Am Acad Dermatol. 2021;85(4):893–900. doi: 10.1016/j.jaad.2021.03.016. [DOI] [PubMed] [Google Scholar]