Abstract
Background
Suspected upper gastrointestinal bleeding (SUGIB) is a common issue during ICU stay. In the absence of specific guidelines on the indication and timing of esophagogastroduodenoscopy (EGD), there is substantial variability in EGD indication depending on accessibility and clinical presentation. This study aimed to investigate factors associated with the need for per-EGD hemostatic therapy and to create a score predicting therapeutic benefit of emergency bedside EGD in ICU patients with SUGIB.
Methods
We conducted a retrospective study in our ICU to identify factors associated with the need for hemostatic procedure during EGD performed for SUGIB. From this observational cohort, we derived a score predicting the need for hemostasis during EGD, the SUGIBI score. This score was subsequently validated in a retrospective multicenter cohort.
Results
Two hundred fifty-five patients not primarily admitted for GI bleeding who underwent a bedside EGD for SUGIB during their ICU stay were analyzed. The preeminent EGD indication were anemia (79%), melena (19%), shock (14%), and hematemesis (13%). EGD was normal in 24.7% of cases, while primary lesions reported were ulcers (23.1%), esophagitis (18.8%), and gastritis (12.5%). Only 12.9% of patients underwent hemostatic endotherapy during EGD. A SUGIBI score < 4 had a negative predictive value of 95% (91–99) for hemostatic endotherapy [AUC of 0.81; 0.75–0.91 (p < 0.0001)]. The SUGIBI score for predicting the need for an EGD-guided hemostatic procedure was next validated in a multicenter cohort with an AUC of 0.75 (0.66–0.85) (p < 0.0001), a score < 4 having a negative predictive value of 95% (92–97).
Conclusions
Our study shows that the therapeutic usefulness of bedside emergency EGD for SUGIB in critically ill patients is limited to a minority of patients. The SUGIBI score should help clinicians stratify the probability of a therapeutic EGD.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13613-024-01250-0.
Keywords: Gastrointestinal bleeding, Esophagogastroduodenoscopy, Hemostasis, Endotherapy, «Stress» ulcer
Background
Suspicion of upper gastrointestinal bleeding (UGIB) is a common situation in critically ill patients during ICU stay [1–4]. Bleeding may be overt, characterized by exteriorized hematemesis, melena, or hematochezia, and lead to hemodynamic instability [5]. However, UGIB is often suspected in other nonspecific situations, without any exteriorized bleeding, as many critically ill patients experience progressive decrease in hemoglobin levels and require RBC transfusion during ICU stay. Besides multifactorial «stress» ulceration associated with critical illness, UGIB in ICU can be of multiple origins, such as esophagitis, gastritis, esophageal varices (EV), or any other lesion responsible for UGIB [5–9]. More rarely, the bleeding originates from the lower gastrointestinal tract.
While the management of acute gastrointestinal bleeding in patients presenting to the emergency department is well established with international guidelines [10, 11] there is no consensus regarding the management of suspected UGIB (SUGIB) occurring during an ICU stay. Despite some heterogeneity in local practices and access to endoscopy procedures, bedside esophagogastroduodenoscopy (EGD) is frequently performed in ICU patients [12]. It serves both a diagnostic and potential therapeutic role, with hemostatic procedure such as hemostatic clips, vasoconstrictor injection, or EV ligation if required. However, certain superficial mucosal lesions such as esophagitis, nasogastric tube (NGT)-associated ulcerations, or gastritis usually do not require endoscopic hemostatic treatment. Currently there is no available data on the incidence of hemostatic endotherapy during EGD performed for SUGIB in critically ill patients. On the other hand, performing an EGD on a critical patient is a procedure with potential risks [13–15], and substantial human and material costs [16]. Therefore, in case of high probability of no EGD hemostatic procedure, the EGD benefits must be weighed against its inherent costs and risks.
Our study aimed to first describe the results of EGD performed in the ICU for SUGIB during ICU stay in patients admitted for another reason than acute UGIB, in terms of diagnostic and therapeutic performances. Second, we analyzed predicting factors associated with an EGD hemostasis and proposed a simple stratification score based on clinical items allowing intensivists to predict the probability of therapeutic EGD hemostasis and subsequently help reconsider performing an EGD. The performances of this score were then evaluated in a multicentric validation cohort.
Methods
Patients and data collection
For the derivation cohort, we conducted a retrospective, monocentric study in an 18-bed ICU in a university hospital. Our ICU is a tertiary center for gastrointestinal bleeding (GIB) in Paris, France, and has 24/7 EGD access. Using the administrative hospital database (PMSI), we screened all patients who underwent a bedside EGD in our ICU between January 2015 and October 2021. For the validation cohort, we used data from 3 teaching centers’ ICUs: Besançon and Tenon university hospitals, from January 2015 to December 2022, and Saint-Antoine hospital (same as the derivation cohort) with patients from November 2021 to December 2022. We excluded patients admitted to the ICU for upper or lower GIB, hemorrhagic shock, or in whom EGD was performed for any other reason than suspicion of upper GIB (SUGIB). SUGIB occurring less than 10 days after an endoscopic retrograde cholangiopancreatography (ERCP) or scheduled EGD were also excluded. In the case of multiple EGDs during ICU stay, only the first was analyzed for the score derivation and validation. The flowchart is provided in Additional file 1: Figure S1. Demographic, clinical, biological, and endoscopic data were collected, as well as outcomes (in-ICU and in-hospital mortality and length of stay). Patients received written information that data extracted from their medical charts could be used for research. According to French legislation, the database was anonymized and registered by the CNIL (N°2226507), and the project received approval from the French intensive care society ethical committee (CE SRLF 23-049).
Local practice for stress ulcer prevention and EGD procedure
According to our ICU policy (derivation cohort), no systematic ulcer prevention is given to patients [17, 18]. We use proton-pump inhibitors (PPIs) only in patients with two or more risk factors of «stress» ulcer amongst the following: previous history of ulcer, antiplatelet drugs, curative anticoagulation, or acute kidney injury (AKI) or in case of multiorgan failure. All patients with SUGIB receive PPIs at a dose of 8 mg/h before EGD, and Octreotide (25–50 µg/h) is also given only in patients with known cirrhosis or suspected portal hypertension. An erythromycin infusion (250 mg over 30 min) is performed 30–60 min before endoscopy if no contraindication. According to our local policy, all bedside in-ICU EGDs are performed under general anesthesia and after endotracheal intubation (ETI) if not already intubated for another reason [19–22], by an experienced gastroenterologist.
Statistics
Results are reported as means (± SD) or median (IQR) for continuous variables and as percentages for qualitative variables. To assess associations between patient characteristics and EGD-guided hemostatic procedures, we first performed univariate analyses based on the Mann–Whitney or chi-square test as appropriate. To identify independent predictors of EGD-guided hemostatic procedure, a multivariable logistic regression model included variables with p-values less than 0.05 by univariate analysis. The model's goodness of fit was assessed using the Hosmer–Lemeshow test and the discrimination by the area under the receiver operating characteristic curve (ROC AUC). All tests were two-sided, and p-values less than 0.05 were considered statistically significant. Statistics were performed using R (https://www.R-project.org/) software, and graphical representations using GraphPad Prism 9.00 (GraphPad Software Inc.®). The Transparent Reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD) guidelines were applied [23].
Results
Patient characteristics at baseline and during ICU stay—derivation cohort
We identified 431 ICU patients who underwent bedside EGD during the inclusion period. One hundred seventy-six patients were excluded, primarily because admitted for acute GIB or hemorrhagic shock (n = 133) or other indication than SUGIB (n = 37). Two hundred and fifty-five patients were included in our study (the flowchart is provided in Additional file 1: Figure S1), mainly men (65.9%), with a mean age of 64 ± 15 years. 11.9% had a previous history of ulcers, and 17.6% had known cirrhosis. Before ICU admission, 27.8% were on antiplatelet therapy, 18.5% had curative anticoagulants, and 32.7% had PPIs. The main reason for ICU admission was respiratory failure (35.3%), followed by sepsis/septic shock (22.4%) and neurological disorders (16.5%). The mean SAPS II at admission was 51 ± 19. During ICU stay, 66.7% had sepsis, 75% were mechanically ventilated (disregarding intubation solely for EGD), 56.4% required vasopressors, and 34.1% received renal replacement therapy (RRT) (hemodialysis only). The median ICU length of stay was 12 [6–23] days, and ICU mortality was 27.4%. Table 1 summarizes patient characteristics at baseline and during ICU stay.
Table 1.
Patient characteristics at baseline and during ICU stay—derivation cohort
| Baseline patient characteristics (n = 255) | |
|---|---|
| Age (years, mean ± SD) | 64 ± 15 |
| Male, n (%) | 168 (65.9) |
| Medical history, n (%) | |
| Ulcer | 29 (11.4) |
| Cirrhosis | 45 (17.6) |
| Esophageal varices | 5 (2) |
| Cardiovascular comorbidity | 134 (52.5) |
| CKD | 33 (12.9) |
| Chronic RRT | 7 (2.7) |
| Cancer/hematological malignancies | 81 (31.8) |
| Digestive surgery | 64 (25.1) |
| Diabetes mellitus | 51 (20) |
| COPD/asthma | 31 (12.2) |
| HIV | 6 (2.4) |
| Medication, n (%) | |
| Antiplatelet drugs | 71 (27.8) |
| Anticoagulants | 47 (18.5) |
| NSAIDs | 14 (5.5) |
| Steroids | 32 (12.6) |
| Chemotherapy | 29 (11.4) |
| PPIs | 83 (32.7) |
| ICU admission cause, n (%) | |
| Sepsis/septic shock | 57 (22.4) |
| Cardiac arrest/cardiogenic shock | 13 (5.1) |
| Respiratory | 90 (35.3) |
| Neurologic | 42 (16.5) |
| Metabolic | 26 (10.2) |
| Others | 27 (10.6) |
| Admission SAPSII (mean ± SD) | 51 ± 19 |
| ICU stay characteristics and treatment, n (%) | |
| Sepsis | 170 (66.7) |
| Invasive mechanical ventilation | 191 (74.9) |
| RRT | 87 (34.1) |
| Vasopressors | 144 (56.4) |
| Anticoagulant | 71 (27.8) |
| Steroids | 54 (21.2) |
| Antiplatelets | 52 (20.4) |
| Outcome | |
| ICU LOS (days, mean ± SD) | 17.5 ± 17.6 |
| Hospital LOS (days, mean ± SD) | 35 ± 34.5 |
| In-ICU mortality, n (%) | 70 (27.4) |
| In-hospital mortality, n (%) | 87 (34.1) |
SD standard deviation, CKD chronic kidney disease, RRT renal replacement therapy, COPD chronic obstructive pulmonary disease, NSAID non-steroidal anti-inflammatory drug, PPI proton pump inhibitor, ICU intensive care unit, SAPSII simplified acute physiology score II, LOS length of stay
EGD indication, procedure, and results
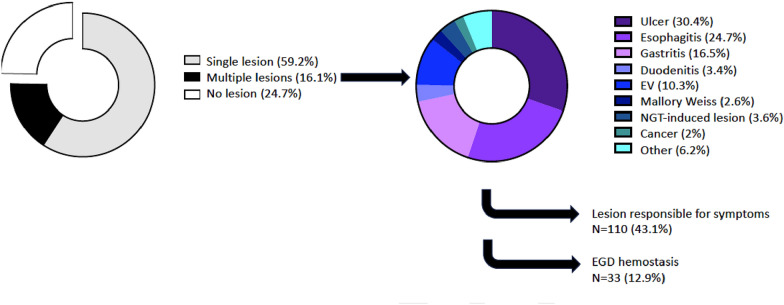
EGD for SUGIB was performed 8.3 ± 13 days after ICU admission. The procedure was primarily motivated by anemia in 79.6% of cases, followed by melena (19.2%), hemodynamic instability (14.5%), and hematemesis/blood in the nasogastric tube (NGT) (13.5%). In 61 patients, ETI was performed specifically for the procedure. Notably, no complication related to airway management was observed in patient requiring ETI. EGD was reported as normal in 24.7% of cases, whereas a single lesion was identified in 59.2% and multiple lesions in 16.1% of cases. The main EGD findings were ulcers (23.1%), esophagitis (18.8%), gastritis (12.5%), and EV (7.8%) (Table 2 and Fig. 1). Ultimately, an endoscopically identified lesion was considered responsible for UGIB in 43.1% of cases. Per-EGD endotherapy was performed in 33 patients (12.9%), using mainly hemostatic clipping (n = 19, 7.5% of patients, 58% of hemostatic procedures), epinephrine local instillation (n = 16, 6.3% of patients, 48% of hemostatic procedures) or EV ligation (n = 14, 5.5% of patients, 42% of hemostatic procedures) (Table 2). Twenty-four (9.4%) patients required a second EGD, 9 (3.5%) had a colonoscopy or rectosigmoidoscopy, 5 (2%) had an arterial embolization and 5 (2%) hemostatic surgery. After EGD, PPI treatment was recommended in 70.2% of cases. During ICU stay, patients received 2.2 ± 3.5 RBC packs, 0.5 ± 2 platelet units/10 kg, and 0.4 ± 1.7 units of fresh frozen plasma (FFP) (Additional file 1: Table S1). Biological parameters on EGD day are reported in Additional file 1: Table S2.
Table 2.
EGD indication, procedure, and results
| EGD (n = 255) | |
|---|---|
| Time from admission (days, mean ± SD) | 8.3 ± 13 |
| EGD indication, n (%)* | |
| Anemia | 203 (79.6) |
| Melena | 49 (19.2) |
| Hemodynamic instability | 37 (14.5) |
| Hematemesis/blood in NGT | 35 (13.7) |
| Hematochezia | 35 (13.7) |
| Intubation solely for EGD, n (%) | 61 (23.9) |
| EGD results, n (%) | |
| Normal | 62 (24.3) |
| Ulcer | 59 (23.1) |
| EV | 20 (7.8) |
| Esophagitis | 48 (18.8) |
| Mallory–Weiss | 5 (2) |
| Gastritis | 32 (12.5) |
| NGT-induced lesion | 7 (2.7) |
| Other | 12 (4.7) |
| Multiple lesions | 41 (16.1) |
| Lesion considered responsible for GI bleeding | 110 (43.1) |
| Hemostatic procedure, n (%)** | 33 (12.9) |
| Hemostatic clip | 19 (58) |
| Epinephrine instillation | 16 (48) |
| EV ligature | 14 (42) |
| Other (Hemospray™/Gold probe™/APC) | 5 (15) |
EGD esophagogastroduodenoscopy, NGT nasogastric tube, EV esophageal varices, EBO endobrachyesophagus, GI gastrointestinal, APC Argon plasma coagulation. *Some patients had EGD for multiple indications. **Some patients received multiple means of hemostasis during EGD
Fig. 1.
EGD findings and distribution. EGD esophagogastroduodenoscopy, EV esophageal varices, NGT nasogastric tube, UGIB upper gastrointestinal bleeding
Factors associated with hemostatic endotherapy procedures
Next, we analyzed the differences between patients with or without EGD-guided hemostatic procedures. In univariate analysis, we observed that patients requiring EGD hemostasis were more often men (81.8% vs. 63.5%, p = 0.04) and more frequently had a history of cirrhosis (36.4% vs. 14.9%, p = 0.006), smoking (66.7% vs. 46%, p = 0.04), and daily alcohol intake (57.6% vs. 37.4%, p = 0.04). Patients who required hemostasis per EGD had more frequent RRT (48.5% vs. 32%, p = 0.008). Regarding EGD indication, hematemesis/blood in NGT (36.4% vs. 10.4%, p < 0.0001), hematochezia (30.3% vs. 11.3%, p = 0.003), and shock (42.4% vs. 10.4%, p < 0.0001), were more frequent in the group with per EGD hemostasis. Conversely, absence of blood exteriorization was three times more frequent in the group without therapeutic hemostasis (21.2% vs. 64%, p < 0.0001). Interestingly, the type of lesion was similar in both groups except for EV (24.2% vs. 5.4%, p = 0.0002). Biological parameters on which the EGD was performed were similar in both groups except for platelet count and prothrombin time, which were significantly lower in patients requiring EGD hemostasis (Additional file 1: Table S2). Ultimately, patients with per-EGD hemostasis received PPI medication, RBC, platelet, and FFP transfusions more often. Nevertheless, both groups had similar ICU mortality and length of stay (Additional file 1: Table S3). In multivariate analysis, history of cirrhosis (OR 3.1; 1.2–8.4, p = 0.02) and hemodynamic instability indicating EGD (OR 4.9; 1.9–13, p = 0.0009) were significantly associated with hemostatic endotherapy. Conversely, no exteriorized bleeding was negatively associated with hemostatic endotherapy (OR 0.25; 0.09–0.66, p = 0.007) (Fig. 2 and Additional file 1: Table S4).
Fig. 2.
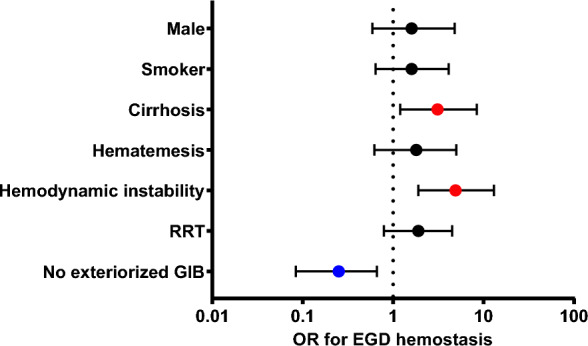
Multivariate analysis: predictors of hemostatic endotherapy. EGD esophagogastroduodenoscopy, OR odds ratio, RRT renal replacement therapy, GIB gastro intestinal bleeding. The dots represent the odds ratio; colored dots are used when the 95% confidence interval for the OR does not include 1. The line through each dot corresponds to the 95% confidence interval. Variables with p < 0.05 entered in the maximal model for multivariate analysis. Goodness of fit (Hosmer–Lemeshow statistic) p = 0.4, Turj r squared = 0.23. Calibration (AUC-ROC) 0.84; p-value < 0.001
Derivation of the SUGIBI score
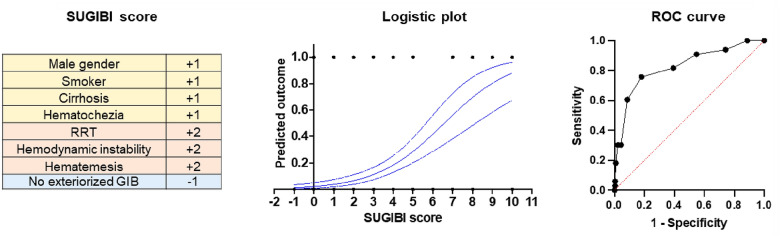
Based on the differences between patients who required EGD hemostasis and those who did not, and after best subsets logistic regression analyses, we build the SUspected GIB in Icu score (Additional file 1: Figure S2). In the SUGIBI score, male gender, smoking, history of cirrhosis and hematochezia were attributed one point. RRT, hemodynamic instability (with no patent alternative etiology) and/or hematemesis indicating EGD were attributed two points. No external bleeding was associated with one negative point. This score had an AUC of 0.81; 0.75–0.91 (p < 0.0001) for predicting the need for an EGD-guided hemostatic procedure. More interestingly, a SUGIBI score < 4 had a negative predictive value for the need for hemostatic endotherapy of 95% (91–99) (Fig. 3). The performance of the Blatchford bleeding score to predict need for endotherapy (AUC: 0.64; 0.53–0.74; p = 0.012) in this ICU context, was significantly lower compared to the SUGIBI score (p < 0.0001) (Additional file 1: Figure S3).
Fig. 3.
The SUGIBI score. The SUGIBI score with a threshold < 4 has a negative predictive value of 95% (0.91 to 0.99). Area under the ROC curve: 00.81; 0.75–0.91 (p < 0.0001). EGD esophagogastroduodenoscopy, RRT renal replacement therapy, GIB gastrointestinal bleeding, ROC receiver operating curve
Performances of the SUGIBI score in the validation cohort
The flowcharts and patient characteristics of the validation cohort are provided in Additional file 1: Figure S4 and Additional file 1: Table S5, respectively. Three hundred and thirty-four patients from 3 centers were used as the SUGIBI score validation cohort. They were mainly men (70.4%) with an age of 62.4 ± 15 years, and were of similar severity compared to the derivation cohort (admission SAPS II of 50 ± 19, ICU mortality of 29.6%). Amongst the 334 EGD performed for SUGIB, 108 (32%) were described as normal, 154 (46.1%) identified a lesion considered responsible for the bleeding, and 32 (9.6%) had a hemostatic endotherapy procedure. Forty-six patients (13.8%) were intubated (or re-intubated) solely for the EGD, with three cardiac arrests occurring during these procedures. In the validation cohort, the SUGIBI score had an AUC of 0.75 (0.66–0.85) (p < 0.0001) for predicting the need for an EGD-guided hemostatic procedure. A score < 4 had a negative predictive value of 95% (92–97).
Discussion
This study reports two main findings. First, we found that EGD performed during ICU stay in critically ill patients for SUGIB identified upper GI lesions in 75% of cases, of which 43.5% were deemed responsible for the bleeding, and ruled out the diagnosis of UGIB in 25% of cases in the derivation cohort, and in 29% of the overall population (derivation and validation cohorts). Therefore, EGD is a valuable diagnostic tool and may help guide PPI or octreotide continuation or withdrawal [24, 25]. Interestingly, critically ill patients with UGIB that occurred during the ICU stay had similar sources of bleeding than patients admitted to the hospital specifically for GIB, as previously reported [5, 26]. However, in the overall population of our study (derivation and validation cohorts), a hemostatic procedure was performed in only 11%, of cases and, therefore, that EGD had no direct therapeutic value in 89% of patients.
The second finding of this study is that patients requiring an EGD-guided hemostatic procedure are significantly different from those who do not. Therefore, we propose a simple score based on clinical criteria, able to identify patients with a low probability of needing EGD hemostasis, with a negative predictive value > 90%. In 2000, Blatchford et al. published the Blatchford score to stratify the probability of need for treatment in patients admitted to the ER for UGIB, and since then other scores have been proposed [27, 28]. Our study reveals that similar items are valid in the ICU SUGIB context, such as hemodynamic instability, exteriorized bleeding, and liver disease (cirrhosis). Nevertheless, some criteria such as tachycardia, decrease in hemoglobin, or increased urea, are ineffective in the context of critically ill patients because of multiple confounding factors related to the high prevalence of multiorgan failure and numerous putative causes of anemia [29] and tachycardia [30], highlighting the interest of a specific score for ICU patients. In addition, our study unveils some unexpected factors associated with the need for an EGD hemostasis, such as male gender and smoking status. This could be explained by the pathophysiology of «stress» ulcers, whose primary driver is mucosal ischemia and is, consequently, more frequent in high cardiovascular-risk patients [28, 31].
Hence, we propose that patients with a SUGIBI score < 4 might have a delayed EGD, assuming PPI treatment until the EGD. Indeed, if we applied the SUGIBI score to our cohort, 459 EGDs without hemostatic intervention could have been avoided, representing a substantial gain in caregiver time and spared costs. Moreover, in our series, general anesthesia and intubations performed solely for the EGD would have been avoided in 74 patients. Although no intubation failure or significant complication was reported in our derivation cohort, it is well established that such airway management procedures in full stomach conditions are at risk of complications [32–34], as illustrated in the validation cohort where three cardiac arrests related to the procedure were reported. In many centers, EGD is not available 24/7, and sometimes such a procedure requires transferring the patients to another hospital. Therefore, the SUGIBI score may help to better stratify the patients and reduce endotherapy-free EGDs performed for SUGIB by reconsidering the need for emergency EGD in case of a score < 4. Conversely, in the case of a SUGIBI score ≥ 4, we believe that EGD must be performed without delay, given the substantial probability of hemostatic endotherapy.
This study has several limitations. First, the monocenter derivation cohort was performed in a high volume/highly experienced GIB center with 24/7 EGD availability. Therefore, our local practice of having broad EGD indications cannot be generalized. Nevertheless, such design was decided on purpose to overcome indication bias. Surprisingly, we observed that in the 2 other centers the percentage of endotherapy was identical to our center, suggesting that despite differences in recruitment and practice, the proportion of patients requiring endotherapy for EGD is consistently around 10%. Second, our local practice is to perform any bedside EGD under general anesthesia, which often leads to (re)intubating the patient. This is an at-risk procedure, with a 10 to 15% complication rate [35–37]. As no complication was reported in our derivation cohort and only 3 in the validation cohort, we suspect an underreporting bias. Moreover, our conclusion on reducing intubation numbers using the SUGIBI score could not be extrapolated to centers not systematically performing intubation for EGD. Last, this score is a dynamic index, as the clinical status of a patient with active GIB is unstable and must be reassessed daily before ruling out the need for a putatively therapeutic EGD.
Conclusion
Our study shows that the therapeutic usefulness of bedside emergency EGD for SUGIB in critically ill patients is limited to a minority of patients. The SUGIBI score could help clinicians stratify the probability of a therapeutic EGD. In case of a SUGIBI score < 4, EGD might be postponed or reconsidered whereas in patients with a SUGIBI score ≥ 4, EGD should be performed without delay.
Supplementary Information
Additional file 1. Supplemental Tables 1-5 and Supplemental figures 1-4.
Acknowledgements
We thank all the gastroenterologist and the endoscopy team for their valuable 24/7 expertise and collaboration, especially Pr. Xavier Dray, Dr. Ulriikka Chaput and Dr. Nicolas Carbonell.
Abbreviations
- AKI
Acute kidney injury
- APC
Argon plasma coagulation
- AUC
Area under the curve
- CKD
Chronic kidney disease
- CNIL
Commission nationale informatique et liberté
- COPD
Chronic obstructive pulmonary disease
- EBO
Endobrachyesophagus
- EGD
Esophagogastroduodenoscopy
- ETI
Endotracheal intubation
- EV
Esophagial varices
- FFP
Fresh frozen plasma
- ICU
Intensive care unit
- IQR
Interquartile range
- LOS
Length of stay.
- NGT
Nasogastric tube
- NSAID
Non-steroidal anti-inflammatory drugs
- PMSI
Programme de médicalisation des systèmes d’information
- PPI
Proton pump inhibitor
- RBC
Red blood cells
- ROC
Receiving operating curve
- RRT
Renal replacement therapy
- SAPSII
Simplified acute physiology score II
- SD
Standard deviation
- SUGIB
Suspected upper gastrointestinal bleeding
- UGE
Upper gastrointestinal endoscopy
- UGIB
Upper gastrointestinal bleeding
Author contributions
All authors met authorship criteria and participated significantly to the study: JJ and VP were involved in conception, design and data collection. JJ wrote the article. Regarding the validation cohort data gathering: TV and GP collected the medical records for the center “Besancon” and AB and MF for the center "Tenon" respectively. TU, MG, HAO, BG, NC and EM provided critical revisions to the manuscript. All authors read and approved the final manuscript.
Funding
No funding.
Availability of data and materials
The full dataset is available from the corresponding author, on request.
Declarations
Ethics approval and consent to participate
The project received approval from the French intensive care society ethical committee (CE SRLF 23-049).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cook DJ, Fuller HD, Guyatt GH, Marshall JC, Leasa D, Hall R, Canadian Critical Care Trials Group et al. Risk factors for gastrointestinal bleeding in critically ill patients. N Engl J Med. 1994;330(6):377–381. doi: 10.1056/NEJM199402103300601. [DOI] [PubMed] [Google Scholar]
- 2.Cook D, Heyland D, Griffith L, Cook R, Marshall J, Pagliarello J, Canadian Critical Care Trials Group Risk factors for clinically important upper gastrointestinal bleeding in patients requiring mechanical ventilation. Crit Care Med. 1999;27(12):2812–2817. doi: 10.1097/00003246-199912000-00034. [DOI] [PubMed] [Google Scholar]
- 3.Kumar S, Ramos C, Garcia-Carrasquillo RJ, Green PH, Lebwohl B. Incidence and risk factors for gastrointestinal bleeding among patients admitted to medical intensive care units. Frontline Gastroenterol. 2017;8(3):167–173. doi: 10.1136/flgastro-2016-100722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krag M, Perner A, Wetterslev J, Wise MP, Borthwick M, Bendel S, et al. Prevalence and outcome of gastrointestinal bleeding and use of acid suppressants in acutely ill adult intensive care patients. Intensive Care Med. 2015;41(5):833–845. doi: 10.1007/s00134-015-3725-1. [DOI] [PubMed] [Google Scholar]
- 5.Lewis JD, Shin EJ, Metz DC. Characterization of gastrointestinal bleeding in severely ill hospitalized patients. Crit Care Med. 2000;28(1):46–50. doi: 10.1097/00003246-200001000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Ovenden C, Plummer MP, Selvanderan S, Donaldson TA, Nguyen NQ, Weinel LM, et al. Occult upper gastrointestinal mucosal abnormalities in critically ill patients. Acta Anaesthesiol Scand. 2017;61(2):216–223. doi: 10.1111/aas.12844. [DOI] [PubMed] [Google Scholar]
- 7.Ashktorab H, Russo T, Oskrochi G, Latella G, Massironi S, Luca M, et al. Clinical and endoscopic outcomes in coronavirus disease-2019 patients with gastrointestinal bleeding. Gastro Hep Adv. 2022;1(4):487–499. doi: 10.1016/j.gastha.2022.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin TA, Wan DW, Hajifathalian K, Tewani S, Shah SL, Mehta A, et al. Gastrointestinal bleeding in patients with coronavirus disease 2019: a matched case–control study. Am J Gastroenterol. 2020;115(10):1609–1616. doi: 10.14309/ajg.0000000000000805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maury E, Tankovic J, Ebel A, Offenstadt G, Parisian Group of the Upper Gastrointestinal Bleeding S An observational study of upper gastrointestinal bleeding in intensive care units: is Helicobacter pylori the culprit? Crit Care Med. 2005;33(7):1513–1518. doi: 10.1097/01.CCM.0000168043.60624.3E. [DOI] [PubMed] [Google Scholar]
- 10.Laine L, Barkun AN, Saltzman JR, Martel M, Leontiadis GI. ACG Clinical guideline: upper gastrointestinal and ulcer bleeding. Am J Gastroenterol. 2021;116(5):899–917. doi: 10.14309/ajg.0000000000001245. [DOI] [PubMed] [Google Scholar]
- 11.Gralnek IM, Stanley AJ, Morris AJ, Camus M, Lau J, Lanas A, et al. Endoscopic diagnosis and management of nonvariceal upper gastrointestinal hemorrhage (NVUGIH): European Society of Gastrointestinal Endoscopy (ESGE) guideline—update 2021. Endoscopy. 2021;53(3):300–332. doi: 10.1055/a-1369-5274. [DOI] [PubMed] [Google Scholar]
- 12.Lee YC, Wang HP, Wu MS, Yang CS, Chang YT, Lin JT. Urgent bedside endoscopy for clinically significant upper gastrointestinal hemorrhage after admission to the intensive care unit. Intensive Care Med. 2003;29(10):1723–1728. doi: 10.1007/s00134-003-1921-x. [DOI] [PubMed] [Google Scholar]
- 13.Blero D, Deviere J. Endoscopic complications–avoidance and management. Nat Rev Gastroenterol Hepatol. 2012;9(3):162–172. doi: 10.1038/nrgastro.2012.3. [DOI] [PubMed] [Google Scholar]
- 14.Rolanda C, Caetano AC, Dinis-Ribeiro M. Emergencies after endoscopic procedures. Best Pract Res Clin Gastroenterol. 2013;27(5):783–798. doi: 10.1016/j.bpg.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Verschoore T, Vandecandelaere S, Vandecandelaere P, Vanderplancke T, Bergs J. Risk factors for complications and mortality related to endoscopic procedures in adults. Acta Gastroenterol Belg. 2016;79(1):39–46. [PubMed] [Google Scholar]
- 16.Bytzer P. Cost-effectiveness of gastroscopy. Ital J Gastroenterol Hepatol. 1999;31(8):749–760. [PubMed] [Google Scholar]
- 17.Selvanderan SP, Summers MJ, Finnis ME, Plummer MP, Ali Abdelhamid Y, Anderson MB, et al. Pantoprazole or placebo for stress ulcer prophylaxis (POP-UP): randomized double-blind exploratory study. Crit Care Med. 2016;44(10):1842–1850. doi: 10.1097/CCM.0000000000001819. [DOI] [PubMed] [Google Scholar]
- 18.Alhazzani W, Guyatt G, Alshahrani M, Deane AM, Marshall JC, Hall R, et al. Withholding pantoprazole for stress ulcer prophylaxis in critically ill patients: a pilot randomized clinical trial and meta-analysis. Crit Care Med. 2017;45(7):1121–1129. doi: 10.1097/CCM.0000000000002461. [DOI] [PubMed] [Google Scholar]
- 19.Smischney NJ, Seisa MO, Kumar M, Deangelis J, Schroeder DR, Diedrich DA. Determinants of endotracheal intubation in critically ill patients undergoing gastrointestinal endoscopy under conscious sedation. J Intensive Care Med. 2019;34(6):480–485. doi: 10.1177/0885066617736256. [DOI] [PubMed] [Google Scholar]
- 20.Rehman A, Iscimen R, Yilmaz M, Khan H, Belsher J, Gomez JF, et al. Prophylactic endotracheal intubation in critically ill patients undergoing endoscopy for upper GI hemorrhage. Gastrointest Endosc. 2009;69(7):e55–e59. doi: 10.1016/j.gie.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaudhuri D, Bishay K, Tandon P, Trivedi V, James PD, Kelly EM, et al. Prophylactic endotracheal intubation in critically ill patients with upper gastrointestinal bleed: a systematic review and meta-analysis. JGH Open. 2020;4(1):22–28. doi: 10.1002/jgh3.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perisetti A, Kopel J, Shredi A, Raghavapuram S, Tharian B, Nugent K. Prophylactic pre-esophagogastroduodenoscopy tracheal intubation in patients with upper gastrointestinal bleeding. Proc (Bayl Univ Med Cent) 2019;32(1):22–25. doi: 10.1080/08998280.2018.1530007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Localio AR, Stack CB. TRIPOD: a new reporting baseline for developing and interpreting prediction models. Ann Intern Med. 2015;162(1):73–74. doi: 10.7326/M14-2423. [DOI] [PubMed] [Google Scholar]
- 24.Jean-Baptiste S, Messika J, Hajage D, Gaudry S, Barbieri J, Duboc H, et al. Clinical impact of upper gastrointestinal endoscopy in critically ill patients with suspected bleeding. Ann Intensive Care. 2018;8(1):75. doi: 10.1186/s13613-018-0423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim JH, Kim JH, Chun J, Lee C, Im JP, Kim JS. Early versus late bedside endoscopy for gastrointestinal bleeding in critically ill patients. Korean J Intern Med. 2018;33(2):304–312. doi: 10.3904/kjim.2016.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forgerini M, Urbano G, Nadai TR, Zapata-Cachafeiro M, Kemp R, Mastroianni PC. Epidemiological profile of patients with non-variceal upper gastrointestinal bleeding secondary to peptic disease in a Tertiary Referral Brazilian Hospital. Arq Gastroenterol. 2021;58(2):202–209. doi: 10.1590/s0004-2803.202100000-36. [DOI] [PubMed] [Google Scholar]
- 27.Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet. 2000;356(9238):1318–1321. doi: 10.1016/S0140-6736(00)02816-6. [DOI] [PubMed] [Google Scholar]
- 28.Witting MD, Magder L, Heins AE, Mattu A, Granja CA, Baumgarten M. ED predictors of upper gastrointestinal tract bleeding in patients without hematemesis. Am J Emerg Med. 2006;24(3):280–285. doi: 10.1016/j.ajem.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Hayden SJ, Albert TJ, Watkins TR, Swenson ER. Anemia in critical illness: insights into etiology, consequences, and management. Am J Respir Crit Care Med. 2012;185(10):1049–1057. doi: 10.1164/rccm.201110-1915CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGinley A, Pearse RM. A national early warning score for acutely ill patients. BMJ. 2012;345:e5310. doi: 10.1136/bmj.e5310. [DOI] [PubMed] [Google Scholar]
- 31.Silen W, Merhav A, Simson JN. The pathophysiology of stress ulcer disease. World J Surg. 1981;5(2):165–174. doi: 10.1007/BF01658279. [DOI] [PubMed] [Google Scholar]
- 32.Higgs A, McGrath BA, Goddard C, Rangasami J, Suntharalingam G, Gale R, et al. Guidelines for the management of tracheal intubation in critically ill adults. Br J Anaesth. 2018;120(2):323–352. doi: 10.1016/j.bja.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 33.Lapinsky SE. Endotracheal intubation in the ICU. Crit Care. 2015;19:258. doi: 10.1186/s13054-015-0964-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Russotto V, Myatra SN, Laffey JG, Tassistro E, Antolini L, Bauer P, et al. Intubation practices and adverse peri-intubation events in critically ill patients from 29 countries. JAMA. 2021;325(12):1164–1172. doi: 10.1001/jama.2021.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lapinsky SE. Endotracheal intubation in the ICU. Crit Care. 2015;19(1):258. doi: 10.1186/s13054-015-0964-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Divatia JV, Khan PU, Myatra SN. Tracheal intubation in the ICU: life saving or life threatening? Indian J Anaesth. 2011;55(5):470–475. doi: 10.4103/0019-5049.89872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simpson GD, Ross MJ, McKeown DW, Ray DC. Tracheal intubation in the critically ill: a multi-centre national study of practice and complications. Br J Anaesth. 2012;108(5):792–799. doi: 10.1093/bja/aer504. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplemental Tables 1-5 and Supplemental figures 1-4.
Data Availability Statement
The full dataset is available from the corresponding author, on request.